Abstract
Background
This is an update of a Cochrane Review published in 2014. Chronic non‐specific low back pain (LBP) has become one of the main causes of disability in the adult population around the world. Although therapeutic ultrasound is not recommended in recent clinical guidelines, it is frequently used by physiotherapists in the treatment of chronic LBP.
Objectives
The objective of this review was to determine the effectiveness of therapeutic ultrasound in the management of chronic non‐specific LBP. A secondary objective was to determine the most effective dosage and intensity of therapeutic ultrasound for chronic LBP.
Search methods
We performed electronic searches in CENTRAL, MEDLINE, Embase, CINAHL, PEDro, Index to Chiropractic Literature, and two trials registers to 7 January 2020. We checked the reference lists of eligible studies and relevant systematic reviews and performed forward citation searching.
Selection criteria
We included randomised controlled trials (RCTs) on therapeutic ultrasound for chronic non‐specific LBP. We compared ultrasound (either alone or in combination with another treatment) with placebo or other interventions for chronic LBP.
Data collection and analysis
Two review authors independently assessed the risk of bias of each trial and extracted the data. We performed a meta‐analysis when sufficient clinical and statistical homogeneity existed. We determined the certainty of the evidence for each comparison using the GRADE approach.
Main results
We included 10 RCTs involving a total of 1025 participants with chronic LBP. The included studies were carried out in secondary care settings in Turkey, Iran, Saudi Arabia, Croatia, the UK, and the USA, and most applied therapeutic ultrasound in addition to another treatment, for six to 18 treatment sessions. The risk of bias was unclear in most studies. Eight studies (80%) had unclear or high risk of selection bias; no studies blinded care providers to the intervention; and only five studies (50%) blinded participants. There was a risk of selective reporting in eight studies (80%), and no studies adequately assessed compliance with the intervention.
There was very low‐certainty evidence (downgraded for imprecision, inconsistency, and limitations in design) of little to no difference between therapeutic ultrasound and placebo for short‐term pain improvement (mean difference (MD) −7.12, 95% confidence interval (CI) −17.99 to 3.75; n = 121, 3 RCTs; 0‐to‐100‐point visual analogue scale (VAS)). There was also moderate‐certainty evidence (downgraded for imprecision) of little to no difference in the number of participants achieving a 30% reduction in pain in the short term (risk ratio 1.08, 95% CI 0.81 to 1.44; n = 225, 1 RCT). There was low‐certainty evidence (downgraded for imprecision and limitations in design) that therapeutic ultrasound has a small effect on back‐specific function compared with placebo in the short term (standardised mean difference −0.29, 95% CI −0.51 to −0.07 (MD −1.07, 95% CI −1.89 to −0.26; Roland Morris Disability Questionnaire); n = 325; 4 RCTs), but this effect does not appear to be clinically important. There was moderate‐certainty evidence (downgraded for imprecision) of little to no difference between therapeutic ultrasound and placebo on well‐being (MD −2.71, 95% CI −9.85 to 4.44; n = 267, 2 RCTs; general health subscale of the 36‐item Short Form Health Survey (SF‐36)). Two studies (n = 486) reported on overall improvement and satisfaction between groups, and both reported little to no difference between groups (low‐certainty evidence, downgraded for serious imprecision). One study (n = 225) reported on adverse events and did not identify any adverse events related to the intervention (low‐certainty evidence, downgraded for serious imprecision). No study reported on disability for this comparison.
We do not know whether therapeutic ultrasound in addition to exercise results in better outcomes than exercise alone because the certainty of the evidence for all outcomes was very low (downgraded for imprecision and serious limitations in design). The estimate effect for pain was in favour of the ultrasound plus exercise group (MD −21.1, 95% CI −27.6 to −14.5; n = 70, 2 RCTs; 0‐to‐100‐point VAS) at short term. Regarding back‐specific function (MD − 0.41, 95% CI −3.14 to 2.32; n = 79, 2 RCTs; Oswestry Disability Questionnaire) and well‐being (MD −2.50, 95% CI −9.53 to 4.53; n = 79, 2 RCTs; general health subscale of the SF‐36), there was little to no difference between groups at short term. No studies reported on the number of participants achieving a 30% reduction in pain, patient satisfaction, disability, or adverse events for this comparison.
Authors' conclusions
The evidence from this systematic review is uncertain regarding the effect of therapeutic ultrasound on pain in individuals with chronic non‐specific LBP. Whilst there is some evidence that therapeutic ultrasound may have a small effect on improving low back function in the short term compared to placebo, the certainty of evidence is very low. The true effect is likely to be substantially different. There are few high‐quality randomised trials, and the available trials were very small. The current evidence does not support the use of therapeutic ultrasound in the management of chronic LBP.
Plain language summary
Ultrasound therapy for chronic low back pain
Background It is common for people to feel pain in their lower back. When the cause of pain is unknown, we say that the pain is ‘non‐specific’. Pain that lasts for more than three months is considered to be 'chronic'. Chronic non‐specific low back pain can be disabling. It can cause people to miss work. Often, people with chronic non‐specific back pain seek medical care. Ultrasound therapy is the use of sound waves (vibrations) to treat medical problems. It is commonly used to treat low back pain. A healthcare provider rubs a hand‐held machine against the skin on the lower back. The machine produces vibrations that go through the skin. The aim is to deliver heat and energy to body parts under the skin, to reduce pain and speed up recovery. This Cochrane Review aimed to find out whether ultrasound is effective for treating chronic non‐specific low back pain, and whether it causes any unwanted effects. Specifically, we wanted to know if ultrasound affected the following outcomes: pain, people feeling restricted in their daily life by pain, satisfaction with the treatment, well‐being, disability, and other unwanted effects. What did we look for? We looked for studies published up to January 2020 that: • were randomised controlled trials, medical studies where people are randomly put into one of two or more treatment groups. This type of study provides the most reliable evidence about whether a treatment makes a difference; • included people with chronic non‐specific low back pain who were aged 18 years or older; • compared ultrasound (either alone or with another treatment) with a placebo (fake treatment) or other treatments for chronic non‐specific low back pain. What did we find? We found 10 studies that included a total of 1025 people treated for chronic non‐specific low back pain. Most people in the studies had mild to moderate back pain, which means they may have found daily activities painful. They were treated in outpatient hospital departments or clinics, where they typically had six to 18 sessions of ultrasound therapy. Study participants were then followed for a period of time after the treatment (usually a few days or weeks). Studies compared ultrasound to one or more of the following: placebo (five studies), no treatment (one study), electrical pulses (one study), manipulation of the spine (one study), osteopathy (one study), and laser therapy (one study). Three studies compared ultrasound with exercise to exercise alone. None of the studies was commercially funded. Key results There is little to suggest that ultrasound is an effective treatment for people with non‐specific chronic low back pain. Ultrasound compared with placebo We do not know whether ultrasound reduces average pain intensity because this has been studied in too few people, in studies that gave varying answers and were poorly conducted. Ultrasound probably makes little or no difference to the number of people in whom pain is reduced by 30% or more in the short term (i.e. less than three months after the start of the study). Ultrasound probably makes little or no difference to people’s well‐being. It may make little or no difference to how much people feel restricted by their back pain in daily life, or to how satisfied people are with their treatment. Ultrasound may have little or no impact on unwanted effects. We do not know whether ultrasound affects disability since no studies investigated this. Ultrasound with exercise compared with exercise alone We do not know whether ultrasound affects the outcomes of interest in this review because either no studies investigated them, or because the studies that did were imprecise or poorly conducted. Certainty of the evidence Based on the studies we found, there was mostly low‐ to very low‐certainty evidence that ultrasound makes little or no difference to pain and well‐being compared to placebo. For all the other outcomes and comparisons, we are less confident in the results we reported. This is because studies were too imprecise or were poorly conducted.
Summary of findings
Background
Description of the condition
Low back pain (LBP) is the most frequent self‐reported type of musculoskeletal pain. It is often recurrent and has important socioeconomic consequences. Estimates of the prevalence of LBP vary considerably between studies and reach 33% for point prevalence, 65% for one‐year prevalence, and 84% for lifetime prevalence (Henschke 2015). Chronic non‐specific LBP and its resulting disability have become an enormous health and socioeconomic problem (Maher 2017).
Low back pain is defined as pain and discomfort in the lumbosacral region, below the last rib and above the gluteal crease. According to the recommended diagnostic triage, three types of LBP can be defined: 1) non‐specific LBP; 2) LBP with nerve root symptoms; and 3) LBP resulting from serious pathology (e.g. malignancy, fracture, ankylosing spondylitis). Non‐specific LBP, in which there is no recognised patho‐anatomical cause, is usually a benign, self‐limiting condition. Using the traditional classification system, LBP is also categorised according to its duration as acute (shorter than six weeks), subacute (six to 12 weeks), and chronic (longer than 12 weeks) (Krismer 2007; Waddell 2004).
The main objectives of treatment for LBP are for the patient to return to their desired level of activity and participation and to prevent chronic complaints and recurrences (Bekkering 2003). The fact that there are many types of treatment for LBP, each of which has multiple subcategories, is testament that no single approach has been able to demonstrate its superiority (Haldeman 2008). The evidence shows that the effectiveness of some interventions is supported (e.g. exercise) (Saragiotto 2016), whilst other interventions are not effective for LBP (e.g. traction) (Gay 2001; Wegner 2013). This situation makes it very challenging for clinicians, policymakers, insurers, and patients to make decisions regarding which treatment is the most appropriate for chronic LBP.
Description of the intervention
Ultrasound is commonly used for musculoskeletal disorders by health professionals such as physiotherapists, osteopaths, chiropractors, and sports therapists. However, the effectiveness of ultrasound for musculoskeletal problems remains controversial. Previous systematic reviews on the effects of ultrasound therapy for different musculoskeletal disorders found that there are few studies on this topic and that there is a dearth of evidence regarding its usefulness in the treatment of shoulder disorders, degenerative rheumatic disorders, and myofascial pain (Chou 2017). The evidence to determine the effectiveness and safety of ultrasound in low back pain is insufficient (Qaseem 2017).
Therapeutic ultrasound is proposed to deliver energy to deep tissue sites through ultrasonic waves, to produce increases in tissue temperature or non‐thermal physiologic changes (Allen 2006). Unlike ultrasound for medical imaging (which transmits ultrasonic waves and processes a returning echo to generate an image), therapeutic ultrasound is a one‐way energy delivery which uses a crystal sound head to transmit acoustic waves at 1 or 3 MHz and at amplitude densities between 0.1 W/cm² and 3 W/cm² (Allen 2006; Robertson 2006).
Therapeutic ultrasound can be delivered in two modes, continuous or pulsed. Continuous ultrasound involves the delivery of non‐stop ultrasonic waves throughout the treatment period, whilst in pulsed ultrasound the delivery is intermittently interrupted (Robertson 2006). Continuous ultrasound is traditionally used for its thermal effects. Pulsed ultrasound is thought to minimise the thermal effects; however, it is not possible to truly isolate the thermal and non‐thermal effects, as both effects occur with ultrasound application (Robertson 2006).
