Uses a theory-based approach to tailor clinical materials and training programmes, creating city-based Community Advisory Boards, and user-based user panels to ensure that tailoring matches user needs, municipal services and coproduction of health.
Tests the added value of embedding and implementing primary healthcare activity within municipal-based adoption mechanisms and support systems, and community-based communication campaigns.
Has a longer time frame (18 months) than is traditionally used in implementation studies, to assess longer term impacts.
Gives considerable emphasis to process evaluation, developing logic models to document the fidelity of all implementation strategies, and to identify, the drivers and barriers and facilitators to successful implementation and scale-up.
Due to municipal-based political and technical considerations, we are unable to randomise the involved municipal areas. We adopt a quasiexperimental design, optimising comparator municipal areas for confounding, and by using propensity score matching.
Introduction
This paper outlines the protocol for a quasiexperimental study1 to test the implementation of primary healthcare (PHC)-based measurement, advice and treatment for heavy drinking and comorbid depression at the municipal level in three Latin American countries, Colombia, Mexico and Peru (Scale-up of Prevention and Management of Alcohol Use Disorders and Comorbid Depression in Latin America (SCALA) study).
Heavy drinking is a cause of considerable disability, morbidity and mortality.2 Heavy drinking is a causal factor for some communicable diseases (including TB and HIV/AIDS), for many non-communicable diseases (NCDs, including cancers, cardiovascular diseases and gastrointestinal diseases) and for many mental and behavioural disorders, including depression, dementias and suicide.3 4
In PHC settings, two-fifths of people with heavy drinking have depression, with risks of incident depression higher for heavier as opposed to lighter drinkers.5 In addition to its role in the aetiology of depression, heavy drinking is associated with worsening the depression course, including suicide risk, impaired social functioning and impaired healthcare utilisation.6
Heavy drinking is also a major contributor to global health inequalities, with alcohol-related harm aggravated by lower socioeconomic status7 and extending beyond the individual drinker to families, communities, health systems and the wider economy. Tackling the multiple individual and societal level harms caused by heavy drinking is essential for achieving global targets of reducing deaths from NCDs by 25% between 2010 and 2025,8 more so as risk of exposure to harmful use of alcohol increases with increasing socioeconomic status.9 In line with tackling harm due to lower socioeconomic status, United Nations Sustainable Development Goals include Target 3.5, to strengthen the prevention and treatment of harmful use of alcohol, with two proposed indicators: coverage of treatment interventions (pharmacological, psychosocial and rehabilitation and aftercare services) for harmful use of alcohol; and per capita alcohol consumption.10 11
Countries in Latin America have the highest alcohol-attributable disease burden after Eastern Europe and sub-Saharan Africa, with particularly high risks in alcohol-attributable traffic injury including violence.12 The burden of alcohol-attributable diseases in Latin America lead to marked economic costs, with numerous calls to implement effective and cost-effective policies.13
A robust and extensive body of literature demonstrates the range of evidence-based strategies that can be implemented to reduce heavy drinking in healthcare settings.14 Questionnaire-based measurement and brief advice programmes delivered in PHC are effective15 and cost-effective16 17 in reducing heavy drinking. In addition to brief advice, treatment for heavy drinking includes cognitive behavioural therapy and pharmacotherapy, both of which are found to be effective in reducing heavy drinking.18 Were the proportion of eligible patients receiving advice and treatment for heavy drinking to increase to 30% of eligible patients, the prevalence of harmful use of alcohol could decrease by between 10% and 15% across OECD (Organisation for Economic Co-operation) and member countries.19 However, to date, measurement and brief advice and treatment programmes have failed to achieve widespread take-up.19
Two systematic reviews20 21 and two multicountry studies22–24 have demonstrated that the proportion of PHC patients whose alcohol consumption is measured, and of heavy drinking patients given advice can be increased by providing training and support to PHC providers, although from very low baseline levels, and with effects not generally sustained over the longer term. Moreover, while there has been some previous research in countries of Latin America,25–30 most implementation work to date has been undertaken in high-income countries. The SCALA study will build on previous evidence31 to fast-track scale-up research and practice in Latin American PHC settings.
Out of a range of implementation frameworks that include a sequential approach for scale-up, and that provide practical guidance for how to work with organisations, health systems and communities to implement and scale-up best practices,32–39 we adopt the Institute for Healthcare Improvement’s Framework for going to Full Scale, which identifies adoption mechanisms and support systems for use across sequential steps, and describes the implementation methods that can be used at each step.40
SCALA seeks to address three specific barriers to sustained implementation of PHC-based measurement, advice and treatment for heavy drinking. The first barrier recognises that most PHC-based programmes focus on providers alone, whereas successful implementation of health interventions within complex health system demands addressing a range of underlying structural and support systems.40 Phase IV of the WHO study on the identification and management of alcohol-related problems in primary care concluded that embedding PHC-based measurement and brief advice programmes within the frame of supportive community and municipal environments might lead to improved outcomes,41 although this has never been formally evaluated. Similar conclusions were reached by the European Optimising Delivery of Healthcare Interventions (ODHIN) study42 and the US-based Substance Abuse and Mental Health Services Administration Screening, Brief Intervention and Referral to Treatment initiative.43–45
The second barrier is that standard cut-off points for the frequently used alcohol measurement instrument, Alcohol Use Disorders Identification Test, 3-item consumption version (AUDIT-C)46 (commonly a score of five for both men and women, or five for men and four for women) to trigger advice are too low,47 being equivalent to an average daily alcohol consumption of about 20 g of alcohol (around two standard drinks) or less.48 Practitioners may well find it problematic to give advice at such levels, which would also have huge time implications, with one in three or four patients being eligible for advice in many countries, under this criterion.24 49 We have argued to adopt similar models to blood pressure, where cut-off points for managing raised blood pressure are often determined by levels of blood pressure at which treatment has shown to be effective.50 51 Similarly, cut-off points for brief advice could be the baseline levels of alcohol consumption found in the randomised controlled trials that have investigated the effectiveness of PHC-delivered brief advice. In the first Cochrane review of the topic that focused on PHC, mean baseline levels were 313 g of alcohol per week,52 equivalent to an AUDIT-C cut-off of 8.48
The third and final barrier concerns the cost of implementing measurement and brief-advice for heavy drinking in PHC setting. Although, alcohol advice and treatment programmes can lead to substantial reductions in healthcare costs,16 freeing considerable numbers of working age people from alcohol-related diseases,19 their initial implementation can require a significant time-commitment on the part of providers, in terms of both initial training requirements and the time taken to deliver advice in routine practice. The largest part of the costs of implementing measurement and brief advice for heavy drinking in PHC settings are directly caused by the time spent by the healthcare providers delivering this intervention.53 Moreover, this large amount of time is experienced by healthcare providers as an important barrier to deliver routine measurement and brief advice to their patients.54 As evidence suggests that shorter sessions of brief advice are not less effective compared with longer sessions,52 55 56 it seems that reducing the time spent by healthcare professionals in preparing for these sessions could be a viable strategy to increase the overall adoption and implementation of alcohol measurement and brief advice at PHC level.
Given the strong comorbidity between heavy drinking and depression, our protocol includes screening for depression for those patients identified as heavy drinkers, with appropriate referral or PHC support for treatment.57–59
In the SCALA study, we implement three interventions (independent variables) for the PHCU:
Intensity of clinical package and training (standard, vs short, vs none).
Training of providers (present, vs absent).
Community integration and support (municipal action present, vs absent).
The main outcome (dependent variable) is the cumulative proportion of the adult (aged 18+ years) population registered with the PHCU that has their alcohol consumption measured within the 18-month implementation test period (defined as coverage). Three hypotheses are to be tested.
Hypothesis 1
Municipal action leads to more sustainable coverage. After 18 months, the difference in coverage between municipal action present and municipal action absent for those PHCU that receive training is larger than after 12 months.
Hypothesis 2
In the absence of municipal action, PHCU that have received training obtain higher coverage than PHCU that do not receive training.
Hypothesis 3
In the presence of municipal action, the short clinical package and short training do not lead to less measurement coverage than the standard clinical package and standard training.
