Abstract
Background
In order to minimise publication bias, authors of systematic reviews often spend considerable time trying to obtain unpublished data. These include data from studies conducted but not published (unpublished data), as either an abstract or full‐text paper, as well as missing data (data available to original researchers but not reported) in published abstracts or full‐text publications. The effectiveness of different methods used to obtain unpublished or missing data has not been systematically evaluated.
Objectives
To assess the effects of different methods for obtaining unpublished studies (data) and missing data from studies to be included in systematic reviews.
Search methods
We identified primary studies comparing different methods of obtaining unpublished studies (data) or missing data by searching the Cochrane Methodology Register (Issue 1, 2010), MEDLINE and EMBASE (1980 to 28 April 2010). We also checked references in relevant reports and contacted researchers who were known or who were thought likely to have carried out relevant studies. We used the Science Citation Index and PubMed 'related articles' feature to identify any additional studies identified by other sources (19 June 2009).
Selection criteria
Primary studies comparing different methods of obtaining unpublished studies (data) or missing data in the healthcare setting.
Data collection and analysis
The primary outcome measure was the proportion of unpublished studies (data) or missing data obtained, as defined and reported by the authors of the included studies. Two authors independently assessed the search results, extracted data and assessed risk of bias using a standardised data extraction form. We resolved any disagreements by discussion.
Main results
Six studies met the inclusion criteria; two were randomised studies and four were observational comparative studies evaluating different methods for obtaining missing data.
Methods to obtain missing data
Five studies, two randomised studies and three observational comparative studies, assessed methods for obtaining missing data (i.e. data available to the original researchers but not reported in the published study).
Two studies found that correspondence with study authors by e‐mail resulted in the greatest response rate with the fewest attempts and shortest time to respond. The difference between the effect of a single request for missing information (by e‐mail or surface mail) versus a multistage approach (pre‐notification, request for missing information and active follow‐up) was not significant for response rate and completeness of information retrieved (one study). Requests for clarification of methods (one study) resulted in a greater response than requests for missing data. A well‐known signatory had no significant effect on the likelihood of authors responding to a request for unpublished information (one study). One study assessed the number of attempts made to obtain missing data and found that the number of items requested did not influence the probability of response. In addition, multiple attempts using the same methods did not increase the likelihood of response.
Methods to obtain unpublished studies
One observational comparative study assessed methods to obtain unpublished studies (i.e. data for studies that have never been published). Identifying unpublished studies ahead of time and then asking the drug industry to provide further specific detail proved to be more fruitful than sending of a non‐specific request.
Authors' conclusions
Those carrying out systematic reviews should continue to contact authors for missing data, recognising that this might not always be successful, particularly for older studies. Contacting authors by e‐mail results in the greatest response rate with the fewest number of attempts and the shortest time to respond.
Plain language summary
Methods for obtaining unpublished data
This methodology review was conducted to assess the effects of different methods for obtaining unpublished studies (data) and missing data from studies to be included in systematic reviews. Six studies met the inclusion criteria, two were randomised studies and four were observational comparative studies evaluating different methods for obtaining missing data.
Five studies assessed methods for obtaining missing data (i.e. data available to the original researchers but not reported in the published study). Two studies found that correspondence with study authors by e‐mail resulted in the greatest response rate with the fewest attempts and shortest time to respond. The difference between the effect of a single request for missing information (by e‐mail or surface mail) versus a multistage approach (pre‐notification, request for missing information and active follow‐up) was not significant for response rate and completeness of information retrieved (one study). Requests for clarification of methods (one study) resulted in a greater response than requests for missing data. A well‐known signatory had no significant effect on the likelihood of authors responding to a request for unpublished information (one study). One study assessed the number of attempts made to obtain missing data and found that the number of items requested did not influence the probability of response. In addition, multiple attempts using the same methods did not increase the likelihood of response.
One study assessed methods to obtain unpublished studies (i.e. data for studies that have never been published). Identifying unpublished studies ahead of time and then asking the drug industry to provide further specific detail proved to be more fruitful than sending of a non‐specific request.
Those carrying out systematic reviews should continue to contact authors for missing data, recognising that this might not always be successful, particularly for older studies. Contacting authors by e‐mail results in the greatest response rate with the fewest number of attempts and the shortest time to respond.
Background
Description of the problem or issue
Reporting bias arises when dissemination of research findings is influenced by the nature and direction of results. Publication bias (the selective publication of research studies as a result of the strength of study findings), time‐lag bias (the rapid or delayed publication of results depending on the results) and language bias (the publication in a particular language depending on the nature and direction of the results) are typical types of reporting bias (Higgins 2009).
Publication bias, especially, is a major threat to the validity of systematic reviews (Song 2000; Sterne 2008). Hopewell et al examined the impact of grey literature (literature which has not formally been published) in meta‐analysis of randomised trials of healthcare interventions and found that published trials tend to be larger and show an overall greater treatment effect than trials from grey literature (Hopewell 2007). Not making an attempt to include unpublished data in a systematic review can thus result in biased larger treatment effects (Higgins 2009).
