Abstract
Background
GM‐CSF (granulocyte macrophage colony‐stimulating factor) is a growth factor that is used to supplement culture media in an effort to improve clinical outcomes for those undergoing assisted reproduction. It is worth noting that the use of GM‐CSF‐supplemented culture media often adds a further cost to the price of an in vitro fertilisation (IVF) cycle. The purpose of this review was to assess the available evidence from randomised controlled trials (RCTs) on the effectiveness and safety of GM‐CSF‐supplemented culture media.
Objectives
To assess the effectiveness and safety of GM‐CSF‐supplemented human embryo culture media versus culture media not supplemented with GM‐CSF, in women or couples undergoing assisted reproduction.
Search methods
We used standard methodology recommended by Cochrane. We searched the Cochrane Gynaecology and Fertility Group Trials Register, CENTRAL, MEDLINE, Embase, CINAHL, LILACS, DARE, OpenGrey, PubMed, Google Scholar, and two trials registers on 15 October 2019, checked references of relevant papers and communicated with experts in the field.
Selection criteria
We included RCTs comparing GM‐CSF (including G‐CSF (granulocyte colony‐stimulating factor))‐supplemented embryo culture media versus any other non‐GM‐CSF‐supplemented embryo culture media (control) in women undergoing assisted reproduction.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcomes were live birth and miscarriage rate. The secondary outcomes were clinical pregnancy, multiple gestation, preterm birth, birth defects, aneuploidy, and stillbirth rates. We assessed the quality of the evidence using GRADE methodology. We undertook one comparison, GM‐CSF‐supplemented culture media versus culture media not supplemented with GM‐CSF, for those undergoing assisted reproduction.
Main results
We included five studies, the data for three of which (1532 participants) were meta‐analysed. We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the live‐birth rate when compared to using conventional culture media not supplemented with GM‐CSF (odds ratio (OR) 1.19, 95% confidence interval (CI) 0.93 to 1.52, 2 RCTs, N = 1432, I2 = 69%, low‐quality evidence). The evidence suggests that if the rate of live birth associated with conventional culture media not supplemented with GM‐CSF was 22%, the rate with the use of GM‐CSF‐supplemented culture media would be between 21% and 30%.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the miscarriage rate when compared to using conventional culture media not supplemented with GM‐CSF (OR 0.75, 95% CI 0.41 to 1.36, 2 RCTs, N = 1432, I2 = 0%, low‐quality evidence). This evidence suggests that if the miscarriage rate associated with conventional culture media not supplemented with GM‐CSF was 4%, the rate with the use of GM‐CSF‐supplemented culture media would be between 2% and 5%.
Furthermore, we are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the following outcomes: clinical pregnancy (OR 1.16, 95% CI 0.93 to 1.45, 3 RCTs, N = 1532 women, I2 = 67%, low‐quality evidence); multiple gestation (OR 1.24, 95% CI 0.73 to 2.10, 2 RCTs, N = 1432, I2 = 35%, very low‐quality evidence); preterm birth (OR 1.20, 95% CI 0.70 to 2.04, 2 RCTs, N = 1432, I2 = 76%, very low‐quality evidence); birth defects (OR 1.33, 95% CI 0.59 to 3.01, I2 = 0%, 2 RCTs, N = 1432, low‐quality evidence); and aneuploidy (OR 0.34, 95% CI 0.03 to 3.26, I2 = 0%, 2 RCTs, N = 1432, low‐quality evidence). We were unable to undertake analysis of stillbirth, as there were no events in either arm of the two studies that assessed this outcome.
Authors' conclusions
Due to the very low to low quality of the evidence, we cannot be certain whether GM‐CSF is any more or less effective than culture media not supplemented with GM‐CSF for clinical outcomes that reflect effectiveness and safety. It is important that independent information on the available evidence is made accessible to those considering using GM‐CSF‐supplemented culture media. The claims from marketing information that GM‐CSF has a positive effect on pregnancy rates are not supported by the available evidence presented here; further well‐designed, properly powered RCTs are needed to lend certainty to the evidence.
Plain language summary
Growth factor‐supplemented culture media for women undergoing assisted reproduction
Review question
Does culture media containing the growth factor GM‐CSF (granulocyte macrophage colony‐stimulating factor) improve the chances of a pregnancy and live‐born baby, and reduce the risk of miscarriage, twin or triplet pregnancy, premature birth, birth defects, genetic problems in the baby, and stillbirth?
Background
Assisted reproduction includes processes whereby a woman's eggs and a man's sperm are combined to achieve fertilisation outside of the body. Embryos are placed in a solution called culture medium to support the growing embryo until it can be replaced into the woman's uterus. Culture medium supplemented with GM‐CSF is widely available in clinics and is often offered as an 'add‐on' to an in vitro fertilisation (IVF) cycle in an effort to improve the success rates of treatment. Using GM‐CSF‐supplemented culture medium can make IVF more expensive.
Study characteristics
The evidence is current to October 2019. We obtained data from three randomised controlled trials (a type of study in which participants are randomly assigned to one of two or more treatment groups) of 1532 infertile women undergoing IVF or intracytoplasmic sperm injection (ICSI), a specialised form of IVF whereby the sperm is injected into the egg. We compared GM‐CSF‐supplemented culture media versus culture media not supplemented with GM‐CSF for those undergoing assisted reproduction.
What the review found
Low‐quality evidence reveals that we are uncertain whether GM‐CSF‐containing culture media makes any difference to the live‐birth rate when compared to using culture media not containing GM‐CSF. This suggests that if the rate of live birth associated with culture media not containing GM‐CSF is 22%, the rate with the use of GM‐CSF‐containing culture media would be between 21% and 30%. Low‐quality evidence also reveals that we are uncertain whether GM‐CSF‐containing culture media makes any difference to miscarriage when compared to using culture media not containing GM‐CSF. This suggests that if the miscarriage rate associated with culture media not containing GM‐CSF is 4%, the rate with the use of GM‐CSF‐containing culture media would be between 2% and 5%. Low‐quality evidence for pregnancy, birth defects, and genetic problems with the baby, and very low‐quality evidence for twin or triplet pregnancies, and premature birth, reveals that we are uncertain whether GM‐CSF‐containing culture media makes any difference to these outcomes when compared to culture media not containing GM‐CSF. Two studies looked at stillbirth, but as no stillbirths occurred in either study, we were unable to analyse this outcome.
Overall conclusions
Due to the very low to low quality of the evidence, we cannot be certain whether GM‐CSF is any more or less effective or harmful than culture media not supplemented with GM‐CSF. It is important that independent information on the available evidence is made accessible to those considering using GM‐CSF‐supplemented culture media. In the meantime, more large studies are needed to increase the certainty of our conclusions.
Summary of findings
Summary of findings 1. GM‐CSF‐supplemented culture media compared to culture media not supplemented with GM‐CSF for women undergoing assisted reproduction.
| GM‐CSF‐supplemented culture media compared to culture media not supplemented with GM‐CSF for women undergoing assisted reproduction | |||||
| Patient or population: women undergoing assisted reproduction Setting: fertility clinics Intervention: GM‐CSF‐supplemented culture media Comparison: culture medium not supplemented with GM‐CSF | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with culture media not supplemented with GM‐CSF | Risk with GM‐CSF‐supplemented culture media | ||||
| Live birth or ongoing pregnancy | Study population | OR 1.19 (0.93 to 1.52) | 1432 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| 223 per 1000 | 254 per 1000 (210 to 303) | ||||
| Miscarriage | Study population | OR 0.75 (0.41 to 1.36) | 1432 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | |
| 36 per 1000 | 27 per 1000 (15 to 48) | ||||
| Clinical pregnancy | Study population | OR 1.16 (0.93 to 1.45) | 1532 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | |
| 263 per 1000 | 293 per 1000 (250 to 342) | ||||
| Multiple gestation | Study population | OR 1.24 (0.73 to 2.10) | 1432 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| 36 per 1000 | 44 per 1000 (26 to 72) | ||||
| Preterm birth | Study population | OR 1.20 (0.70 to 2.04) | 1432 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| 36 per 1000 | 43 per 1000 (25 to 70) | ||||
| Birth defects | Study population | OR 1.33 (0.59 to 3.01) | 1432 (2 RCTs) | ⊕⊕⊝⊝ LOW3 | |
| 14 per 1000 | 18 per 1000 (8 to 40) | ||||
| Aneuploidy | Study population | OR 0.34 (0.03 to 3.26) | 1432 (2 RCTs) | ⊕⊕⊝⊝ LOW 5 | |
| 3 per 1000 | 1 per 1000 (0 to 9) | ||||
| Stillbirth | Study population | ‐ | 1432 (2 RCTs) | ‐ | |
| See comment6 | See comment6 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded once for inconsistency, as the included studies report differing directions of point estimates: one supports the intervention, and one does not support the intervention. 2Downgraded once for imprecision as broad confidence intervals and a low number of included studies, at least one of which is very small. 3Downgraded twice for imprecision as very broad confidence intervals and a low number of included studies. 4Downgraded once for risk of bias. One included study had an unclear risk of selection bias, performance bias, and detection bias due to limited information available from published abstract. 5Downgraded twice for imprecision as included studies had so few reported incidences of aneuploidy that the point estimate is not precise and has very broad confidence intervals.
6No stillbirths occurred in either arm of the included studies, therefore the result is inestimable.
Background
Description of the condition
Assisted reproduction provides the opportunity to have a family for those unable to become pregnant spontaneously for a variety of reasons, including; infertility; those in single‐sex relationships; single women; and those using surrogates. Assisted reproduction is often referred to as a 'cycle', reflecting its stepwise process. It involves a series of procedures from ovarian stimulation and oocyte collection, to mixing the gametes, culturing and assessing the quality of ensuing embryos, and replacing embryos into the uterus of the woman. The success of assisted reproduction is a culmination of all the elements of the cycle, and is in part due to the ability to culture human embryos in vitro using culture media capable of supporting the developing embryo. GM‐CSF (granulocyte macrophage colony‐stimulating factor)‐supplemented culture media was developed in an effort to improve this particular part of the cycle, leading to better outcomes for those undergoing in vitro fertilisation (IVF).
GM‐CSF‐supplemented culture media can be described as an assisted reproduction 'add‐on'. Add‐ons are optional extras to an assisted reproduction cycle, which are sometimes novel interventions or therapies that have shown some promise in initial studies, or have been around for many years, but have not yet been proven to be effective through randomised controlled trials (RCTs). GM‐CSF‐supplemented culture media is one such add‐on, often provided at an additional cost to the IVF cycle (Heneghan 2016).
For the purposes of this review, any culture media containing GM‐CSF may be compared in a randomised controlled trial (RCT) against any culture media not containing GM‐CSF. We addressed the efficacy and safety of GM‐CSF‐supplemented culture media when compared to culture media not containing GM‐CSF. The primary outcomes were live birth and miscarriage.
Description of the intervention
GM‐CSF (also known as colony‐stimulating factor (CSF)‐2) and granulocyte colony‐stimulating factor (G‐CSF or CSF‐3) belong to the CSF family. They are a group of cytokines known for their role in haemopoietic cell proliferation, differentiation, and activation, as well as being an apoptosis suppressor (Rahmati 2015). Their involvement in reproduction was initially investigated in the 1970s in human placenta‐conditioned media (Burgess 1977). Amongst the CSF group, GM‐CSF is the most widely studied, and its extensive research on assisted reproduction has led to the development of new embryo culture media supplemented with human recombinant GM‐CSF. EmbryoGen and BlastGen are examples of commercially available sequential culture media containing GM‐CSF.
GM‐CSF is a cytokine that is produced by the oestrogen‐primed epithelial cells in the female reproductive tract (Robertson 1992). It is maximally expressed at the luminal and glandular epithelial cells of the endometrium in the secretory phase, and in the lining of the fallopian tube during the late proliferative and early mid‐secretory phases of the menstrual cycle (Giacomini 1995; Zhao 1994). Later during implantation, GM‐CSF is produced by the chorionic villi cells and the maternal decidua (Jokhi 1994). In response to local inflammatory stimuli, GM‐CSF acts by stimulating and activating mature monocytes, granulocytes, macrophages, and dendritic cells which promote chemotactic, phagocytic, and cytotoxic actions as well as antigen‐presenting properties needed in the immunomodulation of early pregnancy and embryogenesis (Baldwin 1992; Robertson 2007).
How the intervention might work
The control of the immunological environment during early pregnancy involves a series of autocrine and paracrine signalling between the maternal fetal interface (Robertson 1994; Robertson 2007; Wegmann 1992). Several studies have suggested an association between recurrent pregnancy loss and infertility and the dysregulation of growth factors and cytokines (Hambartsoumian 1998; Torry 2007; Vuorela 2000). In studies of genetically GM‐CSF‐deficient mice, there was a reduced inner cell mass observed which resulted in delayed blastocyst formation, increased fetal resorption in late gestation, decreased fetal size, and greater postnatal mortality (Robertson 1999). Other murine studies have also supported that GM‐CSF is crucial in optimal fetal growth and survival, as animal models lacking GM‐CSF expression experience more pregnancy losses and impaired long‐term survival of the newborn animals (Savion 2002; Seymour 1997).
The initial studies of growth factor supplementation of culture media are limited mostly to animal models, but have largely revealed improved blastocyst development rates, Lighten 1998; Sjöblom 1992; Sjöblom 1999; Spanos 2000; Yu 2012, and increased implantation and birth rates (Block 2003; Lim 2006; Roudebush 2004; Sjöblom 2005). The use of growth factor supplementation in human culture media has been limited, as it is costly to produce, and there are concerns about adverse effects (Richter 2008). Most growth factors are anti‐apoptotic, that is they inhibit programmed cell death. If not controlled, adverse effects may occur, as apoptosis is a crucial phenomenon in embryogenesis. Inhibition of apoptosis may lead to abnormal embryo development such as the well‐documented 'large offspring syndrome' that occurs in mice models (Lazzari 2002; Young 2001).
Early studies on human embryos have revealed that those cultured in GM‐CSF‐supplemented culture media had more viable inner cell masses and reduced apoptosis. This could potentially contribute to improved fetal viability (Sjöblom 1999; Sjöblom 2002). Supplementation of culture media with GM‐CSF is reported to be safe for human embryos; there are no increases or changes in ploidy rates or embryonic chromosomes (Agerholm 2010). Furthermore, initial RCTs in women revealed an improvement in the clinical pregnancy and live‐birth rates of those women randomised to culture of their embryos in GM‐CSF‐supplemented culture media (Mignini 2013; Sfontouris 2013; Tevkin 2014; Ziebe 2013). There were no major and minor birth abnormalities (Mignini 2013; Sfontouris 2013; Tevkin 2014).
Why it is important to do this review
GM‐CSF‐supplemented culture media is widely commercially available and is offered to women undergoing assisted reproduction worldwide. It is often considered an 'add‐on', or supplementary therapy, given alongside standard IVF in an effort to improve success rates (Heneghan 2016). There is currently no up‐to‐date systematic review of RCTs on this topic, and the one published systematic review relied on non‐randomised studies and studies where oocytes rather than women were randomised (Siristatidis 2013). The available RCTs were small with differing results and did not provide certainty as to what should be done in practice. Use of GM‐CSF can carry an additional cost to women undergoing IVF. It was therefore important to distil the available RCT evidence in a meaningful way to provide information on the effectiveness and safety of this intervention for women, clinicians, and embryologists, and regulatory and advisory bodies such as the Human Fertilisation and Embryology Authority (HFEA).
Objectives
To assess the effectiveness and safety of GM‐CSF‐supplemented human embryo culture media versus culture media not supplemented with GM‐CSF, in women or couples undergoing assisted reproduction.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished RCTs. We included cross‐over studies for completeness, but only pooled data from the first phase in meta‐analyses because this study design is not valid in the context of infertility trials (Vail 2003). We excluded quasi‐ and pseudo‐randomised trials. There was no limitation on language, publication date, or publication status.
Types of participants
Women undergoing IVF or intracytoplasmic sperm injection (ICSI) for any cause of infertility, using autologous or donor oocytes. Women undergoing IVF or ICSI with a background of recurrent miscarriage or recurrent implantation failure were also included.
Types of interventions
We included all studies that compared GM‐CSF (including G‐CSF)‐supplemented embryo culture media versus any other non‐GM‐CSF‐supplemented embryo culture media (control).
Types of outcome measures
Primary outcomes
Live birth per woman randomised, defined as a live baby born after 20 weeks' gestation. We used ongoing pregnancy, defined as clinical pregnancy of 12 or more weeks' gestation, as a surrogate for live birth in cases where studies did not report live birth.
Miscarriage per woman randomised. The definition used was miscarriage of clinical pregnancy.
Secondary outcomes
Clinical pregnancy per woman randomised, defined as presence on ultrasound scan of one or more gestational sacs, or definitive signs of clinical pregnancy. This included ectopic pregnancy. Note that multiple gestational sacs were counted as one clinical pregnancy.
Multiple gestation per woman randomised.
Preterm birth per woman randomised (defined as birth before 37 weeks' gestation).
Birth defects (defined as any structural anomaly present at birth that may interfere with function depending upon the organ or structure involved).
