Abstract
Corrosion in water-distribution systems is a costly problem and controlling corrosion is a primary focus of efforts to reduce lead (Pb) and copper (Cu) in tap water. High chloride concentrations can increase the tendency of water to cause corrosion in distribution systems. The effects of chloride are also expressed in several indices commonly used to describe the potential corrosivity of water, the chloride-sulfate mass ratio (CSMR) and the Larson Ratio (LR). Elevated CSMR has been linked to the galvanic corrosion of Pb whereas LR is indicative of the corrosivity of water to iron and steel. Despite the known importance of chloride, CSMR, and LR to the potential corrosivity of water, monitoring of seasonal and interannual changes in these parameters is not common among water purveyors. We analyzed long-term trends (1992–2012) and the current status (2010–2015) of chloride, CSMR, and LR in order to investigate the short and long-term temporal variability in potential corrosivity of US streams and rivers. Among all sites in the trend analyses, chloride, CSMR, and LR increased slightly, with median changes of 0.9 mg L− 1, 0.08, and 0.01, respectively. However, urban-dominated sites had much larger increases, 46.9 mg L− 1, 2.50, and 0.53, respectively. Median CSMR and LR in urban streams (4.01 and 1.34, respectively) greatly exceeded thresholds found to cause corrosion in water distribution systems (0.5 and 0.3, respectively). Urbanization was strongly correlated with elevated chloride, CSMR, and LR, especially in the most snow-affected areas in the study, which are most likely to use road salt. The probability of Pb action-level exceedances (ALEs) in drinking water facilities increased along with raw surface water CSMR, indicating a statistical connection between surface water chemistry and corrosion in drinking water facilities. Optimal corrosion control will require monitoring of critical constituents reflecting the potential corrosivity in surface waters.
Keywords: Water quality trends, Urbanization, Drinking water, Environmental assessment, Road salt, Lead
Graphical Abstract
1. Introduction
Increasing chloride concentrations and specific conductance in U.S. rivers have been linked to urbanization and particularly to road salt application in snow affected areas (Conway, 2007, Corsi et al., 2015, Kaushal et al., 2005, Novotny et al., 2008, Tu, 2013). The most dramatic increases have resulted in the doubling of chloride concentrations in some urban streams in snow-affected areas of the U.S. since 1990 (Corsi et al., 2015). Road salt reaches waterways through surface runoff (Corsi et al., 2010) as well as by entering the shallow aquifers where it is released slowly in groundwater discharge (Ledford et al., 2016). Severely elevated chloride concentrations are detrimental to aquatic organisms and can increase the mobility of metals and other bioactive compounds (Duan and Kaushal, 2015, Nelson et al., 2009, Novotny et al., 1998). By one estimate, the long-term changes in specific conductance could endanger the utility of surface drinking water supplies in heavily developed areas of the northeastern U.S. (Kaushal et al., 2005).
In water distribution systems, elevated chloride concentration can increase the rate of release of lead into the water. High chloride concentration promotes galvanic corrosion of Pb-bearing materials (Ng and Lin, 2016, Willison and Boyer, 2012, AWWARF-TZW, 1996, DeSantis et al., 2009, DeSantis and Schock, 2014) and pitting corrosion of copper (Shalaby et al., 1989, Lytle and Schock, 2008, Schock and Lytle, 2011). Numerous computations, modeling efforts, and field observations of the solubility of lead and copper, suggest that chloride and sulfate are not significant in terms of aqueous complexation or solubility-controlling passivating solids (AWWARF-TZW, 1996, Schock and Lytle, 2011, DeSantis and Schock, 2014). However, elevated chloride and sulfate concentrations, and changes in chloride and sulfate levels have been associated with increasing corrosion and subsequent release of Pb and iron (Edwards and Triantafyllidou, 2007, AWWARF-TZW, 1996, Lytle et al., 2004, Schock and Lytle, 2011). The mode of action is likely to be most important in non-equilibrium sampling conditions such as the 6 +-hour stagnation associated with sampling for the Lead and Copper Rule in the U.S. (e-CFR, 2017b). The corrosive effects of chloride are also expressed in several indices used to determine the potential corrosivity of water to different materials, such as the chloride-sulfate mass ratio (CSMR) and the Larson Ratio (LR, defined as the sum of equivalents of chloride and sulfate divided by equivalents of bicarbonate (Larson and Skold, 1958, Schock and Lytle, 2011)).
Incidents of elevated Pb concentrations in drinking water have been traced to changes in CSMR (Edwards and Triantafyllidou, 2007). Elevated CSMR was found in the finished water of the Flint, Michigan, USA water utility during the period when elevated Pb concentrations were observed in the distribution system (Pieper et al., 2017). The World Health Organization notes that CSMR can be an important variable in determining the potential for corrosion problems in water distribution systems (World Health Organization, 2017). Furthermore, a link between Pb contamination of drinking waters and elevated chloride from road salt has been proposed in several recent incidents (url: http://www.reuters.com/investigates/special-report/usa-water-lead/, accessed 06–08-2017; url: http://www.fresnobee.com/news/nation-world/national/article59227723.html, accessed 06–08-2017), and emphasizes the growing need to understand how anthropogenic sources of chloride can affect the potential corrosivity of water supplies.
Because of the strong link between corrosion and elevated Pb or Cu concentration in tap water, distribution systems serving > 50,000 customers are required to proactively implement “…corrosion control treatment that minimizes the lead and copper concentrations at users’ taps while ensuring that the treatment does not cause the water system to violate any national primary drinking water regulations” (e-CFR, 2017a). Smaller and medium systems are also required to implement corrosion control treatment if Pb or Cu concentrations in tap water exceed the 90th percentile action levels (e-CFR, 2017b). Corrosion control techniques can include alkalinity and pH adjustment of source water or the addition of corrosion inhibitors such as silicate and orthophosphate. The addition of orthophosphate has been widely adopted in the US (McNeill and Edwards, 2002), and it has been estimated that 95% of public water supplies in the United Kingdom add orthophosphate as a corrosion inhibitor (Hayes and Skubala, 2009). The approach to corrosion control depends to a large degree upon the initial chemical composition of the source water. So, as surface water chemistry evolves due to anthropogenic effects, the nature and extent of corrosion and the steps used to control it will also need to evolve. However, the water quality monitoring requirements at drinking water facilities may not be sufficient to capture changes in chloride, CSMR and LR.
At the national level in the U.S., the monitoring of water quality parameters by drinking water facilities is guided by the Lead and Copper Rule of the Safe Drinking Water Act (e-CFR, 2017b). Typically, when required to do regulatory water quality parameter monitoring, the measurements are composed of conductivity, temperature, pH and alkalinity, plus orthophosphate or silicate if those chemicals are added as part of the corrosion control treatment. Entry points to the distribution system are monitored every 2 weeks, while distribution system water quality parameter monitoring can vary widely depending on whether either the Pb or Cu Action Level is exceeded. So, despite the known importance of chloride, sulfate, CSMR, and LR to the potential corrosion of drinking water distribution system materials, there are no regulatory requirements associated with the corrosion of distribution materials to routinely monitor for these parameters in the source water or at the entry point to the distribution system. Thus, without any sampling beyond the minimum requirements of the law, operators, especially in small- and medium-sized public water systems, may be unaware of possible short- or long-term changes in the potential corrosivity of source waters.
We examined trends in chloride concentration, CSMR, and LR in surface waters in the conterminous US during the period 1992–2012 to better understand how changes in surface water chemistry have influenced overall potential corrosivity. We then conducted a status assessment on the same parameters at a larger number of sites from 2010 to 2015 to describe the geographic distribution and land cover influences on potential corrosivity. This approach allowed us to look at the long-term trends as well as seasonal differences in potential corrosivity. Finally, we analyzed the U.S. Environmental Protection Agency (EPA) Safe Drinking Water Information System (SDWIS) database of Pb action level exceedances (ALEs) in drinking water monitoring samples and related the occurrence of these exceedances to source water chemistry. Knowledge of larger-scale drivers to the corrosivity of source waters can help water purveyors ensure they have adequate monitoring and treatment programs in place.
2. Materials and methods
2.1. Data sources and handling
Water quality data (chloride, sulfate, alkalinity, and temperature) were acquired from national, state, and local sources. Data obtained from these sources were used in several ways. First, stations with adequate water quality and streamflow data were included in trend analysis spanning the period 1992–2012. Second, the same parameters were analyzed for central tendencies, seasonal patterns, and relationship to land cover during the period 2010–2015, hereafter called the status assessment. Finally, the water quality monitoring locations were merged with the EPA SDWIS (https://www3.epa.gov/enviro/facts/sdwis/search.html, accessed October, 2016) to investigate water quality monitoring stations with drinking water intakes in the catchment and to relate surface water quality to Pb ALEs at drinking water facilities.
Because the data originated from a variety of sources, it was necessary to harmonize parameter names, reporting units, and remark codes across all sources. The parameters were harmonized to chloride, expressed in mg Cl L− 1, sulfate expressed as mg SO42 − L− 1, alkalinity expressed as mg CaCO3 L− 1, and temperature expressed as degrees Celsius. In cases where parameter names or units could not be determined, the observations were excluded from analysis.
CSMR was calculated as
where Cl− is the chloride concentration, expressed as mg Cl L− 1 and SO42 − is sulfate concentration expressed as mg SO42 − L− 1. CSMR > 0.5 occurring in drinking water facilities is considered to have the potential to promote galvanic corrosion of leaded connections in the distribution system (Nguyen et al., 2011). However, much less is known about how raw surface water CSMR can influence corrosion.