In order to minimise publication bias, authors of systematic reviews often spend considerable time trying to obtain unpublished data. These include data from studies conducted but not published (unpublished data) as either an abstract or full‐text paper, as well as missing data (data available to the original researchers but not reported) in published abstracts or full‐text publications. Types of data commonly missing from published papers include details of allocation concealment and blinding, information about loss to follow‐up and standard deviations. This is different from data that are 'missing' because the original researchers do not have them, but might be able to get them (e.g. a specific subgroup analysis not done by the original researchers, but which could be carried out retrospectively in response to a request from systematic review authors) or data that are missing because they were never collected by the original researchers and are not retrievable by other means (e.g. patient’s quality of life at specific time points).
Often, the search for and retrieval of unpublished and missing data delays the time to review completion.
Description of the methods being investigated
Different methods are used to search for and obtain unpublished data or missing data from studies to be included in systematic reviews.
Authors of systematic reviews informally contact colleagues to find out if they know about unpublished studies (Greenhalgh 2005). In addition, formal requests for information on completed but unpublished studies, as well as ongoing studies, are sent to researchers (authors of identified included studies of the relevant review), experts in the field, research organisations and pharmaceutical companies (Lefebvre 2008; Song 2000). Some organisations might set up websites for systematic review projects, listing the studies identified to date and inviting submission of information on studies not already listed (Lefebvre 2008).
Prospective clinical trial registries, both national and international, are also searched to identify ongoing studies. Plus, registries of grey literature are searched to identify unpublished studies.
In order to obtain details about missing data (data available to the original researchers but not reported) authors of systematic reviews contact the authors of studies included in the review by telephone, e‐mail or letters by post.
How these methods might work
Approaching researchers for information about completed but never published studies has had varied results, ranging from being willing to share information to no response (Greenhalgh 2005). The willingness of investigators of located unpublished studies to provide data may depend upon the findings of the study, where more favourable results may be shared more willingly (Smith 1998).
Why it is important to do this review
The effectiveness of the different methods used to obtain unpublished or missing data has not been systematically evaluated. This review will systematically evaluate these effects and will thus assist authors of reviews in improving their efficiency in conducting their reviews.
Objectives
To assess the effects of different methods for obtaining unpublished studies (data) and missing data from studies to be included in systematic reviews.
Methods
Criteria for considering studies for this review
Types of studies
Primary studies comparing different methods of obtaining unpublished studies (data) or missing data. We excluded studies without a comparison of methods.
Types of data
All relevant studies in the healthcare setting.
Types of methods
Any method designed to obtain unpublished studies (data) or missing data (i.e. data available to researchers but not reported).
Types of outcome measures
Primary outcomes
Methods to obtain missing data (data available to researchers but not reported in the published study).
Proportion of missing data obtained as defined and reported by authors.
Methods to obtain unpublished studies (data for studies that have never been published).
Proportion of unpublished studies (data) obtained as defined and reported by authors.
Secondary outcomes
Methods to obtain missing data (data available to the original researchers but not reported in the published study).
Completeness (extent to which data obtained answers to the questions posed by those seeking the data) of missing data obtained.
Type (e.g. outcome data, baseline data) of missing data obtained.
Time taken to obtain missing data (i.e. time from when efforts start until data are obtained).
Number of attempts (as defined by the authors) made to obtain missing data.
Resources required.
Methods to obtain unpublished studies (data for studies that have never been published).
Time taken to obtain unpublished studies (i.e. time from when efforts start until data are obtained).
Number of attempts (as defined by the authors) made to obtain unpublished studies (data).
Resources required.
Search methods for identification of studies
To identify studies we carried out both electronic and manual searches. All languages were included.
Electronic searches
We searched the Cochrane Methodology Register (CMR) (Issue 1, 2009) using the search terms in Appendix 1. We searched MEDLINE and Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations using OVID (1950 to 10 February 2009) (Appendix 2) and adapted these terms for use in EMBASE (1980 to 2009 Week 06) (Appendix 3). We conducted an updated search in EMBASE, MEDLINE and the Cochrane Methodology Register on 28 April 2010.
Searching other resources
We also checked references in relevant reports (Horsley 2011) and contacted researchers who were known or who were thought likely to have carried out relevant studies. We used the Science Citation Index and PubMed 'related articles' feature to identify any additional studies identified by the sources above (19 June 2009).
Data collection and analysis
Selection of studies
Two authors independently screened titles, abstracts and descriptor terms of the electronic search results for relevance based on the criteria for considering studies for this review. We obtained full‐text articles (where available) of all selected abstracts and used an eligibility form to determine final study selection. We resolved any disagreements through discussion.
Data extraction and management
Two authors independently extracted data using a standardised data extraction form. We resolved any disagreements by discussion.
Data extracted included the following.
Administrative details for the study ‐ identification; author(s); published or unpublished; year of publication; year in which study was conducted; details of other relevant papers cited.
Details of study ‐ study design; inclusion and exclusion criteria; country and location of the study.
Details of intervention ‐ method(s) used to obtain unpublished or missing data.
Details of outcomes and results ‐ proportion, completeness and type of unpublished or missing data; time taken to obtain unpublished or missing data; number of attempts made and resources required.
Assessment of risk of bias in included studies
Two authors independently evaluated the risk of bias of included studies, which included both the inherent properties of the study and the adequacy of its reporting.
For randomised studies comparing different methods to obtain data we assessed the following criteria, based on The Cochrane Collaboration's 'Risk of bias' tool and classified as adequate, inadequate or unclear:
generation of the allocation sequence;
concealment of the allocation sequence;
blinding of the participants, personnel and outcome assessor.
