Abstract
Background
Home haemodialysis is associated with improved survival and quality of life in uncontrolled studies. However, relative benefits and harms of home versus in‐centre haemodialysis in randomised controlled trials (RCTs) are uncertain.
Objectives
To evaluate the benefits and harms of home haemodialysis versus in‐centre haemodialysis in adults with end‐stage kidney disease (ESKD).
Search methods
The Cochrane Renal Group's Specialised Register was searched up to 31 October 2014.
Selection criteria
RCTs of home versus in‐centre haemodialysis in adults with ESKD were included.
Data collection and analysis
Data were extracted by two investigators independently. Study risk of bias and other patient‐centred outcomes were extracted. Insufficient data were available to conduct meta‐analyses.
Main results
We identified a single cross‐over RCT (enrolling 9 participants) that compared home haemodialysis (long hours: 6 to 8 hours, 3 times/week) with in‐centre haemodialysis (short hours: 3.5 to 4.5 hours, 3 times/weeks) for 8 weeks in prevalent home haemodialysis patients. Outcome data were limited and not available for the end of the first phase of treatment in this cross‐over study which was at risk of bias due to differences in dialysate composition between the two treatment comparisons.
Overall, home haemodialysis reduced 24 hour ambulatory blood pressure and improved uraemic symptoms, but increased treatment‐related burden of disease and interference in social activities. Insufficient data were available for mortality, hospitalisation or dialysis vascular access complications or treatment durability.
Authors' conclusions
Insufficient randomised data were available to determine the effects of home haemodialysis on survival, hospitalisation, and quality of life compared with in‐centre haemodialysis. Given the consistently observed benefits of home haemodialysis on quality of life and survival in uncontrolled studies, and the low prevalence of home haemodialysis globally, randomised studies evaluating home haemodialysis would help inform clinical practice and policy.
Plain language summary
Home versus in‐centre haemodialysis for end‐stage kidney disease
Numerous studies observing people who are treated with haemodialysis at home show they have better quality of life and survival, but such analyses are not randomised (that is, participants treated with home haemodialysis may be younger and have fewer health problems that explain the improvements in outcomes observed).
Home haemodialysis may also increase burden of treatment for patients and families and risks complications associated with dialysis vascular access.
We investigated whether home haemodialysis improves clinical outcomes compared with haemodialysis treatment in a hospital or clinic setting (in‐centre haemodialysis) when participants are randomly assigned to different treatment settings.
We found that only one study that involved nine patients had compared home haemodialysis with in‐centre haemodialysis. There was insufficient information to understand the effects of home haemodialysis on survival or need for hospital admission in this study. Home haemodialysis may improve blood pressure and physical symptoms, but may increase the burden of care for families and patients. Given the potential benefits of home haemodialysis in non‐randomised studies, larger randomised trials of home haemodialysis could help inform clinical care and policy.
Background
Description of the condition
Patients with end‐stage kidney disease (ESKD) require dialysis or kidney transplantation to remove accumulated solutes and fluid. Haemodialysis is the most common form of dialysis worldwide, used by over 90% of people with ESKD in the United States (USRDS 2010) and between 60% and 80% of patients in Australia and New Zealand (ANZDATA 2009). While the number of people receiving haemodialysis is increasing by approximately 2% to 4% per year (ANZDATA 2009; USRDS 2010), and despite improvements in dialysis technologies, the annual mortality of haemodialysis patients is approximately 15%, although may be improving if adjustments for baseline comorbidities are considered. Mortality rates, particularly due to cardiovascular causes in adults treated with dialysis are 30 to 50 times that of the general population (de Jager 2009; Roberts 2011) and have remained unchanged over the last decade (USRDS 2010).
Dialysis is associated with a high symptom burden (Davison 2006; Davison 2010) including depression (Hedayati 2006), fatigue (Jhamb 2009), sexual dysfunction (Navaneethan 2010; Vecchio 2010), disordered sleep (Unruh 2008) and pain. People on dialysis are often unable to work (fewer than 10% to 25% are employed, although many are retired or were not working before commencing dialysis) (Kutner 1991; van Manen 2001) and experience markedly lower quality of life than kidney transplant recipients and the general population (Bremer 1989; Evans 1985).
Description of the intervention
Haemodialysis is usually performed in hospital or dialysis clinics (in‐centre haemodialysis), where dialysis and needle insertion are generally provided by nursing or technical staff and treatments consist of three sessions/week for 3.0 to 5.5 hours per session. Home haemodialysis, where people perform the haemodialysis procedure at home, has been available for decades although the numbers of people with ESKD dialysing at home is currently low. Home haemodialysis now represents 12% to 25% of all haemodialysis in Australia and New Zealand (ANZDATA 2009) whereas approximately 4000 of the nearly 400,000 prevalent patients on haemodialysis (∼1%) in the United States perform haemodialysis at home (USRDS 2010). This is in contrast to the 1970s when about half of all patients on haemodialysis did dialysis at home (Agar 2009).
Home haemodialysis requires one to four months training for patients at a specialised clinic or hospital‐based training program. The haemodialysis machine is installed in the patient's home during this time, potentially requiring modifications to accommodate the machine and provide an appropriate water and power supply. Home haemodialysis, where one dialysis machine is provided per patient, allows flexible treatment schedules and longer treatment times than conventional in‐centre haemodialysis. Dialysis sessions can be long (6 to 10 hours, 3 to 7 times/week) or short and frequent (1.5 to 2 hours, 5 to 7 times/week) and treatment schedules can be changed to suit other patient commitments. Sleeping during long‐slow dialysis overnight reduces the need to dialyse during the day and may facilitate return to employment. Technical assistance can be provided to patients at home, when needed, for machine maintenance and supply of consumables. Blood tests are usually monitored at a frequency determined by the dialysis provider and the frequency of routine assessment visits to hospital nephrology staff with home haemodialysis is variable and may be as infrequent as yearly (McGregor 2000). A dialysis helper (often a spouse or family member) is usually required in the home during dialysis in case the individual needs assistance.
