Abstract
Background
Huntington's disease (HD) is an autosomal dominant neurodegenerative disease with an average onset between the fourth and fifth decade of life; it leads to death 15 to 20 years after the onset of symptoms. Although several drugs seem effective in controlling the incapacitating manifestations of HD, no specific therapy is known. The present review aims at analysing the best available data on therapeutic interventions investigated with the goal of modifying the progression of the disease as measured in terms of survival, disability or progression of HD core symptoms.
Objectives
Evaluate the effectiveness of therapeutic interventions aimed at modifying disease progression in HD.
Search methods
The search strategy developed for the Movement Disorders Group was undertaken. The Cochrane Controlled Trials Register, Medline, EMBASE and Clinical Trials Database of the United States National Institute of Health were thoroughly searched until December 2007.
Selection criteria
All randomised, double‐blinded, placebo‐controlled clinical trials of therapeutics investigated with the goal of modifying disease progression in HD were included. Participants should have genetically confirmed diagnosis of HD or compatible symptoms and a family history. Trials had a follow‐up duration of more than three months and at least ten participants. All pharmacological and non‐pharmacological interventions were included.
Data collection and analysis
Two reviewers independently assessed the eligibility of identified trials. The methodological quality was assessed and eligible data were registered onto standardised forms. An intention‐to‐treat analysis was conducted, when feasible. If data were not available in the original publication, the principal investigator of the trial was contacted for further information. A meta‐analysis was to be conducted when possible; otherwise, a descriptive summary of the results was provided. The software Revman 5.0.15 was used for statistical analysis.
Main results
Eight trials were included involving a total of 1366 HD patients. The duration of the studies ranged between 30 and 144 weeks (median: 52 weeks). The following interventions were selected: vitamin E, Idebenone, Baclofen, Lamotrigine, creatine, coenzyme Q10 + Remacemide, ethyl‐eicosapentanoic acid and Riluzole. No trials produced positive results for the selected efficacy outcome measures. A descriptive summary of the trials is provided. The selected interventions were found to be generally safe and well tolerated.
Authors' conclusions
Only pharmacological interventions were included and none proved to be effective as a disease‐modifying therapy for HD. Further trials with greater methodological quality should be conducted using more sensitive biological markers. Pre‐symptomatic mutation carriers should be included in future studies.
Plain language summary
Interventions to delay progression of Huntington's disease
Huntington´s disease (HD) is an autosomal dominant neurodegenerative disease for which no cure is currently available. We proposed to assess the effectiveness of interventions aimed at modifying disease progression and evaluate the methodological quality of the corresponding clinical trials. We selected eight trials comprising a total of 1366 participants. The results show that no intervention demonstrated an effect in modifying disease progression in HD.
Background
Huntington's disease (HD) also known as Huntington's chorea was first described by the eponymous North American physician George Huntington in 1872 (Lanska 1995). It is characterised by choreiform movements, progressive dementia and psychiatric manifestations (depression, psychosis, apathy, irritability). Choreiform movements consist of involuntary, rapid, irregular, jerky motor actions including facial twitching or writhing and twitching of distal extremities and more generalised forms that impair gait (Ropper 2005). HD is an autosomal dominant inherited disease meaning that the child of an affected patient has a 50% chance of developing the disease. The causative gene is present on chromosome 4 and encodes a protein known as huntingtin (TH s DCR Group 1993). This protein gradually accumulates within brain cells causing damage and cell death in certain brain areas, specifically, the basal ganglia and neocortex. The rate of huntingtin accumulation is associated with the number of repeats of a specific sequence of three nucleic acids (CAG repeats). A greater number of repeats are associated with an earlier disease onset (Kieburtz 1994).
HD is found throughout the world and in all ethnic groups. Global prevalence of HD is estimated to be 4‐5 per one million people. In western countries it is estimated to be 8‐10 per 100.000 people. There is no gender predominance. HD usually begins between the age of 30 and 50 and leads to death 15 to 20 years after the onset of neurological symptoms(Ropper 2005). An early onset variant exists (Juvenile HD or Westphal variant) and typically begins in adolescence. In contrast with the adult‐onset form, it presents with particular motor features of slowness of movement (bradykinesia) and increased muscular tone (rigidity type). Additionally, Juvenile HD has a more severe course with an average survival time of 5 to 10 years (Ropper 2005).
No specific pharmacological therapy is known for HD. It is also known that some drugs seem to be active in controlling some of its major troublesome symptoms (chorea, psychosis, depression) (Bonelli 2004). Although it is difficult to separate a disease‐modifying effect from a pure symptomatic one, the present review aims at covering the best quality data available about therapeutic interventions studied with the goal of modifying disease progression as measured with survival, disability or progression of functional impairment.
Objectives
Evaluation of the effectiveness of therapeutic interventions aimed at modifying disease progression in HD
Methods
Criteria for considering studies for this review
Types of studies
All trials investigated were randomised controlled parallel clinical trials of therapeutics investigated with the goal of modifying disease progression in HD. In addition, all trials had a follow‐up duration of more than three months and more than ten patients. Crossover trials were only included if the results of the first part of the trial before crossing‐over were accessible.
Types of participants
Participants should have had a genetically confirmed diagnosis of HD or the presence of clinical features of HD and a positive HD family history. All disease variants and all ages of disease‐onset were included, as well as all possible concomitant therapies.
Types of interventions
All pharmacological and non‐pharmacological interventions used with the objective of modifying disease progression in HD, including brain surgery (fetal neural transplants or other neurosurgical procedures).
Types of outcome measures
Primary outcomes
The time to a clinically relevant disease stage considered as a consensual clinical marker of disease progression was used for primary outcome. The most definite clinical endpoint is death (or survival). Alternatives accepted were: neuropsychiatric symptoms onset, diagnosis of dementia, postural instability, falls, wheelchair confinement, bedridden status, retirement on grounds of ill‐health or loss of autonomy. These outcomes could be measured as a binary variable or as time‐to‐event variable.
Secondary outcomes
The considered outcome measure was decline in functional capacity as measured by a validated or consensually used scale. Other alternatives were symptomatic progression regarding the three main symptom complex of HD, specifically: progression of motor signs, progression of neuropsychiatric symptoms and progression of cognitive decline, all measured by validated scales or scales accepted by consensus. Safety outcomes measures were also considered, specifically: 1) tolerability measured by withdrawal from trials and 2) safety measured by the incidence and type of adverse effects, the occurrence of adverse effects leading to withdrawal and serious adverse effects defined as any adverse event that is life‐threatening or results in death, hospitalisation or prolonged incapacity/disability.