For non‐randomised studies comparing different methods to obtain data we assessed the following criteria and reported whether they were adequate, inadequate or unclear:
how allocation occurred;
attempt to balance groups by design;
use of blinding.
Based on these criteria, we assessed studies as being at 'high', 'low' or 'moderate' risk of bias.
Dealing with missing data
If any of the data were insufficient or missing, we sought data from the contact author of the empirical study using e‐mail. This was successful for one of the two studies (Higgins 1999) for which we contacted the authors.
Data synthesis
Due to significant differences in study design, it was not possible to carry out a meta‐analysis of the included studies. Therefore the results of the individual studies are presented descriptively, reporting individual study effect measures and 95% confidence intervals where available.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Of 4768 identified abstracts and titles, we selected 18 potentially eligible publications, referring to 15 studies, for detailed independent eligibility assessment (Figure 1).
1.
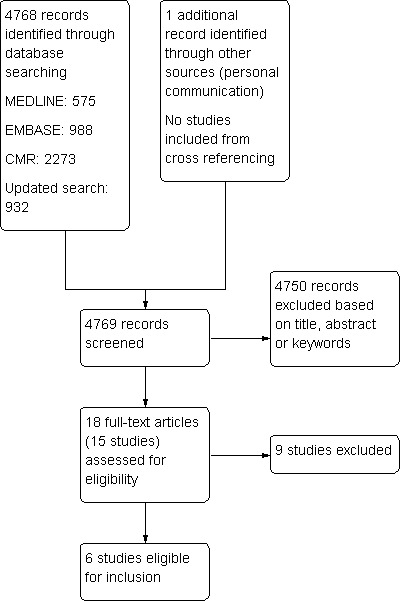
Study flow diagram.
Included studies
Six studies (Brown 2003; Gibson 2006; Guevara 2005; Higgins 1999; Milton 2001; Shukla 2003) met the inclusion criteria. Of these five were published as abstracts for Cochrane Colloquia and one as a full paper (Gibson 2006). Only one observational comparative study evaluated the effects of different methods to obtain unpublished data (Shukla 2003). The other five studies, two randomised studies (Higgins 1999; Milton 2001) and three observational comparative studies (Brown 2003; Gibson 2006; Guevara 2005), evaluated different methods for obtaining missing data. Table 1 provides a summary of the interventions studies and outcomes measured.
1. Summary of interventions and outcomes.
| Study ID | Comparison | Outcome measures |
| Methods for obtaining missing data (data available to researchers but not reported in the published study) | ||
| Brown 2003 | E‐mail versus letter providing a semi‐personalised information retrieval sheet | Additional information retrieved through contact with trial authors Costs incurred |
| Gibson 2006 | E‐mail versus letter versus both | Proportion of responders over time Response rates United States compared to other countries |
| Guevara 2005 | E‐mail versus letter versus fax | Response rate Time to response |
| Higgins 1999 | Single request for missing information (by e‐mail or surface mail) versus multistage approach involving pre‐notification, request for missing information and active follow‐up | Whether contact is established Whether appropriate information is obtained |
| Milton 2001 | Using a well‐known signatory versus an unknown researcher on the cover letter of a mailed questionnaire | Response of clinical trial investigators to requests for information |
| Methods for obtaining unpublished data (data for studies that have never been published) | ||
| Shukla 2003 | Identifying unpublished studies ahead of time and then asking industry to provide further specific detail versus general request by letter for unpublished studies | Unpublished information obtained from the drug industry |
Excluded studies
Nine studies (Bohlius 2003; Eysenbach 2001; Hadhazy 1999; Hetherington 1987; Kelly 2002; Kelly 2004; McGrath 1998; Reveiz 2004; Wille‐Jorgensen 2001) did not meet the inclusion criteria as there was no comparison of different methods of obtaining missing data.
Risk of bias in included studies
Brown 2003, Gibson 2006, Guevara 2005 and Shukla 2003, the four observational, comparative studies, did not report on the methodology used and therefore assessments of risk of bias for these studies are incomplete.
We assessed risk of bias for the two randomised studies by looking at the methods used for allocation sequence generation, allocation concealment and blinding. Allocation concealment was adequate for Higgins 1999 and unclear for Milton 2001. Allocation sequence generation and blinding were not reported.
Effect of methods
Methods to obtain missing data
Five of the six studies assessed methods for obtaining missing data (i.e. data available to the original researchers but not reported in the published study).
Proportion of missing data obtained as defined and reported by authors
All five studies provided information on the proportion of missing data obtained.
Brown 2003 used a non‐randomised design to compare contacting 112 authors (of 139 studies) via 39 e‐mails and 73 letters. The study was designed as a comparative study but data per study arm were not reported. Twenty‐one replies (19%) were received. One study published in the period 1980‐1984 elicited no response, nine 1985‐1989 studies elicited two responses, 41 1990‐1994 studies elicited six responses, 38 1995‐1999 studies elicited eight responses and 21 2000‐2002 studies elicited four responses.
