Abstract
Background:
High-throughput metabolomics has been used cross-sectionally to evaluate differential metabolic profiles associated with individuals who are obese.
Objectives:
This study longitudinally assessed the cord-blood metabolome to explore if metabolic signatures of obesity at age 3–5 are apparent at birth.
Methods:
In a nested case control design, metabolomics analysis was performed on umbilical cord blood of 25 children who became obese at age 3–5 years, compared to 25 sex-matched non-obese children enrolled as part of an ongoing birth cohort. Logistic regression models were used to identify significant metabolites, adjusting for maternal pre-pregnancy obesity.
Results:
Children who were obese by age 3–5 years had elevated levels of medium and long chain fatty acids including stearate, oleate, and palmitate at birth. Children with obesity were also more likely to have elevated levels of acetaminophen metabolites at birth, specifically: 3-(N-acetyl-L-cystein-S-yl) acetaminophen, 2-hydroxyacetaminophen sulfate, 2-methoxyacetaminophen glucuronide, and p-acetamidophenyl glucuronide.
Conclusion:
Although the observed increases in lipids are consistent with previous metabolomic studies of obesity, this study is the first to report associations between acetaminophen metabolites and obesity in children; however, we lack mechanistic insights for this link. Larger human studies with longer follow-up and laboratory-controlled animal experiments are needed to clarify associations.
Keywords: metabolomics, acetaminophen, epidemiology, children, obesity
INTRODUCTION
The prevalence of childhood obesity in the United States has increased from 10% to 17% in the last 30 years1. Obesity at younger ages tends to persist into adulthood and is often found concurrent with many metabolic diseases.2,3 In adults, obesity is a consistent risk factor for type 2 diabetes, cardiovascular disease and liver disease, it is associated with reduced quality of life and premature mortality.4 Genetic differences only account for about 5% of the variation in obesity risk5. While the etiology of obesity is complex, a significant component is driven by complex gene-environment interactions in early life which alter metabolic programming.6–8 Biochemical signatures related to in utero exposures may be apparent at birth, and exploration of these signatures may reveal insights into metabolic pathways involved in the etiology of obesity.
Blood chemistry testing has been used for many years as a clinical measure of metabolic health, but high-throughput “metabolomic” profiling techniques that are now available have made possible simultaneous, agnostic identification, and quantification of hundreds of small molecules, metabolites, and protein products. At different ages, many studies have investigated the metabolic profile associated with obesity and its comorbidities. Those studies found significant differences in metabolites including species of acylcarnitines, branched-chain amino acids (BCAAs) and other amino acid species, as well as lipid species like non-esterified fatty acids (NEFAs), mono and poly-unsaturated fatty acids (MUFAs and PUFAs), phospholipids (PL), phosphatidylcholines (PC), as well as short, medium, and long-chain free fatty acids (FFAs).9–15 The transient nature of many species of metabolites, and cross-sectional or case-control design of many of these studies has made cause-and-effect between altered metabolites and the onset of metabolic disease difficult to infer. Since umbilical cord-blood is collected prior to clinical disease manifestation, temporal sequence is maintained, and causal inference is enhanced. Currently, only a few umbilical cord blood metabolomics assessments relating to obesity have been published.16,17
Reported here are umbilical cord blood metabolite profiles associated with childhood obesity at ages 3–5 years. The metabolomics array revealed 46 metabolites at significantly higher levels in the cord-blood of children with obesity including 25 lipid species similar to those identified in individuals affected by metabolic diseases.10–15 Additionally, an unexpected association was found between acetaminophen metabolites at birth and childhood obesity.
MATERIALS AND METHODS
Metabolomics Analysis
Study Participants
Study participant recruitment methods are described in detail elsewhere18. Expectant mothers who were 18 years or older, spoke English and intended to receive care within the Duke Obstetrics or Durham Regional Hospital were recruited. Of 601 eligible pregnant women were consented and enrolled between April 2005 and June 2009, 590 participants remained at delivery when umbilical cord blood samples were obtained. Children were contacted to collect growth data once every two years. Characteristics of the study population are presented in Table 1. The study protocol was approved by the Duke University Institutional Review Board.
