Abstract
Background
A consumer model of health supports that people undergoing elective surgery should be informed about the past operative performance of their surgeon before making two important decisions: 1. to consent to the proposed surgery, and 2. to have a particular doctor perform the surgery. This information arguably helps empower patients to participate in their care. While surgeons' performance data are available in some settings, there continues to be controversy over the provision of such data to patients, and the question of whether consumers should, or want to, be provided with this information.
Objectives
To assess the effects of providing a surgeon's performance data to people considering elective surgery on patient‐based and service utilisation outcomes.
Search methods
For the original review, we searched: the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2009, Issue 4); MEDLINE (Ovid) (1950 to 28 September 2009); EMBASE (Ovid) (1988 to 28 September 2009); PsycINFO (Ovid) (1806 to 28 September 2009); CINAHL (EBSCO) (1982 to 20 October 2009); Current Contents (Ovid) (1992 to 23 November 2009); and ProQuest Dissertations and Theses (1861 to 20 October 2009).
For this update, we searched: CENTRAL (2009 to 3 March 2014); MEDLINE (Ovid) (2009 to 3 March 2014); EMBASE (Ovid) (2009 to 3 March 2014); PsycINFO (Ovid) (2009 to 9 March 2014); CINAHL (EBSCO) (2009 to 9 March 2014), Current Contents (Web of Science) (November 2009 to 21 March 2014), and ProQuest Dissertations and Theses (2009 to 21 March 2014). We applied no language restrictions.
Selection criteria
Randomised controlled trials (RCTs), cluster RCTs, quasi‐RCTs and controlled before and after studies (CBAs), in which an individual surgeon's performance data were provided to people considering elective surgery. We considered the CBAs for inclusion from 2009 onwards.
Data collection and analysis
Two review authors (AH, SH) independently assessed all titles, abstracts, or both of retrieved citations. We identified no studies for inclusion. Consequently, we conducted no data collection or analysis.
Main results
We found no studies that met the inclusion criteria; therefore, there are no results to report on the effect of the provision of a surgeon's performance data for people considering elective surgery.
Authors' conclusions
We found no studies reporting the impact of the provision of a surgeon's performance data for people considering elective surgery. This is an important finding in itself. While the public reporting of a surgeon's performance is not a new concept, the efficacy of this data for individual patients has not been empirically tested. A review of qualitative studies or new primary qualitative research may be useful to determine what interventions are currently in use and explore the attitudes of consumers and professionals towards such interventions.
Keywords: Humans; Professional Competence; Elective Surgical Procedures; Elective Surgical Procedures/standards; Specialties, Surgical; Specialties, Surgical/standards
Plain language summary
Provision of a surgeon's performance data for people considering elective surgery
Review question
We reviewed the evidence about the effect of providing information about a surgeon's performance to people who are thinking of having elective surgery. Elective surgery is defined as "surgery of a non emergency nature; although recommended, it can be scheduled in advance without affecting the health of the patient or the expected result of the procedure" (Dox 2004, p. 452).
Background
Measuring the performance of surgeons is generally thought to be a good practice that will result in better surgical results. Providing information about the performance of individual surgeons is more controversial and it is not clear what effect giving consumers this information might have. We wanted to discover whether there was any evidence about the effect of making data about a surgeon's performance available to people who are thinking about having elective surgery, compared with people making similar decisions without this information.
Key results
There have been studies on ways of collecting and reporting information about the performance of surgeons, but we did not find any studies published before March 2014 that looked at the effect of this information on consumers.
This lack of evidence may reflect the practical difficulties and ethical issues involved in researching this topic. For example, surgeons might not be willing to take part in such studies. There might also be legal or ethical problems with providing only some patients with information about a surgeon's performance. However, it would be helpful to have more information to inform debate on this topic. Qualitative studies are needed that explore the attitudes of consumers and professionals towards providing this type of information, and their beliefs about potential effects.
Background
This review assessed the effects of providing a surgeon's performance data to people considering elective surgery.
Reporting information about the performance of health organisations and health professionals is not a new concept. Data have been reported for since the early 1990s and there is some agreement that public reporting of the quality of health care is inevitable (Marshall 2002; Bolsin 2012). While it is generally supported that performance data will continue to be published, there is still no consensus about how the information is or should be used (Marshall 2000; Ketelaar 2011).
Although governments and organisations are reviewing the public reporting of performance of health organisations and health professionals, their priorities and reporting strategies do not necessarily help individual patients. While the macro public reporting of data and its effects are important to the research field, we must also consider what is important for individual patients who are considering elective surgery in everyday practice. Keogh 2004 suggested that the "measurement of outcomes from medical or surgical interventions is part of good practice, but publication of individual doctors' results remains controversial" (p. 450). In this review, we sought to assess the effects of providing data about a specific surgeon's performance, on an individual considering undertaking an elective surgical procedure. The data provided to the individual in this situation may or may not be part of a data set that is publicly reported. This review is in contrast to another Cochrane review that aims to assess the effects of public release of performance data on consumers, providers and purchasers of health care (Ketelaar 2011).
People's requirements for performance data about a surgeon
It is important to consider whether people need or want information about their doctor's performance in order to make decisions about their prospective surgery. In 2001, the Foundation for Accountability (FACCT) in the US found that while consumers were still largely unaware of indicators of care, they would seek information to help choose a doctor and to determine if the correct care was being suggested (FACCT 2001). FACCT also reported that participants ranked factual data about a physician as the most useful category of information for choosing a physician (FACCT 2001, p. 7). Ireson 2002 stated "consumers want understandable information that is meaningful, timely and trustworthy" (p. 49). Bozic 2013 reported "46% of patients were able to find useful information to compare outcomes among surgeons" in a study of people undergoing orthopaedic surgery, but where they found this information was not explored (p. 1867). Opinion continues to vary about whether people want, seek or value information about the surgeon's operative performance when they consider elective surgery (Schneider 1998; Beresford 2001). This review assumes that patients require information about a surgeon's performance to make informed decisions about their care. Good clinical practice supports that "patients must be given technical information that is clear and unbiased to ensure that their preferences are based on fact and not misconception" (Say 2003, p. 4).
A consumer perspective
This review assumed that patients are consumers of health care and should be able to exercise informed choice about their provider, treatment and services (Irvine 1999). Furthermore, this consumer perspective emphasises that, as partners in decision making, patients have rights: they will potentially consider a range of different service providers; the doctor will give information about the proposed treatment; and the person will make decisions based on this information (Hogg 1999). Goldfield 2003 (p. 2) suggested that one use of doctor's profiling is "to provide patients with information to guide their choice of doctor and to assess the quality of services they are receiving". In addition, the law in a number of countries now recognises the personal autonomy of people making healthcare decisions, and requires they be provided with information about any material risks faced in using healthcare services. The greatest requirement of disclosure is usually when the treatment or procedure is elective.