How the intervention might work
Ultrasound refers to vibrations that are essentially the same as sound waves but of a higher frequency, beyond the range of human hearing. Therapeutic ultrasound is assumed to have thermal and mechanical effects on the target tissue, which result in an increased local metabolism, circulation, extensibility of connective tissue, and tissue regeneration (Robertson 2006).
When acoustic energy is absorbed as it penetrates soft tissues, it causes molecules to vibrate under repeated cycles of compression waves and rarefaction waves. The higher the intensity of the ultrasonic beam and the more continuous the emission of acoustic waves, the more vigorous the molecular vibration or kinetic energy. The more vigorous the microfriction, the more frictional heat is generated in the tissue (Dyson 1976). Tissue heating is presumed to enhance tissue cell metabolism, which in turn is believed to promote soft‐tissue healing. Tissue heating is clearly of value in numerous clinical conditions, through mechanisms of pain relief and improving tissue flexibility, but the evidence does not fully support the use of ultrasound as an efficient thermal intervention (Watson 2008).
Historically, ultrasound has been widely employed for its thermal effects, but it has been argued more recently that the 'non‐thermal' effects of this energy form are more effective (Watson 2008). The physical mechanisms thought to be involved in producing these non‐thermal effects include cavitation and acoustic streaming (micromassage). Cavitation is triggered by the absorption of acoustic energy and begins when minute gas pockets that infiltrate most biological fluids develop into microscopic bubbles, thus causing cavities in these fluids and the surrounding soft tissues. Under the sustained influence of acoustic radiation, these microscopic bubbles expand and contract (pulsate or oscillate) at the same carrier frequency at which the acoustic waves are produced. Microstreaming is the minute flow of fluid in the vicinity of the pulsating bubbles and is triggered by stable cavitation. These two phenomena are proposed to cause increased cell permeability and to affect the course of cell growth, which in turn can improve tissue healing (O'Brien 2007).
Why it is important to do this review
There remains little evidence to support the use of most passive physical therapies, including ultrasound for low back pain (Chou 2017). The previous version of this review was the first to evaluate the effectiveness of therapeutic ultrasound for individuals with chronic LBP (Ebadi 2011). The current version updates the literature search as well as the review methods as per current recommendations from Cochrane Back and Neck, Furlan 2015, and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Objectives
The objective of this review was to determine the effectiveness of therapeutic ultrasound in the management of chronic non‐specific low back pain (LBP). A secondary objective was to determine the most effective dosage and intensity of therapeutic ultrasound for chronic LBP.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised controlled trials (RCTs), published in any language, that evaluated the use of therapeutic ultrasound as a treatment in people with chronic LBP for inclusion in this systematic review. We only included studies with a follow‐up longer than one day.
Types of participants
We included studies if they recruited adult patients with chronic non‐specific LBP. We excluded studies of postoperative patients and individuals in whom a specific cause for their LBP had been determined (e.g. vertebral fracture, malignancy).
Types of interventions
We included all RCTs comparing ultrasound (either alone or in combination with exercise) with placebo or other interventions for chronic LBP. We excluded studies if ultrasound was one part of a treatment package and if it was not possible to determine the effectiveness of ultrasound alone; for example, we did not include a study that compared aerobic exercise + home exercise with hot pack + ultrasound + TENS (transcutaneous electrical nerve stimulation), but included a study comparing an exercise programme with ultrasound to the same exercise programme without ultrasound.
Types of outcome measures
Primary outcomes
Primary outcome measures were:
symptoms (e.g. pain),
back‐specific functional status (e.g. measured with the Roland Morris Questionnaire, Oswestry Disability Index),
overall improvement or satisfaction with treatment,
well‐being (e.g. quality of life measured with the 36‐item Short Form Health Survey (SF‐36), 12‐item Short Form Health Survey (SF‐12), EuroQol)
disability (e.g. ability to perform activities of daily living, return‐to‐work status, work absenteeism) (Furlan 2015).
Adverse events were also assessed to determine harms associated with therapeutic ultrasound.
Secondary outcomes
Secondary outcome measures included:
lumbar range of motion
muscle strength
endurance
Timing of the outcome measures
We defined the timing of outcome measurements as short term (postintervention or less than three months postrandomisation), intermediate term (from three months to less than six months postrandomisation), and long term (from six months to one year postrandomisation).
Search methods for identification of studies
Electronic searches
The following databases were searched by Cochrane Back and Neck to 7 January 2020 with no language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, to Issue 1, 2020), searched using CRS Web
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (OvidSP, 1946 to 7 January 2020)
Embase (1980 to 2020)
Cumulative Index to Nursing and Allied Health Literature (CINAHL/CINAHL Plus, 1981 to 7 January 2020)
Physiotherapy Evidence Database (PEDro) (7 January 2020)
Index to Chiropractic Literature (ICL) (7 January 2020)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) (7 January 2020)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) (7 January 2020)
Search strategies are in line with Furlan 2015 methods guideline and are shown in Appendix 1.
Searching other resources
To supplement the electronic search strategy, we screened reference lists from relevant publications and reviews and used Science Citation Index to perform citation tracking of the RCTs identified by the first step. We also contacted experts in the field of therapeutic ultrasound to identify other relevant articles that may have been missed by the electronic search.
Data collection and analysis
Selection of studies
Two review authors (SE, NH) independently screened the titles and abstracts of all retrieved studies to identify those that were potentially relevant. Any disagreements over study selection were resolved by discussions. We obtained the full texts of those studies deemed potentially relevant, and assessed these for inclusion in the review. In cases of disagreement, a third review author (MvT) was consulted.
Data extraction and management
We used a standardised data extraction form to extract data from the included papers. The extracted data included study characteristics (e.g. country, recruitment modality, study funding, risk of bias), participant characteristics (e.g. number of participants, age, sex, severity of LBP), description of the experimental and control interventions, co‐interventions, duration of follow‐up, outcomes assessed, and results. The same two review authors (SE, NH) who conducted the study selection independently extracted the data. Any disagreements were discussed and a third review author (MvT) was consulted if necessary.
Assessment of risk of bias in included studies
Two review authors (SE, NH) independently assessed the risk of bias in each included study using the updated Cochrane Back and Neck Review Group criteria (Furlan 2015). The 13 criteria are shown in Table 3, and the criteria for a judgement of 'yes' are presented in Table 4. The criteria in both tables are based on the criteria in the updated Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In cases of disagreement, a third review author (MvT) was consulted. The study by the lead author of this review, Ebadi 2012, was assessed by two researchers with experience in low back pain research (see Acknowledgements). We assessed risk of bias for the included studies for each ‘Risk of bias’ domain.
1. Sources of risk of bias*.
| Bias domain | Source of bias | Possible answers |
| Selection | (1) Was the method of randomisation adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the participant blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the dropout rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they had been allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of the suggestion of selective outcome reporting? | Yes/No/Unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/No/Unsure |
| Performance | (10) Were co‐interventions avoided or similar? | Yes/No/Unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/No/Unsure |
*From Furlan 2015.
2. Criteria for a judgement of 'yes' for the sources of risk of bias*.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they were invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the individuals included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the participants or if the success of blinding was tested amongst the participants and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested amongst the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored 'yes' if the success of blinding was tested amongst the outcome assessors and it was successful, or:
|
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias, a 'yes' is scored (NB these percentages are arbitrary, not supported by literature). |
| 7 | All randomised participants are reported/analysed in the group to which they were allocated by randomisation for the most important moments of effect measurement (minus missing values) irrespective of non‐compliance and co‐interventions. |
| 8 | All results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgement. |
| 9 | Groups must be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of participants with neurological symptoms, and value of main outcome measure(s). |
| 10 | If there were no co‐interventions, or they were similar between the index and control groups. |
| 11 | The review author determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number, and frequency of sessions for both the index intervention and the control intervention(s). For example, physiotherapy treatment is usually administered for several sessions, therefore it is necessary to assess how many sessions each participant attended. For single‐session interventions (e.g. surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. For example:
|
*From Furlan 2015.
Measures of treatment effect
We analysed continuous outcomes by calculating the mean difference (MD) in follow‐up scores with 95% confidence intervals (CI) when studies used the same outcome measure, or the standardised mean difference (SMD) with 95% CI when studies used different outcome measures for the same construct. We calculated the risk ratio (RR) as the effect measure for dichotomous outcomes. For each treatment comparison, an effect size and a 95% CI were calculated and displayed as forest plots. All analyses were conducted in Review Manager 5 (Review Manager 2014).
Unit of analysis issues
In cases where a trial evaluated two or more interventions (plus a control arm), two separate 'pair‐wise' comparisons were made. This was necessary to avoid including data for controls more than once in the same comparison. In this case we divided the control group into equal parts whilst assuming equal incidence in these groups. Suitable multiple treatment arms were grouped (e.g. arms that evaluated different dosages of therapeutic ultrasound), whilst irrelevant trial arms were excluded.
We considered cluster‐RCTs and cross‐over trials as eligible for the review and planned to include them in the analyses along with individually randomised studies. For cluster‐RCTs, we planned that estimates adjusted for the intracluster correlation would be extracted and used in the meta‐analysis. For cross‐over trials, we planned that estimates from paired analyses or from the first treatment phases would be extracted where possible and used in the meta‐analysis. However, no cluster‐RCTs or cross‐over studies were included in the review.
Dealing with missing data
In the case of missing data, we made multiple attempts to contact the corresponding authors of the studies. Where data were reported in a graph and not in a table or the text, we estimated the means and standard deviations from them. Where standard deviations were not reported, we planned to estimate these from the confidence intervals or other measures of variance if possible. If the standard deviations for follow‐up measurements were missing, we planned to use the standard deviation for that measure at baseline for subsequent follow‐up measurements. Finally, if no measure of variation was reported anywhere in the text, we would estimate the standard deviation based upon other studies with a similar population and risk of bias. Standard deviations were available for all included studies, and no missing data were identified.
Assessment of heterogeneity
Prior to meta‐analysis, we assessed clinical heterogeneity of the included RCTs by considering whether the studies were similar in setting, participants, interventions, and outcomes. We evaluated methodological heterogeneity by examining the variability in study design and risk of bias. Where studies within a comparison were considered to be clinically and methodologically heterogenous, we performed a random‐effects meta‐analysis in order to account for differences in the included studies in patient population and application of the intervention. Following meta‐analysis, we inspected forest plots visually to detect heterogeneity. We assessed statistical heterogeneity using the Chi² test and the I² statistic. We considered an I² from 0% to 40% to indicate that heterogeneity might not be important; 30% to 60% moderate heterogeneity; 50% to 90% substantial heterogeneity; and 75% to 100% considerable heterogeneity.
Assessment of reporting biases
For each included study we attempted to identify the clinical trial registration record and published protocol. Where these were available, we compared the methods to those in the final published report. Where the trial registration or protocol were not available, this was noted in the Characteristics of included studies tables and assessed as at unclear risk of bias.
