Abstract
Background
Advance community distribution of misoprostol for preventing or treating postpartum haemorrhage (PPH) has become an attractive strategy to expand uterotonic coverage to places where conventional uterotonic use is not feasible. However, the value and safety of this strategy remain contentious. This is an update of a Cochrane Review first published in 2012.
Objectives
To assess the effectiveness and safety of the strategy of advance misoprostol distribution to pregnant women for the prevention or treatment of PPH in non‐facility births.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth Trial Register, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (19 December 2019), and reference lists of retrieved studies.
Selection criteria
We included randomised, cluster‐randomised or quasi‐randomised controlled trials of advance misoprostol distribution to pregnant women compared with usual (or standard) care for the prevention or treatment of PPH in non‐facility births. We excluded studies without any form of random design and those that were available in abstract form only.
Data collection and analysis
At least two review authors independently assessed trials for inclusion, extracted data and assessed the risk of bias in included studies. Two review authors independently assessed the certainty of the evidence using the GRADE approach.
Main results
Two studies conducted in rural Uganda met the inclusion criteria for this review. One was a stepped‐wedge cluster‐randomised trial (involving 2466 women) which assessed the effectiveness and safety of misoprostol distribution to pregnant women compared with standard care for PPH prevention during non‐facility births. The other study (involving 748 women) was a pilot individually randomised placebo‐controlled trial which assessed the logistics and feasibility of community antenatal distribution of misoprostol, as well as the effectiveness and safety of self‐administration of misoprostol for PPH prevention. Only 271 (11%) of women in the cluster‐randomised trial and 299 (40%) of the women in the individually randomised trial had non‐facility births. Data from the two studies could not be meta‐analysed as the data available from the stepped‐wedge trial were not adjusted for the study design. Therefore, the analysed effects of advance misoprostol distribution on PPH prevention largely reflect the findings of the placebo‐controlled trial. Neither of the included studies addressed advance misoprostol distribution for the treatment of PPH.
Primary outcomes
Severe PPH was not reported in the studies. In both the intervention and standard care arms of the two studies, no cases of severe maternal morbidity or death were recorded among women who had a non‐facility birth.
Secondary outcomes
Compared with standard care, it is uncertain whether advance misoprostol distribution has any effect on blood transfusion (no events, 1 study, 299 women), the number of women not using misoprostol (2% in the advance distribution group versus 4% in the usual care group; risk ratio (RR) 0.50, 95% confidence interval (CI) 0.13 to 1.95, 1 study, 299 women), the number of women not using misoprostol correctly (RR 4.86, 95% CI 0.24 to 100.46, 1 study, 290 women), inappropriate use of misoprostol (RR 4.97, 95% CI 0.24 to 102.59, 1 study, 299 women) or maternal transfer or referral to a health facility (RR 0.66, 95% CI 0.11 to 3.91, 1 study, 299 women). Compared with standard care, it is uncertain whether advance misoprostol provision increases the number of women experiencing minor adverse effects: shivering/chills (RR 1.84, CI 95% 1.35 to 2.50, 1 study, 299 women), fever (RR 1.87, 95% CI 1.16 to 3.00, 1 study, 299 women), or diarrhoea (RR 3.92, 95% CI 0.44 to 34.64, 1 study, 299 women); major adverse effects: placenta retention (RR 1.49, 95% CI 0.25 to 8.79, 1 study, 299 women) or hospital admission for longer than 24 hours (RR 0.99, 95% CI 0.66 to 15.73, 1 study, 299 women) after non‐facility birth. For all the outcomes included in the 'Summary of findings' table, we assessed the certainty of the evidence as very low, according to GRADE criteria.
Authors' conclusions
Whilst it might be considered reasonable and feasible to provide advance misoprostol to pregnant women where there are no suitable alternative options for the prevention or treatment of PPH, the evidence on the benefits and harms of this approach remains uncertain. Expansion of uterotonic coverage through this strategy should be cautiously implemented either in the context of rigorous research or with targeted monitoring and evaluation of its impact.
Plain language summary
Advance provision of misoprostol to pregnant women for preventing and treating excessive blood loss after birth
We set out to determine the safety and effectiveness of giving pregnant women a medication called misoprostol to keep, so they have it ready to prevent or treat excessive bleeding immediately after birth.
What is the issue?
The medications oxytocin and ergometrine are commonly used to help reduce blood loss in the first 24 hours after giving birth. These require a trained health professional to be present as they are given by injection immediately after the birth. They also need to be kept in the refrigerator to remain effective.
Misoprostol is another medication that helps the womb to contract strongly after birth and reduce excess bleeding. It can be given by mouth and does not need refrigeration. This makes it easier to use than oxytocin and ergometrine, in parts of the world where refrigeration and trained health professionals are not readily available. The main side effects of misoprostol are generally self‐limiting and do not require treatment with further medication.
Why is this important?
Excessive blood loss, or postpartum haemorrhage, remains the leading cause of maternal death worldwide. Most of these deaths occur in remote settings in Africa and Asia, where resources are poor and home births without a skilled birth attendant are common.
Having misoprostol available for use by pregnant women and community and lay health workers could be a way of avoiding excessive blood loss and death after giving birth. Misoprostol may, however, cause harm to women and their babies if used for other purposes such as to start labour before its natural onset.
What evidence did we find?
We searched for evidence on 19 December 2019. We identified two studies from rural Uganda involving 3214 women who were randomised (assigned by chance) to receive and keep misoprostol tablets or receive standard care for preventing excessive bleeding after birth. However, only 570 of the women enrolled in these studies gave birth outside of a health facility, which is what we were investigating.
We were unable to analyse most of the information from one study as it was not separated out by birth setting (health facility versus non‐facility) and not well adjusted for the type of study design. Therefore, the analysed information in our review largely reflects the findings of one study.
No serious maternal ill health or deaths were reported in the two studies. One of the main outcomes of the review, blood loss of at least 1000 mL, was not reported. Other results were from one of the studies (299 women) that used a placebo (dummy pill) in the group who did not receive misoprostol. The certainty of the evidence was very low and the findings were variable. It is unclear whether giving women misoprostol in advance affected the number of women who used misoprostol, used it correctly and appropriately, or were referred to a health facility. The number of women who experienced side effects, and newborns with poor outcomes, was not clearly different between those who received misoprostol in advance and those who received standard care.
What does this mean?
Although this update supports the feasibility of a strategy of giving women misoprostol tablets to use after birth outside of a health facility, the evidence on the benefits of this approach remains uncertain. Efforts to scale up this strategy as part of reducing maternal deaths in remote regions should be done cautiously through targeted monitoring and evaluation, or with large‐scale research to resolve the uncertainties.
Summary of findings
Summary of findings 1. Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for prevention of postpartum haemorrhage.
| Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for prevention of postpartum haemorrhage | ||||||
| Patient or population: women in the third stage of labour Settings: non‐facility birth settings Intervention: advance misoprostol distribution/provision to pregnant women for postpartum self‐administration Comparison: usual (or standard) care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual (or standard) care | Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration | |||||
| Severe postpartum haemorrhage | See comment | See comment | Not estimable | 299 (1 study) | Not reported | Not reported. |
| Serious maternal morbidity | See comment | See comment | Not estimable | 299 (1 study) | ⊕⊝⊝⊝ very low1,2 | No cases of severe maternal morbidity were recorded among women who had non‐facility births. |
| Maternal death | See comment | See comment | Not estimable | 299 (1 study) | ⊕⊝⊝⊝ very low1,2 | No cases of maternal death were recorded among women who had non‐facility births. |
| Blood transfusion | See comment | See comment | Not estimable | 299 (1 study) | ⊕⊝⊝⊝ very low1,2 | No cases of blood transfusion were recorded among women who had non‐facility births. |
| Women not using/receiving misoprostol at birth | 40 per 1000 | 20 per 1000 (5 to 79) | RR 0.50 (0.13 to 1.95) | 299 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Inappropriate use (or misuse) of misoprostol | 0 per 1000 | 0 per 1000 (0 to 0) | RR 4.97 (0.24 to 102.59) | 299 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Maternal transfer or referral to a health facility | 20 per 1000 | 13 per 1000 (2 to 79) | RR 0.66 (0.11 to 3.91) | 299 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 We downgraded (by one level) for serious indirectness because the usual (or standard) care was advance distribution and postpartum self‐administration of inactive study drug. 2 We downgraded (by two levels) for very serious imprecision because there were no events and the sample size was small. 3 We downgraded (by two levels) for very serious imprecision because of few events, small sample size and wide confidence intervals.
Background
Description of the condition
Postpartum haemorrhage (PPH) remains the leading cause of maternal mortality worldwide (Khan 2006; Say 2014). It accounts for nearly a quarter of all maternal deaths globally, approximately 125,000 deaths every year (Selo‐Ojeme 2002). Most of these deaths occur in resource‐poor settings in Africa and Asia, where effective methods of PPH prevention and treatment are largely not feasible or practicable because many births still occur at home (Geller 2006; Gupta 2010). Recording of material death in these remote regions is poor, so it is unlikely that the estimated mortality burden of PPH is largely underestimated. The problem of PPH in low‐resource settings is further worsened by the high incidence of anaemia in pregnant women and lack of safe and effective blood transfusion services (Hamilton 2009; Rohilla 2010; Wandabwa 2008). As a result, the condition of a woman giving birth in such settings could deteriorate rapidly even with modest postpartum blood loss. PPH is also associated with severe maternal morbidity including renal failure, the need for massive blood transfusion, coagulation deficiencies and emergency hysterectomy (Amaral 2011; Hazra 2004). Efforts to achieve target 3.1 of the third Sustainable Development Goal (to reduce the global maternal mortality ratio to less than 70 per 100,000 live births by 2030) (UN 2015) must therefore include aggressive interventions to reduce PPH‐related death, especially in countries with high mortality rates.