How the intervention might work
Epidemiological studies indicate that home haemodialysis is associated with improved survival compared with in‐centre haemodialysis (Charra 1992; Mailloux 1988; McGregor 2000; Nitsch 2010; Saner 2005;Woods 1996). Home haemodialysis may improve outcomes through longer treatment times; longer dialysis duration is associated with lower mortality, which persists even when adjusted for haemodialysis dose (the amount of solute clearance) (Held 1991; Marshall 2006; Saran 2006). Home haemodialysis has also been associated with improved blood pressure (BP) and phosphorus levels, rehabilitation, quality of life, and other positive aspects of self‐care (autonomy, preferred self‐identity) (Cases 2011; Walsh 2005). However, because such observational cohort studies are uncontrolled or confounded by indication (patients on home haemodialysis are generally younger, more often male, and with fewer comorbidities), this evidence of an association is weak. Recent randomised controlled trials (RCTs) have demonstrated the superiority of more frequent haemodialysis on composite outcomes of death or change in ventricular mass (FHN Trial Group 2010), and frequent nocturnal dialysis (6 hours, 5 to 6 times/week) improves left ventricular mass, phosphorus control (Walsh 2010) and some components of quality‐of‐life compared with conventional in‐centre haemodialysis (Walsh 2006). Home haemodialysis can achieve both more frequent dialysis and longer dialysis times; suggesting improved survival is possible when compared with in‐centre haemodialysis.
Why it is important to do this review
Home haemodialysis has long been available, potentially providing improved treatment flexibility, better quality of life, and lower mortality. On the other hand the increased responsibilities of patients and their families may have negative effects on overall health, including a potentially greater need for hospitalisations for vascular access and lower quality of life due to increased treatment burden. If home haemodialysis is to be promoted and funded, it is necessary to summarise critically the available study evidence for the efficacy and safety of home haemodialysis, and highlight research questions that need additional investigation in future RCTs.
Objectives
To evaluate the benefits and harms of home haemodialysis versus in‐centre haemodialysis in adults with ESKD.
Methods
Criteria for considering studies for this review
Types of studies
We considered all RCTs and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) of home haemodialysis compared with in‐centre haemodialysis in people with ESKD treated with renal replacement therapy (RRT) for inclusion. Non‐randomised studies were not eligible for inclusion. Cross‐over studies were considered.
Types of participants
All adults with ESKD. Participants could be established on haemodialysis or peritoneal dialysis and then randomised to haemodialysis (home or in‐centre haemodialysis).
Participants could also have chronic kidney disease (CKD) with expectation to commence haemodialysis within a time frame designated by the study investigators.
Types of interventions
We included studies in which participants were randomised to home haemodialysis or in‐centre haemodialysis. Haemodialysis could be provided using any dialysis machine, dialysate, blood or dialysate flow rate, membrane type, dialysis dose (urea clearance), or vascular access type (central venous catheter, or arteriovenous fistula or graft).
Home haemodialysis was defined as any type of haemodialysis, haemodiafiltration, or haemofiltration carried out by the patient, technician, or nurse, at home. We included any duration of dialysis and any frequency in either treatment arm.
In‐centre haemodialysis included dialysis provided in a hospital unit, a private dialysis provider, or a satellite dialysis unit in which nursing or technical staff provide dialysis care.
Studies of haemodialysis performed at self‐care facilities other than home were not considered. RCTs evaluating peritoneal dialysis as a home dialysis modality were not included.
Types of outcome measures
-
Mortality and cardiovascular events
All‐cause mortality
Cardiovascular mortality (fatal myocardial infarction, fatal stroke, sudden death, heart failure)
Composite endpoints of major adverse cardiovascular events and death
Non‐cardiovascular mortality
Non‐fatal myocardial infarction
Non‐fatal stroke
Revascularisation
Hospitalisation (all‐cause, cardiovascular cause, unrelated to vascular access)
Quality of life: we planned to consider and tabulate where necessary all reports of quality of life outcomes using any instrument. Meta‐analyses were to be conducted when sufficient studies report quality of life outcomes using a single instrument including measures of depression, household financial stress. We will planned to assess end‐of‐treatment employment status (employed, unemployed, not eligible for employment)
Symptoms during dialysis (intradialytic cramping, hypotension, nausea, vomiting, headache)
-
Complications secondary to vascular access
Hospitalisation due to vascular access complication or procedure (≥ 1 event)
Access‐related bacteraemia (≥ 1 event)
Insertion or replacement of dialysis central venous catheter (≥ 1 event)
Vascular access intervention including surgery or percutaneous intervention (≥ 1 event)
Vascular access angiogram (≥ 1 event)
Time to access failure; access failure (≥ 1 event)
BP (pre‐dialysis systolic and diastolic BP at end‐of‐treatment) (mm Hg)
Change in predialysis BP (mm Hg)
Number and dose of BP medications at end‐of‐treatment
Change in number of BP medications at end‐of‐treatment
Left ventricular mass (described using any diagnostic tool including magnetic resonance imaging or echocardiography (g; g/m²), considered separately according to diagnostic tool and summarized using standardized mean differences)
Haemoglobin at end‐of‐treatment (g/dL; m/m²)
Serum phosphorus at end‐of‐treatment (mg/dL)
Serum calcium at end‐of‐treatment (mg/dL)
Serum beta₂ microglobulin at end of study
Serum calcium by phosphorus product at end‐of‐treatment (mg²/dL²)
Number and dose of phosphorus binding agents at end‐of‐treatment
Number and dose of BP lowering agents at end‐of‐treatment
Number and dose of any other drugs at end‐of‐treatment
Change in number of BP lowering drugs at end of treatment
Dose of vitamin D compounds at end‐of‐treatment
Dose of epoetin at end‐of‐treatment (units or units/kg) calculated as erythropoietin‐equivalent doses
Parathyroidectomy
Wait‐listing for kidney transplant
Recovery time
Waking hours free for living
Hospital‐acquired infection rate.
We planned to tabulate other adverse events reported in the available studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register through contact with the Trials' Search Co‐ordinator to 1 November 2014 using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the specialised register are identified through search strategies for CENTRAL, MEDLINE and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Letters seeking information about unpublished or incomplete studies were sent to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may have been relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that might have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. We sought unpublished disaggregated data for outcomes among subgroups of patients on home or in‐centre haemodialysis from study investigators of potentially eligible studies. Disagreements were resolved by consultation with all authors.
Two independent authors used standardised data forms to extract the following data.