Historically, pre‐operative information provided to a patient has focused on describing the surgical procedure and suggesting questions for patients to ask about the procedure. This process is often framed by legal requirements to enable a patient to consent to the procedure (NHMRC 2004; UKDH 2004; CHF 2013). The performance of the attending surgeon is rarely the focus of such pre‐operative information. Yet Clarke 2004 (p. 12) argues "information about the performance ability of surgeons is a necessary component of the disclosure of the reasonably foreseeable risks of a surgical intervention". Data on a surgeon's performance provide a patient with information about the risks of having a procedure performed by a particular surgeon (Clarke 2004; Marasco 2005).
While this review advocates a consumer perspective, it also recognises that a patient's choice of surgeon may be limited by several factors. These include the ability to pay for services, the willingness of the health practitioner to provide the service, mixed public/private healthcare systems, the urgency of treatment and the complexities of the doctor‐patient relationship. Diversity across consumer groups also makes it difficult to describe the views and choices of 'ordinary' consumers or patients across different countries. Despite these difficulties, a consumer perspective advocates patient choice, with the ability to question and make fully informed decisions about treatment options. This perspective supports a patient's right to explore questions about their treating surgeon's performance.
Performance data
Issues affecting the reporting of an individual surgeon's operative performance are complex, and include: professional culture, organisational policies and procedures, understanding how much information the patient actually requires, access to resources, available data, patient co‐morbidities, risk analysis and scientific presentation (Poloniecki 1998; Gilfillan 2003; Goldfield 2003). While league tables and report cards on performance can theoretically reveal to the public the extent to which health insurance plans, healthcare providers and institutions are meeting goals, the challenge is to develop measures that are applicable and useful for consumers (Christianson 2010). Importantly, the key stakeholders, including surgeons and consumers, need transparency on the reasons for publishing the data and assurances that such data are valid, accurate and appropriately reported (Exworthy 2010; Dahlke 2014). The data's usefulness depends on many factors including the ability to cater for case mix, and whether the publication is designed to facilitate patient choice or show consistency of standards (Keogh 2004).
There is limited guidance on what information should be given to patients about a surgeon's past operative performance. Cuschieri 2000 suggested that essential data about the surgeon's past performance when operating on similar cases are: surgical outcomes (technical aspects of procedure), morbidity data (as related to the specific diagnosis) and mortality data (as related to the specific procedure). The scope of this review is constrained by reliance on the data presented in different studies. Quality issues associated with the performance data provided to patients are outside the scope of this review. However, data that informs patients about a surgeon's past performance operating on similar cases—surgical outcomes, morbidity and mortality—are considered essential.
In the context of a consumer model of health care, patients should be informed about the surgeon's past operative performance before making two important decisions:
to consent to the proposed surgery, and
to have a particular doctor perform the surgery.
The intervention we considered in this review is the provision of information about the surgeon's past operative performance. The primary outcomes of this intervention concern the decision to consent for the proposed surgery and to have a particular doctor perform that surgery. As these decisions may be compromised by the relative urgency of the surgery during a non‐elective or emergency scenario, this review only considers people requiring elective surgery.
We acknowledged before commencing the review that few relevant trials were likely to exist. Patients' information requirements in everyday practice are still being debated and it is not surprising that the provision of information about the performance of individual surgeons is controversial. Such controversy only increases the importance of reliably determining the effects of such information provision for people considering elective surgery.
This review updates the previous version of the review (Henderson 2010).
Objectives
To assess the effects of providing a surgeon's performance data to people considering elective surgery on patient‐based and service utilisation outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), cluster‐RCTs, quasi‐RCTs and controlled before and after studies (CBAs). To be included, CBA studies needed to meet the following criteria (added for the 2014 update of this review):
to have at least two intervention sites and two control sites;
the timing of the periods for study for the control and intervention groups needed to be comparable (i.e. the pre‐ and post‐intervention periods of measurement for the control and intervention groups should be the same); and
the intervention and control groups needed to be comparable on key characteristics.
Types of participants
People of any age preparing for a planned elective surgical procedure that would be undertaken by a surgeon (an accredited specialist practitioner in surgery) as reported in the study. This included people who had a legal guardian; in this case, the legal guardian would be provided with the surgeon's performance data.
We excluded people undergoing emergency procedures.
Types of interventions
The intervention under consideration was the provision of a surgeon's performance data to people considering a planned elective surgical procedure. Definitions used for this review were as follows:
surgeon: "a health practitioner who specialises in surgery" (Dox 2004, p. 452);
surgery: the "medical specialty concerned with the treatment of disease, injury, or deformity by means of manual and instrumental operations" (Dox 2004, p. 452);
elective surgery: "surgery of a non emergency nature; although recommended, it can be scheduled in advance without affecting the health of the patient or the expected result of the procedure" (Dox 2004, p. 452). In this review, the elective surgery must have been undertaken as an inpatient in any hospital or day surgery unit;
surgeon's operative performance is assessed by audit of successful surgical outcome, mortality and morbidity of the population of patients they have treated. This approach tries to avoid issues impacting patient outcomes that are related to other process and organisational factors.
The active intervention was to include the presentation of summary data about the surgeon's performance to a person considering elective surgery, and could have included:
the number of times the surgeon had completed the specific surgical procedure;
their currency in completing the particular surgical procedure;
the number of adverse events reported by the surgeon for technical aspects of completing the surgical procedure;
the health outcomes of similar patients treated by the particular surgeon undertaking the specific surgical procedure.
We excluded:
post‐procedure interventions;
interventions relating to all non‐elective care.
Types of outcome measures
We intended to compare outcomes for participants who were provided with information about their surgeon's past operative performance for the planned elective surgical procedure with outcomes for others for whom this did not occur. We intended to extract all reported outcomes and categorise them as described in Primary outcomes; Secondary outcomes.
Primary outcomes
Consumer‐oriented outcomes
Patient involvement in care process (e.g. decision making about the choice of surgeon).
Health service delivery‐oriented outcomes
Service utilisation (e.g. undertake surgery as proposed).
Secondary outcomes
Consumer‐oriented outcomes
Knowledge and understanding (e.g. patient's knowledge about their surgeon's performance).
Patient involvement in care process (e.g. seeking a second medical opinion).
Evaluation of care (e.g. patient satisfaction with the information provided).
Health status and well‐being (e.g. pre‐operative anxiety that was due to learning about the surgeon's performance, pre‐operative confidence about the surgical process and outcome, person's involvement in their care).
Health service delivery‐oriented outcomes
Health economic outcomes (e.g. costs of specific interventions, number of consultations before the procedure).
Adverse outcomes
Any adverse outcomes or harms identified in the included studies.
Search methods for identification of studies
Electronic searches
We developed search strategies using combinations of controlled vocabulary and free‐text terms (the latter restricted to the title, abstract and keyword fields) in order to retrieve a high volume of references. We adjusted the original search strategies published in the protocol to broaden the scope of captured citations (Henderson 2007). For the first iteration of this review (Henderson 2010), we searched:
the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2009, Issue 4);
MEDLINE (Ovid) (1950 to 28 September 2009);
EMBASE (Ovid) (1988 to 28 September 2009);
PsycINFO (Ovid) (1806 to 28 September 2009);
CINAHL (EBSCO) (1982 to 20 October 2009);
Current Contents (Ovid) (1992 to 23 November 2009);
ProQuest Dissertations and Theses (1861 to 20 October 2009).
