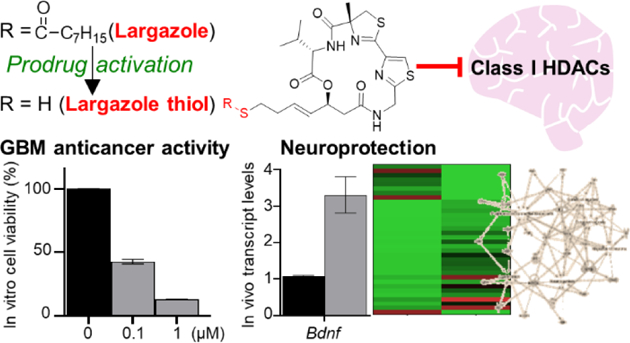
Abstract
Largazole is a potent class I selective histone deacetylase (HDAC) inhibitor prodrug with anticancer activity against solid tumors in preclinical models. Largazole possesses in vitro activity against glioblastoma multiforme (GBM) cells and sufficiently crosses the blood-brain barrier based on measurement of the active species, largazole thiol, to achieve therapeutically relevant concentrations in the mouse brain. The effective dose resulted in pronounced functional responses on the transcript level based on RNA-sequencing and RT-qPCR, revealing desirable expression changes of genes related to neuroprotection, including Bdnf and Pax6 upregulation, extending the applicability of largazole to the treatment of brain cancer and neurodegenerative disorders. The largazole-induced modulation of Pax6 unifies both activities since Pax6 expression suppresses GBM proliferation and invasion and inversely correlates with GBM tumor grade, while it is also implicated in neurogenesis, neuronal plasticity and cognitive ability. Our results suggests that largazole could be repurposed for diseases of the brain.
Keywords: Largazole, histone deacetylases, BDNF, PAX6, brain cancer, neuroprotection
Graphical Abstract
Introduction
Glioblastoma multiforme (GBM; WHO grade IV glioma) is the most common, highly aggressive, and treatment resistant primary malignant brain tumor in adults.1 Investigations of the mechanisms underlying the pathogenesis of GBM via RNA sequencing demonstrated the involvement of both dysregulated genetic and epigenetic mechanisms where altered expressions and/or sequences of histone deacetylases (HDACs)-coding genes have been reported.2 Analysis of RNA-sequencing data from The Cancer Genome Atlas (TCGA) revealed significant increase in the expression levels of HDACs 1–3 (class I) and HDAC 7 (class IIa) in high grade gliomas.3 Histone deacetylases (HDACs) are validated drug targets for cancer therapy as the association of epigenetic changes, including loss of acetylation, with cancer malignancies and the overexpression of class I HDACs in cancers were readily documented.4 This was further supported by the FDA approval of four HDAC inhibitors for cancer therapy, including the pan-HDAC inhibitors vorinostat (SAHA) and belinostat (PXD101) for some T-cell lymphomas, and panobinostat (LBH589) for multiple myeloma (Figure 1), and the class I selective HDAC inhibitor romidepsin (FK228) for cutaneous T-cell lymphoma.5 The inhibition of HDACs results in hyperacetylation of histones, open chromatin structure and subsequently modulation of gene expression, in addition to direct and indirect actions on cell cycle, apoptosis, autophagy, angiogenesis, and signaling pathways, also due to modulating acetylation status of non-histone proteins to modulate protein-protein interactions.5
Figure 1.
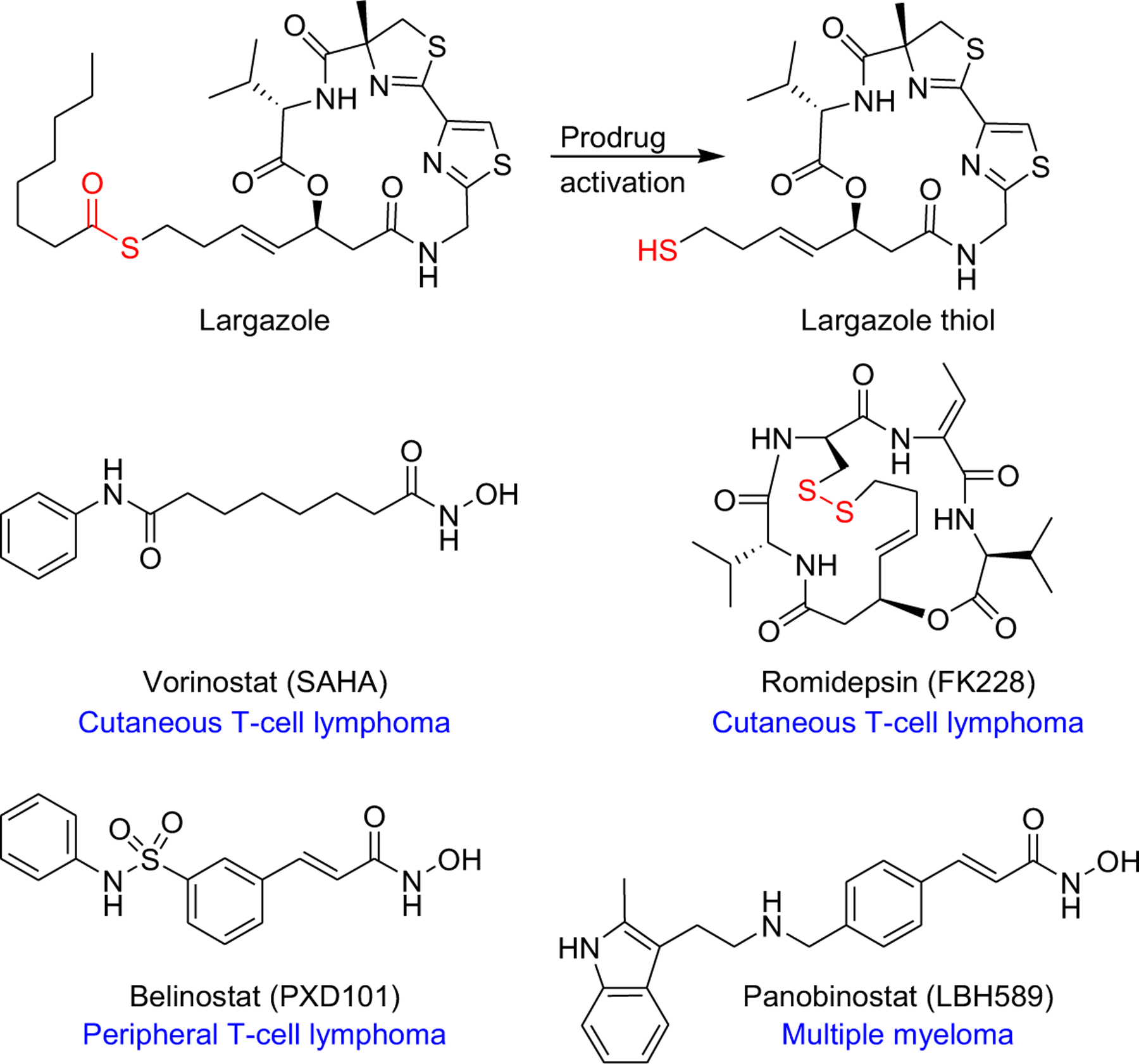
Structures of largazole, largazole thiol, and the four FDA approved HDAC inhibitors. Atoms labeled in red indicate the site of prodrug activation.