Definition and incidence
According to the World Health Organization (WHO), PPH is defined as bleeding from the genital tract of 500 mL or more within the first 24 hours of birth of the baby (WHO 2017). The application of this definition is complicated, as it is extremely difficult to accurately estimate blood loss in clinical practice (Rath 2011; Yoong 2010). Meticulous estimation of blood loss is not convenient or practical outside controlled trial settings since blood is often mixed with amniotic fluid and dispersed in perineal pads, towels and linens. In addition, the clinical value of a timely diagnosis of PPH through accurate quantification of blood loss is yet to be determined (WHO 2012). This cut‐off has also been criticised on the basis of the clinical relevance of postpartum blood loss of 500 mL in women with normal haemoglobin (Hb) levels before birth (Bloomfield 1990). Consequently, alternative cut‐off levels have been suggested; these include 600 mL (Beischer 1986), 1000 mL (Burchell 1980) and 1500 mL (Mousa 2002). However, there is a general agreement that postpartum blood loss in excess of 1000 mL should be regarded as severe PPH. Some researchers have proposed the estimation of Hb concentration 24 to 48 hours after birth as a more objective measure, but this also depends on the Hb level during pregnancy and labour. For most births, visual assessment of blood loss is the usual practice, which results in many cases of PPH remaining undetected and makes accurate determination of incidence difficult. Apart from this ‘quantitative’ definition of PPH, it can also be qualitatively defined as any degree of postpartum blood loss that is enough to compromise a woman’s haemodynamic condition. This definition is more relevant in low‐resource settings where the pre‐delivery Hb profile of many women is poor.
A systematic review of the prevalence of PPH, using 120 data sets and involving close to four million women, showed an overall prevalence of 6% of all births (Carroli 2008). However, studies that measured blood loss objectively, as opposed to subjectively, showed higher prevalence. The prevalence also depends on whether the data were derived from observational studies or clinical trials. Higher prevalence was observed in randomised clinical trial settings where meticulous estimation of blood loss is expected. Considerable variations were also recorded for different regions of the world — ranging from 2.55% in Asia to 10.45% in Africa — although the rates were comparable for Europe (6.38%), Latin America and the Caribbean (8.90%), Northern America (6.37%) and Oceania (7.68%). However, these regional differences are not a direct reflection of the magnitude and risk of PPH‐related maternal death, which are largely determined by the availability of skilled birth attendants and facilities needed to save women’s lives. The risk of maternal death from PPH in low‐income countries is estimated to be one in 1000 births, compared with less than one in 100,000 births in high‐income countries such as the UK (CMACE 2011).
Causes and risk factors
Failure of the uterus to contract properly after childbirth (uterine atony) is the leading cause of PPH. Other common causes are genital tract laceration and retention of placental tissues. Rare causes include coagulation disturbances and uterine inversion. Recognised predisposing factors to uterine atony include prolonged or augmented labour, a large baby or multiple fetuses, high parity and use of halogenated anaesthetic agents prior to delivery (Tsu 2004). An important predisposing factor across all causes of PPH is anaemia in pregnancy and labour. Anaemia increases the risk of dying from PPH because blood loss that could readily be tolerated by women with normal Hb levels could be fatal for an anaemic woman. In spite of the long list of risk factors in the literature, most PPH cases occur in women without any identifiable risk factors and thus preventive measures are recommended for all women giving birth (WHO 2007).
Description of the intervention
Misoprostol for the third stage of labour
The use of an effective uterotonic for the prevention of PPH during the third stage of labour is recommended for all births (WHO 2018). Oxytocin is the recommended uterotonic agent for PPH prevention in settings where multiple uterotonic options are available (WHO 2018). However, oxytocin is administered parenterally and therefore requires the presence of birth attendants skilled in safe injection practices and the availability of sterile needles and syringes. In addition, it is less stable in tropical climates and requires refrigeration to maintain its potency. These requirements have confined its use to settings where skilled professionals attend birth.
Misoprostol, a synthetic prostaglandin E1 analogue, is another potent uterotonic with a remarkable advantage over conventional uterotonics as it is stable at room temperature, can be administered through multiple routes and has a long shelf‐life. In addition, it can be used in women with hypertensive disorders in pregnancy, in contrast to its ergot‐based counterpart. Its minor side effects, particularly fever and shivering, are dose related, self‐limiting and often do not require any medication. Its relative efficacy and safety compared with placebo and other conventional uterotonics in both hospital and community settings has been well documented (Alfirevic 2007; Derman 2006b; Hoj 2005; Mobeen 2011; Walraven 2005). Although it is not as effective as oxytocin or ergometrine in preventing PPH within the context of active management of the third stage of labour (AMTSL) (Gülmezoglu 2001; Gülmezoglu 2007), these attributes make it a useful alternative in situations where there is no access to conventional uterotonics or AMTSL. In settings where skilled health personnel are not present to administer injectable uterotonics, the WHO recommends the administration of oral misoprostol by community health workers and lay health workers (WHO 2017).
Advance distribution or provision of misoprostol for the third stage of labour
Although there is a general consensus that misoprostol is a feasible option to prevent or treat PPH in the absence of a skilled health personnel, its life‐saving potential can only be maximised if it is accessible to women who need it at the point of birth. Efforts by health ministries and non‐governmental organisations to increase coverage in countries such as Afghanistan, India, Indonesia, Nepal and Nigeria have therefore focused on improving the distribution systems to peripheral health centres and providing support and training for paramedical and lay health workers (Chandhiok 2006; Ejembi 2010; Rajbhandari 2010; Sanghvi 2010). For women who are likely to deliver outside a health facility for any reason, providing misoprostol in advance of labour and birth seems to be a reasonable choice. One of the tested methods of advance provision include community‐based distribution of misoprostol to traditional birth attendants (TBAs) and community health workers (Rajbhandari 2010). These individuals are, to various extents, educated on the route, dosage and timing of administration of misoprostol; they are also instructed on how to administer its prophylactic dose to all women giving birth under their care. Where appropriate low technology exists to diagnose PPH (Prata 2005a), they are also trained to use misoprostol for PPH treatment (Prata 2005b). In order to guarantee access in situations where women deliver at home alone or in the presence of a family member, advance distribution of misoprostol to pregnant women themselves during the last trimester for self‐administration postpartum is an interesting approach that has been promoted (Potts 2010a; Sanghvi 2010).
How the intervention might work
Every year an estimated 60 million women give birth outside health facilities, mainly at home, and up to 52 million births occur without a skilled birth attendant (UNICEF 2009). Although the achievement of skilled birth attendance for all is embedded in the promotion and strengthening of institutional births everywhere in the world, the reality is that financial, social and geographic access to facility care is presently beyond the reach of many women in resource‐poor settings and will inevitably remain so at least in the medium term. Therefore, for any preventive and treatment strategy for PPH to have an impact on a global scale in the short to medium term, it should be feasible at the community level and not require a skilled professional at birth. For these reasons, efforts to increase coverage of uterotonics for the management of third stage of labour has expanded to finding a uterotonic option for use in situations in which skilled personnel are unavailable, such as home birth settings. Where injectable uterotonics cannot be applied, the International Federation of Gynaecology and Obstetrics (FIGO), International Confederation of Midwives (ICM) and WHO endorse the use of misoprostol by health workers trained in its use for prevention of PPH (ICM‐FIGO 2006; ICM‐FIGO 2007; WHO 2017). The attributes of misoprostol make it applicable by lower‐level providers and motivated pregnant women themselves for management of the third stage of labour in home births. There is evidence to suggest that community‐based prevention and treatment of maternal health issues could complement a health facility strategy (Costello 2004; Prata 2009). The use of misoprostol by lay health workers for preventing and treating PPH within the community has the potential to prevent severe disease and save healthcare costs. Using a mathematical model, Pagel and colleagues demonstrated that complementing health facility strengthening with advance community‐based distribution of misoprostol to pregnant women through outreach antenatal care and village female volunteers has the potential to reduce maternal death by 24% and 36%, respectively (Pagel 2009). However, these estimates have been criticised on the grounds of the models' reliance on the presumed effectiveness of misoprostol in reducing PPH‐related mortality in home births and its disregard for the implementation processes and costs needed to achieve and sustain uterotonic coverage (Braunholtz 2010; Ronsmans 2010).
Why it is important to do this review
Expanding access to an easy‐to‐use uterotonic in places where the third stage of labour is currently being managed without a uterotonic has generated a lot of interest among international health developmental agencies. Advance distribution of misoprostol through the community or health facility to providers such as auxiliary nurses or unskilled birth attendants (such as community health workers and TBAs), or to women themselves for self‐administration at the time of home birth, has become an attractive strategy to increase uterotonic coverage to places where injectable uterotonics is not feasible (Ejembi 2010; Geller 2014; Rajbhandari 2010; Sanghvi 2010; Smith 2014a; Smith 2014b). However, providing access to misoprostol using this strategy remains a subject of controversy (Potts 2010a; Potts 2010b; WHO 2009; WHO 2010; WHO 2012). Advance provision of misoprostol to pregnant women for self‐administration has the potential to save lives where no uterotonic coverage exists but also presents the risk of inappropriate use before birth. Misoprostol is a potent abortifacient and labour‐induction agent and, depending on the timing of administration, could result in a miscarriage or unintended early delivery following antenatal misuse. There are also concerns about uterine rupture if given at PPH‐prevention dose in the third trimester. Besides its potential for being misused, it is unclear if relatives of women attending birth could adequately manage the side effects, particularly fever and shivering, that often accompany misoprostol treatment when given at home. There are also questions on whether community‐based advance distribution of misoprostol truly reduces PPH‐related maternal mortality, to justify its scaling up in relevant settings. In order to assess the comparative risks and benefits of a strategy of advance misoprostol provision to pregnant women when used for prevention or treatment of PPH, a systematic review was considered necessary. This is an update of a Cochrane Review first published in 2012.