Search methods for identification of studies
Electronic searches
The review was drawn on the search strategy developed for the Movement Disorders Group as a whole. We identified relevant trials in the following electronic databases:
Cochrane Controlled Trials Register (Central/CCTR in The Cochrane Library, Issue 4, 2007);
MEDLINE (1966 to December 2007);
EMBASE (1974 to December 2007);
Clinical Trials Database of the United States National Institute of Health (December 2007)
For MEDLINE and Cochrane Controlled Trials Register, a MeSH search using the following search strategy was conducted: 1.Huntington/all subheadings 2.diet therapy 3.drug therapy 4.prevention and control 5.rehabilitation 6.surgery 7.therapy 8.psychology 9.mortality 10.#1 AND #2 11.#1 AND #3 12.#1 AND #4 13.#1 AND #5 14.#1 AND #6 15.#1 AND #7 16.#1 AND #8 17.#1 AND #9 18.10‐17 19.#18 AND Limit: clinical trial 20. in humans
No language restriction was applied. If necessary, the translation of the original article to Portuguese was done.
Searching other resources
We also:
Searched reference lists of identified trials and HD review articles;
Hand searched the Movement Disorders Journal and abstract books of international congresses of movement disorders and dementia;
Personally contacted other researchers in the field;
Contacted drug manufactures to obtain additional information on trials identified in other sources or unpublished trials.
Data collection and analysis
Two reviewers (Mestre T, Ferreira J) independently assessed selection criteria in the identified studies. Disagreements concerning inclusion were resolved by consensus between the two reviewers and a third party (Coelho M).
The selected studies were independently assessed for methodological quality according to the Cochrane Collaboration handbook (Cochrane 2008). A record was made of the following items: randomisation methods, treatment and assessment blinding, comparability of treatment groups in terms of demographic and clinical characteristics (age of onset, disease duration and severity, CAG repeat expansion, co‐morbidity, concomitant medication), inclusion and exclusion criteria, number of drop‐outs or lost to follow‐up and corresponding causes, duration of follow‐up, definition of outcomes, use of validated scales and description of adverse events. The Jadad score was used as a global measure for methodological quality (Jadad 1996). We excluded trials with a Jadad score <3.
Possible sources of bias were taken into consideration, namely: 1) selection bias, including randomisation and random difference between groups due to a small sample size; 2) performance bias; 3) attrition bias; 4) detection bias, including modifications to validated rating scales; 5) selective reporting of results.
Eligible data were independently registered onto standardised forms by two reviewers (Mestre T, Ferreira J) and cross‐checked for accuracy. Disagreements regarding inclusion were resolved by consensus between reviewers (Mestre T, Ferreira J) and a third party (Coelho M). An intention‐to‐treat analysis was conducted, whenever feasible. Thus data concerning the number of patients with each outcome event in each allocated treatment group was thoroughly sought, regardless of compliance and irrespective of patient ineligibility or exclusion after randomisation. If data were not available in the original publications, the principal investigator of the trial was contacted for further information. When considered relevant, the effect of missing outcomes from patients excluded after randomisation was evaluated by a best‐ and worst‐case sensitivity analysis
The various rating scales used were dichotomised using each author's own criteria for benefit or no benefit. If these criteria were not described, benefit was defined as any delay in disease progression; no benefit was defined as the absence of a delay in disease progression. To dichotomise results, individual patient data were requested if the results were presented as mean values for groups. Thus, if possible, the results were reported as odds ratios with 95 % confidence intervals. Outcomes presented as time‐to‐event data were analysed using a logrank analysis (Cochrane 2008). The results were expressed as a Hazard Ratio (HR) with 95 % confidence intervals.
A meta‐analysis was conducted if feasible. For time‐to‐event data, a modified Peto odds ratio fixed‐effect method was used. For dichotomous data, the Peto odds ratio fixed‐effect method was adopted (Cochrane 2008). Heterogeneity between trial results was tested using a standard chi‐squared test (p < 0.05). A descriptive summary of the results is provided since it was not possible to pool outcome data from different studies. A subgroup analysis for CAG repeat number was considered but not performed due to the reduced number of trials with this information. Statistical analysis was performed using the statistical tools incorporated in the software Revman 5.0.15 (Revman 2008)
Results
Description of studies
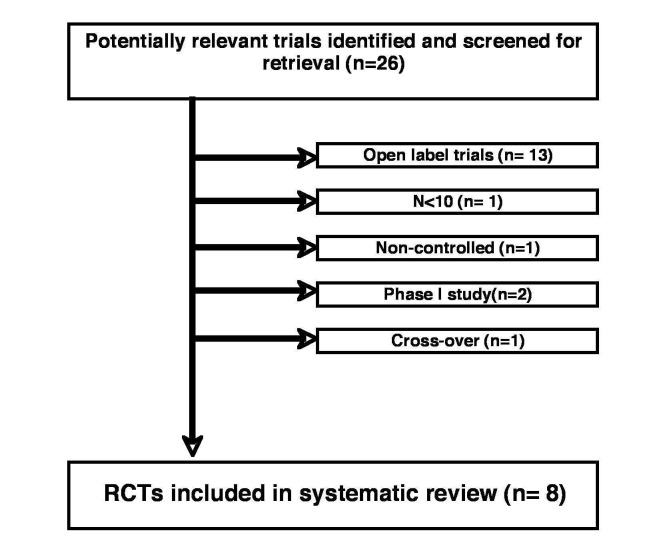
Eight trials were included comparing various pharmacological interventions with placebo in a total of 1366 HD patients. The initial search strategy resulted in 26 studies (1583 HD patients) appearing as eligible from the available information extracted from the corresponding titles and abstracts. Later, trials were excluded on the basis of open label design (n = 13; 178/11 % of patients), absence of control (n = 1; 7 HD patients), crossover design (n = 1; 10 HD patients), small sample, i.e., n < 10 (n = 1; 7 HD patients) and phase I study (n = 2; 15 HD patients) (Figure 1). All surgical interventions were excluded due to an open label design.
