Abstract
Background
Publication of complete trial results is essential if people are to be able to make well‐informed decisions about health care. Selective reporting of randomised controlled trials (RCTs) is a common problem.
Objectives
To systematically review studies of cohorts of RCTs to compare the content of trial reports with the information contained in their protocols, or entries in a trial registry.
Search methods
We conducted electronic searches in Ovid MEDLINE (1950 to August 2010); Ovid EMBASE (1980 to August 2010); ISI Web of Science (1900 to August 2010) and the Cochrane Methodology Register (Issue 3, 2010), checked reference lists, and asked authors of eligible studies to identify further studies. Studies were not excluded based on language of publication or our assessment of their quality.
Selection criteria
Published or unpublished cohort studies comparing the content of protocols or trial registry entries with published trial reports.
Data collection and analysis
Data were extracted by two authors independently. Risk of bias in the cohort studies was assessed in relation to follow up and selective reporting of outcomes. Results are presented separately for the comparison of published reports to protocols and trial registry entries.
Main results
We included 16 studies assessing a median of 54 RCTs (range: 2 to 362). Twelve studies compared protocols to published reports and four compared trial registry entries to published reports. In two studies, eligibility criteria differed between the protocol and publication in 19% and 100% RCTs. In one study, 16% (9/58) of the reports included the same sample size calculation as the protocol. In one study, 6% (4/63) of protocol‐report pairs gave conflicting information regarding the method of allocation concealment, and 67% (49/73) of blinded studies reported discrepant information on who was blinded. In one study unacknowledged discrepancies were found for methods of handling protocol deviations (44%; 19/43), missing data (80%; 39/49), primary outcome analyses (60%; 25/42) and adjusted analyses (82%; 23/28). One study found that of 13 protocols specifying subgroup analyses, 12 of these 13 trials reported only some, or none, of these. Two studies found that statistically significant outcomes had a higher odds of being fully reported compared to nonsignificant outcomes (range of odds ratios: 2.4 to 4.7). Across the studies, at least one primary outcome was changed, introduced, or omitted in 4‐50% of trial reports.
Authors' conclusions
Discrepancies between protocols or trial registry entries and trial reports were common, although reasons for these were not discussed in the reports. Full transparency will be possible only when protocols are made publicly available or the quality and extent of information included in trial registries is improved, and trialists explain substantial changes in their reports.
Keywords: Publication Bias, Clinical Protocols, Clinical Protocols/standards, Cohort Studies, Double-Blind Method, Random Allocation, Randomized Controlled Trials as Topic, Randomized Controlled Trials as Topic/methods, Randomized Controlled Trials as Topic/standards, Registries, Registries/standards
Plain language summary
Comparison of protocols and registry entries to published reports for randomised controlled trials
The non‐reporting of a piece of research and the selective reporting of only some of its findings has been identified as a problem for research studies such as randomised trials and systematic reviews of these. If the decision about what to report and what to keep unpublished is based on the results obtained in the trial, this will lead to bias and potentially misleading conclusions by users of the research. One way to see if there might be discrepancies between what was planned or done in a trial and what is eventually reported is to compare the protocol or entry in a trial registry for the trial with the content of its published report. This might reveal that changes were made between the registration and planning of the trial and its eventual analysis. Any such changes should be described in the published report, to reassure readers and others who will use the trial's results that the risk of bias has been kept low.
This Cochrane methodology review examines the reporting of randomised trials by reviewing research done by others in which the information in protocols or trial registry entries were compared to that in the published reports for groups of trials, to see if this detected any inconsistencies for any aspects of the trials. We included 16 studies in this review and the results indicate that there are often discrepancies between the information provided in protocol and trial registry entries and that contained in the published reports for randomised trials. These discrepancies cover many aspects of the trials and are not explained or stated in the published reports.
Background
Full publication of complete trial results is essential if clinicians, patients, policy makers and others are to make well‐informed decisions about health care. The phenomenon whereby reports of studies are not submitted or published because of the strength and direction of the trial results has been termed ‘publication bias’ (Dickersin 1987; Hopewell 2009). An additional and potentially more serious threat to the validity of evidence‐based healthcare is selective reporting of results. If the decisions about which results to publish are based on the strength or direction of those results, it will result in bias. The selective reporting of outcomes, termed ‘outcome reporting bias (ORB)’, has been defined as the selection for publication of a subset of the original recorded variables from a trial based on the results (Hutton 2000; Williamson 2005a). Therefore, data available in published reports may be subject to bias (Tannock 1996; Hahn 2000; Chan 2008a). This type of bias will not only impact upon the interpretation of the individual randomised controlled trial (RCT) but also the results of any systematic review for which the trial is eligible.
Details of how an RCT will be conducted, including the outcomes to be measured and reported should be included in its protocol and, due to the varying quality of protocols and the need for transparency, the SPIRIT initiative (Standard Protocol items for Randomized Trials) has been established to produce a set of guidelines for the preparation of protocols (Chan 2008b). This should lead to improvements in the quality of protocols, which will make it easier to carry out a critical evaluation of a trial's results and to compare what was done with what was originally planned.
The case for clinical trial registration has been advocated for several decades (Simes 1986) and, in 2004, the International Committee of Medical Journal Editors (ICMJE) announced that its member journals would not consider a trial for publication unless it had been registered in a trial registry (De Angelis 2004). The ICMJE will accept registration of clinical trials in any of the primary registers that participate in the World Health Organisation's (WHO) International Clinical Trials Registry Platform (ICTRP) (Ghersi 2009). To enhance transparency of research, the International Clinical Trials Registry Platform, based at WHO, produced a minimum trial registration dataset of 20 items (see http://www.who.int/ictrp/network/trds/en/).
An earlier review (Dwan 2008) focused on the selective reporting of outcomes from among the complete set that were originally measured within a study. This helped to highlight the recent attention in the scientific literature on the problems associated with incomplete outcome reporting, and there is little doubt that non‐reporting of pre‐specified outcomes has the potential for bias (Chan 2004a; Williamson 2005b; Dwan 2008; Kirkham 2010).
Description of the problem or issue
However, selective reporting is not restricted to selective reporting of outcomes. Different measures of the same outcome may be selectively reported based on the results or an endpoint score might be reported instead of the change from baseline or vice versa. There may also be selective reporting of multiple analyses of the same data; for example, per protocol analyses may be reported rather than intention to treat analyses; or only first period results might be reported in cross over trials. Furthermore, a continuous outcome may be converted to binary data, with the choice of cut‐off selectively chosen from several different cut‐offs examined. Analyses may also be selectively reported from multiple time points (Williamson 2005b). Subgroup analyses are often undertaken in trials, although often not pre‐specified (Wang 2007) and the complete data are not always reported, with subgroup analyses with statistically significant results being more likely to be reported (Hahn 2000; Chan 2008c).
More broadly, discrepancies in any aspect of a trial (such as changes to the trial methodology) can occur between the preparation of the protocol or trial registry entry and publication of the trial's findings. Adherence to the trial protocol is important and any substantial changes to the protocol should be submitted to an ethics committee and described in the trial report.
The validity of a trial can more easily be judged with full disclosure of protocols (Chan 2008a) and by consulting the information in trial registries. Several journals now require submission of reports of trials to be accompanied by the trial protocol, and some publish this along with the manuscript. When conducting a systematic review, it is important to assess any discrepancy between the protocol and trial report, and to examine its potential to introduce bias.
Description of the methods being investigated
Adherence to what was described in trial protocols and entries in trial registries is investigated in this review for RCTs in humans (individuals or groups of people). Comparing what was planned in the original trial protocol or on a trial registry with what was actually reported in the subsequent publications provides information on adherence to the protocol or trial registry. However, if the trialists did intend to do something that was stated in the trial registry or protocol but this proved not to be possible, this would not be seen as non‐adherence to their original plan if a legitimate reason was declared in the trial report or when that report was submitted for publication.
Why it is important to do this review
To date, no systematic review has summarised the evidence from cohort studies that have compared protocols or trial registries to published articles for RCTs. A previous review (Dwan 2008) considered only cohort studies that looked at differences in outcome measures between the protocol and published report. This Cochrane methodology review considers all differences identified between protocols or trial registries and published reports, to provide evidence of non‐adherence to the intentions in the protocol or registry entry. It includes descriptive data relating in particular to outcome reporting bias, within study selective reporting bias, and other discrepancies. We highlight priority areas for establishing guidelines for improving reporting standards.
Objectives
To assess the reporting of RCTs, by reviewing research that used cohorts of RCTs to compare the content of the published reports of these trials with the information stated in
their protocols, or
their entries in a trial registry.
To assess whether these differences are related to trial characteristics, such as sample size, source of funding or the statistical significance of results.
Methods
Criteria for considering studies for this review
Types of studies
We sought any published or unpublished cohort study comparing protocols or trial registry entries to published reports of RCTs for any aspect of trial design or analysis. Published reports include any report in a peer reviewed journal resulting from the RCT, although the definition of a 'published report' may vary across cohorts. All published reports that a cohort study considered in their comparison will be considered in this review, i.e. any publication of the included trials, not just the report including the primary outcome. Cohort studies that only compared conference abstracts to a protocol or trial registry entry will not be included, due to the lack of sufficient space in the abstract to allow the level of reporting that would allow adherence to be assessed.
Cohorts containing exclusively RCTs are eligible. If studies included a mixture of RCTs and non‐RCTs but reported data separately for the two types of study, we used the findings for the RCTs. If studies included a mixture of RCTs and non‐RCTs but did not report these study designs separately, we contacted the authors for data on the RCTs alone.
Types of data
We included data regarding differences between the protocol (as defined in the cohort study) or the trial registry entry and the published report. Trial characteristics (including sample size and source of funding), and any assessment of the quality of the included RCTs (however measured in the cohort study) were extracted and reported. We recorded the definition of the "protocol" used for each cohort study, in particular whether they examined the original protocol or an amended version.
Types of methods
We recognise that eligible studies might not compare all aspects of the protocol or trial registry to the trial report. Therefore, we include any study that examines any difference between protocol or trial registry and the published report.
Types of outcome measures
Differences between the protocol or trial registry entry and the published report for any aspect of the included trials. These include:
a. All specified outcomes, and whether designated as primary or secondary, and whether reflecting efficacy or harm
b. Methodological features, including but not limited to randomisation, blinding, allocation concealment
c. Statistical analysis
d. Sample size and sample size calculations
e. Funding
f. Any other aspect.
Search methods for identification of studies
We conducted electronic searches and checked reference lists to identify studies. Studies were not excluded based on language of publication or our assessment of their quality.
Electronic searches
Literature searches were conducted in Ovid MEDLINE (1950 to August 2010); Ovid EMBASE (1980 to August 2010); ISI Web of Science (1900 to August 2010) and the Cochrane Methodology Register (Issue 3, 2010). See Appendix 1 for more details.
Searching other resources
Articles were sought through known item searching (i.e. studies that were already to known to the authors of this review through previous work and familiarity with the research area), with articles citing those references being retrieved for screening. Authors of studies that are deemed eligible for inclusion were contacted to ask if they knew of any other relevant published or unpublished studies.
Data collection and analysis
Selection of studies
The titles and abstracts of all reports identified using the search strategy detailed above were independently screened by two authors (KD and MB). The full‐text for all records identified as potentially eligible was retrieved and reviewed for eligibility by the same two authors, using the inclusion criteria listed in the protocol for this review. There were no disagreements between the authors. If there had been, these would have been resolved through discussion or by a third author (PRW).
Data extraction and management
One author (KD) extracted all relevant data from the eligible studies and recorded this on a specifically designed form, and a second author (LC) assessed the accuracy of data extraction. There were no discrepancies in data extraction. If there had been, these would have been resolved through discussion or by a third author (PRW). Data extraction included:
Study characteristics: author names, institutional affiliation, country, contact address, language of publication, type of document, and whether the study is a comparison of protocols or trial registry entries to published reports.
Population: journals in which the assessed RCTs were published, trial registry, definition of protocol, medical specialty area, number of RCT reports included in the comparison.
Reporting quality: comparisons made between protocol or trial registry and published report.
Discrepancies, similarities, completeness of reporting, non‐reporting, and factors of particular interest (i.e.sample size and source of funding).
Information on the statistical significance (i.e. p‐value above or below 0.05), and perceived importance (as decided by the authors of the cohort study) or direction of results.
RCT quality: score on any quality assessment scale, and name of quality assessment scale used. This will depend on how each cohort study assessed the quality of trials reviewed.
Assessment of risk of bias in included studies
An assessment of the risk of bias for each included cohort study was made independently by two authors (KD and LC) using the following criteria:
1. Was there complete or near complete follow up (after data analysis) of all of the RCTs in the cohort?
Yes, percentage of follow up to be recorded, including number of unpublished studies.
No
Unclear
2. Were cohort studies free of selective reporting?
Yes (i.e. all comparisons stated in the methods section were fully reported)
No (i.e. not all of the comparisons that were stated in the methods section were fully reported)
Unclear
Each criterion was assigned an answer of yes, no or unclear, corresponding to a low, high or unclear risk of bias within the cohort study, respectively. There were no disagreements between the authors on this assessment. If there had been, these would have been resolved through discussion or by a third author (PRW).
Measures of the effect of the methods
Discrepancies between protocols or trial registries and trial reports were sought and reported using the following framework.
Discrepancies regarding outcomes were considered, when possible, as follows:
Primary outcome stated in the protocol or trial registry is the same as in the published report;
Primary outcome stated in the protocol or trial registry is downgraded to secondary in the published report;
Primary outcome stated in the protocol or trial registry is omitted from the published report;
A non primary outcome in the protocol or trial registry is changed to primary in the published report;
A new primary outcome that was not stated in the protocol or trial registry (as primary or secondary) is included in the published report;
The definition of the primary outcome was different (although the same variable) in the protocol or trial registry compared to the published report.
