Abstract
Background
Subfertility affects 15% to 20% of couples trying to conceive. In vitro fertilisation (IVF) is one of the assisted reproduction techniques developed to improve chances of achieving pregnancy. In the standard IVF method with controlled ovarian hyperstimulation (COH), growth and development of multiple follicles are stimulated by using gonadotrophins, often combined with a gonadotrophin‐releasing hormone (GnRH) agonist or antagonist. Although it is an established method of conception for subfertile couples, the treatment is expensive and has a high risk of adverse effects. Studies have shown that IVF in a natural cycle (NC) or a modified natural cycle (MNC) might be a promising low risk and low cost alternative to the standard stimulated IVF treatment since the available dominant follicle of each cycle is used. In this review, we included available randomised controlled studies comparing natural cycle IVF (NC and MNC) with standard IVF.
Objectives
To compare the efficacy and safety of natural cycle IVF (including both NC‐IVF and MNC‐IVF) with controlled ovarian hyperstimulation IVF (COH‐IVF) in subfertile couples.
Search methods
An extended search including of the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, ClinicalTrials.gov, conference abstracts in the Web of Knowledge, the World Health Organization International Trials Registry Platform search portal, LILACS database, PubMed and the OpenSIGLE database was conducted according to Cochrane guidelines. The last search was on 31st July 2013.
Selection criteria
All randomised controlled trials (RCTs) comparing either natural cycle IVF or modified natural cycle IVF versus standard IVF in subfertile couples were included.
Data collection and analysis
Data selection and extraction and risk of bias assessment were carried out independently by two authors (TA and AC). The primary outcome measures were live birth rate and ovarian hyperstimulation syndrome (OHSS) rate per randomised woman. We calculated Mantel‐Haenszel odds ratios for each dichotomous outcome and either the mean difference or the standardised mean difference (SMD) for continuous outcomes, with 95% confidence intervals (CIs). A fixed effect model was used unless there was substantial heterogeneity, in which case a random effects model was used.
Main results
Six randomised controlled trials with a total of 788 women were included. The largest of these trials included 396 women eligible for this review.
No evidence of a statistically significant difference was found between natural cycle and standard IVF in live birth rates (OR 0.68, 95% CI 0.46 to 1.01, two studies, 425 women, I2= 0%, moderate quality evidence). The evidence suggests that for a woman with a 53% chance of live birth using standard IVF, the chance using natural cycle IVF would range from 34% to 53%. There was no evidence of a statistically significant difference between natural cycle and standard IVF in rates of OHSS (OR 0.19, 95% CI 0.01 to 4.06, one study, 60 women, very low quality evidence), clinical pregnancy (OR 0.52 95% CI 0.17 to 1.61, 4 studies, 351 women, I2=63%, low quality evidence), ongoing pregnancy (OR 0.72, 95% CI 0.50 to 1.05, three studies, 485 women, I2=0%, moderate quality evidence), multiple pregnancy (OR 0.76, 95% CI 0.25 to 2.31, 2 studies, 527 women, I2=0%, very low quality evidence), gestational abnormalities (OR 0.44 95% CI 0.03 to 5.93, 1 study, 18 women, very low quality evidence) or cycle cancellations (OR 8.98, 95% CI 0.20 to 393.66, 2 studies, 159 women, I2=83%, very low quality evidence). One trial reported that the oocyte retrieval rate was significantly lower in the natural cycle group (MD ‐4.40, 95% CI ‐7.87 to ‐0.93, 60 women, very low quality evidence). There were insufficient data to draw any conclusions about rates of treatment cancellation. Findings on treatment costs were inconsistent and more data are awaited. The evidence was limited by imprecision. Findings for pregnancy rate and for cycle cancellation were sensitive to the choice of statistical model: for these outcomes, use of a fixed effect model suggested a benefit for the standard IVF group. Moreover the largest trial has not yet completed follow up, though data have been reported for over 95% of women.
Authors' conclusions
Further evidence from well conducted large trials is awaited on natural cycle IVF treatment. Future trials should compare natural cycle IVF with standard IVF. Outcomes should include cumulative live birth and pregnancy rates, the number of treatment cycles necessary to reach live birth, treatment costs and adverse effects.
Keywords: Female; Humans; Pregnancy; Pregnancy Rate; Fertilization in Vitro; Fertilization in Vitro/methods; Fertilization in Vitro/statistics & numerical data; Infertility, Female; Infertility, Female/therapy; Live Birth; Live Birth/epidemiology; Menstrual Cycle; Menstrual Cycle/physiology; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/epidemiology; Ovulation Induction; Ovulation Induction/methods; Patient Compliance; Patient Compliance/statistics & numerical data; Randomized Controlled Trials as Topic
Plain language summary
Natural cycle in vitro fertilisation for subfertile couples
Review question: To determine whether in vitro fertilisation (IVF) in a natural cycle is a good alternative to standard IVF for subfertile couples.
Background: Assisted reproduction techniques such as IVF can help subfertile women to achieve a pregnancy. In IVF, an egg is fertilised in a laboratory and placed back in the woman's uterus. Different IVF protocols have been developed since the first IVF in 1978 including natural cycle IVF (without hyperstimulation of the ovaries), modified natural cycle IVF (with low dose ovarian hyperstimulation) and IVF with controlled ovarian hyperstimulation. The aim of this systematic review was to assess the efficacy and safety of natural cycle IVF and modified natural cycle IVF compared with controlled ovarian hyperstimulation IVF in subfertile couples.
Study characteristics: Six trials were included, with a total of 788 women undergoing an IVF treatment. The evidence is current to 31st July 2013. The largest trial in the review (with 396 women) has not yet reported full results.
Key points: The evidence suggested that for a woman with a 53% chance of live birth using standard IVF, the chance using natural cycle IVF ranges from 34% to 53%. No significant difference was found in rates of clinical pregnancy, ongoing pregnancy, multiple pregnancy, incidence of ovarian hyperstimulation syndrome, gestational abnormalities or cancellations of treatment. However findings were imprecise for all outcomes and further evidence from larger studies is awaited. There was evidence from single studies that a lower number of oocytes was retrieved in the natural cycle group. Findings on cost‐effectiveness were inconsistent.
Quality of evidence: Quality ratings for the evidence ranged from very low to moderate, the main limitation being imprecision due to insufficient data. When the review authors checked the effect of using an alternative method of analysis the findings suggested higher rates of clinical pregnancy with standard IVF than with natural cycle IVF.
Summary of findings
Summary of findings for the main comparison. Natural cycle IVF versus standard IVF for subfertile couples.
| DRAFT Natural cycle IVF versus standard IVF for subfertile couples | ||||||
| Patient or population: Subfertile couples Settings: Assisted reproductive technology Intervention: Natural cycle IVF versus standard IVF | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard IVF | Natural cycle IVF | |||||
| Live birth per woman | 530 per 1000 | 434 per 1000 (342 to 532) | OR 0.68 (0.46 to 1.01) | 425 (2 studies) | ⊕⊕⊕⊝ Moderate1 |
|
| OHSS per woman | 67 per 1000 | 13 per 1000 (1 to 225) | OR 0.19 (0.01 to 4.06) | 60 (1) | ⊕⊝⊝⊝ Very low2 |
|
| Clinical pregnancy per woman | 207 per 1000 | 119 per 1000 (42 to 295) | OR 0.52 (0.17 to 1.61) | 351 (4 studies) | ⊕⊕⊝⊝ Low1, 2, 3,4 | |
| Ongoing pregnancy per woman | 494 per 1000 | 416 per 1000 (328 to 508) | OR 0.72 (0.5 to 1.05) | 485 (3 studies) | ⊕⊕⊕⊝ Moderate1,2 |
|
| Multiple pregnancy per woman | 26 per 1000 |
20 per 1000 (7 to 58) |
OR 0.76 (0.25 to 2.31) |
527 (2 studies) |
⊕⊝⊝⊝ Very low2 |
|
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Serious imprecision: confidence intervals compatible with no difference between the interventions or with substantial benefit from standard IVF 2 Very serious imprecision, did not describe methods of allocation concealment or sequence generation in all cases 3 High risk of attrition bias in one study
4 Substantial inconsistency (I2=63%), findings sensitive to choice of statistical model
Background
Description of the condition
Subfertility is defined as not achieving pregnancy after a period of 12 months of intercourse with the same partner without contraception. Subfertility affects 15% to 20% of couples trying to conceive (Evers 2002; Heineman 2011). Assisted reproduction techniques (ART) have been developed to improve the chance of achieving pregnancy. In vitro fertilisation (IVF) is one approach, where an oocyte and spermatozoa are merged in a laboratory setting before being implanted in the uterus. Although initially IVF was used mostly for women with tubal subfertility (Leeton 1982), the indications were soon expanded to include couples with menstrual cycle disorders, tubal abnormalities and male subfertility as well unexplained subfertility (Heineman 2011). For IVF, stimulating follicle growth and retrieval of the oocytes are necessary, for which several different methods are used. The first successful IVF treatment was performed in 1978 in an unstimulated natural cycle. Although pregnancies did occur with early natural cycle IVF (NC‐IVF), the success rates were low secondary to luteinising hormone (LH) surges which induced ovulation and resulted in cancellations (Rongieres‐Bertrand 1999). The introduction of controlled ovarian hyperstimulation (COH) IVF (COH‐IVF) led to it becoming the standard ovarian stimulation method because of the improved success rates (Pelinck 2009).
Although COH‐IVF increases pregnancy rates, it also meant an increase in costs and complications, mainly due to ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies. Up to 10 or more oocytes could be retrieved, however the oocyte best suited for fertilisation based on morphology could be selected, which may have improved the success rate of the treatment (Rosen 2008; Wang 2011). Other technical improvements such as cryopreservation and vitrification (Geraedts 2012) have meant that oocytes could be preserved so that women do not have to repeat the full COH treatment when implantation of a fresh embryo fails. Initially IVF had live birth rates of less than 16% per transfer (Naaktgeboren 1985), but now most clinics are reporting live birth rates of 20% to 25% per started cycle for women under the age of 40 years (Heineman 2011).
With the development of gonadotrophin‐releasing hormone (GnRH) antagonists, a new IVF treatment was developed, known as modified natural cycle IVF (MNC‐IVF), with fewer complications and risks compared to COH protocols (Rongieres‐Bertrand 1999). Because of this improvement in ovarian stimulation and also improved laboratory techniques such as the culture media, NC‐IVF has again been considered as an option. However, more MNC or NC treatments are likely to be necessary in order to obtain pregnancy rates comparable to COH‐IVF (Pelinck 2009). Overall, the treatment costs might be lower in NC‐IVF and MNC‐IVF compared to COH‐IVF, but it may cost the woman more effort to reach pregnancy because of the lower pregnancy rate per treatment and the need to repeat treatment cycles. On the other hand, the side effects of the hormone treatment and the emotional distress of stimulated IVF are often perceived as unacceptable and people seem to prefer the simplicity and short duration of a low stimulation treatment (Hojgaard 2001; Verberg 2008).
Description of the intervention
In both NC‐IVF and MNC‐IVF, the treatment cycles of women with a normal menstrual function are monitored in order to measure the follicle structure and endometrial morphology. When the follicle reaches an estimated size of 15 to 20 mm, human chorionic gonadotrophin (hCG) is administered intramuscularly and final maturation of the oocyte is thereby induced (Nargund 2001). The oocyte is then retrieved by aspirating the follicle under vaginal ultrasound guidance.
The potential advantages of both NC‐IVF and MNC‐IVF are the following:
the almost complete absence of multiple pregnancies;
the very low risk of ovarian hyperstimulation syndrome;
the reduced length of stimulation;
the reduction in both physical and emotional stress as ovarian stimulation is not used or only used as a very low‐dose protocol;
the reduced costs;
no resting cycle is needed following a failed cycle (Pelinck 2002).
Cryopreservation after MNC‐IVF and NC‐IVF is generally not possible, so there are no embryos available for freezing. Therefore NC‐IVF and MNC‐IVF may be preferable for couples who object to embryo freezing for cultural or religious reasons, or where cryopreservation of embryos is illegal.
The potential disadvantages of NC‐IVF include:
a higher cancellation rate (due to premature LH surges);
the lowered chances of a live birth per started cycle;
the lowered chances of embryo transfer after a thawed cycle.
