Abstract
Background
This is an updated version of the original Cochrane review published in Issue 1, 2007.
Epilepsy is a disorder with recurrent epileptic seizures. Corticosteroids have been used in the treatment of children with epilepsy and have significant adverse effects. Their efficacy and tolerability have not been clearly established.
Objectives
To determine the efficacy, in terms of seizure control, improvements in cognition and in quality of life and tolerability of steroids compared to placebo or other antiepileptic drugs in children with epilepsy, excluding epileptic spasms.
Search methods
We searched the following databases: The Cochrane Epilepsy Group Specialized Register (1 August 2014); CENTRAL, (The Cochrane Library Issue 7, July 2014); MEDLINE (1946 to 1 August 2014); EMBASE (1966 to December 2004); Database of Abstracts of Reviews of Effectiveness (DARE; Issue 3 of the database published in The Cochrane Library Issue 7, July 2014); ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform ICTRP (1 August 2014).
We checked the reference lists of retrieved studies for additional reports of relevant studies.
Selection criteria
All randomised controlled trials of administration of corticosteroids to children (less than 16 years) with epilepsy.
Data collection and analysis
For this update two review authors independently selected trials for inclusion and extracted data. Outcomes included cessation of seizures, reduction in seizure frequency, improvement in cognition, quality of life and adverse effects of steroids.
Main results
A single RCT was included that recruited five children in a double blind cross‐over trial. One child was withdrawn prematurely from the study and another had infantile spasms and hence was excluded from further analysis. Adrenocorticotrophin hormone (ACTH 4‐9) was administered. Of the three children analysed, one showed a reduction in seizures of 25% to 50% at both the low and higher doses of corticosteroids compared to placebo; one child showed a reduction in seizures at the higher dose only and one child showed no reduction in seizures at either dose. No adverse effects were reported.
Authors' conclusions
Since the last version of this review no new evidence has been found for the efficacy of corticosteroids in treating childhood epilepsies. Clinicians using steroids in childhood epilepsies, other than for epileptic spasms, should take this into account before using these agents.
Plain language summary
Corticosteroids including ACTH (adrenocorticotrophin hormone) for childhood epilepsy other than epileptic spasms
Background
We wanted to assess whether corticosteroids including ACTH are an effective treatment for children with epilepsy. Corticosteroids are sometimes used as an additional therapy to antiepileptic drugs in children with uncontrolled epilepsy. The role of corticosteroids in children with epilepsy is yet to be established.
Study characteristics
The evidence is current to August 2014. One study was included in this review. In total, data from five children, with uncontrolled seizures, aged between one and 11 years old were assessed. The duration of this study was two months; one month for the corticosteroid treatment and one month for the placebo (inactive) treatment. One of the five children was withdrawn from the study and another had epileptic spasms which meant we could not include their data in our analysis. Therefore, we were only able to compare data for three children. All three received both of the study treatments and the frequency of seizures they experienced while taking each treatment was compared.
Key results
One child showed a 25% to 50% reduction in seizure frequency whilst being treated with low dose corticosteroids compared to the placebo treatment. This child plus another child both showed a 25% to 50% reduction in seizure frequency whilst being treated with the higher dose of corticosteroids compared to the placebo treatment. A third child showed no reduction in seizures with either of the doses. As only one study was found which included data from three out of five participants, further statistical analysis was not possible. No conclusions can be drawn regarding the role of corticosteroids in children with epilepsy.
Quality of evidence
The evidence from the one study included in this review was rated as low quality due to the small sample size. There is a need for larger, well‐designed studies.
Summary of findings
for the main comparison.
| ACTH 4‐9 compared with placebo for children with intractable seizures | |||
|
Patient or population: children with intractable seizures Settings: Sweden Intervention: ACTH 4‐9 Comparison: placebo | |||
| Outcomes | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Seizure frequency: Two out of five children had some improvement in seizure frequency. One child has unchanged seizure frequency and one child withdrew from the study and other had Infantile spasm. |
3 (1) |
⊕⊕⊝⊝ low | In spite of a reasonably good study design, there were only three children included in the analysis of this study and a very short follow‐up assessment. Therefore, the study is unlikely to be adequately powered to detect significant differences in treatment groups. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Background
Description of the condition
Epilepsy is defined as the occurrence of recurrent epileptic seizures. An epileptic seizure is defined as a manifestation of epileptic, i.e. excessive or hypersynchronous, usually self limited activity of neurons in the brain (Blume 2001). Epilepsy comprises many different syndromes and has an estimated incidence of 150 per 100,000 per year during the first year of life, 60 per 100,000 per year at ages five to nine years and 4 to ‐50 per 100,000 per year in older children. Its prevalence is 4 to 5 per 1000 in the developed world (Forsgren 2004). Up to 20% of patients continue to have seizures despite treatment with antiepileptic drugs (Deonna 2005).
