Abstract
Background
First trimester abortions especially cervical dilation and suction aspiration are associated with pain, despite various methods of pain control.
Objectives
Compare different methods of pain control during first trimester surgical abortion.
Search methods
We searched multiple electronic databases with the appropriate key words, as well as reference lists of articles, and contacted professionals to seek other trials.
Selection criteria
Randomized controlled trials comparing methods of pain control in first trimester surgical abortion at less than 14 weeks gestational age using electric or manual suction aspiration. Outcomes included intra‐ and postoperative pain, side effects, recovery measures and satisfaction.
Data collection and analysis
Two reviewers independently extracted data. Meta‐analysis results are expressed as weighted mean difference (WMD) or Peto Odds ratio with 95% confidence interval (CI).
Main results
We included forty studies with 5131 participants. Due to heterogeneity we divided studies into 7 groups:
Local anesthesia: Data was insufficient to show a clear benefit of a paracervical block (PCB) compared to no PCB or a PCB with bacteriostatic saline. Pain scores during dilation and aspiration were improved with deep injection (WMD ‐1.64 95% CI ‐3.21 to ‐0.08; WMD 1.00 95% CI 1.09 to 0.91), and with adding a 4% intrauterine lidocaine infusion (WMD ‐2.0 95% CI ‐3.29 to ‐0.71, WMD ‐2.8 95% CI ‐3.95 to ‐1.65 with dilation and aspiration respectively).
PCB with premedication: Ibuprofen and naproxen resulted in small reduction of intra‐ and post‐operative pain.
Analgesia: Diclofenac‐sodium did not reduce pain.
Conscious sedation: The addition of conscious intravenous sedation using diazepam and fentanyl to PCB decreased procedural pain.
General anesthesia (GA): Conscious sedation increased intraoperative but decreased postoperative pain compared to GA (Peto OR 14.77 95% CI 4.91 to 44.38, and Peto OR 7.47 95% CI 2.2 to 25.36 for dilation and aspiration respectively, and WMD 1.00 95% CI 1.77 to 0.23 postoperatively). Inhalation anesthetics are associated with increased blood loss (p<0.001).
GA with premedication: The COX 2 inhibitor etoricoxib, the non‐selective COX inhibitors lornoxicam, diclofenac and ketorolac IM, and the opioid nalbuphine were improved postoperative pain.
Non‐pharmacological intervention: Listening to music decreased procedural pain.
No major complication was observed.
Authors' conclusions
Conscious sedation, GA and some non‐pharmacological interventions decreased procedural and postoperative pain, while being safe and satisfactory to patients. Data on the widely used PCB is inadequate to support its use, and it needs to be further studied to determine any benefit.
Plain language summary
Pain control in first trimester surgical abortion.
Multiple methods of pain control in first trimester surgical abortion at less than 14 weeks gestational age using electric or manual suction aspiration are available, and appear both safe and effective. Pain control methods can be divided in local anesthesia, conscious sedation, general anesthesia and non‐pharmacological methods. Data to support the benefit of the widely used local aneathetic is inadequate. While general anesthesia achieved complete pain control during the procedure, other forms of anesthesia such as conscious sedation with a paracervical block improved postoperative pain control.
Summary of findings
Background
Elective abortions are among the most common outpatient surgical procedures performed on women with an estimated 46 million performed yearly worldwide (WHO 2003). Nearly 90% are performed in the first trimester before 13 weeks gestation (Strauss 2007). A major complication occurs in less than 1 in 100 women and mortality is around 0.7 in 100,000 (Hakim‐Elahi 1990; Bartlett 2004; Koonin 2000). Although the case‐fatality‐rate has decreased since the 1970s, anesthesia‐related events continue to be the leading cause of morbidity (Lawson 1994).
Anesthesia is important for women undergoing an abortion since most will experience pain with the procedure. Key factors that influence the choice of anesthesia or analgesia include effectiveness, safety, side effects, and costs. Other crucial factors include patient preference, practitioner choice or bias, facility resources and medical indications (Maltzer 1999).
Pain perception is a complex phenomenon comprised of both physical and psychosocial elements and their interaction, and varies considerably between women (Stubblefield 1989). The physical pain women experience with abortion most likely originates from the S2 to S4 parasympathetic fibers (the Frankenhäuser plexus) that innervate the cervix and the lower part of the uterine body (Scott 1976; Smith 1991). In addition, the fundus and lower part of uterine body are innervated by sympathetic (Maltzer 1999) fibers from T10 to L1 via the inferior hypogastric nerve, and the ovarian plexus (Maltzer 1999).
Additionally, psychological (affective, motivational, interpretive), and social (context, support) features play into pain perception (Borgotta 1997). Increased pain with abortion has been associated with young age, nulliparity, less education, anxiety, depression, "moral problems" (with the procedure), a retroverted uterus, and dysmenorrhea (Belanger 1989, Glantz 2001). A history of prior vaginal delivery correlates well with decreased pain (Belanger 1989)). Data on the relationship between pain and gestational age, as well as the amount of cervical dilation performed, has been conflicting (Belanger 1989; Borgotta 1997; Smith 1979).
Due to this complex nature, effective management of abortion‐related pain requires a combination of pharmacological and non pharmacological means (Maltzer 1999). Pharmacological methods include local anesthetic, non‐steroidal anti‐inflammatory medications (NSAID) narcotics, anxiolytics, sedatives, and/or hypnotics. Concerns regarding general anesthesia stem from its association with greater costs and personnel and increased morbidity and mortality based on observational data that includes cases until the mid 1980s (Maltzer 1999; Raeder 1992; Grimes 1979; Lawson 1994). Therefore, it is less frequently used in the United States (Lichtenberg 2001).
Non pharmacological aspects of pain have a considerable impact on pain perception (Maltzer 1999). Active participation in one's own pain management, and control over the life situation have been found to be beneficial (Belanger 1989).
Unfortunately, despite these advances, many patients still find surgical abortion extremely uncomfortable; 78‐97% report at least moderate procedural pain (Stubblefield 1989; Belanger 1989; Smith 1979; Rawling 1998). Therefore, optimizing pain control should be a goal in every procedure. Opinions may vary how much pain reduction is clinically relevant. Strategies designed to reduce abortion‐related pain have great public health importance considering the large numbers of women who undergo first trimester surgical abortions. This review will examine the existing randomized controlled trials to compare the effect of different methods of pain control during first trimester surgical abortion on patient perceived pain, satisfaction, side effects, and safety. The review will investigate preemptive as well as intra operative analgesia, focusing on pharmacological methods administered via mucosal (oral, vaginal, intrauterine, buccal/sublingual), intramuscular, or intravenous routes, but also include non‐pharmacological methods.
Objectives
To compare the effect of different methods of pharmacological and non pharmacological pain control administered prior to or during first trimester surgical abortion (< 14 weeks gestation, electric or manual suction aspiration) on patient perceived pain, satisfaction, side effects, and safety.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials, including placebo controlled in any language.
Types of participants
Pregnant women undergoing first trimester surgical abortion at less than 14 weeks gestational age using electric or manual suction aspiration.
Types of interventions
Any type of pharmacological pain control administered via mucosal (oral, vaginal, intrauterine, buccal/sublingual), intramuscular, or intravenous routes or non‐pharmacological pain control prior to or during a first trimester surgical abortion at less than 14 weeks gestational age using electric or manual suction aspiration.
Types of outcome measures
The main outcome is patient reported effectiveness of pain control on perceived pain during and immediately post abortion using validated scales, e.g. visual analogue, CAT, and Likert scales, categorical or dichotomous assessment (yes versus no). Additional outcomes are adverse effects, and side effects (including if the method of pain control causes pain), as well as patient satisfaction.
Search methods for identification of studies
See: Cochrane Fertility Regulation Group search strategy
Electronic searches We searched the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and POPLINE for articles for trials of pain control in first trimester surgical abortion at less than 14 weeks gestational age using electric or manual suction aspiration. Electronic literature search of 'Cochrane Central Register of Controlled Trials (4th quarter, 2007), MEDLINE (1950 to January 2008), EMBASE (1974 to January 2008), and POPLINE (1927 to December 2007) used the following respective search strategies:
The Cochrane Controlled Trials Register: (surgical abortion or abortion or surgical abortion or manual suction aspiration or electric suction aspiration) and (analges* or epidural or lidocaine or fentanyl or NSAID or general anesthesia or narcot* or sedat* or anxiolyt* or pain control or midazolam or diazepam or ibuprofen or NSAID or vicodin or percocet or morphine or propofol or nitrous oxide) and (first trimester)
MEDLINE: (analges* OR epidural OR lidocaine OR fentanyl OR NSAID OR general anesthesia OR narcot* OR sedat* OR anxiolyt* OR pain control OR midazolam OR diazepam OR ibuprofen OR NSAID OR vicodin OR percocet OR morphine OR propofol OR nitrous oxide) AND (surgical abortion OR abortion OR surgical abortion OR manual suction aspiration OR electric suction aspiration) AND first trimester AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR "clinical trial" [tw] OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR "latin square" [tw] OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh])
EMBASE: ((induced abortion! or suction(w)aspiration or surgical(w)abortion) and first(w)trimester) and ((pain(w)control or pain(w)relief) or analgesic agent! or anesthesia! or nonsteroid antiinflammatory agent)
POPLINE: (abortion/first trimester termination) & (analges*/anesthes*/(pain & (control/relief)))
There was no language preference in the application of the search.
Other sources: We contacted professionals in the field to seek other trials, including unpublished or ongoing trials we might have missed with the electronic search. Reference lists of articles retrieved were also searched.
Data collection and analysis
Selection of studies:
The primary reviewer evaluated the articles identified from the literature search described above. An additional reviewer evaluated articles in question for inclusion, and in some cases inclusion was based on additional information received from the study's author. Trials under consideration were evaluated for appropriateness for inclusion and methodological quality, including the study design, randomization method, group allocation concealment, and exclusion after randomization without consideration of their results.
A quality score for concealment of allocation has been assigned to each trial using the criteria described in the Cochrane Handbook:
(A) adequate concealment of the allocation
(B) unclear whether adequate concealment of the allocation
(C) inadequate concealment of allocation
(D) allocation concealment not used
Trials scoring A, B, and C were included in this review. The few studies that scored C were included due to their overall importance to the review.
First trimester abortion was defined as an abortion at < 14 weeks gestation. In order for a study to be included, the procedure had to be either electric or manual suction aspiration as opposed to sharp curettage. If the authors did not state the gestational age, but suction aspiration only was performed, we assumed based on common practice that the gestational age was in the first trimester and therefore, included the study. If the authors specified that they performed terminations in the 1st trimester, but did not state that the procedure was performed by suction aspiration versus sharp curettage, and the study was performed in a time period and region where suction aspiration was the predominant procedure method, we assumed that the study used suction aspiration as well, and therefore, included the study. If a sharp curettage check was preformed after suction aspiration, we included the study and noted this step in the individual included study table. If the authors did not state gestational age and did not specify that suction aspiration was performed, or if the procedure was a sharp curettage only, we did not include the study.
Studies were not included if the authors did not measure pain; the primary outcome of this review. Studies were also not included if the only pain‐related outcome reported was need for postoperative analgesics, but pain itself was not measured.
Data extraction and management
Data extraction was performed independently by two of the reviewers. In case of discrepancies, this was resolved by consensus. Attempts were made to obtain additional information from authors if clarifications were needed. Data was then entered in RevMan 5.
Data in the present review has been based on the analytic method (e.g., intention‐to‐treat, per‐protocol) used in the trial report. The main focus was on procedural‐related pain. Given that randomization usually occurred just prior to the procedure and that follow‐up was only for the immediate postoperative time period, exclusions after randomization and loss‐to‐follow‐up were not a significant problem.
Measures of treatment effect
Data was processed using the RevMan software. Peto odds ratios using a fixed‐effects model with 95% confidence interval (CI) were calculated for all dichotomous outcomes. Weighted mean differences (WMD) using a a random‐effects model with 95% confidence intervals were used for continuous outcomes. The data from 11‐point visual or verbal pain scales was treated as continuous data to allow comparisons to 10 cm scales.
Data synthesis
A few studies reported their results as graphs (Suprapto 1984; Hall 1997; Lindholm 1994; Marc 2007) or medians (Heath 1989; Dahl 2000; Kan 2004; Kan 2006; Wong 2002) only. Thus, we could not extract data for comparisons, and only descriptions of their results were included. In other studies percentages were reported and we calculated the number of patients based on the total number of participants per group (Barneschi 1985; Bonnardot 1987; Raeder 1992). If the mean and the standard error of the mean (SEM) were reported, we calculated the standard deviation (SD) using the formula standard error (SE) x square root of N (Bone 1988; Collins 1985; Hackett 1982). If the mean and the CI were reported we calculated the SD using the formula square root of N x (upper limit ‐ lower limit)/3.92 for 95% CI (Phair 2002). Some studies reported categorical outcomes with 3 groups. If deemed appropriate, the result groups were dichotomized for outcomes including pain, side effects or satisfaction to allow for analysis (Collins 1985; Dahl 2000; Phair 2002; Suprapto 1984; Wong 2002). In case a study compared more than 2 groups, we selected 2 groups at a time for comparison in Revman.
We attempted to contact study authors with missing or unclear data. We found the studies to be included in this review to be extremely heterogeneous and thus, could not perform a single meta‐analysis. However, it was possible to group trials into 7 groups:
Group 1: local anesthesia
Group 2: paracervical block with premedication
Group 3: analgesia per os only
Group 4: conscious sedation
Group 5: general anesthesia
Group 6: general anesthesia with premedication
Group 7: non‐pharmacological interventions
For the purpose of this review, conscious sedation was defined as a drug‐induced depression of consciousness during which patients responded purposefully to verbal commands (spontaneous respiration with no interventions needed to maintain a patent airway). With deep sedation, the ability to independently maintain ventilation may be impaired and patients may require assisted ventilation. General anesthesia was defined as drug‐ induced loss of consciousness. Patients are not arousable, not even by painful stimulus. Frequently patients will require assistance in maintaining an open airway, possibly including positive pressure ventilation. Cardiovascular function may be affected (Steele 2005).
The primary outcome, pain, was assessed at different time points depending on the type of anesthesia used. With local anesthesia, pain was usually assessed during and sometimes after the procedure. In the sedation and general anesthesia groups, pain was assessed postoperatively. Instruments used to assess pain varied; some were dichotomous, others categorical. Some used visual or verbal analogue scales in a continuous way. This further made direct comparison of studies more difficult and increased data heterogeneity.
While many studies used a 11‐point VAS (visual/verbal analog scale), some used a 100mm VAS (Edelman 2004; Edelman 2006; Kan 2004; Kan 2006; Dahl 2000; Li 2003; Liu 2005; Suprapto 1984). In order to facilitate comparability, if possible the 100mm VAS was converted to a 11‐point VAS by dividing the results by 10 (Edelman 2004; Edelman 2006; Li 2003; Liu 2005).
Within groups we ordered studies by anesthesia technique, substance and within these subgroups by no intervention/placebo versus intervention. In most instances we chose to create subcategories within an outcome for doses and route of administration rather than different times of outcome assessment. Side effects were listed as subcategories when deemed appropriate for better overview.
Co‐interventions were heterogeneous as well, and participants were not randomized to them. They are briefly summarized in the results section and the most important are presented in the Summary of findings table 1; Table 2; Table 3. An individual and detailed description can be found in the included study tables. They may have affected the results and may have even introduced bias. However, due to their heterogeneity we did not include them in our data analysis
Summary of findings 2. General anesthesia.
| Study | Fentanyl$ | Alfentanil $ | Midazolam/diazepam/lorazepam (benzodiazepine)* | Propofol* | Ketamine* | Methohexital (barbiturate)* | Thiopental (barbiturate)* | Etomidate# | Halothane # | Enflurane# | Trichlorethylene# | N2O2 |
| Barneschi | x | x | x | x | x | x | c | |||||
| Bonnardot | x | c | x | x | ||||||||
| Boysen 1989 | c | x | x | x | ||||||||
| Boysen 1990 | c | x | x | |||||||||
| Collins | x | c | x | c | ||||||||
| Hackett | x | c | x | c | ||||||||
| Hall | x | c | ||||||||||
| Jakobsson 1991 | x | X (vs fent vs placebo) | c | x | c | |||||||
| Jakobsson 1993 | x | x | x | x | x | |||||||
| Jakobsson 1995 | x | x | x | x | c | |||||||
| Lindholm | x | X vs fent vs NS | c | |||||||||
| Ogg | x | c | X | x | ||||||||
| Raeder | x | x | x | x | ||||||||
| Rossi | x | x | x | x | c |
Opiates$ , Sedative hypnotic agents*, Inhalational anesthetic#
x= control or intervention, c= co‐treatment
Summary of findings 3. General anesthesia with premedication.
| Study | Fentanyl$ | Alfentanil$ | Midazolam (benzodiazepine)* | Propofol* | Thiopental (barbiturate)* | Desflurane# | Enflurane# | N2O2# | Opioid | Non‐selective COX | Selective COX |
| Bone | x | c | c | c | Nalbuphine vs fentanyl | ||||||
| Dahl | c | c | c | c |
Paracetamol with codeine | Paracetamol (COX 3) vs placebo | |||||
| Heath | c | c | c | dihydrocodeine vs placebo | |||||||
| Hein 1999 | c | c | c | Paracetamol vs placebo | |||||||
| Hein 2001 | c | c | c | Lornoxicam | Paracetamol | ||||||
| Jakobsson 1996 | c | c | c | Sodium‐diclofenac vs ketorolac vs potassium‐diclofenac vs NaCl | |||||||
| Liu | c | c | c | Etoricoxib vs placebo (COX 2) |
Opiates$, Sedative hypnotic agents*, Inhalational anesthetic#
x= control or intervention, c= co‐treatment
Results
Description of studies
Results of the search
The searches resulted in 49 articles in the Cochrane Central Register of Controlled Trials, 217 in MEDLINE, 92 EMBASE, and 2367 in POPLINE.
Included studies
Forty studies met inclusion criteria with a total of 5131 participants. Please see table for details. Based on type of pain control they were divided in the following groups:
Group 1: local anesthetics, local anesthesia technique, premedication and paracervical block (PCB)
Ten studies with 1527 participants investigating local anesthesia met inclusion criteria.
Several studies compared local anesthetics. Kan et al compared PCB with 1% lignocaine with no PCB (Kan 2004). Glantz et al compared 14ml of 1% chloroprocaine with bacteriostatic saline (Glantz 2001). Wiebe et al 1992 compared 10ml 2% carbonated lidocaine and 2mg atropine /50ml with 2% plain lidocaine and 2mg atropine/50ml (Wiebe 1992). In a study in 1995 Wiebe et al added a third arm with 20ml 0.25% bupivacaine, which was compared to 20ml 1% buffered and plain lidocaine (Wiebe 1995). In 1996 she compared 20ml 1% with 0.5% lidocaine (Wiebe 1996).
Some studies compared different techniques of PCBs, such as deep versus regular injection of 1% lidocaine (3cm versus 1.5cm and a total of 16 versus 10ml) (Cetin 1997) and lidocaine with epinephrine (20ml 1% lidocaine 1‐1.5 inch versus 10ml 2% lidocaine 0.5 inch; atropine 2mg/50ml added to lidocaine) (Wiebe 1992). Others studied injection at different sites of the paracervical area (3, 5, 7 and 9 o'clock versus 4 and 8 o'clock) with 14ml of either 1% chloroprocaine or bacteriostatic (0.9% benzyl alcohol) saline (Glantz 2001), or injection of 10ml 1% lignocaine at the vaginal vault versus the cervix (Kan 2004). Of note, even though some paracervical blocks included injecting local anesthesia in the anterior or posterior lip of the cervix, in all other studies the main portion of the local anesthestic was injected at the vaginal vault around the cervix. Phair et al studied the effect of no waiting versus waiting 3‐5 minutes between the injection of 12ml 1% buffered lidocaine and dilation (Phair 2002). Wiebe et al had a two step study; the first step was about waiting time, but randomization was not adequate as it was by day of the procedure (Wiebe 1995). The second step of this study investigated the influence of a slow versus fast injection.
Edelman at al studied the effect of intrauterine lidocaine 10ml of 1% and 5ml of 4 % versus placebo given in addition to the PCB with 10ml of 1% lidocaine in 2 different studies (Edelman 2004; Edelman 2006). Li et al compared the topical application to the cervix (directly and via Hegar dilators) of 10ml 2% lignocaine jelly with KY jelly (Li 2006).
Group 2: PCB with premedication
Three studies with 434 participants investigated the effect of premedication, such as ibuprofen 600mg per os (Wiebe 1995), lorazepam 1mg per os (Wiebe 2003) or naproxen sodium 550mg per os (Suprapto 1984) followed by a PCB with 1% lidocaine (20ml in Wiebe 1995).
Since patients are awake during the procedure under these different local anesthetic techniques outcomes included pain with dilation, aspiration and post‐procedure. Some studies also measured pain with the paracervical/cervical block application (Glantz 2001; Kan 2004).
The predominant study instruments used to measure pain were visual and verbal analogue scales; some 11‐point, some 100mm. Additional outcomes were anxiety, satisfaction, sedation, side effects, difficulty of the procedure, and varied between the studies.
Group 3: analgesia alone
One study with 100 participants investigated diclofenac sodium 50mg combined with 200mcg misoprostol versus misoprostol alone (Li 2003).
Group 4: conscious sedation
Three studies with 274 participants investigated conscious sedation. Kan et al compared entonox (50:50 mixture of nitrous oxide in oxygen) with air after administering 2mg midazolam (another 1mg if sedation inadequate) and 25mcg fentanyl IV. In this study, patients did not receive a PCB (Kan 2006). Wong on the other hand compared conscious sedation with midazolam 2mg and fentanyl 25mcg IV with placebo after administering a PCB to all participants (Wong 2002). Wells et al compared local cervical block alone with a PCB combined with intravenous sedation with diazepam and fentanyl in 2 of their four arms (Wells 1992).
Group 5: general anesthesia (Table 2)
Fourteen studies with 1812 participants investigated general anesthesia. One of them compared general anesthesia using propofol and alfentanil with conscious sedation using midazolam, alfentanil and PCB with 20ml mepivacaine (Raeder 1992). Hall compared GA using propofol with GA and PCB combined. General anesthesia studies used either fentanyl or alfentanil as opiates for pain control. Four studies investigated inhalational anesthetics, specifically halothane (Barneschi 1985; Collins 1985), enflurane (Hackett 1982) and trichloethylene (Ogg 1983) and compared them to various sedative/hypnotic agents. All studies included at least one sedative/hypnotic agent. Ten studies included propofol (Bonnardot 1987; Boysen 1989; Boysen 1990; Hall 1997; Jakobsson 1991; Jakobsson 1993; Jakobsson 1995; Lindholm 1994; Raeder 1992; Rossi 1995), 9 studies included a barbiturate (5 methohexital (Boysen 1990; Collins 1985; Hackett 1982; Jakobsson 1993; Ogg 1983), 5 thiopental (Barneschi 1985; Boysen 1989; Jakobsson 1991; Jakobsson 1993; Jakobsson 1995), 4 ketamine (Barneschi 1985; Bonnardot 1987; Jakobsson 1993; Rossi 1995), 3 benzodiazepine midazolam (Bonnardot 1987; Raeder 1992; Rossi 1995) and 1 etomidate (Boysen 1989).
In studies with general anesthesia, pain was usually assessed postoperatively, either as a dichotomous or categorical variable. Other outcomes included typical side effects such as pain with injection, nausea, vomiting, and apnea, various tests of recovery and time until discharge.
Group 6: general anesthesia with premedication (Table 3)
Seven studies with 770 participants investigated the influence of premedication with various analgesics on postoperative pain after general anesthesia. Most studies included a cyclooxygenase inhibitor (COX); COX 3 ‐ paracetamol (Dahl 2000; Hein 1999; Hein 2001), COX 2 etoricoxib (Liu 2005), non‐selective COX inhibitor ketoroloac (Jakobsson 1996), sodium‐ and potassium‐diclofenac (Jakobsson 1996) and lornoxicam (Hein 2001). Other studies investigated opioids such as nalbuphine (Bone 1988), dihydrocodeine (Heath 1989), paracetamol with codeine (Dahl 2000). In 5 out of 7 studies, general anesthesia was achieved with propofol (Dahl 2000; Heath 1989; Hein 1999; Hein 2001; Liu 2005), in one of them enflurane (Bone 1988) and in another desflurane (Liu 2005) was added. Thiopental was used in the 2 other studies (Bone 1988; Jakobsson 1996). All but one (Liu 2005) included either fentanyl (Bone 1988; Hein 1999; Hein 2001) or alfentanil (Dahl 2000; Heath 1989; Jakobsson 1996) for anesthesia.
Group 7: non‐pharmacological intervention
Four very different studies with 214 participants investigated non‐pharmacological interventions. In a recent study, the effect of hypnosis was investigated compared to standard care in patients who all received a PCB (Marc 2007). Shapiro et al compared 3 groups; one control and two treatment arms with self‐administered methoxyflurane (0.5 volume % with 5l oxygen per minute) and stereophonic headphones with music chosen by patient (Shapiro 1975). A further study compared provision of sensory information (3 minute audio taped message containing orienting information as well as nine sensations related to abortion, and identified by over 50% of women in a previous pilot study) with provision of general information. They also compared PCB versus PCB plus intravenous sedation with diazepam and fentanyl (Wells 1992). Wells et al 1989 compared 4 groups: Relaxation exercise for 10 minutes prior to the procedure versus pleasant imagery (beach or mountain), 7 minute practice session prior to the procedure versus analgesic imagery, 8 minute practice session prior to the procedure versus attention control no instruction in a technique but advise to use coping strategy that worked in a previous painful experience and 10‐15 minutes prior to the procedure (Wells 1989). All participants received a PCB.
Within the different anesthetic groups medications, doses, technique and route of administration and timing varied, as did at what time and with which study instrument pain was assessed. This minimized the option for meta‐analysis.
Co‐interventions were either given to all participants, i.e. if a certain preoperative anxiety score was measured or were optional per patient request. They included cervical ripening with laminaria or misoprostol (not optional), premedication/anesthesia induction with either an anxiolytic, benzodiazepine or opiate. The most important ones are included in summary of findings table 1 to 3. They were too heterogeneous to be listed in a meaningful way here in the text, but they are described in detail in the included study tables.
Studies were conducted in Europe as well as in North America. Many were hospital based; others were conducted in freestanding abortion clinics, especially in North America. In several studies a sharp curettage check was performed after suction evacuation (Jakobsson 1991; Jakobsson 1995; Cetin 1997; Glantz 2001; Miller 1996a; Marc 2007; Wiebe 1995). Included studies had been published in English, French and Italian.
Excluded studies
Twenty‐nine studies were excluded. Please see tables for details.
Risk of bias in included studies
Information regarding randomization and allocation concealment obtained from the publications and written correspondence with the authors proved these two areas to be adequate in most of the included studies.
Allocation concealment was adequate in 23 included studies and unclear in 14 studies. Three studies had inadequate allocation concealment. They had a research assistant draw up the syringes, or used an envelope but did not designate it as opaque.
Randomization was described in 25 of the studies; most often computer randomization. In 16 studies, the authors stated that they did randomize, but not how this was performed. The lack of information on randomization and allocation concealment likely derives from the fact that many of these publications were from the 1970s, 1980s and early 1990s.
Blinding: Patients were blinded in many studies. The surgeons and anesthesiologists could not always be blinded due to the individual study designs,. which may have introduced bias. However, assessors of postoperative outcomes were usually, blinded.
Follow‐up and exclusions: Due to the short follow‐up period until discharge after surgery, loss to follow‐up did not occur. In several studies patients were excluded after inappropriate inclusion; more often data collection was incomplete. This made true intention to treat analysis more difficult.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Local anesthesia.
| Local anasthetisia | WMD | ||||||
| Treatment | Control | Notes, co‐treatments | Dilation | Aspiration | Post‐op | Satisfaction | |
| LOCAL ANESTHETICS | |||||||
| Paracervical block (PCB) v placebo/no treatment | |||||||
| Glantz 2001 | Chloroprocaine 1% | Bacteriostatic saline (0.9% benzyl alcohol) | 14 ml, 2 sites (4, 8) | ‐0.5 (pain with PCB) ns | ‐1.5 ns | ‐1.9 | |
| Glantz 2001 | Chloroprocaine 1% | Bacteriostatic saline (0.9% benzyl alcohol) | 14 ml, 4 sites (3, 5, 7, 9), | ‐1.3 (pain with PCB) | ‐1.7 | ‐1.3 ns | |
| Kan 2004 | Lignocaine 1% | No treatment | 10 ml, 2 sites (4, 8), 2.5 cm deep, Co‐treatment: conscious sedation Small trial Also other active arm. Only medians reported. |
ns | ns | ns | |
| Various local anesthetics | |||||||
| Wiebe 1992 | Carbonated lidocaine 2% | Plain lidocaine 2% | 10 ml, with 2mg atropin/50ml, No delay, 3 to 6 sites (12, 3, 6 or 12, 2, 4, 6, 8, 10 o'clock) ½ in. deep, no waiting. All participants: premedication with 1mg lorazepam sublingual 30 minutes prior to procedure per patient request. |
‐0.8 | ‐0.4 | ||
| Wiebe 1995 | Carbonated lidocaine 1% | Plain lidocaine 1% | 20ml, (10ml injected in 4 to 6 sites around the cervix and 5ml each between 3 and 4 o'clock and between 8 and 9 o'clock, 1 inch deep, no waiting. All participants: premedication with lorazepam 0.5‐1mg SL per patient request 30 minutes prior to procedure |
‐0.96 ns | ‐0.05 ns | ||
| Wiebe 1996 | Lidocaine 0.5% | Lidocaine 1% | 20 ml. Some patients received preoperative laminaria, lorazepam or ibuprofen. |
0.2 ns | |||
| Wiebe 1995 | Lidocaine 1% | Bupivacaine 0.25% | 20 ml, as in other groups | ‐0.24 ns | |||
| Local anesthesia technique | |||||||
| Depth of paracervical block | |||||||
| Cetin 1997 | Deep injection (1ml superficially and 3ml 3cm deep at 4, 6, 8, and 10 o'clock position; total of 16ml) | Regular injection (1.5cm deep at same 4 positions) | 16ml 1% lidocaine. All participants: 5mg oral diazepam 60 minutes prior to procedure if preprocedural anxiety of 6 or more (rated by physician not performing procedure). After 2 minute wait, cervical dilation. Vacuum aspiration followed by sharp curette. |
‐0.8 | ‐0.9 | ||
| Wiebe 1992 |
Superficially to blanch the mucous membrane: 1ml injected at 6 sites (12, 2, 4, 6, 8 and 10 o'clock). Then 3‐4ml injected 1 to 1.5 inches deep at 4 sites (4, 6, 8, and 10 o'clock). Total of 20ml 1% plain lidocaine with 1mg atropin/50ml. |
½ inch deep at the reflection of the vagina off the cervix. 3 to 6 sites (12, 3, 6 or 12, 2, 4, 6, 8, 10 o'clock). 10ml 2% plain lidocaine with 2mg atropin/50ml. |
No delay All participants: premedication with 1mg lorazepam sublingual 30 minutes prior to procedure per patient request. |
‐2.4 | ‐1.0 | ||
| Paracervical block 4 sites v 2 sites | |||||||
| Glantz 2001 | 4 sites bacteriostatic saline (3, 5, 7, 9) | 2 sites bacteriostatic saline (4, 8) | 14ml, Also chloroprocaine in 2 groups | 0.8 (pain with PCB) ns | 0.1 ns | ‐0.5 ns | |
| Glantz 2001 | 4 sites 1% chloroprocaine (3, 5, 7, 9) | 2 sites 1% chloroprocaine (4, 8) | 14ml, Also saline placebo in 2 groups | 0 (pain with PCB) ns | ‐0.1 ns | 0.1 ns | |
| Waiting v no waiting paracervical block | |||||||
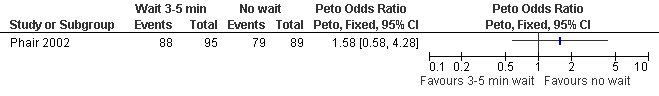
| Phair 2002 | Waiting 3‐5 mins | No waiting | 12ml 1% buffered lidocaine at 12 (superficially, cervix), 4 and 8 o'clock (1‐2cm deep, paracervical). Co‐treatment: fentanyl IV and or diazepam per patient request |
‐0.7 | ‐0.2 ns | ‐0.1 ns | 1.58 ns |
| Slow v fast injection paracervical block | |||||||
| Wiebe 1995 | Fast 30 secs | Slow 60 secs | Lidocaine 1%, 20 ml, no waiting Factorial design Outcome: pain with injection |
0.62 (pain with PCB) ns | |||
| Intrauterine infusion | |||||||
| Edelman 2004 | Lidocaine 10ml, 1% | Saline placebo 10ml | All participants: premedication with 800mg ibuprofen, and if requested, 5mg diazepam. Paracervical block with 10ml of 1% lidocaine (1ml 1% nonbuffered lidocaine on the anterior and posterior lip of the cerivx and then 4.5ml of 1% lidocaine paracervical at the 4‐ and 8‐o'clock postitions). 100mm VAS |
‐0.3 ns | ‐0.4 ns | 0.7 ns | ‐0.1 ns |
| Edelman 2006 | Lidocaine 5 ml, 4% | Saline placebo 5ml | Co treatment: ibuprofen 800 mg, cervical lidocaine 1% 10 ml, 4 sites, diazepam mg if requested 100mm VAS |
‐2 | ‐2.8 | ‐0.5 ns | 0.5 ns |
| Topical | |||||||
| Li 2006 | Lignocaine yelly 2% 3 ml ?applied to cervix?, to dilator and speculum |
Placebo gel | Co‐treatment: All subjects: cervical priming with 400micrg misoprostol prior to the procedure (1‐2 hours in multiparous, 3‐5 hours in nulliparous subjects). Premedication with 5mg diazepam po and 1mg/kg pethidine IM 15‐30 minutes prior to the procedure. Rescue pain medication with pethidine repeat dose IM. | ‐0.42 ns | ‐0.87 | ‐0.51 marginal sig | |
| Cervical block | |||||||
| Kan 2004 | Cervical, 2 sites (4, 8), 2.5 cm deep, Lignocaine 1%, 10ml | No treatment | 10ml, Co‐treatment: All patients: 400mcg misoprostol vaginally for cervical priming 3‐6hrs prior to the procedure. Conscious sedation with 2mg midazolam and 25mcg fentanyl IV 5 minutes prior to cervical dilation. Pethidine IM as needed for additional analgesia. Small trial Also other active arm. Only medians reported. |
ns | ns | ns | |
| Cervical v paracervical | |||||||
| Kan 2004 | Cervical 2.5 cm deep | Paracervical, 2.5 cm deep | Lidocaine 1%, 10ml , 2 sites (4, 8) Co‐treatment: conscious sedation (details see other arm) Small trial Also no treatment arm. Only medians reported. |
ns | ns | ns | |
Summary of findings 4. Quality of evidence.
| Author (year) | Randomization | Randomization unclear | Allocation concealment |
Allocation concealment unclear |
Allocation concealment Inadequate |
Blinding |
| Barneschi | computer | 1 envelope | Participants and outcome assessor= double blind | |||
| Bone | 1 | 1 | Double blind | |||
| Bonnardot (1987) | Table of numbers | 1 Opaque envelopes | Outcome assessor | |||
| Boysen (1989) | 1 | 1 | ||||
| Boysen (1990) | 1 | 1 | Outcome assessor | |||
| Cetin | Computer | 1 | Unclear | |||
| Collins | 1 | 1 | Unclear | |||
| Dahl | Random number list | 1 | Double blind | |||
| Edelman 2004 | Computer | 1 identical study syringes | Double blind | |||
| Edelman 2006 | computer | 1 identical study syringes | Double blind | |||
| Glantz | Permuted block technique | 1opaque sealed envelopes | ||||
| Hackett | 1 | 1 | Outcome assessor | |||
| Hall, G | 1 | 1 closed envelopes | Investigators blinded | |||
| Heath | 1 | 1 | Double blind | |||
| Hein 1999 | computer | 1 nurse drew envelope | Double blind | |||
| Hein 2001 | 1 | 1 envelope | Double blind | |||
| Jakobsson 1991 | computer | 1 envelope | Patient and outcome assessor = double blind | |||
| Jakobsson 1993 | computer | 1 envelope | Patient and outcome assessor = double blind | |||
| Jakobsson 1995 | computer | 1 envelope | Patient and outcome assessor = double blind | |||
| Jakobsson 1996 | computer | 1 sealed envelope | Double blind | |||
| Kan 2004 | computer | 1 opaque envelope | Double blind | |||
| Kan 2006 | computer | 1 sealed envelope | Double blind | |||
| Li 2003 | computer | 1 sealed envelope | Double blind | |||
| Li 2006 | computer | 1 sealed envelope | Double blind | |||
| Lindholm | 1 | 1 (identical looking ampoules delivered by pharmacy) | Anesthesiologist | |||
| Liu | 1 | 1 sealed opaque envelopes | Double blind | |||
| Marc | computer | 1 sealed opaque envelopes | ||||
| Ogg | 1 | 1 | Outcome assessor | |||
| Phair | computer | 1 opaque envelopes | NO BLINDING POSSIBLE | |||
| Raeder | computer | 1 sealed envelopes | Double blind | |||
| Rossi | 1 | 1 | Unclear | |||
| Shapiro | 1 | 1 | Unclear | |||
| Suprapto | 1 | 1 | Double blind | |||
| Wells 1989 | 1 | 1 | nurses, counselors, physicians and technicians | |||
| Wells 1992 | computer | 1 envelopes | Outcome assessor | |||
| Wiebe 1992 | computer | 1 The nurse drew up the syringes | Some phases double blind | |||
| Wiebe 1995 | computer | 1 opaque envelopes | Double blind for some phases | |||
| Wiebe 1996 | 1 | 1 assistant drew up the syringes | Double blind | |||
| Wiebe 2003 | computer | 1 opaque envelopes | Double blind | |||
| Wong 2002 | computer | 1 opaque envelopes | Double blind | |||
| TOTAL | 2 4 | 16 | 23 | 14 | 3 |
Forty trials with a total of 5131 participants were included in this review. Due to the variety of interventions, trials were grouped as listed below. Secondary to heterogeneity, most of them could not be combined for meta‐analysis. In studies involving general anesthesia, pain could only be assessed postoperatively. Data on side effects were combined for some comparisons. Major complications with any of the methods were rarely mentioned and if so, they were included in the table of included studies. Data on additional outcomes such as time until discharge or satisfaction was reported, if measured. Due to study heterogeneity, we decided not to report individual results on the multitude of tests used to assess for recovery. Some data regarding general anesthetics was only of historical interest, and therefore not all results were reported. This includes various tests of recovery and some of the side effects.
Wiebe 1992 and Wells 1992 had 2 study steps each and Wiebe 1995 had 4 study steps/phases. Some of these belonged to different anesthesia groupings, thus the sum of all studies listed below exceeds 41 included trials.
Group 1: local anesthesia technique, local anesthetics, premedication and PCB
We included 12 studies with 1961 participants investigating local anesthesia with or without premedication.
Local anesthetics (Comparison 1)
Bacteriostatic saline PCB was compared with local anesthetics in one study (Glantz 2001;Figure 1, Figure 2, Figure 3). Glantz et al found better pain control during injection of the paracervical block (WMD ‐0.90 95% CI ‐1.78 to ‐0.02), during aspiration (WMD ‐1.50 95% CI ‐2.45 to ‐0.55) and postoperative (WMD ‐1.5 95% CI ‐2.54 to ‐0.46, N = 79) pain control with 1% chloroprocaine injected at 2 or 4 sites compared to bacteriostati saline. In the subanalysis for each site PCB and aspiration were only less painful with a 4 site injection while postoperative pain was only less after a 2 site injection. Pain with dilation was not studied. Kan et al did not observe a difference in pain with either dilation, aspiration or postoperatively when comparing PCB using lignocaine with no injection in patients with conscious sedation (Kan 2004) (only medians reported, N = 135).
1.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.1 Pain with paracervical block or dilation comparing local anesthetics with bacteriostatic normal saline.
2.
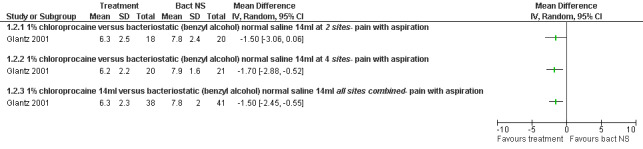
Forest plot of comparison: 2 Local anesthetics, outcome: 2.2 Pain with aspiration comparing local anesthetics with bacteriostatic normal saline.
3.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.3 Pain postpoperatively comparing local anesthetics with bacteriostatic normal saline.
A PCB with buffered 2% lidocaine was more effective at controlling pain with cervical dilation and at the end of the procedure than plain 2% lidocaine (WMD ‐0.80 95% CI ‐0.89 to ‐0.71, WMD ‐0.40 95% CI ‐0.49 to ‐0.31, N = 167) (Wiebe 1992, Figure 4, Figure 5). Buffered 1% lidocaine improved pain with aspiration compared to plain 1% lidocaine (WMD ‐0.96 95% CI ‐1.67 to ‐0.25, N = 124), but not postoperative pain (Wiebe 1995, Figure 6). Pain control with aspiration did not differ when comparing lidocaine 0.5% with 1%, or 1% lidocaine with 0.25% bupivacaine (Wiebe 1996; Wiebe 1995, Figure 7, Figure 8).
4.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.4 Pain with dilation comparing 2% buffered lidocaine with 2% plain lidocaine.
5.
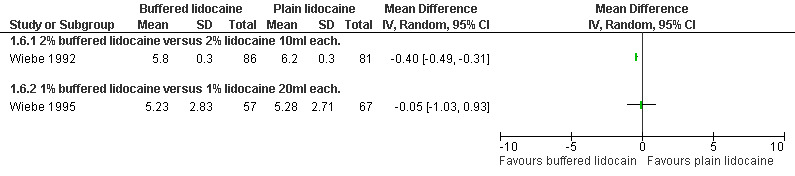
Forest plot of comparison: 2 Local anesthetics, outcome: 2.6 Pain at end of procedure comparing buffered lidocaine with plain lidocaine.
6.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.5 Pain with aspiration comparing 1% buffered lidocaine with 1% plain lidocaine 20ml each.
7.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.7 Pain with aspiration comparing 0.5% lidocaine with 1% lidocaine 20ml each.
8.