Aneuploidy (defined as any genetic disorder diagnosed during pregnancy or at the time of birth).
Stillbirth (defined as a baby born with no signs of life after 20 completed weeks of pregnancy).
Search methods for identification of studies
We searched for relevant studies with no language or date restriction in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist.
Electronic searches
We designed search strategies for the following databases:
Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials; ProCite platform, searched 15 October 2019 (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL); Ovid platform, searched 15 October 2019 (Issue 9; 2019) (Appendix 2);
MEDLINE; Ovid platform, searched from 1946 to 15 October 2019 (Appendix 3);
Embase; Ovid platform, searched from 1980 to 15 October 2019 (Appendix 4);
CINAHL (Cumulative Index to Nursing and Allied Health Literature), Ebsco platform, searched from 1961 to 15 October 2019 (Appendix 5);
LILACS (Latin American and Caribbean Health Science Information database) (lilacs.bvsalud.org/en/), Web platform, searched 15 October 2019 (Appendix 6).
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in Section 4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). The Embase and CINAHL search strategies are combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN; www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials (Web platforms, searched 15 October 2019) included:
trial registers for ongoing and registered trials: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx) (Appendix 7);
DARE (Database of Abstracts of Reviews of Effects) on the Cochrane Library (onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.htm) (Appendix 8);
Web of Knowledge (wokinfo.com) (Appendix 9);
OpenGrey (www.opengrey.eu/) for unpublished literature from Europe (Appendix 10);
PubMed and Google Scholar (for recent trials not yet indexed in the major databases) (Appendix 11 and Appendix 12).
Searching other resources
We handsearched reference lists of included and excluded studies retrieved by the search, and communicated with experts in the field to inquire after any additional studies.
We did not perform a separate search for adverse effects of GM‐CSF‐supplemented culture media. We considered adverse effects described in the studies only.
Data collection and analysis
Selection of studies
All review authors independently undertook assessment of eligibility of all studies identified by the search using Covidence (Covidence). We retrieved the full‐text publications of potentially eligible studies. Three review authors (SA, JM, and AP) screened the full texts to identify studies for inclusion, and recorded reasons for exclusion of the excluded studies in the 'Characteristics of excluded studies' table. Any disagreements were resolved by discussion or consultation with another review author.
Data extraction and management
Two review authors (SA and JM) independently extracted data on study characteristics and primary and secondary outcomes from the included studies using a data extraction form designed and piloted by the review authors. We included the following characteristics of included studies in the data extraction form:
methods;
participants;
interventions;
outcomes, including adverse events;
funding source for studies.
Any disagreements or discrepancies were resolved by discussion. Where there were multiple publications for a study, we used the main trial report as the reference and obtained additional details from secondary papers, which appear as subreferences. We corresponded with study investigators for further information on study methods and results as required. This correspondence is documented in the 'Characteristics of included studies' table and in Appendix 13.
Assessment of risk of bias in included studies
Two review authors (SA and JM) independently assessed the included studies for methodological quality and undertook data extraction according to the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed selection bias (random sequence generation and allocation concealment), attrition bias (incomplete outcome data), reporting bias (selective reporting), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors), and other biases (other problems that could put a trial at high risk of bias). Our judgements are presented and described in the 'Risk of bias' table in Characteristics of included studies. Any disagreements were resolved by discussion.
Measures of treatment effect
We summarised the effects and adverse events related to the intervention as odds ratios (ORs) using a fixed‐effect model. We presented 95% confidence intervals (CIs) for all outcomes to evaluate the precision of the estimate. We considered the clinical relevance of the results from the meta‐analysis of each comparison, taking into account the precision of the estimate. When adding data from individual studies to comparisons, we considered whether the rates of events in both the intervention and control arm reflect current practice. For example, we explored major discrepancies in direction and magnitude of effect in the Results section, and these are reflected in our 'Risk of bias' assessment.
Unit of analysis issues
The denominator for all outcomes was the number of women randomised. We did not use per‐cycle data.
We counted multiple births (e.g. twins or triplets) as one live‐birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis and attempted to obtain missing data from the primary investigators (Appendix 13). We assumed that participants who dropped out after randomisation (e.g. because of cycle cancellation), or who were lost to follow‐up or withdrew, did not achieve clinical pregnancy or live birth. We made no other assumptions.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic, considering an I2 statistic greater than 50% to indicate substantial heterogeneity (Higgins 2019). Where there was significant heterogeneity, we undertook planned subgroup analyses to explore this in more detail.
Assessment of reporting biases
We reduced the potential impact of publication and reporting bias by performing a comprehensive search for eligible studies and looking for duplication of data. We decided to construct a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) if there were 10 or more studies included in an analysis. When possible, we used published protocols and prospective trial registration web pages for included studies to investigate selective reporting (i.e. comparisons of outcomes listed in the study protocol versus outcomes reported in papers).
Data synthesis
We performed meta‐analyses where data were available from multiple studies investigating the same treatment, and the outcome was measured in a standard way between the studies. We used a fixed‐effect model. We undertook meta‐analysis according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions for the following comparison (Higgins 2019).
Studies that include GM‐CSF supplementation in human embryo culture media versus any other non‐GM‐CSF‐supplemented human embryo culture media.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for all outcomes when data were available to determine the separate effect between the following subgroups.
Studies including only women with recurrent implantation failure, defined as the failure to achieve a clinical pregnancy after transfer of at least four good‐quality embryos in a minimum of three fresh or frozen cycles (Coughlan 2014), versus studies not including women with recurrent miscarriage.
Studies using single‐step culture media versus studies using sequential culture media.
Studies including only women with donor oocytes versus studies using autologous oocytes.
Studies including only women with recurrent miscarriage (loss of three or more consecutive pregnancies before 20 weeks' gestation) versus studies not including women with recurrent miscarriage.
Studies replacing embryos at cleavage stage (day 2 or 3) versus studies replacing embryos at blastocyst stage (day 5).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias (we defined low risk of bias studies as those with low risk of bias in at least the following two domains: random sequence generation and allocation concealment);
a random‐effects model had been adopted;
the summary effect measure was risk ratio rather than OR.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table to evaluate the overall quality of the body of evidence for the main review outcomes (live birth, miscarriage, clinical pregnancy, multiple gestation, preterm birth, birth defects, aneuploidy, stillbirth) using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias) (Table 1) (GRADEpro GDT). We justified and documented judgements about the quality of the evidence (high, moderate, low, and very low) and incorporated this information into the reporting of the results for each outcome. The 'Summary of findings' table compared GM‐CSF‐supplemented embryo culture media versus any other non‐GM‐CSF‐supplemented embryo culture media (control).
Results
Description of studies
Results of the search
The following databases were systematically searched by Marian Showell, the Information Specialist at Cochrane Gynaecology and Fertility, on 15 October 2019: Cochrane Gynaecology and Fertility Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL. In addition, the 2019 European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM) conference abstracts were handsearched by review author SA on 28 October 2019. In addition, a Google search using the terms 'GM‐CSF, culture media, RCT, and live birth' was undertaken on 28 October 2019.
The search returned 452 records, 151 of which were duplicates. This left 301 titles and abstracts for screening, which was undertaken by all co‐authors using the online software Covidence (Covidence). Each record was screened by two review authors at every stage. We considered 28 papers to be eligible for full‐text screening. We excluded 23 full texts for the following reasons: 11 were the wrong study design; seven were trial registry information only, without data; three were duplicates of included studies; one was an animal study; and one was the wrong intervention. We considered five studies to be eligible for inclusion in the review (Rose 2020; Sbracia 2014; Zafardoust 2017; Zavvar 2016; Ziebe 2013), of which three could be used in meta‐analysis (Rose 2020; Sbracia 2014; Ziebe 2013). The two studies that were not included in meta‐analysis were conference abstracts, and the data could not be extracted reliably without further information from the study authors (Zafardoust 2017; Zavvar 2016); unfortunately we were unable to contact the authors of these studies to obtain the needed clarification. The PRISMA figure illustrates the flow of studies through the review (Figure 1).
1.
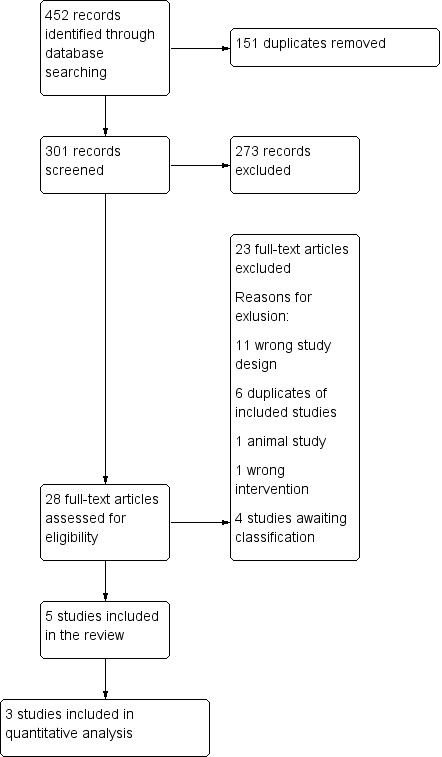
PRISMA study flow diagram.
Included studies
Five studies were eligible for inclusion in the review. Two of these were conference abstracts that could not be included in meta‐analysis because data could not be reliably extracted (Zafardoust 2017; Zavvar 2016). The remaining three studies included two fully published and peer‐reviewed papers, Rose 2020; Ziebe 2013, and one conference abstract (Sbracia 2014).
The largest study was undertaken in Europe; the trial was co‐ordinated from the Netherlands, and participants were recruited from 14 fertility clinics in Sweden and Denmark (Ziebe 2013). Ziebe 2013 included a total of 1332 participants, of whom 654 were randomised to the intervention arm and 678 were randomised to the control arm. The study was sponsored, co‐ordinated, and authored by the worldwide market‐leading manufacturer of GM‐CSF‐supplemented culture media. Women in the intervention arm had all of their embryos cultured in GM‐CSF‐supplemented culture medium at a concentration of 2 ng/mL from fertilisation through to embryo transfer. Women randomised to the control arm of the study had all of their embryos cultured in an IVF culture medium that did not contain GM‐CSF from fertilisation through to embryo transfer. Both IVF and ICSI were undertaken, and a maximum of two embryos were transferred on day 3 in a fresh embryo transfer cycle. The inclusion criteria for the study were as follows: women aged 25 to 39 years, women who had a regular menstrual cycle of 21 to 35 days, women treated with a standard gonadotropin‐releasing hormone (GnRH) agonist or antagonist protocol, and women with three or more follicles with a diameter of 14 mm on the day of human chorionic gonadotropin (hCG) administration, including a leading follicle of 17 mm. The exclusion criteria were: previous participation in the study; use of assisted hatching; use of non‐ejaculated sperm; medical conditions or genetic disorders prohibiting IVF/ICSI or interfering with the interpretation of results; use of investigational drugs within 30 days before oocyte retrieval; severe chronic disease of relevance for reproduction; and oocyte donation.
Rose 2020 was a smaller, single‐centre study undertaken in a fertility clinic in Australia. A total of 100 women were randomised, 50 to the intervention arm and 50 to the control arm of the study. There were no dropouts. Rose 2020 was a cross‐over RCT, but the published data were from the first phase of the trial prior to cross‐over. The study was sponsored by the worldwide market‐leading manufacturer of GM‐CSF‐supplemented culture media. The same company also funded two co‐authors of the study for statistical support. The women in this study underwent fresh embryo transfer following IVF or ICSI. The women in the intervention arm had all of their embryos cultured in GM‐CSF‐supplemented culture medium from fertilisation through to embryo transfer on day 5. The concentration of GM‐CSF in the intervention culture medium was 2 ng/mL, and the medium was changed on day 3 following observation, scoring, and washing, to the next phase of sequential fresh culture medium with the same concentration of GM‐CSF. The control culture medium did not contain GM‐CSF, and similarly, day 3 embryos were observed, scored, washed and then transferred to a fresh sequential culture medium. All trial participants had a day 5 embryo transfer, apart from one woman in the control arm and two in the intervention arm who underwent day 3 embryo transfer. Participants underwent single‐embryo transfer, except four women in the control arm and six women in the intervention arm, who underwent double‐embryo transfer.
The inclusion criteria were: patients must have previously had consecutive transfer of two or more embryos without a positive pregnancy outcome OR have had a history of at least one previous pregnancy loss OR a previous history of poor embryo development (< 20% of embryos developing on the time at day 3 or no blastocysts above grade 2 on day 5). Other additional inclusion parameters included a maternal age between 25 and 41 years, the use of a standard GnRH agonist or antagonist protocol, and three or more follicles of > 14 mm as seen by transvaginal ultrasound before the day of hCG administration. Exclusion criteria included: a need for surgical sperm retrieval (except in cases of previous vasectomy), the use of another investigational drug within 30 days of oocyte retrieval, and/or the presence of a severe chronic disease that could impact the IVF cycle or reproductive outcomes.
Sbracia 2014 was another small, single‐centre RCT, undertaken in a fertility clinic in Italy. The study was written as an abstract for an international conference. The authors reported that there was no funding for the study. A total of 100 women were randomised, 50 to the intervention arm and 50 to the control arm of the study. The women in the intervention arm had all of their embryos cultured in GM‐CSF‐supplemented culture media at a concentration of 2 ng/mL from fertilisation through to embryo transfer. Women in the control arm of the study had all of their embryos cultured in a medium not containing GM‐CSF from fertilisation to embryo transfer. The brand name of the control culture medium was not disclosed in the paper. Fresh embryo transfer of up to a maximum of three embryos following ICSI was undertaken in all cycles in both the intervention and control arms of the study. The inclusion criteria were: women with recurrent implantation failure, three or more consecutive failed IVF cycles with a total of at least 8 good embryos replaced in the uterus, and women aged 40 or less. The exclusion criteria were: women aged over 40, chromosomal defects in the couple, metabolic diseases (diabetes, etc.), and other genetic diseases (thalassaemia, cystic fibrosis, etc.).
Both Zafardoust 2017 and Zavvar 2016 were eligible for inclusion, however data could not be reliably extracted for meta‐analysis. Zafardoust 2017 was a conference abstract which outlined that it was a single‐centre RCT undertaken at a fertility clinic in Iran. The study included couples undergoing frozen embryo transfer following an ICSI cycle with their own gametes. Couples were randomised to either have their frozen embryos thawed and cultured in a test medium containing 2 ng/mL of GM‐CSF, or a control medium not containing GM‐CSF. Couples were eligible for inclusion in the study if the female partner was < 40 years old, had at least four good‐quality embryos after thawing (grade A), and had not had more than one previous embryo transfer. Couples were excluded from entering the study if they needed ICSI cycles requiring pre‐implantation genetic diagnosis, if the female partner had an anatomic disorder of the uterus, or one or more hydrosalpinges. The abstract outlines that 90 women were randomised, and 10 were excluded from the final analysis due to various reasons, however the original numbers of women randomised to each group are not disclosed. The outcome of interest reported by the study was clinical pregnancy, which is reported as two percentages, alongside a P value. However, it was not clear which percentage belonged to which arm of the study, therefore it was impossible to extract any meaningful data for meta‐analysis. Review author SA attempted to contact two of the authors of this study by email on three separate occasions for clarification of these issues, but unfortunately no response was forthcoming.
Zavvar 2016 was also a conference abstract, which outlined a single‐centre RCT undertaken in a fertility clinic in Iran. Zavvar 2016 sought to compare the outcomes of women undergoing ICSI who were randomised to receive an embryo culture medium containing 2 ng/mL GM‐CSF or to a culture medium not containing GM‐CSF. The inclusion criterion was women who produced only immature oocytes in spite of stimulation with gonadotropins. No exclusion criteria were described. The day of embryo transfer and length of time embryos were exposed to the intervention or control media were not described. The outcome of interest, the clinical pregnancy rate, was reported as percentages alongside a P value. However, it was not possible to identify which result was associated with which arm of the study, therefore we could not reliably include data from this study. Review author SA attempted to contact the authors of this study on two separate occasions by email, but unfortunately no response was received.
Excluded studies
We excluded 23 studies following full‐text screening. Eleven studies were the wrong study design, and were excluded for the following reasons: Agerholm 2010 was a phase I safety study and did not replace embryos; Fawzy 2019, Shapiro 2003, Sjoblom 1998, Sjoblom 1999, Sjoblom 1999a, and Sjoblom 2001 randomised oocytes opposed to women or couples; Kinoshita 2019 was a retrospective study; Min 2017 and Sfontouris 2013 were observational studies; and Siristatidis 2013 was a systematic review. Scarpellini 2011 was excluded because it did not study the intervention we were interested in, and Siqueira 2016 was excluded because it was an animal study. Six studies were duplicate references of included studies: Rose 2020, Sbracia 2014, Zafardoust 2017, and Zavvar 2016. Four studies are awaiting classification because the nature of the study and whether women or oocytes was randomised was unclear (ISRCTN94726536; NCT01689428; NCT01689454; NCT02651285).