Natural cycle IVF mimics the body's natural processes, such as alterations to the endometrium in preparation for implantation. The treatment is physically less demanding than the COH treatment, and usually no resting cycle is necessary after a failed treatment. The treatment can therefore be repeated in the following cycle. However, because only one oocyte is retrieved, and therefore only one embryo is implanted, the pregnancy rates per woman per cycle are low at 6% to 7% (Pelinck 2002; Zayed 1997).
How the intervention might work
There are two types of natural cycle IVF.
1. Natural cycle‐IVF (NC‐IVF)
In NC‐IVF no drugs are administered
From the moment the follicle approaches maturity (follicle size 10 mm approximately), the oocyte is monitored and the retrieval date is planned
Ovulation triggering with hCG administration is given when the follicle size is 15 to 20 mm or when the serum estradiol rises, or both (Pelinck 2009)
In the case of LH surge (measured in urine), either cancellation or advancement of oocyte retrieval occurs (Zayed 1997)
2. Modified natural cycle‐IVF (MNC‐IVF)
In MNC‐IVF gonadotrophin protocols are used to stimulate follicular growth. Different protocols start administering follicle stimulating hormone (FSH) at different stages in the cycle but all protocols use a similar short stimulation period of two to six days
After up to six days of ovarian stimulation or when the largest follicle reaches a diameter of 14 mm (number of days varies according to differing protocols), then GnRH antagonists are administered to suppress LH secretion (in order to prevent premature ovulation)
When the leading follicle reaches a size of at least 15 to 20 mm, ovulation is triggered in the same manner as in COH‐IVF but only one oocyte is fully grown and retrieved
Regardless of the immediate pituitary recovery after discontinuing the GnRH antagonist, luteal phase support improves pregnancy rates for MNC‐IVF (Chavez‐Badiola 2011).
Both MNC‐IVF and NC‐IVF have oocyte retrieval performed in the same manner as COH‐IVF; that is, with vaginal ultrasound and usually under mild sedation.
In COH‐IVF, FSH is administered to stimulate the growth of five to 15 follicles. To prevent early oocyte maturation caused by premature LH production, a GnRH agonist is used for suppressing the pituitary release of both LH and FSH. Down‐regulation by continuous administration first causes LH and FSH hypersecretion followed by depletion of the pituitary store and desensitisation after approximately 10 days.
In the 'long protocol' down‐regulation begins in the cycle prior to the treatment cycle.
In the 'short' or 'flare‐up protocol' the GnRH agonist is administered from day one of the treatment cycle.
In the 'ultrashort protocol' only three doses of the agonist are used (Elder 2011).
As an alternative to the GnRH agonist, a GnRH antagonist can be used to prevent the LH surge in COH. The antagonist binds to and immediately blocks receptors in the pituitary, directly inhibiting the release of gonadotrophins. Different protocols for GnRH antagonist administration using different doses are used, varying from multiple‐dose fixed (0.25 mg daily from day six to seven of stimulation) to single‐dose (single administration of 3 mg on day seven to eight of stimulation) (Al‐Inany 2011).
When two or more follicles reach a size of 18 to 20 mm (Heineman 2011), hCG is administered for the final maturation. Finally, 34 to 36 hours after hCG administration, the oocyte retrieval procedure is performed. This is done transvaginally under vaginal ultrasound guidance and usually with mild sedation.
All of these IVF techniques aim to retrieve one or more oocytes suitable for fertilisation.
Why it is important to do this review
When choosing between different IVF protocols, couples need to balance the benefits and harms. Standard protocol IVF is thought to be associated with higher birth rates than the natural cycle treatments, but it is closely linked with complications such as OHSS and multiple pregnancies. Evidence from studies has suggested that natural cycle IVF is a low‐risk, low‐cost (to the patient) and patient‐friendly procedure, although results have often been based on small study populations. Furthermore, studies comparing NC‐IVF, MNC‐IVF and COH‐IVF report different outcomes. Based on previous studies, natural cycle IVF seems a low‐risk and low‐cost procedure, preferred by women and physicians (Pelinck 2009; Pistorius 2006; Reyftmann 2007). This review evaluated the evidence from randomised controlled trials on NC‐IVF, MNC‐IVF and COH‐IVF.
Objectives
To compare the efficacy and safety of natural cycle IVF (including both NC‐IVF and MNC‐IVF) with controlled ovarian hyperstimulation IVF (COH‐IVF) in subfertile couples.
Methods
Criteria for considering studies for this review
Types of studies
In this review we included only truly randomised controlled trials (RCTs) comparing natural cycle IVF with COH‐IVF. In this review, natural cycle IVF included both NC‐IVF and MNC‐IVF. Crossover trials were included but only the data from the first phase were included in meta‐analyses.
Types of participants
Inclusion criteria
No age restriction
Subfertile women and couples undertaking an IVF treatment
Both male and female factor subfertility
Both nulliparous and multiparous women
With or without a previous IVF treatment
Exclusion criteria
Donor oocytes
Frozen embryo transfer
Animal studies
Types of interventions
1. All trials comparing either NC‐IVF or MNC‐IVF with COH‐IVF were eligible for inclusion.
2. All trials comparing different protocols of MNC‐IVF were also eligible for inclusion.
Types of outcome measures
Primary outcomes
Effectiveness: live birth rate per woman, defined as the delivery of one or more living fetuses after 20 completed weeks of gestation.
Adverse effect: ovarian hyperstimulation syndrome (OHSS) per woman.
Secondary outcomes
Effectiveness:
pregnancy rate per woman defined as the successful implantation of a fetus, confirmed by the visualisation of a gestational sac. Cumulative measures will be preferred
ongoing pregnancies per woman, defined as the confirmed presence of a gestational sac and a fetal heart beat after 12 weeks gestation;
number of oocytes retrieved per woman;
time from start of treatment to live birth;
number of cycles required to conceive per woman
Adverse effects:
multiple pregnancies per woman
lack of embryos for cryopreservation;
cycle cancellation rates per woman;
gestational abnormalities (ectopic pregnancy, fetal growth disorders, preterm births and miscarriages) per woman;
cancellation of the treatment, due to patient motivation or adverse effects;
cost effectiveness, evaluating the total costs to reach pregnancy in the different IVF treatments.
Cumulative measures of effectiveness were preferred, due to the difference in number of oocytes retrieved, and because a COH‐IVF treatment is a much greater burden for the patient and therefore cannot be repeated as often as a natural cycle IVF treatment.
Search methods for identification of studies
We searched for all published and unpublished randomised controlled trials, studying either NC‐IVF or MNC‐IVF versus COH‐IVF. We used the following search strategies, in consultation with the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator. We applied no language restrictions.
Electronic searches
We searched the following databases to 31 July 2013:
Menstrual Disorders and Subfertility Group Specialised Register (MDSG) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL) (Ovid) (Appendix 2);
MEDLINE® In‐Process & Other Non‐Indexed Citations, MEDLINE® Daily and MEDLINE® (Ovid) (Appendix 3);
EMBASE (Ovid) (Appendix 4);
PsycINFO (Ovid) (Appendix 5);
CINAHL (EBSCOhost) Appendix 6).
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials: 'ClinicalTrials.gov', a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home) and World Health Organization International Clinical Trials Registry Platform search portal (http://www.who.int/trialsearch/Default.aspx) (Appendix 7).
Conference abstracts in the Web of Knowledge (http://wokinfo.com/) (Appendix 8).
LILACS database as a source of trials from the Portuguese and Spanish‐speaking world (htpp://regional.bvsalud.org/php/index.php?lang=en) (choose ’LILACS’ in ’all sources’ drop‐down box).
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) (Appendix 9).
OpenSIGLE database for grey literature from Europe (http://opensigle.inist.fr/).
We used EndNote to manage the search results. The MEDLINE randomised controlled trial filter was the Cochrane highly sensitive search strategy for identifying randomised controlled trials, which is found in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), whereas the EMBASE filter has been developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Searching other resources
In order to obtain additional relevant data, we examined reference lists of eligible articles and contacted the study authors where necessary. Professor Cindy Farquhar and Dr Astrid Cantineau acted as experts on different IVF treatments, and we requested additional information about unpublished trials from the authors. We handsearched non‐indexed journals in collaboration with the Cochrane Menstrual Disorders and Subfertility Group Trials Search Co‐ordinator.
Data collection and analysis
Selection of studies
TA and AC independently scanned the titles and abstracts of the articles retrieved by the search. Those judged to be irrelevant were removed while the full texts of potentially eligible articles were retrieved and independently examined by the two authors. They assessed the full‐text articles according to the inclusion criteria and selected those eligible for inclusion in the review. Any doubts or disagreements regarding the inclusion of an article were discussed with CF in order to reach an acceptable compromise.
Data extraction and management
The authors designed and pilot tested a data extraction form. We included the following characteristics of included studies in the extraction form: methods, participants, interventions and outcomes. Both authors trained with the extraction form using a representative sample of the studies to be reviewed and, in case of disagreement, achieved a consensus in consultation with a third author. Where necessary, we modified the extraction form. If studies were reported in more than one publication, we extracted data from the different reports directly into a single data extraction form so that no data would be missed.
Assessment of risk of bias in included studies
The Cochrane Collaboration’s recommended tool for assessing risk of bias is a domain‐based evaluation (Higgins 2011). Assessments were made for the following domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias.
These assessments were:
high risk of bias;
unclear risk of bias;
low risk of bias.
Measures of treatment effect
For dichotomous data (for example live birth rate, pregnancy rate, ongoing pregnancy rate, failure to freeze embryos, cycle cancellation, cancellation of treatment, OHSS, multiple pregnancies, number of cycles required to conceive, gestational abnormalities or cumulative pregnancy or live birth rate) we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). For continuous data (for example number of oocytes retrieved per woman or time from start of treatment to live birth), if all studies reported exactly the same outcomes we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scales (for example cost effectiveness) we calculated the standardised mean difference (SMD). We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% confidence intervals for all outcomes. Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available that may facilitate similar analyses of included studies (for example test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking account of legitimate differences.
Unit of analysis issues
The unit of primary analysis was per woman randomised to the intervention or control groups. For the primary analysis, we counted multiple live births as one live birth event. From the included crossover study, we only used the data up to the crossover point.
Dealing with missing data
Where relevant data were missing from one of the included studies, we tried to contact the original investigator to request the missing data. If the missing data were unobtainable, the authors determined whether the data were missing at random or not and were adjusted accordingly (Higgins 2011). The potential impact was reported in the 'Discussion' section. Where live birth was mentioned as an outcome measure but not reported in the results section, we assumed an (ongoing) pregnancy did not occur. For the secondary outcomes, we only analysed the available data. When assumptions were made, we performed sensitivity analyses to assess how sensitive results were to reasonable changes.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity and when study participants, interventions and outcomes were judged to be sufficiently similar, we conducted a meta‐analysis to provide a meaningful summary. We assessed statistical heterogeneity by visually inspecting the plot and using the I² statistic. We interpreted the results of the I² statistic according to the Higgins 2011; an I2 greater than 50% was judged to indicate substantial heterogeneity.
Assessment of reporting biases
In order to minimise the impact of reporting biases, we conducted an extensive search for eligible articles and we carefully inspected the included articles for reporting biases, such as publication bias, duplication bias or outcome reporting bias. Where possible, we compared outcomes reported in final published studies with preplanned outcomes reported in published protocols, and contacted the original investigator where necessary. We included unpublished data by searching for it or by contacting the original investigator. We did not construct a funnel plot since there were only five included trials.
Data synthesis
We used a fixed‐effect model to calculate pooled ORs and 95% CIs. If moderate to considerable heterogeneity was identified, we used a random‐effects model. Where some studies measured multiple cycles and some reported only one cycle data were pooled but were stratified according to whether multiple or single cycles were reported.
Subgroup analysis and investigation of heterogeneity
We considered clinical differences between the studies where heterogeneity was found. If more data had been available, we would have conducted subgroup analyses in the following subgroups in order to investigate heterogeneous results:
Cause of subfertility, grouped by unexplained subfertility, male factor subfertility, tubal disease and ovulation disorder.
Age, < 38 years or > 38 years.
Prior treatment, if the patient had had an IVF treatment before.
Intervention, grouped by NC‐IVF or MNC‐IVF
However, data were too few for the planned subgroup analysis to be feasible, except for type of intervention.