1.
TRIAL DESIGN:
The eight included trials were randomised, placebo‐controlled, double blind studies with a parallel design. One trial had a factorial design with three arms with different combinations of two active interventions and one with placebo (CARE‐HD). Four trials were conducted in a single centre (Peyser CE; Ranen NG; Shoulson I; Verbessem P) and the remaining trials were conducted in a multicentric fashion. The patients included in the trials received treatment or placebo during 30 to 144 weeks (median: 52 weeks). All trials were published between 1989 and 2007 in the English language.
PARTICIPANTS:
In the included trials, the age of the participants had a median value of 45.5, ranging from 39.4 to 49.8 years of age. The disease duration had a median value of 5.1, ranging from 2.9 to 8.0 years. In two trials there was no data concerning age of participants (Peyser CE) and/or disease duration (Peyser CE; Puri BK). A confirmatory genetic diagnosis (CAG repeat) was only available in four trials (CARE‐HD; EHDN; Kremer B; Puri BK) with the median value being 45 CAG repeats in both treatment and placebo groups.
INTERVENTIONS:
The following interventions were found: Baclofen (Shoulson I), coenzyme Q10+Remacemide (CARE‐HD), creatine (Verbessem P) , ethyl‐eicosapentanoic acid (Puri BK), Idebenone (Ranen NG), Lamotrigine (Kremer B), Riluzole (EHDN), vitamin E (Peyser CE).
OUTCOME MEASURES:
Outcome measures changed across trials. The most frequently used primary outcome measure was a change in functional capacity using the Total Function Capacity (TFC)‐UHDRS (Table 1) (CARE‐HD; Kremer B; Shoulson I). The remaining trials defined as primary outcome measure the control of motor symptoms, namely chorea, using the reduced form of the UHDRS‐motor component, Total Motor Score‐4 (TMS‐4) (Puri BK) or an improvement in muscle strength expressed as the change in static and dynamic torque (Verbessem P). Two trials used composite outcomes, Activity of Daily Living (ADL)‐UHDRS and Quantified Neurological Examination (QNE) (Ranen NG), and a combined score of the TMS and TFC of UHDRS (Table 1) (EHDN). The primary outcome was not clearly stated in one trial (Peyser CE). No trial used a time‐to‐event variable as an outcome measure.
1. Unified Huntington's Disease Rating Scale (UHDRS).
| COMPONENTS | domains evaluated |
| Motor Assessment | oculomotor function, dysarthria, chorea, dystonia, gait, postural stability. |
| Cognitive Assessment | phonetic verbal fluency test, Symbol Digit Modalities Test, the Stroop Test. |
| Behavioral Assessment | affect, thought content, coping styles. |
| Functional Assessment | Independence Scale (checklist of common daily tasks), Total Functional Capacity (TFC). |
| HSG 1996 | |
Risk of bias in included studies
RANDOMISATION:
In three trials a comprehensive description of the methodology used in randomisation could be found. The following methods were used: random number table (Peyser CE), computer‐generated stratification by investigator (CARE‐HD) and computer‐generated stratification by centre (EHDN). Three trials failed to give a complete description in the original article, stating only the use of stratification by age and stage of illness (Shoulson I), blocking and stratification (Puri BK) and stratification by age and gender (Kremer B). In one trial, the authors considered a quasi‐randomisation classification since randomisation was not followed until the completion of patients´ recruitment (Verbessem P).
ALLOCATION:
Allocation was adequately concealed in four trials (EHDN; Kremer B; Puri BK; Verbessem P). The remaining trials were unclear about allocation concealment (CARE‐HD; Peyser CE; Ranen NG; Shoulson I). The authors decided to include the latter trials.
SAMPLE SIZE CALCULATION:
Sample size calculation procedures were described in seven trials (CARE‐HD; EHDN; Kremer B; Peyser CE; Puri BK; Ranen NG; Shoulson I). In one trial (Verbessem P) a post‐hoc sample size analysis was conducted.
DATA INCLUDED:
Five trials performed data analysis on an intention‐to‐treat basis exclusively (CARE‐HD; Kremer B; Ranen NG; Verbessem P). In two trials (EHDN; Puri BK) both an intention‐to‐treat and a per‐protocol analysis were performed. For these only the results from the intention‐to‐tract analysis were included as defined in the methodology. In two trials (Peyser CE; Shoulson I), only a per‐protocol inclusion of data was performed. The contact with the authors was unfruitful.
Effects of interventions
Unless otherwise indicated, results are expressed as mean±standard deviation.
In the trial of Peyser CE with Vitamin E, 3000 UI/day, n = 81, at the end of one year of follow‐up a deterioration of the main outcome variables was observed, although in a non significant fashion: 1. Quality of life as measured by the ADL scale of the Baltimore HD project (‐2.2 ± 7.3 treatment group vs. ‐1.1 ± 7.6 placebo group; p = 0.55); 2. motor evaluation as measured by the QNE (‐2.9 ± 11.8 treatment group vs. ‐2.3 ± 7.5 placebo group; p = 0,78) and 3. cognitive function as measured by the Mini‐Mental Status Examination (MMSE) score (1.2 ± 3.5 treatment group vs. 0.0 ± 2.6 placebo group; p = 0,1). A post‐hoc analysis of the patients with better baseline motor performance score (QNE ≤ 45) tended to improve slightly with vitamin E (p = 0.02).
In a trial with Idebenone, 270 mg/day, n = 100, (Ranen NG) no treatment effect was observed for the primary outcome variables: 1. ADL‐UHDRS (2.9 ± 3.3 treatment group vs. 3.1 ± 4.9 placebo group; p = NS) and 2. QNE (4.9 ± 7.5 treatment group vs. 5.1 ± 5.0 placebo group; p = NS) and motor impairment scale, a sub‐component of QNE (1.4 ± 2.0 treatment group vs. 1.6 ± 2.0 placebo group; p = NS). No treatment effect was observed in cognitive measures: MMSE, Buschke Selective Reading test, Benton Visual Retention test, Grooved Pegboard, Wechsler Adult Intelligence Scale (WAIS), Trail Making test and Stroop test. A cohort with the less affected patients (QNE < 45) was also analysed and produced similar non‐significant differences between treatment and placebo groups.