Table 1:
Characteristics of Study Participants
| Obese (n=25) |
Non-Obese (n=25) |
P-value | |
|---|---|---|---|
| Maternal Characteristics | Mean (SD) or N (%) | ||
| Pre-pregnancy BMI, kg/m2 | 35.57 (7.82) | 26.27 (8.12) | 0.002 |
| Maternal Smoking, N (%) | 0.07 | ||
| Yes | 12 (50) | 6 (25) | |
| No | 12 (50) | 18 (75) | |
| Gestational Weight Gain, kg | 12.23 (6.86) | 16.94 (6.69) | 0.02 |
| Maternal Age, years | 30.2 (8.19) | 30.3 (7.03) | 0.97 |
| Chronic Hypertension, N (%) | 0.64 | ||
| Yes | 3 (12) | 2 (8) | |
| No | 22 (88) | 23 (92) | |
| Pregnancy Hypertension, N (%) | 1.00 | ||
| Yes | 1 (4) | 1 (4) | |
| No | 24 (96) | 24 (96) | |
| Diabetes, N (%) | 1.00 | ||
| Yes | 8 (32) | 8 (32) | |
| No | 17 (68) | 17 (68) | |
| Gestational Diabetes, N (%) | 0.68 | ||
| Yes | 3 (12) | 4 (16) | |
| No | 22 (88) | 21 (84) | |
| Preeclampsia, N (%) | 0.29 | ||
| Yes | 3 (12) | 1 (4) | |
| No | 22 (88) | 24 (96) | |
| Delivery Route, N (%) | 0.16 | ||
| Vaginal | 11 (41) | 16 (61) | |
| Caesarian | 14 (59) | 9 (39) | |
| Ethnicity, N (%) | |||
| African American | 17 (68) | 8 (32) | 0.01 |
| Caucasian | 7 (28) | 17 (68) | |
| Child Characteristics | |||
| Gestational Age, weeks | 38.3 (1.66) | 38.3 (2.25) | 0.96 |
| Birth Weight, grams | 3256 (665) | 3250 (563.7) | 0.97 |
| Breastfeeding, N (%) | 0.16 | ||
| Yes | 11 (44) | 12 (48) | |
| No | 10 (40) | 4 (16) | |
| Missing | 4 (16) | 9 (36) | |
| Caloric Intake, kCal | 1630 (464) | 1642 (414) | 0.94 |
| WHZ-Age 1 | 1.27 (1.3) | 0.23 (1.1) | 0.01 |
| Sex, N (%) | 1.00 | ||
| Male | 14 (56) | 14 (56) | |
| Female | 11 (44) | 11 (44) | |
| Age at Assessment, N (%) | 0.0003 | ||
| 3 years | 7 (14) | 1 (2) | |
| 4 years | 10 (20) | 2 (4) | |
| 5 years | 8 (16) | 22 (44) | |
Maternal and early childhood characteristics for 50 children recruited via NEsT cohort. This analysis includes 25 children who became obese by age 3–5 and 25 who were not classified as obese (non-obese) by age 3–5. Categories that do not sum to 25 reflect missing questionnaire data. Obese= (Weight Hight Z-Score ≥ 95%). Non-Obese= (Weight Height Z-Score < 95%)
Childhood obesity
Twenty-five children classified as “obese” (Weight-Height-Zscore ≥ 95%) and 25 classified as “non-obese” (Weight-Height-Zscore < 85%) were selected at age 3–5 years. Weight and height was obtained from a combination of medical records and measurement by staff during study visits, the latter using a Seca® stadiometer and Tanita BWB-800 scale. Children were chosen from amongst the first 100 participants enrolled into the cohort at the time of analysis. These did not differ from the remaining 540 participants with respect to ethnicity (p= 0.20), gestational weight gain (p= 0.40), and maternal cigarette smoking (p= 0.76). They did however differ with respect to maternal pre-pregnancy obesity (p= 0.02), but this was adjusted for in regression models.