Forest plot of comparison: 2 Local anesthetics, outcome: 2.8 Pain with aspiration comparing 1% lidocaine with 0.25% bupivacaine 20ml each.
Local anesthesia technique (Comparison 2)
Deep injection achieved better pain control than regular injection for cervical dilation and aspiration when combining the results of 2 studies with a total of 113 patients (WMD ‐1.64 95% CI ‐3.21 to ‐0.08 and WMD 1.00 95% CI 1.09 to 0.91) (Cetin 1997; Wiebe 1992, Figure 9; Figure 10).
9.
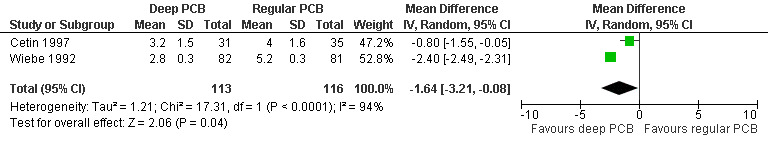
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.1 Pain with dilation comparing a deep paracervical block with a regular injection technique.
10.
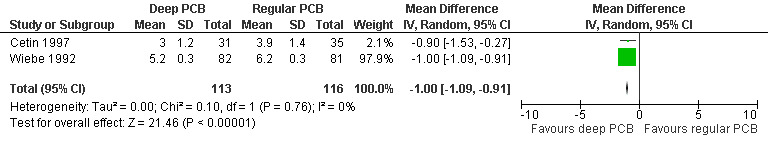
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.2 Pain with aspiration comparing a deep paracervical block with a regular injection technique.
Pain with PCB injection, aspiration and postoperatively did not differ when comparing a 4 site (3‐5‐7‐9 o’clock) with a 2 site (4‐8 o’clock) injection (Glantz 2001, Figure 11; Figure 12; Figure 13). Similarly it did not differ when comparing a cervical block with lignocaine injected at 4 and 8 o’clock into the cervix versus the vaginal vault in patients with conscious sedation (Kan 2004). Kan et al only reported medians and thus the actual data could not be abstracted.
11.
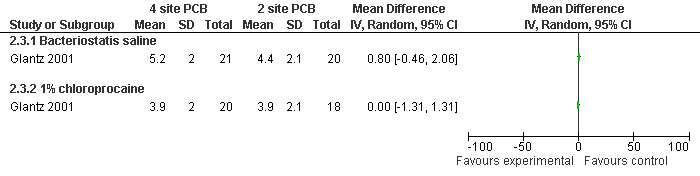
Forest plot of comparison: 2 Local anesthesia technique, outcome: 2.3 Pain with PCB placement comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB.
12.
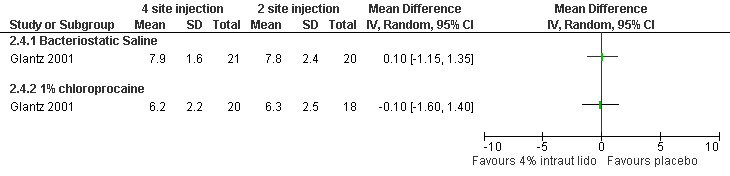
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.3 Pain with aspiration comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB of 1% chloroprocaine.
13.
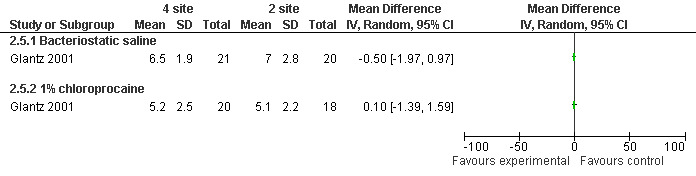
Forest plot of comparison: 2 Local anesthesia technique, outcome: 2.5 Pain postoperatively comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB.
Waiting 3 minutes between PCB and dilation improved pain with cervical dilation (WMD ‐0.7 95% CI ‐1.37 to ‐0.03, N = 194), but not with aspiration or postoperative pain (Phair 2002, Figure 14, Figure 15; Figure 16). Of note in the original article no significant results were described. Since only confidence intervals were reported, we calculated standard deviation as described in the data synthesis section, and obtained these results.
14.
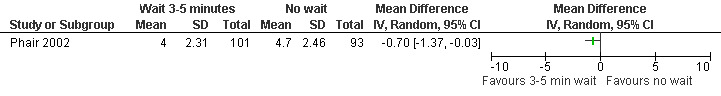
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.4 Pain with dilation comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
15.
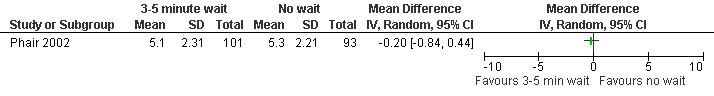
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.5 Pain with aspiration comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
16.

Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.6 Pain postpoperatively comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
Slow versus fast injection did not alter the pain experience with PCB injection (Wiebe 1995; Figure 17).
17.

Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.7 Pain with injection comparing fast injection with slow injection of buffered lidocaine.
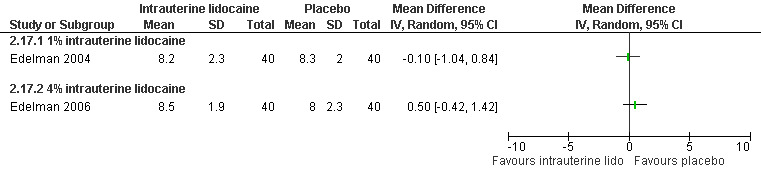
A 1 % intrauterine lidocaine infusion plus PCB was not more effective in controlling pain with cervical dilation or aspiration as compared to PCB with intrauterine placebo, but a 4% intrauterine lidocaine infusion plus PCB was (WMD ‐2.0 95% CI ‐3.29 to ‐0.71, WMD ‐2.8 95% CI ‐3.95 to ‐1.65, N = 80 each study). In addition, postoperative pain (30 minutes) was less after a 4% intrauterine lidocaine infusion, but results were not statistically significant (Edelman 2004; Edelman 2006, Figure 18; Figure 19; Figure 20).
18.
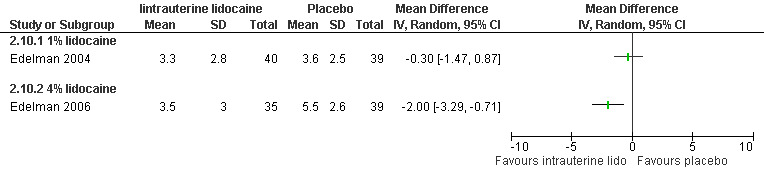
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.8 Pain with dilation comparing intrauterine lidocaine with placebo.
19.
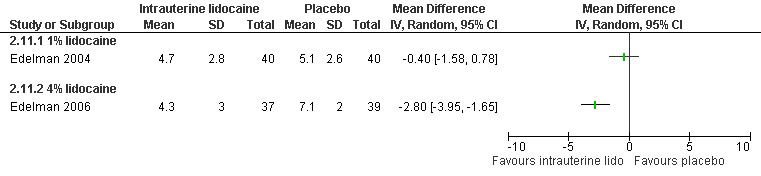
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.9 Pain with aspiration comparing intrauterine lidocaine with placebo.
20.
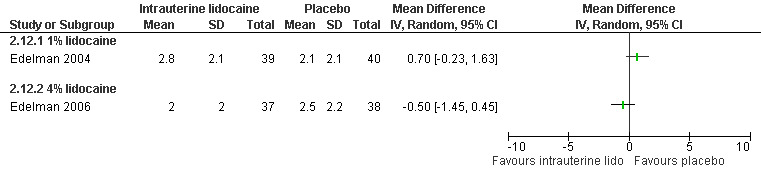
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.10 Pain 30 min postoperatively comparing intrauterine lidocaine with placebo.
Topical lignocaine gel compared to KY jelly did not alter pain with cervical dilation or postoperative pain, but alleviated pain with aspiration (WMD ‐0.87 95% CI ‐1.60 to ‐0.14, N = 131) (Li 2006, Figure 21; Figure 22; Figure 23; Figure 24).
21.

Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.11 Pain with dilation comparing 2% lignocaine gel 10ml with KY jelly 10ml.
22.

Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.12 Pain with aspiration comparing 2% lignocaine gel 10ml with KY jelly 10ml.
23.
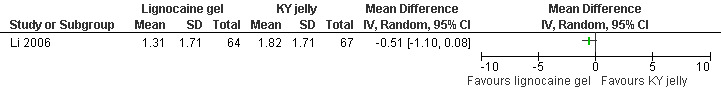
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.13 Pain postpoperatively comparing 2% lignocaine gel 10ml with KY jelly 10ml.
24.

Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.14 Satisfaction with pain control comparing lignocaine gel with KY jelly.
PCB with premedication (Comparison 3)
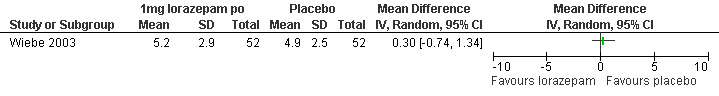
Ibuprofen, given 30 minutes preoperatively, improved pain control with aspiration and postoperatively compared to placebo (WMD ‐0.78 95% CI ‐1.52 to ‐0.04, WMD ‐0.93 95% CI ‐1.62 to ‐0.24, N 193) (Wiebe 1995, Figure 25; Figure 26), while lorazepam, given 1 hour preoperatively, did not make a difference (Wiebe 2003, Figure 27). Naproxen, given 1‐2 hours preoperatively, decreased pain compared to placebo (max pain during procedure p<=0.001, 15 minutes postoperatively p<=0.0001, 30 minutes postoperatively p<=0.002) (Suprapto 1984). Respective values for naproxen versus no drug were p<=0.001 with abortion and p=0.059 30 minutes postoperatively. No significant difference was found difference between placebo and no‐drug group. Only the graphs with mean pain scores were presented in the article (Suprapto 1984).
25.

Forest plot of comparison: 3 Paracervical block with premedication, outcome: 3.1 Pain with aspiration comparing ibuprofen 600mg po with placebo in addition to PCB.
26.
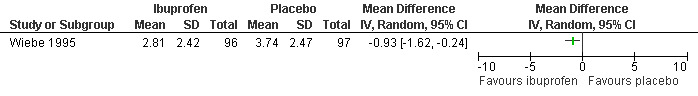
Forest plot of comparison: 3 Paracervical block with premedication, outcome: 3.2 Pain postpoperatively comparing ibuprofen 600mg po with placebo in addition to PCB.
27.
Forest plot of comparison: 3 Paracervical block with premedication, outcome: 3.3 Pain with aspiration comparing 1mg lorazepam po with placebo in addition to PCB.
Additional outcomes and sub‐analysis results reported in studies for comparisons 1, 2 and 3:
Lorazepam received per patient request did not affect pain in patients undergoing the procedure with a PCB (Wiebe 1992; Wiebe 1995). It also did not significantly impact anxiety in a RCT Wiebe 2003.
Subanalysis for nulliparity versus multiparity was performed, and showed significantly lower pain scores with multiparity on arrival in the OR, with cervical manipulation/dilation and overall intraoperatively. Multiparous women were significantly more satisfied; type of anesthesia did not alter satisfaction (Li 2006).
Many studies did not study patient satisfaction but in those that did satisfaction was high in both study arms (Edelman 2004; Edelman 2006; Phair 2002; Kan 2004; Figure 28; Figure 29).
28.
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.15 Satisfaction with the abortion experience comparing intrauterine lidocaine with placebo.
29.
Forest plot of comparison: 1 Local anesthesia technique, outcome: 1.16 Satisfaction with the abortion experience comparing deep with regular PCB injection technique.
Group 2: analgesia alone (Comparison 4)
One study investigated diclofenac sodium 50mg, given 4 hours preoperatively, combined with 200mcg misoprostol compared to misoprostol alone and did not find differences in pain control with aspiration or postoperatively, or with acceptability of pain control (Li 2003, Figure 30, Figure 31, Figure 32). If broken down in nulliparous and multiparous, there was significant less pain with diclofenac sodium in multiparous women during the procedure (mean 58 (SD 27) versus 63(27)).
30.
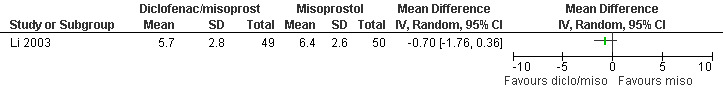
Forest plot of comparison: 4 Analgesia per os only, outcome: 4.1 Pain with aspiration comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200cmg po.
31.

Forest plot of comparison: 4 Analgesia per os only, outcome: 4.2 Pain postoperatively comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200mcg po.
32.

Forest plot of comparison: 4 Analgesia per os only, outcome: 4.3 Acceptability of pain control comparing diclofenac sodium/misoprostol po with misoprostol po.
Group 3: conscious sedation (Comparison 5)
Three studies investigated conscious sedation. Pain with aspiration and 1 hour postoperatively did not differ when comparing entonox (50:50 mixture of nitrous oxide in oxygen) with air when added to conscious sedation with midazolam and fentanyl (Kan 2006). Only median and 95% CI were reported. Anxiety, satisfaction level as well as side effects (nausea, dizziness, dry mouth and drowsiness) did not significantly vary between groups (Kan 2006).
While pain with aspiration and postoperatively did not differ comparing conscious sedation using midazolam 2mg and fentanyl 25mcg IV with placebo after administering a PCB to all participants (only medians were reported), satisfaction was higher with conscious sedation (Peto OR 3.69 95% CI 1.63 to 8.36, N = 100) (Wong 2002, Figure 33). No difference was observed in sedation. Postoperative more dizziness (p=0.015) and drowsiness (p<0.001) was noted in the conscious sedation group. Multiple regression showed that sedation (decreased, p=0.008) and gestational age (increased, p=0.024) affected pain (Wong 2002). A second study compared PCB and conscious IV sedation using diazepam and fentanyl with PCB alone (Wells 1992). In this study, which does not report SDs, women with IV sedation reported less pain (Mean 4.54 versus 6.30, p=0.003 (F (1.8)=9.40) N = 84). Pain intensity further correlated with subjective distress (r=0.74, p>0.001) and behavioral distress (r=0.54, p<0.001) (Wells 1992, Figure 34).
33.
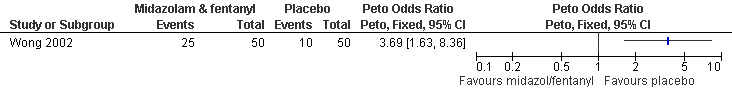
Forest plot of comparison: 5 Conscious sedation, outcome: 5.1 Satisfaction comparing conscious sedation with placebo.
34.

Forest plot of comparison: 5 Conscious sedation, outcome: 5.2 Pain with aspiration comparing PCB and IV sedation with PCB.
Group 4: general anesthesia (Comparison 6)
14 studies investigated general anesthesia.
Inhalation anesthetics:
Four studies included inhalation anesthetics (Barneschi 1985; Collins 1985; Hackett 1982; Ogg 1983). Halothane did not change postoperative reported pain compared to alfentanil when added to methohexitone (Collins 1985). For the forest plots we dichotomized the 3 groups by combining nil and slight pain versus moderate/severe pain (Collins 1985, Figure 35). Adding halothane, enflurane or fentanyl to thiopental did not affect postoperative pain (Barneschi 1985, Figure 36, Figure 37). Trichlorethylene did not change pain control compared to methohexital (Ogg 1983, Figure 38). Enflurane compared to fentanyl did not affect pain when added to methohexitone (Hackett 1982). The data cannot be shown in graph due to inaccurate numbers in the publication that could not be successfully verified with the author (Hackett 1982).
35.

Forest plot of comparison: 6 General anesthesia, outcome: 6.1 Postoperative pain comparing halothane and alfentanil.
36.

Forest plot of comparison: 6 General anesthesia, outcome: 6.2 Postoperative pain comparing thiopental and fentanyl with thiopental and halothane.
37.

Forest plot of comparison: 6 General anesthesia, outcome: 6.3 Postoperative pain comparing thiopental and fentanyl with thiopental and enflurane.
38.

Forest plot of comparison: 6 General anesthesia, outcome: 6.4 Postoperative pain comparing trichlorethylen with total IV (methohexital) anesthesia.
Side effects of inhalation anesthetics: Higher blood loss was noted with inhalation anesthetics, such as enflurane (Hackett 1982) and halothane (Collins 1985) per reported study results. Since no CI was given we could not recalculate this (Figure 39).
39.
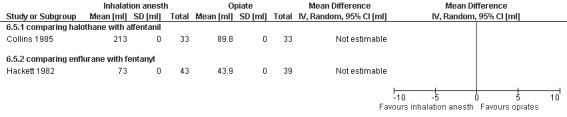
Forest plot of comparison: 6 General anesthesia, outcome: 6.5 Blood loss comparing inhalational anesthetics with opiates.
Data on nausea and vomiting was controversial; less with halothane compared to fentanyl (Collins 1985), more with enflurane compared to fentanyl (Hackett 1982), no difference with trichlorethylene (Ogg 1983,Figure 40 ). Halothane anesthesia was associated with more cough compared to alfentanil given for maintenance after methohexitone induction. However it was associated with less limb movement. Since only ranges were given, we could not recalculate the statistics (Collins 1985).
40.
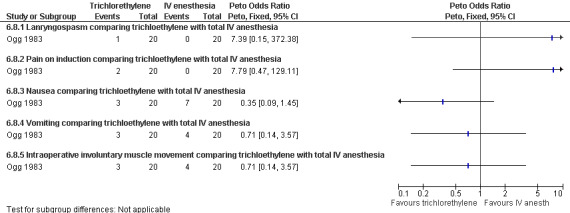
Forest plot of comparison: 6 General anesthesia, outcome: 6.8 Side effects comparing trichloethylene with total IV anesthesia.
Laryngospasm, pain on induction, and intraoperative muscle movement did not differ between trichloethylene and total IV anesthesia (Ogg 1983,Figure 40 ). Severe anesthesia complications, as well as apnea (Collins 1985) did not differ (Collins 1985; Hackett 1982, Figure 41, Figure 42). Anesthesia with volatile agents was considered safe and reliable (Barneschi 1985).
41.

