Abstract
Background
Structured treatment interruptions (STI) of antiretroviral therapy (ART) have been investigated as part of novel treatment strategies, with different aims and objectives depending on the populations involved. These populations include: 1) patients who initiate ART during acute HIV infection; 2) patients with chronic HIV infection, on ART, with successfully suppressed viremia; and 3) patients with chronic HIV infection and treatment failure, with persistent viremia due to multi‐drug resistant HIV (Hirschel 2001; Deeks 2002; Miller 2003).
In an earlier Cochrane review (Pai 2005), we had summarized the evidence about the effects of STI in chronic suppressed HIV infection. In this review, we summarize the evidence on STI in patients with chronic unsuppressed HIV infection due to drug‐resistant HIV. Unsuppressed HIV infection describes those patients who cannot suppress viremia, due to the presence of multi‐drug‐resistant virus. It is also referred to as treatment failure. Drug resistance is identified by the presence of resistant mutations at baseline.
STI as a treatment strategy in HIV‐infected patients with chronic unsuppressed viremia involves interrupting ART in controlled clinical settings, for a pre‐specified duration of time. These interruptions have various aims, including the following: 1) to allow wild virus to re‐emerge and replace the resistant mutant virus, with the hope of improving the efficacy of a subsequent ART regimen; 2) to halt development of drug resistance and to preserve subsequent treatment options; 3) to alleviate treatment fatigue and reduce drug‐related adverse effects; and 4) to improve quality of life (Miller 2003; Montaner 2001; Vella 2000;).
Objectives
The objective of our systematic review was to synthesize the evidence on the effect of structured treatment interruptions in adult patients with chronic unsuppressed HIV infection.
Search methods
We included all available intervention studies (randomized controlled trials and non‐randomized trials) conducted in HIV‐infected patients worldwide. We searched nine databases, covering the period from January 1996 to February 2006. We also scanned bibliographies of relevant studies and contacted experts in the field to identify unpublished research, abstracts and ongoing trials.
In the first screen, a total of 3186 potentially eligible citations from nine databases and sources were identified, of which 2047 duplicate citations were excluded. The remaining 1139 citations were examined in detail, and we further excluded 951 citations that were modeling studies, animal studies, case reports, and opinion pieces. As shown in Figure 01, 188 citations were identified in the second screen as relevant for full‐text screening. Of these, 60 basic science studies, editorials and abstracts were excluded and 128 full‐text articles were retrieved. In the third screen, all full‐text articles were examined for eligibility in our review. These were subclassified into three categories: 1) chronic suppressed HIV infection; 2) chronic unsuppressed HIV infection; and 3) acute HIV infection. Studies were further excluded if their abstracts did not contain enough information for inclusion in our reviews. A total of 62 studies were finally classified into chronic suppressed, acute, and chronic unsuppressed categories. Of these, 17 trials met the eligibility criteria for this review.
Selection criteria
Inclusion criteria All available randomized or non‐randomized controlled trials investigating planned treatment interruptions among patients with chronic unsuppressed HIV infection. Early pilot non‐randomized prospective studies on treatment interruptions of fixed and variable durations were also included. Relevant abstracts on randomized controlled trials were also included if they contained sufficient information.
Exclusion criteria Editorials, reviews, modeling studies, and basic science studies were excluded. Studies on STI among patients with chronic suppressed HIV infection were summarized in a separate review. Studies on STI in primary HIV infection were beyond the scope of this review.
Data collection and analysis
Two reviewers independently extracted data, evaluated study eligibility and quality. Disagreements were resolved in consultation with a third reviewer.
A total of seventeen studies on STI were included in our review. However, due to significant heterogeneity across studies (i.e. in study design, populations, baseline characteristics, and reported outcomes; and in reporting of measures of effect, hazard ratios, and risk ratios), we considered it inappropriate to perform a meta‐analysis.
Main results
In early pilot non‐randomized trials, a pattern was evident across studies. During treatment interruption, a decline in CD4 cell counts, increase in viral load, and a shift in the level of genotypic drug resistance towards more of a wild‐type HIV virus was reported. This suggests that STI may be used to increase drug susceptibility to an optimized salvage regimen upon treatment re‐initiation. These studies generated useful data and hypotheses that were later tested in randomized controlled trials.
Randomized controlled trials rated high on quality. Of the eight randomized controlled trials reviewed, seven had been completed while one was ongoing and remains blinded. Of the seven completed randomized controlled trials, six have reported consistent virologic and immunologic patterns, and found no significant benefit in virologic response to subsequent ART in the STI arm, compared to the control arm. In addition, the largest completed randomized trial reported greater numbers of clinical disease progression events and evidence of prolonged negative impact on CD4 cell counts in the STI arm (Beatty 2005; Benson 2004; Deeks 2001; Lawrence 2003; Walmsley 2005; Ruiz 2003).
The single RCT with divergent findings from the others (GigHAART), reporting a significant virologic and immunologic benefit due to STI, was different in prescribing a shorter STI duration and a salvage ART regimen of 8‐9 drugs. There were also differences in the patient population characteristics with this study, targeting those with very advanced HIV disease (Katlama 2004).
Although we await the unblinded results of the eighth RCT (OPTIMA), the evidence so far does not support STI in the setting of chronic unsuppressed HIV infection with antiretroviral treatment failure (Brown 2004; Holodniy 2004; Kyriakides 2002; Singer 2006).
Authors' conclusions
The current available evidence primarily supports a lack of benefit of STI before switching therapy in patients with unsuppressed HIV viremia despite ART. There is evidence of harm in attempting STI in patients with relatively advanced HIV disease, due to the associated CD4 cell decline and the increased risk of clinical disease progression. At this time, there is no evidence to recommend the use of STI in this clinical category of patients with treatment failure.
Plain language summary
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults
Structured treatment interruptions (STI) of antiretroviral therapy (ART) have been under investigation as an alternative strategy in the management of chronic HIV infection since 1999. The investigation of treatment interruptions (also called "drug holidays") was initially driven by patients seeking to alleviate treatment fatigue, reduce drug‐related toxicities, and improve quality of life. In patients whose ART treatment fails, treatment interruption has been implemented in order to halt progression of HIV drug resistance on the failing regimen, thereby preserving subsequent treatment options. In patients with multi‐drug resistance, some early studies suggested that ART interruption could improve subsequent response to ART. More recently, STI has been proposed as a means of reducing the cost of HIV treatment in resource‐limited settings. We conducted a systematic review of evidence regarding STI in persons with chronic, unsuppressed HIV infection and multi‐drug resistance. Based on the completed trials we reviewed, the vast majority of evidence suggests greater harm than benefit from interrupting ART in patients with chronic HIV infection, who have treatment failure with multi‐drug resistant HIV. The evidence does not support the use of STI with such patients.
Background
Structured treatment interruptions (STI), a novel antiretroviral therapy (ART) strategy, involve taking a planned break from therapy (sometimes called a "drug holiday"), under pre‐specified conditions, in a supervised clinical setting. This strategy of ART management has been under investigation since 1999, when the first case report of STI was reported (Lisziewicz 1999).
STI have been attempted in three main settings of HIV infection: 1) subsequent to early ART initiation during acute or primary HIV infection, 2) in chronic controlled HIV infection (suppressed viremia on ART), and 3) in chronic uncontrolled HIV infection (unsuppressed viremia due to treatment failure). In these three distinct categories, STI is utilized with different aims and objectives, clinically, immunologicly, and virologicly, depending on patient populations (Hirschel 2001; Lisziewicz 2002; Lori 2001; Miller 2003).
In an earlier Cochrane review, we summarized the available evidence on STI in chronic suppressed HIV infection (Pai 2005). In the present review, we evaluate and synthesize evidence on the utility of STI in chronic unsuppressed infection.
Chronic unsuppressed HIV infection is a common outcome in patients in whom first‐line and second‐line regimens have failed, and there is evidence of resistance to all three initial ART drug classes: nucleoside reverse transcriptase inhibitor (NRTI); non‐nucleoside reverse transcriptase inhibitor (NNRTI); and protease inhibitor (PI). In these patients, treatment failure may be a consequence of a substantial period of prior treatment with what is now considered suboptimal therapy (e.g., mono‐ or dual drug regimens) and poor patient adherence (Benson 2001; Bonhoeffer 2000; Deeks 2002; Foli 2004; Montaner 2005). Remaining on a failing regimen with persistent viremia results in the development of progressive drug mutations and resistance to ART (Deeks 2002; Moss 2001; Oxenius 2003). There are data to suggest that drug‐resistant viruses may be less fit (i.e. have lower replication capacity) than wild‐type viruses (Deeks 2002; Miller 2003; Walmsley 2002).
STI have been explored in this setting with various aims. These are to do the following: 1) alleviate treatment fatigue; 2) reduce drug related adverse effects; 3) improve quality of life; (Jager 2002; Flepp 2001) 4) halt progression of resistance and preserve subsequent treatment options (Dorman 2000; Vella 2000); and 5) allow wild‐type virus to replace the resistant mutant virus, with the hope of improving efficacy of subsequent ART regimens (Deeks 2002; Miller 2001; Montaner 2005). When treatment interruptions are attempted, it is important to monitor clinical disease progression, and immunologic and virologic response (Deeks 2002; Dybul 2002; Flepp 2001; Hirschel 2001;)
For the past few years, several non‐randomized studies and randomized trials have been conducted in the setting of setting of chronic HIV infection with ART failure due to multi‐drug resistant viruses (Deeks 2001; Ghosn 2005; Jaafar 2004; Katlama 2004; Lawrence 2003; Ruiz 2003). In this context, the effect of STI in these trials needs to be synthesized.
Objectives
The objective of our systematic review was to synthesize the evidence on the effects of structured treatment interruptions (STI), in adult patients with chronic unsuppressed HIV infection despite ART (treatment failure with drug‐resistant virus) using all available trials.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria All available randomized or non‐randomized controlled trials investigating planned treatment interruptions among patients with chronic unsuppressed HIV infection. Early pilot non‐randomized prospective studies on treatment interruptions of fixed and variable durations were also included. Relevant abstracts on randomized controlled trials were also included if they contained sufficient information. Exclusion criteria Editorials, reviews, modeling studies, and basic science studies were excluded. Studies on STI among patients with chronic suppressed HIV infection were summarized in a separate review. Studies on STI in primary HIV infection were beyond the scope of this review.
Types of participants
HIV‐infected adults with chronic unsuppressed viremia (drug‐resistant HIV, treatment failure) as part of randomized controlled trials (RCTs) or non‐randomized trials evaluating effects of STI. Types of comparison groups In RCTs, comparison/control groups were HIV‐infected adults with chronic unsuppressed viremia, on continuous ART, with no period when not receiving therapy (or no STI).
Types of interventions
In RCTs, single planned interruptions of pre‐specified (fixed) duration have been attempted in clinical settings, with close monitoring of clinical, virologic, immunologic, and drug‐related parameters. In early pilot non‐randomized trials, treatment interruptions of variable durations were attempted. Common reasons for interruptions or discontinuations were treatment failure or drug‐related toxicities. Early reported observational studies were case series on patients observed prospectively while interrupting ART, rather than with treatment interruption being mandated by the protocol.
