Abstract
Background
Burkitt lymphoma (BL) is an important cancer found mostly in children but uncertainty remains as to the most effective form of management. In endemic areas, late‐stage presentation as a result of delayed access to treatment compounds the situation.
Objectives
To assess the evidence for chemotherapy, surgery, radiotherapy and immunotherapy in the treatment of children with endemic BL.
Search methods
We updated and re‐ran the searches in the following electronic databases from the time of the first publication; the Cochrane Controlled Trials Register (CENTRAL) (Issue 1, 2011); MEDLINE (January 2011); EMBASE (January 2011); and the clinical trials registry (up to January 2011) to identify relevant trials. In addition, we also updated the search of the US clinical trials register for on‐going and completed trials up to January 2011. We also updated the search terms and used the Cochrane filter for identifying randomised trials in MEDLINE.
Selection criteria
We included randomised controlled trials (RCTs) of any duration. We included studies conducted in children with a confirmed diagnosis of BL. We did not restrict studies by geographical location or by language of publication. We considered any therapeutic intervention. The primary outcome was overall survival.
Data collection and analysis
Two review authors assessed studies for relevance. We assessed studies that met the entry criteria for study quality. We independently extracted data and entered the data into Review Manager (RevMan). In this update, two review authors independently assessed citations from the updated search and reviewed abstracts for relevance.
Main results
We included one new study in this update. In total, 13 trials involving 1824 participants met the inclusion criteria for this review however, data in usable format were only available in 10 trials (732 participants). Inadequate reporting of study methodology was a common feature of the trials preventing thorough assessment of study quality. We were unable to pool data for any of the outcomes due to the differences between the interventions assessed in the studies. Eight studies aimed to induce remission; overall survival did not differ significantly between treatment groups. Five studies aimed to maintain remission. In two out of three studies reporting survival, this was substantial but the difference was not statistically significant between treatment groups. Less aggressive treatment schedules appear to produce similar effects with less adverse event profiles.
Authors' conclusions
This review notes a preference in more recent studies for less aggressive care options for treatment of BL. However, the evidence for the relative effectiveness of interventions to treat BL is not strong as studies were small, underpowered and prone to both systematic and random error. We included one additional trial without change of conclusions.
Plain language summary
Therapeutic interventions for Burkitt lymphoma in children
Burkitt lymphoma is an important cancer, particularly in children. It is a fast growing tumour but also very sensitive to chemotherapy. It presents a challenge in endemic areas due to late presentation and an often incomplete complement of drugs available for treatment. Different regimens are in use for treatment with varied success rates. This review aims to evaluate these treatments to assess their effectiveness especially for later stages. The review identified 13 trials involving 1824 participants. However, data presentable for the review were only available in 10 trials with 732 participants. The data were difficult to collate because of the quality of the study methods and the reporting of the results; outcome measures differed between trials and they were mainly small‐sized trials. No significant differences in overall survival were seen between studies aimed at inducing remission. Adverse events reported were mostly due to infections and reductions in blood cell counts. The more recent studies were focused on using less intensive treatment regimens as they could provide similar responses with lower risk of adverse effects.
Background
Description of the condition
Endemic Burkitt lymphoma (BL) is the most common childhood cancer in Africa (Goldstein 1990). In 1958 Dennis Burkitt first described this cancer in Ugandan children in East Africa (Burkitt 1958). Epidemiological observations show a spread of the tumour in areas approximately 15 degrees north and south of the equator. In this area, this cancer presents predominantly as jaw swellings. The World Health Organization (WHO) classification criteria groups BL in the subgroup of mature B‐cell neoplasms (Harris 2000). This categorisation of BL includes the classic form of BL and a variant, Burkitt‐like lymphoma (BLL). In addition, three subcategories; endemic, non‐endemic and immunodeficiency‐associated have been defined to reflect the main clinical and genetic subtypes of the disease (Harris 2000). Current WHO classification describes it as a small non‐cleaved cell lymphoma. These epidemiological and clinical characteristics distinguish endemic BL from other variants of small non‐cleaved cell lymphoma (SNCCL) (Magrath 1987).
HIV‐associated BL, on the other hand represents an AIDS‐defining criterion as it was observed to occur more frequently in those with HIV infection than the normal population (Knowles 2003). Patients were younger, with higher mean CD4 counts (usually > 200 cells/μl) compared with other HIV+ patients with diffuse large B‐cell type non‐Hodgkin’s lymphoma (NHL) (Knowles 2003; Martinez‐Maza 2002). Sites of predilection include lymph nodes, bone marrow and extranodal sites, most often in the abdomen. HIV is not believed to be directly involved in lymphomagenesis (the growth and development of lymphoma) but rather via cytokine deregulation, chronic antigenic stimulation and decreased immune surveillance (Martinez‐Maza 2002).
As its description suggests, sporadic BL occurs with no specific geographic or climatic association. It accounts for 1% to 2% of lymphomas in adults (Blum 2004).
Tumour incidence is estimated to be around 1:10,000 children (Goldstein 1990), accounting for most cases of NHL in some series. It is more common in males, with a sex ratio of about 2:1. Peak occurrence is seven years (range 5 to 16 years) (Oguonu 2002; Ziegler 1981).
The hypothesis of endemic BL comprises three components: (1) an early infection by the Epstein‐Barr virus resulting in transformation and immortalisation of B‐lymphocytes (Sugimoto 2004); (2) in areas of high malaria prevalence, this process is enhanced with suppression of T‐cell function (Goldstein 1990). However, these may only result in a malignant change in the presence of translocation between chromosomes t(8;14), t(2;8), and t(8;22). In this new site, c‐Myc oncogene activity is lost, resulting in uncontrolled proliferation of B‐cells (Bernheim 1981).
Diagnosis is often based on histology and cytological examination of biopsy specimens, although the gold standard for the diagnosis is considered to be immunophenotyping which shows the presence of the t(8;14) (q24;q32) and its variants or c‐Myc rearrangement (Harris 2000). The clinical staging of the disease proposed by Ziegler and Magrath is widely accepted (Ziegler 1974).
Description of the intervention
Endemic BL is very sensitive to chemotherapy (Smeland 2004; Ziegler 1972), although other variants are less so. Surgery is rarely required to reduce symptomatic tumour bulk (Magrath 1974a; Magrath 1989). The prognosis following effective chemotherapy is estimated to exceed 80% event‐free survival for limited‐stages disease (stages A, B, AR, A = solitary tumour limited to the face, B = multiple tumour on the face, AR = intra‐abdominal tumour which is 90% resectable). Radiotherapy (Norlin 1971) and immunotherapy (Magrath 1974) have been tried as interventions and have shown variable degrees of success. The average cost of treatment is about US$164 (Meremikwu 2005).
Why it is important to do this review
Overall, mortality from endemic BL remains high despite the fact that the disease is potentially curable. Limited access to health care, late presentation and higher relapse rates with late‐stage disease contribute to these high mortality rates (Olweny 1980). Improving access to effective chemotherapy in endemic areas would contribute considerably to the reduction of the mortality rate. Several regimens have been introduced (Cairo 2003b). However, there is little consensus on the best regimen for the tumour at various stages. This systematic review aims to assess the various interventions in order to provide the best options for these patients. This update has involved an updated search for trials, revisions in the methods and formatting of the structure.
Objectives
To assess the relative effects of chemotherapy, surgery, radiotherapy and immunotherapy in the treatment of endemic BL.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐randomised controlled trials (QRCTs) (e.g. alternate allocation, allocation by date of birth or days of the week). We intended to analyse QRCTs separately, not combined with properly randomised trials, and to report these in the results section stating problems with the studies in the discussion. However, we did not find any such trials.
Types of participants
In the protocol for this review, we stated that we would include trials of children aged up to 20 years with cancer confirmed by clinical and histopathologic features to be BL, irrespective of HIV/AIDS status. We also stated that we would restrict studies to those conducted in areas of endemic malaria. However, we revised the latter criterion because we agreed that the primary concern of the review was assessment of interventions in people with a confirmed diagnosis of BL. We did record geographical location of the trials.
Types of interventions
Cyclophosphamide monotherapy versus combination therapy.
Any other single drug chemotherapy versus combination therapy.
One type of combination therapy versus another combination therapy.
Chemotherapy versus radiotherapy.
Chemotherapy plus radiotherapy versus radiotherapy.
Chemotherapy versus chemotherapy plus immunotherapy.
Chemotherapy versus chemotherapy plus surgery.
Types of outcome measures
Primary outcomes
Overall survival.
Secondary outcomes
Event‐free survival (central nervous system residuals).
Overall remission rate (complete and partial).
Relapse rate (> six months).
Toxicity and adverse events.
Quality of life.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Haematological Malignancies Specialised Register (for search strategy see Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) (see Appendix 2).
MEDLINE (see Appendix 3).
EMBASE (seeAppendix 4).
We updated and re‐ran the searches for this update as follows (most recent search in brackets): CENTRAL (Issue 1, 2011); MEDLINE (January 2011); EMBASE (Janurary 2011). We used the new Cochrane search filter: the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximizing version (2008 revision) (Lefebre 2008). We updated the search for the clinical trials registry (www.clinicaltrials.gov) up to January 2011. In addition, we updated the search of the US clinical trials register for on‐going and completed trials until January 2011.
Data collection and analysis
Selection of studies
In the first publication of this review, two review authors (JO and SR) independently screened titles and abstracts for possible relevance. Disagreements were resolved by discussion. Based upon the agreed selections made, the review authors obtained full text versions for assessment. The review authors further assessed studies that met the inclusion criteria by this screening for eligibility criteria such as availability of the primary outcome measure. In the event of incomplete information, we tried to correspond with the study contact author for clarification.
In this update, JO and NS independently screened identified titles and abstracts and we resolved disagreements by discussion. We also reviewed the full texts of potentially eligible articles. We included one additional study.
Data extraction and management
Two review authors independently extracted data using a pre‐tested extraction form and we resolved differences by discussion. We extracted the following data.
General characteristics: author, title, source, year of publication, contact details, funding.
Trial details: setting, accrual period, method of randomisation, concealment of allocation, blinding of outcomes assessments, inclusion criteria, exclusion criteria, sample size, baseline characteristics, co‐treatments, withdrawals/drop outs, use of intention‐to‐treat (ITT) analysis, outcomes available.
Interventions: chemotherapy regimens, doses, timing, radiotherapy regimens, type of immunotherapy, surgery.
Where possible, we grouped, and pooled results for each outcome of relevance to the review, by comparison type.
Cyclophospamide monotherapy versus combination therapy.
A particular single drug chemotherapy versus any combination therapy.
Combination therapy A versus combination therapy B.
Any chemotherapy versus radiotherapy.
Any chemotherapy versus same chemotherapy plus immunotherapy.
Any chemotherapy versus same chemotherapy plus surgery.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias as described in the Cochrane Handbook (Higgins 2008). We judged risk of bias within each included study in relation to the following domains; sequence generation, allocation concealment, blinding and incomplete outcome data using ratings of 'Yes ' (low risk of bias); 'No' (high risk of bias) and 'Unclear'' (unknown risk of bias). We resolved disagreements by discussion. We used a question sheet covering the following areas.