Specimen collection and processing
Within minutes of delivery, umbilical cord blood (UCB) was collected into a K3EDTA tube by puncturing the umbilical vein. The specimens were kept at 4°C for up to 2 hours, before they were centrifuged at 2,000 rpm for 10 minutes in the laboratory to isolate plasma and buffy coat. Aliquots were frozen and stored at −80°C prior to analysis.
Metabolomic Profiling
Two hundred microliter aliquots were shipped to Metabolon (Durham, NC) for metabolite analysis using a combination of LC and GC mass spectrometry platforms. Sample preparation and mass spectrometry procedures have been published elsewhere.19 Metabolites were identified using Metabolon’s Precision Metabolomics Biochemical Reference Library ™. Analyses identified 384 metabolites and the data was presented in area counts. Since exposure to acetaminophen was not ubiquitous the raw area counts were dichotomized into “exposed” or “unexposed”. Individuals were classified as “exposed” for each metabolite if they had detectable levels of that metabolite. For lipids, raw area counts were re-scaled so that the median equaled 1 and absent values were assigned the minimum value. These data were then log2-transformed to minimize the effects of outliers, and fold difference was generated (2log2 mean cases/ 2log2 mean controls). Metabolite concentrations for children with and without obesity were compared using regression models and metabolites p≤0.05 were considered significant.
Statistical Analysis
Covariates
To identify potential confounding factors, we compared children with and without obesity with respect to pre-pregnancy maternal BMI, maternal gestational weight gain, maternal cigarette smoking, maternal age, maternal hypertension, maternal diabetes, delivery route, sex, ethnicity, birth weight, gestational age, breastfeeding for three months or more, and postnatal daily caloric intake. Pre-pregnancy obesity was calculated using self-reported height and weight (to nearest kg) at last menstrual period and analyzed as a continuous variable. The use of self-reported pre-pregnancy weight has been previously validated.20 Maternal gestational weight was assessed at each visit and weight gain was calculated as the difference between final recorded weight (up to 7 days before delivery) and self-reported pre-pregnancy weight. Ethnicity and maternal cigarette smoking during pregnancy were collected by self-report. Smoking status was verified by cotinine levels in infant cord blood samples. Maternal age, delivery route, infant sex, and birthweight were collected from medical records as were maternal morbidity data including gestational and type 2 diabetes and hypertension. Gestational age was determined based on the last menstrual period unless a 14+ day difference was detected in developmental stage using ultrasonography. Breast-feeding data (yes/no) were self-reported for every month for the first year of life, and then dichotomized at 3 months. Caloric intake for the child was assessed using two 24-hour dietary recalls during postnatal visits.
Modeling Approach
Logistic regression models were used to compare metabolite concentrations of children with and without obesity at age 3–5 years controlling for potential confounding variables. Concentrations of acetaminophen metabolites were dichotomized as “exposed” if acetaminophen was detected or “unexposed” otherwise. Figure 1 shows the mean difference in the level of each of the acetaminophen metabolites for children who were obese vs those who were not by ages 3–5 years. Data are presented here continuously and non-detectable levels were imputed with ½ the minimum detected value for each metabolite21. Additionally, the number of children exposed is reflected below the x-axis of each plot. Lipid metabolites were also analyzed continuously using logistic regression models. Of the factors considered for confounding, only maternal pre-pregnancy obesity changed the odds ratios by greater than 10% and was included in models. Additionally, we explored pathway enrichment using IPA (Qiagen Inc.).
Figure 1: Mean Difference between Children with and without Obesity.