Discrepancies regarding trial methodology were considered, when possible, as follows:
The method of randomisation, blinding and allocation concealment stated in the published report was different in the protocol or trial registry;
The method of randomisation, blinding and allocation concealment was stated in the protocol or trial registry but not stated in the published report.
Discrepancies regarding statistical analysis were considered, when possible, as follows:
Per protocol analyses reported rather than intention to treat analyses, with the analysis used in the published report being different to what was stated in the protocol or trial registry;
First period results in cross over trials only reported instead of the appropriate results, with the analysis used in the published report being different to what was stated in the protocol or trial registry;
Endpoint score reported instead of change from baseline or vice versa, with the analysis used in the published report being different to what was stated in the protocol or trial registry;
Continuous outcome converted to binary, with the cut off used in the published report being different to what was stated in the protocol or trial registry;
Analyses at multiple time points stated in the protocol or trial registry differ to those included in the published report;
Subgroup analyses in the published report are different from those stated in the protocol or trial registry.
Discrepancies regarding sample size and sample size calculations were considered, when possible, as follows:
Sample size and sample size calculation stated in the published report was different to that in the protocol or trial registry;
Sample size and sample size calculation was stated in the protocol or trial registry but not stated in the published report.
Discrepancies regarding funding were considered, when possible, as follows:
Source of funding in the published report was different in the protocol or trial registry;
Source of funding was stated in the protocol or trial registry but not stated in the published report.
Unit of analysis issues
The unit of analysis in all the cohort studies was the RCT for which a paired protocol or trial registry entry and a published report was compared.
Dealing with missing data
If any data were perceived to be missing, whether this is information on characteristics of the cohort study or results regarding the included RCTs, the correspondence author of the cohort study was contacted for further information. If we did not receive a reply, we contacted their co‐authors.
Assessment of heterogeneity
Heterogeneity of included cohort studies is discussed narratively.
Assessment of reporting biases
We assessed the selective reporting of results in the cohort studies by comparing the methods section of the included cohort study to its results section, and completing a table of all comparisons reported by each cohort study. Authors of included studies were contacted to ask if they reported all comparisons that they looked at and if they knew of any other cohort studies that may be eligible to be included in this review, to limit publication bias.
Data synthesis
This review provides a descriptive summary of the findings of the included cohort studies because they were too diverse to combine in a meta‐analysis, due to the population of included RCTs in each cohort study and the different aspects of RCTs that they investigated. The cohort studies that have compared protocols to published reports are considered separately from the cohort studies that compared trial registry entries to published reports.
Subgroup analysis and investigation of heterogeneity
We planned to explore the following factors in subgroup analyses, assuming enough studies were identified, as we believed that these were plausible explanations for heterogeneity: small sample size versus large sample size (as defined in the included cohort study); industry funding versus public funding of the RCTs; and significant results versus non‐significant results.
Sensitivity analysis
We did not plan or undertake any sensitivity analyses.
Results
Description of studies
Results of the search
The electronic search strategies identified 4480 citations (Cochrane Methodology Register 479; EMBASE 1579; MEDLINE 1140 and Web of Science 1282). One of the studies that we already knew about was not found in this search (Hahn 2002). Our searching of conference proceedings identified one further study (You 2010) and contact with authors located a further study (Djulbegovic 2010). We did not find any additional studies by screening the reference lists of eligible studies. When we screened the titles and abstracts, 4413 citations were excluded as they were not relevant. This is shown in the PRISMA flow diagram (Figure 1).
1.
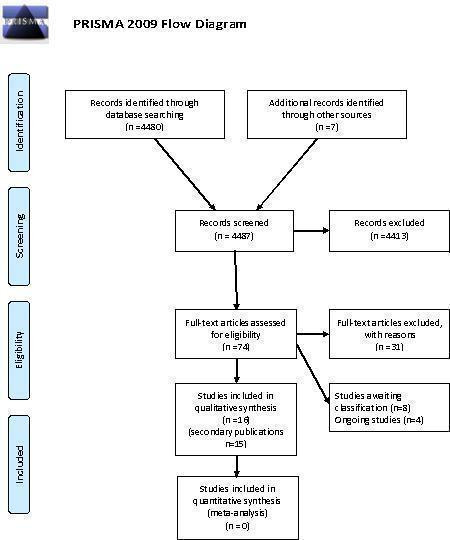
PRISMA 2009 Flow Diagram
We know of four ongoing studies (McKenzie 2010; Rasmussen 2010; Chan 2010; Urrutia 2010).
Included studies
After screening titles and abstracts, we obtained and assessed the full text for 70 citations. Sixteen studies were deemed eligible for inclusion, with 31 associated publications. The median sample size was 54 RCTs (range: 2 to 362).
There are eight studies awaiting classification (Chappell 2005; Djulbegovic 2009; Djulbegovic 2010; Ghersi 2006; Jureidini 2008; Mhaskar 2009; Smyth 2010;You 2010) as we were only able to find abstracts for these. They are likely to be eligible and we have contacted the authors for more information, so that they can be considered for inclusion in future updates of this review. Information on these studies is included in the table of Characteristics of studies awaiting classification.
Of the 16 included studies, 12 compared protocols to published reports (Al‐Marzouki 2008; Blumle 2008; Chan 2004a; Chan 2004b; Gandhi 2005; Hahn 2002; Pich 2003; Scharf 2006; Shapiro 2000; Soares 2004; Vedula 2009; von Elm 2008) and four compared registry entries to published reports (Bourgeois 2010; Charles 2009; Ewart 2009; Mathieu 2009).
Comparison of protocols to published reports
Ten of the 12 included cohort studies only considered RCTs and two considered a mixture of RCTs and other studies (Hahn 2002; Vedula 2009). Data were available for the RCTs included in the Hahn 2002 cohort study for outcomes. In the Vedula 2009 cohort, 19 of the 21 included studies were RCTs and, for the data on comparisons, 11 (92%) of the 12 included studies were RCTs and the other was an uncontrolled open label trial.
One cohort study followed up protocols that had been peer reviewed for publication by the Lancet (Al‐Marzouki 2008). Five cohort studies followed up a cohort of protocols approved by an ethics committee (Blumle 2008; Chan 2004a; Hahn 2002; Pich 2003; von Elm 2008). Chan 2004b considered protocols funded by the Canadian Institute of Health Research (CIHR), Gandhi 2005 looked at the National Institute of Health (NIH) funded RCTs for people with HIV and Vedula 2009 examined trials of gabapentin funded by Pfizer and Warner‐Lambert’s subsidiary, Parke‐Davis. One cohort study (Scharf 2006) looked at studies that used the Common Toxicity Criteria version 2.0 on the National Cancer Institute (NCI) Clinical Data Update System (CDUS). One cohort study investigated trials that served as the subject of a single study Clinical Alert (an advisory issued by the National Institutes of Health in the USA) for which the journal article was published (Shapiro 2000) and another followed up trials conducted by the Radiation Therapy Oncology Group (RTOG) since its establishment (Soares 2004). The definition of the protocol version used for the comparison with the published report are included in the Characteristics of included studies table and included: a summary on the Lancet's website (Al‐Marzouki 2008); the original protocol obtained from the ethics committee (Hahn 2002; Pich 2003) or the authors (Shapiro 2000); and protocols, amendments and correspondance (Blumle 2008; Chan 2004a; Chan 2004b; Gandhi 2005; Soares 2004; Vedula 2009; von Elm 2008). The version of the protocol used was not stated in one cohort study (Scharf 2006).
Comparison of trial registry entries to published reports
All four included studies that compared the content of a trial registry entry with the subsequent report of the research considered RCTs only.
One cohort study followed up drug trials listed on ClinicalTrials.gov (Bourgeois 2010). One cohort study searched MEDLINE for superiority RCTs published in six high impact factor general medical journals and then looked for registration details of these trials (Charles 2009). One cohort study looked for registration information on RCTs published in consecutive issues of five major medical journals (Ewart 2009). One cohort study searched MEDLINE via PubMed for reports of RCTs in three medical areas (cardiology, rheumatology, and gastroenterology) indexed in 2008 in the ten general medical journals and specialty journals with the highest impact factors (Mathieu 2009).
Excluded studies
Thirty one studies were excluded, the majority of which did not compare protocols or trial registry entries to publications (Characteristics of excluded studies).
Risk of bias in included studies
Risk of bias was assessed by considering the follow up of RCTs in the included cohort studies and selective reporting by the authors of the cohort studies.
Incomplete outcome data
Comparison of protocols to published reports
Nine cohort studies followed up all protocols, or had less than 10% loss to follow up in their cohort, and were deemed at low risk of bias (Blumle 2008,Chan 2004a; Chan 2004b; Gandhi 2005; Pich 2003; Scharf 2006; Shapiro 2000; Soares 2004; von Elm 2008). Three studies, with loss to follow up either greater than 10% or not reported, were deemed at high risk of bias (Al‐Marzouki 2008; Hahn 2002; Vedula 2009). Details are included in the risk of bias table for each study.
Comparison of trial registry entries to published reports
Three cohort studies did not follow up all trials in their cohort (with loss to follow up either greater than 10% or not reported) and were therefore deemed at high risk of bias (Charles 2009; Ewart 2009; Mathieu 2009). Details are included in the risk of bias table for each study. For one cohort study, follow up was unclear and authors have been contacted for more information (Bourgeois 2010).
Selective reporting
Comparison of protocols to published reports
Ten cohort studies reported all outcomes stated in their methods section and were therefore deemed at low risk of bias (Al‐Marzouki 2008; Chan 2004a; Chan 2004b; Gandhi 2005; Hahn 2002; Scharf 2006; Shapiro 2000; Soares 2004; Vedula 2009; von Elm 2008). One study did not report all outcomes stated in their methods section and was therefore deemed at high risk of bias (Blumle 2008). Details are included in the risk of bias table for each study and more information on outcomes are included in the results tables. In one cohort study, it was unclear whether any other comparisons had been made between protocols and published reports (Pich 2003).
Comparison of trial registry entries to published reports
Three cohort studies reported all outcomes stated in their methods section and were therefore deemed at low risk of bias (Bourgeois 2010; Charles 2009; Mathieu 2009). One study did not report all outcomes stated in their methods section and was therefore deemed at high risk of bias (Ewart 2009). Details are included in the risk of bias table for each study and more information on outcomes are included in the results tables.
Other potential sources of bias
No other potential sources of bias were identified.
Effect of methods
Comparison of protocols to published reports
Eligibility criteria
Three studies compared eligibility criteria (Blumle 2008; Gandhi 2005; Shapiro 2000) and found that between 0% and 63% of RCTs reported all eligibility criteria in the published reports that were stated in the protocol. Two of these studies (Blumle 2008; Gandhi 2005) found that there were differences between the protocol and published report (19% (6/32) and 100% (52/52)) and one study (Blumle 2008) found that in 86% of RCTs, new eligibility criteria were included in the published report which were not stated in the protocol (Table 1).
1. Differences between protocol and published reports: eligibility criteria.
| Studies that compared protocols to published reports | |
| Blumle 2008 | Eligibility criteria (EC) identical: 0/52 Differences in EC reporting: 100% (52/52); missing (96%) or modified (88%) in the publication, 86% were added in the publications |
| Gandhi 2005 | Subjective clinical criteria identical: 31% (10/32) Enrolment criteria: 34% (11/32) reported all, 31% (10/32) listed fewer than 50% of the eligibility criteria; 19% (6/32) disclosed less than a quarter of the actual enrolment criteria and no information was available for 5 trials. |
| Shapiro 2000 | 82% of protocol eligibility criteria were reported in methods papers, 63% in journal articles and 19% in clinical alerts Definition of disease (criteria that define clinical parameters of the disease being studied): 100% Precision (criteria that render the study population more homogeneous for the purposes of the trial): 66% Safety (criteria that exclude persons thought to be unduly vulnerable to harm from the study therapy): 57% Legal and ethical (criteria needed to ensure that research satisfies legal and ethical norms of human experimentation): 52% Administrative (criteria that ensure the smooth functioning of the trial): 17% |
Methodological information
Two studies compared methodological information (Chan 2004a; Soares 2004). Chan 2004a considered blinding, allocation concealment and sequence generation; six of 102 trials had adequate allocation concelament according to the trial publication and 96 of 102 trials had unclear allocation concealment. According to the protocols, 15 (16%) of these 96 trials had adequate allocation concealment, 80 (83%) had unclear concealment, and one of the 96 trials had inadequate concealment. In 6% (4/63) of trials that specified the method of allocation concealment, the protocol and the publication gave conflicting information on which method was used. In 79% (81/102) of trials, the publication gave no information on how the allocation sequence was generated; 20% of these (16/81) described adequate sequence generation in the protocol (Pildal 2005). Blinding was mentioned in the protocol for 72% (73/102) of trials and no publication reported a protocol change relevant to blinding. There was an exact match between the global terms used to describe blinding in 75% of the trials with blinding (55/73) and 32% (23/73) had an exact match of the key trial personnel who were described as blinded. Discrepant (but not necessarily contradictory) global terms were used to describe blinding in 22% (16/73) of trials with blinding, and, in 67% (49/73), there was discrepant information on who was blinded (Hrobjartsson 2009). Soares 2004 found that although all trials had adequate allocation concealment according to the protocol, this was reported in only 41% (24/59) of the papers (Table 2).