Sensitivity analysis
We conducted sensitivity analyses to examine whether the conclusions were affected by different assumptions, and therefore the decisions regarding the eligibility and analysis of the studies. Where possible, we analysed results to test for differences with the following adjustments:
if another analysis method (risk ratio) was used;
studies with high risk of bias were excluded;
studies with a large sample size were excluded;
the early studies of IVF (pre‐1990) were excluded, as ovulation stimulation protocols were still being developed and natural cycle IVF success rates may be lower than current natural cycles.
In cases of high heterogeneity we used the random‐effects model and compared the results with those using the fixed‐effect model;
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies.
Results of the search
The search was conducted in July 2013. Searching each database as stated in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; and Appendix 9 resulted in a total of 864 articles of which EndNote removed 247 duplicates, leaving 617 articles. After screening the title and abstract, a total of 36 appeared eligible for the review (Table 2). Seven further duplicates were removed. The remaining 29 articles were retrieved in full text or as an abstract, protocol or clinical trial report. Seventeen reports that did not meet our inclusion criteria were excluded. Of the 12 reports that met our criteria, five were abstracts or preliminary results of a published full text and one (Zhang 2013) was an ongoing study. We unsuccessfully tried to contact the author for additional information. One study was a conference presentation (Bensdorp 2013) and further data are awaited. Overall, we included six studies. See: the study flow diagram (Figure 1).
1. Number of articles.
| Initial search result | After screening | |
| CENTRAL | 151 | 16 |
| EMBASE | 127 | 2 |
| MEDLINE | 110 | 3 |
| PsycINFO | 15 | 0 |
| MDSG | 28 | 3 |
| Clinicaltrials | 114 | 2 |
| CINAHL | 7 | 1 |
| WEBOFKN | 66 | 9 |
| TOTAL | 617 | 36 |
1.
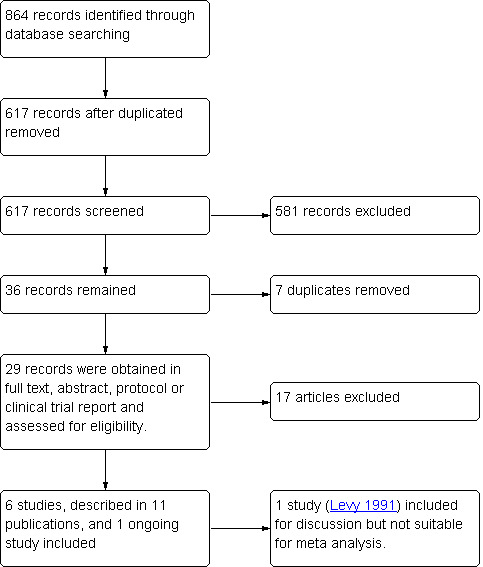
Study flow diagram.
Included studies
A total of six randomised controlled trials, described in 11 publications, were eligible for inclusion. The total number of participants was 788. The studies were conducted in China, Denmark, Italy, the United Kingdom, The Netherlands and the United States. One of the included abstracts (Levy 1991) did not report data suitable for meta‐analysis. Attempts to contact the authors for clarification were unsuccessful, so per woman data were not available. The largest study (Bensdorp 2013) accounted for over half the participants in the review (396); this study had three intervention arms of which only two are included in this review. Bensdorp 2013 has not completed follow up, but has reported preliminary findings for over 95% of women.
Participants
The studies used differing inclusion and exclusion criteria with respect to participant age, treatment indication and cause of subfertility. For details, see Characteristics of included studies. One trial (Morgia 2004) only included women who were poor responders in previous IVF cycles. This could influence their findings because poor responders are less likely to reach pregnancy in any IVF protocol.
Interventions
A variety of protocols were used in the included trials. In the natural cycles, no treatment was given in four studies (Ingerslev 2001; Levy 1991; MacDougal 1994; Morgia 2004), whereas in one study (Lou 2010) human menopausal gonadotrophin (HMG) 150 IU/day was given intramuscularly as a modified natural cycle protocol. In Bensdorp 2013 a GnRH antagonist was used and FSH was continued up to the day of ovulation triggering.
In the stimulated cycles, Ingerslev 2001 and MacDougal 1994 used clomiphene citrate. In one study (Levy 1991), ovarian hyperstimulation was started with luteal phase initiated GnRH suppression followed by HMG administration. Morgia 2004 used a GnRH agonist (0.05 mg buserelin) from the first day of the menstrual cycle in combination with 600 IU FSH (Metrodin HP, Serono, Italy) from the third day of the menstrual cycle as the stimulation protocol. In one study (Lou 2010), a GnRH agonist (triptorelin 0.1 mg/day subcutaneously) was used in combination with recombinant FSH (Gonal‐F®; Merck Sereno, Geneva, Switzerland) 150 to 300 IU/day as the stimulation protocol. In Bensdorp 2013, controlled ovarian hyperstimulation was started with 150 IU FSH. Treatment was continued until at least 2 follicles > 18mm had developed. Ovulation was induced by 10.000 IU human chorionic gonadotropin hormone (hCG).
Primary outcomes
Effectiveness: 2/6 included studies reported live birth rate per woman (Bensdorp 2013; MacDougal 1994).
Adverse effects: 1/6 included studies reported ovarian hyperstimulation syndrome (OHSS) per woman (Lou 2010).
Secondary outcomes
Effectiveness:
4/6 included studies reported clinical pregnancy rates per woman. Two reported cumulative pregnancy ( Ingerslev 2001; Morgia 2004) and two reported pregnancy after a single cycle (Lou 2010; MacDougal 1994).
3/6 included studies reported ongoing pregnancy (Bensdorp 2013, Lou 2010; MacDougal 1994);
2/6 included studies reported number of oocytes retrieved per woman (Lou 2010; MacDougal 1994). In one trial (Ingerslev 2001), the number of oocytes retrieved after multiple treatment cycles per woman was given. E‐mails were sent to the author to request the data from the first cycle only
0/6 included studies reported time from start of treatment to live birth;
Adverse effects:
2/6 included studies reported multiple pregnancies per treatment (Bensdorp 2013; MacDougal 1994)
0/6 included studies reported failure to cryopreserve embryos;
2/6 included studies reported cycle cancellation rates per woman (MacDougal 1994; Morgia 2004). Re‐analysis was required in one study (Morgia 2004) for results that were reported as percentages rather than cycle cancellations per treatment. In one trial (Ingerslev 2001), cycle cancellation rates were only reported after multiple treatment cycles per woman. E‐mails were sent to the author to request the data from only the first cycle
2/6 included studies reported gestational abnormalities (ectopic pregnancy, fetal growth disorders, preterm births and miscarriages) per woman (Lou 2010; MacDougal 1994). Data for Bensdorp 2013 are awaited.
1/6 included studies reported cancellation of the treatment (Lou 2010);
2/6 included studies reported cost effectiveness: Bensdorp 2013 reported directed medical costs per ongoing pregnancy and Lou 2010 reported the sum of the treatment medication in yuan. Data for Bensdorp 2013 on cost per birth of healthy singleton are awaited.
Excluded studies
Seventeen studies were excluded from the analysis. For details, see Characteristics of excluded studies. Of these studies, 10 were not truly randomised controlled trials (Adams 2004; Bassil 1999; Groenewoud 2012; Hojgaard 2001; Jancar 2009; Lee 2008; Paulson 1990; Pistorius 2006; Reyftmann 2007; Schimberni 2011). One study (Belaid 2005) compared assisted hatching versus no assisted hatching. Four studies (Karimzadeh 2012; Kim 2009; Rama Devi 2011; Strohmer 1997) compared two different controlled ovarian hyperstimulation protocols for IVF. One study included females of proven fertility for an egg donation program (Mirkin 2004) and one study (Vidal 2013) compared interventions in endometrial preparation for oocyte donation rather than in IVF.
Risk of bias in included studies
The risk of bias of the included trials was judged (see Characteristics of included studies). For details, see Figure 2; Figure 3. We tried to contact authors of the included studies for additional data on allocation, incomplete outcome data and selective reporting. We received additional information from the authors of two trials (MacDougal 1994; Morgia 2004), but for three studies (Ingerslev 2001; Levy 1991; Lou 2010) we failed to receive any information.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
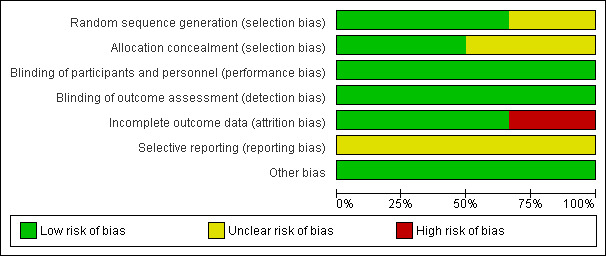
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Four of the six included trials generated the random sequence by computer or internet and were rated as at low risk of bias in this domain. Two of the studies did not describe what method was used for sequence generation and were rated as at unclear risk of bias.
Allocation concealment
One trial (Bensdorp 2013) used remote allocation and was rated as at low risk of bias. Of the other five included trials, three reported the use of envelopes (Ingerslev 2001; MacDougal 1994; Morgia 2004). After contacting the authors, two confirmed that the envelopes were numbered and opaque (MacDougal 1994; Morgia 2004). One trial (Ingerslev 2001) stated that the envelopes were sealed. Attempts were made to contact the authors for specification. In two trials (Levy 1991; Lou 2010) the method of allocation concealment was not stated and therefore was judged as unclear of bias. Attempts were made to contact the authors.
Blinding
We judged that the lack of blinding would only affect the cancellation of the treatment due to patient motivation. Because no other outcomes were likely to be influenced by blinding, all trials were judged low risk of performance and detection bias related to other outcomes.
Incomplete outcome data
In two trials (Lou 2010; MacDougal 1994) all randomised participants were included in analysis and in two trials (Bensdorp 2013; Levy 1991) 94‐99% of participants were included in analysis. These studies were rated as at low risk of attrition bias. One trial (Ingerslev 2001), published as an abstract in 1998, stated that 167 patients participated in the study, whereas the full‐text article published in 2001 stated that 35 of these patients were enrolled in a pilot study and were excluded from the final analysis; this study was rated as at high risk of attrition bias. In one trial (Morgia 2004) 16% of women in the natural cycle group refused their treatment assignment. This study was rated as at high risk of attrition bias.
Selective reporting
We could not obtain a protocol for five of the included studies, therefore it was unclear whether they reported all expected outcomes. Attempts were made to obtain protocols from the authors. Bensdorp 2013 has published a protocol but has reported preliminary results only, and so the risk of selective reporting in this study was rated as unclear.
Other potential sources of bias
No other source of potential bias was detected in any of the included studies.
Effects of interventions
See: Table 1
1. Natural cycle versus standard IVF
Primary outcomes
Effectiveness
1.1 Live birth rate per woman
See: Analysis 1.1; Figure 4
1.1. Analysis.
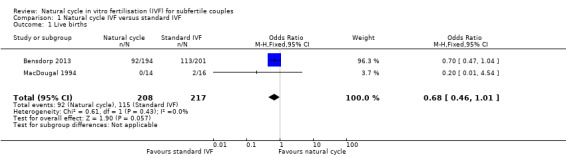
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 1 Live births.
4.
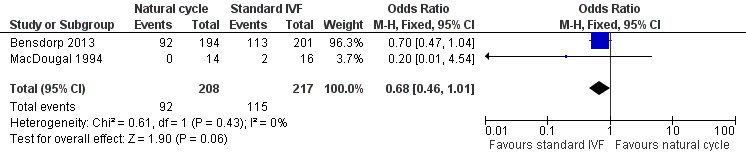
Forest plot of comparison: 1 Natural cycle IVF versus standard IVF, outcome: 1.1 Live births.
Two trials (Bensdorp 2013; MacDougal 1994) reported this outcome. Bensdorp 2013 reported the cumulative live birth rate after modified natural cycles compared with standard IVF. MacDougal 1994 reported the live birth rate after a single cycle of natural cycle compared with standard IVF. There was no significant difference in the live birth rate between the natural cycle and the standard IVF groups (OR 0.68, 95% CI 0.46 to 1.01, 425 women, I2=0%).