Data synthesis
Where possible, we combined the outcome measures from the individual RCTs through meta‐analysis provided sufficient clinical and methodological homogeneity existed between studies. Where SMD was calculated, the estimates were re‐expressed in the scale of the most representative study and presented in the 'Summary of findings' tables (Higgins 2011).
We used the GRADE approach to interpret findings (Schünemann 2017), and employed GRADEpro GDT, GRADEpro GDT, to import data from Review Manager, Review Manager 2014, to create 'Summary of findings' tables. According to the GRADE approach, the certainty of the evidence reflects the extent to which we are confident that an estimate of the effect is correct. Evidence certainty for a specific outcome was based on the assessment of five principal domains: 1) limitations in study design or execution (i.e. risk of bias), 2) consistency of results, 3) directness of evidence (i.e. generalisability), 4) precision (sufficient data with narrow confidence intervals), and 5) other reasons such as publication bias (Appendix 2). The review authors agreed on GRADE evidence ratings following discussion and referral to the GRADE Handbook (GRADE Handbook).
'Summary of findings'
We created 'Summary of findings' tables for two main comparisons: therapeutic ultrasound compared to placebo (Table 1) and therapeutic ultrasound plus exercise compared to exercise alone (Table 2). These tables provide outcome‐specific information at short‐term follow‐up concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the following outcomes rated as particularly important to patient care and decision‐making: pain intensity, back‐specific functional status, overall improvement or satisfaction, well‐being, disability, and adverse events.
Summary of findings 1. Therapeutic ultrasound versus placebo.
| Therapeutic ultrasound compared to placebo for chronic low back pain | ||||||
|
Patient or population: adults with chronic low back pain Settings: secondary care Intervention: therapeutic ultrasound Comparison: placebo ultrasound | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic ultrasound | |||||
|
Pain intensity Scale: VAS (100‐point scale; higher scores mean increased pain) Follow‐up: short term (post‐treatment) |
The mean pain intensity in the control groups ranged from 30.7 to 78.9. | The mean pain intensity in the intervention groups was 7.12 points lower (17.99 lower to 3.75 higher). | MD −7.12 mm (−17.99 to 3.75) | 121 (3 RCTs) | ⊕⊝⊝⊝ very low1,2,3 | Estimate is not clinically important and is very uncertain. |
|
Pain intensity Scale: VAS (number of responders at 30% reduction of pain) Follow‐up: short term (post‐treatment) |
439 per 1000 | 474 per 1000 (356 to 633) |
RR 1.08 (0.81 to 1.44) | 225 (1 RCT) |
⊕⊕⊕⊝ moderate1 | Estimate is not clinically important and suggests there is probably little to no difference between groups. |
|
Back‐specific functional status Scale: Functional Rating Index, Roland‐Morris Disability Questionnaire, or Oswestry Disability Questionnaire (higher scores mean worse function) Follow‐up: short term (post‐treatment) |
*The mean Roland‐Morris Disability Questionnaire score in the control group for the most representative study, Licciardone 2013, is 4 (SD 3.7). | The mean back‐specific functional status in the intervention groups was 1.07 points lower (1.89 lower to 0.26 lower) using the Roland‐Morris Disability Questionnaire. | SMD −0.29 (−0.51 to −0.07) | 325 (4 RCTs) | ⊕⊕⊝⊝ low1,3 | Estimate is not clinically important and suggests there may be a slight reduction in the ultrasound group. |
| Overall improvement or satisfaction | 2 studies reported on this outcome but did not provide data in a form that permitted pooling. Both studies reported no meaningful difference between groups on satisfaction in the short term (post‐treatment). | Not estimable | 486 (2 RCTs) |
⊕⊕⊝⊝ low4 | Estimate is not clinically important and suggests there may be little to no difference between groups. | |
|
Well‐being Scale: general health subscale of SF‐36 (higher scores mean worse well‐being) Follow‐up: short term (post‐treatment) |
The mean well‐being scores in the control groups ranged from 55.2 to 77. | The mean well‐being score in the intervention groups was 2.71 points lower (9.85 lower to 4.44 higher). | MD −2.71 (−9.85 to 4.44) | 267 (2 RCTs) |
⊕⊕⊕⊝ moderate1 | Estimate is not clinically important and suggests there is probably little to no difference between groups. |
| Disability | No trials were identified that reported on this outcome. | |||||
| Adverse events | 1 study measured adverse events following treatment and reported a total of 7/118 (5.9%) adverse events in the ultrasound group and 4/107 (3.7%) events in the placebo group (Licciardone 2013). 3 of these events (2 in ultrasound group, 1 in placebo group) were considered to be serious adverse events, however none of the reported events was judged as related to the intervention. | — | 225 (1 RCT) | ⊕⊕⊝⊝ low5 | Estimate is not clinically important and suggests there may be little to no difference between groups. | |
| *Of the included trials for this outcome, we chose the study that was a combination of the most representative study population and the lowest risk of bias (Licciardone 2013). This figure represents the mean outcome in the control group of this particular study. CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level for imprecision due to small sample size. 2Downgraded one level for unexplained statistical inconsistency (I² = 77%). 3Downgraded one level for limitations in study design, two studies with unclear selection bias. 4Downgraded two levels for serious imprecision, no data available for meta‐analysis. 5Downgraded two levels for serious imprecision, few events and small sample size.
Summary of findings 2. Therapeutic ultrasound plus exercise versus exercise alone.
| Therapeutic ultrasound plus exercise compared to exercise alone for chronic low back pain | ||||||
|
Patient or population: adults with chronic low back pain Settings: secondary care Intervention: therapeutic ultrasound plus exercise Comparison: exercise | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise | Therapeutic ultrasound plus exercise | |||||
|
Pain intensity Scale: VAS (100‐point scale; higher scores mean increased pain) Follow‐up: short term (post‐treatment) |
The mean pain intensity in the control groups ranged from 28.3 to 30.5. | The mean pain intensity in the intervention groups was 21.1 points lower (27.6 lower to 14.5 lower). | MD −21.07 (−27.60 to −14.54) | 70 (2 RCTs) | ⊕⊝⊝⊝ verylow1,2 | Estimate is not clinically important and is very uncertain. 1 study did not provide data in a form to permit pooling. |
|
Pain intensity Scale: VAS (number of responders at 30% reduction of pain) |
No trials were identified that reported on this outcome. | |||||
|
Back‐specific functional status Scale: Oswestry Disability Questionnaire (higher scores mean worse function) Follow‐up: short term (post‐treatment) |
The mean back‐specific functional status in the control groups ranged from 8.2% to 18%. | The mean back‐specific functional status in the intervention groups was 0.41 lower (3.14 lower to 2.32 higher). | MD −0.41 (−3.14 to 2.32) | 79 (2 RCTs) | ⊕⊝⊝⊝ verylow2,3 | Estimate is not clinically important and is very uncertain. |
| Overall improvement or satisfaction | No trials were identified that reported on this outcome. | |||||
|
Well‐being Scale: general health subscale of SF‐36 (higher scores mean worse well‐being) Follow‐up: short term (post‐treatment) |
The mean well‐being scores in the control groups ranged from 64.2 to 66.8. | The mean well‐being score in the intervention groups was 2.50 points lower (9.53 lower to 4.53 higher). | MD −2.50 (−9.53 to 4.53) | 79 (2 RCTs) | ⊕⊝⊝⊝ verylow2,3 | Estimate is not clinically important and is very uncertain. |
| Disability | No trials were identified that reported on this outcome. | |||||
| Adverse events | No trials were identified that reported on this outcome. | |||||
| Abbreviations: CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level for imprecision, small sample size. 2Downgraded two levels for serious limitations in design; both included studies had a high risk of performance and detection bias and an unclear risk of selection bias. 3Downgraded two levels for serious imprecision; small sample size, and the resulting estimate had a wide 95% confidence interval which includes both potential harm and potential benefit from the intervention.
Subgroup analysis and investigation of heterogeneity
A secondary objective of this review was to determine the most effective dosage and intensity of therapeutic ultrasound for chronic LBP. Where a sufficient number of studies (i.e. > 10) were included in a meta‐analysis, we would perform subgroup analyses based on dosage (W/cm²) and intensity (number and duration of treatment sessions). We also planned to perform subgroup analyses to determine the effect of study methods, risk of bias, and clinical differences. However, as only 10 studies were included in the review, subgroup analyses were not performed.
Sensitivity analysis
We planned to perform sensitivity analyses to exclude studies at high risk of selection and reporting bias from the analyses.
Results
Description of studies
Results of the search
Our search strategy identified 2778 references from electronic databases and 46 records from additional sources (Figure 1). After removal of duplicates, 2824 unique articles were screened based on title and abstract for potential relevance. We retrieved the full texts of 66 trials deemed potentially relevant and screened these for inclusion in the review. We checked the reference lists of previous reviews on ultrasound but this did not result in the identification of any further relevant studies. Two review authors (SE, NH) agreed on the inclusion of five new trials for this update, which were added to the five trials from the original review.
1.
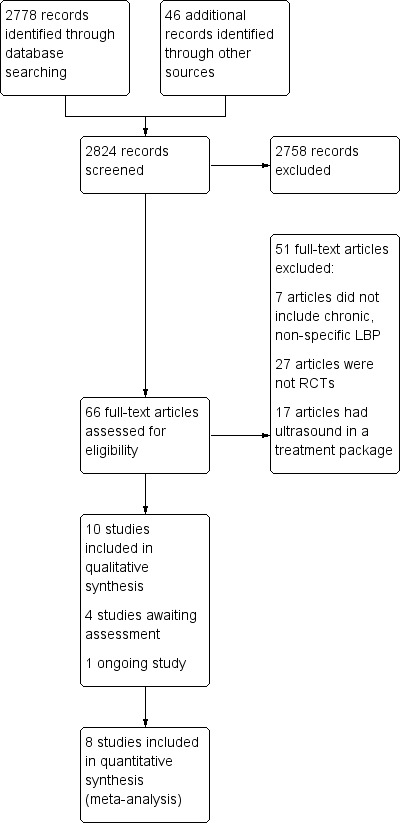
Study flow diagram.
We classified four trials as awaiting assessment and one trial as an ongoing study (for details, see Characteristics of studies awaiting classification; Characteristics of ongoing studies).
Included studies
We included nine studies published in English and one Croatian study (which was translated by a native speaker) in the review. Outcome measures and intervention details are described below as well as in the Characteristics of included studies table. The 10 included studies reported on a total of 1025 participants. The included studies had mostly small sample sizes, with only three studies having more than 25 participants per treatment arm (Abdel‐Aziem 2012; Licciardone 2013; Mohseni‐Bandpei 2006). All studies were performed in secondary care settings, usually in outpatient physiotherapy departments. Three studies were from Turkey (Durmus 2010a; Durmus 2010b; Durmus 2013); two were from Iran (Ansari 2006; Ebadi 2012); two were from Saudi Arabia (Abdel‐Aziem 2012; Khan 2013); and one each were from Croatia (Grubisic 2006), the UK (Mohseni‐Bandpei 2006), and USA (Licciardone 2013). No study reported a conflict of interest with regard to funding.