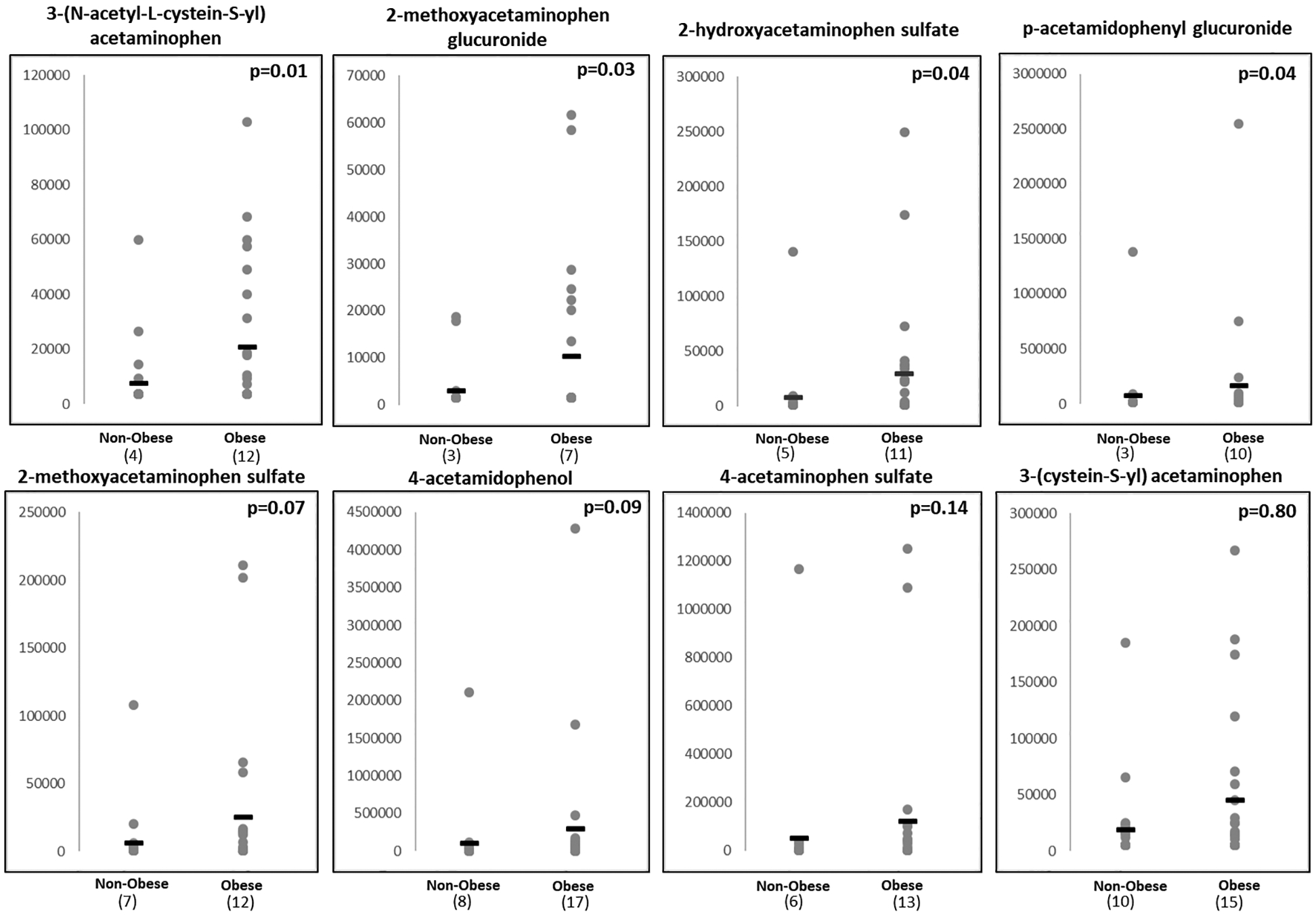
Shown are 8 metabolites of acetaminophen measured through agnostic metabolomics array (Metabolon Inc.) P-values calculated using logistic regression corrected for maternal pre-pregnancy BMI.
Y-axis = arbitrary units derived from Area Under the Curve (AUC) values. Non-detect values were imputed as ½ the lowest detectable level for each metabolite and number of children with detectable levels is reflected below each plot.