The Larson Ratio (LR) is defined as the ratio of equivalents of chloride plus sulfate divided by equivalents of bicarbonate (Larson and Skold, 1958, Schock and Lytle, 2011). Aqueous solutions with LR > 0.3 have the potential to cause corrosion to iron and steel. LR was calculated as
where Cl−, SO42 −, and Alk are the concentrations of chloride, sulfate, and alkalinity, respectively, expressed as mg Cl− L− 1, mg SO42 − L− 1, and mg CaCO3 L− 1, respectively. Concentrations were divided by molecular mass and multiplied by valence charge to convert to equivalents. Even though the LR calculation has equivalents of HCO3− in the denominator, we assumed that alkalinity provided a reasonable estimation of HCO3− because: (1) carbonate buffering is the primary source of alkalinity in most surface waters; and, (2) the pH range of natural waters is typically low enough that the carbonate buffering system is dominated by HCO3−. For example, in our status assessment dataset, the median and 90th percentile pH observation was 7.78 and 8.35, respectively (n = 4385). At those pH values, HCO3 − would be > 99.0% of the measured alkalinity. For both the CSMR and LR, constituent values below the detection limit were extremely rare (< 0.03% of the observations) and so were excluded from the analysis because doing so was not expected to affect the results.
Stream discharge data, which is required for trend analysis, originated primarily from the U.S. Geological Survey (USGS) streamgage network and was obtained from the USGS National Water Information System (NWIS, (U.S. Geological Survey, 2016)). For several sites (n = 3), U.S. Army Corps of Engineer gage data was used. In most cases the water quality and stream discharge data originated from the same station or the same location. Otherwise, water quality and stream discharge data were matched using the National Hydrography Dataset (NHDPlus) V2 Medium Resolution dataset (McKay et al., 2012). An indexing algorithm was used to match monitoring stations on streamflow lines in NHDPlus and then to locate nearby streamgage stations. Water quality monitoring stations were matched to gaging stations if the reported basin area of each was within 10% and if basic metrics of data coverage were met by the gaging station. More detailed descriptions of the gage matching routines are given in Oelsner et al. (2017).
2.2. Trend analysis
Trend analysis was performed using the Weighted Regression on Time, Discharge, and Seasons (WRTDS) model implemented as the EGRETci package in R (Hirsch et al., 2015, Hirsch et al., 2010). WRTDS uses weighted linear regression of a calibration dataset to estimate daily concentration throughout time, discharge (streamflow), and seasonal dimensions. Individual calibration data points are weighted based on their proximity to estimated values in the model domain. That is, a calibration data point will be weighted heavily when it is used to estimate concentration for points of similar discharge, season, and year. The weighting scheme therefore allows features such as seasonality or concentration-discharge relationships to change through time. WRTDS has been used in previous trend analyses of nutrients and major ions (Corsi et al., 2015, Sprague et al., 2011) and performed well in a comparison of methods used to compute decadal water-quality loads (Lee et al., 2016). The weighting proximity follows a tricube function and parameters were assigned in the WRTDS package with the half-window width set to 10 years, 1 or 2 natural log units (for sites with drainage areas greater or < 250,000 km2, respectively), and 0.5 years in the time, streamflow, and seasonal dimensions, respectively.
WRTDS requires daily discharge values throughout the period of trend analysis and a relatively high density of water quality observations. For this study, sites were required to have at least four samples per year in 70% of the years in the trend period (i.e. 14 of 20 years). Additionally, at least four samples per year were required in the first two and last two years in the trend period. The trend period was nominally 1992–2012, although the beginning year (yS) and ending year (yE) in the trend analysis were allowed to vary by one year such that yS could be 1992 or 1993 and yE could be 2011 or 2012. Sites were required to have at least one sample per quarter in at least 70% of the years included in the analysis. Sites were also required to pass a high-flow screen to ensure that a representative number of samples were collected at high flow. All sites that passed the screening criteria were included in the trend analysis. No sites in Alaska or Hawaii passed and so the trend analysis technically pertains to rivers in the conterminous U.S. The criterion, guided by sensitivity analyses, are described in greater detail in Oelsner et al. (2017). The screened and finalized data were then used in the subsequent trend analyses in this manuscript (Whiddon and Stets, 2017).
The flow-normalized concentrations produced by WRTDS were examined for trends between 1992 and 2012. Flow-normalization is intended to remove random variation in concentration due to random variation in discharge (Hirsch et al., 2010). Briefly, flow normalization uses the concentration associated with the mean discharge for each day of the model simulation. Therefore changes in concentration over time are expected to be representative of changes occurring at mean discharge conditions rather than describing temporal variability due to changes in flow.
Trend detection was performed using differences in the bootstrapped flow-normalized concentration estimates between yS and yE. Bootstrapping in WRTDS was performed using predefined temporal blocks which were meant to: (1) preserve the temporal residual structure in the model; (2) avoid oversampling periods of time with unusually large numbers of water quality samples; and, (3) preserve flow-related dependencies present in groups of samples (Hirsch et al., 2015). Briefly, each bootstrap replicate created an estimate of annual flow-normalized concentration in yS and yE. The difference in flow-normalized estimates between yS and yE, Δc, could be either positive or negative. Hypothesis testing was implemented using likelihood analysis with the proportion of bootstrap estimates with Δc > 0 and Δc < 0 used to generate likelihood estimates. Models with > 85% likelihood that Δc was either positive or negative were considered to have strong evidence for a trend, models with likelihoods of 0.7 to 0.85 were considered to have some evidence for trends, and models with likelihoods < 0.7 were considered to have a low likelihood of trends. For each site, 100 bootstrap replicates were performed. More detail on the bootstrapping method is given in Hirsch et al. (2015).
Model output was checked visually for fit, residual structure, and residual bias. Models failing quality checks were discarded and were not used in the analysis. Among sites with sufficient data to run the WRTDS trend model, a total of 11 and 1 sites failed to pass quality checks for CSMR and LR, respectively. After data screening and model quality checks, 141 sites were considered suitable for chloride trend analysis, 74 sites were found to be suitable for CSMR trend analysis, and 76 sites for LR trend analysis (Table S1).
2.3. Long-term changes in concentration in cold versus warm seasons
In addition to the WRTDS-based trend analysis, a comparison was made of raw chloride, CSMR, and LR data between the period 1991–1993 and 2011–2012, which are referred to as 1992 and 2012 in the analysis, respectively. The long-term trend analysis sites were used for the comparison. The comparison was made for when reported water temperature was < 4 °C and > 20 °C. The analysis was meant to capture how the parameters have changed in cold seasons versus warmer seasons. Previous studies have found that chloride concentrations have increased most dramatically during the winter (Corsi et al., 2015), but increases in warm seasons could have a greater effect on corrosion because corrosion rates are strongly positively linked to temperature (Volk et al., 2000). Because of the non-normal distribution of parameter values, the Wilcoxon Rank Sum test was used to test for differences seasonally and through time. The Wilcoxon test statistic (W) was reported along with the p value.
2.4. Current status assessment
Sites included in the status assessment were required to have at least eight seasonally distributed samples collected per year in at least four years during the period 2010–2015. A total of 320, 248, and 103 sites passed the screen for chloride, CSMR, and LR, respectively. The sites were then categorized on the basis of land use and geographic region, described below.
2.5. Regional and land use categories
Land use in each watershed was classified according to coverage of National Land Cover Dataset (NLCD) 2011 land use types in the watershed (Homer et al., 2012). The extent of agricultural development was calculated as the sum of NLCD land cover types 81 and 82; urban development was calculated as the sum of NLCD land cover types 21–24 (Homer et al., 2012). Both land use categories were converted to percentage of basin area. Land use in watersheds was classified based on the extent of urban and agricultural development such that watersheds with < 5% urban and < 25% agricultural development were considered “Undeveloped”; watersheds with > 50% agricultural and < 10% urban development were considered “Agricultural”; watersheds with > 25% urban development and < 25% agricultural development were considered “Urban”; and, all other combinations were considered to be “Mixed” development. For cases in which the NLCD 2011 dataset indicated 0% development (i.e. urban or agricultural) in a watershed, the value was recoded to 0.1% for use in analyses.
The status assessment sites were also categorized by four predefined regions meant to express differences in snowfall: Northern Tier, Southern Tier, Interior West, and Pacific (Fig. S1). The Northern Tier states encompass the most snow-affected states in the Eastern and Midwestern U.S. Southern Tier states are warmer states in the Eastern and Central parts of the U.S. Interior West includes states of the Great Plains and Rocky Mountains, and the Pacific region includes West Coast states along with Nevada and Idaho (Fig. S1). Annual snowfall was estimated using average annual precipitation from the Parameter-elevation Relationship on Independent Slopes Model (PRISM, http://prism.oregonstate.edu/, accessed June 2017) multiplied by average percent of precipitation as snow (McCabe and Wolock, 2010) using raster-based zonal statistics in ArcMap (ESRI, 2011). Estimated annual snowfall (1981–2010) for the Northern Tier region was 178 mm and the Southern Tier region was 12 mm. The Interior West and Pacific Regions have relatively high estimated annual snowfall, 65 and 119 mm, respectively, but the regions are marked by a large degree of variability and geographic separation between the areas of heaviest snowfall and urbanized areas, which are more likely to use road salt.