Methods and analysis
The study is a quasiexperimental design,1 comparing changes in measurement and assessment for alcohol consumption and comorbid depression, and, if needed, advice and/or referral for treatment between PHCUs in intervention municipal areas and PHCUs in similar control municipal areas. In 2017, prior to a grant application, we published a preprotocol for a three-country study to test the scale-up of PHC-based programmes to identify and manage the harmful use of alcohol and comorbid depression.60 Since the application, and during the grant negotiation and planning phase, the design of the study has changed considerably, essentially moving from a two-arm design to a four-arm design, and changing the primary outcome measure to the proportion of the adult population registered with a PHCU that has their alcohol consumption measured, online supplementary file S1, online supplementary box S1. With all changes approved by the concerned ethics committee, this paper outlines the final protocol for a quasiexperimental study to test the implementation of PHC-based measurement, advice and treatment for heavy drinking and comorbid depression at the community level in three Latin American countries, Colombia, Mexico and Peru (SCALA study).
bmjopen-2020-038226supp001.pdf (761KB, pdf)
bmjopen-2020-038226supp002.pdf (2.9MB, pdf)
Intervention municipal areas are investigator-selected from Bogotá (Colombia), Mexico City (Mexico) and Callao—Lima (Peru). Control municipal areas are investigator-selected in the same cities, on the basis of comparability with the intervention municipal area in terms of socioeconomic and other characteristics which impact on drinking, healthcare and survival, comparable community mental health services and sufficient geographical separation to minimise spill over effects from the intervention municipal area. Randomised selection of the municipal areas was not feasible due to organisational limitations. Municipal areas are chosen as a scalable implementation unit at mesosystem level that can be replicated as the intervention is scaled-up,40 given their jurisdictional responsibilities for prevention and healthcare services.
Within each intervention municipal area, a local Community Advisory Board (CAB) is created of key stakeholders, including representatives of local and regional government, directors of PHC services, non-governmental organisations active in providing counselling and treatment services for alcohol and mental health, academic experts and local media. The CABs meet regularly during the course of the study, giving advice on tailoring materials for local use, giving advice on adoption mechanisms, support systems and communication campaigns to support the action and preparing for sustainability and scale-up at the end of the action.
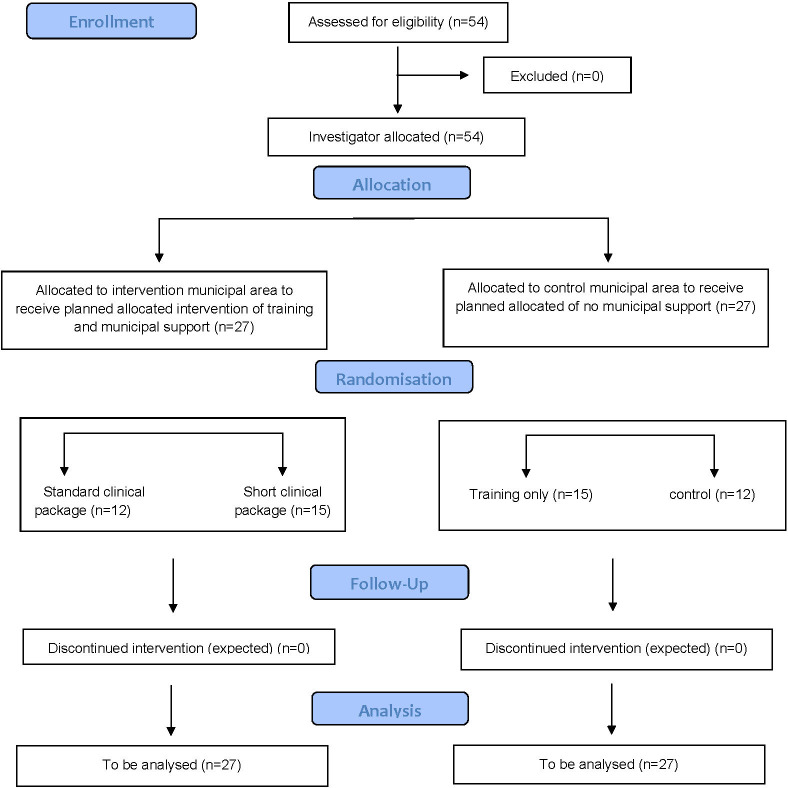
The units of allocation and analysis, that is, study participants, are 54 PHCUs and the providers working in them. Within each PHCU, eligible providers include any fully trained healthcare provider working in the PHCU and involved in medical and/or preventive care. Within each PHCU, individual providers decide themselves whether or not to participate in the study; those who do sign an informed consent for their participation. Based on the five-country ODHIN study, we estimate that approximately two-fifths of providers will consent to join the study.61 The overall study design is summarised in figure 1. Fifty-four PHCU are invited to join the study until 27 are achieved within each of the two municipal areas (intervention and control) across the three countries (nine per municipal area within each of the three countries).
Figure 1.
Study flow diagram.
Within each intervention municipal area, a user panel (UP) is created of providers and patients drawn from the PHC centres to advise on the tailoring of patient and provider materials and on provider training programmes.
For the first 6 months of the 18-month implementation and test period, a four-arm design is adopted, figure 2. Within the comparator municipal area, 12 PHCUs out of the 27 are randomly allocated to control (Arm 1), and 15 are allocated to receive short training to implement a short clinical package (Arm 2). Within the intervention municipal area, in which all 27 PHCU receive municipal action, 15 PHCUs are randomly allocated to receive short training to implement a short clinical package (Arm 3), and 12 PHCUs are allocated to receive standard training to implement a standard clinical package (Arm 4). Random allocation was undertaken using Excel random number generator.
Figure 2.
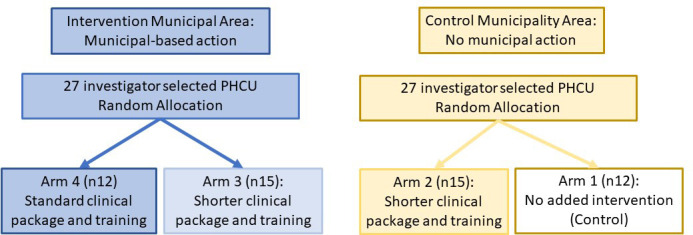
Study design for the first 6 months of the 18-month implementation period.PHCU, primary healthcare units.
The clinical package comprises measurement instruments, patient information and advice material and provider guidance material, with the differences between the standard and short clinical materials are described in online supplementary file S1, online supplementary table S1, with references. Online supplementary file S1, online supplementary table S1 also lists the material used in control Arm 1. The standard material is essentially that used in common clinical practice60 and the short version a simplified version deliverable in practice during a short period of time. The packages include measurement instruments and patient advice material for comorbid depression implemented with patients with an AUDIT-C score of 8+. Online supplementary file S1, online supplementary table S1 summarises the differences between the standard and short versions of the training programme.
The standard and short care pathways that are implemented are summarised in online supplementary file S1, online supplementary figures S1 and S2.
Essentially, in all arms, PHC providers are asked to measure the alcohol consumption of all adult patients who consult for whatever reason using AUDIT-C. The three AUDIT-C questions are included in a paper tally sheet completed by the provider, in which the providers document the outcome of the consultation (advice given, patient referred, etc). The local researchers visit each PHCU on a 2–4 weekly basis to collect completed tally sheets and deliver new tally sheets as required. The local researchers collect information on the total number of adult patients (aged 18+ years) registered with each PHCU and the monthly number of total adult consultations with each provider. Patients who score <8 with AUDIT-C are given a patient information leaflet. Patients who score 8+ with AUDIT-C are assessed and manged as appropriate for depression, and are advised to reduce their alcohol consumption, unless there are clinical indications for referral. Arm 4 differs from Arm 3 in having a lengthier assessment, if indicated, and a longer session of advice giving.
By Month 6, Hypothesis 3, that is, non-superiority of Arm 4 (standard package with municipal action and standard training) over Arm 3 (short package with municipal action and short training) will be tested. In the presence of clinical equivalence of a relative difference of the primary outcome, that is, the cumulative coverage of patients whose alcohol consumption is measured, of less than 10%, Arm 4 will be replaced by Arm 3 from month 8 onwards, figure 3.
Figure 3.