The trial with Baclofen, 60 mg/day, n = 60, (Shoulson I) produced no significant change in the modified TFC (mTFC)‐UHDRS between groups when comparing baseline to the end of trial at 30 weeks (‐2.13 ± 1.61 treatment group vs. 1.32 ± 1.16 placebo group; p = NS). In fact, a trend toward a deleterious effect of Baclofen on the yearly change of the mTFC score was reported: 0.85 ± 0.64 treatment group vs. 0.53 ± 0.46 placebo group; p = 0.053. However, controlling data for age, stage of disease and inheritance pattern resulted in non significant results (p = 0.14).
In the trial with Lamotrigine 400 mg/day, n = 64 (Kremer B), a deterioration of functional capacity, both in treatment and placebo groups, was observed as measured by TFC‐UHDRS (‐1.89 ± 2.46 treatment group vs. 2.11 ± 1.99 placebo group; p = NS). Similarly, no difference between groups was found in the median QNE: 13.5 (range: 8‐17) treatment group vs. 17 (range: 4‐42) placebo group; p = 0.84. However, a trend toward a beneficial effect in median chorea reduction was reported: 2.5 (range: 1‐16) treatment group vs. 4 (range: 2‐19) placebo group; p = 0.08. No significant difference between groups was found in the pre‐selected neuropsychiatric measures (MMSE, Wechsler Adult Intelligence Scale‐Revised (WAIS‐R), WMS, Paired associates, paragraph recall, word fluency tests and Beck Depression Inventory) as well as in Klove Grooved Pegboard, finger‐tapping tests. In the trial of Verbessem P studying creatine 5 g/day, n = 42, an improvement of muscular strength was assessed using as primary outcome the measurement of static and dynamic force. Other measures were used: bimanual coordination skill and cardiorespiratory fitness. No significant difference between groups was found, as well as in UHDRS scales of motor function, cognitive function and functional capacity.
In a trial with coenzyme Q10 and/or Remacemide, 300‐600 mg/day and/or 400‐600 mg/day, n = 347 (CARE‐HD), no treatment effect was found in terms of a decrease in the deterioration of functional capacity as measured by TFC‐UHDRS: 0.35 (95 % CI: ‐0.12, 0.82) coenzyme Q10 group vs. 0.00 (95 % CI: ‐0.47, 0.47) Remacemide group. However, coenzyme Q10 showed a nominally significant effect in slowing functional decline as measured by the functional assessment checklist of UHDRS (22 % slowing, p = 0.02). A trend was also found for a smaller decline in the TFC‐UHDRS among coenzyme Q10‐treated patients when compared with coenzyme Q10 non‐treated patients (2.4 ± 2.14 coenzyme Q10‐treated patients vs. 2.74 ± 2.28 coenzyme Q10 non‐treated patients; p = 0.15). A trend toward a beneficial effect of coenzyme Q10 was also reported for behaviour (p = 0.08) and cognitive tests (Stroop test word reading (p = 0.09) and colour naming (p = 0.01), and the brief test of attention (p = 0.02). In the Remacemide group, a similar report was made for chorea using the motor scale of UHDRS (p = 0.06).
In the trial conducted by Puri BK (n = 135) no effect of ethyl‐eicosapentanoic acid 2 g/day, was observed in the primary outcome measure: change in the TMS‐4 (UHDRS) at 12 months. Similar results were obtained for the secondary outcome measures, i.e., the TMS‐4 (UHDRS) at 6 months, the cognitive, behavioural, and functional scales of UHDRS and the Rockland Simpson Dysknesia Score. However, a per‐protocol exploratory analysis produced a significant beneficial result in the TMS‐4 (UHDRS) for ethyl‐eicosapentanoic acid over placebo at 12 months (p = 0.046). An interaction of treatment with the number of CAG repeats was reported (p = 0.044) with better treatment effects for lower numbers of CAG repeat.
In the most recently published trial (EHDN) conducted with Riluzole, 100 mg/day, n = 537, no significant difference was found between groups for the primary outcome, i.e., the mean change of an algorithm‐specified combined score of the TMS and TFC‐UHDRS: 29.8 ± 26.8 treatment group vs. 28.2 ± 25.8 placebo group; p = 0.66. Presenting the scales separately produced similar results: TMS‐UHDRS: 30.1 ± 28.6 treatment group vs. 28.3 ± 27.2 placebo group; p = NS. TFC‐UHDRS: ‐4.6 ± 4.2 treatment group vs. 4.4 ± 4.1 placebo group, p = NS. No beneficial effect was observed using other outcome measures (chorea score, cognitive, behavioural, functional, independence and global clinical impression scales of UHDRS and Beck Depression Inventory).
All but three trials (Peyser CE; Ranen NG; Verbessem P) reported adverse events. Regarding serious adverse events, they were reported in four trials (EHDN; CARE‐HD; Kremer B; Puri BK), and two other reported the absence of serious adverse events (Ranen NG; Shoulson I). In two trials there was no reference to serious adverse events (Peyser CE; Verbessem P).
In the eight trials, there were a total of 164 (19%) drop‐outs in the treatment group and 85 (17.4 %) in the placebo group. A special reference to the high number of drop‐outs in the trial of Riluzole (EHDN),158 (29.4 %) out of 537 participants (treatment 106; control 52); 42 of those participants withdrew informed consent and 40 patients withdrew from study due to the initiation of anti‐choreic medication. In terms of causes of drop‐outs, in seven trials (CARE‐HD; EHDN; Kremer B; Peyser CE; Puri BK; Ranen NG; Shoulson I) drop‐outs were due to adverse events in 43/164 cases in the treatment group with a median per‐trial frequency of 40.0 % (range: 0‐57.1 %) and in 12/85 cases in the placebo group with a median per‐trial frequency of 14.3 % (range: 0‐28.6 %). In one trial no comprehensive description of adverse events was found in the original article (Verbessem P).