Risk of bias in included studies
Allocation
We assessed the risk of selection bias for the three studies included in meta‐analysis to be low (Figure 2) (Rose 2020; Sbracia 2014; Ziebe 2013), as random sequence generation was described in detail and considered to be adequate to achieve a truly random sequence. Rose 2020 described how 50 cards with 'control' and 50 with 'BlastGen' written on them were placed in sealed envelopes by a person unrelated to the trial. They were shuffled several times, and the envelopes were then numbered and opened in consecutive order by the embryologist when an eligible participant was scheduled for egg retrieval. Sbracia 2014 described how participants were randomised using a computer‐generated number sequence; however, allocation concealment was not described, therefore we deemed this study to be at unclear risk. Ziebe 2013 described how they used a computer‐generated randomisation list in blocks of four for each individual clinic in order to maintain balance between the treatment groups at each site. Allocation concealment was described in detail and considered to be at low risk on the basis that each study site received a list of study‐specific consecutive patient ID numbers and a corresponding number of identical‐looking randomised bottles of test and control media that were individually labelled with the corresponding study‐specific ID numbers. On site, the lowest number on the list was always allocated to any new patient recruited at the time of informed consent signature. Consequently, the clinician, embryologist, and participant were all blinded to the allocation.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Zafardoust 2017 and Zavvar 2016 could not be included in meta‐analysis, but were considered to be at unclear risk of selection bias because no description of randomisation or allocation concealment was provided.
Blinding
We considered Rose 2020 and Ziebe 2013 to be at low risk for performance and detection bias. Rose 2020 described how clinicians, sonographers, statisticians, and participants were completely blinded to the intervention. Ziebe 2013 described how participants and investigators, including clinicians and embryologists, were blinded to treatment allocation. Following email correspondence, we established that the clinicians performing the ultrasound scans were blinded to the treatment allocation at all times.
Sbracia 2014 did not provide any description of blinding of participants, personnel, or outcome assessors, and was therefore assessed as at unclear risk of bias of performance and detection bias. We judged Zafardoust 2017 and Zavvar 2016 to be at unclear risk of performance and detection bias because there was no description of who, if anyone, was blinded.
Incomplete outcome data
We assessed Rose 2020 and Sbracia 2014 as at low risk of attrition bias. Both studies reported no dropouts. We considered Ziebe 2013 to be at high risk of attrition bias because despite all dropouts being accounted for, the reasons given were not included within the predefined exclusion criteria. For example, no oocytes retrieved, no semen sample, no fertilisation, no embryo transfer, and "non‐includable after randomisation" were given as reasons for exclusion after randomisation, however none of these were listed as exclusion criteria. We contacted the authors to obtain accurate intention‐to‐treat (ITT) data for both arms of the study, which they were able to provide.
We considered Zafardoust 2017 to be at high risk of attrition bias because 10 women were not included in the final analysis, with no reasons provided. We considered this to be a high rate of attrition in a small study. Unfortunately we were unable to use data from this study as the data could not be reliably extracted. We considered Zavvar 2016 to be at unclear risk of attrition bias as dropouts were not described. We could not include data from this study because it was unclear how many participants were included in the analysis.
Selective reporting
We rated Rose 2020, Sbracia 2014, and Ziebe 2013 as being at low risk of reporting bias because the study authors confirmed via email correspondence that they had reported all outcomes as per their prospective clinical trials registrations (NCT02305420, NCT01718210, and NCT00565747, respectively).
We rated Zafardoust 2017 as being at high risk of reporting bias. The available abstract did not report data on miscarriage, multiple pregnancy, and beta human chorionic gonadotropin (BHCG) levels, which are secondary outcomes noted on the prospective clinical trials register (Zafardoust 2017). We attempted to contact the study authors to establish if further trial data were available, but received no response. We rated Zavvar 2016 as being at unclear risk of reporting bias. We had no access to a protocol or an online clinical trial registry.
Other potential sources of bias
We have been in extensive contact with the authors of Ziebe 2013 via email to clarify various numbers from their published study. The co‐authors of this study have been very forthcoming in answering all of our queries and have offered clear explanations of how various numbers are reached in their paper. However, we have assessed Ziebe 2013 as at high risk of bias for this domain because the numbers published in the paper differ from those published in this review, that is we discovered through correspondence that some participants were inaccurately described as miscarriages opposed to biochemical pregnancy losses. We also asked for individual participant data in relation to those babies that suffered aneuploidy or birth defects, or both. On reviewing the data, we discovered that some women underwent termination of pregnancy in light of aneuploidy or birth defects, which had not been included in their aneuploidy or birth defect data. We also discovered that one baby had been classified as having a birth defects, when in fact it was reported as having immature lungs secondary to prematurity. In addition, the reporting of multiple pregnancies in the paper is very confusing. We clarified all of these issues through correspondence, which is summarised in Appendix 13. One co‐author of this review has written a letter, which has been published, outlining the concerns regarding the statistical analysis presented in the paper (Farquhar 2015). Examples of concerns include the adjustment of sample size and the increase of concentration of human serum albumin following interim analysis, and the reporting of 'ongoing implantation rate' as number of transferred embryos opposed to per woman.
Effects of interventions
See: Table 1
GM‐CSF‐supplemented culture media versus culture media not supplemented with GM‐CSF for women undergoing assisted reproduction
A total of five studies undertook this comparison. Three of these studies (1532 participants) reported data that could be included in meta‐analysis (Rose 2020; Sbracia 2014; Ziebe 2013).
Primary outcomes
1.1 Live birth
Two studies (N = 1432) provided live‐birth data (Rose 2020; Ziebe 2013). We obtained ITT live‐birth data following correspondence with the authors of Ziebe 2013 (see Appendix 13). There were 179 live births reported amongst the 704 women randomised to the GM‐CSF arm, and 162 live births amongst the 728 women randomised to the control arm. No studies reported ongoing pregnancy as a proxy to live birth.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the live‐birth rate when compared to using conventional culture media not supplemented with GM‐CSF (odds ratio (OR) 1.19, 95% confidence interval (CI) 0.93 to 1.52, 2 RCTs, N = 1432, I2 = 69%, low‐quality evidence) (Analysis 1.1; Figure 3).
1.1. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 1: Live birth
3.

Forest plot of comparison: 1 GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, outcome: 1.1 Live birth.
The evidence suggests that if the rate of live birth associated with conventional culture media not supplemented with GM‐CSF was 22%, the rate with the use of GM‐CSF‐supplemented culture media would be between 21% and 30%.
1.2 Miscarriage
Two RCTs (N = 1432) provided miscarriage data (Rose 2020; Ziebe 2013). The authors of both studies were able to clarify that the miscarriages were of clinical pregnancies. Based on correspondence, we were able to remove terminations of pregnancy that had been classified as miscarriage in these two studies (Appendix 13). Terminations of pregnancy as a result of aneuploidy or birth defect are accounted for in Analysis 1.6 and Analysis 1.7. All miscarriages were first‐trimester losses, apart from one in the control arm of Rose 2020, which was a midtrimester loss at 17 weeks' gestation. There were 19 miscarriages amongst the 704 women randomised to the GM‐CSF arm, and 26 miscarriages amongst the 728 women randomised to the control arm.
1.6. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 6: Birth defects
1.7. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 7: Aneuploidy
It is unclear whether use of GM‐CSF‐supplemented culture media makes any difference to miscarriage rate when compared to conventional culture media not supplemented with GM‐CSF (OR 0.75, 95% CI 0.41 to 1.36, 2 RCTs, N = 1432, I2 = 0%, low‐quality evidence) (Analysis 1.2; Figure 4).
1.2. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 2: Miscarriage
4.

Forest plot of comparison: 1 GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, outcome: 1.2 Miscarriage.
The evidence suggests that if the miscarriage rate associated with conventional culture media not supplemented with GM‐CSF was 4%, the rate with the use of GM‐CSF‐supplemented culture media would be between 2% and 5%. It is worth noting that these figures are per woman randomised, hence the apparently very low miscarriage rates. They do not include miscarriages that occurred before the diagnosis of a clinical pregnancy on ultrasound scan, otherwise known as biochemical pregnancy losses.
Secondary outcomes
1.3 Clinical pregnancy
Three studies (N = 1532) reported clinical pregnancy rates (Rose 2020; Sbracia 2014; Ziebe 2013). Both Rose 2020 and Ziebe 2013 describe how an ultrasound scan was performed at seven weeks’ gestation in order to diagnose clinical pregnancy. Information on the methods of Sbracia 2014 was limited, as the study is only available as a conference abstract, and we received no response to our emails to the authors of the study. The authors of Sbracia 2014 describe pregnancy rate as their primary outcome, however there are no further details as to what stage pregnancy was diagnosed, and whether they were clinical pregnancies diagnosed with ultrasound. The authors report an "implantation rate", which we have taken to mean a biochemical pregnancy rate. Consequently, for the purposes of this review, we have assumed the pregnancy rate in Sbracia 2014 to be clinical.
There were 221 clinical pregnancies amongst the 754 women randomised to the GM‐CSF arm, and 205 clinical pregnancies amongst the 778 women randomised to the control arm.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the clinical pregnancy rate when compared to using a conventional culture medium not supplemented with GM‐CSF (OR 1.16, 95% CI 0.93 to 1.45, 3 RCTs, N = 1532 women, I2 = 67%, low‐quality evidence) (Analysis 1.3; Figure 5).
1.3. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 3: Clinical pregnancy
5.

Forest plot of comparison: 1 GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, outcome: 1.3 Clinical pregnancy.
The evidence suggests that if the clinical pregnancy rate associated with conventional culture media not supplemented with GM‐CSF was 26%, the rate with the use of GM‐CSF‐supplemented culture media would be between 25% and 34%.
1.4 Multiple gestation
Two studies (N = 1432) reported multiple gestation rate (Rose 2020; Ziebe 2013). The multiple gestation rate was clarified following correspondence with authors of both studies (Appendix 13). The authors of Ziebe 2013 also detail the incidence of monozygotic and dizygotic twins, but in this review we did not differentiate between types of twins. The authors of Rose 2020 report single‐embryo transfer as standard, but explain that four women in the control arm and six women in the intervention arm received double‐embryo transfer (Appendix 13). The authors of Ziebe 2013 describe how a maximum of two embryos were replaced per woman with a mean embryo transfer rate of 1.51 for the control arm and 1.49 for the GM‐CSF arm. There was one triplet pregnancy in the intervention arm of the study by Ziebe 2013. The remaining multiple gestations reported here were all twins.
There were 31 women with a multiple gestation amongst the 704 women randomised to the GM‐CSF arm, and 205 women with a multiple pregnancy amongst the 728 women randomised to the control arm.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the multiple pregnancy rate when compared to use of a conventional culture medium not supplemented with GM‐CSF (OR 1.24, 95% CI 0.73 to 2.10, 2 RCTs, N = 1432, I2 = 35%, very low‐quality evidence) (Analysis 1.4).
1.4. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 4: Multiple gestation
The evidence suggests that if the multiple gestation rate associated with conventional culture media not supplemented with GM‐CSF was 4%, the rate with the use of GM‐CSF‐supplemented culture media would be between 3% and 7%.
1.5 Preterm birth
Two studies (N = 1432) reported the preterm birth rate, defined as the birth of a baby (or babies in the case of multiple pregnancy) under 37 weeks' gestation, per woman randomised (Rose 2020; Ziebe 2013). Preterm birth was detailed in the published study by Ziebe 2013. For singletons, the preterm birth data were easily extractable. For women with multiple gestations, the authors of Ziebe 2013 report gestational age at birth with a standard deviation, therefore we clarified these data with the study authors to establish the number of preterm births (Appendix 13). We sought preterm birth data through correspondence with the authors of Rose 2020 (Appendix 13). We counted twins and triplets that were born before 37 weeks as one event for this outcome, as we undertook ITT analysis.
There were 30 women with a preterm birth amongst the 704 women randomised to the GM‐CSF arm, and 26 women with a preterm birth amongst the 728 women randomised to the control arm.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the preterm birth rate when compared to using a conventional culture medium not supplemented by GM‐CSF (OR 1.20, 95% CI 0.70 to 2.04, 2 RCTs, N = 1432, I2 = 76%, very low‐quality evidence) (Analysis 1.5).
1.5. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 5: Preterm birth
The evidence suggests that if the preterm birth rate associated with conventional culture media not supplemented with GM‐CSF was 4%, the rate with the use of GM‐CSF‐supplemented culture media would be between 3% and 7%.
1.6 Birth defects
The authors of two studies (N = 1432) were able to provide details on birth defects following correspondence (Appendix 13) (Rose 2020; Ziebe 2013). The authors of Rose 2020 explained that there was one baby with multiple birth defects, which was detected antenatally (this participant was classified as experiencing a miscarriage in the published study, but we have clarified that this was a termination of pregnancy, and it has therefore been removed from the miscarriage group in this review). The authors of Ziebe 2013 provided details on 22 infants who were born with defects (three participants underwent termination of pregnancy for birth defects). We did not count any infants as having both birth defects and aneuploidy, but rather divided them into one of the two groups. We are not aware of twins within the birth defects group, and have assumed all data to be per woman randomised.
Thirteen women had a baby with a birth defect amongst the 704 women randomised to the GM‐CSF arm, and 10 women had a baby with a birth defect amongst the 728 women randomised to the control arm.
We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the rate of birth defects when compared to using a conventional culture medium not supplemented by GM‐CSF (OR 1.33, 95% CI 0.59 to 3.01, I2 = 0%, 2 RCTs, N = 1432, low‐quality evidence) (Analysis 1.6).
The evidence suggests that if the birth defect rate associated with conventional culture media not supplemented with GM‐CSF was 1%, the rate with the use of GM‐CSF‐supplemented culture media would be between 1% and 4%.
1.7 Aneuploidy
Two studies (N = 1432) provided data regarding aneuploidy after we inquired about this outcome (Rose 2020; Ziebe 2013). The authors of Rose 2020 described how one baby had a trisomy, which was detected antenatally, and Ziebe 2013 reported that one baby had a trisomy detected antenatally.
No women had a baby with aneuploidy amongst the 704 women randomised to the GM‐CSF arm, and two women had a baby with aneuploidy amongst the 728 women randomised to the control arm. We are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the rate of aneuploidy when compared to using a conventional culture medium not supplemented by GM‐CSF (OR 0.34, 95% CI 0.03 to 3.26, I2 = 0%, 2 RCTs, N = 1432, low‐quality evidence) (Analysis 1.7).
The evidence suggests that if the aneuploidy rate associated with conventional culture media not supplemented with GM‐CSF was 0.3%, the rate with the use of GM‐CSF‐supplemented culture media would be between 0% and 0.9%.
1.8 Stillbirth
Two studies (N = 1432) reported stillbirth (Rose 2020; Ziebe 2013). Following correspondence, the authors of Rose 2020 provided data on stillbirth that were not published (Appendix 13). There were no stillbirths reported in either arm of the study, hence the OR was not estimable (Analysis 1.8). The average rate of stillbirth ranges from approximately 4 per 1000 total births in high‐income countries to approximately 28 per 1000 total births in low‐income countries such as sub‐Saharan Africa (Lawn 2016), therefore the stillbirth rate in this review is better than average for the high income countries.
1.8. Analysis.

Comparison 1: GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF, Outcome 8: Stillbirth
Planned additional analyses
We did not need to undertake a funnel plot to explore the possibility of small‐study effects as there were only three included studies in the quantitative analysis.
Subgroup analyses
1) Studies including only women with recurrent implantation failure. Two studies were defined as including women with "poor prognosis" as a result of previous recurrent implantation failure. The definition of recurrent implantation failure (the failure to achieve a clinical pregnancy after transfer of at least four good‐quality embryos in a minimum of three fresh or frozen cycles (Coughlan 2014)) was met by only one of these studies (Sbracia 2014). When examining this study alone, the only outcome it informs is clinical pregnancy. The low‐quality evidence suggests that GM‐CSF‐supplemented culture media may slightly improve pregnancy rates when compared to culture media not supplemented by GM‐CSF (OR 2.45, 95% CI 1.00 to 6.02, 1 RCT, N = 100, low‐quality evidence).
2) Single‐step versus sequential culture media. A single‐step culture medium supplemented with GM‐CSF would involve culturing the embryos in one medium following fertilisation all the way through to blastocyst embryo replacement if required. The included study that cultured embryos through to blastocyst utilised a sequential culture medium supplemented with GM‐CSF (Rose 2020), therefore we were unable to undertake this subgroup analysis. Correspondence with Cooper Surgical revealed that a single‐step culture medium supplemented by GM‐CSF is yet to obtain its CE mark (certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area), and for this reason is not yet available in Europe from this company.
3) Donor versus autologous oocytes. No included studies utilised donor oocytes, so a subgroup analysis was not possible.
4) Studies including only women with recurrent miscarriage. No included studies involved only women who had experienced recurrent miscarriage, so a subgroup analysis was not possible.
5) Studies replacing embryos at cleavage stage versus blastocyst stage. We know that Rose 2020 was the only study that definitely replaced all embryos at day 5, thereby satisfying the criterion of blastocyst stage transfer. The authors of Ziebe 2013 describe how they undertook all day 3 embryo transfers, which classifies this study as cleavage stage transfer. The authors of Sbracia 2014 did not describe whether they undertook cleavage stage or blastocyst stage transfer. However, they do report using EmbryoGen as the intervention culture media, which is a culture medium licensed to culture embryos to day 3, therefore we have assumed for the sake of subgroup analysis that Sbracia 2014 is classified as a cleavage stage transfer study.