Prenatal Assessment of Acetaminophen Exposure
A separate cohort assembled between 2009 and 2011 was used to determine if therapeutic maternal acetaminophen use during pregnancy was associated with childhood obesity at ages 3 and 5 years. Inclusion and exclusion criteria and recruitment methods for the cohort were similar to the first cohort, and have been previously described.22 Pregnant women were recruited during their first prenatal visit at one of five prenatal/delivery clinics in Durham, NC. These analyses are limited to 681 of 1700 total participants who remained in the cohort at age 3 or 5 years and responded to questions about intake of pharmaceuticals including acetaminophen during pregnancy. These 681 were similar to those not included with respect to ethnicity (age 3: Black p=0.24, White p=0.21, and Other p=1.00, and at age 5: Black p=0.07, White p=1.00, and Other p=1.00), gestational weight gain (p= 0.61, p=0.74 at ages 3 and 5 respectively), cigarette smoking (p=0.41 at age 3 and p=0.88 at age 5) and sex distribution (p=0.28 and p=0.69 at ages 3 and 5), but differed with respect to maternal pre-pregnancy obesity (p=0.01 and p=0.001 at ages 3 and 5 respectively). The effects of maternal pre-pregnancy obesity were adjusted for in logistic regression models.
The use of pharmaceuticals during pregnancy was queried for six categories: cough/cold, pain relief, sleep aid, migraine, back pain, and other. Acetaminophen use was assessed as a binary variable where “1” reflected a single reported use, and “0” referred to no usage. Child obesity status was calculated using Weight-Height(z) (WHz) scores at ages 3 and 5 years, and was defined in this cohort in the same manner as previously described. Logistic regression models were used to adjust for pre-pregnancy obesity.
RESULTS
Table 1 shows that study participants were similar with respect to gestational age, birth weight, breastfeeding, caloric intake, and sex. They did differ by ethnicity (p=0.01), gestational weight gain (p=0.02), and maternal pre-pregnancy obesity (p=0.002). Of these factors, only maternal pre-pregnancy obesity was also found to be related to cord-blood metabolite levels.
Of 384 metabolites analyzed, 68 were found statistically different in children who became obese by age 3–5 years. (full list in Supplementary Table 1) Children with obesity had significantly elevated lipid species, including linoleate, myristate, oleate, palmitate, stearate, caprate, and species of acylcarnitines (P<0.05) compared to non-obese children. These associations were adjusted for maternal pre-pregnancy BMI, but models remained unchanged when maternal cigarette smoking, gestational weight gain, maternal hypertension, maternal diabetes, gestational diabetes, delivery route, ethnicity, sex, birthweight, and offspring caloric intake were included. IPA analysis revealed associations with biosynthesis of glycine, creatine, glutathione and palmitate.
The analysis revealed that of the eight metabolites of acetaminophen measured, four were significantly elevated in children who became obese by age 3–5 years. The odds ratio (OR) for 3-(N-acetyl-L-cystein-S-yl) acetaminophen was 10.42 (95% Confidence Interval (CI) 1.67–65.26; p=0.01). Two to three-fold increases in risk of obesity were also observed in children with higher concentrations of 2-hydroxyacetaminophen sulfate, 2-methoxyacetaminophen glucuronide, and p-acetamidophenyl glucuronide (p<0.05). The association of 4-methoxyacetaminophen sulfate and 4-acetamidophenol with obesity were borderline significant (p=0.07 and p=0.09). These relationships remained unchanged after adjusting for pre-pregnancy maternal BMI. (Table 2) Associations in the same direction, albeit non-significant, were found between 4-acetaminophen sulfate (OR=3.20; p=0.14), 3(cysteine-S-yl)acetaminophen (OR=1.20; p=0.80) and obesity.
Table 2:
Odds Ratios and 95% Confidence Intervals for the Association between Acetaminophen Metabolites and Childhood Obesity
| Odds Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| 3-(N-acetyl-L-cystein-S-yl) acetaminophen | 10.42 | (1.67 – 65.26) | 0.01 |
| 2-methoxyacetaminophenglucuronide | 9.06 | (1.19 – 68.97) | 0.03 |
| 2-hydroxyacetaminophen sulfate | 5.94 | (1.07 – 33.10) | 0.04 |
| p-acetamidophenyl glucuronide | 6.09 | (1.05 – 35.33) | 0.04 |
| 2-methoxyacetaminophen sulfate | 4.54 | (0.89 – 23.33) | 0.07 |
| 4-acetamidophenol | 3.65 | (0.82 – 16.33) | 0.09 |
| 4-acetaminophen sulfate | 3.20 | (0.67 – 15.27) | 0.14 |
| 3-(cystein-S-yl) acetaminophen | 1.20 | (0.23 – 4.93) | 0.80 |
Adjusted for maternal pre-pregnancy BMI.