Objectives
To assess the effectiveness and safety of the strategy of advance misoprostol distribution to pregnant women for the prevention or treatment of postpartum haemorrhage in non‐facility births.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised and quasi‐randomised controlled trials evaluating the effectiveness and safety of a strategy of advance misoprostol provision to pregnant women for the prevention or treatment of postpartum haemorrhage (PPH) in non‐facility births. We considered studies for inclusion whether randomisation was conducted at the level of the individual or cluster. We excluded studies without any form of random design and those that were available only in abstract form.
Types of participants
We included women giving birth outside a health facility regardless of the reasons behind such choice. This includes women giving birth in their own home or other birth homes manned by unskilled birth attendants, but excludes home births attended by skilled attendants.
Types of interventions
Experimental interventions assessed included any strategy of advance provision of misoprostol for the prevention or treatment of PPH in non‐facility births. This includes community‐based advance distribution/provision of misoprostol:
directly to pregnant women themselves (through lay health workers — e.g. community health workers, female volunteers or traditional birth attendants — or a facility‐based/outreach antenatal care programme) for postpartum self‐administration in home births;
directly to pregnant women themselves (through lay health workers or a facility‐based/outreach antenatal care programme) for postpartum administration by an unskilled birth attendant (e.g. a family member or lay health worker).
We excluded studies without any form of advance distribution/provision of misoprostol for the sole purpose of routine or emergency care for PPH in non‐facility births. We considered studies regardless of the timing of provision, content and extent of training for end‐users, and package, dose or route of administration of misoprostol. Comparator interventions were usual (or standard) care within any given setting, regardless of the way this was defined. This includes situations where usual (or standard care) is non‐use of uterotonic after non‐facility birth or advance provision of another uterotonic (e.g. Uniject) or placebo was used to denote usual care.
For the prevention of PPH, we considered the following comparisons:
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care;
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum administration by an unskilled birth attendant versus usual (or standard) care;
comparison of two different strategies of advance misoprostol distribution/provision to pregnant women for the prevention of PPH.
For the treatment of PPH, we considered the following comparisons:
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care;
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum administration by an unskilled birth attendant versus usual (or standard) care;
comparison of two different strategies of advance misoprostol distribution/provision to pregnant women for the treatment of PPH.
We excluded studies without any of the above comparisons.
Types of outcome measures
We included studies in the review whether or not they reported the following outcome measures of interest.
Primary outcomes
Severe PPH (at least 1000 mL, or as defined by authors)
Serious maternal morbidity (organ failure, hysterectomy, intensive care unit admission, or as defined by authors)
Maternal death
Maternal outcomes
PPH (at least 500 mL, or as defined by authors) (only where intervention is used for PPH prevention)
Maternal anaemia within 24 to 48 hours postdelivery (Hb concentration less than 9 g/dL, or as defined by authors)
Blood transfusion
Use of additional intervention(s) to stop bleeding
Women not using/receiving misoprostol at birth
Women not using/receiving misoprostol correctly at birth
Inappropriate use (or misuse) of misoprostol (as defined by authors)
Maternal transfer or referral to a health facility
Any adverse effect reported
Minor adverse effects (e.g. low‐grade fever, nausea, vomiting, diarrhoea, or as defined by authors)
Major adverse effects (e.g. hyperpyrexia, uterine rupture, any adverse effect requiring treatment/hospital referral, or as defined by authors)
Infant outcomes
Stillbirth
Early neonatal death (within the first seven days)
Neonatal death (within 28 days)
Neonatal transfer or referral to a health facility for special care
Acceptability of intervention
Woman's dissatisfaction with the intervention
Provider's dissatisfaction with the intervention
Where studies reported outcomes of women giving birth in both health facility and non‐health facility, we only extracted data for non‐facility births. Where outcome data were not disaggregated by place of birth, we contacted the trial authors to provide disaggregated data on non‐facility births. Data were considered unusable where disaggregated data cannot be provided.
Search methods for identification of studies
The search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (19 December 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register — including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service — please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (on 19 December 2019), using the search methods described in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Oladapo 2012. For this update, the following methods (based on a standard template used by Cochrane Pregnancy and Childbirth) were used for assessing the reports that were identified as a result of the updated search.
Selection of studies
Four review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the other review authors. Data from the two included studies were not combined as they were not in a format that was appropriate for meta‐analysis.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; the study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. For the stepped‐wedge cluster‐randomised trial, we assessed whether there was any baseline imbalance, loss of clusters, comparability with individually randomised trials, and trial adjustment for clustering and time.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessment of the certainty of the evidence using the GRADE approach
For this update, we used the GRADE approach (as outlined in the GRADE handbook) to assess the certainty of the evidence for the following outcomes:
severe PPH (at least 1000 mL, or as defined by authors);
serious maternal morbidity (organ failure, hysterectomy, intensive care unit admission, or as defined by authors);
maternal death;
blood transfusion;
women not using/receiving misoprostol at birth;
inappropriate use (or misuse) of misoprostol;
maternal transfer or referral to a health facility.
We planned to separately assess the certainty of evidence for the above outcomes for the main comparisons, with respect to PPH prevention and treatment:
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care;
any strategy of advance misoprostol distribution/provision to pregnant women for postpartum administration by an unskilled birth attendant versus usual (or standard) care;
comparison of two different strategies of advance misoprostol distribution/provision to pregnant women.
For this update, however, the certainty of evidence was assessed only for the comparison of any strategy of advance misoprostol distribution/provision to pregnant women for postpartum self‐administration with usual (or standard) care, with respect to PPH prevention.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create the 'Summary of findings' table. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
There were no continuous data analysed in this review. If we obtain such data in future updates, we will analyse them using the mean difference if outcomes are measured in the same way between trials. We plan to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We included a stepped‐wedge cluster‐randomised trial. However, we did not synthesise along with the included individually randomised trial because the stepped‐wedge trial did not adjust for time as a potential confounder. In future updates, we plan to synthesise the relevant information in cluster‐randomised and individually randomised trials. We will consider it reasonable to combine the results from both trial designs if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not include cross‐over trials in this review.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, we will use sensitivity analyses to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing. Should meta‐analysis be feasible in future updates, we will carry out analyses for all outcomes on an intention‐to‐treat basis (i.e. we will attempt to include all participants randomised to each group in the analyses), where possible.
Assessment of heterogeneity
We did not assess statistical heterogeneity as we did not perform a meta‐analysis of data from included studies. In future updates, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if I² is greater than 30% and either Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 30%), we will explore it using pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
Data from only one of the included studies could be individually presented as risk ratios, with 95% confidence intervals. We were unable to analyse data from the other included study because it was not separated out by birth setting (facility versus non‐facility), not appropriately adjusted for the stepped‐wedge design, and because most of the data obtained by correspondence with study authors lacked denominators by study arms. In future updates, we will carry out statistical analysis using Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data, where it is reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials' populations and methods were judged to be sufficiently similar.
If in future updates there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful; if it is, we will use random‐effects analysis to produce it.
We will carry out the following subgroup analyses:
birth setting (home versus other non‐facility birth setting);
birth attendant (woman or family member versus lay health worker).
The following primary outcomes will be used in subgroup analyses:
severe PPH (at least 1000 mL, or as defined by authors);
serious maternal morbidity (organ failure, hysterectomy, intensive care unit admission, or as defined by authors);
maternal death.
We will assess subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not perform any sensitivity analysis as data from the two included studies were not meta‐analysed. In future updates, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies (i.e. those at high risk of bias) being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1
1.
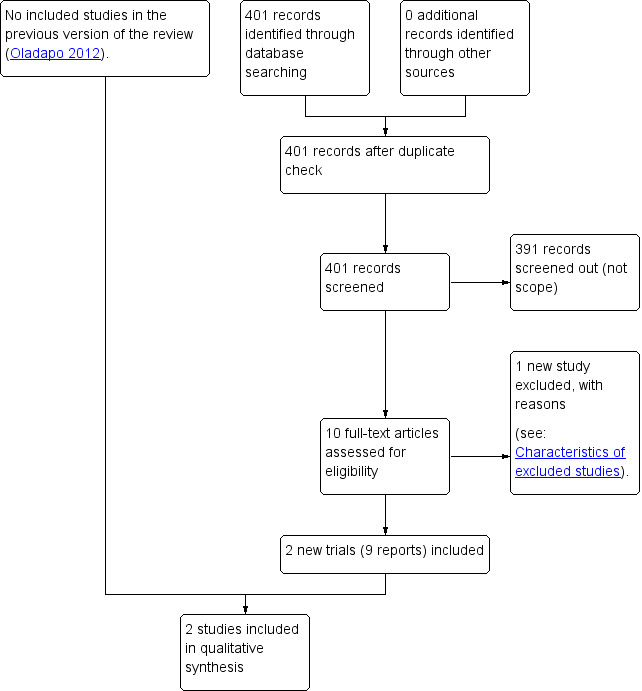
Study flow diagram
The updated search retrieved 10 trial reports to assess. Two studies (nine reports) met the inclusion criteria for this review. One new study was excluded (Abbas 2019). See: Characteristics of excluded studies.
Included studies
Two studies (Ononge 2015; Weeks 2015) conducted in rural Uganda met the inclusion criteria for this review. Ononge 2015 was a stepped‐wedge cluster‐randomised trial that assessed the effectiveness and safety of misoprostol distribution to pregnant women versus standard care for the prevention of PPH during non‐facility births. Weeks 2015 was a pilot individually randomised trial, designed to assess the logistics and feasibility of community antenatal distribution of misoprostol but which also assessed the effectiveness and safety of self‐administration of misoprostol using a placebo‐controlled design. Ononge 2015 was conducted over a 14‐month period (February 2013 to March 2014) and was funded by Wellcome Trust. Weeks 2015 enrolled participants over a period of two months (23 May 2012 to 17 July 2012) and was funded by the Bill and Melinda Gates Foundation. The research teams for both studies reported no conflicts of interests. See: Characteristics of included studies.