2. Differences between protocol and published reports: methods of randomisation, allocation, concealment or blinding.
| Studies that compared protocols to published reports | |
| Chan 2004a | 94% (96/102) trials had unclear allocation concealment according to the trial publication. According to the protocols, 15 of these 96 trials had adequate allocation concealment (16%, 95% CI 9% to 24%), 80 had unclear concealment (83%, 74% to 90%), and one had inadequate concealment. One was inadequate in both protocol and publication. Both were adequate in four. Unclear in protocol and adequate in publication in four Eighty one of the 102 trial publications (79%) gave no information on how the allocation sequence was generated; 16 of these 81 trials (20%, 12% to 30%) described adequate sequence generation in the protocol. No protocols or trial publications reported inadequate methods of sequence generation. Numbered coded vehicles was the most frequently applied method according to the protocols (26 of 102) but had the lowest rate of appearance in the trial publications (three of 26). In 39 of the 102 trials (38%) neither the protocols nor the publications provided any information on attempts to conceal the allocation. In four trials, the protocol and the publication gave conflicting information on which method was used. In 42 of the 55 double blind studies (76%), a security system for emergency code breaking was described in the protocol but mentioned in only one publication. Table in the paper includes differences in methods of allocation concealment. Blinding was mentioned for 73 of the 102 trials (72%; 95% CI: 62% to 80%) in the protocols alone (5), in the publications alone (9), or in both (59). No publication reported a protocol change relevant to blinding 55/73 (75%) exact match between the global terms used to describe blinding. 23/73 (32%) exact match of the key trial persons described as blinded 2/73 (3%) used overtly contradictory global terms to describe blinding 1/73 (1%) provided overtly contradictory information on who was blinded 16/73 (22%) used discrepant (but not necessarily contradictory) global terms to describe blinding 49/73 (67%) had discrepant information on who was blinded |
| Soares 2004 | All trials had adequate allocation concealment (through central randomisation), this was reported in only 24 (41%) of the papers. |
Authors
One study compared authors included in the protocol to the published report (Chan 2004a) and concluded that ghost authorship in industry‐initiated trials is very common with the company statistician listed only in the protocol in 23% (10/44) of trials (Table 3). Only five protocols explicitly identified the author of the protocol, but none of these individuals, all of whom were company employees were listed as authors of the publications or were thanked in the acknowledgments, although one protocol had noted that the 'author of this protocol will be included in the list of authors' (Gøtzsche 2007).
3. Differences between protocol and published reports: Authors (post hoc).
| Studies that compared protocols to published reports | |
| Chan 2004a | Company statistician listed only in the protocol: 10/44 (23%) Only five protocols explicitly identified the author of the protocol, but none of these individuals, all of whom were company employees were listed as authors of the publications or were thanked in the acknowledgments, although one protocol had noted that the 'author of this protocol will be included in the list of authors'. |
Funding
One study compared protocols and reports for information about funding. Chan 2004a found that 50% (22/44) of protocols stated that the sponsor either owned the data or needed to approve the manuscript, but such conditions for publication were not stated in any of the trial reports (Gøtzsche 2006) (Table 4).
4. Differences between protocol and published reports: source of funding.
| Studies that compared protocols to published reports | |
| Chan 2004a | 50% (22/44) protocols stated that the sponsor either owned the data or needed to approve the manuscript, but such conditions for publication were not stated in any of the trial reports. According to the protocols, the sponsor had access to accumulating data during 16 trials, eg, through interim analyses and participation in data and safety monitoring committees. Such access was disclosed in only 1 corresponding trial article. An additional 16 protocols noted that the sponsor had the right to stop the trial at any time, for any reason; this was not noted in any of the trial publications. Constraints on the publication rights were described in 40 (91%) of the protocols, and 22 (50%) noted that the sponsor either owned the data, needed to approve the manuscript, or both. None of the constraints were stated in any of the trial publications. |
Sample size
Four studies compared sample size (Chan 2004a; Chan 2004b; Pich 2003; Soares 2004). In summarising these results, the denominators differ because they are dependent on whether the particular component was mentioned in the publication. In the Chan 2004a study; 18% (11/62) of trials described sample size calculations fully and consistently in both the protocol and the publication, whilst six presented a power calculation in the publication but not in the protocol. In 13% (4/31) the power calculation was based on an outcome other than the one used in the protocol; the value of delta was different in 18% (6/33); the estimated standard deviation was different in 21% (3/14); and there were discrepancies in the power in 21% (7/34) and sample size in 27% (8/30). Publications for 24% (8/34) of trials reported components (delta, outcome measure, estimated event rates, estimated standard deviation, alpha, power) that had not been pre‐specified in the protocol. None of the publications mentioned any amendments to the original sample size calculation. Chan 2004b noted that 36 studies reported a power calculation; two trials used a different outcome from the protocol and one trial introduced a power calculation that had not been in protocol. A priori sample size calculations were performed in 76% (44/58) of the trials in the Soares 2004 study, but this information was given in only 16% (9/58) of the published reports. End points were clearly defined, and errors were prespecified in 76% (44/58) and 74% (43/58) trials, respectively, but only reported in 10% (6/58) of the papers.
In the Pich 2003 study, 45% (64/143) of RCTs had a recruitment rate lower than expected; 27% (39/143) was as expected, and it was higher than expected in 24% (34/143). In one of 143 trials, the recruitment period was not closed, and no information was available for five. (Table 5)
5. Differences between protocol/registry entry and published reports: sample size and sample size calculation.
| Studies that compared protocols to published reports | |
| Chan 2004a | 11/62 trials (18%) described sample size calculations fully and consistently in both the protocol and the publication 4/38 (11%) power calculation based on an outcome other than the one used in the protocol; 6/33 (18%) delta different; 3/14 (21%) estimated SD different; 7/34 (21%) power; 8/30 (27%) sample size; 16/34 (47%) discrepancies in any component of sample size 6 presented power calculation in the publication but not in the protocol 18/34 (53%) unacknowledged discrepancies between protocols and publications were found for sample size calculations Publications for eight trials reported components that had not been pre‐specified in the protocol 30 subsequently recruited a sample size within 10% of the calculated figure from the protocol; 22 trials randomised at least 10% fewer participants than planned as a result of early stopping (n=3), poor recruitment (2), and unspecified reasons (17); and 10 trials randomised at least 10% more participants than planned as a result of lower than anticipated average age (1), a higher than expected recruitment rate (1), and unspecified reasons (8). A calculated sample size was as likely to be reported accurately in the publication if there was a discrepancy with the actual sample size compared with no discrepancy (11/32 v 14/30). None of the publications mentioned any amendments to the original sample size calculation. |
| Chan 2004b | 36 studies reported a power calculation 2/36 (6%) used a different outcome from the protocol 1/36 (3%) introduced a power calculation that had not been in protocol. |
| Pich 2003 | 45% (64/143) recruitment rate was lower than expected; 27% (39/143) was as expected, and in 24% (34/143) was higher than expected. In 1 out of 143 clinical trials (1%) the recruitment period was not closed, and no information was available for 5 out of 143 trials (3%) |
| Soares 2004 | a priori sample size calculations were performed in 44 (76%) trials, but this information was given in only nine of the 58 published papers (16%). End points were clearly defined, and errors were prespecified in 44 (76%) and 43 (74%) trials, respectively, but only reported in six (10%) of the papers. |
| Studies that compared registry entries to published reports | |
| Charles 2009 | 5% (10/215) did not report any sample size calculation 89% (31/35), the data for sample size calculation were given. For 52% (16/35) articles the reporting of the assumptions differed from the design article. (not clear if this is a comparison from trial registry or just other articles) For 96/113 registered articles (85%), an expected sample size was given in the online database and was equal to the target sample size reported in the article in 46/96 of these articles (48%). The relative difference between the registered and reported sample size was greater than 10% in 18 articles (19%) and greater than 20% in five articles (5%). The parameters for the sample size calculation were not stated in the online registration databases for any of the trials. |
Statistical analyses
Four studies compared the statistical analysis plan stated in the protocol with the published report (Chan 2004a; Scharf 2006; Soares 2004; Vedula 2009). In the Chan 2004a study, 99% (69/70) of parallel trials were designed and reported as superiority trials and one trial was stated to be an equivalence trial in the protocol but reported as a superiority trial in the publication, with no explanation given for the change. Unacknowledged discrepancies between protocols and publications were found for methods of handling protocol deviations (44%; 19/43) and missing data (80%; 39/49), primary outcome analyses (60%; 25/42) and adjusted analyses (82%; 23/28). Interim analyses were described in 13 protocols but mentioned in only five (38%) corresponding publications. A further two trials reported interim analyses in the publications, despite the protocol explicitly stating that there would be none. Scharf 2006 found that 27% (6/22) of studies did not identify any criteria adverse effect system and 33.3% (4/12) did not specify an adverse effect evaluation schedule. An intention to treat analysis was used in 83% (48/58) of studies in the Soares 2004 cohort but we need to clarify with the authors if a comparison was made between protocols and published reports. A statistical analysis plan was included in the internal company research report for 60% (12/20) of trials in the Vedula 2009 cohort, but they could not determine the relationship between the date of the statistical analysis plan, the protocol and the research report for 60% (3/5) published trials that had such a plan. Therefore, they could not assess the timing of the observed changes from the protocol‐defined outcomes (Table 6).
6. Differences between protocol and published reports: analysis plan.
| Studies that compared protocols to published reports | |
| Chan 2004a | One trial was stated to be an equivalence trial in the protocol but was reported as a superiority trial in the publication; no explanation was given for the change. 39/49 protocols and 42/43 publications reported the statistical test used to analyse primary outcome measures. The method of handling protocol deviations was described in 37 protocols and 43 publications. The method of handling missing data was described in 16 protocols and 49 publications. Unacknowledged discrepancies between protocols and publications were found for methods of handling protocol deviations (19/43) and missing data (39/49), primary outcome analyses (25/42), subgroup analyses (25/25), and adjusted analyses (23/28). Interim analyses were described in 13 protocols but mentioned in only five corresponding publications. An additional two trials reported interim analyses in the publications, despite the protocol explicitly stating that there would be none. A data monitoring board was described in 12 protocols but in only five of the corresponding publications. |
| Scharf 2006 | 6/22 (27%) did not identify any criteria adverse effects (AE) system 4/12 (33%) not specify AE evaluation schedule |
| Soares 2004 | 40/58 (69%) of these trials used an intention to treat analysis. This number was increased to 48/58 (83%) after verification by the Radiation Therapy Oncology Group. |
| Vedula 2009 | A statistical analysis plan was included in the internal company research report for 5/12 (42%) published trials and for 7/8 (88%) unpublished trials. They were unable to determine the date of the statistical‐analysis plan relative to the protocol and research report for 3/5 (60%) published trials that had such a plan, so they cannot assess the timing of the changes from the protocol‐defined outcomes that we observed. |
Subgroup analyses
Two studies compared subgroup analyses specified in protocols and those included in published reports (Al‐Marzouki 2008; Chan 2004a). Al‐Marzouki 2008 found that only 49% (18/37) of trials mentioned subgroup analysis in the protocols, but 76% (28/37) reported such an analysis in the report of the trial. Among the 51% (19/37) of trials with no prespecified subgroup analyses in the protocol, subgroup analyses were undertaken in 58% (11/19). None gave the reason for these analyses. In the 18 trials in which subgroup analyses were prespecified in the protocol, 61% (11/18) had at least one unreported subgroup analysis or at least one new subgroup analysis. Chan 2004a found that of 13 protocols specifying subgroup analyses, 12 of these 13 trials reported only some, or none, of these in the publication. Nineteen of the trials with published subgroup analyses reported at least one that was not pre‐specified in the protocol and four trials claimed that the subgroup analyses were pre‐specified, even though they did not appear in the protocol (Table 7).
7. Differences between protocol and published reports: subgroup analyses.
| Studies that compared protocols to published reports | |
| Al‐Marzouki 2008 | Only 18/37 trials (49%) mentioned subgroup analysis in the protocols, but 28/37 (76%) reported it. Only one protocol gave the reason for subgroup selection. None specified the total number of subgroups. Among the 19 trials with no prespecified subgroup analyses in the protocol, subgroup analyses were done in 11 (58%). None gave the reason for these analyses. In the 18 trials in which subgroup analyses were prespecified in the protocol, 11 (61%) had at least one unreported subgroup analysis or at least one new subgroup analysis. |
| Chan 2004a | Overall, 25 trials described subgroup analyses in the protocol (n=13) or publication (20). All had discrepancies between the two documents. Twelve of the trials with protocol specified analyses reported only some (n=7) or none (5) in the publication. Nineteen of the trials with published subgroup analyses reported at least one that was not pre‐specified in the protocol. Protocols for 12 of these trials specified no subgroup analyses, whereas seven specified some but not all of the published analyses. Only seven publications explicitly stated whether the analyses were defined a priori; four of these trials claimed that the subgroup analyses were pre‐specified even though they did not appear in the protocol. |
Outcomes
Table 8 includes results for differences in outcomes for six studies (Al‐Marzouki 2008; Chan 2004a; Chan 2004b; Hahn 2002; Vedula 2009; von Elm 2008). Three studies (Chan 2004a; Chan 2004b; Vedula 2009) found that the primary outcome was the same in the protocol as in the publication for 33% (11/21) to 67% (32/48) of RCTs and one study found that it was the same for secondary outcomes in one of 12 trials (Vedula 2009). Four studies (Al‐Marzouki 2008; Chan 2004a; Chan 2004b; Vedula 2009) considered the downgrading of a primary outcome from the protocol to a secondary outcome in the published report, and found that this happened in 5% (2/37) to 34% (26/76) of RCTs. All six studies considered primary outcomes that were included in protocols and omitted from published reports and found that this occurred in between 13% (6/48) and 42% (5/12) of RCTs. One study (Al‐Marzouki 2008) found that secondary outcomes were omitted in 86% (32/37) of the published reports for the RCTs. The studies found that outcomes that had not been included in the protocol were included in the published reports for between 11% (11/101) and 50% (6/12) of RCTs and two studies (Al‐Marzouki 2008; Vedula 2009) found that this occurred in 33% (4/12) and 86% (32/27) of RCTs for secondary outcomes. Three studies considered outcomes that were upgraded from secondary in the protocol to primary in the published report and found that this occurred in between 9% (4/45) and 19% (12/63) of RCTs in two studies (Chan 2004a; Chan 2004b). The third study reported that this occurred for 18% (5/28) of outcomes but did not report this as a proportion of the RCTs (Vedula 2009).