Discussion
None of the selected trials showed effectiveness for the defined primary outcome measures. However, in some trials non significant results closely achieving the pre‐specified level of statistical significance were reported, and the authors defined those as trends. In the trial CARE‐HD a trend of coenzyme Q10 for a reduced decline in functional capacity, cognitive and behaviour dimensions was reported, as well as a lesser slowing of functional capacity for the coenzyme Q10‐treated patients was documented to be significant. These inconclusive preliminary results have prompted an additional trial to confirm or disclaim a potential beneficial effect of coenzyme Q10 that is ongoing (2CARE). In the trial by Puri BK, the authors reported a trend for the beneficial effect of ethyl‐eicosapentanoic acid in TMS‐4‐UHDR, the selected primary outcome measure, but only in the per‐protocol analysis. This was not confirmed in the intention‐to‐treat analysis. In fact, recent trials with ethyl‐eicosapentanoic acid robustly showed a lack of effect for the same outcome measure after a 6‐month treatment period (Miraxion‐Europe; TREND‐HD).
Regarding the definition of outcome measures, the included trials only used variables defined as secondary outcome measures for the present systematic review, namely, decline in functional capacity or quality of life. Time‐to‐an‐event variables were not integrated in the selected trials. Additionally, no trial focused on asymptomatic HD mutation carriers, who might be considered ideal candidates for studies assessing the delaying effect on disease onset or progression with a given intervention, as they will develop symptoms later in life. However, the discovery of more sensitive and specific biological markers is needed in HD, not only for a more sensitive and thus early clinical diagnosis, but also to decrease sample size in future clinical trials. These tools will provide a better means on determining the potential clinical benefit of newly discovered interventions. Research has been conducted in recent years to identify better markers of disease onset (Paulsen JS, TRACK‐HD).
Authors' conclusions
Implications for practice.
No intervention has proven a disease‐modifying effect for Huntington's disease.
Implications for research.
The current review emphasises the need to adapt trial design. Trials on asymptomatic HD mutation carriers are desirable. Improved clinimetric scales and biological markers for the assessment of disease progression are required in order to increase the sensitivity in which clinical trials capture a disease‐modifying effect.
What's new
| Date | Event | Description |
|---|---|---|
| 11 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 23 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CARE‐HD.
| Methods | 120‐week double‐blind, four‐arm parallel study. Method of randomisation: computer generated stratification by investigator. Results presented for each arm of the trial. Intention‐to‐treat data analysis. Location: 25 centres (USA). | |
| Participants | 347 patients, 37 drop‐outs. 176 male and 171 female patients completed the trial. Mean age of participants: 47.9±10,5 years. Mean duration of disease: 4,9±3,1 years. Inclusion criteria: HD (genetically established or positive family history) TFC‐UHDRS≥7, disease stage I or II. Exclusion criteria: Coenzyme Q10 use in previous 3 months, unstable medical or psychiatric disease. | |
| Interventions | Coenzyme Q10, 300‐600mg/day (87 patients), remacemide, 400‐600mg/day (86 patients) or Coenzyme Q10+remacemide , 300‐600mg/day+400‐600mg/day (87 patients) and placebo ‐ identical pills (87 patients). No titration. | |
| Outcomes | Primary: deteriorarion in TFC‐UHDRS. Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EHDN.
| Methods | 144‐week double‐blind, unbalanced parallel study (treatment:placebo ‐ 2:1). Method of randomisation: computer generated stratification by centre. Results presented for each arm of the trial. Intention‐to‐treat and per‐protocol data analysis. Location: 44 centres (Europe). | |
| Participants | 537 patients with 158 drop‐outs. 271 male and 266 female patients. Mean age of participants: 45,5±9.7 years. Mean duration of disease: 5,5±4.4 years. Inclusion criteria: adult (25‐65 years old), genetically established HD, independently ambulatory with a TFC‐UHDRS≥8, total motor score‐UHDRS≥5, monitor compliance, caregiver to witness consent. Exclusion criteria: comorbid or co‐medication that would interfere study results, pregnancy, breast‐feeding, inadequate contraception, prior exposure to riluzole, contraindication to riluzole, serum markers of hepatic function>2xULN (alanine aminotransferase, aspartate aminotransferase and bilirubin) and anti‐choreic medication within 1 month of baseline visit. | |
| Interventions | Oral Riluzole 100 mg/day (357 patients) and placebo ‐ identical pills ‐ (180 patients). No titration. | |
| Outcomes | Primary: composite score of TMS‐UHDRS and TFC‐UHDRS. Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kremer B.
| Methods | 120‐week double‐blind, parallel study. Method of randomisation: stratification by age and gender. Results presented for each arm of the trial. Intention‐to‐treat data analysis. Location: 7 centres (Canada). |
|
| Participants | 64 patients, 9 drop‐outs. 23 male and 32 female patients completed the trial. Mean age of participants: 44,7± 3,8 years. Mean duration of disease: 2,9 ±0.7 years. Inclusion criteria: HD diagnosis defined by the appearance of motor abnormalities, disease duration<5 years, presence of neurological abnormalities (e.g., chorea, gait abnormalities, dysarthria) and expanded CAG repeat. Exclusion criteria: heart, kidney or liver disease, Diabetes Mellitus, unable to cope cognitively with trial requirements, <18 years, use of antidepressants or neuroleptics, ongoing depression or psychosis on recruitment. | |
| Interventions | Oral Lamotrigine 400 mg/day (maximum), increase every 2 weeks for 12 weeks (no dosage described) (33 patients) and placebo (31 patients). | |
| Outcomes | Primary: Change in TFC‐UHDRS.
Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Peyser CE.
| Methods | 52‐week double‐blind, parallel study. Method of randomisation: random number table. Results presented for each arm of the trial. Per‐protocol data analysis. Location: 1 centre (USA). | |
| Participants | 81 patients, 8 drop‐outs. Mean age of participants: no data. Mean duration of disease: no data. Inclusion criteria: HD (clinical diagnosis and positive family history), mild to moderate disease (mild to moderate dementia, MMSE>15, ambulatory care), reliable family member for information and medication compliance. Exclusion criteria: none stated. | |
| Interventions | Oral Vitamin E 3000 UI/day (40 patients) and placebo, identical capsules (33 patients). No titration. | |
| Outcomes | Change in quality of life (ADL scale of the Baltimore HD project). Change in motor abnormalities (QNE). Change in cognitive function (MMSE, digit span, Controlled Oral Word Association Test, Design Fluency Test, Trail Making Test, CERAD Verbal Learning Test, Luria Sequential Hand Position Test, Wisconsin Card Sorting Test, Benton Visual Retention Test, Motor Go/No‐Go Test, Stroop Word Test). | |
| Notes | Negative for efficacy measures. Post‐hoc analysis produced positive results for patients with QNE<45. All patients were given anti‐oxidants vitamin A and C. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Puri BK.