One study with three arms compared ultrasound to no treatment and electrical stimulation (Durmus 2010b); three studies compared ultrasound plus exercise to exercise alone (Durmus 2010b; Durmus 2013; Khan 2013); five studies compared therapeutic ultrasound to placebo ultrasound (i.e. application of ultrasound with the machine turned off) (Ansari 2006; Durmus 2010a; Ebadi 2012; Grubisic 2006; Licciardone 2013); and one study each compared ultrasound to spinal manipulation (Mohseni‐Bandpei 2006), laser (Abdel‐Aziem 2012), or osteopathic manual treatment (Licciardone 2013). All studies except for two, Ansari 2006; Licciardone 2013, used stretching or strengthening exercise as an additional intervention to ultrasound therapy, whilst Durmus 2010a also provided hot packs to both groups.
All studies used 1 MHz continuous ultrasound at intensities between 1 W/cm² and 2.5 W/cm². The duration of intervention was diverse amongst studies. Two studies, Ansari 2006; Ebadi 2012, used Gray’s formula for calculation of the application time (Allen 2006), whilst the other studies applied ultrasound for 5 to 10 minutes. The number of treatment sessions varied amongst studies, from 6 sessions, Mohseni‐Bandpei 2006, to 18 sessions, Durmus 2010b; Durmus 2013. Pain intensity and back‐specific function were the most common outcomes measured in all studies.
We originally excluded the trial by Licciardone 2013 from the review due to insufficient data. We contacted the trial authors, and they provided data for this version of the review.
Excluded studies
We excluded 51 studies at the full‐text screening phase (see Characteristics of excluded studies table). The most common reasons for exclusion were that studies were not RCTs (n = 27) (Allen 2006; Borman 2003; Chipchase 2003; Cloonan 1987; Draper 1993; Foster 1999; Gorbunov 1997; Greenough 2009; Hamm 2003; Kiralp 2009; Leistner 1989; Lopes 2009; Morrisette 2004; Nordin 1999; Onel 1993; Pensri 2005; Poitras 2005; Poitras 2008; Roman 1960; Rush 1994; Sahin 2004; Scott 2010; Si 2005; Tajali 2009; Tander 2005; Wagner 1995; Wiesinger 1997); the ultrasound therapy was used as part of a combination treatment (n = 17) (Bertocco 2002; Brockow 1997; Gurer 2005; Haas 2004; Hurwitz 2002; Jia 2003; Koes 1992a; Koes 1992b; Koes 1993; Koldas 2008; Kumar 2009a; Kumar 2009b; Kumar 2010; Li 2007; Timm 1994; Tonev 2010; Whitman 2006); and the effect of the ultrasound could not be separated from that of other therapies, or participants had specific causes of low back pain (such as spinal stenosis) (n = 7) (Acar 2012; Charlusz 2010; Fiore 2011; Goren 2010; Nwuga 1983; Santiesteban 1984; Unlu 2008).
Risk of bias in included studies
The 'Risk of bias' assessments of all included studies are provided in the Characteristics of included studies table and summary graphs in Figure 2 and Figure 3. Poor reporting of trial methodology in most of the included studies resulted in unclear risk of bias across domains. We considered only two studies to be at low risk of bias (Ebadi 2012; Licciardone 2013), with the other eight studies assessed as at unclear risk of bias.
2.
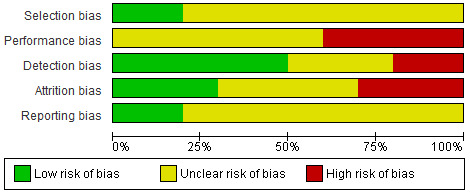
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
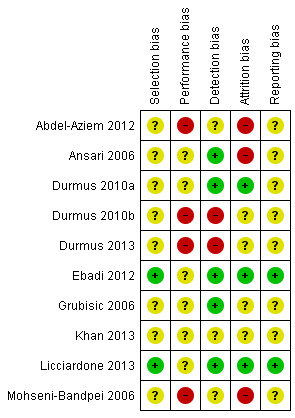
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only four included studies clearly described the randomisation procedure, and only two reported a concealed allocation procedure (Ebadi 2012; Licciardone 2013). Most studies did not report sufficient details on either the method of randomisation or of allocation and were thus judged as at unclear risk of bias for these items. Overall, the risk of selection bias was considered to be low in two studies, Ebadi 2012; Licciardone 2013, and unclear in the other eight studies.
Blinding
Participants were blinded to group allocation in five studies through the use of placebo ultrasound (i.e. application of ultrasound with the machine turned off or output set to zero) (Ansari 2006; Durmus 2010a; Ebadi 2012; Grubisic 2006; Licciardone 2013). In three studies that compared ultrasound with other treatments (Durmus 2010b; Durmus 2013; Mohseni‐Bandpei 2006), blinding of participants was not carried out. In no study was the care provider blinded to group allocation. Because the primary outcome measure in all studies was self‐reported, the risk of outcome assessor bias was low in the studies in which participants were blinded. Overall, the risk of performance bias was high in four studies, Abdel‐Aziem 2012; Durmus 2010b; Durmus 2013; Mohseni‐Bandpei 2006, and unclear in six studies. The risk of detection bias was low in five studies (Ansari 2006; Durmus 2010a; Ebadi 2012; Grubisic 2006; Licciardone 2013), high in two studies (Durmus 2010b; Durmus 2013), and unclear in three studies.
Incomplete outcome data
In six studies (Durmus 2010a; Durmus 2010b; Durmus 2013; Ebadi 2012; Licciardone 2013; Mohseni‐Bandpei 2006), dropout rates were explained and acceptable. The rate of dropout in the study by Ansari 2006 was 30% of the (already very small) sample size, which renders the study as at high risk of attrition bias. In three studies (Ansari 2006; Durmus 2010b; Durmus 2013), participants who dropped out were excluded from the analysis. Three studies reported that an intention‐to‐treat analysis had been performed (Durmus 2010a; Ebadi 2012; Licciardone 2013). Overall, we considered three studies to be at high risk of attrition bias, Abdel‐Aziem 2012; Ansari 2006; Mohseni‐Bandpei 2006, and three studies as at low risk of attrition bias (Durmus 2010a; Ebadi 2012; Licciardone 2013).
Selective reporting
Only two studies reported registration of the trial prior to commencement (Ebadi 2012; Licciardone 2013). One of these studies also had a published protocol available (Ebadi 2012). Overall, we considered two studies to be at low risk of reporting bias (Ebadi 2012; Licciardone 2013), and the other eight included studies as at unclear risk of reporting bias.
Other potential sources of bias
None of the studies reported on compliance with the intervention. Four studies controlled for co‐interventions (Ansari 2006; Durmus 2013; Ebadi 2012; Licciardone 2013), and all of the included studies assessed their outcomes at similar time intervals for all groups.
Effects of interventions
Therapeutic ultrasound versus placebo
The results for this comparison are presented in Table 1. Five studies compared therapeutic ultrasound with placebo ultrasound and reported on outcomes in the short term (Ansari 2006; Durmus 2010a; Ebadi 2012; Grubisic 2006; Licciardone 2013). No studies reported on outcomes at intermediate‐ or long‐term follow‐up.
Primary outcomes
Four studies provided short‐term (postintervention) data on pain intensity for this comparison (Durmus 2010a; Ebadi 2012; Grubisic 2006; Licciardone 2013). There was very low‐certainty evidence from three studies that therapeutic ultrasound results in little to no change in pain intensity when compared to placebo in the short term (mean difference (MD) −7.12, 95% confidence interval (CI) −17.99 to 3.75; n = 121; 3 RCTs; Figure 4; Analysis 1.1) (Durmus 2010a; Ebadi 2012; Grubisic 2006). We downgraded the evidence by three levels due to limitations in study design, imprecision, and inconsistency. One study reported a responder analysis of participants achieving a 30% reduction in pain intensity in the short term (Licciardone 2013). There was moderate‐certainty evidence that therapeutic ultrasound results in little to no difference in the proportion of responders in the short term compared to placebo (risk ratio 1.08, 95% CI 0.81 to 1.44; n = 225; 1 RCT; Analysis 1.2). We downgraded the evidence by one level due to imprecision.
4.

Forest plot of comparison: 1 Ultrasound versus placebo ultrasound, outcome: 1.1 Pain (VAS) short term [mm].
1.1. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 1: Pain (VAS) short‐term
1.2. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 2: Pain (VAS responder analysis 30% reduction) short‐term
Four studies provided short‐term (postintervention) data on back‐specific functional status (Ansari 2006; Durmus 2010a; Ebadi 2012; Licciardone 2013). No studies reported on intermediate‐ or long‐term follow‐up for this outcome. There was low‐certainty evidence that therapeutic ultrasound improves back‐specific functional status when compared to placebo in the short term (standardised mean difference (SMD) −0.29, 95% CI −0.51 to −0.07; converted MD −1.07, 95% CI −1.89 to −0.26; n = 325; 4 RCTs; Figure 5; Analysis 1.3). We downgraded the evidence by two levels due to limitations in study design and imprecision.
5.

Forest plot of comparison: 1 Ultrasound versus placebo ultrasound, outcome: 1.3 Back‐specific functional status (various scales) short term.
1.3. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 3: Back‐specific functional status (various scales) short‐term
One study measured satisfaction with back care in the short term and reported no difference between groups (Licciardone 2013). Another study reported a participant‐reported measure of overall improvement in the short term and reported no difference between groups (Grubisic 2006). Neither study reported data in a form that permitted meta‐analysis of these outcomes.
Two studies reported short‐term (postintervention) data on well‐being, measured using the general health subscale of the SF‐36 (Durmus 2010a; Licciardone 2013). There was moderate‐certainty evidence of little to no difference in well‐being in the short‐term between therapeutic ultrasound and placebo (MD −2.71, 95% CI −9.85 to 4.44; n = 267; 2 RCTs; Analysis 1.4). We downgraded the evidence by one level due to imprecision.
1.4. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 4: Well‐being (general health subscale of SF‐36) short‐term
None of the studies included in this comparison reported on disability.
Only one study measured adverse events following treatment and reported a total of 7/118 (5.9%) adverse events in the ultrasound group and 4/107 (3.7%) events in the placebo group (Licciardone 2013). Three of these events (two in the ultrasound group, one in the placebo group) were considered to be serious adverse events; however, none of the reported adverse events was determined to be related to the intervention.
The effect sizes for all outcomes were not considered to be clinically important.
Secondary outcomes
A 'Summary of findings' table for secondary outcomes for this comparison is shown in Appendix 3.
Three studies provided short‐term (postintervention) data on lumbar flexion range of motion (ROM) (Ansari 2006; Ebadi 2012; Grubisic 2006). There was very low‐certainty evidence of little to no difference between therapeutic ultrasound and placebo (SMD 0.18, 95% CI −0.62 to 0.98; converted MD 3.2 millimetres, 95% CI −11.1 to 17.5; n = 89; 3 RCTs; Analysis 1.5). We downgraded the evidence for this outcome by three levels due to limitations in design, serious imprecision, and inconsistency.