Description of the intervention
Steroids, including prednisolone, prednisone, adrenocorticotrophin hormone (ACTH), methylprednisolone and hydrocortisone have been used in the treatment of various seizure disorders, including epileptic spasms, Landau‐Kleffner Syndrome, Lennox‐Gastaut syndrome, childhood myoclonic epilepsies and myoclonic astatic epilepsy. They have also been used to treat various forms of non‐convulsive status epilepticus, fugues, absence status, atonic status and complex partial status epilepticus (Chutorian 1968; Marescaux 1990; Oguni 2002; Tsuru 2000). Gelastic seizures have also been treated with ACTH (Go 1999). In addition, they have been used to treat early and explosive onset of epileptic encephalopathy, including Rasmussen’s encephalitis where there may be an autoimmune or post‐infectious inflammatory cause (Gupta 2005). Whilst their benefit in the treatment of epileptic spasms is well established (Hancock 2013; Lux 2005) their role in other types of epilepsy remains unclear. They are widely accepted as having significant adverse effects including increased risk of infection, glycosuria, electrolyte imbalance, suppression of the hypothalamus and pituitary, psychosis, weight gain, hypertension, increased risk of nephrocalcinosis, brain atrophy and subdural effusion (Riikonen 1986; Satoh 1982).
How the intervention might work
Prednisone is a synthetic corticosteroid. It has no substantial biological effects until converted via hepatic metabolism to prednisolone. Prednisolone is a synthetic glucocorticoid, a derivative of cortisol and is the active metabolite of the drug prednisone. Hydrocortisone is the pharmaceutical term used for cortisol, the principal glucocorticosteroid hormone secreted by the adrenal cortex in response to stimulation by ACTH. Strictly speaking ACTH is not a steroid; it is a 39 amino‐acid polypeptide secreted by the anterior pituitary, which when released stimulates the adrenal gland to produce cortisol. There are two 'ACTH' preparations in widespread use; ACTH i.e. adrenocorticotrophin hormone and tetracosactrin. ACTH is a natural bovine‐derived product administered as a daily intramuscular injection. Tetracosactrin is a synthetic analogue of corticotrophic hormone. However, in the UK, because of the concerns regarding bovine spongiform encephalopathy (BSE) ACTH has been withdrawn from the market. The mechanism or mechanisms underlying the antiepileptic action of steroids is unknown. Suggestions include alterations in neurochemical transmission as a result of alterations in serotonin turnover or gamma amino butyric acid (GABA) uptake. These effects may be mediated through the glucocorticoid or mineralocorticoid receptors, or both. Data on the age, sex, and site‐dependent expression of these in human brains are lacking (Watzka 2000). Deflazacort is a methyloxazoline derivative of prednisolone. Overall, deflazacort has demonstrated equivalent efficacy to that of prednisolone and other oral steroids in a variety of conditions. When compared with other oral steroids, such as betamethasone and prednisone, deflazacort has shown a lower incidence of steroid‐induced adverse effects, such as bone loss, glucose intolerance, Cushing’s syndrome and growth velocity suppression (Campbell 2003).
Why it is important to do this review
Current evidence based on some prospective and retrospective studies suggests steroids have some role in treating children with epilepsy. However, there is a paucity of high quality randomised controlled trials assessing the efficacy of steroids in this population.
In this updated review, we investigated the efficacy and tolerability of steroids in the treatment of the childhood epilepsies (excluding epileptic spasms) and their effect on seizures, cognition, quality of life and adverse effects.
Objectives
To determine the efficacy, in terms of seizure control, improvements in cognition and in quality of life and tolerability of steroids compared to placebo or other antiepileptic drugs in children with epilepsy, excluding epileptic spasms.
Methods
Criteria for considering studies for this review
Types of studies
(1) Randomised studies with adequate or quasi (e.g. days of the week) methods of randomisation. (2) Parallel group or cross‐over studies. (3) No minimum treatment period.
We included all randomised controlled trials (RCTs) of the administration of corticosteroids to children with epilepsy. Definition of RCT: trials in which participants were prospectively allocated to treatment groups by a random or quasi random process.
Types of participants
Children (less than 16 years) with any seizure type and any type of epilepsy other than epileptic spasms. For the purpose of this review, we assumed a clinical diagnosis of epilepsy had been made for any participant that was entered into the trial.
Types of interventions
Administration of steroids by oral, intravenous, intramuscular or subcutaneous route. (1) Any trial that compared steroid therapy against placebo treatment. (2) Any trial that compared steroid therapy against no therapy. (3) Any trial that compared steroid therapy against another therapy. Therapies included: 'Steroids': ACTH (including tetracosactrin); hydrocortisone; prednisone or prednisolone; methyl prednisolone; dexamethasone; deflazacort (derivative of prednisolone).
Types of outcome measures
Primary outcomes
Efficacy measures
The proportion of children who achieved complete cessation of seizures.
The proportion of children with 25%, 50% or 75% reduction in seizure frequency in the treatment period and for up to three months post‐treatment period compared to the prerandomised baseline period for the entire group, and for each specific seizure type groups.
Tolerability measures
Adverse effects were documented.
Secondary outcomes
Tolerability measures
An improvement in electroencephalogram (EEG) if prerandomisation EEG was suggestive of continuous seizure types (status epilepticus).
Any improvement in cognition and quality of life outcome using a validated score.
Search methods for identification of studies
Electronic searches
We searched the following databases. There were no language restrictions.
Cochrane Epilepsy Group Specialized Register (1 August 2014) using the search strategy set out in Appendix 1.
CENTRAL (The Cochrane Library Issue 7, July 2014) using the search strategy set out in Appendix 2.
MEDLINE (Ovid) (1946 to 1 August 2014) using the search strategy set out in Appendix 3.
EMBASE (1966 to December 2004) using the search terms set out in Appendix 4. (This was searched for the original version of this review but not for subsequent updates because EMBASE can no longer be accessed by the authors or the Cochrane Epilepsy Group.)