| Methods | 52‐week double‐blind, parallel study. Method of randomisation: stratified by blocks of four. Results presented for each arm of the trial. Intention‐to‐treat and per‐protocol data analysis. Location: 6 centres (USA and Canada). | |
| Participants | 135 patients with 14 drop‐outs. 68 male and 67 female patients completed the trial. Mean age of participants: 49,5±9,1 years. Mean duration of disease: no data. Inclusion criteria: symptomatic HD genetically confirmed or with family history of HD. Functional stage I/II. Independence scale score of 50 ‐ 90. Available caregiver between 30 and 70 years of age. Exclusion criteria: depot neuroleptics. | |
| Interventions | Oral Ethyl‐EPA 2g/day (67 patients) and placebo ‐ identical pills (68 patients). No titration. | |
| Outcomes | Primary: TMS‐4 score (UHDRS) at 12 months.
Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ranen NG.
| Methods | 52‐week double‐blind, parallel study. Method of randomisation: not stated. Results presented for each arm of the trial. Intention‐to‐treat data analysis. Location: 1 centre (USA). | |
| Participants | 100 patients, 9 drop‐outs. 44 male and 47 female patients (per‐protocol cohort). Mean age of participants: 42.1±11.7 years. Mean age of disease onset: 5,0±0.4 years. Inclusion criteria: mild‐moderate HD (family history, chorea/rigidity, MMSE>14), relative or companion to serve as informant and to supervise medication. Exclusion criteria: current major depression, other CNS disease, active medical illness, alcohol or substance abuse, psychosis, MRI evidence of striatal infarction. CNS‐acting medication (antipsychotics, CoQ, vitamin E, BZD, barbiturics, antioxidants, CNS‐acting antihypertensives), except stable use of nortriptyline (depression) and carbamazepine (mania or irritability), chloral hydrate up to twice a week (sleep), but not within 72h of screening/visits. | |
| Interventions | Oral Idebenone 270 mg/day (48 patients) and placebo, identical pills (43 patients). No titration. | |
| Outcomes | Primary: Change in the composite of ADL‐UHDRS and QNE (chorea, eye movement, motor impairment scales).
Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shoulson I.
| Methods | 30‐week double‐blind, in parallel study (12 weeks follow‐up). Method of randomisation: stratification by age and stage of ilness. Results presented for each arm of the trial. Per‐protocol data analysis. Location: 1 centre (USA). | |
| Participants | 60 patients, 11 drop‐outs. 24 male patients and 25 female patients. Mean age of participants: 39,4±10.4 years. Mean duration of disease: 5,3± 3.3 years. Inclusion criteria: HD (unexplained movement disorder and positive family history), >18 years of age, early stage disease (functional stages I‐II), satisfactory general medical health, person for support and company. Exclusion criteria: serious psychiatric illness (major depression with antidepressant medication, psychotic illness requiring antipsychotics, active suicidal behavior). | |
| Interventions | Ora Baclofen 60 mg/day (30 patients) and placebo (30 patients). No titration. | |
| Outcomes | Change in mTFC‐UHDRS. | |
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Verbessem P.
| Methods | 52‐week double‐blind, in parallel study. Method of randomisation: unbalanced randomisation (Creatine:Placebo ‐ 2:1). Full random allocation was not possible. Results presented for each arm of the trial. Intention‐to‐treat data analysis. Location: 1 centre (Belgium). | |
| Participants | 42 patients, 3 drop‐outs. 15 male and 26 female patients completed the trial. Mean age of participants: 49,8±2,2 years. Mean duration of disease: 8,0±1,5 years. Inclusion criteria: HD (genetic diagnosis), functional stages I‐III, stable medication for at least 3 months. Exclusion criteria: history of renal pathology, existing albuminuria, prior creatine use. | |
| Interventions | Oral Creatine 5 g/day (87 patients) and placebo, identical in appearance and taste (30 patients). No titration. | |
| Outcomes | Primary: change in static and dynamic force.
Secondary:
|
|
| Notes | Negative for efficacy measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ADL ‐ Activities of daily living. BZD ‐ Benzodiazepine. CAG ‐ Glutamine. CERAD ‐ Consortium to Establish a Registry for Alzheimer’s Disease. CNS ‐ Central Nervous System. EPA ‐ Eicosapentaenoic acid. HD ‐Huntington's disease. MMSE ‐ Mini‐Mental State Examination. MRI ‐ Magnetic Ressonance Imaging. mTFC ‐ modified Total Functioning Capacity. PET ‐ Positron Emission Tomography. QNE ‐ Quantified Neurological Examination. TFC ‐ Total Functioning Capacity. TMS ‐ Total Motor Score. UHDRS ‐Unified Huntington Disease Revised Scale. WAIS‐R ‐ Wechsler Adult Intelligence Scale‐revised.
WMS ‐ Wechsler Memory Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bachoud‐Lévi AC (a) | open‐label study |
| Bachoud‐Lévi AC (b) | open‐label study |
| Bachoud‐Lévi AC (c) | open‐label study |
| Beister A | open‐label study |
| Bender A | open‐label study |
| Bloch J | phase I study |
| Bonelli RM (a) | open‐label study |
| Bonelli RM (b) | open‐label study |
| Caro A | Crossover |
| Dubinsky R | phase I study |
| Feigin A | open‐label study |
| Fink JS | open‐label study |
| Hauser RA | non‐controlled |
| Puri BK (b) | n<10 |
| Rosas HD | open‐label study |
| Tabrizi SJ (a) | open‐label study |
| Tabrizi SJ (b) | open‐label study |
| Thomas M | open‐label study |
Characteristics of studies awaiting assessment [ordered by study ID]
DIMOND.
| Methods | 12‐week, randomised, double‐blind, parallel study. |
| Participants | 90 HD patients (clinical features of HD and a confirmatory family history of HD, and/or genetically confirmed HD), able to take medication (capsules) by mouth. Exclusion criteria: unstable medical illness, pregnant or lactating women of those intending to become pregnant during the study period. |
| Interventions | Dimebon 60 mg/day vs placebo. |
| Outcomes | Primary: safety and tolerability. Secondary endpoint: efficacy on cognitive, motor, and overall function in subjects with Huntington's Disease. Assess the pharmacokinetics of Dimebon. |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
2CARE.