1.5. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 5: Flexion ROM (various scales) short‐term
Two studies provided short‐term (postintervention) data on lumbar extension ROM (Ansari 2006; Ebadi 2012). There was very low‐certainty evidence of little to no difference between therapeutic ultrasound and placebo (SMD −0.33, 95% CI −0.85 to 0.19; converted MD −3.1 millimetres, 95% CI −7.9 to 1.8; n = 58; 2 RCTs; Analysis 1.6). We downgraded the evidence for this outcome by three levels due to limitations in design and serious imprecision.
1.6. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 6: Extension ROM (various scales) short‐term
None of the studies included in this comparison reported on muscle strength as an outcome.
One study assessed muscle endurance in the short term using the Biering‐Sorensen test (Ebadi 2012). There was low‐certainty evidence of little to no difference between therapeutic ultrasound and placebo (MD −11.00, 95% CI −34.94 to 12.94; n = 39; 1 RCT; Analysis 1.7). We downgraded the evidence for this outcome by two levels due to serious imprecision.
1.7. Analysis.

Comparison 1: Ultrasound versus placebo ultrasound, Outcome 7: Trunk extensor muscle endurance (Biering‐Sorensen test) short‐term
Therapeutic ultrasound plus exercise versus exercise alone
The results for this comparison are presented in Table 2. Three small (total n = 109) studies compared therapeutic ultrasound in addition to an exercise programme with an exercise programme alone in the short term (Durmus 2010b; Durmus 2013; Khan 2013). No studies reported on outcomes at intermediate‐ or long‐term follow‐up.
Primary outcomes
Three studies provided short‐term (postintervention) data on pain intensity measured with the visual analogue scale (VAS). One of the studies reported an effect in favour of the exercise‐only group (Durmus 2010b), but only presented data graphically, which did not allow for pooling. There was very low‐certainty evidence that ultrasound plus exercise resulted in slightly less pain than exercise alone in the short term (MD −21.1, 95% CI −27.60 to −14.54; n = 70; 2 RCTs; Figure 6; Analysis 2.1). We downgraded the evidence by three levels for serious limitations in study design and for imprecision.
6.

Forest plot of comparison: 2 Ultrasound plus exercise versus exercise alone, outcome: 2.1 Pain (VAS) short term.
2.1. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 1: Pain (VAS) short‐term
Two studies provided short‐term (postintervention) data on back‐specific functional status measured with the Oswestry Disability Questionnaire (Durmus 2010b; Durmus 2013). There was very low‐certainty evidence of little to no difference between ultrasound plus exercise and exercise alone in the short term (MD −0.41, 95% CI −3.14 to 2.32; n = 79; 2 RCTs; Analysis 2.2). We downgraded the evidence by three levels for limitations in study design and for serious imprecision.
2.2. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 2: Back‐specific functional status (Oswestry) short‐term
Two studies reported on short‐term (postintervention) well‐being through the general health subscale of the SF‐36 (Durmus 2010b; Durmus 2013). There was very low‐certainty evidence of little to no difference in well‐being in the short term between ultrasound plus exercise and exercise alone (MD −2.50, 95% CI −9.53 to 4.53; n = 79; 2 RCTs; Analysis 2.3). We downgraded the evidence by three levels for limitations in study design and for serious imprecision.
2.3. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 3: Well‐being (general health subscale of SF‐36) short‐term
None of the studies included in this comparison reported on overall improvement or satisfaction, disability, or adverse events.
The effect sizes for all outcomes were not considered to be clinically important.
Secondary outcomes
The GRADE ratings for the secondary outcomes for this comparison are reported in Appendix 4.
Two studies also provided short‐term (postintervention) data on flexion ROM measured with the Lumbar Schober method. There was very low‐certainty evidence of little to no difference between ultrasound plus exercise and exercise alone (MD 0.02, 95% CI −0.52 to 0.56; n = 79; 2 RCTs; Analysis 2.4). We downgraded the evidence by three levels for serious limitations in study design and imprecision.
2.4. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 4: Flexion ROM (Schober test) short‐term
One study reported on short‐term (postintervention) back flexor and extensor muscle strength and endurance. There was very low‐certainty evidence that therapeutic ultrasound plus exercise compared to exercise alone results in little to no difference in trunk flexor strength (MD 0.65, 95% CI −0.23 to 1.53; n = 40; 1 RCT); slightly more trunk flexor endurance (MD 4.00, 95% CI 1.09 to 6.91; n = 40; 1 RCT); and slightly more trunk extensor endurance (MD 37.35, 95% CI 33.29 to 41.41; n = 40; 1 RCT) (Analysis 2.5; Analysis 2.6; Analysis 2.7). We downgraded the evidence by three levels for serious limitations in study design and imprecision.
2.5. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 5: Trunk flexor strength short‐term
2.6. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 6: Trunk flexor endurance short‐term
2.7. Analysis.

Comparison 2: Ultrasound plus exercise versus exercise alone, Outcome 7: Trunk extensor endurance short‐term
Therapeutic ultrasound versus other treatments
Five studies compared therapeutic ultrasound with other treatments for chronic LBP (Abdel‐Aziem 2012; Durmus 2010b; Durmus 2013; Licciardone 2013; Mohseni‐Bandpei 2006). All studies reported outcomes in the short term (postintervention), and one study reported on pain and back‐specific functional status at six months' follow‐up (Mohseni‐Bandpei 2006).
One study compared ultrasound to electrical stimulation and reported on pain intensity (MD 2.50, 95% CI −3.67 to 8.67; n = 39; 1 RCT); back‐specific functional status (MD 1.88, 95% CI 0.00 to 3.76; n = 39; 1 RCT); and flexion ROM (MD 0.08, 95% CI −0.77 to 0.93; n = 39; 1 RCT) in the short term (Durmus 2010b). We do not know the effect of therapeutic ultrasound compared to electrical stimulation because for all of these outcomes the certainty of the evidence was very low (Analysis 3.1; Analysis 3.2; Analysis 3.3) (Durmus 2010b). We downgraded the evidence for these outcomes by three levels for serious imprecision and serious limitations in design.
3.1. Analysis.

Comparison 3: Ultrasound versus electrical stimulation, Outcome 1: Pain (SF‐36) short‐term
3.2. Analysis.

Comparison 3: Ultrasound versus electrical stimulation, Outcome 2: Back‐specific functional status (Oswestry) short‐term
3.3. Analysis.

Comparison 3: Ultrasound versus electrical stimulation, Outcome 3: Flexion ROM (Schober test) short‐term
One study compared ultrasound to laser and reported on pain intensity (MD 0.40, 95% CI 0.16 to 0.64; n = 150; 1 RCT) and flexion ROM (MD 0.48, 95% CI 0.25 to 0.71; n = 150; 1 RCT) in the short term (Abdel‐Aziem 2012). We do not know the effect of therapeutic ultrasound compared to laser because for both of these outcomes the certainty of the evidence was very low (Analysis 4.1; Analysis 4.2). We downgraded the evidence for these outcomes by three levels for imprecision and serious limitations in design.
4.1. Analysis.

Comparison 4: Ultrasound versus laser, Outcome 1: Pain (VAS) short‐term
4.2. Analysis.

Comparison 4: Ultrasound versus laser, Outcome 2: Flexion ROM (Schober test) short‐term
One study compared ultrasound to phonophoresis and reported on pain intensity (MD 0.10, 95% CI −0.68 to 0.88; n = 40; 1 RCT); back‐specific functional status (MD 1.30, 95% CI −0.47 to 3.07; n = 40; 1 RCT); well‐being (MD −10.75, 95% CI −21.83 to 0.33; n = 40; 1 RCT); flexion ROM (MD 0.25, 95% CI −0.33 to 0.83; n = 40; 1 RCT); muscle strength (MD −0.01, 95% CI −0.91 to 0.89; n = 40; 1 RCT); and endurance (MD 2.10, 95% CI −0.70 to 4.90; n = 40; 1 RCT) in the short term (Durmus 2013). We do not know the effect of therapeutic ultrasound compared to phonophoresis because for all of these outcomes the certainty of the evidence was very low (Analysis 5.1 to Analysis 5.7). We downgraded the evidence for these outcomes by three levels for serious imprecision and serious limitations in study design.
5.1. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 1: Pain (VAS) short‐term
5.7. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 7: Trunk extensor endurance short‐term
One study compared ultrasound to spinal manipulative therapy and reported on pain intensity, back‐specific functional status, flexion ROM, and extension ROM in the short term (Mohseni‐Bandpei 2006). There is low‐certainty evidence that spinal manipulation results in lower pain intensity than therapeutic ultrasound in the short term (post‐treatment) (MD −16.50, 95% CI −27.55 to −5.45; n = 112; 1 RCT; Analysis 6.1). Spinal manipulation also resulted in improved back‐specific functional status (MD −7.80, 95% CI −13.19 to −2.41; n = 112; 1 RCT); flexion ROM (MD −10.00, 95% CI −14.37 to −5.63; n = 112; 1 RCT); and extension ROM (MD −4.00, 95% CI −6.77 to −1.23; n = 112; 1 RCT) when compared with therapeutic ultrasound. There was also low‐certainty evidence of little to no difference between spinal manipulation and therapeutic ultrasound at six months' follow‐up for pain intensity (MD −15.10, 95% CI −32.84 to 2.64; n = 73; 1 RCT) and back‐specific functional status (MD −5.20, 95% CI −13.05 to 2.65; n = 73; 1 RCT) (Analysis 6.5; Analysis 6.6). We judged the evidence for these outcomes to be of low certainty due to limitations in study design and imprecision.
6.1. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 1: Pain (VAS) short‐term
6.5. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 5: Pain (VAS) at 6 months follow‐up
6.6. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 6: Back‐specific functional status (Oswestry) at 6 months follow‐up
One study compared ultrasound to osteopathic manual treatment and reported on pain intensity using a responder analysis in the short term (Licciardone 2013). There is moderate‐certainty evidence that therapeutic ultrasound results in fewer participants reaching a 30% improvement in pain intensity compared to osteopathic manual treatment in the short term (RR 0.75, 95% CI 0.59 to 0.95; n = 233; 1 RCT; Analysis 7.1). We judged the evidence for this outcome to be of moderate certainty due to imprecision.
7.1. Analysis.

Comparison 7: Ultrasound versus osteopathic manual treatment, Outcome 1: Low back pain reduction (threshold >= 30%)
Sensitivity analysis
We planned sensitivity analyses that would exclude studies at high risk of selection and reporting bias from the analyses. However, only two studies had a low risk of selection and reporting bias (Ebadi 2012; Licciardone 2013). The estimates from these studies were consistent with the pooled estimates in the comparison between therapeutic ultrasound and placebo (see Analysis 1.1; Analysis 1.3).