Database of Abstracts of Reviews of Effectiveness (DARE) (Issue 3 of the database, published in The Cochrane Library Issue 7, July 2014) using the search strategy set out in Appendix 2.
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform ICTRP (1 August 2014) using the search terms "corticosteroids and epilepsy''.
Searching other resources
We reviewed the reference lists from identified trials for other relevant articles and conference proceedings.
Data collection and analysis
Selection of studies
For the updated searches trials were independently assessed for inclusion by three review authors (VM, NG, CF). Results were compared and any disagreements resolved by discussion. There was no blinding of authorship or results. All randomised controlled trials were considered. All non‐English studies were also considered and a translation obtained, where required.
Data extraction and management
We planned for two authors to independently extract data from the identified studies. The results were cross‐checked and any disagreements were discussed and resolved.
For each trial which met our inclusion criteria, we obtained the following information.
(1) Patient factors (a) Age. (b) Sex. (c) Seizure type(s) and epilepsy type if provided. (d) Number and description of background drugs. (e) Number of seizures prior to randomisation. (f) Presence of neurological deficit/signs at baseline. (g) EEG results at baseline. (h) Neuroimaging CT/MRI scans. (i) Metabolic investigations.
(2) Treatment data (a) Type of steroid, its dose, mode of administration and duration of treatment per treatment group. (b) Total number of individuals allocated to each group.
(3) Outcomes Efficacy, tolerability and adverse events, as listed above per randomised group.
(4) Methods
(a) Duration of study. (b) Type of intervention used. (c) Confounding variables controlled for. (d) Sequence generation and allocation concealment. (e) Method of blinding. (f) Other risk of bias concerns.
Assessment of risk of bias in included studies
We planned for two review authors to independently assess the risk of bias using the 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) in which risk of bias is rated as either high, low or unclear. Each quality element of the study was considered independently.
Measures of treatment effect
We planned to present effect estimates as the mean difference (MD) or standardised mean difference (SMD) in the event of variation across outcome measures. Alternatively, if studies had reported dichotomous data we planned to present risk ratios (RR) using 95% confidence intervals (CI).
Unit of analysis issues
In the event of unit of analysis issues being present across the included studies (e.g. cross‐over studies, cluster randomised or repeated measures) we planned to:
1. determine whether the methods in such studies was conducted appropriately;
2. combine extracted effect sizes from such studies through a generic inverse variance meta‐analysis.
Dealing with missing data
We planned to contact the study authors to request any missing data and to determine if data were missing at random or not.
Assessment of heterogeneity
We expected to see differences in patient and control groups across the included studies. We also expected to see differences in outcome measures due to the number of measures available within research.
Two authors (VM, NG) planned to assess visually the clinical and methodological heterogeneity of the included studies, and planned to carry out I2 and Chi2 tests where applicable to assess statistical heterogeneity. A Chi2 P value of less than 0.10 and an I2 of greater than 50% indicates statistical heterogeneity.
Assessment of reporting biases
In addition to published data, we planned to search for unpublished data through contacting study authors and experts in this field, in order to avoid the effects of publication bias.
Data synthesis
In the event that two or more studies assessed the same corticosteroid within the same patient population we planned to combine the data in a meta‐analysis. However, as only one study was identified, this was not possible.
Subgroup analysis and investigation of heterogeneity
We planned to stratify subgroup analyses by type of corticosteroid used and duration of treatment. In the event of statistical heterogeneity, we planned to carry out a random‐effects meta‐analysis.
Sensitivity analysis
In the event of any inconsistencies or peculiarities being identified, we planned to carry out a sensitivity analysis.
Results
Description of studies
The literature search of Cochrane Epilepsy Group Specialized Register, CENTRAL, MEDLINE, EMBASE and DARE found nine randomised controlled trials.
Only a single study was identified as suitable for inclusion in the original review published in January 2007. It was reported by Pentella (Pentella 1982) and evaluated ACTH 4‐9 analogue (ORG 2766) in five children using a double blind, placebo controlled, cross‐over design.
Results of the search
The updated searches found 27 studies. After initial screening, all 27 studies were excluded due to irrelevance. For further details please see Figure 1.
1.
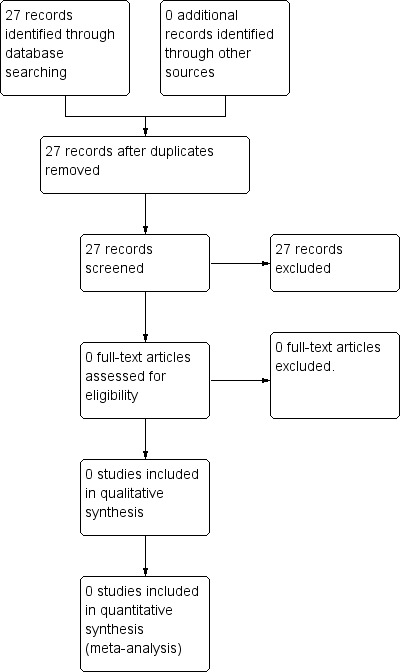
Study flow diagram (updated searches)
Included studies
The included study (Pentella 1982) was a prospective double blind, placebo controlled, cross‐over trial of ACTH analogue 4‐9 in five children with intractable seizures. Participants were aged between 1.5 and 11 years and had a history of multiple seizure types and mental retardation or developmental delay. Other treatments, including antiepileptic drugs (AEDs) as well as ketogenic diet were continued unchanged throughout the study period. The trial lasted nine weeks during which ACTH 4‐9 was administered for four weeks and a placebo for four weeks with a week lapse between the medications. It was concluded that one week was sufficient for a 'wash out' period. ACTH 4‐9 was given in a dose of 5 mg a day for the first two weeks and 10 mg a day for the next two weeks. Both the recipients and assessors were blinded. The outcomes were reported only during the nine week study period. EEGs were recorded prior to the study and during the last week of each month of therapy. One child was withdrawn from the study due to worsening seizures on placebo administration. One child had infantile (epileptic) spasms and we therefore excluded him/her from the analysis of this review.