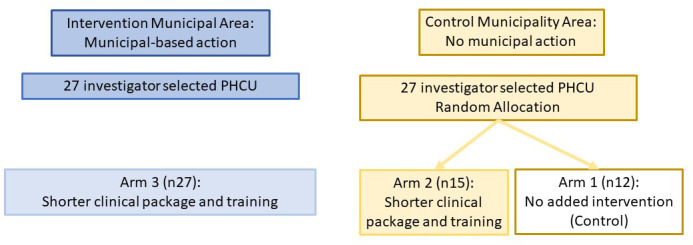
Study design from month 8 onwards, assuming no superiority of Arm 4 over Arm 3 during first 6 months of implementation. PHCU, primary healthcare units.
The municipal integration and support inputs to Arms 3 and 4 within the intervention municipal area are summarised in online supplementary file S1, online supplementary table S2, with references. Municipal integration and support comprises:
Creation of local CAB of local stakeholders to advise on tailoring of materials, support local implementation and review drivers of successful action.
Appointment of local project champion to advocate for successful implementation of programmes.
Implementation of five evidence-based adoption mechanisms.
Implementation of five evidence-based support systems.
Implementation of community-based communication campaigns.
Tailoring
The CABs and UPs review and tailor relevant materials of the clinical package and training courses and of the municipal integration and support inputs within the seven domains of: (1) local and national guideline factors; (2) individual healthcare provider factors; (3) patient factors; (4) interactions between different professional groups; (5) incentives and resources; (6) capacity for organisational change and (7) social, political and legal factors.62–64
The study timetable is summarised in figure 4. The data management plan, as submitted to the European Commission, is available as online supplementary file S2.
Figure 4.
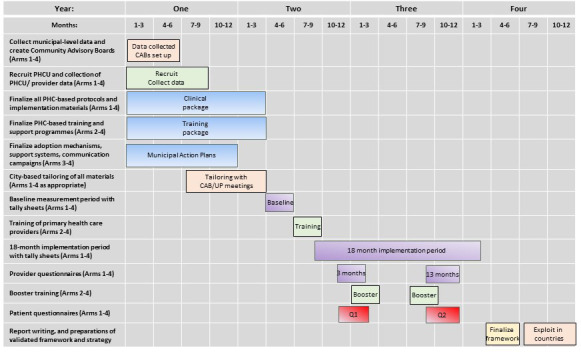
Study timetable.
Data collection and instruments
During set-up phase for Arms 1–4
Municipal level information
At the level of the municipal area (or, when not available, at whole city, regional or country level), the following information will be collected from routinely available data on sociodemographic factors, alcohol and mental health data, health system structures, quality of life, sustainable governance and values, online supplementary file S1, online supplementary table S3.
PHCU and provider level information
All contacted PHCU, including those who did and did not agree to be part of the study, will provide information on the following:
Numbers of registered patients, divided into age 0 to 17 years and 18+ years.
Numbers and professions of provider staff (including physicians, nurses, nurse technicians, midwifes, psychologists, social workers and others).
At recruitment, PHC providers will provide data on their:
Age.
Gender.
Profession (doctor, nurse, practice assistant, etc).
Time worked in the PHC.
Data on their attitudes and experiences to working with patients with heavy drinking and comorbid depression (online supplementary file S1, online supplementary table S4).
Since we are unable to randomise the municipal areas involved, we will use propensity score matching (PSM) based on data collected at the level of the municipal area and the PHCU, to take into account potential confounding variables between control and intervention municipal areas, and minimise bias on account of these.
During 1-month baseline measurement period for Arms 1–4
Provider-based measurement and assessment of alcohol consumption and comorbid depression and record of advice and treatment given (tally sheets)
Based on the validated methodology of the ODHIN project,22 24 PHC providers will be asked to document activity by completing anonymous paper tally sheets that record eligible patients’ (aged 18+ years) AUDIT-C scores,65 and, if administered (as documented in online supplementary file S1, online supplementary table S1), AUDIT-10,66 Patient Health Questionnaire (mental disorders), 2-item version (PHQ-2)67 and PHQ-968 scores, and the advice or treatment given to each patient. The tally sheets will record the age, sex and educational level of the patient, the latter as a proxy measure of socioeconomic status. PHCUs will return data on the number of adult (aged 18+ years) consultations per provider for the 1-month baseline measurement period. Tally sheets will be delivered to the PHCU to be distributed to the participating providers at the beginning of the 1-month baseline measurement period and collected at the end of the period, with no other contact during the period.
During training prior to implementation for Arms 2–4
Providers will complete a short questionnaire after the initial training sessions. The questionnaires, which are adapted based on specific training contents (standard or short package), will assess the participants’ experience of the training, measuring satisfaction with the components of the training aspects, as well as their perceived utility. Two measures included in the main provider questionnaires, Short Alcohol and Alcohol Problems Perception questionnaire (SAAPPQ)69 and self-efficacy,70 will be included in order to assess the specific impact of the training, independent of the effect of the implementation of the intervention.
During 18-month implementation period for Arms 1–4
Provider-based measurement and assessment of alcohol consumption and comorbid depression and record of advice and treatment given (tally sheets)
The same mechanism, for tally sheets used during the baseline measurement period will continue for each calendar month of the 18-month implementation period. Tally sheets will be delivered monthly to each PHCU to distribute to participating providers. Completed tally sheets will be collected at the end of each month. Following training in Arms 2–4, and municipal support in Arms 3–4, each provider determines use and completion of the tally sheets, with no additional prompting. Monthly data will be collected and reported with accumulation of coverage over time. Formal reporting will be undertaken at baseline, and for coverage achieved by month 12 and by month 18 of the 18 month implementation and test period. Tally sheets will include an identifying code of the provider, PHCU, country and study arm, but no identifying code of the patient. Data will be extracted and sent to the project’s data warehouse at Technical University Dresden on a monthly basis.
Extended tally sheets
As part of quality control, in all four arms at two time points, during the 18-month implementation and test period (months 3 and 15), providers will complete extended tally sheets on two separate days in each month. The extended tally sheets will include an identifying code of the provider but no identifying code of the patient. The extended tally sheet will include additional information from the patient on alcohol knowledge,71 social norms72 and health literacy73 applied to alcohol, as it informs the content of advice given; and, additional information from the provider on contextual characteristics that informed their advice giving. The extended tally sheets will include a consent form for the patient and self-completed additional questions for the patient to complete, once the consultation has ended.
Self-completed additional questions by patient
On two separate days, during months 3 and 13, coinciding with and following the consultation with the provider using the extended tally sheet, patients who are able to read and write will be invited to give consent to self-complete additional questions to the extended tally sheet in the waiting room before leaving the PHCU, handing the completed tally sheet and questions to a researcher in attendance. No patient identifying information will be included in the questionnaires. Six domains, serving as quality control, will be included:
AUDIT-C.65
PHQ-2.67
Experiences of the consultation.
Views on being asked about alcohol consumption.
Health literacy73 as it applies to alcohol.
Exposure to communication and media campaigns on alcohol.
On each day, 270 patient questionnaires will be collected across all PHCUs, with up to 1080 (540 during each of months 3 and 13) questionnaires completed in total across the 4 days.
Provider-based attitudes and experiences
At two time points during the 18-month implementation period (months 3 and 13), providers will provide data on their attitudes and experiences to working with patients with heavy drinking and comorbid depression, online supplementary file S1, online supplementary file S2.
Providers will complete a short questionnaire after each of the booster training sessions that they attended (at months 4 and 8). The specific content, number and timing of the training-related questionnaires will depend on the study arm: Arm 2 and 3 participants will fill in one questionnaire after the booster session; while Arm 4 participants will fill in two after each of the two booster sessions.
Observations
The training sessions with the PHC providers, and the meetings of the CABs will be observed by a neutral observer in order to take note of additional possible barriers in the implementation of the protocol that emerge through the training sessions and meetings. Participant responsiveness will also be observed.
Economic data for return-of-investment analyses
Within SCALA, we will conduct return-on-investment (RoI) analyses, by assessing for each EURO invested in scaling up delivery of screening and brief interventions in PHC in Columbia, Mexico and Peru, how many EUROs will be saved by reductions in future healthcare utilisation. The return of investment will be defined as the (return on investment=(gain from investment–cost of investment)/cost of investment). For details on the data required for RoI analyses, online supplementary file S1, online supplementary table S5.
For the RoI analyses, the effects of increased coverage of alcohol brief advice among PHC patients will be modelled using effect sizes from previous meta-analyses.52 74 To translate the reduced intake of alcohol into health gains, we will calculate alcohol-attributable fractions for major disease and injury categories. These fractions will then be applied to the cost data outlined in online supplementary file S1, online supplementary table S5 to estimate the alcohol-attributable costs per disease category.