HDAC inhibitors have not yet shown significant clinical activity against solid tumors, including GBM where various combination regimens have been studied, due limitations including toxicity as well as insufficient concentration of drug within the tumor due to low brain uptake.2 Several studies investigated the brain bioavailability of the four marketed HDAC inhibitors and revealed poor blood-brain barrier (BBB) penetration.2,6–8 Nevertheless, the compromised BBB in GBM facilitates the access of drugs to the brain tumor tissue, but which is not the case in other CNS conditions.2,9
Several studies proved the role of histone acetylation in regulating memory consolidation as the lack of histone acetyl transferase (HAT) may contribute to impaired memory function associated with neurodegenerative disorders.10 Excessive HDAC activity has been also reported in mice as well as in post-mortem brain samples of Alzheimer’s disease (AD) patients.11 In particular, HDAC2 (class I) was reported to be upregulated and its knockdown was found to restore disturbed synaptic plasticity and memory deficits in mouse models of AD.11,12 In line with these findings, it was suggested that targeted inhibition of class I HDACs could potentially ameliorate cognitive decline associated with neurodegenerative disorders such as AD.10 Among the FDA approved HDAC inhibitors, SAHA has been extensively studied in several animal models of AD and age-associated cognitive decline and was reported to rescue spatial and early contextual memory impairment.10,13 Furthermore, several studies utilizing in vivo models of Huntington’s disease (HD) demonstrated improved motor and behavioral impairments, molecular biomarkers of disease progression and reversed aberrant neuronal differentiation in response to pan-HDAC inhibitors SAHA and LBH589.14,15 However, the results have been controversial, and beneficial effects were not robustly observed across various studies, largely due to limited brain bioavailability of these agents.6 Collectively, these data indicate the need and promise of a brain-penetrant class I selective HDAC inhibitor for GBM and non-cancer CNS diseases.
Largazole is a prodrug, characterized by the presence of a thioester moiety, which upon hydrolysis liberates largazole thiol as the most potent natural class I selective HDAC inhibitor discovered from marine cyanobacteria (Figure 1).16–19 Of particular interest, it potently inhibits HDAC2, the key regulator of cognition-enhancing genes, at picomolar concentrations (Ki 0.07 nM).19,20 Anticancer studies of largazole demonstrated in vivo solid tumor anticancer (colon) and anti-invasive properties (triple negative breast cancer).18,21 Largazole was also shown to exhibit potent antiproliferative effects against sympathetic nervous system cancer cells, including IMR-32 neuroblastoma cells;16 data which we corroborated in SH-SY5Y neuroblastoma cells (IC50 102 nM, Supplementary Figure S1). The antiproliferative effects of largazole in the NCI-60 screen demonstrated a wide spectrum of activity, including CNS tumor cell lines,18 indicating largazole’s potential against cancers of both peripheral and central nervous system, including brain cancers, with brain penetration being the requisite. Compared to other clinical and preclinical stage class I HDAC inhibitors, largazole possesses prominent features which include: (1) superior in vitro potency against class I HDACs;19 (2) a scaffold amenable to generate next-generation inhibitors with class I isoform selectivity;22 (3) modulation of activity profiles through alteration of prodrug properties as well as opportunity for conjugation to targeting agents; (4) oral bioavailability indicating that it can successfully pass intestinal membranes,23 which was not the case for the disulfide prodrugs such as present in the only approved class I HDAC inhibitor, romidepsin;23 and (5) scalable synthesis.24 The lack of nonspecific toxicity in vivo would allow to test largazole for other indications where cellular reprogramming would have a beneficial effect.25,26 These differentiating features of largazole may overcome limitations presented by current HDAC inhibitor therapies and other most advanced HDAC inhibitors in clinical trials. Here we describe the anticancer effects of largazole in GBM in vitro, brain bioavailability and its potential neuroprotective effects through modulation of expression of genes implicated in neuroprotection in vitro and in vivo, identifying opportunities for repurposing of this preclinical candidate.
Results and Discussion
Initial in vitro testing validated the anticancer activity of largazole against two GBM cancer cell lines, SF-268 and SF-295 (Figure 2A). Largazole potently inhibited the proliferation of both cell lines with IC50 values of 62 and 68 nM, respectively. We then investigated the effects of largazole on target genes involved in neuroprotection/neurogenesis. One key target is BDNF, a brain-derived neurotrophic factor implicated in synaptic plasticity, neuronal development, behavioral adaptations, and cognition.27 Reduced expression of BDNF has been associated with neurodegenerative disorders such as Alzheimer’s, Huntington’s, and Parkinson’s diseases.28 SF-268 and SF-295 cells were both treated with largazole at the IC90 dose (0.3 μM) for 12 h and BDNF expression was assessed by RT-qPCR, revealing a 1.4-fold increase (Figure 2B).