One site from each pre-defined region was also selected for further analysis of the temporal and seasonal dynamics of chloride and potential corrosivity. The sites were selected on the basis of the regular sampling regime and to broadly cover a variety of climates and land use categories. The selected sites were: (1) Connecticut River at Thompsonville, CT (USGS gage 01184000), which represented a snow-dominated Northern Tier site which is likely to be subject to inputs of road salt; (2) Altamaha River near Everett City, GA (USGS gage 02226160), a mixed-use Southern Tier site that was selected to represent sites without a large snowmelt signal in warmer climates typical of the Southern Tier of the U.S.; (3) Platte River at Louisville, NE (USGS gage 06805500), an Interior West site located in the Great Plains region of the interior US; and, (4) the Yakima River at Kiona, WA, a relatively undeveloped site in the Pacific region which receives hydrologic inputs primarily from relatively pristine mountainous headwaters as well as irrigation return flows from agricultural fields (Ebberts et al., 2003). Site selection was not meant to be exhaustive, but rather to provide additional context into the variety of seasonal patterns and processes driving potential corrosivity at the individual monitoring locations.
2.6. Water quality and Pb exceedances
We also explored the degree to which source water characteristics relate to Pb exceedances in drinking water systems. Median chloride, CSMR, and LR data collected from 1992 to 2012 were compared with the number of Pb ALEs at drinking water facilities with surface intakes located upstream of the monitoring locations. Direct connections between finished water (i.e. after all water treatments) and Pb ALEs have been documented (Dodrill and Edwards, 1995, Edwards and Triantafyllidou, 2007). However, less is known about how raw surface water characteristics relate to corrosion in drinking water facilities when treatment does not substantially change some key corrosion related parameters. Many of the water quality properties of surface water change during the treatment process before entering the distribution system. Alkalinity and pH adjustments are common along with the addition of coagulants to reduce organic carbon. Therefore, a more direct connection is expected between the water quality characteristics of finished water and corrosion in water distribution systems. Nevertheless, intakes using surface water with elevated chloride, CSMR, or LR could produce finished water that is potentially corrosive. Typical drinking water treatment techniques do not remove chloride and therefore high chloride concentrations should be conserved throughout the treatment. Elevated CSMR can be offset to some extent by treatments, for example, by the addition of alum (aluminum sulfate) as a coagulant (Edwards and Triantafyllidou, 2007) and LR would be affected by pH and alkalinity adjustments.
Drinking water facilities monitor Pb concentrations in tap water under the 1991 Lead and Copper Rule (e-CFR, 2017b). A Pb ALE is triggered when at least 10% of the samples included in a monitoring period have Pb concentrations > 0.015 mg L− 1. ALEs are reported in the EPA SDWIS database (url: https://www3.epa.gov/enviro/facts/sdwis/search.html, accessed October 28, 2016). Pb ALEs were linked to water quality monitoring data using the USGS Public Supply Database (PSDB, (Price and Maupin, 2014)). The PSDB was used to create lists of surface drinking water intakes upstream of each monitoring station used in the trend analysis. The analysis was restricted to monitoring stations that met the following criteria. First, we used monitoring stations that were part of the trend analysis to ensure that water quality samples were collected throughout the period of analysis. Monitoring stations were required to have at least 5 surface drinking water intakes on the stream network to ensure a large enough sample size to conduct the analysis. The upstream watersheds were required to be 30,000 km2 or less to increase the likelihood that water quality conditions at the monitoring location were related to conditions at the drinking water intake. The data screen was passed by 39 monitoring sites. It was then determined how many public water system Pb ALEs had been reported among the upstream surface drinking water intakes during the period 1992 to 2016.
We analyzed the results based on the reporting of an ALE, which is a categorical variable (i.e. presence versus absence). Therefore, logistic regression was used to evaluate whether median chloride, CSMR, or LR were statistically predictive of the occurrence of multiple associated ALEs at upstream drinking water distribution systems. Water quality monitoring stations were coded based upon the number of ALEs per upstream drinking water intake: sites with one or less associated ALEs per 10 drinking water intakes were coded 0; sites with > 1 associated ALE per 10 drinking water intakes were coded 1. Three logistic regressions were developed to facilitate the assessment of the probability of a site having > 1 associated ALE per 10 drinking water intakes as a function of either chloride, CSMR, or LR. Logistic regression was run using the glm function in the R Software package, version 3.3.1 (R Core Team, 2016) and significance was tested at the p = 0.05 level.
2.7. Other statistical analyses
Correlation analysis involving water quality data often contains large outliers and has a non-normal distribution. Therefore non-parametric Spearman correlation was used when analyzing water quality data and model results in relation to land use. In several figures we also presented a best-fit line to demonstrate the relationship between chloride, CSMR, and/or LR and land use. Those fits were drawn using Robust Regression to provide conservative best-fit lines with low sensitivity to outliers in the dataset. Robust regression was performed using the quantreg library in the R Software Package, version 3.3.1 (R Core Team, 2016).
Differences in chloride, CSMR, and LR among the broad land use categories were determined using the non-parametric Kruskal-Wallis test followed by the Dunn’s pairwise post-hoc multiple comparison test. These tests were implemented using the R package dunn.test (Dinno, 2017).
3. Results
3.1. Trends
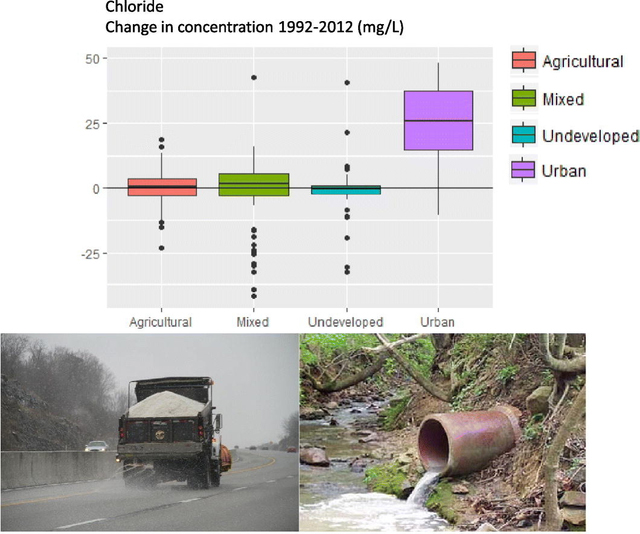
Between 1992 and 2012, a high likelihood of positive trends were found in 64 out of 141 monitoring stations analyzed for chloride concentration, 45 out of 74 monitoring stations analyzed for CSMR, and 32 of 76 monitoring stations analyzed for LR (Table 1). Maps of trend locations and trend results appear in the Supplemental Information (Fig. S2–S4). Among trend locations, median chloride concentration was 37.1 mg L− 1 with a slight increase, between 1992 and 2012. Similar results were found for CSMR and Larson Ratio as well (Table 1). Land cover strongly influenced the observed trends with urban sites having much larger increases in all three parameters than other land use types (Table 1). For example, the median change in chloride concentration at urban sites was equivalent to an 88% increase since 1992.
Table 1.
Summary of trend results by constituent. The number of stations with the specified trend result are listed along with the median change 1992–2012 and the 25th to 75th percentile of change. Magnitude of change (Δ) is mg L− 1 for chloride and change in ratio for CSMR and LR.
| Δ chloride (n = 141) | Δ CSMR (n = 74) | Δ LR (n = 76) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trend result |  |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| # of stations | 49 | 6 | 13 | 9 | 64 | 16 | 3 | 6 | 4 | 45 | 28 | 6 | 6 | 4 | 32 |
| Land use | Median (25th–75th pctile) | Median (25th–75th pctile) | Median (25th–75th pctile) | ||||||||||||
| All sites | 0.9 | (− 2.9–5.9) | 0.08 | (− 0.01–0.29) | 0.01 | (− 0.20–0.23) | |||||||||
| Undeveloped | − 0.4 | (− 2.6–2.5) | 0.03 | (0.05–0.10) | − 0.10 | (− 0.33–0.04) | |||||||||
| Agricultural | 0.5 | (− 3.3–3.7) | 0.03 | (− 0.04–0.10) | 0.03 | (− 0.03–0.31) | |||||||||
| Mixed | 1.4 | (− 6.7–5.7) | 0.03 | (− 0.09–0.27) | 0.04 | (− 0.11–0.15) | |||||||||
| Urban | 46.9 | (24.2–53.8) | 2.50 | (1.84–3.29) | 0.53 | (0.37–0.82) | |||||||||
 - Decreasing, high likelihood.
- Decreasing, high likelihood.
 - Decreasing, some likelihood.
- Decreasing, some likelihood.
 - Trend not likely.
- Trend not likely.
 - Increasing, high likelihood.
- Increasing, high likelihood.
 - Increasing, some likelihood.
- Increasing, some likelihood.