Participants
In Ononge 2015, 2466 pregnant women were recruited from antenatal clinics of participating health facilities. These women had reached 28 weeks or more of gestation, and had no plans to leave the district during the pregnancy, birth or in the immediate postpartum period. The trial excluded women who had a planned elective caesarean section, a previous caesarean section scar, and those who could not be accessed after a home birth due to poor terrain. The data for 271 women (11% of participants) who had non‐facility births were not separately presented in the report but were provided by the study authors, where available.
In Weeks 2015, 748 pregnant women who were at 34 weeks of gestation or more were recruited from one regional referral hospital and three large health centres. All women living in the 200 villages that were served by the participating health facilities and had active village health teams were eligible to participate. Women with a known allergy to misoprostol or other prostaglandins, and unanticipated minors less than 18 years of age, were excluded. However, women at risk of experiencing a childbirth complication were not excluded. The data for 299 women (40% of participants) who had non‐facility births were separately presented in the report, and additional data were also provided by the study authors.
Interventions
In Ononge 2015 a stepped‐wedge design was used: women in the intervention period were given 600 micrograms (mcg) of oral misoprostol at enrolment to the study to self‐administer after childbirth if delivery happened outside a health facility, or when there was no oxytocin at the health facility. Three tablets of misoprostol (200 mcg each, for a total of 600 mcg) in aluminium foil were packaged in a plastic envelope. Specific instructions were given to women to ensure safe self administration of oral misoprostol. Standard of care (control) at the time of the trial was that women who delivered at home received no uterotonic.
In Weeks 2015, women were given a small purse with a string that could be hung around the neck. It contained a packet with three foil‐packed tablets (either 600 mcg of misoprostol or identical placebo) along with both pictorial and written instructions on how to take the tablets.
Outcomes
Ononge 2015 reported the following outcomes: PPH (defined as a drop in maternal haemoglobin (Hb) by 2 g/dl or more from the prenatal haemoglobin (primary outcome)), postpartum anaemia (defined as Hb less than 11 g/dl when assessed within seven days and Hb less than 12 g/dl if assessed after the seventh day after childbirth), place of birth, use of any uterotonics for PPH prevention, referral to a health facility after childbirth, blood transfusion, maternal death, self‐reported side effects related to misoprostol use, appropriate use of misoprostol and its acceptability to women.
Weeks 2015 reported the following outcomes: Hb fall of over 20% lower than the prenatal Hb (primary outcome), mean change in Hb, rate of postnatal anaemia, rate of poor maternal and fetal health, appropriate use of study medication, self‐reported side effects, self‐assessed blood loss, quality‐of‐life measures, and safety outcomes (including transfer to hospital, surgical intervention, blood transfusion, maternal death).
Regarding primary outcomes specified for this review, neither of the included studies reported severe PPH. For the review's secondary outcomes, data from Ononge 2015 were unusable for data analysis (after contacting study authors) for all outcomes except for minor adverse effects (fever and diarrhoea). Weeks 2015 did not provide data (after contacting study authors) on the following outcomes: PPH at least 500 mL, or as defined by authors; maternal anaemia within 24 to 48 hours post‐delivery; neonatal transfer or referral to a health facility; women's dissatisfaction with the intervention, and provider's dissatisfaction with intervention. For more information about included studies, see Characteristics of included studies.
Excluded studies
We excluded four studies (Abbas 2019; Derman 2006a; Rajbhandari 2010; Sanghvi 2010). In Abbas 2019 and Derman 2006a, the intervention did not include direct or indirect advance provision of misoprostol to pregnant women themselves; misoprostol was provided to traditional birth attendants and auxillary midwives, respectively, for the management of PPH among women who gave birth under their care. Although Rajbhandari 2010 and Sanghvi 2010 distributed misoprostol in advance to pregnant women for PPH prevention, both studies were excluded because they did not meet our review criteria for study design (see Characteristics of excluded studies).
Risk of bias in included studies
The overall risk of bias was moderate for Weeks 2015 and high for Ononge 2015 (see Figure 2 and Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For methods of randomisation, we assessed both studies to be at low risk of bias because random allocation was based on computer‐generated numbered sequence. For allocation concealment, we assessed Weeks 2015 as being at low risk of bias because identical misoprostol and placebo tablets were packed in sequentially numbered, labelled manila coin envelopes which had unique packet IDs and were sealed in purses. The trial packs (in the purses) were distributed in sequential order to the next randomised woman. Study participants were instructed not to open the manila envelope until time of birth. We assessed the methods of allocation concealment in Ononge 2015 to be at high risk of bias because the stepped‐wedge trial design meant both participants and the research team knew in advance the assigned allocation.
Blinding
The risks of performance and detection bias were low for Weeks 2015 as women, providers, and outcome assessors were blinded to study group assignment. For the second study the risks of performance and detection bias were high as "it was not possible to blind the intervention to the caregivers, research team or study participants" (Ononge 2015).
Incomplete outcome data
We considered both included studies to be at high risk of attrition bias. In Ononge 2015, 12% of women in the intervention group, and 20% of women in the control group, were lost to follow‐up and primary outcome data were not available. For Weeks 2015, the proportion of women who did not have primary outcome data measured was 35% in the intervention and 46% in the control arms.
Selective reporting
We considered the risk of reporting bias to be low for both included studies because the pre‐specified outcomes in both were reported. Additionally, the study authors were able to provide data for a number of outcomes which were not reported in the study reports, suggesting adherence to specified outcomes in their original study protocols.
Other potential sources of bias
Both studies were considered to be at high risk of other biases. Weeks 2015 was implemented as a pilot study without sample size calculation and used "per protocol analysis" for Hb outcomes and "modified intention‐to‐treat" analysis for other outcomes. Ononge 2015 did not adjust for time despite using a stepped‐wedge cluster‐randomised trial design.
Effects of interventions
See: Table 1
Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for the prevention of postpartum haemorrhage
Primary outcomes
Severe postpartum haemorrhage (at least 1000 mL, or as defined by authors)
Neither of the two included studies (Ononge 2015; Weeks 2015) reported data on severe PPH.
Serious maternal morbidity (organ failure, hysterectomy, intensive care unit admission, or as defined by authors)
Both studies (Ononge 2015; Weeks 2015) reported no serious maternal morbidity among women who had non‐facility births in the intervention and standard‐care arms of the studies (Analysis 1.1) (very low‐certainty evidence).
1.1. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 1: Severe maternal morbidity
Maternal death
Weeks 2015 reported one maternal death from PPH after facility birth (in a woman who received but did not take misoprostol), and no maternal death in either arm of the trial for non‐facility births (Analysis 1.2) (very low‐certainty evidence). Ononge 2015 registered four maternal deaths in the control clusters during the study period; three occurred at the health facility and the fourth occurred at home following alleged domestic violence while the woman was still pregnant.
1.2. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 2: Maternal death
Secondary outcomes
Maternal outcomes
Postpartum haemorrhage (at least 500 mL, or as defined by authors)
This outcome was not reported by Weeks 2015. The PPH data as reported by Ononge 2015 were not usable as the study presented overall rates without disaggregating by birth setting.
Maternal anaemia within 24 to 48 hours post‐delivery (Hb concentration less than 9 g/dL, or as defined by authors)
This outcome was not reported as pre‐specified for Weeks 2015, while the data reported by Ononge 2015 were not usable. However, Weeks 2015 reported maternal anaemia defined as Hb less than 9 g/dL within five days after childbirth. Based on this definition, it Is uncertain whether advance misoprostol distribution has any effect on the number of women with maternal anaemia between intervention (10.3%) and control (7.5%), because the certainty of the evidence is very low (risk ratio (RR) 1.37, 95% confidence interval (CI) 0.52 to 3.62; 1 study, 177 women; Analysis 1.3).
1.3. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 3: Maternal anaemia
Blood transfusion
There were no cases of blood transfusion among women who had a non‐facility birth in either study (Ononge 2015; Weeks 2015) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 4: Blood transfusion
Use of additional intervention(s) to stop bleeding
From the data reported by Weeks 2015 on use of additional uterotonics after non‐facility births, it is uncertain whether advance misoprostol distribution has any effect on the use of additional uterotonics, because the certainty of evidence is very low (RR 0.74, 95% CI 0.17 to 3.27; 1 study, 299 women; Analysis 1.5). Ononge 2015 did not report this outcome.
1.5. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 5: Use of additional uterotonics
Women not using/receiving misoprostol at birth
From the data reported by Weeks 2015 (where women received placebo tablets in advance in the usual‐care arm), it is uncertain whether advance misoprostol distribution has any effect on the number of women not using misoprostol after non‐facility birth (intervention: 2.0%; control: 4.0%) (RR 0.50, 95% CI 0.13 to 1.95; 1 study, 299 women; very low‐certainty evidence; Analysis 1.6). Ononge 2015 reported that 34.8% of women who received misoprostol and had a non‐facility birth did not use the medication after childbirth. No data were reported for women in the control group.
1.6. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 6: Women not using/receiving misoprostol at birth
Women not using/receiving misoprostol correctly at birth
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution has any effect on the number of women not using misoprostol correctly during non‐facility birth (RR 4.86, 95% CI 0.24 to 100.46; 1 study, 290 women; very low‐certainty evidence; Analysis 1.7). Ononge 2015 did not report this outcome.
1.7. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 7: Women not using/receiving misoprostol correctly at birth
Inappropriate use of misoprostol
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution decreases inappropriate use of the medication after non‐facility birth (RR 4.97, 95% CI 0.24 to 102.59; 1 study, 299 women; very low‐certainty evidence; Analysis 1.8). Ononge 2015 did not report this outcome.