Two studies, one cleavage stage transfer, Ziebe 2013, and one blastocyst stage transfer, Rose 2020, reported on the outcome of live birth. The subgroup analysis for both cleavage stage and blastocyst stage transfer did not alter the finding from the pooled meta‐analysis. In other words, for both subgroup analyses and the main pooled meta‐analysis, we are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the live‐birth rate when compared to using conventional culture media not supplemented with GM‐CSF. The quality of the evidence of the subgroup analyses is low given that only one study informs each analysis.
The same two studies that reported on live birth also reported on miscarriage (Rose 2020; Ziebe 2013), one cleavage and one blastocyst stage transfer. The subgroup analyses did not change the outcome of the main meta‐analysis. In other words, we are uncertain whether GM‐CSF‐supplemented culture media makes any difference to the miscarriage rate when compared to using conventional culture media not supplemented with GM‐CSF. The quality of the evidence of the subgroup analyses is low given that only one study informs each analysis.
The outcome clinical pregnancy is informed by three included studies. Two studies undertook cleavage stage transfers (Sbracia 2014; Ziebe 2013), and one undertook blastocyst stage transfer (Rose 2020). The subgroup analyses did not alter the results of the main pooled meta‐analysis. Low‐quality evidence remains of uncertainty as to whether GM‐CSF‐supplemented culture media makes any difference to the clinical pregnancy rate when compared to using a conventional culture medium not supplemented with GM‐CSF.
The outcome multiple gestation is informed by two included studies, one of which undertook cleavage stage transfers, Ziebe 2013, and the other blastocyst stage transfers, Rose 2020. The subgroup analyses did not alter the results of the main pooled meta‐analysis. Low‐quality evidence remains of uncertainty as to whether GM‐CSF‐supplemented culture media makes any difference to the multiple gestation rate when compared to using a conventional culture medium not supplemented with GM‐CSF. We downgraded the quality of the evidence from moderate for the main meta‐analysis, to low, given that only one study informs each subgroup analysis.
Sensitivity analyses
We decided to undertake three sensitivity analyses for the primary outcomes to determine whether our conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. The analyses were as follows.
1) If eligibility was restricted to studies without high risk of bias (studies at low risk of bias were defined as those with low risk of bias in at least the following two domains: random sequence generation and allocation concealment). For live birth and miscarriage, our two primary outcomes, both studies included in the meta‐analysis were low risk according to our definition, therefore we did not need to undertake this sensitivity analysis.
2) If a random‐effects model had been adopted. We applied the random‐effects model to both of our primary outcomes, and it did not alter the conclusions of the review.
3) If the summary effect measure was risk ratio rather than OR. We altered the summary effect measure to risk ratio for both live birth and miscarriage, however it did not alter the conclusions of the review.
Discussion
Summary of main results
Despite GM‐CSF‐supplemented culture media being commercially available for a number of years, there were very few RCTs from around the world with data that could be included in this review. The three trials included in meta‐analysis, Rose 2020; Sbracia 2014; Ziebe 2013, involved a total 1532 women, of whom the vast majority (1332 women) were from Ziebe 2013, a trial designed, conducted, and written by Cooper Surgical, one of the global market leaders in culture media supplemented with GM‐CSF. Having said this, there was transparency in communication with the authors of Ziebe 2013; this review contains ITT data and further details on methods as a result of the authors' willingness to share information (Appendix 13).
For the primary and secondary outcomes assessed, including live birth, miscarriage, clinical pregnancy, multiple gestation, preterm birth, birth defects, and aneuploidy, due to very low‐ to low‐quality evidence we cannot be certain whether GM‐CSF is any more or less effective than culture media not supplemented with GM‐CSF for clinical outcomes that reflect effectiveness and safety (Table 1). We were unable to undertake analysis of stillbirth, as there were no events in either study arm; however, the lack of events in either arm supports the hypothesis that there is no advantage or disadvantage to using culture media supplemented by GM‐CSF versus culture media not supplemented by GM‐CSF.
Overall completeness and applicability of evidence
The evidence for this review was dominated by one large, multicentre European RCT, which was designed and conducted by industry. However, despite concerns about the equipoise of the trial designers, co‐ordinators, and data analysts, the study appears to be well executed overall. There is without doubt a number of flaws in the design of the study, in particular the statistical analysis of the results presented in the paper (Farquhar 2015); however, we sought ITT data from the authors, which means the data are as transparent as possible (Appendix 13). The three studies included in the quantitative analysis have a number of similarities as described below, but most notable is the concentration of GM‐CSF within the intervention culture media, which is the same across all studies. This possibly reflects the dominance of one particular company who supplied the intervention culture media for all of the included studies. On balance, the available data represent women or couples attending for assisted reproduction with their own gametes, on a single fresh embryo transfer cycle. Two hundred of the 1532 included women were considered to be 'poor prognosis', with recurrent implantation failure or a history of poor embryo development. Further studies including frozen cycles, donor oocytes, and cumulative embryo transfer data from one cycle are required to make the evidence more broadly applicable to the types of women or couples attending for assisted reproduction.
The studies included in the quantitative analysis were conducted in high‐income countries, as defined by the World Bank (World Bank). Two included studies were undertaken in the upper‐middle‐income country of Iran (Zafardoust 2017; Zavvar 2016), however no data could be reliably extracted from these studies, therefore the data available for this review were based solely on those residing in high‐income countries. There was a mixture of IVF and ICSI across the three studies included in quantitative analysis. All three studies undertook fresh embryo transfers, and no cumulative embryo transfer data were available from subsequent frozen embryo transfer cycles.
The intervention culture media contained the same concentration of GM‐CSF in all three studies, which makes the intervention arm more homogenous in terms of what participants received than the control culture media, which were described in less detail. Rose 2020 describes a control of "standard embryo culture media", whilst the authors of Sbracia 2014 do not provide any information on the control medium. The authors of Ziebe 2013 describe using "EmbryoAssist without cytokine", a culture media manufactured by Cooper Surgical. The potential variation in the control culture media makes the result more generalisable to 'real‐world' practice, where individual clinics use a variety of culture media 'as standard'.
The inclusion of studies with variations in day of embryo transfer, poor‐ and good‐prognosis patients, variation in underlying medical conditions of participants, and numbers of embryo transferred helps make the results of this review generalisable.
The two studies that could not be included in the quantitative analysis, Zafardoust 2017; Zavvar 2016, were in some ways different to the studies included in the quantitative analysis. For example, Zafardoust 2017 utilised frozen embryos, and Zavvar 2016 included women in whom immature oocytes were retrieved in spite of stimulation with gonadotropins. Both studies included the same concentration of GM‐CSF in the intervention culture media (2 ng/mL), which were supplied by Cooper Surgical, the supplier of all of the intervention culture media across all included studies. Both Zafardoust 2017 and Zavvar 2016 undertook ICSI. It may be possible to include data from these two studies in the future, if we are able to make contact with the authors to clarify issues regarding data and methods. Both studies reported that there was no significant difference in the pregnancy rate between the GM‐CSF and conventional culture media arms of their studies.
Quality of the evidence
Overall, the quality of the evidence using the GRADE approach is low or very low for all outcomes (Table 1). Live birth, clinical pregnancy, multiple gestation, and preterm birth were all downgraded once for inconsistency. There were differing point estimates in the included studies, with one supporting GM‐CSF‐supplemented culture media, and one supporting the control culture medium. The point estimates have broad confidence intervals, and in many cases the I2 result is high, representing a high degree of heterogeneity between trials.
Live birth, miscarriage, multiple gestation, preterm birth, birth defects, and aneuploidy were all downgraded for imprecision. We downgraded live birth once and the remaining outcomes twice. These outcomes had point estimates with broad or very broad confidence intervals, from a low number of included studies, at least one of which was very small in terms of number of participants. We downgraded clinical pregnancy once for risk of bias, as all studies that inform this outcome have high risk of other bias, and one study was at unclear risk of selection, performance, and detection bias. The unclear risk of bias in this study was due mainly to a lack of information from the available published abstract.
We were unable to undertake meta‐analysis of the results for stillbirth because there we no occurrences of stillbirth in either arm of the study in the one trial that reported this outcome.
Regarding risk of bias, both Rose 2020 and Ziebe 2013 were considered to be overall low risk of bias. Rose 2020 was low risk in all domains except for other bias, which was rated high risk because although the study appears to be well run, the sample size of 100 participants is small, and is unlikely to be powered to detect meaningful difference between the groups in terms of clinical outcomes. We considered Ziebe 2013 to be at low risk of selection, performance, detection, and reporting bias, but at high risk of attrition and other bias. Sbracia 2014 was rated as having high risk of other bias and unclear risk of selection, performance, and detection bias.
Potential biases in the review process
We aimed to identify all eligible studies for inclusion in this review, and contacted authors of all five included studies on many occasions in an effort to include as much information as possible. The authors of two studies were forthcoming with further study information, which helped us to acquire a full picture of the study outcomes, as well as providing information needed to assess and establish risk of bias (Appendix 13).
Agreements and disagreements with other studies or reviews
We found one published systematic review examining GM‐CSF‐supplemented culture media for women undergoing assisted reproduction (Siristatidis 2013). This review undertook a search of studies published between 1966 and 2012. Siristatidis 2013 included all study designs except case series and case reports. The primary outcome was live birth per woman/couple. Secondary outcomes were clinical pregnancy per woman/couple (defined as evidence of fetal heart on ultrasound at seven weeks), miscarriage rate (defined as the number of miscarriages divided by the number of clinical pregnancies), fertilisation rate (rate of oocytes fertilised per oocytes retrieved), and laboratory parameters, such as progression of embryos to blastocyst stage, blastocyst performance and hatching, and chromosomal abnormalities of the embryos. The search yielded 152 records, 112 of which were discarded. Six of the remaining 41 studies were considered eligible for inclusion in the review, four of which were RCTs and two prospective observational studies. The review by Siristatidis 2013 has one RCT in common with our review (Ziebe 2013). The other three RCTs included in Siristatidis 2013 were excluded here because they randomised oocytes (Shapiro 2003; Sjoblom 2001), or no embryos were replaced in women (Agerholm 2010).
The authors of Siristatidis 2013 concluded that most of the included studies trend towards favouring the supplementation of culture media with GM‐CSF in terms of good‐quality embryos reaching the blastocyst stage, improved hatching initiation and number of cells in the blastocyst, and reduction in cell death. However, no statistically significant differences were found in implantation and pregnancy rates in all but one trial, which reported favourable outcomes in terms of implantation and live birth. The authors of Siristatidis 2013 go on to propose properly conducted and adequately powered RCTs to further validate and extrapolate the current findings. The quality of included studies is deemed by the Siristatidis 2013 authors to be "average".
Our review adds two further RCTs, and has the advantage of conducting a meta‐analysis and undertaking a 'Risk of bias' assessment, as well as applying GRADE to the findings of the review. The fact that three of the included RCTs in Siristatidis 2013 randomised oocytes or did not replace embryos into the women means that interpreting the data and applying it to real‐world clinical situations is almost impossible, as the trial design is not adequate for phase III clinical trials assessing clinical outcomes.
We received email correspondence from a reader of the protocol for this review who expressed concerns that we were planning to include studies that used GM‐CSF or G‐CSF as the intervention medium (Appendix 14). The author expressed concerns about this decision as they felt those media were not homogenous enough to be included in one group. This review only found studies that included GM‐CSF as the intervention medium and we explained that we would look into this issue for the re‐run of the review in the coming years.
Authors' conclusions
Implications for practice.
As is the case with most in vitro fertilisation (IVF) add‐ons, granulocyte macrophage colony‐stimulating factor (GM‐CSF)‐supplemented culture media is already widely in use in many fertility clinics across the world, well before the arrival of this systematic review of randomised trials. Despite GM‐CSF being available commercially for a number of years, there are still only five randomised trials, only three of which have data to extract for meta‐analysis, and two of which are extremely small and not adequately powered to detect a meaningful clinical differences between groups. It is also notable that two of the three studies included in quantitative analysis were sponsored by industry; however, the risk of bias in these studies appears to be low (Figure 2).
The findings of this review reveal that overall there is very low‐ to low‐quality evidence to suggest that GM‐CSF supplemented culture media is no more or less effective or harmful than culture media not supplemented with GM‐CSF for the following outcomes: live birth, miscarriage, clinical pregnancy, multiple gestation, preterm birth, birth defects, and aneuploidy. The evidence for stillbirth was also low quality, but we were unable to undertake meta‐analysis as there were no events in either arm of the studies.
Given these findings, clinicians, embryologists and women/couples considering using GM‐CSF‐supplemented culture media during an assisted reproduction cycle should be aware that the available evidence neither supports, nor opposes its use. It would appear important that independent information on the available evidence is made accessible to those considering using GM‐CSF‐supplemented culture media, particularly in the face of strong marketing information. GM‐CSF‐supplemented culture media is marketed as being recommended for those who have experienced recurrent clinical and biochemical pregnancy loss; recurrent implantation failure; unexplained infertility; and advanced maternal age (Cooper Surgical 2020a). However, the available evidence from randomised controlled trials (RCTs) only includes two small studies (N = 200), which included women aged 40 or over, or women who had experienced recurrent miscarriage or implantation failure (Rose 2020; Sbracia 2014). The claim that GM‐CSF has a positive effect on implantation and pregnancy rates is simply not supported by the available evidence presented here (Cooper Surgical 2020a).
Implications for research.
The question of whether GM‐CSF‐supplemented culture media has any advantage over culture media not supplemented by GM‐CSF urgently requires further high‐quality, independent, adequately powered RCTs to add to this systematic review. The ideal RCT would pre‐register a protocol, recruit women/couples attending for assisted reproduction, of all ages and backgrounds, with all types of infertility, undergoing IVF or intracytoplasmic sperm injection (ICSI). It would be powered highly enough to be able to detect whether particular subgroups would benefit from this type of culture media, for example those with previous failed IVF attempts. Women, clinicians, embryologists, and those assessing outcomes would be blinded to the intervention. After randomisation, women would remain in the group to which they had been allocated for the sake of data analysis, regardless of whether they had received the allocated intervention, or whether they dropped out, in order to maintain the effects of randomisation. Data would be analysed using intention‐to‐treat, maintaining the denominator as all women who were randomised to that arm of the study, for all outcomes, including pregnancy, miscarriage, and live birth. The study would be registered on a trials registry beforehand, and the protocol published, including which statistical analyses were planned. The study would ideally be designed and conducted, and data analysed by independent researchers, who hold equipoise regarding the efficacy of GM‐CSF‐supplemented culture media.
History
Protocol first published: Issue 12, 2019 Review first published: Issue 7, 2020
Acknowledgements
We would like to thank Jasmine Lee for her contribution to the protocol draft.
We thank Rik van Eekelen, Demián Glujovsky, Debbie Blake, Mohamed Youssef, Eleonora Uphoff, Co‐ordinating Editor Madelon van Wely, and the editors who gave their time to peer review the protocol and review.
Thank you to Marian Showell, Information Specialist, for her time and expertise with the search of the literature.
Special thanks to Elena Kostova, Managing Editor, for her help in getting this review through all stages to publication.