Study design: parallel or cross‐over, risks of bias, duration, sample size, non‐randomised co interventions, location of study, number of centres
Participants: source, time on dialysis, previous dialysis modality, age, gender, ethnicity, comorbidities (hypertension, diabetes mellitus, cardiovascular disease), baseline biochemistry and haemoglobin), occupational status, quality of life scores, educational attainment, medications used, distance from dialysis centre, interdialytic weight gain
Interventions: make and model of dialysis machine, dialysis duration/week, dialysis duration/session, number of dialysis sessions/week, dialysis membrane type (synthetic, cellulose, modified cellulose), dialysis flow rate, dialysis composition (acetate/bicarbonate), dialysis membrane reuse, ultrafiltration (volume/rate)
Outcomes: as described in Types of outcome measures.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
We made explicit judgements regarding whether studies were at high risk of bias according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For dichotomous outcomes (mortality, cardiovascular events, hospitalisation, vascular access adverse events) results were to be expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (quality of life scale, BP, doses of medication, haemoglobin, biochemical variables), the mean difference (MD) was to be used, or the standardised mean difference (SMD) if different scales had been used. We planned to be cautious when providing summary estimates of treatment when high‐level heterogeneity between studies cannot be explained.
Meta‐analysis of change scores
We planned to combine change‐from‐baseline and final value scores (e.g. left ventricular mass, BP, haemoglobin, serum phosphorus, calcium) in a meta‐analysis using the (unstandardized) MD method (Higgins 2011). End‐of‐treatment values and change‐from‐baseline scores were to be placed in subgroups for clarity and pooled using random effects meta‐analysis.
Imputing standard deviation
We planned to impute change‐from‐baseline SD using an imputed correlation coefficient when sufficient data are available. We planned to conduct sensitivity analyses if possible to evaluate the effect of imputing missing SD data in our meta‐analysis.
Unit of analysis issues
We planned to include only data from the first period of treatment in cross‐over studies (Higgins 2011). Data in different metrics were be analysed by converting reported values to SI units. The final results were to be presented in International System (SI) units with conventional units in parentheses.
Dealing with missing data
If possible, data for each prespecified outcome were to be evaluated regardless of whether the analysis is based on intention‐to‐treat or completeness to follow‐up. In particular, dropout rates will be investigated and reported in detail (e.g. drop‐out due to discontinuation of dialysis modality, treatment failure, death, transplantation, withdrawal of consent or loss to follow‐up). When data were unavailable or not reported in an extractable format, we contacted the original investigators to request the missing data. We planned to assess all studies for risks of bias due to incomplete reporting of results.
Assessment of heterogeneity
We planned to test for heterogeneity with the Cochran Q test which follows a Chi² distribution with N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance. The extent of heterogeneity was to be assessed with I², which ranges between 0% and 100% and expresses the proportion of between group variability that is attributable to heterogeneity rather than chance (Higgins 2003). I² values of above 75% are typically held to signify extreme heterogeneity, whereas 25% and 50% correspond to low and medium levels of heterogeneity, respectively.
Assessment of reporting biases
We planned to test for asymmetries in the inverted funnel plots (i.e. for systematic differences in the effect sizes between more precise and less precise studies) using the original and modified Egger tests (Egger 1997) and the Begg and Mazumdar correlation test (Begg 1994). There are many potential explanations for why an inverted funnel plot may be asymmetric, including chance, heterogeneity, publication and reporting bias (Terrin 2005). We planned to refrain from judging funnel plot asymmetries based on visual inspection as this has been shown to be misleading in empirical research (Lau 2006). Publication bias was also be evaluated by testing the robustness of the results according to publications, namely publication as a full manuscript in a peer reviewed journal versus studies published as abstracts/text/letters/editorials.
Data synthesis
Data were to be pooled using the random‐effects model but the fixed‐effect model was also to be used to ensure robustness of the model chosen and susceptibility to outliers. We planned to qualitatively summarise data where insufficient data are available for meta‐analysis. Qualitative review was to be conducted for adverse events and quality of life outcomes.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis were to be used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age and haemodialysis methods. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy. Adverse effects were to be tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. If possible, the risk difference (RD) with 95% CI were to be calculated for each adverse effect, either compared to no treatment or to another agent.
Heterogeneity was to be investigated by analysing the data using subgroups according to the following parameters:
-
Population characteristics
Presence or absence of co‐morbidities (diabetes, hypertension, dyslipidaemia, smoking, obesity, family history of cardiovascular disease, baseline cardiovascular disease); percentage of patients with these co‐morbidities in each study
Age (adult, paediatric)
Gender
Mean systolic BP
Ethnicity (White, Afro‐American, Asian, other)
Time on dialysis (less than three years versus three years or more)
-
Intervention characteristics
Duration of home haemodialysis/session
Duration of home haemodialysis/week
Prescribed blood flow rate
Prescribed dialysis dose (Kt/V)
Hospital versus satellite clinic comparator
Acetate or bicarbonate dialysis
Number of home haemodialysis sessions/week
Duration of intervention (less than 6 months, 6 to 12 months, more than 12 months)
Treatment dropout rate
If sufficient studies were available, we planned to conduct sensitivity analysis based on allocation concealment, blinding of participants, investigators and outcome assessors, attrition (above or below 10%), ITT analysis, and premature discontinuation of the study.
We planned to perform univariate meta‐regression according to previously described methods if sufficient studies were identified (Palmer 2007).
Sensitivity analysis
Sensitivity analyses were to be undertaken to explore the robustness of findings to key decisions in the review process. These were to be determined as the review process takes place (Higgins 2011). Sensitivity analyses were to be undertaken to explore the influence of a study's risk of bias on the results.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified above
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Results
Description of studies
Results of the search
A search of the Cochrane Renal Group's Specialised Register (31 October 2014) retrieved 57 reports (Figure 1). We excluded seven records (five studies) on review of the title and abstract because they did not report comparison of home haemodialysis versus in‐centre haemodialysis (Cargill 2002; Fagugli 2001a; Gutman 1984; Raj 2000; Shand 1993).
1.
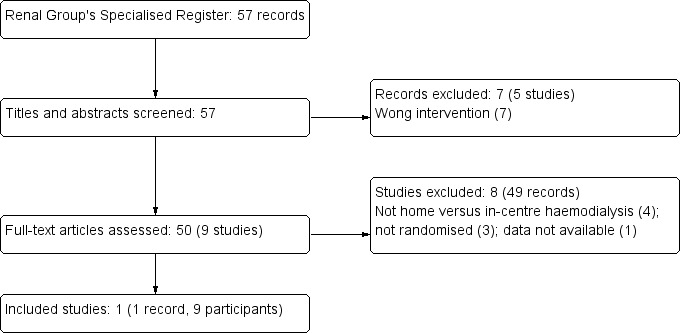
Flow diagram showing study selection process
We examined 50 full‐text reports (9 studies). We excluded 49 reports (8 studies) because they did not report an appropriate intervention (Walsh 2006; FHN DAILY Trial 2007; FHN NOCTURNAL Trial 2007; Zellweger 2004) or were not randomised (Chauveau 1999; De Smet 2007; Kraus 2007). One study was not available for full‐text analysis (Castro 1971). Although participants in Walsh 2006 were allocated to either conventional in‐centre haemodialysis or frequent nocturnal haemodialysis and included some patients treated at home, the randomised interventions were not in‐centre versus home haemodialysis; therefore this study was not included in the systematic review. In FHN NOCTURNAL Trial 2007, comparing nocturnal haemodialysis six times/week with conventional haemodialysis three times/week, dialysis treatment in both arms was carried out primarily at home and the study was not eligible for inclusion.