| Trial name or title | Coenzyme Q10 in Huntington's Disease. |
| Methods | 5‐year randomised, double‐blind, parallel study. |
| Participants | Clinical features of HD and confirmatory family history of HD and/or genetically confirmed HD. ≥16 years of age. |
| Interventions | Coenzyme Q10 2400 mg/day vs placebo. |
| Outcomes | Primary: change in total functional capacity. Secondary: change in other UHDRS scores, tolerability and safety, time to decline in TFC by 2 and 3 points. |
| Starting date | March 2008. |
| Contact information | Huntington Study Group (1‐800‐487‐7671). |
| Notes |
DOMINO.
| Trial name or title | A Multi‐Center, Double‐Blind, Pilot Study of Minocycline in Huntington's Disease. |
| Methods | 18‐month, randomised, double‐blind, parallel study. |
| Participants | Clinical features of HD and a confirmatory family history of HD and/or genetically confirmed HD. 18 years or older. |
| Interventions | Minocycline 200 mg/day vs. placebo. |
| Outcomes | Primary: change in TFC‐UHDRS, and to assess futility of further study of the drug. Secondary: safety/tolerability. |
| Starting date | January 2006. |
| Contact information | Huntington Study Group (1‐800‐487‐7671). |
| Notes |
Contributions of authors
Protocol ‐ Ferreira J, Mestre T, Sampaio C. Literature search ‐ Ferreira J, Mestre T, Coelho M. Literature selection ‐ Ferreira J, Mestre T, Coelho M. Papers quality assessment ‐ Ferreira J, Mestre T. Data collection from papers ‐ Mestre T, Ferreira J. Interpretation of data ‐ Mestre T, Ferreira J, Coelho M, Rosa MM, Sampaio C. Review writing ‐ Mestre T, Ferreira J, Coelho M, Rosa MM, Sampaio C.
Sources of support
Internal sources
Movement disorders Cochrane Review Group, Portugal.
Neurological Clinical Research Unit, Institute of Molecular Medicine, Portugal.
External sources
No sources of support supplied
Declarations of interest
Mestre T, Ferreira J, Coelho M, Rosa MM were investigators in a clinical trial to investigate the efficacy of ethyl‐eicosapentanoic acid for the treatment of HD.
New
References
References to studies included in this review
CARE‐HD {published data only}
- The Huntington study group. A randomized, placebo‐controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology 2001;57(3):397‐404. [DOI] [PubMed] [Google Scholar]
EHDN {published data only}
- European Huntington Disease Initiative Study Group. Riluzole in Huntington´s disease: a 3 year, randomised controlled study. Annals of Neurology 2007;62(3):262‐272. [DOI] [PubMed] [Google Scholar]
Kremer B {published data only}
- Kremer B, Clark CM, Almqvist EW, Raymond LA, Graf P, Jacova C, et al. Influence of lamotrigine on progression of early Huntington disease: a randomized clinical trial. Neurology 1999;53(5):1000‐1011. [DOI] [PubMed] [Google Scholar]
Peyser CE {published data only}
- Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, et al. Trial of d‐alpha‐tocopherol in Huntington's disease. Am J Psychiatry 1995;152(12):1771‐1775. [DOI] [PubMed] [Google Scholar]
Puri BK {published and unpublished data}
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, et al. Ethyl‐EPA in Huntington disease: a double‐blind, randomized, placebo‐controlled trial. Neurology 2005;65(2):286‐92. [DOI] [PubMed] [Google Scholar]
Ranen NG {published data only}
- Ranen NG, Peyser CE, Coyle JT, Bylsma FW, Sherr M, Day L, et al. A controlled trial of idebenone in Huntington's disease. Mov Disord 1996;11(5):549‐554. [DOI] [PubMed] [Google Scholar]
Shoulson I {published data only}
- Shoulson I, Odoroff C, Oakes D, Behr J, Goldblatt D, Caine E, et al. A controlled clinical trial of baclofen as protective therapy in early Huntington's disease. Ann Neurol 1989;25(3):252‐259. [DOI] [PubMed] [Google Scholar]
Verbessem P {published data only}
- Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Leemputte M, et al. Creatine supplementation in Huntington's disease: a placebo‐controlled pilot trial. Neurology 2003;61(7):925‐930. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bachoud‐Lévi AC (a) {published data only}
- Bachoud‐Lévi A, Bourdet C, Brugières P, Nguyen JP, Grandmougin T, Haddad B, Jény R, Bartolomeo P, Boissé MF, Barba GD, Degos JD, Ergis AM, Lefaucheur JP, Lisovoski F, Pailhous E, Rémy P, Palfi S, Defer GL, Cesaro P, Hantraye P, Peschanski M. Safety and tolerability assessment of intrastriatal neural allografts in five patients with Huntington's disease. Exp Neurol 2000;161(1):194‐202. [DOI] [PubMed] [Google Scholar]
Bachoud‐Lévi AC (b) {published data only}
- Bachoud‐Lévi AC, Rémy P, Nguyen JP, Brugières P, Lefaucheur JP, Bourdet C, Baudic S, Gaura V, Maison P, Haddad B, Boissé MF, Grandmougin T, Jény R, Bartolomeo P, Dalla Barba G, Degos JD, Lisovoski F, Ergis AM, Pailhous E, Cesaro P, Hantraye P, Peschanski M. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet 2000;356(9246):1975‐9. [DOI] [PubMed] [Google Scholar]
Bachoud‐Lévi AC (c) {published data only}
- Bachoud‐Lévi AC, Gaura V, Brugières P, Lefaucheur JP, Boissé MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, Cesaro P, Hantraye P, Peschanski M. Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: a long‐term follow‐up study. Lancet Neurology 2006;5(4):303‐309. [DOI] [PubMed] [Google Scholar]
Beister A {published data only}
- Beister A, Kraus P, Kuhn W, Dose M, Weindl A, Gerlach M. The N‐methyl‐D‐aspartate antagonist memantine retards progression of Huntington's disease. J Neural Transm Suppl 2004;68:117‐22. [DOI] [PubMed] [Google Scholar]
Bender A {published data only}
- Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T. Creatine supplementation lowers brain glutamate levels in Huntington's disease. J Neurol. 2005;252(1):36‐41. [DOI] [PubMed] [Google Scholar]