Allocation sequence generation: Was treatment randomised?
Allocation concealment: Was the allocation to trial arm adequately concealed?
Blinding: Was the knowledge of intervention adequately prevented for participants, personnel and outcome assessors?
Incopmplete outcome data: Reporting of withdrawals, dropouts and loss to follow‐up (attrition bias).
Yes (ITT analysis available and drop‐out rate less than 10% in all treatment groups).
Unknown.
No (drop‐out rate more than 10% or more than 5% difference in drop‐out rates between treatment groups, or ITT analysis not available).
Note: The figures of 10% and 5% were arbitrarily chosen.
Additional quality criteria recorded.
Is there any reason to suppose that follow‐up assessments were not the same for the compared treatment groups?
Were outcome assessments made blinded to assigned treatment?
Were treatments other than the randomised ones similar in both treatment groups?
Are results available from a full publication, or only an abstract?
Measures of treatment effect
We calculated odds ratios (OR) in the analysis of dichotomous data (e.g. adverse events, and remission rates), and mean difference (MD) or generic inverse variance for continuous outcomes (e.g. some toxicities). Where possible, we pooled time‐to‐event data by using hazard ratios as described by Parmer (Parmer 1998).
Dealing with missing data
We screened the studies to determine if all study participants were accounted for in the results reported (ITT analysis). Where this was not so, we assessed if the authors provided reasons for this. We also contacted authors of primary studies, where valid addresses were available, for additional information on reported findings.
Data synthesis
We intended to use fixed‐effect methods. However, where a meta‐analysis was not possible, we provided a description of the data and their results.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis of the impact of interventions in HIV/AIDS‐related BL but there were no data for this. We also were unable to assess heterogeneity between the interventions as treatment protocols were dissimilar.
Sensitivity analysis
We undertook sensitivity analyses by each of the main quality criteria, and by an overall quality assessment, wherever the data were adequate.
Results
Description of studies
Results of the search
We identified 4958 references from the initial electronic literature search, of which we retrieved 41 references for full text scrutiny. We also identified one reference from additional searching. There were three duplicates. From a total of 39 full text papers, we identified 32 unique studies. Of these, 20 did not meet the review‐entry criteria (details of these studies are given in the table Characteristics of excluded studies). Twelve studies conducted between 1971 and 2003 met the entry criteria of the initial review. Full details of the characteristics of these studies are provided in the table Characteristics of included studies.
The updated search produced 6,600 references from which we reviewed 22 potentially eligible articles. We included one new study in this update (Patte 2007). A detailed summary of the chemotherapy regimens assessed are provided in Table 1.
1. Chemotherapy regimens.
| Study ID | Common treatment | Intervention 1 | Intervention 2 |
| Anderson 1983 | C: 1.2 g/m2 IV on day 1; O: 2.0 mg/m2 (max 2 mg) IV on days 3,10,17 and 24; MTX: 6.25 mg/m2 IT on days 5, 31, and 34; P: 15 mg/m2 (max 60 mg) orally qds on days 3 to 30 decreasing to zero on days 31 to 33. Radiation therapy. Tumour excision attempted in patients with localised disease. Laparotomy and biopsy in patients with non‐localised disease. | COMP
Induction: MTX 300 mg/m2 IV on day 12. Maintenance: C: 1 g/m2 IV on day 1; O: 1.5 mg/m2 IV on days 1 and 4; MTX: 6.25 mg/m2 IT on day 1 (excluded from 1st maintenance cycle), then 300 mg/m2 IV on day 15. Repeat maintenance cycle every 28 days. |
LSA2 L2 (modified).
Induction: DAU; 60 mg/m2 IV on days 12 and 13. Consolidation: CYT; 100 mg/m2 IV 5 days on, 2 days off x 2 weeks; THIO; 50 mg/m2 orally 8 to 12 hrs post CYT injection; ASP; 6000 IU/m2 IM daily x 14 days post CYT and THIO; MTX; 6.25 mg/m2 IT x2 doses 3 days apart, 2 to 3 days after last dose of ASP; CAR; 60 mg/m2 IV single dose given 2 to 3 days after completion of MTX. Maintenance: THIO: 300 mg/m2 orally on days 1 to 4, 600 mg/m2 IV on day 5; H: 2.4 g/m2 orally on days 1 to 4; DAU: 45 mg/m2 orally on day 5,;CYT: 150 mg/m2 IV days 1 to 5; O: 2.0 mg/m2 (max 2 mg) IV on day 5; MTX: 6.25 mg/m2 IT x 2 doses 3 days apart. Repeat maintenance cycles 1 to 5. |
| Brecher 1997 | None. | A
Prespecified duration:
Induction: C: on day 1 (dose not specified); MTX: on days 24 and 31 (dose not specified); O: weekly (x 5 weeks dose not specified); P: daily x 4 weeks (dose not specified). Consolidation (22 weeks): C: days 52 and 102; MTX: on days 74,81,124, and 131; O: I hour prior to each MTX. Maintenance: (11 weeks): O and MTX on days 174 and 216. CNS prophylaxis: Ara‐C, MTX and H. |
B
Duration determined by clinical response. Induction: fractionated C,O and DOX. Infusion phase: sequential continuous infusion of MTX and Ara‐C (pending mucosal and bone marrow recovery). Repeat induction and infusion x 4 with dose of Ara‐C being doubled with each course. CNS prophylaxis: MTX and Ara‐C. |
| Cairo 2003 | Prephase: C: 0.3 g/m2 IV; O: 1 mg/m2 IV on day1; P: 60 mg/m2 IV or orally in 2 fractions on days 1 to 7; MTX+HYD+Ara‐C: 30 mg IT on days 1,3 and 5. Induction: COPADM 1 (started 1 week after day 1 of prephase). O: 2 mg/m2 (max 2 mg) IV;high‐dose MTX: 8 g/m2 IV x 4 hours on day1; CFR: 15 mg/m2 every 6 hours orally on days 2 to 4; MTX+HYD+Ara‐C: 30 mg IT on day 2,4 and 6; DOX: 60 mg/m2 IV on day 2; C: 0.5 g/m2 IV (in 2 fractions) on days 2 to 4; P: 60 mg/m2 IV or orally on days 1 to 6. COPADM 2 similar to COPADM1 except for: 2nd O dose: 2 mg/m2 (max 2 mg) IV on day 6; C: 1 g/m2 IV (in 2 fractions) on days 2 to 4. |
Reduced intensity Similar to standard dose except for consolidation drugs are given at 2/3 the standard doses and deletion of M2 to 4 maintenance. | Standard dose
Consolidation: (x 2 courses).
Ara‐C: 50 mg/m2 CI x 12 hours on days 1 to 5 (8 pm to 8 am); high‐dose Ara‐C 3 g/m2 IV x 3 hours on days 2 to 5 (8 am to 11 am); VP‐16: 200 mg/m2 IV on days 2 to 5 (2 pm to 4 pm). Maintenance (monthly alternating courses). M1: O: 2 mg/m2 (max 2 mg) IV; high‐dose MTX: 8 g/m2 IV x 4 hours on day 1; CFR: 15 mg/m2 every 6 hours orally on days 2 to 4; P: 60 mg/m2 orally on days 1 to 5; MTX+HYD+Ara‐C: 30 mg IT on day 2; C: 0.5 g/m2 IV on days 1 and 2; DOX: 60 mg/m2 IV on day 2. M2/M4: VP‐16: 150 mg/m2 IV on days 1 to 3; Ara‐C: 100 mg/m2 SC (in 2 fractions) on days 1 to 5. M3: similar to M1 but without high‐dose MTX and IT. |
| Magrath 1973 | IV C 40 mg/kg x 2 doses 2 weeks apart. | Lomustine (70 mg/m2) administered orally. | No treatment. |
| Magrath 1976 | IV C 40 mg/kg x 2 doses (a third dose was given when complete remission was not achieved with the standard 2 doses). | 0.5 ml of freshly constituted BCG suspension administered by scarification. | No treatment. |
| Olweny 1976 | None. | C: 40 mg/kg IV on day 1,repeated after 2 weeks or as soon as toxicity is abated. | C: 30 mg/kg IV on day 1; O: 2 mg/m2 IV on day 1; MTX: 15 mg/m2 orally on days 1 to 3. This is repeated 12 to 14 days later. |
| Patte 1991 | Reduction phase: C: 0.3 g/m2 IV; O: 1 mg/m2 IV on day 1; P: 2 mg/kg orally on days 1 to 7; MTX+HYD: 15 mg/m2 IT on day 1. Induction: COPADM 1 (started 1 week after day 1 of prephase). O: 2 mg/m2 IV;high‐dose MTX: 3 g/m2 IV x 3 hours on day 1; CFR: 15 mg/m2 every 6 hours orally on days 2 to 4; MTX+HYD: 15 mg IT on days 2 and 6; DOX: 60 mg/m2 IV on day 2; C: 0.5 g/m2 IV (in 2 fractions) on days 2 to; P: 2 mg/kg IV or orally on days 1 to 6. COPADM 2 similar to COPADM 1 except for: addition of 2nd O dose: 2 mg/m2 IV on day 6; C: 1 g/m2 IV (in 2 fractions) on days 2 to 4. CYM: high‐dose MTX 3 g/m2 IV x 3 hours on day 1; CFR: 15 mg/m2 every 6 hrs orally on days 2 to 4; Ara‐C: 100 mg/m2 CI on days 2 to 6; Ara‐C+HYD: 30 mg/m2 IT on day 6. Maintenance (monthly alternating courses) M1: O: 2 mg/m2 IV; high‐dose MTX 3 g/m2 IV x 3hours on day 1; CFR: 15 mg every 6 hours orally on days 2 to 4; P: 2 mg/kg orally on days 1 to 5; MTX+HYD: 15 mg IT on day 2; C: 0.5 g/m2 IV on days 1 and 2; DOX: 60 mg/m2 IV on day 2. M2: Lomustine: 60 mg/m2 orally on day 28,; Ara‐C: 100 mg/m2 SC (in 2 fractions) on days 28 to 31; Ara‐C+HYD: 30 mg/m2 IT on day 28; THIO: 150 mg/m2 orally on days 28 to 31. |
Long arm: CYM 1, Mini‐BACT: Lomustine: 60 mg/m2 orally on day 1; Ara‐C: 100 mg/m2 CI on days 2 to 6; THIO: 150 mg/m2 orally on days 2 to 6; C: 0.5 g/m2 IV on days 2 to 4. M1, M2, M1, M2. | Short arm: CYM 1 and 2, M1. |
| Patte 2007 | Prephase COP + IT MTX. Maintenance: O, P, AD, high‐dose MTX given if 20% response at day 7 after COP. Leucovorin rescue treatment. NB. There is a second stage which involves subgroup of main interventions to receive either M1 (C1g/m2, O, P, AD, high‐dose MTX, or not. |
1A: COPADM1: (Cyclophosphamide 1.5mg/m2) on days 2 and 6; CYM: (CYT IV on days 2 to 6 and IT CYT on day 6): M1 C 1g/m2, O, P, AD, high‐dose MTX. | 1B: COPADM2 (double dose of C is given, O, P, AD. high‐dose MTX) on days 2 and 6, CYM (CYT IV on days 2 to 6 and IT CYT on day 6). |
| Sullivan 1991 | Induction: C: 1.2 g/m2 IV on day 1, repeat on day 1 of weeks 7 and 14; O: 2.0 mg/m2 (max 2.0mg) IV on days 2 or 3 weekly x 4 then 1.0 mg/m2 IV given 1 hour before MTX infusion; P: 60 mg/m2 (max 60 mg) orally daily from day 1 x 28 days; MTX: 2 6‐hour infusion starting from week 3, starting dose 50 mg/kg increasing to 100 mg/kg then to 200 mg/kg throughout the rest of the treatment, given as 2 doses every 7 weeks during induction and consolidation and every 6 weeks during maintenance; CFR: 15 mg IV 3 hourly x 9 doses then 15 mg 6 hourly x 8 doses after each MTX infusion. CNS prophylaxis: CYT: 45 mg/m2 on D1 and 2, MTX 15 mg/m2 on day 3 starting on day 2 of induction, subsequently given as triple therapy (CYT 60 mg/m2, MTX 15 mg/m2 (max 15 mg, HYD 30 mg/m2) at week 6 and 14, then 24 hours before each pair of MTX infusions. Maintenance: triple therapy, IV MTX, IV O. |