Forest plot of comparison: 6 General anesthesia, outcome: 6.6 Anesthetic complications comparing halothane and alfentanil.
42.
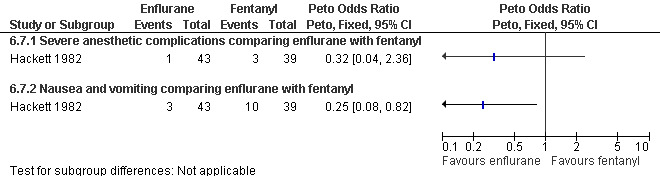
Forest plot of comparison: 6 General anesthesia, outcome: 6.7 Side effects comparing enflurane with fentanyl.
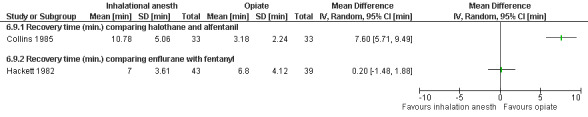
Recovery time after halothane was longer compared to alfentanil (WMD 7.6 95% CI 5.71 to 9.49, N 66) (Collins 1985), while enflurane and fentanyl did not differ (Hackett 1982, Figure 43 ). Memory function as part of the recovery testing did not differ between groups (Ogg 1983). Anesthesia with volatile agents was considered safe and reliable (Barneschi 1985).
43.
Forest plot of comparison: 6 General anesthesia, outcome: 6.9 Recovery time comparing inhalation anesthetics with opiates.
Sedatives, hypnotics and opiates
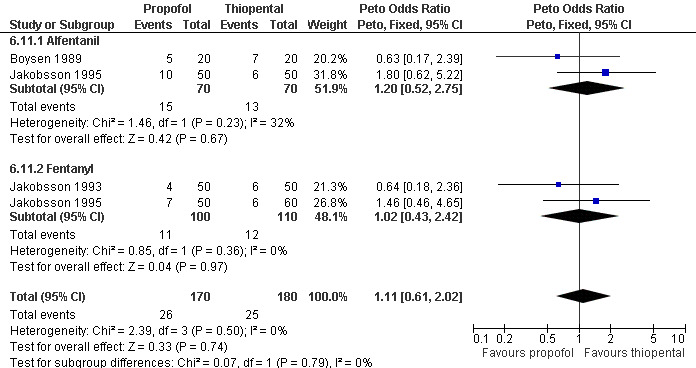
Ten studies included propofol. Postoperative pain did not differ comparing propofol with etomidate (Boysen 1989, Figure 44). In a meta‐analysis of 3 studies with 350 patients comparing propofol and thiopental, no differences in postoperative pain were measured regardless of adding fentanyl or alfentanil (Jakobsson 1993; Jakobsson 1995; Boysen 1989, Figure 45).
44.

Forest plot of comparison: 6 General anesthesia, outcome: 6.10 Postoperative pain comparing propofol with etomidate.
45.
Forest plot of comparison: 6 General anesthesia, outcome: 6.11 Postoperative pain comparing propofol with thiopental.
Propofol was associated with decreased postoperative pain compared to methohexital (Peto OR 0.28 95% CI 0.10 to 0.80, N 100) (Jakobsson 1993, Figure 46). However, Boysen et al 1990 showed a trend towards the reverse, and when combining both studies there was no significant difference in postoperative pain (Boysen 1990; Jakobsson 1993).
46.
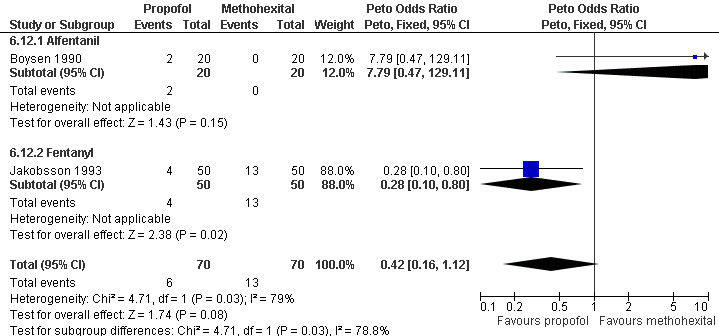
Forest plot of comparison: 6 General anesthesia, outcome: 6.12 Postoperative pain comparing propofol with methohexital.
Midazolam and propofol when added to fentanyl did not differ in reported postoperative pain (Rossi 1995, Figure 47).
47.

Forest plot of comparison: 6 General anesthesia, outcome: 6.13 Postoperative pain comparing propofol and fentanyl with midazolam and fentanyl.
Combination of propofol and alfentanil achieved better postoperative pain control compared to 0.5mg/kg ketamine and 0.25mg/kg midazolam (Peto OR 0.18 95% CI 0.07 to 0.47, N 100); the trend when using 1mg/kg ketamine and 0.1mg/kg midazolam was not significant (Bonnardot 1987, Figure 48). Ketamine was associated with more postoperative pain than fentanyl when added to propofol (Peto OR 7.13 95% CI 2.99‐17.0, N 100) (Jakobsson 1993, Figure 49). Even though Rossi et al (Rossi 1995) did not confirm this, the association remained significant in the meta‐analysis (Peto OR 4.66 95% CI 2.16 to 10.06, N 180) (Figure 49). The combination of ketamine and diazepam compared to thiopental and fentanyl did not change pain (Barneschi 1985, Figure 50).
48.
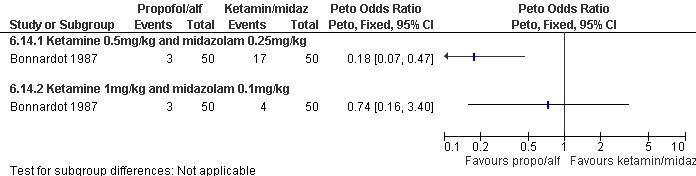
Forest plot of comparison: 6 General anesthesia, outcome: 6.14 Postoperative pain comparing propofol and alfentanil with ketamine and midazolam.
49.
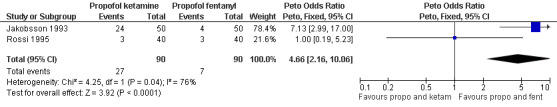
Forest plot of comparison: 6 General anesthesia, outcome: 6.15 Postoperative pain comparing propofol and ketamine with propofol and fentanyl.
50.
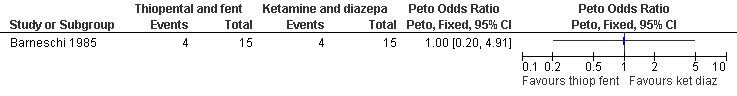
Forest plot of comparison: 6 General anesthesia, outcome: 6.16 Postoperative pain comparing thiopental and fentanyl with ketamine and diazepam.
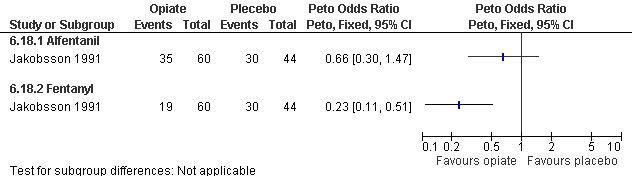
Adding alfentanil to propofol reduced postoperative pain (Peto OR 0.16 95% CI 0.06 to 0.40, N 100) (Bonnardot 1987, Figure 51). In Jakobsson et al 1991 adding alfentanil compared to placebo did not change pain, but fentanyl did (Peto OR 0.23 95% CI 0.11 to 0.51, N 208) (Jakobsson 1991, Figure 52). However, by the time of discharge the pain was the same. Adding alfentanil to propofol postoperative pain was higher compared to fentanyl (Peto OR 1.96 95% CI 1.07 to 3.6, N 210) (Jakobsson 1991; Jakobsson 1995, combined in a meta‐analysis, Figure 53). In the arm with thiopental, pain did not differ (Jakobsson 1995, Figure 54). At 30 minutes postoperatively pain was less in the fentanyl (p<0.05) and alfentanil (p<0.01) group compared to placebo (Lindholm 1994). Pain intensity was equal among the groups at 120 and 180 minutes. Only a graph but no raw data was available (Lindholm 1994).
51.

Forest plot of comparison: 6 General anesthesia, outcome: 6.17 Postoperative pain comparing propofol and alfentanil with propofol.
52.
Forest plot of comparison: 6 General anesthesia, outcome: 6.18 Postoperative pain comparing placebo with alfentanil and fentanyl.
53.
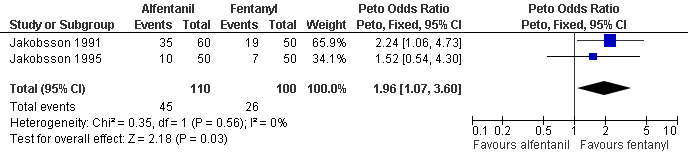
Forest plot of comparison: 6 General anesthesia, outcome: 6.19 Postoperative pain comparing alfentanil with fentanyl when added to propofol.
54.

Forest plot of comparison: 6 General anesthesia, outcome: 6.20 Postoperative pain comparing alfentanil with fentanyl when added to thiopental.
One study investigated if a PCB added to GA altered postoperative pain control (Hall 1997). Pain and postoperative pain medication consumption did not change. Only a graph but no raw data was available. It further did not alter nausea, or time until discharge (no absolute numbers or only medians given in article).
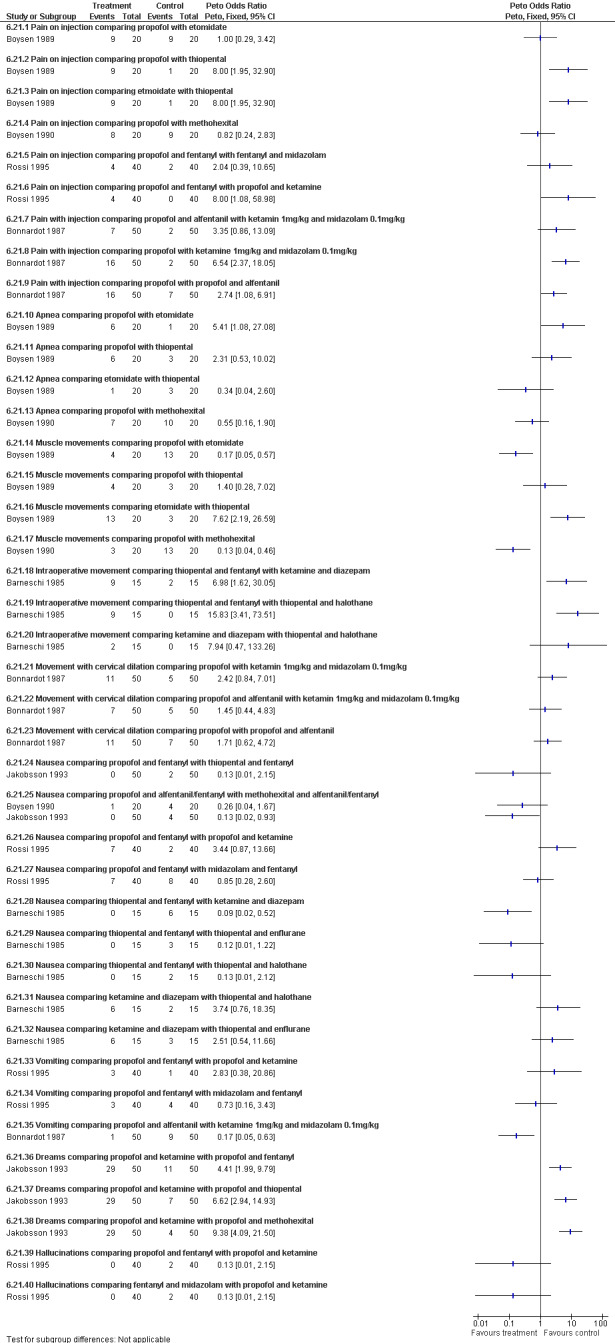
Side effects of Sedatives, hypnotics and opiates (Figure 55): Various side effects comparing propofol with other sedative hypnotic agents and inhalation anesthetics were measured. Please see figures for complete list of Peto ORs and CIs.
55.
Forest plot of comparison: 6 General anesthesia, outcome: 6.21 Side effects comparing propofol with other sedative hypnotic agents.
Increased pain on injection was associated with propofol and etomidate compared to thiopental (Boysen 1989) and ketamine (Bonnardot 1987), as well as with fentanyl versus ketamine added to propofol (Rossi 1995). Propofol alone caused more pain with injection compared to alfentanil and propofol (Bonnardot 1987).
Propofol was associated with increased apnea compared to etomidate but not thiopental or methohexital (Boysen 1989; Boysen 1990).
Muscle movement was increased with etomidate compared to propofol and thiopental and methohexital compared to propofol (Boysen 1989; Boysen 1990). Intraoperative movement was further increased with thiopental and fentanyl compared to ketamine and diazepam as well as thiopental and halothane (Barneschi 1985).
Nausea was decreased with propofol compared to methohexital in one study (Jakobsson 1993), but not another (Boysen 1990). It was also decreased with thiopental and fentanyl compared to ketamine and diazepam (Barneschi 1985). Vomiting was decreased with propofol and alfentanil compared to ketamine and midazolam (Bonnardot 1987).
Dreams were increased with ketamine compared to other sedative hypnotics (Jakobsson 1993).
Nausea, vomiting, laryngospasm and overall complications did not differ when comparing alfentanil or fentanyl with each other or placebo (Jakobsson 1991; Lindholm 1994; Figure 56). Propofol induction dose was significantly lower in the alfentanil group compared to fentanyl (p<0.05; only medians given). The total propofol dose required, and the number of people moving to surgical stimulus was significantly lower in both the fentanyl and alfentanil group compared to the normal saline control group (p<0.01), while recovery measures were improved in the alfentanil group compared to the NS control group (Lindholm 1994).
56.
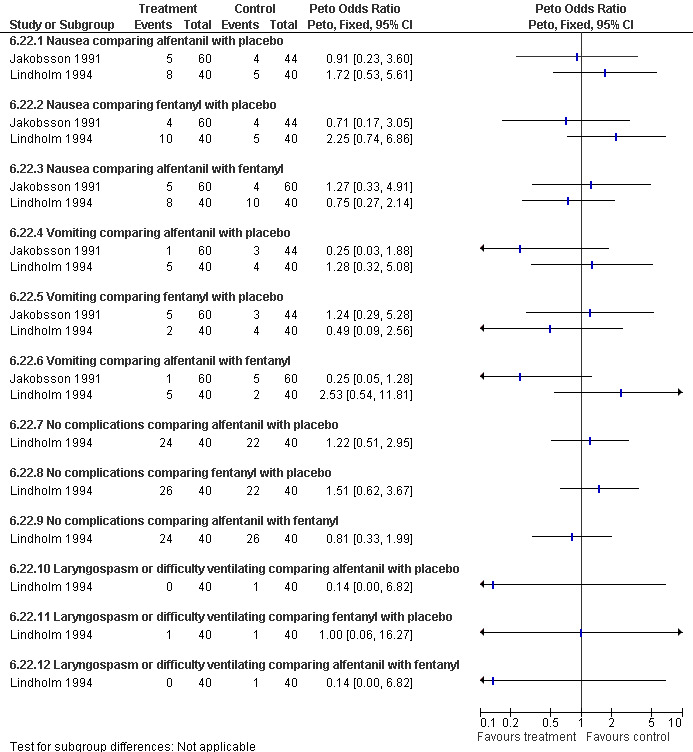
Forest plot of comparison: 6 General anesthesia, outcome: 6.24 Side effects comparing propofol and placebo with propofol and either alfentanil or fentanyl.
Time to discharge was shorter after propofol compared to thiopental in the meta‐analysis of 2 studies (WMD 14.69 95% CI 24.95 to 4.43, N 200) (Jakobsson 1993; Jakobsson 1995; Figure 57). Adding an opioid compared to placebo did not alter the time (Jakobsson 1991). Per study reports: Overall propofol was associated with a better recovery compared to etomidate and thiopental (Boysen 1989), and a similar recovery compared to methohexital (Boysen 1990). Pain significantly correlated to prolonged time until hospital discharge (Jakobsson 1991).
57.

Forest plot of comparison: 6 General anesthesia, outcome: 6.23 Time to discharge.
Recovery was faster in propofol/fentanyl group compared to ketamine/fentanyl and fentanyl/midazolam as assessed per Steward score (only medians reported) (Rossi 1995). Speed and quality of psychomotor and sensory tests was significantly better in the propofol groups compared to the ketamine groups (Bonnardot 1987), as it was in the thiopentane group compared to ketamine (Barneschi 1985).
Conscious sedation versus general anesthesia
Only one study with 59 patients directly compared conscious sedation with general anesthesia (Raeder 1992). With conscious sedation pain with dilation and aspiration was higher as assessed by the anesthesiologist (Peto OR 14.77 95% CI 4.91 to 44.38, and Peto OR 7.47 95% CI 2.2 to 25.36, Figure 58; Figure 59). However, postoperative patient reported pain was decreased (WMD 1.00 95% CI 1.77 to 0.23, Figure 60). Risk for apnea was reduced with conscious sedation (Peto OR 0.10 95% CI 0.02 to 0.46, Figure 61), and duration of sleep shorter (WMD 9.5 95% CI 11.5 to 7.5, Figure 62). Except for better p‐deletion score (a test in which patients are shown a sheet of randomly written letters, and are instructed to delete with a pen all p’s as fast and accurately as possible during a 3 minute period) 30 min after the procedure in the general anesthesia group, there was no difference in the recovery functions between the groups, as per reported results (Raeder 1992).
58.

Forest plot of comparison: 6 General anesthesia, outcome: 6.24 Pain with dilation comparing conscious sedation and PCB with general anesthesia.
59.

Forest plot of comparison: 6 General anesthesia, outcome: 6.25 Pain with aspiration comparing conscious sedation and PCB with general anesthesia.
60.

Forest plot of comparison: 6 General anesthesia, outcome: 6.26 Postoperative pain comparing conscious sedation and PCB with general anesthesia.
61.

Forest plot of comparison: 6 General anesthesia, outcome: 6.27 Apnea incidence comparing conscious sedation and PCB with genereal anesthesia.
62.

Forest plot of comparison: 6 General anesthesia, outcome: 6.28 Duration of sleep comparing conscious sedation and PCB with general anesthesia.
Group 5: general anesthesia with premedication (Comparison 7)
Seven studies investigated the influence of premedication with various analgesics (selective or non‐selective COX inhibitor or opioids) on postoperative pain after general anesthesia (mostly propofol and fentanyl or alfentanil).
The selective COX 3 inhibitor paracetamol given as a suppository at the end of the procedure did not improve pain control compared to placebo (Hein 1999; Figure 63). Even when adding codeine to the paracetamol suppository and giving it 1 hour preoperatively, pain did not improve compared to placebo (Dahl 2000; Figure 64). The non‐selective COX inhibitor lornoxicam significantly decreased postoperative pain compared to paracetamol dosed orally (Peto OR 0.36 95% CI 0.17 to 0.78, N 140), which in turn did not change pain compared to placebo. All test drugs were given 1 hour before anesthesia (Hein 2001; Figure 65; Figure 66). Diclofenac IM and ketorolac IM both decreased postoperative pain compared to NaCl when given 10‐20 minutes before the anesthesia (Peto OR 0.37 95% CI 0.14 to 0.92, and Peto OR 0.32 95% CI 0.12 to 0.81, N 100) (Jakobsson 1996; Figure 67), and did not differ when compared to each other. Diclofenac orally was associated with more postoperative pain compared to ketorolac IM (Peto OR 3.17 95% CI 1.24 to 8.13, N 100), and did not improve pain control compared to NaCl (Jakobsson 1996). The COX 2 inhibitor etoricoxib, given 30‐60 minutes preoperatively did not improve pain control immediately postoperative, but by the time of discharge (WMD 0.7 95% CI ‐1.2 to ‐0.2, N 40) (Liu 2005; Figure 68).
63.
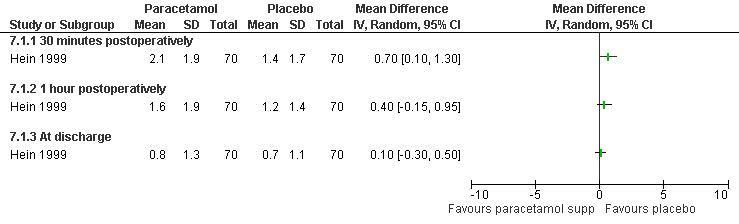
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.1 Postoperative pain comparing paracetamol supp with placebo.
64.
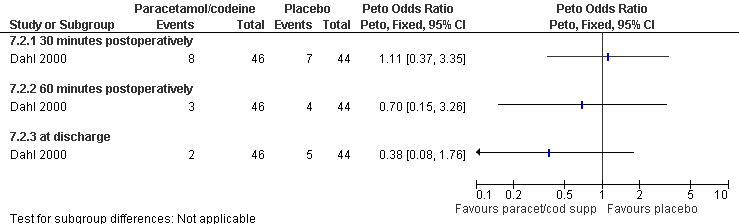
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.2 Postoperative pain comparing paracetamol/codeine supp with placebo.
65.

Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.3 Postoperative pain comparing paracetamol po with placebo.
66.

Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.4 Postoperative pain comparing paracetamol po with lornoxicam.
67.
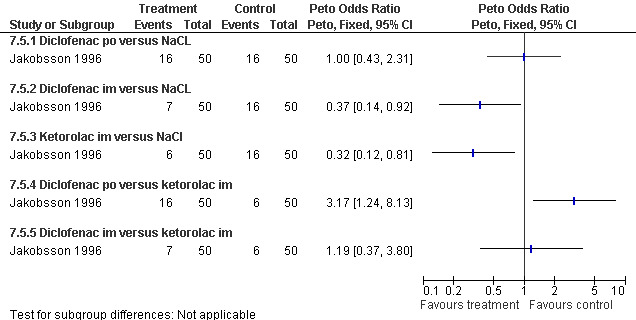
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.5 Postoperative pain comparing diclofenac with ketorolac and with NaCl.
68.
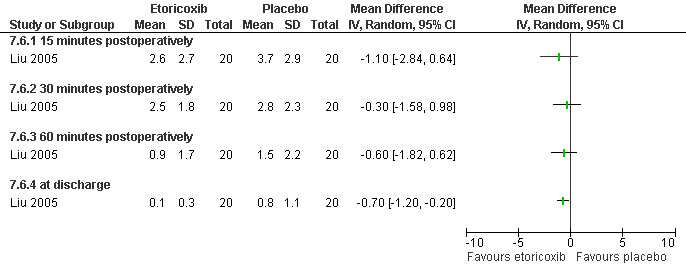
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.6 Postoperative pain comparing etoricoxib with placebo.
Side effects (Figure 69) comparing COX inhibitors with placebo did not show any difference regarding antiemetic requirements, nausea, vomiting, anxiety or satisfaction except for diclofenac IM decreasing nausea and anxiety compared to NaCl (Peto OR 0.13 95% CI 0.02 to 0.93 and Peto OR 0.29 95% CI 0.09 to 0.94, N 100) (Jakobsson 1996). Ketorolac decreased anxiety compared to Nacl (OR 0.29 95% CI 0.09 to 0.94, N 100) (Jakobsson 1996). Time to discharge was the same in all groups (Hein 1999; Hein 2001; Jakobsson 1996; Liu 2005; Figure 70).
69.
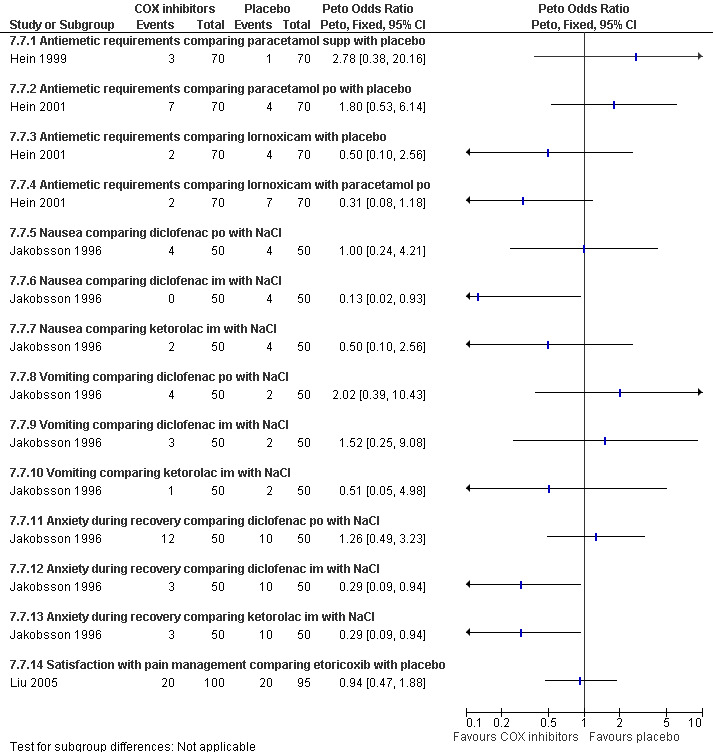
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.7 Side effects comparing COX inhibitors with placebo as premedication for general anesthesia.
70.
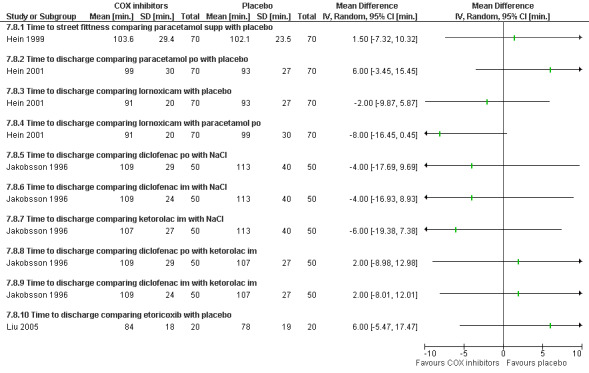
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.8 Recovery time comparing COX inhibitors with placebo as premedication for general anesthesia.
Nalbuphine achieved better 1 hour postoperative pain control than fentanyl (Peto OR 0.21 95% CI 0.05 to 0.86, N 40); after 2 hours the difference was not significant anymore (Bone 1988; Figure 71). The incidence of postoperative pain and nausea was the same when comparing dihydrocodeine po with placebo (Heath 1989). Only medians were reported in that study.
71.
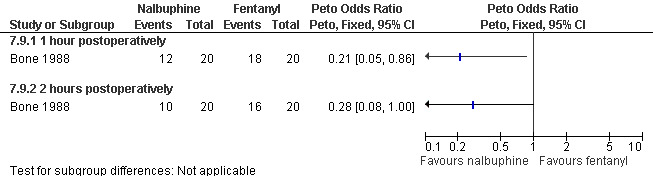
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.9 Postoperative pain comparing Nalbuphine with fentanyl.
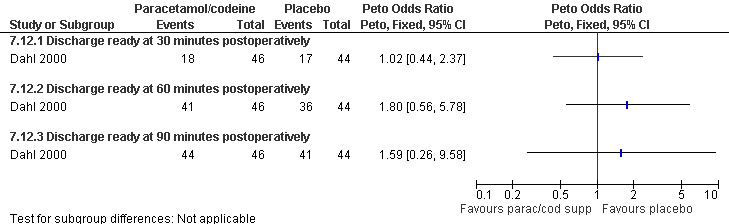
Side effects: Nausea and recovery (reaction time) did not differ between nalbuphine and fentanyl (Bone 1988; Figure 72). Of note, nausea was reported as a mean despite the fact that it was a categorical measurement with a score between 1‐3. Paracetamol with codeine suppository compared to placebo did not change nausea or awakeness/sleepiness at most time points measured, except for more women being sleepy at 30 minutes postoperatively after paracetamol with codeine (Peto OR 3.17 95% CI 1.39 to 7.23) and less fully awake (Peto OR 0.35 95% CI 0.15 to 0.79, N 90) (Dahl 2000; Figure 73). Time to discharge was not affected (Dahl 2000; Figure 74).
72.
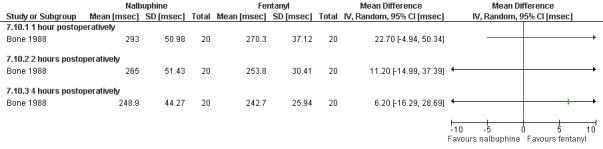
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.10 Recovery (reaction time) comparing nalbuphine with fentanyl.
73.
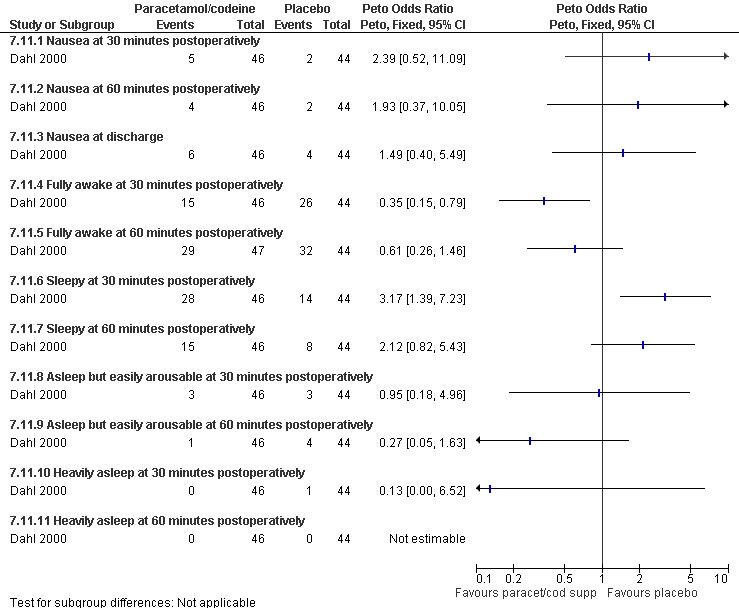
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.11 Side effects comparing paracetamol/codeine supp with placebo.
74.
Forest plot of comparison: 7 General anesthesia with premedication, outcome: 7.12 Recovery time (discharge ready) comparing paracetamol/codeine supp with placebo.
Group 6: Non‐pharmacological intervention (Comparison 9)
Four very different studies investigated non‐pharmacological interventions. In patients with a PCB, hypnosis did not did not change the level of comfort during the procedure compared to standard care; however, it decreased the requests for nitrous oxide (Peto OR 0.12 95% CI 0.03 to 0.54) (Marc 2007; Figure 75; Figure 76). Listening to stereo music compared to self‐administration of methoxyflurane decreased pain with aspiration (Peto OR 0.17 95% CI 0.04‐0.63, N 98). No statistics were reported in the study and thus results were dichotomized to enter them into Revman (Shapiro 1975; Figure 77). Providing sensory (3 minute audio taped message containing orienting information as well as nine sensations related to abortion and identified by over 50% of women in a previous pilot study) compared to general information did not affect procedural pain or distress (Wiebe 1992). Relaxation did not change procedural or postoperative pain in patients with local anesthesia compared to pleasant or analgesic imagery, or a control group (Wells 1989)
75.

Forest plot of comparison: 8 Non pharmacological interventions, outcome: 8.1 Level of comfort during procedure comparing hypnosis with control group.
76.