For the current update, we did not find any new studies to include in this review.
Excluded studies
In previous searches, 22 studies were excluded at full‐text review.
A number of prospective observational studies (Dooley 1989; Sinclair 2003; Snead 1983; Willig 1980) and a few retrospective studies (Charuvanij 1992; Chutorian 1968; Oguni 2002; Verhelst 2005; Yamatogi 1979; You 2008) were found for the use of steroids in epilepsy. In addition, a number of retrospective and prospective studies of the efficacy of steroids in continuous spike waves in slow‐wave sleep (CSWS), including Landau‐Kleffner syndrome (Buzatu 2009; Lerman 1991; Sinclair 2005) and Rasmussen's syndrome (Hart 1994), were found.
Six randomised trials reported the use of short courses of steroids in patients with recent onset epileptic seizures and neurocysticercosis (Garg 2006; Kalra 2003; Kishore 2007; Mall 2003; Prakash 2006; Singhi 2004; Singla 2011), with the primary aim of treating the lesion. In no study was it possible to ascertain the effect of steroids on epilepsy independent of the effect of steroids and antiparasitic treatment on the underlying cause. Therefore, these studies were excluded from further analysis. Grosso 2008 compared hydrocortisone versus deflazacort (derivative of prednisolone) in drug resistant epilepsy of childhood. This study did not meet the criteria for inclusion in this review as it compared one form of steroid against the other in terms of efficacy, and seizure relapse rate rather than steroids against another therapy or no therapy. It was not possible to ascertain change in seizure frequency due to steroid treatment as both groups received one or other form of steroid throughout the duration of trial; therefore, we also excluded this study.
In the updated searches, 27 studies were found. However, after initial screening of titles and abstracts, all studies were excluded due to irrelevance. The results of the updates search only are detailed in the Figure 1.
Risk of bias in included studies
The single RCT we found was a double blind, placebo controlled, cross‐over trial (Pentella 1982). However, only three children could be included in the analysis of this review as one child was withdrawn from the study before receiving the study medication and another had epileptic spasms. Although the included study had potential bias through missing data, the reasons for exclusions were explained and seems unlikely to have had a significant effect on the outcomes. This study had good methods of blinding. Despite this, the small sample size may introduce bias as it is unlikely that this study is adequately powered to detect statistical significance. Therefore, Pentella 1982 was rated as having an overall high risk of bias. Further information regarding the risk of bias assessment are detailed in the Characteristics of included studies section.
Effects of interventions
See: Table 1
From the one study included in this review (Pentella 1982), the only outcome measure of interest was seizure frequency. On 5 mg ACTH 4‐9 a day, one child with psychomotor and myoclonic seizures had a 40% reduction in psychomotor seizures and a 47% reduction in myoclonic seizure compared to placebo. Another child, with akinetic seizures, had a 9% reduction in seizures compared to placebo. On the 10 mg a day dose the first child had a 30% reduction in psychomotor seizures compared to placebo and the second child had a 34% reduction in akinetic seizures compared to placebo. A third child had tonic‐clonic seizures and did not show any reduction in frequency of seizures on either dose of ACTH 4‐9 compared to placebo.
Therefore, the overall reduction in seizure frequency of more than 25% and less than 50% occurred in one child at the low dose and in two children at the higher dose. The reduction in psychomotor seizures and myoclonic seizures was more than 25% and less than 50% in one child on low dose. The reduction in psychomotor seizures and akinetic seizures was more than 25% and less than 50% in one child each on higher dose. The child with tonic‐clonic seizures had no reduction in seizure frequency on either.
There were no differences in the EEGs before the study and during the trial period. No side effects were reported when on ACTH 4‐9 analogue.
Further analysis was not performed.
Discussion
Summary of main results
The updated review identified only one RCT which was already included in the original review. This study found some small improvement in two out of three children who took part in the study and the third child showed no improvement.
Overall completeness and applicability of evidence
Excluding epileptic spasms, there is no reliable evidence from randomised controlled trials to support the use of steroids in childhood epilepsy. Evidence is lacking concerning the efficacy of steroids in controlling seizures (including status epilepticus), in improving cognition and in improving quality of life. Moreover, there are no reliable data from randomised controlled studies concerning the potential adverse effects of using steroids to treat childhood epilepsy.
Quality of the evidence
Although the identified study was rated as low risk of bias, it was of low quality due to the very small number of participants and short duration of follow‐up assessments. Therefore, no robust conclusions can be drawn from this.
Possible reasons for the lack of a good evidence base for the use of steroids to treat childhood epilepsies, other than epileptic spasms, include the heterogeneous nature of the population of intractable childhood epilepsies, making patient selection difficult; the wide availability of alternative treatments which are usually used in preference to steroids because of the perceived risks of steroid treatment; and the inherent difficulties of undertaking clinical trials in children.
Potential biases in the review process
None identified.