1.8. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 8: Inappropriate use (or misuse) of misoprostol (as defined by authors)
Maternal transfer or referral to health facility
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution reduces maternal transfer or referral to a health facility after non‐facility birth (RR 0.66, 95% CI 0.11 to 3.91; 1 study, 299 women; very low‐certainty evidence; Analysis 1.9). Ononge 2015 reported no cases of maternal transfer or referral to a health facility in either the intervention or control group.
1.9. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 9: Maternal transfer or referral to a health facility
Minor adverse effect: shivering/chills
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases the reported cases of shivering/chills after non‐facility birth (RR 1.84, 95% CI 1.35 to 2.50; 1 study, 299 women; very low‐certainty evidence; Analysis 1.10). Ononge 2015 did not report this outcome.
1.10. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 10: Minor adverse effect ‐ shivering/chills
Minor adverse effect: fever
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases the occurrence of fever after non‐facility birth (RR 1.87, 95% CI 1.36 to 3.00, 1 study, 299 women; very low‐certainty evidence; Analysis 1.11). The data as reported by Ononge 2015 were not usable as the study presented overall rates without disaggregating by birth setting.
1.11. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 11: Minor adverse effect ‐ fever
Minor adverse effect: diarrhoea
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases the occurrence of diarrhoea after non‐facility birth (RR 3.92, 95% CI 0.44 to 34.64; 1 study, 299 women; very low‐certainty evidence; Analysis 1.12). The data as reported by Ononge 2015 were not usable as the study presented overall rates without disaggregating by birth setting.
1.12. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 12: Minor adverse effect ‐ diarrhoea
Major adverse effect:placental retention
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases the risk of placental retention after non‐facility birth (RR 1.49, 95% CI 0.25 to 8.79; 1 study, 299 women; very low‐certainty evidence; Analysis 1.13). Ononge 2015 did not report this outcome.
1.13. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 13: Major adverse effect ‐ placental retention
Major adverse effect:hospital admission more than 24 hours
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases hospitalisation for longer than 24 hours after non‐facility birth (RR 0.99, 95% CI 0.06 to 15.73, 1 study, 299 women; very low‐certainty evidence; Analysis 1.14). Ononge 2015 did not report this outcome.
1.14. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 14: Major adverse effect ‐ hospital admission > 24 hours
Infant outcomes
Stillbirth
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advance misoprostol distribution increases the risk of stillbirth after non‐facility birth (RR 0.33, 95% CI 0.01 to 8.06, 1 study, 299 women; very low‐certainty evidence; Analysis 1.15). The other study (Ononge 2015) reported data in an unusable format.
1.15. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 15: Stillbirth
Early neonatal death
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advanced misoprostol distribution increases the risk of early neonatal death after non‐facility birth (very low‐certainty evidence; Analysis 1.16). Ononge 2015 reported data in an unusable format.
1.16. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 16: Early neonatal death
Neonatal death (within 28 days)
From the data reported by Weeks 2015 (where women received in advance either misoprostol or placebo), it is uncertain whether advanced misoprostol distribution increases the risk of neonatal death after non‐facility birth (RR 0.20, 95% CI 0.01 to 4.10, 1 study, 299 women; very low‐certainty evidence; Analysis 1.17). Ononge 2015 did not assess this outcome.
1.17. Analysis.

Comparison 1: Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention, Outcome 17: Neonatal death (within 28 days)
Neonatal transfer or referral to a health facility for special care
Weeks 2015 did not report this outcome. Ononge 2015 reported data in an unusable format.
Acceptability of the intervention
Woman's dissatisfaction with the intervention
This outcome was not reported by the included studies (Ononge 2015; Weeks 2015).
Provider's dissatisfaction with the intervention
This outcome was not reported by the included studies (Ononge 2015; Weeks 2015).
Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for the treatment of postpartum haemorrhage
No study addressing the treatment of PPH met our inclusion criteria.
Discussion
Advance distribution of misoprostol to pregnant women as an intervention for prevention or treatment of postpartum haemorrhage (PPH) is complex. This is essentially because its effectiveness and safety reflect a combination of what occurs at two principal levels: distribution of misoprostol to lay individuals before it is needed; and its correct use at the time of birth. Although there is existing evidence to support the efficacy of misoprostol in reducing PPH risk when administered by providers with minimal or no formal education (Derman 2006b; Mobeen 2011; Walraven 2005), a wide‐scale distribution of the drug within the community (particularly to pregnant women themselves) well ahead of the time of use introduces further complexities. Community‐based educational programmes that often accompany the intervention may expose potential end users to other indications for misoprostol (e.g. the induction of labour), and having the drug when not yet needed could carry potential risk for unintended use. Using misoprostol intended for PPH prevention for other indications within this context could put the women at risk as a result of incompatible dose and route of administration for such indications.
Summary of main results
Evidence from two trials suggests that advance misoprostol distribution to pregnant women for self‐administration during non‐facility birth does not increase the risk of severe maternal morbidity or death compared to usual (or standard) care. There were no reliable data on quantifiable blood loss. Compared to usual care, the available evidence suggests there may be no increase in the risks of blood transfusion or maternal transfer or referral to a health facility. While a strategy of advance misoprostol distribution appears to reduce the proportion of women not using misoprostol (or any uterotonic) during non‐facility childbirth, it remains uncertain whether misoprostol is correctly or appropriately used during non‐facility birth. Although advance misoprostol provision to pregnant women (and its use) may increase the common side effects associated with this medication, such as shivering/chills and fever, the evidence for this was uncertain. Given the very low quality of the evidence, it is uncertain whether advance misoprostol distribution to pregnant women for postpartum self‐administration has any impact on adverse fetal and newborn outcomes. None of the included studies reported data on pre‐specified outcomes to assess acceptability.
Overall completeness and applicability of evidence
Despite the wide interest in this subject area, our review only identified two trials conducted in the same setting over a similar period of time. The findings of both trials were, however, insufficient to address the review objectives as important outcomes pre‐specified for this review were not reported or could not be used. While both trials investigated the feasibility and impact of advance misoprostol provision to participants from settings with high rates of non‐facility births, consistent and unbiased assessment of outcomes appear problematic. In these trials, misoprostol was given to pregnant women through health facilities serving catchment areas, meaning that the strategies only targeted women who have antenatal contacts with the health system but who may not deliver in a facility. Only one of the two studies was designed to test a strategy of advance misoprostol distribution against the existing standard of care. The individually‐randomised pilot trial applied advance provision and self‐administration of inert tablets to denote usual care, on the assumption that women giving birth outside of a health facility would not receive misoprostol (or any uterotonic). However, this assumption masks the impact of advance provision of misoprostol with the addition of specific instructions on postpartum use as a strategy, especially as it relates to non‐clinical outcomes such as increased uterotonic coverage. Given that outcome data in our review largely reflect the findings of this pilot study, the findings of our review cannot be generalised to settings where there are insurmountable barriers to antenatal contacts, and where the introduction of misoprostol distribution would require community‐based implementation approaches. The very low certainty of the evidence on important outcomes implies that the findings cannot be generalised to settings where advance misoprostol distribution is included as a core component of a national maternal mortality and morbidity‐reduction programme.
Quality of the evidence
The certainty of evidence for reported outcomes was very low; therefore the body of evidence identified does not allow robust conclusions regarding the comparative effects and safety of advance misoprostol provision to pregnant women. Two studies with important methodological differences and design limitations (which precluded a meta‐analysis) have been included in the review. The risk of bias for Weeks 2015 was generally low (except for attrition bias), whereas for Ononge 2015 the risk of bias was high for allocation concealment, blinding, attrition, and other biases regarding lack of adjustment for time for the presented results. Nonetheless, the results were generally consistent for similar outcomes reported. Although the study that reported the data used in the review (Weeks 2015) was by itself at overall low risk of bias, the overall certainty of evidence for important outcomes is very low because we downgraded for indirectness (relating to the comparison arm) and imprecision (as a result of no or few events, small sample size, and wide confidence intervals).
Potential biases in the review process
We minimised potential biases by the use of comprehensive search strategies and restriction of the study design to randomised trials and trials with imperfect method of random allocation (quasi‐randomised). Since the concept and implementation of a strategy of advance misoprostol distribution for PPH prevention and treatment only became prominent in the 2000s, it is unlikely that our search strategies failed to identify any relevant studies. We limited the studies considered for inclusion to those with some form of random component in their allocation of intervention as this represents a crucial step to reduce recruitment bias and misleading observations in a multi‐faceted intervention of this type. While we acknowledge the rarity of randomised trials for a relatively new subject, the rollout of this strategy requires significant investment, logistical management, time and considerable resources, and so it should be supported by high‐quality evidence that outlines the trade‐offs between its benefits and risks.