Forest plot of comparison: 8 Non pharmacological interventions, outcome: 8.2 N2O request comparing hypnosis with a control group.
77.
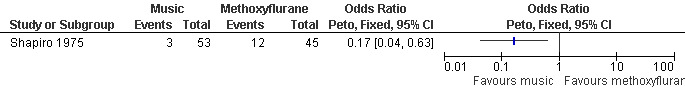
Forest plot of comparison: 8 Non pharmacological interventions, outcome: 8.3 Pain with aspiration comparing music with methoxyflurane.
Discussion
Summary of main results
Various methods of pain control for first trimester surgical abortion have been studied. Local anesthesia, IV sedation, general anesthesia and some forms of non‐pharmacological pain control have been found to effectively decrease pain during and after the procedure while being safe and satisfactory to patients. Data on the effect of a PCB is heterogeneous and very limited. While one small study showed a pain reduction with injection and aspiration when using 1% chloroprocaine compared to normal saline for a 4 point PCB (Glantz 2001), another study did not show a benefit of a PCB compared to no PCB (Kan 2004). Data on buffered lidocaine compared to non‐buffered lidocaine in the PCB was conflicting (Wiebe 1992; Wiebe 1995). Pain with cervical dilation was improved with deep injection of the paracervical block (Cetin 1997; Wiebe 1992), waiting 3 minutes between PCB and dilation (Phair 2002), and with adding a 4% intrauterine lidocaine infusion to PCB (Edelman 2006). All but waiting 3 minutes also decreased pain with aspiration. Premedication with ibuprofen and naproxen (p<0.001) improved intra‐ and post‐operative pain (Wiebe 1995; Suprapto 1984). The addition of conscious IV sedation using diazepam and fentanyl to PCB decreased pain with the procedure (Wells 1992). Adding a PCB to general anesthesia has not been shown to reduce postoperative pain (Hall 1997).
In regard to general anesthesia, a shift from inhalational anesthetics to sedatives and hypnotics has decreased procedure related blood loss. Propofol has been shown to be superior to ketamine in multiple studies, and shortened time until discharge compared to some other hypnotics. Adding opioids to general anesthesia has been found to be beneficial for postoperative pain. No major complications were observed in any study.
Overall completeness evidence
Various countries, decades of years, settings in which the procedure was provided and pain management options have been represented by the included studies. Methods of pain control varied widely and were often combined regimens. In order to synthesize the data, we grouped the included trials as mentioned previously. Trials were too heterogeneous regarding combination of medications, doses, and routes of administration, to be combined in a large meta‐analysis. Therefore, we focused on the primary outcome of pain and were unable to draw firm conclusion on side effects or complications. In addition, the nature of general anesthesia, which achieves complete pain control during the surgery, challenges the ability to compare it to any other form of anesthesia. Pain during deep conscious sedation and general anesthesia can only be assessed by an observer and cannot be patient reported which decreases comparability to other forms of anesthesia.
Quality of evidence (Table 4)
Randomization and allocation concealment were not specified in one third and one half of the studies respectively. This likely derives from the fact that many publications were from the 1970s to early 1990s. Three studies (Hein 1999; Wiebe 1992; Wiebe 1996) had inadequate allocation concealment, but were included due to their overall importance to the review. Only Wiebe 1992 had significant results of decreased pain with a deep PCB, which should be treated with skepticism.
Several studies reported incomplete data, and some of the general anesthesia literature did not contain detailed gynecologic information (e.g. type of procedure sharp curettage versus suction). Not all authors could be successfully contacted to obtain missing information.
Some studies had statistically significant results, however, they only detected a small change in pain (WMD <1) (Cetin 1997, Li 2006, Liu 2005, Phair 2002, Wiebe 1992, Wiebe 1995), or did not measure the amount in pain reduction they had determined in their power calculations (i.e. Phair 2002). Of note Phair et al did not originally report any statistical signficant results, but in our reanalysis, pain with dilation was reduced. This raises the questions of quality of evidence, and points out that clinical significant pain reduction is hard to determine.
Applicability of evidence
Severe complications are rare; therefore, none of the included studies were powered to detect these. Due to short follow up, delayed side effects may have been missed. However, most of the medications studied do not have a long half life.
Recommendations by the WHO on safe abortion (WHO 2003) as well as by the Royal College of Obstetricians and Gynaecologists (RCOG 2004) favor local/IV sedation, over general anesthesia. These recommendations are based on data from the 1970‐1980s. Overall the case‐fatality‐rate from general anesthesia has decreased since the 1970s to 0.7 from 4.1 in 100,000 in 1972, but anesthesia‐related events continued to be the leading cause of morbidity between 1977 and 1987 (Lawson 1994). However, one large abortion‐related study did not show a significant difference in complications between local and general anesthesia (Hakim‐Elahi 1990). Data from the closed claims project of the American society of anesthesiologists has shown a decrease in the percentage of death and brain damage claims related to respiratory events between the period 1970‐1979 and the period 1990‐1994 from 56% to 39% (Cheney 1999). This is thought to be due to improved monitoring including pulse oximetry and capnography (Cheney 1999). There is a paucity of an up‐to date observational data summary on risks of general anesthesia in first trimester surgical abortions.
In any case, in order to prevent complications, the provider must have a profound respect for the continuum from anxiolysis to unconsciousness. It is imperative that patients be monitored appropriately by qualified personnel who are knowledgeable about pharmacokinetics and pharmacodynamics and who are experienced in airway management resuscitation (Steele 2005). Therefore, the setting in which abortions take place strongly affects available resources and risks from anesthesia.
Authors' conclusions
Implications for practice.
Methods of pain control including local anesthesia, IV sedation, general anesthesia and non‐pharmacological methods for first trimester surgical abortion have been studied. Many have been found to effectively decrease pain during and after the procedure while being safe and satisfactory to patients. No major complications were observed in any study. Many patients still find the procedure extremely uncomfortable due to pain with cervical dilation and aspiration, unless given general anesthesia. Given how widely used the PCB is, the paucity of data supporting the benefit of a PCB as shown in this review is surprising and concerning.
Given these findings, factors such as women’s preference, medical risk factors for anesthesia complications, setting and resources availability should be considered when choosing a method of pain control. Trials were too heterogeneous to be combined in a large meta‐analysis.
Considering the small WMD of some significant results, as well as the quality of evidence the strongest evidence supports:
1) Data on the effect of a PCB and buffered lidocaine are conflicting. PCB with local anesthetic such chloroprocaine reduced pain with PCB injection, cervical dilation and aspiration in only one small study, and only when injected at 4 sites, but not when injected at only 2 sites. Another study did not show any benefit of a PCB over no PCB. A deep injection technique seems to reduce pain with cervical dilation and aspiration. Strong evidence supports adding intrauterine 4% lidocaine, but one must be prepared for patients reporting lidocaine exposure sumptoms (i.e. ear ringing).
2) Conscious sedation combined with PCB do not achieve the same pain control as general anesthesia during the procedure, but improved postoperative pain control.
3) General anesthesia ideally consists of a combination of propofol (methohexital, etomidate and thiopentane had very similar results, but have fallen out of favour in many places by now for procedural pain control) with an opioid for postoperative pain control.
4) Premedication for general anesthesia: lornoxicam, IM ketorolac or diclofenac.
Implications for research.
Future studies should aim for using the same outcomes and study instruments to measure pain in order to increase comparability. In order to establish as to whether the PCB is effective of not, a well designed and large study is needed, comparing PCB to a no treatment arm rather than comparing it to placebo, given that the injection of the PCB itself is painful. More studies should try to compare local anesthesia with conscious sedation and general anesthesia regarding pain during and after the procedure as well as regarding side effects, time until discharge, and satisfaction. The nature of general anesthesia, which achieves complete pain control during the surgery, challenges direct comparison to any other form of anesthesia.
Newer observational data on risks of general anesthesia will further help to improve its adequate risk perception. Such data may revise current recommendations.
History
Protocol first published: Issue 3, 2007 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 11 January 2009 | Amended | Reviewers comments implemeted. |
| 15 October 2008 | Amended | Implemented the reviewers comments. |
| 16 June 2008 | Amended | Converted to new review format. |
| 28 January 2007 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
Dr. P. Thorborg provided clinical expertise in anesthesia related questions
Translators: Jana Jarosch (Russian), Chiara Ghetti (Italian), Annemarie Renner (French)
Data and analyses
Comparison 1. Local anesthetics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain with paracervical block or dilation comparing local anesthetics with bacteriostatic normal saline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 1% chloroprocaine versus bacteriostatic (benzyl alcohol) normal saline 14ml at 2 sites‐ pain with PCB | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1% chloroprocaine versus bacteriostatic (benzyl alcohol) normal saline 14ml at 4 sites‐ pain with PCB | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 1% chloroprocaine 14ml versus bacteriostatic (benzyl alcohol) normal saline 14ml all sites combined ‐ pain with PCB | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain with aspiration comparing local anesthetics with bacteriostatic normal saline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1% chloroprocaine versus bacteriostatic (benzyl alcohol) normal saline 14ml at 2 sites‐ pain with aspiration | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1% chloroprocaine versus bacteriostatic (benzyl alcohol) normal saline 14ml at 4 sites‐ pain with aspiration | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 1% chloroprocaine 14ml versus bacteriostatic (benzyl alcohol) normal saline 14ml all sites combined‐ pain with aspiration | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pain postpoperatively comparing local anesthetics with bacteriostatic normal saline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 1% chloroprocaine 14ml versus bacteriostatic (benzyl alcohol) normal saline 14ml at 2 sites‐ pain postop | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1% chloroprocaine 14ml versus bacteriostatic (benzyl alcohol) normal saline 14ml at 4 sites‐ pain postop | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 1% chloroprocaine 14ml versus bacteriostatic (benzyl alcohol) normal saline 14ml all sites combined‐ pain postop | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain with dilation comparing 2% buffered lidocaine with 2% plain lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Pain with aspiration comparing 1% buffered lidocaine with 1% plain lidocaine 20ml each | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Pain at end of procedure comparing buffered lidocaine with plain lidocaine | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 2% buffered lidocaine versus 2% lidocaine 10ml each. | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 1% buffered lidocaine versus 1% lidocaine 20ml each. | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Pain with aspiration comparing 0.5% lidocaine with 1% lidocaine 20ml each | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Pain with aspiration comparing 1% lidocaine with 0.25% bupivacaine 20ml each | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.1. Analysis.
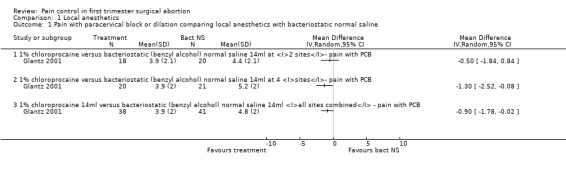
Comparison 1 Local anesthetics, Outcome 1 Pain with paracervical block or dilation comparing local anesthetics with bacteriostatic normal saline.
1.2. Analysis.
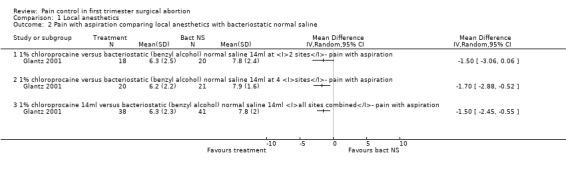
Comparison 1 Local anesthetics, Outcome 2 Pain with aspiration comparing local anesthetics with bacteriostatic normal saline.
1.3. Analysis.
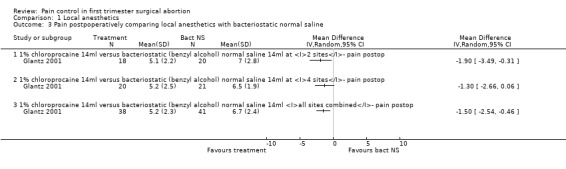
Comparison 1 Local anesthetics, Outcome 3 Pain postpoperatively comparing local anesthetics with bacteriostatic normal saline.
1.4. Analysis.

Comparison 1 Local anesthetics, Outcome 4 Pain with dilation comparing 2% buffered lidocaine with 2% plain lidocaine.
1.5. Analysis.
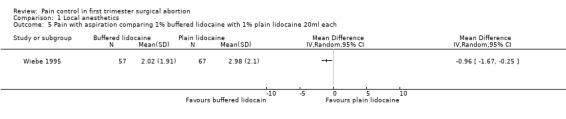
Comparison 1 Local anesthetics, Outcome 5 Pain with aspiration comparing 1% buffered lidocaine with 1% plain lidocaine 20ml each.
1.6. Analysis.
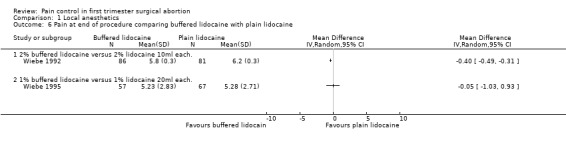
Comparison 1 Local anesthetics, Outcome 6 Pain at end of procedure comparing buffered lidocaine with plain lidocaine.
1.7. Analysis.
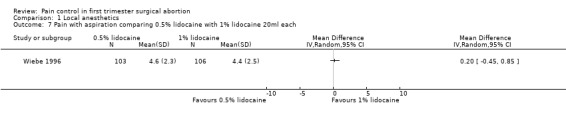
Comparison 1 Local anesthetics, Outcome 7 Pain with aspiration comparing 0.5% lidocaine with 1% lidocaine 20ml each.
1.8. Analysis.
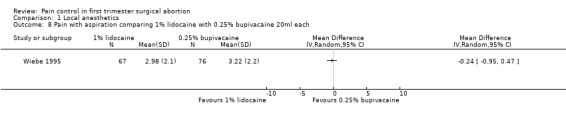
Comparison 1 Local anesthetics, Outcome 8 Pain with aspiration comparing 1% lidocaine with 0.25% bupivacaine 20ml each.
Comparison 2. Local anesthesia technique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain with dilation comparing a deep paracervical block with a regular injection technique | 2 | 229 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.21, ‐0.08] |
| 2 Pain with aspiration comparing a deep paracervical block with a regular injection technique | 2 | 229 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.09, ‐0.91] |
| 3 Pain with PCB placement comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Bacteriostatis saline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1% chloroprocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain with aspiration comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Bacteriostatic Saline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1% chloroprocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pain postoperatively comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Bacteriostatic saline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 1% chloroprocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain with dilation comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Pain with aspiration comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Pain postpoperatively comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Pain with injection comparing fast injection with slow injection of buffered lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Pain with dilation comparing intrauterine lidocaine with placebo | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 1% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 4% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Pain with aspiration comparing intrauterine lidocaine with placebo | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 1% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 4% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Pain 30 min postoperatively comparing intrauterine lidocaine with placebo | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 1% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 4% lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Pain with dilation comparing 2% lignocaine gel 10ml with KY jelly 10ml | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Pain with aspiration comparing 2% lignocaine gel 10ml with KY jelly 10ml | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Pain postpoperatively comparing 2% lignocaine gel 10ml with KY jelly 10ml | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Satisfaction with pain control comparing lignocaine gel with KY jelly | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Satisfaction with the abortion experience comparing intrauterine lidocaine with placebo | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 1% intrauterine lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 4% intrauterine lidocaine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Satisfaction with the abortion experience comparing deep with regular PCB injection technique | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
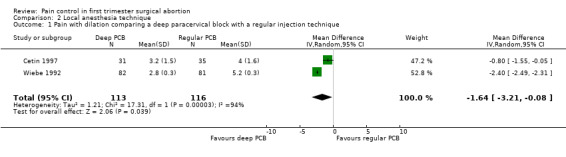
Comparison 2 Local anesthesia technique, Outcome 1 Pain with dilation comparing a deep paracervical block with a regular injection technique.
2.2. Analysis.
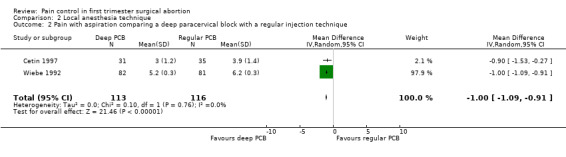
Comparison 2 Local anesthesia technique, Outcome 2 Pain with aspiration comparing a deep paracervical block with a regular injection technique.
2.3. Analysis.
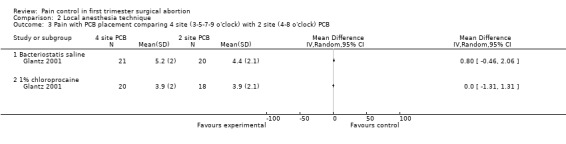
Comparison 2 Local anesthesia technique, Outcome 3 Pain with PCB placement comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB.
2.4. Analysis.
Comparison 2 Local anesthesia technique, Outcome 4 Pain with aspiration comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB.
2.5. Analysis.
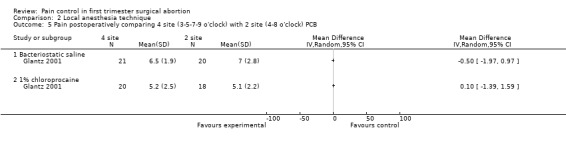
Comparison 2 Local anesthesia technique, Outcome 5 Pain postoperatively comparing 4 site (3‐5‐7‐9 o'clock) with 2 site (4‐8 o'clock) PCB.
2.6. Analysis.
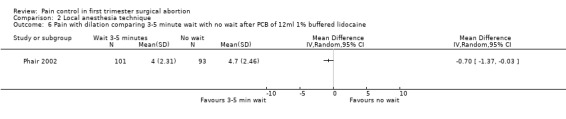
Comparison 2 Local anesthesia technique, Outcome 6 Pain with dilation comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
2.7. Analysis.
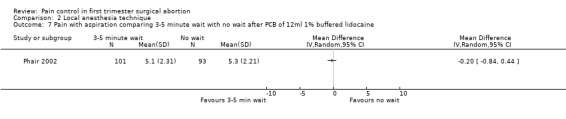
Comparison 2 Local anesthesia technique, Outcome 7 Pain with aspiration comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
2.8. Analysis.
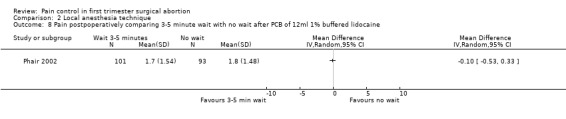
Comparison 2 Local anesthesia technique, Outcome 8 Pain postpoperatively comparing 3‐5 minute wait with no wait after PCB of 12ml 1% buffered lidocaine.
2.9. Analysis.

Comparison 2 Local anesthesia technique, Outcome 9 Pain with injection comparing fast injection with slow injection of buffered lidocaine.
2.10. Analysis.
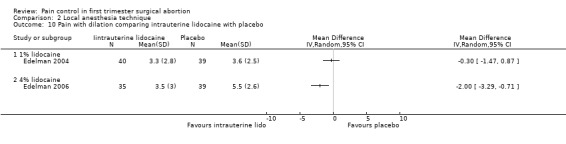
Comparison 2 Local anesthesia technique, Outcome 10 Pain with dilation comparing intrauterine lidocaine with placebo.
2.11. Analysis.
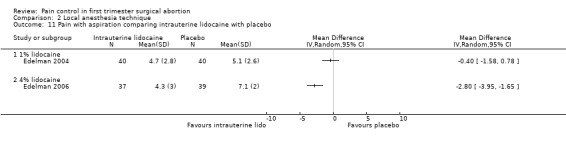
Comparison 2 Local anesthesia technique, Outcome 11 Pain with aspiration comparing intrauterine lidocaine with placebo.
2.12. Analysis.
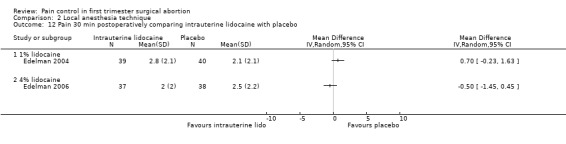
Comparison 2 Local anesthesia technique, Outcome 12 Pain 30 min postoperatively comparing intrauterine lidocaine with placebo.
2.13. Analysis.
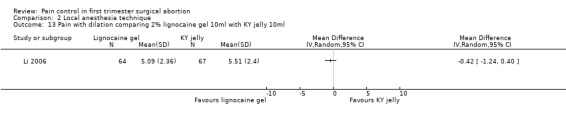
Comparison 2 Local anesthesia technique, Outcome 13 Pain with dilation comparing 2% lignocaine gel 10ml with KY jelly 10ml.
2.14. Analysis.
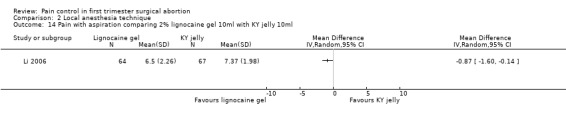
Comparison 2 Local anesthesia technique, Outcome 14 Pain with aspiration comparing 2% lignocaine gel 10ml with KY jelly 10ml.
2.15. Analysis.
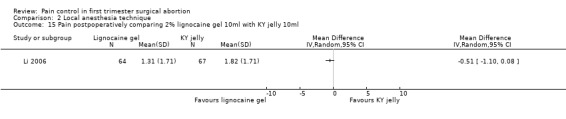
Comparison 2 Local anesthesia technique, Outcome 15 Pain postpoperatively comparing 2% lignocaine gel 10ml with KY jelly 10ml.
2.16. Analysis.
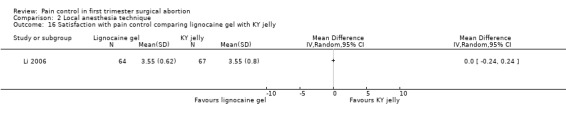
Comparison 2 Local anesthesia technique, Outcome 16 Satisfaction with pain control comparing lignocaine gel with KY jelly.
2.17. Analysis.
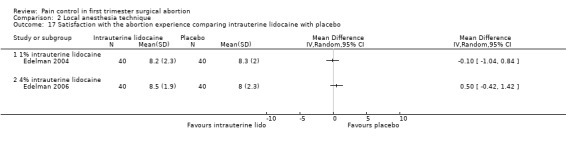
Comparison 2 Local anesthesia technique, Outcome 17 Satisfaction with the abortion experience comparing intrauterine lidocaine with placebo.
2.18. Analysis.
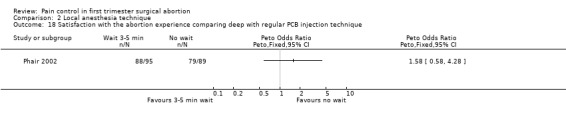
Comparison 2 Local anesthesia technique, Outcome 18 Satisfaction with the abortion experience comparing deep with regular PCB injection technique.
Comparison 3. Paracervical block with premedication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain with aspiration comparing ibuprofen 600mg po with placebo in addition to PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Pain postpoperatively comparing ibuprofen 600mg po with placebo in addition to PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Pain with aspiration comparing 1mg lorazepam po with placebo in addition to PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
3.1. Analysis.
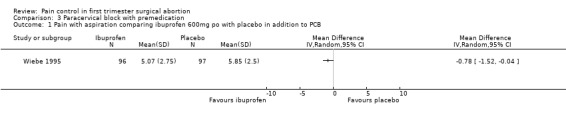
Comparison 3 Paracervical block with premedication, Outcome 1 Pain with aspiration comparing ibuprofen 600mg po with placebo in addition to PCB.
3.2. Analysis.
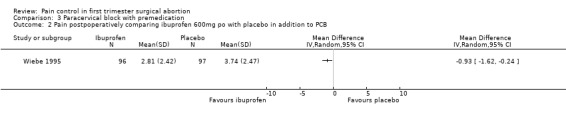
Comparison 3 Paracervical block with premedication, Outcome 2 Pain postpoperatively comparing ibuprofen 600mg po with placebo in addition to PCB.
3.3. Analysis.
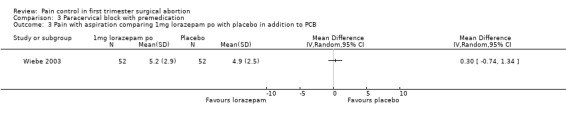
Comparison 3 Paracervical block with premedication, Outcome 3 Pain with aspiration comparing 1mg lorazepam po with placebo in addition to PCB.
Comparison 4. Analgesia per os only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain with aspiration comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200cmg po | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Pain postoperatively comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200mcg po | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Acceptability of pain control comparing diclofenac sodium/misoprostol po with misoprostol po | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
4.1. Analysis.
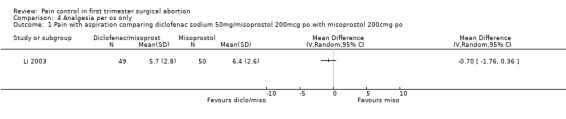
Comparison 4 Analgesia per os only, Outcome 1 Pain with aspiration comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200cmg po.
4.2. Analysis.
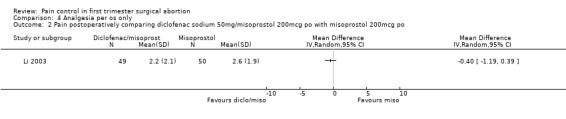
Comparison 4 Analgesia per os only, Outcome 2 Pain postoperatively comparing diclofenac sodium 50mg/misoprostol 200mcg po with misoprostol 200mcg po.
4.3. Analysis.
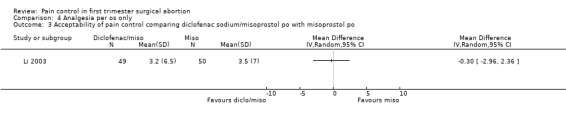
Comparison 4 Analgesia per os only, Outcome 3 Acceptability of pain control comparing diclofenac sodium/misoprostol po with misoprostol po.
Comparison 5. Conscious sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction comparing conscious sedation with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Pain with aspiration comparing PCB and IV sedation with PCB | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
5.1. Analysis.

Comparison 5 Conscious sedation, Outcome 1 Satisfaction comparing conscious sedation with placebo.
5.2. Analysis.
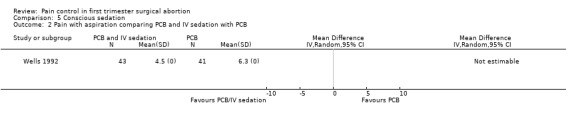
Comparison 5 Conscious sedation, Outcome 2 Pain with aspiration comparing PCB and IV sedation with PCB.
Comparison 6. General anesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain comparing halothane and alfentanil | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Postoperative pain comparing thiopental and fentanyl with thiopental and halothane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Postoperative pain comparing thiopental and fentanyl with thiopental and enflurane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Postoperative pain comparing trichlorethylen with total IV (methohexital) anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Blood loss (ml) comparing inhalational anesthetics with opiates | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 comparing halothane with alfentanil | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 comparing enflurane with fentanyl | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Anesthetic complications comparing halothane and alfentanil | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Side effects comparing enflurane with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7.1 Severe anesthetic complications comparing enflurane with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Nausea and vomiting comparing enflurane with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Side effects comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8.1 Lanryngospasm comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Pain on induction comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Nausea comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Vomiting comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Intraoperative involuntary muscle movement comparing trichloethylene with total IV anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Recovery time (min.) comparing inhalation anesthetics with opiates | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Recovery time (min.) comparing halothane and alfentanil | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Recovery time (min.) comparing enflurane with fentanyl | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Postoperative pain comparing propofol with etomidate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 11 Postoperative pain comparing propofol with thiopental | 3 | 350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.61, 2.02] |
| 11.1 Alfentanil | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.52, 2.75] |
| 11.2 Fentanyl | 2 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.43, 2.42] |
| 12 Postoperative pain comparing propofol with methohexital | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.16, 1.12] |
| 12.1 Alfentanil | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.79 [0.47, 129.11] |
| 12.2 Fentanyl | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.10, 0.80] |
| 13 Postoperative pain comparing propofol and fentanyl with midazolam and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14 Postoperative pain comparing propofol and alfentanil with ketamine and midazolam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14.1 Ketamine 0.5mg/kg and midazolam 0.25mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Ketamine 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Postoperative pain comparing propofol and ketamine with propofol and fentanyl | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.66 [2.16, 10.06] |
| 16 Postoperative pain comparing thiopental and fentanyl with ketamine and diazepam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17 Postoperative pain comparing propofol and alfentanil with propofol | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18 Postoperative pain comparing placebo with alfentanil and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18.1 Alfentanil | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Postoperative pain comparing alfentanil with fentanyl when added to propofol | 2 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [1.07, 3.60] |
| 20 Postoperative pain comparing alfentanil with fentanyl when added to thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 21 Side effects comparing propofol with other sedative hypnotic agents | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 21.1 Pain on injection comparing propofol with etomidate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Pain on injection comparing propofol with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Pain on injection comparing etmoidate with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.4 Pain on injection comparing propofol with methohexital | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.5 Pain on injection comparing propofol and fentanyl with fentanyl and midazolam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.6 Pain on injection comparing propofol and fentanyl with propofol and ketamine | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.7 Pain with injection comparing propofol and alfentanil with ketamin 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.8 Pain with injection comparing propofol with ketamine 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.9 Pain with injection comparing propofol with propofol and alfentanil | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.10 Apnea comparing propofol with etomidate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.11 Apnea comparing propofol with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.12 Apnea comparing etomidate with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.13 Apnea comparing propofol with methohexital | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.14 Muscle movements comparing propofol with etomidate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.15 Muscle movements comparing propofol with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.16 Muscle movements comparing etomidate with thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.17 Muscle movements comparing propofol with methohexital | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.18 Intraoperative movement comparing thiopental and fentanyl with ketamine and diazepam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.19 Intraoperative movement comparing thiopental and fentanyl with thiopental and halothane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.20 Intraoperative movement comparing ketamine and diazepam with thiopental and halothane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.21 Movement with cervical dilation comparing propofol with ketamin 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.22 Movement with cervical dilation comparing propofol and alfentanil with ketamin 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.23 Movement with cervical dilation comparing propofol with propofol and alfentanil | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.24 Nausea comparing propofol and fentanyl with thiopental and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.25 Nausea comparing propofol and alfentanil/fentanyl with methohexital and alfentanil/fentanyl | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.26 Nausea comparing propofol and fentanyl with propofol and ketamine | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.27 Nausea comparing propofol and fentanyl with midazolam and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.28 Nausea comparing thiopental and fentanyl with ketamine and diazepam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.29 Nausea comparing thiopental and fentanyl with thiopental and enflurane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.30 Nausea comparing thiopental and fentanyl with thiopental and halothane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.31 Nausea comparing ketamine and diazepam with thiopental and halothane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.32 Nausea comparing ketamine and diazepam with thiopental and enflurane | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.33 Vomiting comparing propofol and fentanyl with propofol and ketamine | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.34 Vomiting comparing propofol and fentanyl with midazolam and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.35 Vomiting comparing propofol and alfentanil with ketamine 1mg/kg and midazolam 0.1mg/kg | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.36 Dreams comparing propofol and ketamine with propofol and fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.37 Dreams comparing propofol and ketamine with propofol and thiopental | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.38 Dreams comparing propofol and ketamine with propofol and methohexital | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.39 Hallucinations comparing propofol and fentanyl with propofol and ketamine | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.40 Hallucinations comparing fentanyl and midazolam with propofol and ketamine | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Side effects comparing propofol and placebo with propofol and either alfentanil or fentanyl | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 22.1 Nausea comparing alfentanil with placebo | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Nausea comparing fentanyl with placebo | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Nausea comparing alfentanil with fentanyl | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.4 Vomiting comparing alfentanil with placebo | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.5 Vomiting comparing fentanyl with placebo | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.6 Vomiting comparing alfentanil with fentanyl | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.7 No complications comparing alfentanil with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.8 No complications comparing fentanyl with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.9 No complications comparing alfentanil with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.10 Laryngospasm or difficulty ventilating comparing alfentanil with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.11 Laryngospasm or difficulty ventilating comparing fentanyl with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.12 Laryngospasm or difficulty ventilating comparing alfentanil with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Time to discharge | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Time to discharge (min.) comparing propofol with thiopental | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐14.69 [‐24.95, ‐4.43] |
| 23.2 Time to discharge (min.) comparing propofol and placebo with propofol and alfentanil | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐24.87, 6.87] |
| 23.3 Time to discharge (min.) comparing propofol and placebo with propofol and fentanyl | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐16.50, 20.50] |
| 24 Pain with dilation comparing conscious sedation and PCB with general anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 25 Pain with aspiration comparing conscious sedation and PCB with general anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 26 Postoperative pain comparing conscious sedation and PCB with general anesthesia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27 Apnea incidence comparing conscious sedation and PCB with genereal anesthesia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 28 Duration of sleep (min.) comparing conscious sedation and PCB with general anesthesia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
6.1. Analysis.
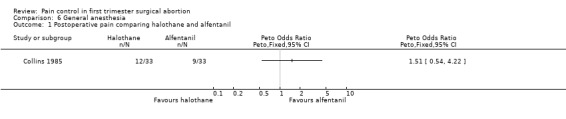
Comparison 6 General anesthesia, Outcome 1 Postoperative pain comparing halothane and alfentanil.
6.2. Analysis.
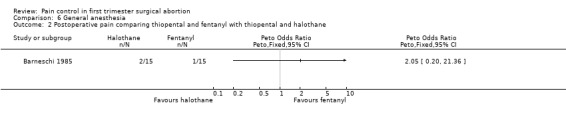
Comparison 6 General anesthesia, Outcome 2 Postoperative pain comparing thiopental and fentanyl with thiopental and halothane.
6.3. Analysis.
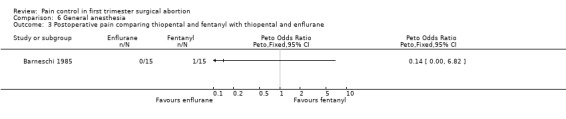
Comparison 6 General anesthesia, Outcome 3 Postoperative pain comparing thiopental and fentanyl with thiopental and enflurane.
6.4. Analysis.
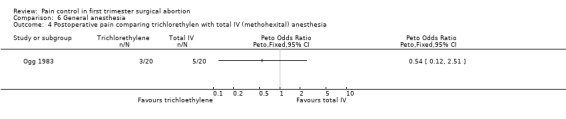
Comparison 6 General anesthesia, Outcome 4 Postoperative pain comparing trichlorethylen with total IV (methohexital) anesthesia.
6.5. Analysis.
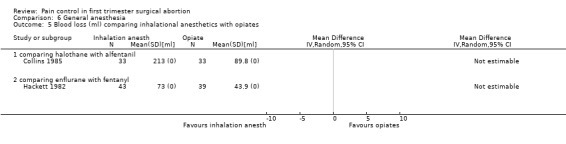
Comparison 6 General anesthesia, Outcome 5 Blood loss (ml) comparing inhalational anesthetics with opiates.
6.6. Analysis.
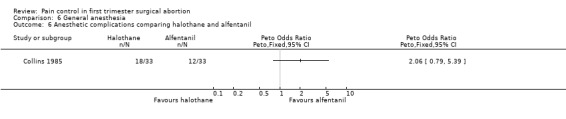
Comparison 6 General anesthesia, Outcome 6 Anesthetic complications comparing halothane and alfentanil.
6.7. Analysis.
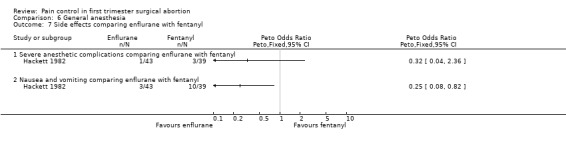
Comparison 6 General anesthesia, Outcome 7 Side effects comparing enflurane with fentanyl.
6.8. Analysis.