Bloch J {published data only}
- Bloch J, Bachoud‐Lévi AC, Déglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugières P, Boisse MF, Baudic S, Cesaro P, Hantraye P, Aebischer P, Peschanski M. Neuroprotective gene therapy for Huntington's disease, using polymer‐encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther 2004;15(10):968‐75. [DOI] [PubMed] [Google Scholar]
Bonelli RM (a) {published data only}
- Bonelli RM, Heuberger C, Reisecker F. Minocycline for Huntington's disease: an open label study. Neurology. 2003;60(5):883‐4. [DOI] [PubMed] [Google Scholar]
Bonelli RM (b) {published data only}
- Bonelli RM, Hödl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington's disease: a 2‐year study on minocycline. Int Clin Psychopharmacol 2004;19(6):337‐42. [DOI] [PubMed] [Google Scholar]
Caro A {published data only}
- Caro A, Caro S. Vitamin E in treatment of Huntington's chorea. Br Med J 1978;1(6116):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubinsky R {published data only}
- Dubinsky R, Gray C. CTYE‐I‐HD: phase I dose finding and tolerability study of cysteamine (Cystagon) in Huntington's disease. Mov Disord 2006;21(4):530‐533.. [DOI] [PubMed] [Google Scholar]
Feigin A {published data only}
- Feigin A, Kieburtz K, Como P, Hickey C, Claude K, Abwender D, Zimmerman C, Steinberg K, Shoulson I. Assessment of coenzyme Q10 tolerability in Huntington's disease. Mov Disord 1996;11(3):312‐323. [DOI] [PubMed] [Google Scholar]
Fink JS {published data only}
- Fink JS, Schumacher JM, Ellias SL, Palmer EP, Saint‐Hilaire M, Shannon K, Penn R, Starr P, VanHorne C, Kott HS, Dempsey PK, Fischman AJ, Raineri R, Manhart C, Dinsmore J, Isacson O. Porcine xenografts in Parkinson's disease and Huntington's disease patients:preliminary results. Cell Transplant 2000;9(2):273‐8. [DOI] [PubMed] [Google Scholar]
Hauser RA {published data only}
- Hauser RA, Furtado S, Cimino CR, Delgado H, Eichler S, Schwartz S, Scott D, Nauert GM, Soety E, Sossi V, Holt DA, Sanberg PR, Stoessl AJ, Freeman TB. Bilateral human fetal striatal transplantation in Huntington's disease. Neurology 2002;58(5):687‐95. [DOI] [PubMed] [Google Scholar]
Puri BK (b) {published data only}
- Puri BK, Bydder GM, Counsell SJ, Corridan BJ, Richardson AJ, Hajnal JV, Appel C, Mckee HM, Vaddadi KS, Horrobin DF. MRI and neuropsychological improvement in Huntington disease following ethyl‐EPA treatment. Neuroreport. 2002;13(1):123‐6.. [DOI] [PubMed] [Google Scholar]
Rosas HD {published data only}
- Rosas HD, Koroshetz WJ, Jenkins BG, Chen YI, Hayden DL, Beal MF, Cudkowicz ME. Riluzole therapy in Huntington's disease (HD). Mov Disord 1999;14(2):326‐30. [DOI] [PubMed] [Google Scholar]
Tabrizi SJ (a) {published data only}
- Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AH, Warner TT. Creatine therapy for Huntington's disease: clinical and MRS findings in a 1‐year pilot study. Neurology 2003;Jul 8;61(1):141‐2.. [DOI] [PubMed] [Google Scholar]
Tabrizi SJ (b) {published data only}
- Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AH, Warner TT. High‐dose creatine therapy for Huntington disease: a 2‐year clinical and MRS study. Neurology 2005;64(9):1655‐6. [DOI] [PubMed] [Google Scholar]
Thomas M {published data only}
- Thomas M, Ashizawa T, Jankovic J. Minocycline in Huntington´s disease: a pilot study. Movement disorders 2004;20(4):510‐511. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
DIMOND {published data only}
References to ongoing studies
2CARE {published data only}
- Coenzyme Q10 in Huntington's Disease.. Ongoing study March 2008..
DOMINO {published data only}
- A Multi‐Center, Double‐Blind, Pilot Study of Minocycline in Huntington's Disease.. Ongoing study January 2006..
Additional references
Bonelli 2004
- Bonelli, RM, Wenning GK, Kapfhammer HP. Huntington's disease: present treatments and future therapeutic modalities. Int Clin Psychopharmacol 2004;19 (2):51‐62. [DOI] [PubMed] [Google Scholar]
Cochrane 2008
- www.cochrane.org/resources/handbook.
HSG 1996
- Huntington Study Group. Unified Huntington's disease rating scale: Reliability and consistency. Movement Disorders 1996;11(2):136‐42. [DOI: 10.1002/mds.870110204] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials 1996;17((1)):1‐12. [DOI] [PubMed] [Google Scholar]
Kieburtz 1994
- Kieburtz, K, et al. Trinucleotide repeat length and progression of illness in Huntington's disease. J Med Genet, 1994;1 (11):872‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanska 1995
- Lanska, DJ. George Huntington and hereditary chorea. J Child Neurol 1995;10 (1):46‐8. [DOI] [PubMed] [Google Scholar]
Miraxion‐Europe
- European Huntingotn Disease Network. World Congress in Huntington's disease. 2007.
Paulsen JS
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, McDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington's disease decades before diagnosis: The Predict HD study. J Neurol Neurosurg Psychiatry 2008;79(8):874‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Revman 2008
- Review Manager (RevMan) [MacOSX]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Ropper 2005
- Ropper AH, Brown RH. Principles of Neurology. 8th Edition. McGraw‐Hill, 2005. [Google Scholar]
TH s DCR Group 1993
- Group, TH.s.DCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosome. Cell 1993;72:971‐83. [DOI] [PubMed] [Google Scholar]
TRACK‐HD
- http://www.track‐hd.net.
TREND‐HD
- The Huntington Study group. TREND‐HD ‐ A Trial of Ethyl‐EPA (Miraxion™) in Treating Mild to Moderate Huntington's Disease. World Congress in Huntington's disease. 2007.