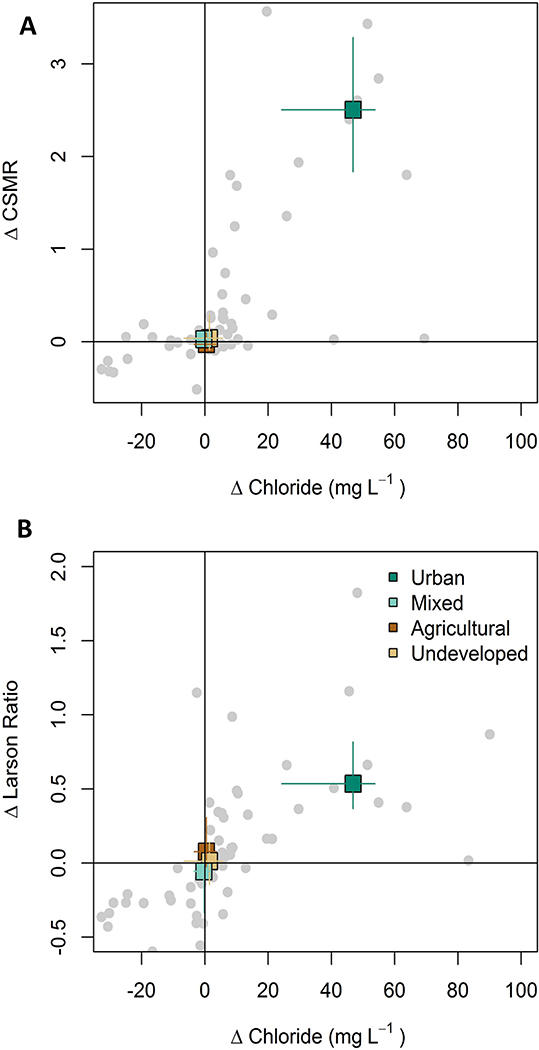
Strongly positive correlations were evident between trends in chloride and trends in CSMR (ρ = 0.66, p < 0.0001) and LR (ρ = 0.71, p < 0.0001, Fig. 1A, B). This finding suggests that increases in the potential corrosivity of surface waters, as indicated by CSMR and LR, were related to the changes in chloride at these sites. Land cover categories showed distinct patterns with urban sites having concomitant increases in chloride and potential corrosivity while relatively small and less consistent changes were found at sites in other land use categories (Fig. 1A, B). A small number of sites had significant increases in CSMR (n = 14) or LR (n = 8), but not chloride (not shown). In these cases, changes in potential corrosivity could arise from decreasing alkalinity or changes in sulfate concentrations. For example, several sites with the largest increases in sulfate concentration also had increasing LR (Fig. S5) although no similar relationship was observed among either sulfate and CSMR trends (Fig. S5) or alkalinity and LR trends (Fig. S5).
Fig. 1.
Relationship between modeled flow-normalized change between 1992 and 2012 for chloride concentration versus A.) chloride-sulfate mass ratio (CSMR), and B.) Larson Ratio. All stations are shown as gray dots. The median and 25th–75th percentile are plotted as colored squares and lines, respectively. Square colors correspond to land use classification.
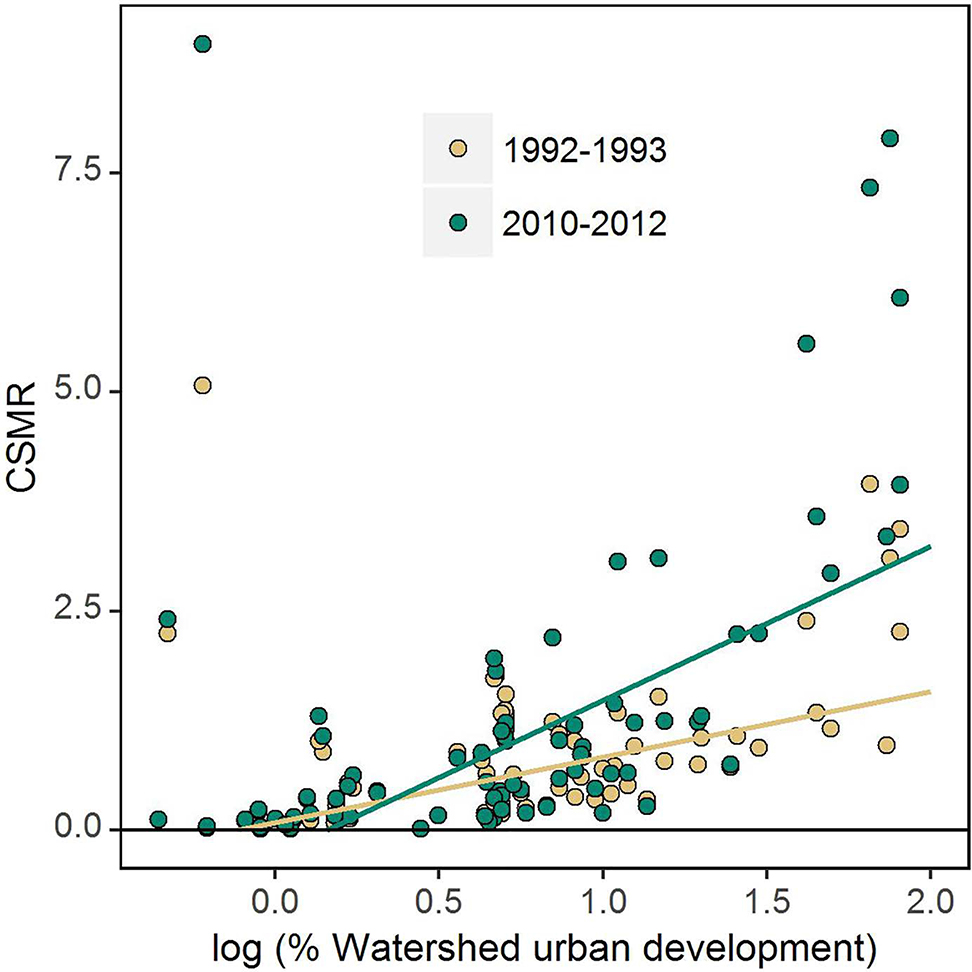
The effects of development were evident across a gradient of urbanization. Annual flow-normalized CSMR was positively related to percent urban development in a watershed in 1992 but that relationship became stronger and more pronounced by 2012 (Fig. 2). The correlation between CSMR and watershed development was significant at both time periods (ρ > 0.5, p < 0.0001 for both, Fig. 2). Urbanization was also related to LR, but only when watersheds with < 3% urbanization were excluded. High LR was present in several undeveloped watersheds leading to a non-significant correlation between LR and urbanization when the entire dataset was used (ρ = 0.05, p > 0.5, not shown). However, when the analysis was limited to watersheds with at least 3% urban development, significant correlations existed for both time periods 1992 (ρ = 0.29, p = 0.04) and 2012 (ρ = 0.43, p = 0.001).
Fig. 2.
Relationship between chloride-sulfate mass ratio (CSMR) and percent urban development in the watershed. The x-axis is expressed on a logarithmic scale. Flow-normalized CSMR is displayed for 1992–1993 (yellow dots) and 2010–2012 (green dots). The lines were drawn using robust regression and are meant to display the general relationship between the variables. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
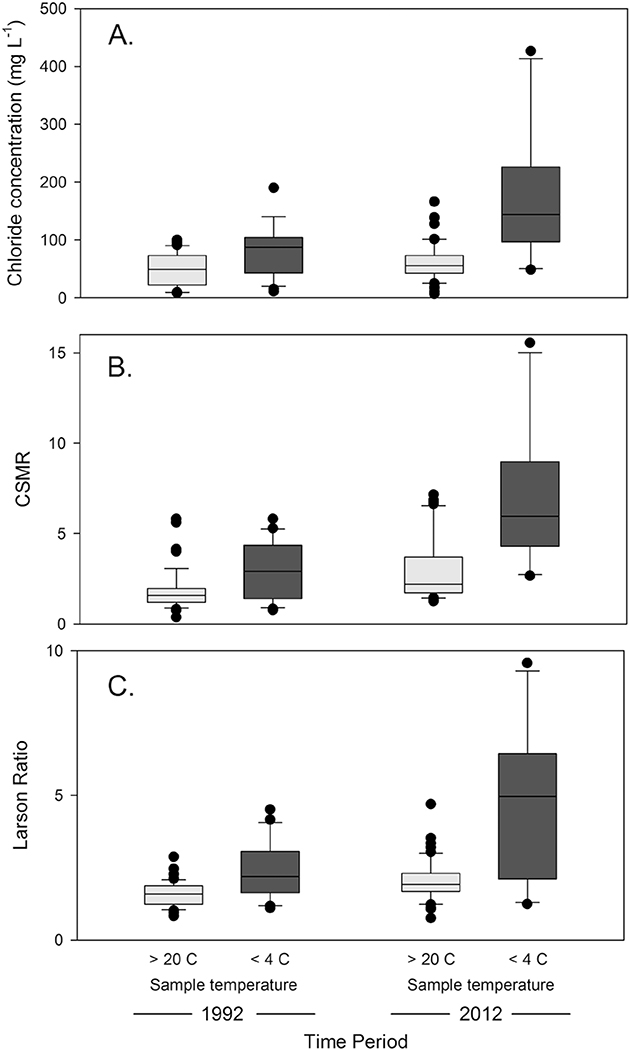
Among urban sites, samples collected during cold seasons, when water temperatures were < 4 °C (i.e. “cold samples”), had significantly higher chloride concentration, CSMR, and LR than samples collected when water temperatures were > 20 °C (i.e. “warm samples”, Fig. 3A–C). Temporal increases were observed in both seasons. Median chloride concentration increased from 87 to 144 mg L− 1 from 1992 to 2012 in cold samples collected from urban sites (Fig. 3A, W = 48, p = 0.005). Median CSMR increased from 2.9 to 6.0 from 1992 to 2012 in cold samples (Fig. 3B, W = 28, p < 0.001). Median LR increased from 2.2 to 5.0 from 1992 to 2012 in cold samples (Fig. 3C, W = 50, p = 0.005). In warm samples, significant increases were found in CSMR and LR between 1992 and 2012, but not chloride concentration. Median chloride concentration in warm samples was 49 mg L− 1 in 1992 and 56 mg L− 1 in 2012 (Fig. 3A, W = 1251, p = 0.17). Median CSMR increased from 1.6 to 2.2 in warm samples (Fig. 3B, W = 689, p < 0.001), and median LR increased from 1.6 to 1.9 (Fig. 3C, W = 844, p < 0.001). A similar analysis for other land use categories showed inconsistent trends and seasonality (Table S2). Most notably, raw values of CSMR increased between 1992 and 2012 at sites in Mixed land use in both warm and cold seasons and LR increased at sites in Undeveloped land use in both seasons (Table S2).