8. Differences between protocol/registry entry and published report: outcomes.
| Study | Outcome stated in the protocol or trial registry is the same as in the published report | Primary outcome stated in the protocol or trial registry is downgraded to secondary in the published report | Outcome stated in the protocol or trial registry is omitted from the published report | A non primary outcome in the protocol or trial registry is changed to primary in the published report | A new outcome that was not stated in the protocol or trial registry (as primary or secondary) is included in the published report | Other information on outcomes |
| Studies that compared protocols to published reports | ||||||
| Al‐Marzouki 2008 | 5% (2/37) | primary: 14% (5/37) secondary: 86% (32/37) |
primary: 22% (8/37) secondary: 86% (32/37) |
|||
| Blumle 2008 | 128/299 No primary outcomes stated in publications | |||||
| Chan 2004a | primary: 47% (36/76) | 34% (26/76) | primary: 26% (20/76) | 19% (12/63) | primary: 17% (11/63) | 71% (70/99) and 60% (43/72) had at least 1 unreported efficacy or harm outcome, respectively 62% (51/82) of trials had major discrepancies in primary outcomes |
| Chan 2004b | primary: 67% (32/48) | 23% (11/48) | primary:13% (6/48) | 9% (4/45) | primary:18% (8/45) | 42/48 (88%): at least 1 unreported efficacy outcome; 16/26 (62%) at least 1 unreported harm outcome; 40% (19/48) of the trials contained major discrepancies in the specification of primary outcomes |
| Hahn 2002 | all outcomes in RCTs: 100% (2/2) (4 outcomes) |
all outcomes in RCTs: 100% (2/2) (10 outcomes) |
40% (6/15) stated which outcome variables were of primary interest | |||
| Vedula 2009 | primary: 33% (4/12) (11/21 outcomes) secondary: 8% (1/12) (55/180 outcomes) |
17% (2/12) (4/21 outcomes) |
primary: 42% (5/12) (6/21 primary outcomes and 122/180 secondary outcomes) |
(5/28 outcomes) |
primary: 50% (6/12) (12/28 outcomes) secondary: 33% (4/12) |
For 67% (8/12) reported trials, the primary outcome defined in the published report differed from that described in the protocol 17% (2/12) failed to distinguish between primary and secondary |
| von Elm 2008 | primary: 26% (24/92) (preliminary results) |
primary: 11% (11/101) (preliminary results) |
||||
| Studies that compared registry entries to published reports | ||||||
| Bourgeois 2010 | primary: 82% (70/85) | |||||
| Charles 2009 | Only compared report to design article | |||||
| Ewart 2009 | Primary:69% (76/110) Secondary:30% (33/110) |
5% (5/110) | Primary: 18% (20/110) Secondary: 44% (48/110) |
3% (3/110) | Primary: 9% (10/110) Secondary:49% (54/110) |
In 31% (34/110), a primary outcome had been changed In 70% (77/110), a secondary outcome had been changed 42% (20/48) of excluded studies did not record a primary outcome, or the outcome recorded was too vague to use in the registry |
| Mathieu 2009 | primary:69% (101/147) | 4% (6/147) | primary: 10% (15/147) | primary:15% (22/147) | 18% (42/234): registered
with no or an unclear description of the primary outcome. 31% (46/147): some evidence of discrepancies between the outcomes registered and the outcomes published 3% (4/147): different timing of assessment |
|
Two studies (Chan 2004a; Chan 2004b) found that statistically significant outcomes had higher odds of being fully reported compared to nonsignificant outcomes (range of odds ratios: 2.4 to 4.7).
Factors associated with discrepancies
Table 9 includes the results for factors associated with differences between protocols and published reports. One study suggested that statistical significance of the results could be associated with differences in the primary outcome between protocols and published reports (Vedula 2009). Three studies found that statistical significance was associated with complete reporting (Chan 2004a; Chan 2004b; von Elm 2008).
9. Factors associated with differences between protocol/registry entry and published reports.
| Study | Statistical significance | Funding | Sample size | Other |
| Studies that compared protocols to published reports | ||||
| Blumle 2008 | No correlation between funding and selective reporting of eligibility criteria could be determined | No correlation between sample size and selective reporting of eligibility criteria could be determined | Study design, multicentre, number of treatment groups | |
| Chan 2004a | Statistically significant outcomes had a higher odds of being fully reported compared with nonsignificant outcomes for both efficacy (pooled odds ratio, 2.4; 95% confidence interval [CI], 1.4‐4.0) and harm (pooled odds ratio, 4.7; 95% CI, 1.8‐12.0) data | Regression coefficient 0.34 SE 0.29, p=0.23 | Regression coefficient ‐0.17 SE 0.11, p=0.11 | Number of study centres (p=0.03) |
| Chan 2004b | Fully versus incompletely reported Efficacy outcomes: OR 2.7 (95% CI 1.5–5.0) Harm outcomes: OR 7.7 (95% CI 0.5–111) |
Prevalence of major discrepancies: Jointly funded 35% (7/20) CIHR funded 43% (12/28) |
Published in a general medical journal; speciality journal; Investigators responded to follow‐up survey | |
| Vedula 2009 | Trials that presented findings that were not significant (P≥0.05) for the protocol‐defined primary outcome in the internal documents either were not reported in full or were reported with a changed primary outcome. The primary outcome was changed in the case of 5/8 published trials for which statistically significant differences favoring gabapentin were reported For 3/4 studies in which the primary outcome was unchanged, statistically significant results were reported. For the remaining study, with nonsignificant findings, the results were published as part of a pooled analysis. For five of the eight studies with a changed primary outcome, statistically significant findings were reported, and four of the five were published as full‐length articles |
|||
| von Elm 2008 | OR 4.1 (95% CI 1.8 to 9.7) for complete reporting (preliminary results) | OR 1.0 (95% CI 0.3 to 4.0) for complete reporting (preliminary results) | This was considered for full publication | Time to event versus other, primary versus secondary, efficacy versus harm |
| Studies that compared registry entries to published reports | ||||
| Bourgeois 2010 | Industry‐funded trials reported positive outcomes in 85.4% of publications, compared with 50.0% for government‐funded trials and 71.9% for nonprofit or nonfederal organization –funded trials (P 0.001). Trials funded by nonprofit or nonfederal sources with industry contributions were also more likely to report positive outcomes than those without industry funding (85.0% vs. 61.2%; P=0.013). Differences in primary outcome reporting was associated with funding source: industry 8.7% (4/46), government 40.0% (4/10), nonprofit/nonfederal 24.1% (7/29) (p=0.03). |
|||
| Charles 2009 | differences between the assumptions and the results were large and small in roughly even proportions, whether the results were significant or not. | The size of the trial and the differences between the assumptions for the control group and the results did not seem to be substantially related (rho=0.03, 95% CI −0.05 to 0.15). | ||
| Ewart 2009 | Although not part of our research question, we noted that there were almost no differences in outcomes when comparing trials funded by pharmaceutical companies with those that had noncommercial sponsorship | |||
| Mathieu 2009 | For the 46 articles with a discrepancy between the registry and the published article, the influence of this discrepancy could be assessed only in half (23/46). Among them, 19 of 23 (82.6%) had a discrepancy that favored statistically significant results (ie, a new, statistically significant primary outcome was introduced in the published article or a nonsignificant primary outcome was omitted or not defined as the primary outcome in the published article). | General medical and speciality journals | ||
In one study, no correlation between funding or sample size and selective reporting of eligibility criteria could be determined (Blumle 2008). Chan 2004a found that a change in the primary outcome was not associated with funding or sample size. Chan 2004b found major discrepancies in 35% (7/20) of jointly funded (industry and the Canadian Institue of Health Research) and 43% (12/28) of CIHR funded RCTs. von Elm 2008 found that funding was not associated with complete reporting.
Comparison of trial registry entries to published reports
Eligibility criteria, methodological information, authors, funding, statistical analyses and subgroup analyses
None of the cohort studies that compared trial registry entries to published reports considered differences in eligibility criteria, methodological information, authors, funding, statistical analyses or subgroup analyses.
Sample size
One study (Charles 2009) compared sample size from trial registry to published report (Table 5) and found that, of 96 trials where an expected sample size was given in the online databse, the sample size was the same in 48% (46/96) of RCTs. Ten of 215 trials (5%) did not report and sample size calculation. They also found that the parameters for the sample size calculation were not included in trial registries.
Outcomes
Table 8 includes results for the three studies that compared differences in outcomes between trial registry entry and published reports (Bourgeois 2010; Ewart 2009; Mathieu 2009). These studies found that the primary outcome was the same in the trial registry as in the publication for 69% (76/110 and 101/147) to 82% (70/85) of RCTs, and one study found it was the same for secondary outcomes in 30% (33/110) of RCTs (Ewart 2009). Two studies (Ewart 2009; Mathieu 2009) considered the downgrading of an outcome that was a primary in the trial registry but which was included as a secondary outcome in the published report, and found that this happened in 4% (6/147) and 5% (5/110) of RCTs. These studies also considered primary outcomes that were included in trial registries and omitted from published reports and found that this occurred in 10% (15/147) and 18% (20/110) of RCTs. One study (Ewart 2009) found that secondary outcomes were omitted in 44% (48/110) of published reports. Both studies also found that outcomes that had not been included in the trial registry were included in the published reports for 9% (10/110) and 15% (22/147) of RCTs, and one study (Ewart 2009) found that this occurred in 49% (54/110) of RCTs for secondary outcomes. Ewart 2009 considered outcomes that were upgraded from secondary in the trial registry to primary in the published report, and found that this occurred in 3% (3/110) of RCTs.
Factors associated with discrepancies
Table 9 includes the results for factors associated with differences between registry entries and published reports. Two studies considered statistical significance; one found that the size of the trial and the differences between the assumptions for the control group and the results did not seem to be substantially related (rho=0.03, 95% confidence interval: −0.05 to 0.15) (Charles 2009). Another study found that 83% (19/23) had a discrepancy that favoured statistically significant results (ie, a new, statistically significant primary outcome was introduced in the published article or a nonsignificant primary outcome was omitted or not defined as the primary outcome in the published article) (Mathieu 2009). Two studies investigated funding; one study found that industry funding was associated with reporting of positive outcomes for the new drug (Bourgeois 2010) and another study found that there was no difference in outcomes in industry and non‐industry funded trials (Ewart 2009). Charles 2009 found that the size of the trial and the differences between the assumptions for the control group and the results did not seem to be substantially related.
Explanation of discrepancies
Twelve studies did not comment on the reasons for discrepancies (Shapiro 2000; Scharf 2006; Pich 2003; Hahn 2002; Gandhi 2005; Ewart 2009; Charles 2009; von Elm 2008; Vedula 2009; Mathieu 2009; Soares 2004; Bourgeois 2010). Two studies stated that no reasons for discrepancies were given in any of the trial reports within the cohort (Al‐Marzouki 2008; Blumle 2008). Two studies sent questionnaires to trialists to determine reasons for discrepancies. Chan 2004b found that among 78 trials with any unreported outcomes (efficacy or harm or both) they received 24 survey responses (31%) that provided reasons for not reporting outcomes for efficacy (23 trials) or harm (ten trials) in their published articles. The most common reasons for not reporting efficacy outcomes were lack of statistical significance (7/23 trials), journal space restriction (7/23) and lack of clinical importance (7/23). Similar reasons were provided for harm data. Chan 2004a found that the most common reason given by 29 investigators for not reporting efficacy outcomes included a lack of clinical importance (18 trials) and a lack of statistical significance (13 trials). These two reasons were also provided by five of 11 survey respondents for harm outcomes. Investigators for three of six studies with unreported primary outcomes provided reasons for omission: to be submitted for future publication (two trials) and not relevant for published article (one trial).
Discussion
Summary of main results
The results for the comparisons of published reports with both trial registries and protocols indicate that there are often discrepancies between the plans for a trial and what is eventually published, for many aspects of RCTs. Explanations for these are not stated in the published reports. The majority of research has focused on discrepancies in outcomes and its association with statistical significance.
Sixteen studies were included in this Cochrane methodology review, with 12 comparing protocols to published reports and four comparing trial registry entries to published reports. Three studies focused on discrepancies in eligibility criteria; two focused on methods of randomisation, allocation concealment and blinding; one focused on authors; two focused on funding; six focused on sample size and sample size calculation; five focused on the analysis plan and nine focused on outcomes.
This review shows that there are many different discrepancies between protocols and trial registry entries and the subsequent published reports. However, we have not identified any study that has reported a comparison of all three sources; protocols, trial registries and published reports in the same cohort of RCTs but we know of one ongoing study that is investigating this (Chan 2010). This is important, in part because it will identify whether information in trial registries is updated when protocol amendments are made, and whether reasons are included to justify these changes.
The full statistical analysis plan is often not included in the protocol and unless this information is obtained from the trialist it would be difficult to tell if any changes had been made to it. Several studies found that there were discrepancies between what was written about the statistical analyses in the protocol or trial registry entry and what was in the published report.
The SPIRIT initiative (Chan 2008b) will produce guidelines to standardise protocols, which could have an impact on the information to be included in trial registries. Trial registration should be enforced, and should include all 20 recommended items from the WHO minimum data set (WHO 2006) and allow changes to be documented with reasons and dates for these changes. However, Moja 2009 found that compliance of information in trial registries is unsatisfactory and largely incomplete even though many agree that transparency is paramount (see Implications for systematic reviews and evaluations of healthcare for further information on this study). The studies that have compared trial registries to published reports are more recent and have been facilitated by the ICMJE requirements in 2004 that trials would have to be registered before they commenced if researchers wanted to publish in their journals (De Angelis 2004).
The updated CONSORT statement now advises (in item 3b) that important changes to methods after trial commencement (such as eligibility criteria) should be included in the published report along with the reasons for these changes. Furthermore, item 6b in the CONSORT statement advises that any changes to trial outcomes after the trial commenced, and the reasons for these changes, should be included. No other items state that changes to other aspects, for example statistical analysis, should be reported. However, CONSORT urges completeness, clarity, and transparency of reporting, which simply reflects the actual trial design and conduct (Schulz 2010). CONSORT 2010 also now requires authors to include details of trial registration in the abstract of a randomized trial (Schulz 2010).