Included studies
We included one eligible study that involved nine participants (McGregor 2001). McGregor 2001 reported the effects of in‐centre haemodialysis for 3.5 hours to 4.5 hours, 3 times/week compared with long hours home haemodialysis for 6 to 8 hours, 3 times/week dialysed to the same eKt/Vurea among 9 home haemodialysis patients in a single centre cross‐over RCT. Participants were aware of allocated treatment, and outcome assessment was performed by assessors blinded to treatment allocation and sequence. Eligible participants had been on home haemodialysis for more 6 months, were dialysing more than 6 hours 3 times/week and were not receiving antihypertensive drugs (with a predialysis BP less than 160/90 mm Hg) before entry into the study. Follow‐up in each phase lasted for 8 weeks.
Risk of bias in included studies
Risk of bias in the included study is described in Characteristics of included studies, Figure 2 and Figure 3. Study methodology data not reported in the published study were obtained from the investigators on request. Allocation concealment methods were assessed as low risk (indicating that investigators were unlikely to anticipate treatment allocation of future enrolled participants) and had low risk methods for random sequence generation. Due to the nature of the interventions, the study could blind participants or investigators to treatment assignment. However, the study blinded outcome assessors to treatment allocation and sequence and reported no loss to follow‐up. The study reported all clinically‐relevant outcomes but had a clinically‐relevant mismatch in treatment interventions (acetate versus bicarbonate buffer) which may have reduced comparability between interventions. The study reported longer hours of haemodialysis in the home haemodialysis versus short hours of haemodialysis in the in‐centre arm, potentially introducing differences in treatment effects based on treatment duration. However, this was not considered to be a potential source of bias because longer‐hours dialysis might be considered a feature of home haemodialysis due to the increased treatment schedule flexibility of home‐based therapy.
2.
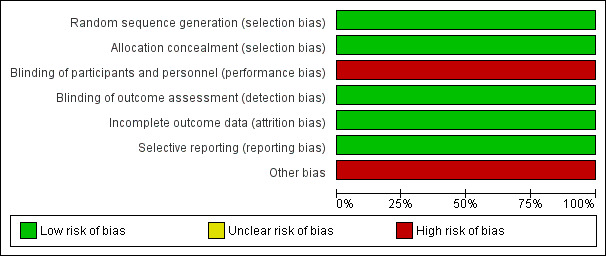
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
Study data could not be extracted for meta‐analysis because end of first phase study data were not reported and were not available from the investigators. All planned subgroup and sensitivity analyses could not be undertaken.
Mortality and hospitalisation
No mortality events or hospitalisation were reported.
Quality of life
After 8 weeks of home haemodialysis, patients reported fewer uraemia‐associated symptoms and less physical distress as measured by the Kidney Disease Quality of Life (KDQOL) instrument compared with eight weeks of shorter hours in‐centre haemodialysis. Patients also reported that home haemodialysis interfered with social activities more and placed a larger burden on their families.
Data for depression were not available.
Cardiac structure and function
There was no statistical difference in echocardiographic measures of left ventricular mass index, left ventricular ejection fraction, left atrial size, or indices of diastolic function between groups reported in the study.
Blood pressure
Patients were included only if they were not on antihypertensive medication. Symptomatic hypotensive episodes were more frequent with short‐hours in‐centre haemodialysis and 24‐hour ambulatory BP was lower with home haemodialysis at eight weeks.
Anaemia
Haematocrit levels were similar between treatment groups at end of follow‐up.
Calcium and phosphorus metabolism
Predialysis serum phosphorus levels were similar between treatment groups at eight weeks.
Other outcomes
No other outcome data were available.
Discussion
Summary of main results
One small RCT compared the effects of shorter hours in‐centre haemodialysis with longer hours home haemodialysis in nine prevalent home haemodialysis patients. Outcome data were limited and not available for the end of the first phase of treatment in this cross‐over study which was at risk of bias due to differences in dialysate composition between the two treatment comparisons.
Study data suggested longer hours (6 to 8 hours/sessions, 3 times/week) home haemodialysis lowers BP and reduces risks of symptomatic intradialytic hypotension and may improve uraemia‐related symptoms and physical suffering. However patients reported increased interference with social activities with home haemodialysis and a greater treatment burden for families. Effects on mortality and hospitalisation were uncertain and home haemodialysis showed similar effects on cardiac function, serum phosphorus levels and haematocrit compared with shorter hours in‐centre haemodialysis (3.5 to 4.5 hours/sessions, 3 times/week) in this short study.
Overall completeness and applicability of evidence
Randomised data comparing home haemodialysis with in‐centre haemodialysis were limited to a small, single centre study. Study participants were treated with home haemodialysis before enrolment and were not taking antihypertensive agents. Existing experience of home haemodialysis in the study population may have resulted in treatment effects on quality of life, treatment and symptom‐burden that differ systematically from in‐centre haemodialysis patients. Because a small minority of US and European haemodialysis patients are currently treated on home haemodialysis, the effects on quality of life may not be generalisable beyond the study setting.
Study participants tended to be a highly selected group of younger patients (mean age 48 years) without pre‐existing cardiovascular disease and diabetes, which reduced the generalisability of the study to other haemodialysis settings where patients are older and frequently experience multiple comorbid conditions (ANZDATA 2009; USRDS 2010).
Treatment effects were evaluated over eight weeks and there were insufficient data for patient‐relevant outcomes including mortality, hospitalisation, cardiovascular events, and dialysis vascular access complications to enable meta‐analysis.
The study compared longer hours of haemodialysis at home with shorter hours of haemodialysis in‐centre; it remains uncertain whether treatment effects between groups resulted from the location of the treatment or treatment duration.
A generalised and similar increase in physical health scores in prevalent in‐centre haemodialysis patients randomly allocated to either nocturnal haemodialysis at home (6 times/week) or conventional home haemodialysis (3 times/week) in FHN NOCTURNAL Trial 2007 suggests the location of treatment (based at home) rather than treatment frequency may contribute to improved quality of life.