Fig. 3.
Boxplots of raw observations of (A.) chloride concentration (mg L− 1), (B.) chloride-sulfate mass ratio (CSMR), and (C.) Larson Ratio (LR) for samples collected at monitoring stations dominated by urban land cover. Samples were categorized as being collected in cold periods (< 4C) and warm periods (> 20C) during 1991–1993 and 2011–2013 (listed as 1992 and 2012 on the figure, respectively).
3.2. Status
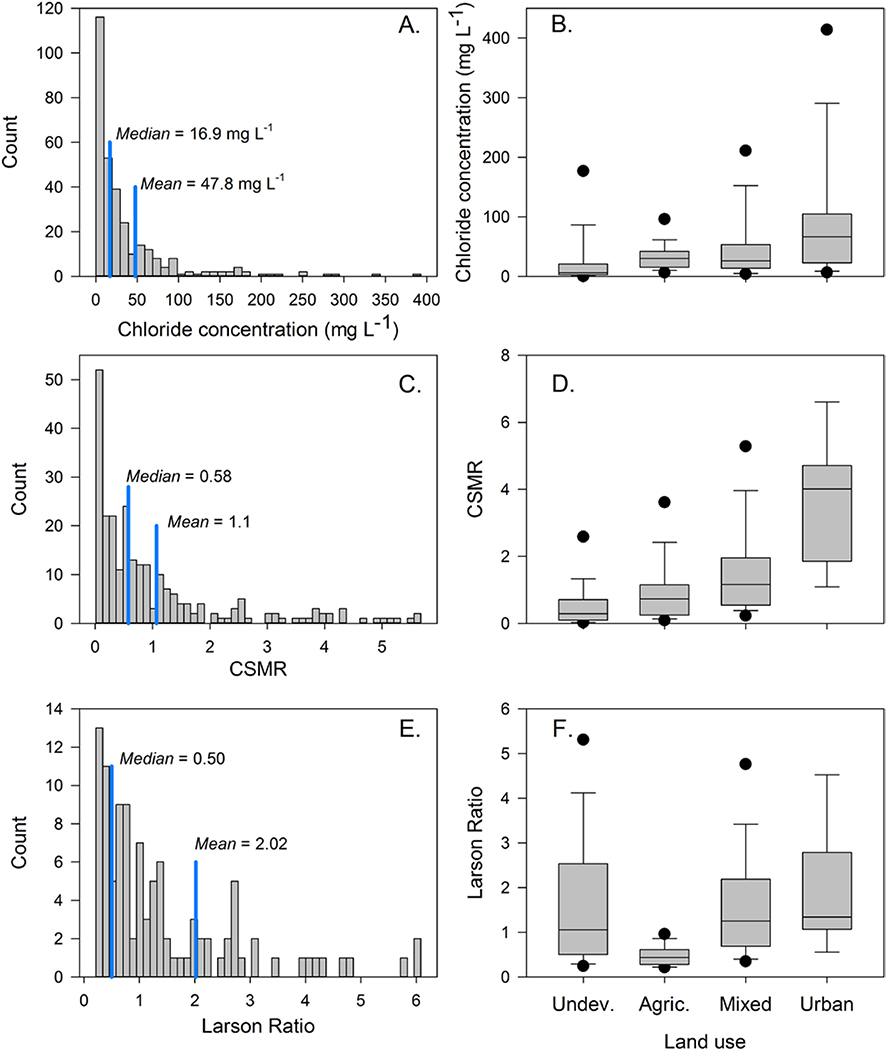
Among all sites analyzed as part of the 2010–2015 status assessment chloride had mean and median concentrations of 47.8 and 16.9 mg L− 1, respectively (Fig. 4A). Mean and median CSMR were 1.1 and 0.6, respectively (Fig. 4C), while mean and median LR was 2.0 and 0.5, respectively (Fig. 4E). Sites in urban land use had the highest chloride concentration and CSMR (Fig. 4B, D, F; Table S3). This is consistent with the observed effects of urbanization on stream chloride concentration and hence potential corrosivity (Fig. 2). LR was also highest in urban sites, but the difference between urban and mixed land use was minimally significant (p < 0.1, Table S3) and the difference between urban and undeveloped sites was not significant (Table S3).
Fig. 4.
Histograms (A., C., and E.) and boxplots (B., D., and F.) of the median chloride concentration (mg L− 1), chloride-sulfate mass ratio (CSMR) and Larson Ratio for all sites included in the status assessment. Histograms express results for all sites while the boxplots express results for each parameter categorized by land use. The land use categories include undeveloped (Undev.), agricultural (Agric.), mixed, and urban.
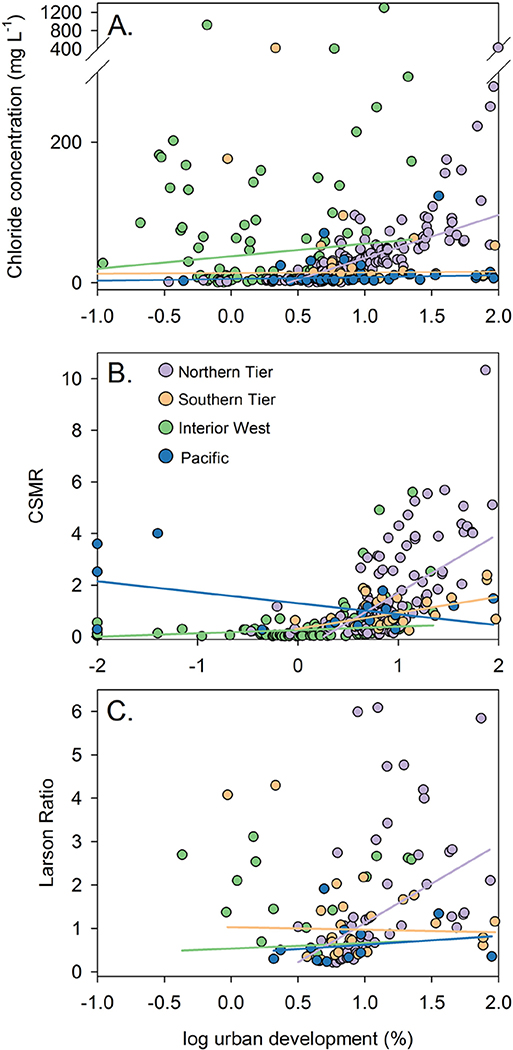
Sites located in the Northern tier region (Fig. S1) had a strongly positive relationship between urban development and chloride concentration, CSMR, and LR (ρ > 0.6 and p < 0.001 for each, Fig. 5A–C). In the Southern Tier region urbanization was not correlated with any of the parameters analyzed (ρ < 0.25 and p > 0.2 for each, Fig. 5A–C). A significant correlation existed between urbanization and chloride concentrations in the Pacific Region (ρ = 0.54, p < 0.001, Fig. 5A), but not CSMR or LR (ρ < 0.25, p > 0.4 for each, Fig. 5B–C). In the Interior West region, significant correlations existed between urbanization and chloride concentration and CSMR (ρ > 0.4, p < 0.001 for each, Fig. 5A–B), but not LR (ρ = 0.0, p > 0.99, Fig. 5C), although the range of urbanization in the Interior West region was relatively limited (< 1–22%).
Fig. 5.
Relationships between watershed percent urban development expressed on a log scale and median (A.) chloride concentration (mg L− 1), (B.) chloride-sulfate mass ratio (CSMR), and (C.) Larson Ratio for sites included in the status assessment. Points represent results for individual sites colored by region. Lines express the robust regression fit between urbanization and each parameter color-coded by region.