Gibson 2006 used a non‐randomised design to compare contacting authors by e‐mail, letter or both. Two hundred and forty‐one studies (40%) had missing or incomplete data. They were unable to locate 95 authors (39%). Of the remaining 146 authors, 46 authors (32%) responded to information requests. The response rate differed by mode of contact ‐ letter (24%), e‐mail (47%) and both (73%). Response was significantly higher with e‐mail compared to using letters (hazard ratio 2.5; 95% confidence interval (CI) 1.3 to 4.0). Combining letter and e‐mail had a higher response rate, however, it was not significantly different from using e‐mail alone (reported P = 0.36). The combination of methods (letter plus e‐mail follow‐up) rather than multiple contacts using the same method was more effective for eliciting a response from the author. Response rates from US authors did not differ from those of other countries. The older the article, the less likely the response.
Guevara 2005 used a non‐randomised design to compare e‐mail versus letter versus fax. Fifteen authors (60%) responded to information requests. E‐mail resulted in fewer attempts and a greater response rate than post or fax. Authors of studies published after 1990 were as likely to respond (67% versus 50%, reported P = 0.45) as authors of studies published earlier. Similarly, corresponding authors were no more likely to respond (58% versus 9%, reported P = 0.44) than secondary authors, although few secondary authors were contacted.
Higgins 1999 used a randomised comparison of single request for missing information (by e‐mail or surface mail) (n = 116) versus a multistage approach involving pre‐notification, request for missing information and active follow‐up (n = 117) and found no significant difference between the two groups (risk ratio (RR) 1.04; 95% CI 0.74 to 1.45) in response rate.
Milton 2001 compared, using a randomised design, the response of clinical trial investigators to requests for information signed by either Richard Smith (RS), editor of the British Medical Journal (n = 96), or an unknown researcher (n = 48) and found no significant differences between signatory groups in response rates. By three weeks, 34% in the former and 27% in the unknown researcher's group had responded (odds ratio (OR) 1.35; 95% CI 0.59 to 3.11). No baseline data had been provided by three weeks. By the end of the study, at five weeks, 74% and 67% respectively had responded (OR 1.42; 95% CI 0.62 to 3.22) and 16 out of 53 studies in the RS group and five out of 27 authors in the unknown researcher's group had provided baseline data (OR 1.90; CI 0.55 to 6.94).
Completeness of data
One of the five studies assessed the extent to which data obtained answers to the questions posed by those seeking the data.
Higgins 1999 compared, using a randomised design, the completeness of information retrieved between study arms (single request for missing information (by e‐mail or surface mail) (n = 116) versus multistage approach involving pre‐notification, request for missing information and active follow‐up (n = 117)) and found no significant difference between the two study methods.
Type of missing data obtained
Two of the five studies assessed the type of missing data obtained.
Brown 2003 used a non‐randomised design to compare contacting 112 authors (of 139 studies) via 39 e‐mails and 73 letters and received 21 replies (19%), of which nine provided relevant outcome and quality data, one provided additional data on study quality only and one provided information regarding duplicate publications. Eleven studies provided no useful information. Data per study arm were not reported.
Guevara 2005 used a non‐randomised design to compare e‐mail versus letter versus fax and reported that requests for clarification of methods resulted in a greater response (50% versus 32%, P = 0.03) than requests for missing data. Once again, data per study arm were not reported.
Time taken to obtain missing data
Two of the five studies assessed the time taken to obtain missing data (i.e. time from when efforts start until data are obtained).
Gibson 2006 used a non‐randomised design to compare e‐mail versus letter versus fax and reported that the time to respond differed significantly by contact method (P < 0.05): e‐mail (3 +/‐ 3 days; median one day), letter (27 +/‐ 30 days; median 10 days) and both (13 +/‐ 12 days; median nine days).
Guevara 2005 used a non‐randomised design to compare e‐mail versus letter versus fax and reported that e‐mail had a shorter response time than post or fax.
Number of attempts made to obtain missing data
One of the five studies assessed the number of attempts made to obtain missing data.
Gibson 2006 used a non‐randomised design to compare e‐mail versus letter versus fax and reported that the number of items requested per authors averaged two or more. The number of items requested did not influence the probability of response. In addition, multiple attempts using the same methods did not increase the likelihood of response.
Resources required
One of the five studies assessed the resources required to obtain missing data.
Brown 2003 used a non‐randomised design to compare contacting 112 authors (of 139 studies) via 39 e‐mails and 73 letters and reported total costs of 80 GBP for printing and postage. Cost was not reported per study arm.
Methods to obtain unpublished studies
One of the six included studies assessed methods to obtain unpublished studies (i.e. data for studies that have never been published).
Proportion of unpublished studies (data) obtained as defined and reported by authors
Shukla 2003, using a non‐randomised design, assessed two different approaches to seek unpublished information from the drug industry. The outcome of a general request letter was compared with efforts to identify unpublished data and then contacting the industry to provide further specific detail. With the first approach, no unpublished information was obtained. With the second approach, relevant unpublished information was obtained for four of five of the systematic reviews (in the form of manuscripts or oral/poster presentations).
No information was available for the following secondary outcome measures.
Time taken to obtain unpublished studies (i.e. time from when efforts start until data are obtained).
Number of attempts (as defined by the authors) made to obtain unpublished studies (data).
Resources required.
