Abstract
Background
Researchers have criticised epilepsy care for adults for its lack of impact, stimulating the development of various service models and strategies to respond to perceived inadequacies.
Objectives
To assess the effects of any specialised or dedicated intervention beyond that of usual care in adults with epilepsy.
Search methods
For the latest update of this review, we searched the Cochrane Epilepsy Group Specialized Register (9 December 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11), MEDLINE (1946 to June 2013), EMBASE (1988 to June 2013), PsycINFO (1887 to December 2013) and CINAHL (1937 to December 2013). In addition, we contacted experts in the field to seek information on unpublished and ongoing studies, checked the websites of epilepsy organisations and checked the reference lists of included studies.
Selection criteria
We included randomised controlled trials, controlled or matched trials, cohort studies or other prospective studies with a control group, and time series studies.
Data collection and analysis
Two review authors independently selected studies, extracted all data, and assessed the quality of all included studies.
Main results
Our review included 18 different studies of 16 separate interventions, which we classified into seven distinct groups. Most of the studies have methodological weaknesses, and many results from other analyses within studies need to be interpreted with caution because of study limitations. Consequently, there is currently limited evidence for the effectiveness of interventions to improve the health and quality of life in people with epilepsy. It was not possible to combine study results in a meta‐analysis because of the heterogeneity of outcomes, study populations, interventions and time scales across the studies.
Authors' conclusions
Two intervention types, the specialist epilepsy nurse and self management education, have some evidence of benefit. However, we did not find clear evidence that other service models substantially improve outcomes for adults with epilepsy. It is also possible that benefits are situation specific and may not apply to other settings. These studies included only a small number of service providers whose individual competence or expertise may have had a significant impact on outcomes. At present it is not possible to advocate any single model of service provision.
Keywords: Adult; Humans; Delivery of Health Care; Delivery of Health Care/methods; Epilepsy; Epilepsy/nursing; Epilepsy/therapy; Neurology; Outcome and Process Assessment, Health Care; Patient Education as Topic; Patient Education as Topic/methods; Randomized Controlled Trials as Topic; Self Care; Self Care/methods
Plain language summary
Care delivery and self management strategies for adults with epilepsy
Evidence for the effectiveness of care interventions for adults with epilepsy is still unclear.
This review compared the effectiveness of a range of interventions, including specialist nurses and management strategies, in improving outcomes for adults with epilepsy. We identified seven distinct intervention types, with varying amounts of evidence to support them. While included studies did show some benefit from specialist epilepsy nurses and self management education, other intervention types lack evidence of effectiveness. This is compounded by the poor quality methods of some studies and by the complex nature of the interventions, whose impact may vary according to where they take place. Based on this evidence, it is not possible to advocate any specific intervention type in the care of adults with epilepsy.
Background
Description of the condition
Epilepsy is spectrum of disorders in which an individual may experience seizures that are unpredictable in frequency (England 2012). Researchers have identified at least 40 different seizure types (Berg 2010). While most people can control seizures well with medications and other treatment options, epilepsy can pose challenges to autonomy and in social, school and work situations. Not only do people with seizures tend to have more physical problems (ranging from fractures and bruising to—rarely—an increased risk of sudden death) but people with epilepsy face significant challenges in how others perceive (or misperceive) their condition, which can lead to the stigmatisation of people with epilepsy (Bandstra 2008). As a result, they may experience a lack of social support, social isolation, embarrassment, fear and discrimination (England 2012). Epilepsy affects around 50 million people worldwide, with around 80% of all cases in developing countries (WHO 2012). Epilepsy is most common in children and older adults (Betts 1992; Sander 1990).
Description of the intervention
The self management of epilepsy refers to a wide range of health behaviours and activities that an individual can learn and adapt in order to promote seizure control and enhance well‐being (Austin 1997). Self management of any condition typically entails a partnership between users and service providers (Clark 2008). Various dedicated models of service provision exist to improve care networks and self education (Clark 2010; Fitzsimons 2012; SIGN 2003; SIGN 2005). Services may include specialist epilepsy outpatient clinics, nurse‐based liaison services between primary (GP) and secondary/tertiary (hospital‐based) care and specialist epilepsy multidisciplinary community teams (Clark 2010; Fitzsimons 2012; SIGN 2003; SIGN 2005). Services may also include input from social care or the voluntary sector (Clark 2010; SIGN 2003; SIGN 2005) and target specific groups, such as people with learning disabilities.
How the intervention might work
Specialist or dedicated models of care, care networks, or self education and self management may improve the quality of care, promote more systematic multidisciplinary follow‐up of individuals and enhance communication among professionals, patients and other services (Fitzsimons 2012). Importantly, care should enable people with epilepsy to cope with all aspects of the disease through improved self education and self management skills (Clark 2008; Fitzsimons 2012).
Why it is important to do this review
Different researchers have criticised epilepsy care as having limited impact by not fully addressing all the health and social needs of people suffering from it (Betts 1992; Chappell 1992; Elwyn 2003; Thapar 1996). In order to improve the quality of care for adults with epilepsy, the aim of this review is to systematically update the evidence from studies investigating the effectiveness of these service models compared to non‐specialist services. This systematic review is an update of the Cochrane Review previously published in 2009 (Bradley 2008).
Objectives
To assess the effects of any specialised or dedicated intervention beyond that of usual care in adults with epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
We included several study types in the review, as the interventions considered were highly variable and complex. We based our inclusion criteria for studies on those used by the Cochrane Effective Practice and Organisation of Care Review Group (EPOC). We included all randomised controlled, controlled or matched trials, cohort or other prospective studies with a control group, and time series studies.
Types of participants
We considered studies that included anyone aged over 16 years with any diagnosis of new or recurrent epilepsy eligible for this review. We included studies incorporating epilepsy with other long‐term conditions if they reported results separately for each condition.
Types of interventions
We included any intervention, including a specialised or dedicated team or individual for the care of epilepsy patients, whether based:
in hospital (e.g. a specialist epilepsy clinic);
in the community (e.g. a dedicated team focusing on epilepsy treatment);
in general practice (e.g. a specialist epilepsy nurse);
elsewhere (e.g. social worker, the voluntary sector);
on education or counselling with content specific to epilepsy for improved self management;
as a care network combining any of these elements.
Types of outcome measures
The outcomes we considered are:
seizure frequency and severity;
appropriateness and volume of medication prescribed (including evidence of drug toxicity);
participants' reported knowledge of information and advice received from professionals;
participants' reports of health and quality of life;
objective measures of general health status;
objective measures of social or psychological functioning (including the number of days spent on sick leave/absence from school or work, and employment status);
costs of care or treatment.
We assessed all outcome measures for reliability and validity (i.e. for clinical relevance and whether validated tools were used for outcome measurement). If trials misused measures (e.g. children's scales used on adults), we planned to investigate their effect on study results by a sensitivity analysis.
Search methods for identification of studies
We searched the following databases.
Cochrane Epilepsy Group Specialized Register (9 December 2013). See Appendix 1 for details of search strategy for the latest update.
Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11). See Appendix 2 for details of search strategy for the latest update.
MEDLINE (Ovid) (1946 to June 2013). See Appendix 3 for details of search strategy.
EMBASE (1988 to June 2013). See Appendix 4 for details of search strategy.
PsycINFO (EBSCOhost 1887 to December 2013). See Appendix 5 for details of search strategy.
CINAHL (EBSCOhost 1937 to December 2013). See Appendix 6 for details of search strategy.
Finally we contacted experts in the field to seek information on unpublished and ongoing studies, checked the websites of epilepsy organisations and checked the reference lists of included studies.
We should note that we undertook this review at the same time as another Cochrane review update of care delivery and self management strategies for children with epilepsy (Lindsay 2015), and we used the same search strategy for both reviews.
Data collection and analysis
Selection of studies
We screened papers in two stages. At stage 1, two review authors (PM and BL in the original review, PM and NF in the updated review), independently screened all titles and abstracts identified by the searches for relevance. We only excluded papers that were clearly irrelevant at this stage. At stage 2, two review authors (PM and BL in the original review, PM and NF in the updated review) independently screened the full text, identified relevant studies and assessed eligibility of studies for inclusion, resolving any disagreements by discussion.
Data extraction and management
The same review authors extracted the following types of data.
Study characteristics, including place of publication, date of publication, population characteristics, setting, and detailed nature of intervention, comparator and outcomes. A key purpose of these data is to define unexpected clinical heterogeneity in included studies independently from analysis of results.
Results of included studies with respect to each of the main outcomes indicated in the review question, including data on outcomes not considered and assessing the possibility of selective reporting of results for particular outcomes.
For the original systematic review, we based our judgement regarding the quality of included studies on explicit criteria used by the Cochrane Effective Practice and Organisation of Care Review Group (EPOC) (http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf). For the update, we assessed the risk of bias (see below). We resolved any disagreements when extracting data or assessing their quality by discussion. If reports provided inadequate information, we contacted authors for further information.
Assessment of risk of bias in included studies
Two authors (PB and NF) independently assessed every trial using a simple form following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as all included studies prospectively compared interventions with control populations. In view of this, we assessed the following domains as having a high, low or unclear risk of bias.
Sequence generation.
Allocation concealment.
Blinding (of participants, personnel and outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
In addition, we conducted an overall 'Risk of bias' assessment based on the information required to assess the above.
Assessment of heterogeneity
We assessed clinical heterogeneity between studies by reviewing the differences across trials. There was considerable clinical heterogeneity in the trials, so we did not consider it appropriate to run any meta‐analyses. Had we combined the results of any trials in a meta‐analysis, we would have investigated heterogeneity with an I2 test. If the results had shown heterogeneity, we would have investigated the cause.
Data synthesis
If studies had been of a suitable quality and sufficiently homogeneous to combine in a meta‐analysis, we would have used (standardised) mean differences for continuous variables and relative risks (including Mantel Haenzsel analysis) for dichotomous variables, using either a random‐effects or fixed‐effect model. For future updates of this review, if the data allows, we will consider sensitivity analyses based on the risk of bias.
Results
Description of studies
Results of the search
In the original review, initial searches identified over 4000 papers, including duplicates. Of 29 potentially eligible studies, we finally included 16 trials that evaluated 14 different interventions (Adamolekun 1999; Davis 2004; Gilliam 2004; Helde 2005; Helgeson 1990; May 2002; McAuley 2001; Mills 1999a; Mills 1999b; Morrow 1990; Peterson 1984; Ridsdale 1997; Ridsdale 1999; Ridsdale 2000; Thapar 2002; Warren 1998) (Figure 1). The updated searches yielded 2438 additional papers including duplicates, two of which we included (Aliasgharpour 2013; DiIorio 2011) (Figure 2). Hence, the updated review includes 18 different studies of 16 separate interventions.
1.
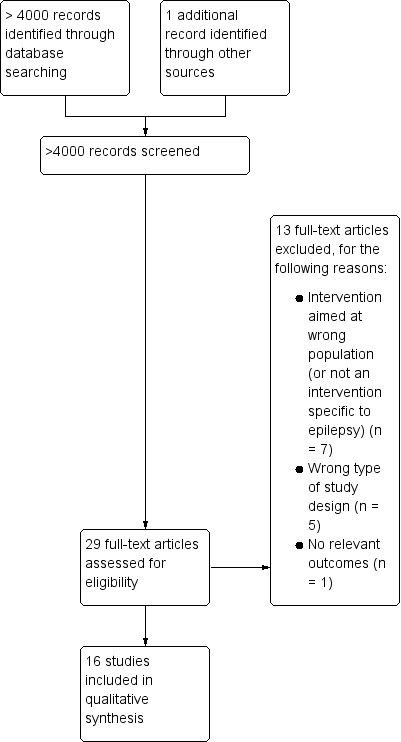
Study flow diagram (original searches).
2.
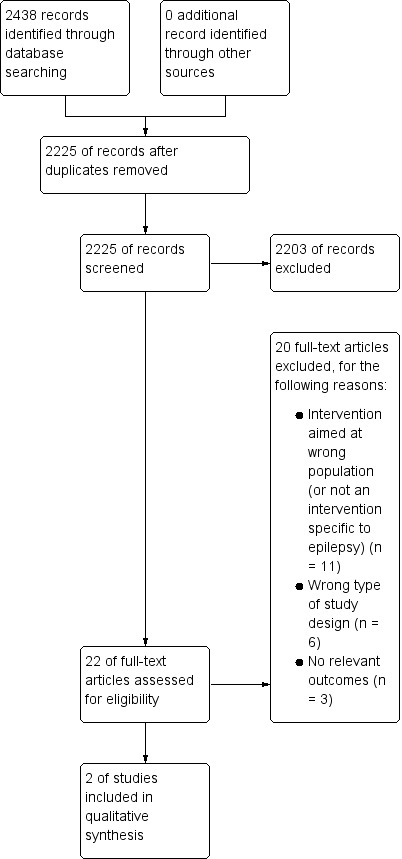
Study flow diagram (updated searches).
Included studies
While all the included studies investigated specialist care, the exact nature of this care varied between the studies. We therefore found it helpful to classify the included studies according to the type of specialist care under investigation. This produced a classification of seven intervention types.
Self management education.
Strategies to improve patient compliance.
Self management through screening.
Alternative models of outpatient care delivery.
Specialist nurse practitioners.
Behavioural interventions.
Guideline implementation and patient intervention.
We summarise information about each individual intervention in Appendix 7.
Self management education
Four trials evaluated the effect of self management education in adults with epilepsy (Aliasgharpour 2013; DiIorio 2011; Helgeson 1990; May 2002). Helgeson 1990 recruited participants from among those insured by Kaiser Permanente in California. May 2002 took place in 22 epilepsy centres across Germany, Austria and Switzerland. DiIorio 2011 was an online epilepsy self management programme to assist people with taking medication, managing stress and improving sleep quality in Atlanta, USA. Finally, Aliasgharpour 2013 evaluated an educational programme to improve self management in Zanjan, Iran.
Helgeson 1990 evaluated a two‐day psycho‐educational treatment programme (Sepulveda Epilepsy Education, also known as the Seizures and Epilepsy Education programme, or SEE) in 38 adults with epilepsy who were also prescribed antiepileptic drugs (AEDs). Participants were randomly assigned to either the SEE programme (n = 20) or to a waiting list control group (n = 23). Participants completed questionnaires before the programme and four months after completion. Investigators then invited waiting list control group members to attend the programme at four months. Questionnaires included questions about anxiety and depression, seizures, coping with epilepsy and self efficacy.
May 2002 evaluated a two‐day educational programme (the Modular Service Package Epilepsy, or MOSES) in adults with epilepsy. Two hundred forty‐two participants were randomly assigned to the MOSES programme (n = 113) or to a waiting list control group (n = 129). Participants completed questionnaires before the programme and six months after completion of the programme. Investigators then invited waiting list control group members to attend the MOSES programme at six months. Questionnaires included measures of knowledge, coping with epilepsy, seizure frequency, contentedness with AED therapy, depressive mood and an evaluation of MOSES.
DiIorio 2011 evaluated a six‐week WebEase programme, in 192 participants who voluntarily enrolled to participate after obtaining information about the study, either from healthcare professionals, online clinical research matching services, family, friends or online epilepsy and research sites. Following completion of a baseline assessment, only the first participant who enrolled to the programme was randomly assigned. Thereafter participants were allocated alternatively to either the intervention (WebEase) (n = 96) or a waiting list control group (n = 96). After six weeks (when the intervention group had completed WebEase), those in the waiting list control began the programme as well. Participants completed three questionnaire assessments, at baseline, 6 weeks (when only the intervention group had completed WebEase), and 12 weeks (when both groups had completed WebEase). At each assessment, investigators assessed measures of medication adherence, stress, sleep quality, self management, self efficacy, knowledge, and quality of life. All participants received a gift voucher for an online retailer at the end of their participation in the study.
Aliasgharpour 2013 evaluated an educational programme with the aim of increasing patient self management. The programme consisted of four sessions over one month to groups of four to six participants. In total, 66 participants were randomised to either the educational programme (n = 33) or to a control group (n = 33) who received the usual epilepsy care and support offered by the clinic. The control group also received two brief courtesy telephone calls as a control for attention. Investigators carried out assessments via questionnaire at baseline and at one month. Questionnaires included general measures of demographic details and of disease (i.e. type of convulsions, seizure frequency, time since the last seizure and the number of antiepileptic drugs taken). Trialists measured self management using the Epilepsy Self Management Scale (ESMS).
Strategies to improve patient compliance
Three trials evaluated the effect of strategies to improve patient compliance (Adamolekun 1999; Peterson 1984; Thapar 2002). One recruited participants from general practices in the United Kingdom (Thapar 2002), another from outpatients attending an Australian hospital clinic (Peterson 1984) and the third from the population of the Zvimba health district in rural Zimbabwe (Adamolekun 1999).
A three‐arm cluster‐randomised trial based in general practices in Greater Manchester, England, Thapar 2002 studied the impact of a 'prompt and reminder card' on the care of people with epilepsy. The study included 1275 participants from 82 practices, stratified according to size then allocated to one of three groups using a random number table. Intervention group 1 (n = 368) gave participants the responsibility of keeping the cards (patient‐held card group), and intervention group 2 (n = 515) had the cards placed into patients' records at the practice (doctor‐held card group), while the control group (n = 392) did not use cards.
In their study of outpatients attending a hospital clinic in Hobart, Australia, Peterson 1984 used a range of strategies to improve patient compliance with anticonvulsant therapy. Fifty‐three adults aged between 18 and 74 years entered the trial. Subjects were allocated by coin toss to the control group receiving usual care (n = 26) or the intervention group receiving a package of strategies to improve compliance (n = 27). Outcome measures focused on patient compliance as measured by plasma anticonvulsant levels, prescription refill frequency and appointment keeping.
Adamolekun 1999 evaluated the impact of healthcare worker and patient education on care in their study of epilepsy in rural Zimbabwe. As the team did not establish a control group for the first part of the study on health worker education, we excluded this part from this review. We included the second part of the project: studying the impact of information pamphlets on patient management in 400 participants. Health facilities (a district hospital, a mine hospital, 3 rural hospitals and 20 rural health centres) were randomised to one of two groups. The intervention group received patient information pamphlets for distribution to patients with epilepsy and their relatives at clinic visits. Control facilities did not receive the pamphlets. Impact was measured at six months after receipt of the information, by between‐group comparisons of clinic attendance, seizure frequency and mean serum drug levels.
Self management through screening
One trial based in a university hospital in the USA evaluated the effect of physicians' use of a risk profile (the Adverse Effects Profile, or AEP) on adverse effects of antiepilepsy drugs and on participants' reported subjective health status (Gilliam 2004). Trialists recruited participants attending an epilepsy clinic if their scores on the AEP were 45 or more. In total, 62 adults with epilepsy participated. The AEPs of participants randomised to the intervention group (n = 32) were available to their physicians, while the control group's (n = 30) physicians did not have access. At the end of the four‐month trial, investigators re‐assessed participants' AEPs as well as the changes in seizure rates, and each subject completed the Quality of Life in Epilepsy Inventory (QOLIE‐89) questionnaire.