Maintenance regimen for 2 months. | Maintenance regimen for 6 months. |
| Ziegler 1971 | Unclear.unclear | MTX 25 mg/m2 IT alternating with Ara‐C 50 mg /m2 IT every 4 days. Two doses of each drug were given. | No intrathecal therapy. |
| Ziegler 1972a | None. | C: 40 mg/kg IV at 2‐weekly intervals for 6 doses. | TRIKE schedule C: 40 mg/kg followed in two weeks by O: 1.4 mg/m2 IV on day 1 and MTX 15 mg/m2 orally on days 1 to 4; Ara‐C: two weeks later; 250 mg/m2 CI daily x 3 days. |
AD: adriamycin (doxorubicin) Ara‐C: cytosine arabinoside ASP: asparaginase BCG: Bacille‐Calmette‐Guerin C: cyclophosphamide CAR: carmustine CFR: citrovorum factor rescue CI: continuous infusion CNS: central nervous system COMP: cyclophosphamide, oncovin, methotrexate and prednisolone COP: cyclophosphamide, oncovin and prednisolone CYM: CYT plus high‐dose MTX CYT: cytarabine DAU: daunorubicin DOX: doxorubicon H: hydroxyurea HYD: hydrocortisone IM: intramuscular IT: intrathecal IV: intravenous MTX: methotrexate O: oncovin (vincristine) P: prednisolone qds: four times daily THIO: thioguanine
Included studies
Participants
Thirteen trials involving 1824 participants met the inclusion criteria for the review (Anderson 1983; Brecher 1997; Cairo 2003a; Magrath 1973; Magrath 1976; Neequaye 1990; Olweny 1976; Olweny 1977; Patte 1991; Patte 2007; Sullivan 1991; Ziegler 1971; Ziegler 1972a). The The average age reported for participants with BL varied between six and 10 years. Five of the13 studies recruited a mixed population which included a subgroup of participants with BL (Anderson 1983; Brecher 1997; Cairo 2003a; Patte 2007; Sullivan 1991).The disposition of the trial populations differed according to the aims of the trial. Four studies sought to assess the effects of treatment in people who were in remission from BL following successful treatment with cyclophosphamide (Magrath 1973; Magrath 1976; Neequaye 1990; Olweny 1977). Ziegler 1971 required participants to be free from malignant pleocytosis (an increase in white blood cells in the cerebrospinal fluid) on entry to the study. Results were however, not available in an analysable format for three trials. Twenty percent of the participants in the Anderson 1983 trial had BL, but results were presented as either localised or non‐localised disease subgroups for all tumour types. Cairo 2003a presented results based on intervention groups and in Patte 2007, 62% were participants diagnosed with BL but results were also presented by intervention group. The review's outcomes are based on data from 10 trials involving 732 participants.
The diagnosis of BL was confirmed by pathology in all the studies with the exception of Sullivan 1991 where this was not reported. Different tumour staging methods featured in the trials. Murphy classification was used in four trials (Brecher 1997; Patte 1991; Patte 2007; Sullivan 1991); the staging method by Ziegler was used in four trials (Olweny 1976; Olweny 1977; Ziegler 1971; Ziegler 1972a); the Rappaport system was applied in Anderson 1983; Cairo 2003a used REAL; Magrath 1976 used the WHO staging method; and in two studies the method was not reported (Magrath 1973; Neequaye 1990).
Interventions
Eight studies compared two combination chemotherapy regimens differing in terms of the component drugs given, or the duration of therapy (Anderson 1983; Brecher 1997; Cairo 2003a; Magrath 1973; Patte 1991; Patte 2007; Sullivan 1991; Ziegler 1971). Two studies evaluated cyclophosphamide versus combination therapy (Olweny 1976; Ziegler 1972a). Two studies analysed the use of immunotherapy compared with no treatment (Magrath 1976; Neequaye 1990). All the studies involving chemotherapy used cyclophosphamide. Consolidation phases were also described in several trials. One trial assessed the effects of irradiation therapy following remission (Olweny 1977). Table 1 outlines the component drugs, doses and frequency of administration for the chemotherapy trials.
Outcomes
Outcome results were based on 10 trials including 732 participants. The primary outcome of the review (overall survival by treatment group) was reported in seven trials (Brecher 1997; Magrath 1976; Neequaye 1990; Olweny 1976; Olweny 1977; Patte 1991; Ziegler 1972a). Event‐free survival was reported in four studies (Brecher 1997; Magrath 1976; Patte 1991; Sullivan 1991. Relapse was reported in nine studies (the exception was Sullivan 1991); and toxicity was reported in three studies (Magrath 1976; Patte 1991; Sullivan 1991). Quality of life scores were not reported in any study.
Risk of bias in included studies
The studies were reported as being randomised, however, the methods for generating the allocation sequence were described in only seven of these trials (Figure 1). It was generally not clear if there were sufficient efforts at concealment of allocation to study groups. However, based on the text, the description in two studies could be judged as adequate (Patte 1991; Patte 2007). The method used in two studies was not sufficiently explained to indicate whether the procedure would have generated a satisfactorily randomised schedule ("prepared cards" were used) (Olweny 1976; Olweny 1977). While the comparison of the head‐to‐head active treatments in the chemotherapy trials could have been done with the interventions 'masked', the difference in type and frequency of the component treatments would have been problematic in devising double‐dummy schedules. Intention‐to‐treat analysis was reported in only two of the studies (Anderson 1983; Brecher 1997), and in the remainder it was either unclear or, not done.
1.
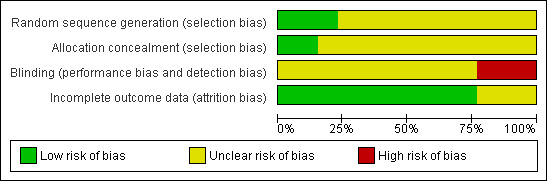
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Cyclophosphamide monotherapy versus combination therapy (Olweny 1976; Ziegler 1972a).
Any other single drug chemotherapy versus combination therapy (no studies).
One type of combination therapy versus another combination therapy (Anderson 1983; Brecher 1997; Cairo 2003a; Patte 1991; Patte 2007; Sullivan 1991).
Chemotherapy versus radiotherapy (no studies).
Chemotherapy plus radiotherapy versus radiotherapy (no studies).
Chemotherapy versus chemotherapy plus immunotherapy (no studies).
Chemotherapy versus chemotherapy plus surgery (no studies).
An analysis of the study interventions generated a number of new comparisons.
Chemotherapy versus no treatment (Magrath 1973; Ziegler 1971).
Immunology versus no treatment (Magrath 1976; Neequaye 1990).
Radiotherapy versus no treatment (Olweny 1977).
The differences between the interventions meant that we were unable to combine data statistically, according to our prespecified methods. We identified two principal aims of the assembled studies and we divided the results section between those which aimed to induce remission from BL, and studies which aimed to maintain remission following successful response to an intervention administered prior to randomisation.
Studies aiming to induce remission
Five studies reported data according to this study aim (Brecher 1997; Olweny 1976; Patte 1991; Sullivan 1991; Ziegler 1972a). The results could not be combined in a meta‐analysis due to significant heterogeneity in the drugs and protocols used.
Overall survival
This was reported in four studies (Brecher 1997; Olweny 1976; Patte 1991; Ziegler 1972a). The median evaluation period ranged from 19 to 41 months. Survival rates were not significantly different in the two arms in all studies. An overview of the deaths is presented in Table 2.
2. Percentage deaths (remission‐inducing studies).
| Study | Comparison | Rx 1 | Rx 2 /control |
| Brecher 1997 | Prespecified duration (1) versus duration determined by clinical response (2). | 29 | 21 |
| Olweny 1976 | C: 40 mg/kg IV on day 1,repeated after 2 weeks or as soon as toxicity is abated (1) versus C: 30 mg/kg IV on day 1; O: 2 mg/m2 IV on day 1; MTX: 15 mg/m2 orally on days 1 to 3. This is repeated 12 to 14 days later (2). | 58 | 33 |
| Patte 1991 | Long‐duration chemotherapy (1) versus short‐duration chemotherapy (2). | 10 | 12 |
| Ziegler 1972a | C (1) versus TRIKE (2). | 50 | 28 |
C: cyclophosphamide IV: intravenous MTX: methotrexate O: oncovin (vincristine)
Event‐free survival
This was reported in three trials (Brecher 1997; Patte 1991; Sullivan 1991). A significant difference in two years event‐free survival rates was reported in the Brecher 1997 trial (P = 0.027) while the two arms in the Patte 1991 study reported an average of 88%. The rates at > two years was reported to be 65% and 61% for both study arms in the Sullivan 1991 study. A median period between two years and 41 months was used in Sullivan 1991 and Patte 1991 respectively while a log rank method was used to assess event‐free survival in Brecher 1997 (Table 3).
3. Percentage event‐free survival (remission‐inducing studies).
| Study | Comparison | Rx 1 | Rx 2/control |
| Brecher 1997 | Prespecified duration (1) versus duration determined by clinical response (2). | 35 | 21 |
| Patte 1991 | Long‐duration chemotherapy (1) versus short‐duration chemotherapy (2). | 11 | 13 |
| Sullivan 1991 | Two‐month (1) versus six‐month (2) duration of maintenance chemotherapy. | 6 | 10 |
| Ziegler 1971 | IT chemotherapy versus no treatment. | 50 | 40 |
IT: intrathecal
Remission rate
This was reported in three studies (Brecher 1997; Olweny 1976; Patte 1991). All showed rates above 80% with no differences between groups (see Table 4).