We searched all databases from their start date. We applied no language restrictions and retrieved no citations required translation into English. The original search strategies were reported in the first iteration of the review (Henderson 2010).
Before updating the review in 2014, we reviewed the original search strategies. We adopted the same search strategies, with the exception of the Current Contents database, with minor changes to improve efficiency. We broadened the search strategy to include CBAs in an attempt to increase the likelihood of finding studies. We searched Current Contents using the Web of Science interface and updated the original search string accordingly.
For this update, we searched:
CENTRAL (The Cochrane Library, 2014, Issue 2);
MEDLINE (Ovid) (2009 to 3 March 2014);
EMBASE (Ovid) (2009 to 3 March 2014);
PsycINFO (Ovid) (2009 to 9 March 2014);
CINAHL (EBSCO) (October 2009 to 9 March 2014);
Current Contents (Web of Science) (2009 to 21 March 2014);
ProQuest Dissertations and Theses (2009 to 21 March 2014).
We applied no language restrictions and retrieved no citations that required translation into English. We presented search strategies for each database for the 2014 review in Appendices 1 to 7.
Searching other resources
We reviewed the bibliographies of relevant papers.
Data collection and analysis
Selection of studies
Two review authors (AH, SH) independently screened all titles, abstracts or both of the studies identified by the electronic search to determine if the studies were relevant. Two review authors (AH, SH) planned to retrieve all potentially relevant studies in full text for independent assessment against the inclusion criteria for inclusion in this review. We planned to list excluded studies and note the reasons for exclusion in the 'Characteristics of excluded studies' table. We planned to resolve any disagreements about whether to include a study by discussion and consensus between review authors. Figure 1 outlines the screening and selection process.
1.
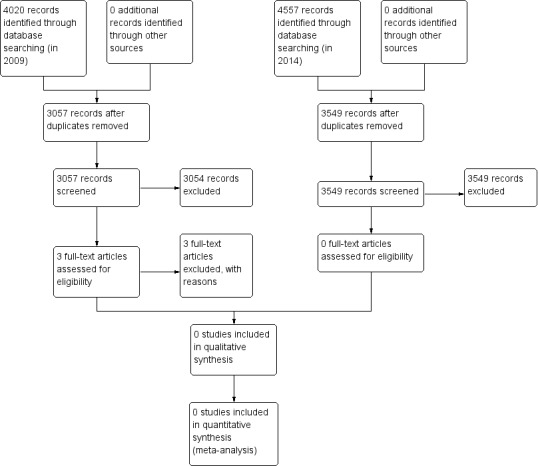
Study flow diagram.
Data extraction and management
We intended to develop, pilot test and refine a data extraction matrix to compare key areas across all studies. Two review authors (AH, SH) planned to independently extract the data for the matrix from each included study and then compare the data. The study matrix would collate the following information about each study.
Ethical approval.
Study funding source.
Declaration of interest of primary investigators.
Study participants' (people who were planning elective surgery) data including:
inclusion and exclusion criteria;
diagnostic groups;
proposed elective surgical procedure;
co‐morbidities;
medical prognosis;
ability to interpret information (first language, language spoken at home, level of education);
demographic data (age, ethnicity, gender, socioeconomic status);
private or public health cover.
Study setting. The intervention could have taken place in a variety of locations in different countries; for example:
hospital;
outpatient unit;
perioperative unit;
surgeon's private consulting rooms.
Study methods:
objective;
primary investigators' declaration of interest;
funding source;
design;
randomisation procedure;
recruitment;
informed consent;
blind assessment of outcomes;
total number of people approached;
number of people agreed to participate;
methods of analysis;
follow‐up.
Data presented to participants:
source of the data used;
content;
sample size;
validity and reliability of the data;
if the data were adjusted for case mix and risk factors/co‐morbidities;
currency of the data;
if the data were publicly available.
Timing of the intervention:
the provision of information about the surgeon's past operative performance must have been conducted in the pre‐operative period.
Intervention media:
intervention media could have included verbal, written, audio and video methods. A single or a combination of intervention media may have been used. Information may have been given to the patient to take home;
method of dissemination (numerical, graphical, pictorial).
Provider of information:
the operating surgeon;
the operating surgeon's designated healthcare representative (e.g. other medical staff qualified to provide this information, nurse);
a third party (e.g. general practitioner, insurer).
Co‐interventions (e.g. included with routine information about the surgery).
Control intervention:
pre‐operative information about the proposed elective surgery that did not include specific information about the surgeon's performance;
number of participants in control group.
Outcomes:
type of outcome;
measurement tool (including validity);
timing and frequency of assessment;
assessor.
Results
Conclusions (as stated by the study authors) were intended to be stated.
Limitations of study, and other remarks.
We intended to resolve any disagreement on the data extracted from the individual studies by discussion and consensus between review authors. One review author planned to enter all extracted data into Review Manager 5 (RevMan 2012), and a second review author planned to check entries for accuracy against the data extraction sheets. We planned to resolve disagreements by discussion; if we could not reach agreement, a third review author (to be appointed) would have decided.
Assessment of risk of bias in included studies
We intended to assess and report on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the guidelines of the Cochrane Consumers and Communication Review Group (Ryan 2013), which recommend the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting and other sources of bias. We intended to consider blinding separately for different outcomes where appropriate (e.g. blinding may have the potential to affect subjective versus objective outcome measures differently). We intended to judge each item as being at high, low or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provide a quote from the study report and a justification for our judgement for each item in the 'Risk of bias' table.
We intended to deem studies at the highest risk of bias if they were scored at high or unclear risk of bias for either the sequence generation or allocation concealment domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
In all cases, two review authors intended to assess independently the risk of bias of included studies, and resolve any disagreements by discussion to reach consensus. We intended to contact study authors for additional information about the included studies or for clarification of the study methods as required. We intended to incorporate the results of the risk of bias assessment into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment the risk of bias of included studies and a judgement about the internal validity of the review's results.
We planned to assess included quasi‐RCTs using the 'Risk of bias' tool, rating them at a high risk of bias on the random sequence generation item of the tool.
If we had included cluster RCTs in the review, we would have assessed and reported the risk of bias associated with the additional domain of selective recruitment of cluster participants (as described in Ryan 2013).
We would have assessed included CBA studies against the same criteria as RCTs but reported them at high risk of bias on both the random sequence generation and allocation sequence concealment items. We would have excluded CBA studies where the groups were not reasonably comparable at baseline.
Measures of treatment effect
For dichotomous outcomes, we intended to analyse data based on the number of events and the number of people assessed in the intervention and comparison groups. We intended to use these to calculate the risk ratio (RR) and 95% confidence interval (CI). For continuous measures, we intended to analyse data based on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI. If the MD was reported without individual group data, we intended to use this to report the study results. If more than one study had measured the same outcome using different tools, we intended to calculate the standardised mean difference (SMD) and 95% CI using the inverse‐variance method in Review Manager 5 (RevMan 2012).