Comparison 6 General anesthesia, Outcome 8 Side effects comparing trichloethylene with total IV anesthesia.
6.9. Analysis.
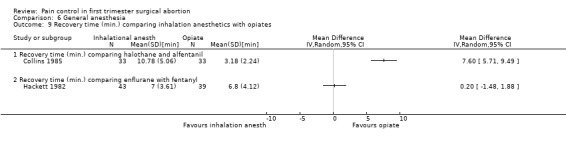
Comparison 6 General anesthesia, Outcome 9 Recovery time (min.) comparing inhalation anesthetics with opiates.
6.10. Analysis.
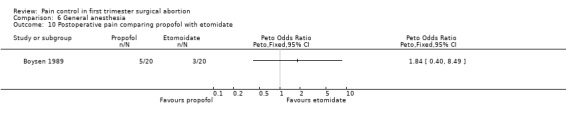
Comparison 6 General anesthesia, Outcome 10 Postoperative pain comparing propofol with etomidate.
6.11. Analysis.
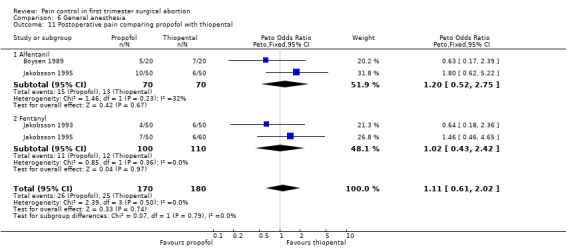
Comparison 6 General anesthesia, Outcome 11 Postoperative pain comparing propofol with thiopental.
6.12. Analysis.
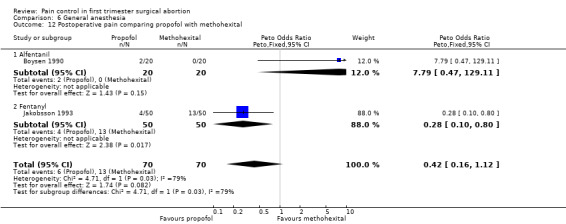
Comparison 6 General anesthesia, Outcome 12 Postoperative pain comparing propofol with methohexital.
6.13. Analysis.
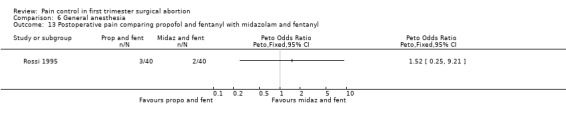
Comparison 6 General anesthesia, Outcome 13 Postoperative pain comparing propofol and fentanyl with midazolam and fentanyl.
6.14. Analysis.
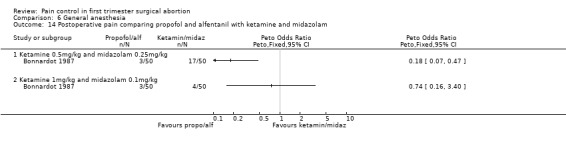
Comparison 6 General anesthesia, Outcome 14 Postoperative pain comparing propofol and alfentanil with ketamine and midazolam.
6.15. Analysis.
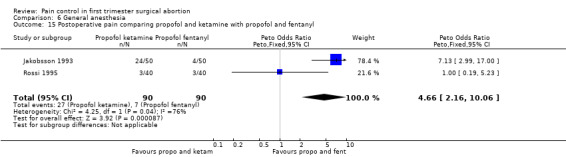
Comparison 6 General anesthesia, Outcome 15 Postoperative pain comparing propofol and ketamine with propofol and fentanyl.
6.16. Analysis.
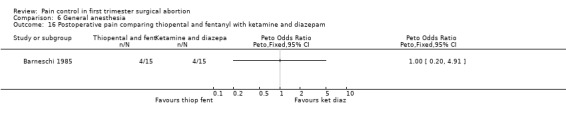
Comparison 6 General anesthesia, Outcome 16 Postoperative pain comparing thiopental and fentanyl with ketamine and diazepam.
6.17. Analysis.
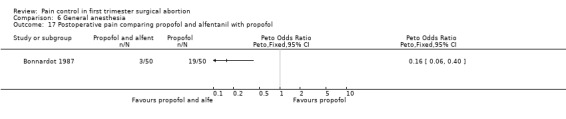
Comparison 6 General anesthesia, Outcome 17 Postoperative pain comparing propofol and alfentanil with propofol.
6.18. Analysis.
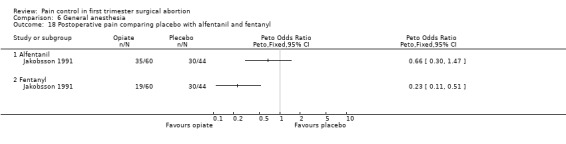
Comparison 6 General anesthesia, Outcome 18 Postoperative pain comparing placebo with alfentanil and fentanyl.
6.19. Analysis.
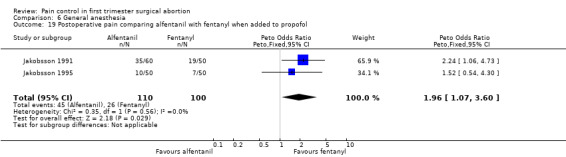
Comparison 6 General anesthesia, Outcome 19 Postoperative pain comparing alfentanil with fentanyl when added to propofol.
6.20. Analysis.
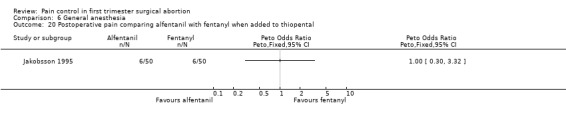
Comparison 6 General anesthesia, Outcome 20 Postoperative pain comparing alfentanil with fentanyl when added to thiopental.
6.21. Analysis.