4. Percentage remission (remission‐inducing studies).
| Study | Comparison | Rx 1 | Rx 2/control |
| Brecher 1997 | Prespecified duration (1) versus duration determined by clinical response (2). | 81 | 89 |
| Patte 1991 | Long‐duration chemotherapy (1) versus short‐duration chemotherapy (2). | 87 | 89 |
| Olweny 1976 | Irradiation (1) versus no treatment (2). | 83 | 84 |
Relapse
Four studies reported this outcome (Brecher 1997; Olweny 1976; Patte 1991; Ziegler 1972a). No significant differences between the treatment groups were reported in these studies. The later studies using more complex combination therapy tended to report lower rates of relapse in both groups (see Table 5).
5. Percentage relapse (remission‐inducing studies).
| Study | Comparison | Rx 1 | Rx2/control |
| Brecher 1997 | Prespecified duration (1) versus duration determined by clinical response (2). | 15 | 15 |
| Olweny 1976 | C: 40 mg/kg IV on day 1, repeated after 2 weeks or as soon as toxicity is abated (1) versus C: 30 mg/kg IV on day 1; O: 2 mg/m2 IV on day 1; MTX: 15 mg/m2 orally on days 1 to 3. This is repeated 12 to 14 days later (2). | 53 | 62 |
| Patte 1991 | Long‐duration chemotherapy (1) versus short‐duration chemotherapy (2). | 11 | 10 |
| Ziegler 1972a | C (1) versus TRIKE (2). | 80 | 61 |
C: cyclophosphamide IV: intravenous MTX: methotrexate O: oncovin (vincristine)
Toxicity
Two studies reported this (Brecher 1997; Patte 1991). In Brecher 1997, toxicity was predominantly haematologic in both arms; this event, as well as the number of infections were significantly higher in treatment group two. Participants in treatment group one had more neurotoxic events. There were four recorded deaths; two in each group. These were due to infection and metabolic causes. Toxic events reported in Patte 1991 were mostly haematologic and due to infections. These occurred largely before randomisation. There were no significant differences in toxicity profile post‐randomisation (Table 6).
6. Percentage toxicity (remission‐inducing studies).
| Study | Comparisons | Rx1 | Rx2/ control | Type of toxicity |
| Brecher 1997 | Prespecified duration (1) versus duration determined by clinical response (2) | 74 | 96 | Grades 3 and 4 toxicity; haematologic, neurotoxic, infections. |
| Patte 1991 | Long‐duration chemotherapy (1) versus short‐duration chemotherapy (2) | 0 | 4 | Haematologic, multi‐organ failure, infections. |
| Patte 2007 | 1A versus 1B and 2A versus 2B | Infections and stomatitis (graded 1 to 4). |
Studies aiming to maintain remission
There were five studies in this category (Magrath 1973; Magrath 1976; Neequaye 1990; Olweny 1977; Ziegler 1971). They all had remission induced before randomisation to study interventions. Two studies evaluated chemotherapy (Magrath 1973; Ziegler 1971), two immunotherapy (Neequaye 1990; Magrath 1976), and one study (Olweny 1977) radiotherapy. All these were compared against no treatment. Again, we were unable to perform a meta‐analysis for all study outcomes evaluated.
Overall survival
This was reported in three studies (Magrath 1976; Neequaye 1990; Olweny 1977). There were marked differences between the intervention groups in two studies. The median follow‐up period varied from 19 to 21.5 weeks (Olweny 1977), 19 to 27.2 weeks (Neequaye 1990), to 33 weeks (Neequaye 1990). The findings from Neequaye 1990 suggested a numerically higher survival benefit in the control group (14% versus 31%), whereas Olweny 1977 reported a numerically higher survival rate in irradiation‐treated participants versus those not receiving treatment. An overview of survival rates are presented in Table 7.
7. Percentage deaths (remission‐maintaining studies).
| Study | Comparison | Rx 1 | Rx 2/control |
| Magrath 1976 | BCG (1) versus no treatment (2) | 29 | 26 |
| Neequaye 1990 | TF (1) versus no treatment (2) | 14 | 31 |
| Olweny 1977 | Irradiation (1) versus no treatment (2) | 27 | 9 |
BCG: Bacille‐Calmette‐Guerin TF: transfer factor
Event‐free survival
No studies reported event‐free survival.
Relapse
Five studies reported relapse (Magrath 1973; Magrath 1976; Olweny 1977; Magrath 1973; Ziegler 1971). Neequaye 1990 did not report on the site of relapse while the others reported central nervous system relapse (Table 8).
8. Percentage relapse (remission‐maintaining studies).
| Study | Comparison | Rx 1 | Rx 2/control | Type of relapse |
| Neequaye 1990 | TF (1) versus no treatment (2) | 28 | 54 | Not stated |
| Magrath 1976 | BCG (1) versus no treatment (2) | 52 | 58 | CNS |
| Magrath 1973 | Lomustine (1) versus no treatment (2) | 38 | 38 | CNS: |
| Olweny 1977 | Irradiation (1) versus no treatment (2) | 54 | 36 | CNS: |
| Ziegler 1971 | IT chemotherapy (MTX) (1) versus no treatment (2) | 50 | 40 | CNS: |
BCG: Bacille‐Calmette‐Guerin CNS: central nervous system IT: intrathecal MTX: methotrexate TF: transfer factor
Toxicity
Olweny 1977 reported deaths in three participants in the treatment group; cause of deaths was not confirmed as it occurred at home. There was one death in the control group of unknown cause.
Discussion
Overview of findings
With one additional study, 13 studies have now been included involving 1824 participants without change of conclusion. The results presented are still based on ten studies with 732 participants. Data from Anderson 1983 and Cairo 2003a could not be analysed as they were presented as proportions and in Patte 2007, results are presented as per comparison groups. We maintained the original format of the review to present results based on the overall intent of the studies; induction of remission or maintaining remission (patients were randomised after remission had been induced; details of induction were sometimes incomplete). In all studies, we were hampered in assessing the risk of bias by unclear description of the methods used (stated and implied, or both), the definition of study targets and reporting of results. Studies on interventions for inducing remission appear to be better reported and indicate some evidence of effect for our assessed outcomes at the two‐year, follow‐up time point. The interpretation of the evidence must however consider the context of the variations in study designs and endpoints. The outcomes for this review were not uniformly reported in the studies, thereby making combination of results and their subsequent analysis difficult.
Context of studies
As in the original version of this review, relatively little has changed with BL in terms of the age, sex, disease presentation and progression. The reclassification and definition of standard methods of diagnosis provided better ways of understanding the disease process and has improved the potential for pooling resources and participants in multicentre trials. These are likely to provide better estimates of the effects of the interventions. This also means that the findings of these results gain a more global applicability. There appears to be no new research activities in areas that were classically described as endemic for the disease. There, the main research interest also appears to shift towards comparisons of the effectiveness of lower dosage versions of regimens that have been shown to have effect (Patte 2007), with the aim to evaluate their performance in comparative survival and a lower adverse‐effect profile. This is encouraging as it addresses one of the observations of the original review that the use of regimens are more likely to be determined by the quality of supportive care available. The trials also involved other subsets of NHL. This could influence the results if the effect of disease characteristics on outcomes were not considered. Burkitt lymphoma has been reported in children with HIV/AIDS but this is not represented as either their serology status was not reported or those patients with HIV were excluded (Sullivan 1991).
Methodological limitations
The lack of data on HIV/AIDS‐related BL prevented subgroup analysis. Most studies were described as randomised but methods were often not described. Blinding was not undertaken for many of the interventions and the quality of reporting of these trials was also generally quite poor. The pattern of reporting shows an emphasis on overall survival, remission or relapse rates and event‐free survival; no study described the quality of life of the survivors.
Closing remarks
This update identified one new study on the treatment of BL (Patte 2007). However, as observed previously, a detailed analysis of the data was not possible due to the format in which the data were presented. There is some evidence on the effect of these treatment combinations but the size and direction may be difficult to determine due to differences in reporting formats. The improved characterisation of cancers is invaluable as it may be influential to the use of treatment regimens for various types and stages of disease as seen in the more recent studies and perhaps reflect on reporting of trials. Further updates of the review may consider the revising definitions of the disease and perhaps focus on broader categories currently in use in literature. Newer studies appear to focus on revisions of existing treatment protocols (Patte 2007); a trend towards balancing between efficacy and reduction in morbidity and adverse events.
Authors' conclusions
Implications for practice.
Existing treatment protocols show encouraging responses in terms of response and survival rates, perhaps due to better designs. The use of less‐intensive protocols appear to produce similar responses compared with standard regimens with possibly lower adverse‐event profiles. Issues around the context of the level of supportive care available, participant selection criteria and the quality of life of study participants are other determinants to the widespread use of treatment regimens. Efforts to increase access should also consider raising awareness on the disease and sources of treatment since early‐stage disease is associated with better outcomes with existing drugs. There is little data on treatment options for relapse.
Implications for research.
The progress in research in this field has focused on better characterisation of the disease process but comparatively less progress has been reported on improvements to treatment regimens and outcomes. The current trend appears to be the evaluation of the efficacy of current and perhaps new drug combinations in terms of induction and maintenance of remission. This can be deduced from the Patte 1991 and Patte 2007 studies. Other issues would relate to clear data reporting on outcomes to help with comparisons between studies. There has been some interest in the use of immunotherapeutic agents such monoclonal antibodies in patients with B‐cell malignancies (Kasamon 2004). Results suggest a possible role in BL but these are based on small observations (Akbayram 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2011 | New search has been performed | Review updated; new search, conclusions not changed |
| 10 March 2011 | New citation required but conclusions have not changed | Updated search, one additional trial included, substantial revision of the text |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 6 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful for the receipt of the Jim Milan Memorial Award which funded the travel and subsistence of Dr Okebe while at the UKCC, and which helped us to complete this review. We are extremely grateful to Dr Jane Dennis, Julie Millener and Joanne Abbott of the Cochrane Developmental, Psychosocial and Learning Problems Group for kind and continual assistance in locating and retrieving trial reports on our behalf. We would also like to thank staff of the Cochrane Haematological Malignancies Group (CHMG) for editorial support.
Toby Lasserson contributed to the first version of the review by contributing to the write‐up and constructing tables and figures.
Appendices
Appendix 1. Cochrane Haematological Malignancies Specialised Register search strategy
Burkit*
Burkitt Lymphoma [MESH]
1 or 2
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Burkitt Lymphoma explode all trees
#2 burkit*in All Text
#3 MeSH descriptor lymphoma, b‐cell explode all trees
#4 (african* in All Text near/2 lymphom*in All Text)
#5 (lymphom* in All Text and b‐cell* in All Text)
#6 (b‐lymphocyte* in All Text and burkit* in All Text)
#7 (lymphom* in All Text and small in All Text and noncleaved‐cell*in All Text)
#8 (lymphom* in All Text and small in All Text and non‐cleaved‐cell*in All Text)
#9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8)
Clinical Trials
Appendix 3. MEDLINE search strategy
MEDLINE (OVID) was searched using the following terms below together with the optimally sensitive search strategy developed by the Cochrane Collaboration for the identification of RCTs.