Overall completeness and applicability of evidence
Although not every included cohort study investigated all aspects of RCTs, between the 16 included studies identified, all aspects listed in the protocol of this review have been considered. These studies have been conducted in different countries and cover a wide variety of RCTs, and despite the included studies being heterogeneous they have broadly similar conclusions in that there are often discrepancies between protocols or trial registry entries and published reports.
Quality of the evidence
The majority of included cohort studies had a low risk of bias for follow up. However, the authors of some cohort studies were not given permission by authors of some included studies to access their protocols, which raises the issue of whether discrepancies may differ in these RCTs. Some cohort studies excluded RCTs that were not registered or those where a primary outcome was not explicitly identified or registered. Again, in such instances, it would be impossible to know if there were any changes made between protocol or trial registry and the published report and discrepancies may be more or less prevalent in these cases.
Although many of the included cohort studies were deemed at low risk of bias for selective reporting as the outcomes stated in the methods section were fully reported, there are many outcomes that could have been measured and were not, which is a missed opportunity. For example, only one included study addressed authorship and only two addressed methods such as allocation concealment. Authors have been contacted to check that all comparisons have been reported.
Limitations
There are limitations to this review. For example, eight studies are still awaiting assessment and should contribute more information to the body of evidence when this review is updated. There were also problems in combining studies to provide overall summary estimates and so the results of the studies had to be discussed narratively.
Potential biases in the review process
No potential biases have been identified during the review process.
Agreements and disagreements with other studies or reviews
In a previous review (Dwan 2008), publication bias and outcome reporting bias were considered and it was found that statistically significant outcomes were more likely to be fully reported. That review also identified discrepancies in the primary outcome between the protocol and published report for five included cohort studies. This Cochrane methodology review has updated that information and shows that discrepancies in outcomes occur frequently, with no explanation of the changes in the published reports.
Other studies, which were not eligible for this review have compared information submitted to the Food and Drug Administration and regulatory agencies to published reports and have also identified discrepancies (Bardy 1998; Melander 2003; Rising 2008; Turner 2008). These studies were excluded because they did not compare protocols or trial registry entries to published reports. Information submitted to the Food and Drug Administration and regulatory agencies may also differ to protocols and trial registry entries although we know of no study that has considered this.
Authors' conclusions
Implication for methodological research.
It would be of interest to see what effect the differences between protocol and registry entries and subsequent reports might have on the conclusions presented in trial reports and the impact this has on the decisions people make after reading those reports. Few of the authors of the cohort studies in this review asked the original trialists for reasons for discrepancies (Chan 2004a; Chan 2004b). One study, which is awaiting classification, found that trialists seemed generally unaware of the implications for the evidence base of not reporting all outcomes and protocol changes (Smyth 2010). Future work might also involve looking at a cohort of systematic reviews, and contacting the authors of included RCTs to obtain the protocols for these studies, to see if there are any discrepancies and to examine how this impacts on the conclusions of the reviews.
Acknowledgements
The authors would like to thank Sarah Chapman and Natalie Yates for their help with the search strategy, Mike Clarke for his valuable input and the referees for their helpful comments which improved the review.
Appendices
Appendix 1. Search Strategy
OvidSP MEDLINE (1950 to August 2010)
1 Clinical Protocols/
2 protocol$.ti,ab.
3 regist$.ti,ab.
4 Registries/
5 or/1‐4
6 Randomized Controlled Trials as Topic/
7 clinical trials as topic/
8 rct.ti,ab.
9 rcts.ti,ab.
10 (randomized or randomised).ti,ab.
11 trial$.ti,ab.
12 or/6‐11
13 "Bias (Epidemiology)"/
14 publication bias/
15 (unreported or "incompletely reported" or "partially reported" or "fully reported" or "not reported" or "non‐report$" or missing or omission or omit$ or "not publish$").ti,ab.
16 ((selectiv$ or suppress$ or non$ or bias$) adj5 (report$ or publish$ or publication$)).ti,ab.
17 or/13‐16
18 (discrepan$ adj5 (protocol$ or regist$)).ti,ab.
19 (compar$ adj8 publication$ adj8 protocol$).ti,ab.
20 (compar$ adj8 protocol$ adj8 publication$).ti,ab.
21 (publication$ adj8 protocol$ adj8 compar$).ti,ab.
22 (publication$ adj8 compar$ adj8 protocol$).ti,ab.
23 (protocol$ adj8 publication$ adj8 compar$).ti,ab.
24 (protocol$ adj8 compar$ adj8 publication$).ti,ab.
25 (compar$ adj8 publication$ adj8 regist$).ti,ab.
26 (compar$ adj8 regist$ adj8 publication$).ti,ab.
27 (publication$ adj8 regist$ adj8 compar$).ti,ab.
28 (publication$ adj8 compar$ adj8 regist$).ti,ab.
29 (regist$ adj8 publication$ adj8 compar$).ti,ab.
30 (regist$ adj8 compar$ adj8 publication$).ti,ab.
31 or/18‐30
32 5 and 12 and 17
33 12 and 31
34 32 or 33
35 cochrane database of systematic reviews.jn.
36 34 not 35
OVIDSP EMBASE (1980 to August 2010)
1 Clinical Protocols/
2 protocol$.ti,ab.
3 regist$.ti,ab.
4 Register/
5 or/1‐4
6 randomized controlled trial/
7 clinical trial/
8 rct.ti,ab.
9 rcts.ti,ab.
10 (randomized or randomised).ti,ab.
11 trial$.ti,ab.
12 or/6‐11
13 publishing/
14 (unreported or "incompletely reported" or "partially reported" or "fully reported" or "not reported" or "non‐report$" or missing or omission or omit$ or "not publish$").ti,ab.
15 ((selectiv$ or suppress$ or non$ or bias$) adj5 (report$ or publish$ or publication$)).ti,ab.
16 or/13‐15
17 (discrepan$ adj5 (protocol$ or regist$).ti,ab.
18 (compar$ adj8 publication$ adj8 protocol$).ti,ab.
19 (compar$ adj8 protocol$ adj8 publication$).ti,ab.
20 (publication$ adj8 protocol$ adj8 compar$).ti,ab.
21 (publication$ adj8 compar$ adj8 protocol$).ti,ab.
22 (protocol$ adj8 publication$ adj8 compar$).ti,ab.
23 (protocol$ adj8 compar$ adj8 publication$).ti,ab.
24 (compar$ adj8 publication$ adj8 regist$).ti,ab.
25 (compar$ adj8 regist$ adj8 publication$).ti,ab.
26 (publication$ adj8 regist$ adj8 compar$).ti,ab.
27 (publication$ adj8 compar$ adj8 regist$).ti,ab.
28 (regist$ adj8 publication$ adj8 compar$).ti,ab.
29 (regist$ adj8 compar$ adj8 publication$).ti,ab
30 or/17‐29
31 5 and 12 and 16
32 12 and 30
33 31 or 32
34 “cochrane database of systematic reviews”.jn.
35 “Cochrane database of systematic reviews (online)”.jn.
36 34 or 35
37 33 not 36
Cochrane Methodology Register Issue 3 2010 (Wiley InterScience (Online))
#1 (protocol* OR regist*):ti in Methods Studies
#2 (protocol* OR regist*):ab in Methods Studies
#3 #1 OR #2
#4 (randomised OR randomized OR rct OR rcts OR trial*):ti in Methods Studies
#5 (randomised OR randomized OR rct OR rcts OR trial*):ab in Methods Studies
#6 #4 OR #5
#7 "bias in trials":kw in Methods Studies
#8 ("study identification" next "publication bias"):kw in Methods Studies
#9 (unreported OR "incompletely reported" OR "partially reported" OR "fully reported" OR "not reported" OR "non reported" OR "non‐reported" OR "non reporting" OR "nonreporting" OR missing OR omission OR "not published" OR "not publishing"):ti in Methods Studies
#10 (unreported OR "incompletely reported" OR "partially reported" OR "fully reported" OR "not reported" OR "non reported" OR "non‐reported" OR "non reporting" OR "nonreporting" OR missing OR omission OR "not published" OR "not publishing"):ab in Methods Studies
#11 omit*:ti in Methods Studies
#12 omit*:ab in Methods Studies
#13 ((selectiv* OR suppress* OR non* OR bias*) NEAR/5 (report* OR publish* OR publication*)):ti in Methods Studies
#14 ((selectiv* OR suppress* OR non* OR bias*) NEAR/5 (report* OR publish* OR publication*)):ab in Methods Studies
#15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#16 (discrepan* NEAR/5 (protocol* OR regist*)):ti in Methods Studies
#17 (discrepan* NEAR/5 (protocol* OR regist*)):ab in Methods Studies
#18 (compar* NEAR/8 publication* NEAR/8 protocol*):ti in Methods Studies
#19 (compar* NEAR/8 publication* NEAR/8 protocol*):ab in Methods Studies
#20 (compar* NEAR/8 publication* NEAR/8 regist*):ti in Methods Studies
#21 (compar* NEAR/8 publication* NEAR/8 regist*):ab in Methods Studies
#22 #16 OR #17 OR #18 OR #19 OR #20 OR #21
#23 #3 AND #6 AND #15
#24 #6 AND #22
#25 #23 OR #24
Web of Science (1900 to August 2010)
#1 TS=protocol*
#2 TS=registr*
#3 #1 OR #2
#4 TS=Randomi?ed Controlled Trials
#5 TS=rct*
#6 #4 OR #5
#7 TS="Bias (Epidemiology)"
#8 TS=publication bias
#9 TS=(unreported or "incompletely reported" or "partially reported" or "fully reported" or "not reported" or "non‐report*" or missing or omission or omit* or "not publish*")
#10 TS=((selective* or suppress* or non* or bias*) adj5 (report* or publish* or publication*))
#11 #7 OR #8 OR #9 OR #10
#12 TS=(discrepan* SAME (protocol* or registr*))
#13 TS=(compare* SAME publication* SAME protocol*)
#14 TS=(compare* SAME protocol* SAME publication*)
#15 TS=(publication* SAME protocol* SAME compar*)
#16 TS=(publication* SAME compar* SAME protocol*)
#17 TS=(protocol* SAME publication* SAME compar*)
#18 TS=(protocol* SAME compar* SAME publication*)
#19 TS=(compar* SAME publication* SAME registr*)
#20 TS=(compare* SAME registr* SAME publication*)
#21 TS=(publication* SAME registr* SAME compar*)
#22 TS=(publication* SAME compar* SAME registr*)
#23 TS=(registr* SAME publication* SAME compar*)
#24 TS=(registr* SAME compar* SAME publication*)
#25 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24
#26 #3 AND #6 AND #11
#27 #11 AND #25
#28 #26 OR #27
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Marzouki 2008.
| Methods | Checked consistency between protocols that had been peer reviewed and accepted for publication in the Lancet as of June 2007 and published reports. Investigators were contacted and databases searched to identify published reports | |
| Data | 71 RCTs; permission to use 64; 37 published (50 reports) | |
| Comparisons | Comparison of protocols accepted by the Lancet to published reports | |
| Outcomes | Subgroup analysis; outcomes | |
| Notes | Protocol definition: summary published on the Lancet's website (need to check with author if they had access to the full protocol) Published reports: published reports |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | Permission was only given to use 64/71 protocols (10% loss to follow up) |
| Selective reporting? | Yes | All outcomes mentioned were reported |
Blumle 2008.
| Methods | The protocols of clinical research projects submitted to the research ethics committee of the University of Freiburg (Germany) in 2000 were analysed. Several databases were searched and investigators contacted for published reports (Published between 2000 and 2006). | |
| Data | Completed RCT protocols: 103/225 Published RCTs: 54/103 Analysed RCTs: 52 with 78 publications |
|
| Comparisons | Comparison of protocols to published reports | |
| Outcomes | Study characteristics, including, study design; single/multicentre status; national/international study; sample size; length of enrolment; source of funding; number of prespecified primary outcomes and eligibility criteria | |
| Notes | Protocol definition: submitted study protocols, amendments, progress reports and related correspondence (eg, committee decisions) Published reports: articles published in scientific journals that provide adequate information on at least the objectives of the study as well as on its methods and results. Conference abstracts and review articles were excluded. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | 52/54 published RCTs were compared (4% loss to follow up) |
| Selective reporting? | No | Abstract and poster only available for data on comparisons. Factors not fully reported. |
Bourgeois 2010.
| Methods | An observational study of safety and efficacy trials for anticholesteremics, antidepressants, antipsychotics, proton‐pump inhibitors, and vasodilators conducted between 2000 and 2006. The objective was to describe characteristics of drug trials listed in ClinicalTrials.gov and examine whether the funding source of these trials is associated with favourable published outcomes. Published reports were found by searching online databases, an online results registry and reports available through company websites, contacing investigators or pharmaceutical companies up to 2010. |
|
| Data | 362/546 trials published | |
| Comparisons | Comparison of trial registry entries to published reports | |
| Outcomes | Primary outcome, funding source. Detailed information on study conduct and quality were not assessed. |
|
| Notes | Trial registry: clinicaltrials.gov Published reports: If more than 1 publication was identified, they chose the publication that most closely fit the study description in the record |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Unclear | Not all published trials seem to be compared to the registry entires (being checked with the author) |
| Selective reporting? | Yes | Data on primary outcome is included in published report. |
Chan 2004a.