Discussion
Summary of main results
Ten small RCTs (n = 1025) met the inclusion criteria for this review (Abdel‐Aziem 2012; Ansari 2006; Durmus 2010a; Durmus 2010b; Durmus 2013; Ebadi 2012; Grubisic 2006; Khan 2013; Licciardone 2013; Mohseni‐Bandpei 2006). There was very low‐certainty evidence (n = 121; 3 RCTs) that therapeutic ultrasound results in little to no difference in pain intensity compared with placebo in the short term, and moderate‐certainty evidence of little to no difference in the number of responders (with 30% reduction in pain intensity) in the short term. There was low‐certainty evidence from four trials (n = 325) that therapeutic ultrasound improves back‐specific function compared with placebo in the short term, but this difference was not clinically significant (MD = 1.07 points on Roland‐Morris Disability Questionnaire). There was also moderate‐certainty evidence of little to no difference between therapeutic ultrasound and placebo on well‐being in the short term. None of the studies included in this comparison reported on disability. Only one study reported on adverse events (Licciardone 2013), with no events judged to be related to the intervention.
There was very low‐certainty evidence that therapeutic ultrasound plus exercise resulted in lower pain intensity than exercise alone in the short term; however, this difference was not clinically significant (MD 21.1 points on VAS). There was also very low‐certainty evidence of little to no difference in back‐specific functional status and well‐being when ultrasound plus exercise was compared to exercise alone. None of the studies included in this comparison reported on overall improvement or satisfaction, disability, or adverse events.
Overall completeness and applicability of evidence
Most of the available RCTs on therapeutic ultrasound for chronic LBP are small and at unclear risk of bias. There is a lack of evidence to support the use of therapeutic ultrasound in chronic LBP, with studies suggesting little to no difference in outcomes compared to placebo and worse outcomes compared to other active or passive interventions. The reported data were most often assessed in the short term, and the lack of intermediate‐ and long‐term outcome assessment restricts our ability to comment on whether any effects of therapeutic ultrasound manifest over a longer time period. In most of the included studies, therapeutic ultrasound was evaluated in combination with some form of exercise therapy, which limits any conclusions on the effectiveness of ultrasound as a uni‐modal treatment. Not all recommended outcome measures for studies on LBP (such as pain and back‐specific function) were measured by all studies (Furlan 2015). The reporting of ultrasound application parameters and dose was inconsistent in the included studies, which meant that no conclusions on the most effective dose could be made. No study reported on calibration of the ultrasound device prior to or between treatment sessions.
Quality of the evidence
The small sample sizes in the included studies led to the downgrading of the evidence (i.e. imprecision) for most of the treatment comparisons. As a result, there was mostly low‐ to very low‐certainty evidence for the outcomes in the comparisons of interest to this review. Most studies were affected by poor reporting; were not registered prior to commencement; or did not publish protocols, which made assessment of the risk of bias difficult. Whilst most studies blinded the participant or outcome assessor, no study was able to appropriately blind the caregiver (therapist). In addition, there was a lack of information from most studies about compliance with therapeutic ultrasound or adverse events.
Potential biases in the review process
We made all attempts to reduce the bias involved with the review process. Where review authors were also authors of an included study, external reviewers were consulted to apply the eligibility criteria, extract the data, and perform the 'Risk of bias' assessment. In the case of missing data, we made attempts to obtain the information from the authors of included studies.
Agreements and disagreements with other studies or reviews
This review is in agreement with previous systematic reviews on the effects of ultrasound therapy, which identify a lack of high‐quality evidence and limited support for ultrasound as an effective intervention for chronic musculoskeletal disorders (Chou 2017; Qaseem 2017). The latest guidelines on the management of chronic LBP recommend that clinicians do not offer ultrasound for managing low back pain with or without sciatica (NICE 2016).
Authors' conclusions
Implications for practice.
There is a lack of large, high‐quality studies that have investigated the effect of therapeutic ultrasound for chronic low back pain (LBP). Despite various outcome measures being used by the studies in this review to highlight different facets of chronic LBP, the evidence to support the use of therapeutic ultrasound in practice is limited. Effect sizes are small, and any improvements do not appear to be clinically meaningful. There is very limited information available on adverse events following therapeutic ultrasound. Other active and passive interventions are likely to result in better outcomes than therapeutic ultrasound for individuals with chronic LBP.
Implications for research.
Whilst further research is likely to have an important impact on our confidence in the estimates of effect of therapeutic ultrasound, there is little to suggest that this research will uncover a benefit from therapeutic ultrasound that is clinically meaningful for individuals with chronic LBP. In order to identify whether therapeutic ultrasound has any clinically important effect on chronic LBP and to investigate the implications of varying dose, intensity, and application type, randomised controlled trials with low risk of bias and adequate sample size would be required. Future trials would need to include long‐term outcome measurements, record any potential adverse effects, and consider the cost‐effectiveness of ultrasound treatment compared with usual care in order to improve the evidence base. However, based on the findings of this systematic review, further research in the field of chronic LBP in other areas would likely be of greater value.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2020 | New citation required and conclusions have changed | The review now includes 10 randomised controlled trials with updated GRADE ratings; the conclusions have changed as a result of new included studies. The current evidence remains at risk of bias; however, there is more certainty of little to no effect of the intervention. |
| 7 January 2020 | New search has been performed | New search was performed and methods updated according to Furlan 2015. |
History
Protocol first published: Issue 6, 2011 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 3 April 2014 | New search has been performed | One study added as awaiting classification. See Published notes for details. |
Acknowledgements
The authors would like to thank Rachel Couban for assistance in developing the electronic search strategy. The authors would also like to thank Steven Kamper and Zoe Michaleff for their assistance in assessing the risk of bias and data extraction for one included study. The authors would also like to thank Katina Aleksovska for her assistance in assessing the risk of bias and data extraction for the study in Croatian.
The authors would also like to thank the following people for their peer review: Terry P Corbin (Consumer Editor, Cochrane Back and Neck group), Arianne P Verhagen (University of Technology Sydney, Graduate School of Health, Discipline of Physiotherapy, Sydney, Australia), Professor Mark Hancock (Faculty of Medicine and Health Sciences, Macquarie University), and Marilyn Walsh (consumer reviewer).
Appendices
Appendix 1. Search strategies
CENTRAL
Last searched 7 January 2020
1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
2 lumb* NEAR pain AND CENTRAL:TARGET
3 lumbago AND CENTRAL:TARGET
4 "back pain" OR backache* AND CENTRAL:TARGET
5 coccyx or coccydynia or sciatic* or spondylosis AND CENTRAL:TARGET
6 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Spine EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Spinal Diseases EXPLODE ALL AND CENTRAL:TARGET
9 MESH DESCRIPTOR Spinal Fusion EXPLODE ALL AND CENTRAL:TARGET
10 facet NEAR joint* AND CENTRAL:TARGET
11 lumb* NEAR vertebra* AND CENTRAL:TARGET
12 stenosis NEAR spine AND CENTRAL:TARGET
13 stenosis NEAR root AND CENTRAL:TARGET
14 stenosis NEAR spinal AND CENTRAL:TARGET
15 slipped NEAR disc* AND CENTRAL:TARGET
16 slipped NEAR disk* AND CENTRAL:TARGET
17 degenerat* NEAR disc* AND CENTRAL:TARGET
18 degenerat* NEAR disk* AND CENTRAL:TARGET
19 herniat* NEAR disc* AND CENTRAL:TARGET
20 herniat* NEAR disk* AND CENTRAL:TARGET
21 prolaps* NEAR disc* AND CENTRAL:TARGET
22 prolaps* NEAR disk* AND CENTRAL:TARGET
23 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 AND CENTRAL:TARGET
24 MESH DESCRIPTOR Ultrasonic Therapy EXPLODE ALL AND CENTRAL:TARGET
25 MESH DESCRIPTOR Ultrasonics EXPLODE ALL AND CENTRAL:TARGET
26 ultrasound OR ultrasonic* AND CENTRAL:TARGET
27 #24 OR #25 OR #26 AND CENTRAL:TARGET
28 #27 AND #23 AND CENTRAL:TARGET
29 (2018 OR 2019 OR 2020):YR AND CENTRAL:TARGET
30 #28 AND #29 AND CENTRAL:TARGET
March 2018 search in CRS web. Some truncation was revised.
1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
2 lumb* NEAR pain AND CENTRAL:TARGET
3 lumbago AND CENTRAL:TARGET
4 "back pain" or backache* AND CENTRAL:TARGET
5 coccyx or coccydynia or sciatic* or spondylosis AND CENTRAL:TARGET
6 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Spine EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Spinal Diseases EXPLODE ALL AND CENTRAL:TARGET
9 MESH DESCRIPTOR Spinal Fusion EXPLODE ALL AND CENTRAL:TARGET
10 facet NEAR joint* AND CENTRAL:TARGET
11 lumb* NEAR vertebra* AND CENTRAL:TARGET
12 stenosis NEAR (spine or root or spinal) AND CENTRAL:TARGET
13 slipped NEAR (disc* or disk*) AND CENTRAL:TARGET
14 degenerat* NEAR (disc* or disk*) AND CENTRAL:TARGET
15 herniat* NEAR (disc* or disk*) AND CENTRAL:TARGET
16 displace* NEAR (disc* or disk*) AND CENTRAL:TARGET
17 prolap* NEAR (disc* or disk*) AND CENTRAL:TARGET
18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
19 MESH DESCRIPTOR Ultrasonic Therapy EXPLODE ALL AND CENTRAL:TARGET
20 MESH DESCRIPTOR Ultrasonics EXPLODE ALL AND CENTRAL:TARGET
21 ultrasound or ultrasonic* AND CENTRAL:TARGET
22 #19 OR #20 OR #21
23 #18 AND #22
24 #23 AND (2017 TO 2018:YR)
January 2017 search strategy using CRS stand alone. The strategy was revised.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 back pain or backache
#3 (lumb* next pain) or lumbago
#4 coccyx or coccydynia or sciatica or spondylosis
#5 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 facet near joint*
#9 lumb* near vertebra*
#10 MeSH descriptor: [Spinal Fusion] explode all trees
#11 stenosis near (spine or root or spinal)
#12 slipped near (disc* or disk*)
#13 degenerat* near (disc* or disk*)
#14 herniat* near (disc* or disk*)
#15 displace* near (disc* or disk*)
#16 prolap* near (disc* or disk*)
#17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Ultrasonic Therapy] explode all trees 839
#19 MeSH descriptor: [Ultrasonics] explode all trees 298
#20 ultrasound or ultrasonic*
#21 #18 or #19 or #20
#22 #17 and #21
#23 #22 Publication Year from 2013 to 2017, in Trials
MEDLINE
Last searched 7 January 2020. Line 15 was revised.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 random*.ti,ab.
5 placebo.ab,ti.
6 drug therapy.fs.
7 trial.ab,ti.
8 groups.ab,ti.
9 or/1‐8
10 (animals not (humans and animals)).sh.
11 9 not 10
12 dorsalgia.tw,kf.
13 exp Back Pain/
14 backache.tw,kf.
15 ((lumb* adj pain) or (back adj pain)).tw,kf.
16 coccyx.tw,kf.
17 coccydynia.tw,kf.
18 sciatic*.tw,kf.
19 exp sciatic neuropathy/
20 spondylosis.tw,kf.