Types of outcome measures
1) Immunologic outcomes a. absolute change in CD4 cell count or percent change in CD4 cell count (cells/mm3) from baseline b. absolute change in CD8 T‐cell count or percent change in CD8 T‐cell specific response from baseline
2) Virologic outcomes a. absolute change in plasma HIV RNA viral load (VL) or percent change in plasma HIV RNA viral load (VL) from baseline (reported as copies/mL or log10 copies/mL) b. drug resistance: change in HIV genotypic patterns including mutations associated with ART drug resistance
3) Clinical outcomes a. progression of disease events (e.g., AIDS‐defining events) b. death c. non‐serious AIDS defining events
4) drug‐related outcomes a. adverse drug effects and toxicities b. antiretroviral drug levels
5) Quality of life outcomes
Search methods for identification of studies
The search strategy covered the period from the year January 1996 to February 2006. Searches were performed using the Cochrane HIV/AIDS Review Group search strategy.
Search string The MEDLINE search string is reproduced below:
(("HIV"[MESH]) OR ("Acquired Immunodeficiency Syndrome"[MESH]) OR (HIV‐1 [TW]) OR (HAART [TW]) OR (AIDS [TW]) OR ("Antiretroviral Therapy, Highly Active"[MESH])) AND (((structured treatment interruption*) OR (Structured therapeutic interruption*) OR (structured intermittent therapy) OR (scheduled treatment interruption*) OR (drug holiday*) OR (planned interruption*) OR (treatment interruption*) OR (strategic treatment interruption*) OR (intermittent therapy) OR (intermittent treatment)))
Databases and sources A total of nine databases and sources were searched:
Cochrane Controlled Trials Register (CENTRAL) Medline EMBASE BIOSIS Web of Science AIDSLINE (via NLM Gateway) ACTIS (AIDS Clinical Trials Information Service) Database of Abstracts of Reviews of Effectiveness (DARE) Proceedings and abstracts from AIDS conferences, global meetings (via NLM Gateway) In addition, bibliographies of included studies and relevant review articles were also searched. Experts in the field were contacted to identify unpublished research and ongoing trials.
Data collection and analysis
Study search
Figure 1 shows the search strategy for included studies. In the first screen, a total of 3186 potentially eligible citations from nine databases and sources were identified, of which 2047 duplicate citations were excluded. The remaining 1139 citations were examined in detail, and we further excluded 951 citations that were modeling studies, animal studies, case reports, and opinion pieces.
1.
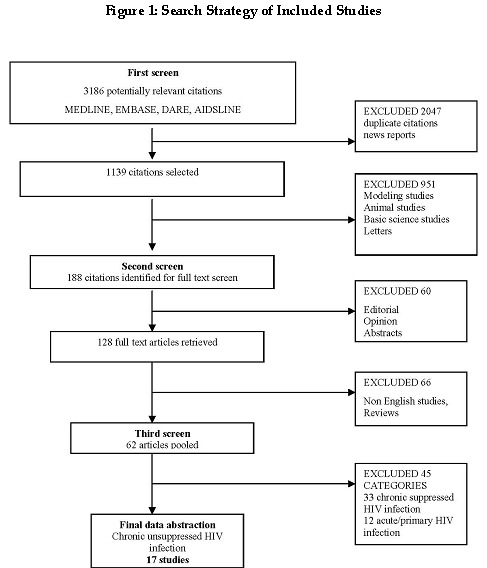
Figure 1: Search Strategy of Included Studies.
As shown in Figure 1, 188 citations were identified in the second screen as relevant for full‐text screening. Of these, 60 basic science studies, editorials and abstracts were excluded and 128 full‐text articles were retrieved. Please refer to the table of excluded studies.
In the third screen, all full‐text articles were examined for eligibility in our review. These were subclassified into three categories: 1) chronic suppressed HIV infection; 2) chronic unsuppressed HIV infection; and 3) acute HIV infection. Studies were further excluded if their abstracts did not contain enough information for inclusion in our reviews. A total of 62 studies were finally classified into chronic suppressed, acute, and chronic unsuppressed categories. Of these, 17 trials met the eligibility criteria for this review.
Study Eligibility Two reviewers independently screened titles and abstracts, and evaluated study eligibility. Reviewers were not blinded to the publication details. Articles identified as relevant in the first screen were evaluated in greater detail in the second screen of full‐text articles. Using our eligibility criteria of randomized trials, non‐randomized trials, and abstracts of important RCTs, a final set of 17 studies were selected for the review. Independent data abstraction was done by two reviewers. Disagreements were resolved in consultation with a third reviewer. Methodological quality of all the included studies was assessed by both reviewers. The inter‐rater reliability was high (kappa=0.8).
Results
Description of studies
Our search strategy identified a total of 17 studies. These studies were classified by study designs into randomized controlled trials (N=8) and non‐randomized trials (N=9). See Table: Characteristics of included studies.
A) Randomized Controlled Trials: Eight RCTs have been conducted to date. These trials attempted the strategy of using a single STI of pre‐determined duration (with a range of 2 to 4 months), prior to switching ART, in persons with treatment failure. Safety parameters were usually set up so that if patients fared poorly with large declines in CD4 counts, clinicians were allowed to intervene earlier with re‐initiation of ART. Sample sizes in the completed trials varied from 46 to 274. In the ongoing OPTIMA trial the targeted sample size is 504. The mean number of antiretroviral drugs in the study‐prescribed ART regimens varied from 4‐8 drugs. Eligibility criteria with respect to CD4 cell count and entry HIV RNA level differed across trials (Table 1). Presence of HIV drug resistance was determined either by history of prior treatment failure or by genotypic testing for evidence of multi‐drug resistant (MDR) virus. Varying durations of follow up (24 weeks‐48 months) were reported. Besides reporting the effect of STI on surrogate markers (including CD4 and viral load) of HIV disease, in some cases the effects on clinical end‐points were also determined and recorded. See Table of included studies. B) Non‐randomized trials: Nine non‐randomized studies in this category investigated the impact of treatment interruptions of varying durations (8 weeks to 3 months). Treatment interruptions were attempted because of patients developing drug toxicities or because of treatment failure. The studies were small, with sample sizes ranging from 9 to 77 patients. These studies were characterized by the absence of comparison groups, hence of inferior methodological quality. Eligibility criteria varied across studies, and have been summarized elsewhere (Table 2). The impact of treatment interruption on virologic, immunologic and clinical outcomes was documented. In a few studies, patients were also followed after resumption of therapy, after the treatment interruption. See Table of included studies.
1. Randomized controlled trials.
| Study, year | Sample size | STI duration | Eligibility criteria | Baseline | End points |
| Deeks, 2001 | 16 | 12 weeks | VL: 2500* 6 months; CD4:100 cells/mm3 above PI pre‐therapy levels; | CD4 302(258‐355) VL 4.5(4.0‐4.6) | p=plasma VL,CD4, s= drug susceptibility, viral replicative capacity |
| Ruiz, 2003 RETROGENE | 46 | 12 weeks | VL.1,000 cop/ml ART: 3 drug combo with PI* 6mo, MUT 8(5‐13) VL.1,000 cop/ml ART: 3 drug combo with PI* 6mo, MUT 8(5‐13) | CD4 383(84‐783) VL 4.3(3.2‐5.3) ART: 5.73 years, 77% MDR resistant Age: 32(25‐43) | p=vl<50 copies/ml, s=reversion,cd4 decline, resistance mutations |
| Lawrence, 2003 CPCRA 064; Lawrence CPCRA 064 2005 | 270; 274 | 16 weeks; follow up 24 months | VL>5,000 CD4: na ART: evidence of MDR virus, stable ART | CD4 153( 52‐281) VL 5.0(4.5‐5.5) 2.2NRTI+2.5PI+ 1.0NNRTI MUT 10.8 2.2NRTI+2.5PI+ 1.0NNRTI MUT 10.8 | p=Progression to AIDS or death; s= Genotype, VL, CD4+, QOL |
| Katlama, 2004 ANRS 097 | 68 | 8 weeks | VL>75,000 CD4<200 Mut:13(6‐18) | CD4 28(2‐216) VL 5.3 5NRTI+2NNRTI +7PI MUT11(5‐14) | p=Virological response = > 1.0 log reduction in VL at wks 12, 24; s= Toxicity, genotype, PI plasma concentrations |
| Benson, 2004 ACTG A5086 | 41 | 16 weeks | VL>10,000 CD4:>150 ART: one virologic failure | CD4 226 VL 38,000 cop/ml | p=Proportion with VL < 400 at 48 weeks; s= VL < 50 at 24, 48, 64 weeks. |
| Beatty, 2005 | 30 | 16 weeks | VL>500 CD4: na ART: resistant >2PI+2NRTI+1NNRTI | CD4 47 VL 4.72 ART2PI+2NRTI+1NNRTI | p=viral suppression s=CD4 |
| Walmsley, 2005 CTN 164 | 134 | 12 weeks | VL>1,000 OI prophylaxis if CD4<200 ART: 2 ART available in salvage regimen | CD4 343 VL 3.9 ART PI+NRTI+NNRTI | p= VL<50 at least once or one log VL at least once s=CD4, VL |
| Singer, 2006 OPTIMA | 504 (ongoing study target) | 3 months | VL>2,500 cop/ml CD4<300 Age>18 yrs Failure two drug classes On ART for 3 months HIV serologic diagnosis | CD4 111 VL 5.0 ART PI+NRTI+NNRTI | p=Time to AIDS or death s= Toxicity, illness, QOL, CD4, VL, economics |
| Brown, 2004 OPTIMA | 255 (interim report) | 24 weeks | VL>2,500 cop/ml CD4<300 Age>18 yrs Failure two drug classes On ART for 3 months HIV serologic diagnosis | CD4 111 VL 5.0 ART PI+NRTI+NNRTI | p=Non serious and serious aids defining events s= QOL, CD4, VL, economics p=Time to AIDS or death s= Toxicity, illness, QOL, CD4, VL, |
2. Non Randomized studies.
| Study, year | Sample size | Eligibility criteria | Baseline | TI duration | Outcomes post STI |
| Verhofstade, 1999 | 9 | VL (15,924‐997.361) NRTI regimen CD4: na | CD4 na VL na | unclear | Reversion: na VL rebound (77%) Loss of mutations (88%) |
| Miller, 2000 | 48 | Median ART: 9 drugs (4‐13) TI episodes: 49 Median age: 37.7 yrs | CD4155(2‐777) VL 5.07(2.7‐6.7), | 121days( 54‐322) | Reversion 68%, VL rebound (0.7logcop/ml), CD4 decline by (89 cells/l) |
| Youle, 2000 | 35 | VL>50,000 log cop/ml, 3/4 drug regimen*6 month | CD4 125(36‐254) VL540,000 (72,000‐750,000) | 28 days( 0‐275) | Reversion: na VL rebound: present CD4 decline 42 cells/l |
| Izopet,2000 | 38 | VL>5,000c/ml, ART*6mo, age 41years, CDC stage A(12), B(6), C(20). | CD4 188(6‐732) VL 4.6logcop/ml(3.7‐6.2) | 3 months | Reversion 61% Viral rebound 0.4log cop/ml, CD4 decline 43 cells/l |
| Delauggerre, 2001 | 20 | Tmt failure VL>10,000*36 mo, 16/20 CDC stage C, 3 drug classes used; Median ART 73mon(29‐118) | Median CD4 200 cells/mm3, Median VL 160,000 c/ml, Mutations median 10 CDC stage C | 8 weeks (4‐24 weeks) | Reversion 55% VL rebound: na CD4 decline: na |
| Halfon, 2003 | 11 | Multi drug failure, 2 primary mutations at baseline; | CD4 314(22‐1510) VL 4.45(2.9‐5.7) | 3 months | Reversion: 2/11 pts Viral rebound: 3/11 pts >300,000 8/11 pts>70,000 CD4 decline: na |
| Deeks, 2003 | 24 | VL>2500 copies*6mo, >12mo * PI regimen*12mo, Stable ART* 4months | CD4:218(80‐218) VL 4.6(3.9‐5.1 | 20 weeks | Reversion: present VL rebound: 0.76 log cop/ml, CD4 decline 84 cells/l |
| Jaafar,2004 | 77 | VL>1,000 RNA copies, CD4>200,ART* 6month, | CD4: 483(200‐919) VL: 4.0(3.5‐5.5) | 3 months | Reversion 87% VL rebound: 1.01 log cop/ml. CD4 decline: 95 cells |
| Ghosn, 2005 | 23 | VL>30,000 c/ml, ART>>3 drug classes Mu: 1.3TAM+1NNRTI+2PI Baseline mut: 17(9‐28) | CD4:43(1‐372) VL 5.14(4.51‐5.69) | 24 weeks (12‐37 weeks) | Reversion 70% Viral rebound not significant CD4 decline not significant |
Risk of bias in included studies
Methodological quality of RCTs was assessed by using two methods.