For CBA studies, we intended to report appropriate effect measures for dichotomous outcomes (RR, adjusted RR) and continuous outcomes (relative per cent change post intervention, SMD).
Unit of analysis issues
For cluster‐RCTs, we intended to check for unit‐of‐analysis errors. If we had found errors, and sufficient information was available, we intended to re‐analyse the data using the appropriate unit of analysis, by taking account of the intracluster correlation (ICC). We intended to obtain estimates of the ICC by contacting authors of included studies, or impute ICC using estimates from external sources. If it were not possible to obtain sufficient information to re‐analyse the data, we intended to report effect estimates and annotate unit‐of‐analysis error.
Dealing with missing data
Had we found applicable studies with missing data, we intended to attempt to contact study authors to request missing data. For participant data, we intended to, where possible, conduct analysis on an intention‐to‐treat basis; otherwise, we planned to report data as reported. We intended to report the levels of loss to follow‐up and assess this as a source of potential bias.
Had we identified applicable missing outcome or summary data, we intended to impute missing data where possible and report any assumptions in the review. We intended to investigate, through sensitivity analyses, the effects of any imputed data on pooled effect estimates.
Assessment of heterogeneity
Had we found studies that we considered similar enough to allow pooling of data using meta‐analysis, we intended to assess the degree of heterogeneity by visual inspection of forest plots and by examining the Chi2 test for heterogeneity. We intended to quantify heterogeneity using the I2 statistic. We planned to consider an I2 value of 50% or more to represent substantial levels of heterogeneity, but we intended to interpret this value depending on the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi2 test (Higgins 2011).
Had we detected substantial clinical, methodological or statistical heterogeneity across included studies, we planned not to report pooled results from meta‐analysis but planned to use a narrative approach to data synthesis. In this event, we intended to explore possible clinical or methodological reasons for this variation by grouping similar studies to explore differences in intervention effects.
Assessment of reporting biases
We intended to assess reporting bias qualitatively based on the characteristics of the included studies (e.g. if we identified only small studies that indicated positive findings).
If we had identified sufficient studies (at least 10) for inclusion in the review, we intended to construct a funnel plot to investigate small‐study effects, which may indicate the presence of publication bias. We intended to test formally for funnel plot asymmetry, with the choice of test made based on advice in Higgins 2011, and planned to discuss the various reasons for funnel plot asymmetry when interpreting the results.
Data synthesis
The primary analysis intended to compare outcome data for participants for whom the routine pre‐operative consultation included a planned and formal presentation of information about the surgeon's performance record, with data for participants for whom this did not occur.
Once we had extracted the data, we intended to complete a narrative presentation of all studies structured according to the intervention. If enough studies existed, we planned to undertake further stratification by:
study participants' characteristics: diagnosis, proposed surgical procedure, co‐morbidities, medical prognosis, language spoken at home, first language, age, ethnicity, gender, socioeconomic status, public or private health insurance;
intervention media;
timing of the intervention.
We considered it unlikely that a meta‐analysis would be possible due to an expected lack of studies. If we had found studies, we anticipated that there would be heterogeneity in the interventions, data used and outcomes. If studies were sufficiently similar in design, interventions and outcomes, we intended to undertake a meta‐analysis using a random‐effects model.
Consumer participation
To ensure that this review was relevant to people preparing for elective surgery it was important that consumers were involved in the key stages of the review. We intended to establish a consumer panel consisting of consumers from The Cochrane Collaboration and relevant consumer organisations if we identified studies for inclusion in the review.
Results
Description of studies
Results of the search
The searches that we conducted in 2009 identified 4020 citations, which included 963 duplicate records, leaving 3057 unique records. Two review authors reviewed all citations. We removed 2704 citations from consideration based upon their title and a further 350 citations following review of the abstract. We reviewed three studies in full text because their abstracts suggested that access to a surgeon's data had been made available to patients in these studies. We excluded these three studies as their content or design did not meet the inclusion criteria of our review (see Characteristics of excluded studies table).
The updated searches that we conducted in 2014 identified 4557 citations, which included 1008 duplicate records, leaving 3549 unique records. Both review authors (AH, SH) reviewed all citations. We removed 3478 citations from consideration based upon their title. We removed the abstracts of the remaining 71 citations and included no studies in the updated review.
Figure 1 shows the study flow diagram.
Included studies
We identified no studies for inclusion in the updated review.
Risk of bias in included studies
We identified no studies that met the inclusion criteria for the review.
Effects of interventions
We identified no studies that met the inclusion criteria for the review.
Discussion
Although the search strategies returned a rich and varied literature, we identified no studies that met this review's inclusion criteria. Patients should have appropriate information to enable them to participate in decisions about their surgery (Clarke 2004; Jha 2006). Information about an individual surgeon's performance is relevant to patients in this context. Some of the challenges underpinning the provision of such information include:
public reporting of performance data for organisations, units and surgeons;
issues with the published data's accuracy, relevance and usability;
ethical issues associated with publishing comparative data for surgeons; and
the potential use of a surgeon's data in the consent process.
While this search revealed no studies that met this review's inclusion criteria, there may be more in the broader qualitative literature that could inform the design of an intervention and its evaluation.
Public reporting of performance data for organisations, units and surgeons
Public reporting of data remains controversial. Report cards are used to provide performance outcome data at different levels of health services provision such as hospital, unit or individual doctor. The literature suggests that report cards documenting hospital‐ and unit‐level outcome performance can be used in a proactive way to monitor quality and help drive improvements; however, we found no studies examining the effects of directly providing a surgeon's performance data to an individual patient. Rather, the background literature confirms challenges that continue to impact the public disclosure of individual surgeon's performance data (Ghalandarpoorattar 2012; Marjoua 2012).
Proponents of report cards argue that they provide consumers with information that allows them to have greater choice about their surgeon or health provider (Clarke 2004; Keogh 2004; Mukamel 2004; Marasco 2005; Walton 2007). However, there is no evidence of consumer uptake in line with this argument. For example, one excluded study that reviewed public reporting of risk‐adjusted coronary bypass graft mortality rates in New York concluded that "...despite a decade of experience, we found no evidence that purchasers or patients are using these reports to drive market share to higher‐performing providers" (Jha 2006, p. 854). This is consistent with previous reports, such as Schneider 1998, which found that "public reporting of mortality outcomes in Pennsylvania has had virtually no direct impact on patients' selection of hospitals or surgeons" (p. 1642).
Issues with the published data's accuracy, relevance and usability
Questions of relevance, accessibility and usefulness confront the reporting of performance data. Are the statistics accurate? Are patients able to interpret league tables of risk‐adjusted mortality figures? Can patients make sense of the information they are given? Is the information current (since data "typically becomes available one to three years after the observation period"; Jha 2006, p. 845)? Are patients likely to misinterpret the data? How can the confidentiality of data presented to patients be maintained? These questions all raise valid issues for further research and are factors that may have deterred specific research in the area. Walton 2007 suggested that current reporting is intended for a number of different audiences, and people considering elective surgery are only one of many stakeholder groups. Hence, the information provided may not be suitable for their particular needs.