21 lumbago.tw,kf.
22 exp Spondylosis/
23 or/12‐22
24 Ultrasonics/
25 Ultrasonic therapy/
26 ultrasonic*.tw,kf.
27 ultrasound.tw,kf.
28 or/24‐27
29 11 and 23 and 28
30 limit 29 to yr=2018‐2020
31 limit 29 to ed=20180316‐20200107
32 30 or 31
Search 16 March 2018. Some truncation was revised.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 random*.ti,ab.
5 placebo.ab,ti.
6 drug therapy.fs.
7 trial.ab,ti.
8 groups.ab,ti.
9 or/1‐8
10 (animals not (humans and animals)).sh.
11 9 not 10
12 dorsalgia.tw,kf.
13 exp Back Pain/
14 backache.tw,kf.
15 ((lumb* or back) adj pain).tw,kf.
16 coccyx.tw,kf.
17 coccydynia.tw,kf.
18 sciatic*.tw,kf.
19 exp sciatic neuropathy/
20 spondylosis.tw,kf.
21 lumbago.tw,kf.
22 exp Spondylosis/
23 or/12‐22
24 Ultrasonics/
25 Ultrasonic therapy/
26 ultrasonic*.tw,kf.
27 ultrasound.tw,kf.
28 or/24‐27
29 11 and 23 and 28
30 limit 29 to yr=2017‐2018
31 limit 29 to ed=20170127‐20180316
32 30 or 31
27 January 2017 search strategy. Added line 25 and .tw,kf. fields searched instead of.mp.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 random*.ti,ab.
5 placebo.ab,ti.
6 drug therapy.fs.
7 trial.ab,ti.
8 groups.ab,ti.
9 or/1‐8
10 (animals not (humans and animals)).sh.
11 9 not 10
12 dorsalgia.tw,kf.
13 exp Back Pain/
14 backache.tw,kf.
15 ((lumb* or back) adj pain).tw,kf.
16 coccyx.tw,kf.
17 coccydynia.tw,kf.
18 sciatica.tw,kf.
19 exp sciatic neuropathy/
20 spondylosis.tw,kf.
21 lumbago.tw,kf.
22 exp Spondylosis/
23 or/12‐22
24 Ultrasonics/
25 Ultrasonic therapy/
26 ultrasonic*.tw,kf.
27 ultrasound.tw,kf.
28 or/24‐27
29 11 and 23 and 28
30 limit 29 to yr=2013‐2017
31 limit 29 to ed=20131010‐20170127
32 30 or 31
EMBASE
Last searched 7 January 2020. The RCT filter was revised.
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 randomization/
9 random*.ti,ab.
10 placebo?.ti,ab.
11 allocat*.ti,ab.
12 assign*.ti,ab.
13 blind*.ti,ab.
14 (cross‐over or crossover).ti,ab.
15 (compare or compared or comparing or comparison or comparative).ti,ab.
16 ((controlled adj7 study) or (controlled adj7 design)).ti,ab.
17 ((singl* adj7 mask*) or (doubl* adj7 mask*) or (trebl* adj7 mask*) or (tripl* adj7 mask*)).ti,ab.
18 trial.ti,ab.
19 or/1‐18
20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
21 human/ or normal human/ or human cell/
22 20 and 21
23 20 not 22
24 19 not 23
25 dorsalgia.tw,kw.
26 back pain.tw,kw.
27 exp BACKACHE/
28 (lumb* adj pain).tw,kw.
29 coccyx.tw,kw.
30 coccydynia.tw,kw.
31 sciatic*.tw,kw.
32 sciatica/
33 exp ISCHIALGIA/
34 spondylosis.tw,kw.
35 lumbago.tw,kw.
36 exp Low back pain/
37 or/25‐36
38 ultrasound/
39 ultrasonic*.tw,kw.
40 ultrasound.tw,kw.
41 or/38‐40
42 24 and 37 and 41
43 limit 42 to yr=2018‐2020
44 limit 42 to dd=20180316‐20200107
45 43 or 44
Last searched 16 March 2018. Some truncation was revised.
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 randomization/
9 random*.ti,ab.
10 placebo?.ti,ab.
11 allocat*.ti,ab.
12 assign*.ti,ab.
13 blind*.ti,ab.
14 (cross‐over or crossover).ti,ab.
15 (compare or compared or comparing or comparison or comparative).ti,ab.
16 (controlled adj7 (study or design or trial)).ti,ab.
17 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
18 trial.ti,ab.
19 or/1‐18
20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
21 human/ or normal human/ or human cell/
22 20 and 21
23 20 not 22
24 19 not 23
25 dorsalgia.tw,kw.
26 back pain.tw,kw.
27 exp BACKACHE/
28 (lumb* adj pain).tw,kw.
29 coccyx.tw,kw.
30 coccydynia.tw,kw.
31 sciatic*.tw,kw.
32 sciatica/
33 exp ISCHIALGIA/
34 spondylosis.tw,kw.
35 lumbago.tw,kw.
36 exp Low back pain/
37 or/25‐36
38 ultrasound/
39 ultrasonic*.tw,kw.
40 ultrasound.tw,kw.
41 or/38‐40
42 24 and 37 and 41
43 limit 42 to yr=2017‐2018
44 limit 42 to dd=20170127‐20180316
45 43 or 44
27 January 2017 search strategy. The RCT filter and line 28 were revised, line 32 was added, and .tw,kw. field was searched instead of.mp.
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 randomization/
9 random*.ti,ab.
10 placebo?.ti,ab.
11 allocat*.ti,ab.
12 assign*.ti,ab.
13 blind*.ti,ab.
14 (cross‐over or crossover).ti,ab.
15 (compare or compared or comparing or comparison or comparative).ti,ab.
16 (controlled adj7 (study or design or trial)).ti,ab.
17 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
18 trial.ti,ab.
19 or/1‐18
20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
21 human/ or normal human/ or human cell/
22 20 and 21
23 20 not 22
24 19 not 23
25 dorsalgia.tw,kw.
26 back pain.tw,kw.
27 exp BACKACHE/
28 (lumb* adj pain).tw,kw.
29 coccyx.tw,kw.
30 coccydynia.tw,kw.
31 sciatica.tw,kw.
32 sciatica/
33 exp ISCHIALGIA/
34 spondylosis.tw,kw.
35 lumbago.tw,kw.
36 exp Low back pain/
37 or/25‐36
38 ultrasound/
39 ultrasonic*.tw,kw.
40 ultrasound.tw,kw.
41 or/38‐40
42 24 and 37 and 41
43 limit 42 to yr=2013‐2017
44 limit 42 to dd=20131010‐20170127
45 43 or 44
CINAHL
Last searched 7 January 2020. In 2018 some truncations were revised.
S59 S57 OR S58
S58 S56 AND EM 20180316‐20200107
S57 S56 Limiters ‐ Published Date: 20180301‐20200131
S56 S50 and S55
S55 S51 or S52 or S53 or S54
S54 "ultrasonic"
S53 "ultrasound"
S52 (MH "Ultrasonics")
S51 (MH "Ultrasonic Therapy")
S50 S48 AND S28
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumb* N2 vertebra*
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatic*"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumb* N5 pain
S33 lumb* W1 pain
S32 "backache" or back pain
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square 142
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
27 January 2017 search strategy
S59 S57 OR S58
S58 S56 AND EM 20131010‐20170127
S57 S56 Limiters ‐ Published Date: 20131001‐20170131
S56 S50 and S55
S55 S51 or S52 or S53 or S54
S54 "ultrasonic"
S53 "ultrasound"
S52 (MH "Ultrasonics")
S51 (MH "Ultrasonic Therapy")
S50 S48 AND S28
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumb* N2 vertebra*
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumb* N5 pain
S33 lumb* W1 pain
S32 "backache" or back pain
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
PEDro
Last searched 7 January 2020
Abstract and title: ultrasound AND
Problem: pain AND
Body Part: lumbar spine, sacroiliac joint or pelvis AND
Method: (blank) AND
New records added since: 16/03/2018
Index to Chiropractic Literature (ICL)
Last searched 7 January 2020. In 2018 the search was revised.
S1 Subject:\"Sciatica\" OR Subject:\"Back Pain\" OR Subject:\"Low Back Pain\", Peer Review only
S2 All Fields:back pain OR All Fields:lumbago OR All Fields:sciatica, Peer Review only
S3 Subject:\"Ultrasonic Therapy\" OR Subject:\"Ultrasonics\", Peer Review only
S4 All Fields:ultrasound OR All Fields:ultrasonic*, Peer Review only
S5 Subject:\"Sciatica\" OR Subject:\"Back Pain\" OR Subject:\"Low Back Pain\", Peer Review only OR All Fields:back pain OR All Fields:lumbago OR All Fields:sciatica, Peer Review only
S6 Subject:\"Ultrasonic Therapy\" OR Subject:\"Ultrasonics\", Peer Review only OR All Fields:ultrasound OR All Fields:ultrasonic*, Peer Review only
S7 Subject:\"Sciatica\" OR Subject:\"Back Pain\" OR Subject:\"Low Back Pain\", Peer Review only OR All Fields:back pain OR All Fields:lumbago OR All Fields:sciatica, Peer Review only AND Subject:\"Ultrasonic Therapy\" OR Subject:\"Ultrasonics\", Peer Review only OR All Fields:ultrasound OR All Fields:ultrasonic*, Peer Review only
S8, Year: from 2013 to 2017, Peer Review only
S9 Subject:\"Sciatica\" OR Subject:\"Back Pain\" OR Subject:\"Low Back Pain\", Peer Review only OR All Fields:back pain OR All Fields:lumbago OR All Fields:sciatica, Peer Review only AND Subject:\"Ultrasonic Therapy\" OR Subject:\"Ultrasonics\", Peer Review only OR All Fields:ultrasound OR All Fields:ultrasonic*, Peer Review only AND, Year: from 2018 to 2020, Peer Review only
ClinicalTrials.gov
Last searched 7 January 2020.
Conditions: back pain AND Other terms: ultrasound
First posted from 03/16/2018 to 01/07/2020
Last searched 16 March 2018.
Conditions: back pain AND Interventions: ultrasound
First posted from 1/27/2017 to 03/16/2018
27 January 2017 search strategy.
Conditions: back pain AND
Interventions: ultrasound
First received from 10/10/2013 to 1/27/2017
ICTRP
Last searched 7 January 2020
Basic search: Back pain AND ultrasound
Appendix 2. The GRADE approach to evidence synthesis
We will categorise the certainty of evidence as follows.
High (⊕⊕⊕⊕): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊕⊕⊕⊖): further research is likely to have an important impact in the confidence in the estimate of effect.
Low (⊕⊕⊖⊖): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low (⊕⊖⊖⊖): any estimate of effect is very uncertain.
The evidence available to answer each subquestion will be graded on the domains as follows.
1. Risk of bias
Limitations in the study design and implementation may bias the estimates of the treatment effect. Our confidence in the estimate of the effect and in the following recommendation decreases if studies suffer from major limitations. We examined all studies with regard to the following five types of biases.