Process evaluation
As the intervention is embedded in a complex system involving actions and actors at different levels (individual, organisational and municipal), a thorough process evaluation will be carried out to complement and better understand the outcomes. Through the process evaluation, the implementation with its fidelity and adaptation will be assessed, along with the drivers of scale-up and contextual factors influencing the implementation, the drivers and the outcomes. This will be achieved in four blocks: driver diagram creation; barriers and facilitators analysis; assessment of implementation, mechanisms of impact and context and further contextual and policy analysis.
Key informant interviews
A number of individual or group interviews will be undertaken throughout the project with key stakeholders—providers, UP members, CAB members, municipal and PHC-based clinical leaders, project partners and any other people involved in the implementation of the SCALA project. Depending on the stakeholder and their involvement in the project, the topics of the interviews will cover topics such as the necessary adaptation to the protocol; the experience of implementing the programme in PHC practice and the perception of the municipal support and the community campaigns.
Driver diagrams
Driver diagrams75 will be used in order to describe the intervention and its causal assumptions, providing the theory of change through displaying what contributes to intervention aim and what are the relationships between primary drivers, secondary drivers and specific change ideas/activities. The initial general driver diagram, online supplementary file S1, online supplementary figure S3, will be modified based on local contexts and adapted throughout the duration of the project in order to understand how scale up varies in the different cities.
Barriers and facilitators assessment
Factors influencing the implementation of the SCALA protocol will be assessed before the implementation, as well as monitored throughout. The anticipated barriers and facilitators to implementation will be assessed through development of evaluation tool based on literature review76–78 and implementation framework,62 with subsequent refinement and adaptation to the local context through focus group discussions and workshops with the CABs. The aim of the tool is to identify the barriers that would have to be addressed and monitored throughout implementation and the facilitators that would incentivise and engage providers and the primary healthcare unit (PHCU) managers in uptake and scaling up of the SCALA protocol. The experienced barriers and facilitators will be further monitored through meeting observations, provider questionnaires and interviews, as well as interviews with other involved stakeholders (eg, CAB members, PHCU managers).
Implementation, mechanisms of impact and context
The factors influencing the progress from scale-up to outcomes will be identified and documented based on UK Medical Research Council guidance,79 analysing factors within five groups: (1) description of intervention and its causal assumptions, (2) implementation, (3) mechanisms of impact, (4) context and (5) outcomes. All aspects of the intervention will be taken into consideration: the intervention, intervention tailoring, training, training tailoring, as well as the municipal action, consisting of the CABs and the communication campaign, combining both quantitative and qualitative methods in order to obtain a comprehensive picture of the integration and interaction of included variables. A detailed description of the topics of interest and accompanied methods is presented in online supplementary file S1, online supplementary table S6.
The five groups will be assessed as follows:
Description of the intervention. The description of the intervention and its causal assumptions draws from the previously described driver diagram.
Implementation. Delivery of the training will be assessed though document analysis (reports from training), observation and self-reports from the trainers. Delivery of the intervention will be assessed through document analysis, interviews with patients and providers. The areas of focus will be fidelity, adaptation, dose and reach. Implementation of the CAB meetings and community action will be assessed mainly through document analysis, as well as key informant interviews.
Mechanisms of impact. The following three areas will be covered: participant responses to the intervention, mediators and unintended consequences. Mechanisms of impact of intervention delivery will be assessed through patient and providers’ questionnaires. The patient interviews will focus on their responsiveness to the intervention, specifically looking at perceived acceptability. In order to evaluate participants’ responses to the training, a post-training questionnaire examining satisfaction with the training and perceived utility of training sessions will be applied, triangulated with data from observation and trainers’ self-report. Additionally, providers’ self-efficacy will be tested as potential mechanism of impact that links the implementation to the outcomes. Mechanisms of impact of the CAB meetings and community action will be examined through key informant interviews and questionnaires. Specific focus will be placed on perceptions and mechanisms of actions of the communication campaign, examining its effect on attitudes and social norms of both providers and patients.
Context. Contextual factors that should be considered in order to better understand the success of the intervention will be assessed through meeting observation, document analysis and provider questionnaires, as well as stakeholder interviews, with the main focus primarily on individual and organisational level characteristics of the context. For the training evaluation, context will be assessed through observation and trainers’ self-report. Context of municipal level actions will be assessed through key informant interviews. Additionally, contextual and policy factors on national and municipal levels will be assessed as described below.
Outcomes. The data collected through process evaluation will be combined with the outcomes and presented within the Reach, Effectiveness, Adoption, Implementation and Maintenance framework,80–82 evaluating SCALA’s impact across the dimensions of reach, effectiveness, adoption, implementation and maintenance.
Contextual and policy factors
Based on methodology of Ysa et al,83 contextual and policy factors on national and municipal level will be identified through document analysis and key informant interviews. The main variables considered for contextual analysis will be: (1) available data similar to that of the OECD better life initiative;84 (2) Sustainable Governance Indicators85 and (3) World Values Survey data.86 For policy analysis, the information sought will be for a for alcohol policy-related strategies, action plans, legislation and evaluations, both on country and municipal levels. The existing contextual and policy factors will be mapped onto the test of the scale-up of the SCALA package to describe and identify those factors on national and municipal level that might influence going to full scale beyond the tested scalable units.
Outcomes
Primary outcome
The primary outcome will be the cumulative proportion of the number of adults (aged 18+ years) registered with the PHCU that have their alcohol consumption measured with a completed AUDIT-C instrument during the study period (coverage). The number of adults registered is provided by the administrative office of the PHCU and includes all adult patients covered by the PHCU, whether or not they consult during the 18-month implementation test period.
Secondary outcomes
Proportion of consulting patients who have their alcohol consumption measured by AUDIT-C: calculated as the number of adults who have their alcohol consumption measured by AUDIT-C divided by the total number of adults who consult the PHCU during the same time period per participating provider and per PHCU.
At risk population receiving advice and/or treatment for heavy drinking: calculated as the number of adults with an AUDIT-C score of 8+ who receive brief advice and/or referral for their heavy drinking divided by the total number of patients with an AUDIT-C score of 8+ per participating provider and per PHCU. Information will also be collected on the number of patients with an AUDIT-C score of <8 who receive brief advice and/or treatment for their heavy drinking.
Proportion of patients with AUDIT-C score of 8+ who receive assessment for depression: calculated as the number of consulting adults with an AUDIT-C score of 8+ who complete PHQ-2 divided by the total number of patients with an AUDIT-C score of 8+ per participating provider and per PHCU.
At risk population receiving advice and/or treatment for comorbid depression: calculated as the number of adults with a PHQ-2 score of 3+ who receive a patient leaflet and/or referral for their depression divided by the total number of patients with a PHQ-2 score of 3+ per participating provider and per PHCU.
Provider attitudes: attitudes of the participating providers will be measured by the SAAPPQ.65 The responses will be summed within the two scales of role security and therapeutic commitment. Individual missing values for any of the items in a domain will be assigned the mean value of the remaining items of the domain before summation.
Statistical tests of key hypotheses
Primary study goal
Multilevel regression analyses will be undertaken at 12 months’ time of the implementation test period, using cumulative results at months 1 to 12, and at 18 months’ time using cumulative results months 1 to 18. Both analyses will include covariates of country and results during baseline month, analysed at the levels of the PHCU by study arm, taking into consideration the hierarchical nature of the data. For any PHCU that drops out during the study, outcome values for subsequent measurement points will be set at the last value obtained.
Hypothesis 1
Municipal action leads to more sustainable coverage among PHCU that receive training. We will compare results on primary outcome after 18 months with results after 12 months between Arm 3 versus Arm 2 via regression.
Dependent variables:
For each PHCU, cumulative results of months 1–18 of number of patients whose alcohol consumption is measured with AUDIT-C per 1000 registered patients; and cumulative results of months 1–12 per 1000 registered patients.
Random effects:
Country as random intercept (test for inclusion).
Independent variables:
Proportion of consulting patients who have their alcohol consumption measured with a completed AUDIT-C instrument during the baseline measurement month.
-
Condition:
Municipal action (yes vs no).