Agreements and disagreements with other studies or reviews
There is an increasing number of non‐randomised studies evaluating the impact of advance misoprostol distribution to pregnant women for PPH prevention or treatment (or both) in non‐facility births (Geller 2014; Haver 2016; Parashar 2018; Rajbhandari 2017; Smith 2014a; Smith 2014b). The majority of these studies suggest that the potential benefit in terms of increased coverage for uterotonic use for PPH prevention outweighs the potential risk of misoprostol misuse (Parashar 2018; Smith 2014a; Smith 2014b). However, the inherent biases in non‐randomised study designs means that the certainty of evidence derived from these studies would be similar to the evidence generated from our review. Two such non‐randomised studies exemplify this notion: firstly, Sanghvi 2010, a non‐randomised experimental control study that tested a strategy of community education on PPH prevention accompanied by advance provision of misoprostol directly to pregnant women for postpartum self‐administration. This study, which was conducted in rural Afghanistan, essentially focused on demonstrating that community distribution of misoprostol is a safe and feasible strategy to ensure that misoprostol reached nearly every woman in need. Study participants were mostly women who gave birth in their own home but also included those who gave birth at a health facility or the home of a midwife or traditional birth attendant. Thus, women in the study received labour and birth care from either a lay or skilled healthcare provider. Similar to the studies included in our review, the study found that all women who used misoprostol in the intervention areas did so correctly and uterotonic coverage (misoprostol and/or oxytocin) was achieved in 96.2% of women in the intervention areas compared with 25.7% in the comparison areas. Outcomes related to blood loss (such as PPH and severe PPH) and indicators of severe maternal morbidity were not reported in the study. The only maternal death recorded occurred in the intervention area in a woman who had eclampsia. However, as a result of the lack of sequence generation and allocation concealment that are inherent limitations of such studies, the study was at high risk of selection bias, as demonstrated by the significant differences in a number of baseline characteristics that were unadjusted for in the presented outcome data. There was also no information on baseline measure of outcomes for study participants or clusters to evaluate an unconfounded impact of the intervention. These shortcomings are likely to generate very low certainty of evidence for few of the outcomes that were pre‐specified for this review (maternal death, women not using misoprostol at birth and any adverse effects).
Another uncontrolled study has assessed the feasibility of community‐based distribution of misoprostol to pregnant women for PPH prevention in Nepal (Rajbhandari 2010). The study, which used a pre‐ and post‐intervention design, observed an increase in the overall uterotonic coverage (mainly due to increased use of misoprostol during home births) from 11.6% at baseline to 74.2% post‐intervention. This study only measured programme performance by assessing change in uterotonic coverage and could not reliably address the morbidity and mortality impact of the intervention. Apart from the fact that no patient‐important outcomes were reported in this study, its findings cannot be taken as reliable evidence to drive a strategy of advance community distribution of misoprostol due to the inherent flaws (such as biases at multiple levels and the effects of unmeasured co‐interventions) in the study design.
Authors' conclusions
Implications for practice.
Although advance misoprostol provision to pregnant women where there are no suitable alternative care options is a reasonable and feasible undertaking, evidence on its beneficial impact remains uncertain. While it is reassuring that concerns about potential harms could not be confirmed by the available evidence in this review, expansion of uterotonic coverage through this strategy should, nonetheless, be cautiously implemented either in the context of rigorous research or with targeted programme monitoring and evaluation to assess its impact.
Implications for research.
The question on the effectiveness and safety of advance distribution of misoprostol to pregnant women for the prevention or treatment of postpartum haemorrhage (PPH) in non‐facility births is yet to be addressed with certainty. In view of continuing interest to scale up this strategy, implementation in the context of rigorous research to address persistent uncertainties regarding comparative benefits and harms remain relevant. In this context, well‐designed and sufficiently large controlled studies should be used to assess the effectiveness of the strategy in terms of its ability to reduce PPH and its complications, in addition to assessing the feasibility of misoprostol reaching the end users. To be more generalisable, a community‐based strategy that combines misoprostol distribution through facility‐based and community outreach programmes, and randomisation of pregnant women at the cluster level, is most desirable. Clusters with high levels of non‐facility births would be ideal to enable intention‐to‐treat analysis and impact evaluation at the population level. Baseline assessment of relevant outcomes and characteristics to enable appropriate matching of clusters before randomisation should be an essential step to ensure comparability of such clusters. Outcome measures of interest should include innovative approaches to measure patient‐important outcomes (e.g. severe PPH, severe maternal morbidity, adverse effects, satisfaction with the intervention) and outcomes relevant to policy makers (e.g. resource utilisation), to allow future evaluation of the trade‐offs between benefits, risks and costs and to guide future recommendation of this strategy.
What's new
| Date | Event | Description |
|---|---|---|
| 19 December 2019 | New search has been performed | The search was updated and 10 reports identified. We added a 'Summary of findings' table, in compliance with updated Cochrane methodology. |
| 19 December 2019 | New citation required but conclusions have not changed | Two eligible studies were included. Whilst the previous version of the review contained no studies, the new data are insufficient to change the conclusions. |
History
Protocol first published: Issue 9, 2011 Review first published: Issue 2, 2012
Acknowledgements
The World Health Organization, Jennifer Blum, Edgardo Abalos and Babasola O Okusanya retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication. We acknowledge Bukola Fawole (deceased), a co‐author of the first version of this review, for his contributions to the assessment of study eligibility for this update.
This review is supported by funding from the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, World Health Organization to Cochrane Pregnancy and Childbirth (University of Liverpool). HRP supports and co‐ordinates research on a global scale, synthesises research through systematic reviews of literature, builds research capacity in low‐ and middle‐income countries and develops dissemination tools to make efficient use of ever‐increasing research information. In addition to its co‐sponsors, the International Planned Parenthood Federation (IPPF) and UNAIDS are both members of HRP’s governing body.
As part of the pre‐publication editorial process, this review has been commented on by three peers (and editor and two referees who are external to the editorial team), a member of our international panel of consumers and our Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Natalia Novikova; Jorge E Tolosa, Professor of Obstetrics and Gynecology, St Luke's University Health Network, USA.
Appendices
Appendix 1. Search methods ‐ ICTRP and ClinicalTrials.gov
ICTRP
postpartum h(a)emorrhage
third AND stage AND labo(u)r AND bleeding
ClinicalTrials.gov
Advanced search
Interventional studies | postpartum hemorrhage
Interventional studies | third stage | labor
Interventional studies | bleeding | labor
Data and analyses
Comparison 1. Advance misoprostol distribution/provision to pregnant women for postpartum self‐administration versus usual (or standard) care for PPH prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Severe maternal morbidity | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Maternal death | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Maternal anaemia | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.52, 3.62] |
| 1.4 Blood transfusion | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Use of additional uterotonics | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.17, 3.27] |
| 1.6 Women not using/receiving misoprostol at birth | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 1.95] |
| 1.7 Women not using/receiving misoprostol correctly at birth | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.86 [0.24, 100.46] |
| 1.8 Inappropriate use (or misuse) of misoprostol (as defined by authors) | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.24, 102.59] |
| 1.9 Maternal transfer or referral to a health facility | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.91] |
| 1.10 Minor adverse effect ‐ shivering/chills | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.35, 2.50] |
| 1.11 Minor adverse effect ‐ fever | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.16, 3.00] |
| 1.12 Minor adverse effect ‐ diarrhoea | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.44, 34.64] |
| 1.13 Major adverse effect ‐ placental retention | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.79] |
| 1.14 Major adverse effect ‐ hospital admission > 24 hours | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.73] |
| 1.15 Stillbirth | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 1.16 Early neonatal death | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.17 Neonatal death (within 28 days) | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ononge 2015.
| Study characteristics | ||
| Methods | Stepped‐wedged cluster‐randomised trial design | |
| Participants | 2466 pregnant women from antenatal clinics of participating health facilities, at 28 weeks or more of gestation, and who had no plans to leave the district during pregnancy, childbirth or in the immediate postpartum period, consented to participate. Women who had a planned elective caesarean section, a previous caesarean section scar, and those who could not be accessed after a home birth due to poor terrain were excluded. 1036 and 1430 women were recruited into the intervention and control arms of the study, respectively. However, only 271 out of 2466 women recruited into the study had non‐facility births. | |
| Interventions | Women in the intervention period were given 600 mcg of oral misoprostol at enrolment to the study to self‐administer after childbirth if birth happened outside a health facility, or when there was no oxytocin at the health facility. Three tablets of misoprostol (200 mcg each, for a total of 600 mcg) in aluminium foil were packaged in a plastic envelope. Specific instructions were given to women to ensure safe self‐administration of oral misoprostol. Standard of care (control) at the time of the trial was that women who gave birth at home received no uterotonic. | |
| Outcomes | PPH, defined as a drop in maternal haemoglobin by 2 g/dl or more lower than the prenatal haemoglobin (primary outcome), postpartum anaemia (defined as Hb < 11 g/dl when assessed within 7 days and Hb < 12 g/dl if assessed after the seventh day after childbirth), place of birth, use of any uterotonics for PPH prevention, referral to a health facility after childbirth, blood transfusion, maternal death, self‐reported side effects related to misoprostol use, appropriate use of misoprostol and its acceptability to women. | |
| Notes | The study was conducted between February 2013 and March 2014 (14 months). The authors declared no conflicts of interest. The study was funded by Wellcome Trust (grant number 087540). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence for starting the intervention was determined before the start of the study using computer‐generated number sequence. |
| Allocation concealment (selection bias) | High risk | The stepped‐wedge trial design meant every woman at the same period of the trial received what was offered and known to the research team. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding. According to the report, “it was not possible to blind the intervention to caregivers, research team or study participants". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessors. Additional data were collected only among women in the intervention group. The report states that "specific to the intervention group, we also assessed the timing of swallowing misoprostol, and its acceptability to women". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 17% of participants were lost to follow‐up: 12% of the baseline number of women in the intervention cluster, and 20% of those in the control cluster, were lost to follow‐up and primary outcome data were not available. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | No adjustment was made for time as required for a stepped‐wedge cluster‐randomised study design. |
Weeks 2015.