Comparison 6 General anesthesia, Outcome 21 Side effects comparing propofol with other sedative hypnotic agents.
6.22. Analysis.
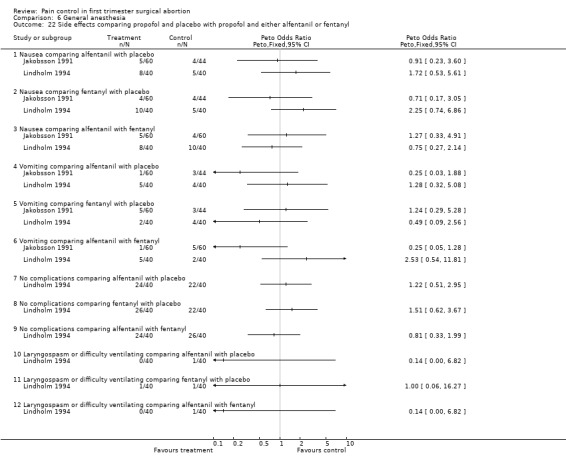
Comparison 6 General anesthesia, Outcome 22 Side effects comparing propofol and placebo with propofol and either alfentanil or fentanyl.
6.23. Analysis.
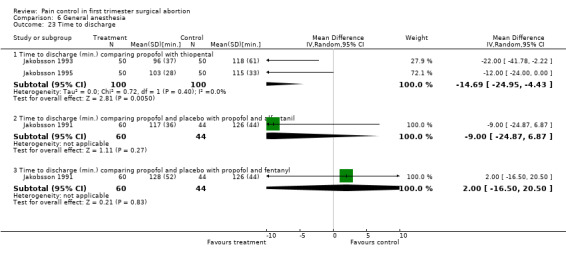
Comparison 6 General anesthesia, Outcome 23 Time to discharge.
6.24. Analysis.
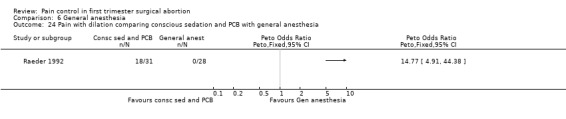
Comparison 6 General anesthesia, Outcome 24 Pain with dilation comparing conscious sedation and PCB with general anesthesia.
6.25. Analysis.
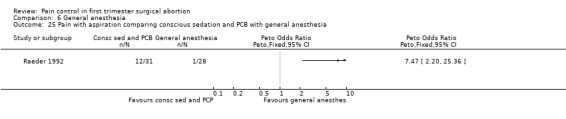
Comparison 6 General anesthesia, Outcome 25 Pain with aspiration comparing conscious sedation and PCB with general anesthesia.
6.26. Analysis.
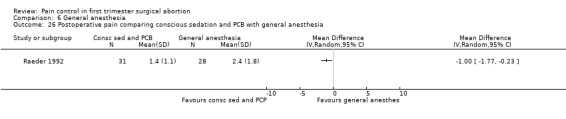
Comparison 6 General anesthesia, Outcome 26 Postoperative pain comparing conscious sedation and PCB with general anesthesia.
6.27. Analysis.
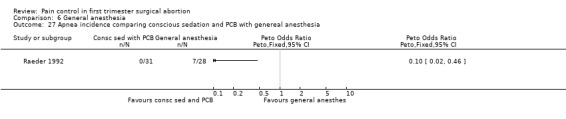
Comparison 6 General anesthesia, Outcome 27 Apnea incidence comparing conscious sedation and PCB with genereal anesthesia.
6.28. Analysis.
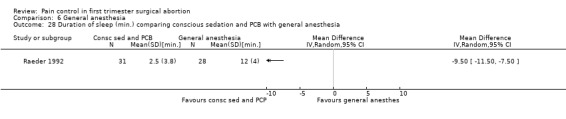
Comparison 6 General anesthesia, Outcome 28 Duration of sleep (min.) comparing conscious sedation and PCB with general anesthesia.
Comparison 7. General anesthesia with premedication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain comparing paracetamol supp with placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 30 minutes postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1 hour postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At discharge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Postoperative pain comparing paracetamol/codeine supp with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 at discharge | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Postoperative pain comparing paracetamol po with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Postoperative pain comparing paracetamol po with lornoxicam | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Postoperative pain comparing diclofenac with ketorolac and with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5.1 Diclofenac po versus NaCL | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Diclofenac im versus NaCL | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Ketorolac im versus NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Diclofenac po versus ketorolac im | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Diclofenac im versus ketorolac im | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Postoperative pain comparing etoricoxib with placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 15 minutes postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 30 minutes postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 60 minutes postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 at discharge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Side effects comparing COX inhibitors with placebo as premedication for general anesthesia | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7.1 Antiemetic requirements comparing paracetamol supp with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Antiemetic requirements comparing paracetamol po with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Antiemetic requirements comparing lornoxicam with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Antiemetic requirements comparing lornoxicam with paracetamol po | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Nausea comparing diclofenac po with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Nausea comparing diclofenac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Nausea comparing ketorolac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Vomiting comparing diclofenac po with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Vomiting comparing diclofenac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.10 Vomiting comparing ketorolac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.11 Anxiety during recovery comparing diclofenac po with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.12 Anxiety during recovery comparing diclofenac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.13 Anxiety during recovery comparing ketorolac im with NaCl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.14 Satisfaction with pain management comparing etoricoxib with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Recovery time (min.) comparing COX inhibitors with placebo as premedication for general anesthesia | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Time to street fittness comparing paracetamol supp with placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Time to discharge comparing paracetamol po with placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Time to discharge comparing lornoxicam with placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Time to discharge comparing lornoxicam with paracetamol po | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Time to discharge comparing diclofenac po with NaCl | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Time to discharge comparing diclofenac im with NaCl | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Time to discharge comparing ketorolac im with NaCl | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Time to discharge comparing diclofenac po with ketorolac im | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Time to discharge comparing diclofenac im with ketorolac im | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Time to discharge comparing etoricoxib with placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Postoperative pain comparing Nalbuphine with fentanyl | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.1 1 hour postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 2 hours postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Recovery (reaction time (msec.)) comparing nalbuphine with fentanyl | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 1 hour postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 2 hours postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 4 hours postoperatively | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Side effects comparing paracetamol/codeine supp with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 11.1 Nausea at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Nausea at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Nausea at discharge | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Fully awake at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Fully awake at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Sleepy at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.7 Sleepy at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.8 Asleep but easily arousable at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.9 Asleep but easily arousable at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.10 Heavily asleep at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.11 Heavily asleep at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Recovery time (discharge ready) comparing paracetamol/codeine supp with placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12.1 Discharge ready at 30 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Discharge ready at 60 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Discharge ready at 90 minutes postoperatively | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
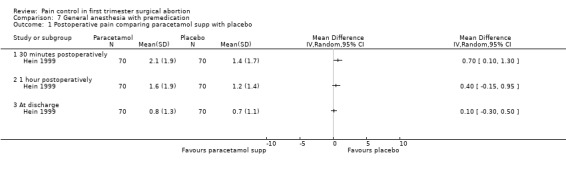
Comparison 7 General anesthesia with premedication, Outcome 1 Postoperative pain comparing paracetamol supp with placebo.
7.2. Analysis.
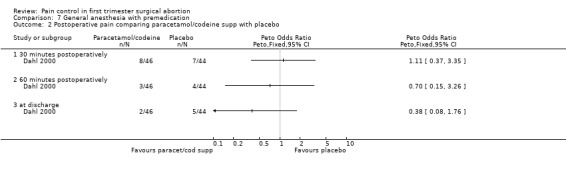
Comparison 7 General anesthesia with premedication, Outcome 2 Postoperative pain comparing paracetamol/codeine supp with placebo.
7.3. Analysis.
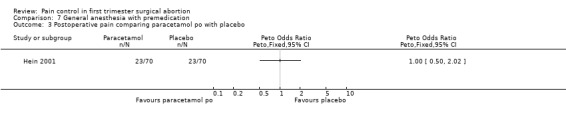
Comparison 7 General anesthesia with premedication, Outcome 3 Postoperative pain comparing paracetamol po with placebo.
7.4. Analysis.
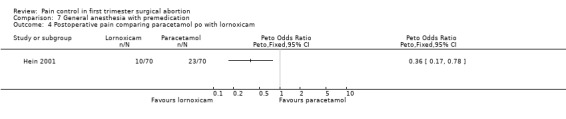
Comparison 7 General anesthesia with premedication, Outcome 4 Postoperative pain comparing paracetamol po with lornoxicam.
7.5. Analysis.
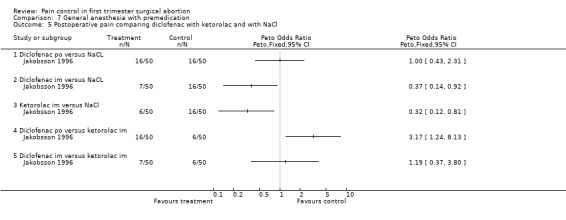
Comparison 7 General anesthesia with premedication, Outcome 5 Postoperative pain comparing diclofenac with ketorolac and with NaCl.
7.6. Analysis.
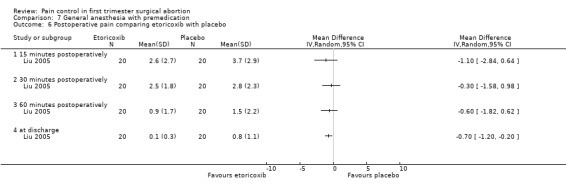
Comparison 7 General anesthesia with premedication, Outcome 6 Postoperative pain comparing etoricoxib with placebo.
7.7. Analysis.
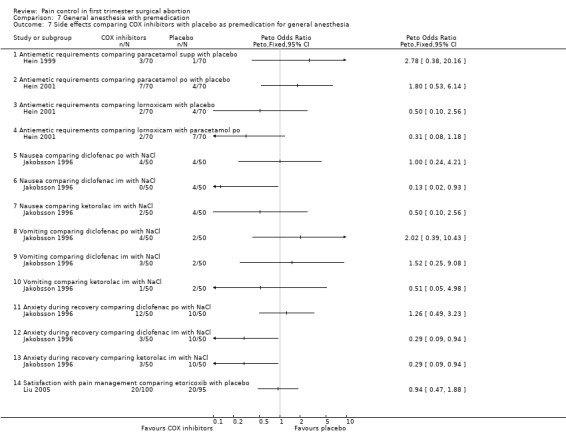
Comparison 7 General anesthesia with premedication, Outcome 7 Side effects comparing COX inhibitors with placebo as premedication for general anesthesia.
7.8. Analysis.
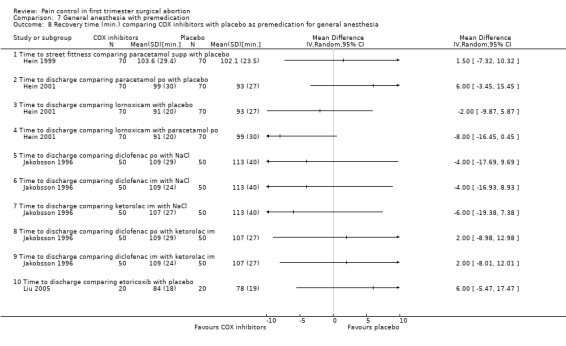
Comparison 7 General anesthesia with premedication, Outcome 8 Recovery time (min.) comparing COX inhibitors with placebo as premedication for general anesthesia.
7.9. Analysis.
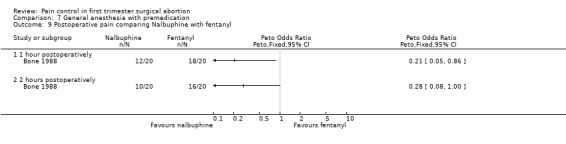
Comparison 7 General anesthesia with premedication, Outcome 9 Postoperative pain comparing Nalbuphine with fentanyl.
7.10. Analysis.
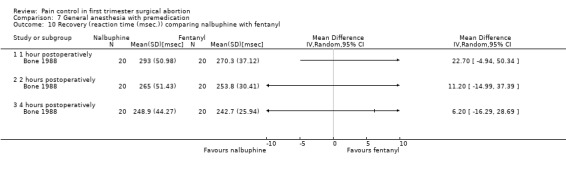
Comparison 7 General anesthesia with premedication, Outcome 10 Recovery (reaction time (msec.)) comparing nalbuphine with fentanyl.
7.11. Analysis.
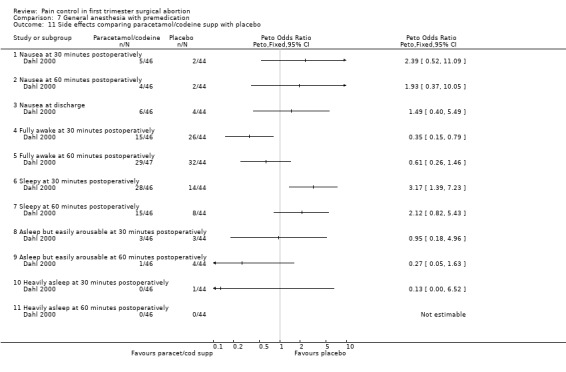
Comparison 7 General anesthesia with premedication, Outcome 11 Side effects comparing paracetamol/codeine supp with placebo.
7.12. Analysis.
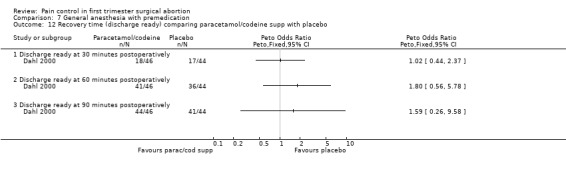
Comparison 7 General anesthesia with premedication, Outcome 12 Recovery time (discharge ready) comparing paracetamol/codeine supp with placebo.
Comparison 8. Non pharmacological interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Level of comfort during procedure comparing hypnosis with control group | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 N2O request comparing hypnosis with a control group | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Pain with aspiration comparing music with methoxyflurane | 1 | Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (mean) with aspiration comparing ralxation versus pleasant imagery versus analgesic imagery versus contro | Other data | No numeric data |
8.1. Analysis.
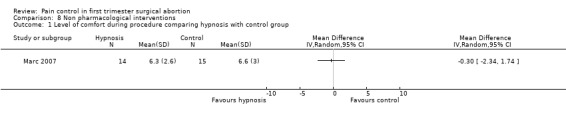
Comparison 8 Non pharmacological interventions, Outcome 1 Level of comfort during procedure comparing hypnosis with control group.
8.2. Analysis.
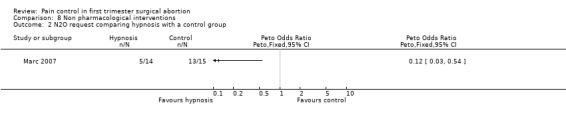
Comparison 8 Non pharmacological interventions, Outcome 2 N2O request comparing hypnosis with a control group.
8.3. Analysis.
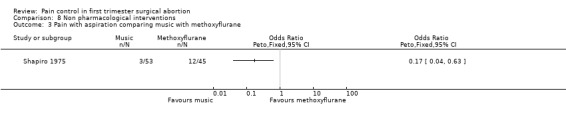
Comparison 8 Non pharmacological interventions, Outcome 3 Pain with aspiration comparing music with methoxyflurane.
8.4. Analysis.
Comparison 8 Non pharmacological interventions, Outcome 4 Pain (mean) with aspiration comparing ralxation versus pleasant imagery versus analgesic imagery versus contro.
| Pain (mean) with aspiration comparing ralxation versus pleasant imagery versus analgesic imagery versus contro | ||||
|---|---|---|---|---|
| Study | relaxation | pleasant imagery | analgesic imagery | control |
| Wells 1989 | 6.77 | 5.45 | 7.36 | 5.76 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barneschi 1985.
| Methods | Randomized controlled trial. Hospital in Firenze, Italy. | |
| Participants | 60 pregnant women. Age: 17‐44 years. ASA I or II. Elective pregnancy termination. Gestational age 7‐12 weeks. | |
| Interventions | Group 1: fentanyl 1.5mcg/kg IV two minutes prior to induction with thiopentone sodium 5mg/kg IV; maintenance with N2O 60% in O2 via mask and assisted breathing. Group 2: ketamine 2mg/kg and diazepam 0.1mg/kg IV; maintenance with N2O 60% in O2 via mask and assisted breathing. Group3: thiopentone sodium 5mg/kg IV; maintenance with N2O60% in O2 and enflurane 2.5% after complete cervical dilation via mask and assisted breathing Group 4: thiopentone sodium 5mg/kg IV; maintenance with N2O60% in O2 and halotane 2% after complete cervical dilation via mask and assisted breathing All participants: premedication with atropine 0.02mg/kg IM 45 minutes prior to the procedure. Vacuum aspiration with or without sharp curettage. | |
| Outcomes | Psychomotor recovery time with Zazzo's test of "deux barrages" and the matrix attentive test. Anesthesia time. Intraoperative side‐effects: introperative movement. Postoperative side‐effects: strong abdominal pain, headache, nausea, vomiting, agitation at awakening (all yes/no). | |
| Notes | No power analysis done. Randomization method and blinding not further described. Per e‐mail communication with Dr. Barneschi: Study length 4 months. Randomization with computer tables. Allocation concealment with envelope technique. Blinding of participants and outcome assessor. Vacuum aspiration. No industry sponsorship. No major complication reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bone 1988.
| Methods | Randomized double‐blind controlled trial. Leicester Royal Infirmary, Leicester, UK | |
| Participants | 40 pregnant women. Age: 16‐40 years. ASA I and II. Gestational week up to 12 weeks. Exclusion criteria: history of asthma, drug allergy or sensitivity to opioids. | |
| Interventions | Group 1: nalbuphine 0.25 mg/kg IV. Group 2: fentanyl 1.5mcg/kg IV. All participants: No premedication. One minute after opioid anesthesia was induced in both groups with thiopentone 3‐4mg/kg. Maintenance with Bain breathing system with 66% nitrous oxide in oxygen and supplementation with enflurane as needed. Syntocinon 10units IV at onset of cervical dilation. Postoperative analgesia with paracetamol 1g orally 6 hourly. | |
| Outcomes | Postoperative pain reported on a 10cm horizontal linear analog scale at 1, 2 and 4 hours postoperatively. Duration of anesthesia, dose of thiopentone, and maximum concentration of enflurane administration. Time until recovery of consciousness (name, date of birth and correct address) and psychomotor function. Patient assessment as asleep, awake, and calm or awake and restless on four different occasions (pre‐operatively, 1, 2, and 4 hours postoperatively). Postoperative nausea. Need for postoperative analgesics. | |
| Notes | No power calculations. Unclear study length, method of randomization and allocation concealment. Unclear if the procedure was vacuum versus sharp curettage. The author could not be succeessfully contacted to clarify unclear information. Outcome assessor in recovery room was blinded. No major complication reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bonnardot 1987.
| Methods | Randomized controlled trial. Hospital Tenon, Paris, France. January to October 1986. | |
| Participants | 200 pregnant women. ASA I or II. | |
| Interventions | Group 1: propofol 2mg/kg. Group 2: ketamine 0.5mg/kg and midazolam 0.25mg/kg. Group 3: propofol 2mg/kg and alfentanil 4mcg/kg. Group 4: ketamine 1mg/kg and midazolam 0.1mg/kg. 2 successive series of 2 groups (1 and 2, and 3 and 4): All participants premedicated with midazolam 0.25mg/kg orally. | |
| Outcomes | Side effects including postopoperative pain (yes/no) and vomiting (yes/no), pain with injection, visual disturbances and rash were secondary outcomes. Primary outcomes: clinical revocery with 4 psychomotor and sensory tests (Newman, Bourdon, Horatz and neursensorial test). Vital signs. | |
| Notes | Per e‐mail communication with Dr. Maillart, the co‐author: randomization with table of numbers, allocation concealment with opaque envelopes, blinding of outcomes assessor, gestational age less than 10 weeks, vacuum evacuation. No power calculations. No major complication reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Boysen 1989.
| Methods | Randomized controlled trial. University of Copenhagen, Denmark. | |
| Participants | 60 pregnant women. Age: 18‐44 years. Healthy women. Gestational age up to 12 weeks. | |
| Interventions | Group 1: thiopental 4mg/kg Group 2: etomidate 0.3mg/kg Group 3: propofol 2.5mg/kg for induction of anesthesia. All participants: No premedication. Alfentanil 15mcg/kg after induction. Additional 1/4 of the induction anesthetic as needed. Oxytocic drugs. All under assisted ventilation with 100% oxygen with a face mask. | |
| Outcomes | Postoperative side ffects including pain (yes/no) and vomiting. Dosage requirements. Vital signs. Pain on injection of anesthetic. Recovery: Steward score, coin counting test (CCT), continuous auditory reaction time test (CART). Need for postoperative analgesics. Other side effects: apnea, involuntary muscle movements. | |
| Notes | No power calculations. Unclear study length, method of randomization and allocation concealment. Unclear if the procedure was vacuum versus sharp curettage. The author could not be succeessfully contacted to clarify unclear information. Outcome assessor was blinded. Apnea occured in 9, 1 and 3 patients of the propofol, etomidate and thiopental group respectively. There was no significant diffference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boysen 1990.
| Methods | Randomized controlled trial. University of Copenhagen, Denmark. | |
| Participants | 40 pregnant women. Age: 22‐34 years. Healthy women. Gestational age up to 12 weeks. | |
| Interventions | Group 1: methohexital 2mg/kg IV Group 2: propofol 2.5mg/kg IV for induction of anesthesia All participants: No premedication. All patients received alfentanil 15mcg/kg after induction. Both groups received 25% of induction dose for maintenance as needed. Oxytocic drugs. Assisted ventilation with 100% oxygen with a face mask. | |
| Outcomes | Postoperative side ffects including pain (yes/no), nausea, vomiting and headache. Dosage requirements, duration of surgery and anesthesia. Vital signs. Intraoperative side effects including pain on injection of anesthetic, apnea, involuntary muscle movements, hiccuping, bronchospasm and coughing. Recovery: Coin counting test (CCT), continuous auditory reaction time test (CART). | |
| Notes | Unclear study length, method of randomization and allocation concealment. Outcome assessor blinded but not anesthesiologist. Unclear if the procedure was vacuum versus sharp curettage, but assume vacuum given year of publication. Pain was recorded if patient spontaneously complained about it. Patients were not systematically asked about pain. The author could not be succeessfully contacted to clarify unclear information. No power calculations. Apnea occured in 10 and 7patients of the methohexital and propofol group respectively. There was no significant diffference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cetin 1997.
| Methods | Randomized controlled trial. Randomization with computerized, random number generation carried out outside the familiy planning unit. Per power analysis 33 patients per group required. Cumhuriyet University, School of Medicine, Sivas, Turkey. Study length: May 1996 to November 1996. | |
| Participants | 66 pregnant women. Singleton intrauterine pregnancy at 6‐11 weeks of gestational age by LMP confirmed bv transvaginal ultrasound. | |
| Interventions | Group 1: deep injection (superficially 1ml, 3ml 3cm deep at 4, 6, 8, and 10 o'clock position; total of 16ml) of PCB Group 2: regular injection (1.5cm deep at same 4 positions) of 10ml 1% lidocaine local anesthetic for paracervical block. All participants: 5mg oral diazepam 60 minutes prior to procedure if preprocedural anxiety of 6 or more (rated by physician not performing procedure). After 2 minute wait, cervical dilation. Vacuum aspiration followed by sharp curette. | |
| Outcomes | Pain associated with dilation and curettage was rated by patient on a verbal analog scale of 0 to 10 (0 = no pain, 10 = severe pain) after the procedure. Anxiety score, procedure time, basal cervical dilation and dilation increased obtained. Follow‐up visit 4‐6weeks after the procedure to assess late complications and to perform a gynecological exam. | |
| Notes | Physician not performing procedure rated preprocedural anxiety (scale 1‐10). Otherwise blinding is not commented on. Unclear how many got diazepam and in which group they were. The author could not be succeessfully contacted to clarify unclear information. No adverse effects, no major complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Collins 1985.
| Methods | Randomized controlled trial. St. Thomas Hospital London, UK. | |
| Participants | 66 pregnant women. Age: 16‐38 years old. ASA 1 or 2. Gestational age <12weeks | |
| Interventions | Group 1: general anesthesia with 1.5% halothane and 70% nitrous oxide in oxygen for maintenance after methohexitone induction. Group 2: general anesthesia with 7mcg/kg alfentanil followed by methohexitone induction and maintenance with alfentanil 0.1‐0.2mg increments as needed and 70% nitrous oxyde in oxygen. All participants: Oxytocin 5 units IV at onset of cervical dilation. Vacuum aspiration. | |
| Outcomes | Postoperative abdominal pain (none, slight or moderate/severe). Need for postoperative analgesia. Blood loss as measured by collection of all blood and the aspirate which was then processed and measured in a standardized way as described by Garrioch, Gilbert and Plantevin 1981. Blood volume was determined using the alkaline haematin method of Hallberg and Nielsson (1964). Ansethetitc morbidity: duration, hiccup, cough, laryngeal stridor, apnea, limb movement. Recovery time, nausea and vomiting. | |
| Notes | Randomization and Allocation concelament not described in detail. Statistical analysis using the group sequential design, in groups of 20 patients. In case of moderate or sever nausea the anesthetic was considered a "failure" for a particular patient. The trial was stopped after 66 patients, since blood loss was significantly higher with halothane use. One patient in the alfentanil and no patient in the halothane group had apnea; defined as no spontaneoeus respirations for more than 30 seconds. No power analysis described. Study length, method of randomization and allocation concealment as well as details of blinding are not described. Author could not successfully be contacted regarding missing information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dahl 2000.
| Methods | Randomized controlled double‐blind trial. Randomization made by pharmacy from a list of random numbers. Per power analysis done to detect reduction in postoperative rescue analgesics from 40 to 20% 46 patients are needed in each group. Hospital in Oslo, Norway. | |
| Participants | 100 pregnant women. ASA I or II. Exclusion criteria: cardiac, pulmonary, or renal disease, extreme obesity, allergy to opioids, or paracetamol. | |
| Interventions | Group 1: paracetamol/codeine 800/60mg suppository 1hr prior to surgery. Groups 2: placebo suppository, identically looking and at the same time All participants: Premedication with midazolam 0.08mg/kg. Induction of anestesia for all with propofol 1.5mg/kg and alfentanil 15mcg/kg. Maintenance of anesthesia with 60% nitrous oxide in oxygen and incremental doses of propofol as eeded. Vacuum aspiration. Rescue analgesic for postoperative pain: ketobemidone 1‐2mg IV. | |
| Outcomes | Pain level measured with visual analog scale (VAS; 0 = no pain, 100 = worst pain ever) and the verbal pain score (VPS; no pain, slight pain, medium pain, strong pain) at 30 and 60 minutes postoperatively as well as before discharge. Need for rescue analgesics, occurrence of nausea/vomiting, degree of sedation at 30 and 60 minutes postoperatively as well as before discharge. Vital signs, duration of surgery, total amount propofol needed. | |
| Notes | Unclear study length, method of allocation concealment. Unclear what the gestational age was, but assume that it was first trimester given that vacuum curettage was used. 100 patients recruited, but 10 exlcuded due to violation of the protocol (insertion of an IUD, use of NSAIDS). Results are only reported for the remaining 90. No major complications reported. The author could not be succeessfully contacted to clarify unclear information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Edelman 2004.
| Methods | Randomized double‐blind placebo‐controlled trial. Computer generated block randomization sequence (block size 20), through an inverstigator not involved with recruitement. Study syringes were identically looking and prepared by the study nurse and coordinator who labeled them with consecutive numbers. Subjects were also enrolled with consecutive numbers. The Investigator was blinded. Per power analysis 40 women needed per study group. Planned Parenthood at Columbia Willamette in Portland, Oregon, USA. Study length: July 2002 to February 2003. | |
| Participants | 80 pregnant women. Age: 18 years old or older. Good general health. English speaking. Gestational age: Less than 11 weeks by LMP confirmed by ultrasound. Body weight more than 100lbs (approx 45kg) | |
| Interventions | Group 1: intrauterine infusion of 10ml 1% lidocaine (maximum total lidocaine dose of 200mg). Groups 2: intrauterine infusion of 10ml of sterile saline. All participants: premedication with 800mg ibuprofen. If requested, 5mg diazepam. Paracervical block with 10ml of 1% lidocaine (1ml 1% nonbuffered lidocaine on the anterior and posterior lip of the cerivx and then 4.5ml of 1% lidocaine at the 4‐ and 8‐o'clock postitions). Five experienced surgeons performed all abortions with an electric vacuum pump after dilation of the cervix. | |
| Outcomes | Pain rated by subjects using a 100mm visual analog scale (VAS; anchors: 0 = none, 100 mm = worst imaginable) at several points in time: 1) before the procedure started (anticipatory pain); 2) following speculum insertion; 3) after intrauterine infusion; 4) following dilation; 5) after suction aspiration; and 6) 30 minutes later in the recovery room. Assessment of overall satisfaction level regarding the abortion experience using the VAS, before discharge. Subjects performed all VAS scales concurrently with each step and not from memory. | |
| Notes | Serum lidocaine were drawn in a subset (n=10) of subjects to obtain safety data. No patient developed overt symptoms of lidocaine toxycity. One protocol violation in lidocaine group with an inadvertant enrollement of a patient who was not premedicated with ibuprofen. Another patient in the lidocaine group received IV narcotics prior to reaspiration. Her 30 minute pain score was conducted after her reaspiraiton. Analysis in accordance with intent to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Edelman 2006.
| Methods | Randomized double‐blind placebo‐controlled trial. Computer generated block randomization sequence (block size 20), through an inverstigator not involved with recruitement. Study syringes were identically looking and prepared by the study nurse and coordinator who labeled them with consecutive numbers. Subjects were also enrolled with consecutive numbers. The Investigator was blinded. Per power analysis 40 women needed per study group. Planned Parenthood at Columbia Willamette in Portland, Oregon, USA. Study length: November 2003 to December 2004. | |
| Participants | 80 pregnant women. Age: 18 years old or older (mean 26). Good general health. English speaking. Gestational age: Less than 11 weeks by LMP confirmed by ultrasound. Body weight more thn 100lbs (approx 45kg) | |
| Interventions | Group 1: intrauterine infusion of 5ml of 4% lidocaine (maximum total lidocaine dose of 300mg). Group 2: intrauterine infusion of 5ml of sterile saline. All participants: premedication with 800mg ibuprofen and if requested 5mg diazepam (per recommendation of safety monitoring committee to raise seizure threshold). Paracervical block with 10ml of 1% lidocaine (1ml 1% nonbuffered lidocaine on the anterior and posterior lip of the cerivx and then 4.5ml of 1% lidocaine at the 4‐ and 8‐o'clock postitions). Five experienced surgeons performed all abortions with an electric vacuum pump after dilation of the cervix. | |
| Outcomes | Pain rated by subjects using a 100mm visual analog scale (VAS; anchors: 0 = none, 100 mm = worst imaginable) at several points in time: 1) before the procedure started (anticipatory pain); 2) following speculum insertion; 3) after intrauterine infusion; 4) following dilation; 5) after suction aspiration; and 6) 30 minutes later in the recovery room. Assessment of overall satisfaction level regarding the abortion experience using the VAS, before discharge. Subjects performed all VAS scales concurrently with each step and not from memory. | |
| Notes | Serum lidocaine were drawn in a subset (n=8) of subjects to obtain safety data. Interim analysis after 37 subjects studied because of concerns of lidocaine toxicity. Some subjects had lidocaine side effects (including ear ringing, perioral numbness and tingling), but none had overt symptoms of severe toxicity (i.e. seizuers, cardiac arrest or loss of consciousness) or toxic serum range of lidocaine. Safety monitoring committee recommended diazepam for all subsequent subjects to raise seizure threshold. 3 women withdrew from the trial prior to receiving study medication; 2 were in the lidocaine groups the other in the saline group. One protocol violation in the saline group with the inadvertent enrollment of a subject without premedication with ibuprofen, but diazepam only. Analysis in accordance with intent to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Glantz 2001.
| Methods | Randomized controlled trial. Randomization by permuted block technique in sets of four, with blocks randomly ordered by use of a random number table. Ordered opaque sealed envelopes used. Allocation verification done. Double‐blind regarding solution injected, but not regarding injection technique. Per power analys 18 patients per group required and a total of 72. Planned Parenthood of the Rochester Syracuse Region, NY, USA. Study length: October 1997 to January 1999. | |
| Participants | 82 pregnant women. Age: 18 years and older. Gestational age: 7‐12 weeks by LMP and phyisical exam, with sonogram if needed. Exclusion criteria: significant medical condition increasing risk for complication from procedure in clinic setting | |
| Interventions | Group 1: paracervical block at 3‐5‐7‐9 o'clock with 5‐2‐2‐5ml of 1% chloroprocaine. Group 2: paracervical block at 3‐5‐7‐9 o'clock with 5‐2‐2‐5ml of bacteriostatic (0.9% benzyl alcohol) saline. Group 3: paracervical block at 4‐8o'clock with 7‐7ml of 1% chloroprocaine. Group 4: paracervical block at 4‐8o'clock with 7‐7ml of bacteriostatic (0.9% benzyl alcohol) saline. All participants: Laminaria insertion the day prior to the procedure. Iboprofen 600mg po 1hr prior to the procedure. No po or IV sedation. Procedure started 3 minutes after paracervical block administration. Mechanical vauum aspiration followed by sharp curettage and a final pass with suction. | |
| Outcomes | Patient reported pain on a 10 point box scale (0 = no pain, 10 = worst imaginable pain) with: laminaria insertion, paracervical block administration (measured immediately after administration), aspiration (measured immediately after procedure), recovery room recollection of pain associated with the abortion procedure. Dysmenorrhea prior to conception, anxiety regarding the procedure measured on a 10 point box scale and associated with painful laminaria insertion. | |
| Notes | Analysis included 79 patients, since 2 changed their mind just after randomization and before receiving the PCB. Another patient turned out to have a gestational age of 15wks after the PCB was administered. Dysmenorrhea was also associated with and more painful paracervical block administration and aspiration. No anesthetic complication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hackett 1982.
| Methods | Randomized controlled trial at St Thomas' Hospital London, UK. Outcome assessor in recovery room was blinded. | |
| Participants | 82 pregnant women. ASA group I or II. Gestational age <12 weeks. | |
| Interventions | Group 1 (enflurane): Induction with methohexitone 2mg/kg. Maintenance with 70% nitrous oxide in oxygen and 3% enflurane until cervical dilation completed. Group 2 (fentanyl): fentanyl 1.5mcg/kg IV followed by methohexitone 2mg/kg. Maintenance with 70% nitrous oxide in oxygen and incremental doses of methohexitone. All participants: Vacuum aspiration (Karmen curettage). | |
| Outcomes | Blood loss, duration of operation, minor complications such as coughing, salivation, hiccup, laryngeal stridor, and limb movements. Recovery observations: Recovery of consciousness assessed by time of first eye opening in response to their name. Nausea, vomiting, abdominal pain (nil, slight, moderate/severe) and need for medication (yes/no) after operation during the first hour and each subsequent hour until discharge. | |
| Notes | Unclear study length, method of randomization and allocation concealment. Unclear who was blinded other than the recovery room outcome assessor. Group sequential design to determine trial size. Anestethic agent was considered a failure if moderate to severe nausea occured. The upper boundary was crossed after 74 patients indicating a statistical significnt difference between the anesthesia groups at the 5% level using a one‐sided test. The author states that the 82 patients were included; 43 in the enflurane and 39 in the fentanyl group. However, patients table 2 with abdominal pain add up to 47 and 110% in the enflurane group. Severe complications, defined as delaying the procedure, occured in 4 patients; respiratory depression in one patient in the fentanyl group, prolonged coughing with salivation in one patient in the enflurane group and 2 patients in the fentanyl group. The author could not be succeessfully contacted to clarify unclear information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hall 1997.
| Methods | Randomized trial. Outpatient clinic of the Department of Obstetrics and Gynecology at the Karolinska Hospital in Stockholm, Sweden. No power calculation. | |
| Participants | 200 pregnant women. Age: older than 18. Outpatient clinic, legal 1st trimester surgical abortion. | |
| Interventions | Group 1: preoperative vaginal prostaglandin (PG) (PGE1, gemeprost 1mg given vaginally 3hrs preoperatively) for cervical softening, surgery in general anesthesia (GA) Group 2: preoperative PG and surgery in GA and paracervical block (PCB, 10 and 10ml lidocaine 10mg/ml) Group 3: surgery in GA Group 4: GA and PCB. Premedication with morphine 5‐10mg IM 60‐90 minutes preoperatively. GA with propofol 2mg/kg IV, 60% nitrous oxide in oxygen, breathed spontaneously by mask and isoflurane supplementation as needed. Dilation of the cervix as needed followed by vacuum suction curettage and 10IU oxytocin IV. Postoperatively oral medications per patient request: paracetamol 500mg and codeine phosphate 30mg for pain or thiethylperazin 6.5mg for nausea. |
|
| Outcomes | Pain preoperatively, and postoperatively (at 1, 2, 3 and 4 hrs); consumption of analgesics postoperatively; nausea; time interval to discharge home. Study instrument: Visual analog scale (VAS; 10cm from "no pain" to "worst pain ever"; no nausea to extreme nausea) for pain and nausea. | |
| Notes | Per e‐mail communication with Dr. Persson, the coauthor, the study length was 4 months. Patients were randomized and allocation concealment was with closed envelopes. All investigators were blinded; not the operating gynecologist and not the nurses . Women in the two groups receiving PG were significantly younger, of lower gravity and parity than women in the two groups not receiving PG. The discussion does not include this being a possible confounder of the lower pain perception and analgesia need in the latter two groups. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Heath 1989.
| Methods | Randomized controlled double‐blind trial. Power analysis done to detect 10% reduction in postoperative pain with 80% power. Study underpowered, since 600 patients would have been required per their discussion. Hospital in Portsmouth, UK. | |
| Participants | 40 pregnant women. Primiparous, gestational age up to 12 weeks. Day‐case vaginal termination. | |
| Interventions | Group 1: controlled‐released dihydrocodeine 60mg with 20ml of water orally 1hour before surgery. Group 2: placebo with 20ml of water orally 1hour before surgery. All participants: anesthesia induction with alfentanil 7mcg/kg and propofol 2.5mg/kg. Anesthesia maintenance in all with propofol infusion at 9mcg/kg/hour. Patients breathed 70% nitrous oxide in oxygen via a Bain system. Oxytocin as needed. Escape analgesia postoperatively with 1g paracetamol 4 hourly orally per patient request. | |
| Outcomes | Pain and nausea assessed hourly throughout their admission with a 10cm visual analog scale. A questionnaire after discharge assessed pain and nausea upon arrival at home. | |
| Notes | One anesthesist administered all the anesthetics whilst another performed all the study assessments. Unclear study length, method of randomization and allocation concealment. Unclear if the procedure was suction or sharp curettage, but given gestational age and time of publication we assume it was vacuum curettage. Unclear if any loss after randomization. The author could not be succeessfully contacted to clarify unclear information. 3 patients required atropine to correct bradycardia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hein 1999.