1 lymphoma, b‐cell/ 2 burkitt lymphoma/ 3 non‐hodgkin lymphoma/ 4 burkit$.tw,kf,ot. 5 (african$ adj2 lymphom$).tw,kf,ot. 6 (lymphoma$ and b‐cell$).tw,kf,ot. 7 (b‐lymphocytes$ and burkit$).tw,kf,ot. 8 (b‐lymphocyt$ adj3 leuk?em$).tw,kf,ot. 9 or/1‐8 10 randomized controlled trial.pt. 11 controlled clinical trial.pt. 12 randomized.ab. 13 placebo.ab. 14 drug therapy.fs. 15 randomly.ab. 16 trial.ab. 17 groups.ab. 18 or/10‐17 19 humans.sh. 20 18 and 19 21 9 and 20 22 limit 21 to ed=20060301‐20080810
Appendix 4. EMBASE search strategy
1 lymphoma, b‐cell/ 2 burkitt lymphoma/ 3 nonhodgkin lymphoma/ 4 burkit$.tw. 5 (african$ adj2 lymphom$).tw. 6 (lymphoma$ and b‐cell$).tw. 7 (b‐lymphocytes$ and burkit$).tw. 8 (b‐lymphocyt$ adj3 leuk?em$).tw. 9 or/1‐8 10 Clinical trial/ 11 Randomized controlled trial/ 12 RANDOMIZATION/ 13 SINGLE BLIND PROCEDURE/ 14 DOUBLE BLIND PROCEDURE/ 15 CROSSOVER PROCEDURE/ 16 PLACEBO/ 17 Randomi?ed controlled trial$.tw. 18 RCT.tw. 19 Random allocation.tw. 20 Randomly allocated.tw. 21 Allocated randomly.tw. 22 (allocated adj2 random).tw. 23 Single blind$.tw. 24 Double blind$.tw. 25 ((treble or triple) adj blind$).tw. 26 Placebo$.tw. 27 PROSPECTIVE STUDY/ 28 or/10‐27 29 Case study/ 30 Case report.tw. 31 Abstract report/ or letter/ 32 or/29‐31 33 28 not 32 34 animal/ 35 human/ 36 34 not 35 37 33 not 36 38 9 and 37 39 limit 38 to yr="2004 ‐ 2008"
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1983.
| Methods | Randomised, parallel group, multicentre trial (North America). Method of randomisation: described as an "adaptive randomisation plan to ensure a satisfactory balance of factors hat were potentially important in the prognosis.." ITT: yes. Withdrawals: stated. | |
| Participants | Baseline characteristics: 234 participants eligible, 47 (20%) classified as undifferentiated BL. Male:female: 184:50. Mean age not reported. 211 randomised (COMP ‐105, LSA2‐L2 protocol‐ 106) Tumour staging: Rappaport.
Diagnosis: histopathological confirmation of childhood NHL. Entry criteria: < 18 years of age; no previous treatment for NHL; biopsy‐confirmed NHL. Exclusion criteria: not stated. |
|
| Interventions | COMP versus LSA2‐L2 treatment protocol (modified). SeeTable 1 for details of treatment protocol. Treatment duration: 18 months. Follow‐up: two to four years. |
|
| Outcomes | Overall survival (12 to 24 months). Failure‐free survival at 24 months. Relapse rate. Adverse events and toxicity. | |
| Notes | Additional 23 participants followed during course of study but not randomly allocated to treatment group. Full text publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as an "adaptive randomisation plan to ensure a satisfactory balance of factors hat were potentially important in the prognosis.." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported on failure‐free survival, and adverse events/toxicity and accounted for those not included in the analysis. |
Brecher 1997.
| Methods | Randomised, parallel group, multicentre trial (North America). Analysis stated as by ITT. Withdrawals: stated. | |
| Participants | Baseline characteristics: 106 male, 17 female, all participants had stage III disease (Regimen A: 65; Regimen B: 58). Mean age: 8.6 years range 2.3 to 20.3 years.
Tumour staging: Murphy classification.
Diagnosis: histo‐cytologic diagnosis of SNCCL. Entry criteria: newly‐diagnosed stage III SNCCL, pathology confirmed. Exclusion criteria: not stated. |
|
| Interventions | Regimen A: (COMP given for induction, consolidation with the first three dugs; maintenance with O and MTX and nervous‐system prophylaxis, all given at prespecified intervals) versus Regimen B:(duration of treatment being determined by clinical response). Treatment duration: 9 months. Follow‐up: 3 to 8 years. |
|
| Outcomes | Overall survival (complete response). Event‐free survival. Remission rate. Relapse rate. | |
| Notes | Full text publication. Study type: remission induction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was reported that one participant in regimen A was "inevaluable for response". |
Cairo 2003a.
| Methods | Randomised, parallel group, multicentre trial (Europe). ITT: unclear. Withdrawals: unclear. | |
| Participants | Baseline characteristics: 241 participants screened (BL and BLL: 51.3%), 195 randomised (standard dose: 96; reduced intensity: 99).
Median age: 8 years (range: 1 to 19).
Tumour staging: REAL.
Diagnosis: not reported. Entry criteria: ≤ 21 years; advanced disease (bone marrow and/or CNS, B‐cell NHL (large‐cell lymphoma, BL and BLL)). Exclusion criteria: not described. |
|
| Interventions | A standard‐treatment course consisting of a consolidation phase and 4 courses of maintenance regimens compared with a reduced‐intensity regimen similar to standard dose except that the consolidation drugs are given at two‐thirds of the standard doses and no maintenance phase was given (see.Table 1). Tretament duration: not reported. Follow‐up: median 3.25 years. |
|
| Outcomes | Overall survival. Event‐free survival. | |
| Notes | Unpublished conference abstract. Study type: remission induction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described; perhaps due to constraints of the size of the abstract. |
Magrath 1973.
| Methods | Randomised, parallel group. ITT: unclear. Withdrawals: stated. | |
| Participants | Baseline characteristics: 35 participants recruited (interim analysis). No other details reported.
Mean age: not reported.
Tumour staging: not reported.
Diagnosis: not reported. Entry criteria: In remission from BL following treatment with C. |
|
| Interventions | Lomustine (70 mg/m2) administered orally once versus no treatment post treatment with C (two doses, 40 mg/kg IV, two weeks apart). Treatment duration: 2 weeks. Follow‐up: unclear. |
|
| Outcomes | CNS relapse. | |
| Notes | Unpublished conference abstract. Study type: remission maintenance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only time of relapse mentioned; proportions of patients with this outcome not stated. |
Magrath 1976.
| Methods | Randomised, parallel group, single‐centre trial (Uganda). ITT: unclear. Withdrawals: stated. | |
| Participants | Baseline characteristics: screening population: 80; 48 participants randomised (40 evaluated: BCG: 21; control: 19; male:female: BCG 15:6; control: 14:5).
Mean age: BCG: 10 years; control: 6 years.
Tumour staging: WHO classification.
Diagnosis: histopathology. Entry criteria: untreated BL; remission two weeks after treatment with C. |
|
| Interventions | BCG versus no treatment. Participants randomised if in remission two weeks after the last dose of C. Follow‐up: median 1.75 years. |
|
| Outcomes | Relapse. Overall survival. Event‐free survival. Toxicity. | |
| Notes | Participants recruited if in remission. Study type: remittance maintenance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be "randomised by stage". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unlikely: therapy involved scarification. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
Neequaye 1990.
| Methods | Randomised, parallel group, single‐centre study (Ghana). Method of randomisation: "assigned by a randomised numbered list". Allocation concealment: unclear. Blinding: no. ITT: unclear. Withdrawals: stated. | |
| Participants | Baseline characteristics: 46 participants screened and given 3 courses of C (27 children randomised (male:female: 16:11)).
Median age: 9 years.
Tumour staging: unclear.
Diagnosis: histopathology. Entry criteria: stage III BL; adequate response to C. Exclusion criteria: not reported. |
|
| Interventions | Monthly intramuscular TF versus no treatment. Trial was stopped prematurely due to exhausted stocks of intervention agent. Study duration: maximum of one year. Follow‐up: median 3.3 years. |
|
| Outcomes | Relapse. Overall survival. | |
| Notes | Full text publication. Study type: remittance maintenance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Late failures rates were accounted for. |
Olweny 1976.
| Methods | Randomised, parallel group, single‐centre trial (Kenya). Withdrawals: stated. | |
| Participants | Baseline characteristics: 40 participants randomised.
Mean age: not reported.
Tumour staging: Ziegler and Magrath.
Diagnosis: histopathology. Entry criteria: confirmed BL. |
|
| Interventions | C versus COM. Treatment duration was for 2 weeks (or as soon as toxicity abated). A third dose was given to 3 participants in C group and in one participant in COM group. Follow‐up: unclear. |
|
| Outcomes | Relapse. Overall survival. Response (remission, reduction in tumour size or no response). | |
| Notes | Full text publication. Study type: remission induction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated as "previously prepared random cards". |
| Allocation concealment (selection bias) | Low risk | The use of these cards also suggests that this may be adequate. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported. |
Olweny 1977.
| Methods | Randomised, parallel group, single‐centre trial (Kenya). ITT: no. Withdrawals: stated. | |
| Participants | Baseline characteristics: 25 participants randomised; 22 participated.
Median age: irradiation: 8 years; no irradiation: 5 years (range: 4 to 14).
Tumour staging: Ziegler and Magrath.
Diagnosis: histology or cytology. Entry criteria: BL free of CNS involvement in remission post treatment with C, MTX, or O. |
|
| Interventions | Irradiation versus no irradiation. Dose given was 20 to 24 Gy (0.7 to 0.75 Gy per fraction). Treatment duration: 2 x 5 days. Follow‐up: 1.6 years. |
|
| Outcomes | Relapse. Overall survival. | |
| Notes | Full text publication. a follow‐up on Olweny 1976. Study type: remittance maintenance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as "previously prepared random cards". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors made adjustments for the number that were unable to make the trip to the treatment venue. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were described for all participants. |
Patte 1991.
| Methods | Randomised, parallel group, multicentre trial (Europe). ITT: stated that all were included in the analysis. Withdrawals: stated. | |
| Participants | Baseline characteristics: 216 eligible (166 randomised; long duration: 84; short duration: 82).
Age range: 6 months to 17 years.
Tumour staging: Murphy.