1. The Jadad scale, a validated quality assessment instrument (Jadad 1996) 2. The Cochrane method for adequacy of concealment of allocation (Clarke 2001)
The Jadad instrument (Jadad 1996) includes the following components. Each component gets a score of one. I. Was the study described as randomized? II. If so, was allocation concealed? III. Was the study described as double blind? IV. Were the outcome assessors blinded? V. Was there a description of withdrawals and dropouts?
Concealment of treatment (intervention) allocation is assessed with standard Cochrane criteria. This is divided into four categories, and each is graded A, B, C, or D. These were: A. adequate: if computer‐generated random numbers were used B. unclear: if methods were not described C. inadequate: if sequences such as consecutive series of patients, case record numbers, date of birth, date of admission were used D. not used/not reported
Methodological quality of included trials:
A. Randomized controlled trials: All 8 trials were of high quality. All were randomized and open‐label due to concerns about the nature and safety of the intervention. Outcome assessors were blinded in a majority of the trials. A complete description of withdrawals and dropouts during the flow of the trial was available in all trials. Concealment of treatment allocation was reported in four trials (Katlama 2004; Lawrence 2003; Singer 2006; Walmsley 2005). Overall Jadad score: 4/5.
Concealment was unclear in the other trials (Beatty 2005; Benson 2004; Deeks 2001; Ruiz 2003) Overall Jadad score: 3/5.
B. Non‐randomized trials: Nine non‐randomized trials were open label, unblinded single arm trials. A complete reporting of withdrawals and dropouts was available, but the methodological quality of these studies was poor (Deeks 2003; Delaugerre 2001; Ghosn 2005; Halfon 2003; Izopet 2000; Jaafar 2004; Miller 2000; Verhofstede 1999; Youle 2000). Overall Jadad score: 1/5.
Effects of interventions
A. Randomized controlled trials An early RCT (Deeks 2001) was followed by three landmark trials: the Retrogene study, CPCRA 064, and GigHAART (Katlama 2004; Lawrence 2003; Ruiz 2003). The ACTG A 5086 trial and CTN 164 trials recently reported results similar to the CPCRA 064 and Retrogene studies (Benson 2004; Walmsley 2005). A small trial by Beatty et al used a new class of drug in the prescribed salvage regimen (Beatty 2005). The OPTIMA trial is ongoing and the results remain blinded to randomization assignment. (Brown 2004; Holodniy 2004; Kyriakides 2002; Singer 2006). The designs of the trials have been tabulated, and the individual trial results elucidated.
See Table 1 for baseline comparisons.
A.1. Beatty 2005 reported data from a randomized study in 30 participants that investigated a 16‐week STI followed by Enfuvirtide (ENF) ‐based optimized salvage regimen. The control group was administered an immediate ENF regimen. In the STI arm, treatment interruption resulted in a drop in CD4 by 27 cells/mm and viral rebound by 0.4 log copies/ml. After resuming salvage therapy, the percent of patients achieving an undetectable viral load (HIV RNA <75 copies/ml was greater) was 53% in the immediate ENF arm (controls) compared to 36% in the STI arm. There was no virologic benefit at 24 weeks associated with use of STI prior to switching to an optimized salvage therapy with ENF. The main predictor of treatment response was reported to be baseline phenotypic susceptibility score.
A.2. Benson 2004 reported data on the AACTG A5086 study, in triple‐class experienced patients, with failure of at least two regimens. Forty‐one participants were enrolled and randomized to a sixteen week STI arm (n=21) with resumption of salvage therapy or to a continuous treatment arm. This study was stopped early due to poor recruitment.
A.3. Deeks 2001, a small pilot study, randomized sixteen patients either to a single STI of 12 weeks duration, or to continued therapy. This was an early randomized study in the field of STI. They reported that STI resulted in a shift in viral population from drug‐resistant to drug‐sensitive, an increase in plasma HIV RNA levels, and a decrease in CD4 cell counts. The authors concluded that the return of wild virus during interruption increased the ability of virus to replicate, and also depleted peripheral blood CD4 cells.
A.4. Katlama 2004: This trial reported results in 68 patients who were randomized to either an immediate switch to a GigHAART regimen or a single 8‐week STI followed by a GigHAART regimen. Differentiating features of this trial were use of a regimen of 8‐9 drugs, a patient population with very advanced HIV disease, and a shorter duration of STI (2 months). This study was also closed early to accrual.
In contrast to the other RCTs, the findings in this trial indicated a virologic and immunologic benefit from STI. A better virologic success rate (defined as a minimum 1 log reduction in HIV RNA at 12 weeks) was reported in the STI arm compared to the control arm (62% vs. 26% success, respectively; p=0.007). A favorable change from baseline CD4 cell count in the STI arm, (+ 51 versus +7, p=0.047) and a decrease in log HIV RNA by 1.91 as compared to log 0.37 in the control arm (p=0.013) was reported at 24 weeks. In multivariate analysis, significant predictors of virologic success were treatment interruption, reversion to wild‐type virus, adequate plasma drug concentration, and use of lopinavir. This is the only randomized clinical trial to report a significant and sustained difference between the treatment arms in virologic and immunologic response due to STI (Katlama 2004) A.5. Lawrence 2003 and Lawrence 2005 reported results from the RCT of largest sample size (N=274). In this trial, participants were randomized to a single STI of 16 weeks duration, followed by optimized regimen, (STI arm, N=140). The control arm was immediately switched to an optimized regimen (control arm, N=134). The study was closed to accrual early per recommendations by the Data and Safety Monitoring Board.
The initial results published in 2003 were based on data from N=270 patients, with a median follow‐up of 12 months. Post‐STI and after ART resumption, both arms reported a sustained reduction in HIV RNA of 0.8 log copies/ml. In the STI arm, a significant decrease in CD4 counts was noticed as compared to the control arm (p<0.001). In the STI arm, a higher rate of clinical disease progression was reported (hazard ratio 2.57) (1.2 to 5.5, p=0.01) as compared to control arm. In a multivariate model, the significant predictors of clinical disease progression were baseline CD4 count (hazard ratio=1.38) (1.11‐1.72) and treatment group (hazard ratio 2.74) (1.25‐5.98). There were no differences between groups with respect to adherence, prophylaxis for opportunistic infections and quality of life measures (Lawrence 2003)
In the 2005 abstract, the authors presented the follow‐up data from all participants (N=274) with a median follow‐up of 36 months. During the STI, 55% of the STI arm lost at least half of the mutations present at randomization; 26% lost all mutations. The final results showed no significant difference between the STI and control arms in virologic response during the follow‐up periods after treatment was reinitiated in the STI arm. However, there was a sustained negative impact of STI on CD4 cell counts with treatment arm differences for the follow‐up periods 0 to 4, 5 to 24, and after 24 months of 84.3 (p < 0.0001), 47.0 (p = 0.0001), and 42.8 (p = 0.07), favoring the control arm. Although the number of clinical events (progression of disease or death) were higher in the STI arm than in the control arm, the overall difference was no longer significant with a median follow‐up of 36 months (Lawrence 2005)
The authors concluded that in this patient setting, there was no clinical, virologic, immunologic or quality of life benefit from STI. Furthermore, STI was associated with a significantly unfavorable CD4 response that persisted well after treatment re‐initiation. Therefore, they recommend continuing treatment with an optimized ART regimen and avoiding the use of STI (Lawrence 2003; Lawrence 2005)
In the STI arm, the median increase in CD4 was 10 cells/mm3 as compared to 17.5 cells/mm3 in the control arm. In the STI arm, the median change in HIV RNA was ‐0.65 log copies/mL as compared to ‐1.15 copies/mL in the control arm. In STI arm, viral reversion was reported in 15/18 (83%) patients. The authors concluded that STI did not lead to improvement in virologic response nor in the sustained disappearance of drug resistance mutations.
A.6. Ruiz 2003 reported results of an RCT of small sample size (N=46), in which patients were randomly assigned to receive a 5‐drug salvage regimen after a single 12 week STI (STI arm, N=22), or to immediately switch to this 5‐drug salvage regimen (control arm, N=24). At week 48, there were no differences in viral load (p=0.619) between interrupted and non‐interrupted groups. In the STI arm, a decrease (‐11) in CD4 cell count from baseline was reported, compared to an increase (+63) in the control arm; however, the difference was not significant (p=0.734). In 35% of the participants in the STI arm, viral reversion took place, which did not affect virologic response. The investigators concluded that prior to switching salvage regimens, attempting STI failed to provide any immunologic or virologic benefit.
A.7. Walmsley 2005 reported data from the CTN 164 trial, involving 134 participants and aimed to assess the benefit of an STI before switching to an optimized treatment regimen. Participants were randomized to a 12 week STI followed by optimized salvage therapy (STI arm) or to an immediate switch to optimized salvage therapy (IS arm). If the participant's health deteriorated in the STI arm, treatment was initiated early. The trial was stopped early to accrual due to slow recruitment. The original target sample size was 200; however, data on 134 participants were reported. Participants were followed for 60 weeks post‐randomization.
The primary outcome of this trial was to detect the proportion of patients who achieve a reduction in viral load <50 copies/ml after at least three months on salvage therapy. This endpoint was achieved by 55% participants in the STI arm and 69% participants in the IS arm (p=0.11). Although the reported reductions in HIV RNA in both STI and control arms, were similar from baseline, (‐1.7 log cop/ml) the differences in CD4 counts between the two arms, i.e., IS (+95) and STI (+25) arms were significant (p<0.04). Therefore, there was a statistically lower CD4 cell count rise but similar load reduction at 60 weeks (Walmsley 2005) To conclude, a 12‐week STI prior to switching salvage therapy did not improve outcomes.
A.8. The OPTIMA trial is still ongoing and remains blinded to randomization assignment. The study is designed with a targeted sample size of 504 participants (Kyriakides 2002).
In Singer 2006, the design of the OPTIMA trial is elucidated. This trial compares three month fixed duration STI arms (called "ART drug‐free period" or ARTDFP) to no ART drug‐free period arms. It also compares ARTDFP followed by optimized standard ART (<4 drugs) or mega‐ART (>5 drugs) salvage regimens. It aims to evaluate differences between mega‐ART and standard ART on virologic response, and to assess differences in clinical outcomes (aids or death, and aids related events), and quality of life between the two (ARDFP and non ARDFP) arms. Clinicians are allowed to terminate the ARTDFP earlier than 3 months, if they find that patients are deteriorating.
Brown 2004 presented preliminary reports on the OPTIMA trial, showing available data in 255 participants, with a median follow‐up of 11 months. These results remain blinded to the randomization arm, so no conclusions as to the utility of STI or mega‐HAART can yet be made from this trial.