Thank you to the authors of studies who provided additional information and data at our request, and to those women who participated in the trials which have contributed to this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
PROCITE platform
Searched 15 October 2019
Keywords CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "ovarian stimulation" or "ovarian stimulation controlled ovarian stimulation" or "ovulation induction" or "ovulation stimulation" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "poor prognostic patients" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "embryo culture" or "embryo culture media" or "blastocyst culture technique" or "blastocyst media" or "blastocyst" or "culture" or "Culture‐Media" or "culture techniques" or Title CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "embryo culture" or "embryo culture media" or "blastocyst culture technique" or "blastocyst media" or "blastocyst" or "culture" or "Culture‐Media" or "culture techniques"
AND
Keywords CONTAINS "granulocyte colony‐stimulating factor" or "granulocyte macrophage colony stimulating factor" or "GM‐CSF" or "G‐CSF" or "Embryogen" or "Sage culture medium" or Title CONTAINS "granulocyte colony‐stimulating factor" or "granulocyte macrophage colony stimulating factor" or "GM‐CSF" or "G‐CSF" or "Embryogen" or "Sage culture medium" (54 records)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL)
OVID platform
Searched 15 October 2019 (Issue 9, September 2019)
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (2207) 2 vitro fertili?ation.tw. (2997) 3 ivf‐et.tw. (583) 4 ivf.tw. (5555) 5 icsi.tw. (2657) 6 intracytoplasmic sperm injection$.tw. (1133) 7 assisted reproduct$.tw. (1459) 8 ovulation induc$.tw. (1108) 9 (ovari$ adj2 stimulat$).tw. (2278) 10 superovulat$.tw. (205) 11 ovarian hyperstimulation.tw. (1427) 12 COH.tw. (397) 13 infertil$.tw. (6380) 14 subfertil$.tw. (698) 15 (ovari$ adj2 induction).tw. (61) 16 blastocyst$.tw. (1240) 17 embryo$.tw. (6590) 18 (recurrent adj3 miscarriage$).tw. (268) 19 (recurrent adj3 abortion$).tw. (231) 20 (recurrent adj3 implantation$).tw. (180) 21 implantation failure$.tw. (452) 22 or/1‐21 (15673) 23 exp colony‐stimulating factors/ or exp granulocyte‐macrophage colony‐stimulating factor/ or exp macrophage colony‐stimulating factor/ (3300) 24 (CSF‐2 or CSF‐3).tw. (98) 25 (Colony stimulating adj3 factor$).tw. (3135) 26 granulocyte macrophag$.tw. (1092) 27 (Culture* adj5 growth factor*).tw. (40) 28 GM CSF.tw. (1674) 29 gcsf.tw. (262) 30 gmcsf.tw. (103) 31 g csf.tw. (2805) 32 (EmbryoGen or BlastGen or blastogen or Leucomax).tw. (24) 33 SAGE 1‐step.tw. (4) 34 or/23‐33 (7699) 35 22 and 34 (153)
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 15 October 2019
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (40326) 2 vitro fertili?ation.tw. (22322) 3 ivf‐et.tw. (2249) 4 ivf.tw. (22695) 5 icsi.tw. (8019) 6 intracytoplasmic sperm injection$.tw. (6901) 7 assisted reproduct$.tw. (14151) 8 ovulation induc$.tw. (4066) 9 (ovari$ adj2 stimulat$).tw. (6795) 10 superovulat$.tw. (3348) 11 ovarian hyperstimulation.tw. (4929) 12 COH.tw. (1664) 13 infertil$.tw. (58082) 14 subfertil$.tw. (4898) 15 (ovari$ adj2 induction).tw. (284) 16 blastocyst$.tw. (21461) 17 embryo$.tw. (341298) 18 (recurrent adj3 miscarriage$).tw. (2188) 19 (recurrent adj3 abortion$).tw. (2372) 20 (recurrent adj3 implantation$).tw. (523) 21 implantation failure$.tw. (1479) 22 or/1‐21 (431419) 23 exp colony‐stimulating factors/ or exp granulocyte‐macrophage colony‐stimulating factor/ or exp macrophage colony‐stimulating factor/ (63409) 24 (CSF‐2 or CSF‐3).tw. (395) 25 (Colony stimulating adj3 factor$).tw. (35409) 26 granulocyte macrophag$.tw. (19010) 27 (Culture* adj5 growth factor*).tw. (5038) 28 GM CSF.tw. (18309) 29 gcsf.tw. (600) 30 gmcsf.tw. (472) 31 g csf.tw. (14257) 32 (EmbryoGen or BlastGen or blastogen or Leucomax).tw. (35) 33 SAGE 1‐step.tw. (2) 34 or/23‐33 (90883) 35 22 and 34 (1884) 36 randomized controlled trial.pt. (491373) 37 controlled clinical trial.pt. (93315) 38 randomized.ab. (457040) 39 randomised.ab. (91296) 40 placebo.tw. (206997) 41 clinical trials as topic.sh. (188692) 42 randomly.ab. (319649) 43 trial.ti. (206046) 44 (crossover or cross‐over or cross over).tw. (81967) 45 or/36‐44 (1306338) 46 exp animals/ not humans.sh. (4627546) 47 45 not 46 (1201919) 48 35 and 47 (48)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 15 October 2019
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (67279) 2 vitro fertili?ation.tw. (29584) 3 icsi.tw. (15791) 4 intracytoplasmic sperm injection$.tw. (9347) 5 ivf.tw. (39523) 6 assisted reproduct$.tw. (21962) 7 ovulation induc$.tw. (5543) 8 (ovari$ adj2 stimulat$).tw. (10800) 9 superovulat$.tw. (3777) 10 ovarian hyperstimulation.tw. (7354) 11 COH.tw. (2380) 12 infertil$.tw. (81510) 13 subfertil$.tw. (6767) 14 (ovari$ adj2 induction).tw. (330) 15 blastocyst$.tw. (28689) 16 embryo$.tw. (378546) 17 (recurrent adj3 miscarriage$).tw. (3806) 18 (recurrent adj3 abortion$).tw. (3314) 19 (recurrent adj3 implantation$).tw. (1112) 20 implantation failure$.tw. (3091) 21 or/1‐20 (500910) 22 exp colony stimulating factor/ (5954) 23 exp granulocyte macrophage colony stimulating factor/ (37514) 24 exp colony stimulating factor 1/ (12734) 25 (CSF‐2 or CSF‐3).tw. (635) 26 (Colony stimulating adj3 factor$).tw. (41992) 27 granulocyte macrophag$.tw. (21016) 28 (Culture* adj5 growth factor*).tw. (5955) 29 GM CSF.tw. (25621) 30 gcsf.tw. (2478) 31 gmcsf.tw. (1648) 32 g csf.tw. (23305) 33 SAGE 1‐step.tw. (14) 34 (EmbryoGen or blastGen or blastogen or Leucomax).tw. (420) 35 or/22‐34 (101247) 36 Clinical Trial/ (954250) 37 Randomized Controlled Trial/ (572677) 38 exp randomization/ (84808) 39 Single Blind Procedure/ (36997) 40 Double Blind Procedure/ (164394) 41 Crossover Procedure/ (61141) 42 Placebo/ (330524) 43 Randomi?ed controlled trial$.tw. (213916) 44 Rct.tw. (34381) 45 random allocation.tw. (1919) 46 randomly.tw. (420773) 47 randomly allocated.tw. (33522) 48 allocated randomly.tw. (2487) 49 (allocated adj2 random).tw. (810) 50 Single blind$.tw. (23587) 51 Double blind$.tw. (197107) 52 ((treble or triple) adj blind$).tw. (1025) 53 placebo$.tw. (293366) 54 prospective study/ (557816) 55 or/36‐54 (2331046) 56 case study/ (65015) 57 case report.tw. (385105) 58 abstract report/ or letter/ (1076178) 59 or/56‐58 (1516287) 60 55 not 59 (2278531) 61 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5836378) 62 60 not 61 (2120418) 63 21 and 35 and 62 (179)
Appendix 5. CINAHL search strategy
Ebsco platform
Searched from 1961 to 15 October 2019
S58 S34 AND S57 16 S57 S56 NOT S55 598,106 S56 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 625,300 S55 S53 NOT S54 163,549 S54 MH (human) 1,982,918 S53 S50 OR S51 OR S52 185,486 S52 TI (animal model*) 2,818 S51 MH (animal studies) 107,579 S50 MH animals+ 85,636 S49 AB (cluster W3 RCT) 302 S48 MH (crossover design) OR MH (comparative studies) 236,274 S47 AB (control W5 group) 94,594 S46 PT (randomized controlled trial) 86,203 S45 MH (placebos) 11,435 S44 MH (sample size) AND AB (assigned OR allocated OR control) 3,699 S43 TI (trial) 95,545 S42 AB (random*) 269,798 S41 TI (randomised OR randomized) 93,431 S40 MH cluster sample 3,898 S39 MH pretest‐posttest design 38,357 S38 MH random assignment 55,470 S37 MH single‐blind studies 12,785 S36 MH double‐blind studies 42,502 S35 MH randomized controlled trials 86,500 S34 S21 AND S33 76 S33 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 8,753 S32 TX SAGE 1‐step 18 S31 TX (Culture* N5 growth factor*) 607 S30 TX (EmbryoGen or BlastGen or blastogen or Leucomax) 1 S29 TX g csf 2,195 S28 TX gmcsf 34 S27 TX gcsf 96 S26 TX GM CSF 824 S25 TX granulocyte macrophag* 1,373 S24 TX (Colony stimulating N3 factor*) 4,099 S23 TX (CSF‐2 or CSF‐3) 2 S22 (MM "Colony‐Stimulating Factors+") OR (MM "Granulocyte Colony‐Stimulating Factor") OR (MM "Granulocyte‐Macrophage Colony‐Stimulating Factor") 3,955 S21 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S17 OR S18 OR S19 OR S20 32,225 S20 TX implantation failure* 5,559 S19 TX recurrent N3 implantation* 159 S18 TX recurrent N3 abortion* 205 S17 TX recurrent N3 miscarriage* 434 S16 TX embryo* 22,469 S15 TX blastocyst* 2,163 S14 TX subfertil* 893 S13 TX infertil* 16,457 S12 TX COH 234 S11 TX ovarian hyperstimulation 825 S10 TX superovulat* 84 S9 TX ovulation induc* 1,856 S8 TX assisted reproduct* 4,780 S7 TX intracytoplasmic sperm injection* 878 S6 TX vitro fertili?ation 6,908 S5 (MM "Embryo Transfer") 1,079 S4 TX ovar* N3 hyperstimulat* 825 S3 TX ovari* N3 stimulat* 978 S2 TX IVF or TX ICSI 4,883 S1 (MM "Fertilization in Vitro") 3,376
Appendix 6. LILACS search strategy
Web platform
Searched 15 October 2019
colony stimulating factor and embryo*
colony stimulating factor and ivf*
Appendix 7. ClinicalTrials.gov and WHO ICTRP search strategy
Web platform
Searched 15 October 2019
csf* and embryo*
csf* and ivf*
colony stimulating factor and embryo
colony stimulating factor and ivf
Appendix 8. DARE search strategy
Web platform
Searched 15 October 2019
csf* and embryo*
csf* and ivf*
colony stimulating factor and embryo
colony stimulating factor and ivf
Appendix 9. Web of Knowledge search strategy
Web platform
Searched 15 October 2019
csf* and embryo*
csf* and ivf*, limited by Web of Science Categories REPRODUCTIVE BIOLOGY, OBSTETRICS GYNECOLOGY and MEDICINE RESEARCH EXPERIMENTAL
Appendix 10. OpenGrey search strategy
Web platform
Searched 15 October 2019
csf* and embryo*
csf* and ivf*
Appendix 11. PubMed search strategy
Web platform
Searched 15 October 2019
(colony stimulating factor [title] OR csf [title] AND embryo* [Title/Abstract] OR ivf* [Title/Abstract] limited by clinical trials and randomised controlled trial
Appendix 12. Google Scholar search strategy
Web platform
Searched 15 October 2019
(csf AND embryo* OR ivf*)
(colony stimulating factor AND embryo*)
Appendix 13. Correspondence with authors of included studies
Correspondence with the authors of Ziebe 2013
Amendment #1 for Clinical Investigation Report, DK001 The effect of granulocyte‐macrophage colony stimulation factor (GM‐CSF) during in vitro culture of human embryos on subsequent implantation rates (DK001) Rep1.02.03.05
Issued by: Senior Clinical Coordinator, Bibi Munding Rasmussen Consultant, Sandra Maher. This confidential document is the property of Cooper Surgical. Permission has been granted by Cooper Surgical to publish this correspondence.
Background: The Cochrane organization is working on a review concerning GM‐CSF containing culture media and are including the DK001 study, published in “Fertility and Sterility" (Ziebe et al. 2013). In relation to this they have approached Søren Ziebe, Professor in Clinical Embryology and principal investigator for the DK001 study, for information on the Intend‐to treat (ITT) population and some clarifications.
Information requested and answers:
1) Please could you let us know the clinical pregnancy rate (ongoing implantation rate at 7 weeks) per woman randomised? i.e. twin or triplet pregnancy would be n=1.
Answer provided by e‐mail 2019.Dec.06; Søren Ziebe (Rigshospitalet, DK) and Bibi Munding Rasmussen (Cooper Surgical ‐ ORIGIO a/s): Based on the intend‐to‐treat (ITT) data analysis, the clinical outcome gestational week 7 per woman randomised was as follows:
1. Clinical pregnancy rate (CPR): GM‐CSF: 28.9% (189/654) Control: 25.8% (175/678)
2. Ongoing CPR: GM‐CSF: 26.5% (173/654) Control: 23.5% (159/678)
Further information: Please notice that the objective of the DK001 study was to evaluate effect of GM‐CSF in embryo culture medium on ongoing implantation rate (OIR), which is the reason why the publication is focusing on OIR's and supported with data on ongoing clinical pregnancy rates gestational week 7 and 12. The primary endpoint OIR gestational week 7 was chosen based on previous studies indicating a protective effect of GM‐CSF on culture‐induced embryo stress. Ziebe et al. (2013) report data on the per‐protocol (PP) population, which is the subset of ITT patients fulfilling inclusion/exclusion criteria, who had an embryo transfer Day 3 and did not violate the protocol in a way that could impair evaluation of the primary endpoint, ongoing OIR gestational week 7.
For a comparison of the ITT‐ and PP‐population, we suggest presenting data per embryo transfer cycle (all transfer patients):
ITT‐population:
3. Clinical pregnancy rate per transfer (CPR): GM‐CSF: 32.2% (189/587) Control: 28.9% (175/605)
4. Ongoing CPR per transfer: GM‐CSF: 29.5% (173/587) Control: 26.3% (159/605)
PP‐population:
5. Clinical pregnancy rate per transfer (CPR): GM‐CSF: 32.4% (183/564) Control: 29.2% (171/585)
6. Ongoing CPR per transfer: GM‐CSF: 29.6% (167/564) Control: 26.5% (155/585)
Please refer to the enclosed Table A, for further information on clinical pregnancy data for both the ITT‐ and PP‐population, calculated per embryo transfer cycle and including data on the primary outcome OIR.
References: 1a) and 1b)
1b) Please can you confirm whether the clinical pregnancies at 7 weeks were all scanned at this stage?
The scanning gestational week 7 was defined like this: week 7 plus 0‐6 days; and day 0 calculated as "the day of oocyte aspiration" minus "14 days”.
Out of total 364 ITT‐ patients with a clinical pregnancy (GM‐CSF: 189; Control: 175), there were 37 patients where the ultrasound scanning gestational week 7 was performed either too early (GM‐CSF: 9; Control: 4) or too late (GM‐CSF: 12; Control: 12).
None of the patients were undergoing ultrasound scanning before gestational week 6, and the cases where the scanning was performed too late were all performed within gestational week 8‐10. Moreover, there have been no overestimations of clinical pregnancy rate gestation week 7, as majority of deviations were due to scanning performed later than gestational week 7. This is further supported by the fact that the number of miscarriages observed gestational week 7 were similar in the two groups (GM‐CSF: 14; Control: 17), while the miscarriages observed gestational week 12 were higher in the control group (GM‐CSF: 3; Control: 10).
Please refer to the enclosed Table B, for an overview of deviations concerning the ultrasound scanning gestational week 7 for both the ITT‐ and PP‐population.
Reference: 2a)
2) Please could you confirm how allocation was concealed? For example, who performed the randomisation? Was it done electronically or with envelopes? Who held the master randomisation list?
Answer provided by e‐mail 2019.Dec.06; Søren Ziebe (Rigshospitalet, DK) and Bibi Munding Rasmussen (CooperSurgical ‐ ORIGIO a/s): Randomization was performed by Cooper Surgical ‐ ORIGIO a/s, and based on a randomisation list per clinic generated automatically using www.randomization.com. Each study site received a list of study specific consecutive patient ID numbers (e.g. clinic 1: 01001, 01002, 01003, 01004 etc.) and a corresponding number of identically looking randomized bottles of test and control media individually labelled with the corresponding study specific patient ID numbers. On site the lowest number on the list was always allocated to any new patient recruited, at the time of informed consent signature. Therefore, both the clinician, embryologist and patient, was blinded to the treatment allocation. The master randomisation list was held by ORIGIO a/s. All data analysis was performed externally by a Clinical Research Organization (CRO) and performed on blinded data. CORRECTION: During the interim analysis the statistician was blinded at all times to the media, which were presented as Medium A and Medium B. For the final statistical analyses, the codes for blinding were broken after database lock and patient classification.
Reference: 2c)
3) Please could you clarify whether those who performed the ultrasound scans were blinded to the interventions?
Answer provided by e‐mail 2019.Dec.06; Søren Ziebe (Rigshospitalet, DK) and Bibi Munding Rasmussen (Cooper Surgical ‐ ORIGIO a/s): Yes, also the clinicians performing the ultrasound scans were blinded to the treatment allocation at all times.
Reference: 2c)
4) Please clarify the number of twins in the intervention group. Under table 1 you write that there were 2x27 sets of twins for the intervention group. However, in table 1 you write that 58 twins were born which equals 29 sets of twins.
Answer provided by e‐mail 2019.Dec.06; Søren Ziebe (Rigshospitalet, DK) and Bibi Munding Rasmussen (CooperSurgical – ORIGIO a/s): Apologize for the inconsistency in reporting. The correct numbers are as follows: GM‐CSF: 58 twin births, corresponding to 29 sets of twins; Control: 50 twin births, corresponding to 25 sets of twins. CORRECTION: As stated under the answer for question 1), Ziebe et al. (2013) report data on the PP population, which is the subset of ITT patients fulfilling the inclusion/exclusion criteria, who had an embryo transfer Day 3 and did not violate the protocol in a way that could impair evaluation of the primary endpoint, ongoing implantation week 7.