Alternative care delivery in outpatient clinics
Prior to 1984, there was no specialist unit for epilepsy patients in Cardiff and South Wales, UK so epilepsy patients would most likely be referred to neurology. Morrow 1990 therefore undertook a randomised controlled trial to evaluate the outpatient activities of a specialist epilepsy unit. Individuals referred to hospital with confirmed or suspected epilepsy were submitted for randomisation to the Epilepsy Unit or to a standard neurology clinic. Because the referring physician did not always grant permission for randomisation, the study recruited 64 non‐randomised and 232 randomised individuals. We have therefore treated the study as a controlled before‐and‐after study (intervention, n = 130; control, n = 102) rather than a randomised trial. Outcome assessors evaluated participants at 3, 6 and 12 months. Outcome measures were seizure control, antiepileptic medication, use of other health resources (such as GP consultations), receipt of advice and counselling, patient satisfaction and the Hospital Anxiety and Depression Scale (HAD).
Specialist nurse practitioners
Seven studies reporting on five mutually exclusive study populations evaluated the effects of specialist nurse practitioners (Helde 2005; Mills 1999a; Mills 1999b; Ridsdale 1997; Ridsdale 1999; Ridsdale 2000; Warren 1998). Six studies took place in the UK, four in patients of general practices in southeast England (Mills 1999a; Mills 1999b; Ridsdale 1997; Ridsdale 1999), one in hospitals based in the same region (Ridsdale 2000) and one in a regional epilepsy clinic in northern England (Warren 1998). The remaining study took place in a neurology clinic in Norway (Helde 2005).
Mills 1999a studied the effect of a primary care‐based epilepsy nurse from the perspective of patients in 14 general practices in southeast England. Practices were allocated to either intervention or control to ensure similar distributions of size, doctor:patient ratio, socioeconomic status and mean distance from the local general hospital. The study had 574 participants aged 16 years or over with epilepsy (intervention, n = 278; control, n = 296). Intervention group members received information, advice and support from the epilepsy nurse, who also liaised with other professionals and provided education for staff. Participants filled in a self completion questionnaire based on the Living With Epilepsy survey instrument at baseline and after one year. Outcome measures included seizure frequency, AED use, information provision and attitudes to care. Secondary measures included patient preferences and the effect of epilepsy and treatment on everyday life.
Following the completion of the Mills 1999a study, during the second year, the specialist epilepsy nurse worked with participants who had been in the original control group of seven GP practices. Mills 1999b reported on follow‐up of 394 participants after two years, comparing participants who had accessed the specialist epilepsy nurse (n = 195) with those who had not (n = 194), regardless of their original group allocation. The same self completion questionnaire used at the end of year one was sent out again at the end of year two. Two hundred forty participants responded to both baseline and year two questionnaires: 60.9% of baseline respondents and 40.3% of the 595 participants with epilepsy in the 14 practices at the start of the trial.
Two papers from the UK based Epilepsy Care Evaluation Group reported outcomes from a trial based in six general practices in southern England (Ridsdale 1997;Ridsdale 1999). Two hundred fifty‐one adults with epilepsy (aged 17 to 90) were randomised either to specialist nurse based in general practice (n = 127) or usual care (n = 124). Criteria for exclusion were other severe illness (e.g. terminal cancer), severe psychological illness (e.g. active psychosis or severe depression) and low IQ (i.e. associated with learning disability or dementia). Ridsdale 1997 reported on knowledge of epilepsy, depression and anxiety scores at six months, which they assessed using validated questionnaires before and after the intervention. Ridsdale 1999 reported on patient attendance rate, nurse perception of appropriateness of medical management, and patients' perceptions of level of advice they had received on epilepsy at six months.
A third paper by Ridsdale 2000 reported on nurse specialists in the hospital‐based care of people with newly diagnosed epilepsy. This trial recruited individuals aged 17 or over from the neurology clinics of five hospitals in southeast England. The intervention matched that of the earlier trials (Ridsdale 1997; Ridsdale 1999), but the study was in the hospital setting, with a specialist epilepsy nurse giving two consultations, three months apart. People with learning disability were again excluded. One hundred two participants were randomised to the intervention (n = 54) or usual care (n = 48). Like Ridsdale 1997, the 2000 study measured knowledge of epilepsy, depression and anxiety scores at six months, assessed by validated questionnaires before and after the intervention.
Warren 1998 evaluated an epilepsy nurse specialist case manager who worked in a regional epilepsy clinic in northern England. The nurse complemented the work of the clinic doctors and replaced them in some aspects of care. Warren 1998 recruited 322 people with epilepsy, aged 16 or over, and then randomised them to the intervention (n = 154) or standard care (n = 168). The sample of participants included patients with learning disabilities, and the study authors stated that they excluded 20 for being unable to complete questionnaires; however, in 19 of these instances, their caregiver completed the questionnaire instead. The caregivers of 248 other participants with epilepsy also completed questionnaires. Warren 1998 reported on a wide range of outcomes, including: seizure frequency; anxiety and depression; impact of epilepsy (functioning); knowledge of epilepsy scores; impact on medical management; psychosocial outcomes for patients and caregivers; patient and general practitioner satisfaction with clinic care; use of other hospital services at six months; and costs of treatment.
Helde 2005 recruited 114 adults with epilepsy who attended a neurology clinic at a hospital in Trondheim, Norway into their randomised controlled trial. Using computer‐generated block randomisation, the trial allocated participants to either the intervention group (n = 58), which received counselling and teaching from a specialist epilepsy nurse, or to the control group (n = 56), which continued to receive standard care. Investigators measured primary outcomes using the QOLIE‐89, which they administered two years after recruitment to the trial. In addition, three months after this, each participant gave the clinic a general satisfaction rating by completing a Visual Analogue Scale.
Behavioural interventions
McAuley 2001 evaluated the impact of a structured exercise programme on behavioural and clinical outcomes in a group of adults with epilepsy in Ohio, USA. Twenty‐eight participants aged 16 to 60 years participated in the study, but authors did not describe the source of these participants or recruitment methods. Subjects were randomised to the intervention group (n = 17) or to a control group (n = 11), which received no additional exercise. Trialists conducted baseline physiological evaluations prior to the commencement of the exercise programme, including body composition, maximum oxygen consumption, strength and cardiovascular endurance. They also assessed seizure frequency over the previous four weeks, and monthly after baseline up to 12 weeks, by review of the patients' seizure calendars. All participants also provided AED concentrations (via blood test) and completed the QOLIE‐89 at baseline and 12 weeks.
Guideline implementation and patient information
In primary care settings in Tayside, Scotland, UK, Davis 2004 carried out a three‐arm randomised controlled trial of the use of epilepsy guidelines by general practitioners. General practitioners from 68 general practices were randomised to an intensive intervention (24 practices), an intermediate intervention (22 practices) or control (22 practices). A copy of a nationally developed clinical guideline was posted in all practices. The intermediate intervention group also received interactive, accredited workshops, and dedicated, structured protocol documents. The intensive intervention group received all the elements of the other two arms with the addition of a nurse specialist who supported and educated practices in the establishment of epilepsy review clinics. The primary patient outcome measure was the 36‐item Short Form Health Survey (SF‐36), a general quality of life instrument. Secondary patient outcome measures were epilepsy specific, including the nature and perceived severity of seizures, perceived adverse drug effects, the impact of epilepsy on participants' lives, and their sense of mastery. The study also used the Epilepsy Surgery Inventory 55 Survey (ESI‐55), a cognitive function test. Investigators measured all patient outcomes from completed questionnaires. In total, 3284 participants received a questionnaire, and 1133 entered the study by completing a baseline questionnaire, a response rate of 56%. Of these 1133, 399 participants were in the intensive intervention group, 364 in the intermediate intervention group and 370 in the control group.
Excluded studies
We summarise the characteristics of excluded studies in Characteristics of excluded studies. Three studies assessed interventions that were not specific to epilepsy but were rather generic psychological or mindfulness techniques applied to the epilepsy population (Lundgren 2006; Lundgren 2008; Pramuka 2007). DiIorio 2009 was a feasibility study of an epilepsy self management intervention by telephone, and we excluded it primarily because, as noted by the authors of this study, "the design of the study was not developed to test the efficacy of the intervention". However, the authors later adapted the programme for the Internet (WebEase), and we included the report on that study in the review (DiIorio 2011).
Risk of bias in included studies
We only judged three studies to be at low overall risk of bias: two studies of specialist nurse practitioners (Aliasgharpour 2013; Helde 2005), and one study of self management education (Warren 1998). We considered six studies to be at high risk of bias: one of the four studies of self management education (Helgeson 1990); one of the three studies to improve patient compliance (Adamolekun 1999); two of the seven studies of specialist nurse practitioners (Mills 1999a; Mills 1999b); the sole study of behavioural interventions (McAuley 2001); and the study of alternative care delivery in outpatient clinics (Morrow 1990). We deemed the remaining nine studies to be have an unclear risk overall: two of the four studies of self management education (DiIorio 2011;May 2002); two studies of strategies to improve patient compliance (Peterson 1984; Thapar 2002); the only study of self management through screening (Gilliam 2004); three of the seven studies of specialist nurse practitioners (Ridsdale 1997;Ridsdale 1999; Ridsdale 2000); and the only study of guideline implementation and patient information (Davis 2004). We detail the assessments for each study in the Characteristics of included studies section and summarise them in Figure 3 and Figure 4 .
3.
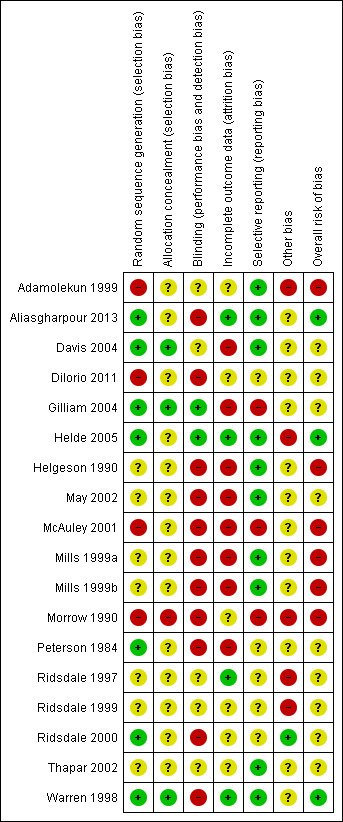
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
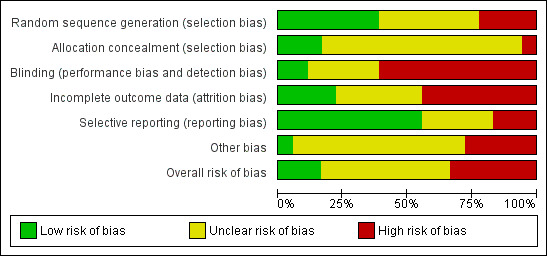
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We considered the risk of bias for random sequence generation to be unclear for six studies due to a lack of information (Helgeson 1990, May 2002, Mills 1999a, Mills 1999b, Ridsdale 1997, Ridsdale 1999). We also considered Thapar 2002 to have an unclear risk because on the one hand, there were a much higher number of participants in the doctor‐held card group (n = 515) than either the patient‐held card group (n = 368) or the control group (n = 392), which could indicate that the randomisation failed (and carried a high risk of bias). On the other hand, given the cluster‐randomisation design, the imbalance could equally have indicated that there were a greater number of larger sized general practices (in terms of patient numbers as opposed to numbers of general practitioners) in this group, making the overall risk unclear.
We considered the risk of bias for random sequence generation to be low in seven studies because the process appeared to be methodologically sound (Aliasgharpour 2013, Davis 2004, Gilliam 2004, Helde 2005, Peterson 1984, Ridsdale 2000, Warren 1998). We judged the other four studies to be at high risk of bias: Adamolekun 1999 because it was unclear if the intervention and control sites were determined by randomisation or convenience; DiIorio 2011 because it consecutively assigned participants to intervention and control groups; McAuley 2001 because it did not provide details of randomisation, and the numbers of participants between arms were imbalanced (17 in exercise group and 11 in control), suggesting randomisation may have failed; and Morrow 1990 because only 78% of participants were successfully randomised, since both the referring physician and the consultant to whom the subject was referred had to agree that the arm to which the subject was randomised was appropriate.
Allocation concealment
There was a lack of information about treatment allocation in 14 studies (Adamolekun 1999;Aliasgharpour 2013;DiIorio 2011;Helde 2005;Helgeson 1990;May 2002;McAuley 2001;Mills 1999a;Mills 1999b;Peterson 1984;Ridsdale 1997;Ridsdale 1999;Ridsdale 2000;Thapar 2002), so we judged these studies to carry an unclear risk of bias for allocation concealment. We considered the majority of the studies where there was adequate information (n = 3) to be at low risk of bias (Davis 2004;Gilliam 2004;Warren 1998), with only one study considered to be at high risk of bias because there was considerable variation in the size of the intervention and comparison arms and because this was clearly caused by failed randomisation (Morrow 1990). Two other studies (McAuley 2001; Thapar 2002) with unclear risk of bias also had imbalances in the size of treatment arms, which may have been due to a lack of randomisation.
Blinding
Blinding was rare across the studies. Only Gilliam 2004 was double blind in that clinicians and participants were both blinded. Helde 2005 blinded neither clinicians nor participants, but independent research assistants, blinded to treatment allocation, conducted (and presumably analysed) the interviews. Thus, we considered these two studies to be at low risk of bias. We judged 11 studies to be at high risk of bias because of a lack of blinding (Aliasgharpour 2013;DiIorio 2011;Helgeson 1990;May 2002;McAuley 2001;Mills 1999a;Mills 1999b;Morrow 1990;Peterson 1984; Ridsdale 2000;Warren 1998), and 5 studies to be at unclear risk due to a lack of information (Adamolekun 1999;Davis 2004;Ridsdale 1997;Ridsdale 1999;Thapar 2002).
Incomplete outcome data
Overall, dropout rates across the studies were high, and we considered eight studies to be at high risk of attrition bias (Davis 2004;Gilliam 2004;Helgeson 1990;May 2002;McAuley 2001;Mills 1999a;Mills 1999b;Peterson 1984). We considered the risk of bias to be low in four studies (Aliasgharpour 2013;Helde 2005;Ridsdale 1997;Warren 1998) and unclear in a further six studies (Adamolekun 1999; DiIorio 2011; Morrow 1990; Ridsdale 1999; Ridsdale 2000; Thapar 2002). In Ridsdale 1999 22% of participants did not respond at the end of the study. While those who responded did not differ to the non‐responders with respect to key baseline characteristics, it is still unclear if bias could have been introduced. In Ridsdale 2000 dropout was relatively low, but participants lost to follow‐up were significantly younger and at baseline reported not having had a recent epileptic attack, so it was unclear as to the extent, if any, of the risk of bias. In DiIorio 2011 we judged the risk of bias to be unclear because whereas the dropout rate was 24%, investigators did conduct a completer versus non‐completer analysis and an intention‐to‐treat analysis. In Thapar 2002, we considered the risk of bias to be unclear because data from medical records were available for almost all of the enrolled participants (92%), but questionnaires were available for fewer of them (74%). There was a lack of relevant information about dropout rates in Adamolekun 1999 and Morrow 1990, so we assessed the risk of bias to be unclear.
Selective reporting
The majority of studies appeared to report all of the outcomes they planned to. Hence for ten studies (Adamolekun 1999;Aliasgharpour 2013; Davis 2004;Helde 2005;Helgeson 1990;May 2002;Mills 1999a;Mills 1999b;Thapar 2002;Warren 1998), the risk of bias was low. We considered the risk of bias to be high for three studies (Gilliam 2004;McAuley 2001;Morrow 1990). This was because certain outcomes referred to in the Methods were not reported by Adamolekun 1999 and Morrow 1990; in Gilliam 2004 the opposite was the case—outcomes not referred to in the Methods were reported in the Results. In McAuley 2001, although authors stated the study lasted 12 weeks, they reported the outcome measuring physical self concept and self esteem at 16 weeks with no explanation as to why this was the case. Information about selective reporting was insufficient for four studies (Peterson 1984;Ridsdale 1997;Ridsdale 1999;Ridsdale 2000) and hence the risk of bias in these studies was considered to be unclear. The risk of bias was also deemed to be unclear for DiIorio 2011 because while all outcomes detailed in the methods were referred to in the results, not all values were presented for these analyses.
Other potential sources of bias
Most of the studies had other potential risks of bias. The most common reason resulting in high or unclear risk of bias was lack of reporting on power calculations and required sample size. This occurred in 12 studies (Adamolekun 1999;DiIorio 2011;Gilliam 2004;Helde 2005;Helgeson 1990;May 2002;McAuley 2001;Mills 1999b;Morrow 1990;Peterson 1984;Ridsdale 1997;Ridsdale 1999). Davis 2004 and Thapar 2002 did report power calculations and the required sample size, although the numbers of participants in each group fell short of that target. On the other hand, Warren 1998 reported a required sample size, but it was not clear if this was the result of a power calculation. For the most part, where reported, no differences in baseline characteristics were apparent, exceptions being between treatment arms in four studies (Aliasgharpour 2013, Helde 2005, Mills 1999a, Mills 1999b) and between randomised and non‐randomised participants in Morrow 1990. Nevertheless, the potential risk of bias was deemed unclear due to these uncertainties. Other potential biases that resulted in studies being deemed at high risk of bias were present in Adamolekun 1999;Helgeson 1990;Morrow 1990;Ridsdale 1997 and Ridsdale 1999. In Adamolekun 1999 it was unclear if pre and postintervention periods for study and control sites were the same and if control sites were comparable with respect to health system, level of care, setting of care and educational level among participants. Statistical methods did not account for outcomes that may have varied according to the individual clinics. We also considered that there was a possibility of contamination in this study, as patient information could easily have been distributed to control sites. Helgeson 1990 reported no details of power calculations or required sample size. Furthermore, the intervention group completed the pre‐assessment questionnaire immediately before participating in the programme, whereas the control group participants were sent the questionnaire by post one week earlier. Similarly, Morrow 1990, did not report the power calculations or the required sample size, and there were also significant differences at baseline between participants who were randomised and not randomised. Ridsdale 1997 and Ridsdale 1999 also failed to report power calculations and sample size, and in addition, participants in the intervention group were told that they would attend a 'neurology clinic', which they may have interpreted as specialist care. This belief may have potentially improved participant outcomes over and above the effects of the intervention from the epilepsy nurse specialist.
Effects of interventions
The presentation of results varied considerably between trials and we have been unable to report statistics in an optimal way because of the limitations of the data presented. We considered reporting all continuous outcomes as mean difference (MD), but several trials had baseline measures which would require imputing pre‐post correlation. Moreover, given that the populations, interventions, study design, treatment settings and outcome measures differed for each trial, we concluded that meta‐analysis of the results, even within the same type of outcome, would be inappropriate. We have therefore presented the results of the trials narratively. Thus, all results are presented as originally reported, with standard errors transformed to standard deviations. We have only presented the findings reported that could be considered to match the pre‐defined outcomes of our review. A simple descriptive summary of the results, highlighting where there were significant differences between groups over time, are presented in Table 1, Table 2, Table 3,Table 4, Table 5, Table 6 and Table 7.