Ranganathan 2009 showed that patients appear to make limited use of the available physician's performance data. This excluded study invited 6611 active and retired General Electric employees to view a physician's quality ratings website. Only 376 participants registered, yielding a registration rate of 5.7% (Ranganathan 2009, p. 75). Furthermore, Walton 2007 suggested that any assumption that patients are able to choose their surgeon based on the performance data provided may not adequately consider the patient‐doctor relationship or reflect the true level of patient empowerment.
Ethical issues associated with publishing comparative data for surgeons
A key ethical concern associated with publishing comparative performance data is that surgeons may be encouraged to avoid high‐risk patients (Burack 1999; Marasco 2005; Guru 2009; Maytham 2011Hannan 2012). Keogh 2004 also suggested that "publication of individuals' results remains controversial because of the potential, unintended negative effects and increasing recognition that individuals' results are strongly influenced by institutional influences that may impinge differently on different individuals" (p. 453). Keogh's comments, made in 2004, continue to be supported in the current literature (Guru 2009; Marjoua 2012). While the unresolved debate on the provision of individual surgeon's data, especially comparative data, continues, it potentially discourages research in the review area.
The potential use of a surgeon's data in the consent process
One of the compelling arguments for the provision of an individual surgeon's performance data to a person considering elective surgery revolves around its relevance to informed consent. Clarke 2004 (see also Clarke 2007) strongly advocates access to an individual surgeon's performance data by people considering surgery as a provision for informed consent. However, there is little agreement in this area and Freckelton 2007 observed that "report cards for doctors and for health institutions at this stage have no formal legal status, although, like a variety of other documents, they have the potential to be utilised in an evidentiary sense as a yardstick [i.e. standard comparison] for delineating acceptable and actionable conduct" (p. 279). One review of interventions to promote informed consent for people undergoing a surgical procedure did not highlight any interventions for the provision of a surgeon's performance data (Kinnersley 2013). Any requirement to provide an individual surgeon's performance data to a person considering elective surgery as part of the informed consent process remains controversial.
Summary of main results
The absence of any included studies limited this review and the discussion was informed by the review of general background literature identified by the search strategies. Many complex issues exist that may have hindered the conduct of research to date. An opportunity exists to establish a formal research agenda to understand better the factors affecting, and the impact that the provision of a surgeon's data may have on, people considering elective surgery.
Authors' conclusions
Implications for practice.
This review did not identify any randomised or quasi‐randomised trials or controlled before and after studies providing individual surgeons' performance data to people considering elective surgery. The core issues pertaining to this review have not yet been the subject of any descriptive or analytical studies. No implications for practice can be stated, as there are no data to support how the provision of surgeon's performance data for people considering elective surgery may influence their involvement in their care and decision making about the choice of surgeon.
Implications for research.
Surgeons' performance data have been reported in a variety of ways since the mid‐1990s; however, we identified no studies assessing the effects of such reporting for people considering elective surgery. The absence of relevant studies may suggest that such research is difficult to undertake. This indicates that the first question to be addressed is "What type of studies should be completed to explore the outcomes associated with an intervention that provides people considering elective surgery with the surgeon's performance data?" While randomised studies are normally the preferred research method, there are several barriers that might render the application of such a rigorous approach unrealistic. Potential problems arising from any proposed trial or study could be:
would surgeons be prepared to participate in a study?
how would randomisation of participants be completed?
are there ethical issues regarding the provision of the surgeon's performance data to intervention patients and not to control patients?
are there any legal ramifications for the consent process for such as a study(i.e. not providing all the available information for a patient to make an informed choice)?
how would the intervention information (data) be maintained in a confidential manner?
Initial qualitative studies could further the discourse, determine what interventions are currently in use and explore the attitudes of consumers and professionals towards such interventions along with their beliefs about potential effects.
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2015 | New search has been performed | The database searches employed a similar, although slightly broader, strategy to that of the original protocol. All search strategies were updated to incorporate controlled before and after (CBA) studies. This update used the Web of Science rather than Ovid to search Current Contents which reflected a wider scope. All search strategies sought updated records since 2009. The 2014 updated review has not identified any studies to inform the discussion on the provision of a surgeon's performance data for people considering elective surgery. The authors' conclusions and associated implications for practice and research remain unchanged. |
| 26 January 2015 | New citation required but conclusions have not changed | No new studies were added in the update. |
Acknowledgements
We thank Dr. Megan Prictor and Dr. Sue Cole (Managing Editors, Cochrane Consumers and Communication Review Group) and Mr John Kis‐Rigo (current Trials Search Co‐ordinator) for their support to complete the 2014 update of the review.
For the 2010 review, we acknowledge the editors and staff of the Cochrane Consumers and Communication Review Group, particularly Dr. Sophie Hill, Dr. Megan Prictor, Dr. Dell Horey, Professor Sandy Oliver, Ms. Judy Stoelwinder (former Trials Search Co‐ordinator) and Mr. John Kis‐Rigo (current Trials Search Co‐ordinator), for their assistance. The 2010 review was supported by the facilitators at the UK Cochrane Centre's Review Completion Course (which we attended in February 2010); we thank them for their patience and assistance with writing this review. We acknowledge the Emirates Academy of Hospitality Management, Dubai, for providing study leave to AH to attend the UK Cochrane Centre's Review Completion Course in 2010.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Searched 9 March 2014 (72 records)
1. [mh disclosure]
2. disclos*:ti,ab
3. consultation:kw
4. "patient information":kw
5. "interpersonal communication":kw
6. "self report":kw
7. "informed consent":kw
8. ((patient* or client* or consumer* or customer*) near/5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*)):ti,ab
9. {or #1‐#8}
10. (quality and "health care"):kw
11. (outcome and assessment):kw
12. "quality control":kw
13. competence:kw
14. "job performance":kw
15. report‐card* or score‐card* or scorecard*
16. performance* next (data or information or report* or audit* or monitor* or rate* or rating* or measure* or indicat* or assessment*)
17. (ranking* or grading* or credential* or outcome*‐data or outcome*‐report* or success‐rate*):ti,ab
18. (operative or surgical or surgeon* or clinical) next (performance* or competenc*)
19. {or #10‐#18}
20. (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*):ti,ab,kw
21. #19 and #20
22. (individual or particular or specific) near/2 (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)
23. #21 or #22
24. [mh "specialties, surgical"]
25. (surgery or surgical*):ti,ab,kw
26. [mh "surgical procedures, operative"]
27. {or #24‐#26}
28. #9 and #23 and #27
Appendix 2. MEDLINE (Ovid) search strategy
Searched 3 March 2014 (719 records)
1. exp disclosure/
2. disclos*.tw.