Agreements and disagreements with other studies or reviews
There have been case reports of using different steroids in treating various forms of non‐convulsive status epilepticus, fugues, absence status, atonic status and complex partial status epilepticus (Chutorian 1968; Marescaux 1990; Oguni 2002; Tsuru 2000). ACTH has also been used to treat gelastic seizures (Go 1999). In addition to this, they have been used to treat early and explosive onset of epileptic encephalopathy where there may have been an autoimmune or postinfectious inflammatory cause (Gupta 2005). In this regard, the study identified in the updated review yielded consistent findings in terms of the broader literature in this field.
Authors' conclusions
Implications for practice.
Since the last version of this review no new RCTs have been found examining the efficacy or tolerability of corticosteroids in treating childhood epilepsies. Clinicians using steroids in childhood epilepsies, other than for epileptic spasms, should take this into account before using these agents.
Implications for research.
There are several implications for research. (1) Adequately powered multicentre randomised controlled trials of corticosteroids should be carried out in childhood epilepsies of different types including non‐convulsive status. (This is also recognized in the NICE guideline in 2004 (NICE 2004).) (2) Studies need to consider carefully, clinically relevant efficacy measures, taking into account the various reasons why steroids are given in the childhood epilepsies. (3) Studies should incorporate validated measures of quality of life and cognition. (4) Studies should be of sufficient duration to be clinically relevant and to enable adverse effects to be detected.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2014 | New citation required but conclusions have not changed | No new studies included. Conclusions remain the same. |
| 1 August 2014 | New search has been performed | The searches were updated on 1 August 2014. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 13 March 2013 | New search has been performed | Searches updated 8 March 2013. |
| 10 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the support from the Cochrane Epilepsy Group in updating this review.
This review was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 (steroid*) or (hydrocortisone*) or (prednisolone*) or (dexamethasone) [REFERENCE] [STANDARD]
#2 (methyl prednisolone) or (methylprednisolone) or (ACTH) or (adrenocorticotropic hormone) or (tetracosactrin) [REFERENCE] [STANDARD]
#3 MeSH DESCRIPTOR Steroids Explode All WITH AD AE AG AN AI BI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PH PO RE ST SD TU TO UR [REFERENCE] [STANDARD]
#4 MeSH DESCRIPTOR Hydrocortisone Explode All WITH AD AE AG AA AN AI BI BL CF CS CH CL CT DF DU EC GE HI IM IP ME PK PD PH PO RE SE ST SD TU TO UR [REFERENCE] [STANDARD]
#5 MeSH DESCRIPTOR Prednisolone Explode All WITH AD AE AG AA AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR [REFERENCE] [STANDARD]
#6 MeSH DESCRIPTOR Dexamethasone Explode All WITH AD AE AG AA AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR [REFERENCE] [STANDARD]
#7 MeSH DESCRIPTOR Methylprednisolone Explode All WITH AD AE AG AA AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR [REFERENCE] [STANDARD]
#8 MeSH DESCRIPTOR Adrenocorticotropic Hormone Explode All WITH AD AE AG AA AN AI BI BL CF CS CH CL CT DF DU DE EC GE HI IM IP ME PK PD PH PO RE SE ST SD TU TO UR [REFERENCE] [STANDARD]
#9 MeSH DESCRIPTOR Cosyntropin Explode All WITH AD AE AG AA AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR [REFERENCE] [STANDARD]
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 [REFERENCE] [STANDARD]
Appendix 2. CENTRAL and DARE search strategy
#1 (epilep* or seizure* or convulsion*)
#2 MeSH descriptor Epilepsy explode all trees
#3 MeSH descriptor Seizures explode all trees
#4 (non‐convulsive status epilepticus)
#5 (absence status epilepticus)
#6 (complex partial status epilepticus)
#7 MeSH descriptor Status Epilepticus explode all trees
#8 (status epilepticus)
#9 (electrical status epilepticus) and (slow wave sleep)
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 (steroid*) or (hydrocortisone*) or (prednisolone*) or (dexamethasone)
#12 (methyl prednisolone) or (methylprednisolone) or (ACTH) or (adrenocorticotropic hormone) or (tetracosactrin)
#13 MeSH descriptor Steroids explode all trees
#14 MeSH descriptor Hydrocortisone explode all trees
#15 MeSH descriptor Prednisolone explode all trees
#16 MeSH descriptor Dexamethasone explode all trees
#17 MeSH descriptor Methylprednisolone explode all trees
#18 MeSH descriptor Adrenocorticotropic Hormone explode all trees
#19 MeSH descriptor Cosyntropin explode all trees
#20 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
#21 (#10 AND #20)
Appendix 3. MEDLINE search strategy
The following search strategy was used in updating the searches from March 2013 onwards. It is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2009.
1. (randomized controlled trial or controlled clinical trial).pt. or (randomized or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Epilepsy/
8. exp Seizures/
9. (epilep$ or seizure$ or convuls$).tw.
10. 7 or 8 or 9
11. exp Pre‐Eclampsia/ or exp Eclampsia/
12. 10 not 11
13. (electrical status epilepticus and slow wave sleep).tw.
14. 12 or 13
15. steroid$.tw.
16. *Steroids/
17. hydrocortisone$.tw.
18. *Hydrocortisone/
19. prednisolone.tw.
20. *Prednisolone/
21. dexamethasone.tw.
22. *Dexamethasone/
23. methylprednisolone.tw.
24. *Methylprednisolone/
25. ACTH.tw.
26. adrenocorticotropic hormone.tw.