1. Seizure frequency and severity.
| Study | Intervention type | Outcome(s) measured | Outcome time | Findings |
| Adamolekun 1999 | Patient compliance – information pamphlets | Seizure frequency per month | 6 months | No statistically significant difference between groups |
| Gilliam 2004 | Self management through screening ‐ Adverse Effects Profile | Seizure frequency per month | 4 months | No statistically significant difference between groups although seizure frequency decreased in intervention group and increased in control group |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Seizure frequency per month | 4 months | No statistically significant difference between groups |
| May 2002 | Self management education ‐ MOSES | Seizure frequency (as measured on a scale of 0 to 5, i.e. no seizures in past six months to one or more seizure per day) | 6 months | Statistically significant reduction in seizure frequency (improvements ≥2 points on seizure frequency scale) in favour of intervention vs control |
| McAuley 2001 | Behavioural intervention ‐ structured exercise programme | Seizure frequency from previous 4 weeks | 12 weeks | No apparent difference between groups; however, no formal statistical tests are reported |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | One or more seizure attacks in last year | 1 year | No statistically significant difference between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | One or more seizure attacks per month in last year | 1 year | No statistically significant difference between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | One or more seizure attacks in last year | 2 years | No statistically significant difference between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | One or more seizure attacks per month in last year | 2 years | No statistically significant difference between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Seizure frequency in the last three months | 3, 6, 12 months | Seizure frequency reduced to zero in intervention group by 12 months (statistically significant over time) and to one in control group (not statistically significant over time) |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Proportion of participants who were seizure free | 3, 6, 12 months | Differences between groups but were not statistically significant at 12 months (but favoured intervention at 3 and 6 months) |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Proportion of participants who experienced a 50% reduction in seizure activity from baseline | 3, 6, 12 months | Differences between groups but were not statistically significant at 12 months (but favoured intervention at 3 and 6 months) |
| Peterson 1984 | Patient compliance ‐ combination of compliance‐improving strategies | Median number of seizures in preceding 6 months | 6 months | Seizure frequency reduced significantly in the intervention group but not in the control group; not reported if differences between groups |
| Ridsdale 2000 | Specialist nurse practitioner ‐ hospital | Number of months since last seizure | 6 months | No statistically significant difference between groups |
| Thapar 2002 | Patient compliance ‐ prompt and reminder card | Recording of seizure frequency | 1 year | No statistically significant difference between the control and the doctor‐held groups and the control and the patient‐held groups |
| Thapar 2002 | Patient compliance ‐ prompt and reminder card | Self‐reported seizure frequency | 1 year | No statistically significant difference between the control and the doctor‐held groups and the control and the patient‐held groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Seizure frequency (more than one seizure per month, one or fewer seizures per month, or seizure‐free) | 6 months | No statistically significant difference between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
2. Appropriateness and volume of medication prescribed (including evidence of drug toxicity).
| Study | Intervention type | Outcome(s) measured | Outcome time | Findings |
| Adamolekun 1999 | Patient compliance – information pamphlets | Drug compliance | 6 months | No statistically significant difference between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Self reported medication adherence | 12 weeks | Statistically significant improvement in favour of intervention group |
| Gilliam 2004 | Self management through screening ‐ Adverse Effects Profile | AED dose changes | 4 months | Statistically significantly greater number of dose changes in intervention group compared with control group |
| Gilliam 2004 | Self management through screening ‐ Adverse Effects Profile | Adverse events profile relative improvement | 4 months | Mean improvement in adverse events profile scores was statistically significantly greater in intervention group vs control |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Hazardous medical self management practices subscale | 4 months | Statistically significant group‐time interaction effects in favour of intervention vs control |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Compliance (as measured by blood antiepileptic drug levels) | 4 months | In a subset of the study population, statistically significant increase in compliance in favour of intervention group |
| May 2002 | Self management education ‐ MOSES | Tolerability of antiepileptic drug treatment | 6 months | Statistically significant improvement in tolerability in favour of intervention group over time |
| McAuley 2001 | Behavioural intervention ‐ structured exercise programme | Variation in AED concentrations (measured by serum carbamazepine, phenytoin, and valproic acid concentrations, as applicable) | 12 weeks | No apparent differences between intervention and control groups; however, no formal statistical tests are reported |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Taking one type of antiepileptic drug | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Feel very well controlled by drug | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Report very important to take tablets exactly as prescribed | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Report never missing taking their antiepileptic drugs | 1 year | Statistically significantly in favour of intervention group |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Side effects from drugs (in past month) | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Taking one type of antiepileptic drug | 2 years | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Feel very well controlled by drug | 2 years | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Report very important to take tablets exactly as prescribed | 2 years | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Report never miss taking antiepileptic drugs | 2 years | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Side effects from drugs (in past month) | 2 years | No statistically significant differences between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Number and type of antiepileptic drugs or the number of drugs prescribed per patient | During study period | No statistically significant difference between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Reduction in the percentage of drug concentrations outside the reference range | 6 and 12 months | Statistically significant reduction in the percentage of drug concentrations outside the reference range in intervention vs control at both time points |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Adverse drug effects (ADRs) | 3, 6, 12 months | Statistically significant reduction in the percentage of ADRs in the intervention group at 6 and 12 months |
| Peterson 1984 | Patient compliance ‐ combination of compliance‐improving strategies | Compliance in terms of plasma level of antiepileptic drugs | 6 months | Statistically significant differences in mean plasma levels/dose for phenytoin and carbamazepine but not sodium valproate. Plasma levels of phenytoin, carbamazepine, and sodium valproate substantially increased within the intervention but not control group |
| Peterson 1984 | Patient compliance ‐ combination of compliance‐improving strategies | Prescription refill frequency | 6 months | Statistically significant in favour of intervention; over time, compliance increased in intervention group but not control group |
| Peterson 1984 | Patient compliance ‐ combination of compliance‐improving strategies | Clinic attendance | 6 months | No statistically significant difference between groups |
| Ridsdale 1997 | Specialist nurse practitioner ‐ general practice | Appropriateness of medication supplied | 6 months | 11.1% of intervention patients required changes; no data reported for control |
| Ridsdale 1997 | Specialist nurse practitioner ‐ general practice | Increase serum concentration monitoring | 6 months | Statistically significant increase in serum monitoring over time in intervention group compared with control group |
| Thapar 2002 | Patient compliance ‐ prompt and reminder cards | Proportion of patients taking only one antiepileptic drug (monotherapy) | 1 year | No statistically significant difference between either intervention group and control |
| Thapar 2002 | Patient compliance ‐ prompt and reminder cards | Checking of phenytoin levels | 1 year | No statistically significant difference between either intervention group and control |
| Thapar 2002 | Patient compliance ‐ prompt and reminder card | Side effects from medication | 1 year | Statistically significantly higher levels of side effects in doctor‐held card group vs control and patient‐held card group vs control group |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Self reported non‐compliance with medication | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Attendance at epilepsy clinic | 6 months | No statistically significant differences between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
AED: antiepileptic drug.
3. Patients’ reported knowledge of information and advice received from professionals.
| Study | Intervention type | Outcome(s) measured | Outcome time | Findings |
| Study | Intervention type | Outcome(s) measured | Outcome time | Findings |
| DiIorio 2011 | Self management education ‐ WebEase | Knowledge about epilepsy | 12 weeks | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Fear of death and brain damage due to seizures | 4 months | Statistically significant decrease in level of fear in favour of intervention vs control |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Extent of overall misinformation and misconceptions regarding epilepsy | 4 months | Statistically significant decrease in overall level of misinformation and misconceptions regarding epilepsy in favour of intervention vs control |
| May 2002 | Self management education ‐ MOSES | Epilepsy knowledge | 6 months | Statistically significant increase in level of knowledge in favour of intervention vs control over time |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Discussed epilepsy topics with GP | 1 year | Of 11 topics, patients in the intervention group were statistically significantly more likely to have discussed 4 topics with primary care staff and 2 topics with hospital staff |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Discussed epilepsy topics with GP | 2 years | Of 11 topics, patients in the intervention group were statistically significantly more likely to have discussed 8 topics with primary care staff and 2 topics with hospital doctors |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Number of information items offered to participants | 1 year | Groups were not compared with each other but there was an increase in number of items offered over time in intervention group but not control group |
| Ridsdale 1999 | Specialist nurse practitioner ‐ general practice | Knowledge of epilepsy | 6 months | No statistically significant differences between groups |
| Ridsdale 2000 | Specialist nurse practitioner ‐ general practice | Knowledge of epilepsy | 6 months | No statistically significant differences between groups although improved over time in intervention group |
| Ridsdale 2000 | Specialist nurse practitioner ‐ hospital | Advice provided on epilepsy‐related topics | 6 months | Of 9 topics, patients in the intervention group were statistically significantly more likely to have received enough advice on 8 topics with primary care staff |
| Thapar 2002 | Patient compliance ‐ prompt and reminder card | Information provision from professionals | 1 year | Participants in doctor‐held card group were statistically significantly less satisfied with information provision about epilepsy Compared with the control group but not in the patient‐held card group where there were no differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Medical knowledge of epilepsy | 6 months | Statistically significant difference in level of knowledge in favour of intervention |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Social knowledge of epilepsy | 6 months | No statistically significant differences between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
4. Patients’ reports of health and quality of life.
| Study | Intervention type | Outcome type | Outcome time | Findings |
| Study | Intervention type | Outcome type | Outcome time | Findings |
| Aliasgharpour 2013 | Self management education | Self management levels | 1 month | Statistically significantly higher levels of self management in intervention group vs control group; there were statistically significant differences over time in the intervention group but not control group |
| Davis 2004 | Guideline implementation and patient information | General quality of life (SF‐36) | 6 months to 1 year | No statistically significant differences between groups |
| Davis 2004 | Guideline implementation and patient information | Epilepsy specific quality of life | 6 months to 1 year | No statistically significant differences between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Perceived stress | 12 weeks | No statistically significant differences between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Sleep quality | 12 weeks | No statistically significant differences between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Self management | 12 weeks | No statistically significant differences between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Self efficacy | 12 weeks | No statistically significant differences between groups |
| DiIorio 2011 | Self management education ‐ WebEase | Quality of life | 12 weeks | No statistically significant differences between groups |
| Gilliam 2004 | Self management through screening ‐ Adverse Effects Profile | Quality of life in Epilepsy (QOLIE‐89) | 4 months | No statistically significant differences between groups |
| Helde 2005 | Specialist nurse practitioner ‐ neurology | Quality of life in Epilepsy (QOLIE‐89) | 2 years | No statistically significant differences between groups overall but some statistically significant improvements reported for individual domains over time in both groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Acceptance of disability | 4 months | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Depression | 4 months | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Anxiety | 4 months | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Self efficacy ‐ general | 4 months | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Self efficacy ‐ social | 4 months | No statistically significant differences between groups |
| Helgeson 1990 | Self management education ‐ Sepulveda Epilepsy Education | Overall psychosocial functioning | 4 months | No statistically significant differences between groups |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Coping with epilepsy | 6 months | Statistically significant increase in coping with epilepsy in intervention group vs control group over time |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Restriction in daily living | 6 months | Statistically significant decrease in restriction in daily living intervention group over time but no statistically significant differences between groups |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Mobility and leisure behaviour | 6 months | No statistically significant differences between groups but statistically significant improvement over time in intervention group |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Epilepsy‐related fear | 6 months | Statistically significant decrease in epilepsy related fear in intervention group over time but no statistically significant differences between groups |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Stigma | 6 months | No statistically significant differences between groups or over time |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | SF‐36 physical functioning | 6 months | No statistically significant differences between groups or over time |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | SF‐36 mental functioning | 6 months | No statistically significant differences between groups or over time |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Self esteem | 6 months | No statistically significant differences between groups or over time |
| May 2002 | Self management education ‐ Sepulveda Epilepsy Education | Depression | 6 months | No statistically significant differences between groups or over time |
| McAuley 2001 | Behavioural intervention ‐ structured exercise programme | Quality of life in Epilepsy (QOLIE‐89) | 12 weeks | Statistically significant improvement over time (overall) in intervention group and statistically significant improvements reported for individual domains over time in both groups; no formal statistical tests between groups are reported |
| McAuley 2001 | Behavioural intervention ‐ structured exercise programme | Mood State including tension, depression, anger, vigour and confusion | 12 weeks | Statistically significant improvement over time for vigour; no formal statistical tests between groups are reported at end of study but it is noted that there were statistically significant differences between groups at baseline |
| McAuley 2001 | behavioural intervention ‐ structured exercise programme | Self esteem | 12 weeks | No statistically significant differences over time in either group; no formal statistical tests between groups are reported |
| McAuley 2001 | Behavioural intervention ‐ structured exercise programme | Physical self concept and vigour | 12 and 16 weeks | Statistically significant difference over time in intervention group overall and for the following domains: physical activity, coordination, endurance and strength; no formal statistical tests between groups are reported |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | 10 questions about quality of life | 1 year | Intervention group statistically significantly more likely to report an effect for three items: Epilepsy affects future plans and ambitions, Epilepsy affects overall health, Epilepsy affects standard of living |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Feel stigmatised due to epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Feel unhappy about life as a whole | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | 10 questions about quality of life | 2 years | Intervention group statistically significantly more likely to report an effect for three items: Epilepsy impacts on overall health, the way individuals feel about themselves and the impact of epilepsy on their social life/activities |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Feel stigmatised due to epilepsy | 2 years | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Feel unhappy about life as a whole | 2 years | No statistically significant differences between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Hospital Anxiety and Depression Scale | 12 months | Groups were not compared but no statistically significant change over time in either group was reported |
| Ridsdale 1999 | Specialist nurse practitioner ‐ general practice | Depression | 6 months | No statistically significant differences between groups in patients who had had a seizure but statistically significantly reduced risk of depression in patients reporting no seizures |
| Ridsdale 2000 | Specialist nurse practitioner ‐ hospital | Anxiety | 6 months | No statistically significant differences between groups |
| Ridsdale 2000 | Specialist nurse practitioner ‐ hospital | Depression | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Self rated health status (quality of life) as measured by EuroQoL | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Weighted health status (quality of life) as measured by EuroQoL | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Social functioning | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Social outcomes | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Anxiety | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Depression | 6 months | No statistically significant differences between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
SF‐36: 36‐item Short Form Health Survey; QOLIE‐89: Quality of Life in Epilepsy Inventory.
5. Objective measures of general health status.
| Study | Intervention type | Outcome type | Outcome time | Findings |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Long‐term health problems | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Injury as a result of epilepsy attack (in past year) | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Injury as a result of epilepsy attack (in past year) | 2 years | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Injuries from seizures | 6 months | No statistically significant differences between groups |
Note: Presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
6. Objective measures of social or psychological functioning (including the number of days spent on sick leave/absent from school and work, and employment status).
| Study | Intervention type | Outcome type | Outcome time | Findings |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Social activities | 1 year | No statistically significant differences between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Employment status | 1 year | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Number of days absent from work | 6 months | No statistically significant differences between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
7. Costs of care or treatment.
| Study | Intervention type | Outcome type | Outcome time | Findings |
| Adamolekun 1999 | Patient compliance – information pamphlets | Patient non‐attendance at clinic | 6 months | No statistically significant difference between groups in magnitude of the change in attendance |
| Gilliam 2004 | Self management through screening ‐ Adverse Effects Profile | Mean number of clinic visits | 4 months | Significantly greater number of clinic visits were recorded by intervention group vs control group |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Seen GP for any reason | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Seen GP for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Seen hospital doctor for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Admitted to hospital for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Attended A&E department for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999a | Specialist nurse practitioner ‐ general practice | Regular arrangement to see GP for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Seen GP for any reason | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Seen GP for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Seen hospital doctor for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Admitted to hospital for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Attended A&E department for epilepsy | 1 year | No statistically significant differences between groups |
| Mills 1999b | Specialist nurse practitioner ‐ general practice | Regular arrangement to see GP for epilepsy | 1 year | No statistically significant differences between groups |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Number of outpatient clinic visits | 1 year | Numerically there were a greater number of visits to the epilepsy clinic than to the neurology clinic, but groups were not formally compared with each other |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Visits to outpatient clinic doctor | 1 year | Numerically there were a greater number of visits to the clinic doctor in the specialist unit than in the neurology clinic, but groups were not formally compared with each other |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | GP consultations | 1 year | Numerically the number of GP consultations by the neurology clinic patients was higher than the epilepsy clinic patients, but groups were not formally compared with each other |
| Morrow 1990 | Alternative care delivery in outpatient clinics – specialist epilepsy unit | Inpatient days | 1 year | Numerically the number of inpatients days by the neurology clinic patients was higher than the epilepsy clinic patients, but groups were not formally compared with each other |
| Peterson 1984 | Patient compliance ‐ combination of compliance‐improving strategies | Clinic appointment keeping | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | ≥ 1 GP consultations | 6 months | A smaller proportion of intervention patients saw their GP once or more than did control patients but this difference was not statistically significant; |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Number of GP consultations | 6 months | Intervention patients had statistically significantly fewer consultations than the control group |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visits to general practice nurse | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visits made by district nurse | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visits made by health visitor | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visits made by CPN | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visits to outpatient clinic doctor | 6 months | Intervention patients made statistically significantly fewer visits to the outpatient clinic doctor than did control patients |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Specialist outpatient clinic psychiatrist consultation | 6 months | Numerically intervention patients made more visits to outpatients clinics than did patients to the control group but groups were not formally compared with each other |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Specialist outpatient clinical psychologist consultation | 6 months | Numerically intervention patients made more visits to outpatients clinics than did patients to the control group but groups were not formally compared with each other |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Specialist inpatient admission | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | EEG | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | CT scan | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | MR scan | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Other outpatient consultation | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Other inpatient admission | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Other day‐patient visit | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Visit to A&E | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Primary healthcare cost per patient | 6 months | Primary care costs were statistically significantly reduced in intervention group |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Secondary healthcare cost per patient | 6 months | No statistically significant differences between groups |
| Warren 1998 | Specialist nurse practitioner ‐ regional epilepsy clinic | Total healthcare cost per patient | 6 months | No statistically significant differences between groups |
| Warren 19988 | Specialist nurse practitioner ‐ regional epilepsy clinic | Blood level estimation for antiepileptic drugs | 6 months | No statistically significant differences between groups |
Note: presented findings only include studies reporting on outcome of interest. All numerical data (including P values), where available, reported in text of report
A&E: accident and emergency department; CT: computed tomography; EEG: electroencephalogram; MR: magnetic resonance.
Seizure frequency and severity
See Table 1 for summarised results on seizure frequency and severity.
Self management education
In the evaluation of SEE (Helgeson 1990), seizure frequency (defined as average monthly seizure frequency during previous four months) decreased in both groups. At baseline, mean (standard deviation, SD) seizure frequency was 2.47 (SD 3.98) in the intervention group and 2.14 (SD 4.72) in the control, and after four months it was 2.32 (SD 4.01) and 2.05 (SD 4.73), respectively. However, there were no significant differences between groups over time (P = 0.129). All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
For MOSES (May 2002), seizure frequency (as measured on a scale of 0 to 5, i.e. no seizures in past six months to one or more seizure per day) improved significantly between groups over time (P = 0.041). At six months 19% of the intervention group improved two or more points on the seizure frequency scale, compared to 7.2% of the control group. Seizure frequency deteriorated in 4.8% of the control group (two or more points on the scale) compared to 1.8% of the MOSES group. The percentage of people with zero to two seizures in the previous six months increased in the MOSES group from 35.4% to 50.4% (+ 15.0%) and in the control group from 38.7% to 45.8%, (+ 7.1%). The percentage of people with a high seizure frequency (weekly or daily seizures in the past six months) decreased in the intervention group from 24.7% to 18.6% (− 6.1%); and in the control group from 17.9% to 15.6% (− 2.3%).
DiIorio 2011 did not report seizure frequency and severity for WebEase, nor did Aliasgharpour 2013 for the educational programme on self management.