| Methods | Randomized controlled double blind trial. Nurse otherwise not involved in study drew the randomized envelope. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. | |
| Participants | 140 pregnant women. ASA I‐II group. Elective termination of pregnancy. | |
| Interventions | Group 1: 1gm paracetamol suppository. Group 2: placebo suppository. All participants: no premedication. Induction of anesthesia with 0.1mg fentanyl and propofol. Maintenance with nitrous oxide in oxygen 2:1 and additional small doses of propofol as needed. Spontaneous breathing, assisted ventilation as needed. 5 units oxytocin at the end of the procedure. All patients: Vacuum aspiration. Rescue medication for postoperative pain diclofenac 100mg supp. | |
| Outcomes | Postoperative pain measured with a 10cm visual analog scale (0cm=no pain, 10cm=unbearable pain), measured 30 and 60 minutes postoperatively and at discharge. Analgesic and antiemetic requirements. Time until street fittness. | |
| Notes | Per e‐mail communication with Dr. Jakobsson: Gestational age 7‐12 weeks. Computer‐based randomization with envelopes. The study lasted over 2.5 months. All procedures were uneventful and no complications were noted. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Hein 2001.
| Methods | Randomized controlled double blind trial. Allocation concealment with envelope technique done by a nurse otherwise not involved in study. Per power analysis 70 needed per group to achieve power of 80%. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. | |
| Participants | 210 pregnant women. ASA I‐II group. Elective termination of pregnancy. | |
| Interventions | Group 1: paracetamol 1gm per os 1 hour preoperatively. Group 2: lornoxicam 8mg per os 1 hour preoperatively. Group 3: placebo. All participants: no other premedication. Induction of anesthesia with 0.1mg fentanyl and 2‐2.5mg/kg propofol. Maintenance with nitrous oxide in oxygen 2:1 and additional small doses of propofol (20‐30mg) as needed. Spontaneous breathing, assisted ventilation as needed. Some patients received laminaria preoperatively. 5 units oxytocin at the end of the procedure. All patients: Vacuum aspiration. Rescue medication for postoperative pain diclofenac 100mg supp. | |
| Outcomes | Postoperative pain on 100mm visual analog scale (0=no pain, 100=unbearable pain) measured at 30 and 60 minutes postoperatively and at discharge. Analgesics postoperatively. Antiemetics. Time to discharge | |
| Notes | Unclear method of randomization. For statistics pain was dichotomized. No complications noted. Per e‐mail communication gestational age 8‐13 weeks, and no industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jakobsson 1991.
| Methods | Randomized controlled trial. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. | |
| Participants | 165 pregnant women. ASA I group. Elective ambulatory termination of pregnancy. Gestational age: 7‐12 weeks. Patient age: 17‐44. | |
| Interventions | Group 1: alfentanil 0.5mg. Group 2: fentanyl 0.1mg. Group 3: placebo (saline). All participants: anesthesia induced with propofol and thereafter nitrous oxide in oxygen 2:1. Additional boluses of propofol 10‐30mg as needed. Paracetamol and diclofenac for postoperative pain on patient request. | |
| Outcomes | Peroperative: Complications during anesthesia, such as laryngospasm. Surgeons opinion about quality of anesthesia. Amount propofol required. Postoperative: Complaint of pain to nurse (yes/no). Request for analgesia. Nausea, vomiting. Patient self ‐assessment: qustionnaire just prior to discharge, asking about postoperative pain (slight versus intense) and emesis. | |
| Notes | Per e‐mail communication with Dr. Jakobsson: Study lasted approximately 3 months. Blinding of patient and outcome assessor. Randomization per computer. Allocation concealment with envelope technique. Gestational age 7‐12 weeks. Power analysis done. All underwent vacuum aspiration followed by sharp curettage check. One patient in the placebo group was excluded because of pronounced postoperative beeding. Data of 164 patients was analyzed. Laryngospasm in 2 patients in the placebo and in one patient in the alfentanil group. Regurgitation requiring intubation in one patient in the placebo group. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jakobsson 1993.
| Methods | Randomized controlled trial. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. Recovery room outcome assessor was blinded. | |
| Participants | 200 pregnant women. ASA I group. Termination of pregnancy. Age: 18‐45 years. Exclusion criteria: weight <45kg and >90kg. | |
| Interventions | Group 1: propofol 20mg IV followed by ketamine 20mg IV just prior to induction of anesthesia. Group 2: propofol 20mg and fentanyl 0.1mg IV 3‐4 minutes before induction of anesthesia. Group 3: thiopentone 50mg and fentanyl 0.1mg IV 3‐4 minutes before induction of anesthesia. Group 4: methohexitone 20mg and fentanyl 0.1mg IV 3‐4 minutes before induction of anesthesia. All participants: spontaneous breathing of nitrous oxide in oxygen 1:2; assisted if needed. Maintenance with increments of the respective induction agent as needed. Postoperative analgesia with paracetamol or diclofenac and morphine as rescue. Dixyrazine 5mg IV as needed for nausea. Vacuum aspiration. | |
| Outcomes | Postoperative pain (yes/no). Time to discharge. Awareness with recall, dreams, nausea, vomiting, side effects such as anxiety and psychomimetic effects. | |
| Notes | No peroperative complications. Per e‐mail communication with Dr. Jakobsson: Study lasted approximately a few months. Blinding of patient and outcome assessor. Randomization per computer. Allocation concealment with envelope technique. Gestational age 7‐14 weeks. No power analysis done. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jakobsson 1995.
| Methods | Randomized controlled trial. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. | |
| Participants | 400 pregnant women. ASA I group. Elective ambulatory termination of pregnancy. Gestational age: 7‐13 weeks. | |
| Interventions | Group 1: alfentanil 0.5 mg 1‐2 minutes before induction with thiopentone Group 2: alfentanil 1.0 mg 1‐2 minutes before induction with thiopentone Group 3: alfentanil 0.5 mg 1‐2 minutes before induction with propofol Group 4: alfentanil 1.0 mg 1‐2 minutes before induction with propofol Group 5: fentanyl 0.05 mg 1‐2 minutes before induction with thiopentone Group 6: fentanyl 0.1 mg 1‐2 minutes before induction with thiopentone Group 7: fentanyl 0.05 mg 1‐2 minutes before induction with propofol Group 8: fentanyl 0.1 mg 1‐2 minutes before induction with propofol All participants: no premedication. Bolus doses of thiopentone 50mg or propofol 20mg in addition to nitrous oxide in oxygen 2:1 as needed for maintenance. Breathing assisted only if necessary. 5 IU oxytocin at end of procedure. Postoperative analgesia with paracetamol 1 g per rectum and additional central acting analgesics as needed. | |
| Outcomes | Spontanous complaints of pain, emesis, need for analgesics and antiemetics during recovery period. Total dose of induction agent. Time to discharge. Questionnaire prior to discharge asking about memories from the procedure, dreams during anesthesia, grading of postoperative pain (pain versus severe pain), emesis, and anxiety. | |
| Notes | Per e‐mail communication with Dr. Jakobsson: Study lasted approximately 3‐4 months. Blinding of patient and outcome assessor. Randomization per computer. Allocation concealment with envelope technique. Gestational age 7‐13 weeks. All underwent vacuum aspiration, followed by sharp curettage check. Power analysis done. No major peroperative complications. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jakobsson 1996.
| Methods | Randomized controlled double‐blind trial. Karolinska Institute at Danderyds Hospital, Denderyd, Sweden. | |
| Participants | 200 pregnant women. ASA I group. | |
| Interventions | Group 1: 75mg sodium‐diclofenac IM. Group 2: 30mg ketorolac IM. Group 3: 50mg potassium‐diclofenac per os. Group 4: 4.2ml NaCl IM All groups given 10‐20min prior to anesthesia. No other premedication. All participants: 0.5mg alfentanil followed by thiopentone and nitrous oxide in oxygen 2:1. Maintenance with 25‐50mg thiopentone as needed. Ventilation assisted if necessary. Postoperative pain medication with 1g paracetamol per rectum per patient request. Rescue pain medication with 3‐5mg morphine IV. | |
| Outcomes | Postoperative pain (no pain, pain, intense pain). Postoperative emesis. Request for postoperative pain medication and antiemetics. Peri‐operative complications. Dreams during anesthesia. Anxiety during recovery. | |
| Notes | Per e‐mail communication with Dr. Jakobsson: Computer randomization. Sealed envelopes. Elective pregnancy termination. Study length 2‐3 months. Gestational age 7‐12weeks. No power analysis done. No industry sponsorship. No major complications reported. The patients who received oral diclofenac were told that the pill may not contain an active substance. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kan 2004.
| Methods | Prospective randomized controlled double‐blind trial. Computer generated randomization list. Opaque, sequentially numbered envelopes with allocation information. Per power calculations 40 patients needed in each arm. Queen Mary Hospital of University of Hong Kong in Hong Kong, China. Study length: July 2002 to April 2003. | |
| Participants | 135 pregnant women. Maternal age >16yrs. Normal general and gynecological examination. Gestational age: equal or less than 12 weeks. Size of the uterus on pelvic examination compatible with estated gestational age. Exclusion criteria: allergy or contraindication to lignocaine or prostaglandins. History of severe respiratory, cardiac or liver disease, myasthenia gravis, and psychiatric conditions requiring medication. | |
| Interventions | Stratification by parity (nulliparous or multiparous). Randomization into 3 groups. Group 1: paracervical block (PCB) with 10 mL of 1% lignocaine injected at the 4 and 8 o'clock positions of the vaginal vault 2.5cm beneath the mucosa (5ml each). Group 2: Cervical block with 10 mL of 1% lignocaine injected at the 4 and 8 o'clock positions of the cervix 2.5cm beneath the mucosa (5ml each) Group 3: no PCB. All participants: 400mcg misoprostol vaginally for cervical priming 3‐6hrs prior to the procedure. Prophylactic antibiotics. Conscious sedation with 2mg midazolam and 25mcg fentanyl IV 5 minutes prior to cervical dilation. Pethidine IM as needed for additional analgesia. Vacuum aspiration (Karmen catheter with an electrical vacuum machine). | |
| Outcomes | Pain scores measured by 100‐point visual analog scale at three points in time: (a) before the operation (following insertion of intravenous catheter), (b) just after the operation to rate pain during PCB, cervical dilation and during suction evacuation (=primary outcome), and (c) 1hr after suction evacuation. Secondary outcome measures included: satisfaction levels rated by patient prior to discharge home, sedation level and difficulty of the operation rated by the surgeon, additional analgesia, and adverse effects of PCB. Pain and anxiety were measured with a 100mm linear visual analog scale (VAS: 0 = none, 100 = most severe/painful). Sedation was measured with a sedation scale proposed by Ramsey et al. (1974) (6 very detailed defined levels with increasing sedation from level 1 to 6). Satisfaction levels were excellent, satisfactory, fair and unsatisfactory. | |
| Notes | One patient excluded from study since the uterus was found to be enlarged to 14 weeks of gestation in the operation theater. 134 remaining patients were analyzed. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kan 2006.
| Methods | Double‐blind, randomized controlled trial. Randomization by computer generated randomization list, and allocation by sealed envelope. Per power calculations 40 patients needed in each arm. University of Hong Kong, at Queen Mary Hospital, Hong Kong, China. Study length: May 2004 to January 2005. | |
| Participants | 90 pregnant women. Inclusion criteria: Maternal age >16yrs, normal general and gyecological examination, gestational age up to 12wks, size of the uterus on pelvic examination compatible with the estimated duration of pregnancy. Exclusion criteria: history of severe respiratory or cardiac disease, severe and recurrent liver disease, myasthenia gravis, psychiatric conditions requiring medication or disorders that constitute contraindications to the use of prostaglandins. History of entotox use, uperr respiratory infection, sinus blockage, recent history of middle ear or inner ear surgery or histpry of bone marrow suppression. | |
| Interventions | Group 1: entonox (50:50 mixture of nitrous oxide in oxygen) via face mask and T‐piece breathing circuit. Group 2: air. The the patient was instructed to inhale the study medication. Maintenance by inhalation as needed. All participants: 400mcg misoprostol vaginally 3‐6hrs prior to procedure. Prophylactic antibiotics. Conscious sedation with 2mg midazolam (another 1mg if sedation inadequate) and 25mcg fentanyl IV. Vacuum aspiration. | |
| Outcomes | Pain scores during venipuncture, insertion of intravenous cannula and during vaginal examination were assessed before the procedure using a 100mm linear VAS (0 = no pain, 100 = worst possible pain). Pain scores during the procedure, and 1 hr after the procedure were assessed 1 hr after the procedure using a VAS. Basal anxiety levels assessed using a state anxiety questionnaire and visual analog scale (VAS). Preprocedural anxiety was assessed by a nurse. Sedation measured by doctor using the Ramsay et al (1974) sedation scale. Post‐operative side‐effects (including nausea, vomiting, dreams, parasthesia, dizziness, dry mouth, memory of operation and euphoria) and satisfaction level assessed 1 hr after the procedure using a questionnaire, as were anxiety and satisfaction level. | |
| Notes | No paracervical block given. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Li 2003.
| Methods | Double‐blind randomized controlled trial. Computer generated randomization schedule, medication in sealed, numbered opaque envelope and plastic bag. Per power analysis 45 patients needed in each arm. University of Hong Kong, at Queen Mary Hospital, Hong Kong, China. | |
| Participants | 100 pregnant women; 70 multiparous and 30 nulliparous women. Healthy women, gestational age 7‐12wks. Exlusion criteria: significant medical diseases, medical contraindication to the use of NSAIDs or diclofenac sodium, misoprostol or lorazepam. | |
| Interventions | Group 1: arthotec (misoprostol 200mcg and diclofenac sodium 50mg, a non steroidal anti‐inflammatory drug=NSAID) Group 2: cytotec (misoprostol 200mcg) both administered 4hrs prior to procedure. All participants: lorazepam 1mg sublingually 30 min prior to procedure, rescue pain medication with pethidine25mg IV. Vacuum aspiration. | |
| Outcomes | Pain score during procedure was assessed immediately after procedure. Further pain score was assessed 1hr after the procedure. Measurement instrument: visual analogue scale (100mm linear; 0 = no pain, 100 = most severe pain) given by RN blinded to study group assignment. Further outcomes measured: Preoperative side effects of medication, cervical priming effect, subject's acceptabiliy of the pain control method (assessed 1 hr after the procedure). | |
| Notes | Unclear study length. 70 multiparous women and 30 nulliparous were randomized seperately. Half life of diclofenac sodium is 1‐2hrs. No paracervical block. One patient excluded from analysis since the operation was done under general anaesthesia upon her request. No intention to treat analysis, but not possible given that with general anesthesia there is no intraoperative pain measurable. No serious complications observed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Li 2006.
| Methods | Prospective double‐blind randomized placebo‐controlled trial. Computer randomization and allocation with sealed envelopes. Per power analysis for which a decrease of pain level by 0.5 standard deviations chosen as being acceptable 64 patients needed in each arm. Family Planning Association of Hong Kong, China. Study length: April to June 2005. | |
| Participants | 140 pregnant women. Inclusion criteria: Maternal age: older than 16 years. Gestational age 7‐10 weeks at day of procedure. Normal general and gynecological exam. Uterine size corresponding to gestational age or ultrasound dating. Being ethnic Chinese and Cantonese speaking. No history of complicated medical or surgical problems for which operation in community‐based day‐care setting wa contraindicated. Exlusion criteria: Known allergy to lognocaine or prostaglandin or medical contraindication to the use of prostaglandins. | |
| Interventions | Group 1: 2% lignocaine jelly, 3ml applied to cervix, 7ml applied to Hegar dilators and vaginal speculum 1 minute prior to procedure. Group 2: KY jelly (placebo) applied in same fashion. All subjects: cervical priming with 400micrg misoprostol prior to the procedure (1‐2 hours in multiparous, 3‐5 hours in nulliparous subjects). Premedication with 5mg diazepam po and 1mg/kg pethidine IM 15‐30 minutes prior to the procedure. Rescue pain medicatioin with pethidine repeat dose IM. Suction evacuation with electrical vacuum machine. | |
| Outcomes | Pain on an 11‐point verbal analog scale from 0 to 10 (0 = no pain at all, 10 = intolerable pain) preoperative with pethidine injection, on arrival in the operating room for preoperative pain (i.e. from misoprostol), with cervical manipulation and/or dilation, immdiately after operation for overall intraoperative pain and 1 hour after operation. Before discharge satisfaction toward pain control was assessed (0 = totally unsatisfied to 5 excellent). In addition the surgeon graded the level of patient sedation preoperatively and intraoperatively according to standard scale from 1 to 6, described by Ramsay et al 1974. | |
| Notes | Subanalysis for nulliparity versus multiparity was performed. Due to missing part of data 6 patients were excluded in the lignocaine and 3 in the KY jelly group. Results for excluded patients not available. Analysis only includes 64 patients for the lignocaine and 67 patients for the KY Jelly group. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lindholm 1994.
| Methods | Randomized controlled trial. The study solutions were prepared by the local hospital pharmacy and delivered in blinded identical looking ampoules. Anesthesiologists were blinded as well (pupil size was not assessed intraoperatively). Odense University Hospital, Odense, Denmark. | |
| Participants | 120 pregnant women. Gestational age <12 weeks. Healthy patients. Exclusion criteria: allergy to trial drugs, on medications likely to influence the course of anesthesia, more than 20% overweight. | |
| Interventions | Group 1: normal saline 0.03ml/kg Group 2: fentanyl 1.5mcg/kg Group 3: alfentanil 15mcg/kg bolus injection. All participants: Premedication with lorazepam 2mg orally. 2 minutes after study medication induction of anesthesia with propofol 2mg/kg IV with lignocaine to lessen pain of injection. Additional increments of propofol 0.5mg/kg IV as needed for induction and maintenance of anesthesia. Alfentanil 100mcg/kg IV as rescue medication if more than four incremental doses of propofol required. Ergometrine 0.5mg IV during operation. Postoperative analgesics were paracetamol 1g for moderate pain, pethidine 75mg IM for severe pain. Vacuum aspiration. | |
| Outcomes | Postoperative pain intensity assessed with 10cm visual analog scale, anchored with "no pain" at 0 and "worst pain imaginable" at 10cm at 30, 120 and 180 minutes. Need for postoperative analgesics. Duration of induction, vital signs, induction dose and total dose of propofol, need for alfentanil, quality of induction movement of patient with surgery stimulus. Recovery: open eyes on command, give birthdate, cooperation score. Complications and side effects during and after the operation. | |
| Notes | Unclear randomization. No power analysis mentioned. The author could not be succeessfully contacted to clarify unclear information. Laryngospasm occured in 1 patient in the fentanyl group. One patient in the control group was difficult to ventilate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Liu 2005.
| Methods | Randomized placebo‐controlled double‐blind trial. Allocation using sealed opaque envelopes. Power calculations done for a power of 0.8 and alpha=0.05 to detect a 20% difference in opioid usage between groups. KK Women's and Children's Hospital, Singapore. | |
| Participants | 40 pregnant women. ASA I and II, elective first trimester termination of pregnancy in an ambulatory surgical center. Exclusion criteria: asthma, gastritis, coagulation disorder, renal impairment, allergy to NSAIDs or COX‐2 inhibitors, history of long‐tern analgesc use, or use of any agent that may influence the analgesic response. | |
| Interventions | Group 1: etoricoxib 120mg orally with 20ml plain water Group 2: placebo both 30‐60 minutes prior to surgery. All participants: general anesthesia with IV propofol 2.0‐2.5mg‐kg for induction and oxygen nitrous mixture (40:60) and Desfluran (end tidal) 1% at fresh gas flow of 3l/min. Postoperatively per patient request of if verbal analog scale (VAS) for pain >50mm IV fentanyl bolus of 25mcg every 15 minutes until comfortable or VAS<50mm. | |
| Outcomes | Main outcome: post‐operative need for fentanyl. Post‐operative pain assessed through a blinded observer using a 100mm verbal analogue score (VAS; 0=no pain and 100=the worst imaginable pain). Time points were emergence from general anesthesia, 15 minutes, 30 minutes, and 60 minutes post‐operatively and at discharge. Further outcomes: side effects (nausea, vomiting gastric pain, heart burn, or dizziness), time to first drink, time to step‐down, time between step‐down and discharge, and patient satisfaction scores (100‐point analogue scale; 0 = very bad experience and 100 = excellent experience) assessed by blinded observer. Via telephone pain sores were assessed at 6 hours and 24 hours post‐operatively as well as need for rescue analgesia with acetaminophen 1g every 6 hours. | |
| Notes | Unclear study length, and randomization method. Unclear if vacuum or sharp curettage. The author could not be succeessfully contacted to clarify unclear information. No major complication reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Marc 2007.
| Methods | Open randomized pilot study. Computer generated list of random numbers in blocks of 6 and 4. Allocation concealment with sealed opaque envelpes. Study period: August to September 2003. Family Planning Clinic of the Hospital Saint‐Francois d'Assise, University Laval in Quebec City, Canada. | |
| Participants | 30 pregnant women. Age 18 yrs or older, Gestational age: between 6 and <14weeks, elective abortion. Exclusion criteria: non french speaker, referal from hospital outside of Quebec City, medical condition requiring preplanned IV sedation, daily use of any illegal drug. | |
| Interventions | Group 1: Hypnosis Group 2: standard care. All participants: paracervical block with total of 12ml 0.5% lidocaine on the cervix at 12 o'clock, and at 4 and 8 o'clock at 1‐2cm depth. Vacuum aspiration followed by sharp curettage. Nitrous oxide in oxygen 1: 1 via nose mask per patient request. Intracervcal laminaria 4‐12 hours prior to surgery for gestational age of 9 weeks or more, stenosis or surgical history of the cervix, age less than 20 zears, gravidity of 5 or more. | |
| Outcomes | Self reported pain and anxiety measured with a 11‐point verbal numerical scale (0 = no pain/not unpleasant at all/not anxious, 10 = the most intense pain/unpleasantness/anxious possible) preoperatively, with insertion of the speculum, with maximum dilation, suction and end of curettage as well as in recovery. Use of N2O (dichotomous variable). | |
| Notes | 47 patients eligible, 17 refuse to participate, 30 are randomized. One patient in the hypnosis group was excluded after randomization and before hypnotic intervention since IV sedation had been already scheduled. Study may be underpowered to detect difference in anxiety and pain. No double‐blinding due to hypnosis, may introduce bias. Inability to differentiate between specific and non‐specific effect of hypnosis. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ogg 1983.
| Methods | Randomized controlled trial with additional 2 non‐randomized control groups Outcome assessor of side effects was blinded. Addenbrooke's Hospital, Cambridge, UK. | |
| Participants | 60 participants. 40 were randomized to the 2 trial arms. Healthy women. Age 16‐40. Gestational age <12 weeks. Additional, non‐randomized control groups of 10 women each for memory function: 1) Inpatients awaiting minor gynecological surgery who had received no general anesthesia. 2) Ten female nurses | |
| Interventions | Group 1 (trichlorethylen): fentanyl 1.5mcg/kg IV followed by methohexitone 1% 1.5mg/kg with 5ml lignocaine. Nitrous oxide 2 5l and oxygen 3l per minute. Trichloethylene 0.5‐1%. Spontaneous respiration was maintained via coaxial Bain system. Group 2 (total intravenous group, fentanyl): fentanyl 1.5 mcg/kg IV followed by methohexitone 1% 1.5mg/kg with lignocaine 0.1%. Supplements of methohexitone 0.25mg/kg as needed. Spontaneous respiration was maintained Mary Catterral mask. | |
| Outcomes | Drugs administered, duration of anesthesia, immediate recovery time. Side effects during anesthesia and for 2 hours postoperatively including pain on induction and postoperatively (yes/no), as well as hiccoughs, laryngospasm, vomiting, involuntary muscle movements during anesthesia and nausea, vomiting, shivering, dizziness, headache, drowsiness and tearfulness in recovery. Heart rate and mean systolic blood pressure. Memory function. | |
| Notes | Unclear study length, method of randomization and allocation concealment. Unclear who was blinded other than assessor of side effects. Unclear if suction curettage or sharp curettage was used for the procedure. Given that at that time suction curettage was predominantly used, we decided to include this study. No power analysis. Small sample size. Pain not measured in detail, only yes versus no, and it is not clear if it was self reported. The author could not be succeessfully contacted to clarify unclear information. Laryngospasm in one patient in the trichlorethylene group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Phair 2002.
| Methods | Prospective, randomized, non‐blinded (cannot blind waiting versus non waiting) trial. Per power calculations sample size of 196 needed to detect 1cm difference in pain. Columbia Willamette Planned Parenthood, Portland, Oregon, USA. Study length: May 2000 to June 2001 | |
| Participants | 199 pregnant women. Age 18 yrs or older. Gestational age <11wks. Good general health. English language comprehension. Exclusion criteria: major psychiatric symptoms as well as PID, intravenous sedation or misoprostol. | |
| Interventions | Group 1: 3‐5 minute wait between injection and dilation of the cervix Group 2: no wait. All participants: paracervical block (PCB) with total of 12ml 1% buffered lidocaine at 12 (superficially), 4 and 8 o'clock (1‐2cm deep). Diazepam 5mg po and /or fentanyl 100mcg IV as additional pain medication per patient request. Vacuum aspiration. | |
| Outcomes | Self reported pain and measured with a 10cm visual analog scale with dilation, aspiration and post procedure as well as anticipated pain. Satisfaaction at the end of the procedure (very satisfied, somewhat satisfied, netrual, somewhat dissatisfied, very dissatisfied). | |
| Notes | Per e‐mail communication: randomization with computer generated random numbers in blocks of 50. Allocation concealment with sequentially numbered opaque envelopes. 194 women completed the study and were analysed. 2 excluded for administration of extra sedative medications and 2 lost to follow‐up. One woman chose to discontinue. No major complications reported. Subanalysis per fentanyl use. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Raeder 1992.
| Methods | Randomized double‐blind (except for intraoperative assessment by anesthesiologist), controlled trial. Hospital in Baerum, Sykehus, Norway | |
| Participants | 59 pregnant women. Inclusion criteria: first trimester abortion, weight 50‐80kg, ASA I or II. | |
| Interventions | Group 1: regional anesthesia (group R). Midazolam 0.1mg/kg IV and alfentanil 0.01mg/kg IV followed by paracervical bock with 20ml mepivicaine 20mg/ml with adrenaline 0.005mg/ml. Patient breathed spontaneous and assisted with oxygen as needed. Group 2: general anesthesia (groups G). Alfentanil 0.01mg/kg IV, followed by bolus of propofol 2.0mg/kg. Patients breathed 75% nitrous oxide in oxygen spontaneously by mask and were assisted as needed for dropping oxygen saturation. | |
| Outcomes | Anesthesia data: patient discomfort during anesthesia induction, cervical dilation and uterine curettage. Duration of anesthesia, duration of sleep, apnea. Vital signs. Side effects. Overall evaluation of the procedure by the anesthesiologist, gynecologist and patient. Postoperative function (at 15, 30, 60, 120 and 180 minutes postoperatively): wakefulness, cooperation, postoperative amnesia, drinking, voiding, walking at different time intervals, p‐deletion score, Maddox Wing Score. Postoperative side effects: Pain, nausea, headache (postoperatively at 0‐15, 15‐60, 60‐120, 120‐180 minutes) rated on visual analog scale (0 = no pain to 100 = extreme pain) and blurred vision. Postoperative questionnaire (sent 5 days postoperatively): discomfort and side‐effects during hospital stay, travel home and at home, wakefulness, everyday function. | |
| Notes | Per e‐mail communication with the author: Randomization using a table of random numbers generated by computer. Allocation concealment: sealed envelopes. Vacuum aspiration. The investigators of postoperative patient function were blinded. No power analysis. 88 consecutive patients were asked to participate, 21 refused, 8 met exclusion criteria. No patient exlcuded after inclusion. No signs of mepivicaine toxicity. Apnea, defined as oxygen saturation of less than 85% in more than seconds, in 25% of patients with propofol (none in regional group; statistically significant p<0.0001). Patient discomfort with cervical dilation and uterine curettage in the general anesthesia group was likely assessed by the anesthesiologist and not patient reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rossi 1995.
| Methods | Randomized controlled trial. Surgical day‐case hospital, Napoli, Italy. | |
| Participants | 120 pregnant women. ASA I and II. Elective pregnancy termination. Age: 18‐40. Gestational age: 6 to 10 weeks. Exclusion criteria: allergies to study medications, hypertension, psychiatric problems, drug dependance. | |
| Interventions | Group 1: fentanyl 0.005mg/kg and midazolam 0.2mg/kg. Group 2: fentanyl 0.005mg/kg and propofol 2.5mg/kg. Group 3: ketamine 0.5mg/kg and propofol 2.0mg/kg. All participants: Premedication with atropin 0.007mg/kg 5 minutes prior to anesthesia induction. Maintenance with 70% nitrous oxide in oxygen after cervical dilation via spontaneous‐assisted ventilation. Additional increments of 1/4 dose of anesthetics given as needed. Vacuum aspiration (Karman method). | |
| Outcomes | Postoperative pain (yes/no). Anesthesia: Quality and rapidity of some neurofunctional aspects of recovery using the Steward Score and the Coin Counting Test respectively. Side effects including pain with anesthesia injection, and postoperive rash, halluzinations, tremor, nausea and vomiting, headache, unpleasant dreams. | |
| Notes | Unclear study length, method of randomization and allocation concealment. Unclear who was blinded. Unclear if pain was self reported. The author could not be succeessfully contacted to clarify unclear information. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shapiro 1975.
| Methods | Randomized controlled trial. University Hospital in Miami, USA. | |
| Participants | 144 pregnant women. Gestational age: 6‐12 weeks. | |
| Interventions | Group 1: control Group 2: self‐administered methoxyflurane (0.5 volume % with 5l oxygen per minute) Group 3: stereophonic headphones with music chosen by patient. All participants: premedication with Valium 10mg orally 1‐2 hours preoperatively. Paracervical block with 20ml of 1% carbocaine. Vacuum aspiration. | |
| Outcomes | Pain with the procedure scored by patient, physician, nurse and counseler (scoring by staff/patient: 0 = no observalble signs of pain/"absolutely no pain or minimal cramping"; 1+ = some movement or grimace during the procedure/"I had some pain"; 2+ = moderate amount of pain/"It really hurt ‐‐ very uncomfortable"; 3+ = severe pain, no relief/"very painful ‐‐excruciating"). Amnesia. | |
| Notes | Unclear study length, method of randomization and allocation concealment. Not clear which pain assessment shown in results; self reported versus staff assessed. The author could not be succeessfully contacted to clarify unclear information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Suprapto 1984.
| Methods | Double‐blind placebo controlled randomized study. Family planning clinic in Hagerstown, Maryland, USA. | |
| Participants | 137 pregnant women. Age: 16‐35years. First trimester elective abortion. General good health. | |
| Interventions | Group 1: single dose naproxen sodium 550mg orally 1‐2 hours prior to abortion Group 2: placebo Group 3: no drug. All participants: paracervical block with 1% lidocaine followed by vacuum aspiration. | |
| Outcomes | Pain during abortion (assessed in recovery room), 15 an 30 minutes postoperatively, assessed with visual analogue scale (0 = no pain to 99 = severe pain). Any adverse effects. Estimated blood loss. | |
| Notes | No major complication observed. Unclear study length, method of randomization and allocation concealment. The author could not be succeessfully contacted to clarify unclear information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wells 1989.
| Methods | Randomized controlled trial. Blinding: nurses, counsellors, physicians and technicians were unaware of the interventions that were tested. Free standing private reproductive health clinic in the metropolitan area of New England, USA. | |
| Participants | 40 participants. English speaking. Mentally competent. Uncomplicated first trimester abortion. Gestational age 5 ‐ 11 weeks. | |
| Interventions | Group 1: relaxation ecxercise for 10 minutes prior to the procedure Group 2: pleasant imagery (beach or mountain) 7 minute practice session prior to the procedure Group 3: analgesic imagery 8 minute practice session prior to the procedure Group 4: attention control no instruction in a technique but advise to use coping strategy that worked in a previous painful experience; 10‐15 minutes prior to the procedure . All participants received local anesthesia. | |
| Outcomes | Pain sensation measured with a 10cm graphic rating scale, distress measured with a 10cm graphic rating scale at worst (assessed at the end or the procedure) as well as in the recovery area. Time participants used the technique during the procedure. Length or procedure, length of time in recovery area, analgesics for the first 24 hours after abortion. | |
| Notes | Out of 145 patients 43 agreed to participate. One did not meet inclusions criteria. Per e‐mail communication with Ms Wells: randomized study, but does not recall method of randomization and allocation concealment. She herself did all the interventions and assessed the outcomes. Study length was 3‐4 months in 1985‐1986. Study participants underwent vacuum aspiration. Pilot study, undepowered. No industry sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wells 1992.
| Methods | Randomized controlled trial. 2x2 factorial design: first factor type of information provided before the abortion, second factor type of anesthesia. Free standing private reproductive health clinic in the metropolitan area of New England, USA. | |
| Participants | 84 pregnant women. Inclusion criteria: more than 18 years old, able to read and understand English. Age 18 to 44 years. Gestational age: first trimester. | |
| Interventions | First factor Group 1: provision of sensory information (3 minute audio taped message containing orienting information as well as nine sensations related to abortion and identified by over 50% of women in a previous pilot study) First factor Group 2: provision of general information Second factor Group 1: local cervical block Second factor Group 2: local cervical block and intravenous sedation with diazepam and fentanyl. | |
| Outcomes | Pain intensity during abortion measured on a 10 cm VAS with anchors of "no sensation" and "the most intense sensation imaginable". Subjective distress measured on a visual analog scale of 10 cm with anchors of "not bad at all " and " most intense bad feeling possible for me". Distress checklist; a 7 item observational checklist tapping four categories of behavior ‐ facial expression, posture, vocalization and verbalization. State anxiety measured with the STAI (State ‐ trait anxiety inventory) | |