B. Non‐randomized studies: Non‐randomized studies, of sample sizes from 9‐77, attempted unplanned and planned interruptions of fixed and variable durations (9‐49 days), with documentation of effects of interruption. These were small pilot studies that helped in the development of hypotheses and rationale for attempting STI in treatment failure category. Characteristics of participants and effects of interruptions and ART resumption are summarized in Table 2. The effects of STI after the resumption of ART are documented in the subsequent section.
See Table 2for baseline comparisons.
Summary of results across studies:
The principal findings of individual studies have been reported below.
B.1. Deeks 2003 reported results of their study of 24 participants. A single STI of variable duration (median 20 weeks) was attempted. The authors suggest that post ART resumption, viral suppression (<200 HIV RNA copies/ml) could be possible on a regimen with one active drug.
B.2. Delaugerre 2001, in their prospective study in 20 participants, reported results of a fixed 8‐week treatment interruption. On ART resumption, rapid detection of drug mutations was noted. Duration of interruption emerged as a significant predictor of viral reversion. There was a greater genotypic reversion in protease inhibitor‐treated patients, as compared to nucleoside analogue‐resistant patients.
B.3. Ghosn 2005, in their REVERT study of 23 participants, investigated the impact of interruptions of 24 weeks median duration (12‐37). In this single‐arm study, participants interrupted therapy and resumed salvage GigHAART therapy on reversion of resistance mutations to at least two drug classes. At baseline, CD4 count was 43(1‐372), and VL was 5.14(4.51‐5.69), with evidence of genotypic resistance. At the end of STI, an increase (70%) in viral susceptibility to three drugs in 70% (16/23) participants was reported. AIDS‐defining events in 66% participants were also reported. Significant viral reversions led to no virologic and immunologic benefit. The authors concluded that in patients failing therapy, i.e., with low CD4<200, reversion of resistance mutations was not successful in restoring the efficacy of a salvage regimen, and was clinically deleterious.
B.4. Halfon 2003 reported results of a 3‐month single treatment interruption in 11 patients. A complete reversion of mutant to wild‐type virus and a sharp reduction in the number of mutations was seen in 45% of participants.
B.5. Izopet 2000 reported results of a 3‐month fixed single treatment interruption followed by salvage therapy in 38 participants. Treatment interruption led to a median increase in HIV RNA of 0.4 log10, a median decrease in CD4 cell count of 43 cells/mm3, and a shift to wild‐type virus in 61% patients. This study suggested in patients where HIV disease was not very advanced, a three‐month treatment interruption could be considered in optimizing salvage therapy. The genotypic shift was best predicted by clinical stage of HIV infection.
B.6. Jaafar 2004 investigated a fixed 3‐month interruption in 77 patients. In a multivariate model, the factor associated with a genotypic shift was a high nadir CD4 cell count (OR 9.15). The predictors of virologic response to ART resumption were genotypic shift (OR 11.68), a shorter time on failing ART (OR 3.6), and a lower HIV RNA level before treatment resumption (OR 2.83). Sustained virologic response was best predicted by use of a NNRTI in NNRTI‐naïve patients (OR 4.89). The authors reported no significant differences in viral rebound and CD4 cell loss during STI between patients with and without a genotypic shift.
B.7. Miller 2000 conducted the first prospective cohort study, the Frankfurt cohort, and reported on the effects of resuming ART, post‐interruption. Forty‐eight participants were studied, in whom treatment interruptions of variable durations were undertaken, with documentation of outcomes. During treatment interruption, participants experienced a shift toward genotypic reversion in drug resistance within the virus population, and CD4 decline. Predictors of improvement in response to ART resumption in a multivariate model were: 1) pre‐treatment VL (hazard ratio 0.33); 2) genotypic shift (hazard ratio 5.22); and 3) new drugs in the ART regimen (hazard ratio 2.12).
B.8. Verhofstede 1999 investigated the effects of STI of variable durations in nine patients with high viral loads at baseline. Interruptions led to replacement of the mutant virus by wild‐type virus fourteen days after treatment interruption.
B.9 Youle 2000, in their study of 35 participants, reported on interruptions of variable duration. Treatment interruption resulted in a significant decline in CD4 cell count (median CD4 cell count 125 cells/mm3 at baseline dropping to 83 cells/mm3, p<0.008).
Discussion
We have summarized the currently available evidence on the effects of structured treatment interruption in patients with drug‐resistant virus and chronic unsuppressed HIV disease, despite being on HAART.
A. Non‐randomized trials: Non‐randomized trials investigated the impact of fixed and variable durations of interruptions, but were limited by small sample sizes (<100) and the absence of control groups. Durations of follow‐up were short. Overall, however, a pattern was evident across studies.
An improvement in drug susceptibility, with genotypic shift towards wild‐type virus, and a decline in CD4 and viral rebound were reported as a result of STI. In two studies, significant viral reversions failed to restore efficacy of salvage ART (Ghosn 2005; Jaafar 2004). Predictors of genotypic shift were: 1) high baseline CD4 (Miller 2000); 2) high nadir CD4 (Jaafar 2004); 3) clinical stage of HIV infection (Izopet 2000); and 4) duration of interruption (Delaugerre 2001). The progression of HIV infection was hypothesized to be a factor of increased viral fitness of re‐emergent wild‐type virus, and its effect on the immune system.
Responses after ART resumption were reported in a few studies. Viral suppression was better restored in those participants resuming therapy containing at least one active drug (Deeks 2003; Ghosn 2005). Predictors of favorable response to ART resumption were: 1) a shorter time on the failing regimen (Jaafar 2004); 2) a lower HIV level before treatment resumption (Jaafar 2004); 3) genotypic shift (Jaafar 2004; Miller 2000); and 4) an increased number of drugs in the optimized regimen (Miller 2000).
The ART salvage regimen used post‐STI played a major role in affecting response to resumption after interruption of therapy. This offered a clue to the introduction of newer drug classes with significant antiviral activity in multi‐drug resistant patients. Ghosn 2005 also concluded that in patients failing therapy, reversion of resistant mutations within viral populations failed to restore ART efficacy. As discussed by Ghosn 2005, the number of active drugs in the salvage regimen was important in deciding the ART post‐resumption. The issue of use of active drugs was first raised by Deeks 2003. Miller 2000 had similarly suggested the role of continued treatment in the setting of treatment failure in the light of risks of CD4 decline during treatment interruption.
Frequent monitoring of patients undergoing treatment interruption in controlled settings was recommended by all authors. A need for randomized studies of large sample sizes was also raised by the trials. B. Randomized controlled trials
Evidence from three landmark trials (CPCRA 064, Retrogene, GigHAART trials)
Similar results were reported in RCT by Lawrence 2003 and Ruiz 2003, with a significant decrease in CD4 cell counts and rise in HIV RNA during STI, and no virologic or immunologic benefit from STI.
However, divergent results were reported in the RCT by Katlama 2004. These divergent results were a greater virologic success, increase in CD4 cell count and greater log reduction in STI arm post STI compared to the no‐STI control arm. In a multivariate model, the major predictors of virologic success were treatment interruption, reversion to wild‐type virus, adequate plasma drug concentration, and use of lopinavir (Katlama 2004)
Potential factors contributing to the difference in results between these trials include the differences in sample size, baseline characteristics of participants, STI durations, and the prescribed ART salvage regimens as enumerated below.
1) Sample size: Two RCTs (Katlama 2004; Ruiz 2003) had small sample sizes, (46, 68), while one RCT (Lawrence 2003; Lawrence 2005) had a moderately‐sized sample of 274.
2) Study populations: The GigHAART trial (Katlama 2004) targeted patients with advanced HIV disease and low baseline CD4 cell counts (median CD4 = 27 cells/mm3), while CPCRA 064 trial (Lawrence 2003) had no CD4 entry criteria and enrolled patients with moderate HIV disease and median CD4 of 153 cells/mm3. The Retrogene study (Ruiz 2003) participants had less advanced disease and higher baseline CD4 counts (median CD4 =339 cells/mm3).
3) STI duration: STI durations also varied across these trials. The GigHAART study attempted 2 months STI; the Retrogene study, 3 months; and CPCRA 064, 4 months. (Katlama 2004; Lawrence 2003; Ruiz 2003)
4) ART regimen: While drugs in the optimized ART regimens were similar in the Retrogene and CPCRA 064 trials (i.e., 4‐5 drug regimens), they were different in GigHAART trial (i.e., 8‐9 drugs including hydroxyurea). This could also explain differences in responses to STI post ART resumption in the GigHAART trial (Katlama 2004; Lawrence 2003; Ruiz 2003)
Evidence from other randomized trials (ACTG 5086, CTN 164, and others)
In Benson 2004 and Deeks 2001, the sample size of the studies were small, however, no significant virologic and immunologic benefit were reported. The ACTG 5086 trial was stopped prematurely due to poor recruitment. Due to a small sample size (41), the study was underpowered to detect differences between the two groups (STI and continuous therapy) arm.
Another larger trial, the CTN 164 with a sample size 134 was closed early to accrual due to poor recruitment (Walmsley 2005). In 67 participants in STI arm, a pattern of no significant immunologic and virologic benefit was reported. Although the study was not powered to examine clinical endpoints, there were a greater number of AIDS defining events noted in the STI arm. In summary, there was no benefit accrued to STI and some suggestion of harm.
Beatty 2005 reported trial results using Enfuvirtide (ENF) in the salvage regimen subsequent to attempting a 16 week STI. As a result of STI, an expected drop in CD4 in STI arm and viral rebound, was noted. But post resumption of Enfuvirtide based regimen, the reduction in VL to undetectable was less compared to control arm. There was no virologic benefit, and treatment response was best predicted by phenotypic susceptibility at baseline. This study also was limited by a small sample size (47), and a short study follow‐up of 24 weeks. This study challenged the speculation of better response with new antiretrovirals (ENF). However, the results reported suggested no virologic benefit due to STI. Currently ongoing trial OPTIMA trial is currently ongoing and the primary endpoint results remain blinded as to randomization assignment. Therefore, conclusions as to the utility of STI and/or mega‐HAART in this patient population cannot yet be made from this trial (Holodniy 2004; Kyriakides 2002).
Authors' conclusions
Implications for practice.
Based on the results of recently reported trials, there were reports of no significant virologic or immunologic benefit due to STI, before switching to an optimized salvage regimen in patients with chronic unsuppressed HIV infection (treatment failure) and multi‐drug resistant HIV. There is strong evidence that STI leads to CD4 decline during treatment interruption and is likely to have a prolonged negative impact on CD4 response even after treatment is resumed. This appears to translate into an increased risk of progression of disease events, although the difference in risk between those who undergo a single STI and those remaining on continuous salvage therapy may wane over time after ARVs have been resumed.
To conclude, based on the studies we reviewed, there is no evidence to support the use of STI in this patient population.
Implications for research.
A standardized method for reporting of all important outcomes (measures of effect such as hazard ratios, risk ratios) needs to be emphasized. This will aid comparability across trials. Use of CONSORT guidelines for reporting of trials will be useful in this regard. Evaluation of success of STI strategies in terms of in terms of consistent definitions of primary and secondary end points will greatly aid comparability across trials.