Maternal Smoking, Breastfeeding 3+ months. Caloric Intake, Gestational Age, Birthweight, Sex, Race, Maternal Hypertension, Maternal Diabetes, Delivery Route, and Child Age at Obesity Assessment were not significant confounders.
To identify potential sources of acetaminophen exposure, the association between acetaminophen intake during pregnancy and obesity in children at age 3–5 years was examined. As expected, maternal obesity was strongly associated with child obesity status at both ages 3 and 5 years, but no association was found with self-reported maternal acetaminophen intake during pregnancy. The odds ratios of the WHz for exposed and unexposed individuals was 1.07 (95%CI 0.29 – 4.02) (p=0.91) and 0.86 (95%CI 0.12 – 6.11) (p=0.86) at ages 3 and 5 years, respectively. No associations were found when stratified by sex [Boys age 3: 1.28 (95%CI 0.44–3.73; p=0.77); Boys age 5: 1.70 (95%CI 0.49–5.52; p=0.43; Girls age 3: 1.18 (95%CI 0.39–3.54); p=0.66; Girls age 5: 0.70 (95%CI 0.23–2.13; p=0.53)]. Additionally, we explored the possibility that women with obesity take acetaminophen more often during pregnancy but found no association. The mean difference in BMI in between mothers who reported they did not take acetaminophen during pregnancy vs those who reported they did, was 0.82kg/m2 (95%CI: (−0.17–1.80) p=0.10).
DISCUSSION
In a nested case-control study of 25 children with obesity and 25 children without obesity at age 3–5 years, we undertook a metabolomics analysis to identify differential metabolite profiles in umbilical cord blood at birth. We found elevated concentrations of 3-(N-acetyl-L-cystein-S-yl) acetaminophen, 2-hydroxyacetaminophen sulfate, 2-methoxyacetaminophen glucuronide, p-acetamidophenyl glucuronide, 2-methoxyacetaminophen sulfate, and 4-acetamidophenol, metabolites of the analgesic/antipyretic medication acetaminophen, in children who became obese at age 3–5 years. Associations between childhood obesity and acetaminophen metabolites were of a similar magnitude and direction as those found in a similar size study nested within the Project Viva Cohort of Massachusetts, USA. That study investigated the differential metabolic profile associated with rapid post-natal weight gain at age 0–6 months. These associations were comparable even though the average prenatal BMI of mothers whose children became obese in Project Viva was much lower (27.9kg/m2) compared to the prenatal BMI (35.5kg/m2) found in this population. Notably, associations were identified in both studies despite very different ethnic distributions, with 50% Caucasian, 31% African American and 15% Hispanic in Project Viva, compared to 68% African American, and 28% Caucasian in the cohort analyzed here.16
In addition to acetaminophen metabolites, we also found elevated levels of lipid species associated with childhood obesity at ages 3–5 years. Similar lipid species have previously been linked to obesity, diabetes and/or nonalcoholic fatty liver disease (NAFLD) in adults and children.10–12,14,23 For instance, children born to mothers with gestational diabetes have been shown to have elevations in the same free fatty acids found in this study (myristate, palmitate, stearate, oleate, and linoleate). The specificity of these associations to diabetes is unclear since maternal diabetes was not found to contribute to elevated lipids in our population23. It is therefore possible that elevations in these metabolites are an outcome of the obesity phenotype which may be apparent at birth. This makes these lipids a potential target for discovering biomarkers of early detection of obesity and intervention for those at greatest risk.
Age at which obesity is assessed may also play a role in discovery of more disease specific biomarkers. Isganaitis et al. who investigated metabolomic signatures associated with elevated postnatal growth trajectories by six months of age, did not find elevated fatty acid signatures, but instead uncovered enrichment in “Tryptophan Metabolism” and “Excitatory neural signaling through 5HTR4/6/7 and serotonin16.” Cases in the present study were also significantly larger at age one (1.27 WHz Cases, 0.23 WHz controls), but because of sample size restrictions metabolite analysis at age one could not be examined.