27. Adrenocorticotropic Hormone/
28. corticotropin.tw.
29. tetracosactrin.tw.
30. *Cosyntropin/
31. exp Adrenal Cortex Hormones/
32. corticosteroids.tw.
33. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32
34. 6 and 14 and 33
The search strategy below is the original MEDLINE strategy that was used for earlier versions of this review:
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. exp Randomized Controlled Trials/
4. exp Random Allocation/
5. exp Double‐Blind Method/
6. exp Single‐Blind Method/
7. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ab,ti.
8. exp PLACEBOS/
9. placebo$.ab,ti.
10. random$.ab,ti.
11. epilep$.tw.
12. exp EPILEPSY/
13. seizure$.tw.
14. exp SEIZURES/
15. convulsion$.tw.
16. non‐convulsive status epilepticus.tw.
17. complex partial status epilepticus.tw.
18. absence status epilepticus.tw.
19. exp Status Epilepticus/
20. status epilepticus.tw.
21. electrical status epilepticus.tw.
22. slow wave sleep.tw.
23. 21 and 22
24. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
25. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 23
26. 24 and 25
27. steroid$.tw.
28. *STEROIDS/
29. hydrocortisone$.tw.
30. *HYDROCORTISONE/
31. prednisolone.tw.
32. *PREDNISOLONE/
33. dexamethasone.tw.
34. *DEXAMETHASONE/
35. methyl prednisolone.tw.
36. methylprednisolone.tw.
37. *METHYLPREDNISOLONE/
38. ACTH.tw.
39. adrenocorticotropic hormone.tw.
40. *Corticotropin/
41. tetracosactrin.tw.
42. *Cosyntropin/
43. 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42
44. 43 and 26
46. exp animals/ not humans.sh.
47. 44 not 46
Appendix 4. EMBASE search terms
EMBASE was searched using the following search terms: "epileptic seizure", "seizure", "epilepsy", "status epilepticus", "non‐convulsive status epilepticus", "complex partial status epilepticus", "absence status epilepticus", "electrical status epilepticus during slow wave sleep" and "steroids", including hydrocortisone, prednisolone, prednisone, dexamethasone, methyl prednisolone; ACTH and Tetracosactrin.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pentella 1982.
| Methods | Randomised, double blind, placebo controlled, cross‐over trial | |
| Participants | 3 children with epilepsy | |
| Interventions | ACTH 4‐9 | |
| Outcomes | 25% to 50% reduction in overall seizure frequency in those on low dose, in 50% of those on high dose | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not state how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Although a code is mentioned, no detail is given about this. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researchers were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 60% of randomised participants could be included in the analysis. Therefore, there was 40% missing data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study unavailable. |
| Other bias | High risk | Very low sample size (5 participants) and therefore it is unlikely that this study was adequately powered to detect statistical significance. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Buzatu 2009 | This was a retrospective trial looking at efficacy and tolerability of steroids in epileptic syndromes with continuous spike‐waves during slow‐wave sleep (CSWS). |
| Charuvanij 1992 | This was a retrospective cohort study in which 21 children suffering from intractable seizures other than infantile spasms were treated with intramuscular ACTH. |
| Chutorian 1968 | This was a cohort study. |
| Dooley 1989 | This was a prospective cohort study in which corticotropin was used to treat 17 children with intractable epilepsy other than infantile spasms. |
| Garg 2006 | Trial included epileptic seizures due to a specific disorder such as neurocysticercosis and the results in such a population cannot be generalized to children with epilepsy. |
| Grosso 2008 | Trial compares one form of steroid against other. It’s not possible to ascertain the change in seizure frequency due to steroid treatment as both groups received one or other form of steroid throughout the duration of trial. |
| Hart 1994 | This was a cohort study which described a temporary improvement in seizure control in 10 of the 17 patients receiving corticosteroids. |
| Kalra 2003 | Co‐intervention the effect of which confounded effect of steroids. Not all the patients in the trial had a diagnosis of epilepsy. |
| Kishore 2007 | Trial included symptomatic epileptic seizures due to a specific disorder such as neurocysticercosis and the results in such a population cannot be generalized to children with epilepsy. |
| Lerman 1991 | Case series of only four children with epilepsy being treated with corticosteroids. |
| Mall 2003 | Not all participants were diagnosed with epilepsy and there was a large number of adult participants whose results could not be separated from those of the children. |
| Oguni 2002 | This was a retrospective study of 81 patients with myoclonic astatic epilepsy to investigate the most effective treatment and long‐term seizure and intellectual prognosis. |
| Prakash 2006 | Trial included epileptic seizures due to a specific disorder such as neurocysticercosis and the results in such a population cannot be generalized to children with epilepsy. |