Trials designed with adequate power to determine differences in clinical endpoints and follow up of longer durations will help assess the true clinical impact of interventions over time. These will also help assess safety, efficacy and applicability of STI. Two large trials, CPCRA and OPTIMA, have been designed with primary clinical endpoints to explore these questions (Brown 2004; Lawrence 2003; Singer 2006). The unblinded results from the OPTIMA study are eagerly awaited (Brown 2004; Holodniy 2004; Kyriakides 2002; Singer 2006). Overall evidence
To conclude, based on the studies we reviewed, the evidence in this category primarily supports a lack of benefit of STI before switching therapy, in patients with unsuppressed HIV viremia despite ART. There is no evidence that viral reversion of resistance during STI results in an improvement in response to subsequent antiretroviral therapy. There is no net virologic or immunologic benefit accrued to STI before switching to an optimized salvage regimen. Importantly, there is evidence of harm from STI in patients with relatively advanced HIV disease, including an immediate decline in CD4 cell count during the STI period, followed by a prolonged negative impact on CD4 recovery, even after ART re‐initiation. This suggests an increased risk of clinical disease progression although the difference in risk of a single STI compared with continuous salvage therapy may wane over time once ART is resumed.
Based on the studies we reviewed, the vast majority of evidence suggests greater harm than good from interrupting ART, in chronically HIV‐infected individuals who have treatment failure with multi‐drug resistant HIV. The evidence does not support the use of STI in this setting.
What's new
| Date | Event | Description |
|---|---|---|
| 16 March 2011 | Review declared as stable | This review will no longer be updated. |
History
Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 30 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the Cochrane HIV/AIDS Review Group in San Francisco for their constant support and encouragement. We acknowledge inputs from Dr Lidia Ruiz, and Dr Christine Katlama in responding to our questions and clarifications via email. We also acknowledge the support of the training grant from the Fogarty AIDS International Training Program (grant 1‐D43‐TW00003‐16) at the University of California, Berkeley. We thank Dr Madhukar Pai for his feedback on the draft of this manuscript.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beatty 2005.
| Methods | N=30 Randomized Open label Fup: 24 weeks w/o: reported | |
| Participants | Eligibility:‐ VL>500 ART: 2PI+2NRTI+1NNRTI Baseline CD4 47 cells/mm3 Baseline VL 4.72 log copies/ml Baseline ART: 2PI+2NRTI+1NNRTI | |
| Interventions | 16 wks STI | |
| Outcomes | STI arm:
CD4 decline: 27 cells/mm3
VL rebound: +0.4log cop/ml Post ART resumption STI arm:‐ VL decline < 75 cop/ml in 36% participants Control arm continuous ART: VL decline <74 cop/ml in 53% participants |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Benson 2004.
| Methods | ACTG A 5086 trial N=41 Randomized Open label fup: 48 weeks w/o: reported | |
| Participants | Eligibility :‐ VL>10,000 copies/ml CD4:>150 cells/mm3 ART: one virologic failure Baseline CD4 226 cells/mm3 Baseline VL 38,000 cop/ml | |
| Interventions | 16 wks STI | |
| Outcomes | STI arm:
CD4 increase of + 10 cells/mm3
VL decline ‐0.65 log copies/ml Control arm: CD4 increase of +17.5 cells/mm3 VL decline of ‐1.15 log copies/ml |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Brown 2004.
| Methods | OPTIMA trial N=289 Randomized Open label fup: 24 weeks w/o: reported | |
| Participants | Eligibility:‐ VL>2,500 cop/ml CD4<300 Age>18 yrs Failure two drug classes On ART for 3 months HIV serologic diagnosis | |
| Interventions | 3 months STI | |
| Outcomes | Differences between STI and control arms: Events: Greater Non serious AIDS events than AIDS events in STI arm. Quality of life: no differences between arms | |
| Notes | Currently ongoing trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Deeks 2001.
| Methods | N=16 Randomized, Open label, Fup =24 weeks, w/o d/o: reported | |
| Participants | Eligibility:‐ VL >2500 copies/ml for 6 months, ART: 35 weeks on PI regimen Age: median 38 years(37‐45), Sex: 100% male Baseline CD4 302(258‐355), Baseline VL 4.5(4.0‐4.6), | |
| Interventions | 12 wks STI | |
| Outcomes | STI arm: CD4 decline: ‐ 128 cells/cubic mm. VL rebound : + 0.84 log copies/ml (0.27‐1.07) Adverse effects: Pneumocystic carinii pneumonia, peripheral neuropathy, thrombocytopenia | |
| Notes | Continued treatment during interruption associated with sustained clinical benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Deeks 2003.
| Methods | N= 24 Non randomized, Fup=109 weeks, w/o d/o reported | |
| Participants | Eligibility:‐ VL >2500 copies for 6months; ART: PI* 12 months, Stable ART for 4 months; ART duration 27 weeks (25‐33) Baseline CD4 218 (80‐298); Baseline VL 4.6 (3.9‐5.1); | |
| Interventions | 20 wks TI | |
| Outcomes | STI arm: CD4 decline: 108 cells/mm3 VL rebound: + 0.76 log cop/ml Adverse events: Pneumocystis carinii pneumonia, thrombocytopenia; peripheral neuropathy | |
| Notes | Durable viral suppression achieved with use of one fully active drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Delaugerre 2001.
| Methods | N=20 Non randomized Fup=unclear | |
| Participants | Eligibility:‐ Treatment failure VL>10,000 copies/ml for 36 months, CDC stage C 80% patients; Median ART 73months(29‐118) Median CD4 200 cells/mm3; Baseline median VL 160, 000 cop/ml | |
| Interventions | 8 wks TI (4‐24 wks) | |
| Outcomes | STI arm: Viral reversion: 55% participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Ghosn 2005.
| Methods | REVERSE study N=23 Non randomized FUP=24 weeks w/o d/o=unclear | |
| Participants | Eligibilty:‐ VL>30,000 copies/ml, ART>3 drugs, Mutations: 1.3TAM+1NNRTI+2PI Baseline mutations: 17(9‐28) Baseline CD4:43(1‐372) Baseline VL 5.14(4.51‐5.69) | |
| Interventions | 24 wks TI (12‐37 wks) | |
| Outcomes | STI arm: CD4 decline: no significant decline Viral rebound: no significant rebound Viral reversion: 70% patients Drug susceptibility : Increased in 16/23 participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Halfon 2003.
| Methods | N=11 Non Randomized Fup: not reported, w/o d/o: reported | |
| Participants | Eligibility: ‐ HAART failure Baseline primary mutations: minimum two Baseline mean CD4 314 (22‐1510) Baseline mean HIV RNA 4.45 (2.9‐5.7) | |
| Interventions | 3 month TI | |
| Outcomes | CD4 decline: present in all participants VL rebound: VL<70,000 cop/ml in 8/11 participants; VL >300,000 cop/ml in 3/11 participants Viral Reversion: 2 participants Mutations: 2 participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Izopet 2000.
| Methods | N=38 Non randomised, Fup=6 months, w/o: reported | |
| Participants | Eligibility:‐ HIV RNA >5000 copies/ml, Age: 41 years, Sex: 82% males ART : ART for >6months, NRTI + PI used, Baseline CD4 median 188(6‐732) Baseline VL 4.6(3.7‐6.2); | |
| Interventions | 3 month TI | |
| Outcomes | CD4 decline: 43 cells/mm3 VL rebound: 0.4 log copies/ml Viral reversion:61% participants | |
| Notes | Clinical stage predicts viral reversion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Jaafar 2004.
| Methods | N=77 Non randomized Fup: na w/o d/o: na | |
| Participants | Eligibility:‐ VL >1,000 copies/ml, CD4>200 cells/mm3, Potent ART >6months ART: NNRTI+NRTI+PI for 59 months; Baseline CD4 483 (200‐919) Baseline VL 4.0 (3.0‐5.5); | |
| Interventions | 3 month TI | |
| Outcomes | CD4 decline: 132 cells/mm3; VL rebound: 2.7 log copies/ml, | |
| Notes | Predictors of virological response in a multivariate model: 1.genotypic shift OR 11.68; 2. short time on failing ART OR 3.6; 3. lower HIV RNA Level 2.83; 4. salvage regimen OR 3.69; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Katlama 2004.
| Methods | ANRS 097 trial N=68 Randomized Open Fup: 56 weeks w/o d/o: reported | |
| Participants | Eligibility :‐ VL>75,000 CD4: na ART: evidence of MDR virus; regimen >2PI + 2 NRTI +2NNRTI, duration: 6.2 years, Age>15 years. STI group: N=31, median age 41.3 years 97% males, Control group: N=34 median age 38.6 years, 97% males, | |
| Interventions | 8 wks STI Post STI ART: GIGHAART= 3‐4 NRTI + HU+ 1NNRTI + 3 PI; | |
| Outcomes | CD4 decline ‐10 cells/mm3(‐130‐15) in STI gp
CD4 cell count differences at week 24:
CD4 increase STI gp +51
CD4 increase control gp +7(p=0.047*)
CD4 cell count differences at week 48/52:
CD4 increase in STI gp +69
CD4 increase in control gp +7(*p=0.04) VL rebound VL rebound STI gp: +0.16 log copies(‐0.55 to 1.10) VL differences between gps at week 12: VL decline STI gp ‐1.91 (‐3.28 to +3.2) VL decline Control gp ‐0.37 (‐3.47 to ‐.76) (p=0.008) VL differences between gps at week 24: VL decline STI gp ‐1.08(‐3.63‐0.33) VL decline Control gp ‐0.29(‐3.6 to 0.45) (p =0.013) Viral reversion: STI gp 52% Loss of mutations: 1 class 11% 2 classes 26% 3 classes 1%. Adverse effects: STI group 3/9 pts Control gp 3/15 pts Deaths: 2 pts in both STI and control gps |
|
| Notes | 8 week STI followed by multidrug salvage regimen associated with better virological response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lawrence 2003.
| Methods | CPCRA 064 trial N=270 Randomized Open Fup= 11.6 months prematurely terminated w/o d/o reported | |
| Participants | Eligibility:‐ HIV infection >13 yrs,HIV RNA >5,000 copies/ml, MDR virus, stable ART regime, STI N= 138 STI median age 45 years 8.7%, Females, 132 control group, 43.9 years mean age, 9.8% Baseline CD4 153 cells/mm3 Baseline VL 5.0 copies/ml ART: 2.2 NNRTI +2.5PI+1.0NNRTI Mut: 10.8 | |
| Interventions | 4 month STI | |
| Outcomes | CD4 differences b/w control and STI gps:
0‐4 months:85 cells/mm3 ( p<0.001)
5‐8 months: 47 cells/mm3 (p<0.001)
9‐20 months: 31 cells/mm3 (p<0.11)
Control group mean CD4 counts higher by 85 cells p<0.001 VL differences b/w STI and Control gps: 0‐4 months: +1.2 log cop/ml higher in STI gp (p<0.001), 4‐20 months: 0.8 log cop/ml suppressed both gps Viral reversion: 64% pts A/E: no difference between gps Death: 8 pts both gps (HR=2.57(1.2‐5.5) (p=0.01) Disease events: STI gp 17 pts Control gp 5 pts HR=6.04(1.8‐20.8) Quality of life at 2 months: STI gp: SF12 score +1.7 Control gp: SF12 score ‐0.4 |
|
| Notes | Continue with an optimized ARV regimens and avoid the use of STI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lawrence 2005.
| Methods | CPCRA 064 trial N=274 Randomized Open Fup= 36 months | |
| Participants | Eligibility:‐ HIV infection >13 yrs,HIV RNA >5,000 copies/ml, MDR virus, stable ART regime, STI N= 138 STI median age 45 years 8.7 Baseline CD4 153 cells/mm3 Baseline VL 5.0 log copies/ml, ART: 2.2 NNRTI +2.5PI+1.0NNRTI Mut: 10.8 | |
| Interventions | 4 month STI | |
| Outcomes | Differences in CD4 between STI and control groups:‐
0‐4 months= 84 cells
5‐24 months =47.0 cells
24 months =42.8 cells STI arm: Loss of all mutations in 26% participants Loss of half mutations in 55% participants. Disease progression events: STI arm: 91 events Control arm: 71 events Deaths: STI arm 30 events Control arm 33 events Quality of life: no differences in both arms Adherence: no differences in both arms. |
|
| Notes | No benefit of STI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Miller 2000.