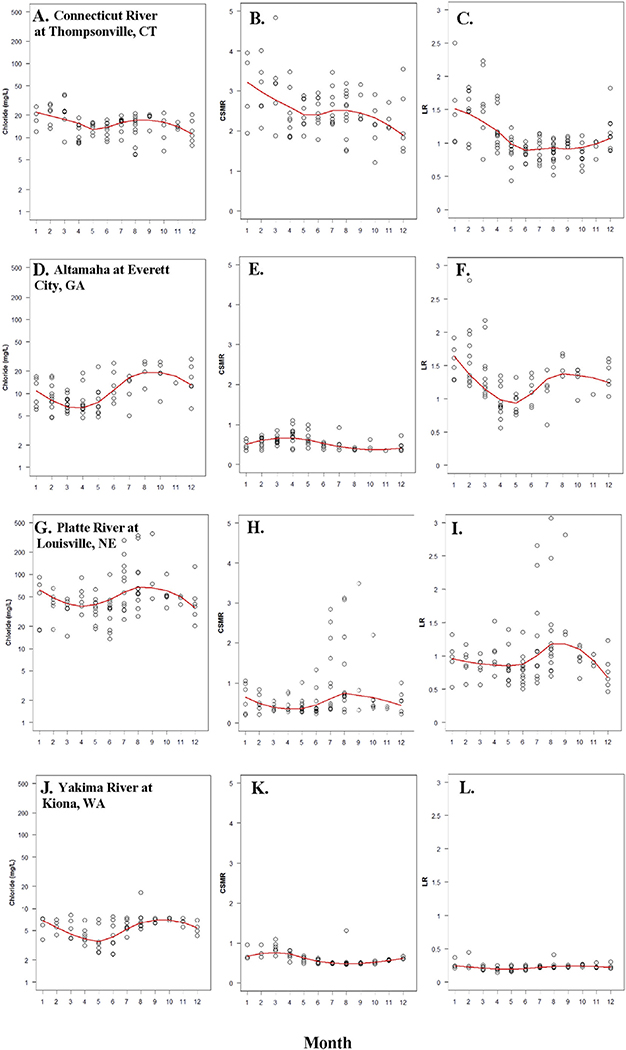
In order to explore the seasonality of chloride and the potential corrosivity, water quality data from several individual streams were examined in greater detail. One example was chosen from each region and displayed in Fig. 6. For the Northern Tier region the Connecticut River at Thompsonville, CT was examined. Chloride concentrations peaked and showed high variability from January through March and then decreased through July with a smaller secondary peak in August and September (Fig. 6A). CSMR and LR followed a similar seasonal pattern with CSMR commonly exceeding 3.0 and LR exceeding 1.5 from January to March. A secondary peak in CSMR, but not LR, was evident during August–September (Fig. 6B, C). The Southern Tier example was the Altamaha River near Everett City, GA which had peak chloride concentrations during the period August–December (Fig. 6D). CSMR showed relatively little seasonality and remained < 1.0 for most observations (Fig. 6E). LR showed a seasonal pattern with peak values > 1.5 during the January–March and a smaller peak August–December (Fig. 6F). The Platte River at Louisville, NE was displayed as the example from the Interior West region and had overall elevated chloride concentrations which peaked at > 100 mg L− 1 during July–September (Fig. 6G). CSMR and LR were generally < 1.0 except during the period July–September in which peak values were > 2.0 for both indices (Fig. 6H, I). Peak values of chloride, CSMR, and LR occurred during an unusually dry period in 2012 when streamflow during days of sample collection were below the first percentile of annual daily flow for the period 1953–2014 (not shown, flow data from USGS NWIS, http://dx.doi.org/10.5066/F7P55KJN). The example for the Pacific region was the Yakima River at Kiona, WA which had chloride concentrations generally < 10 mg L− 1 which were lowest during April–June (Fig. 6J). CSMR and LR were generally < 1.0 and < 0.5, respectively, with relatively little seasonality (Fig. 6K, L).
Fig. 6.
Individual results (circles) by month and monthly medians (lines) for samples collected 2010–2015 at four monitoring stations included in the status assessment. One site was chosen from each region: the Connecticut River from the Northern Tier region (A.–C.); the Altamaha River from the Southern Tier region (D.–F.); the Platte River from the Interior West region (G.–I.), and; the Yakima River from the Pacific region (J.–L.). Results are displayed for chloride concentration (A., D., G., and J.); CSMR (B., E., H., and K.); and LR (C., F., I., L.).
3.3. Empirical relationship to Pb exceedances
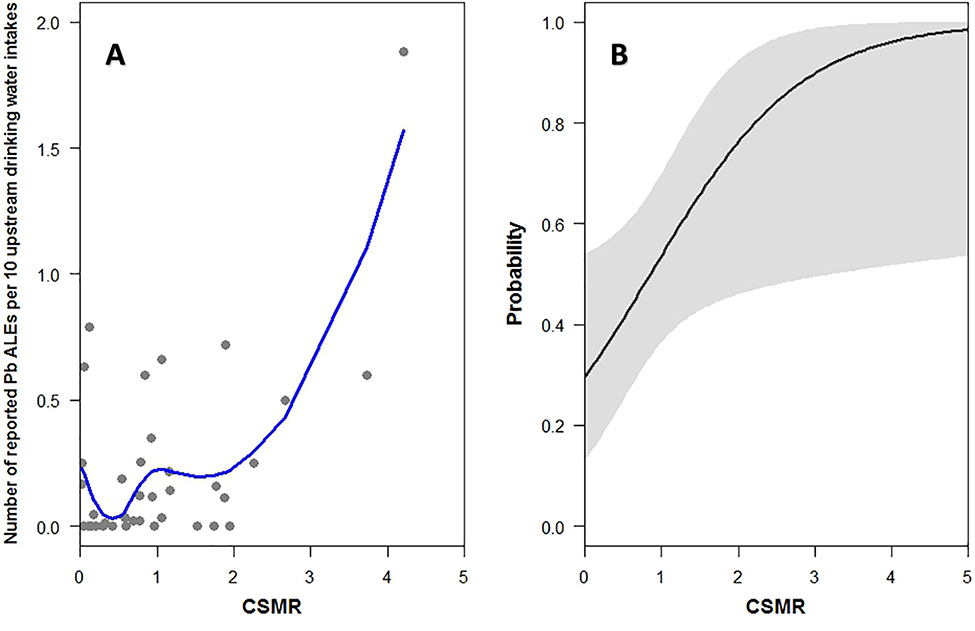
The occurrence of Pb ALEs at drinking water intakes upstream of water quality monitoring stations showed some correlation to chloride, CSMR, and LR. The relationships were characterized by a large degree of scatter and 13 of the 39 monitoring stations included in the analysis had no reported Pb ALEs at drinking water intakes upstream (Fig. 7A, Fig. S6). The logistic regression analysis found that CSMR was significantly predictive of the presence of multiple Pb ALEs per 10 surface drinking water intakes upstream of the monitoring location (p < 0.05, Table 2). The probability of having multiple ALEs was generally < 50% upstream from sites with CSMR values of < 1.0, but increases to > 90% at sites with median CSMR values above 3.0 (Fig. 7B). The uncertainty in the overall fit was relatively large and the 95% confidence intervals were nearly 25% above and below the logistic fit, indicating a high degree of variability in the relationship (Fig. 7B, Table 2). Neither chloride nor LR were predictive (p = 0.46 and p = 0.43, respectively, Table 2, Fig. S6). It is acknowledged that treatment process and distribution system characteristics are often the controlling factors affecting the presence of Pb in water systems, but it is also important to determine the degree to which source water characteristics can be linked to presence of lead in drinking water.
Fig. 7.
A.) Plot of the median (1992–2012) chloride-sulfate mass ratio (CSMR) at water quality monitoring stations on the x-axis versus the number of reported lead action-level exceedances (Pb ALEs) per 10 upstream surface drinking water intakes on the y-axis. Open circles show results for individual monitoring stations and red line is a best-fit locally weighted regression (LOESS). B.) Logistic regression showing the predicted probability of having multiple reported Pb ALEs per 10 surface upstream of monitoring stations with the given chloride-sulfate mass ratio (CSMR).
Table 2.
Logistic regression models on the probability of observing more than one lead action level exceedance (Pb ALE) per 10 surface drinking water intakes upstream of water quality monitoring stations. The models tested median (1992–2012) chloride concentration (mg L− 1), chloride-sulfate mass ratio (CSMR), and Larson Ratio (LR) as predictor variables.
| Predictor variable | Slope | p-Value | Accuracya | Akaike information criterion |
|---|---|---|---|---|
| Chloride | 0.004 | 0.461 | 0.436 | 57.34 |
| CSMR | 1.02 | 0.037 | 0.667 | 51.88 |
| LR | − 0.113 | 0.426 | 0.538 | 57.32 |
Accuracy – the sum of true positives and true negatives divided by total observations. True positives are cases with more than one ALE per 10 surface water intakes that have a predicted probability > 0.5. True negatives are the analogous negative case.
4. Discussion
This study found that chloride and several indices of the potential corrosivity of surface water, CSMR and LR, increased particularly in urban areas between 1992 and 2012 (Table 1). The increases in potential corrosivity were strongly linked with increasing chloride concentration (Fig. 1). In a relatively small number of sites, increasing CSMR or LR was caused by decreasing alkalinity or changes to SO42 − concentration rather than increasing chloride concentration. However, the changes in CSMR and LR at such sites were mostly small (0.1 to 0.3 between 1992 and 2012) and less likely to be the drivers of major trends and distribution of potential corrosivity. The statistical connection between surface water CSMR and the occurrence of elevated Pb levels in tap water (Fig. 7) is suggestive of a connection between surface water quality and potential corrosivity in distribution systems. The analysis was marked by high uncertainty (Fig. 7A–B, Table 2), consistent with the reality that corrosion in drinking water facilities is governed by a number of processes and water quality parameters. Lead release and corrosivity toward distribution systems and premise plumbing are affected to a large extent by water treatment such as (but not restricted to) coagulation, addition of corrosion inhibitors, pH and alkalinity adjustment, disinfection, and organic matter removal. Therefore, further investigation of any linkages between surface water quality and lead occurrence in tap water would need to account for differences in the water treatments in place at individual utilities and a fuller description of raw surface water quality. However, the findings underscore the need to better understand environmental drivers of corrosion in drinking water facilities as a step toward adapting protocols to achieve optimal corrosion control. Understanding the short-term (i.e. seasonal) variability and long-term changes (i.e. trends) in chloride, CSMR, and LR are important to understanding corrosion and implementing successful strategies to control it.