Please notice, that the numbers stated below Table 1 in the publication by Ziebe et al. (2013) is number of embryos implanted (ongoing) as dizygotic twins (2x27 for GM‐CSF group and 2x20 for the control group) and number of embryos implanted (ongoing) as monozygotic twins (2x4 for GM‐CSF group and 2x3 for the control group). Therefore, these numbers are including the triplet pregnancy in the GM‐CSF group resulting from transfer of two embryos, which in principal is due to implantation of 2 dizygotic twins and 2 monozygotic twins, although one of the twins is forming part of both twin groups. In other words, the difference in numbers reported by Ziebe et al. in Table 1 and below the table, is due to the fact that the table is presenting gestational age and birth weight according to whether the children born was a result of a singleton, twin or triplet live birth; while the ongoing implantation of twins stated below the table is counting the triplet pregnancy as 4 ongoing implantations in the GM‐CSF group (dizygotic twins: 1x2; monozygotic twins: 1x2).
Please have an overview of dizygotic and monozygotic twins born after twin pregnancies: Number of twins born in the PP‐population: GM‐CSF: 58 twins, corresponding to 29 sets of twins (2x26 dizygotic twins, 2x3 monozygotic twins). Control: 46 twins, corresponding to 23 sets of twins (2x20 dizygotic twins, 2x3 monozygotic twins). Number of twins born in the ITT‐population: GM‐CSF: 60 twins, corresponding to 30 sets of twins (2x27 dizygotic twins, 2x3 monozygotic twins). Control: 48 twins, corresponding to 24 sets of twins (2x21 dizygotic twins, 2x3 monozygotic twins). When including the triplet pregnancy as 4 embryos implanted due to 1 set of dizygotic twins and 1 set of monozygotic twins, the number of embryos implanted (ongoing) as twins in the GM‐CSF groups changes to 2x27 dizygotic twins, 2x4 monozygotic twins, as stated below Table 1 in the publication by Ziebe et al. (2013). Please refer to the enclosed Table A, for an overview of children born for both the ITT‐ and PP‐population.
Reference: 3a), 3b) and 3c)
5) Please could you confirm that you have published all the outcomes you set out to assess?
Answer provided by e‐mail 2019.Dec.06; Søren Ziebe (Rigshospitalet, DK) and Bibi Munding Rasmussen (CooperSurgical – ORIGIO a/s): Yes
Reference: 2c)
6) Number of live births.
ITT‐population: 7. Live birth rate per transfer: GM‐CSF: 28.6% (168/587) Control: 24.0% (145/605)
PP‐population: 8. Live birth rate per transfer: GM‐CSF: 28.9% (163/564) Control: 24.1% (141/585)
Please refer to the enclosed Table A, for further information on clinical outcome for both the ITT‐ and PP‐population, calculated per embryo transfer cycle.
References: 1a), 1b) and 1c)
7) Number of stillbirths.
There were no stillbirths, neither in the GM‐CSF nor in the control group. Please refer to the enclosed Table A, for further information on clinical outcome for both the ITT‐ and PP‐population, calculated per embryo transfer cycle.
Reference: 1d)
8) Number of ectopic pregnancies.
ITT‐population: 9. Ectopic pregnancies per transfer: GM‐CSF: 0.7% (4/587) Control: 0.3% (2/605)
PP‐population: 10. Ectopic pregnancies per transfer: GM‐CSF: 0.7% (4/564) Control: 0.3% (2/585)
Please refer to the enclosed Table A, for further information on clinical outcome for both the ITT‐ and PP‐population, calculated per embryo transfer cycle.
References: 1a) and 1b)
9a) Miscarriage of clinical preg (up to 20/40).
ITT‐population: 11. Late pregnancy loss (>week 12) per transfer: GM‐CSF: 0.3% (2/587) Control: 0.7% (4/605)
PP‐population: 12. Late pregnancy loss (>week 12) per transfer: GM‐CSF: 0.4% (2/564) Control: 0.7% (4/585)
Please refer to the enclosed Table A, for further information on clinical outcome for both the ITT‐ and PP‐population, calculated per embryo transfer cycle.
References: 1a) and 1b)
9b) We are interested in miscarriage of clinic pregnancy, so this is miscarriages of those women who were pregnant at 7/40 following evidence of gestational sac on scan, which you kindly clarified was all of these women. GM‐CSF 189 were pregnant at 7/40. Minus 168 live births = 21 pregnancy losses. We know there were 17 miscarriages in this group, plus 2 terminations of pregnancy. This makes 19 pregnancy losses. Can you please clarify the other 2 pregnancy losses. I'm guessing they weren't ectopics as these are generally picked up before 7 weeks' gestation? Control group 175 women were pregnant at 7/40. Minus 145 live birth= 30 pregnancy losses. We know there were 27 pregnancy losses, plus 4 termination of pregnancies. This totals 31 pregnancy losses. Please could you kindly clarify?
Please know that in order to be able to calculation live birth based on clinical pregnancies week 7 minus miscarriages and ectopic pregnancies, you need to account for 5 mistakes in how results of the scanning gestational week 7 were reported.
GM‐CSF: 2 out of the 4 patients with an ectopic pregnancy were not accounted for as clinical pregnancy week 7. CONTROL: 3 out of the 27 patients with miscarriage week 7 were not accounted for as clinical pregnancy week 7. All 5 patients were forming part of both the ITT‐ and PP‐population. As these mistakes are causing similar underestimations of clinical pregnancy and implantation rate week 7 in both groups, without affecting the endpoints or other outcome parameters of the study, we did not make a correction, Please have an overview of data, where these 5 patients have been accounted for:
| ITTGM‐CSF | ITT Control | PP GM‐CSF | PP Control | |
| Clinical pregnancy week 7 | 191 (189+2) | 178 (175+3) | 185 (183+2) | 174 (171+3) |
| Miscarriages </= week 12 | 17 | 27 | 16 | 27 |
| Miscarriages/elective terminations > week 12 | 2 | 4 | 2 | 4 |
| Ectopic pregnancies | 4 | 2 | 4 | 2 |
| Live birth | 168 | 145 | 163 | 141 |
References: 1e
10) Multiple pregnancies
ITT‐population:
13. Twin pregnancies per transfer: GM‐CSF: 5.1% (30/587) Control: 4.0% (24/605)
14. Triplet pregnancies per transfer: GM‐CSF: 0.2% (1/587) Control: 0.0% (0/605)
PP‐population:
15. Twin pregnancies per transfer: GM‐CSF: 5.1% (29/564) Control: 3.9% (23/585)
16. Triplets pregnancies per transfer: GM‐CSF: 0.2% (1/564) Control: 0.0% (0/585)
Please refer to the enclosed Table A, for further information on clinical outcome for both the ITT‐ and PP‐population, calculated per embryo transfer cycle.
Reference: 3a), 3b) and 3c)
11a) You mentioned terminations of pregnancy as a result of malformations. Please could you confirm whether these were isolated defects or trisomies? If at all possible we'd love a breakdown of reasons for termination. We are particularly interested in aneuploidy and birth defects.
The study was not powered to draw conclusions concerning malformations, but rates were similar without any trends in the two groups (GM‐CSF or control). The number and types of miscarriage/elective termination due to malformations after gestational week 12 are list in the table next page.
11b) Your paper states that there were abnormalities detected within 1 week of birth in 11/654 in the intervention group and 8/678 in the control group. Please can we confirm whether these number contain the termination of pregnancy babies or not? If possible, please could we have a breakdown of the aneuploidies/abnormalities for all 11 and 8 babies in these groups?
Please know that the abnormalities reported as detected within 1 week of birth, does not include the termination of pregnancy babies:
| GM‐CSF | Control |
| Elective terminations >week 12 | |
| 1. fetus with acrania | 1. dead fetus without further information |
| 2. fetus with myelomeningocele: a developmental congenital disorder caused by incomplete closing of the neural tube | 2. fetus with multiple malformations (trisomy for chromosome 13) |
| 3. fetus with hypocephalus and myelomeningocele | |
| 4. fetus due to risk of serious physical disorder due to injury or illness during prenatal development | |
| Live births | |
| 1. fetus with hypospadia glandis/penis arcuatus | 1. Preterm birth week 27. The child died due to immature lungs |
| 2. fetus with hypospadia glandis | 2. Hypospadia without specifications |
| 3. fetus with retentio testis unilateralis | 3. Mild hypospadia |
| 4. fetus with ductus arteriosus persistens | 4. Retentio testis unilateralis |
| 5. fetus with stenosis arteriae pulmonalis congenita | 5. Heart failure and chromosome deletion (Steno Fallots tetralogy) |
| 6. fetus with defectus septi ventriculorum cordis/defectus septi atriorum cordis | 6. Twins with defectus septi atriorum cordis and one of the twins also had ductus arteriosus persistens |
| 7. fetus with defectus septi atriorum cordis/ductus arteriousus persistens | 7. Palatoschisis unilateralis without specifications |
| 8. fetus with hernia diaphragmatica congenita | 8. Congenital malformation in the hip without specifications |
| 9. fetus with luxatio coxae congenita unilateralis | |
| 10. fetus with talipes equinovarus | |
| 11. fetus with congenital malformation in one foot without specifications | |
All cases were forming part of both the ITT‐ and PP‐population.
12) Please can we check that all the twins and triplets delivered <37 week’s gestation? Preterm birth is one of our outcomes and I can see that you’ve documented preterm birth for singletons, but we need to add the multiple gestation pregnancies too. Please can you confirm?
(N.B. twin and triplet numbers represent actual numbers of infants)
| ITT population, singletons | PP population, singletons | |||
| Gestational age | GM‐CSF | CONTROL | GM‐CSF | CONTROL |
| >/=wk 37+0 | 124 | 112 | 120 | 110 |
| >/=wk 32+0 and <wk 37+0 | 9 | 8 | 9 | 7 |
| <wk 32+0 | 4 | 1 | 4 | 1 |
| TOTAL | 137 | 121 | 133 | 118 |
| ITT population, twins | PP population, twins | |||
| >/=wk 37+0 | 30 | 26 | 30 | 24 |
| >/=wk 32+0 and <wk 37+0 | 28 | 18 | 26 | 18 |
| <wk 32+0 | 2 | 4 | 2 | 4 |
| TOTAL | 60 | 48 | 58 | 46 |
| ITT population, triplets | PP population, triplets | |||
| >/=wk 37+0 | ||||
| >/=wk 32+0 and <wk 37+0 | 3 | 3 | ||
| <wk 32+0 | ||||
| TOTAL | 3 | 0 | 3 | 0 |
Reference: 2b)
Table A: Overview of clinical data for ITT‐ and PP‐population
| DK001 | ITT | PP | ||
| GM‐CSF | CONTROL | GM‐CSF | CONTROL | |
| #Patients randomized | 654 | 678 | ||
| #Transfer cycles | 587 | 605 | 564 | 585 |
| #Embryos transferred | 874 | 913 | 838 | 882 |
| Embryos transferred (mean) | 1.49 | 1.51 | 1.49 | 1.51 |
| #Ongoing implantation w.7 | 204 | 181 | 197 | 176 |
| Ongoing implantation w.7 (%) | 23.3+/‐1.4 | 19.8+/‐1.3 | 23.5+/‐1.5 | 20.0+/‐1.3 |
| #Ongoing implantation w.12 | 199 | 170 | 193 | 165 |
| Ongoing implantation w.12 (%) | 22.8+/‐1.4 | 18.6+/‐1.3 | 23.0+/‐1.5 | 18.7+/‐1.3 |
| #Clinical Pregnancies w.7 | 189 | 175 | 183 | 171 |
| Clinical Pregnancy rate w.7 (%) | 32.2 | 28.9 | 32.4 | 29.2 |
| #Ongoing clinical pregnancies w.7 | 173 | 159 | 167 | 155 |
| Ongoing clinical pregnancy rate w.7 (%) | 29.5+/‐1.9 | 26.3+/‐1.8 | 29.6+/‐1.9 | 26.5+/‐1.8 |
| #Clinical pregnancies w.12 | 171 | 155 | 166 | 151 |
| Clinical Pregnancy rate w.12 (%) | 29.1 | 25.6 | 29.4 | 25.8 |
| #Ongoing clinical pregnancies w.12 | 170 | 149 | 165 | 145 |
| Ongoing clinical pregnancy rate w.12 (%) | 29.0+/‐1.9 | 24.6+/‐1.8 | 29.3+/‐1.9 | 24.8+/‐1.8 |
| #live birth | 168 | 145 | 163 | 141 |
| Live birth rate (%) | 28.6+/‐1.9 | 24.0+/‐1.7 | 28.9+/‐1.9 | 24.1+/‐1.8 |
| #Stillborn children | 0 | 0 | 0 | 0 |
| #Liveborn children | 200 | 169 | 194 | 164 |
| #Singletons | 137 | 121 | 133 | 118 |
| #Twins | 60 | 48 | 58 | 46 |
| Dizygotic | 54 | 42 | 52 | 40 |
| Monozygotic | 6 | 6 | 6 | 6 |
| #Triplets* | 3 | 0 | 3 | 0 |
| #Pos. hCG | 222 | 226 | 214 | 218 |
| #Early pregnancy loss (</=w.12) | 52 | 77 | 49 | 73 |
| #Biochemical | 31 | 48 | 29 | 44 |
| #Ectopic | 4 | 2 | 4 | 2 |
| #Miscarriage (loss w.7‐12) | 17 | 27 | 16 | 27 |
| #Miscarriage observed week 7 | 14 | 17 | 14 | 17 |
| #Miscarriage observied week 12 | 3 | 10 | 2 | 10 |
|
#Late pregnancy loss (>w.12) Miscarriage/elective termination due to malformations |
2 | 4 | 2 | 4 |
*Including 1 set of monozygotic twins
Table B: Overview of deviations concerning the ultrasound scanning gestational week 7 for ITT‐ and PP‐population
| DK001 | ITT | PP | ||
| GM‐CSF | CONTROL | GM‐CSF | CONTROL | |
| #Clinical pregnancies w.7 | 189 | 175 | 183 | 171 |
| Time of ultrasound scanning: | ||||
| #Week 7 plus 0‐6 days | 168 | 159 | 164 | 155 |
| Week 6 | 9 | 4 | 8 | 4 |
| Weeks 8‐10 | 12 | 12 | 11 | 12 |
References:
1. Statistically analysis report, DK001 TFL FINAL FULL STUDY 19 MAR2012
a) Table 30
b) Table 31
c) Table 34
d) Table 61
e) Note to File, Rep1.02.03.05
2. Clinical Investigation Report DK001, Rep1.02.03.05
a) Appendix D. 1
b) Appendix D. 10
c) Appendix J
3. Final derived data Excel DK001:
a) DK001_Outcome_Stage 1
b) DK001_Outcome_Stage 2
c) DK001_BIRTHS
Correspondence with the authors of Rose 2020
1) Please could you clarify how long the embryos were exposed to the intervention culture medium?
They were exposed to GM‐CSF containing media for 5 days ‐ Embryogen from Day 0‐3 and Blastgen from Day 3‐5
2) Please could you clarify on what day embryos were replaced?
3) Were they all Single embryo transfers? Or multiple?
All trial participants had a single fresh embryo transfer apart from one in the standard group and three in the EmbryoGen®/BlastGen™ group who did not achieve fertilisation and 1 participant in the standard group and two in the EmbryoGen®/BlastGen™ group who did not have an embryo of sufficient quality to transfer. All trial participants had a day 5 embryo transfer apart from one in the standard media group and two in the BlastGen group who transferred one embryo on day 3 due to delayed embryo development. Couples who insisted on transferring two embryos, once embryo quality was known, were included in the study but were recorded as having a trial variation (four in the standard group and six in the EmbryoGen®/BlastGen™ group).
4) Please could you share the inclusion and exclusion criteria? If you have a protocol this would be fantastic to see.
This will be further explained in the paper;
The inclusion criteria selected for a poor prognosis patient population. Patients must have previously had consecutive transfer of two or more embryos without a positive pregnancy outcome OR have had a history of at least 1 previous pregnancy loss OR a previous history of poor embryo development (<20% of embryos developing on the time at Day 3 or no blastocysts above grade 2 on Day 5). Other additional inclusion parameters included a maternal age between 25 and 41 years, the use of a standard GnRH agonist or antagonist protocol and three or more follicles of >14mm as seen by transvaginal ultrasound before the day of human Chorionic Gonadotrophin (hCG) administration. Exclusion criteria included a need for surgical sperm retrieval (except in cases of previous vasectomy), the use of another investigational drug within 30 days of oocyte retrieval and/or the presence of a severe chronic disease that could impact the IVF cycle or reproductive outcomes.
5) Please could you clarify that it was exactly 50 participants in each group? Were there any drop outs?
See the flow chart in the paper
6) We are interested in miscarriage. Please can I clarify the following numbers with you to check we have it accurately. I note one late miscarriage in the control medium group. Please can you confirm if this was pre or post 20 weeks, or if it was a stillbirth? I note that the clinical pregnancy rate is possibly written up wrong in your paper (i.e. they have been written up in the wrong order perhaps?).
live birthGMCSF 11/50 Control medium 17/50 Clinical preg rate GMCSF 13/50 Control medium 20/50 Ongoing clinical pregnancy rate GMCSF 11/50 Control medium 18/50 Miscarriage of clinical preg GMCSF 2/50 Control medium 3/50
This is correct ‐ the control group had higher pregnancy rates but this was not statistically significant. The late miscarriage was at 17 weeks.