In a sensitivity analysis, the findings remained non‐significant when a risk ratio was calculated rather than an odds ratio.
Adverse effect
1.2 Ovarian hyperstimulation syndrome (OHSS) per woman
See: Analysis 1.2; Figure 5
1.2. Analysis.
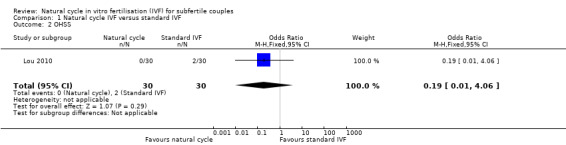
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 2 OHSS.
5.
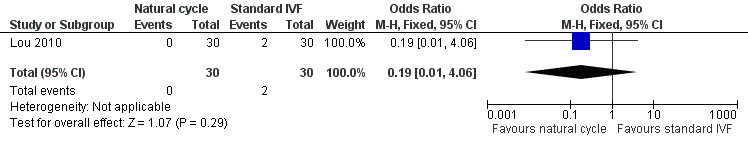
Forest plot of comparison: 1 Natural cycle IVF versus standard IVF, outcome: 1.2 OHSS.
There was only one trial (Lou 2010) that reported this outcome. There was no significant difference between the groups (OR 0.19, 95% CI 0.01 to 4.06, 60 women).
No sensitivity analysis could be done.
Secondary outcomes
Effectiveness
1.3 Pregnancy rate per woman
See: Analysis 1.3; Figure 6
1.3. Analysis.
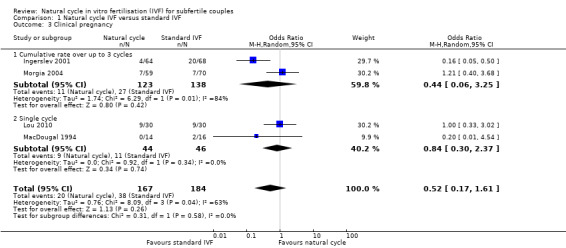
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 3 Clinical pregnancy.
6.
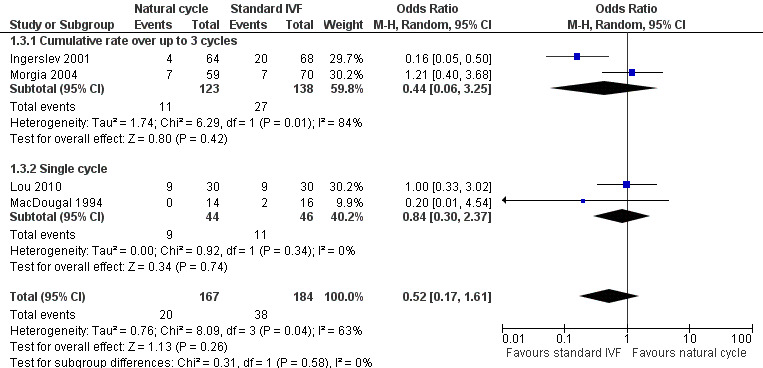
Forest plot of comparison: 1 Natural cycle IVF versus standard IVF, outcome: 1.3 Clinical pregnancy.
Four studies reported clinical pregnancy rate. Two reported cumulative pregnancy (Ingerslev 2001; Morgia 2004) and two reported pregnancy after a single cycle (Lou 2010; MacDougal 1994).
When a fixed effect model was used, there was a significantly lower pregnancy rate in the natural cycle group (OR 0.51, 95% CI 0.28 to 0.92, four studies, 351 women, I2=63%), with substantial heterogeneity. When a random effects model was used, there was no significant different between the groups (OR 0.52, 95% CI 0.17 to 1.61, four trials, 351 women, I2=63%)
The analysis was stratified by number of cycles (multiple versus single). Using a random effects model, there was no significant difference between the groups in the cumulative pregnancy rate over up to three cycles (OR 0.44, 95% CI 0.06 to 3.25, two studies, 261 women, I2=84%). Nor was there any significant difference in the rate after a single cycle (OR 0.84, 95% CI 0.30 to 2.37, two studies, 890 women, I2=0%). The high heterogeneity for this analysis was attributable to a single study (Ingerslev 2001), and exclusion of this study in a sensitivity analysis reduced the I2 measure to 0%. The reason for the heterogeneity was unclear.
Sensitivity analysis
When relative risk (RR) was used in a sensitivity analysis, rather than odds ratio (OR), no statistically significant difference in pregnancy rates per woman was found (random effects model: RR 0.60, 95% CI 0.23 to 1.53).
One trial (Lou 2010) did not describe the method of allocation concealment and was removed in a sensitivity analysis. The result showed no statistically significant difference in pregnancy rates per woman (random effects model: OR 0.39, 95% CI 0.08 to 1.89)).
When the trial with the biggest sample size was removed in a sensitivity analysis, no statistically significant difference in pregnancy rates per woman was found (random effects model: OR 0.36, 95% CI 0.09 to 1.51).
None of the included trials were published before 1990, therefore this sensitivity analysis could not be done.
In a further sensitivity analysis, we assessed the effect of including the largest trial in the review (Bensdorp 2013) in this analysis of clincial pregnancy, using their data for ongoing pregnancy (because data for clinical pregnancy are not yet available for this trial). Findings were similar to the main analysis, and remained sensitive to choice of statistical model, though heterogeneity was reduced somewhat (I2=52%).
Subgroup analysis by treatment type
In subgroup analysis by treatment type, when a fixed effect model was used, there was a significantly lower pregnancy rate in the unmodified natural cycle group compared to the standard IVF group (OR 0.39, 95% CI 0.19 to 0.80, three studies, 291 women, I2= 69%), with substantial heterogeneity. When a random effects model was used, there was no significant difference between the groups (OR 0.39, 95% CI 0.08 to 1.89, three studies, 291 women, I2=63%). Nor was there a significant difference in pregnancy rate between the modified natural cycle group and the standard IVF group, although there was only one relevant study (OR 1.00, 95% CI. 0.33 to 3.02, 60 women).
1.4 Ongoing pregnancies
See: Analysis 1.4 and Figure 7.
1.4. Analysis.
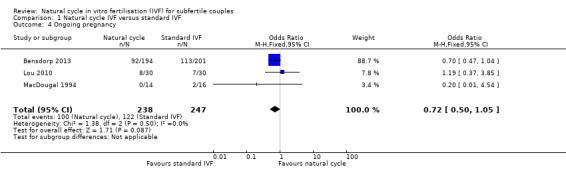
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 4 Ongoing pregnancy.
7.
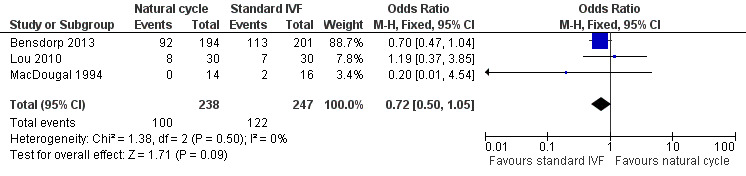
Forest plot of comparison: 1 Natural cycle IVF versus standard IVF, outcome: 1.4 Ongoing pregnancy.
Three trials reported ongoing pregnancies after one cycle (Lou 2010; MacDougal 1994) or multiple cycles (Bensdorp 2013) of treatment.
There was no evidence of a statistically significant difference in ongoing pregnancy rates per woman between natural cycle and standard IVF (OR 0.72, 95% CI 0.50 to 1.05, three studies, 485 women, I2=0%).
Sensitivity analysis
The findings remained non‐significant when a risk ratio was calculated rather than an odds ratio.
Because only two trials recorded this outcome after one cycle, there was no point in excluding one of them in a sensitivity analysis.
None of the included trials were published before 1990, therefore this sensitivity analysis could not be done.
1.5 Number of oocytes retrieved per woman
See: Analysis 1.5.
1.5. Analysis.
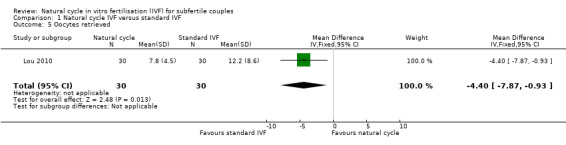
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 5 Oocytes retrieved.
There was only one trial (Lou 2010) with this outcome, which reported that significantly fewer oocytes were retrieved in the natural cycle group (MD ‐4.40, 95% CI ‐7.87 to ‐0.93, 60 women). One trial (Ingerslev 2001) recorded the number of oocytes retrieved after multiple treatment cycles. One trial (MacDougal 1994) stated a mean of 1 with a standard deviation of 0, so the outcome was not estimable.
No sensitivity analysis could be done.
1.6 Time from start of treatment to live birth
Time from start of treatment to live birth was not reported in any of the included trials.
1.7 Number of cycles required to conceive per woman
No trials reported this outcome
Adverse effects
1.8 Multiple pregnancies per woman
See: Analysis 1.8.
1.8. Analysis.
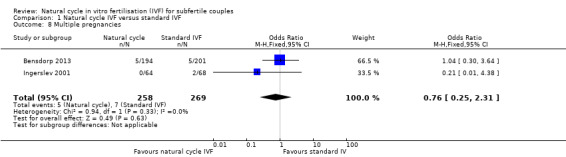
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 8 Multiple pregnancies.
Two trials (Bensdorp 2013; Ingerslev 2001) reported events for this outcome. Both these trials administered multiple cycles. There was no significant difference between the groups in multiple pregnancy rate (OR 0.76, 95% CI 0.25 to 2.31, 527 women, I2=0%). Two trials (MacDougal 1994; Morgia 2004) reported no events in either the natural cycle treatment or the stimulated cycle treatment groups.
1.9 Gestational abnormalities per woman
See: Analysis 1.9. There was only one trial (Lou 2010) which reported this outcome (OR 0.44, 95% CI 0.03 to 5.93, 18 women). Two trials (MacDougal 1994; Morgia 2004) had no events and therefore could not be included in analysis. Data for Bensdorp 2013 are awaited.No sensitivity analysis could be done.
1.9. Analysis.
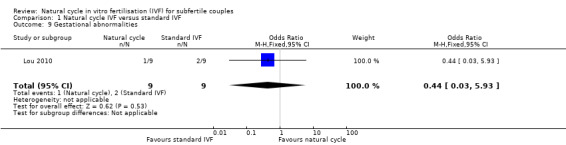
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 9 Gestational abnormalities.
1.10 Cryopreservation of embryos
The number of embryos frozen was not reported in any of the included trials.
1.11 Cycle cancellation rates per woman
See: Analysis 1.11 and Figure 8.
1.11. Analysis.
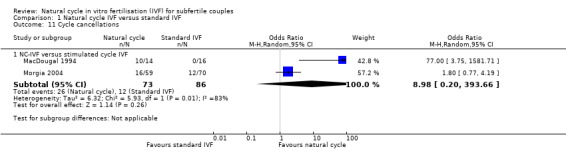
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 11 Cycle cancellations.
8.
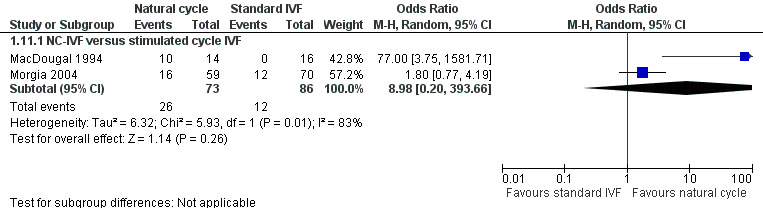
Forest plot of comparison: 1 Natural cycle IVF versus standard IVF, outcome: 1.11 Cycle cancellations.
Two trials reported cycle cancellation rates. Higher cycle cancellation rates were associated with natural cycle treatment compared to the stimulated treatment (OR 3.10, 95% CI 1.49 to 6.45, 159 women; I2 = 83%). The considerable heterogeneity was probably caused by the different treatment protocols and the differing populations. MacDougal 1994 enrolled women ≤ 38 years of age with > one year infertility and spontaneous ovulatory cycles, and used a clomiphene citrate protocol; whereas Morgia 2004 enrolled women ≤ 43 years of age with regular menstrual cycles, primary infertility and poor ovarian reserve, and used a microdose GnRH analogue flare protocol. Because of this considerable heterogeneity, we used a random‐effects model, which showed no statistically significant difference in cycle cancellation rates (OR 8.98, 95% CI 0.20 to 393.66, 159 women; I2 = 83%).