-
Covariate:
Proportion of consulting patients who have their alcohol consumption measured with a completed AUDIT-C instrument during the baseline measurement month.
It is postulated that coverage for Arm 3 will be significantly higher than for Arm 2.
Hypothesis 2
Training leads to higher coverage than no training. For both months 1–12 and months 1–18, compare cumulative coverage as per primary outcome between Arms 1 and 2 via multilevel regression analyses.
Dependent variable
-
Cumulative results months 1–12, and cumulative results months 1–18 of number of patients whose alcohol consumption is measured with AUDIT-C per 1000 registered patients with
PHCU.
Random effects:
Country as random intercept (test for inclusion).
Independent variables:
-
Condition:
Training (Arm 2 vs Arm 1).
-
Covariate:
Proportion of consulting patients who have their alcohol consumption measured with a completed AUDIT-C instrument during the baseline measurement month.
It is postulated that coverage for Arm 2 will be significantly higher than for Arm 1.
Hypotheses 3
In the presence of municipal action, the short clinical package and short training do not lead to less coverage than the standard clinical package and standard training. In the presence of clinical equivalence of a relative difference of cumulative coverage of patients screened by less than 10% by month 6, the difference between Arm 3 (all 15 PHCU across the three countries) and Arm 4 (all 12 PHCU across the three countries) will be assessed with regression analyses. If Arm 4 is not superior to Arm 3, both arms will be collapsed into Arm 3 (shorter package) from month 8 onwards.
Dependent variable:
Cumulative results months 1 to 6 per 1000 patients.
Random effects:
Country as random intercept (test for inclusion).
Independent variables:
-
Condition:
Length of clinical package (longer=Arm 4 vs shorter=Arm 3).
-
Covariate:
Proportion of consulting patients who have their alcohol consumption measured with a completed AUDIT-C instrument during the baseline measurement month.
It is postulated that Arm 4 is not significantly superior to Arm 3.
Sample size calculations for main hypothesis
As the outcome of the primary study goal is predicted to be Arm 3>Arm 2>Arm 1, we compared both Arm 2>Arm 1, and Arm 3>Arm 2.
Our power calculations are based on the following assumptions: given an average size of a PHCU of approximately 15 000 adults, with an average of 1500 new consultations per month, we expect a cumulative coverage after 12 months of 0.0325 of the registered adult population to have had their alcohol consumption measured in the control condition (Arm 1) (data extrapolated from month 3 and month 9 assessments of control group from ODHIN study;22 24 Anderson, personal communication). For the short clinical package and short training (Arm 2), we expect this to increase to 0.075 (data extrapolated from month 3 and month 9 assessments of training group from ODHIN study;22 24 Anderson, personal communication). Although the WHO Phase IV study predicts an additional beneficial impact of municipal support,41 precise empirical data is not available—however, we consider an estimate for Arm 3, with municipal support, to be 0.15, a proportion that would need to be achieved to consider municipal support to be worthwhile. To detect the difference between Arm 2 and Arm 1, assuming a design effect of 15 PHCUs (clusters) across the three municipal areas in Arm 2, with 15 000 patients (items), and 12 PHCUs (clusters) in Arm 1, with 15 000 patients (items), with an ICC for PHCUs of 0.03 (data from ODHIN study;22 24 Anderson, personal communication) we would have 82% power at a significance level of 5%.87 For the difference between Arm 3 and Arm 2 (15 PHCUs/clusters in each arm), we would have 96.5% power.
Patient and public involvement
Patients were not involved in the design of the study but are involved in the tailoring processes. Existing literature suggests that most patients find it acceptable for PHC providers to ask about their drinking using validated measurement instruments, and support the delivery of brief advice to those drinking above recommended levels.88–96 However, the majority of the evidence to date draws on research conducted in Europe, and thus the findings are potentially less transferable to Latin American populations. In order to ensure the design and content of the intervention package, including related outcome measures, are appropriate for implementation in the target SCALA sites, we work closely with patients in each city to tailor patient materials. Within the intervention municipal areas in each of the three countries, UPs are created with representatives of patients from the PHC centres. As part of the tailoring process, people and patients within the UPs have the opportunity to comment on the materials and information designed for use by patients. The results of the study will be disseminated directly to patients and the public through information made available via the PHCUs.
Discussion
The study has several features worth mentioning. It:
Uses a theory-based approach62–64 to tailoring clinical materials and training programmes, creating city-based CAB, and user-based UPs to ensure that tailoring matches user needs, municipal services97 and coproduction of health.98
Sets a higher cut-off score for AUDIT-C (8+) than is commonly used to trigger advice-giving, matching definitions of heavy drinking99 100 and similar to baseline levels of alcohol consumption in PHC-based trials to reduce heavy drinking.52 We set the same cut-offs for men and women, based on epidemiological evidence,101 and to minimise unintended consequences of using different cut-offs for men and women.102 We recognise the importance of comorbid depression by building in identification, management and referral mechanisms.57–59
Tests for non-superiority of implementing a standard measurement and 5 min brief advice intervention with 6 hours of training, compared with implementing a shorter 1 min brief advice intervention with 3 hours of training, taking into account that brief advice is as effective and cost-effective as more extended advice or treatment in reducing heavy drinking55 103 104 and the need for very brief clinical and training programmes for time-constrained providers.
Tests the added value of embedding and implementing PHC activity within municipal-based adoption mechanisms and support systems,40 and communication campaigns over and above training programmes solely directed to PHC providers.
Has a longer time frame (18 months) than is traditionally used in implementation studies,105 106 to assess longer term impacts.
Gives considerable emphasis to process evaluation,79 developing logic models to document the fidelity of all implementation strategies, and to identify, the drivers and barriers and facilitators to successful implementation and scale-up, and the political and economic contextual factors that might influence scale-up.
There are some limitations to the study design. A trial with random assignment of municipal areas is not feasible due to municipal-based political and technical considerations. As we are unable to randomise the involved municipal areas, we adopt a quasiexperimental design,1 trying to optimise control municipal areas for confounding, and by using PSM. While full comparisons via randomisation, and thus establishment of causality, are not possible, together with the qualitative evaluation component of the study, we will be able to clearly identify the mechanisms which were crucial in leading to the outcomes. According to a recent seven-item checklist for classifying quasiexperimental studies for Cochrane reviews,107 our approach is, nevertheless, ranked as a strong design, online supplementary table S7.
Although our focus on embedding PHC activity within supportive municipal actions is hypothesised to increase measurement and brief activity over and above that previously demonstrated, such an approach also brings risks. Municipal and national governments change; and, thus health priorities may change. Although our approach minimises the need for extra resources (and in some jurisdictions, could be resource saving),19 it is not resource free. Funding constraints could limit future scale-up and sustainability.
We have based our protocol adopted on a model of transdisciplinary research to promote sustainability. Such a model identifies, structures, analyses and deals with specific problems in a way that grasps the complexity of problems;108 it takes into account the diversity of real-world and scientific perceptions of problems; and develops knowledge and practices that promote what is generally accepted to be the common good.109 As such, we include municipalities and health systems as stakeholders to form explicitly orchestrated and managed ecosystems that cross-organisational boundaries. Municipal areas and health systems create an engagement platform that provides the necessary environment, including people and resources, for sustainability.
Ethics and dissemination
This protocol outlines a quasiexperimental study1 to test the extent to which embedding PHC-based measurement and brief advice activity within supportive municipal action leads to improved scale-up of an intervention package, with more patients having their alcohol consumption measured, and with heavy drinkers receiving subsequent appropriate advice and treatment. It is not envisaged that there will be any substantial protocol modifications during the course of the study. Any modification to the protocol will be described in all scientific publications.
All participating PHCUs and participating PHC providers sign an informed consent form for participation with the country-based research team. Selected patients at two separate time points sign an informed consent form with the country-based research team to provide additional anonymised information following a consultation with a PHC provider. The consent forms are included within Annexe Data Management Plan. All data collection, processing, and sharing procedures will adhere to national and international laws including the General Data Protection Regulation (EU Regulation 2016/679), as described within the Annexe Data Management Plan.
All materials are publicly available on the project website: https://www.scalaproject.eu/. According to the SCALA data management plan, by default, all quantitative data sets generated in the course of the SCALA study will be made openly available through the UK Data Service on publication of the results (http://www.data-archive.ac.uk/). Prior to publication, all data will be formatted to meet UK Data Service requirements.