| Study characteristics | ||
| Methods | Individually randomised, parallel, double‐blind, placebo controlled, pilot trial | |
| Participants | The study included 748 pregnant women, at 34 or more weeks of gestation, from one regional referral hospital and three large health centres. All women living in the 200 villages that were served by the participating health facilities and had active village health teams were eligible to participate. Women with a known allergy to misoprostol or other prostaglandins, and unanticipated minors aged less than 18 years, were excluded. Women at risk of experiencing a childbirth complication were not excluded. 374 women were recruited into the intervention arm and 374 women were recruited into the control arm. Only 299 out of 748 women recruited into the study had non‐facility births. | |
| Interventions | Women were given a small purse with a string that could be hung around the neck, containing a packet with three foil‐packed tablets (containing either 600 mcg of misoprostol or identical placebo) along with both pictorial and written instructions on how to take the tablets. | |
| Outcomes | Haemoglobin fall of over 20% lower than the prenatal haemoglobin (primary outcome), mean change in haemoglobin, rate of postnatal anaemia, rate of poor maternal and fetal health, appropriate use of study medication, self‐reported side effects, self‐assessed blood loss, quality‐of‐life measures, and safety outcomes (including transfer to hospital, surgical intervention, blood transfusion, maternal death). | |
| Notes | The study was designed as a pilot trial to assess the feasibility of a larger trial, sample size was not formally calculated, and recruitment lasted two months (23 May 2012 to 17 July 2012). The study was funded by the Bill and Melinda Gates Foundation. The authors declared no competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment packets were consecutively numbered according to a computer‐generated block randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | This is not clearly described in the report. The following additional information was obtained through correspondence with the study authors: completely identical misoprostol and placebo tablets were professionally prepared. The tablets were then packed in sequentially numbered, labelled manila coin envelopes with unique packet IDs and sealed in purses. The trial packs (in the purses) were distributed in sequential order to the next randomised woman. Participants were instructed not to open the manila envelope until time of birth. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received a small purse containing three foil‐packed tablets (either misoprostol 600 mcg tablets or identical placebo) which were consecutively numbered and identical. Both women and providers were blinded to study group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In addition to blinding of providers, the measurement of all haemoglobin outcomes was unlikely to be affected by blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of women who did not have primary outcome data (haemoglobin outcomes) measured was 35% in the intervention and 46% in the control arms. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Unclear risk | There was no predetermined sample size estimation. This pilot study was not followed by a definitive study. |
Hb: haemoglobin PPH: postpartum haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbas 2019 | This double‐blind individually randomised controlled trial explored the efficacy, safety, and feasibility of equipping traditional birth attendants with 800 μg of misoprostol to treat PPH occurring during home birth in rural Pakistan. The study intervention did not include direct or indirect advance provision of misoprostol to pregnant women themselves; misoprostol was provided to traditional birth attendants for the prevention and treatment of PPH among women who gave birth under their care. |
| Derman 2006a | This community‐based placebo‐controlled randomised trial tested the efficacy and feasibility of misoprostol administered by 25 auxiliary nurse midwives undertaking home and sub‐centre deliveries in rural India. The study intervention did not include direct or indirect advance provision of misoprostol to pregnant women themselves; misoprostol was provided to auxiliary nurse midwives for the prevention of PPH among women who gave birth under their care. |
| Rajbhandari 2010 | This was an uncontrolled study of the feasibility of community‐based distribution of misoprostol through community volunteers working under government services to pregnant women for PPH prevention in Nepal. The study used a pre‐ and post‐intervention design and was excluded on the basis of non‐random study design. |
| Sanghvi 2010 | This was a non‐randomised experimental control study that tested a strategy of community education on PPH prevention accompanied by advance provision of misoprostol directly to pregnant women for postpartum self‐administration. The study had no random element whatsoever (e.g. alternate allocation) in its design to be regarded as a quasi‐randomised trial. No systematic method (albeit inadequate) was reported for assigning provinces and districts to intervention and comparison areas. This limitation was acknowledged by the study authors who noted that "although we recognized the limitations of non‐randomised trials, several logistical reasons prevented use of a randomised design." |
PPH: postpartum haemorrhage
Differences between protocol and review
For this update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). We limited the scope of the review to strategies of advance misoprostol distribution/provision directly to pregnant women for postpartum self‐administration or its application by lay health workers, and amended the review title accordingly. The previous version of the review included a comparison on advance misoprostol distribution to lay health workers for use during childbirth for women under their care. We have added a 'Summary of findings' table in this update.
Contributions of authors
For this update, all authors assessed the eligibility of studies for inclusion. Jennifer Blum and Babasola O Okusanya performed data extraction. Olufemi T Oladapo and Babasola O Okusanya prepared the draft of the review update and Jennifer Blum and Edgardo Abalos reviewed and revised the draft for intellectual content. Olufemi T Oladapo has overall responsibility for maintaining the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, World Health Organization, Switzerland
This review is supported by funding to Cochrane Pregnancy and Childbirth (University of Liverpool)
Declarations of interest
Olufemi T Oladapo received financial support from the World Health Organization (Geneva, Switzerland), as part of the evidence‐base preparation for the update of "WHO recommendations for prevention and treatment of postpartum haemorrhage" for the first version of the review that was published in 2012. Olufemi T Oladapo was a paid employee of the World Health Organization during the review update.
Jennifer Blum previously worked at an organization that received funding to carry out research on misoprostol for PPH indications for many years and was an investigator on many studies of misoprostol for the prevention and treatment of PPH.
Edgardo Abalos: none known.
Babasola O Okusanya: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ononge 2015 {published data only}
- Mirembe F, PACTR201303000459148. Prevention of post partum haemorrhage: role of self administered misoprostol. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201303000459148 (first received 19 November 2012).
- Ononge S, Campbell OMR, Kaharuza F, Lewis JJ, Fielding K, Mirembe F. Effectiveness and safety of misoprostol distributed to antenatal women to prevent postpartum haemorrhage after child-births: a stepped-wedge cluster-randomized trial. BMC Pregnancy and Childbirth 2015;15:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weeks 2015 {published data only}ISRCTN70408620
- Ditai J, Frye LJ, Durocher J, Byrne ME, Ononge S, Winikoff B, et al. Achieving community-based postpartum follow up in eastern Uganda: the field experience from the MamaMiso Study on antenatal distribution of misoprostol. BMC Research Notes 2017;10(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye L, Durocher J, Weeks A, Ditai J, Ononge S, Faragher B, et al. On the trail of misoprostol in the community: a secondary analysis of self-administered misoprostol for the prevention of postpartum hemorrhage in Uganda. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E354-E355. [Google Scholar]
- ISRCTN70408620. A study of self-administered misoprostol to prevent bleeding after childbirth in the community (MamaMiso). isrctn.com/ISRCTN70408620 (first received 28 December 2011).
- NCT01510574. A study of self-administered misoprostol to prevent bleeding after childbirth in the community (Mama Miso). clinicaltrials.gov/show/NCT01510574 (first received 16 March 2012).
- Weeks A, Ditai J, Ononge S, Faragher B, Mirembe F, Byamugisha J, et al. Self-administered misoprostol to prevent bleeding after homebirths in Uganda: a placebo-controlled randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2013;120:76. [Google Scholar]
- Weeks AD, Ditai J, Ononge S, Durocher J, Faragher B, Byamugisha J, et al. PL.03 self-administration of misoprostol to prevent bleeding after homebirths in Uganda: a pilot placebo-controlled, randomised trial. Archives of Disease in Childhood: Fetal and Neonatal Edition 2013;98(Suppl 1):A55. [Google Scholar]
- Weeks AD, Ditai J, Ononge S, Faragher B, Frye LJ, Durocher J, et al. The MamaMiso study of self-administered misoprostol to prevent bleeding after childbirth in rural Uganda: a community-based, placebo-controlled randomised trial. BMC Pregnancy and Childbirth 2015;15(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbas 2019 {published data only}
- Abbas DF, Jehan N, Diop A, Durocher J, Byrne ME, Zuberi N, et al. Using misoprostol to treat postpartum hemorrhage in home deliveries attended by traditional birth attendants. International Journal of Gynaecology and Obstetrics 2019;144(3):290-6. [CENTRAL: CN-01707705] [PMID: ] [DOI] [PubMed] [Google Scholar]
Derman 2006a {published data only}
- Derman R, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368:1248-53. [DOI] [PubMed] [Google Scholar]
- Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low-risk women delivering in rural India. International Journal of Gynecology & Obstetrics 2008;101(1):94-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267-71. [DOI] [PubMed] [Google Scholar]
- Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal-Fetal & Neonatal Medicine 2008;21(8):559-64. [DOI] [PubMed] [Google Scholar]
- Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91-6. [PubMed] [Google Scholar]
- Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double-blind placebo-controlled trial. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S141-2. [DOI] [PubMed] [Google Scholar]
- Moss NM. Oral misoprostol for prevention of postpartum hemorrhage (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community-based randomised controlled trial in rural India. Journal of Maternal-Fetal & Neonatal Medicine 2009;22(1):24-8. [DOI] [PubMed] [Google Scholar]