Sensitivity analysis
When RR was used in a sensitivity analysis, rather than OR, there was no statistically significant difference found in cycle cancellation rates (RR 4.66, 95% CI 0.26 to 84.85, 159 women; I2 = 77%).
Because only two trials recorded this outcome, there was no point in excluding one of them in a sensitivity analysis.
None of the included trials were published before 1990.
1.12 Cost effectiveness
See: Analysis 1.12.
1.12. Analysis.
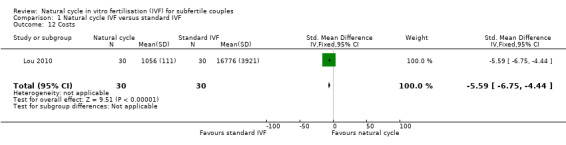
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 12 Costs.
No studies reported cost‐effectiveness, defined as the total cost to reach pregnancy. One study (Bensdorp 2013) reported the direct medical costs to achieve ongoing pregnancy (singleton and multiple), which were €9,838 in the modified natural cycle group and €5,723 in the IVF single embryo transplant group. One study (Lou 2010) reported the cost of medication, and found that costs (to achieve pregnancy) were significantly lower in the natural cycle group (SMD ‐5.59, 95% CI ‐6.75 to ‐4.44, 60 women). Data on the total cost to reach a healthy singleton birth are awaited for Bensdorp 2013.
1.13 Cancellation of the treatment
There was only one trial (Lou 2010) that reported cancellation due to patient motivation, but because there were no events data were not estimable.
Discussion
Summary of main results
No evidence of a statistically significant difference was found between natural cycle and standard IVF in live birth rates. Findings suggest that for a woman with a 53% chance of live birth using standard IVF, the chance using natural cycle IVF would range from 34% to 53%. Nor was there evidence of a statistically significant difference between natural cycle and standard IVF in rates of OHSS, clinical pregnancy, ongoing pregnancy, multiple pregnancy, gestational abnormalities or cycle cancellations. One trial reported the number of oocytes retrieved per woman, and found that the rate was significantly lower in the natural cycle group. There was insufficient data on cancellation of treatment due to patient motivation or adverse effects. Findings on treatment costs were inconsistent and more data are awaited. Findings for pregnancy rate and for cycle cancellation were sensitive to the choice of statistical model, and suggested a benefit for the standard IVF group when a fixed effect model was used.
The lack of fully‐reported large scale RCTs and the use of different treatment protocols in different trials made it difficult to draw definite conclusions.
The trial judged not suitable for meta‐analysis (Levy 1991) reported significantly higher pregnancy rates in COH‐IVF compared to NC‐IVF, and significantly higher cycle cancellation rates in NC‐IVF compared to COH‐IVF. Other data that we judged important but not suitable for pooling can be found in Table 3.
2. Additional data.
| Ingerslev 2001 | Levy 1991 | |||
| Natural cycle | Stimulated cycle | Natural cycle | Stimulated cycle | |
| Cycles | 114 | 111 | 22 | 26 |
| Clinical pregnancy rate | 4 | 20 | 0 | 6 |
| Oocytes retrieved | 68 (0.92 ± 0.40) | 174 (1.83 ± 1.15) | ||
| Cycle cancellations | 40 | 16 | 6 | 1 |
Overall completeness and applicability of evidence
Of the six included trials, live birth rate was reported as an outcome in only two studies (Bensdorp 2013 ; MacDougal 1994), and OHSS in only one (Lou 2010). Four trials (Levy 1991; Lou 2010; MacDougal 1994; Morgia 2004) reported clinical pregnancy rate, and three (Bensdorp 2013, Lou 2010; MacDougal 1994) reported ongoing pregnancy. The number of oocytes retrieved was reported in two studies (Lou 2010; MacDougal 1994) but because MacDougal 1994 reported a standard deviation of 0, data were not estimable and could not be pooled. The time from start of treatment to live birth was not reported in any of the included trials. No trials reported number of cycles to conceive per woman. Multiple pregnancies were reported in four trials (Bensdorp 2013; Ingerslev 2001; MacDougal 1994; Morgia 2004) but MacDougal 1994 and Morgia 2004 reported no events so these data were not estimable and could not be pooled. The number of embryos frozen was not reported in any of the included trials. Cycle cancellation was reported in four of the included trials (Levy 1991; Lou 2010; MacDougal 1994; Morgia 2004), but Lou 2010 reported no events which made the data not estimable. Gestational abnormalities were reported in three trials to date (Lou 2010; MacDougal 1994; Morgia 2004), but MacDougal 1994 and Morgia 2004 reported zero events so the data were not estimable and could not be pooled. Cancellation of treatment due to patient motivation was reported in one study (Lou 2010), but there were no events recorded so the data were not estimable. Also, because this outcome was only recorded in one study, the data could not be pooled. Treatment costs were reported in only two studies (Bensdorp 2013; Lou 2010, and did not include full costs to pregnancy or live birth. Data for Bensdorp 2013 are awaited for some of these outcomes.
These results could be applicable to fertility clinics as in most studies the participants were similar to most women having a first cycle of IVF. The study by Morgia included women who were poor responders only, which is not a general subfertile population. Two studies did not include frequently used stimulation protocols for IVF; stimulation with clomiphene citrate in IVF cycles is not generally recommended.
As the data are currently limited, the results from this review are unable to be translated into clinical practice.
Quality of the evidence
Five trials reported data suitable for meta‐analysis. Almost all trials were judged as at low risk of bias for random sequence generation, but three failed to adequately describe allocation concealment. Two were rated as at high risk of attrition bias, due to the apparent failure to report the outcomes of one group of participants (Ingerslev 2001) and the refusal of 16% of women in one group to accept their treatment assignment (Morgia 2004). There was high heterogeneity for some analyses, possibly due to different inclusion criteria for the participants and the difference in IVF protocols used for controls.
It is helpful for trials to report cumulative pregnancy and live birth rates, due to the difference in number of oocytes retrieved, and because a COH‐IVF treatment is a much greater burden for the couple and therefore cannot be repeated as often as a natural cycle IVF treatment. Moreover in clinical practice, it is not common to give just one IVF treatment. Therefore, trials reporting cumulative rates provide a more realistic comparison.
The overall quality of the evidence was rated using GRADE methods and ranged from very low to moderate. The main limitation was imprecision. Bensdorp 2013 has reported preliminary data only and has yet to complete follow up, but data were available for over 95% of women randomised .
Potential biases in the review process
We based our definition of the natural cycle and the standard treatment on the literature and clinical expertise. We conducted the search, extracted data and excluded studies according to that definition. This may have introduced bias. We aimed to retrieve all eligible studies, however unpublished studies may not have been identified. Data were incomplete for Bensdorp 2013 and full results are awaited.
Because of the small number of studies, we did not construct a funnel plot. Therefore we were unable to visually estimate the existence of other studies or publication bias. Because of the small amount of data, we were also unable to subgroup the data as we stated in the protocol.
Finally, using computation from percentages to create dichotomous data may have introduced bias.
Agreements and disagreements with other studies or reviews
Other reviews on the subject show similar results (Loutradis 2007; Loutradis 2008; Pandian 2010; Pelinck 2009; Reyftmann 2007); they conclude that natural cycle IVF seems promising but that there is insufficient information for definite conclusions. Optimistic data on natural cycle IVF treatment have been published, but more data from good quality trials are needed and further data from Bensdorp 2013are also awaited.
Authors' conclusions
Implications for practice.
We wanted to provide a clear overview of the differences between natural cycle IVF and standard IVF so subfertile couples could judge which treatment suited their preferences. Because the five included trials in the meta‐analysis (one included study was not suitable) used different protocols and different study populations, we could not come to clear conclusions. Because of the difference in subfertility causes, there is no IVF treatment ideal for all couples. The situation and personal preferences of the women should therefore be taken into consideration and women should be well informed when choosing a specific treatment. For couples with male factor subfertility, which is 20% of all causes of subfertility (Sharlip 2002), natural cycle IVF may be particularly suitable. Treating a healthy female in a couple with male factor subfertility can be considered a psychological burden for the male and both a psychological and physical burden for the female.
Implications for research.
Large scale randomised controlled trials are required comparing natural cycle IVF with standard IVF. Outcomes should be cumulative live birth rates, the number of treatment cycles per woman necessary to reach live birth, treatment costs and adverse effects of the treatment. Different treatment protocols and different causes of subfertility should be subgrouped within the same trial so a sensitivity analysis can be conducted. The data should be measured per woman, so a clear overview can be given on cumulative pregnancy rates of different IVF treatments. Only then is it fair to compare improvements in outcomes with the different IVF treatments.
Acknowledgements
We would like to thank the Cochrane Menstrual Disorders and Subfertility Group, in particular Marian Showell (Trials Search Co‐ordinator) for writing and running the search and Vanessa Jordan (New Zealand Cochrane Fellow) for answering questions.
We would also like to thank Dr Jane MacDougall and Dr Marco Sbracia for providing additional information, and Professor Hans Jakob Ingerslev for his response.