Strategies to improve patient compliance
In an evaluation of the combination of compliance‐improving strategies (Peterson 1984), seizure frequency was defined as median seizure frequency during the previous six months. Investigators observed a significant reduction in seizures in the intervention group (median 6 seizures at baseline and 2.5 at six months follow‐up, P < 0.01) but not in the control group (median 4 seizures at baseline, 3.5 at six months, P > 0.10). However, investigators did not report if significant differences occurred between groups. Investigators reported that the reduction of seizure levels in the intervention group correlated with each patient's increased plasma level/dose ratio (P < 0.01).
The evaluation of patient pamphlets by Adamolekun 1999 did not report baseline seizure frequency (defined as data on seizure frequency per month obtained from clinic epilepsy registers). However at the end of the study (six months), there were no differences in reported mean (SD) seizure frequency between groups (intervention 0.78 SD 2.03 vs control 0.38 SD 0.85, P = 0.8784). Interpretation of all results of this evaluation warrants caution because of the weak study design (see Risk of bias in included studies).
At one year, an evaluation of a prompt and reminder card showed significant differences in recording of seizure frequency (defined as seizure frequency recorded in medical notes in the previous year) in doctor‐held card practices (57.4% vs 42.8%, OR 1.82, 95% CI 1.23 to 2.69, P = 0.003), but not in patient‐held card practices (44.6% vs 42.8%, OR 1.16, 95% CI 0.76 to 1.77, P = 0.49) compared to the control group (Thapar 2002). There were no significant changes with the control group in the proportion of seizure‐free participants (defined as participants self reporting as seizure‐free in the previous year) in doctor‐held card practices (56.0% vs 51.5%, OR 1.33, 95% CI 0.83 to 2.13, P = 0.24), or in patient‐held card practices (58.1% vs 51.5%, OR 1.47, 95% CI 0.88 to 2.46, P = 0.38).
Self management through screening
The evaluation of effect of self management through screening for adults with epilepsy (Gilliam 2004) reported a decrease in seizure frequency (defined as average monthly seizure frequency during previous four months) in the intervention group (− 17.2%) and an increase in the usual care group (+ 5.6%). However there was no significant difference between groups (P = 0.71).
Alternative care delivery in outpatient clinics
At 12 months, an evaluation of a specialist epilepsy unit in hospital outpatients found no significant difference between groups in seizure frequency (as defined by any seizure in the last three months or the proportion of participants who were seizure‐free or who had experienced a 50% reduction in seizure activity from baseline) (Morrow 1990). Authors reported that there were a median of 0 seizures at 6 and 12 months in the intervention group and 1 seizure at 6 and 12 months in the control group. However, there were significant improvements over time in the intervention group (baseline median 3 seizures, P < 0.001) but not in the control group (baseline median 2 seizures, P > 0.05). Furthermore, while investigators did not report any significant between‐group differences at 12 months, they did report significant differences in the proportion of participants who were seizure‐free or who had experienced a 50% reduction in seizure activity from baseline at three months (P < 0.05) and six months (P < 0.01). They did not specify the precise proportion of participants at any time point. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
At six months, an evaluation of a hospital‐based epilepsy nurse, in a study involving a substantial minority of people with learning disability, considered seizure frequency (Warren 1998). Trialists asked participants to categorise the frequency of seizures in the previous six‐month interval as more than one seizure per month, one or fewer seizures per month, or seizure‐free. They did not find a difference between the two groups in the first six months after the intervention (P = 0.494).
At both one year (Mills 1999a) and two years (Mills 1999b), an evaluation of a primary care‐based specialist epilepsy nurse showed no significant changes between groups in seizure frequency, defined either as one or more epilepsy attacks in the previous year or one or more epilepsy attacks per month in the past year. In Mills 1999a, there was a slight increase in the intervention group over time (+ 0.7% and + 0.8%, respectively) and in the control group (+ 3.9% and + 0.8%, respectively); differences between arms were not statistically significant with regard to one or more epilepsy attacks in the previous year (P = 0.69) or one or more epilepsy attacks per month in the past year (P = 0.91). It is noticeable that the proportion of participants at baseline for both outcomes was lower in the intervention group (32.1% and 16.0%, respectively) than the control group (43.3% and 21.2%, respectively). Mills 1999b only reported the odds ratios between those who had accessed the specialist epilepsy nurse with those who had not (one or more epilepsy attacks in the previous year: OR 1.02, 95% CI 0.45 to 2.30, P = 0.97; and (one or more epilepsy attacks per month in the past year: OR 1.02, 95% CI 0.35 to 2.97, P = 0.98). All results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
An evaluation of a hospital‐based epilepsy nurse in outpatients found no significant difference in seizure frequency as measured by number of months since last seizure (Ridsdale 2000). In this study, at six months the median was 6.5 months in the intervention group and 4.9 months in the control group (P > 0.05).
The evaluations of an epilepsy nurse based in general practice did not report on seizure frequency (Ridsdale 1997, Ridsdale 1999), nor did the evaluation of a specialist nurse based in a neurology clinic (Helde 2005).
Behavioural interventions
At 12 weeks, an evaluation of an outpatient exercise programme showed no apparent difference in seizure frequency (defined as seizure frequency from previous four weeks by reviewing participant notes) (McAuley 2001) between intervention and control groups. However, these results should be interpreted with caution as only seven participants had active seizures at baseline, two participants were excluded because of increased seizure frequency, and no participants who were seizure‐free developed seizures during the trial.
Guideline implementation and patient information
The evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment did not report seizure frequency (Davis 2004).
Appropriateness and volume of medication prescribed (including evidence of drug toxicity)
See Table 2 for summarised results on appropriateness and volume of medication prescribed.
Self management education
At four months, an evaluation of the SEE programme showed significant differences between groups for hazardous medical self management practices as measured on a subscale of the SEE 50‐item questionnaire,between groups over time (P < 0.0001) (Helgeson 1990). The trial used a subgroup (n = 26) to evaluate the effect of SEE on compliance with antiepileptic drug (AED) treatment. The intervention group showed significantly increased compliance (as measured by blood AED levels) compared to the control group (percentage change score intervention + 70%, control − 18%, P < 0.05). Helgeson 1990 does not offer an explanation of how this subset was chosen, so these results should be interpreted with caution. Likewise, all results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
May 2002 also saw improvements in MOSES for the tolerability of AED treatment, as rated from 0 (no side effects) to 4 (severe side effects, not tolerable). At baseline the mean (SD) score in the intervention group was 2.20 (SD 0.86) compared to 2.03 (SD 0.85) in the control group, and at six months, the respective scores were 2.05 (SD 0.88) and 2.10 (SD 0.82). Authors reported the difference between groups over time to be statistically significant (P < 0.05).
In their evaluation of WebEase, DiIorio 2011 measured medication adherence using the Medication Adherence Scale (MAS), an eight‐item measure of self report medication‐taking behaviours. At 12 weeks, investigators observed a significant improvement in the WebEase group compared to the control group (P = 0.049).
The evaluation of the educational programme on self management by Aliasgharpour 2013 did not report on the appropriateness and volume of medication prescribed.
Strategies to improve patient compliance
At six months, an evaluation of the combination of compliance‐improving strategies reported increases in plasma levels for all AEDs, which resulted in significant differences for two of these AEDs (phenytoin and carbamazepine) at the end of the study (Peterson 1984). At six months, phenytoin mean (SD) plasma levels/dose were 9.9 (SD 3.2) in the intervention arm and 7.1 (SD 4.6) in the control arm (P < 0.05). Mean (SD) carbamazepine plasma levels/dose were 9.9 (SD 3.2) in the intervention arm and 7.1 (SD 4.6) in the control arm (P < 0.05). While mean (SD) sodium valproate plasma levels/dose did not differ between groups at 12 weeks (intervention 14.9 SD 2.7; control 20.2 SD 7.9 P > 0.1), levels were lower in the intervention group at baseline (P < 0.01). Hence plasma levels substantially increased within the intervention arm for phenytoin (P = 0.07), carbamazepine (P < 0.02) and sodium valproate (P < 0.02), but investigators did not identify significant increases in the control group (P > 0.2). The study also showed significant differences for prescription refill frequency (defined by dates set in participants' prescription record book) in the intervention group. Compliance increased from 48% to 88% (P < 0.01) in the intervention group, compared to a decrease of 58% to 50% in the control group (P > 0.10). At six months, the differences were significant between groups (P < 0.01). It was not possible from these results alone to judge whether the intervention was associated with clinical improvement, but there was a corresponding statistically significant decrease in seizure frequency. Investigators did not observe any significant changes in measures of clinic appointment keeping. However, they only reported the baseline measures (intervention 59% vs control 65%) while the median number of clinic appointments for both the intervention and control groups during the six‐month study period was 2.5.
There were no differences in antiepileptic drug compliance (defined by undetectable plasma phenobarbitone concentration) at six months between groups in the evaluation of a patient pamphlet for trained primary healthcare workers (Adamolekun 1999). Reported findings were 0% in the intervention group vs 5.3% in the control group. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
The evaluation of the prompt and reminder card by Thapar 2002 reported no significant differences in the proportion of participants taking only one antiepileptic drug during the intervention year (doctor‐held 69.7%, patient‐held 70.1%, control 71.1%) or checking of phenytoin levels (doctor‐held 28.7%, patient‐held 39.2%, control 31.5%). However, the participants in doctor‐held card practices reported a greater number of side effects (defined by patients in the previous year) than the control group (49.3% vs 43.6%, OR 1.54, 95% CI 1.10 to 2.17, P = 0.013), as did participants in the patient‐held care practices (50.8% vs 43.6%, OR 1.60, 95% CI 1.10 to 2.32, P = 0.016).
Self management through screening
The evaluation of the effect of self management through screening for adults with epilepsy reported significant differences between groups at four months in AED dose changes, as defined by any participant‐recorded dose change (intervention 65.6%, control 13.3%; RR 2.8, 95% CI 1.7 to 4.8, P < 0.0001) (Gilliam 2004). However, the study record gave no information on whether proposed medication management changes were appropriate. Nevertheless, the mean percent improvement in Adverse Event Profile (AEP) score was 25% in the intervention group vs 5% in the control, which was significantly different (P = 0.01).
Alternative care delivery in outpatient clinics
Over the 12‐month study period, an evaluation of a specialist epilepsy unit in hospital outpatients reported (although with no detailed data) that there was no significant difference between groups in the number and type of antiepileptic drugs or the number of drugs prescribed per participant (Morrow 1990). There was, however, a significant reduction in the percentage of drug concentrations outside the reference range in intervention vs control (P < 0.001). This fell from 55% of all participants at baseline to 26% in the intervention group but remained "essentially unchanged" in the control group (proportions not reported). Alongside this finding, there was also a reduction in adverse drug reactions (ADRs) in the intervention group from 40% to 45% at baseline to around 25% at 12 months, whereas in the control group, the proportion remained unchanged at around 40% to 45% (data only reported graphically, P < 0.001). The proportion of ADRs was lowest at three months in the control group but then began to rise back to baseline levels, whereas in the intervention group, the lowest level was recorded at six months, at which point the difference between groups was also significant (P < 0.05). All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
The evaluation of a hospital‐based epilepsy nurse by Warren 1998, which included a minority of participants with learning disabilities, found that there was no difference between study and intervention groups with respect to self management, as measured by self reported non‐compliance with medication (intervention 46%, control 35%, P = 0.130) and attendance at epilepsy clinic (intervention 84%, control 92%, P = 0.085).
At both one year (Mills 1999a) and two years (Mills 1999b), an evaluation of a primary care‐based specialist epilepsy nurse reported five outcomes relating to the appropriateness of medication. For four of these ('taking one type of antiepileptic drug', 'feel very well controlled by drug', 'report very important to take tablets exactly as prescribed' and 'reporting side effects from drugs'), there were no significant differences between intervention and control groups at one year (Mills 1999a) or between those who had accessed the specialist epilepsy nurse and those who had not at two years (Mills 1999b). Intervention participants were, however, significantly less likely than controls to have reported never missing taking their antiepileptic drugs (OR 0.48, 95% CI 0.24 to 0.94, P = 0.032) at two years. There was no significant difference for this outcome between those who had accessed the specialist epilepsy nurse and those who had not (Mills 1999b). All results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
At six months, the evaluation of a general practice‐based epilepsy nurse reported on the 'appropriateness of medication supplied' (Ridsdale 1997). This outcome was in fact a measure of the number of occasions when the specialist nurse felt that medication plans could be improved and noted this in the patient record. The trial reported that the epilepsy nurse found that 11.1% of participants required medication management changes. However, authors did not give any information on whether these proposed changes were or were not appropriate, and there was no control group comparison. This trial also reported an increase in measurement of serum levels in the last six months between arms (intervention 29% to 66%, control 23% to 17%, P < 0.01). However, increased serum concentration monitoring was not necessarily clinically desirable, and it was not clear what implications this had for the appropriateness of medication supplied.
The remaining three studies did not report on the appropriateness and volume of medication prescribed (Helde 2005; Ridsdale 1999; Ridsdale 2000).
Behavioural interventions
The evaluation of an outpatient exercise programme reported that in all 19 participants taking AEDs, there was < 26% coefficient of variation in AED concentrations (measured by serum carbamazepine, phenytoin, and valproic acid concentrations, as applicable) over 12 weeks (McAuley 2001).The authors state that this suggests little or no impact of the exercise intervention between groups over time, but they report no formal statistical tests. However, these results should be interpreted with caution, as the study collected only 80% of possible samples.
Guideline implementation and patient information
The evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment did not report on the appropriateness and volume of medication prescribed (Davis 2004).
Reported knowledge of information and advice received from professionals
See Table 3 for summarised results on reported knowledge of information and advice received from professionals
Self management education
At four months, an evaluation of the SEE programme showed significant differences between groups in terms of fear of death and brain damage due to seizures (P < 0.05) and the extent of overall misinformation and misconceptions about epilepsy (P < 0.01) (Helgeson 1990). Changes were also reported to be significant over time (P < 0.05 in both instances). Hence, investigators saw significant group x time interaction effects for these two measures (P < 0.05 and P < 0.001, respectively). All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
The evaluation of MOSES showed significant improvements at six months in the intervention group for the primary outcome of epilepsy knowledge (P < 0.0001) (May 2002). The study also evaluated the effect of the interaction between the group and time, reporting significant differences for group x time (P < 0.001) and time (P < 0.001).
In DiIorio 2011's evaluation of WebEase, there were no significant differences between groups after 12 weeks (P = 0.077).
The evaluation of the educational programme on self management by Aliasgharpour 2013 did not report on knowledge of information and advice received from professionals.
Strategies to improve patient compliance
The evaluation of the prompt and reminder card by Thapar 2002 found that participants in the doctor‐held card group were significantly less satisfied with information provision about epilepsy compared to the control group (P = 0.006). There were no significant differences between the patient‐held card group and control group (P = 0.943). Satisfaction at baseline was 67.7%, 64.4% and 65.1% in the control, doctor‐held and patient‐held groups, respectively, whereas at one year it was 76.1%, 66.0% and 76.2%, respectively. Peterson 1984 did not assess reported knowledge of information and advice received from professionals in their evaluation of an intervention combining compliance‐improving strategies, nor did Adamolekun 1999 in their evaluation of information pamphlets.
Self management through screening
Gilliam 2004 did not evaluate or report participant's knowledge of the information and advice received from professionals.
Alternative care delivery in outpatient clinics
Morrow 1990 did not report any significant differences in the number of information items offered to participants over 12 months in either the intervention group or control group in their evaluation of a specialist epilepsy unit in hospital outpatients. In participants who were re‐assessed, the number of items offered increased from 1.1 at baseline to 2.5 in the intervention group (P < 0.001). In the control group it remained stable (1.1 at baseline and 1.2 at 12 months, P > 0.05). However, the study did not compare the intervention and control groups to each other. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
At six months, Warren 1998 (which included a minority of participants with learning disabilities) reported that medical knowledge of epilepsy improved in the group receiving the intervention of a hospital‐based epilepsy nurse (P = 0.035). Investigators did not find any significant differences in terms of social knowledge of epilepsy (intervention mean 15.3 SD) 2.5, control mean 14.9, SD 2.3, P = 0.368).
Mills 1999a's evaluation of a primary care‐based specialist epilepsy nurse found that at one year, participants in the intervention group were significantly more likely to have discussed 4 out of 11 topics with primary care staff (P = 0.004 to P = 0.048) and 2 out of 11 topics with hospital staff (P = 0.020 to P = 0.048). The study investigators adjusted these results for baseline value of outcome variable and gender in a multiple regression model. However, as only 50.9% of participants responded to both baseline and follow‐up questionnaires, these results should be interpreted with caution. At one year (Mills 1999a), an analysis of those participants who actually saw the epilepsy nurse (as opposed to those who did not) were significantly more likely to have discussed 10 of 11 epilepsy topics with either their GP or hospital doctor (P values not reported). The study investigators adjusted these results for baseline value of outcome variable in a multiple regression model. However, as this analysis was not based on comparison groups from the original study and does not reflect the impact of those not wishing to see the epilepsy nurse, these results should be interpreted with caution. At two years (Mills 1999b), of 11 topics, participants who had accessed the specialist epilepsy nurse were significantly more likely to have discussed 8 topics with primary care staff (P = 0.001 to P = 0.037) and 2 topics with hospital doctors (P = 0.031 to P = 0.040) than those who had not accessed the specialist nurse. The study investigators adjusted these results for baseline value of outcome variable, seizure frequency in the last year and other long‐term illness in a multiple regression model. However, as this analysis was not based on comparison groups from the original study, but rather on a 40% response rate to baseline and follow‐up questionnaires, and as it did not reflect the impact of those not wishing to see the epilepsy nurse, these results should be interpreted with caution. Indeed, all results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
An evaluation of an epilepsy nurse based in general practice measured knowledge using the Knowledge of Epilepsy questionnaire (Ridsdale 1999). Authors stated that overall, there were no significant differences in knowledge scores between groups at six months, but they do not provide further information (e.g. scores or statistical tests).
At six months, the evaluation of a hospital‐based specialist nurse by Ridsdale 2000 found that of nine topics, participants in the intervention group were significantly more likely to have received enough advice on eight topics with primary care staff (P < 0.01 to P = 0.05). This study also found no difference in epilepsy knowledge scores between control and intervention groups (P values ranged from 0.49 to 0.73), except in those whose score lay in the lowest quartile at the start of the study. In this group, knowledge scores did improve (median in intervention group from 38.2 to 42.7, median in control group from 36.0 to 37.2, P < 0.01).
Neither Helde 2005 nor Ridsdale 1997 evaluated reported knowledge of information and advice received from professionals in their studies of specialist nurse interventions.
Behavioural interventions
McAuley 2001 did not evaluate the impact on reported knowledge of information and advice received from professionals in their study of a structured exercise programme.
Guideline implementation and patient information
Davis 2004 did not evaluate reported knowledge of information and advice received from professionals in their study of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment.
Reported health and quality of life
See Table 4 for summarised results on participants' reported health and quality of life.
Self management education
At four months, an evaluation of the SEE programme showed no significant changes in measures of acceptance of disability, depression, anxiety, self efficacy, or overall psychosocial functioning (Helgeson 1990). Analysis using repeated‐measures ANOVA showed that changes in the groups could be considered significant over and above changes seen in both groups due to time alone. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
At 12 weeks, the evaluation of MOSES reported no significant differences between groups for measures of coping with epilepsy, restriction in daily living, mobility and leisure behaviour, epilepsy‐related fear, stigma, SF‐36 mental and physical functioning, self esteem or depression (May 2002). There were, however, significant differences over time for coping with epilepsy (P < 0.001), restriction in daily living (P < 0.0001), mobility and leisure behaviour (P < 0.001) and epilepsy‐related fear (P < 0.05). Effects were significant for group x time for coping with epilepsy (P < 0.01) and restriction in daily living (P < 0.0001).