| Sinclair 2003 | Retrospective cohort study of 28 children older than 1 year with intractable epilepsy treated with steroids. |
| Sinclair 2005 | Retrospective review of 10 patients (8 with LKS and 2 with CSWS), only 8/10 had some seizures. |
| Singhi 2004 | Co‐intervention, the effect of which confounded effect of steroids. |
| Singla 2011 | Trial included epileptic seizures due to a specific disorder such as neurocysticercosis and the results in such a population cannot be generalized to children with epilepsy. |
| Snead 1983 | This study compared ACTH verses prednisone. |
| Verhelst 2005 | This was a retrospective review of 32 children with intractable epilepsy other than infantile spasm. |
| Willig 1980 | Prospective cohort study in which 7 children were treated with ACTH fragment (ACTH 4‐10). |
| Yamatogi 1979 | This was a cohort study in which 45 people with Lennox syndrome treated with ACTH were assessed to determine the immediate and long‐term effects and the various factors affecting them were investigated by a follow‐up study. |
| You 2008 | A retrospective assessment of 41 children to evaluate the efficacy and safety of adjunctive prednisolone therapy in children with cryptogenic epileptic encephalopathy, other than infantile spasms, and to determine its prognosis. |
Contributions of authors
Dr Neti A Gayatri, Dr Colin D Ferrie and Dr Helen Cross were involved with all aspects of the original review including protocol design, data collection and analysis. Dr Neti A Gayatri was primarily responsible for writing the original review and Dr Vishal Mehta for updating the current review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Health Research (NIHR), UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Pentella 1982 {published data only}
- Pentella K, Bachman DS, Sandman CA. Trial of an ACTH‐4‐9 analogue (ORG 2766) in children with intractable seizures. Neuropediatrics 1982;13(2):59‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Buzatu 2009 {published data only}
- Buzatu M, Bulteau C, Altuzarra C, Dulac O, Bogaert P. Corticosteroids as treatment of epileptic syndromes with continuous spike‐waves during slow‐wave sleep. Epilepsia 2009;50(Suppl 7):68‐72. [DOI] [PubMed] [Google Scholar]
Charuvanij 1992 {published data only}
- Charuvanij A, Ouvrier RA, Procopis PG, Antony JH, Fagan ER. ACTH treatment for intractable seizures of childhood. Brain Development 1992;14(2):102‐6. [DOI] [PubMed] [Google Scholar]
Chutorian 1968 {published data only}
- Chutorian AM, Gold AP, Low NL. Steroid therapy of non‐infantile (childhood) myoclonic epilepsy. Neurology 1968;18(3):304‐5. [PubMed] [Google Scholar]
Dooley 1989 {published data only}
- Dooley JM, Camfield PR, Goulden KJ, Macken SR. Low dose alternate‐day corticotropin therapy in the treatment of childhood seizures. American Journal of Diseases of Children 1989;143(11):1263‐5. [DOI] [PubMed] [Google Scholar]
Garg 2006 {published data only}
- Garg RK, Potluri N, Kar AM, Singh MK, Shukla R, Agrawal A, et al. Short course of prednisolone in patients with solitary cysticercus granuloma: a double blind placebo controlled study. Journal of Infection 2006 Jul;53(1):65‐9. [DOI] [PubMed] [Google Scholar]
Grosso 2008 {published data only}
- Grosso S, Farnetani M, Mostardini R, Cordelli D, Berardi R, Balestri P. A comparative study of hydrocortisone versus deflazacort in drug‐resistant epilepsy of childhood. Epilepsy Research 2008 Sep;81(1):80‐5. [DOI] [PubMed] [Google Scholar]
Hart 1994 {published data only}
- Hart YM, Cortez M, Andermann F, Hwang P, Fish DR, Dulac O, et al. Medical treatment of Rassmusen's syndrome (chronic encephalitis and epilepsy): effect of high dose steroids of immunoglobulins in 19 patients. Neurology 1994;44(6):1030‐6. [DOI] [PubMed] [Google Scholar]
Kalra 2003 {published data only}
- Kalra V, Dua R, Kumar V. Efficacy of albendazole and short‐course dexamethasone treatment in children with 1 or 2 ring‐enhancing lesions of neurocysticercosis: a randomised controlled trial. Journal of Pediatrics 2003;143(1):111‐4. [DOI] [PubMed] [Google Scholar]
Kishore 2007 {published data only}
- Kishore D, Misra S. Short course of oral prednisolone on disappearance of lesion and seizure recurrence in patients of solitary cysticercal granuloma with single small enhancing CT lesion: an open label randomized prospective study. Journal of the Association of Physicians of India 2007 Jun;55:419‐24. [PubMed] [Google Scholar]
Lerman 1991 {published data only}
- Lerman P, Lerman‐Sagie T, Kivity S. Effect of early corticosteroid therapy for Landau‐Kleffner Syndrome. Developmental medicine and child neurology 1991;33(3):257‐60. [DOI] [PubMed] [Google Scholar]
Mall 2003 {published data only}
- Mall RK, Agarwal A, Garg RK, Kar AM, Skukla R. Short course of prednisolone in Indian patients with solitary cysticercus granuloma and new‐onset seizures. Epilepsia 2003;44(11):1397‐401. [DOI] [PubMed] [Google Scholar]
Oguni 2002 {published data only}
- Oguni H, Tanaka T, Hayashi K, Funatsuka M, Sakauchi M, Shirakawa S, et al. Treatment and long‐term prognosis of myoclonic‐astatic epilepsy of early childhood. Neuropediatrics 2002;33(3):122‐32. [DOI] [PubMed] [Google Scholar]
Prakash 2006 {published data only}