Ministries of Health at municipal and country levels are represented in the CAB created in each intervention municipality to facilitate scale-up at municipal and country levels, once the implementation strategy is validated. SCALA works closely with the Pan American Health Organization (PAHO), with the principal investigator form Mexico being a Collaborating Centre with PAHO, to facilitate scale-up at Latin American levels, once the implementation strategy is validated.
Supplementary Material
Footnotes
Twitter: @MantheyJakob
Contributors: EJL, PA, MP, AOD, AG, BS, APG, HdV, GNR, DK, IVB, FB, JMT, AS, APDL, EFSK, SM, JM, LM, HLP, GR, CS and JR contributed to the Grant Application, on which this protocol is based and adapted; commented on drafts of the manuscript and read and approved the final version. EJL drafted the first version of the paper, and revised the paper based on author’s feedback and comments. PA prepared the paper and material for submission and undertook the submission process. PA undertook random allocation generation. APG and JMT assigned PHCU to arms in Colombia; GNR and APDL assigned PHCU to arms in Mexico; MP and IVB assigned PHCU to arms in Peru.
Funding: The research leading to these results or outcomes has received funding from the European Horizon 2020 Programme for research, technological development and demonstration under Grant Agreement no. 778048—Scale-up of Prevention and Management of Alcohol Use Disorders and Comorbid Depression in Latin America (SCALA). Participant organisations in SCALA can be seen at: www.scalaproject.eu. The views expressed here reflect those of the authors only and the European Union is not liable for any use that may be made of the information contained therein. The Funder was not involved in the study design. The funder will not be involved in the collection, analysis, interpretation of data and preparations of any publication.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Ethics approval: The Ethics Committee of the Technical University of Dresden gave final ethical approval for the SCALA project on 12 April 2019, EK90032018.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Adamou B, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. 2nd ed Michigan: Houghton Mifflin, 2002. [Google Scholar]
- 2.World Health Organization Global status report on alcohol and health 2018. Geneva, Switzerland: WHO Press, 2018. [Google Scholar]
- 3.Rehm J, Gmel GE, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017;112:968–1001. 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odlaug BL, Gual A, DeCourcy J, et al. Alcohol dependence, co-occurring conditions and attributable burden. Alcohol Alcohol 2016;51:201–9. 10.1093/alcalc/agv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellos S, Skapinakis P, Rai D, et al. Longitudinal association between different levels of alcohol consumption and a new onset of depression and generalized anxiety disorder: results from an international study in primary care. Psychiatry Res 2016;243:30–4. 10.1016/j.psychres.2016.05.049 [DOI] [PubMed] [Google Scholar]
- 6.Boden JM, Fergusson DM. Alcohol and depression. Addiction 2011;106:906–14. 10.1111/j.1360-0443.2010.03351.x [DOI] [PubMed] [Google Scholar]
- 7.Probst C, Roerecke M, Behrendt S, et al. Socioeconomic differences in alcohol-attributable mortality compared with all-cause mortality: a systematic review and meta-analysis. Int J Epidemiol 2014;43:1314–27. 10.1093/ije/dyu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontis V, Mathers CD, Rehm J, et al. Contribution of six risk factors to achieving the “25×25” NCD mortality reduction target. Lancet 2014. [DOI] [PubMed] [Google Scholar]
- 9.Kontis V, Mathers CD, Bonita R, et al. Regional contributions of six preventable risk factors to achieving the "25×25" non-communicable disease mortality reduction target: a modelling study. Lancet Glob Health 2015;3:e746–57. 10.1016/S2214-109X(15)00179-5 [DOI] [PubMed] [Google Scholar]
- 10.United Nations General Assembly Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. Available: http://www.un.org/en/ga/ncdmeeting2011/ [Accessed 01 Feb 2017].
- 11.GBD 2015 SDG Collaborators Measuring the health-related sustainable development goals in 188 countries: a baseline analysis from the global burden of disease study 2015. Lancet 2016;388:1813–50. 10.1016/S0140-6736(16)31467-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shield K, Manthey J, Rylett M, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health 2020;5:e51–61. 10.1016/S2468-2667(19)30231-2 [DOI] [PubMed] [Google Scholar]
- 13.United States of America, University of Washington . Economic dimensions of noncommunicable diseases in Latin America and the Caribbean: policy agenda considerations 143 George Alleyne. Available: http://iris.paho.org/xmlui/bitstream/handle/123456789/28501/9789275119051_eng.pdf?sequence=1&isAllowed=y
- 14.Levin C, Chisholm D. Cost-effectiveness and affordability of interventions, policies, and platforms for the prevention and treatment of mental, neurological, and substance use disorders : Patel V, Chisholm D, Dua T, et al., Mental, neurological, and substance use disorders. Disease control priorities. 3rd ed Washington, DC: World Bank, 2015. [PubMed] [Google Scholar]
- 15.O’Donnell A, Anderson P, Newbury-Birch D, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol and Alcoholism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angus C, Thomas C, Anderson P, et al. Estimating the cost-effectiveness of brief interventions for heavy drinking in primary health care across Europe. Eur J Public Health 2017;27:345–51. 10.1093/eurpub/ckw122 [DOI] [PubMed] [Google Scholar]
- 17.Rehm J, Barbosa C. The cost-effectiveness of therapies to treat alcohol use disorders. Expert Rev Pharmacoecon Outcomes Res 2018;18:43–9. 10.1080/14737167.2018.1392241 [DOI] [PubMed] [Google Scholar]
- 18.Rehm J, Shield KD, Gmel G, et al. Modeling the impact of alcohol dependence on mortality burden and the effect of available treatment interventions in the European Union. Eur Neuropsychopharmacol 2013;23:89–97. 10.1016/j.euroneuro.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 19.Organisation for Economic Co-operation and Development Tackling harmful alcohol use: economics and public health policy. Paris: Organisation for Economic Co-operation and Development, 2015. [Google Scholar]
- 20.Anderson P, Laurant M, Kaner E, et al. Engaging general practitioners in the management of hazardous and harmful alcohol consumption: results of a meta-analysis. J Stud Alcohol 2004;65:191–9. 10.15288/jsa.2004.65.191 [DOI] [PubMed] [Google Scholar]
- 21.Keurhorst M, Glind vande, Bitarello do Amaral-Sabadini M, et al. Determinants of successful implementation of screening and brief interventions for hazardous and harmful alcohol consumption in primary healthcare. A systematic review and meta-regression analysis. Addiction Addiction 2015;110:877–900. [Google Scholar]
- 22.Anderson P, Gual T, Coulton S, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: nine month outcomes of the ODHIN five country cluster randomized factorial trial. Annals Fam Pract 2017. [Google Scholar]
- 23.Anderson P, Kaner E, Wutzke S, et al. Attitudes and managing alcohol problems in general practice: an interaction analysis based on findings from a who Collaborative study. Alcohol Alcohol 2004;39:351–6. 10.1093/alcalc/agh072 [DOI] [PubMed] [Google Scholar]
- 24.Anderson P, Bendsten P, Spak F, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the ODHIN five country cluster randomized factorial trial' addiction. Addiction 2016;111:1935–45. [DOI] [PubMed] [Google Scholar]
- 25.Serrano CC, Martínez RD, Martha P, et al. La intervención eficaz del médico General en El tratamiento de bebedores cuyo hábito alcohólico representa un riesgo para SU salud O Ya les HA ocasionado algún daño. (preliminary results of a prospective double blind clinical trial). Salud mental 1992;15. [Google Scholar]
- 26.Hoffman KA, Beltrán J, Ponce J, et al. Barreras para implementar El despistaje, intervenciones breves Y referencia al tratamiento POR problemas de consumo de alcohol Y otras drogas en hospitales que atienden personas que viven Con El VIH/SIDA en El Perú. Rev Peru Med Exp Salud Publica 2016;33:432–7. [DOI] [PubMed] [Google Scholar]
- 27.Gelberg L, Natera Rey G, Andersen RM, et al. Prevalence of substance use among patients of community health centers in East Los Angeles and Tijuana. Subst Use Misuse 2017;52:359–72. 10.1080/10826084.2016.1227848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto-Brandt G, Huidobro RP, Artigas DH, et al. Evidencia de validez en Chile del alcohol, smoking and substance involvement screening test (assist). Adicciones 2013;26:291–302. [DOI] [PubMed] [Google Scholar]
- 29.Costa PHAda, Mota DCB, Cruvinel E, et al. [A methodology to implement preventive actions against harmful drug use in the context of primary health care in Latin America]. Rev Panam Salud Publica 2013;33:325–31. 10.1590/s1020-49892013000500003 [DOI] [PubMed] [Google Scholar]
- 30.Natera R, et al. Final narrative report of the bi-national assist screening and quit using drugs intervention trial (quit) Tijuana/Los Angeles. Mexico: National Institute on Psychiatry de la Fuente Muniz, 2014. [Google Scholar]
- 31.Kaner E. Brief alcohol intervention: time for translational research. Addiction 2010;105:960–1. 10.1111/j.1360-0443.2009.02848.x [DOI] [PubMed] [Google Scholar]
- 32.Eccles M, Grimshaw J, Walker A, et al. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol 2005;58:107–12 10.1016/j.jclinepi.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 33.Norton W, Mittman B. Scaling-up health Promotion/Disease prevention programmes in community settings: barriers, facilitators, and initial recommendations. West Hartford: The Patrick and Catherine Weldon Donaghue Medical Research Foundation, 2010. [Google Scholar]
- 34.Woolf SH, Johnson RE. The break-even point: when medical advances are less important than improving the fidelity with which they are delivered. Ann Fam Med 2005;3:545–52. 10.1370/afm.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massoud R. An approach to rapid scale-up using HIV/AIDS treatment and care as an example. Geneva: World Health Organization, 2004 [Google Scholar]
- 36.Cooley L, Kohl R. Scaling up—from vision to large-scale change: a management framework for practitioners. Washington: Management Systems International, 2006 [Google Scholar]
- 37.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamey G. Scaling up global health interventions: a proposed framework for success. PLoS Med 2011;8:e1001049. 10.1371/journal.pmed.1001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamou B. Guide for monitoring scale-up of health practices and interventions: measure evaluation, 2013. Available: https://www.measureevaluation.org/resources/publications/ms13-64 [Accessed 04 Sep 2016].