Appendices
Appendix 1. CENTRAL search strategy
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <1977 ‐ present>
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1579) 2 vitro fertili?ation.tw. (1336) 3 ivf‐et.tw. (256) 4 ivf.tw. (1925) 5 icsi.tw. (679) 6 intracytoplasmic sperm injection$.tw. (414) 7 assisted reproduct$.tw. (392) 8 ovulation induc$.tw. (455) 9 (ovari$ adj2 stimulat$).tw. (750) 10 superovulat$.tw. (134) 11 ovarian hyperstimulation.tw. (549) 12 COH.tw. (121) 13 infertil$.tw. (1804) 14 subfertil$.tw. (132) 15 (ovari$ adj2 induction).tw. (26) 16 (stimulat$ adj3 cycle$).tw. (351) 17 (embryo$ or blastocyst$).tw. (2115) 18 or/1‐17 (5663) 19 natural.tw. (5056) 20 (modified adj5 cycle$).tw. (49) 21 MNC IVF.tw. (1) 22 NCIVF.tw. (0) 23 NC‐IVF.tw. (0) 24 unstimulated.tw. (307) 25 or/19‐24 (5387) 26 18 and 25 (151)
Appendix 2. EMBASE search strategy
Embase <1980 ‐ present>
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (48345) 2 in vitro fertili?ation.tw. (19220) 3 icsi.tw. (8227) 4 intracytoplasmic sperm injection$.tw. (5736) 5 ivf.tw. (21578) 6 assisted reproduct$.tw. (11542) 7 intrauterine insemination$.tw. (2224) 8 ovulation induc$.tw. (4130) 9 (ovari$ adj2 stimulat$).tw. (6205) 10 superovulat$.tw. (2898) 11 ovarian hyperstimulation.tw. (4762) 12 COH.tw. (1287) 13 infertil$.tw. (49635) 14 subfertil$.tw. (3984) 15 (ovari$ adj2 induction).tw. (260) 16 embryo$.tw. (280998) 17 blastocyst$.tw. (17376) 18 (stimulat$ adj3 cycle$).tw. (3768) 19 or/1‐18 (360701) 20 natural$.tw. (446575) 21 (modified adj5 cycle$).tw. (738) 22 NCIVF.tw. (2) 23 NC‐IVF.tw. (9) 24 MNC‐IVF.tw. (15) 25 unstimulated.tw. (15518) 26 simple protocol.tw. (786) 27 no stimulation.tw. (1502) 28 'not stimulated'.tw. (2468) 29 or/20‐28 (466658) 30 19 and 29 (10077) 31 Clinical Trial/ (875792) 32 Randomized Controlled Trial/ (338076) 33 exp randomization/ (60804) 34 Single Blind Procedure/ (17075) 35 Double Blind Procedure/ (113402) 36 Crossover Procedure/ (36349) 37 Placebo/ (213772) 38 Randomi?ed controlled trial$.tw. (84127) 39 Rct.tw. (10976) 40 random allocation.tw. (1213) 41 randomly allocated.tw. (18390) 42 allocated randomly.tw. (1869) 43 (allocated adj2 random).tw. (717) 44 Single blind$.tw. (13064) 45 Double blind$.tw. (134437) 46 ((treble or triple) adj blind$).tw. (304) 47 placebo$.tw. (185409) 48 prospective study/ (226861) 49 or/31‐48 (1310623) 50 case study/ (18825) 51 case report.tw. (240081) 52 abstract report/ or letter/ (860278) 53 or/50‐52 (1114196) 54 49 not 53 (1274596) 55 30 and 54 (592) 56 (2010$ or 2011$ or 2012$ or 2013$).em. (3491034) 57 55 and 56 (148)
Appendix 3. MEDLINE search strategy
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 ‐ present>
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (31238) 2 vitro fertili?ation.tw. (15921) 3 ivf‐et.tw. (1817) 4 ivf.tw. (15269) 5 icsi.tw. (5150) 6 intracytoplasmic sperm injection$.tw. (4649) 7 assisted reproduct$.tw. (8118) 8 ovulation induc$.tw. (3251) 9 (ovari$ adj2 stimulat$).tw. (4565) 10 superovulat$.tw. (2831) 11 ovarian hyperstimulation.tw. (3583) 12 COH.tw. (1028) 13 infertil$.tw. (39391) 14 subfertil$.tw. (3230) 15 (ovari$ adj2 induction).tw. (211) 16 (stimulat$ adj3 cycle$).tw. (3075) 17 (embryo$ or blastocyst$).tw. (256993) 18 or/1‐17 (316114) 19 natural.tw. (314184) 20 (modified adj5 cycle$).tw. (577) 21 MNC IVF.tw. (10) 22 NCIVF.tw. (1) 23 NC‐IVF.tw. (2) 24 unstimulated.tw. (14127) 25 'not stimulated'.tw. (2480) 26 no stimulation.tw. (1418) 27 or/19‐26 (332104) 28 18 and 27 (6456) 29 randomized controlled trial.pt. (341704) 30 controlled clinical trial.pt. (85284) 31 randomized.ab. (259371) 32 randomised.ab. (50942) 33 placebo.tw. (145229) 34 clinical trials as topic.sh. (162693) 35 randomly.ab. (189401) 36 trial.ti. (110617) 37 (crossover or cross‐over or cross over).tw. (55740) 38 or/29‐37 (857169) 39 exp animals/ not humans.sh. (3767737) 40 38 not 39 (790885) 41 28 and 40 (233)
Appendix 4. PsycINFO search strategy
PsycINFO <1806 ‐ present>
1 random.tw. (37235) 2 control.tw. (289345) 3 double‐blind.tw. (16666) 4 clinical trials/ (6576) 5 placebo/ (3391) 6 exp Treatment/ (538852) 7 or/1‐6 (819614) 8 natural cycle$.tw. (34) 9 7 and 8 (15)
Appendix 5. MDSG search strategy
Keywords CONTAINS "natural cycle" or "natural cycles" or "modified ICSI" or "modified natural cycle" or "unstimulated ovaries" or Title CONTAINS "natural cycle" or "natural cycles" or "modified ICSI" or "modified natural cycle" or "unstimulated ovaries"
Appendix 6. CINAHL search strategy
<inception ‐ present>
1 (MH "Fertilization in Vitro") OR "ivf"
2 TX intracytoplasmic sperm injection
3 TX icsi
4 S1 OR S2 OR S3
5 TX natural cycle
6 TX modified cycle
7 TX unstimulated cycles
8 S5 OR S6 OR S7
9 S4 AND S8
Appendix 7. ISI Web of Knowledge search strategy
<inception ‐ present>
Natural cycle AND IVF or ICSI
Modified cycle AND IVF or ICSI
Appendix 8. clinicaltrials.gov and WHO portal for ongoing trials search strategy
<inception ‐ present>
Keywords included:
Natural cycle
Modified cycle
Unstimulated cycle
Appendix 9. PubMed search strategy
<inception ‐ present>
((((((((("Fertilization in Vitro"[Mesh]) AND "Sperm Injections, Intracytoplasmic"[Mesh]) AND "Ovulation Induction"[Mesh]) OR ivf[tw]) OR Fertilization in Vitro[tw]) OR icsi[tw]) OR (stimulated cycle[tw] OR stimulated cycles[tw])) AND (natural cycle[tw] OR natural cycle/mild[tw] OR natural cyclers[tw] OR natural cycles[tw])) OR (modified cycle[tw] OR modified cycles[tw])) OR (unstimulated cycle[tw] OR unstimulated cycles[tw]) AND Randomized Controlled Trial[ptyp]
Data and analyses
Comparison 1. Natural cycle IVF versus standard IVF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live births | 2 | 425 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.46, 1.01] |
| 2 OHSS | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |
| 3 Clinical pregnancy | 4 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.17, 1.61] |
| 3.1 Cumulative rate over up to 3 cycles | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.06, 3.25] |
| 3.2 Single cycle | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.37] |
| 4 Ongoing pregnancy | 3 | 485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 5 Oocytes retrieved | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐7.87, ‐0.93] |
| 8 Multiple pregnancies | 2 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.25, 2.31] |
| 9 Gestational abnormalities | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.03, 5.93] |
| 11 Cycle cancellations | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 NC‐IVF versus stimulated cycle IVF | 2 | 159 | Odds Ratio (M‐H, Random, 95% CI) | 8.98 [0.20, 393.66] |
| 12 Costs | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐5.59 [‐6.75, ‐4.44] |
| 13 Subgroup analysis: Clinical pregnancy rate by intervention | 4 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.17, 1.61] |
| 13.1 NC IVF versus stimulated cycle IVF | 3 | 291 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.89] |
| 13.2 MNC‐IVF versus stimulated cycle IVF | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.33, 3.02] |
1.13. Analysis.
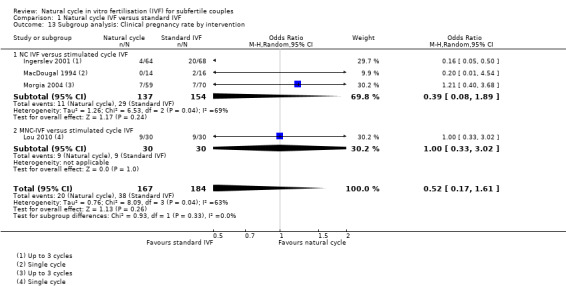
Comparison 1 Natural cycle IVF versus standard IVF, Outcome 13 Subgroup analysis: Clinical pregnancy rate by intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bensdorp 2013.
| Methods | Multicentre randomised controlled trial (17 centres in the Netherlands): trial acronym INeS 603 couples randomised, of whom 395 were randomised to comparisons of interest in current review Conducted Jan 2009 to Feb 2011 Follow‐up 12 months |
|
| Participants | Included: Couples with female aged between 18 and 38 years, diagnosed with unexplained or mild male subfertility, failure to conceive within at least 12 months of unprotected intercourse and a poor prognosis. A poor prognosis was defined as a chance of spontaneous pregnancy within 12 months below 30% or failure to conceive within at least 3 years of unprotected intercourse. Mild male subfertility was defined as pre‐wash total motile sperm count above 10 million or a post‐wash total motile sperm count above 1 million Excluded: Women with PCOS/anovulatory cycles, severe endometriosis, double sided tubal pathology or serious endocrine illness |
|
| Interventions | 1. Modified natural cycle (MNC) IVF x six cycles: the oocyte that developed spontaneously was used for IVF, minimally modified with a GnRH antagonist to prevent untimely ovulations, together with FSH to prevent collapse of the follicle When a lead follicle with a mean diameter of at least 14 mm was observed, daily injections of 0.25 mg of a GnRH‐antagonist together with 150 IU FSH were started. GnRH‐antagonist was continued up to and including the day of ovulation triggering. FSH was continued up to the day of ovulation triggering (n=195 randomised, 194 analysed) 2. IVF with elective single‐embryo transfer (SET) x 3 cycles, plus cryo‐cycles within 12 months. Controlled ovarian hyperstimulation after down‐regulation with a GnRH agonist in a long protocol with a mid luteal start or with a fixed start antagonist protocol starting on day two. Controlled ovarian hyperstimulation was started with 150 IU FSH. Treatment was continued until at least 2 follicles > 18mm had developed. Ovulation was induced by 10.000 IU human chorionic gonadotropin hormone (hCG). (n=203 randomised, 201 analysed) Findings were evaluated over one year of follow up, within which time some women in each group underwent cycles in addition to their allocated treatment, as follows: 1. MNC‐IVF group (n=194) Allocated treatment: MNC‐IVF 640 cycles Additional treatment: IUI 58 cycles, IVF MNC/SET/double embryo transfer (DET): 34 cycles, IVF SET 34 cycles, IVF DET<7 cycles, cryo cycles: 9 2. IVF‐SET group (n=201) Allocated treatment: IVF‐SET 303 cycles, frozen cycles 147, Additional treatment: IUI cycles 35, IVF MNC/SET/DET 4 cycles, IVF SET 1 cycle [3. The study also included a group undergoing IUI with COH x 6 cycles (n=207 randomised, 207 analysed). This group were not included in the current review] |
|
| Outcomes | Birth of healthy singleton (term, birth weight >5th percentile, no congenital anomalies, normal development up to 6 weeks), multiple pregnancy, clinical pregnancy, ongoing pregnancy, time to pregnancy, neonatal and pregnancy complications, cost‐effectiveness | |
| Notes | Funding: Netherlands Organization for Health Research and Development (ZonMw) and Zorgverzekeraars Nederland (ZN) In this review we reported data from the 2013 ESHRE slide presentation, which are 95% complete. Follow up is incomplete for 7/194 in the MNC group and 8/201 in the standard IVF group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central internet‐based randomisation programme |
| Allocation concealment (selection bias) | Low risk | Central internet‐based randomisation programme |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed by ITT. Incomplete follow‐up for 7/194 in MNC group and 8/201 in standard IVF group (4%) |
| Selective reporting (reporting bias) | Unclear risk | Reported only in abstract/slide presentation so far and not all outcomes reported yet (numbers inconsistent between the presentations) |
| Other bias | Low risk | Baseline characteristics of two groups were similar. Reported only as abstract/slide presentation but no evidence of likely bias |
Ingerslev 2001.
| Methods | Randomised controlled trial (block randomisation, five patients in each block) Performed between August 1 and December 31, 1997 Informed consent obtained |
|
| Participants | Fertility Clinic and Perinatal Epidemiological Research Unit, Department of Obstetrics and Gynaecology, Aarhun University Hospital, Skejby Sygehus, Aarhus, Denmark As stated in the article: among 564 couples waiting for IVF or ICSO treatment, 196 were invited to participate in the study, fulfilling the following criteria: female age < 35, unexplained infertility, tubal factor or due to severe male factor with indication for ICSI, regular menstrual cycle, presence of two ovaries and no previous IVF treatment. Of these, 29 did not respond, 35 were enrolled in a pilot study so 132 couples participated in the present study. Unstimulated group:
Clomiphene citrate group:
|
|
| Interventions | Unstimulated cycle IVF versus stimulated cycle IVF Unstimulated cycle group (64) received no treatment. When the dominant follicle reached a diameter of ≥17 mm, HCG (Pregnyl®; 5000 IU) was given for a timed oocyte retrieval 35 ‐ 36 hours later. The stimulated group (68) received clomiphene citrate (Clomivid®; Astra, Denmark) 100 mg from cycle day 3‐7. When the dominant follicle reached a diameter ≥ 20 mm, HCG (Pregnyl®; 5000 IU) was given for a timed oocyte retrieval 35 ‐ 36 hours later. |
|
| Outcomes | Oocyte aspiration Oocyte harvested Oocytes fertilised Cycles with embryo transfer Total number of embryos transferred Live intrauterine pregnancy rate per started cycle Live intrauterine pregnancy rate per embryo transfer Implantation rate. |
|
| Notes | Author was unable to provide additional information, contact author again for update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation was used, with five patients in each block. Does not state method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope method was used, does not state opaque |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results of 35 pilot patients are not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias were found |
Levy 1991.