Figure 2.
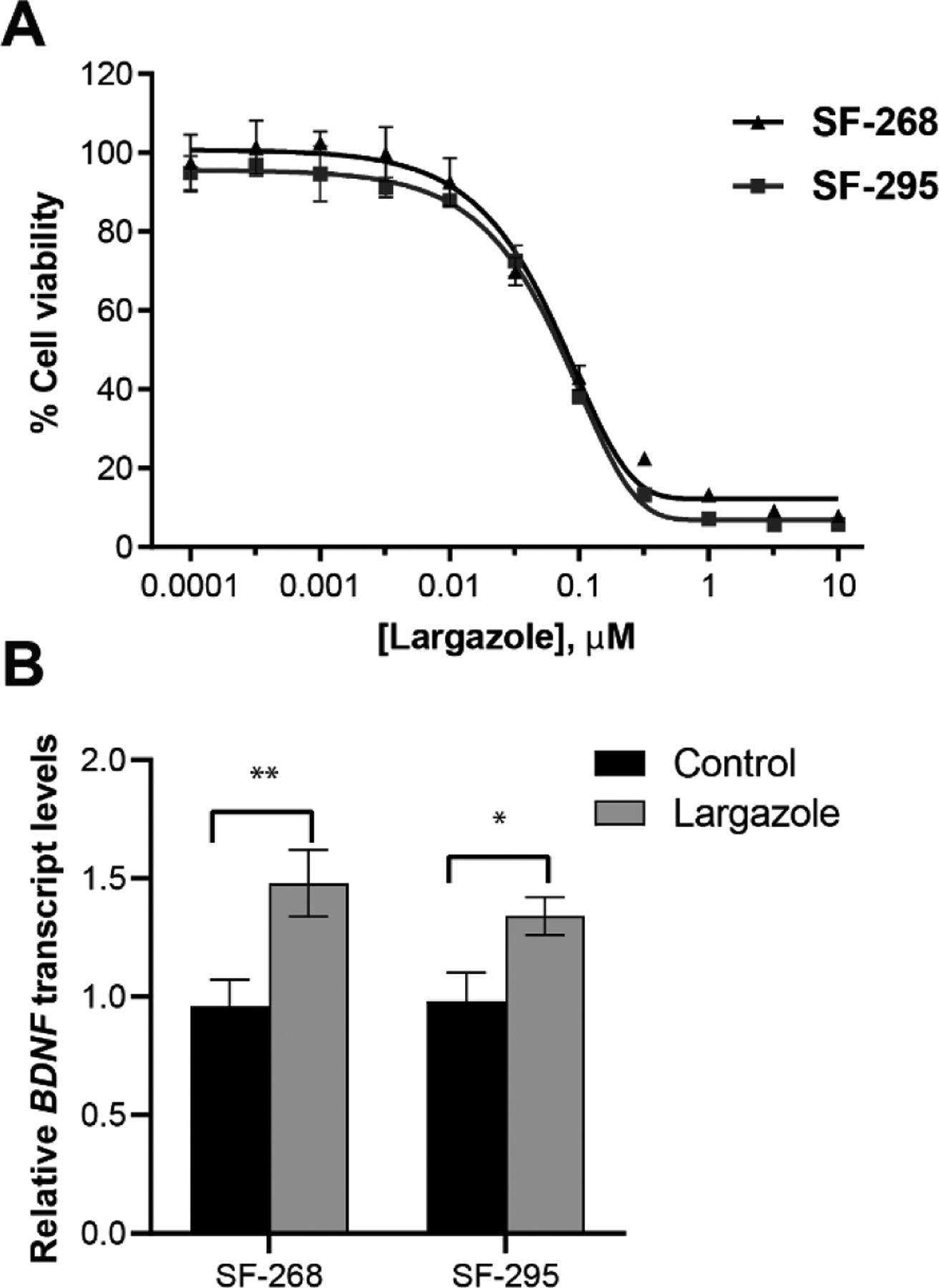
The effects of largazole on proliferation and BDNF gene expression of glioblastoma cells (SF-268 and SF-295). A. The antiproliferative activity of largazole assessed by MTT assay. B. BDNF transcript levels in response to largazole treatment (0.3 μM; 12 h) assessed by RT-qPCR (TaqMan; GAPDH was the endogenous control). Error bars represent SD, n = 3. The asterisks denote significance of p < 0.05 relative to solvent control using two-tailed unpaired t test (* denotes p ≤ 0.05, ** denotes p ≤ 0.01)
We then investigated the ability of largazole to cross the BBB, indirectly by assessing histone hyperacetylation using Western blot analysis, and directly by quantifying levels of largazole thiol in non-tumor bearing mouse brain by HPLC-MS using multiple reaction monitoring (MRM), as described in Yu et al.29 Initial intraperitoneal (ip) dosing studies identified 5 mg/kg as sufficient to induce slight hyperacetylation in the brain and pronounced effects at 50 mg/kg after 4 h, suggesting that largazole (presumably the active thiol form) is highly brain-penetrant (Figure 3A). An ip dose of 50 mg/kg resulted in largazole thiol concentrations near the IC90 of the GBM in vitro activity after 4 h (300 nM) with time-dependent decrease to concentrations near the IC50 after 24 h (~80 nM, Figure 3B). A lower ip dose (10 mg/kg) or oral administration (50 mg/kg) followed a similar trend with highest levels near the IC50 achieved in both cases after 4 h (Figure 3B). Therefore, these data set the stage for future brain-tumor efficacy studies since effective concentrations to retard GBM cell growth can be achieved, even if the BBB is not compromised as in our model system, extending the applicability of largazole to GBM but also non-cancer CNS diseases. Acute toxicity studies aimed at determining the maximum tolerated dose (MTD) indicated that single ip dosing with largazole was well tolerated up to 200 mg/kg. Repeated dosing below the MTD (and above our highest assay concentration of 50 mg/kg) on four consecutive days, using 60 mg/kg (x 4) and 75 mg/kg (x 4) did also not result in toxicity.