The urban streams included in the status assessment had high and increasing CSMR and LR, which may lead to a high potential to cause corrosion problems in water distribution systems. Median CSMR and LR of urban sites in the status assessment were 4.01 and 1.34, respectively (Fig. 4). By comparison, CSMR > 0.5 and LR > 0.3 in the finished waters of distribution systems are potentially corrosive and can result in elevated Pb concentrations in tap water (Dodrill and Edwards, 1995, Edwards and Triantafyllidou, 2007, Nguyen et al., 2011, Schock and Lytle, 2011, U.S. Environmental Protection Agency, 2016). These thresholds do not necessarily directly reflect source water because water treatment can change many of the water quality parameters linked with corrosion (alkalinity, chloride, pH, CSMR, LR, etc.). It is also important to note that some of the most urbanized sites in our dataset are small streams with watersheds confined to urban areas and are not used as drinking water intakes. However, Pb ALEs were much more common upstream of monitoring stations with CSMR > 1.0 (Fig. 7), a level which was greatly exceeded at times even in larger systems such as the Connecticut and Platte Rivers (Fig. 6).
Road salt usage has been established as a major contributor to chloride increases in urban streams. Because of this, chloride trends have been most dramatic in cold-weather months, especially in snow-affected areas of the U.S. (Chapra et al., 2009, Corsi et al., 2015, Kaushal et al., 2005, Norrström, 2005). The strong relationships between chloride trends and those of CSMR and LR (Fig. 1) suggest that trends in potential corrosivity would have many of the same spatial and temporal characteristics as trends in chloride. This expectation is consistent with findings of the evolving seasonality of potential corrosivity indices in urban streams (Fig. 3) and the regional differences in the effect of urbanization on corrosivity (Fig. 5). In addition to the large increases in chloride-related potential corrosivity in cold samples, a smaller but statistically significant increase also occurred in warm samples (Fig. 3). Increases observed during warm periods suggest longer-term trends in chloride in either the shallow groundwater systems of snow-affected regions (Ledford et al., 2016) or other chloride sources to urban areas, such as septic systems or wastewater discharge (Chapra et al., 2009, Coles et al., 2012, Sprague et al., 2007), which occur regardless of the use of road salt. Temperature is an important influence on corrosion rates (Volk et al., 2000) and so the increases in warm samples, though small in magnitude, may have a disproportionate effect on the corrosivity of surface water to distribution systems.
Beyond the large trends observed in urban, snow-affected areas (Fig. 3; Corsi et al. (2015); Kaushal et al. (2005)), the seasonality in chloride, CSMR, and LR were highly variable by site and defied simple categorization (Fig. 6). Elevated chloride, CSMR, and LR was evident during the winter in snow-affected areas (Fig. 6A–C), but at other sites peak values were observed in summer or during droughts (Fig. 6D–I), indicating a hydrologic influence on the indices of potential corrosivity. For example, while corrosivity peaked during January–March in the Connecticut River, the Platte River experienced highest corrosivity during late summer and fall (Fig. 6). Additionally, the highest values of CSMR and LR in the Platte River occurred in 2012 and were collected on days for which streamflow in the South Platte River was below the 1 percentile of flows for the period 1953–2014 (Fig. 6, not shown). So, although long-term increases in potential corrosivity are evident at sites affected by upstream urbanization, site-specific conditions make it difficult to generalize seasonal patterns in corrosivity. This finding underscores the need for effective water quality monitoring in order to optimize corrosion control strategies at drinking water facilities.
Despite its importance to drinking water facilities, water quality monitoring protocols do not emphasize characterization of the seasonal or long-term changes in surface water corrosivity or metal release. In the U.S., the Lead and Copper Rule sets the national guiding principles of detecting corrosivity toward lead and copper in drinking water systems. Thus, corrosion driven by changes in chloride concentration, CSMR, or LR would not be detected under regulatory requirements or typical voluntary monitoring. Therefore, long-term, seasonal, and event-driven changes in corrosivity are not worked into plans for corrosion optimization, at least not at the national level.
The role of chloride trends in urban areas stood out as an important driver of indices of potential corrosivity in this national-scale study, but individual intakes could be affected by changes in a number of water quality parameters or in hydrologic characteristics. As noted above, a small number of sites had significant increases in CSMR or LR without concurrent increases in chloride (Fig. 1). Sulfate concentrations have decreased in surface waters of the U.S. as a result of efforts to combat acid deposition (Clow and Mast, 1999, Stets et al., 2014, Stoddard et al., 1999), which could lead to increasing CSMR. Additionally, hydrologic modification has led to alkalinity decreases in some areas due to calcium carbonate formation in reservoirs and trans-basin water diversions (Hu et al., 2015, Stets et al., 2014). Furthermore, as observed in the Platte River example (Fig. 6), interannual variability in corrosivity due to droughts or floods could be significant but are not currently part of the monitoring protocols associated with drinking water facilities. Therefore, while road salt and urbanization influences were important in this study because of the magnitude of effects, the drivers of changes in corrosivity are likely to be numerous and site-specific.
5. Conclusions
The evolving and seasonally diverse nature of water quality in U.S. surface waters emphasizes the need for adequate monitoring as a basis for optimal corrosion control. As made clear in these analyses, water quality parameters potentially linked to corrosion in drinking water facilities is changing over time and those changes are spatially heterogeneous and driven by a number of factors. Therefore, the ability of water purveyors to be aware and respond to changes in the potential corrosivity of surface waters will depend upon observations made at adequate time intervals and including the appropriate suite of water quality parameters.
Supplementary Material
Acknowledgments
Support for this study was provided by the USGS National Water Quality Program. We thank Neil Dubrovsky, Lori Sprague, and Jeff Deacon for their contributions to the concepts and approaches in this study. We also thank Gretchen Oelsner, Melissa Riskin, Laura DeCicco, and Elizabeth Whiddon for their role in gathering the necessary data, running the trend analyses, and creating the data infrastructure that made interpretation possible. James Falcone analyzed land cover types for the basins of interest. We especially thank the field technicians, their funding agencies, and the local, state, and national monitoring networks that have been collecting the critical observational data that is used to manage our resources and allows researchers to draw important conclusions about a complicated and rapidly changing environment. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the U.S. EPA. Any mention of products or trade names does not constitute recommendation for use by the U.S. EPA.
References
- AWWARF-TZW, Internal Corrosion of Water Distribution Systems, (Second ed.), AWWA Research Foundation/DVGW-TZW, Denver, CO: (1996) [Google Scholar]
- Chapra SC, Dove A, Rockwell DC, Great lakes chloride trends: long-term mass balance and loading analysis, J. Great Lakes Res, 35 (2) (2009), pp. 272–284, 10.1016/j.jglr.2008.11.013 [DOI] [Google Scholar]
- Clow DW, Mast MA, Long-term trends in stream water and precipitation chemistry at five headwater basins in the northeastern United States, Water Resour. Res, 35 (2) (1999), pp. 541–554, 10.1029/1998WR900050 [DOI] [Google Scholar]
- Coles JF, et al. , Effects of Urban Development on Stream Ecosystems in Nine Metropolitan Study Areas Across the United States (2012) (138 pp.)
- Conway TM, Impervious surface as an indicator of pH and specific conductance in the urbanizing coastal zone of New Jersey, USA, J. Environ. Manag, 85 (2) (2007), pp. 308–316, 10.1016/j.jenvman.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Corsi SR, Graczyk DJ, Geis SW, Booth NL, Richards KD, A fresh look at road salt: aquatic toxicity and water-quality impacts on local, regional, and national scales, Environ. Sci. Technol, 44 (19) (2010), pp. 7376–7382, 10.1021/es101333u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi SR, De Cicco LA, Lutz MA, Hirsch RM, River chloride trends in snow-affected urban watersheds: increasing concentrations outpace urban growth rate and are common among all seasons, Sci. Total Environ, 508 (2015), pp. 488–497, 10.1016/j.scitotenv.2014.12.012 [DOI] [PubMed] [Google Scholar]
- DeSantis MK, Schock MR, Ground truthing the ‘conventional wisdom’ of lead corrosion control using mineralogical analysis, Proc. AWWA Water Quality Technology Conference, New Orleans, LA, November 16–20 (2014) [Google Scholar]
- DeSantis MK, Welch MM, Schock MR, Mineralogical evidence of galvanic corrosion in domestic drinking water pipes, Proc. AWWA Water Quality Technology Conference, Seattle, WA, November 15–19 (2009) [Google Scholar]