| Methods | Prospective randomised crossover study | |
| Participants | George Washington University Medical Center, Washington, DC As stated in the abstract, 31 IVF‐ET candidates with regular ovulatory menstrual cycles and no male factor have enrolled thus far |
|
| Interventions | Natural cycle versus stimulated cycle IVF In the natural cycle, 4000 IU hCG was given in an effort to precede the endogenous LH surge In the stimulated cycle, luteal phase initiated GnRH suppression was followed by human menopausal gonadotropin (10.000 IU) administration |
|
| Outcomes | Pregnancy rates, cancellation rates, oocyte retrieval and fertilisation rate | |
| Notes | Stated as ongoing. Attempts to contact any of the authors failed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not state method of randomisation. No further information obtained |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Thirty‐one patients included, 16 patients underwent natural cycle and 13 underwent the stimulated cycle; 2 patients are missing; 94% of participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias were found |
Lou 2010.
| Methods | Prospective, randomised controlled trial Performed between August 2006 and April 2008 Informed consent obtained Sixty women randomised |
|
| Participants | Ruijin Hospital, Shanghai, China Inclusion criteria as stated:
|
|
| Interventions | Modified natural cycle IVF versus controlled ovarian hyperstimulation IVF The modified natural cycle treatment as stated: If the serum estradiol concentration was < 50 pg/ml, HMG 150 IU/day was given IM, by a nurse or doctor, starting on the third day of the menstrual cycle; patients whose serum estradiol concentration was > 50 pg/ml were removed from the study. The number and size of ovarian follicles in were monitored by transvaginal ultrasonography on the second day of stimulation. No gonadotrophin agonist or antagonist was given at any time during the treatment cycle. The COH treatment as stated: A GNRH agonist (triptorelin 0.1 mg/day SC) was self‐administered by the patients from day 21 of the menstrual cycle (7 days after ovulation), before the IVF cycle. Recombinant FSH (Gonal‐F®; Merck Sereno, Geneva, Switzerland) 150 – 300 IU/day was then self administered by the patients from day 2 of the menstrual cycle, at which time the dose of GNRH agonist was reduced to 0.05 mg/day. On the second day of the menstrual cycle and on alternate days subsequently, the number and size of ovarian follicles in the patients were monitored by transvaginal ultrasonography and measurement of serum estradiol was carried out. The daily dose of recombinant FSH was adjusted according to the serum estradiol level and the number and size of ovarian follicles. If the rate of development follicles was greater or less than expected, the FSH dose was decreased or increased, respectively, by 75 IU/day. In both groups, HCG 10000 IU was administered at a predetermined time of the day on which two or more follicles ≥ 17 mm in diameter |
|
| Outcomes | Implantation rate Clinical pregnancy rate Successful pregnancies Number of oocytes retrieved Medication cost |
|
| Notes | No additional data from the author were obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated set of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias were found |
MacDougal 1994.
| Methods | Randomised controlled trial Informed consent obtained |
|
| Participants | Hallam Medical Centre, The London Women’s Clinic, The Middlesex Hospital and King’s College Hospital, London, United Kingdom 30 patients with the following inclusion criteria:
|
|
| Interventions | Natural cycle versus clomiphene citrate stimulated cycles The natural cycle group (n=14) received no treatment, whereas the clomiphene citrate group received 100 mg during cycle day 2‐6. All patients had an ultrasound scan (US) on day 2 and 7, followed by daily scans once the leading follicle reached a size of 14 mm in diameter. Serum LH and E2 concentrations were measured daily from day 7 of the cycle. When the mean diameter of the dominant follicle reached 17 mm, hCG, 5000 IU, was administered and US‐directed oocyte collection was performed 35 hour later. |
|
| Outcomes | Number of patients reaching oocyte recovery Numbers of oocytes collected and fertilised Embryos transferred Clinical pregnancy rate Multiple pregnancy rate |
|
| Notes | Author stated: patients were randomised using computer generated numbers to assign patients to treatment arm with concealment in brown sealed opaque envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer selected random numbers were used |
| Allocation concealment (selection bias) | Low risk | After contacting the author, she stated the use of 'brown paper opaque envelopes that were numbered individually' |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias were found |
Morgia 2004.
| Methods | Randomised controlled trial Performed between January 2000 and July 2002 Informed consent received |
|
| Participants | Bioroma Center, Rome, Italy One hundred and forty women with the following inclusion criteria:
|
|
| Interventions | Natural cycle IVF versus IVF with controlled ovarian hyperstimulation The natural cycle treatment as stated: The follicle size was monitored by transvaginal ultrasound scan daily from the 7th day of the cycle to measure follicular structures and endometrial thickness and morphology. When a follicle reached 16 mm in diameter, ovulation was triggered with hCG, 10,000 IU (Profasi HP 5000, Serono, Italy). The COH treatment as stated: Patients undergoing controlled ovarian hyperstimulation with the microdose GnRH analog flare protocol group were treated with 0.05 mg buserelin (Suprefact; Hoechst, Berlin, Germany) SC twice daily from the 1st day of the menstrual cycle and FSH, 600 IU (Metrodin HP, Serono, Italy) daily from the 3rd day of the menstrual cycle. Follicle size was measured daily by ultrasound and plasma levels of E2 were measured from the 7th day of stimulation. From this stage, the dose of pFSH was adjusted, depending on the individual response of each patient. When at least 2 follicles reached 16 mm in diameter, ovulation was triggered with hCG, 10,000 IU (Profasi HP 5000, Serono, Italy). |
|
| Outcomes | Number of oocytes retrieved Pregnancy rate per cycle Pregnancy rate per transfer Implantation rate |
|
| Notes | Author stated: For randomisation we used a list of random numbers in sealed opaque envelopes given to patients Re‐analysis was required for data per woman Pregnancy rate: Out of 59 NC patients, 40.7% had a transfer so (59 x 40.7) / 100% = 24 transfers Out of 24 transfers, 4.2% got pregnant so (24 x 4.2) / 100% = 1 pregnancy in NC Out of 70 COH patients, 71.4% had a transfer so (70 x 71.4) / 100% = 50 transfers Out of 50 transfers, 4.0% got pregnant so (50 x 4.0) / 100% = 2 pregnancies in COH Cycle cancellation: Out of 59 NC patients, 72.9% had an oocyte retrieval; therefore we assume 27.1% had a cycle cancellation. So (59 x 27.1) / 100% = 16 cycle cancellations for NC Out of 70 COH patients, 82.8% had an oocyte retrieval; therefore we assume 17.2% had a cycle cancellation. So (70 x 17.2) / 100% = 12 cycle cancellations for COH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomised according to a computer generated number sequence at the time that their cycle was scheduled |
| Allocation concealment (selection bias) | Low risk | After contacting the author, he stated the use of a 'list of random numbers in sealed, opaque envelopes given to patients' |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | The outcomes are not likely to be influenced by any lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by any lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seventy women were randomly allocated to each group: 11 women assigned to the natural‐cycle group refused the randomization and chose another treatment. Thus attrition rate of 16% in one group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias were found |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2004 | This is a cohort study |
| Bassil 1999 | This is a cohort study |
| Belaid 2005 | Not a comparison of interest; study compares assisted hatching versus no assisted hatching |
| Groenewoud 2012 | This publication is a study protocol. It compares NC‐frozen thawed embryo transfer (FET) versus artificial cycle (AC)‐FET, not a comparison of interest |
| Hojgaard 2001 | This is a retrospective study |
| Jancar 2009 | Consecutive women were used, so not a randomised controlled trial |
| Karimzadeh 2012 | Study compares 2 stimulation protocols: clomiphene citrate/gonadotropin/antagonist versus microdose GnRH agonist flare protocols, so not a comparison of interest |
| Kim 2009 | This study compares 2 different stimulation protocols: minimal stimulation using the GnRH antagonist cetrorelix and 150 IU recombinant human FSH (rhFSH; Gonal‐F, Merck Serono SA) versus FSH 225 IU/day in combination with cetrorelix (Cetrotide) 0.25 mg/day when the mean diameter of the lead follicle reached 13 to14 mm |
| Lee 2008 | This is a retrospective study |
| Mirkin 2004 | This study included females of proven fertility, so not a study population of interest. The outcome measures are in gene expression, so no outcomes of interest |
| Paulson 1990 | This is a study on unstimulated cycle IVF, not a comparison of interest |
| Pistorius 2006 | This study uses questionnaires, so it is not a randomised controlled trial |
| Rama Devi 2011 | This study compares 2 stimulation protocols, clomiphene citrate in combination with FSH versus the standard long IVF protocol with a GnRH agonist, so not a comparison of interest |
| Reyftmann 2007 | This is a review of the literature on natural cycle IVF |
| Schimberni 2011 | Treatments were assigned to patients according to admission date, so not a randomised controlled trial |
| Strohmer 1997 | This study compares two different stimulation protocols, an ultrashort gonadotrophin‐releasing hormone agonist versus a modified suppression protocol, so not a comparison of interest |
| Vidal 2013 | This study compares a GnRHa agonists versus GnRH antagonists in endometrial preparation for oocyte donation, so not a comparison of interest |
Characteristics of ongoing studies [ordered by study ID]
Zhang 2013.
| Trial name or title | IVF Clinical Trial of Two Different Treatment Protocols |
| Methods | Randomised controlled trial |
| Participants | New Hope Fertility Center, New York, New York, United States Inclusion criteria:
Exclusion criteria:
|
| Interventions | IVF protocol and minimal stimulation IVF protocol |
| Outcomes | Primary outcome parameter: Live birth Secondary outcome parameters: Biochemical pregnancy, Clinical pregnancy, Ongoing pregnancy, Multiple pregnancy rate, Miscarriage rate, Fertilisation rate, Number of oocytes, Number of embryos |
| Starting date | December 2008 |
| Contact information | Henriette Julien, MD, info@ivfclinicaltrial.com |
| Notes | Estimated completion date: January 2014 |
Differences between protocol and review
Because of the small amount of data, we were unable to subgroup the data as we stated in the protocol.
During the exclusion process, we realised we did not report important exclusion criteria in the protocol, so we added them in the review:
donor oocytes;
frozen embryo transfer;
animal studies.
Contributions of authors
Thomas Allersma and Astrid Cantineau extracted data. Thomas Allersma entered the data and wrote the review. Cindy Farquhar helped drafting the review, acted as a clinical expert and commented on the review. Astrid Cantineau acted as a clinical expert and commented on the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
No declaration of interests.