3. "referral and consultation"/
4. informed consent/
5. ((patient* or client* or consumer* or customer*) adj5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*)).tw.
6. or/1‐5
7. quality of health care/
8. "outcome and process assessment (health care)"/
9. "outcome assessment (health care)"/
10. quality assurance health care/
11. quality indicators health care/
12. clinical competence/
13. (report card* or score card* or scorecard* or (performance adj (data or information or report* or audit* or monitor* or rate* or rating* or measure* or indicat* or assessment*))).tw.
14. (ranking or grading or credential* or outcome* data or outcome* report* or success rate*).tw.
15. ((operative or surgical or surgeon* or clinical) adj (performance* or competenc*)).tw.
16. or/7‐15
17. (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*).tw.
18. 16 and 17
19. ((individual or particular or specific) adj2 (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)).tw.
20. or/18‐19
21. exp specialties surgical/
22. exp surgical procedures operative/
23. (surgery or surgical).tw.
24. or/21‐23
25. 6 and 20 and 24
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. random*.tw.
29. placebo*.tw.
30. drug therapy.fs.
31. trial.tw.
32. groups.ab.
33. clinical trial.pt.
34. evaluation studies.pt.
35. research design/
36. follow up studies/
37. prospective studies/
38. cross over studies/
39. comparative study.pt.
40. (experiment* or intervention*).tw.
41. (pre test or pretest or post test or posttest).tw.
42. (preintervention or postintervention).tw.
43. (cross over or crossover or factorial* or latin square).tw.
44. (assign* or allocat* or volunteer*).tw.
45. (control* or compar* or prospectiv*).tw.
46. (impact* or effect? or chang* or evaluat*).tw.
47. or/26‐46
48. 25 and 47
49. 48 and (2009* or 2010* or 2011* or 2012* or 2013* or 2014*).ed,ep,dc.
Appendix 3. EMBASE (Ovid) search strategy
Searched 3 March 2014 (2699 records)
1. interpersonal communication/
2. information/
3. patient information/
4. disclos*.tw.
5. self report/
6. voluntary reporting/
7. consultation/
8. informed consent/
9. ((patient* or client* or consumer* or customer*) adj5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*)).tw.
10. or/1‐9
11. health care quality/
12. outcome assessment/
13. exp competence/
14. performance/ or job performance/
15. quality control/
16. (report card* or score card* or scorecard* or (performance adj (data or information or report* or audit* or monitor* or rate* or rating* or measure* or indicat* or assessment*))).tw.
17. (ranking or grading or credential* or outcome* data or outcome* report* or success rate* or mortality rate*).tw.
18. ((operative or surgical or surgeon* or clinical) adj (performance* or competenc*)).tw.
19. or/11‐18
20. (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*).tw.
21. surgeon/
22. or/20‐21
23. 19 and 22
24. ((individual or particular or specific) adj2 (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)).tw.
25. or/23‐24
26. exp surgery/
27. (surgery or surgical).tw.
28. or/26‐27
29. 10 and 25 and 28
30. randomized controlled trial/
31. single blind procedure/ or double blind procedure/
32. crossover procedure/
33. random*.tw.
34. trial.tw.
35. placebo*.tw.
36. ((singl* or doubl*) adj (blind* or mask*)).tw.
37. (experiment* or intervention*).tw.
38. (pre test or pretest or post test or posttest).tw.
39. (preintervention or postintervention).tw.
40. (cross over or crossover or factorial* or latin square).tw.
41. (assign* or allocat* or volunteer*).tw.
42. (control* or compar* or prospectiv*).tw.
43. (impact* or effect? or chang* or evaluat*).tw.
44. or/30‐43
45. 29 and 44
45 and (2009* or 2010* or 2011* or 2012* or 2013* or 2014*).dd,em.
Appendix 4. PsycINFO (Ovid) search strategy
Searched 9 March 2014 (50 records)
1. disclos*.ti,ab,hw,id.
2. (communication or consultation or information or informed consent or informed choice or accountability).ti,ab,hw,id.
3. ((patient* or client* or consumer* or customer*) adj5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*)).ti,ab,id.
4. or/1‐3
5. exp quality of services/
6. clinical audit/
7. exp competence/
8. job performance/
9. quality control/
10. (report card* or score card* or scorecard* or (performance adj (data or information or report* or audit* or monitor* or rate* or rating* or measure* ndicat* or assessment*))).ti,ab,id.
11. ((operative or surgical or surgeon* or clinical) adj (performance* or competenc*)).ti,ab,id.
12. (ranking or grading or credential* or outcome* data or outcome* report* or success rate* or mortality rate*).ti,ab,id.
13. mortality rate/
14. or/5‐13
15. (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*).ti,ab,id.
16. surgeons/
17. or/15‐16
18. 14 and 17
19. ((individual or particular or specific) adj2 (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)).ti,ab,id.
20. or/18‐19
21. exp surgery/
22. (surgery or surgical).ti,ab,hw,id.
23. or/21‐22
24. 4 and 20 and 23
25. random*.ti,ab,hw,id.
26. (experiment* or intervention*).ti,ab,hw,id.
27. trial*.ti,ab,hw,id.
28. placebo*.ti,ab,hw,id.
29. groups.ab.
30. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
31. (pre test or pretest or post test or posttest).ti,ab,hw,id.
32. (preintervention or postintervention).ti,ab,hw,id.
33. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
34. (assign* or allocat* or volunteer*).ti,ab,hw,id.
35. (control* or compar* or prospectiv*).ti,ab,hw,id.
36. (impact* or effect? or chang* or evaluat*).ti,ab,hw,id.
37. exp experimental design/
38. ("0430" or "0450" or "0451" or "1800" or "2000").md.
39. or/25‐38
40. 24 and 39
41. 40 and (2009* or 2010* or 2011* or 2012* or 2013* or 2014*).up.
Appendix 5. CINAHL (EBSCO) search strategy
Searched 9 March 2014 (35 records)
1. disclos*
2. TI (patient* or client* or consumer* or customer*) and TI (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*)
3. MH communication
4. MH access to information
5. MH physician‐patient relations
6. MH consent+
7. s1 or s2 or s3 or s4 or s5 or s6
8. MH quality of health care
9. MH quality assurance
10. MH quality assessment
11. MH clinical indicators
12. MH "outcomes (health care)"
13. MH outcome assessment
14. MH surgeons evaluation
15. MH treatment outcomes+
16. MH clinical competence
17. report card* or score card* or scorecard*
18. performance N1 (data or information or report* or audit* or monitor* or rate* or rating* or measure* or indicat* or assessment*)
19. ranking or grading or credential* or outcome* data or outcome* report* or success rate*
20. (operative or surgical or surgeon* or clinical) N1 (performance* or competenc*)
21. (individual or particular or specific) N2 (surgeon* or neurosurgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)
22. s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21
23. MH surgery, operative+
24. surgery or surgical
25. s23 or s24
26. s7 and s22 and s25
27. "randomi?ed controlled trial*" or PT randomized controlled trial
28. MH Experimental Studies+
29. MH Random Assignment
30. MH Comparative Studies
31. MH Crossover Design
32. MH Placebos
33. MH Quantitative Studies
34. MH Quasi‐Experimental Studies+
35. PT Clinical Trial
36. AB (random* or trial or groups or placebo* or assign* or allocat* or volunteer* or factorial* or experiment* or control* or compar* or intervention* or chang* or evaluat* or impact* or effect or effects) or TI (random* or trial or groups or placebo* or assign* or allocat* or volunteer* or factorial* or experiment* or control* or compar* or intervention* or chang* or evaluat* or impact* or effect or effects)
37. s27 or s28 or s29 or s30 or s31 or s32 or s33 or s34 or s35 or s36
38. s26 and s37
39. s38
40. s39 and EM 200910
Appendix 6. Current Contents Connect (Web of Science) search strategy
Searched 21 March 2014 (930 records)
The original protocol search strategy searched Ovid. This update searched the Web of Science which reflected a wider scope and included the following indexes; SBS, CM, LS, AH Timespan=2009‐2014.