7) Any information you can give us on how the randomisation sequence was generated, how allocation was concealed, who exactly was blinded, was the person performing ultrasound blinded? Did you report all outcomes you assessed?
50 cards with control and 50 with BlastGen™ written on them were placed in concealed envelopes by a person unrelated to the trial. They were shuffled several times and the envelopes were marked from 1‐100 in consecutive order. When the eligible patient was scheduled for an egg retrieval the embryologist took the next envelope by sequence and the random assignment was made. Clinicians, sonographers and patients were completely blinded, embryologists were partly blinded. We report all outcomes as per our prospective clinical trials registration (ClinicalTrials.gov Identifier: NCT02305420). Patients were guaranteed to have BlastGen™ in 1 of 2 cycles (if they didn't conceive in their first). They were blind as to which cycle the BlastGen™ media was used.
8) Finally, we took this study from the abstract at ESHRE 2019. Have you published the full study elsewhere?
This paper was accepted for publication by RBM online on the 14th of January 2020 and should be in available for advanced online publication in the next week or so.
Thank you so much for your thorough reply to my questions. I look forward to seeing the full paper in the coming weeks. I just want to clarify one thing. Is this a cross‐over study? The results here, are they all from the first phase of the study or are some taken from the second phase?
You are correct that this is only the first phase, and not a crossover.
I spotted in table 2 that you mention 2 sets of twins in the control group. Please can I confirm this? Am I right in thinking there were no twins in the intervention arm? The other outcomes we are interested include preterm birth rate per woman randomised (<37 weeks’ gestation), birth defects per woman randomised, aneuploidy per woman randomised, stillbirth per woman randomised (>20 weeks’ gestation). Thank you for any further information you can provide us.
In terms of the additional information, although we did not report them in our trial I have looked through our records and pulled the data out. Answered below:
Yes two sets of twins, both in the standard medium group (none in BlastGen™ arm).
Preterm birth rate per woman randomised (<37 weeks’ gestation)
9 cases of preterm birth (2 sets of twins), Standard = 8, BlastGen™ =1
Birth defects per woman randomised
One birth defect noted in the standard group (genetic). The child was born mosaic for a trisomy 18 and no obvious phenotype present as yet.
Aneuploidy per woman randomised
No PGT was done as part of the trial.
Stillbirth per woman randomised (>20 weeks’ gestation)
One case of stillbirth (Standard Group) born at 21+3 weeks (termination, fetal abnormalities, we have no genetic results except the amino/harmony was normal). This would mean minus one miscarriage from the standard group.
Thank you for your reply. Just for clarity, we will classify these results as follows:
AneuploidyGM‐CSF 0/50 Control 1/50 (mosaic T18)
Stillbirth (we do not consider TOPs to be stillbirths, or miscarriages. However that particular baby will be counted as birth defect) GMCSF 0/50 Control 0/50
Birth defect GM‐CSF 0/50 Control 1/50
Preterm birth GM‐CSF 1/50 Control 6/50
Multiple gestation GM‐CSF 0/50 Control 2/50
Yes that all looks correct and makes sense. Thanks for clarifying.
Appendix 14. Other correspondence
The following email was received regarding the protocol of this study. In light of this email, we will alter the protocol for future iterations of this review to remove studies that use 'GCSF' as an intervention opposed to GM‐CSF. We did no find or include any studies that used G‐CSF as an intervention.
Dear Sarah
I’ve just read with interest your protocol for the Cochrane Review on use of GM‐CSF in IVF. I’m very pleased to see this is happening and would like to thank you for taking this on. It promises to be an important and valuable study and I’ll look forward to seeing the outcome.However I have one major concern. The protocol states in the methods section:“We will include all studies that compare GM‐CSF (including G‐CSF)‐supplemented embryo culture media versus any other non‐GM‐CSF‐supplemented embryo culture media (control).”This is very worrying and if indeed this will be done, it threatens the validity and usefulness of the study.
GM‐CSF and G‐CSF are two entirely different cytokines, with no homology in their protein or DNA sequence, no relatedness in their tertiary structure, and only very limited relatedness in their biological function. Although their names are similar this is very misleading. Their synthesis in the female reproductive tract is regulated differently, and they act via different receptors in their respective target cells. While there is strong biological and preclinical rationale for evaluating GM‐CSF in human embryo development, there is almost no rationale for G‐CSF.
It does not make any sense at all to pool the clinical studies on these two cytokines!
I very much hope you will reconsider the protocol in light of this.
I would be very happy to provide further information on these cytokines, and/or discuss the points above by email or phone/zoom with you and/or Dr Farquhar if that helps.
Best wishes,
Sarah Robertson, PhD FAA
The University of Adelaide
Data and analyses
Comparison 1. GM‐CSF‐supplemented culture medium versus culture medium not supplemented with GM‐CSF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.93, 1.52] |
| 1.2 Miscarriage | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.41, 1.36] |
| 1.3 Clinical pregnancy | 3 | 1532 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.93, 1.45] |
| 1.4 Multiple gestation | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.73, 2.10] |
| 1.5 Preterm birth | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.70, 2.04] |
| 1.6 Birth defects | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.59, 3.01] |
| 1.7 Aneuploidy | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.03, 3.26] |
| 1.8 Stillbirth | 2 | 1432 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rose 2020.
| Study characteristics | ||
| Methods |
Study design: single‐centre randomised controlled trial (RCT). The study was cross‐over in design, but only data from the first phase of the study, prior to cross‐over, are included in the review. Country: Australia Setting: fertility clinic, Adelaide Sponsorship source: EmbryoGen/BlastGen media was supplied by ORIGIO, a Cooper Surgical company. A grant from Cooper Surgical also funded MLH and EJK for statistical support. Fertility SA and The Robinson Institute, University of Adelaide provided in kind support from staff and affiliates providing time and expertise. Trials registry number: NCT02305420 (prospectively registered) |
|
| Participants |
Inclusion criteria: the inclusion criteria selected for a poor‐prognosis patient population. Patients must have previously had consecutive transfer of 2 or more embryos without a positive pregnancy outcome OR have had a history of at least 1 previous pregnancy loss OR a previous history of poor embryo development (< 20% of embryos developing on the time at day 3 or no blastocysts above grade 2 on day 5). Other additional inclusion parameters included a maternal age between 25 and 41 years, the use of a standard gonadotrophin‐releasing hormone (GnRH) agonist or antagonist protocol, and 3 or more follicles of > 14 mm as seen by transvaginal ultrasound before the day of human chorionic gonadotropin administration. Exclusion criteria: exclusion criteria included a need for surgical sperm retrieval (except in cases of previous vasectomy), the use of another investigational drug within 30 days of oocyte retrieval, and/or the presence of a severe chronic disease that could impact the in‐vitro fertilisation (IVF) cycle or reproductive outcomes. Number of participants randomised to each arm of the study: 50 to intervention arm, 50 to control arm. Dropouts: none Group differences: participants were matched for age, body mass index (BMI), and smoking status. A greater number of participants in the intervention group underwent intracytoplasmic sperm injection (ICSI) opposed to IVF versus the control group (37/50 versus 29/50). Fresh or frozen cycle?: fresh cycle IVF or ICSI?: IVF and ICSI Length of time exposed to intervention medium: 5 days Trade name and concentration of granulocyte macrophage colony‐stimulating factor (GM‐CSF) in intervention medium: EmbryoGen days 0 to 3 followed by BlastGen days 3 to 5 Trade name of control medium: "Standard embryo culture medium" Day of embryo transfer: day 5 (apart from 1 in the control group and 2 in the intervention group who transferred 1 embryo on day 3 due to delayed embryo development) Number of embryos transferred: single‐embryo transfer. "Couples who insisted on transferring two embryos, once embryo quality was known, were included in the study but were recorded as having a trial variation (four in the standard group and six in the intervention group).” |
|
| Interventions |
Intervention: GM‐CSF‐supplemented culture medium Control: Sydney IVF medium |
|
| Outcomes |
|
|
| Notes | Email correspondence with all additional data outlined in Appendix 13. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "50 cards with control and 50 with BlastGen written on them were placed in opaque sealed envelopes by a person unrelated to the trial. They were shuffled several times and the envelopes were marked from 1‐100 in consecutive order." |
| Allocation concealment (selection bias) | Low risk | "When the eligible patient was scheduled for an egg retrieval the embryologist took the next envelope by sequence and the random assignment was made." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Clinicians, sonographers, statisticians, and patients were completely blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sonographers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts accounted for. |
| Selective reporting (reporting bias) | Low risk | "We reported all outcomes as per our prospective clinical trials registration (ClinicalTrials.gov Identifier: NCT02305420)." |
| Other bias | High risk | There is a potential conflict of interest regarding the funding and administration of this study, as it was granted by the manufacturer of the intervention culture medium. However, the study authors have been very forthcoming with information regarding the trial, and there has been transparency. We assessed the study as at high risk of other bias due to the small sample size of 100 participants. |
Sbracia 2014.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Country: Italy Setting: fertility clinic Sponsorship source: no funding Trials registry number: NCT01718210 (prospectively registered) |
|
| Participants |
Inclusion criteria: women with recurrent implantation failure, 3 or more consecutive failed IVF cycles with a total of at least 8 good embryos replaced in the uterus, women aged 40 or less Exclusion criteria: over 40 years of age, chromosomal defects in the couple, metabolic diseases (diabetes, etc.), other genetic diseases (thalassaemia, cystic fibrosis, etc.) Number of participants randomised to each arm of the study: 50 to the intervention arm and 50 to the control arm Dropouts: none Group differences: not disclosed Fresh or frozen cycle?: fresh cycle IVF or ICSI?: ICSI Length of time exposed to intervention medium: from fertilisation to embryo transfer Trade name and concentration of GM‐CSF in intervention medium: EmbryoGen Trade name of control medium: not disclosed Day of embryo transfer: not disclosed Number of embryos transferred: a maximum of 3 embryos were transferred |
|
| Interventions |
Intervention: GM‐CSF‐supplemented culture medium Control: standard IVF medium |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Emailed 29 October 2019 and 9 January 2020 for further information, but received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised by a computer generated number sequence" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of who, if anyone, was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat data reported, and no dropouts described. |
| Selective reporting (reporting bias) | Low risk | The 2 outcomes reported in the study were outlined on the trials registry ClinicalTrials.gov Identifier NCT01718210. |
| Other bias | High risk | Small sample size of 100 participants |
Zafardoust 2017.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Country: Iran Setting: fertility clinic Sponsorship source: infertility and recurrent abortion treatment centre of Avicenna Trials registry number: www.irct.ir/trial/11703 (registered during recruitment) |
|
| Participants |
Inclusion criteria: couples undergoing treatment with their own gametes, women aged < 40 years old, women with at least 4 good‐quality embryos after thawing (grade A), women who had not had more than 1 previous embryo transfer Exclusion criteria: cycles requiring pre‐implantation genetic diagnosis, women with an anatomic disorder of the uterus or hydrosalpinx/hydrosalpinges Number of participants randomised to each arm of the study: number randomised to each arm before dropout was not disclosed Group differences: the average age between the 2 groups differed Fresh or frozen cycle?: frozen cycles IVF or ICSI?: ICSI Length of time exposed to intervention medium: from embryo thawing through to embryo transfer Trade name and concentration of GM‐CSF in intervention medium: BlastGen 2 ng/mL Trade name of control medium: Vitrolife, USA Day of embryo transfer: not disclosed Number of embryos transferred: not disclosed |
|
| Interventions |
Intervention: GM‐CSF‐supplemented culture medium Control: standard IVF medium |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Emailed on 29 October 2019, 26 November 2019, and 9 January 2020 for further information, but received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how randomisation was undertaken |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation concealment was undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of who, if anyone, was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 women were not included in the final analysis, which is a high rate of attrition. |
| Selective reporting (reporting bias) | High risk | No data on miscarriage, multiple pregnancy, and beta human chorionic gonadotrophin (BHCG) levels, which are secondary outcomes noted on trials register |
| Other bias | High risk | Small sample size of 90 participants |
Zavvar 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Country: Iran Setting: fertility clinic Sponsorship source: not disclosed Trials registry number: not disclosed |
|
| Participants |
Inclusion criteria: women who produce only immature oocytes in spite of stimulation with gonadotropins Exclusion criteria: not disclosed Number of participants randomised to each arm of the study: 31 women were randomised to receive the intervention medium, and 45 were randomised to receive the control medium Group differences: not disclosed Fresh or frozen cycle?: not disclosed IVF or ICSI?: ICSI Length of time exposed to intervention medium: not disclosed Trade name and concentration of GM‐CSF in intervention medium: MediCult 2 ng/mL Trade name of control medium: not disclosed Day of embryo transfer: not disclosed Number of embryos transferred: not disclosed |
|
| Interventions |
Intervention: GM‐CSF‐supplemented culture medium Control: standard IVF medium |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | First author contacted by email for further information on 26 November 2019 and 9 January 2020, but no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We assessed the study as at unclear risk, as we do not have access to number of dropouts or indeed how many participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No access to a protocol or a trial registry entry |
| Other bias | High risk | Small sample size of 76 participants |
Ziebe 2013.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT (14 different clinics) Country: study co‐ordinated in the Netherlands. Participants were recruited from clinics in Sweden and Denmark. Setting: fertility clinics Sponsorship source: supported by ORIGIO, Maløv, Denmark Trials registry number: NCT00565747 (prospectively registered) |
|
| Participants |
Inclusion criteria: women aged 25 to 39 years, women who had a regular menstrual cycle of 21 to 35 days, women treated with a standard gonadotrophin‐releasing hormone(GnRH) agonist or antagonist protocol, women with 3 or more follicles with a diameter of >/= 14 mm on the day of human chorionic gonadotrophin (hCG) administration, including a leading follicle of >/= 17 mm Exclusion criteria: previous participation in the study, use of assisted hatching, use of non‐ejaculated sperm, medical conditions or genetic disorders prohibiting in vitro fertilisation(IVF)/ intracytoplasmic sperm injection (ICSI) or interfering with interpretation of results, use of investigational drugs within 30 days before oocyte retrieval, severe chronic disease of relevance for reproduction, and oocyte donation Number of participants randomised to each arm of the study: 654 women randomised to receive GM‐CSF‐supplemented culture medium, and 678 women randomised to the control culture medium Group differences: no significant between‐group differences in baseline characteristics Fresh or frozen cycle?: fresh IVF or ICSI?: IVF and ICSI Length of time exposed to intervention medium: from fertilisation through to embryo transfer Trade name and concentration of GM‐CSF in intervention medium: ORIGIO 2 ng/mL Trade name of control medium: EmbryoAssist Day of embryo transfer: day 3 Maxiumum number of embryos transferred: 2 |
|
| Interventions |
Intervention: GM‐CSF‐supplemented culture medium Control: standard IVF medium |
|
| Outcomes |
|
|
| Notes | Email correspondence with all additional data outlined in Appendix 13. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation (blocks of four) was computer‐generated individually for each clinic to maintain balance between the treatment groups at each site." |
| Allocation concealment (selection bias) | Low risk | Following email correspondence: "Randomization was performed by ORIGIO a/s, and based on a randomisation list per clinic generated automatically using www.randomization.com. Each study site received a list of study specific consecutive patient ID numbers (e.g. clinic 1: 01001, 01002, 01003, 01004 etc.) and a corresponding number of identically looking randomized bottles of test and control media individually labelled with the corresponding study specific patient ID numbers. On site the lowest number on the list was always allocated to any new patient recruited, at the time of informed consent signature. Therefore, both the clinician, embryologist and patient, was blinded to the treatment allocation. The master randomisation list was held by ORIGIO a/s. All data analysis was performed externally by a Clinical Research Organization (CRO). During the interim analysis the statistician was blinded at all times to the media, which were presented as Medium A and Medium B. For the final statistical analyses, the codes for blinding were broken after database lock and patient classification." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants and investigators were blinded to treatment allocation. Study media were packaged unidentifiably and labelled only with the randomization number. For each new patient recruited, the lowest available randomization number was used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Following email correspondence: "The clinicians performing the ultrasound scans were blinded to the treatment allocation at all times." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All dropouts accounted for, however the reasons given were not included in the predefined exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | The authors confirmed through email correspondence that all outcomes were published. |
| Other bias | High risk | There is a potential conflict of interest regarding the funding and administration of this study as it was granted by the manufacturer of the intervention culture medium. However, the study authors have been very forthcoming with information regarding the trial, and there has been transparency. We assessed this trial as at high risk of other bias because the data provided in private email correspondence differ from the published data. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agerholm 2010 | Wrong study design |
| Fawzy 2019 | Wrong study design |
| Kinoshita 2019 | Wrong study design |
| Min 2017 | Wrong study design |
| Scarpellini 2011 | Wrong intervention |
| Sfontouris 2013 | Wrong study design |
| Shapiro 2003 | Wrong study design |
| Siqueira 2016 | Animal study |
| Siristatidis 2013 | Wrong study design |
| Sjoblom 1998 | Wrong study design |
| Sjoblom 1999 | Wrong study design |
| Sjoblom 1999a | Wrong study design |
| Sjoblom 2001 | Wrong study design |
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN94726536.