Discussion
Despite extensive searches we identified only six studies as eligible for inclusion in this review. Of these, five were published as abstracts and one as a full paper. Due to lack of high‐quality studies the results should be interpreted with caution. Five studies, two randomised studies and three observational comparative studies evaluated different methods for obtaining missing data (e.g. data available to the original researchers but not reported in the published study). Two studies found that correspondence with study authors by e‐mail resulted in the greatest response rate with the fewest number of attempts and the shortest time to respond, when compared with correspondence by fax or letter. Combining letter and e‐mail had a higher response rate, however, it was not significantly different from using e‐mail alone. Another study found that you were more likely to solicit a response from authors whose studies were published more recently. In addition, requests for clarification of the study methods appeared to result in a greater response rate than requests for missing data about the study results.
The effect of a single request for missing information (by e‐mail or surface mail) versus a multistage approach (pre‐notification, request for missing information and active follow‐up) did not appear to affect the rate of response or the completeness of information retrieved; neither did the number of attempts made to obtain missing data or the number of items requested. Interestingly, the use of a well‐known signatory also had no significant effect on the likelihood of authors responding to a request for unpublished information. Only one study evaluated the effects of different methods to obtain unpublished data (e.g. data for studies that have never been published). This found that leg‐work ahead of time to clarify and request the specific unpublished study information required can prove to be more fruitful than sending of a non‐specific request. The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) suggests that review authors also consider contacting colleagues to find out if they are aware of any unpublished studies; we did not find any studies addressing the effectiveness of this approach.
When considering the findings from this review it is important to consider the limitations in the completeness of the available data and how this weakens the strength of any recommendations we are able to draw. The general problem that a large proportion of conference abstracts do not get published in full has been shown by others (Scherer 2007) and it was recently found that about two‐thirds of the research studies presented at Cochrane Colloquia do not get published in full (Chapman 2010). We encountered this problem in this review with five of the six studies being available only as abstracts at Colloquia. They lacked information about the study methodology and detailed results, and were never written up and published in full. Despite attempts to contact the authors of these studies we were only able to obtain additional information for one of the five studies. Ironically, our systematic review is subject to the same problems of obtaining missing data which our review is trying to address. Assessment of risk of bias was also hampered by incomplete data; the four observational studies did not report on the study methods and only one of the two randomised studies reported on the method of allocation concealment. The Brown 2003 study was designed as a comparative study, however only combined results were reported. The study is therefore reported in this review as though it was a non‐comparative study report of the experience of contacting original authors.
Missing and incomplete data continue to be a major problem and potential source of bias for those carrying out systematic reviews. If data were missing from study reports at random then there would be less information around but that missing information would not necessarily be biased. The problem is that there is considerable evidence showing that studies are more likely to be published and published more quickly if they have significant findings (Scherer 2007). Even when study results are published, there is evidence to show that authors are more likely to report significant study outcomes as opposed to non‐significant study outcomes (Kirkham 2010). The findings from our review support the current recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) that those carrying out systematic reviews should continue to contact authors for missing data, recognising that this might not always be successful, particularly for older studies. In the absence of being able to contact authors to obtain missing data, review authors should also consider the potential benefits of searching prospective clinical trial registries and trial results registers for missing data. For example, in 2007 the US government passed legislation that the findings for all US government funded research should be included on www.clinicaltrials.gov within one year of study completion, thus making available previously unpublished information. The setting up of websites for systematic review projects, listing the studies identified to date and inviting submission of information on studies not already listed (Lefebvre 2008), has also been proposed as a way of identifying unpublished studies.
Authors' conclusions
Implication for methodological research.
The strength of the evidence included in this review is limited by the completeness of the available data; five of the six studies included in this review lacked information about the study methodology and their results. Despite extensive searching only one study assessed methods for obtaining unpublished data. Further robust, comparative, well‐conducted and reported studies are needed on strategies to obtain missing and unpublished data.
Acknowledgements
We are very grateful to Julian Higgins who provided us with additional information regarding the First Contact study.
Appendices
Appendix 1. Cochrane Methodology Register search strategy
#1 ("study identification" next general) or ("study identification" next "publication bias") or ("study identification" next "prospective registration") or ("study identification" next internet) or ("data collection") or ("missing data") or ("information retrieval" next general) or ("information retrieval" next "retrieval techniques") or ("information retrieval" next "comparisons of methods"):kw in Methods Studies
#2 (request* or obtain* or identify* or locat* or find* or detect* or search or "ask for") NEAR/3 (grey or unpublished or "un published" or "not published"):ti or (request* or obtain* or identify* or locat* or find* or detect* or search or "ask for") NEAR/3 (grey or unpublished or "un published" or "not published"):ab
#3 (request* or obtain* or identify* or locat* or find* or detect* or search or "ask for") NEAR/3 (missing or missed or insufficient or incomplete or lack* or addition*):ti or (request* or obtain* or identify* or locat* or find* or detect* or search or "ask for") NEAR/3 (missing or missed or insufficient or incomplete or lack* or addition*):ab
#4 (missing or incomplete or unpublished or "un published" or "not published") NEAR/3 (data or information or study or studies or evidence or trial or trials):ti or (missing or incomplete or unpublished or "un published" or "not published") NEAR/3 (data or information or study or studies or evidence or trial or trials):ab
#5 (bad or ambiguous or insufficient or incomplete) NEAR/6 report*:ti or (bad or ambiguous or insufficient or incomplete) NEAR/3 report*:ab
#6 (#1 OR #2 OR #3 OR #4 OR #5)
Appendix 2. MEDLINE search strategy
1. ((request$ or obtain$ or identify$ or locat$ or find$ or detect$ or search or ask for) adj3 (grey or unpublished or "un published" or "not published") adj3 (data or information or evidence or study or studies or trial? or paper? or article? or report? or literature or work)).tw.