- Prakash S, Garg RK, Kar AM, Shukla R, Agarwal A, Verma R, et al. Intravenous methyl prednisolone in patients with solitary cysticercus granuloma: a random evaluation. Seizure 2006 Jul;15(5):328‐32. [DOI] [PubMed] [Google Scholar]
Sinclair 2003 {published data only}
- Sinclair DB. Prednisone therapy in pediatric epilepsy. Pediatric Neurology 2003;28(3):194‐8. [DOI] [PubMed] [Google Scholar]
Sinclair 2005 {published data only}
- Sinclair DB, Snyder TJ. Corticosteroids for the treatment of Landau‐Kleffner syndrome and continuous spike‐wave discharge during sleep. Pediatric Neurology 2005;32(5):300‐6. [DOI] [PubMed] [Google Scholar]
Singhi 2004 {published data only}
- Singhi P, Jain V, Khandelwal N. Corticosteroids versus albendazole for treatment of single small enhancing computed tomographic lesions in children with neurocysticercosis. Journal of Child Neurology 2004 May;19(5):323‐7. [DOI] [PubMed] [Google Scholar]
Singla 2011 {published data only}
- Singla M, Prabhakar S, Modi M, Medhi B, Khandelwal N, Lal V. Short course of prednisolone in solitary cysticersus granuloma: a randomized, double‐blind, placebo‐controlled trial. Epilepsia 2011 Oct;52(10):1914‐7. [DOI] [PubMed] [Google Scholar]
Snead 1983 {published data only}
- Snead OC 3rd, Benton JW, Myers GJ. ACTH and prednisone in childhood seizure disorders. Neurology 1983;33(8):966‐70. [DOI] [PubMed] [Google Scholar]
Verhelst 2005 {published data only}
- Verhelst H, Boon P, Buyse G, Ceulemans B, D'Hooghe M, Meirleir LD, et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure 2005;14(6):412‐21. [DOI] [PubMed] [Google Scholar]
Willig 1980 {published data only}
- Willig RP, Lagenstein I. [Therapeutic trial with a fragment of ACTH (ACTH 4‐10) in early childhood epilepsy (author's transl)]. Monatsschrift fur Kinderheilkunde 1980;128(2):100‐3. [PubMed] [Google Scholar]
Yamatogi 1979 {published data only}
- Yamatogi Y, Ohtsuka Y, Ishida T, Ischiba N, Ishida S, Miyake S, et al. Treatment of the Lennox syndrome with ACTH: a clinical and electroencephalographic study. Brain Development 1979;1(4):267‐76. [DOI] [PubMed] [Google Scholar]
You 2008 {published data only}
- You SJ, Jung DE, Kim HD, Lee HS, Kang HC. Efficacy and prognosis of a short course of prednisolone therapy for pediatric epilepsy. European Journal of Pediatric Neurology 2008;12(4):314‐20. [DOI] [PubMed] [Google Scholar]
Additional references
Blume 2001
- Blume WT, Luders HO, Mizrahi E, Tassinari C, Emde Boas W, Engel J Jr. Glossary of descriptive terms for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia 2001;42(9):1212‐8. [DOI] [PubMed] [Google Scholar]
Campbell 2003
- Campbell C, Jacob P. Deflazacort for the treatment of Duchenne Dystrophy: A systematic review. BMC Neurology 2003;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deonna 2005
- Deonna T. Management of epilepsy. Archives of Disease in Childhood 2005;90(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsgren 2004
- Forsgren L. Incidence and prevalence. In: Wallace SJ, Farrell K editor(s). Epilepsy in children. Arnold, 2004:21‐25. [Google Scholar]
Go 1999
- Go T. ACTH treatment for gelastic seizures. Archives of Disease in Childhood 1999;81(3):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2005
- Gupta R, Appleton RE. Corticosteroids in the management of the paediatric epilepsy. Archives of Disease in Childhood 2005;90(4):379‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancock 2013
- Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001770.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lux 2005
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurology 2005;4(11):712‐7. [DOI] [PubMed] [Google Scholar]
Marescaux 1990
- Marescaux C, Hirsch E, Finck S, Maquet P, Schlumberger E, Sellal F, et al. Landau‐Kleffner syndrome: a pharmacologic study of five cases. Epilepsia 1990;31(6):768‐77. [DOI] [PubMed] [Google Scholar]
NICE 2004
- NICE 2004. The epilepsies: diagnosis and management of the epilepsies in children and young people in primary and secondary care. National Institute for Clinical Excellence (NICE) 2004; Vol. Clinical Guideline 20.
Riikonen 1986
- Riikonen R, Simell O, Jaaskelainen J, Rapola J, Perheentupa J. Disturbed calcium and phosphate homeostasis during treatment with ACTH of infantile spasms. Archives of Disease in Childhood 1986;61(7):671‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satoh 1982
- Satoh J, Takeshige H, Hara H, Fukuyama Y. Brain shrinkage and subdural effusion associated with ACTH administration. Brain & Development 1982;4(1):13‐20. [PubMed] [Google Scholar]
Tsuru 2000
- Tsuru T, Mori M, Mizuguchi M, Momoi MY. Effects of high dose intravenous corticosteroid therapy in Landau‐Kleffner syndrome. Pediatric Neurology 2000;22(2):145‐7. [DOI] [PubMed] [Google Scholar]
Watzka 2000
- Watzka M, Bidlingmaier F, Beyenberg S, Henke RT, Clusmann H, Elger CE, et al. Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids 2000;65(12):895‐901. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gayatri 2007
- Gayatri N, Ferrie CD, Cross HHJ. Corticosteroids including ACTH for childhood epilepsy other than epileptic spasms. Cochrane Database of Systematic Reviews 2007, Issue Issue 1. Art. No.: CD005222. DOI:10.1002/14651858.CD005222. pub2.. [DOI: 10.1002/14651858.CD005222.pub2] [DOI] [PubMed] [Google Scholar]