Quality of the evidence
Data comparing home haemodialysis with in‐centre haemodialysis were limited to a single small study whose participants were younger than usual haemodialysis populations. Generally, data could not be extracted from the first treatment phase from the cross‐over study.
Risk of bias was increased by the lack of comparability between treatment interventions (dialysate composition), although the study was otherwise at low risk of bias.
Potential biases in the review process
While this review was conducted using standard Cochrane methodology, potential biases exist in the review process that may limit the validity of the findings. First, only one study was included after searching and assessment according to our inclusion criteria. Therefore, generalisability to most global haemodialysis settings is limited. Secondly, publication bias may exist (i.e. bias caused by lack of publication with neutral or opposite effects). Due to lack of sufficient data, we could not test for potential publication bias. Third, highly relevant clinical outcomes could not be extracted from the included study. Due to the short term nature of the study, patient‐relevant outcomes including death, hospitalisation, and dialysis vascular access outcomes were not reported. Beneficial effects or harms of home haemodialysis may not have been observed due to a lack of reported data. Finally, the included study was conducted in patients already established on home haemodialysis, for whom experiences of different venues for haemodialysis treatment may be systematically different than for patients established on in‐centre haemodialysis.
Agreements and disagreements with other studies or reviews
A 2003 review of home haemodialysis summarised data from 22 cohort studies that compared home versus hospital or satellite unit haemodialysis for people with ESKD (Mowatt 2003). Mowatt 2003 found that people treated with haemodialysis at home experienced better quality of life, although treatment burden for partners was increased. In these uncontrolled analyses, the investigators concluded that patients on home haemodialysis were hospitalised less, survived longer, were more likely to be in full time employment and experienced fewer dialysis‐related complications. However, due to confounding by treatment selection (patients treated at home are likely to be younger and have better functional status) and lack of meta‐analysis in this review, the consistency and effect size of home haemodialysis on clinical outcomes (quality of life, mortality, hospital admission) based on these analyses was uncertain.
Similar to available data for home haemodialysis, a review of cohort studies of daily haemodialysis in adults (14 populations comprising 268 patients) by Suri 2006b found that evidence was limited by small sample sizes for available studies, non‐comparable control groups, bias due to selection and attrition, and insufficient data for potential risks.
In a review of nocturnal haemodialysis, Walsh 2005 included 14 cohort studies that showed improved BP after conversion to nocturnal haemodialysis, although data for mortality were lacking and analyses were at risk of bias due to treatment selection.
Overall, these reviews together with the present review suggest current data for the benefits and harms of differing modalities of haemodialysis treatment (home, in‐centre, frequent, nocturnal, daily) are sparse.
Two recent RCTs comparing increased frequency, duration of haemodialysis or both (6 times/week compared to 3 times/week) (Walsh 2006; FHN DAILY Trial 2007) showing improvements in cardiac function, selected components of quality of life and improved BP control suggest that longer hours' dialysis might improve patient‐relevant outcomes in adults treated with haemodialysis, although dialysis vascular access complications may be more frequent. Home haemodialysis has the potential to increase dialysis frequency and duration through greater treatment flexibility, although uncontrolled studies and the single available RCT suggesting increased treatment burden indicates potential treatment‐hazards for home haemodialysis that warrant further exploration.
Authors' conclusions
Implications for practice.
Currently available randomised data were insufficient to determine the treatment benefits and harms of home haemodialysis on patient‐relevant outcomes.
Implications for research.
Given the existing poor survival and high symptom burden associated with haemodialysis treatment, new modifiable determinants of health and clinical outcomes in this population are needed. Non‐randomised evidence suggested that home haemodialysis was associated with better survival and quality of life that warrants further exploration in RCTs.
The feasibility of a RCT of home haemodialysis could be explored using a vanguard study design to assess if sufficient patients can be randomised, whether centres can successfully train participants for home haemodialysis, and if participants can adhere to allocated treatments (Suri 2006a). A multinational collaborative trial network may be necessary to achieve a RCT of home haemodialysis evaluating the benefits (survival, hospitalisation, quality of life) and harms (dialysis vascular access complications, and treatment burden and durability) of home haemodialysis.
What's new
| Date | Event | Description |
|---|---|---|
| 4 December 2014 | Review declared as stable | As of December 2014 this Cochrane Review is no longer being updated. There have been no new studies published on this topic in the past 12 years and there are currently no registered ongoing studies. |
Notes
As of December 2014 this Cochrane Review is no longer being updated. There have been no new studies published on this topic in the past 12 years and there are currently no registered ongoing studies.
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID) |
|
| EMBASE (OVID) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
McGregor 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | In‐centre HD
Home HD
|
|
| Outcomes |
*Relevant to this review |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation using random number table designed by statistician (data obtained from authors on request) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation assigned by statistician unaware of patient details (data obtained from authors on request) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quality of life, BP, and echocardiography outcomes assessed by investigators unaware of treatment sequence (data obtained from authors on request) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/9 (0%) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes systematically assessed |
| Other bias | High risk | Unmatched interventions (acetate versus bicarbonate buffer) |
BP ‐ blood pressure; HD ‐ haemodialysis; NS ‐ not stated; RCT ‐ randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cargill 2002 | Not home versus in‐centre HD |
| Castro 1971 | Data not available |
| Chauveau 1999 | Not RCT |
| De Smet 2007 | Not RCT |
| Fagugli 2001a | Not home versus in‐centre HD |
| FHN DAILY Trial 2007 | Not home versus in‐centre HD |
| FHN NOCTURNAL Trial 2007 | Not home versus in‐centre HD |
| Gutman 1984 | Not home versus in‐centre HD |
| Kraus 2007 | Not RCT |
| Raj 2000 | Not home versus in‐centre HD |
| Shand 1993 | Not home versus in‐centre HD |
| Walsh 2006 | Not home versus in‐centre HD (requested additional data) |
| Zellweger 2004 | Not home versus in‐centre HD |
HD ‐ haemodialysis; RCT ‐ randomised controlled trial
Contributions of authors
Draft the protocol: SP, GFMS
Study selection: SP, AP
Extract data from studies: SP, AP
Enter data into RevMan: SP, AP
Carry out the analysis: SP
Interpret the analysis: SP, AP, JC, DJ, PS, LF, ML, SH, JH, GFMS
Draft the final review: SP
Review the final review for intellectual content: AP, JC, DJ, PS, LF, ML, SH, JH, GFMS
Disagreement resolution: GFMS
Update the review: SP, AP, GFMS
Sources of support
Internal sources
University of Otago, Christchurch, New Zealand.