| Notes | High refusal rate. 94 women agreed to participate (25% acceptance rate), 84 had complete data. Incomplete data: 3 women could not undergo procedure due to gestational age, in 7 cases there was a lack of a blinded observer.
Per e‐mail communication with Ms Wells: study length was between January and March 1988. Study participants underwent vacuum aspiration. Randomization using block randomization, and allocation concealment with envelopes. The research assistent who collected data during the procedure and in the recovery room were blinded. Gestational age was first trimester. Medication dose was not recorded. Per power analysis using a moderate effect size (as defined by Cohen) with a power of .80 and alpha of .05, 42 per group needed. High refusal rate of 75%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wiebe 1992.
| Methods | Randomized double‐blind (for phase one), controlled trial. Free‐standing urban abortion clinic in British Columbia, Canada. | |
| Participants | 249 pregnant women. Mean age 25‐28 years depending on group, mean gestational age 8.5‐8.7 depending on group. Exclusion criteria: unable to understand or unwilling to sign the consent form. | |
| Interventions | Phase 1 Group 1: 10ml 2% carbonated lidocaine with 2mg atropin/50ml, injected at 3 to 6 sites (12, 3, 6 or 12, 2, 4, 6, 8, 10 o'clock) 1/2 inch deep at the reflection of the vagina off the cervix. Phase 1 Group 2: 10ml 2% plain lidocaine with 2mg atropin/50ml, injected at 3 to 6 sites (12, 3, 6 or 12, 2, 4, 6, 8, 10 o'clock) 1/2 inch deep at the reflection of the vagina off the cervix. Phase 2 Group 1: 10ml 2% plain lidocaine with 2mg atropin/50ml, injected at 3 to 6 sites (12, 3, 6 or 12, 2, 4, 6, 8, 10 o'clock) 1/2 inch deep at the reflection of the vagina off the cervix. Phase 2 Group 2: 20ml 1% plain lidocaine with 1mg atropin/50ml. 1ml injected at 6 sites (12, 2, 4, 6, 8 and 10 o'clock) superficially to blanch the mucous membrane, then 3‐4ml injected 1 to 1.5 inches deep at 4 sites (4, 6, 8, and 10 o'clock). No delay between injection and procedure. All participants: premedication with 1mg lorazepam sublingual 30 minutes prior to procedure per patient request. | |
| Outcomes | Patient reported pain with dilation and procedure measured at end of dilation and at end or procedure. Study instrument: 11 point verbal pain scale (0 = no pain to 10 = worst pain you can imagine). Effect of lorazepam on pain. | |
| Notes | Per e‐mail communication with Dr. Wiebe: study length was few months at the beginning of 1991. Gestational age at that clinic 6‐14 weeks. Study participants underwent vacuum aspiration. Randomization using a table of random numbers generated by computer. Allocation concealment: The nurse drew up the syringes. The nurse was not blinded, but the doctor, couselor and patient were. No power analysis. No information on number of people randomized and discontinued availbable. No complication with deep injection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Wiebe 1995.
| Methods | Randomized double‐blind (blinding of patient, counselor and physician for phase 1 and 2, as well as nurse for phase 1) controlled trial. Phase 3: no blinding, randomization by procedure day. Phase 4: no blinding. Free‐standing urban abortion clinic in British Columbia, Canada. | |
| Participants | Total of 480 pregnant patients for phase 1, 2 and 4. Phase 3 with 139 pregnant women. First trimester. Exclusion criteria: unable to understand or unwilling to sign the consent form. Allergic to lidocaine, bupivacaine or ibuprofen. | |
| Interventions | Phase 1 Group 1: 600g ibuprofen 30 minutes prior to procedure Phase 1 Group 2: placebo 30 minutes prior to procedure All participants in phase 1: paracervical block (PCB) with 20ml 1% lidocaine (10ml injected in 4 to 6 sites around the cervix and 5ml each between 3 and 4 o'clock and between 8 and 9 o'clock, about 1 inch deep from the reflection of the vagina into the lower uterine segment, and as decsribed in their included study from 1992). Phase 2 Group 1: PCB with 20ml plain 1% lidocaine Phase 2 Group 2: PCB with 20ml 1% lidocaine buffered with 2ml 8.4% sodium bicarbonate Phase 2 Group 3: PCB wih 20 ml 0.25% bupivacaine Phase 3: comparison of waiting time 1, 3, 10 or 20 minutes after administrating PCB with bupivacaine Phase 4: fast (30 sec) versus slow (60 sec) injection of buffered lidocaine for PCB on right or left side of cervix (randomized which to speed and side which was first injected). No waiting time. between injection and procedure. All participants: premedication with lorazepam 0.5‐1mg SL per patient request 30 minutes prior to procedure. Vacuum aspiration followed by sharp curettage. | |
| Outcomes | Phase 1: pain with procedure, measured at end of procedure. Pain 30 minutes after the procedure. Phase 2: pain with injection and with procedure. Phase 3: pain with procedure. Phase 4: pain with injection of each side. Pain measured on a verbal 11‐point pain scale (0 = no pain to 10 = worst pain you can imagine) in phase 1 to 3, and with a 10cm long visual anlog pain scale in phase 4. | |
| Notes | Per power analysis 65 patients per group required to detect mean pain score difference of 1 with a standard deviation of 2 at the 0.05 significance level. Not all groups had that many patients. Per e‐mail communication with Dr. Wiebe: randomization using tables of computer‐generated random numbers, allocation concealment with opaque numbered envelopes. Phase 3 was randomized by procedure day, which is not adequate randomization. No major complicaions reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wiebe 1996.
| Methods | Randomized double‐blind, controlled trial. Two free‐standing urban abortion clinics in British Columbia, Canada. | |
| Participants | 209 pregnant women. Maternal age 14 to 42 years. Gestational age 7 to 14 weeks. | |
| Interventions | Group 1: para‐cervical block (PCB) with 20 ml1.0% lidocaine Group 2: PCB with 20ml 0.5% lidocaine. Some patients received preoperative laminaria, lorazepam or ibuprofen. | |
| Outcomes | Pain with procedure measured with a 11‐point verbal pain scale (0 = no pain to 10 = worst pain you can imagine) after the procedure. Anxiety (mild, moderate, severe). | |
| Notes | Per e‐mail communication with Dr. Wiebe: study length was approximately June to December 1995. Exclusion criteria: inability to understand consent, allergy to lidocaine. Study participants underwent vacuum aspiration. Randomization using a table of random numbers generated by computer. Allocation concealment: The research assistant drew up the syringes. No information on number of people randomized and discontinued availbable. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Wiebe 2003.
| Methods | Randomized double‐blind, placebo‐controlled trial. Randomization per computer generated list of random numbers. Allocation with sequentially numbered opaque envelopes. Power analysis done. A difference of 1.5 was chosen to be clinically important. Urban free‐standing urban abortion clinic in British Columbia, Canada. Study length: July 2000 to November 2001. | |
| Participants | 104 pregnant women. Gestational age equal to or less than 14wks. Mean maternal age 25‐27. Excluded women who were unable to understand or unwilling to sign consent form. Allergy to lorazepam. | |
| Interventions | Group 1: 1mg lorazepam orally Group 2: placebo both given 1 hour prior to vacuum aspiration. All participants received local anesthesia. | |
| Outcomes | Anxiety and depression prior to counseling and after counseling. Anxiety and pain during procedure assessed after the procedure. All outcomes were assessed with a 11‐point verbal scale (0 = no pain/no anxiety to 10 = worst pain you can imagine/most anxious you could be). | |
| Notes | Per e‐mail communicaion with Dr. Wiebe: Lorazepam was given orally. No participant 14+0 or more wks of gestational age. Study also had observational arm with 262 pregnant women, in which patients choosing lorazepam were compared to the group not choosing lorazepam. Mild side effects noted in 3 study subjects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wong 2002.
| Methods | Randomized double‐blind, placebo‐controlled study. Computer randomization in blocks of 10. Sealed opaque envelope for allocation. Per power analysis 45 participants needed in each arm to detect a difference mean pain score of 1.5 with a power of 80%. Queen Mary Hospital in Hong Kong China. Study length: September to December 1999. | |
| Participants | 100 pregnant women. Age: >16 years. Gestational age <12 weeks, and uterine size on pelvic exam compatible with estimated gestational age. Normal genereal and gynecological examination. Exlusion criteria: severe and recurrent liver disease, myasthenia gravis, psychiatric conditions requiring medication or contraindication to misoprostol. | |
| Interventions | Group 1: placebo (saline) IV 5 min before cervical dilation Group 2: conscious sedation with midazolam 2mg and fentanyl 25mcg IV All participants: prophylactic antibiotics. 400mcg misoprostol vaginally 3‐6hrs prior to procedure for cervical ripening. Paracervical block with 10ml of 1% lignocaine at 4 and 8 o'clock of the cervix 2 min after study medication. Vacuum aspiration. Rescue pain medication with pethidine 75mg IM. | |
| Outcomes | Pain rated on a 11‐point verbal analog scale (0 = no pain to 10 = intolerable pain) during intravenous catheter insertion, suction evacuation (SE), 5 min and 1 hrs after SE. Sedation (Ramsay scale). Severity (none, mild, moderate, severe) of post‐operative side‐effects (nausea, vomiting, dizziness and drowsiness). Satisfaction level (excellent, satisfactory, fair and unsatisfactory) prior to discharge from the hospital (usually 4 hrs after SE). | |
| Notes | 3 patients in the conscious sedation group and 1 patient in the placcebo group needed pethidine, and the difference was not statistically significant. All patients completed study and were analysed. No major complications reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andolsek 1977 | Pain related to the abortion was not reported. |
| Bonazzi 1987 | Unclear range of gestational age (mean 9.8 with SD of 1.6), unclear if vacuum curettage. The author could not be successsfully contacted. |
| Bonazzi 1994 | Unclear range of gestational age (mean 9.8 with SD of 1.4), unclear if vacuum curettage. The author could not be successsfully contacted. |
| Cade 1993 | Randomization by hospital medical records number does not qualify as randomization. Unclear if procedure was suction curettage versus sharp D&C |
| Ciri 1986 | Pain related to the abortion was not reported. |
| Coad 1986 | Pain is not measured with the procedure, but only as side effect of the anesthesia administration |
| Corli 1984 | Amount of pain is measured in a dichotomous fashion only with clamping of the cervix and at what amount of cervical dilation; not with the procedure itself. The range of the gestational age is unclear, however, given that vacuum curettage was performed we assume it was first trimester. The author could not successfully be contacted to clarify questions. |
| Crawford 1984 | Pain is not an outcome measured |
| Edelist 1987 | Pain with or after the procedure is not measured, but only pain as side effect of the anesthesia administration (meantioned in the discussion). Unclear if vacuum or sharp curettage. Author could not successfully be contacted to clarify questions. |
| Enlund 1996 | Outcome of number of days of sick leave depending on anesthetic used leads to follow up of several days, which is beyond the scope of our review. |
| Eriksson 1995 | Pain with or after the procedure is not measured, but only pain as side effect of the anesthesia administration and postoperative analgesia requirements. Unclear if vacuum or sharp curettage |
| Erkola 1990 | The outcome studied is the treatment of anesthesia side effects. Unclear if the procedure was a dilation and curettage or a vacuum aspiration. |
| Hamar 1989 | Pain related to the abortion was not reported. |
| Jakobbson 1990 | Randomization by date of birth. |
| Kallela 1994 | Pain related to the abortion was not reported. Only assessed need for postoperative analgesics and pain with injection of anesthetic. Author could not successfully be contacted to clarify questions. |
| Lichtenberg 2003 | Pain related to the abortion was not reported. |
| Lowenstein 2006 | Randomization by day of treatment. |
| Matambo 1999 | only letter to editor. |
| Miller 1996 | Not adequately allocated |
| Nielsen 1975 | Not adequately randomized |
| Parkash 1979 | Not randomized. |
| Peters 1978 | Unclear if randomized. Author could not successfully be contacted. |
| Rawling 2001 | Gestational age up to 14.5 weeks. |
| Sanders 1984 | Pain related to the abortion was not reported. |
| Schoeffler 1987 | Pain related to the abortion was not reported. |
| Verma 1985 | Pain related to the abortion was not reported. |
| West 1985 | Pain related to the abortion was not reported. |
| Wiebe 2005 | Gestational age up to 16 weeks as per e‐mail clarification with the author. |
| Willdeck‐Lund 1975 | Not randomized. Tried to contact the author, but he passed away. |
Contributions of authors
Dr. Renner performed the literature search, data collection and analysis, and drafted the protocol.
Dr. Edelman performed data collection and analysis, edited and advised on the protocol, and provided clinical expertise.
Dr. Jensen and Dr. Nichols edited and advised on the protocol, and provided clinical expertise.
Sources of support
Internal sources
-
Dept. of Obstetrics and Gynaecology, Oregon Health and Science University, Portland, USA, Not specified.
Library services.
External sources
No sources of support supplied
Declarations of interest
Dr. Renner has no conflicts of interest Dr. Edelman is a consultant for ScheringPharmaceuticals. Dr. Jensen has served on the speakers bureau for Wyeth, Ortho‐McNeil, Pfizer, and Bayer Healthcare Laboratories. He also is a member of the Wyeth & Berlex Contraceptive Advisory Boards. He has received grant support form Wyeth, Pfizer, Ortho‐McNeil, Symbollon, Warner‐Chilcott, and Bayer Healthcare laboratories. Dr. Nichols has served on the speakers bureau for Organon and Bayer Healthcare. He received research funding from Conceptus (manufacturer of Essure).
Drs. Edelman, Jensen, and Nichols have been involved with several of the studies included in this review. These studies did not receive pharmaceutical funding.
New
References
References to studies included in this review
Barneschi 1985 {published data only}
- Barneschi MG, Calamandrei M, Livi P, Marinelli L, Peduto VA. Anesthesia for outpatient termination of pregnancy: a comparison of anesthetictechniques. Acta Anaesthesiologica Italiana 1985;36(3):411‐7. [PubMed] [Google Scholar]
Bone 1988 {published data only}
- Bone ME, Wilkins CJ, Lew JK. A comparison of propofol and methohexitone as anaesthetic agents for electroconvulsive therapy. European Journal of Anaesthesiology 1988;5(4):279‐86. [PubMed] [Google Scholar]
Bonnardot 1987 {published data only}
- Bonnardot JP, Maillet M, Brulé ML, Deligné P. Ambulatory anesthesia and induced abortion. Comparative study of propofol‐alfentanyl and ketamine‐midazolam combinations [Anesthesie ambulatoire et interruption volontaire de grossesse. Etude comparative des associations propofol‐alfentanil et ketamine‐midazolam]. Annales francaises d'anesthesie et de reanimation 1987;6(4):297‐300. [DOI] [PubMed] [Google Scholar]
Boysen 1989 {published data only}
- Boysen K, Sanchez R, Krintel JJ, Hansen M, Haar PM, Dyrberg V. Induction and recovery characteristics of propofol, thiopental and etomidate. Acta anaesthesiologica Scandinavica 1989;33(8):689‐92. [PUBMED: 2589002] [DOI] [PubMed] [Google Scholar]
Boysen 1990 {published data only}
- Boysen K, Sanchez R, Ravn J, Pedersen E, Krintel JJ, Dyrberg V. Comparison of induction with and first hour of recovery from brief propofol and methohexital anesthesia. Acta anaesthesiologica Scandinavica 1990;34(3):212‐5. [PUBMED: 2343720] [DOI] [PubMed] [Google Scholar]
Cetin 1997 {published data only}
- Cetin M, Cetin A. The role of transvaginal sonography in predicting recurrent preterm labour in patients with intact membranes. European journal of obstetrics, gynecology, and reproductive biology 1997;74(1):7‐11. [PUBMED: 9243192] [DOI] [PubMed] [Google Scholar]
Collins 1985 {published data only}
- Collins KM, Plantevin OM, Whitburn RH, Doyle JP. Outpatient termination of pregnancy: halothane or alfentanil‐supplementedanaesthesia.. British Journal of Anaesthiology 1985;57(12):1226‐31. [DOI] [PubMed] [Google Scholar]
Dahl 2000 {published data only}
- Dahl V, Fjellanger F, Raeder JC. No effect of preoperative paracetamol and codeine suppositories for pain after termination of pregnancies in general anaesthesia. European journal of pain (London, England) 2000;4(2):211‐5. [PUBMED: 10957701] [DOI] [PubMed] [Google Scholar]
Edelman 2004 {published data only}
- Edelman A, Nichols MD, Leclair C, Astley S, Shy K, Jensen JT. Intrauterine lidocaine infusion for pain management in first‐trimester abortions. Obstetrics and gynecology 2004;103(6):1267‐72. [DOI] [PubMed] [Google Scholar]
Edelman 2006 {published data only}
- Edelman A, Nichols MD, Leclair C, Jensen JT. Four percent intrauterine lidocaine infusion for pain management in first‐trimester abortions. Obstetrics and gynecology 2006;107(2 Pt 1):269‐75. [PUBMED: 16449111] [DOI] [PubMed] [Google Scholar]
Glantz 2001 {published data only}
- Glantz JC, Shomento S. Comparison of paracervical block techniques during first trimester pregnancy termination. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2001;72(2):171‐8. [PUBMED: 11166751] [DOI] [PubMed] [Google Scholar]
Hackett 1982 {published data only}
- Hackett GH, Harris MN, Plantevin OM, Pringle HM, Garrioch DB, Avery AJ. Anaesthesia for outpatient termination of pregnancy. A comparison of two anaesthetic techniques. British journal of anaesthesia 1982;54(8):865‐70. [PUBMED: 7104136] [DOI] [PubMed] [Google Scholar]
Hall 1997 {published data only}
- Hall G, Ekblom A, Persson E, Irestedt L. Effects of prostaglandin treatment and paracervical blockade on postoperativepain in patients undergoing first trimester abortion in general anesthesia.. Acta obstetricia et gynecologica Scandinavica 1997;76(9):868‐72. [DOI] [PubMed] [Google Scholar]
Heath 1989 {published data only}
- Heath PJ, Ogg TW. Prophylactic analgesia for daycase termination of pregnancy. A double‐blind study with controlled release dihydrocodeine. Anaesthesia 1989;44(12):991‐4. [PUBMED: 2619027] [DOI] [PubMed] [Google Scholar]
Hein 1999 {published data only}
- Hein A, Jakobsson J, Ryberg G. Paracetamol 1 g given rectally at the end of minor gynaecological surgery is not efficacious in reducing postoperative pain. Acta anaesthesiologica Scandinavica 1999;43(3):248‐51. [PUBMED: 10081528] [DOI] [PubMed] [Google Scholar]
Hein 2001 {published data only}
- Hein A, Norlander C, Blom L, Jakobsson J. Is pain prophylaxis in minor gynaecological surgery of clinical value? a double‐blind placebo controlled study of paracetamol 1 g versus lornoxicam 8 mg given orally. Ambulatory surgery 2001;9(2):91‐4. [PUBMED: 11454488] [DOI] [PubMed] [Google Scholar]
Jakobsson 1991 {published data only}
- Jakobsson J, Davidson S, Andreen M, Westgreen M. Opioid supplementation to propofol anaesthesia for outpatient abortion: a comparison between alfentanil, fentanyl and placebo. Acta anaesthesiologica Scandinavica 1991;35(8):767‐70. [PUBMED: 1763599] [DOI] [PubMed] [Google Scholar]
Jakobsson 1993 {published data only}
- Jakobsson J, Oddby E, Rane K. Patient evaluation of four different combinations of intravenous anaesthetics for short outpatient procedures. Anaesthesia 1993;48(11):1005‐7. [PUBMED: 8285950] [DOI] [PubMed] [Google Scholar]
Jakobsson 1995 {published data only}
- Jakobsson J, Rane K. Anaesthesia for short outpatient procedures. A comparison between thiopentone and propofol in combination with fentanyl or alfentanil. Acta anaesthesiologica Scandinavica 1995;39(4):503‐7. [PUBMED: 7676787] [DOI] [PubMed] [Google Scholar]
Jakobsson 1996 {published data only}
- Jakobsson J, Rane K, Davidson S. Intramuscular NSAIDS reduce post‐operative pain after minor outpatient anaesthesia. European journal of anaesthesiology 1996;13(1):67‐71. [PUBMED: 8829939] [DOI] [PubMed] [Google Scholar]
Kan 2004 {published data only}
- Kan AS, Ng EH, Ho PC. The role and comparison of two techniques of paracervical block for pain reliefduring suction evacuation for first‐trimester pregnancy termination. Contraception 2004;70(2):159‐63. [DOI] [PubMed] [Google Scholar]
Kan 2006 {published data only}
- Kan AS, Caves N, Wong SY, Ng EH, Ho PC. A double‐blind, randomized controlled trial on the use of a 50:50 mixture ofnitrous oxide/oxygen in pain relief during suction evacuation for the firsttrimester pregnancy termination. Human Reproduction 2006;21(10):2606‐11. [DOI] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li CF, Wong CY, Chan CP, Ho PC. A study of co‐treatment of nonsteroidal anti‐inflammatory drugs (NSAIDs) withmisoprostol for cervical priming before suction termination of first trimesterpregnancy. Contraception 2003;67(2):101‐5. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li HW, Wong CY, Lo SS, Fan SY. Effect of local lignocaine gel application for pain relief during suctiontermination of first‐trimester pregnancy: a randomized controlled trial. Human Reproduction 2006;21(6):1461‐6. [DOI] [PubMed] [Google Scholar]
Lindholm 1994 {published data only}
- Lindholm P, Helbo‐Hansen HS, Jensen B, Bulow K, Nielsen TG. Effects of fentanyl or alfentanil as supplement to propofol anaesthesia for termination of pregnancy. Acta anaesthesiologica Scandinavica 1994;38(6):545‐9. [PUBMED: 7976143] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu W, Loo CC, Chiu JW, Tan HM, Ren HZ, Lim Y. Analgesic efficacy of pre‐operative etoricoxib for termination of pregnancy in an ambulatory centre. Singapore medical journal 2005;46(8):397‐400. [PUBMED: 16049609] [PubMed] [Google Scholar]
Marc 2007 {published data only}
- Marc I, Rainville P, Verreault R, Vaillancourt L, Masse B, Dodin S. The use of hypnosis to improve pain management during voluntary interruption ofpregnancy: an open randomized preliminary study. Contraception 2007;75(1):52‐8. [DOI] [PubMed] [Google Scholar]
Ogg 1983 {published data only}
- Ogg TW, Jennings RA, Morrison CG. Day‐case anaesthesia for termination of pregnancy. Evaluation of a total intravenous anaesthetic technique. Anaesthesia 1983;38(11):1042‐6. [PUBMED: 6638451] [DOI] [PubMed] [Google Scholar]
Phair 2002 {published data only}
- Phair N, Jensen JT, Nichols MD. Paracervical block and elective abortion: the effect on pain of waiting betweeninjection and procedure. American Journal of Obstetrics and Gynecology 2002;186(6):1304‐7. [DOI] [PubMed] [Google Scholar]
Raeder 1992 {published data only}
- Raeder JC. Propofol anaesthesia versus paracervical blockade with alfentanil and midazolam sedation for outpatient abortion. Acta anaesthesiologica Scandinavica 1992;36(1):31‐7. [PUBMED: 1539476] [DOI] [PubMed] [Google Scholar]
Rossi 1995 {published data only}
- Rossi AE, Lo Sapio D, Oliva O, Vitale O, Ebano A. Hospital day‐surgery: comparative evaluation of 3 general anesthesia techniques [Day hospital chirurgico: valutazione comparative di tre tecniche di anestesia generale]. Minerva Anestesiologica 1995;61(6):265‐9. [PubMed] [Google Scholar]
Shapiro 1975 {published data only}
- Shapiro AG, Cohen H. Auxiliary pain relief during suction curettage. Contraception 1975;11(1):25‐30. [PUBMED: 1116357] [DOI] [PubMed] [Google Scholar]
Suprapto 1984 {published data only}
- Suprapto K, Reed S. Naproxen sodium for pain relief in first‐trimester abortion. American Journal of Obstetics and Gynecology 1984;150(8):1000‐1. [DOI] [PubMed] [Google Scholar]
Wells 1989 {published data only}
- Wells N. Management of pain during abortion. Journal of advanced nursing 1989;14(1):56‐62. [PUBMED: 2647807] [DOI] [PubMed] [Google Scholar]
Wells 1992 {published data only}
- Wells N. Reducing distress during abortion: a test of sensory information. Journal of Advanced Nursing 1992;17(9):1050‐6. [DOI] [PubMed] [Google Scholar]
Wiebe 1992 {published data only}
- Wiebe ER. Comparison of the efficacy of different local anesthetics and techniques of local anesthesia in therapeutic abortions. American journal of obstetrics and gynecology 1992;167(1):131‐4. [PUBMED: 1442914] [DOI] [PubMed] [Google Scholar]
Wiebe 1995 {published data only}
- Wiebe ER, Rawling M. Pain control in abortion. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 1995;50(1):41‐6. [PUBMED: 7556858] [DOI] [PubMed] [Google Scholar]
Wiebe 1996 {published data only}
- Wiebe ER, Rawling M, Janssen P. Comparison of 0.5% and 1.0% lidocaine for abortions. International Journal of Gynaecology and Obstetrics 1996;55(1):71‐2. [DOI] [PubMed] [Google Scholar]
Wiebe 2003 {published data only}
- Wiebe E, Podhradsky L, Dijak V. The effect of lorazepam on pain and anxiety in abortion. Contraception 2003;67(3):219‐21. [PUBMED: 12618257] [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong CY, Ng EH, Ngai SW, Ho PC. A randomized, double blind, placebo‐controlled study to investigate the use ofconscious sedation in conjunction with paracervical block for reducing pain intermination of first trimester pregnancy by suction evacuation. Human Reproduction 2002;17(5):1222‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andolsek 1977 {published data only}
- Andolsek L, Cheng M, Hren M, Ogrinc‐Oven M, Ng A, Ratnam S, et al. The safety of local anesthesia and outpatient treatment: a controlled study of induced abortion by vacuum aspiration. Studies in family planning 1977;8(5):118‐24. [PUBMED: 867453] [PubMed] [Google Scholar]
Bonazzi 1987 {published data only}
- Bonazzi M, Laveneziana D, Riva A, Vitali C. Clotiazepam vs flunitrazepam in premedication in a day hospital regimen [Clotiazepam vs flunitrazepam nella premedicazione in regime di day‐hospital.]. Minerva anestesiologica 1987;53(12):689‐92. [PUBMED: 2901054] [PubMed] [Google Scholar]
Bonazzi 1994 {published data only}
- Bonazzi M, Riva A, Marsicano M, Prampolini F, Speranza R, Andriolli A, et al. Trazodone versus flunitrazepam in premedication in day‐care surgery [Trazodone versus flunitrazepam nella premedicazione in regime di ricovero giornaliero.]. Minerva anestesiologica 1994;60(3):115‐21. [PUBMED: 8090301] [PubMed] [Google Scholar]
Cade 1993 {published data only}
- Cade L, Ashley J. Prophylactic paracetamol for analgesia after vaginal termination of pregnancy. Anaesthesia and intensive care 1993;21(1):93‐6. [PUBMED: 8447616] [DOI] [PubMed] [Google Scholar]
Ciri 1986 {published data only}
- Ciri F, Berioli M, Bifarini G, Pucci C, Gorietti A, Ballatori E. Various pharmacologic combinations in anesthesia for voluntary abortions in day hospitals [Associazioni farmacologiche diverse nell'anestesia per l'interruzione volontaria della gravidanza in regime di day‐hospital.]. Minerva anestesiologica 1986;52(1‐2):15‐8. [PUBMED: 3736909] [PubMed] [Google Scholar]
Coad 1986 {published data only}
- Coad NR, Mills PJ, Verma R, Ramasubramanian R. Evaluation of blood loss during suction termination of pregnancy: ketaminecompared with methohexitone. Acta anaesthesiologica Scandinavica 1986;30(3):253‐5. [DOI] [PubMed] [Google Scholar]
Corli 1984 {published data only}
- Corli O, Roma G, Bacchini M, Battagliarin G, Piazza D, Brambilla C, et al. Double‐blind placebo‐controlled trial of baclofen, alone and in combination, in patients undergoing voluntary abortion. Clinical therapeutics 1984;6(6):800‐7. [PUBMED: 6391666] [PubMed] [Google Scholar]
Crawford 1984 {published data only}
- Crawford ME, Carl P, Andersen RS, Mikkelsen BO. Comparison between midazolam and thiopentone‐based balanced anaesthesia for day‐case surgery. British journal of anaesthesia 1984;56(2):165‐9. [PUBMED: 6691877] [DOI] [PubMed] [Google Scholar]
Edelist 1987 {published data only}
- Edelist G. A comparison of propofol and thiopentone as induction agents in outpatient surgery. Canadian journal of anaesthesia = Journal canadien d'anesthesie 1987;34(2):110‐6. [PUBMED: 3493852] [DOI] [PubMed] [Google Scholar]
Enlund 1996 {published data only}
- Enlund M, Kobosko P, Rhodin A. A cost‐benefit evaluation of using propofol and alfentanil for a short gynecological procedure. Acta anaesthesiologica Scandinavica 1996;40(4):416‐20. [PUBMED: 8738684] [DOI] [PubMed] [Google Scholar]
Eriksson 1995 {published data only}
- Eriksson H, Haasio J, Korttila K. Comparison of eltanolone and thiopental in anaesthesia for termination of pregnancy. Acta anaesthesiologica Scandinavica 1995;39(4):479‐84. [PUBMED: 7676782] [DOI] [PubMed] [Google Scholar]
Erkola 1990 {published data only}
- Erkola O. Effects of precurarisation on suxamethonium‐induced postoperative myalgia during the first trimester of pregnancy. Acta Anaesthesiologica Scandinavica 1990;34(1):63‐7. [DOI] [PubMed] [Google Scholar]
Hamar 1989 {published data only}
- Hamar O, Garamvolgyi G, Kalman A. Fentanyl‐midazolam‐flumazenil anesthesia for induced abortion. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 1989;30(1):69‐72. [PUBMED: 2572477] [DOI] [PubMed] [Google Scholar]
Jakobbson 1990 {published data only}
- Jakobbson J, Andreen M, Westgren M, Thomasson K. Discomfort after outpatient abortion using paracervical block: a comparison between two opioids and one non‐opioid drug for premedication. Gynecologic and obstetric investigation 1990;30(2):71‐4. [PUBMED: 2245951] [DOI] [PubMed] [Google Scholar]
Kallela 1994 {published data only}
- Kallela H, Haasio J, Korttila K. Comparison of eltanolone and propofol in anesthesia for termination of pregnancy. Anesthesia and analgesia 1994;79(3):512‐6. [PUBMED: 8067557] [DOI] [PubMed] [Google Scholar]
Lichtenberg 2003 {published data only}
- Lichtenberg ES, Hill LJ, Howe M, Heber W, Peipert JF. A randomized comparison of propofol and methohexital as general anesthetics for vacuum abortion. Contraception 2003;68(3):211‐7. [DOI] [PubMed] [Google Scholar]
Lowenstein 2006 {published data only}
- Lowenstein L, Granot M, Tamir A, Glik A, Deutsch M, Jakobi P, Zimmer EZ. Efficacy of suppository analgesia in postabortion pain reduction. Contraception 2006;74(4):345‐8. [DOI] [PubMed] [Google Scholar]
Matambo 1999 {published data only}
- Matambo J, Moodley J, Chigumadzi P. Analgesia for termination of pregnancy. South African medical journal = Suid‐Afrikaanse tydskrif vir geneeskunde 1999; Vol. 89, issue 8:816. [PUBMED: 10488349] [PubMed]
Miller 1996 {published data only}
- Miller L, Jensen MP, Stenchever MA. A double‐blind randomized comparison of lidocaine and saline for cervical anesthesia. Obstetrics and gynecology 1996;87(4):600‐4. [PUBMED: 8602315] [DOI] [PubMed] [Google Scholar]
Nielsen 1975 {published data only}
- Nielsen JB, Madsen OG, Campbell UD. Diazepam as a sedative in induced abortion. Acta obstetricia et gynecologica Scandinavica 1975;54(3):237‐9. [PUBMED: 1099860] [DOI] [PubMed] [Google Scholar]
Parkash 1979 {published data only}
- Parkash A, Do Rosario YP. Effect of anaesthesia on the blood loss in first trimester medical termination ofpregnancies. Journal of Obstetics and Gynaecology India 1979;29(6):1128‐30. [PubMed] [Google Scholar]
Peters 1978 {published data only}
- Peters FD, Hirsch HA. Comparison between intra and para cervical anaesthesia in therapeutic abortion (author's transl) [Vergleich von Intra‐ und Parazervikal‐Anasthesie beim Schwangerschaftsabbruch.]. Geburtshilfe und Frauenheilkunde 1978;38(11):946‐9. [PUBMED: 710880] [PubMed] [Google Scholar]
Rawling 2001 {published data only}
- Rawling MJ, Wiebe ER. A randomized controlled trial of fentanyl for abortion pain. American Journal of Obstetrics and Gynecology 2001;185(1):103‐7. [DOI] [PubMed] [Google Scholar]
Sanders 1984 {published data only}
- Sanders RS, Sinclair ME, Sear JW. Alfentanil in short procedures. A comparison with halothane using etomidate or methohexitone for induction of anaesthesia. Anaesthesia 1984;39(12):1202‐6. [PUBMED: 6440446] [DOI] [PubMed] [Google Scholar]
Schoeffler 1987 {published data only}
- Schoeffler P, Monteillard C, Ginzac G, Forestier B, Haberer JP. The use of isoflurane in brief anesthesia for voluntary interruption of pregnancy [Utilisation de l'isoflurane lors d'anesthesies breves pour interruption volontaire de grossesse.]. Cahiers d'anesthesiologie 1987;35(2):83‐6. [PUBMED: 3607584] [PubMed] [Google Scholar]
Verma 1985 {published data only}
- Verma R, Ramasubramanian R, Sachar RM. Anesthesia for termination of pregnancy: midazolam compared with methohexital. Anesthesia and analgesia 1985;64(8):792‐4. [PubMed] [Google Scholar]
West 1985 {published data only}
- West SL, Moore CA, Gillard M, Browne PD. Anaesthesia for suction termination of pregnancy. Anaesthesia 1985;40(7):669‐72. [PUBMED: 4025771] [DOI] [PubMed] [Google Scholar]
Wiebe 2005 {published data only}
- Wiebe ER, Trouton KJ, Savoy E. Intra‐cervical versus i.v. fentanyl for abortion. Human Reproduction 2005;20(7):2025‐8. [DOI] [PubMed] [Google Scholar]
Willdeck‐Lund 1975 {published data only}
- Willdeck‐Lund G, Zador G. Paracervical block with etidocaine for out‐patient abortion. Acta anaesthesiologica Scandinavica. Supplementum 1975;60:106‐9. [PUBMED: 1101603] [PubMed] [Google Scholar]
Additional references
Bartlett 2004
- Bartlett LA, Berg CJ, Shulman HB, Zane SB, Green CA, Whitehead S, Atrash HK. Risk factors for legal induced abortion‐related mortality in the United States. Obstetrics and Gynecolology 2004;103(4):729‐37. [DOI] [PubMed] [Google Scholar]
Belanger 1989
- Belanger E, Melzack R, Lauzon P. Pain of first‐trimester abortion: a study of psychosocial and medical predictors. Pain 1989;36:339‐50. [DOI] [PubMed] [Google Scholar]
Borgotta 1997
- Borgotta L, Nickinovich D. Pain during early abortion. The Journal of Reproductive Medicine 1997;42:287‐93. [PubMed] [Google Scholar]
Cheney 1999
- Cheney FW. The American Society of Anesthesiologists Closed Claims Project: what have we learned, how has it affected practice, and how will it affect practice in the future?. Anesthesiology 1999;91(2):552‐6. [PUBMED: 10443619] [DOI] [PubMed] [Google Scholar]
Grimes 1979
- Grimes DA, Schulz KF, Cates W, JR, Tyler CW, JR. Local versus general anesthesia: which is safer for performing suction curettage abortions?. American Journal of Obstetrics and Gynecololgy 1979;135:1030‐5. [DOI] [PubMed] [Google Scholar]
Hakim‐Elahi 1990
- Hakim‐Elahi E, Tovell HM, Burnhill MS. Complications of first‐trimester abortion: a report of 170,000 cases. Obstetrics and gynecology 1990;76(1):129‐35. [PUBMED: 2359559] [PubMed] [Google Scholar]
Koonin 2000
- Koonin LM, Strauss LT, Chrisman CE, Parker WY. Abortion surveillance‐‐United States, 1997. Morbidity & Mortality Weekly Report. CDC Surveillance Summaries 2000, Dec 8; Vol. 49, issue 11:1‐43. [PubMed]
Lawson 1994
- Lawson HW, Frye A, Atrash HK, Smith JC, Shulman HB, Ramick M. Abortion mortality, United States, 1972 through 1987. American Journal of Obstetrics and Gynecolology 1994;171:1365‐72. [DOI] [PubMed] [Google Scholar]
Lichtenberg 2001
- Lichtenberg ES, Paul M, Jones H. First trimester surgical abortion practices: a survey of National Abortion Federation members. Contraception 2001;64(6):345‐52. [PUBMED: 11834232] [DOI] [PubMed] [Google Scholar]
Maltzer 1999
- Maltzer DS, Maltzer MC, Wiebe ER, Halvorson‐Boyd G, Boyd C. Pain management. In: Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG editor(s). A clinicinan's guide to medical and surgical abortion. Philadelphia. PA: Churchill Livingstone, 1999:73‐89. [Google Scholar]
Rawling 1998
- Rawling MJ, Wiebe ER. Pain control in abortion clinics. International Journal of Gynaecology & Obstetrics 1998;60(3):293‐5. [DOI] [PubMed] [Google Scholar]
RCOG 2004
- Royal College of Obstetricians and Gynaecologists. The Care of Women Requesting Induced Abortion. http://www.rcog.org.uk/resources/Public/pdf/induced_abortionfull.pdf 2004.
Scott 1976
- Scott J, Huskisson EC. Graphic representation of pain. Pain 1976;2:175‐84. [PubMed] [Google Scholar]
Smith 1979
- Smith GM, Stubblefield PG, Chirchirillo L, McCarthy MJ. Pain of first‐trimester abortion: its quantification and relations with other variables. American Journal of Obstetrics and Gynecology 1979;133:489‐98. [DOI] [PubMed] [Google Scholar]
Smith 1991
- Smith RP, Heltzel JA. Interrelation of analgesia and uterine activity in women with primary dysmenorrhea. A preliminary report. Journal of Reproductive Medicine 1991;36:260‐4. [PubMed] [Google Scholar]
Steele 2005
- Steele M, Nielsen KC, Klein SM. Ambulatory Anesthesia Perioperative Analgesia. McGraw‐Hill, 2005. [ISBN: 0‐07‐141240‐9] [Google Scholar]
Strauss 2007
- Strauss LT, Gamble SB, Parker WY, Cook DA, Zane SB, Hamdan S. Abortion surveillance‐‐United States, 2004. MMWR. Surveillance summaries : Morbidity and mortality weekly report. Surveillance summaries / CDC 2007;56(9):1‐33. [PUBMED: 18030283] [PubMed] [Google Scholar]
Stubblefield 1989
- Stubblefield PG. Control of pain for women undergoing abortion. Supplement for International Journal of Gynaecology & Obstetrics 1989;3:131‐40. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Safe Abortion:Technical and Policy Guidance for Health Systems. http://www.who.int/reproductive‐health/publications/safe_abortion/safe_abortion.pdf 2003. [PubMed]