Figure 3.

The brain bioavailability of largazole. A. Dose-response analysis of histone hyperacetylation in brain (4 h; ip). B. In vivo monitoring of largazole thiol levels in excised whole-brain tissue (error bars represent SEM, n = 2). C. Bdnf expression in brain tissue after 12 h treatment with largazole (ip 50 mg/kg) vs control assessed by RT-qPCR (TaqMan; Actin was the endogenous control). Error bars represent SEM, n = 2. The asterisk denotes significance of p ≤ 0.05 relative to solvent control using two-tailed unpaired t test.
Given the bioavailability of largazole thiol in mice brains and promising toxicity data, we further assessed the effects of largazole on Bdnf expression in vivo 12 h following ip dose of 50 mg/kg largazole. We detected a 3.3-fold increase in Bdnf gene expression based on RT-qPCR (Figure 3C). The upregulation of Bdnf indicates that largazole thiol levels in the brain are sufficient to induce a functional response. To capture functional consequences in the brain on a global level, we then carried out RNA-sequencing, followed by Ingenuity Pathway Analysis (IPA) of differentially expressed genes using cutoff criteria of 2.5-fold change (Exp log ratio 1) and p-value of 0.05 to focus on biologically and statistically significant changes (1220 genes). Aside from cancer, which was detected as a top hit in the IPA list of diseases and functions (Supplementary Figure S2, Table S1), we were interested in investigating the potential applications of largazole in diseases or functions implicated in nervous system development and function, neurological and behavioral diseases. Based on the activation z-scores, a statistical quantity computed for each biological function, the activation states of some diseases related to those categories were predicted and linked to the corresponding genes in the dataset (Tables 1 and 2). Our findings support beneficial effects in response to largazole treatment, since behavioral functions such as cognition as well as several nervous system functions involved in neuroprotection were predicted to increase (Table 1), whereas the activation states of several neurological diseases were decreased (Table 2).
Table 1.
Diseases or functions implicated in nervous system development/behavior predicted to be activated (based on IPA; 2.5-fold cutoff, p < 0.05), many of which are associated with PAX6 and OPRM1.
| Diseases or functions annotation | p-Value | Activation z-score | No. of molecules | Selected genes |
|---|---|---|---|---|
| Development of neurons | 6.12E-17 | 3.763 | 132 | SOD2, PAX6, OPRM1 |
| Morphogenesis of neurons | 3.03E-16 | 2.841 | 108 | NLGN1, PAX6, OPRM1 |
| Neurogenesis | 8.34E-16 | 2.841 | 106 | PAX6, OPRM1, SRGAP2 |
| Development of CNS | 3.37E-11 | 2.092 | 99 | NLGN1, PAX6, S100B |
| Growth of neurites | 4.16E-08 | 3.692 | 69 | PAX6, S100B, CDH2 |
| Outgrowth of neurites | 3.89E-07 | 3.291 | 57 | ERBB4, PAX6, NLGN1 |
| Proliferation of neuronal cells | 4.47E-08 | 3.476 | 78 | CNTN2, PAX6, NLGN1 |
| Coordination | 0.00014 | 3.651 | 29 | ATXN2,CDKL5, ITPR1 |
| Migration of neurons | 0.000207 | 3.001 | 32 | PAX6, CDKL5, IGF1R |
| Differentiation of neurons | 0.000188 | 2.223 | 51 | CNTN2, PAX6, NLGN1 |
| Extension of neurites | 2.16E-06 | 2.474 | 29 | PAX6, ERBB4, ITPR1 |
| Cognition | 4.47E-08 | 2.368 | 70 | CREBBP, PAX6, OPRM1 |
| Learning | 5.55E-08 | 2.267 | 65 | CREBBP, PAX6, OPRM1 |
| Contextual conditioning | 0.000338 | 2.138 | 15 | ERBB4, CDKL5, VDAC1 |
Table 2.
Neurological diseases predicted to be decreased in response to treatment (based on IPA; 2.5-fold cutoff, p < 0.05).
| Diseases or functions annotation | p-Value | Activation z-score | No. of molecules | Selected genes |
|---|---|---|---|---|
| Movement disorders | 1.93E-011 | −3.773 | 149 | OPRM1, PAX6, ATXN2 |
| Seizure disorders | 6.38E-05 | −3.588 | 63 | SYN2, OPRM1, ATXN2 |
| Paraparesis | 0.000152 | −2.236 | 5 | CTSF, NDRG1, PLP1 |
| Ataxia | 0.00015 | −2.775 | 35 | PAX6, SOD2, PLP1 |
| Congenital malformation of brain | 0.00013 | −3.43 | 36 | PAX6, CREBBP, AKT3 |
Interestingly, the top canonical pathway that had a positive z-score and highly correlated with three major neuro categories (nervous system development, neurological diseases, and behavior) was Dopamine-DARPP32 feedback in cAMP signaling with a z-score of 3.838 and p-value of 1.44E-05 (Supplementary Figure S3). DARPP32 is a key mediator of dopamine signaling and a well-known marker of differentiated striatal medium spiny neurons (MSNs), highly vulnerable neurons in HD.30 It is highly expressed in MSNs and modulate their response to dopamine. Mutant mice lacking BDNF were reported to exhibit reduced expression levels of DARPP32.30 Another study demonstrated increased histone acetylation and expression of DARPP32 mRNA and protein levels following in vitro exposure of MSNs to HDAC inhibitors to promote their phenotypic maturation.31 Furthermore, a recent study demonstrated enhanced DARPP32 protein levels, a marker of striatal development, in response to the pan-HDAC inhibitor LBH589 in an animal model of HD.14
Several other relevant canonical pathways were identified in the analysis, including: CREB signaling in neurons and synaptic long term potentiation (LTP) signaling. A recent study ascribed the critical roles of BDNF and CREB signaling in hippocampal LTP and memory formation where SAHA (25 mg/kg ip)was shown to reverse induced-cognitive and synaptic plasticity impairments in vivo through the upregulation of BDNF, tropomyosin-related kinase B (TrkB), and CREB signaling pathways in the hippocampus.32 Moreover, SAHA was previously reported to enhance LTP of excitatory synapses in vitro (0.5 μM) using rat organotypic hippocampal brain slices, but without cognition enhancing effects in vivo – at the same dose that showed functional consequences with largazole (50 mg/kg ip) - attributed to limited brain bioavailability of SAHA.6
Our analysis also identified several regulator effect networks, which demonstrated the neuroprotective potential of largazole (Figure 4). Such networks connect the upstream regulator identified and predicted to be activated in the analysis, through the differentially regulated genes in the dataset, to a relevant phenotype or disease state where it has been implicated. One network involved Nrf2 (NFE2L2), a neuroprotective transcription factor which induces antioxidant and phase II detoxification enzymes upon binding to the antioxidant response element (ARE), which has been reported to prevent or reduce neuronal cell death (Figure 4).33 The Nrf2-ARE pathway is a therapeutic target in neurodegenerative diseases where oxidative stress has been implicated in the etiology.34 Other networks identified include cell cycle, gene expression, and posttranslational modifications, consistent with the established mechanisms of HDAC inhibitors in cancer (Supplementary Figure S4).