| Methods | Frankfurt cohort N=48 Non Randomized Open fup: reported w/o: reported | |
| Participants | Eligibility: Median ART =9 drugs (4‐13) AZT/3TC98% d4T/DDI SQV 84% IDV 88% NVP 72% RTV 65% ZCT Baseline CD4 =155 (2‐777) Baseline VL =5.07(2.7‐6.7) | |
| Interventions | 121 days(54‐322 days) TI | |
| Outcomes | STI gp:
CD4 decline: 89 cells/microlitre (p=0.0001);
VL rebound: 0.7 log copies/ml ( p=0.0001); Reversion: 28/45 participants Model predictors of TI : 1. CD4 cell OR 2.32; 2. prior ART OR=0.66; |
|
| Notes | Predictors of genotypic shift: 1. CD4 cell count, 2. ARV duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Ruiz 2003.
| Methods | RETROGENE study N= 46 patients Randomized Open Fup: 48 weeks w/o d/o: reported | |
| Participants | Eligibility:‐ 3 drug regimens* 6mo tmt failure * previous regimens VL>1,000 cop/ml, Baseline CD4 383 ( 84‐783) Baseline VL 4.3(3.2‐5.3) ART regimens: NRTI + NNRTI + PI duration: 5.73 years 77% MDR resistant STI: N=22 median age 32(25‐43) yrs, 76% males Controls: N= 24 age 35(23‐47) yrs, 73% males, | |
| Interventions | 12 weeks STI | |
| Outcomes | CD4 cell count differences b/w STI and control gps:
No difference between groups (p=0.734) VL <50 cop/ml at 48 weeks: STI gp 45% participants Control gp46% participants (no difference between gps, p=0.619) Viral Reversion: STI gp 35% participants |
|
| Notes | No immunological benefit of STI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Singer 2006.
| Methods | OPTIMA trial N=504 Randomized Open label fup: 24 weeks w/o: reported | |
| Participants | Eligibility:‐ VL>2,500 cop/ml CD4<300 Age>18 yrs Failure two drug classes On ART for 3 months HIV serologic diagnosis | |
| Interventions | 3 months STI | |
| Outcomes | CD4>200: 8% participants
CD4 51‐200: 14% participants
CD4 <50: 46% participants VL: 1 log reduction in (54% ARDFP) participants Model predictors for AIDS events: 1.CD4 salvage therapy ( HR 0.874) 2. VL at 24 wks (HR 1.81) |
|
| Notes | Preliminary report on an ongoing trial. Predictors of AIDS:‐ 1.CD4 at salvage therapy, 2. changes in CD4, VL, 24 weeks after onset of therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Verhofstede 1999.
| Methods | N=9 Non randomized FUP=unclear W/o d/o: unreported | |
| Participants | Eligibility:‐ NRTI regimen, VL (15,924‐997.361) copies/ml, | |
| Interventions | TI unclear duration | |
| Outcomes | VL rebound: 77% participants Loss of mutations: 88% participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Walmsley 2005.
| Methods | CTN 164 trial N=134 Randomized Open label fup: 60 weeks w/o: reported | |
| Participants | Eligibilty :‐ VL>1,000 cop/ml 2 ART remaining in salvage regimen, Baseline CD4 343 cells/mm3 Baseline VL 3.9 log copies/ml,. Baseline ART: PI + NRTI + NNRTI | |
| Interventions | 12 wks STI | |
| Outcomes | CD4 decline: 132 cells/mm3; VL increase: 2.7 log copies/ml, | |
| Notes | Genotypic shift predicted by nadir CD4; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Youle 2000.
| Methods | N=35 Non randomized W/o: unclear Follow up: unclear | |
| Participants | Eligibility:‐ VL>50,000 RNA cop/ml, ART: on PI based 4 drug regimen for 6 months, Pre STI ART duration: >4.4 years Baseline CD4 125 (36‐254) Baseline VL 540,000 (72,000‐750,000) | |
| Interventions | TI 1‐2 months Washout period present | |
| Outcomes | CD4 differences b/w control and STI gps:
0‐4 months:85 cells/mm3 ( p<0.001)
5‐8 months: 47 cells/mm3 (p<0.001)
9‐20 months: 31 cells/mm3 (p<0.11)
Control group mean CD4 counts higher by 85 cells p<0.001 VL differences b/w STI and Control gps: 0‐4 months: +1.2 log cop/ml higher in STI gp (p<0.001), 4‐20 months: 0.8 log cop/ml suppressed both gps Viral reversion: 64% pts A/E: no difference between gps Death: 8 pts both gps (HR=2.57(1.2‐5.5) (p=0.01) Disease events: 17 pts STI gp 5 pts control gp HR=6.04(1.8‐20.8) Quality of life at 2 months: STI gp: SF12 score +1.7 Control gp: SF12 score ‐0.4 |
|
| Notes | 1.Greater loss of CD4 soon after interruption (p=0.03). 2. Faster decline in CD4 within the first three weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2003 | Chronic suppressed HIV infection |
| Altfeld 2002 | Primary HIV Infection |
| Ananworanich 2003 | Chronic suppressed HIV infection |
| Ananworanich 2005 | Chronic suppressed HIV infection |
| Bajaria 2004 | Modelling studies |
| Blankson 2001 | Review |
| Boschi 2004 | Chronic suppressed HIV infection |
| Cardiello 2005 | Chronic suppressed HIV infection |
| Fagard 2003 | Chronic suppressed HIV infection |
| Fagard 2005 | Chronic suppressed HIV infection |
| Fischer 2003 | Chronic suppressed HIV infection |
| Foli 2002 | Chronic suppressed HIV infection |
| Frost 2002 | Chronic suppressed HIV infection |
| Garcia 2003 | Chronic suppressed HIV infection |
| Garcia 2001 | Chronic suppressed HIV infection |
| Garcia 2002 | Review |
| Grinberg 2000 | Review |
| Gulick 2002 | Review |
| Idemyor 2003 | Review |
| Lori 2000 | Review |
| Lori 2000b | Chronic suppressed HIV infection |
| Maroto 2005 | Chronic suppressed HIV infection |
| Martinez‐Picado 2002 | Chronic suppressed HIV infection |
| Metzner 2003 | Chronic suppressed HIV infection |
| Molto 2004 | Chronic suppressed HIV infection |
| Montes 2005 | Chronic suppressed HIV infection |
| Mussini 2005 | Chronic suppressed HIV infection |
| Neumann 1999 | Acute HIV Infection |
| Nuesch 2005 | Chronic suppressed HIV infection |
| Ortiz 2001 | Chronic suppressed HIV infection |
| Oxenius 2002 | Chronic suppressed HIV infection |
| Papasavvas 2004 | Chronic suppressed HIV infection |
| Parienti 2002 | Chronic suppressed HIV infection |
| Plana 2004 | Chronic suppressed HIV infection |
| Ruiz 2001 | Chronic suppressed HIV infection |
| Schweighardt 2002 | Chronid suppressed HIV infection |
| Sommet 2003 | Review |
| Taffe 2002 | Chronic suppressed HIV infection |
| Tuldra 2001 | Chronic suppressed HIV infection |
| Yerly 2003 | Review |
| Yerly 2004 | Chronic suppressed HIV infection |
Characteristics of ongoing studies [ordered by study ID]
Holodniy 2004.
| Trial name or title | OPTIMA |
| Methods | |
| Participants | N(proposed)= 1700 |
| Interventions | 3 months ARTDFP ART drug free period compared to non ART drug free periods in a 4 by 4 factorial design |
| Outcomes | primary outcomes=time to AIDS or death secondary outcomes=toxicity, illness, quality of life, CD4, VL, |
| Starting date | June 2001 |
| Contact information | http://www.optimatrial.org/uk/pandp.html |
| Notes | largest trinational trial (UK, USA, Canada) currently ongoing |
Kyriakides 2002.
| Trial name or title | OPTIMA |
| Methods | |
| Participants | 1700 patients randomized to four treatment arms |
| Interventions | Four treatment arms 1. ARTDFP plus standard ART 2. ARTDFP plus MegaART 3.no ARTDFP plus standard ART 4. no ARDFP plus megaART |
| Outcomes | primary outcomes=time to AIDS or death secondary outcomes= toxicity, illness, quality of life, CD4, VL, cost effectiveness |
| Starting date | June 2001 |
| Contact information | tassosl@mindspring.com |
| Notes |
Contributions of authors
Study concept and design: Pai, Tulsky, Lawrence Acquisition of data: Pai, Tulsky Analysis and Interpretation of data: Pai, Lawrence, Tulsky, Drafting of manuscript: Pai, Lawrence, Tulsky, Reingold Dr. Pai and Dr. Lawrence contributed equally to this review. Critical revision of manuscript for important intellectual content: all authors
Sources of support
Internal sources
Fogarty AIDS International Training program ( AITRP grant 1‐ D43‐TW00003‐16) University of California at Berkeley, USA.
External sources
No sources of support supplied
Declarations of interest
We certify that we have no affiliations or involvements in any organization or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, and expert testimony).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Beatty 2005 {published data only}
- George Beatty, J Lu, P Hunt, W Huang, J Martin, D Kuritzkes, S Deeks. Randomized Pilot Study of Immediate Enfuvirtide‐based Therapy vs a Treatment Interruption followed by Enfuvirtide‐based Therapy in Highly Treatment‐experienced Patients. 12th conference on retroviruses and opportunistic infections. Feb 22‐25, 2005 Boston. [Abstract No 581]
Benson 2004 {published and unpublished data}
- C Benson, G Downey, D V Havlir, F Vaida, M Lederman, R Gulick, M Glesby, S Patel, M Wantman, C Bixby, C Pettinelli, A Rinehart, S Snyder, J Mello, and the ACTG A 5086 Study Team. A 16‐week Treatment Interruption Does Not Improve the Virologic Response to Multidrug Salvage Therapy in Treatment‐experienced Patients: 48‐week Results from ACTG A5086. 11th conference on retroviruses and opportunistic infections. Feb 8‐11 2004 San Francisco.
Brown 2004 {published data only}
- S T Brown, J Singer, A Anis, H Sun, T C Kyriakides, B J Angus, K Swanson, W. Cameron, A. Babiker, M. Holodniy and The OPTIMA Study Team. The impact of non‐AIDS Serious events equals or exceeds that of AIDS outcomes in patients with multi drug resistant HIV disease. IAC 2004 Bangkok. July 2004; Vol. Abstract Q #73.