In addition to lipids, this analysis also found acetaminophen metabolites more often in the umbilical cord blood of children who became obese vs those who did not by ages 3–5 years. Although acetaminophen is regarded as safe and commonly recommended for use as an analgesic during pregnancy, in utero exposure to acetaminophen has been associated with attention deficit hyperactive disorder, asthma, and endocrine disruption.24–28
To date, no mouse studies have been conducted linking in utero exposure to acetaminophen and obesity but, chronic acetaminophen exposure to mice during pregnancy has been shown to result in decreased fetal liver stem cells29. This is thought to result in altered immune function/allergic response later in life. Moreover, this work suggests that the fetal liver may be a target of insult from acetaminophen during pregnancy. Furthermore, acetaminophen hepatoxicity has been shown in mice to correlate with serum levels of liver free fatty acids including palmitate, stearate, and oleate—these lipids have been proposed as biomarkers of subclinical liver damage30. Together, these metabolic signatures suggest it is plausible that in-utero exposure to acetaminophen could result in decreased liver function at birth, and that dysfunction in the primary organ of lipid metabolism may lead to obesity later in childhood.
Since data regarding the use of pharmaceuticals during pregnancy was not collected, and acetaminophen was not recorded in patient medical records as administered at delivery, questionnaire data of self-reported intake of acetaminophen from a larger cohort of women assembled from the same geographic location was evaluated to clarify these findings. No association between childhood obesity and reported gestational acetaminophen use was found. These data suggest that in utero exposure to acetaminophen is not associated with obesity. These findings may be due to chance alone, inaccurate recall in the use of this common pharmaceutical agent, or failure of the questionnaire to fully capture critical windows of exposure. Elucidating the timing during gestation when acetaminophen is associated with obesity risk and mechanism by which this occurs will require further study.
Nesting this case control study within an ongoing birth cohort adds considerable strength to these findings. This design eliminates the temporal ambiguity that plagues traditional case-control study designs as blood samples in which metabolites were measured were collected many years prior to the clinical manifestation of obesity, enhancing causal inference. In addition, the agnostic metabolomic assay revealed differential concentrations of lipid metabolites at birth similar in species and pathway to those identified by other obesity studies.10–15,23
These findings should be interpreted in the context of the study limitations. It is possible that other associations in the comprehensive metabolomics array may have been masked because of limited statistical power. Additionally, although we controlled for maternal obesity, residual confounding by pre-pregnancy obesity or its correlate in this study, African American ethnicity, cannot be excluded as a potential explanation for our findings; however, others have found associations between acetaminophen presence in cord blood and obesity later in life where maternal obesity was less extreme and ethnicity more homogenous16. Despite limited statistical power in the metabolomics analysis and population heterogeneity, this data adds to the evidence relating in-utero exposure to a very common analgesic, acetaminophen with adverse effects in children.
In summary, we observed associations between childhood obesity at age 3–5 years with detectable concentrations of acetaminophen and elevated lipid metabolites in infant cord blood. Replication of these findings in controlled laboratory experiments and larger epidemiologic studies will increase confidence in the association and facilitate inquiry into these relationships.
Supplementary Material
Acknowledgements
We thank the parents and children of the Newborn Epigenetics Study, research nurse Tammy Bishop, and research assistants Stacy Murray, Carole Grenier, Darby Kroyer, Natasha Duggan, and Suba Narasimhan for tracing enrolled participants; data manager Francine Overcash for statistical assistance, and the obstetrics faculty and staff at Duke University and Durham Regional Hospitals, Durham, ND.
Conceived the question: PS. Designed nested case-control study for metabolomic analysis: CH. Conducted statistical analysis: RM. Interpreted the data: PS, CH, SMB. Oversaw specimen handling: SM. Contributed to writing the manuscript: PS, CH, SMB, RM, SM.
This work was supported by R21ESO14947, P30ES025128, T32ES004046, P01ES022831 and USEPA award RD-83543701.