New
References
References to studies included in this review
Bensdorp 2013 {published data only}
- Bensdorp AJ, Slappendel E, Koks C, Oosterhuis J, Hoek A, Hompes P, et al. The INeS study: prevention of multiple pregnancies: a randomised controlled trial comparing IUI COH versus IVF e SET versus MNC IVF in couples with unexplained or mild male subfertility. BMC Women's Health 2009;9:35. [DOI: 10.1186/1472-6874-9-35] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjon‐Kon‐Fat RI, Bensdorp AJ, Maas J, Oosterhuis GJE, et al. An economic analysis comparing IVF with a single embryo transfer and IVF with amodified natural cycle to IUI with hyperstimulation (the INeS trial). European Society of Human Reproduction and Embryology 29th Annual Meeting, Human Reproduction 2013;28 S1:Abstract 0‐171. [Google Scholar]
Ingerslev 2001 {published data only}
- Ingerslev HJ, Hojgaard A, Hindkjaer J, Kesmodel U. A randomized study comparing IVF in the unstimulated cycle with IVF following clomiphene citrate. Human Reproduction (Oxford, England) 2001 Apr;16(4):696‐702. [DOI] [PubMed] [Google Scholar]
- Ingerslev HJ, Hojgaard A, et al. An open randomized study of IVF in natural cycles or with clomiphene citrate in younger patients with selected diagnoses. Human Reproduction 1998;13:50‐1. [Google Scholar]
- Ingerslev HJ, Hojgaard A, et al. Natural cycle and chlomiphene citrate ivf revisited an open randomized study of ivf in natural cycles or with clomiphene citrate in younger patients with selected diagnosis. 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics. 1999:255p. [Google Scholar]
Levy 1991 {published data only}
- Levy MJ, Gindoff P, Hall J, Stillman RJ. The efficacy of natural versus stimulated cycle IVF‐ET. Fertility and Sterility 1991;56:pp.S15‐16. [Google Scholar]
Lou 2010 {published data only}
- Lou HY, Huang XY. Modified natural cycle for in vitro fertilization and embryo transfer in normal ovarian responders. The Journal of International Medical Research 2010;38(6):2070‐6. [DOI] [PubMed] [Google Scholar]
MacDougal 1994 {published data only}
- MacDougall MJ, Tan SL, Hall V, Balen A, Mason BA, Jacobs HS. Comparison of natural with clomiphene citrate‐stimulated cycles in in vitro fertilization: a prospective, randomized trial. Fertility and Sterility 1994;61(6):1052‐7. [DOI] [PubMed] [Google Scholar]
- MacDougall MJ, Tan SL, et al. Natural cycle in‐vitro fertilization Prospective‐ randomized trial comparing unstimulated with stimulated in‐vitro fertilization (abstract). Journal of Reproduction and Fertility 1992;96:20. [Google Scholar]
Morgia 2004 {published data only}
- Aragona C, Sbracia M, et al. VF in poor responder patients: A controlled trial between natural cycle and micro‐dose GnRH analogue flare. Fertility and Sterility 2003;80 Suppl 3:S191, Abstract no: P‐206. [Google Scholar]
- Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, Aragona C. A controlled trial of natural cycle versus microdose gonadotropin‐releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertility and Sterility 2004;81(6):1542‐7. [DOI: 10.1016/j.fertnstert.2003.11.031] [DOI] [PubMed] [Google Scholar]
- Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, Aragona C. IVF in poor responder patients: a controlled trial between natural cycle and micro‐dose GnRH‐a flare. The 20th Annual Meeting of the European Society of Human Reproduction and Embryology 2004:i117p. [Google Scholar]
References to studies excluded from this review
Adams 2004 {published data only}
- Adams SM, Terry V, Hosie MJ, Gayer N, Murphy CR. Endometrial response to IVF hormonal manipulation: comparative analysis of menopausal, down regulated and natural cycles. Reproductive Biology and Endocrinology : RB&E 2004 Apr 30;2:21. [DOI: 10.1186/1477-7827-2-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassil 1999 {published data only}
- Bassil S, Godin PA, Donnez J. Outcome of in‐vitro fertilization through natural cycles in poor responders. Human Reproduction 1999;14(5):1262‐5. [DOI: 10.1093/humrep/14.5.1262] [DOI] [PubMed] [Google Scholar]
Belaid 2005 {published data only}
- Belaid Y, Fanchin R, Du A, Hesters L, Frydman R, Frydman N. Assisted hatching and natural cycle: A prospective and randomized study. Fertility and Sterility 2005;84 Suppl 1:S420. [Google Scholar]
Groenewoud 2012 {published data only}
- Groenewoud ER, Macklon NS, Cohlen BJ. Cryo‐thawed embryo transfer: natural versus artificial cycle. A non‐inferiority trial (ANTARCTICA trial). BMC Women's Health 2012;12:27. [DOI: 10.1186/1472-6874-12-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hojgaard 2001 {published data only}
- Hojgaard A, Ingerslev HJ, Dinesen J. Friendly IVF: patient opinions. Human Reproduction (Oxford, England) 2001;16(7):1391‐6. [DOI] [PubMed] [Google Scholar]
Jancar 2009 {published data only}
- Jancar N, Virant‐Klun I, Bokal EV. Serum and follicular endocrine profile is different in modified natural cycles than in cycles stimulated with gonadotropin and gonadotropin‐releasing hormone antagonist. Fertility and Sterility 2009;92(6):2069‐71. [DOI: 10.1016/j.fertnstert.2009.06.054] [DOI] [PubMed] [Google Scholar]
Karimzadeh 2012 {published data only}
- Karimzadeh MA, Mohammadian F, Mashayekhy M. Comparison of frozen‐thawed embryo transfer outcome in natural cycle and hormone replacement cycle. Human Reproduction 2012;27 Suppl 2:ii226‐7 Abstract number: P‐284. [DOI: 10.1007/s00404-010-1828-z] [DOI] [Google Scholar]
Kim 2009 {published data only}
- Kim CH, Kim SR, Cheon YP, Kim SH, Chae HD, Kang BM. Minimal stimulation using gonadotropin‐releasing hormone (GnRH) antagonist and recombinant human follicle‐stimulating hormone versus GnRH antagonist multiple‐dose protocol in low responders undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertility and Sterility 2009;92(6):2082‐4. [DOI: 10.1016/j.fertnstert.2009.06.005] [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee SJ, Kwon HC, Kim JW, Lee JH, Jung KJ, Jung JY, Ko HS. Comparison of clinical outcome of frozen‐thawed embryo transfer cycles between natural and artificial (hormone‐treated) cycles. Human Reproduction. European Society of Human Reproduction and Embryology. ESHRE 24th Annual Meeting, Barcelona 2008;23:313. [Google Scholar]
Mirkin 2004 {published data only}
- Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri‐implantation endometrium in natural and gonadotropin‐stimulated cycles. The Journal of Clinical Endocrinology and Metabolism 2004;89(11):5742‐52. [DOI: 10.1210/jc.2004-0605] [DOI] [PubMed] [Google Scholar]
Paulson 1990 {published data only}
- Paulson RJ, Sauer MV, Francis MM, Macaso TM, Lobo RA. In vitro fertilization in unstimulated cycles: a clinical trial using hCG for timing of follicle aspiration. Obstetrics and Gynecology 1990;76(5 Pt 1):788‐91. [DOI] [PubMed] [Google Scholar]
Pistorius 2006 {published data only}
- Pistorius EN, Adang EM, Stalmeier PF, Braat DD, Kremer JA. Prospective patient and physician preferences for stimulation or no stimulation in IVF. Human Fertility 2006;9(4):209‐16. [DOI: 10.1080/14647270600560287] [DOI] [PubMed] [Google Scholar]
Rama Devi 2011 {published data only}
- Rama Devi P, Chatterjee C, Rajyalakshmi A, Navatha P, Arshiya F. A friendly IVF protocol. Journal of Obstetrics and Gynecology of India 2011;61(1):77‐80. [Google Scholar]
Reyftmann 2007 {published data only}
- Reyftmann L, Dechaud H, Loup V, Anahory T, Brunet‐Joyeux C, Lacroix N, et al. Natural cycle in vitro fertilization cycle in poor responders. Gynecologie, Obstetrique & Fertilite 2007;35(4):352‐8. [DOI: 10.1016/S1297-9589(07)00072-0] [DOI] [PubMed] [Google Scholar]
Schimberni 2011 {published and unpublished data}
- Schimberni M, Ubaldi F, Giallonardo A, Rienzi L, Morgia F, Sbracia M. A controlled trial between natural cycle versus minimal stimulation in poor responder women: minimal stimulation works better in patients less than 40 years old. Fertility and Sterility 2011;96(3 Suppl):S262 P‐525. [Google Scholar]
Strohmer 1997 {published data only}
- Strohmer H, Chatwani S, Wieser F, Danninger B, Obruca A, Feichtinger W. Prospective randomized study of an ultrashort gonadotrophin‐releasing hormone agonist versus a modified suppression protocol for ovarian stimulation in intracytoplasmic sperm injection cycles. Human Reproduction 1997;12(7):1403‐8. [DOI: 10.1093/humrep/12.7.1403] [DOI] [PubMed] [Google Scholar]
Vidal 2013 {published data only}
- Vidal. Use of Antagonist Versus Agonist GnRH in Oocyte Recipient Endometrium Preparation. 2013.
References to ongoing studies
Zhang 2013 {published data only}
- Zhang JJ, Veen F, Repping S, Wely M, Chang L, Wong S, et al. IVF Clinical Trial of Two Different Treatment Protocols.
Additional references
Al‐Inany 2011
- Al‐Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews (Online) 2011, (5):CD001750. [PUBMED: 21563131] [DOI] [PubMed] [Google Scholar]
Chavez‐Badiola 2011
- Chavez‐Badiola AE, Allahbadla GN. Minimal Stimulation IVF: Milder, Mildest Or Back to Nature. Jaypee Brothers Medical Publisher (P) Ltd, 2011. [Google Scholar]
Elder 2011
- Elder K, Dale B. In vitro fertilization. Cambridge University Press, 2011. [Google Scholar]
Evers 2002
- Evers JLH. Female subfertility. Lancet July 2002;360(9327):151–9. [DOI] [PubMed] [Google Scholar]
Geraedts 2012
Heineman 2011
- Heineman J, Evers JLH, Massuger LFAG, Steegers EAP. Obstetrie en Gynaecology De voortplanting van de mens. Elsevier Gezondheidszorg, 2011. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Leeton 1982
- Leeton J. The management of infertility: where to stop. Clinical Reproduction and Fertility 1982;1(4):249‐59. [PUBMED: 6821241] [PubMed] [Google Scholar]
Loutradis 2007
- Loutradis D, Drakakis P, Vomvolaki E, Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. Journal of Assisted Reproduction and Genetics December 2007;24(12):597‐611. [DOI: 10.1007/s10815-007-9181-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loutradis 2008
- Loutradis D, Vomvolaki E, Drakakis P. Poor responder protocols for in‐vitro fertilization: options and results. Current Opinion in Obstetrics & Gynecology 2008;20(4):374‐8. [DOI] [PubMed] [Google Scholar]
Naaktgeboren 1985
- Naaktgeboren N, Devroey P, Traey E, Wisanto A, Steirteghem AC. Success of in vitro fertilization and embryo transfer in relation to the causes of infertility. Acta Europaea Fertilitatis 1985;16(4):281‐7. [PUBMED: 2933915] [PubMed] [Google Scholar]
Nargund 2001
- Nargund G, Waterstone J, Bland J, Philips Z, Parsons J, Campbell S. Cumulative conception and live birth rates in natural (unstimulated) IVF cycles. Human Reproduction (Oxford, England) 2001;16(2):259‐62. [PUBMED: 11157816] [DOI] [PubMed] [Google Scholar]
Pandian 2010
- Pandian Z, McTavish AR, Aucott L, Hamilton MP, Bhattacharya S. Interventions for 'poor responders' to controlled ovarian hyper stimulation (COH) in in‐vitro fertilisation (IVF). Cochrane Database of Systematic Reviews Jan 20;1. [DOI] [PubMed] [Google Scholar]
Pelinck 2002
- Pelinck MJ, Hoek A, Simons AH, Heineman MJ. Efficacy of natural cycle IVF: a review of the literature. Human Reproduction Update 2002;8(2):129‐39. [PUBMED: 12099628] [DOI] [PubMed] [Google Scholar]
Pelinck 2009
- Pelinck MJ. Modified natural cycle IVF: feasibility and results. http://dissertations.ub.rug.nl/faculties/medicine/2009/m.j.pelinck/?pLanguage=en&pFullItemRecord=ON. Unpublished, 2009. [Google Scholar]
Rongieres‐Bertrand 1999
- Rongieres‐Bertrand C, Olivennes F, Righini C, Fanchin R, Taieb J, Hamamah S, et al. Revival of the natural cycles in in‐vitro fertilization with the use of a new gonadotrophin‐releasing hormone antagonist (Cetrorelix): a pilot study with minimal stimulation. Human Reproduction (Oxford, England) 1999;14(3):683‐8. [PUBMED: 10221695] [DOI] [PubMed] [Google Scholar]
Rosen 2008
- Rosen MP, Shen S, Dobson AT, Rinaudo PF, McCulloch CE, Cedars MI. A quantitative assessment of follicle size on oocyte developmental competence. Fertility and Sterility 2008;90(3):684‐90. [PUBMED: 18249377] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharlip 2002
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertility and Sterility 2002;77(5):873‐82. [PUBMED: 12009338] [DOI] [PubMed] [Google Scholar]
Verberg 2008
- Verberg MF, Eijkemans MJ, Heijnen EM, Broekmans FJ, Klerk C, Fauser BC, et al. Why do couples drop‐out from IVF treatment? A prospective cohort study. Human Reproduction (Oxford, England) 2008;23(9):2050‐5. [PUBMED: 18544578] [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang SX. The past, present, and future of embryo selection in in vitro fertilization: Frontiers in Reproduction Conference. The Yale Journal of Biology and Medicine 2011; Vol. 84, issue 4:487‐90. [PUBMED: 22180687] [PMC free article] [PubMed]
Zayed 1997
- Zayed F, Lenton EA, Cooke ID. Natural cycle in‐vitro fertilization in couples with unexplained infertility: impact of various factors on outcome. Human Reproduction (Oxford, England) 1997;12(11):2402‐7. [PUBMED: 9436673] [DOI] [PubMed] [Google Scholar]