Deeks 2001 {published data only}
- Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral‐drug therapy in HIV‐infected patients with detectable viremia. N Engl J Med 2001;344(7):472‐80. [DOI] [PubMed] [Google Scholar]
Deeks 2003 {published data only}
- Deeks SG, Grant RM, Wrin T, Paxinos EE, Liegler T, Hoh R, et al. Persistence of drug‐resistant HIV‐1 after a structured treatment interruption and its impact on treatment response. Aids 2003;17(3):361‐70. [DOI] [PubMed] [Google Scholar]
Delaugerre 2001 {published data only}
- Delaugerre C, Valantin MA, Mouroux M, Bonmarchand M, Carcelain G, Duvivier C, et al. Re‐occurrence of HIV‐1 drug mutations after treatment re‐initiation following interruption in patients with multiple treatment failure.. Aids. . 2001 Nov 9;;15(16)::2189‐91. [DOI] [PubMed] [Google Scholar]
Ghosn 2005 {published data only}
- Ghosn J, Wirden M, Ktorza N, Peytavin G, Ait‐Mohand H, Schneider L, et al. No benefit of a structured treatment interruption based on genotypic resistance in heavily pretreated HIV‐infected patients.. Aids. 2005 Oct 14;;19(15)::1643‐7.. [DOI] [PubMed] [Google Scholar]
Halfon 2003 {published data only}
- Halfon P, Durant J, Clevenbergh P, Carsenti H, Celis L, Khiri H, et al. Kinetics of disappearance of resistance mutations and reappearance of wild‐type during structured treatment interruptions. Aids 2003;17(9):1351‐61. [DOI] [PubMed] [Google Scholar]
Izopet 2000 {published data only}
- Izopet J, Massip P, Souyris C, Sandres K, Puissant B, Obadia M, et al. Shift in HIV resistance genotype after treatment interruption and short‐term antiviral effect following a new salvage regimen. Aids 2000;14(15):2247‐55. [DOI] [PubMed] [Google Scholar]
Jaafar 2004 {published data only}
- Jaafar A, Massip P, Sandres‐Saune K, Souyris C, Pasquier C, Aquilina C, et al. HIV therapy after treatment interruption in patients with multiple failure and more than 200 CD4+ T lymphocyte count. J Med Virol 2004;74(1):8‐15. [DOI] [PubMed] [Google Scholar]
Katlama 2004 {published data only}
- Katlama C, Dominguez S, Gourlain K, Duvivier C, Delaugerre C, Legrand M, et al. Benefit of treatment interruption in HIV‐infected patients with multiple therapeutic failures: a randomized controlled trial (ANRS 097). Aids 2004;18(2):217‐26. [DOI] [PubMed] [Google Scholar]
Lawrence 2003 {published data only}
- Lawrence J, Mayers DL, Hullsiek KH, Collins G, Abrams DI, Reisler RB, et al. Structured treatment interruption in patients with multidrug‐resistant human immunodeficiency virus. N Engl J Med 2003;349(9):837‐46. [DOI] [PubMed] [Google Scholar]
Lawrence 2005 {published and unpublished data}
- Jody Lawrence, K Huppler Hullsiek, L Thackeray, D Abrams, D Mayers, L Crane, M Jones, J Saldanha, B Schmetter, T Dionne, C Pettinelli, J Baxter, and for the 064 Study Team of the Terry Beirn Community Programs for Clinical Research on AIDS. Final Results of CPCRA 064: A Randomized Trial Examining Structured Treatment Interruption for Patients Failing Therapy with Multi‐drug Resistant HIV. 12th conference on retroviruses and opportunistic infections. Feb 22‐25, 2005 Boston.
Miller 2000 {published data only}
- Miller V, Sabin C, Hertogs K, Bloor S, Martinez‐Picado J, D'Aquila R, et al. Virological and immunological effects of treatment interruptions in HIV‐1 infected patients with treatment failure. Aids 2000;14(18):2857‐67. [DOI] [PubMed] [Google Scholar]
Ruiz 2003 {published data only}
- Ruiz L, Ribera E, Bonjoch A, Romeu J, Martinez‐Picado J, Paredes R, et al. Role of structured treatment interruption before a 5‐drug salvage antiretroviral regimen: the Retrogene Study. J Infect Dis 2003;188(7):977‐85. [DOI] [PubMed] [Google Scholar]
Singer 2006 {published and unpublished data}
- Joel Singer, D Ayers, D Cameron, M Holodniy, S Brown, T Kyriakides, A Babiker, A Anis, M Youle, M Schechter. Predictors of Clinical Response to Salvage Therapy in a Late Salvage Population with Multidrug‐resistant HIV in the OPTIMA Trial. CROI 13th conference on retroviruses and opportunistic infections. Feb 5‐8, 2006 Denver. [Abstract no 526]
Verhofstede 1999 {published data only}
- Verhofstede C, Wanzeele FV, Gucht B, Cabooter N, Plum J. Interruption of reverse transcriptase inhibitors or a switch from reverse transcriptase to protease inhibitors resulted in a fast reappearance of virus strains with a reverse transcriptase inhibitor‐sensitive genotype.. Aids. 1999 Dec 24;;13(18)::2541‐6.. [DOI] [PubMed] [Google Scholar]
Walmsley 2005 {published data only}
- Sharon Walmsley, N LaPierre, M Loutfy, J MacLeod, B Trottier, B Conway, S Trottier, A Thorne, D Zarowny, J Singer, and CTN 164 Study Investigators. CTN 164: A Prospective Randomized Trial of Structured Treatment Interruption vs Immmediate Switching in HIV‐infected Patients Experiencing Virologic Failure on HAART. 12th conference on retroviruses and opportunistic infections Feb 22‐25, 2005; Vol. Feb 22‐25, 2005.
Youle 2000 {published data only}
- Youle M, Janossy G, Turnbull W, Tilling R, Loveday C, Mocroft A, et al. Changes in CD4 lymphocyte counts after interruption of therapy in patients with viral failure on protease inhibitor‐containing regimens. Royal Free Centre for HIV Medicine. Aids 2000;14(12):1717‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 2003 {published data only}
- Alexander TH, Ortiz GM, Wellons MF, Allen A, Grace EJ, 2nd, Schweighardt B, et al. Changes in CD4+ T‐cell differentiation phenotype during structured treatment interruption in patients with chronic HIV‐1 infection. J Acquir Immune Defic Syndr 2003;34(5):475‐81. [DOI] [PubMed] [Google Scholar]
Altfeld 2002 {published data only}
- Altfeld M, Lunzen J, Frahm N, Yu XG, Schneider C, Eldridge RL, et al. Expansion of pre‐existing, lymph node‐localized CD8+ T cells during supervised treatment interruptions in chronic HIV‐1 infection. J Clin Invest 2002;109(6):837‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananworanich 2003 {published data only}
- Ananworanich J, Nuesch R, Braz M, Chetchotisakd P, Vibhagool A, Wicharuk S, et al. Failures of 1 week on, 1 week off antiretroviral therapies in a randomized trial. Aids 2003;17(15):F33‐7. [DOI] [PubMed] [Google Scholar]
Ananworanich 2005 {published data only}
- Ananworanich J, Hirschel B. Interrupting highly active antiretroviral therapy in patients with HIV. Expert Rev Anti Infect Ther 2005;3(1):51‐60. [DOI] [PubMed] [Google Scholar]
Bajaria 2004 {published data only}
- Bajaria SH, Webb G, Kirschner DE. Predicting differential responses to structured treatment interruptions during HAART. Bull Math Biol 2004;66(5):1093‐118. [DOI] [PubMed] [Google Scholar]
Blankson 2001 {published data only}
- Blankson JN, Siliciano RF. Structured therapeutic interruptions: a review. Hopkins HIV Rep 2001;13(1):1, 8‐9, 13. [PubMed] [Google Scholar]
Boschi 2004 {published data only}
- Boschi A, Tinelli C, Ortolani P, Moscatelli G, Morigi G, Arlotti M. CD4+ cell‐count‐guided treatment interruptions in chronic HIV‐infected patients with good response to highly active antiretroviral therapy. Aids 2004;18(18):2381‐9. [PubMed] [Google Scholar]
Cardiello 2005 {published data only}
- Cardiello PG, Hassink E, Ananworanich J, Srasuebkul P, Samor T, Mahanontharit A, et al. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin Infect Dis 2005;40(4):594‐600. [DOI] [PubMed] [Google Scholar]
Fagard 2003 {published data only}
- Fagard C, Oxenius A, Gunthard H, Garcia F, Braz M, Mestre G, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med 2003;163(10):1220‐6. [DOI] [PubMed] [Google Scholar]
Fagard 2005 {published data only}
- Fagard C, Bandelier CY, Ananworanich J, Braz M, Gunthard H, Perneger T, et al. Biphasic decline of CD4 cell count during scheduled treatment interruptions. Aids 2005;19(4):439‐41. [DOI] [PubMed] [Google Scholar]
Fischer 2003 {published data only}
- Fischer M, Hafner R, Schneider C, Trkola A, Joos B, Joller H, et al. HIV RNA in plasma rebounds within days during structured treatment interruptions. Aids 2003;17(2):195‐9. [DOI] [PubMed] [Google Scholar]
Foli 2002 {published data only}
- Foli A, Seminari E, Ravot E, Lisziewicz J, Lori F. Role of hydroxyurea during structured treatment interruptions. J Biol Regul Homeost Agents 2002;16(1):64‐8. [PubMed] [Google Scholar]
Frost 2002 {published data only}
- Frost SD, Martinez‐Picado J, Ruiz L, Clotet B, Brown AJ. Viral dynamics during structured treatment interruptions of chronic human immunodeficiency virus type 1 infection. J Virol 2002;76(3):968‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2003 {published data only}
- Garcia F, Plana M, Arnedo M, Ortiz GM, Miro JM, Lopalco L, et al. A cytostatic drug improves control of HIV‐1 replication during structured treatment interruptions: a randomized study. Aids 2003;17(1):43‐51. [DOI] [PubMed] [Google Scholar]
Garcia 2001 {published data only}
- Garcia F, Plana M, Ortiz GM, Bonhoeffer S, Soriano A, Vidal C, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV‐1 infection. Aids 2001;15(9):F29‐40. [DOI] [PubMed] [Google Scholar]
Garcia 2002 {published data only}
- Garcia 2002. Structured interruptions in antiretroviral treatment: a new therapeutic strategy?. Enferm Infecc Microbiol Clin 2002;20(8):373‐5. [PubMed] [Google Scholar]
Grinberg 2000 {published data only}
- Grinberg L. HAART breaks: structured treatment interruptions. Focus 2000;15(10):5‐6. [PubMed] [Google Scholar]
Gulick 2002 {published data only}
- Gulick RM. Structured treatment interruption in patients infected with HIV: a new approach to therapy. Drugs 2002;62(2):245‐53. [DOI] [PubMed] [Google Scholar]
Idemyor 2003 {published data only}
- Idemyor V. The concept of structured treatment interruptions in the management of patients with human immunodeficiency virus (HIV) disease: where are we currently?. HIV Clin Trials 2003;4(2):79‐83. [DOI] [PubMed] [Google Scholar]
Lori 2000 {published data only}
- Lori F, Maserati R, Foli A, Seminari E, Timpone J, Lisziewicz J. Structured treatment interruptions to control HIV‐1 infection. Lancet 2000;355(9200):287‐8. [DOI] [PubMed] [Google Scholar]
Lori 2000b {published data only}
- Lori F, Lewis MG, Xu J, Varga G, Zinn DE, Jr. , Crabbs C, et al. Control of SIV rebound through structured treatment interruptions during early infection. Science 2000;290(5496):1591‐3. [DOI] [PubMed] [Google Scholar]
Maroto 2005 {published data only}
- Maroto AA, Cabrera MH, Perez‐Arellano JL. HIV‐related thrombocytopenic purpura not relapsing during CD4 cell‐guided treatment interruptions. Aids 2005;19(10):1111‐1112. [DOI] [PubMed] [Google Scholar]