2. ((request$ or obtain$ or identify$ or locat$ or find$ or detect$ or search or ask for) adj3 (missing or insufficient or incomplete or lack$ or addition$) adj3 (data or information or evidence)).tw.
3. ((bad or ambiguous or insufficient or incomplete) adj6 reporting).tw.
4. 1 or 2 or 3
5. (2000$ or 2001$ or 2002$ or 2003$ or 2004$ or 2005$ or 2006$ or 2007$ or 2008$ or 2009$).ep.
6. 4 and 5
Appendix 3. EMBASE search strategy
1. ((request$ or obtain$ or identify$ or locat$ or find$ or detect$ or search or ask for) adj3 (grey or unpublished or "un published" or "not published") adj3 (data or information or evidence or study or studies or trial? or paper? or article? or report? or literature or work)).tw.
2. ((request$ or obtain$ or identify$ or locat$ or find$ or detect$ or search or ask for) adj3 (missing or insufficient or incomplete or lack$ or addition$) adj3 (data or information or evidence)).tw.
3. ((bad or ambiguous or insufficient or incomplete) adj6 reporting).tw.
4. 1 or 2 or 3
5. (2004$ or 2005$ or 2006$ or 2007$ or 2008$ or 2009$).em.
6. 4 and 5
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2003.
| Methods | This was a non‐randomised comparative study. Within the context of 4 systematic reviews on the prevention of NSAID‐induced gastro‐intestinal toxicity trial authors were contacted by e‐mail (preferentially) or letter, providing a semi‐personalised information retrieval sheet. | |
| Data | Context of 4 systematic reviews on the prevention of NSAID‐induced gastro‐intestinal toxicity; 112 authors (of 139 studies) were contacted | |
| Comparisons | E‐mail (n = 39) versus letter (n = 73) providing a semi‐personalised information retrieval sheet. However, the results were not presented separately for each approach. | |
| Outcomes | Additional information retrieved through contact with trial authors Costs incurred |
|
| Notes | This study was published as an abstract | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | Non‐randomised comparison therefore not applicable |
| Allocation sequence generation? | Unclear | Non‐randomised comparison therefore not applicable |
| How allocation occurred? | Unclear | Not reported |
| Attempts to balance groups? | Unclear | Not reported |
| Use of blinding? | Unclear | Not reported |
Gibson 2006.
| Methods | This was a non‐randomised comparative study. The mode of contact and response levels of authors who had been asked to provide missing or incomplete data for a systematic review on diet and exercise interventions for weight loss was examined. | |
| Data | A systematic review on diet and exercise interventions for weight loss | |
| Comparisons | E‐mail versus letter versus both (total n = 146; sample size per study arm not reported) | |
| Outcomes | Proportion of responders over time among the different modes of contact Response rates from United States compared to other countries |
|
| Notes | This study was published as a full‐text paper | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | Non‐randomised comparison therefore not applicable |
| Allocation sequence generation? | Unclear | Non‐randomised comparison therefore not applicable |
| How allocation occurred? | Unclear | Not reported |
| Attempts to balance groups? | Unclear | Not reported |
| Use of blinding? | Unclear | Not reported |
Guevara 2005.
| Methods | This was a non‐randomised comparative study. As part of a Cochrane Review comparing the effects of inhaled corticosteroids to cromolyn, authors of all included trials were contacted to clarify methods and/or to obtain missing outcome data. Authors listed as corresponding authors were contacted by
Remaining authors were contacted if there was no response by the corresponding author |
|
| Data | Cochrane Review comparing the effects of inhaled corticosteroids to cromolyn; study authors of all 25 included trials were contacted to clarify methods and/or to obtain missing outcome data | |
| Comparisons | E‐mail versus letter versus fax (total n = 25; sample size per study arm not reported) | |
| Outcomes | Response rate Time to response |
|
| Notes | This study was published as an abstract | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | Non‐randomised comparison therefore not applicable |
| Allocation sequence generation? | Unclear | Non‐randomised comparison therefore not applicable |
| How allocation occurred? | Unclear | Unclear |
| Attempts to balance groups? | Unclear | E‐mail was the preferred method of contact |
| Use of blinding? | No | No blinding |
Higgins 1999.
| Methods | This was a randomised comparison. Contact persons or authors (primary investigators) of published studies were eligible for the study if (i) the study had been identified as probably or definitely fulfilling the criteria for inclusion in a Cochrane Review, (ii) any information needed to complete the systematic review was missing from the published report, and (iii) a postal, or e‐mail, address was available for them. The reviewers should have completed assessment of studies for inclusion in the review and any data extraction. | |
| Data | Randomised trial of Cochrane Review authors where the reviewer was uncertain how first contact should be made with the investigator of a primary study which was included in the Cochrane Review in order to obtain missing information | |
| Comparisons | Single request for missing information (by e‐mail or surface mail) (n = 116) versus multistage approach involving pre‐notification, request for missing information and active follow‐up (n = 117) | |
| Outcomes |
Primary outcome: Amount of missing information retrieved from the investigator within 12 weeks of sending the original letter. A 4‐point ordinal scale was used:
Secondary outcomes: The time taken to receive some or all of the requested information Any response or acknowledgement from the investigator or someone else involved with the study or its data Cost, in terms of postage and telephone call time |
|
| Notes | This study was called 'First Contact' and was published as an abstract. The study's website is available at http://www.mrc‐bsu.cam.ac.uk/firstcontact/index.html Additional information was obtained from study authors |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | Central randomisation with minimisation to attempt to balance confounders |
| Allocation sequence generation? | Unclear | Central randomisation |
| How allocation occurred? | Unclear | Randomised comparison therefore not applicable |
| Attempts to balance groups? | Unclear | Randomised comparison therefore not applicable |
| Use of blinding? | Unclear | Not reported |
Milton 2001.