- Dinno A, Dunn's Test of Multiple Comparisons Using Rank Sums, R package Version 1.3.4, url: https://cran.r-project.org/web/packages/dunn.test/ (2017) [Google Scholar]
- Dodrill DM, Edwards M, Corrosion Control on the Basis of Utility Experience, American Water Works Association, Denver, CO, U.S. (1995) [Google Scholar]
- Duan S, Kaushal SS, Salinization alters fluxes of bioreactive elements from stream ecosystems across land use, Biogeosciences, 12 (23) (2015), pp. 7331–7347, 10.5194/bg-12-7331-2015 [DOI] [Google Scholar]
- Ebberts JC, Embrey SS, Kelley JA, Concentrations and Loads of Suspended Sediment and Nutrients in Surface Water of the Yakima River Basin, Washington, 199–2000 - With an Analysis of Trends in Concentrations, U.S. Geological Survey Water-Resources Investigations; (2003), (Report 03–4026, 111 pp.) [Google Scholar]
- e-CFR (Electronic Code of Federal Regulations), National Primary Drinking Water Regulations. Title 40 Part 141 Subpart A—General §141.2 Definitions Accessed at, https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=504562c26c4c3387ec017b54b73e88ab&mc=true&n=pt40.25.141&r=PART&ty=HTML#se40.25.141_12 (2017)
- e-CFR (Electronic Code of Federal Regulations), National Primary Drinking Water Regulations. Title 40 Part 141 Subpart I—Control of Lead and Copper, Accessed at, https://www.ecfr.gov/cgi-bin/text-idx?SID=504562c26c4c3387ec017b54b73e88ab&mc=true&node=sp40.25.141.i&rgn=div6 (2017)
- Edwards M, Triantafyllidou S, Chloride-to-sulfate mass ratio and lead leaching to water, J. Am. Water Works Assoc, 99 (7) (2007), pp. 96–109 [Google Scholar]
- ESRI, ArcGIS Desktop: Release 10, Environmental Systems Research Institute, Redlands, CA: (2011) [Google Scholar]
- U.S. Geological Survey, National Water Information System—Web Interface (2016), 10.5066/F7P55KJN, (accessed September 28, 2016, at) [DOI] [Google Scholar]
- Hayes CR, Skubala ND, Is there still a problem with lead in drinking water in the European Union?, J. Water Health, 7 (4) (2009), pp. 569–580 [DOI] [PubMed] [Google Scholar]
- Hirsch RM, Moyer DL, Archfield SA, Weighted regressions on time, discharge, and season (WRTDS), with an application to Chesapeake Bay River Inputs1, J. Am. Water Resour. Assoc, 46 (5) (2010), pp. 857–880, 10.1111/j.1752-1688.2010.00482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch RM, Archfield SA, De Cicco LA, A bootstrap method for estimating uncertainty of water quality trends, Environ. Model. Softw, 73 (2015), pp. 148–166, 10.1016/j.envsoft.2015.07.017 [DOI] [Google Scholar]
- Homer CH, Fry JA, Barnes CA, The National Land Cover Database, U. S. G. Survey, Reston, VA: (2012) [Google Scholar]
- Hu X, Pollack JB, McCutcheon MR, Montagna PA, Ouyang Z, Long-term alkalinity decrease and acidification of estuaries in Northwestern Gulf of Mexico, Environ. Sci. Technol, 49 (6) (2015), pp. 3401–3409, 10.1021/es505945p [DOI] [PubMed] [Google Scholar]
- Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT, Increased salinization of fresh water in the northeastern United States, Proc. Natl. Acad. Sci. U. S. A, 102 (38) (2005), pp. 13517–13520, 10.1073/pnas.0506414102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TE, Skold RV, Laboratory studies relating mineral quality of water to corrosion of steel and cast iron, Corrosion, 14 (6) (1958), pp. 43–46, 10.5006/0010-9312-14.6.43 [DOI] [Google Scholar]
- Ledford SH, Lautz LK, Stella JC, Hydrogeologic processes impacting storage, fate, and transport of chloride from road salt in urban riparian aquifers, Environ. Sci. Technol, 50 (10) (2016), pp. 4979–4988, 10.1021/acs.est.6b00402 [DOI] [PubMed] [Google Scholar]
- Lee CJ, Hirsch RM, Schwarz GE, Holtschlag DJ, Preston SD, Crawford CG, Vecchia AV, An evaluation of methods for estimating decadal stream loads, J. Hydrol. (2016), 10.1016/j.jhydrol.2016.08.059 [DOI] [Google Scholar]
- Lytle DA, Schock MR, Pitting corrosion of copper in high pH and low alkalinity waters, J. Am. Water Works Assoc, 100 (3) (2008), pp. 115–128 [Google Scholar]
- Lytle DA, Sarin P, Snoeyink VL, The effect of chloride and orthophosphate on the release of iron from a drinking water distribution system cast iron pipe, J. Water Supply Res Technol, 54 (5) (2004), pp. 267–281 [Google Scholar]
- McCabe GJ, Wolock DM, Long-term variability in Northern Hemisphere snow cover and associations with warmer winters, Clim. Chang, 99 (2010), pp. 141–153 [Google Scholar]
- McKay L, Bondelid T, Dewald T, Johnston C, Moore RB, Rea A, NHDPlus Version 2: User Guide, url: http://www.horizon-systems.com/nhdplus/NHDplusV2_home.php (2012)
- McNeill LS, Edwards M, Phosphate inhibitor use in US utilities, J. Am. Water Works Assoc, 94 (7) (2002), pp. 57–63 [Google Scholar]
- Nelson SS, Yonge DR, Barber ME, Effects of road salts on heavy metal mobility in two Eastern Washington soils, J. Environ. Eng, 135 (7) (2009), pp. 505–510, 10.1061/(ASCE)0733-9372(2009)135:7(505) [DOI] [Google Scholar]
- Ng D-Q, Lin Y-P, Effects of pH value, chloride and sulfate concentrations on galvanic corrosion between lead and copper in drinking water, Environ. Chem, 13 (4) (2016), pp. 602–610, 10.1071/EN15156 [DOI] [Google Scholar]
- Nguyen CK, Clark BN, Stone KR, Edwards MA, Role of chloride, sulfate, and alkalinity on galvanic lead corrosion, Corrosion, 67 (6) (2011), 10.5006/1.3600449, (065005–065001-065005–065009) [DOI] [Google Scholar]
- Norrström AC, Metal mobility by de-icing salt from an infiltration trench for highway runoff, Appl. Geochem, 20 (10) (2005), pp. 1907–1919, 10.1016/j.apgeochem.2005.06.002 [DOI] [Google Scholar]
- Novotny V, Muehring D, Zitomer DH, Smith DW, Facey R, Cyanide and metal pollution by urban snowmelt: impact of deicing compounds, Water Sci. Technol, 38 (10) (1998), pp. 223–230 [Google Scholar]
- Novotny EV, Murphy D, Stefan HG, Increase of urban lake salinity by road deicing salt, Sci. Total Environ, 406 (1–2) (2008), pp. 131–144, 10.1016/j.scitotenv.2008.07.037 [DOI] [PubMed] [Google Scholar]
- Oelsner GP, Sprague LA, Murphy JC, Zuellig RE, Johnson HM, Ryberg KR, Falcone JA, Stets EG, Vecchia AV, Riskin ML, DeCicco LA, Mills TJ, Farmer WH, Water-quality Trends in the Nation's Rivers and Streams 1972–2012 – Data Preparation, Statistical Methods, and Trend Results, U.S. Geological Survey; (2017), (Scientific Investigations Report 2017-xxxx, xx pp.) [Google Scholar]
- Pieper KJ, Tang M, Edwards MA, Flint Water Crisis Caused By Interrupted Corrosion Control: Investigating “Ground Zero” Home, Environmental Science & Technology, 51 (2017), pp. 2007–2014 [DOI] [PubMed] [Google Scholar]
- Price CV, Maupin MA, Documentation for the U.S. Geological Survey Public-supply Database (PSDB) – A Database of Permitted Public-supply Wells, Surface-water Intakes, and Systems in the United States, U.S. Geological Survey; (2014), (Open-File Report 2014–12, 32 p.) [Google Scholar]
- R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria: (2016) [Google Scholar]
- Schock MR, Lytle DA, Internal corrosion and deposition control, Edzwald JK (Ed.), Water Quality and Treatment: A Handbook on Drinking Water, McGraw-Hill, New York, NY: (2011), pp. 20.21–20.103 [Google Scholar]
- Shalaby AM, Al-Kharafi FM, Gouda VK, A morphological study of pitting corrosion of copper in soft tap water, Corrosion, 45 (7) (1989), pp. 536–547 [Google Scholar]
- Sprague LA, Harned DA, Hall DW, Nowell LH, Bauch NJ, Richards KD, Response of Stream Chemistry during Base Flow to Gradients of Urbanization in Selected Locations Across the Conterminous United States, 2002–04, U. S. G. Survey; (2007) [Google Scholar]
- Sprague LA, Hirsch RM, Aulenbach BT, Nitrate in the Mississippi River and its tributaries, 1980 to 2008: are we making progress?, Environ. Sci. Technol (2011), 10.1021/es201221s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stets EG, Kelly VJ, Crawford CG, Long-term trends in alkalinity in large rivers of the conterminous US in relation to acidification, agriculture, and hydrologic modification, Sci. Total Environ, 488–489 (0) (2014), pp. 280–289, 10.1016/j.scitotenv.2014.04.054 [DOI] [PubMed] [Google Scholar]
- Stoddard JL, et al. , Regional trends in aquatic recovery from acidification in North America and Europe, Nature, 401 (6753) (1999), pp. 575–578 [Google Scholar]
- Tu J, Spatial variations in the relationships between land use and water quality across an urbanization gradient in the watersheds of Northern Georgia, USA, Environ. Manag, 51 (1) (2013), pp. 1–17, 10.1007/s00267-011-9738-9 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, Optimal Corrosion Control Treatment Evaluation Technical Recommendations for Primacy Agencies and Public Water Systems, Office of Water, Washington, DC: (2016), (140 p.) [Google Scholar]
- Volk C, Dundore E, Schiermann J, LeChevallier M, Practical evaluation of iron corrosion control in a drinking water distribution system, Water Res, 34 (6) (2000), pp. 1967–1974, 10.1016/S0043-1354(99)00342-5 [DOI] [Google Scholar]
- Whiddon ET, Stets EG, Corrosivity Index and Streamflow Datasets Used to Evaluate Trends in Potentially Corrosive Source Waters in the Nation's Streams and Rivers: U.S. Geological Survey Data Release, (2017), 1.0.5066/F79S1P74 [Google Scholar]
- Willison H, Boyer TH, Secondary effects of anion exchange on chloride, sulfate, and lead release: systems approach to corrosion control Water Res, 46 (7) (2012), pp. 2385–2394, 10.1016/j.watres.2012.02.010 [DOI] [PubMed] [Google Scholar]
- World Health Organization, Annex 5: Treatment Methods and Performance in Guidelines for Drinking-water Quality, (4th edition) (2017) (Geneva, 631 pp.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.