Martinez‐Picado 2002 {published data only}
- Martinez‐Picado J, Morales‐Lopetegi K, Wrin T, Prado JG, Frost SD, Petropoulos CJ, et al. Selection of drug‐resistant HIV‐1 mutants in response to repeated structured treatment interruptions. Aids 2002;16(6):895‐9. [DOI] [PubMed] [Google Scholar]
Metzner 2003 {published data only}
- Metzner KJ, Bonhoeffer S, Fischer M, Karanicolas R, Allers K, Joos B, et al. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. J Infect Dis 2003;188(10):1433‐43. [DOI] [PubMed] [Google Scholar]
Molto 2004 {published data only}
- Molto J, Ruiz L, Romeu J, Martinez‐Picado J, Negredo E, Tural C, et al. Influence of prior structured treatment interruptions on the length of time without antiretroviral treatment in chronically HIV‐infected subjects. AIDS Res Hum Retroviruses 2004;20(12):1283‐8. [DOI] [PubMed] [Google Scholar]
Montes 2005 {published data only}
- Montes de Oca Arjona M, Perez Cano R, Orozco MJ, Martin Aspas A, Guerrero F, Fernandez Gutierrez Del Alamo C, et al. Absence of favourable changes in circulating levels of interleukin‐16 or beta‐chemokine concentration following structured intermittent interruption treatment of chronic human immunodeficiency virus infection. Clin Microbiol Infect 2005;11(1):57‐62. [DOI] [PubMed] [Google Scholar]
Mussini 2005 {published data only}
- Mussini C, Bedini A, Borghi V, Guaraldi G, Esposito R, Barchi E, et al. CD4 cell‐monitored treatment interruption in patients with a CD4 cell count > 500 x 106 cells/l. Aids 2005;19(3):287‐94. [PubMed] [Google Scholar]
Neumann 1999 {published data only}
- Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, Agut H, et al. HIV‐1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. Aids 1999;13(6):677‐83. [DOI] [PubMed] [Google Scholar]
Nuesch 2005 {published data only}
- Nuesch R, Ananworanich J, Sirivichayakul S, Ubolyam S, Siangphoe U, Hill A, et al. Development of HIV with drug resistance after CD4 cell count‐guided structured treatment interruptions in patients treated with highly active antiretroviral therapy after dual‐nucleoside analogue treatment. Clin Infect Dis 2005;40(5):728‐34. [DOI] [PubMed] [Google Scholar]
Ortiz 2001 {published data only}
- Ortiz GM, Wellons M, Brancato J, Vo HT, Zinn RL, Clarkson DE, et al. Structured antiretroviral treatment interruptions in chronically HIV‐1‐infected subjects. Proc Natl Acad Sci U S A 2001;98(23):13288‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxenius 2002 {published data only}
- Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, Perrin L, et al. Stimulation of HIV‐specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A 2002;99(21):13747‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papasavvas 2004 {published data only}
- Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV‐1 infection. PLoS Med 2004;1(3):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parienti 2002 {published data only}
- Parienti JJ. Cytokine therapy or structured treatment interruptions in HIV infection: which is best?. Expert Opin Pharmacother 2002;3(6):719‐26. [DOI] [PubMed] [Google Scholar]
Plana 2004 {published data only}
- Plana M, Garcia F, Oxenius A, Ortiz GM, Lopez A, Cruceta A, et al. Relevance of HIV‐1‐Specific CD4+ Helper T‐Cell Responses During Structured Treatment Interruptions in Patients With CD4+ T‐Cell Nadir Above 400/mm3. J Acquir Immune Defic Syndr 2004;36(3):791‐799. [DOI] [PubMed] [Google Scholar]
Ruiz 2001 {published data only}
- Ruiz L, Carcelain G, Martinez‐Picado J, Frost S, Marfil S, Paredes R, et al. HIV dynamics and T‐cell immunity after three structured treatment interruptions in chronic HIV‐1 infection. Aids 2001;15(9):F19‐27. [DOI] [PubMed] [Google Scholar]
Schweighardt 2002 {published data only}
- Schweighardt B, Ortiz GM, Grant RM, Wellons M, Miralles GD, Kostrikis LG, et al. Emergence of drug‐resistant HIV‐1 variants in patients undergoing structured treatment interruptions. Aids 2002;16(17):2342‐4. [DOI] [PubMed] [Google Scholar]
Sommet 2003 {published data only}
- Sommet A, Delpierre C, Cuzin L, Jaafar A, Marchou B, Massip P. [Anti‐retroviral treatment interruptions in HIV‐infected adults: causes, clinical, immunological and virological consequences]. Rev Med Interne 2003;24(6):350‐7. [DOI] [PubMed] [Google Scholar]
Taffe 2002 {published data only}
- Taffe P, Rickenbach M, Hirschel B, Opravil M, Furrer H, Janin P, et al. Impact of occasional short interruptions of HAART on the progression of HIV infection: results from a cohort study. Aids 2002;16(5):747‐55. [DOI] [PubMed] [Google Scholar]
Tuldra 2001 {published data only}
- Tuldra A, Fumaz CR, Ferrer MJ, Paredes R, Romeu J, Ruiz L, et al. Psychological impact of structured treatment interruptions in patients with prolonged undetectable HIV‐1 viral loads. Aids 2001;15(14):1904‐6. [DOI] [PubMed] [Google Scholar]
Yerly 2003 {published data only}
- Yerly S, Fagard C, Gunthard HF, Hirschel B, Perrin L. Drug resistance mutations during structured treatment interruptions. Antivir Ther 2003;8(5):411‐5. [PubMed] [Google Scholar]
Yerly 2004 {published data only}
- Yerly S, Gunthard HF, Fagard C, Joos B, Perneger TV, Hirschel B, et al. Proviral HIV‐DNA predicts viral rebound and viral setpoint after structured treatment interruptions. Aids 2004;18(14):1951‐3. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Holodniy 2004 {published data only}
- M. Holodniy, B. Angus, S. Brown, A. Babiker, W. Cameron, B. Gazzard, T. Kyriakides, J. Singer, M. Youle, for the OPTIMA Study Team. Baseline characteristics, antiretroviral resistance profile and clinical events in the OPTIMA trial: A preliminary report.. IAC 2004 Bangkok. July 2004.
Kyriakides 2002 {published data only}
- Kyriakides TC, Babiker A, Singer J, Cameron W, Brown S, Holodniy M, Youle M, Gazzard B, Deyton L, Darbyshire J, Schechter M, and the OPTIMA Team. Design, Rationale and Methods of the OPTIMA Trial. Society for Clinical Trials Annual Meeting. May 12‐15, 2002.
Additional references
Aiuti 2003
- Aiuti F, Giovannetti A. Structured interruptions of therapy: looking for the best protocol. Aids 2003;17(15):2257‐8. [DOI] [PubMed] [Google Scholar]
Benson 2001
- Benson CA. Structured treatment interruption in HIV infection. AIDS Read 2001;11(2):99‐102. [PubMed] [Google Scholar]
Bonhoeffer 2000
- Bonhoeffer S, Rembiszewski M, Ortiz GM, Nixon DF. Risks and benefits of structured antiretroviral drug therapy interruptions in HIV‐1 infection. Aids 2000;14(15):2313‐22. [DOI] [PubMed] [Google Scholar]
Clarke 2001
- Clarke M, Horton R. Bringing it all together: Lancet‐Cochrane collaborate on systematic reviews. Lancet 2001;357(9270):1728. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks SG, Hirschel B. Supervised interruptions of antiretroviral therapy. Aids 2002;16 Suppl 4:S157‐69. [DOI] [PubMed] [Google Scholar]
Dorman 2000
- Dorman KS, Kaplan AH, Lange K, Sinsheimer JS. Mutation takes no vacation: can structured treatment interruptions increase the risk of drug‐resistant HIV‐1?. J Acquir Immune Defic Syndr 2000;25(5):398‐402. [DOI] [PubMed] [Google Scholar]
Dybul 2002
- Dybul M. Structured Treatment Interruption: Approaches and Risks. Curr Infect Dis Rep 2002;4(2):175‐180. [DOI] [PubMed] [Google Scholar]
Flepp 2001
- Flepp M, Schiffer V, Weber R, Hirschel B. Modern anti‐HIV therapy. Swiss Med Wkly 2001;131(15‐16):207‐13. [DOI] [PubMed] [Google Scholar]
Foli 2004
- Foli A, Maserati R, Barasolo G, Castelli F, Tomasoni L, Migliorino M, et al. Strategies to decrease viral load rebound, and prevent loss of CD4 and onset of resistance during structured treatment interruptions. Antivir Ther 2004;9(1):123‐32. [PubMed] [Google Scholar]
Hirschel 2001
- Hirschel B. Planned interruptions of anti‐HIV treatment. Lancet Infect Dis 2001;1(1):53‐9. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jager 2002
- Jager H. [Saving on drugs, reducing side‐effects. Treatment interruptions benefit acute and chronic HIV infected patients]. MMW Fortschr Med 2002;144 Suppl 1:8‐11. [PubMed] [Google Scholar]
Lisziewicz 1999
- Lisziewicz. Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med 1999;340(21):1683‐4. [DOI] [PubMed] [Google Scholar]
Lisziewicz 2002
- Lisziewicz J, Lori F. Structured treatment interruptions in HIV/AIDS therapy. Microbes Infect 2002;4(2):207‐14. [DOI] [PubMed] [Google Scholar]
Lori 2001
- Lori F, Lisziewicz J. Structured treatment interruptions for the management of HIV infection. Jama 2001;286(23):2981‐7. [DOI] [PubMed] [Google Scholar]
Lori 2002
- Lori F, Foli A, Lisziewicz J. Structured treatment interruptions as a potential alternative therapeutic regimen for HIV‐infected patients: a review of recent clinical data and future prospects. J Antimicrob Chemother 2002;50(2):155‐60. [DOI] [PubMed] [Google Scholar]
Miller 2001
- Miller V. Structured treatment interruptions in antiretroviral management of HIV‐1. Curr Opin Infect Dis 2001;14(1):29‐37. [DOI] [PubMed] [Google Scholar]
Miller 2003
- Miller V. Treatment interruptions in the salvage setting: what have we learned?. Res Initiat Treat Action 2003;9(2):17‐9. [PubMed] [Google Scholar]
Montaner 2001
- Montaner 2001. Structured treatment interruptions to control HIV‐1 and limit drug exposure. Trends Immunol 2001;22(2):92‐6. [DOI] [PubMed] [Google Scholar]
Montaner 2005
- Montaner J, Harris M, Hogg R. Structured treatment interruptions: a risky business. Clin Infect Dis 2005;40(4):601‐3. [DOI] [PubMed] [Google Scholar]
Moss 2001
- Moss RB, Jensen FC, Carlo DJ, Wallace MR. The strategy of immunologic control of HIV‐1 with structured treatment interruptions (STIs). Curr Drug Targets Infect Disord 2001;1(1):11‐7. [DOI] [PubMed] [Google Scholar]
Oxenius 2003
- Oxenius A, Hirschel B. Structured treatment interruptions in HIV infection: benefit or disappointment?. Expert Rev Anti Infect Ther 2003;1(1):129‐39. [DOI] [PubMed] [Google Scholar]
Pai 2005
- Pai NP, Tulsky JP, Lawrence J, Colford JM, Jr, Reingold AL. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database Syst Rev. Cochrane Database Syst Rev 2005, Issue (4): CD005482. [DOI] [PubMed] [Google Scholar]
Vella 2000
- Vella S. Structured treatment interruptions and treatment intensification. AIDS Clin Care 2000;12(7):55‐7, 62. [PubMed] [Google Scholar]
Walmsley 2002
- Walmsley S, Loutfy M. Can structured treatment interruptions (STIs) be used as a strategy to decrease total drug requirements and toxicity in HIV infection?. J Int Assoc Physicians AIDS Care (Chic Ill) 2002;1(3):95‐103. [DOI] [PubMed] [Google Scholar]