| Methods | This was a randomised comparison. Authors of eligible RCTs of interventions for essential hypertension published since 1996 and forming part of a methodological systematic review were randomised to receive a mailed questionnaire with a cover letter signed by Richard Smith (RS) or Julie Milton (JM), on stationery appropriate to each. After 3 weeks non‐responders were sent a questionnaire by recorded mail, with the same signatory. After a further 5 weeks, JM attempted to telephone non‐responders, telling authors randomised to RS as signatory that she was calling on his behalf. | |
| Data | Authors of 144 eligible RCTs of interventions for essential hypertension published since 1996 and forming part of a methodological systematic review | |
| Comparisons | Using a well know signatory (n = 96) versus an unknown researcher (n = 48) on the cover letter of a mailed questionnaire | |
| Outcomes | Response of clinical trial investigators to requests for information | |
| Notes | This study was published as an abstract | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | Allocation was performed by an independent statistician |
| Allocation sequence generation? | Unclear | Not reported |
| How allocation occurred? | Unclear | Randomised comparison therefore not applicable |
| Attempts to balance groups? | Unclear | Randomised comparison therefore not applicable |
| Use of blinding? | Unclear | Not reported |
Shukla 2003.
| Methods | This was a non‐randomised comparative study. Over 4 years, for each of 5 systematic reviews of drugs at CCOHTA, 2 different approaches were used to seek unpublished information from the drug industry. With the first approach, a general request letter was sent. With the second approach, unpublished studies were identified ahead of time via handsearching of conference abstracts, review articles and bibliographies of included studies plus electronic searches of BIOSIS Previews. A Google search was also run. Unpublished studies were identified and industry was asked to provide further specific detail. | |
| Data | Five systematic reviews of drugs at CCOHTA. Number of trials not reported. | |
| Comparisons | Identifying unpublished studies ahead of time and then asking industry to provide further specific detail versus general request by letter for unpublished studies | |
| Outcomes | Unpublished information obtained from the drug industry | |
| Notes | This study was published as an abstract | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | Non‐randomised comparison therefore not applicable |
| Allocation sequence generation? | Unclear | Non‐randomised comparison therefore not applicable |
| How allocation occurred? | Unclear | Not reported |
| Attempts to balance groups? | Unclear | Not reported |
| Use of blinding? | Unclear | Not reported |
CCOHTA: Canadian Co‐ordinating Office for Health Technology Assessment NSAID: non‐steroidal anti‐inflammatory drug RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bohlius 2003 | Study that looked at contacting authors to obtain missing data. It was excluded because there was no comparison of different methods of obtaining missing data. |
| Eysenbach 2001 | Study that looked at use of the internet to identify unpublished studies. It was excluded because there was no comparison of different methods of obtaining unpublished studies. |
| Hadhazy 1999 | Study that looked at contacting authors to obtain missing data. It was excluded because there was no comparison of different methods of obtaining missing data. |
| Hetherington 1987 | Study that looked at surveying content experts to identify unpublished studies. It was excluded because there was no comparison of different methods of obtaining unpublished studies. |
| Kelly 2002 | This study contacted authors of studies for individual patient data using letters. It was excluded because there was no comparison of different methods of obtaining missing data. |
| Kelly 2004 | Study that looked at contacting authors to obtain missing data. It was excluded because there was no comparison of different methods of obtaining missing data. |
| McGrath 1998 | Study that looked at contacting authors to obtain missing data. It was excluded because there was no comparison of different methods of obtaining missing data. |
| Reveiz 2004 | Study that looked at surveying content experts to identify unpublished studies. It was excluded because there was no comparison of different methods of obtaining unpublished studies. |
| Wille‐Jorgensen 2001 | Primary authors and/or sponsoring pharmaceutical companies of studies in general surgery which might contain colorectal patients were contacted per mail, e‐mail and/or personal contacts. The responses from the 3 methods were not compared. |
Contributions of authors
Taryn Young (TY) developed and Sally Hopewell (SH) provided comments on the protocol. Both authors reviewed the search results, selected potential studies for inclusion, worked independently to do a formal eligibility assessment and then extracted data from included studies. TY drafted the review with input from SH.
Sources of support
Internal sources
South African Cochrane Centre, South Africa.
UK Cochrane Centre, NHS Research & Development Programme, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Brown 2003 {published data only}
- Brown T, Hooper L. Effectiveness of brief contact with authors. XI Cochrane Colloquium: Evidence, Health Care and Culture; 2003 Oct 26‐31; Barcelona, Spain. 2003.