At 12 weeks, an evaluation of WebEase reported no significant differences between groups for measures of perceived stress, sleep quality, epilepsy self management, self efficacy or quality of life (DiIorio 2011).
In their evaluation of an educational programme on self management, Aliasgharpour 2013 reported that the majority of the participants in the intervention and control reported 'medium' self management at baseline (73.3% and 53.3%, respectively), with those reporting 'high' levels being 10% and 20%, respectively. However, at one‐month follow‐up, those reporting 'high' self management were 76.7% and 10%, respectively (levels of 'medium' were 23.3% and 60.0%, respectively), which constitutes a statistically significant difference (P < 0.001). Hence there were also significant differences over time in the intervention group (P < 0.001) but not in the control group (P = 0.594).
Strategies to improve patient compliance
The evaluation of prompt and reminder cards studied by Thapar 2002 and the evaluation of a combination of compliance‐improving strategies by Peterson 1984 did not report any quality of life measures, nor did the evaluation of patient pamphlets by Adamolekun 1999.
Self management through screening
The evaluation of the effect of self management through screening for adults with epilepsy reported mean change in Quality of Life in Epilepsy (QOLIE‐89); total scores were not significantly different between groups at four months (Gilliam 2004). However, authors did not report numerical results.
Alternative care delivery in outpatient clinics
In the evaluation of a specialist epilepsy unit in hospital outpatients (Morrow 1990), there were no significant changes in the Hospital Anxiety and Depression Scale questionnaire in the intervention or the control group (mean values not accurately specified for either group) at 12 months. Investigators did not compare the two groups with each other for this measure. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
In an evaluation of a hospital‐based epilepsy nurse in a population that included a minority of participants with learning disabilities, Warren 1998 found that there was no difference between study and intervention groups with respect to overall health status as measured by EuroQoL; weighted health status (P = 0.496) or self related health status (P = 0.364). Similarly, there was no significant difference between control and intervention groups in social outcomes at six months (P = 0.385, P = 0.125 after adjustment for sex and employment status) or for any individual domains on the social functioning instrument. Finally, authors did not report any overall difference in anxiety (P = 0.635) and depression (P = 0.500) between groups.
In evaluations of a primary case‐based specialist epilepsy nurse at one year (Mills 1999a) and two years (Mills 1999b), investigators assessed perceived quality of life primarily from 10 questions about the effects of epilepsy and its treatment on daily living. At one year, Mills 1999a reported that those in the intervention group were significantly more likely than those in the control group to report that epilepsy affected their future plans and ambitions (OR 6.19, 95% CI 2.07 to 18.50), overall health (OR 4.28, 95% CI 1.77 to 10.34) and standard of living (OR 2.74, 95% CI 1.05 to 7.16), to a large, moderate or small extent. The reported odds ratios for self reported effects on other areas of everyday life, while greater than one, were not statistically significant. There were no significant interactions between having seen the epilepsy nurse and time since last epilepsy attack on reported quality of life variables. At two years (Mills 1999b), authors reported significant differences between the group of participants who had accessed the specialist epilepsy nurse and those who had not for epilepsy's impact on overall health (OR 2.50, CI 1.23 to 5.08). There were also significant differences between groups with regard to how individuals felt about themselves (OR 2.09, CI 1.01 to 4.33) and the impact on their social life/activities (OR 2.28, CI 1.08 to 4.82). Investigators measured effects by controlling for the same variable at baseline, seizure in the previous year and other long‐term illness. Reported odds ratios for self reported effects on seven other areas of everyday life were greater than one, but not significantly so. Mills 1999a and Mills 1999b also reported two additional questions relating to quality of life in tables (i.e. 'feel stigmatised due to epilepsy' and 'feel unhappy about life as a whole'). At neither point in time did investigators report differences between the intervention and control groups or between the participants who had accessed the specialist epilepsy nurse and those who had not. All results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
An evaluation of an epilepsy nurse based in general practice found no significant changes over time in depression scores at six months if participants had a seizure in this period (P = 0.44) (Ridsdale 1999). For those participants who had had no seizure, investigators did observe a significant difference in depression (P = 0.03).
At six months, an evaluation of a hospital‐based epilepsy nurse in outpatients found no significant difference between control and intervention groups in either anxiety (P = 0.41) or depression (P = 0.27) (Ridsdale 2000).
At two years, Helde 2005 evaluated a hospital‐based specialist epilepsy nurse, showing that there were no significant differences between groups for the Quality of Life in Epilepsy Inventory (QOLIE‐89) (P = 0.58). However, intervention group participants were significantly more likely to have an improved score compared to baseline (P = 0.019). There were also significant improvements from baseline for 3 of 17 sub‐items on the QOLIE‐89 scale in the intervention group. These were: role limitations ‐ physical (intervention P = 0.05, control P = 0.59), health discouragement (intervention P = 0.01, control P = 0.15) and medication effects (intervention P = 0.04, control P = 0.36). Conversely, significant improvements were reported from baseline for 1 of 17 sub‐items on the QOLIE‐89 scale in the control group, namely pain (intervention P = 0.41, control P = 0.04).
The earliest evaluation of an epilepsy nurse based in general practice by Ridsdale 1997 did not report any quality of life measures.
Behavioural interventions
At 12 weeks, an evaluation of an outpatient exercise programme reported no apparent differences between groups for the QOLIE‐89 (overall quality of life), profile of mood states (POMS) or Rosenberg self esteem scales (McAuley 2001) but formal statistical tests were not reported between groups. However QOLIE‐89 scores showed significant improvement over time overall in the intervention group only (intervention P = 0.03, control P = 0.94) and for two of the six individual domains (physical function P = 0.02 and energy/fatigue P = 0.02). Energy/fatigue also significantly improved in the control group (P < 0.01). There were no differences over time in the total POMS score for the control group, but there was a near significant multivariate effect for time for the intervention group (P = 0.05). Of the five POMS subscales, only vigour improved over time in the intervention group (P = 0.03). There were no changes in any of the psychological variables in the control group or global self esteem in the intervention group. Overall physical self description questionnaire (PSDQ, measuring physical self concept and vigour) scores significantly increased in the intervention group (P < 0.05) at weeks 12 and 16 and for 4 of the 11 domains from the PSDQ scale (physical activity, coordination, endurance and strength). The global physical domain was not significantly different at week 12 but had become so by week 16. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Guideline implementation and patient information
At 6 to 12 months, an evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment (Davis 2004) showed no significant difference in SF‐36 scores. Similarly, the study found no significant differences for epilepsy‐related quality of life as measured by a specific instrument.
Objective measures of general health status
See Table 5 for summarised results on objective measures of general health status.
Self management education
Investigators did not report on any outcomes relating to objective measures of general health status in the evaluations of SEE (Helgeson 1990), MOSES (May 2002), WebEase (DiIorio 2011) or the educational programme on self management (Aliasgharpour 2013).
Strategies to improve patient compliance
The evaluations of prompt and reminder cards by Thapar 2002, combination of compliance‐improving strategies by Peterson 1984 and evaluation of patient pamphlets by Adamolekun 1999 did not report outcomes relating to objective measures of general health status.
Self management through screening
Gilliam 2004 did not report on outcomes relating to objective measures of general health status in their evaluation of effects of self management through screening for adults with epilepsy.
Alternative care delivery in outpatient clinics
The evaluations of a specialist epilepsy unit in hospital outpatients did not report outcomes relating to objective measures of general health status (Morrow 1990).
Specialist nurse practitioners
The evaluation of a hospital‐based epilepsy nurse, which included a minority participant population with learning disabilities, found no significant differences in injuries from seizures or other specific types of injuries at six months (Warren 1998). The authors did note, however, that numerically, the injuries tended to be lower in the intervention group, with the proportion of participants suffering any injury being 29% in the intervention and 38% in the control groups (P = 0.240).
In the evaluation of primary care‐based specialist epilepsy nurse, again Mills 1999a reported no significant differences between intervention and control groups in terms of injuries as a result of epilepsy attacks in the previous year, while Mills 1999b observed no significant difference between those who accessed and did not access the specialist nurse at two years. At baseline, the proportion of subjects in the intervention and control groups reporting an injury was 12.8% and 20.0%. At one year, the proportions in both groups fell to 10.8% and 14.8%, respectively (OR = 0.92, 95% CI 0.41 to 2.04, P = 0.84). Investigators did not report the proportions of those who had and had not accessed the specialist epilepsy nurse at two years, but the reported odds ratio was 1.02 (95% CI 0.35 to 2.97, P = 0.98). Mills 1999a also reported other long‐term health problems: the proportions of participants reporting these were 45.0% in the intervention group and 46.5% in the control group at baseline. At one year, the proportions were 51.4% and 44.4%, respectively (OR 1.83, 95% CI 0.95 to 3.51, P = 0.07). Mills 1999b did not report the same outcome for a comparison of those who had accessed the specialist epilepsy nurse and for those who had not at two years. All results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
The other evaluations of specialist nurse practitioners did not measure objective health status, other than reporting on seizures (Helde 2005; Ridsdale 1997;Ridsdale 1999;Ridsdale 2000); see Seizure frequency and severity.
Behavioural interventions
McAuley 2001 did not report outcomes relating to objective measures of general health status (other than seizure frequency) in their evaluation of an outpatient exercise programme.
Guideline implementation and patient information
The evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment did not report outcomes relating to objective measures of general health status (Davis 2004).
Objective measures of social or psychological functioning
See Table 6 for summarised results on objective measures of social or psychological functioning.
Self management education
The evaluations of SEE (Helgeson 1990), MOSES (May 2002), WebEase (DiIorio 2011) and the educational programme on self management (Aliasgharpour 2013) did not report objective measures of social or psychological functioning.
Strategies to improve patient compliance
The evaluations of patient pamphlets (Adamolekun 1999), a prompt and reminder card (Thapar 2002) and compliance‐improving strategies (Peterson 1984) did not report objective measures of social or psychological functioning.
Self management through screening
Gilliam 2004 did not report any objective measures of social or psychological functioning in their evaluation of the effects of self management through screening for adults with epilepsy.
Alternative care delivery in outpatient clinics
At 12 months, the evaluation of a specialist epilepsy unit in hospital outpatients found no significant changes in social activities in either group (P > 0.05). Similarly at 12 months, there were no significant changes in employment status in either group (P > 0.05) (Morrow 1990). All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
At six months, the evaluation of a hospital‐based epilepsy nurse, whose study population included a minority of people with learning disabilities (Warren 1998), considered absence from work as an outcome. Investigators found no difference in the number of days' absence from work in the intervention (67%) and control (65%) groups at six months (P = 0.864).
None of the other specialist nurse interventions measured objective measures of social or psychological functioning (Helde 2005; Mills 1999a; Mills 1999b;Ridsdale 1997; Ridsdale 1999; Ridsdale 2000).
Behavioural interventions
The evaluation of an outpatient exercise programme did not report any objective measures of social or psychological functioning (McAuley 2001).
Guideline implementation and patient information
Davis 2004 did not report any objective measures of social or psychological functioning in their evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment.
Costs of care or treatment
See Table 7 for summarised results on costs of care or treatment.
Self management education
The evaluations of SEE (Helgeson 1990), MOSES (May 2002), WebEase (DiIorio 2011) and the educational programme on self management (Aliasgharpour 2013) did not report on costs of care or treatment.
Strategies to improve patient compliance
Investigators did not see any significant changes at six months in measures of clinic appointment keeping in their evaluation of the combination of compliance‐improving strategies (Peterson 1984). Changes were neither significant over time (P > 0.30) nor between groups (P > 0.20). The proportion of subjects attending all their scheduled appointments after six months was not reported. However, investigators reported that 59% of the intervention group and 65% of the control group attended all scheduled appointments prior to the study commencing.
At six months, an evaluation of a patient pamphlet for trained primary healthcare workers showed improvement in patient default from clinic follow‐up (defined as two consecutive missed appointments after the intervention) (Adamolekun 1999). At six months, the intervention default rate was 22.3% in the intervention group vs 56.3% in the control group. However, the significant difference between the two groups in baseline monthly attendance (P = 0.001) precluded a meaningful comparison at six months. Nevertheless, when comparing the magnitude of the change in attendance over the time period, there was no significant difference between the two groups (P = 0.2678). All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Self management through screening
In their evaluation of the effects of self management through screening for adults with epilepsy, Gilliam 2004 reported that at four months there were significant differences between groups in the mean number of clinic visits (intervention 2.2 SD 0.89 control 1.3 SD 0.54 P < 0.0001).
Alternative care delivery in outpatient clinics
The number of outpatient clinic visits, visits to the outpatient clinic doctor, GP consultations and inpatient days appeared lower in the epilepsy unit participants, but these results cannot be verified as Morrow 1990 did not report any statistical analysis. All results of this evaluation should be interpreted with caution because of the weak study design (see Risk of bias in included studies).
Specialist nurse practitioners
Warren 1998 reported a whole range of healthcare use and cost measures at six months: one or more visits to GP, number of visits to GP, visits to general practice nurse, visits made by district nurse, visits made by health visitor, visits made by community psychiatric nurse (CPN), visits to outpatient clinic doctor, specialist outpatient clinic psychiatrist consultation, specialist outpatient clinical psychologist consultation, specialist inpatient admission, EEG, CT scan, MR scan, blood level estimation for antiepileptic drugs, other outpatient consultation, other inpatient admission, other day‐patient visit and visit to accident & emergency (A&E). The study also assessed primary healthcare cost per patient, secondary healthcare cost per patient and total healthcare cost per patient. While the majority of between‐group comparisons reported no significant differences, the study suggested a significant decrease in outpatient clinic hospital attendance with doctors (P < 0.0001) at six months. Proportionately, more intervention participants visited specialist outpatients clinics for psychiatric (1% vs 0%) or psychological assessments (2% vs 1%) than did participants in the control group, but investigators did not formally compare groups with each other, presumably due to small numbers of events. There was a non‐significant trend in terms of participants' seeing their GP once or more (P = 0.054) which translated into a significant difference upon comparing the number of times between groups (P = 0.028). Investigators reported that primary care costs were significantly reduced in the intervention arm (P = 0.017) although they noted that these costs were a small proportion of the total cost per patient. Numerically participants in the intervention group made more visits to specialist outpatient clinical psychologists and psychiatrists than did participants in the control group, but there were no formal comparisons between groups. The economic analysis had several limitations, as it was based on the economic consequences for a tertiary care (specialist) centre, only considered the consequences for the health service and did not link financial costs to health or other outcomes. However, there is currently no evidence to suggest that specialist epilepsy nurses are more expensive than standard care.
Mills 1999a reported that healthcare use associated with a primary care‐based specialist epilepsy nurse at one year was not significant between groups for any one of the six types of healthcare use measured: saw GP for any reason, saw GP for epilepsy, saw hospital doctor for epilepsy, admitted to hospital for epilepsy, attended A&E department for epilepsy and had regular arrangement to see GP for epilepsy. However, while the healthcare use always decreased after one year in the control group, in the intervention group the proportion who saw their GP for any reason rose from 65.1% to 73.4% as did attendance at A&E (3.8% to 6.6%) and regular arrangements to see GP for epilepsy (15.6% to 16.9%). At two years, Mills 1999b reported no significant differences for the same measures between participants who had accessed the specialist nurse and those who had not. While proportions of participants are not reported, it is worth noting that the odds ratio for seeing a GP for any reason was close to achieving statistical significance (OR 1.97, 95% CI 0.97 to 4.00, P = 0.06). All results of this evaluation from both studies should be interpreted with caution because of the weak study design (see Risk of bias in included studies) and the large number of comparisons made, which increase the likelihood of a significant finding occurring by chance.
The remaining four studies did not report on costs of care or treatment (Ridsdale 1997;Ridsdale 1999;Ridsdale 2000;Helde 2005).
Behavioural interventions
The evaluation of an outpatient exercise programme did not report costs of care or treatment (McAuley 2001).
Guideline implementation and patient information
Davis 2004 did not report costs of care or treatment in their evaluation of a control, intermediate and intensive implementation of a national guideline for epilepsy treatment.
Discussion
Summary of main results
There are 18 different studies of 16 separate interventions included in the review. It was not possible to combine study results in a meta‐analysis because of the heterogeneity of outcomes, study populations, interventions and time scales across the studies. Each study used a unique combination of outcome measures, mostly subjective in nature. No single intervention was found to be consistently effective across the full range of reported outcomes.
Self management education
There is some evidence of effectiveness for self management education in terms of improving the appropriateness and volume of medication prescribed, as three of four studies that studied these interventions reported statistically significant improvement (DiIorio 2011; Helgeson 1990; May 2002). One of four studies showed an improvement in seizure frequency and knowledge (May 2002), but this was not described in the other three studies (Aliasgharpour 2013; DiIorio 2011; Helgeson 1990).
Strategies to improve patient compliance
There was no evidence of overall improvement in the three included studies that evaluated strategies to improve patient compliance (Adamolekun 1999; Peterson 1984; Thapar 2002). Although there were significant differences between groups in terms of plasma levels at six months for phenytoin and carbamazepine (although not for sodium valproate) in Peterson 1984, this was not the case in the other two studies (Adamolekun 1999; Thapar 2002).
Self management through screening
There was no evidence of improvement after self management through screening in the included study that assessed this outcome (Gilliam 2004). Although there were significant differences between groups at four months in AED dose changes, there was no information on whether proposed medication management changes were appropriate.
Alternative care delivery in outpatient clinics
There was no evidence of improvement for any of the outcomes our review considered after alternative care delivery in outpatient clinics in Morrow 1990, with the exception that participants in the intervention group had fewer GP consultations and visits to the outpatient doctor than those in the control group.
Specialist nurse practitioners
There is some evidence of effectiveness for specialist nurse practitioners in terms of improving participants' reported knowledge of information and advice received from professionals, with four of eight studies reporting improvement in at least one category compared to controls (Mills 1999a; Mills 1999b; Ridsdale 2000; Warren 1998). There were few significant differences between groups for any of the other outcomes considered by this review with the exception of Mills 1999a reporting that individuals in the intervention group were significantly more likely than those in the control group to report never missing a dose of their antiepileptic drugs. This study and the follow‐up by Mills 1999b also reported significant differences between groups for 3 out of 10 measures of self reported quality of life. Primary care costs were reported to be significantly reduced in the intervention arm of Warren 1998, in which participants received the intervention in a regional epilepsy clinic.
Behavioural interventions
There was no evidence of improvement at the end of a 12‐week study evaluating a behavioural intervention for any of the outcomes we considered in our the review (McAuley 2001). However, for one outcome, physical self concept and vigour, there were significant differences in the intervention group at 16 weeks but formal statistical comparisons between groups were not reported. It is not clear why investigators measured this outcome at 16 weeks when they reported no other outcome at this time point in a study they described as lasting 12 weeks.
Guideline implementation and patient information
There was no evidence of improvement after guideline implementation and patient information in one included study for any of the outcomes we considered in our review (Davis 2004).
Overall completeness and applicability of evidence
The outcomes that the primary trials covered were generally consistent with the outcomes considered in this review. Not all trials considered patient perceptions, and hardly any trials considered the cost‐effectiveness of services. In addition, they rarely described the long‐term effects of most of the interventions.