Figure 4.
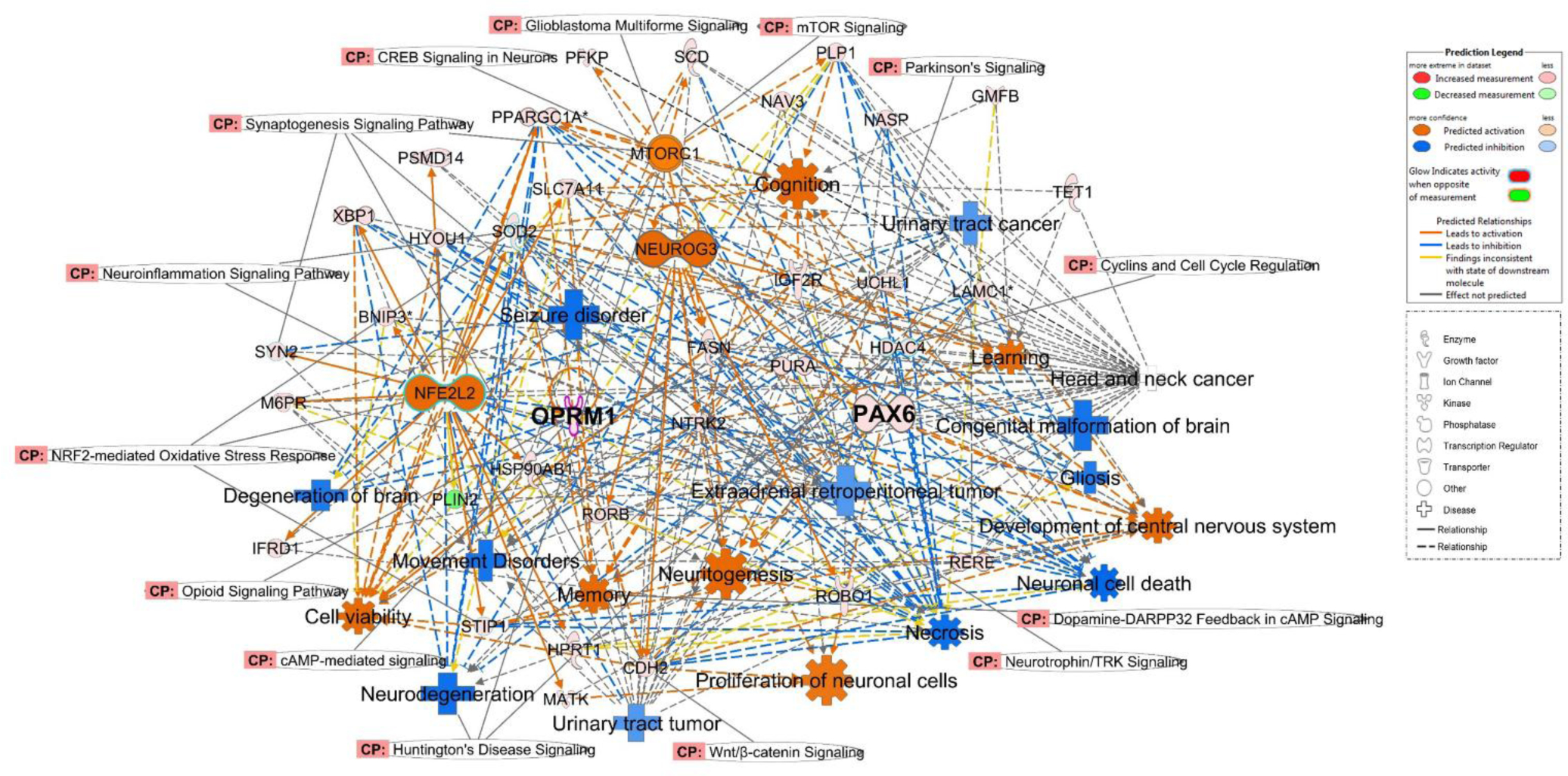
Regulator effect networks affected by largazole, identified through the analysis of RNA-seq data of mice brains (50 mg/kg ip, 12 h treatment) using IPA (2.5-fold cutoff, p < 0.05), showing the anticancer and neuroprotective potential of largazole. CP: canonical pathways. PAX6 and OPRM1 are emphasized in bold face.