Diagnosis: histology, cytology or immunotyping. Entry criteria: Age <17 years, diagnosis of BL, stages III and IV disease. Exclusion criteria: CNS involvement. After 1986, only those with abdominal or head‐neck primary tumours were included. |
|
| Interventions | The interventions differed mainly by the duration d the number of drugs given at each stage of treatment; a 5‐week short course compared with a long (16‐week) course with additional drugs maintenance chemotherapy. Follow‐up: 18 months. |
|
| Outcomes | Remission. Relapse. Toxicity. Overall survival. Event‐free survival. | |
| Notes | Full text publication. Equivalence trial. Study type: remission induction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, balanced block randomisation. |
| Allocation concealment (selection bias) | Low risk | Implied from the description of randomisation from a central office. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes were reported. |
Patte 2007.
| Methods | Multicentre, 4‐arm study. | |
| Participants | Eligibility: non‐immune compromised; < 18 years or 21 years with newly diagnosed B‐cell lymphoma. Tumour staging: non‐resected stages I to III & stage IV, CNS negative (St Jude/ Murphy classification). 762 eligible; 657 randomised; results based on 637. |
|
| Interventions | A factorial design between 4 arms, 2 receiving half‐dose of C in the second induction course with C, O, P, AD (doxorubicon), MTX (1A versus 1B) and 2 not receiving the maintenance course M1 (2A versus. 2B). | |
| Outcomes | Event‐free survival. Overall survival. Failure‐free survival. |
|
| Notes | All participants had a standard pre‐randomisation phase and received COP and COPADM1. Median follow up period 54 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be done at national group level. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Stated as an open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 657 randomised but 20 excluded post randomisation due to wrong classification and lack of clinical data. |
Sullivan 1991.
| Methods | Randomised, parallel group, multicentre trial (North America). ITT: unclear. Withdrawals: stated. | |
| Participants | Baseline characteristics: 168 participants registered, 148 were evaluable, 73 had BL.
Median age: 8.7 years (range 0.7 to 18.7), male:female; 4.4:1.0.
Tumour staging: Murphy.
Diagnosis: unclear (institutional review). Entry criteria: stages III and IV non lymphoblastic NHL, age < 22 years. Exclusion criteria: not stated. |
|
| Interventions | Triple IT (Ara‐C, MTX, HYD) given for short (2 months) versus long (6 months) term as maintenance treatment. Follow‐up: 3 to 7 years. |
|
| Outcomes | Complete remission. Event‐free survival. Toxicity. | |
| Notes | Full text publication. Study type: remission induction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes described |
Ziegler 1971.
| Methods | Randomised, parallel group, chemotherapeutic trial, single‐site trial (Uganda). ITT: unclear. Withdrawals: not stated. | |
| Participants | Baseline characteristics: sample size; 20 evaluated for study, main study apparently focused on an initial 15.
Age range: 3 to 14 years.
Tumour staging: Ziegler.
Diagnosis: unclear. Entry criteria: BL at stages I to III; no evidence of malignant pleocytosis on admission. Exclusion criteria: not stated. |
|
| Interventions | Prophylactic IT versus no treatment. Treatment duration: 4 or 10 days. Follow‐up: participants followed to relapse. |
|
| Outcomes | Relapse rate | |
| Notes | Full publication. The study describes a sequential trial of different drug regimens following relapse in the same set of participants. Induction of remission was by a randomised schedule though outcomes were not described. Study type: remittance maintenance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were said to be "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not described in the text. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding but it was unlikely that blinding was done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relapse rates were described for outcomes and were reported for all participants who relapsed. |
Ziegler 1972a.
| Methods | Randomised, parallel group, single‐site trial (Uganda). Method of randomisation: unclear. Allocation concealment: unclear. ITT: no. Withdrawals: stated. | |
| Participants | Baseline characteristics: sample size: 41 participants, 27 were evaluable for the study.
Age range: 3 to 25 years.
Tumour staging: Ziegler.
Diagnosis: histopathology. Entry criteria: stages III or IV disease, untreated, histopathologic diagnosis. Exclusion criteria: not stated. |
|
| Interventions | Multiple doses of C versus the TRIKE regimen (sequential regimen using C, O, MTX and Ara‐C). Follow‐up: participants followed up until death. |
|
| Outcomes | Relapse. Remission. Overall survival. | |
| Notes | 2:1 allocation was used in randomisation. Full text publication. Randomisation rating: B. Withdrawal bias rating: C. Study type: remission induction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised by stage". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nature and frequency of relapse was reported |
AD: adriamycin (doxorubicin) Ara‐C: cytosine arabinoside BCG: Bacille‐Calmette‐Guerin BL: Burkitt lymphoma BLL: Burkitt‐like lymphoma C: cyclophosphamide CNS: central nervous system COM: cyclophosphamide, oncovin and methotrexate COMP: cyclophosphamide, oncovin, methotrexate and prednisolone Gy: Gray unit (SI unit of absorbed radiation) HYD: hydrocortisone IT: intrathecal ITT: intention‐to‐treat analysis IV: intravenous MTX: methotrexate NHL: non‐Hodgkin's lymphoma O: oncovin (vincristine) P: prednisolone SNCCL: small non‐cleaved cell lymphoma TF: transfer factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adde 1998 | There was no randomisation. |
| Amengual 2008 | This was a case report. |
| Aviles 1983 | The study was done in adults and the tumour type (marginal zone B‐cell lymphoma) is different. |
| Baldissera 2006 | Participants were adults with non‐Burkitt tumours. |
| Blum 2006 | A single‐arm phase 11 study. |
| Boue 2006 | Participants were adults. |
| Cairo 2007 | Results are presented for all groups and not as per type of tumour. |
| Coiffier 1990 | The age group of the study participants was not stated but the tumour type is different and the study was not randomised. |
| Economopoulos 2007 | Excludes participants with BL. |
| Eldar 2009 | Retrospective review. |
| Federico 2006 | Non‐Burkitt tumours, mostly adults in the study. |
| Grogg 2007 | A review article. |
| Gururagan 2000 | This is a review article, not a randomised trial. |
| Hagenbeck 2006 | Age greater than18 years (above cut‐off point for the review). |
| Hainsworth 2005 | No randomisation, tumour type is different. |
| Haioun 1993 | Study was done in adults. |
| Hesseling 2009 | Not randomised; a single‐arm longitudinal study. |
| Hsu 1997 | Age was above the cut‐off point. The study was not randomised, tumour type is different. |
| Kaplan 1991 | The study was done in adults. |
| Kimby 1993 | The tumour type is different. |
| Laver 2005 | Non‐Burkitt tumours. |
| Levine 1991 | Age above cut‐off point; the study was not randomised. |
| Magrath 2009 | Historical review of BL management. |
| Maloney 1994 | Age above cut‐off point; study was not randomised. |
| Maloney 1997 | Age above cut‐off point; study was not randomised. |
| Olweny 1971 | The study was done in adults. |
| Oriol 2005 | Study in adults (over 15 years). |
| Pfreundschuh 2004 | The study was done in adults. |
| Pfreundschuh 2008 | Participnts were 18 years and above, not BL. |
| Reiter 1994a | Not a randomised trial. |
| Reiter 1994b | Not a randomised trial. |
| Sparano 2007 | Opinion in a review. |
| Spina 2005 | Opinion in a review. |
| Sun 2006 | Multiple tumour types. |
| Tilly 2000 | The study was done in adults. |
| Tsurumi 2004 | Multiple neoplasms, age above cut‐off point. |
| Tura 1991 | The study was done in adults. |
| Verdonck 2007 | Non‐Burkitt type tumors. |
| Witzig 2000 | The study was done in adults. |
| Witzig 2002 | The study was done in adults, tumour type is different. |
| Wood 2005 | This was a review. |
BL: Burkitt lymphoma
Differences between protocol and review
In this update, the background has been revised to take into account the classification of the disease and diagnosis methods. The methods section was revised in line with the updates to the Cochrane review format.
Contributions of authors
JO wrote the draft of the review with contributions from MM (discussion), SR (results/statistics). The draft was amended by JO after comments from all review authors. TL edited the final draft and helped with the tables and figures. For the update, JO and NS reviewed the new search and revised the text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Jim Milan Memorial Award, UK.
The Editorial Base of CHMG is funded by the German Ministry of Education and Research (BMBF) FKZ : 01GH0501, Germany.
Declarations of interest
The authors report no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anderson 1983 {published data only}
- Anderson JR, Wilson JF, Jenkin DT, Meadows AT, Kersey J, Chilcote RR, et al. Childhood non‐Hodgkin's lymphoma. The results of a randomised therapeutic trial comparing a 4‐drug regimen (COMP) with a 10‐drug regimen (LSA2‐L2). New England Journal of Medicine 1983;308(10):559‐65. [DOI] [PubMed] [Google Scholar]
Brecher 1997 {published data only}
- Brecher ML, Schwenn MR, Coppes MJ, Bowman WP, Link MP, Berard CW, et al. Fractionated cyclophosphamide and back to back high dose methrotrexate and cytosine arabinoside improves outcome in patients with stage III high grade small non‐cleaved cell lymphomas (snccl): a randomised trial of the pediatric oncology group. Medical and Pediatric Oncology 1997;29(6):526‐33. [DOI] [PubMed] [Google Scholar]
Cairo 2003a {published data only}
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton R, Michon J, et al. Results of a randomised FAB LMB89 international study in children and adolescents (C+A) with advanced (bone marrow [BM] [B‐ALL] and/or CNS) B‐NHL (large cell [LCL], Burkitt's [BM] and Burkitt‐like [BLL]): Pts with L3 leukemia/ CNS‐ have an excellent prognosis. Procdeedings of the American society of Clinical Oncology. 2003; Vol. 22:796.
Magrath 1973 {published data only}
- Magrath IT, Ziegler. Prophylaxis of meningeal Burkitt's lymphoma with CCNU. American Association for Cancer Research 1973;14:67. [Google Scholar]
Magrath 1976 {published data only}
- Magrath IT, Ziegler JL. Failure of BCG immunostimulation to affect the clinical course of Burkitt's Lymphoma. British Medical Journal 1976;1:615‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath IT, Ziegler JL, Bluming AZ. Preliminary results of a randomized trial of BCG immunotherapy in Burkitt's lymphoma. Recent Results in Cancer Research 1974;47:461‐5. [Google Scholar]
- Ziegler JL, Magrath IT. BCG immunotherapy in Burkitt's lymphoma: preliminary results of a randomized clinical trial. National Cancer Institute Monograph 1973;39:199‐202. [PubMed] [Google Scholar]
- Ziegler JL, Magrath IT, Bluming AZ. BCG immunotherapy of Burkitt's lymphoma. Proceedings of the American Association for Cancer Research. 1972; Vol. 13:38.