Rajbhandari 2010 {published data only}
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH, Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. International Journal of Gynecology & Obstetrics 2010;108(3):282–8. [DOI] [PubMed] [Google Scholar]
Sanghvi 2010 {published data only}
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. International Journal of Gynecology & Obstetrics 2010;108(3):276-81. [DOI] [PubMed] [Google Scholar]
Additional references
Alfirevic 2007
- Alfirevic Z, Blum J, Walraven G, Weeks A, Winikoff B. Prevention of postpartum hemorrhage with misoprostol. International Journal of Gynecology & Obstetrics 2007;99:S198–S201. [DOI] [PubMed] [Google Scholar]
Amaral 2011
- Amaral E, Souza JP, Surita F, Luz AG, Sousa MH, Cecatti JG, et al. A population-based surveillance study on severe acute maternal morbidity (near-miss) and adverse perinatal outcomes in Campinas, Brazil: the Vigimoma Project. BMC Pregnancy and Childbirth 2011;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beischer 1986
- Beischer NA, Mackay EV. Obstetrics and the Newborn. Eastbourne: Bailliere Tindall, 1986. [Google Scholar]
Bloomfield 1990
- Bloomfield TH, Gordon H. Reaction to blood loss at delivery. Journal of Obstetrics and Gynaecology 1990;10(Suppl 2):S13-S16. [Google Scholar]
Braunholtz 2010
- Braunholtz D, Graham W, Hussein J. Community-based interventions to reduce maternal mortality. Lancet 2010;375:457. [DOI] [PubMed] [Google Scholar]
Burchell 1980
- Burchell RC. Postpartum haemorrhage. In: Quilligan ES, editors(s). Current Therapy in Obstetrics and Gynaecology. Philadelphia: WB Saunders, 1980. [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics and Gynaecology 2008;22:999–1012. [DOI] [PubMed] [Google Scholar]
Chandhiok 2006
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynecology & Obstetrics 2006;92:170-5. [DOI] [PubMed] [Google Scholar]
CMACE 2011
- Centre for Maternal and Child Enquiries (CMACE). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG: an International Journal of Obstetrics and Gynaecology 2011;118(Suppl 1):1-203. [DOI] [PubMed] [Google Scholar]
Costello 2004
- Costello A, Osrin D, Manandhar D. Reducing maternal and neonatal mortality in the poorest communities. BMJ 2004;329:1166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derman 2006b
- Derman R, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368:1248-53. [DOI] [PubMed] [Google Scholar]
Ejembi 2010
- Ejembi CL, Prata N. Prevention of Postpartum Haemorrhage at Home Births in Five Communities around Zaria, Kaduna State, Nigeria Technical Report. The Population and Reproductive Health Partnership and Venture Strategies Innovations, 2010. [Google Scholar]
Geller 2006
- Geller SE, Adams MG, Kelly PJ, Kodkany BS, Derman RJ. Postpartum hemorrhage in resource-poor settings. International Journal of Gynecology & Obstetrics 2006;92(3):202-11. [DOI] [PubMed] [Google Scholar]
Geller 2014
- Geller S, Carnahan L, Akosah E, Asare G, Agyemang R, Dickson R, et al. Community-based distribution of misoprostol to prevent postpartum haemorrhage at home births: results from operations research in rural Ghana. BJOG: an International Journal of Obstetrics and Gynaecology 2014;21(3):319-25. [DOI] [PubMed] [Google Scholar]
Gupta 2010
- Gupta SD, Khanna A, Gupta R, Sharma NK, Sharma ND. Maternal mortality ratio and predictors of maternal deaths in selected desert districts in Rajasthan a community-based survey and case control study. Women's Health Issues 2010;20(1):80-5. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2001
- Gülmezoglu AM, Villar J, Ngoc NN, Piaggio G, Carroli G, Adetoro L, et al. The WHO multicentre double-blind randomized controlled trial to evaluate the use of misoprostol in the management of the third stage of labour. Lancet 2001;358(9283):689-95. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2007
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000494.pub3] [DOI] [PubMed] [Google Scholar]
Hamilton 2009
- Hamilton AK, Hayton JC. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG: an international journal of obstetrics and gynaecology 2009;116(5):736-7. [DOI] [PubMed] [Google Scholar]
Haver 2016
- Haver J, Ansari N, Zainullah P, Kim YM, Tappis H. Misoprostol for prevention of postpartum haemorrhage of home birth in Afghanistan: program expansion experience. Journal of Midwifery and Women's Health 2016;61(2):196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazra 2004
- Hazra S, Chilaka VN, Rajendran S, Konje JC. Massive postpartum haemorrhage as a cause of maternal morbidity in a large tertiary hospital. Journal of Obstetrics and Gynaecology 2004;24(5):519-20. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Hoj 2005
- Hoj L, Cardosa P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomized double blind clinical trial. BMJ 2005;331:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICM‐FIGO 2006
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Prevention and treatment of post-partum haemorrhage: new advances for low resource settings. Joint statement. www.figo.org/docs/PPH%20Joint%20Statement%202%20English.pdf (accessed 4 June 2011) 2006.
ICM‐FIGO 2007
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Prevention and treatment of post-partum haemorrhage: new advances for low resource settings. International Journal of Gynecology and Obstetrics 2007;97(2):160-3. [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
Mobeen 2011
- Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2002
- Mousa H, Alfirevic Z. Postpartum hemorrhage: survey of maternity units in the United Kingdom. Acta Obstetricia et Gynecologica Scandinavica 2002;81(8):727-30. [DOI] [PubMed] [Google Scholar]
Pagel 2009
- Pagel C, Lewycka S, Colbourn T, Mwansambo C, Meguid T, Chiudzu G, et al. Estimation of potential effects of improved community-based drug provision, to augment health-facility strengthening, on maternal mortality due to post-partum haemorrhage and sepsis in sub-Saharan Africa: an equity-effectiveness model. Lancet 2009;374:1441-8. [DOI] [PubMed] [Google Scholar]
Parashar 2018
- Parashar R, Gupt A, Bajpayee D, Gupta A, Thakur R, Sangwan A, et al. Implementation of community based advanced distribution of misoprostol in Himachal Pradesh (India): lessons and way forward. BMC Pregnancy and Childbirth 2018;18(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Potts 2010a
- Potts M, Prata N, Sahin-Hodoglugil NN. Maternal mortality: one death every 7 min. Lancet 2010;375:1762–3. [DOI] [PubMed] [Google Scholar]
Potts 2010b
- Potts M, Prata N, Sahin-Hodoglugil NN. Misoprostol use in the community to prevent death. Lancet 2010;376:955-6. [Google Scholar]
Prata 2005a
- Prata N, Mbaruku G, Campbell M. Using the kanga to measure postpartum blood loss. International Journal of Gynecology & Obstetrics 2005;89(1):49-50. [DOI] [PubMed] [Google Scholar]
Prata 2005b
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after homebirths in Tanzania. International Journal of Gynecology and Obstetrics 2005;90:51-5. [DOI] [PubMed] [Google Scholar]
Prata 2009
- Prata N, Sreenivas A, Vahidnia F, Potts M. Saving maternal lives in resource-poor settings: facing reality. Health Policy 2009;89:131-48. [DOI] [PubMed] [Google Scholar]
Rajbhandari 2017
- Rajbhandari SP, Aryal K, Sheldon WR, Ban B, Upreti SR, Regmi K, et al. Postpartum hemorrhage prevention in Nepal: a program assessment. BMC Pregnancy and Childbirth 2017;17(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rath 2011
- Rath WH. Postpartum hemorrhage: update on problems of definitions and diagnosis. Acta Obstetricia et Gynecologica Scandinavica 2011;90(5):421-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohilla 2010
- Rohilla M, Raveendran A, Dhaliwal LK, Chopra S. Severe anaemia in pregnancy: a tertiary hospital experience from northern India. Journal of Obstetrics and Gynaecology 2010;30(7):694-6. [DOI] [PubMed] [Google Scholar]
Ronsmans 2010
- Ronsmans C, Huang W. Community-based interventions to reduce maternal mortality. Lancet 2010;375:457. [DOI] [PubMed] [Google Scholar]
Say 2014
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323-33. [DOI] [PubMed] [Google Scholar]
Selo‐Ojeme 2002
- Selo-Ojeme DO. Primary postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2002;22:463–9. [DOI] [PubMed] [Google Scholar]
Smith 2014a
- Smith JM, Baawo SD, Subah M, Sirtor-Gbassie V, Howe CJ, Ishola G, et al. Advance distribution of misoprostol for prevention of postpartum hemorrhage (PPH) at home births in two districts of Liberia. BMC Pregnancy and Childbirth 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2014b
- Smith JM, Dimiti A, Dwivedi V, Ochieng I, Dalaka M, Currie S, et al. Advance distribution of misoprostol for the prevention of postpartum hemorrhage in South Sudan. International Journal of Gynecology & Obstetrics 2014;127(2):183-8. [DOI] [PubMed] [Google Scholar]
Tsu 2004
- Tsu VD, Langer A, Aldrich T. Postpartum hemorrhage in developing countries: is the public health community using the right tools? International Journal of Gynecology & Obstetrics 2004;85(Suppl 1):S42–S51. [DOI] [PubMed] [Google Scholar]
UN 2015
- United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. New York: United Nations, 2015. [Google Scholar]
UNICEF 2009
- United Nations Children and Educational Fund. State of the World's Children. New York: UNICEF, 2009. [Google Scholar]
Walraven 2005
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112:1277–83. [DOI] [PubMed] [Google Scholar]
Wandabwa 2008
- Wandabwa J, Doyle P, Todd J, Ononge S, Kiondo P. Risk factors for severe post partum haemorrhage in Mulago hospital, Kampala, Uganda. East African Medical Journal 2008;85(2):64-71. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Guidelines for the Prevention of Postpartum Haemorrhage. Geneva: World Health Organization, 2007. [Google Scholar]
WHO 2009
- World Health Organization. WHO Statement Regarding the Use of Misoprostol for Postpartum Haemorrhage Prevention and Treatment. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2010
- World Health Organization. Clarifying WHO Position on Misoprostol Use in the Community to Reduce Maternal Death. Geneva: World Health Organization, 2010. [Google Scholar]
WHO 2012
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization 2012. [PubMed]
WHO 2017
- World Health Organization. Managing Complications in Pregnancy and Childbirth: a Guide for Midwives and Doctors. 2nd edition. Geneva: World Health Organization, 2017. [Google Scholar]
WHO 2018
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization, 2018. [PubMed] [Google Scholar]
Yoong 2010
- Yoong W, Karavolos S, Damodaram M, Madgwick K, Milestone N, Al-Habib A, et al. Observer accuracy and reproducibility of visual estimation of blood loss in obstetrics: how accurate and consistent are health-care professionals? Archives of Gynecology and Obstetrics 2010;281(2):207-13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Oladapo 2012
- Oladapo OT, Fawole B, Blum J, Abalos E. Advance misoprostol distribution for preventing and treating postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009336.pub2] [DOI] [PubMed] [Google Scholar]