| Methods | In February 2003, protocols and protocol amendments were identified for randomized trials by reviewing paper files
from clinical studies approved by the Scientific‐Ethical Committees for Copenhagen and Frederiksberg, Denmark, in 1994‐1995. The objective was to study empirically the extent and nature of outcome reporting bias. Trials with at least 1 identified journal article were included in our study cohort. Publication in journals was identified by contacting trialists and by searching MEDLINE, EMBASE, and the Cochrane Controlled Trials Register using investigator names and keywords (final search, May 2003). Study protocols and any amendments and all published articles were reviewed. Data from amendments took precedence over data from earlier protocols. |
|
| Data | RCTs: 102/102 (100%) 122 published articles |
|
| Comparisons | Comparison of protocol to published report | |
| Outcomes | Number and characteristics of reported and unreported trial efficacy and harm outcomes; Statistically significant versus non‐significant outcomes; Consistency of primary and secondary outcomes; outcome in power calculation; sample size calculations and statistical methods; subgroups; blinding, allocation concealment, sequence generation; ghost authorship and prevalence and nature of constraints such that those that exist on the academic freedom of clinical investigators in industry‐initiated randomised trials. | |
| Notes | Protocol definition: includes amendments (7 trials submitted amendments regarding outcomes but none of the published articles for these trials mentioned that an amendment had been made to the study protocol) Published reports: all published articles reporting final results |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | All 102 published RCTs were followed up. |
| Selective reporting? | Yes | All outcomes mentioned in methods sections were reported. |
Chan 2004b.
| Methods | To determine whether outcome reporting bias would be present in a cohort of government (Canadian Institutes of Health Research (Canada)) funded trials subjected to rigorous peer review between 1990‐1998. Databases were searched and principal investigators were surveyed for published reports and information on unreported outcomes. |
|
| Data | RCTs approved for funding: 105 Published RCTs: 48 with 68 publications |
|
| Comparisons | Comparison of protocols to pubished reports | |
| Outcomes | Number and characteristics of reported and unreported trial efficacy and harm outcomes; statistically significant versus non‐significant outcomes; consistency of primary outcomes; outcome in power calculation | |
| Notes | Protocol: Protocol and amendments submitted to CIHR. None
of the publications stated that an amendment had been made to the protocol. Published reports: Any journal article that reported final results was included. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | All published RCTs followed up. There were 57 RCTs not completed or published, most were confirmed through a negative literature search and survey of authors (52) and from negative literature search alone (5) |
| Selective reporting? | Yes | All outcomes mentioned in published report were reported. |
Charles 2009.
| Methods | To assess quality of reporting of sample size calculation, ascertain accuracy of calculations, and determine the relevance of assumptions made when calculating sample size in randomised controlled trials. MEDLINE was searched for all primary reports of two arm parallel group randomised controlled trials of superiority with a single primary outcome published in six high impact factor general medical journals (New England Journal of Medicine, Journal of the American Medical Association (JAMA), The Lancet, Annals of Internal Medicine, BMJ, and PLoS Medicine) between 1 January 2005 and 31 December 2006. All extra material related to design of trials (other articles, online material, online trial registration) was systematically assessed |
|
| Data | 215 RCTs selected 113/215 registered |
|
| Comparisons | Comparison of trial registry to published reports | |
| Outcomes | Target sample size; parameters for sample size calculation. | |
| Notes | Trial registry: Clinicaltrials.gov (77%), controlled‐trials.com (20%), another database (3%) Published reports: The first report that presented the results for the primary outcome was selected. Follow‐up studies were excluded. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | Only 113/215 registered (47% loss to follow up) |
| Selective reporting? | Yes | Outcomes stated in the published report were all reported and this was confirmed by the author. |
Ewart 2009.
| Methods | To investigate the frequency of undisclosed changes in the outcomes of RCTs between trial registration and publication of RCTs published in consecutive issues of 5 major medical journals (Annals of internal medicine, BMJ, JAMA, The Lancet, NEJM) during a 6‐month period. Articles were excluded if they did not have an available trial registry entry, did not have analyzable outcomes, or were secondary publications. | |
| Data | 158 reports of RCTs were reviewed 110 included in the analysis. |
|
| Comparisons | Comparison of trial registry to published report | |
| Outcomes | Primary outcome, secondary outcome Outcomes were counted as unchanged if the authors acknowledged the change and made any statement indicating that the changes were made before any analyses were done. Secondary outcomes were counted as unchanged if the authors said they would be published separately. |
|
| Notes | Trial registries: Australian clinical trials registry (2), clinical trials.gov (112), European clinical trials database (1), International standard randomised controlled trial (30), ntional research register (1), Registration database not listed (11). Archives of the trials registries were not searched to see whether the outcomes had been changed since registration. Rather, they were taken on the day registries were searched. Published reports: If there were multiple reports from the same trial, what appeared to be the main one was used. If multiple reports considered different outcomes of the same study, they were either combined and considered together as one study or considered as separate studies depending on the circumstances. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | Trials were excluded if there was no publicly accessible trial registration recorded in the report; the registration database did not record a primary outcome or the outcome recorded was too vague to make any judgments; and the trial publication was not the main report of the trial results. |
| Selective reporting? | No | Trials were excluded if the registration database did not record a primary outcome or the outcome recorded was too vague to make any judgments; and the trial publication was not the main report of the trial results. This will therefore underestimate selective reporting within this cohort. |
Gandhi 2005.
| Methods | To assess both the impact on generalizability and the disclosure rate of enrollment criteria for 32 major NIH‐funded HIV RCTs in the AIDS Clinical Trial Group (ACTG) and Community Programs for Clinical Research on AIDS (CPCRA) trial networks published 1994‐2004. Access to protocols available through contact with study leaders. | |
| Data | 32 RCTs | |
| Comparisons | Comparison of protocols to published reports | |
| Outcomes | Eligibility criteria | |
| Notes | Protocol definition: full and updated Published reports: journal publications |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | All RCTs conducted were followed up. |
| Selective reporting? | Yes | All outcomes mentioned in methods section were reported |
Hahn 2002.
| Methods | To examine the extent of within‐study selective reporting in clinical research from protocols initiated in 1994 from a Local Research Ethics Committee (UK). Follow up in 1999. Lead researchers for each study from a complete cohort of 56 applications were contacted, asking for their permission to obtain a copy of the original approved LREC submission and for information on the current status of the study. For projects that had been published, a copy of, or reference for, all articles were requested. Co‐researchers were contacted in the absence of a reply from, or at the request of, the lead researcher. |
|
| Data | Applications: 56 Published (15): RCTs 2/15 (13%), non RCTs 2 (13%), uncontrolled trials 2 (13%), case control 1 (7%), surveys 2 (13%), cohort and case control 1 (7%), method evaluation studies 5 (34%) |
|
| Comparisons | Comparison of protocol to published report | |
| Outcomes | Funding source; outcomes; analysis and sample size | |
| Notes | Protocol definition: original approved LREC submission Published reports: all articles |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | 37/40 who replied from 56 applications gave permission for protocols to be used. (34% loss to follow up) |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. However, data were not available separately for RCTs to include in this review for fuding, sample size and analysis plan. |
Mathieu 2009.
| Methods | To assess the proportion of trials registered with results recently published in journals with high impact factors. MEDLINE via PubMed was searched for reports of RCTs in 3 medical areas (cardiology, rheumatology, and gastroenterology) indexed in 2008 in the 10 general medical journals and specialty journals with the highest impact factors (follow up March 2009). Authors were contacted to ask about registration details. If no reply was received, trial registries were searched including the World Health Organization search portal. Studies were excluded if they were registered after the end of the study. |
|
| Data | 323 articles, 147 registered trials | |
| Comparisons | Comparison of trial registry to published report | |
| Outcomes | Primary outcomes and timing of assessment of outcomes. significant versus non‐significant outcomes. (If none was explicitly reported, they used the outcome stated in the sample size estimation. If none was explicitly identified in the text or in the sample size calculation, the article was excluded) |
|
| Notes | Trial register: registered before the end of the trial, with the primary outcome clearly specified. To take into account the amendments and possible changes by the data provider that could occur any time after the initial registration, when feasible, they checked all changes in the protocol that were available using a specific function on the trial registry site. Published reports: all reports of RCTs assessing treatments in 3 medical specialties |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | Studies were excluded if no primary outcome was explicitly identified |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. |
Pich 2003.
| Methods | The objective of this survey was to assess the outcome of all protocols submitted to the Hospital Clinic ethics committee during 1997. Principal investigators were sent a standard questionnaire and when necessary, sponsors, contract research organisations (CROs), or both were also interviewed. |
|
| Data | 158 approved RCT protocols 143/158 RCTs assessed 11/158 never started 4/158 no data available 123 RCTs finished |
|
| Comparisons | Comparison of protocols to published reports | |
| Outcomes | Sample size | |
| Notes | Protocol definition: All information included in the HCEC clinical trials’ database about protocols submitted in 1997 Published reports: completed studies ‐ asked author for data on published studies only (26/123) |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | 143 RCTs assessed |
| Selective reporting? | Unclear | Unclear if any other comparisons were made between protocol and published reports. |
Scharf 2006.
| Methods | The National Cancer Institute (NCI) Clinical Data Update System (CDUS) was searched for studies that used the Common Toxicity Criteria version 2.0 and for which a final study publication was available. They examined whether the published adverse effect data differ from those in the sponsor’s database and from the data collection requirements stated in study protocols which were active between 1998‐2003. | |
| Data | 355 studies identified 213/355 were single‐agent chemotherapeutic studies 24 RCTs published 2 excluded as one only included a subset of patients in the article and one because of adverse effect (AE) reporting that was not in a format translatable to allow comparison with CDUS AE data |
|
| Comparisons | Comparison of protocols to published reports | |
| Outcomes | Adverse effects collection and reporting methods | |
| Notes | Protocol definition: not stated Published reports: published articles in peer reviewed journals |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | All 22 RCTs followed up |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. |
Shapiro 2000.
| Methods | Trials that served as the subject of a single study Clinical Alert for which the journal article was published between January 1988 and September 1994. Clinical Alerts that were based upon the results of several studies were excluded. Eligibility criteria was compared as a test case for the reporting of clinical trial methods. The corresponding author listed on the final journal article was contacted for a full‐text copy of the clinical trial protocol. |
|
| Data | 8 RCTs | |
| Comparisons | Comparison of protocol to published reports | |
| Outcomes | Eligibility criteria; definition of disease; precision; safety; legal and ethical; administrative | |
| Notes | Protocol definition: original obtained from author Published reports: methods paper (if applicable), journal article, and Clinical Alert |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | Protocols and published reports were obtained for all 8 RCTs |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. |
Soares 2004.
| Methods | To determine whether poor reporting of methods in randomised controlled trials reflects on poor methods from the content of reports compared with the design features described in the protocols for all randomised controlled trials from conducted by the Radiation therapy oncology group (RTOG) since its establishment in 1968. | |
| Data | 59 terminated RCTs 56/59 published RCTs (with 58 publications) |
|
| Comparisons | Comparison of protocol to published report | |
| Outcomes | Primary outcomes; allocation concealment; ITT; sample size; alpha and beta | |
| Notes | Protocol definition: original protocols including revisions Published reports: all papers |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | 56 published RCTs followed up |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. |
Vedula 2009.
| Methods | Reporting practices for trials of gabapentin funded by Pfizer and Warner‐Lambert’s subsidiary, Parke‐Davis were examined for off‐label indications (prophylaxis against migraine and treatment of bipolar disorders, neuropathic pain, and nociceptive pain), comparing internal company documents with published reports. | |
| Data | 19/21 were RCTs (2 open label uncontrolled trials); 11 RCTs and 1 open labelled uncontrolled trial published report and protocol available |
|
| Comparisons | Comparison of protocol to published report | |
| Outcomes | Primary and secondary outcomes; P values (all listed outcomes were counted as primary outcomes if no distinction was made between primary and secondary outcomes). Statistical significance Since certain outcomes, such as quality of life, were described separately from primary and secondary outcomes in the protocol, they were counted separately; pharmacokinetic outcomes were not counted. Methodologic quality of the included trials as described in the publications was not systematically assessed. |
|
| Notes | Protocol definition: protocol amendments and the statistical analysis plan was part of the protocol when they were in the main body of the protocol or the appendices (6/12 had amendments). Published reports: For each trial, one published report was selected as the main study report, using the following order of priority: a full‐length study report in a standalone article, a letter to the editor that reported study results, a nonsystematic review with pooled analysis using results from the included trial, and a conference abstract. |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | No | 12/21 published and with protocols available (not clear if the 12 were RCTs) (43% loss to follow up) |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported. |
von Elm 2008.