Neequaye 1990 {published data only}
- Neequaye J, Viza D, Pizza G, Levine P, Vinci C, Ablashi D, et al. Specific transfer factor with activity against Ebstein‐Barr virus reduces late relapse in endemic Burkitt's lymphoma. Anticancer Research 1990;10(5A):1183‐7. [PubMed] [Google Scholar]
Olweny 1976 {published data only}
- Olweny CLM, Katongole‐Mbidde E, Kaddu‐Mukassa A, Atine I, et al. Treatment of Burkitt's lymphoma: randomized clinical trial of single‐agent versus combination chemotherapy. International Journal of Cancer 1976;17(4):436‐40. [DOI] [PubMed] [Google Scholar]
Olweny 1977 {published data only}
- Olweny CLM, Atine I, Kaddu‐Mukassa A, Katongole‐Mbide E, Lwanda SK, Johansson B, et al. Cerebrospinal irradiation of Burkitt's lymphoma. Acta Radiologica: Oncology, Radiation, Physics, Biology 1977;16(3):225‐31. [DOI] [PubMed] [Google Scholar]
Patte 1991 {published data only}
- Patte C, Philip T, Rodary C, Zucker J, Behrendt H, Gentet J, et al. High survival rate in advanced‐stage B‐cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French pediatric oncology society of a randomized trial of 216 children. Journal of Clinical Oncology 1991;9(1):123‐32. [DOI] [PubMed] [Google Scholar]
Patte 2007 {published data only}
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. FAB/LMB96 International Study Committee. Results of the randomized international FAB/LMB96 trial for intermediate risk B‐cell non‐Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 2007;109(7):2773‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 1991 {published data only}
- Sullivan MP, Brecher M, Ramirez I, Ragab A, Hvizdala E, Pullen J, et al. High‐dose Cyclophosphamide‐high‐dose methotrexate with coordinated intrathecal therapy for advanced nonlymphoblastic lymphoma of childhood: results of a pediatric oncology group study. The American Journal of Pediatric Hematology/Oncology 1991;13(3):288‐95. [DOI] [PubMed] [Google Scholar]
Ziegler 1971 {published data only}
- Ziegler JL, Bluming AZ. Intrathecal chemotherapy in Burkitt's lymphoma. British Medical Journal 1971;3(773):508‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 1972a {published data only}
- Ziegler JL, Bluming AZ, Magrath IT, Carbone PP. Intensive chemotherapy in patients with generalized Burkitt's lymphoma. International Journal of Cancer 1972;10(2):254‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adde 1998 {published data only}
- Adde M, Shad A, Venzon D, Arndt C, Gootenberg J, Neely J, et al. Additional chemotherapy agents improve treatment outcome for children and adults with advanced B‐cell lymphomas. Seminars in Oncology 1998;25(2 suppl 4):33‐9. [PubMed] [Google Scholar]
Amengual 2008 {published data only}
- Amengual JE, Zhang X, Ibrahim S, Gardner LB. Regression of HIV‐related diffuse large B‐cell lymphoma in response to antiviral therapy alone. Blood 2008;112(10):4359‐60. [DOI] [PubMed] [Google Scholar]
Aviles 1983 {published data only}
- Aviles A, Delgado S, Huerta‐Guzman J. Marginal zone B cell lymphoma of the parotid glands. European Journal of Cancer part B, Oral Oncology 1996;32B(6):420‐2. [DOI] [PubMed] [Google Scholar]
Baldissera 2006 {published data only}
- Baldissera RC, Nucci M, Vigorito AC, Maiolino A, Simões BP, Lorand‐Metze I, et al. Frontline therapy with early intensification and autologous stem cell transplantation versus conventional chemotherapy in unselected high‐risk, aggressive non‐Hodgkin's lymphoma patients: a prospective randomized GEMOH report. Acta Haematologica 2006;115(1‐2):15‐22. [DOI] [PubMed] [Google Scholar]
Blum 2006 {published data only}
- Blum KA, Johnson JL, Niedzwiecki D, Piro LD, Saven A, Peterson BA, et al. Cancer and Leukemia Group B Study 9153. Prolonged follow‐up after initial therapy with 2‐chlorodeoxyadenosine in patients with indolent non‐Hodgkin lymphoma: results of Cancer and Leukemia Group B Study 9153. Cancer 2006;107(12):2817‐25. [DOI] [PubMed] [Google Scholar]
Boue 2006 {published data only}
- Boué F, Gabarre J, Gisselbrecht C, Reynes J, Cheret A, Bonnet F, et al. Phase II trial of CHOP plus rituximab in patients with HIV‐associated non‐Hodgkin's lymphoma. Journal of Clinical Oncology 2006;24(25):4123‐8. [DOI] [PubMed] [Google Scholar]
Cairo 2007 {published data only}
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, et al. FAB LMB96 International Study Committee. Results of a randomized international study of high‐risk central nervous system B non‐Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007;109(7):2736‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coiffier 1990 {published data only}
- Coiffier B, Brousse N, Peuchmaur M, Berger F, Gisselbrecht C, Bryon P A, et al. For the GELA group (Groupe d'Etude des lymphomas Aggressives). Peripheral T‐cell lymphocytes have a poorer prognosis than B‐cell lymphomas: A prospective study of 361 immunotyped patients treated with LNH‐84 regimen. Journal of the European Society for Medical Oncology 1990;1(1):45‐50. [DOI] [PubMed] [Google Scholar]
Economopoulos 2007 {published data only}
- Economopoulos T, Psyrri A, Dimopoulos MA, Kalogera‐Fountzila A, Pavlidis N, Tsatalas C, et al. CEOP‐21 versus CEOP‐14 chemotherapy with or without rituximab for the first‐line treatment of patients with aggressive lymphomas: results of the HE22A99 trial of the Hellenic Cooperative Oncology Group. Cancer Journal 2007;13(5):327‐34. [DOI] [PubMed] [Google Scholar]
Eldar 2009 {published data only}
- Eldar AH, Futerman B, Abrahami G, Attias D, Barak AB, Burstein Y, et al. Burkitt lymphoma in children: the Israeli experience. Journal of Pediatric Hematology/Oncology 2009;31(6):428‐36. [DOI] [PubMed] [Google Scholar]
Federico 2006 {published data only}
- Federico M, Luminari S, Gobbi PG, Sacchi S, Renzo N, Lombardo M, et al. The length of treatment of aggressive non‐Hodgkin's lymphomas established according to the international prognostic index score: long‐term results of the GISL LA03 study. European Journal of Haematology 2006;76(3):217‐29. [DOI] [PubMed] [Google Scholar]
Grogg 2007 {published data only}
- Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma. Journal of Clinical Pathology 2007;60(12):1365‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gururagan 2000 {published data only}
- Gururagan S, Sposto R, Cairo MS, Meadows A T, Finlay J L. Outcome of CNS disease at diagnosis in disseminated small non‐cleaved cell lymphoma and B‐cell leukaemia: a children's cancer group study. Journal of Clinical Oncology 2000;18(10):2017‐25. [DOI] [PubMed] [Google Scholar]
Hagenbeck 2006 {published data only}
- Hagenbeek A, Eghbali H, Monfardini S, Vitolo U, Hoskin PJ, Wolf‐Peeters C, et al. Phase III intergroup study of fludarabine phosphate compared with cyclophosphamide, vincristine, and prednisone chemotherapy in newly diagnosed patients with stage III and IV low‐grade malignant Non‐Hodgkin's lymphoma. Journal of Clinical Oncology 2006;24(10):1590‐6. [DOI] [PubMed] [Google Scholar]
Hainsworth 2005 {published data only}
- Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco AF. Maximising therapeutic effect of rituximab: maintenance treatment vs. re‐treatment at progression in patients with indolent non‐Hodgkin's lymphoma‐ a randomized phase two trial of the Minie Pearl research network. Journal of Clinical Oncology 2005;23(6):1088‐95. [DOI] [PubMed] [Google Scholar]
Haioun 1993 {published data only}
- Haioun C, Lepage E, Gisselbrecht C, Coiffier B, Bosly A, Tilly H, et al. Comparison of autologous bone marrow transplantation (BMT) with sequential chemotherapy for aggressive non‐Hodgkin's lymphoma in first remission: a study on 464 patients (LNH 87 protocol). 5th International Conference on Malignant Lymphoma. 1993:85a.
Hesseling 2009 {published data only}
- Hesseling P, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28‐day treatment schedule with cyclophosphamide and intrathecal methotrexate. Annals of Tropical Paediatrics 2009;29(1):9‐34. [DOI] [PubMed] [Google Scholar]
Hsu 1997 {published data only}
- Hsu FJ, Casper CB, Czerwinski D, Kwak LW, Liles TM, Syrengelas A, et al. Tumor‐specific idiotype vaccines in the treatment of patients with B‐cell lymphoma‐ long term results of a clinical trial. Blood 1997;89(9):3129‐35. [PubMed] [Google Scholar]
Kaplan 1991 {published data only}
- Kaplan LD, Kahn JO, Crawe S, Northfelt D, Neville P, Grossberg H, et al. Clinical and virologic effects of recombinant human‐granulocyte colony stimulating factor in patients receiving chemotherapy for human immunodeficiency virus‐associated non‐Hodgkin's lymphoma: results of a randomized trial. Journal of Clinical Oncology 1991;9(6):929‐40. [DOI] [PubMed] [Google Scholar]
Kimby 1993 {published data only}
- Kimby E, Mellstedt H. Chlorambucil/prednisolone (CHP) versus CHOP in symptomatic low grade lymphomas. 5th International Conference on Malignant Lymphoma. 1993.