Gibson 2006 {published data only}
- Gibson CA, Bailey BW, Carper MJ, Lecheminant JD, Kirk EP, Huang G, et al. Author contacts for retrieval of data for a meta‐analysis on exercise and diet restriction. International Journal of Technology Assessment in Health Care 2006;22(2):267‐70. [DOI] [PubMed] [Google Scholar]
Guevara 2005 {published data only}
- Guevara J, Keren R, Nihtianova S, Zorc J. How do authors respond to written requests for additional information?. XIII Cochrane Colloquium; 2005 Oct 22‐26; Melbourne, Australia. 2005.
Higgins 1999 {published data only}
- Higgins J, Soornro M, Roberts I, Clarke M. Collecting unpublished data for systematic reviews: a proposal for a randomised trial. 7th Annual Cochrane Colloquium Abstracts, October 1999 in Rome. 1999.
Milton 2001 {published data only}
- Milton J, Logan S, Gilbert R. Well‐known signatory does not affect response to a request for information from authors of clinical trials: a randomised controlled trial. 9th Annual Cochrane Colloquium Abstracts, October 2001 in Lyon. 2001.
Shukla 2003 {published data only}
- Shukla V. The challenge of obtaining unpublished information from the drug industry. XI Cochrane Colloquium: Evidence, Health Care and Culture; 2003 Oct 26‐31; Barcelona, Spain. 2003.
References to studies excluded from this review
Bohlius 2003 {published data only}
- Bohlius J, Langensiepen S, Engert A. Data hunting: a case report. XI Cochrane Colloquium: Evidence, Health Care and Culture; 2003 Oct 26‐31; Barcelona, Spain. 2003.
Eysenbach 2001 {published data only}
- Eysenbach G, Tuische J, Diepgen TL. Evaluation of the usefulness of Internet searches to identify unpublished clinical trials for systematic reviews. Medical Informatics and the Internet in Medicine 2001;26(3):203‐18. [DOI] [PubMed] [Google Scholar]
- Eysenbach G, Tuische J, Diepgen TL. Evaluation of the usefulness of internet searches to identify unpublished clinical trials for systematic reviews. Chinese Journal of Evidence‐Based Medicine 2002;2(3):196‐200. [DOI] [PubMed] [Google Scholar]
Hadhazy 1999 {published data only}
- Hadhazy V, Ezzo J, Berman B. How valuable is effort to contact authors to obtain missing data in systematic reviews. 7th Annual Cochrane Colloquium Abstracts, October 1999 in Rome. 1999.
Hetherington 1987 {published data only}
- Hetherington J. An international survey to identify unpublished and ongoing perinatal trials [abstract]. Controlled Clinical Trials 1987;8:287. [Google Scholar]
- Hetherington J, Dickersin K, Chalmers I, Meinert CL. Retrospective and prospective identification of unpublished controlled trials: lessons from a survey of obstetricians and pediatricians. Pediatrics 1989;84(2):374‐80. [PubMed] [Google Scholar]
Kelly 2002 {published data only}
- Kelley GA, Kelley KS, Tran ZV. Retrieval of individual patient data for an exercise‐related meta‐analysis. Medicine & Science in Sports & Exercise 2002;34(5 (Suppl 1)):S225. [Google Scholar]
Kelly 2004 {published data only}
- Kelley GA, Kelley KS, Tran ZV. Retrieval of missing data for meta‐analysis: a practical example. International Journal of Technology Assessment in Health Care 2004;20(3):296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrath 1998 {published data only}
- McGrath J, Davies G, Soares K. Writing to authors of systematic reviews elicited further data in 17% of cases. BMJ 1998;316:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reveiz 2004 {published data only}
- Reveiz L, Andres Felipe C, Egdar Guillermo O. Using e‐mail for identifying unpublished and ongoing clinical trials and those published in non‐indexed journals. 12th Cochrane Colloquium: Bridging the Gaps; 2004 Oct 2‐6; Ottawa, Ontario, Canada. 2004.
- Reveiz L, Cardona AF, Ospina EG, Agular S. An e‐mail survey identified unpublished studies for systematic reviews. Journal of Clinical Epidemiology 2006;59(7):755‐8. [DOI] [PubMed] [Google Scholar]
Wille‐Jorgensen 2001 {published data only}
- Wille‐Jorgensen. Problems with retrieving original data: is it a selection bias?. 9th Annual Cochrane Colloquium, Lyon. October 2001.
Additional references
Chapman 2010
- Chapman S, Eisinga A, Clarke MJ, Hopewell S. Passport to publication? Do methodologists publish after Cochrane Colloquia?. Joint Cochrane and Campbell Colloquium. 2010 Oct 18‐22; Keystone, Colorado, USA. Cochrane Database of Systematic Reviews, Supplement 2010; Suppl: 14.
Greenhalgh 2005
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 205;331(7524):1064‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hopewell 2007
- Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta‐analyses of randomized trials of health care interventions. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000010.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.MR000026.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smith R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Scherer 2007
- Scherer RW, Langenberg P, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000005.pub3] [DOI] [PubMed] [Google Scholar]
Smith 1998
- Smith GD, Egger M. Meta‐analysis. Unresolved issues and future developments. BMJ 1998;316(7126):221‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2000
- Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technology Assessment 2000;4(10):1‐115. [PubMed] [Google Scholar]
Sterne 2008
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.