Footnotes
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Ogden CL et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA 315, 2292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh AS, Mulder C, Twisk JWR, Van Mechelen W & Chinapaw MJM Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes. Rev 9, 474–488 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Schelbert KB Comorbidities of Obesity. Prim. Care Clin. Off. Pract 36, 271–285 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Katz DA, McHorney CA & Atkinson RL Impact of obesity on health-related quality of life in patients with chronic illness. J. Gen. Intern. Med 15, 789–96 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard C Childhood obesity: are genetic differences involved? Am. J. Clin. Nutr 89, 1494S–1501S (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia Q & Grant SFA The genetics of human obesity. Ann. N. Y. Acad. Sci 1281, 178–90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk SJ, Tellam RL, Morrison JL, Muhlhausler BS & Molloy PL Recent developments on the role of epigenetics in obesity and metabolic disease. Clin. Epigenetics 7, 66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanson JM, Entringer S, Buss C & Wadhwa PD Developmental origins of health and disease: environmental exposures. Semin. Reprod. Med 27, 391–402 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauschert S, Uhl O, Koletzko B & Hellmuth C Metabolomic Biomarkers for Obesity in Humans: A Short Review. Ann. Nutr. Metab 64, 314–324 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Puri P et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 50, 1827–38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiehn O et al. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One (2010). doi: 10.1371/journal.pone.0015234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu F et al. Metabolic Signature Shift in Type 2 Diabetes Mellitus Revealed by Mass Spectrometry-based Metabolomics. J. Clin. Endocrinol. Metab 98, E1060–E1065 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Yi L-Z, He J, Liang Y-Z, Yuan D-L & Chau F-T Plasma fatty acid metabolic profiling and biomarkers of type 2 diabetes mellitus based on GC/MS and PLS-LDA. FEBS Letters 580, (2006). [DOI] [PubMed] [Google Scholar]
- 14.Liu L et al. Fasting Serum Lipid and Dehydroepiandrosterone Sulfate as Important Metabolites for Detecting Isolated Postchallenge Diabetes: Serum Metabolomics via Ultra-High-Performance LC-MS. Clin. Chem 59, (2013). [DOI] [PubMed] [Google Scholar]
- 15.Tulipani S et al. Biomarkers of Morbid Obesity and Prediabetes by Metabolomic Profiling of Human Discordant Phenotypes. Clin. Chim. Acta 463, 53–61 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Isganaitis E et al. Associations of cord blood metabolites with early childhood obesity risk. Int. J. Obes 39, 1041–1048 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivorra C et al. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J. Transl. Med 10, 142 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyo C et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 11, 46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gall WE et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5, e10883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuemmeler BF et al. Association between Prepregnancy Body Mass Index and Gestational Weight Gain with Size, Tempo, and Velocity of Infant Growth: Analysis of the Newborn Epigenetic Study Cohort. Child. Obes 12, 210–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US EPA, O. Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments.
- 22.Liu Y et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 7, 735–46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee JK et al. High oleic/stearic fatty-acid desaturation index in cord plasma from infants of mothers with gestational diabetes. J. Perinatol 34, 357–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avella-Garcia CB et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int. J. Epidemiol 25, dyw115 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Sordillo JE et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J. Allergy Clin. Immunol 135, 441–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Fays L et al. Use of paracetamol during pregnancy and child neurological development. Dev. Med. Child Neurol 57, 718–24 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Mazaud-Guittot S et al. Paracetamol, Aspirin, and Indomethacin Induce Endocrine Disturbances in the Human Fetal Testis Capable of Interfering With Testicular Descent. J. Clin. Endocrinol. Metab 98, E1757–E1767 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Andersen ABT, Farkas DK, Mehnert F, Ehrenstein V & Erichsen R Use of prescription paracetamol during pregnancy and risk of asthma in children: a population-based Danish cohort study. Clin. Epidemiol 4, 33–40 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimi K et al. Prenatal acetaminophen induces liver toxicity in dams, reduces fetal liver stem cells, and increases airway inflammation in adult offspring. J. Hepatol 62, 1085–1091 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Suciu M et al. Acetaminophen-induced liver injury: Implications for temporal homeostasis of lipid metabolism and eicosanoid signaling pathway. Chem. Biol. Interact 242, 335–344 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