Laver 2005 {published data only}
- Laver JH, Kraveka JM, Hutchison RE, Chang M, Kepner J, Schwenn M, et al. Advanced‐stage large‐cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate‐dose methotrexate and high‐dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. Journal of Clinical Oncology 2005;23(3):541‐7. [DOI] [PubMed] [Google Scholar]
Levine 1991 {published data only}
- Levine AM, Wernz JC, Kaplan L, Rodman N, Cohen P, Metroka C, et al. Low‐dose chemotherapy with central nervous system prophylaxis and zidovudine maintenance in AIDS‐ related lymphoma. JAMA 1991;266(1):84‐8. [PubMed] [Google Scholar]
Magrath 2009 {published data only}
- Magrath I. Lessons from clinical trials in African Burkitt lymphoma. Current Opinions in Oncology 2009;21(5):462‐8. [DOI] [PubMed] [Google Scholar]
Maloney 1994 {published data only}
- Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenburg J, Grillo‐lopez A, et al. Phase I clinical trial using escalating single‐dose infusion of chimeric Anti‐CD20 monoclonal antibody (IDEC‐C2B8) in patients with recurrent B‐cell lymphoma. Blood 1994;84(8):2457‐66. [PubMed] [Google Scholar]
Maloney 1997 {published data only}
- Maloney DG, Grillo‐Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC‐C2B8: results of a phase I multiple‐dose trial in patients with relapsed non‐Hodgkin's lymphoma. Journal of Clinical Oncology 1997;15(10):3266‐74. [DOI] [PubMed] [Google Scholar]
Olweny 1971 {published data only}
- Olweny CLM, Ziegler JL. Treatment of histiocytic lymphoma in Ugandan adults. East African Medical Journal 1971;48(10):585‐91. [PubMed] [Google Scholar]
Oriol 2005 {published data only}
- Oriol A, Ribera JM, Brunet S, Potro E, Abella E, Esteve J. Highly active antiretroviral therapy and outcome of AIDS‐related Burkitt's lymphoma or leukemia. Results of the PETHEMA‐LAL3/97 study. Haematologica 2005;90(7):990‐2. [PubMed] [Google Scholar]
Pfreundschuh 2004 {published data only}
- Pfreundschuh M, Trümper L, Kloess M, Schmits R, Feller AC, Rudolph C, et al. Two‐weekly or 3‐weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good prognosis (normal LDH) aggressive lymphomas: results of the NHL‐B1 trial of the DSNHL. Blood 2004;104(3):626‐33. [DOI] [PubMed] [Google Scholar]
Pfreundschuh 2008 {published data only}
- Pfreundschuh M, Zwick C, Zeynalova S, Dührsen U, Pflüger KH, Vrieling T, et al. German High‐Grade Non‐Hodgkin's Lymphoma Study Group (DSHNHL). Dose‐escalated CHOEP for the treatment of young patients with aggressive non‐Hodgkin's lymphoma: II. Results of the randomized high‐CHOEP trial of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group (DSHNHL). Annals of Oncology 2008;19(3):545‐52. [DOI] [PubMed] [Google Scholar]
Reiter 1994a {published data only}
- Reiter A, Tiemann M, Ludwig WD, Wacker HH, Yakisan E, Schrappe M, et al. NHL‐BFM 90 therapy study in treatment of malignant non‐Hodgkin's lymphoma in children and adolescents. Part 1: classification and allocation to strategic therapy groups. BIF study group [Therapiestudie NHL‐BFM 90 zur Behandlung maligner non‐Hodgkin‐Lymphome bei Kindern und Jugendlichen. Teil1: Klassifikation und Einteilung in stategische Therapiegruppen]. Klinische Pädiatrie 1994;206(4):222‐33. [DOI] [PubMed] [Google Scholar]
Reiter 1994b {published data only}
- Reiter A, Schrappe M, Yakistan E, Sauter S, Ebell W, Zimmermann M, et al. NHL‐BFM 90 therapy study in treatment of malignant non‐Hodgkin's lymphoma in children and adolescents. Part 3: an intermediate term analysis of the B‐NHL/B‐ALL. [Therapiestudie NHL‐BFM 90 zur Behandlung maligner non‐Hodkin‐lymphome bei Kindern und Jugendlichen. Teil 3: eine Zwischenanalyse der Therapiegruppe B‐NHL/B‐ALL]. Klinische Pädiatrie 1994;204(4):242‐52. [DOI] [PubMed] [Google Scholar]
Sparano 2007 {published data only}
- Sparano JA. HIV‐associated lymphoma: the evidence for treating aggressively but with caution. Current Opinions in Oncology 2007;19(5):458‐63. [DOI] [PubMed] [Google Scholar]
Spina 2005 {published data only}
- Spina M, Tirelli U. Rituximab for HIV‐associated lymphoma: weighing the benefits and risks. Current Opinions in Oncology 2005;17(5):462‐5. [DOI] [PubMed] [Google Scholar]
Sun 2006 {published data only}
- Sun XF, Zhen ZJ, Lui DG, Xia Y, He YJ, Wang ZH, et al. Improved treatment outcome in Chinese children and adolescents with Burkitt's lymphoma and large cell lymphoma by using the modified B‐non‐Hodgkin's lymphoma‐Berlin‐Frankfurt‐Münster‐90 protocol. European Journal of Haematology 2006;77(5):365‐71. [DOI] [PubMed] [Google Scholar]
Tilly 2000 {published data only}
- Tilly H, Mounier N, Lederlin P, Dupriez B, Sebban C, Bosly A, et al. Randomized comparison of ACVBP and m‐BACOD in the treatment of patients with low‐risk aggressive lymphoma: the LNH87‐1 study. Journal of Clinical Oncology 2000;18(6):1309‐15. [DOI] [PubMed] [Google Scholar]
Tsurumi 2004 {published data only}
- Tsurumi H, Yamada T, Sawada M, Kasahara S, Kanemura N, Kojima Y, et al. Biweekly CHOP or THP‐COP regimens in the treatment of newly diagnosed aggressive non‐Hodgkin's lymphoma. A comparison of doxorubicin and pirarubicin: a randomized phase II study. Journal of Cancer Research and Clinical Oncology 2004;130(2):107‐13. [DOI] [PubMed] [Google Scholar]
Tura 1991 {published data only}
- Tura S, Mandelli F, Mazza P, Pileri S, Gherlinzoni F, Bocchia M, et al. MACOP‐B vs. MACHOP in the treatment of high‐grade non‐Hodgkin's lymphoma. Leukemia 1991;5((suppl 1)):74‐8. [PubMed] [Google Scholar]
Verdonck 2007 {published data only}
- Verdonck LF, Notenboom A, Jong DD, MacKenzie MA, Verhoef GE, Kramer MH, et al. Intensified 12‐week CHOP (I‐CHOP) plus G‐CSF compared with standard 24‐week CHOP (CHOP‐21) for patients with intermediate‐risk aggressive non‐Hodgkin lymphoma: a phase 3 trial of the Dutch‐Belgian Hemato‐Oncology Cooperative Group (HOVON). Blood 2007;109(7):2759‐66. [DOI] [PubMed] [Google Scholar]
Witzig 2000 {published data only}
- Witzig TE. The use of Ibritumomab tiuxetan radioimmunology for patients with relapsed B‐cell non‐Hodgkin's lymphoma. Seminars in Oncology 2000;27(suppl 12):74‐8. [PubMed] [Google Scholar]
Witzig 2002 {published data only}
- Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of Yttrium‐90‐labeled Ibritumomab Tiuxetan radioimmunotherapy versus Rituximab immunotherapy for patients with relapsed or refractory low‐grade, follicular, or transformed B‐cell non‐Hodgkin's lymphoma. Journal of Clinical Oncology 2002;20(10):2453‐63. [DOI] [PubMed] [Google Scholar]
Wood 2005 {published data only}
- Wood C, Harrington W Jr. AIDS and associated malignancies. Cell Research 2005;15(11‐12):947‐52. [DOI] [PubMed] [Google Scholar]
Additional references
Akbayram 2010
- Akbayram S, Doğan M, Akgün C, Erbey F, Caksen H, Oner AF. Use of rituximab in three children with relapsed/refractory Burkitt lymphoma. Targeted Oncology 2010;5(4):291‐4. [DOI] [PubMed] [Google Scholar]
Bernheim 1981
- Bernheim A, Berger R, Lenoi G. Cytogenic studies on African Burkitt's lymphoma cell lines; t(8;14),t(2;8), and t(8;22) translocations. Cancer Genetics and Cytogenetics 1981;3:307‐15. [DOI] [PubMed] [Google Scholar]
Blum 2004
- Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009‐20. [DOI] [PubMed] [Google Scholar]
Burkitt 1958
- Burkitt. Sarcoma involving jaws in African children. British Journal of Surgery 1958;46:218‐23. [DOI] [PubMed] [Google Scholar]
Cairo 2003b
- Cairo MS, Sposto R, Perkins SL, Meadows AT, Hoover‐Regan ML, Anderson JR, et al. Burkitt's and Burkitt‐like lymphoma in children and adolescents: a review of the Children's Cancer Group experience.. British Journal of Haematology 2003;4:660‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldstein 1990
- Goldstein J A, Berstein R L. Burkitt's lymphoma and the role of Ebstein‐ Barr virus. Journal of Tropical Paediatrics 1990;30:114‐9. [DOI] [PubMed] [Google Scholar]
Harris 2000
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller‐Hermelink HK, Vardiman J, et al. The World Health Organization Classification of Hematological Malignancies Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia,November 1997. Modern Pathology 2000;13(2):193‐207. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration, 2008 [updated September 2009]. [Google Scholar]
Kasamon 2004
- Kasamon YL, Swinnen LJ. Treatment advances in adult Burkitt lymphoma and leukemia. Current Opinions in Oncology 2004;16:429‐35. [DOI] [PubMed] [Google Scholar]
Knowles 2003
- Knowles DM. Etiology and pathogenesis of AIDS‐related non‐Hodgkin’slymphoma. Hematology/Oncology Clinics of North America 2003;17:785‐820. [DOI] [PubMed] [Google Scholar]
Lefebre 2008
- Lefebre C, Mnheimer E, Glanville J. Chapter 6:Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Wiley, 2008. [Google Scholar]
Magrath 1974
- Magrath IT, Ziegler JL, Bluming AZ. Preliminary results of a randomized trial of BCG immunotherapy in Burkitt's lymphoma.. Recent results in cancer research 1974;47:461‐5. [Google Scholar]
Magrath 1974a
- Magrath IT, Lwanga S, Carswell W, Harrison N. Surgical reduction of tumor bulk in management of abdominal Burkitt's Lymphoma. British Medical Journal 1974;2:308‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magrath 1987
- Magrath IT. Malignant non‐Hodgkin's lymphomas in children. Hematology/Oncology Clinics of North America 1987;1(4):577‐602. [MEDLINE: ] [PubMed] [Google Scholar]
Magrath 1989
- Magrath IT, Shiramizu B. Biology and treatment of small non‐cleaved cell lymphoma. Biology and treatment of small non‐cleaved cell lymphoma. Biology and treatment of small non‐cleaved cell lymphoma. Oncology 1989;3(11):41‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Martinez‐Maza 2002
- Martinez‐Maza O, Breen EC. B‐cell activation and lymphoma in patients with HIV. Current Opinions in Oncology 2002;14:528‐32. [DOI] [PubMed] [Google Scholar]
Meremikwu 2005
Norlin 1971
- Norlin T, Clifford P, Einhorn J, Einhorn N, Johansson B, Klein G, et al. Conventional and superfractionated radiation therapy in Burkit's lymphoma. Acta Radiologica 1971;10:545‐57. [DOI] [PubMed] [Google Scholar]
Oguonu 2002
- Oguonu T, Emodi I, Kaine W. Epidemiology of Burkitt's lymphoma in Enugu, Nigeria. Annals of Tropical Paediatrics 2002;22(4):369‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olweny 1980
- Olweny CL, Katongole‐Mbidde E, Otim D, Lwanga SK, Magrath IT, Ziegler JL. Long‐term experience with Burkitt's lymphoma in Uganda. International Journal of Cancer 1980;26(3):261‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parmer 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Smeland 2004
- Smeland S, Blystad AK, Kvaloy SO, Ikonomou IM, Delabie J, Kvalheim G, et al. Treatment of Burkitt's/Burkitt‐like lymphoma in adolescents and adults: a 20‐year experience from the Norwegian Radium Hospital with the use of three successive regimens.. Annals of Oncology 2004;15(7):1072‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sugimoto 2004
- Sugimoto M, Tahara H, Ide T, Furuichi Y. Steps involved in immortalization and tumorigenesis of human b‐lymphoblastoid cell lines transformed by Epstein‐Barr virus. Cancer Research 2004;64(10):3361‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ziegler 1972
- Ziegler JL. Chemotherapy of Burkitt's lymphoma. Cancer 1972;30:1534‐40. [DOI] [PubMed] [Google Scholar]
Ziegler 1974
- Ziegler JL, Magrath IT. Pathology Annual. Ioachim H L. New York: Apleton‐Century‐Crofts, 1974. [Google Scholar]
Ziegler 1981
- Ziegler JL. Burkitt's Lymphoma. New England Journal of Medicine 1981;305:735‐45. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Okebe 2006
- Okebe JU, Lasserson TJ, Meremikwu MM, Richards S. Therapeutic interventions for Burkitt's lymphoma in children. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005198.pub2] [DOI] [PubMed] [Google Scholar]