- 40.Barker PM, Reid A, Schall MW. A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci 2016;11:12. 10.1186/s13012-016-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heather N. Who Collaborative project on identification and management of alcohol-related problems in primary health care–report to the world health organisation on phase IV: development of country-wide strategies for implementing early identification and brief intervention in primary health care. Geneva: World Health Organisation, Department of Mental Health and Substance Abuse, 2006. http://www.who.int/substance_abuse/publications/identification_management_alcoholproblems_phaseiv.pdf [Google Scholar]
- 42.Keurhorst M, Heinen M, Colom J, et al. Strategies in primary healthcare to implement early identification of risky alcohol consumption: why do they work or not? A qualitative evaluation of the ODHIN study. BMC Fam Pract 2016;17:70. 10.1186/s12875-016-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babor TF, Del Boca F, Bray JW. Screening, brief intervention and referral to treatment: implications of SAMHSA's SBIRT initiative for substance abuse policy and practice. Addiction 2017;112 Suppl 2:110–7. 10.1111/add.13675 [DOI] [PubMed] [Google Scholar]
- 44.Vendetti J, Gmyrek A, Damon D, et al. Screening, brief intervention and referral to treatment (SBIRT): implementation barriers, facilitators and model migration. Addiction 2017;112 Suppl 2:23–33. 10.1111/add.13652 [DOI] [PubMed] [Google Scholar]
- 45.Singh M, Gmyrek A, Hernandez A, et al. Sustaining screening, brief intervention and referral to treatment (SBIRT) services in health-care settings. Addiction 2017;112 Suppl 2:92–100. 10.1111/add.13654 [DOI] [PubMed] [Google Scholar]
- 46.Jonas DE, Garbutt JC, Brown JM, et al. Screening, behavioral counseling, and referral in primary care to reduce alcohol misuse. Comparative effectiveness review No. 64. Rockville: Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 47.Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol 2016;51:422–7. 10.1093/alcalc/agv127 [DOI] [PubMed] [Google Scholar]
- 48.Rubinsky AD, Dawson DA, Williams EC, et al. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res 2013;37:1380–90. 10.1111/acer.12092 [DOI] [PubMed] [Google Scholar]
- 49.Rehm J, Shield KD, Rehm M, et al. Alcohol consumption, alcohol dependence, and attributable burden of disease in Europe: potential gains from effective interventions for alcohol dependence. Toronto, Canada: Centre for Addiction and Mental Health, 2012. [Google Scholar]
- 50.National Clinical Guideline Centre The clinical management of primary hypertension in adults, 2011. Available: https://www.nice.org.uk/guidance/cg127/evidence/cg127-hypertension-full-guideline3 [Accessed 1 Dec 2016].
- 51.Rehm J, Anderson P, Prieto JAA, et al. Towards new recommendations to reduce the burden of alcohol-induced hypertension in the European Union. BMC Med 2017;15:173. 10.1186/s12916-017-0934-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaner EF, Beyer FR, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2:CD004148. [DOI] [PubMed] [Google Scholar]
- 53.Bray JW, Zarkin GA, Hinde JM, et al. Costs of alcohol screening and brief intervention in medical settings: a review of the literature. J Stud Alcohol Drugs 2012;73:911–9. 10.15288/jsad.2012.73.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groves P, Pick S, Davis P, et al. Routine alcohol screening and brief interventions in general hospital in-patient wards: acceptability and barriers. Drugs Edu Preven Policy 2010;17:55–71. [Google Scholar]
- 55.Platt L, Melendez-Torres GJ, O'Donnell A, et al. How effective are brief interventions in reducing alcohol consumption: do the setting, practitioner group and content matter? findings from a systematic review and metaregression analysis. BMJ Open 2016;6:e011473. 10.1136/bmjopen-2016-011473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaner E, Bland M, Cassidy P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SipS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346:e8501. 10.1136/bmj.e8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson GB, Wray C, McGovern R, et al. Intervention to reduce excessive alcohol consumption and improve comorbidity outcomes in hypertensive or depressed primary care patients: two parallel cluster randomized feasibility trials. Trials 2014;15:235. 10.1186/1745-6215-15-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linde K, Sigterman K, Kriston L, et al. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med 2015;13:56–68. 10.1370/afm.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbody S, Whitty P, Grimshaw J, et al. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA 2003;289:3145–51. 10.1001/jama.289.23.3145 [DOI] [PubMed] [Google Scholar]
- 60.Anderson P, O'Donnell A, Kaner E, et al. Scaling-up primary health care-based prevention and management of heavy drinking at the municipal level in middle-income countries in Latin America: background and protocol for a three-country quasi-experimental study. F1000Res 2017;6:311. 10.12688/f1000research.11173.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson P, Kłoda K, Kaner E, et al. Impact of practice, provider and patient characteristics on delivering screening and brief advice for heavy drinking in primary healthcare: secondary analyses of data from the ODHIN five-country cluster randomized factorial trial. Eur J Gen Pract 2017;23:241–5. 10.1080/13814788.2017.1374365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 2013;8:35 10.1186/1748-5908-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wensing M, Oxman A, Baker R, et al. Tailored implementation for chronic diseases (TICD): a project protocol. Implement Sci 2011;6:103 10.1186/1748-5908-6-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wensing M, Huntink E, van Lieshout J, et al. Tailored implementation of evidence-based practice for patients with chronic diseases. PLoS One 2014;9:e101981 10.1371/journal.pone.0101981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Substance Abuse and Mental Health Services Administration The alcohol use disorders identification test. Available: https://www.integration.samhsa.gov/images/res/tool_auditc.pdf
- 66.Babor TF, Higgins-Biddle JC, Saunders JB. Monteiro Mg audit: the alcohol use disorders identification test guidelines for use in primary care (2nd ed.). Available: https://www.who.int/substance_abuse/publications/audit/en/
- 67.Center for quality assessment and improvement in mental health patient health questionnaire PHQ-2. Available: http://www.cqaimh.org/pdf/tool_phq2.pdf
- 68.US Preventive Service task Force Patient health questionnaire PHQ-9. Available: https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218
- 69.Anderson P, Clement S. The AAPPQ revisited: the measurement of general practitioners' attitudes to alcohol problems. Br J Addict 1987;82:753–9 10.1111/j.1360-0443.1987.tb01542.x [DOI] [PubMed] [Google Scholar]
- 70.Schaufeli WB, Shimazu A, Hakanen J, et al. An ultra-short measure for work engagement: the UWES-3 validation across five countries. Eur J Psychol Ass 2017;35:577–91. [Google Scholar]
- 71.Rosenberg G, Bauld L, Hooper L, et al. New national alcohol guidelines in the UK: public awareness, understanding and behavioural intentions. J Public Health 2018;40:549–56. 10.1093/pubmed/fdx126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung A, Rimal RN. Social norms: a review. Rev Comm Res 2016;4:1–29. [Google Scholar]