Close inspection of the top 37 differentially expressed genes revealed Pax6 and Oprm1 with 3.0- and 4.6-fold increase in expression, respectively. Pax6 is a neuronal transcription factor with neurodevelopmental regulatory functions that is implicated in neurogenesis and neuronal plasticity and plays critical role in cognitive abilities.35 Studies have demonstrated the correlation between Pax6 expression and AD severity where it was reported to influence the expression of genes related to neurodegeneration, including BDNF.36 Additionally, the disabilities of patients with aniridia, a neurological disease caused by PAX6 haploinsufficiency, was reported to parallel the morphological and behavioral abnormalities of Pax6 mutant mice.35,37
Interestingly, aside from the role of Pax6 in neurological and neurodegenerative conditions, it was also shown to act as a tumor suppressor in gliomas. Pax6 expression suppressed the proliferation, invasion and colony formation in GBM cell lines.38 As its expression was shown to inversely correlate with tumor grade, Pax6 was suggested to be a prognostic biomarker in astrocytic glioma.39 Hence, the upregulation of Pax6 by largazole might contribute to its dual actions on cancer cell proliferation and modulation of genes involved in neuroprotection. To our knowledge, no other small molecules were reported to modulate the expression of Pax6 apart from one study describing the protective role of caffeine against impaired neurogenesis.40 Therefore, our data related to the discovery of Pax6 as an indirect therapeutic target for largazole might provide the basis for future studies directed at investigating its effects in Pax6 implicated pathogenesis and unifies both GBM anticancer and non-cancer CNS activities.
Finally, few studies reported elevated methylation of OPRM1 gene in AD and supported neuroprotective role of OPRM1 against β-amyloid peptide (Aβ) neurotoxicity through mTOR signaling.41,42 A recent study demonstrated the involvement of HDAC2 in the suppression of μ-opioid receptor activity and suggested underlying mechanisms of this epigenetic deregulation.43 These studies further supports our analysis where mTOR and opioid signaling (z-scores 1.342 and 3.157, respectively) showed up as top canonical pathways after DARPP32 signaling. Hence, the identification of Oprm1 as a hit in the RNA-seq experiment suggests potential repurposing of largazole for opioid receptor modulation and warrants further investigations.
In conclusion, we demonstrated the GBM activity of largazole in vitro as well as brain bioavailability and functional consequences in vivo. Our data supports the therapeutic applications of largazole in brain cancer and potentially other CNS diseases, especially given the class I HDAC selectivity and superior brain penetration over other clinically used HDAC inhibitors. Although the development of BDNF enhancing drugs is still underway, the discovery of additional new putative targets for the therapeutic intervention of neurodegenerative disorders is equally important. Investigations of the impact of largazole on the global network of genes implicated in CNS diseases identified Pax6 and Oprm1 as other potential targets. Hence, these changes in gene expression illuminated potential repurposing opportunities of largazole, which warrants further investigations in relevant model systems. A “systems” approach using a brain-penetrant class I HDAC inhibitor that leads to downstream polypharmacology through on-target effects to modulate an entire network of genes and proteins might be more efficacious and promising than targeting a single gene for such complex diseases with unmet medical need.
Methods
Cell viability assays.
The cells (SF268, SF295, SH-SY5Y) were seeded in 96-well plates and treated after 24 h with different concentrations of largazole or solvent control. Following 48 h, cell viability was measured using MTT. More details can be found in Supporting Information.
Immunoblot analysis.
Brain samples were homogenized through sonication in PhosphoSafe lysis buffer, centrifuged, and the supernatants were collected and used for immunoblot analysis probing with acetyl histone H3 antibody (Lys9/14). The details of the protocol can be found in Supporting Information.
RNA extraction and RT-qPCR.
RNA was extracted from mouse brain tissues using TRIzol while RNeasy Mini Kit (Qiagen) was used for cellular studies. The qPCR experiment was carried out using ABI 7300 sequence detection system. The details of the protocol can be found in Supporting Information.
RNA-seq.
Illumina RNA library construction and subsequent NextSeq500 sequencing (Illumina) were carried out as described in Supporting Information.
Metabolite analysis.
Analysis of largazole thiol in brains was performed using an HPLC-MS where largazole thiol along with the internal standard harmine were specifically monitored in the samples using MRM as described.23,29 The details of the protocol can be found in Supporting Information
Supplementary Material
ACKNOWLEDGMENT
We thank Yanxia Liu for technical assistance in pilot in vivo studies and Western blot analysis, Yanping Zhang from the Gene Expression and Genotyping Core and Alberto Riva from the Bioinformatics Core of the Interdisciplinary Center for Biotechnology Research (ICBR) for RNA-sequencing and assistance with data analysis.
Funding
This research was supported by the National Institutes of Health grants R01CA172310 and R01CA138544 and the Debbie and Sylvia DeSantis Chair professorship (H.L.) and R50CA211487 (R.R.).
Footnotes
Supporting Information
Experimental procedures for in vitro, in vivo assays, HPLC-MS monitoring methods, additional supplementary figures, table and references. This material is available free of charge via the Internet at http://pubs.acs.org.
H.L. is co-founder of Oceanyx Pharmaceuticals, Inc., which is negotiating licenses for largazole-related patents and patent applications.