Except for the evaluation of specialist epilepsy nurses, the generalisability of any findings may be limited, as the level of detail provided for the interventions varies considerably, and only one study examines each, although we sometimes categorised them within a larger group of similar interventions in this review. In addition, contextual factors such as the intervention setting, the local health system, the reimbursement system, staff training, the nature of participants, the duration of the intervention and evaluation period may have heavily influenced the final results. No trials included a process evaluation to assess how the intervention had been implemented or to investigate any potential barriers to its successful implementation.
Quality of the evidence
The quality of evidence is generally poor. We only considered three studies to be at low risk of bias (Aliasgharpour 2013; Helde 2005; Warren 1998), while we judged six—a third of the total—to carry a high risk (Adamolekun 1999; Helgeson 1990; McAuley 2001; Mills 1999a; Mills 1999b; Morrow 1990). Consequently, there is limited robust evidence for the effectiveness of interventions to improve the health and quality of life of people with epilepsy.
Potential biases in the review process
We did not identify any potential biases in the review process.
Agreements and disagreements with other studies or reviews
The current review is an update of a review we originally conducted in 2006 and revised in 2009 (Bradley 2008). Despite the identification of two additional studies in this version (Aliasgharpour 2013; DiIorio 2011), the overall findings remain largely unchanged. However, three similar reviews have examined psychosocial treatment programmes in epilepsy (Mittan 2009), evidence‐based models of care for people with epilepsy (Fitzsimons 2012) and care delivery and self management strategies for children with epilepsy (Lindsay 2015); the last of these is undergoing an update alongside this update for adults. All reviews have reported that there is no clear evidence that any specific service model substantially improved outcomes for children or adults with epilepsy. Likewise, they also note a lack of evidence for cost‐effectiveness, although Mittan 2009 did calculate that one of the interventions it evaluated (the SEE programme described in Helgeson 1990) was likely to be cost‐effective by virtue of the fact that this was the only intervention to use a large audience format (up to 850 people) for treatment delivery.
Authors' conclusions
Implications for practice.
It is clearly plausible that various innovative service models could improve identified problems in epilepsy care by improving the knowledge and awareness of epilepsy amongst clinicians and patients; timeliness and appropriateness of clinical care and advice including medication; follow‐up and clinical investigation; and poor communication among clinicians and between clinicians and patients.
There are two interventions supported by some evidence of benefit: specialist epilepsy nurses and self management education. Some evidence from the specialist epilepsy nurse evaluations suggests that certain subgroups of people (such as those who do not have frequent seizures) benefit more than others. However, there is still no clear evidence to suggest that alternative service models substantively improve health or quality of life for people with epilepsy, especially in the longer term. Consequently, it is unknown if the models would provide cost‐effective options.
It is also possible that the benefits of these complex interventions are situation‐specific and their benefits are not generalisable to other settings. At the moment, results are based on the activity of a few service providers, whose competence and expertise may also have influenced final outcomes, and the trials do not always clearly define the exact nature of the intervention. It is not always clear how service providers have been trained, for instance.
At present, it is not possible to advocate any model to improve outcomes for people with epilepsy. We need further research to investigate the effectiveness of specialist epilepsy nurses before making such recommendations.
Implications for research.
There is a lack of research on service models to improve outcomes for people with epilepsy, with the possible exception of evaluations of specialist epilepsy nurses and self management education. Generally, the number of studies is small, sometimes with very small participant numbers. There are few high quality studies, so it is likely that the study quality has influenced the final results. In addition, the generalisability of studies is limited.
Further studies are needed that:
offer an improved quality of study design and reporting, particularly in promising areas (e.g. self management education);
improve generalisability (e.g. include a full description of the intervention, a process evaluation, and a multicentred assessment of the benefits for more than one population and service provider);
evaluate the effects of interventions for those subgroups most likely to benefit (e.g. people with newly diagnosed epilepsy, people with learning disabilities);
consider the cost‐effectiveness of service models shown to be beneficial.
To maximise the potential generalisability of future studies and to ensure study quality, we would recommend randomised controlled trials rather than observational studies. Studies should also ensure that they adequately define and describe interventions and that the study design takes into account contextual factors. Where socially complex interventions are under study (e.g. specialist nurses), the trials must include sufficient service providers to ensure that individual characteristics do not bias the results.
What's new
| Date | Event | Description |
|---|---|---|
| 9 December 2013 | New search has been performed | We updated the searches on 9 December 2013. A pre‐publication search was carried out on 26 October 2015. The authors will address these search results at a later stage. It is extremely unlikely that these results will change the existing conclusions. |
| 9 December 2013 | New citation required but conclusions have not changed | Two new studies have been included, and two authors (one original author, PB, and one new author, NF) have extensively re‐written the review to fit the new review format. The conclusions remain unchanged. |
Notes
A pre‐publication search was carried out on 26 October 2015. The authors will address these search results at a later stage. It is extremely unlikely that these results will change the existing conclusions.
Acknowledgements
We would like to thank Jeph Herrin (Flying Buttress) who helped us summarise the results from the studies in the original review. We would also like to thank all the information specialists who conducted the searches for the original and updated review, namely Alison Beamond, Dinah Roberts and Yenal Dundar.
Appendices
Appendix 1. Epilepsy Specialized Register search strategy
#1 MeSH DESCRIPTOR Program Evaluation Explode All WITH EC MT ST SN TD #2 MeSH DESCRIPTOR Delivery of Health Care Explode All WITH CL EC ES EH HI LJ MA MT OG ST SN TD UT #3 MeSH DESCRIPTOR Ambulatory Care Explode All WITH CL EC ES HI LJ MA MT OG PX ST SN TD UT #4 MeSH DESCRIPTOR Outcome and Process Assessment (Health Care) Explode All WITH CL EC ES HI LJ MT OG ST SN TD UT #5 epilep* NEAR4 (centre* OR center*) #6 epilep* NEAR3 specialist* #7 epilep* NEAR2 nurs* #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #9 #8 AND INREGISTER AND >2011:YR
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Epilepsy] explode all trees #2 epilep* #3 (#1 or #2) #4 MeSH descriptor: [Program Evaluation] explode all trees #5 MeSH descriptor: [Delivery of Health Care] explode all trees #6 (#4 or #5) #7 (#3 and #6) #8 MeSH descriptor: [Ambulatory Care] explode all trees #9 (#3 and #8) #10 epilep* near/4 centre*:ti,ab,kw (Word variations have been searched) #11 epilep* near/4 center*:ti,ab,kw (Word variations have been searched) #12 epilep* near/3 specialist*:ti,ab,kw (Word variations have been searched) #13 epilep* near/2 nurs*:ti,ab,kw (Word variations have been searched) #14 MeSH descriptor: [Outcome and Process Assessment (Health Care)] explode all trees #15 (#14 and #3) #16 (#7 or #9 or #10 or #11 or #12 or #13 or #15) from 2012, in Trials
Appendix 3. MEDLINE search strategy
Original review
1. exp EPILEPSY/ 2. epilep$.tw. 3. 1 or 2 4. exp Program Evaluation/ 5. exp "Delivery of Health Care"/ 6. 4 or 5 7. 3 and 6 8. exp Ambulatory Care/ 9. 3 and 8 10. (epilep$ adj4 centre$).ab,ti. 11. (epilep$ adj4 center$).ab,ti. 12. (epilep$ adj3 specialist$).ab,ti. 13. (epilep$ adj2 nurs$).ab,ti. 14. exp "Outcome Assessment (Health Care)"/ 15. 14 and 3 16. 7 or 9 or 10 or 11 or 12 or 13 or 15
Review update
1 exp Epilepsy/ 2 epilep$.mp. 3 1 or 2 4 exp Program Evaluation/ 5 exp "Delivery of Health Care"/ 6 exp Ambulatory Care/ 7 *"Outcome Assessment (Health Care)"/ 8 (program$ adj2 evaluat$).mp. 9 4 or 5 or 6 or 7 or 8 10 3 and 9 11 (epilep$ adj4 (centre$ or center$)).mp. 12 (epilep$ adj3 nurs$).mp. 13 (epilep$ adj3 specialist$).mp. 14 11 or 12 or 13 15 10 and 14 16 limit 15 to yr "2012 ‐Current"
Appendix 4. EMBASE search strategy
Original review
1 exp Epilepsy/ 2 epilep$ 3 1 or 2 4 exp Ambulatory Care/ 5 exp Institutional Care/ 6 exp Community Care/ 7 exp Health Care Delivery/ 8 *Outcomes Research/ 9 (program$ adj2 evaluat$) 10 4 or 5 or 6 or 7 or 8 or 9 11 3 and 10 12 (center$ or centre$) 13 nurs$ 14 specialist$ 15 (epilep$ adj4 (centre$ or center$)) 16 (epilep$ adj3 nurs$) 17 (epilep$ adj3 specialist$) 18 11 or 15 or 16 or 17
Review update
1 exp epilepsy/ 2 epilep$.mp. 3 1 or 2 4 exp ambulatory care/ 5 exp institutional care/ 6 exp community care/ 7 exp health care delivery/ 8 *outcomes research/ 9 (program$ adj2 evaluat$).mp. 10 4 or 5 or 6 or 7 or 8 or 9 11 3 and 10 12 (epilep$ adj4 (centre$ or center$)).mp. 13 (epilep$ adj3 nurs$).mp. 14 (epilep$ adj3 specialist$).mp. 15 12 or 13 or 14 16 11 and 15 17 limit 16 to yr="2012 ‐Current"
Appendix 5. PsycINFO search strategy
Original review
This search was carried out in two phases. The first search was carried out in May 2006 using the following strategy. #10 #1 and #9 #9 #2 or #3 or #4 or #5 or #6 or #7 or #8 #8 specialist* #7 nurs* #6 centre* or center* #5 treatment effectiveness evaluation #4 treatment outcome* #3 health care delivery #2 ambulatory care #1 epilep* The second search was carried out in March 2010 using the EBSCOhost platform for PsycINFO, and the following strategy. S12 S8 or S9 or S10 or S11 S11 S3 and S7 S10 epilep* N3 specialist* S9 epilep* N3 nurs* S8 epilep* N4 center* or epilep* N4 centre* S7 S4 or S5 or S6 S6 MM "Program Evaluation" S5 MM "Health Care Delivery" S4 MM "Outpatient Treatment" S3 S1 or S2 S2 epilep* S1 MM "Epilepsy" or DE "Epileptic Seizures" or DE "Grand Mal Seizures" or DE "Petit Mal Seizures"
Review update (EBSCO host)
S12 S8 OR S9 OR S10 OR S11 Limiters ‐ Publication Year: 2012‐
S11 S3 AND S7
S10 TI epilep* N3 specialist* OR AB epilep* N3 specialist* OR SU epilep* N3 specialist*
S9 TI epilep* N3 nurs* OR AB epilep* N3 nurs* OR SU epilep* N3 nurs*
S8 TI ( epilep* N4 center* or epilep* N4 centre* ) OR AB ( epilep* N4 center* or epilep* N4 centre* ) OR SU ( epilep* N4 center* or epilep* N4 centre* )
S7 S4 OR S5 OR S6
S6 MM "Program Evaluation"
S5 MM "Health Care Delivery"
S4 MM "Outpatient Treatment"
S3 S1 OR S2
S2 epilep*
S1 MM "Epilepsy" OR DE "Epileptic Seizures" OR DE "Grand Mal Seizures" OR DE "Petit Mal Seizures"
Appendix 6. CINAHL search strategy
Original review
This search was carried out in two phases. The first search was carried out in May 2006 using the Ovid platform for CINAHL and the following strategy. 1. exp EPILEPSY/ 2. epilep$.tw. 3. 1 or 2 4. exp Ambulatory Care/ 5. exp Health Care Delivery/ 6. exp Program Evaluation/ 7. exp "Outcomes (Health Care)"/ 8.(epilep$ adj4 (centre$ or center$)).tw. 9. (epilep$ adj3 nurs$).tw. 10. (epilep$ adj3 specialist$).tw. 11. 4 or 5 or 6 or 7 12. 3 and 11 13. 8 or 9 or 10 or 12 The second search was carried out in March 2010 using the EBSCO host platform for CINAHL, and the following strategy. S13 S9 or S10 or S11 or S12 S12 S3 and S8 S11 epilep* N3 specialist* S10 epilep* N3 nurs* S9 epilep* N4 centre* or epilep* N4 center* S8 S4 or S5 or S6 or S7 S7 (MM "Outcomes (Health Care)") S6 (MM "Program Evaluation") S5 (MM "Health Care Delivery") S4 (MM "Ambulatory Care") S3 S1 or S2 S2 epilep* S1 (MH "Epilepsy+")
Review update
S13 S9 OR S10 OR S11 OR S12 Limiters ‐ Published: 20120101‐
S12 S3 AND S8
S11 epilep* N3 specialist*
S10 epilep* N3 nurs*
S9 (epilep* N4 centre*) or (epilep* N4 center*)
S8 S4 OR S5 OR S6 OR S7
S7 (MM "Outcomes (Health Care)")
S6 (MM "Program Evaluation")
S5 (MM "Health Care Delivery")
S4 (MM "Ambulatory Care")
S3 S1 OR S2
S2 epilep*
S1 (MH "Epilepsy+")
Appendix 7. Additional detail about the interventions evaluated
Seizures and Epilepsy Education (SEE) programme(Helgeson 1990)
The SEE programme aims to meet a range of medical education and psychosocial needs. It uses a psychosocial treatment approach, based on the belief that an understanding of epilepsy helps individuals to cope with the condition and its impact. It is described in detail at the Seizures and Epilepsy Education programme website (www.theseeprogram.com/). In summary, the programme includes:
Medical aspects of epilepsy
Why understanding epilepsy is essential
An explanation of what epilepsy is
The diagnosis of epilepsy
Getting the best seizure control possible (medication, side effects, latest evidence)
Other treatments for epilepsy
First aid for epilepsy
How epilepsy may change over time
Social and emotional aspects of epilepsy
Key principles of successful coping (also taught throughout the programme)
Psychological problems of epilepsy
Coping with psychological problems
Family aspects of epilepsy
Social aspects of epilepsy
Epilepsy on the job
Resources and finding help
The programme was delivered over two days in a single weekend. No details of who delivered the programme or the delivery methods were reported.
Modular Service Package Epilepsy (MOSES) (May 2002)
MOSES aims to improve individual participants' knowledge of epilepsy, its consequences, and diagnostic and therapeutic measures, and to improve participants' understanding of psychosocial and occupational problems. Participants are encouraged to cope actively with epilepsy, to live with as few limitations as possible, to participate in treatment and to gain more self‐esteem. MOSES focuses on improving individuals' self‐help potential and on promoting the idea of participants as 'experts' in dealing with their epilepsy. No specific theoretical basis was identified as underpinning the programme. However, the MOSES programme includes cognitive, emotional, and behavioural aims. Aims for the participants are to:
get to know and understand the disease and its consequences;
learn to cope with the disease;
understand the diagnostic and therapeutic measures and to take over an active part in the treatment process;
gain a better understanding of psychosocial problems and occupational aspects;
learn to become autonomous;
become the 'ambassador of one's own disease';
lead an everyday life with as few limitations as possible.
Aims for the trainers are to:
promote the active training of the participants;
support empathic relationships with other participants;
create an interesting and varied learning atmosphere.
The MOSES modules covered the following (one topic per module).
Living with epilepsy.
Epidemiology.
Basic knowledge (causes, pathophysiology, types of seizures).
Diagnostics.
Therapy.
Self‐control.
Prognosis.
Psychosocial aspects.
Network epilepsy (how to find help and information).
The MOSES programme is delivered over two days (14 sessions of one hour) in epilepsy centres or clinics. In the May 2002 study the programme was delivered in small groups (seven to 10 people, maximum 12) and included interactive teaching, discussions and the use of a specially developed workout manual. Healthcare professionals who had been prepared by a MOSES Train‐the‐Trainer seminar (including nurses, social workers, psychologists, occupational therapists and EEG assistants) delivered the programme.
WebEase ‐ Epilepsy Awareness, Support, and Education (Dilorio 2011)
The programme incorporates elements of three different theories: social cognitive theory, the transtheoretical model of behaviour change and motivational interviewing.
Three modules (medication management, stress management and sleep management) are delivered using motivational interviewing principles. The purpose of the modules is to enable participants to assess their current status, reflect on their current behaviours, decide whether or not to change behaviour finally create a goal and action plan to either change or maintain their behaviour.
Alongside the modules is an application called MyLog into which participants enter details about their seizures, medication, stress and sleep at the start of the programme. Thereafter daily information is entered into MyLog every time they log onto the WebEase site and MyLog is also used to provide feedback during module sessions. Associated with the modules are a discussion board (My Voice) and a resource component which includes information on learning strategies and links to other useful sites (e.g. Epilepsy Foundation). Finally, to engage participants in learning about epilepsy, daily poll questions and short quizzes could be accessed from the WebEase homepage.
Participants were asked to use the WebEase programme for six weeks (two weeks in each of the three modules). After initially logging into the WebEase site, participants were first required to complete the MyLog section after which they had access to all the other components of WebEase. Participants were sent weekly reminders to log into the site Access to the programme for participants ended after six weeks.
Educational programme on self‐management in Iran (Aliasgharpour 2013)
The educational programme was delivered to groups of four to six patients. During the first session, education about the medical aspects of epilepsy was provided by a Master's student in nursing who had prior experience of working on a Brain‐Neurology ward in Iran. In the remaining three sessions, self‐management information was provided by the same individual in the following areas: medication, information, seizures, safety, and lifestyle education. In all sessions, information was presented using PowerPoint slides, demonstrations, and case histories of patients facing the challenges of epilepsy. In the first session, patients also received leaflets that contained the content of the educational programme.
Strategies to improve compliance (Peterson 1984)
The intervention consisted of a package of strategies to improve compliance.
Patient counselling on the goals of therapy and the importance of compliance (face‐to‐face and by the use of an educational leaflet).
A special medication container.
Medication and seizure diary.
Prescription refill and appointment‐keeping reminder cards sent by mail.
Information pamphlets to improve patient management in rural Zimbabwe (Adamolekun 1999)
The illustrated pamphlets provided information in the local language on the nature of epilepsy, drug therapy, compliance and seizure management.
Prompt and reminder card (Thapar 2002)
The prompt and reminder cards consisted of two main parts: 'prompts' referred to key clinical information to be recorded; 'reminders' were pieces of evidence used for patient management decisions. The cards were used over a one‐year period.
The Adverse Effects Profile (AEP) (Gilliam 2004)
The AEP was not described in detail in the paper but is available on the journal website (www.neurology.org). Essentially it entails scoring a number of adverse effects from 1 to 4, with 1 being 'never a problem' and 4 being 'always or often a problem'. The 19 adverse effects considered were:
unsteadiness;
tiredness;
restlessness;
feelings of aggression;
nervousness, aggression or both;
headache;
hair loss;
problems with skin (e.g. acne, rash);
double or blurred vision;
upset stomach;
difficulty in concentrating;
trouble with mouth or gums;
shaky hands;
weight gain;
dizziness;
sleepiness;
depression;
memory problems;
disturbed sleep.
Outpatient activities of a specialist epilepsy unit in a Welsh university hospital (Morrow 1990)
In the late 1980s, the specialist unit was staffed by healthcare personnel with an interest in epilepsy, a voluntary education officer and social worker. Patients who attended the unit were routinely provided with seizure cards (which included a seizure diary). Facilities offered to patients included EEG and antiepileptic drug evaluation and monitoring facilities.