| Methods | To study trial outcomes specified in protocols of RCTs on drug interventions submitted to the University of Berne/ CH ethics committee (Switzerland) from 1988‐1998 and reported in subsequent full publications and to estimate publication rate and to investigate factors that are associated with complete reporting (e.g. statistical significance, funding). Full articles published up to 2006 were identified by searching the Cochrane CENTRAL database (issue 02/2006) and by contacting investigators. Trial registries and the internet were searched to determine the status of studies when no other information had been located. |
|
| Data | Total: RCTs 451/1698 (27%) Protocols: 233 (52%) published with 375 corresponding articles |
|
| Comparisons | Comparison of protocols to published reports | |
| Outcomes | Publication rate, study characteristics (study design, sample size, source of funding) associated with publication | |
| Notes | Protocol definition: submitted study protocols, amendments and related correspondence including committee decisions and communications on conduct and completion of studies. Published reports:Publications were included if they reported results from an eligible study |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Follow up? | Yes | 233 protocols available and all had published reports |
| Selective reporting? | Yes | All outcomes stated in the methods section were reported ‐ preliminary data in abstract only and data have not been analysed for sample size and funding but data was extracted. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barcena 2005 | No comparison of registry entries or protocols to published reports |
| Bardy 1998 | No comparison of registry entries or protocols to published reports |
| Berlin 2005 | Narrative description of selective reporting, publication bias and clinical trial registration. No comparison of registry entries or protocols to published reports |
| Chan 2005 | No comparison of registry entries or protocols to published reports |
| Cooper 1997 | No comparison of registry entries or protocols to published reports |
| Cronin 2004 | No comparison of registry entries or protocols to published reports |
| Decullier 2005 | No comparison of registry entries or protocols to published reports |
| Decullier 2006 | No comparison of registry entries or protocols to published reports |
| Decullier 2007 | No comparison of registry entries or protocols to published reports |
| Dickersin 1992 | No comparison of registry entries or protocols to published reports (we have emailed author to confirm and are waiting for their response) |
| Dickersin 1993 | No comparison of registry entries or protocols to published reports (we have emailed author to confirm and are waiting for response) |
| Djulbegovic 2008 | No comparison of registry entries or protocols to published reports |
| Easterbrook 1991 | No comparison of registry entries or protocols to published reports (we have emailed to confirm with author and are waiting for response) |
| Habibzadeh 2006 | No comparison of registry entries or protocols to published reports |
| Haidich 2001 | No comparison of registry entries or protocols to published reports |
| Hall 2007 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Ioannidis 1998 | No comparison of registry entries or protocols to published reports |
| Lee 1998 | Survey on trial registration. No comparison of registry entries or protocols to published reports |
| Liebeskind 1998 | No comparison of registry entries or protocols to published reports |
| Liu 2008 | No comparison of registry entries or protocols to published reports. Only registry information considered (we have emailed author to confirm and are waiting on response) |
| Melander 2003 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Menzel 2007 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Nurbhai 2005 | No comparison of registry entries or protocols to published reports. Only information in trial registry considered. |
| Psaty 2008 | No comparison of registry entries or protocols to published reports |
| Ramsey 2008 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Rasmussen 2009 | No comparison of registry entries or protocols to published reports. Only registration status considered (confirmed by email with author) |
| Rising 2008 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Ross 2009 | No comparison of registry entries or protocols to published reports |
| Simes 1986 | No comparison of registry entries or protocols to published reports |
| Stern 1997 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
| Turner 2008 | No comparison of registry entries or protocols to published reports (confirmed by email with author) |
Characteristics of studies awaiting assessment [ordered by study ID]
Chappell 2005.
| Methods | A survey of randomized controlled trials published in BJOG: An International Journal of Obstetrics and Gynaecology between 2001 and 2004. To measure the degree to which changes in design between protocol and publication may compromise the validity of randomized controlled trial results. |
| Data | 53 randomized trials were identified. Nine authors could not be contacted, and the 44 who responded supplied protocols for 30 trials. Three were not in English and 1 was for the wrong trial, leaving 26 for analysis |
| Comparisons | Comparison of protocols to publications |
| Outcomes | Discrepancies in the primary outcome; intervention; sample size; analysis method in the protocol compared to the published report |
| Notes | Definition of protocol not stated |
Djulbegovic 2009.
| Methods | To establish whether reporting of methods in haematological malignancies RCTs conducted by the NCI cooperative groups (CGs) between 1955 and 2000, which conducts all publicly sponsored RCTs in cancer in the US, reflect the actual methodological quality. |
| Data | 4 CGs under the ageis of NCI conducted 120 hematological malignancies RCTs enrolling 37, 845 patients |
| Comparisons | Comparison of protocols to published reports |
| Outcomes | Methodological quality, expected effect size, sample size calculations, alpha and beta error. |
| Notes | Definition of protocol was not stated |
Djulbegovic 2010.
| Methods | All consecutive phase III RCTs conducted between 1955 and 2000 by three NCI sponsored Cooperative Groups were reviewed |
| Data | 261 RCTs |
| Comparisons | Comparison of protocols to published reports |
| Outcomes | Primary outcome; sample size |
| Notes | Definition of protocol was not stated |
Ghersi 2006.
| Methods | To identify discrepancies in the identity and definition of the primary outcome and to investigate factors associated with the completeness of reporting of the primary outcome from protocols submitted to the CSAHS Ethics review committee (Australia) between 1992‐1996 and their associated published reports. |
| Data | 103 published RCTs from 318 protocols considered. |
| Comparisons | Comparison of protocols to published reports |
| Outcomes | Selective reporting encompassed i) discrepancy in the identity of the primary outcome; ii) discrepancy in the definition of the primary outcome; iii) completeness of reporting of the primary outcome. Protocol related variables that may impact on the outcomes were explored using logistic regression. |
| Notes | The term "protocol" is used as a collective term for the protocol as well as any other documentation submitted to the REC, including protocol amendments. |
Jureidini 2008.
| Methods | To expose selective reporting in study 329 paroxetine in adolescents sponsored by GlaxoSmithKline that would not be apparent without access to documents that only emerged through litigation |
| Data | 1 RCT |
| Comparisons | Comparison of company documents to published reports |
| Outcomes | Outcomes |
| Notes | Original and amended protocols were looked at. The paper is based only on publically available documents |
Mhaskar 2009.
| Methods | An assessment in a cohort of RCTs conducted by Southwest Oncology Group (SWOG) between 1960 and 2003 |
| Data | 117 RCTs with matching protocols available for 105 RCTs involving 129 comparisons (n=58,908 patients) |
| Comparisons | Comparison of protocols to published reports |
| Outcomes | Elements addressing assessment of harms |
| Notes | Definition of protocol not stated |
Smyth 2010.
| Methods | Interviews with trialists |
| Data | 21 RCTs |
| Comparisons | Comparison of trial protocols to published reports |
| Outcomes | Frequency and reasons for outcome reporting bias in clinical trials |
| Notes |
You 2010.
| Methods | Oncology RCTs published from 2005 to 2009, to assess the consistency of analysis and reporting of the primary endpoint from registration to publication |
| Data | 346 RCTs |
| Comparisons | Comparison of registry entries to published reports |
| Outcomes | Discrepancies in primary endpoints and methodology |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Chan 2010.
| Trial name or title | |
| Methods | |
| Data | |
| Comparisons | Comparison of protocols to trial registries and publications |
| Outcomes | |
| Starting date | |
| Contact information | An‐Wen Chan |
| Notes |
McKenzie 2010.
| Trial name or title | Reporting of randomised controlled trials submitted to the Otago ethics committee (New Zealand) |
| Methods | Reviewed all ethics applications submitted to a New Zealand Regional Ethics committee (Otago Ethics Committee) between 1998 and 2002 to assess whether they were RCTs. Publications reporting results of the RCTs were then retrieved by contacting trialists and by searching OVID MEDLINE and the Cochrane Central Register of Controlled trials |
| Data | |
| Comparisons | Comparisons of protocols to publications |
| Outcomes | |
| Starting date | |
| Contact information | Joanne Mckenzie, School of Public Health and Preventive Medicine, Monash University, Australia. |
| Notes |
Rasmussen 2010.
| Trial name or title | |
| Methods | |
| Data | |
| Comparisons | Comparison of trial registry entries to publications |
| Outcomes | |
| Starting date | |
| Contact information | Nicolas Rasmussen, University of New South Wales, Sydney, Australia |
| Notes |
Urrutia 2010.
| Trial name or title | |
| Methods | |
| Data | |
| Comparisons | Comparison of protocols to published reports |
| Outcomes | |
| Starting date | |
| Contact information | Gerard Urrutia, Iberoamerican Cochrane Center |
| Notes |
Differences between protocol and review
The protocol stated that MB would complete the data extraction checks but this was completed by LC.
10 November 2010:
New Authors added: Michaela Blundell and Carrol Gamble.
Rebecca Smyth removed.
Kerry Dwan made Contact Author.
Contributions of authors
All authors were involved in the development of the protocol. KD and MB screened the results from the search strategy to decide which studies would be included. KD and LC extracted data from included studies. KD and PRW carried out the analysis. KD prepared the initial draft for the full review and all other authors commented on it.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, England, UK.
Department of Health Cochrane Review Incentive Scheme 2009
Declarations of interest
Two of the authors of this review (PRW and DGA) are co‐authors of three studies included in the review (Hahn 2002; Chan 2004a; Chan 2004b).
New
References
References to studies included in this review
Al‐Marzouki 2008 {published data only}
- Al‐Marzouki S, Roberts I, Evans S, Marshall T. Selective reporting in clinical trials: analysis of trial protocols accepted by The Lancet. Lancet 2008;372(9634):201. [DOI] [PubMed] [Google Scholar]
Blumle 2008 {published data only}
- Blumle A, Antes G, Schumacher M, Just H, Elm E. Clinical research projects at a German medical faculty: follow‐up from ethical approval to publication and citation by others. Journal of Medical Ethics 2008;34:e20. [DOI] [PubMed] [Google Scholar]
- Blumle A, Meerpohl JJ, Antes G, Elm E. Reporting of eligibility criteria of participants in randomized trials: comparison between study protocols and journal articles [abstract]. 17th Cochrane Colloquium. Singapore, 2009:49‐50.
Bourgeois 2010 {published data only}
- Bourgeois FT, Murthy S, Mandl KD. Outcome Reporting Among Drug Trials Registered in ClinicalTrials.gov. Annals of Internal Medicine 2010;153:158‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2004a {published data only}
- Chan A‐W, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. Journal of the American Medical Association 2004a;291:2457‐65. [DOI] [PubMed] [Google Scholar]
- Chan AW, Altman D. Discrepancies between protocols and publications: evidence of outcome reporting bias in randomised trials [abstract]. XI Cochrane Colloquium: Evidence, Health Care and Culture. Barcelona, Spain, 2003:9.
- Chan AW, Hrobjartsson A, Jørgensen KJ, Gøtzsche PC, Altman DG. Discrepancies in sample size calculations and data analysesreported in randomised trials: comparison of publications with protocols. British Medical Journal 2008;337:a2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Hrobjartsson A, Tendal B, Gotzsche P, Altman D. Pre‐specifying sample size calculations and statistical analyses in randomised trials: comparison of protocols to publications [abstract]. XIII Cochrane Colloquium. Melbourne, Australia, 2005:166.
- Gotzsche P, Hrobjartsson A, Hrobjartsonn A, Haahr M. Constraints on academic freedom in industry‐initiated clinical trials [abstract]. XIII Cochrane Colloquium. Melbourne, Australia, 2005:43.
- Gøtzsche PC, Hrobjartsson A, Johansen KJ, Haahr MT, Altman DG, Chan AW. Constraints on Publication Rights in Industry‐Initiated Clinical Trials. Journal of the American Medical Association 2006;295(14):1645‐6. [DOI] [PubMed] [Google Scholar]
- Gøtzsche PC, Hro´ bjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Ghost Authorship in Industry‐Initiated Randomised Trials. PLoS MEDICINE 2007;4(1):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrobjartsson A, Chan AW, Haahr MT, Gotzsche PC, Altman DG. Selective reporting of positive outcomes in randomised trials ‐ secondary publication. A comparison of protocols with published reports. Ugeskrift for Laeger 2005;167(34):3189‐3191. [PubMed] [Google Scholar]
- Hrobjartsson A, Pildal J, Chan A‐W, Haarh MT, Altman DG, Gotzsche PC. Comparisons of descriptions of blinding in trial protocols and the published reports [abstract]. 16th Cochrane Colloquium: Evidence in the era of globalisation. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen. Freiburg, Germany, 2008; Vol. 102:Suppl VI: 8.
- Hrobjartsson A, Pildal J, Chan AW, Haahr MT, Altman DG, Gøtzsche PC. Reporting on blinding in trial protocols and corresponding publications was often inadequate but rarely contradictory. Journal of Clinical Epidemiology 2009;62(967):e973. [DOI] [PubMed] [Google Scholar]
- Pildal J, Chan AW, Hróbjartsson A, Forfang E, Altman DG, Gøtzsche PC. Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. British Medical Journal 2005;330:1049‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2004b {published data only}
- Chan A‐W, Krleza‐Jeri K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. Canadian Medical Association Journal 2004;171:735‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Krleza‐Jeric K, Schmid I, Altman D. Outcome reporting bias in government‐funded RCTs (comments). Canadian Medical Association Journal 2005;172(7):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Krleza‐Jeric K, Schmid I, Altman D. Selective reporting of results in government‐funded randomised trials [abstract]. 12th Cochrane Colloquium: Bridging the Gaps. Ottawa, Ontario, Canada, 2004:94‐95.
Charles 2009 {published data only}
- Charles P, Giraudeau B, Dechartres A, Baron G, Ravaud P. Reporting of sample size calculation in randomised controlled trials: review. British Medical Journal 2009;338:b1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ewart 2009 {published data only}
- Ewart R, Lausen H, Millian N. Undisclosed changes in outcomes in randomized controlled trials: an observational study. Annals of Family Medicine 2009;7(6):542‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandhi 2005 {published data only}
- Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, Gange SJ, Anastos K, Holman S, Levine A, Greenblatt RM. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS 2005;19(16):1885‐96. [DOI] [PubMed] [Google Scholar]
Hahn 2002 {published data only}
- Hahn S, Williamson PR, Hutton JL. Investigation of within‐study selective reporting in clinical research: follow‐up of applications submitted to a local research ethics committee. Journal of Evaluations of Clinical Practice 2002;8:353‐9. [DOI] [PubMed] [Google Scholar]
Mathieu 2009 {published data only}
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. Journal of theAMA 2009;302(9):977‐84. [DOI] [PubMed] [Google Scholar]
Pich 2003 {published data only}
- Pich J, Carne X, Arnaiz JA, Gomez B, Trilla A, Rodes J. Role of a research ethics committee in follow‐up and publication of results. Lancet 2003;361:1015‐6. [DOI] [PubMed] [Google Scholar]
Scharf 2006 {published data only}
- Scharf O, Colevas AD. Adverse event reporting in publications compared with sponsor database for cancer clinical trials. Journal of Clinical Oncology 2006;24(24):3933‐3938. [DOI] [PubMed] [Google Scholar]
Shapiro 2000 {published data only}
- Shapiro SH, Weijer C, Freedman B. Reporting the study populations of clinical trials: Clear transmission or static on the line?. Journal of Clinical Epidemiology 2000;53:973‐979. [DOI] [PubMed] [Google Scholar]
Soares 2004 {published data only}
- Soares HP, Daniels S, Kumar A, Clarke M, Scott C, Swann S, Djulbegovic B. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. British Medical Journal 2004;328:22‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedula 2009 {published data only}
- Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry‐sponsored trials of gabapentin for off‐label use. New England Journal of Medicine 2009;361(20):1963‐71. [DOI] [PubMed] [Google Scholar]
- Vedula SS, Dickersin K. Reporting of trials of gabapentin. New England Journal of Medicine 2010;362(17):1641‐2. [DOI] [PubMed] [Google Scholar]
von Elm 2008 {published data only}
- Elm E, Rollin A, Blumle A, Senessie C, Low N, Egger M. Selective reporting of outcomes of drug trials? Comparison of study protocols and published articles [abstract]. XIV Cochrane Colloquium. Dublin, Ireland, 2006:47.