1. TS=disclos*
2. TS=(communication or consultation or "patient information" or "informed consent" or "informed choice" or accountability)
3. TS=((patient* or client* or consumer* or customer*) near/5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*))
4. #1 or #2 or #3
5. TS=(quality near/2 (service* or health* or care))
6. TS= (quality near/1 (control or assurance or assessment or indicator*))
7. TS=(performance near/1 (data or information or report* or audit* or monitor* or rate* or rating* or measure* or indicat* or assessment*))
8. TS=((operative or surgical or surgeon* or clinical) near/1 performance*)
9. TS=("clinical audit*" or competence or "job performance" or "report card*" or "score card*" or scorecard* or ranking* or grading or credential* or "outcome* data" or "outcome* report*" or "success rate*" or "mortality rate*")
10. #5 or #6 or #7 or #8 or #9
11. TS=(*surgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*)
12. #10 and #11
13. TS=((individual or particular or specific) near/2 (*surgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*))
14. #12 or #13
15. TS=(*surgery or *surgical*)
16. #4 and #14 and #15
17. TS=(random* or experiment* or intervention* or trial* or placebo* or ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or "pre test" or pretest or "post test" or posttest or preintervention or postintervention or "cross over" or crossover or factorial* or "latin square*" or assign* or allocat* or volunteer* or control* or compar* or prospectiv* or impact* or effect$ or chang* or evaluat*)
18. #16 and #17
Appendix 7. ProQuest Dissertations and Theses search strategy
Searched 21 March 2014 (52 records) (limited to last 5 years)
1. all(disclos* or communication or consultation or "patient information" or "informed consent" or "informed choice" or accountability or ((patient* or client* or consumer* or customer*) n/5 (advis* or warn* or inform* or communicat* or present* or provid* or provision or supply* or give* or giving or discuss*))) and all(performance or competence or card* or "clinical audit*" or scorecard* or ranking* or grading or credential* or "outcome* data" or "outcome* report*" or "success rate*" or "mortality rate*" or (quality n/1 (control or assurance or assessment or indicator*)) or (quality n/2 (service* or health* or care))) and all(*surgeon* or clinician* or physician* or doctor* or specialist* or cardiologist*) and all(*surgery or *surgical*)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jha 2006 | Reviewed surgeons' performance and explored:
The content and design of this study was not relevant to the review question |
| Mukamel 2004 | Compared the selection of cardiac surgeons before (1991) and after (1992) the introduction of quality report card publication in New York State, using retrospective data sets. The authors assumed that patients choose a cardiac surgeon practicing in their referral area. The content and design of this study was not relevant to the review question |
| Ranganathan 2009 | Used an experimental design to evaluate the extent to which participants in a benefit programme used the web to review reporting of physician‐level performance data. The intervention included 3 different letters inviting participants to use the website hosting the performance data; actual use of the performance data was then assessed. The content of this study was not relevant to the review question |
Differences between protocol and review
Following advice from the Cochrane Consumers and Communication Review Group, we adjusted the search strategy that presented in the protocol to broaden the range of captured citations (Henderson 2007). The full search strategies that we used are presented in the Appendices of the 2010 review.
Following advice from the Cochrane Consumers and Communication Review Group, we amended the databases we had planned to search, as follows:
CancerLit ‐ not included;
we searched Current Contents instead of the Science Citation Index expanded;
HMIC: The Health Management Information Consortium ‐ not included.
International Meta Register of Controlled Trials ‐ not included;
Evidenced based Internet healthcare resources ‐ not included;
MEDLINE ‐ added.
We updated the methods in the current iteration of the review to reflect changes to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and Cochrane Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards. The Data collection and analysis section now uses standard text from the Cochrane Consumers and Communication Review Group methods template (CCCRG 2014). Previous methods can be found in the protocol (Henderson 2007), and first iteration of the review (Henderson 2010).
Contributions of authors
AH: reviewed search strategies, screened titles and abstracts, assessed full‐text articles and co‐authored complete review.
SH: reviewed search strategies, completed the searches and co‐authored complete review.
Previous contributions of the authors can be found in the protocol (Henderson 2007), and first iteration of the review (Henderson 2010).
Both AH and SH will be jointly responsible for future updates of this review.
Sources of support
Internal sources
-
Cochrane Consumers and Communication Review Group, Australia.
Assistance in developing and running search strategies. Editorial and administrative support.
External sources
-
School of Nursing and Midwifery, Sunshine Coast University, Australia.
The home institution of Amanda Henderson
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Jha 2006 {published data only}
Mukamel 2004 {published data only}
Ranganathan 2009 {published data only}
Additional references
Beresford 2001
- Beresford N, Seymour L, Vincent C, Moat N. Risks of elective cardiac surgery: what do patients want to know?. Heart 2001;86:626‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolsin 2012
- Bolsin S, Barach P. The role and influence of public reporting of pediatric care outcome data. Progress in Pediatric Cardiology 2012;33:99‐101. [Google Scholar]
Bozic 2013
- Bozic K, Kaufman D, Chan V, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clinical Orthopaedics and Related Surgery 2013;471:1865‐72. [DOI: 10.1007/s11999-012-2640-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burack 1999
CCCRG 2014
- Cochrane Consumers and Communication Review Group. Standard protocol text and additional guidance for review authors, 2014. cccrg.cochrane.org (accessed 8 January 2015).
CHF 2013
- Consumers Health Forum of Australia. Informed consent in healthcare: an issues paper, 2013. www.chf.org.au/pdfs/chf/Informed‐Consent‐Issues‐Paper.pdf (accessed 8 January 2015).