Diaverum Scientific Office, Sweden.
Consorzio Mario Negri Sud, Italy.
External sources
No sources of support supplied
Declarations of interest
Jonathan C Craig: none known
Luc Frantzen: fees for consulting from Fresenius Medical Care
Jorgen Hegbrant: paid an honorarium as a member of the Gambro Safety Board
Susanne Hoischen: None known
David W Johnson: consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speaker's honoraria and research grants from Fresenius Medical Care and is a current recipient of a Queensland Government Health Research Fellowship
Miguel Leal: none known
Andrew R Palmer: none known
Suetonia C Palmer: none known
Giovanni FM Strippoli: none known
Paul Stroumza: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
McGregor 2001 {published data only}
- McGregor DO, Buttimore AL, Lynn KL, Nicholls MG, Jardine DL. A comparative study of blood pressure control with short In‐center versus long home hemodialysis. Blood Purification 2001;19(3):293‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cargill 2002 {published data only}
- Cargill A, Watson AR. An evaluation of telecare to support patients receiving chronic peritoneal dialysis at home [abstract]. Peritoneal Dialysis International 2002;22(1):136. [CENTRAL: CN‐00594560] [PubMed] [Google Scholar]
Castro 1971 {published data only}
- Castro L, Gahl G, Froese P, Kessel M. Comparison of effectiveness and frequency of complications in clinical continuous dialysis and home dialysis. Die Medizinische Welt 1971;22(24):1031. [CENTRAL: CN‐00305035] [Google Scholar]
Chauveau 1999 {published data only}
- Chauveau P, Larroumet N, Desvergenes C, Montoriol J, Combe C, Aparicio M. A 3‐year prospective study of the outcome of patients dialyzed at home or in self‐care units [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A184. [CENTRAL: CN‐00483479] [Google Scholar]
De Smet 2007 {published data only}
- Smet R, Dhondt A, Eloot S, Claus S, Vanholder R. Nocturnal hemodialysis with the Genius® dialysis system [abstract no: FP327]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi128. [Google Scholar]
Fagugli 2001a {published data only}
- Fagugli RM, Reboldi G, Quintaliani G, Pasini P, Ciao G, Cicconi B, et al. Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. American Journal of Kidney Diseases 2001;38(2):371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FHN DAILY Trial 2007 {published data only}
- Chan CT, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, Larive B, et al. Effects of daily hemodialysis on heart rate variability: results from the frequent hemodialysis network (FHN) daily trial. Nephrology Dialysis Transplantation 2014;29(1):168‐78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circulation. Cardiovascular Imaging 2012;5(2):251‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(12):2106‐16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P, et al. Determinants of cardiac autonomic dysfunction in ESRD. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(10):1821‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. Journal of the American Society of Nephrology 2012;23(4):727‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney International 2013;83(5):949‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In‐center hemodialysis six times per week versus three times per week. New England Journal of Medicine 2010;363(24):2287‐300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frequent Hemodialysis Network (FHN). The Frequent Hemodialysis Network multicenter randomized trial of in‐center daily hemodialysis [abstract no: F‐PO009]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A. [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. Progress of the frequent hemodialysis network randomized trial of in‐center daily hemodialysis [abstract no: 61]. American Journal of Kidney Diseases 2007;49(4):A40. [CENTRAL: CN‐00601916] [Google Scholar]
- Greene T, Daugirdas J, Depner T, Gotch F, Kuhlmann M, Kotanko P, et al. Clearance and volume removal values for target dialysis prescriptions in the Frequent Hemodialysis Network (FHN) trials [abstract no: F‐PO003]. Journal of the American Society of Nephrology 2006;17(Abstracts):337A. [CENTRAL: CN‐00602073] [Google Scholar]
- Greene T, Daugirdas JT, Depner TA, Gotch F, Kuhlman M, Frequent Hemodialysis Network Study Group, et al. Solute clearances and fluid removal in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2009;53(5):835‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter P, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning Frequent Hemodialysis Network (FHN) Randomized Trials. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(5):782‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamb M, Tamura MK, Gassman J, Garg AX, Lindsay RM, Suri RS, et al. Design and rationale of health‐related quality of life and patient‐reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purification 2011;31(1‐3):151‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Greene T, Larive B, Mehta RL, Lindsay RM, Depner TA, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney International 2012;82(1):90‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Larive B, Painter P, Craig A, Lindsay RM, Rocco MV, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. American Journal of Kidney Diseases 2011;57(1):101‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network Trials. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1429‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2013;61(2):228‐37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornt DB, Larive B, Rastogi A, Rashid M, Daugirdas JT, Hernandez A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrology Dialysis Transplantation 2013;28(7):1888‐98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. American Journal of Kidney Diseases 2011;57(1):90‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyeva O, Gorodetskaya I, Champagne J, Sherer S, Carter M. Recruitment successes and challenges in the frequent hemodialysis network (FHN) daily trial [abstract no: SU‐FC058]. Journal of the American Society of Nephrology 2007;18(Abstracts):79A. [Google Scholar]
- Sergeyeva O, Gorodetskaya I, Ramos R, Schiller BM, Larive B, Raimann JG, et al. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. Journal of Nephrology 2012;25(3):302‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stokes JB. Consequences of frequent hemodialysis: comparison to conventional hemodialysis and transplantation. Transactions of the American Clinical & Climatological Association 2011;122:124‐36. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney International 2007;71(4):349‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suri RS, Larive B, Hall Y, Kimmel PL, Kliger AS, Levin N, et al. Effects of frequent hemodialysis on perceived caregiver burden in the Frequent Hemodialysis Network trials. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):936‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology 2013;24(3):498‐505. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6‐times‐weekly versus 3‐times‐weekly hemodialysis on depressive symptoms and self‐reported mental health: Frequent Hemodialysis Network (FHN) Trials. American Journal of Kidney Diseases 2013;61(5):748‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FHN NOCTURNAL Trial 2007 {published data only}