| Methods | Prospectively randomised controlled study |
| Participants | 500 women undergoing ICSI and embryo culture Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants are randomly allocated to 1 of 2 groups. Group 1: Participants receive ICSI treatments carried out using ISM1 for embryo culture and transfer. Group 2: Participants receive ICSI treatments carried out using EmbryoGen for embryo culture and transfer. |
| Outcomes |
Primary outcome measure: Implantation rate (defined as the number of implanted embryos per number of transferred embryos) is measured 30 days after embryo transfer. Secondary outcome measure: Pregnancy rate (defined as the number of pregnancies per number of transferred embryos) is measured using ultrasound of gestational chamber 30 days after embryo transfer. |
| Notes | Country of recruitment: Italy Overall trial status 'completed' Publication status 'results overdue' Unable to determine if women or embryos were randomised Emailed contact author 29 October 2019, but received no response |
NCT01689428.
| Methods | Prospective randomised controlled trial |
| Participants | 100 infertile couples with an indication for standard IVF or intracytoplasmic sperm injection. All couples had at least a previous miscarriage and/or biochemical pregnancy experience after assisted reproductive technology (ART) treatment. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: embryo culture in EmbryoGen medium Control: embryo culture in ISM1 medium |
| Outcomes |
Primary outcome measure: Ongoing pregnancy rate after 12 weeks of gestation [ Time Frame: 12 weeks after conception ] Secondary outcome measure: Ongoing implantation rate after 12 weeks of gestation [ Time Frame: 12 weeks after conception ] |
| Notes | Country of recruitment: Italy Unable to determine if women or oocytes were randomised |
NCT01689454.
| Methods | Described as both a prospective randomised controlled trial and an observational cohort study |
| Participants | Infertile couple with an indication for standard IVF or intracytoplasmic sperm injection. All couples had previous implantation failures after ART treatment (minimum 2, maximum 4 embryo replacements). Inclusion criteria:
Exclusion criteria:
|
| Interventions | Control: embryo culture in ISM1 medium Intervention: embryo culture in EmbryoGen medium |
| Outcomes |
|
| Notes | Unable to determine if RCT or cohort study, as methods unclear |
NCT02651285.
| Methods | Randomised parallel assignment trial |
| Participants | 180 women aged 20 to 28 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: granulocyte colony‐stimulating factor (G‐CSF) medium Control: medium without G‐CSF |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Notes | Emailed authors for more information 29 October 2019, 9 January 2020, and 28 January 2020, but received no reply. Unsure of nature of study and whether oocytes or women were randomised |
ICSI: intracytoplasmic sperm injection
Differences between protocol and review
We changed the phrase "assisted reproductive technology (ART)" to "assisted reproduction" in the review title. We feel this is easy to understand and avoids use of an acronym.
We included all outcomes in the 'Summary of findings' table.
Contributions of authors
SA wrote the first draft of the protocol and the review.
SA, AP, JM, and BW undertook screening of abstracts.
SA, JM, and AP undertook screening of full‐text articles.
SA and JM undertook data extraction, 'Risk of bias' assessment, and application of GRADE.
All authors contributed to the edits of the full review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Bath Clinical Society, UK
GBP 1000 was awarded to SA to go toward attendance at a conference to present the findings of this review.
Declarations of interest
SA: none.
JM: none.
BW: none.
AP: none.
CF: none.
New
References
References to studies included in this review
Rose 2020 {published and unpublished data}
- EmbryoGen/Blastgen for Couples With Implantation Problems or Previous Miscarriage (BlastGen) [NCT02305420]. ClinicalTrials.gov 2014.
- Hull ML, Barry ML, Cameron LP, Knight EJ, Norman RJ, Rose RD. A randomised control pilot study comparing Embryogen®/BlastGen™ with standard media culture in poor prognosis couples undertaking IVF. In: Fertility Society of Australia Annual Conference. 1 edition. Vol. 1. Melbourne: Fertility Society of Australia, 2018:85.
- Rose RD, Barry MF, Dunstan EV, Yuen SM, Cameron LP, Knight EJ, Norman RJ, Hull ML. The BlastGen study: A randomised controlled trial of GM-CSF supplemented blastocyst media. Reproductive BioMedicine Online 2020;www.doi.org/10.1016/j.rbmo.2020.01.011. [DOI] [PubMed] [Google Scholar]
Sbracia 2014 {published data only}
- Sbracia M, Morgia F, Torti M, Amodei M, Corizza R, Scarpellini F. Use of GM-CSF supplemented IVF medium in patients with recurrent implantation failure: A controlled trial. In: Human Reproduction. Vol. 29 (Suppl. 1). Oxford University Press, 2014.
- Use of GM-CSF Supplemented IVF Medium in Patients With Recurrent Implantation Failure [NCT01718210]. ClinicalTrials.gov 2012.
Zafardoust 2017 {published data only}
- IRCT2014021611430N4. Iranian Registry of Clinical Trials 2014.
- Zafardoost S. Evalutation of efficacy of GM-CSF in embryo culture for freezed embryos in embryo development and pregnancy outcomes. Iranian Registry of Clinical Trials 17/03/2014;www.irct.ir/trial/11703.
- Zafardoust S, Mohammadzadeh A, Fatemi F, Jouhari S, Karimi A, Shahbakhsh A, Barchinezhad M, Fathi Z, Sadeghi MR. Evaluation of efficacy of GM-CSF in embryo culture for frozen embryos on embryo development and pregnancy outcomes. In: Human Reproduction. Vol. 32 (Suppl. 1). Netherlands Oxford University Press, 2017.
Zavvar 2016 {published data only}
- Zavvar TS, Roustaei H, Torshizi MM, FadaviIslam M, Sabouri E. The effect of embryogen medium on oocyte in vitro maturation, fertilization rate and embryo development. In: Journal of Reproduction and Infertility. Vol. 17 (2 Suppl.2). Netherlands Avicenna Research Institute, 2016:99-100.
Ziebe 2013 {published data only}
- Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, Gabrielsen A, Hnida C, Zobel DP, Munding B, Bendz SH, Robertson SA. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertility & Sterility 2013;99(6):1600-1609. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agerholm 2010 {published data only}
- Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sorensen PD, Ziebe S. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reproductive Biomedicine Online 2010;20(4):477-84. [DOI] [PubMed] [Google Scholar]
Fawzy 2019 {published data only}
- Fawzy M, Emad M, Elsuity MA, Mahran A, Abdelrahman MY, Fetih AN, Abdelghafar H, Sabry M, Nour M, Rasheed SM. Cytokines hold promise for human embryo culture in vitro: results of a randomized clinical trial. Fertility and Sterility 2019;112(5):849-57. [DOI] [PubMed] [Google Scholar]
Kinoshita 2019 {published data only}
- Kinoshita M, Fujita M, Takahashi K. The use of granulocyte macrophage colony-stimulating factor (GM-CSF) containing media for embryo transfer increases the clinical pregnancy and ongoing pregnancy rate. Human Reproduction 2019;34(Suppl 1):368. [Google Scholar]
Min 2017 {published data only}
- Min SH, Ja-Seong K, Hyeyoung L, Hwa-Sook M. Treatment with GM-CSF improves pregnancy rates in poor responders. Human Reproduction 2017;32(Suppl 1). [Google Scholar]
Scarpellini 2011 {published data only}
- Scarpellini F, Sbracia M. The use of G-CSF for implantation failure in IVF: a clinical trial. Fertility and Sterility 2011;96(Suppl 3). [Google Scholar]
Sfontouris 2013 {published data only}
- Sfontouris IA, Lainas GT, Anagnostara K, Kolibianakis EM, Lainas TG. Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on pregnancy rates in patients with multiple unsuccessful IVF attempts. Human Reproduction 2013;28(Suppl 1). [Google Scholar]
Shapiro 2003 {published data only}
- Shapiro B, Richter K, Daneshmand S, Quinn P, Behr B. Granulocyte-macrophage colony-stimulating factor enhances human embryo development to the blastocyst stage: a randomized study. Fertility & Sterility 2003;79(Suppl 2):S15-16. [Google Scholar]
Siqueira 2016 {published data only}
- Siqueira LGB, Tribulo P, Denicol AC, Ortega MS, Negron-Perez VM, Kannampuzha-Francis J, Hansen PJ. Consequences of in vitro production of embryos with or without colonystimulating factor 2 in culture medium on morphometric features of the bovine conceptus at day 86 of gestation. Reproduction, Fertility and Development 2016;28:218. [Google Scholar]
Siristatidis 2013 {published data only}
- Siristatidis C, Vogiatzi P, Salamalekis G, Creatsa M, Vrachnis N, Glujovsky D, Iliodromiti Z, Chrelias C. Granulocyte macrophage colony stimulating factor supplementation in culture media for subfertile women undergoing assisted reproduction technologies: A systematic review. International Journal of Endocrinology 2013;2013:704967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sjoblom 1998 {published data only}
- Sjoblom C, Robertson SA, Wikland M. Granulocyte-macrophage colony-stimulating factor promotes the rate and extent of human blastocyst development in vitro. Human Reproduction 1998;13. [DOI] [PubMed] [Google Scholar]
Sjoblom 1999 {published data only}
- Sjoblom C, Robertson SA, Wikland M. Granulocyte -macrophage colony stimulating factor (GM-CSF) promotes development of human pre-implantation embryos through the alpha-chain of the GM-CSF receptor. In: 11th world congress on in vitro fertilization and human reproductive genetics. 1999.
Sjoblom 1999a {published data only}
- Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Human Reproduction 1999;14(12):3069-76. [DOI] [PubMed] [Google Scholar]
Sjoblom 2001 {published data only}
- Sjoblom C, Wikland M, Robertson S. Granulocyte-macrophage-colony stimulating factor (GM-CSF) promotes inner cell mass blastomere viability in human pre-implantation embryos. Fertility & Sterility 2001;76(3 Suppl 1):S70. [Google Scholar]
References to studies awaiting assessment
ISRCTN94726536 {published data only}
- ISRCTN94726536. Clinical efficiency of a ready-to-use medium for human embryo culture supplemented with growth factors in patients with previous in vitro fertilization failures. isrctn.com/ISRCTN94726536 first received 12 January 2016.
NCT01689428 {published data only}
- NCT01689428. Ism1 Versus EmbryoGen Media for Embryo Culture in Previous Pregnancy Loss Cases. clinicaltrials.gov/ct2/show/NCT01689428 first received 21 September 2012.
NCT01689454 {published data only}
- NCT01689454. ISM1 Versus EmbrioGen Media for Embryo Culture With Previous Failure Implantation in ART Treatments. clinicaltrials.gov/ct2/show/NCT01689454 first received 21 September 2012.
NCT02651285 {published data only}
- NCT02651285. Use of G-CSF Supplemented IVF Medium in Patients Undergoing IVF. clinicaltrials.gov/ct2/show/NCT02651285 first received 8 January 2016.
Additional references
Agerholm 2010
- Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sorensen PD, et al. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reproductive Biomedicine Online 2010;20(4):477-84. [DOI] [PubMed] [Google Scholar]
Baldwin 1992
- Baldwin GC. The biology of granulocyte-macrophage colony-stimulating factor: effects on hematopoietic and nonhematopoietic cells. Developmental Biology 1992;151(2):352-67. [0012-1606] [DOI] [PubMed] [Google Scholar]
Biggers 2000
- Biggers JD. Ethical issues and the commercialization of embryo culture media. Reproductive Biomedicine Online 2000;1:74-6. [DOI] [PubMed] [Google Scholar]
Block 2003
- Block J, Drost M, Monson RL, Rutledge JJ, Rivera RM, Paula-Lopes FF, et al. Use of insulin-like growth factor-I during embryo culture and treatment of recipients with gonadotropin-releasing hormone to increase pregnancy rates following the transfer of in vitro-produced embryos to heat-stressed, lactating cows. Journal of Animal Science 2003;81(6):1590-602. [DOI] [PubMed] [Google Scholar]
Burgess 1977
- Burgess AW, Wilson EMA, Metcalf D. Stimulation by human placental conditioned medium of hemopoietic colony formation by human marrow cells. Blood 1977;49(4):573-83. [PubMed]
Chronopoulou 2015
Cooper Surgical 2020
- Cooper Surgical. Bringing knowledge to life. www.fertility.coopersurgical.com/about-us/ (accessed 11 March 2020).
Cooper Surgical 2020a
- Cooper Surgical. Culture media with GM-CSF. Unique culture media for poor prognosis patients. www.fertility.coopersurgical.com/products/culture-media-with-gm-csf/ (accessed 13 March 2020).
Coughlan 2014
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reproductive BioMedicine Online 2014;28(1):14-38. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 1 Jan 2020. Melbourne, Australia: Veritas Health Innovation.Available at covidence.org.
Dyrlund 2014
Farquhar 2015
- Farquhar C, Vail A, Mourad S. [personal communication to editor]. Letter to: editor of Fertility & Sterility 01 December 2015.
Giacomini 1995
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 Feb 2020. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Hambartsoumian 1998
Heneghan 2016
- Heneghan C, Spencer EA, Bobrovitz N, Collins DR, Nunan D, Pluddemann A, et al. Lack of evidence for interventions offered in UK fertility centres. BMJ 2016;355:i6295. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Jokhi 1994
Lawn 2016
- Lawn JE, Blencowe J, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018):587-603. [DOI] [PubMed] [Google Scholar]
Lazzari 2002
Lighten 1998
Lim 2006
- Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, et al. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in peri-implantation mouse embryos. Journal of Assisted Reproduction and Genetics 2006;23(3):111-9. [DOI] [PMC free article] [PubMed]
Mignini 2013
- Mignini Renzini M, Dal Canto M, Coticchio G, Comi R, Brigante C, Caliari I, et al. Clinical efficiency and perinatal outcome of ART cycles following embryo culture in the presence of GM-CSF in patients with miscarriage or early pregnancy loss history. Human Reproduction (Oxford, England) 2013;28(Suppl 1):i139-49.
Morbeck 2014
Rahmati 2015
Richter 2008
Robertson 1992
Robertson 1994
Robertson 1999
Robertson 2007
Roudebush 2004
- Roudebush WE, Massey JB, Kort HI, Elsner CW, Toledo AA, Mitchell-Leef D, et al. Exposure of preimplantation embryos to platelet-activating factor increases birth rate. Journal of Assisted Reproduction and Genetics 2004;21(8):297-300. [DOI] [PMC free article] [PubMed]
Savion 2002
Seymour 1997
- Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood 1997;90(8):3037-49. [PubMed]
Sfontouris 2013
- Sfontouris I, Lainas G, Anagnostara K, Kolibianakis E, Lainas T. Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on pregnancy rates in patients with multiple unsuccessful IVF attempts. Human Reproduction (Oxford, England) 2013;28(Suppl 1):160-2. [Google Scholar]
Sfontouris 2016
- Sfontouris IA, Martins WP, Nastri CO, Viana IG, Navarro PA, Raine-Fenning N, et al. Blastocyst culture using single versus sequential media in clinical IVF: a systematic review and meta-analysis of randomized controlled trials. Journal of Assisted Reproduction and Genetics 2016;33(10):1261-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siristatidis 2013
- Siristatidis C, Vogiatzi P, Salamalekis G, Creatsa M, Vrachnis N, Glujovsky D, et al. Granulocyte macrophage colony stimulating factor supplementation in culture media for subfertile women undergoing assisted reproduction technologies: a systematic review. International Journal of Endocrinology 2013;2013:704967. [DOI] [PMC free article] [PubMed]
Sjöblom 1992
- Sjöblom C, Wikland M, Robertson SA. Granulocyte-macrophage-colony stimulating factor (GM-CSF) promotes inner cell mass blastomere viability in human pre-implantation embryos. Fertility and Sterility 1992;76(3):S70. [0015-0282]
Sjöblom 1999
Sjöblom 2002
Sjöblom 2005
Spanos 2000
Steptoe 1978
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;2:366. [DOI] [PubMed] [Google Scholar]
Tevkin 2014
- Tevkin S, Lokshin V, Shishimorova M, Polumiskov V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecological Endocrinology 2014;30(Suppl 1):9-12. [DOI] [PubMed]
Torry 2007
- Torry DS, Leavenworth J, Chang M, Maheshwari V, Groesch K, Ball ER, et al. Angiogenesis in implantation. Journal of Assisted Reproduction and Genetics 2007;24(7):303-15. [DOI] [PMC free article] [PubMed]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction (Oxford, England) 2003;18:1000-4. [DOI] [PubMed] [Google Scholar]
Vuorela 2000
Wegmann 1992
- Wegmann TG, Guilbert LJ. Immune signalling at the maternal – fetal interface and trophoblast differentiation. Developmental & Comparative Immunology 1992;16(6):425-30. [0145-305X] [DOI] [PubMed] [Google Scholar]
World Bank
- The World Bank. Countries and economies. www.data.worldbank.org/country (accessed 11 March 2020).
Young 2001
Yu 2012
- Yu Y, Yan J, Li M, Yan L, Zhao Y, Lian Y, et al. Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Human Reproduction (Oxford, England) 2012;27(7):2146-59. [DOI] [PubMed]