Christianson 2010
- Christianson JB, Volmar KM, Alexander J, Scanlon DP. A report card on provider report cards: current status of the health care transparency movement. Journal of General Internal Medicine 2010;25(11):1235‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2004
- Clarke S, Oakley J. Informed consent and surgeons' performance. Journal of Medicine and Philosophy 2004;29(1):11‐35. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke S. Surgeons' report cards, heuristics, biases and informed consent. In: Clarke S, Oakley J editor(s). Informed Consent and Clinician Accountability: the Ethics of Report Cards on Surgeon Performance. Cambridge: Cambridge University Press, 2007:167‐79. [Google Scholar]
Cuschieri 2000
- Cuschieri A. Human reliability assessment in surgery: a new approach for improving surgical performance and clinical outcome. Annals of the Royal College of Surgeons of England 2000;82(2):83‐7. [PMC free article] [PubMed] [Google Scholar]
Dahlke 2014
- Dahlke AR, Chung JW, Holl JL, Ko CY, Rajaram R, Modla L, et al. Evaluation of initial participation in public reporting of American College of Surgeons NSQIP surgical outcomes on Medicare's Hospital Compare website. Journal of the American College of Surgeons 2014;218(3):131‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dox 2004
- Dox IG, Melloni BJ, Melloni JL, Melloni ML, Eisner GM. Melloni's Pocket Medical Dictionary: Illustrated. New York: Parthenon Publishing Group, 2004. [Google Scholar]
Exworthy 2010
- Exworthy M, Smith G, Gabe J, Jones IR. Disclosing clinical performance: the case of cardiac surgery. Journal of Health Organization & Management 2010;24(6):571‐83. [DOI] [PubMed] [Google Scholar]
FACCT 2001
- Foundation for Accountability. Consumers and quality: What do they know? What do they want? Results from FACCT consumer research 1996‐2000, 2001. www.policyarchive.org/collections/markle/index?section=5&id=95534 (accessed 8 May 2014).
Freckelton 2007
- Freckelton I. Doctor's report cards: a legal perspective. In: Clarke S, Oakley J editor(s). Informed Consent and Clinician Accountability: the Ethics of Report Cards on Surgeon Performance. Cambridge: Cambridge University Press, 2007:279‐93. [Google Scholar]
Ghalandarpoorattar 2012
- Ghalandarpoorattar SM, Kaviani A, Asghari F. Medical error disclosure: the gap between attitude and practice. Postgraduate Medical Journal 2012;88:130‐3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gilfillan 2003
- Gilfillan IS. Ranking heart surgeons has pitfalls [Letter]. BMJ 2003;327:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldfield 2003
- Goldfield N, Gnani S, Majeed A. Profiling performance in primary care in the United States. BMJ 2003;326:744‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guru 2009
- Guru V, Naylor C, Fremes S, Teoh K, Tu J. Publically reported provider outcomes: the concerns of cardiac surgeons in a single‐payer system. Canadian Journal of Cardiology 2009;25:33‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannan 2012
- Hannan E, Cozzens K, King S, Walford G, Shah N. The New York State Cardiac Registries history, contributions, limitations, and lessons for future efforts to assess and publicaly report healthcare outcomes. Journal of the American College of Cardiology 2012;59(25):2309‐16. [DOI: 10.1016/j/jacc.2011.12.051] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogg 1999
- Hogg C. Patients, Power and Politics: from Patients to Citizens. London: Sage Publications, 1999. [Google Scholar]
Ireson 2002
- Ireson CI, Ford MA, Hower JM, Schwartz RW. Outcome report cards: a necessity in the health care market. Archives of Surgery 2002;137(1):46‐51. [DOI] [PubMed] [Google Scholar]
Irvine 1999
- Irvine R. Losing patients: health care consumers, power and sociocultural change. In: Grbich C editor(s). Health in Australia: Sociological Concepts and Issues. 2nd Edition. Sydney: Longman, 1999:175‐96. [Google Scholar]
Keogh 2004
- Keogh B, Spiegelhalter D, Bailey A, Roxburgh J, Magee P, Hilton C. The legacy of Bristol: public disclosure of individual surgeons' results. BMJ 2004;329(7463):450‐4. [DOI: 10.1136/bmj.329.7463.450] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ketelaar 2011
- Ketelaar NABM, Faber MJ, Flottorp S, Rygh LH, Deane KHO, Eccles MP. Public release of performance data in changing the behaviour of healthcare consumers, professionals or organisations. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD004538.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnersley 2013
- Kinnersley P, Phillips K, Savage K, Kelly MJ, Farrell E, Morgan B, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009445.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marasco 2005
- Marasco SF, Ibrahim JE, Oakley J. Public disclosure of surgeon‐specific report cards: current status of the debate. ANZ Journal of Surgery 2005;75:1000‐4. [DOI] [PubMed] [Google Scholar]
Marjoua 2012
- Marjoua Y, Butler C, Bozic K. Public reporting of cost and quality information in orthopaedics. Clinical Orthopaedics and Related Research 2012;470:1017‐26. [DOI: 10.1007/s11999-011-2077-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall MN, Shekelle PG, Leatherman S, Brook RH. The public release of performance data: what do we expect to gain? A review of the evidence. JAMA 2000;283:1866‐74. [DOI] [PubMed] [Google Scholar]
Marshall 2002
- Marshall MN, Brook RH. Public reporting of comparative information about the quality of healthcare. Medical Journal of Australia 2002;176(5):205‐6. [DOI] [PubMed] [Google Scholar]
Maytham 2011
- Maytham G, Kessaris N. A change in opinion on surgeon's performance indicators. Interactive Cardiovascular and Thoracic Surgery 2011;12:586‐90. [DOI: 10.1510/icvts.2010.257857] [DOI] [PubMed] [Google Scholar]
NHMRC 2004
- National Health and Medical Research Council. General guidelines for medical practitioners on providing information to patients. www.nhmrc.gov.au/guidelines/publications/e57 (accessed on 04 June 2014) 2004.
Poloniecki 1998
- Poloniecki J. Half of all doctors are below average. BMJ 1998;316:1734‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Review Group. Study quality guide, 2013. cccrg.cochrane.org/author‐resources (accessed 30 March 2014).
Say 2003
- Say RE, Thompson R. The importance of patient preferences in treatment decisions: challenges for doctors. BMJ 2003;327:542‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 1998
- Schneider EC, Epstein AM. Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA 1998;279(20):1638‐42. [DOI] [PubMed] [Google Scholar]
UKDH 2004
- United Kingdom Department of Health. Patient agreement to investigation or treatment, 2004. www.dh.gov.uk/assetRoot/04/01/90/34/04019034.pdf (accessed 11 May 2004).
Walton 2007
- Walton M. Public reports: putting patients in the picture requires a new relationship between doctors and patients. In: Clarke S, Oakley J editor(s). Informed Consent and Clinician Accountability: the Ethics of Report Cards on Surgeon Performance. Cambridge: Cambridge University Press, 2007:65‐75. [Google Scholar]
References to other published versions of this review
Henderson 2007
- Henderson A, Henderson S. Provision of a surgeon's performance data for people considering elective surgery. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006327] [DOI] [Google Scholar]
Henderson 2010
- Henderson A, Henderson S. Provision of a surgeon's performance data for people considering elective surgery. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD006327.pub2] [DOI] [PubMed] [Google Scholar]