REFERENCES
- (1).Ostrom QT; Gittleman H; Liao P; Vecchione-Koval T; Wolinsky Y; Kruchko C; Barnholtz-Sloan JS CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro-Oncology. 2017, 19 (5), v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lee P; Murphy B; Miller R; Menon V; Banik NL; Giglio P; Lindhorst SM; Varma AK; Vandergrift WA; Patel SJ; Das A Mechanisms and Clinical Significance of Histone Deacetylase Inhibitors: Epigenetic Glioblastoma Therapy. Anticancer Res. 2015, 35 (2), 615–625. [PMC free article] [PubMed] [Google Scholar]
- (3).Was H; Krol SK; Rotili D; Mai A; Wojtas B; Kaminska B; Maleszewska M Histone Deacetylase Inhibitors Exert Anti-Tumor Effects on Human Adherent and Stem-like Glioma Cells. Clin. Epigenet 2019, 11 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Nakagawa M; Oda Y; Eguchi T; Aishima S-I; Yao T; Hosoi F; Basaki Y; Ono M; Kuwano M; Tanaka M; Tsuneyoshi M Expression Profile of Class I Histone Deacetylases in Human Cancer Tissues. Oncol. Rep 2007, 18 (4), 769–774. [PubMed] [Google Scholar]
- (5).Eckschlager T; Plch J; Stiborova M; Hrabeta J Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci 2017, 18 (7), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hanson JE; La H; Plise E; Chen YH; Ding X; Hanania T; Sabath EV; Alexandrov V; Brunner D; Leahy E; Steiner P; Liu L; Scearce-Levie K; Zhou Q SAHA Enhances Synaptic Function and Plasticity In Vitro but Has Limited Brain Availability In Vivo and Does Not Impact Cognition. PLoS One 2013, 8 (7), e69964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Singleton WG; Collins AM; Bienemann AS; Killick-Cole CL; Haynes HR; Asby DJ; Butts CP; Wyatt MJ; Barua NU; Gill SS Convection Enhanced Delivery of Panobinostat (LBH589)-Loaded Pluronic Nano-Micelles Prolongs Survival in the F98 Rat Glioma Model. Int. J. Nanomed 2017, 12, 1385–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Warren KE; McCully C; Dvinge H; Tjørnelund J; Sehested M; Lichenstein HS; Balis FM Plasma and Cerebrospinal Fluid Pharmacokinetics of the Histone Deacetylase Inhibitor, Belinostat (PXD101), in Non-Human Primates. Cancer Chemother. Pharmacol 2008, 62 (3), 433–437. [DOI] [PubMed] [Google Scholar]
- (9).Leten C; Struys T; Dresselaers T; Himmelreich U In Vivo and Ex Vivo Assessment of the Blood Brain Barrier Integrity in Different Glioblastoma Animal Models. J. Neuro-oncol 2014, 119 (2), 297–306. [DOI] [PubMed] [Google Scholar]
- (10).Benito E; Dean C; Fischer A; Benito E; Urbanke H; Ramachandran B; Barth J; Halder R; Awasthi A; Jain G; Capece V; Burkhardt S; Navarro-sala M; Nagarajan S; Schütz A; Johnsen SA; Bonn S; Lührmann R; Dean C; Fischer A HDAC Inhibitor – Dependent Transcriptome and Memory Reinstatement in Cognitive Decline Models. J. Clin. Invest 2015, 125 (9), 3572–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Gräff J; Rei D; Guan J-S; Wang W-Y; Seo J; Hennig KM; Nieland TJF; Fass DM; Kao PF; Kahn M; Su SC; Samiei A; Joseph N; Haggarty SJ; Delalle I; Tsai L-H An Epigenetic Blockade of Cognitive Functions in the Neurodegenerating Brain. Nature 2012, 483 (7388), 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Guan JS; Haggarty SJ; Giacometti E; Dannenberg JH; Joseph N; Gao J; Nieland TJF; Zhou Y; Wang X; Mazitschek R; Bradner JE; DePinho RA; Jaenisch R; Tsai LH HDAC2 Negatively Regulates Memory Formation and Synaptic Plasticity. Nature 2009, 459 (7243), 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kilgore M; Miller CA; Fass DM; Hennig KM; Haggarty SJ; Sweatt JD; Rumbaugh G Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2010, 35 (4), 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Siebzehnrübl FA; Raber KA; Urbach YK; Schulze-Krebs A; Canneva F; Moceri S; Habermeyer J; Achoui D; Gupta B; Steindler DA; Stephan M; Nguyen HP; Bonin M; Riess O; Bauer A; Aigner L; Couillard-Despres S; Paucar MA; Svenningsson P; Osmand A; Andreew A; Zabel C; Weiss A; Kuhn R; Moussaoui S; Blockx I; Van der Linden A; Cheong RY; Roybon L; Petersén Å; Von Hörsten S Early Postnatal Behavioral, Cellular, and Molecular Changes in Models of Huntington Disease Are Reversible by HDAC Inhibition. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (37), E8765–E8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hockly E; Richon VM; Woodman B; Smith DL; Zhou X; Rosa E; Sathasivam K; Ghazi-Noori S; Mahal A; Lowden PAS; Steffan JS; Marsh JL; Thompson LM; Lewis CM; Marks PA; Bates GP Suberoylanilide Hydroxamic Acid, a Histone Deacetylase Inhibitor, Ameliorates Motor Deficits in a Mouse Model of Huntington’s Disease. Proc. Natl. Acad. Sci. U. S. A 2003, 100 (4), 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Taori K; Paul VJ; Luesch H Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca Sp. J. Am. Chem. Soc 2008, 130 (6), 1806–1807. [DOI] [PubMed] [Google Scholar]
- (17).Ying Y; Taori K; Kim H; Hong J; Luesch H Total Synthesis and Molecular Target of Largazole, a Histone Deacetylase Inhibitor. J. Am. Chem. Soc 2008, 130 (26), 8455–8459. [DOI] [PubMed] [Google Scholar]