- Elm E, Röllin A, Blümle A, Huwiler K, Witschi M, Egger M. Publication and non‐publication of clinical trials: longitudinal study of applications submitted to a research ethics committee. Swiss Medical Weekly 2008;138:197‐203. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barcena 2005 {published data only}
- Barcena L, Pengel L, Morris PJ. Registry of randomized controlled trials in transplantation. Transplantation 2005;80(11):1525‐34. [DOI] [PubMed] [Google Scholar]
Bardy 1998 {published data only}
- Bardy AH. Bias in reporting clinical trials. British Journal of Clinical Pharmacology 1998;46:147‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 2005 {published data only}
- Berlin JA, Wacholtz MC. Selective reporting, publication bias and clinical trial registry: an industry perspective. International Journal of Pharmaceutical Medicine 2005;19(5‐6):277‐284. [Google Scholar]
Chan 2005 {published data only}
- Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. British Medical Journal 2005;330(7494):753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1997 {published data only}
- Cooper H, DeNeve K, Charlton K. Finding the missing science: the fate ofstudies submitted for review by a human subjects committee. Psychological Methods 1997;2(4):447‐452. [Google Scholar]
Cronin 2004 {published data only}
- Cronin E, Sheldon T. Factors influencing the publication of health research. International Journal of Technology Assessment in Health Care 2004;20:351‐5. [DOI] [PubMed] [Google Scholar]
Decullier 2005 {published data only}
- Decullier E, Lheritier V, Chapuis F. Fate of biomedical research protocolsand publication bias in France: retrospective cohort study. British Medical Journal 2005;331:19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decullier 2006 {published data only}
- Decullier E, Chapuis F. Impact of funding on biomedical research: aretrospective cohort study. BMC public health 2006;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decullier 2007 {published data only}
- Decullier E, Chapuis F. Oral presentation bias: a retrospective cohort study. Journal of Epidemiology and Community Health 2007;61:190‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickersin 1992 {published data only}
- Dickersin K, Min YI, Meinert CL. Factors influencing publication of research results: follow up of applications submitted to two institutional review boards. Journal of the American Medical Association 1992;267:374‐8. [PubMed] [Google Scholar]
Dickersin 1993 {published data only}
- Dickersin K, Min YI. NIH clinical trials and publication bias. Online Journal of Current Clinical Trials 1993;Doc No. 50(53). [PubMed] [Google Scholar]
Djulbegovic 2008 {published data only}
- Djulbegovic B, Kumar A, Soares H, Hozo I, Bepler G, Clarke M, Bennett C. New cancer treatment successes identified in phase 3 randomised controlled trials conducted by the national cancer institute‐sponsored cooperative oncology groups. Archives of Internal Medicine 2008;168(6):632‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Easterbrook 1991 {published data only}
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias inclinical research. Lancet 1991;337:867‐72. [DOI] [PubMed] [Google Scholar]
Habibzadeh 2006 {published data only}
- Habibzadeh F. Impact of mandatory registration of clinical trials on small medical journals: scenario on emerging bias. Croatian Medical Journal 2006;47(1):181‐183. [PMC free article] [PubMed] [Google Scholar]
Haidich 2001 {published data only}
- Haidich AB, Ioannidis JP. Effect of early patient enrolment on the time to completion and publication of randomized controlled trials. American Journal of Epidemiology 2001;154:873‐80. [DOI] [PubMed] [Google Scholar]
Hall 2007 {published data only}
- Hall R, Antueno C, Webber A. Publication bias in the medical literature: A review by a Canadian Research Ethics Board. Canadian Journal of Anesthesia 2007;54(5):380‐388. [DOI] [PubMed] [Google Scholar]
Ioannidis 1998 {published data only}
- Ioannidis JPA. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. Journal of the American Medical Association 1998;279:281‐6. [DOI] [PubMed] [Google Scholar]
Lee 1998 {published data only}
- Lee HK, Lim KH, Park JH, Park KM, Kim HJ, Kim MY, Lee YS, Kim CJ, Chang JS, Shin SG. A survey of industrial perspectives on the Central Pharmaceutical Affairs Council's review of clinical trial protocols and study reports. Journal of Korean Society for Clinical Pharmacology & Therapeutics 1998;6(1):83‐100. [Google Scholar]
Liebeskind 1998 {published data only}
- Liebeskind DS, Kidwell CS, Saver JL. Empiric evidence of publication bias affecting acute stroke clinical trials. Stroke 1999;30(1):92. [DOI] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu XM, Li YP, Wu TX, Liu GJ, Li J. [A survey of the status of funding of registered Chinese trials]. Chinese Journal of Evidence‐based Medicine 2008;8(5):305‐311. [Google Scholar]
Melander 2003 {published data only}
- Melander H, Ahlqvist‐Rastad, Meijer G, Beermann B. Evidence b(i)ased medicine – selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. British Medical Journal 2003;326:1171‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzel 2007 {published data only}
- Menzel S, Uebing B, Hucklenbroich P, Schober O. Evaluation of clinicaltrials following an approval from a research ethics committee. Deutsche Medizinische Wochenschrift 2007;132(44):2313‐7. [DOI] [PubMed] [Google Scholar]
Nurbhai 2005 {published data only}
- Nurbhai M, Grimshaw J, Moja PL, Liberati A, Chan AW, Dickersin K, Krezla‐Jeric K, Moher D. Assessing the quality of information recorded on trial registries [abstract]. XIII Cochrane Colloquium. Melbourne, Australia, 2005:165.
Psaty 2008 {published data only}
- Psaty BM, Kronmal RA. Reporting mortality findings in trials of rofecoxib for alzheimer disease or cognitive impairment: a case study based on documents from rofecoxib litigation. Journal of the American Medical Association 2008;299(15):1813‐1817. [DOI] [PubMed] [Google Scholar]
Ramsey 2008 {published data only}
- Ramsey S, Scoggins J. Commentary: practicing on the tip of an information iceberg? Evidence of underpublication of registered clinical trials in oncology. Oncologist 2008;13(9):925‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2009 {published data only}
- Rasmussen N, Lee K, Bero L. Association of trial registration with the results and conclusions of published trials of new oncology drugs. Trials 2009;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rising 2008 {published data only}
- Rising K, Bacchetti, Bero L. Reporting bias in drug trials submitted to the food and drug administration: review of publication and presentation. PLoS Medicine 2008;5(11):e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2009 {published data only}
- Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross‐sectional analysis. PLoS Medicine 2009;6(9):e1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simes 1986 {published data only}
- Simes RJ. Publication bias: the case for an international registry of clinical trials. Journal of Clinical Oncology 1986;4(10):1529‐1541. [DOI] [PubMed] [Google Scholar]
Stern 1997 {published data only}
- Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. British Medical Journal 1997;315(7109):640‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2008 {published data only}
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358(3):252‐60. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chappell 2005 {published data only}
- Chappell L, Alfirevich Z, Chien P, Jarvis S, Thornton JG. A comparison of the published version of randomized controlled trials in a specialist clinical journal with the original trial protocols [abstract]. International Congress on Peer Review and Biomedical Publication. Chicago, Illinois, USA, 2005:27.
Djulbegovic 2009 {published data only}
- Djulbegovic B, Kumar A, Magazin A, Soares HP. Quality of randomized controlled trials (RCTS) in hematological malignancies: What was reported versus what was done. Haematologica Conference: 14th Congress of the European Hematology Association. Berlin, Germany, 2009.
Djulbegovic 2010 {published data only}
- Djulbegovic B, Kumar A, Magazin A, Schroen AT, Soares H, Hozo I, Clarke M, Sargent DJ, Schell MJ. Optimisim bias leads to inconclusive results ‐ an empirical study. Journal of Clinical Epidemiology 2010 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghersi 2006 {unpublished data only}
- Ghersi D. Issues in the design, conduct and reporting of clinical trials that impact on the quality of decision making. School of Public Health, Faculty of Medicine. University of Sydney 2006.
- Ghersi D, Clarke M, Simes J. Selective reporting of the primary outcomes of clinical trials: a follow‐up study [abstract]. XIV Cochrane Colloquium. Dublin, Ireland, 2006.
Jureidini 2008 {published data only}
- Jureidini JN, McHenry LB, Mansfield PR. Clinical trials and drug promotion: Selective reporting of study 329. International Journal of Risk & Safety in Medicine 2008;20:73‐81. [Google Scholar]
Mhaskar 2009 {published data only}
- Mhaskar R, Kumar A, Soares H, Gardner B, Djulbegovic B. Treatment related harms: what was planned and what was reported? An analysis of Southwest Oncology Group phase III trials [abstract]. 17th Cochrane Colloquium. Singapore, 2009:49.
Smyth 2010 {published data only}
- Smyth RMD, Jacoby A, Altman DG, Gamble C, Kirkham JJ, Williamson PR. Frequency and reasons for outcome reporting bias in clinical trials: interviews with trialists. BMJ 2010 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
You 2010 {published data only}
References to ongoing studies
Chan 2010 {unpublished data only}
- personal communication.
McKenzie 2010 {unpublished data only}
- personal communication.
Rasmussen 2010 {unpublished data only}
- personal communication.
Urrutia 2010 {unpublished data only}
- personal communication.
Additional references
Chan 2004a
- Chan A‐W, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. Journal of the American Medical Association 2004;291:2457‐65. [DOI] [PubMed] [Google Scholar]
Chan 2008a
- Chan AW. Bias, spin and misreporting: time for full access to trial protocols and results. PLoS Medicine 2008;5(11):e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2008b
- Chan A, Tetzlaff J, Altman DG, Gotzsche P, Hrobjartsson A, Krleza‐Jeric K, Laupacis A, Moher D. The SPIRIT initiative: defining standard protocol items for randomised trials. 16th Cochrane Colloquium, Freiburg, Germany (3‐7 October, 2008). German Journal for Evidence and Quality in Health Care. 2008; Vol. 102(Suppl VI):S27.
Chan 2008c
- Chan A‐W, Hrobjartsson A, Jorgensen KJ, Gotzsche PC, Altman DG. Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols. British Medical Journal 2008;337:a2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2004
- Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet 2004;364:911‐2. [DOI] [PubMed] [Google Scholar]
Dickersin 1987
- Dickersin K, Chan S, Chalmers TC, Sacks HS, Smith H Jr. Publication bias and clinical trials. Controlled Clinical Trials 1987;8:343‐53. [DOI] [PubMed] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan A‐W, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 2008;3:e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghersi 2009
- Ghersi D, Pang T. From Mexico to Mali: four years in thehistory of clinical trial registration. Journal of Evidence Based Medicine 2009;2(1):1‐7. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2006
- Gøtzsche PC, Hrobjartsson A, Johansen KJ, Haahr MT, Altman DG, Chan AW. Constraints on Publication Rights in Industry‐Initiated Clinical Trials. Journal of the American Medical Association 2006;295(14):1645‐6. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2007
- Gøtzsche PC, Hrobjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Ghost Authorship in Industry‐Initiated Randomised Trials. PLoS Medicine 2007;4(1):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2000
- Hahn S, Williamson PR, Hutton JL, Garner P, Flynn EV. Assessing the potential for bias in meta‐analysis due to selective reporting of subgroup analyses within studies. Statistics in Medicine 2000;19:3325‐36. [DOI] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrobjartsson 2009
- Hrobjartsson A, Pildal J, Chan AW, Haahr MT, Altman DG, Gøtzsche PC. Reporting on blinding in trial protocols and corresponding publications was often inadequate but rarely contradictory. Journal of Clinical Epidemiology 2009;62(967):e973. [DOI] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Applied Statistics 2000;49:359‐70. [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth RMD, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. British Medical Journal 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Moja 2009
- Moja LP, Moschetti I, Nurbhai M, Compagnoni A, Liberati A, Grimshaw JM, Chan AW, Dickersin K, Krleza‐Jeric K, Moher D, Sim I, Volmink J. Compliance of clinical trial registries with the World Health Organization minimum data set: a survey. Trials 2009;10(56). [DOI] [PMC free article] [PubMed] [Google Scholar]
Pildal 2005
- Pildal J, Chan AW, Hróbjartsson A, Forfang E, Altman DG, Gøtzsche PC. Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. British Medical Journal 2005;330:1049‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simes 1986
- Simes RJ. Publication bias: the case for an international registry of clinical trials. Journal of Clinical Oncology 1986;4:1529‐41. [DOI] [PubMed] [Google Scholar]
Tannock 1996
- Tannock IF. False‐positive results in clinical trials: multiple significance tests and the problem of unreported comparisons. Journal of the National Cancer Institute 1996;88:206‐7. [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — Reporting of subgroup analyses in clinical trials. New England Journal of Medicine 2007;357(21):2189‐2194. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. World Health Organization international clinical trials registry platform. New standards for registration of human medical research. http://www.who.int/mediacentre/news/releases/2006/pr25/en/ (Accessed 27 October 2010) 2006.
Williamson 2005a
- Williamson PR, Gamble C. Identification and impact of outcome selection bias in meta‐analysis. Statistics in Medicine 2005;24:1547‐61. [DOI] [PubMed] [Google Scholar]
Williamson 2005b
- Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta‐analysis. Statistical Methods in Medical Research 2005;14:515‐24. [DOI] [PubMed] [Google Scholar]