Primary care‐based epilepsy nurse (Mills 1999a; Mills 1999b)
The role of the specialist nurse was to provide information, advice and support to patients, liaise between different components of the health service and the wider public sector, and educate primary healthcare teams. One‐on‐one consultations with patients took place either at the practice or in the patient's home.
Hospital‐based nurse‐run specialist‐care clinics (Ridsdale 1997, Ridsdale 1999, Ridsdale 2000)
At the nurse‐run clinics (in hospitals and primary care), seizure frequency and drug management were discussed, individual patient concerns addressed and advice given. A second appointment was offered three months later. In both instances, the nurse used a structured record card to record the advice she gave.
Epilepsy nurse specialist case manager (Warren 1998)
The intervention comprised input from the nurse in the areas of education and of co‐ordination and monitoring of care. The nurse complemented the work of the clinic doctors and replaced them in some aspects of care. At the initial consultation the nurse gave structured information on the specialist nurse role and on epilepsy, a care plan was developed and a 'personal health record' was given to the patient. Follow‐up care, over a six‐month period, was individualised.
Specialist neurology clinic in Norway (Helde 2005)
The intervention was delivered by a single specialist nurse with over 15 years' clinical experience in the care of people with epilepsy. It should be noted that this nurse was also the lead author of the paper. Participants received structured group education provided by a multidisciplinary group of health and social care professionals. The nurse then attended the neurology clinic and telephoned each patient every three months. Participants could also call the nurse if necessary. Nursing care was individualised, and the nurse made appointments with the neurologist as necessary.
Structured exercise programme (McAuley 2001)
The structured exercise programme consisted of three exercise sessions per week for 12 weeks. Programmes were individualised for each participant by an exercise physiologist and lasted for approximately one hour. The exercise programme focused on cardiovascular, strength and flexibility training. To remain in the study intervention group participants had to complete at least 80% of the exercise sessions.
Guideline implementation and patient information (Davis 2004)
The guidelines, Diagnosis and Management of Epilepsy in Adults, were produced by the Scottish Intercollegiate Guidelines Network (SIGN). Randomisation was by location (practices sharing premises were grouped together as a single location) using computer‐generated random numbers. The control group practices received a copy of the guidelines by mail. The intermediate intervention group received the guidelines plus protocol documents and an invitation to an interactive workshop. The intensive intervention group also received input from a nurse specialist in epilepsy, who advised practices and gave information to patients.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamolekun 1999.
| Methods | Controlled before‐and‐after study (6 months follow‐up) | |
| Participants | 400 patients registered with 24 health facilities (a district hospital, a mine hospital, three rural hospitals, and 20 rural health centers) in the Zvimba health district, Zimbabwe. Information on the age and sex of participants not provided |
|
| Interventions | Patient information pamphlets | |
| Outcomes |
|
|
| Funding | Study supported by a Zimbabwe International League Against Epilepsy educational grant | |
| Notes | There were two elements to this study: only the evaluation of the impact of patient information leaflets is included here as this was a controlled before‐and‐after study whereas the other element of the study (to evaluate the effectiveness of primary health workers in the diagnosis and management of epilepsy) did not include a control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐and‐after study, including a sub‐group analysis, compared the effect of patient leaflets with a control group. Not stated whether study and control sites for the sub‐group analysis were determined by randomisation or convenience |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not reported if any of the participants, clinicians or assessors were blinded. Reported outcomes, are, however, derived from medical records and so less likely to be prone to bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant dropout rates were not reported in the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported in the results |
| Other bias | High risk | Power calculations and required sample size were not reported. For the sub‐group analysis, it is likely that pre and postintervention periods for study and control sites were the same, and the study and control sites were comparable with respect to health system, level and setting of care and educational level of participants, but authors did not explicitly state this, nor did they report further details to compare these sites. Statistical methods did not account for the possible non‐independence of outcomes by clinic, which was the unit of study assignment. There was a possibility of contamination as patient information could easily have been distributed to control sites |
| Overall risk of bias | High risk | Lack of clarity about number of included participants (with significant risk of drop out), randomisation, allocation and blinding |
Aliasgharpour 2013.
| Methods | Randomised controlled trial (1 month follow‐up) | |
| Participants | 66 patients from the Neurology Clinic in Zanjan, Iran The majority of participants were aged 18 to 25 years (62%) and 26 to 35 years (27%); 52% were male |
|
| Interventions | Intervention: four educational sessions on epilepsy, including a self management plan Control: usual epilepsy care and support offered by the clinic |
|
| Outcomes | Epilepsy self management levels, measured using the Epilepsy Self Management Scale (ESMS) at baseline and 1 month follow‐up | |
| Funding | Research project approved and funded by Tehran University of Medical Sciences | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | None of the participants, clinicians or assessors appeared to have been blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 90% of participants were included in the analysis. Reasons for dropout reported |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods were reported in the results |
| Other bias | Unclear risk | Power calculations and the required sample size were reported. Investigators reported that there were no statistically significant baseline differences between groups although some noticeable differences were apparent from an examination of the data. There was no obvious possibility of contamination |
| Overall risk of bias | Low risk | There was no blinding but no obvious possibility of contamination, and the majority of data was included in the analysis with reasons for participant dropout also reported |
Davis 2004.
| Methods | Three‐arm cluster randomised trial (12 months follow‐up) | |
| Participants | 68 general practices (1133 patients) in Tayside, Scotland, UK Mean age across the arms ranged from 49 to 50 years; 47% were male |
|
| Interventions | Control: postal dissemination of a nationally developed clinical guideline Intermediate intervention: postal dissemination of guideline plus workshops and protocol documents Intensive intervention: intermediate intervention plus epilepsy nurse specialist to assist practices in the running of epilepsy review clinics |
|
| Outcomes | Primary outcome (SF‐36):
Secondary outcomes (five different epilepsy‐specific instruments, all of which have been previously published):
|
|
| Funding | Support from Glaxo‐Wellcome, Janssen‐Cilag, Novartis, Parke‐Davis, Sanofi, and UCB‐Pharma allowed the provision of hospitality at the workshop sessions | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated cluster‐randomisation of GP practices |
| Allocation concealment (selection bias) | Low risk | A researcher not connected with the trial conducted allocation at randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None of the GP practices (or staff), participants or assessors appeared to have been blinded. For some outcomes (from questionnaires as opposed to medical records), this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates were high in the trial, with only 72% completing the programme |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods were reported in the results |
| Other bias | Unclear risk | Power calculations and the required sample size were reported (although it is noted that the numbers of participants in each group fell short of that desired). The statistical analysis was appropriate for the cluster‐randomised design. There was no obvious possibility of contamination |
| Overall risk of bias | Unclear risk | Lack of clarity regarding blinding and significant levels of dropout |
DiIorio 2011.
| Methods | Randomised controlled trial (6 months follow‐up) | |
| Participants | 194 people recruited through epilepsy‐based websites and forums, online clinical research matching services, and referrals from healthcare professionals in a large southeastern metropolitan area, USA Mean age of participants was 43 years; 68% were male |
|
| Interventions | Intervention: WebEase (Epilepsy Awareness, Support, and Education), an online epilepsy self management programme to assist people with taking medication, managing stress and improving sleep quality Control: waiting list control (control group was put on a waiting list receiving usual care and then received the intervention at a later point in time) |
|
| Outcomes |
|
|
| Funding | Study funded by a grant from the Emory University Research Committee | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were consecutively assigned to intervention and control groups (after random assignment of the first participant) |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Investigators do not report if any of the participants, clinicians or assessors were blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The drop out rate was 24%. However, investigators conducted a completer vs non‐completer analysis and an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes detailed in the Methods were referred to in the Results, although not all values presented |
| Other bias | Unclear risk | No details of power calculations or required sample size were reported. Recruitment took place via the Internet which may have appealed to those people who are computer‐literate. There were no baseline differences reported in the comparison of study groups. There was a risk of contamination as the participants could have known each other |
| Overall risk of bias | Unclear risk | Participants were consecutively assigned to intervention and control groups (after random assignment of the first participant) |
Gilliam 2004.
| Methods | Randomised controlled trial (4 months follow‐up) | |
| Participants | 62 adults with epilepsy from outpatients clinics at Washington University (Missouri), USA Mean age of participants was 39 years; 40% were male |
|
| Interventions | Control: usual care without the Adverse Events Profile (AEP) Intervention: AEP to decrease the risk of antiepilepsy drug (AED) side effects |
|
| Outcomes | Primary outcome:
Secondary outcomes: between‐group differences in the following:
|
|
| Funding | Study supported by National Institutes of Health grant and an unrestricted grant from GlaxoSmithKline | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | A study coordinator centralised allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinicians and participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates were high in the trial, with only 71% completers |
| Selective reporting (reporting bias) | High risk | Two additional outcomes (number of clinic visits and medication dose changes) are reported in the Results which are not described in the methods |
| Other bias | Unclear risk | Power calculations and the required sample size were not reported |
| Overall risk of bias | Unclear risk | Double blind randomised trial with computer‐generated allocation, but there was evidence of selective reporting (but only two outcomes) and a significant dropout rate |
Helde 2005.
| Methods | Randomised controlled trial (2 years follow‐up with general satisfaction measured 3 months after this) | |
| Participants | 114 adult patients attending a neurological clinic in Trondheim, Norway Mean age of participants was 35 and 40 years in intervention and control arms respectively; 42% were male |
|
| Interventions | Intervention: group education programme plus follow‐up teaching and support from an epilepsy nurse Control: "conventional treatment according to individual needs" |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Funding | Study supported by a grant from Glaxo‐SmithKline, Norway | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was coordinated by a research centre, but the authors gave no further details of how the trial conducted randomisation, what blocks it used, or how it concealed allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither clinicians nor participants were blinded. However, interviews were conducted (and presumably analysed) by independent research assistants blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% of participants were included in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes were stated to be outcomes derived from the QOLIE‐89 questionnaire. All scores are reported |
| Other bias | High risk | Small study with only 28 randomised participants. Power calculations and required sample size were not reported. Some differences in baseline characteristics are noted (proportion living alone and receiving one antiepileptic drug) |
| Overall risk of bias | Low risk | Computer generated block randomisation, no blinding, and relatively low levels of dropout with most participants included in the intention‐to‐treat analysis |
Helgeson 1990.
| Methods | Randomised controlled trial (4 months follow‐up) | |
| Participants | 43 patients with epilepsy from adult epilepsy outpatient clinics in California, USA Mean age of participants was 36 and 39 years in intervention and control arms respectively; 26% were male |
|
| Interventions | Intervention: Seizures and Epilepsy Education programme, a 2‐day psychoeducational treatment programme for patients and families Control: waiting list control |
|
| Outcomes |
|
|
| Funding | Epilepsy Foundation of America provided partial financial support through a behavioral science fellowship | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation provided. Participants in the control group were older at the age of onset of seizure disorder (mean 23.39 vs 18.80 years) and had a shorter duration of seizure disorder (mean 15.44 vs 17.40 years) |
| Blinding (performance bias and detection bias) All outcomes | High risk | It is not reported if any of the participants, clinicians or assessors were blinded. The subjective nature of the outcomes (measured by self reported instruments) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 38% of those randomised completed the programme. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported in the results. |
| Other bias | Unclear risk | No details of power calculations or required sample size were reported. The intervention group completed the pre‐assessment questionnaire immediately before participating in the programme, whereas the control group participants were sent the questionnaire by post one week earlier |
| Overall risk of bias | High risk | No details about blinding; significant levels of dropout and completion of questionnaires were not conducted at the same point in time in both arms |
May 2002.
| Methods | Randomised controlled trial (6 months follow‐up) | |
| Participants | 242 patients from 22 epilepsy centres in Germany, Switzerland and Austria Mean age of participants was 38 years; 43% were male |
|
| Interventions | Intervention: Modular Service Package Epilepsy (MOSES), a 2‐day educational programme Control: waiting list control |
|
| Outcomes |
|
|
| Funding | Sanofi‐Synthelabo provided financial support | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation provided. Participants in the control group had a longer duration of epilepsy than those in the intervention group (median 18.2 vs 13.5 years) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors do not report if any of the participants, clinicians or assessors were blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 63% of those randomised completed the programme |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported in the results |
| Other bias | Unclear risk | No details of power calculations or required sample size were reported |
| Overall risk of bias | Unclear risk | Lack of detail about randomisation and allocation (but groups relatively similar at baseline apart from duration of epilepsy); no apparent blinding and a large minority of participants dropped out of the study |
McAuley 2001.
| Methods | Randomised controlled trial (12 weeks follow‐up) | |
| Participants | 28 outpatients with "documented epilepsy" from Ohio, USA Mean age of participants was 39 years; 21% were male |
|
| Interventions | Intervention: supervised exercise programme: 3 exercise sessions per week for 12 weeks Control: current level of activity with no planned intervention |
|
| Outcomes |
|
|
| Funding | Partial funding obtained from Hoechst–Marion Roussel, Glaxo‐Wellcome, and the Ohio State University | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details of randomisation provided. Numbers of participants between arms were imbalanced (17 in exercise group and 11 in control), suggesting randomisation may have failed |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | None of the participants, clinicians or assessors appeared to have been blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates were moderately high in the trial, with 82% completing the programme |
| Selective reporting (reporting bias) | High risk | All outcomes detailed in the methods were reported in the results; however, for one outcome measure (physical self concept and self esteem) results were presented at 16 weeks in this 12‐week study with no explanation as to why |
| Other bias | Unclear risk | Power calculations and required sample size were not reported. However, investigators did not report differences in baseline characteristics. There was a possibility of contamination in the trial, as randomisation does not appear to be conducted by an independent research centre |
| Overall risk of bias | High risk | Randomisation may have failed; there was no blinding and moderately high dropout rates |
Mills 1999a.
| Methods | Controlled before‐and‐after study (1 year follow‐up) Mean age of participants was 53 and 54 years in intervention and control arms respectively; 52% were male |
|
| Participants | 574 patients with epilepsy from 14 general practices in northwest Bristol, England, UK | |
| Interventions | Epilepsy specialist nurse service in primary care | |
| Outcomes | Primary outcomes
Secondary outcomes
All outcomes were derived from self completion questionnaire based on the Living With Epilepsy survey instrument |
|
| Funding | Study funded by Avon Health Authority | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The pre and postintervention periods for study and control practices were the same for the intervention and control groups, and the study and control sites were comparable with respect to distributions of practice size, doctor:population ratio, socio‐economic status, and mean distance from hospital. Practices were not, however, randomised to intervention and control arms |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors do not report if any of the participants, clinicians or assessors were blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates were high: 50.9% completed both baseline and final questionnaires |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported in the results |
| Other bias | Unclear risk | Power calculations and required sample size were reported. Though the unit of allocation was the clinic, statistical analysis did not account for clustering by clinic, and was thus not appropriate. Some significant differences were reported between intervention and control at baseline. There was no obvious possibility of contamination |
| Overall risk of bias | High risk | Quasi‐randomisation, no apparent blinding and significant dropout rate |
Mills 1999b.
| Methods | Controlled before‐and‐after study (2 years follow‐up) | |
| Participants | 394 patients with epilepsy from 14 general practices in northwest Bristol, England; participants had either used or not used the specialist nurse service evaluated by Mills 1999a; all participants had previously been included in Mills 1999a; results are based on 240 patients (120 who saw the epilepsy nurse and 120 who did not) who answered both baseline and 2 year follow‐up questionnaires Mean age of participants was 51 and 54 years in users and non‐users of specialist nurse service respectively; 53% were male |
|
| Interventions | Epilepsy specialist nurse service | |
| Outcomes |
All outcomes were derived from self completion questionnaire based on the Living With Epilepsy survey instrument |
|
| Funding | Study funded by Avon Health Authority | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unlike Mills 1999a, the comparison was now between those people who had used the specialist epilepsy nurse service and those who had not. For these new comparison groups, the pre and postintervention periods for study and control practices were the same |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | It is not reported if any of the participants, clinicians or assessors were blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates were high: 60.9% completed both baseline and final questionnaires. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods were reported in the Results |
| Other bias | Unclear risk | Power calculations and required sample size were not reported (unlike Mills 1999a). Though the unit of allocation was the clinic, statistical analysis did not account for clustering by clinic, and was thus not appropriate. Some significant differences were reported between participants who had either used or not used the specialist nurse service at baseline. There was no obvious possibility of contamination |
| Overall risk of bias | High risk | Quasi‐randomisation, no apparent blinding and significant levels of dropout. |
Morrow 1990.
| Methods | Controlled before‐and‐after trial (12 months follow‐up) although reported as randomised controlled trial (see 'Risk of bias' table for more details) | |
| Participants | 232 patients with epilepsy or suspected epilepsy and referred to further services by their primary care physician (GP) in Glamorgan, Wales Mean age of participants was 30 and 32 years in non‐randomised and randomised participants respectively; 40% were male |
|
| Interventions | Intervention: attendance at a Specialist Epilepsy Unit Control: attendance at a neurology clinic |
|
| Outcomes |
Study author states that information was derived via interview or questionnaire at baseline or review of case notes (after 12 months) |
|
| Funding | No details about funding provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This was described as a randomised controlled trial by the study author but was treated as a controlled before‐and‐after trial for the purposes of this review as only 78% of participants were successfully randomised, with both the referring physician and the consultant to whom referred having case‐by‐case veto over randomisation. |
| Allocation concealment (selection bias) | High risk | There was considerable variation in the size of the intervention (n = 130) and comparison (n = 102) arms. This occurred not only because clinicians had to agree with each referral, but also because they could withdraw participants from the trial at any time. The fact that the comparator arm (usual care) had many fewer participants as a result of the vetoes being exercised suggests a perceived bias against the comparator from those involved in allocating participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Clinicians were not blinded and based on the problems with randomisation and allocation, it would appear they had a strong bias towards the intervention over comparator. Although some outcome measures were derived from medical records and therefore less prone to bias, overall it would appear there was a high risk of bias from the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant dropout rates were not reported in the trial. |
| Selective reporting (reporting bias) | High risk | Although follow‐up measurements were made at 3, 6, and 12 months, it was not clear how these repeated measures were accounted for, if at all; they were reported as a single endpoint. |
| Other bias | High risk | Power calculations and the required sample size were not reported. There were significant differences at baseline between participants who were randomised and not randomised. |
| Overall risk of bias | High risk | Failed randomisation and allocation, a lack of blinding, significant levels of dropout, the reporting of outcomes was problematic, and there were significant differences at baseline between participants who were randomised and not randomised. |
Peterson 1984.
| Methods | Randomised controlled trial (6 months follow‐up) | |
| Participants | 53 individuals with epilepsy attending an outpatient clinic in Hobart, Australia The majority of participants were aged 20 to 39 years (58%) and 40 to 60 years (21%); 57% were male |
|
| Interventions | Intervention: range of strategies to increase compliance with anticonvulsant therapy including counselling, medication container, medication/seizure diary, prescription refill and appointment reminders Control: usual care |
|
| Outcomes |
|
|
| Funding | Information on study funding not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by the flip of a coin |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | With the exception of plasma anticonvulsant levels, all outcomes were self‐reported; it is unclear if the measurement of plasma anticonvulsant levels was conducted in a blinded manner |
| Incomplete outcome data (attrition bias) All outcomes | High risk | End‐of‐study data available for only 74% of subjects |
| Selective reporting (reporting bias) | Unclear risk | Outcomes to be measured are not precisely defined in Methods. Relevant results have been reported, but it is unclear if additional outcomes were collected but not reported |
| Other bias | Unclear risk | No details of power calculations or required sample size were reported. No significant differences in participant characteristics were reported at baseline. There was no obvious possibility of contamination |
| Overall risk of bias | Unclear risk | Single blinding, risk of selective reporting but low levels of dropout |
Ridsdale 1997.