- (18).Liu Y; Salvador LA; Byeon S; Ying Y; Kwan JC; Law BK; Hong J; Luesch H Anticolon Cancer Activity of Largazole, a Marine-Derived Tunable Histone Deacetylase Inhibitor. J. Pharmacol. Exp. Ther 2010, 335 (2), 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hong J; Luesch H Largazole: From Discovery to Broad-Spectrum Therapy. Nat. Prod. Rep 2012, 29 (4), 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bowers A; West N; Taunton J; Schreiber SL; Bradner JE; Williams RM Total Synthesis and Biological Mode of Action of Largazole: A Potent Class I Histone Deacetylase Inhibitor. J. Am. Chem. Soc 2008, 130 (33), 11219–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Law ME; Corsino PE; Jahn SC; Davis BJ; Chen S; Patel B; Pham K; Lu J; Sheppard B; Nørgaard P; Hong J; Higgins P; Kim J-S; Luesch H; Law BK Glucocorticoids and Histone Deacetylase Inhibitors Cooperate to Block the Invasiveness of Basal-like Breast Cancer Cells through Novel Mechanisms. Oncogene 2013, 32 (10), 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kim B; Park H; Salvador LA; Serrano PE; Kwan JC; Zeller SL; Chen QY; Ryu S; Liu Y; Byeon S; Luesch H; Hong J Evaluation of Class i HDAC Isoform Selectivity of Largazole Analogues. Bioorg. Med. Chem. Lett 2014, 24 (16), 3728–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Salvador LA; Park H; Al-Awadhi FH; Liu Y; Kim B; Zeller SL; Chen QY; Hong J; Luesch H Modulation of Activity Profiles for Largazole-Based HDAC Inhibitors through Alteration of Prodrug Properties. ACS Med. Chem. Lett 2014, 5 (8), 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chen Q-Y; Chaturvedi PR; Luesch H Process Development and Scale-up Total Synthesis of Largazole, a Potent Class I Histone Deacetylase Inhibitor. Org. Process Res. Dev 2018, 22 (2), 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lee SU; Kwak HB; Pi SH; You HK; Byeon SR; Ying Y; Luesch H; Hong J; Kim SH In Vitro and in Vivo Osteogenic Activity of Largazole. ACS Med. Chem. Lett 2011, 2 (3), 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Liu Y; Wang Z; Wang J; Lam W; Kwong S; Li F; Friedman SL; Zhou S; Ren Q; Xu Z; Wang X; Ji L; Tang S; Zhang H; Lui EL; Ye T A Histone Deacetylase Inhibitor, Largazole, Decreases Liver Fibrosis and Angiogenesis by Inhibiting Transforming Growth Factor-β and Vascular Endothelial Growth Factor Signalling. Liver Int. 2013, 33 (4), 504–515. [DOI] [PubMed] [Google Scholar]
- (27).Binder DK; Scharfman HE BDNF Mini Review. Growth Factors 2004, 22 (3), 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lima Giacobbo B; Doorduin J; Klein HC; Dierckx RAJO; Bromberg E; de Vries EFJ Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol 2019, 56 (5), 3295–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yu M; Salvador LA; Sy SKB; Tang Y; Singh RSP; Chen Q-Y; Liu Y; Hong J; Derendorf H; Luesch H Largazole Pharmacokinetics in Rats by LC-MS/MS. Mar. Drugs 2014, 12 (3), 1623–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Baydyuk M; Xu B BDNF Signaling and Survival of Striatal Neurons. Front. Cell. Neurosci 2014, 8: 254, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chandwani S; Keilani S; Ortiz-Virumbrales M; Morant A; Bezdecny S; Ehrlich ME Induction of DARPP-32 by Brain-Derived Neurotrophic Factor in Striatal Neurons In Vitro Is Modified by Histone Deacetylase Inhibitors and Nab2. PLoS One 2013, 8 (10), e76842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhang D; Xue B; You J; Zhang B; Chai G Suberoylanilide Hydroxamic Acid Reversed Cognitive and Synaptic Plasticity Impairments Induced by Sevoflurane Exposure in Adult Mice. NeuroReport 2019, 30 (4), 274–279. [DOI] [PubMed] [Google Scholar]
- (33).Chen PC; Vargas MR; Pani AK; Smeyne RJ; Johnson DA; Kan YW; Johnson JA Nrf2-Mediated Neuroprotection in the MPTP Mouse Model of Parkinson’s Disease: Critical Role for the Astrocyte. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (8), 2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Johnson DA; Johnson JA Nrf2 - A Therapeutic Target for the Treatment of Neurodegenerative Diseases. Free Radical Biol. Med 2015, 88, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tran CT; Radyushkin K; Tonchev AB; Piñon MC; Ashery-Padan R; Molnár Z; Davidoff MS; Stoykova A Selective Cortical Layering Abnormalities and Behavioral Deficits in Cortex-Specific Pax6 Knock-out Mice. J. Neurosci 2009, 29 (26), 8335–8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mishra S; Maurya SK; Srivastava K; Shukla S; Mishra R Pax6 Influences Expression Patterns of Genes Involved in Neuro-Degeneration. Ann. Neurosci 2015, 22 (4), 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yogarajah M; Matarin M; Vollmar C; Thompson PJ; Duncan JS; Symms M; Moore AT; Liu J; Thom M; van Heyningen V; Sisodiya SM PAX6, Brain Structure and Function in Human Adults: Advanced MRI in Aniridia. Ann. Clin. Transl. Neurol 2016, 3 (5), 314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Huang BS; Luo QZ; Han Y; Li XB; Cao LJ; Wu LX MicroRNA-223 Promotes the Growth and Invasion of Glioblastoma Cells by Targeting Tumor Suppressor PAX6. Oncol. Rep 2013, 30 (5), 2263–2269. [DOI] [PubMed] [Google Scholar]
- (39).Zhou YH; Tan F; Hess KR; Yung WKA The Expression of PAX6, PTEN, Vascular Endothelial Growth Factor, and Epidermal Growth Factor Receptor in Gliomas: Relationship to Tumor Grade and Survival. Clin. Cancer Res 2003, 9 (9), 3369–3375. [PubMed] [Google Scholar]
- (40).Endesfelder S; Weichelt U; Schiller C; Winter K; von Haefen C; Bührer C Caffeine Protects Against Anticonvulsant-Induced Impaired Neurogenesis in the Developing Rat Brain. Neurotoxic. Res 2018, 34 (2), 173–187. [DOI] [PubMed] [Google Scholar]
- (41).Wang Y; Wang YX; Liu T; Law PY; Loh HH; Qiu Y; Chen HZ μ-Opioid Receptor Attenuates Aβ Oligomers-Induced Neurotoxicity Through MTOR Signaling. CNS Neurosci. Ther 2015, 21 (1), 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Xu C; Liu G; Ji H; Chen W; Dai D; Chen Z; Zhou D; Xu L; Hu H; Cui W; Chang L; Zha Q; Liping LI; Duan S; Wang Q Elevated Methylation of OPRM1 and OPRL1 Genes in Alzheimer’s Disease. Mol. Med. Rep 2018, 18 (5), 4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Liao YH; Wang J; Wei YY; Zhang T; Zhang Y; Zuo ZF; Teng XY; Li YQ Histone Deacetylase 2 Is Involved in μ-Opioid Receptor Suppression in the Spinal Dorsal Horn in a Rat Model of Chronic Pancreatitis Pain. Mol. Med. Rep 2018, 17 (2), 2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