- 73.Sørensen K, Van den Broucke S, Pelikan JM, et al. Measuring health literacy in populations: illuminating the design and development process of the European health literacy survey questionnaire (HLS-EU-Q). BMC Public Health 2013;13:948 10.1186/1471-2458-13-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaner EFS, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;18:CD004148. 10.1002/14651858.CD004148.pub3 [DOI] [PubMed] [Google Scholar]
- 75.Svoronos T, Mate KS. Evaluating large-scale health programmes at a district level in resource-limited countries. Bull World Health Organ 2011;89:831–7 10.2471/BLT.11.088138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derges J, Kidger J, Fox F, et al. Alcohol screening and brief interventions for adults and young people in health and community-based settings: a qualitative systematic literature review. BMC Public Health 2017;17:1–12 https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson M, Jackson R, Guillaume L, et al. Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health 2011;33:412–21 https://doi.org/ 10.1093/pubmed/fdq095 [DOI] [PubMed] [Google Scholar]
- 78.O'Donnell A, Wallace P, Kaner E. From efficacy to effectiveness and beyond: what next for brief interventions in primary care? Front Psychiatry 2014;5:1–8. 10.3389/fpsyt.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical research council guidance. BMJ 2015;350:h1258 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glasgow RE, Klesges LM, Dzewaltowski DA, et al. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res 2006;21:688–94 10.1093/her/cyl081 [DOI] [PubMed] [Google Scholar]
- 82.Harden SM, Gaglio B, Shoup JA, et al. Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: a systematic review. Syst Rev 2015;4:155 10.1186/s13643-015-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ysa T, Colom J, Albareda A. Governance of addictions: European public policies. Oxford: Oxford University Press, 2014. [Google Scholar]
- 84.Organisation for Economic Co-operation and Development Compendium of OECD well-being indicators. OECD better life initiative, 2011. Available: http://www.oecd.org/sdd/47917288.pdf [Accessed 18 Dec 2016].
- 85.Sustainable governance indicators Bertelsmann Stiftung, 2016. Available: https://www.sgi-network.org/2019/ [Accessed 18 Dec 2016].
- 86.Inglehart R, Welzel C. Modernization, cultural change and democracy. New York: Cambridge University Press, 2005. [Google Scholar]
- 87.Donner A, Klar N, PASS16 sample size software . Design and analysis of cluster randomization trials in health research. Arnold. London, 2000. Available: https://www.ncss.com/software/pass/
- 88.Hutchings D, Deborah H, Pual C, et al. Implementing screening and brief alcohol interventions in primary care: views from both sides of the consultation. Prim Health Care Res Develop 2006;7:221–9. [Google Scholar]
- 89.Tam CWM, Leong L, Zwar N, et al. Alcohol enquiry by GPs - Understanding patients' perspectives: a qualitative study. Aust Fam Physician 2015;44:833–8. [PubMed] [Google Scholar]
- 90.Lock CA. Alcohol and brief intervention in primary health care: what do patients think? Prim Health Care Res Develop 2004;5:162–78. 10.1191/1463423604pc194oa [DOI] [Google Scholar]
- 91.Khadjesari Z, Stevenson F, Toner P, et al. ‘I’m not a real boozer’: a qualitative study of primary care patients’ views on drinking and its consequences. J Public Health 2019;41:e185–91 10.1093/pubmed/fdy067 [DOI] [PubMed] [Google Scholar]
- 92.O'Donnell A, Abidi L, Brown J, et al. Beliefs and attitudes about addressing alcohol consumption in health care: a population survey in England. BMC Public Health 2018;18:391. 10.1186/s12889-018-5275-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol 2006;41:306–10. 10.1093/alcalc/agl022 [DOI] [PubMed] [Google Scholar]
- 94.Nilsen P, McCambridge J, Karlsson N, et al. Brief interventions in routine health care: a population-based study of conversations about alcohol in Sweden. Addiction 2011;106:1748–56. 10.1111/j.1360-0443.2011.03476.x [DOI] [PubMed] [Google Scholar]
- 95.Aalto M, Pekuri P, Seppä K. Primary health care professionals' activity in intervening in patients' alcohol drinking: a patient perspective. Drug Alcohol Depend 2002;66:39–43. 10.1016/S0376-8716(01)00179-X [DOI] [PubMed] [Google Scholar]
- 96.O’Donnell A, Hanratty B, Schulte B, et al. Patients’ experiences of alcohol screening and advice in primary care: a qualitative study. BMC Fam Pract 2020;21 10.1186/s12875-020-01142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dietz WH, Solomon LS, Pronk N, et al. An integrated framework for the prevention and treatment of obesity and its related chronic diseases. Health Aff 2015;34:1456–63. 10.1377/hlthaff.2015.0371 [DOI] [PubMed] [Google Scholar]
- 98.Palumbo R. Contextualizing co-production of health care: a systematic literature review. Intl Jnl Public Sec Management 2016;29:72–90. 10.1108/IJPSM-07-2015-0125 [DOI] [Google Scholar]
- 99.Rehm J, Room R, Monteiro M, et al. Alcohol Use : Ezzati M, Lopez AD, Rodgers A, et al., Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva, Switzerland: World Health Organization, 2004: 959–1109. [Google Scholar]
- 100.European Medicines Agency Guideline on the development of medicinal products for the treatment of alcohol dependence, 2010. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-medicinal-products-treatment-alcohol-dependence_en.pdf
- 101.Rehm J, Lachenmeier DW, Room R. Why does society accept a higher risk for alcohol than for other voluntary or involuntary risks? BMC Med 2014;12:189. 10.1186/s12916-014-0189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham JA. Unintended impact of using different inclusion cut-offs for males and females in intervention trials for hazardous drinking. Addiction 2017;112:910–1. 10.1111/add.13760 [DOI] [PubMed] [Google Scholar]
- 103.Aldridge A, Dowd W, Bray J. The relative impact of brief treatment versus brief intervention in primary health-care screening programs for substance use disorders. Addiction 2017;112:54–64. 10.1111/add.13653 [DOI] [PubMed] [Google Scholar]
- 104.Barbosa C, Cowell A, Dowd W, et al. The cost-effectiveness of brief intervention versus brief treatment of screening, brief intervention and referral to treatment (SBIRT) in the United States. Addiction 2017;112 Suppl 2:73–81 10.1111/add.13658 [DOI] [PubMed] [Google Scholar]
- 105.Anderson P, Kaner E, Keurhorst M, et al. Attitudes and learning through practice are key to delivering brief interventions for heavy drinking in primary health care: analyses from the ODHIN five country cluster randomized factorial trial. Int J Environ Res Public Health 2017;14 10.3390/ijerph14020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Funk M, Wutzke S, Kaner E, et al. A multicountry controlled trial of strategies to promote dissemination and implementation of brief alcohol intervention in primary health care: findings of a world Health organization collaborative study. J Stud Alcohol 2005;66:379–88 10.15288/jsa.2005.66.379 [DOI] [PubMed] [Google Scholar]
- 107.Reeves BC, Wells GA, Waddington H. Quasi-experimental study designs series-paper 5: a checklist for classifying studies evaluating the effects on health interventions-a taxonomy without labels. J Clin Epidemiol 2017;89:30–42. 10.1016/j.jclinepi.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jantsch E. Inter- and Transdisciplinary university: a systems approach to education and innovation. Policy Sci 1970;1:403–28. 10.1007/BF00145222 [DOI] [Google Scholar]
- 109.Pohl C, Hirsch Hadorn G. Principles for designing transdisciplinary research (proposed by the Swiss academies of arts and sciences). München: oekom Verlag, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038226supp001.pdf (761KB, pdf)
bmjopen-2020-038226supp002.pdf (2.9MB, pdf)