| Methods | Randomised controlled trial (approximately 6 months follow‐up) | |
| Participants | 251 adults with epilepsy recruited from 6 general practices in the South Thames region of England Mean age of participants was 51 years; 54% were male |
|
| Interventions | Intervention: special epilepsy nurse in primary care Control: usual care |
|
| Outcomes |
|
|
| Funding | Study funded by the Nuffield Provincial Hospitals Trust and the National Society for Epilepsy | |
| Notes | Excluded people with a diagnosis of learning or language disability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None of the participants, clinicians or assessors appeared to have been blinded. For some outcomes (from questionnaires), this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clinical data were extracted from the notes of 92% subjects |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were defined broadly as "Questionnaire responses and recording of key variables extracted from the clinical records before and after the intervention". It is unclear if findings were selectively reported |
| Other bias | High risk | Power calculations and the required sample size were not reported. There was no obvious possibility of contamination. Trialists told participants in the intervention group that they would attend a 'neurology clinic', which may have been interpreted as specialist care. Potentially this belief may have improved patient outcomes over and above the effects of the intervention from the epilepsy nurse specialist |
| Overall risk of bias | Unclear risk | A lack of clarity about randomisation and blinding and moderate levels of dropout |
Ridsdale 1999.
| Methods | Randomised controlled trial (approximately 6 months follow‐up) | |
| Participants | 251 individuals with epilepsy registered with 37 general practitioners in the South Thames region of England Mean age of participants was 51 years; 54% were male |
|
| Interventions | Intervention: special epilepsy nurse in primary care Control: usual care |
|
| Outcomes | Measures of knowledge, anxiety, and depression from a postal questionnaire; patients were sent the questionnaire on two occasions, approximately six months apart | |
| Funding | Study funded by the Nuffield Provincial Hospitals Trust | |
| Notes | Excluded people with a diagnosis of learning or language disability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None of the participants, clinicians or assessors appeared to have been blinded. For some outcomes (from questionnaires), this may have introduced bias, |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22% of participants did not respond at end of study. However, neither those who attended (106/127; 78%) nor participants who responded at stage 2 (196/251; 78%) differed significantly from non‐attenders or non‐responders with respect to key baseline characteristics. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were derived from a "questionnaire that included measures of knowledge, anxiety, and depression". It is unclear if findings were selectively reported. |
| Other bias | High risk | Power calculations and the required sample size were not reported. There was no obvious possibility of contamination. Trialists told participants in the intervention group that they would attend a 'neurology clinic', which may have been interpreted as specialist care. Potentially this belief may have improved patient outcomes over and above the effects of the intervention from the epilepsy nurse specialist. |
| Overall risk of bias | Unclear risk | A lack of clarity about randomisation and blinding and moderate levels of dropout, although it is noted that attenders and responders did not significantly differ from non‐attenders or non‐responders with respect to key baseline characteristics. |
Ridsdale 2000.
| Methods | Randomised controlled trial (6 months follow‐up) | |
| Participants | 92 patients with epilepsy recruited from 5 hospitals in southeast England Mean age of participants was 40 years; 48% were male |
|
| Interventions | Intervention: special epilepsy nurse in secondary care (hospital) Control: usual care |
|
| Outcomes | 'Composite questionnaire' measuring impact on patient knowledge, satisfaction with advice and psychological well‐being; patients were sent the questionnaire on two occasions, at first appointment (baseline) and approximately three months after the second appointment (which was offered three months after initial appointment) | |
| Funding | Study supported by funding from the NHS R&D London Region and East Surrey Health Authority | |
| Notes | Excluded people with a learning or language difficulty making it impossible to complete a questionnaire and people with severe medical or psychological disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in blocks so that patients referred from each hospital were equally likely to receive the offer of active treatment or usual care. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | None of the participants, clinicians or assessors appeared to have been blinded. The subjective nature of the outcomes measured (all by self reported questionnaire) means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 88% of those randomised completed the programme, and reasons for loss to follow‐up are provided. Patients who were lost to follow‐up are reported to be significantly younger (mean age, 31 vs 43 years; P = 0.03), and at baseline reported not having had a recent epileptic attack (mean number of months, 5.8 vs 3.5; P = 0.02) |
| Selective reporting (reporting bias) | Unclear risk | A 55‐item questionnaire was used. It is unclear if findings were selectively reported. |
| Other bias | Low risk | Power calculations and required sample size were reported. There were slightly more males in the control group but no other noticeable differences in participant characteristics at baseline although baseline data is only available for participants who completed questionnaires before and after the intervention. There was no obvious possibility of contamination. |
| Overall risk of bias | Unclear risk | There was block randomisation and a relatively low dropout rate but an apparent lack of blinding. |
Thapar 2002.
| Methods | Randomised controlled trial (12 months follow‐up) | |
| Participants | 1313 adults with epilepsy, recruited from 82 general practices in four areas of Greater Manchester, England Mean age of study responders was 50 years; 48% were male |
|
| Interventions | Intervention group 1: doctor‐held reminder card Intervention group 2: patient‐held reminder card Control group: did not use prompt and reminder cards |
|
| Outcomes | Primary:
Secondary:
|
|
| Funding | Study funded by the Department of Health Implementation of research methods programme (IMP 15/12); design costs of the prompt and reminder card provided by Sanofi Pharmaceuticals | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was generated by a random number table; there were a greater number of participants in the doctor‐held arm than the other two arms, which may suggest randomisation did not work or that this arm included larger sized general practices (in terms of numbers of patients, not general practitioners) |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not reported if any of the participants, clinicians or assessors were blinded. For some outcomes (from questionnaires), this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data from medical records were available for 92% (1210) of the 1313 enrolled participants; questionnaires for 976 (74%) |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the methods were reported in the results |
| Other bias | Unclear risk | Power calculations appropriate for a cluster randomised design and the required sample size were reported. It was calculated that 600 participants in each arm were required. However, this number was not achieved in any arm. There was no obvious possibility of contamination |
| Overall risk of bias | Unclear risk | Despite an apparent lack of blinding, there was block randomisation and a relatively low dropout rate. However, it was unclear if imbalances in the number of participants in each arm of the trial was due to failed randomisation or the numbers of patients of general practices included in each arm |
Warren 1998.
| Methods | Randomised controlled trial (6 months follow‐up) | |
| Participants | 322 adults with epilepsy and their caregivers Mean age of patient responders was 36 years; 51% were male |
|
| Interventions | Intervention: epilepsy nurse specialist providing case management and clinic appointment Control: standard care from clinic doctors |
|
| Outcomes |
|
|
| Funding | Information on study funding not reported | |
| Notes | Included patients with learning disabilities | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was centrally coordinated prior to clinician involvement |
| Allocation concealment (selection bias) | Low risk | Patients were allocated by sealed envelopes inserted into the case notes of eligible participants by an individual independent to the research and clinical teams. There was no obvious possibility of contamination. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding reported. The subjective nature of the vast majority of outcomes measured by self reported questionnaire means this may have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89% of participants completed questionnaires at the end of the study |
| Selective reporting (reporting bias) | Low risk | A great many outcomes were assessed by this report. There is, however, no evidence of selective reporting |
| Other bias | Unclear risk | Authors reported a required sample size, but it was not clear if this was the result of a power calculation |
| Overall risk of bias | Low risk | There was block randomisation, but no blinding and moderate levels of dropout with differences between both responders and non‐responders and between intervention and control groups. However, these differences were accounted for by statistical analysis |
AED: antiepileptic drug; ESMS: Epilepsy Self Management Scale; QOLIE‐89: Quality of Life in Epilepsy Inventory; SF‐36: 36‐item Short Form Health Survey.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ball 2000 | Single‐arm cohort study. No patient‐related outcomes measured (study measured clinic attendance rates) |
| Becú 1993 | No epilepsy‐related outcomes measured (study evaluated effects on depression and schizophrenia) |
| DiIorio 2009 | "[T]he design of the study was not developed to test the efficacy of the intervention" |
| Fraser 1984 | Retrospective study |
| Lundgren 2006 | The interventions are not targeted at managing the primary symptoms of epilepsy, i.e. seizures |
| Lundgren 2008 | The interventions are not targeted at managing the primary symptoms of epilepsy, i.e. seizures |
| Ogata 2000 | No inter‐group comparison |
| OREp 1997 | Survey‐based before‐and‐after study. No control sites |
| Pramuka 2007 | The interventions are not targeted at managing the primary symptoms of epilepsy, i.e. seizures |
| Rasmusson 2005 | No baseline measures for outcomes |
| Sarkissian 1999 | Descriptive before‐and‐after design. No contemporaneous data collection |
Differences between protocol and review
None.
Contributions of authors
PB and BL developed the protocol for this review and developed the final systematic review. NF, PB and BL independently reviewed papers for inclusion using Cochrane EPOC Group criteria. PB led the analysis of included papers. BL wrote the original review and NF wrote the updated review. PB commented on and contributed to the write up of the original and updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
PB: None known.
BL: None known.
NF: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adamolekun 1999 {published data only}
- Adamolekun B, Mielke JK, Ball DE. An evaluation of the impact of health worker and patient education on the care and compliance of patients with epilepsy in Zimbabwe. Epilepsia 1999;40(4):507‐11. [DOI] [PubMed] [Google Scholar]
Aliasgharpour 2013 {published data only}
- Aliasgharpour M, Dehgahn Nayeri N, Yadegary MA, Haghani H. Effects of an educational program on self‐management in patients with epilepsy. Seizure 2013;22(1):48‐52. [DOI] [PubMed] [Google Scholar]
Davis 2004 {published data only}
- Davis J, Roberts R, Davidson DLW, Norman A, Ogston S, Grimshaw JM, et al. Implementation strategies for a Scottish National Epilepsy Guideline in primary care: results of the Tayside Implementation of Guidelines in Epilepsy Randomized (TIGER) Trial. Epilepsia 2004;45(1):28‐34. [DOI] [PubMed] [Google Scholar]
DiIorio 2011 {published data only}
Gilliam 2004 {published data only}
- Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology 2004;62(1):6‐7. [DOI] [PubMed] [Google Scholar]
Helde 2005 {published data only}
- Helde G, Bovim G, Bråthen G, Brodtkorb E. A structured, nurse‐led intervention program improves quality of life in patients with epilepsy: a randomized, controlled trial. Epilepsy and Behavior 2005;7:451‐7. [DOI] [PubMed] [Google Scholar]
Helgeson 1990 {published data only}
- Helgeson DC, Mittan R, Tan SR, Chayasirisobhon S. Sepulveda Epilepsy Education: the efficacy of a psychoeducational treatment programme in treating medical and psychosocial aspects of epilepsy. Epilepsia 1990;31(1):75‐82. [DOI] [PubMed] [Google Scholar]
May 2002 {published data only}
- May TW, Pfäfflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Epilepsia 2002;43(5):539‐49. [DOI] [PubMed] [Google Scholar]
McAuley 2001 {published data only}
- McAuley JW, Long L, Heise J, Kirby T, Buckworth J, Pitt C, et al. A prospective evaluation of the effects of a 12‐week outpatient exercise program on clinical and behavioral outcomes in patients with epilepsy]. Epilepsy and Behavior 2001;2:592‐600. [DOI] [PubMed] [Google Scholar]
Mills 1999a {published data only}
- Mills N, Bachmann MO, Harvey I, McGowan M. Effect of a primary care‐based epilepsy specialist nurse service on quality of care from the patient's perspective: quasi‐experimental evaluation. Seizure 1999;8(1):1‐7. [DOI] [PubMed] [Google Scholar]
Mills 1999b {published data only}
- Mills N, Bachmann MO, Campbell R, Hine I, McGowan M. Effect of a primary care based epilepsy specialist nurse service on quality of care from the patients' perspective: results at two‐years follow‐up. Seizure 1999;8(5):291‐6. [DOI] [PubMed] [Google Scholar]
Morrow 1990 {published data only}
- Morrow J. An assessment of an epilepsy clinic. In: Chadwick D editor(s). Quality of life and quality of care in epilepsy. London: Royal Society of Medicine, 1990:96‐105. [Google Scholar]
Peterson 1984 {published data only}
- Peterson GM, McLean S, Millingen KS. A randomised trial of strategies to improve patient compliance with anticonvulsant therapy. Epilepsia 1984;25(4):412‐7. [DOI] [PubMed] [Google Scholar]
Ridsdale 1997 {published data only}
- Ridsdale L, Robins D, Cryer C, Williams H. Feasibility and effects of nurse run clinics for patients with epilepsy in general practice: randomised controlled trial. British Medical Journal 1997;314(7074):120‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridsdale 1999 {published data only}
- Ridsdale L, Kwan I, Cryer C. The effect of a special nurse on patients' knowledge of epilepsy and their emotional state. British Journal of General Practice 1999;49(441):285‐9. [PMC free article] [PubMed] [Google Scholar]
Ridsdale 2000 {published data only}
- Ridsdale L, Kwan I, Cryer C. Newly diagnosed epilepsy: can nurse specialists help? A randomized controlled trial. Epilepsia 2000;41(8):1014‐9. [DOI] [PubMed] [Google Scholar]
Thapar 2002 {published data only}
- Thapar A, Jacoby A, Richens A, Russel I, Roberts C, Porter E, et al. A pragmatic randomised controlled trial of a prompt and reminder card in the care of people with epilepsy. British Journal of General Practice 2002;52(475):93‐8. [PMC free article] [PubMed] [Google Scholar]
Warren 1998 {published data only}
- Warren E. An evaluation of a Nurse Specialist/Case Manager intervention in the management of epilepsy. Report for the North West Regional Health Authority R&D Directorate 1998.
References to studies excluded from this review
Ball 2000 {published data only}
- Ball DE, Mielke J, Adamolekun B, Mundanda T, McLean J. Community leader education to increase epilepsy attendance at clinics in Epworth, Zimbabwe. Epilepsia 2000;41(8):1044‐5. [DOI] [PubMed] [Google Scholar]
Becú 1993 {published data only}
- Becú M, Becú N, Manzur G, Kochen S. Self‐help epilepsy groups: an evaluation of effect on depression and schizophrenia. Epilepsia 1993;34(5):841‐5. [DOI] [PubMed] [Google Scholar]
DiIorio 2009 {published data only}
Fraser 1984 {published data only}
- Fraser RT, Trejo W, Blanchard W. Epilepsy rehabilitation: evaluating specialized versus general agency outcome. Epilepsia 1984;25(3):332‐7. [DOI] [PubMed] [Google Scholar]
Lundgren 2006 {published data only}
Lundgren 2008 {published data only}
- Lundgren TB, Dahl J, Yardi N, Melin N. Acceptance and Commitment Therapy and yoga for drug‐refractory epilepsy: a randomized controlled trial. Epilepsy and Behavior 2008;13(1):102‐108. [DOI] [PubMed] [Google Scholar]
Ogata 2000 {published data only}
- Ogata A, Amano K. A psychosocial approach to epileptic patients. Epilepsia 2000;41(Suppl 9):36‐8. [DOI] [PubMed] [Google Scholar]
OREp 1997 {published data only}
- Osservatorio Regionale per l'Epilessia (OREp), Lombardy. The contribution of tertiary centers to the quality of the diagnosis and treatment of epilepsy. Epilepsia 1997;38(12):1338‐43. [DOI] [PubMed] [Google Scholar]
Pramuka 2007 {published data only}
Rasmusson 2005 {published data only}
- Rasmusson KA, Hartshorn JC. A comparison of epilepsy patients in a traditional ambulatory clinic and a telemedicine clinic. Epilepsia 2005;46(5):767‐70. [DOI] [PubMed] [Google Scholar]
Sarkissian 1999 {published data only}
- Sarkissian S, Wennberg R. Effects of the acute care nurse practitioner role on epilepsy monitoring outcomes. Outcomes Management for Nursing Practice 1999;3(4):161‐6. [PubMed] [Google Scholar]
Additional references
Austin 1997
- Austin JK, Boer H. Disruption in social functioning and services facilitating adjustment for the child and adult with epilepsy. In: Engel J, Pedley T editor(s). Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott‐Raven, 1997:191‐201. [Google Scholar]
Bandstra 2008
- Bandstra N, Camfield C, Camfield P. Stigma of epilepsy. Canadian Journal of Neurological Sciences 2008;35(4):436–40. [DOI] [PubMed] [Google Scholar]
Berg 2010
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51(4):676‐85. [DOI] [PubMed] [Google Scholar]
Betts 1992
- Betts T. Epilepsy services. What people need, what they want, what they get. Acta Neurologica Scandinavica Supplementum 1992;140:95‐100. [DOI] [PubMed] [Google Scholar]
Chappell 1992
- Chappell B. Epilepsy: patient views on their condition and treatment. Seizure 1992;1(2):103‐9. [DOI] [PubMed] [Google Scholar]
Clark 2008
- Clark NM, Cabana MD, Nan B, Gong ZM, Slish KK, Birk NA, et al. The clinician‐patient partnership paradigm: outcomes associated with physician communication behavior. Clinical Pediatrics 2008;47(1):49‐57. [DOI] [PubMed] [Google Scholar]
Clark 2010
- Clark NM, Stoll S, Youatt EJ, Sweetman M, Derry R, Gorelick A. Fostering epilepsy self management: the perspectives of professionals. Epilepsy Behavior 2010;19(3):255‐63. [DOI] [PubMed] [Google Scholar]
Elwyn 2003
- Elwyn G, Todd S, Hibbs R, Thapar A, Edwards P, Webb A, et al. A 'real puzzle': the views of patients with epilepsy about the organisation of care. BMC family practice 2003;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
England 2012
- England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy and Behavior 2012;25(2):266‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzsimons 2012
- Fitzsimons M, Normand C, Varley J, Delanty N. Evidence‐based models of care for people with epilepsy. Epilepsy and Behavior 2012;23(1):1‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lindsay 2015
- Lindsay B, Bradley P. Care delivery and self management strategies for children with epilepsy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD006245.pub3] [DOI] [PubMed] [Google Scholar]
Mittan 2009
- Mittan RJ. Psychosocial treatment programs in epilepsy: a review. Epilepsy and Behavior 2009;16(3):371‐80. [DOI] [PubMed] [Google Scholar]
Sander 1990
- Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. The Lancet 1990;336(8726):1267‐71. [DOI] [PubMed] [Google Scholar]
SIGN 2003
- Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of epilepsy in adults: A national clinical guideline (no. 70). Edinburgh: Scottish Intercollegiate Guidelines Network, Royal College of Physicians, 2003. [Google Scholar]
SIGN 2005
- Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and Management of Epilepsies in Children and Young People: A National Clinical Guideline (no. 81). Edinburgh: Scottish Intercollegiate Guidelines Network, 2005. [Google Scholar]
Thapar 1996
- Thapar AK. Care of patients with epilepsy in the community: will new initiatives address old problems?. British Journal of General Practice 1996;46(402):37‐42. [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO, Fact sheet 999, Epilepsy. www.who.int/mediacentre/factsheets/fs999/en/index.html (accessed: 16 December 2013).
References to other published versions of this review
Bradley 2008
- Bradley PM, Lindsay B. Care delivery and self‐management strategies for adults with epilepsy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006244.pub2] [DOI] [PubMed] [Google Scholar]