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circulation. Cardiovascular Imaging 2012;5(2):251‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P, et al. Determinants of cardiac autonomic dysfunction in ESRD. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(10):1821‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. Journal of the American Society of Nephrology 2012;23(4):727‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney International 2013;83(5):949‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In‐center hemodialysis six times per week versus three times per week. New England Journal of Medicine 2010;363(24):2287‐300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network randomized trial of home nocturnal hemodialysis [abstract no: F‐PO010]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A. [CENTRAL: CN‐00602072] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network randomized trial of home nocturnal hemodialysis: change of trial design [abstract no: 62]. American Journal of Kidney Diseases 2007;49(4):A40. [Google Scholar]
- Gassman JJ, Rocco M, Beck G, Larive B, Sherer S, Kliger A, et al. Changing a protocol early in a trial's vanguard phase: the Frequent Hemodialysis Network Home Nocturnal Hemodialysis Trial [abstract no: P71]. Clinical Trials 2007;4(4):421. [CENTRAL: CN‐00782507] [Google Scholar]
- Greene T, Daugirdas J, Depner T, Gotch F, Kuhlmann M, Kotanko P, et al. Clearance and volume removal values for target dialysis prescriptions in the Frequent Hemodialysis Network (FHN) trials [abstract no: F‐PO003]. Journal of the American Society of Nephrology 2006;17(Abstracts):337A. [CENTRAL: CN‐00602073] [Google Scholar]
- Greene T, Daugirdas JT, Depner TA, Gotch F, Kuhlman M, Frequent Hemodialysis Network Study Group, et al. Solute clearances and fluid removal in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2009;53(5):835‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter P, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning Frequent Hemodialysis Network (FHN) Randomized Trials. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(5):782‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamb M, Tamura MK, Gassman J, Garg AX, Lindsay RM, Suri RS, et al. Design and rationale of health‐related quality of life and patient‐reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purification 2011;31(1‐3):151‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Greene T, Larive B, Mehta RL, Lindsay RM, Depner TA, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney International 2012;82(1):90‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Larive B, Painter P, Craig A, Lindsay RM, Rocco MV, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. American Journal of Kidney Diseases 2011;57(1):101‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network Trials. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1429‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2013;61(2):228‐37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornt DB, Larive B, Rastogi A, Rashid M, Daugirdas JT, Hernandez A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrology Dialysis Transplantation 2013;28(7):1888‐98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin M, Eggers PW, Larive B, Rocco MV, Stokes JB, Suri RS, et al. Recruitment and training for home hemodialysis: experience and lessons from the nocturnal dialysis trial. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(9):1614‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. American Journal of Kidney Diseases 2011;57(1):90‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Lockridge RS Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: The Frequent Hemodialysis Network Nocturnal Trial. Kidney International 2011;80(10):1080‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JB. Consequences of frequent hemodialysis: comparison to conventional hemodialysis and transplantation. Transactions of the American Clinical & Climatological Association 2011;122:124‐36. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney International 2007;71(4):349‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suri RS, Larive B, Hall Y, Kimmel PL, Kliger AS, Levin N, et al. Effects of frequent hemodialysis on perceived caregiver burden in the Frequent Hemodialysis Network trials. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):936‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology 2013;24(3):498‐505. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6‐times‐weekly versus 3‐times‐weekly hemodialysis on depressive symptoms and self‐reported mental health: Frequent Hemodialysis Network (FHN) Trials. American Journal of Kidney Diseases 2013;61(5):748‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutman 1984 {published data only}
- Gutman RA, Blumenkrantz MJ, Chan YK, Barbour GL, Gandhi VC, Shen FH, et al. Controlled comparison of hemodialysis and peritoneal dialysis: Veterans Administration multicenter study. Kidney International 1984;26(4):459‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kraus 2007 {published data only}
- Kraus M, Burkart J, Hegeman R, Solomon R, Coplon N, Moran J. A comparison of center‐based vs. home‐based daily hemodialysis for patients with end‐stage renal disease. Hemodialysis International 2007;11(4):468‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raj 2000 {published data only}
- Raj DS, Ouwendyk M, Francoeur R, Pierratos A. beta(2)‐microglobulin kinetics in nocturnal haemodialysis. Nephrology Dialysis Transplantation 2000;15(1):58‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shand 1993 {published data only}
- Lynn KL, Buttimore AL, Inkster JA, Divakar D, Mylius AL, Ikram H, et al. Echocardiographic assessment of cardiac effects of erythropoietin in haemodialysis patients [abstract]. 9th Asian Colloquium in Nephrology; 1992 May 17‐21; Seoul, Korea. 1992:183.
- Lynn KL, Richards AM, Buttimore AL, Inkster JA, Bailey RR, Robson RA, et al. Placebo‐controlled study of blood pressure and vasoactive hormones in haemodialysis patients on erythropoietin [abstract]. 9th Asian Colloquium in Nephrology; 1992 May 17‐21; Seoul, Korea. 1992:183.
- Shand BI, Buttimore AL, Hurrell MA, Wells JE, Inkster JA, Bailey RR, et al. Hemorheology and fistula function in home hemodialysis patients following erythropoietin treatment: a prospective placebo‐controlled study. Nephron 1993;64(1):53‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 2006 {published data only}
- Bass A, Ahmed SB, Klarenbach S, Culleton B, Hemmelgarn BR, Manns B. The impact of nocturnal hemodialysis on sexual function. BMC Nephrology 2012;13:67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott‐Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007;298(11):1291‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott‐Douglas N, Quinn RR, et al. Nocturnal hemodialysis lowers blood pressure and reduces left ventricular mass: results of a randomized controlled trial [abstract no: SU‐FC002]. Journal of the American Society of Nephrology 2007;18(Abstracts):67A‐8A. [CENTRAL: CN‐00783714] [Google Scholar]
- Khangura J, Culleton BF, Manns BJ, Zhang J, Barnieh L, Walsh M, et al. Association between routine and standardized blood pressure measurements and left ventricular hypertrophy among patients on hemodialysis. BMC Nephrology 2010;11:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbach S, Tonelli M, Pauly R, Walsh M, Culleton B, So H, et al. Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. Journal of the American Society of Nephrology 2014;25(3):587‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns BJ, Klarenbach S, Walsh M, Quinn R, Tonelli M, Scott‐Douglas N, et al. The impact of nocturnal hemodialysis on quality of life: results of a randomized controlled trial [abstract no: F‐PO891]. Journal of the American Society of Nephrology 2007;18(Abstracts):298A‐9A. [CENTRAL: CN‐00784458] [Google Scholar]
- Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, et al. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney International 2009;75(5):542‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schorr M, Manns BJ, Culleton B, Walsh M, Klarenbach S, Tonelli M, et al. The effect of nocturnal and conventional hemodialysis on markers of nutritional status: results from a randomized trial. Journal of Renal Nutrition 2011;21(3):271‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walsh M, Manns B, Tonelli M, Quinn R, Culleton B. Description of a randomized controlled trial on the effects of nocturnal hemodialysis on left ventricular hypertrophy compared to conventional hemodialysis [abstract no: SA‐PO815]. Journal of the American Society of Nephrology 2005;16:734A‐5A. [CENTRAL: CN‐00583210] [Google Scholar]
- Walsh M, Manns BJ, Klarenbach S, Quinn R, Tonelli M, Culleton BF. The effects of nocturnal hemodialysis compared to conventional hemodialysis on change in left ventricular mass: rationale and study design of a randomized controlled pilot study. BMC Nephrology 2006;7:2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Manns BJ, Klarenbach S, Tonelli M, Hemmelgarn B, Culleton B. The effects of nocturnal compared with conventional hemodialysis on mineral metabolism: A randomized‐controlled trial. Hemodialysis International 2010;14(2):174‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zellweger 2004 {published data only}
- Deziel C, Zellweger M, Comeau R, Kerangueven M, Raymond‐Carrier S, Madore F. Hemodynamic stability during hemodialysis and quality of life with automated blood volume regulation: a prospective, randomized controlled trial.[abstract no: SU‐FC014]. Journal of the American Society of Nephrology 2004;15(Oct):47A. [CENTRAL: CN‐00583226] [Google Scholar]
- Deziel C, Zellweger M, Comeau R, Valleau A, Raymond‐Carrier S, Madore F. Home blood pressure management with automated blood volume regulation in hemodialysis patients: a prospective randomized controlled trial. [abstract no: SU‐PO279]. Journal of the American Society of Nephrology 2004;15(Oct):594A. [CENTRAL: CN‐00583224] [Google Scholar]
- Zellweger M, Deziel C, Bui TN, Madore F. Hemodialysis tolerance is significantly improved with blood volume control (hemocontrol®) when aiming at a better blood pressure control by reducing dry weight [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:334. [CENTRAL: CN‐00509576]
Additional references
Agar 2009
- Agar JW. International variations and trends in home hemodialysis. Advances in Chronic Kidney Disease 2009;16(3):205‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ANZDATA 2009
- McDonald S, Excell L. Method and location of dialysis. In: Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) Registry 2009 report. http://www.anzdata.org.au/anzdata/AnzdataReport/32ndReport/Ch04.pdf (accessed 30 October 2014).
Bremer 1989
- Bremer BA, McCauley CR, Wrona RM, Johnson JP. Quality of life in end‐stage renal disease: a reexamination. American Journal of Kidney Diseases 1989;13(3):200‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cases 2011
- Cases A, Dempster M, Davies M, Gamble G. The experience of individuals with renal failure participating in home haemodialysis: An interpretative phenomenological analysis. Journal of Health Psychology 2011;16(6):884‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Charra 1992
Davison 2006
- Davison SN, Jhangri GS, Johnson JA. Cross‐sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney International 2006;69(9):1621‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davison 2010
- Davison SN, Jhangri GS. Impact of pain and symptom burden on the health‐related quality of life of hemodialysis patients. Journal of Pain & Symptom Management 2010;39(3):477‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jager 2009
Evans 1985
FHN Trial Group 2010
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In‐center hemodialysis six times per week versus three times per week. New England Journal of Medicine 2010; Vol. 363, issue 24:2287‐300. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Hedayati 2006
Held 1991
- Held PJ, Levin NW, Bovbjerg RR, Pauly MV, Diamond LH. Mortality and duration of hemodialysis treatment. JAMA 1991; Vol. 265, issue 7:871‐5. [MEDLINE: ] [PubMed]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated February 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jhamb 2009
- Jhamb M, Argyropoulos C, Steel JL, Plantinga L, Wu AW, Fink NE, et al. Correlates and outcomes of fatigue among incident dialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2009; Vol. 4, issue 11:1779‐86. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Kutner 1991
- Kutner NG, Brogan D, Fielding B. Employment status and ability to work among working‐age chronic dialysis patients. American Journal of Nephrology 1991;11(4):334‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mailloux 1988
- Mailloux LU, Bellucci AG, Mossey RT, Napolitano B, Moore T, Wilkes BM, et al. Predictors of survival in patients undergoing dialysis. American Journal of Medicine 1988;84(5):855‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2006
- Marshall MR, Byrne BG, Kerr PG, McDonald SP. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney International 2006;69(7):1229‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McGregor 2000
- McGregor D, Buttimore A, Robson R, Little P, Morton J, Lynn K. Thirty years of universal home dialysis in Christchurch. New Zealand Medical Journal 2000; Vol. 113, issue 1103:27‐9. [MEDLINE: ] [PubMed]
Mowatt 2003
- Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al. Systematic review of the effectiveness and cost‐effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end‐stage renal failure. Health Technology Assessment (Winchester, England) 2003;7(2):1‐174. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navaneethan 2010
Nitsch 2010
Roberts 2011
- Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. American Journal of Kidney Diseases 2011;58(1):64‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saner 2005
Saran 2006
Suri 2006a
Suri 2006b
- Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, et al. Daily hemodialysis: a systematic review. Clinical Journal of The American Society of Nephrology: CJASN 2006;1(1):33‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Unruh 2008
- Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, et al. Subjective and objective sleep quality in patients on conventional thrice‐weekly hemodialysis: comparison with matched controls from the sleep heart health study. American Journal of Kidney Diseases 2008; Vol. 52, issue 2:305‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
USRDS 2010
- US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010. http://www.usrds.org/atlas10.aspx (accessed 30 October 2014).
van Manen 2001
- Manen JG, Korevaar JC, Dekker FW, Reuselaars MC, Boeschoten EW, Krediet RT, et al. Changes in employment status in end‐stage renal disease patients during their first year of dialysis. Peritoneal Dialysis International 2001; Vol. 21, issue 6:595‐601. [MEDLINE: ] [PubMed]
Vecchio 2010
- Vecchio M, Navaneethan SD, Johnson DW, Lucisano G, Graziano G, Saglimbene V, et al. Interventions for treating sexual dysfunction in patients with chronic kidney disease. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007747.pub2] [DOI] [PubMed] [Google Scholar]
Walsh 2005
- Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health‐related quality of life. Kidney International 2005;67(4):1500‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 2010
Woods 1996
References to other published versions of this review
Palmer 2012
- Palmer SC, Palmer AR, Craig JC, Johnson DW, Stroumza P, Frantzen L, et al. Home versus in‐centre haemodialysis for end‐stage kidney disease. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009535] [DOI] [PMC free article] [PubMed] [Google Scholar]