Selection (random sequence generation, allocation concealment, group similarities at baseline)
Performance (blinding of participants, blinding of healthcare providers)
Attrition (dropouts and intention‐to‐treat analysis)
Measurement (blinding of the outcome assessors and timing of outcome assessment)
Reporting bias (selective reporting)
2. Inconsistency
Inconsistency refers to unexplained heterogeneity in results. Widely differing estimates of the treatment effect (i.e. heterogeneity or variability in results) across studies suggest true differences in underlying treatment effect. Inconsistency may arise from differences in: populations (e.g. drugs may have larger relative effects in sicker populations); interventions (e.g. larger effects with higher drug doses); or outcomes (e.g. diminishing treatment effect with time). We downgraded the certainty of evidence:
by one level when the heterogeneity or variability in results was large (e.g. I² > 80%);
by two levels when the heterogeneity or variability in results was large, and there was inconsistency arising from populations, interventions, or outcomes.
3. Indirectness
Indirect population, intervention, comparator, or outcome; the question being addressed in this systematic review is different from the available evidence with regard to the population, intervention, comparator, or an outcome in the included randomised trial. We downgraded the certainty of evidence:
by one level when there was indirectness in only one area;
by two levels when there was indirectness in two or more areas.
4. Imprecision
Results are imprecise when studies include relatively few participants and few events and thus have wide confidence intervals around the estimate of the effect. In such a case we downgraded the certainty of the evidence because of consequential uncertainty in the results. We considered each outcome separately.
For dichotomous outcomes
We considered imprecision for either of the following two reasons.
There is only one study. When there is more than one study, the total number of events is less than 300 (a threshold rule‐of‐thumb value) (Mueller 2007).
The 95% confidence interval around the pooled or best estimate of effect includes both: a) no effect; and b) appreciable benefit or appreciable harm. The threshold for 'appreciable benefit' or 'appreciable harm' is a relative risk reduction or relative risk increase greater than 25%.
We downgraded the certainty of evidence:
by one level when there was imprecision due to either of the above reasons;
by two levels when there was imprecision due to both of the above reasons.
For continuous outcomes
We considered imprecision for either of the following two reasons.
There is only one study. When there is more than one study, total population size is less than 400 (a threshold rule‐of‐thumb value; using the usual α and β, and an effect size of 0.2 standard deviations, representing a small effect).
The 95% confidence interval includes no effect, and the upper or lower confidence limit crosses an effect size (standardised mean difference) of 0.5 in either direction.
We downgraded the certainty of evidence:
by one level when there was imprecision due to either of the above reasons;
by two levels when there was imprecision due to both of the above reasons.
5. Publication bias
Publication bias is a systematic underestimate or overestimate of the underlying beneficial or harmful effect due to the selective publication of studies. We downgraded the certainty of evidence:
by one level when the funnel plot suggested publication bias.
Appendix 3. Therapeutic ultrasound compared with placebo ‐ secondary outcomes
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic ultrasound | |||||
|
Lumbar flexion ROM (mm; higher numbers mean increased ROM) Follow‐up: short term (post‐treatment) |
The mean flexion ROM in the control group for the most representative study, Ebadi 2012, is 59.8 mm (SD 17.9).* | The mean flexion ROM in the intervention groups was 3.2 mm more (11.1 less to 17.5 more). | SMD 0.18 (−0.62 to 0.98 | 89 (3 RCTs) |
⊕⊝⊝⊝ very low1,2,3 |
Corresponding risk calculated with an SMD of 0.18 (−0.62 to 0.98). Estimate is not clinically important and is very uncertain. |
|
Lumbar extension ROM (mm; higher numbers mean increased ROM) Follow‐up: short term (post‐treatment) |
The mean extension ROM in the control group for the most representative study, Ebadi 2012, is 24.1 mm (SD 9.3).* | The mean extension ROM in the intervention groups was 3.1 mm less (7.9 less to 1.8 more). | SMD −0.33 (−0.85 to 0.19 | 58 (2 RCTs) |
⊕⊝⊝⊝ very low2,4 |
Corresponding risk calculated with an SMD of −0.33 (−0.85 to 0.19). Estimate is not clinically important and is very uncertain. |
| Muscle strength | No trials were identified that reported on this outcome. | |||||
|
Muscle endurance (Biering‐Sorensen test (seconds); higher numbers mean increased endurance) Follow‐up: short term (post‐treatment) |
The mean muscle endurance in the control group was 139.3 seconds. | The mean muscle endurance in the intervention group was 11.0 seconds less (34.9 less to 12.9 more). | MD −11.00 (−34.94 to 12.94) | 39 (1 RCT) |
⊕⊕⊝⊝ low2 |
Estimate is not clinically important and suggests there may be little to no difference between groups. |
| *Of the trials included in this outcome, we chose the study that is a combination of the most representative study population and the lowest risk of bias (Ebadi 2012). This figure represents the mean outcome in the control group of this particular study. CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; ROM: range of motion; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level for unexplained statistical inconsistency (I² = 64%). 2Downgraded two levels for serious imprecision: small sample size, and the resulting estimate has a wide 95% confidence interval which includes both potential harm and potential benefit from the intervention. 3Downgraded one level for limitations in study design, two studies at unclear/high risk of selection bias, attrition bias, and reporting bias. 4Downgraded one level for limitations in study design, one study at unclear risk of selection and reporting bias and high risk of attrition bias.
Appendix 4. Therapeutic ultrasound plus exercise compared with exercise alone ‐ secondary outcomes
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise | Therapeutic ultrasound plus exercise | |||||
|
Lumbar flexion range of motion (ROM) (Schober test (cm); higher numbers mean increased ROM) Follow‐up: short term (post‐treatment) |
The mean flexion ROM in the control groups ranged from 0.25 cm to 0.38 cm. | The mean flexion ROM in the intervention groups was 0.02 cm more (0.52 less to 0.56 more). | MD 0.02 (−0.52 to 0.56) | 79 (2 RCTs) |
⊕⊝⊝⊝ very low1,2 |
Estimate is not clinically important and is very uncertain. |
|
Trunk flexor strength (hand‐held dynamometer (N·m); higher scores mean increased strength) Follow‐up: short term (post‐treatment) |
The mean flexor strength in the control group was 23.2 N·m. | The mean flexor strength in the intervention group was 0.65 N·m more (0.23 less to 1.53 more). | MD 0.65 (−0.23 to 1.53) | 40 (1 RCT) |
⊕⊝⊝⊝ very low1,2 |
Estimate is not clinically important and is very uncertain. |
|
Trunk flexor endurance (sit‐up test (seconds); higher numbers mean increased endurance) Follow‐up: short term (post‐treatment) |
The mean trunk flexor endurance in the control group was 89.3 seconds. | The mean trunk extensor endurance in the intervention groups was 4.00 seconds more (1.09 more to 6.91 more). | MD 4.00 (1.09 to 6.91) | 40 (1 RCT) |
⊕⊝⊝⊝ very low1,2 |
Estimate is not clinically important and is very uncertain. |
|
Trunk extensor endurance (Biering‐Sorensen test (seconds); higher numbers mean increased endurance) Follow‐up: short term (post‐treatment) |
The mean trunk extensor endurance in the control group was 126.7 seconds. | The mean trunk extensor endurance in the intervention groups was 37.35 seconds more (33.29 more to 41.41 more). | MD 37.35 (33.29 to 41.41) | 40 (1 RCT) |
⊕⊝⊝⊝ very low1,2 |
Estimate is not clinically important and is very uncertain. |
| Abbreviations: CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; ROM: range of motion | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded two levels for serious limitations in study design, all studies at unclear/high risk of selection bias, attrition bias, and reporting bias. 2Downgraded one level for imprecision, small sample size.
Data and analyses
Comparison 1. Ultrasound versus placebo ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain (VAS) short‐term | 3 | 121 | Mean Difference (IV, Random, 95% CI) | ‐7.12 [‐17.99, 3.75] |
| 1.2 Pain (VAS responder analysis 30% reduction) short‐term | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.44] |
| 1.3 Back‐specific functional status (various scales) short‐term | 4 | 325 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 1.4 Well‐being (general health subscale of SF‐36) short‐term | 2 | 267 | Mean Difference (IV, Random, 95% CI) | ‐2.71 [‐9.85, 4.44] |
| 1.5 Flexion ROM (various scales) short‐term | 3 | 89 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.62, 0.98] |
| 1.6 Extension ROM (various scales) short‐term | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.19] |
| 1.7 Trunk extensor muscle endurance (Biering‐Sorensen test) short‐term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐11.00 [‐34.94, 12.94] |
Comparison 2. Ultrasound plus exercise versus exercise alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain (VAS) short‐term | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐21.07 [‐27.60, ‐14.54] |
| 2.2 Back‐specific functional status (Oswestry) short‐term | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐3.14, 2.32] |
| 2.3 Well‐being (general health subscale of SF‐36) short‐term | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐9.53, 4.53] |
| 2.4 Flexion ROM (Schober test) short‐term | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.52, 0.56] |
| 2.5 Trunk flexor strength short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.23, 1.53] |
| 2.6 Trunk flexor endurance short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 4.00 [1.09, 6.91] |
| 2.7 Trunk extensor endurance short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 37.35 [33.29, 41.41] |
Comparison 3. Ultrasound versus electrical stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain (SF‐36) short‐term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐3.67, 8.67] |
| 3.2 Back‐specific functional status (Oswestry) short‐term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 1.88 [0.00, 3.76] |
| 3.3 Flexion ROM (Schober test) short‐term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.77, 0.93] |
Comparison 4. Ultrasound versus laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain (VAS) short‐term | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.16, 0.64] |
| 4.2 Flexion ROM (Schober test) short‐term | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 0.48 [0.25, 0.71] |
Comparison 5. Ultrasound versus phonophoresis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain (VAS) short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.68, 0.88] |
| 5.2 Back‐specific functional status (Oswestry) short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.47, 3.07] |
| 5.3 Well‐being (general health subscale of SF‐36) short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐10.75 [‐21.83, 0.33] |
| 5.4 Flexion ROM (Schober test) short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.33, 0.83] |
| 5.5 Trunk flexor strength short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.91, 0.89] |
| 5.6 Trunk flexor endurance short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐0.70, 4.90] |
| 5.7 Trunk extensor endurance short‐term | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 33.19 [29.08, 37.30] |
5.2. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 2: Back‐specific functional status (Oswestry) short‐term
5.3. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 3: Well‐being (general health subscale of SF‐36) short‐term
5.4. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 4: Flexion ROM (Schober test) short‐term
5.5. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 5: Trunk flexor strength short‐term
5.6. Analysis.

Comparison 5: Ultrasound versus phonophoresis, Outcome 6: Trunk flexor endurance short‐term
Comparison 6. Ultrasound versus spinal manipulative therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain (VAS) short‐term | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐16.50 [‐27.55, ‐5.45] |
| 6.2 Back‐specific functional status (Oswestry) short‐term | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐13.19, ‐2.41] |
| 6.3 Flexion ROM short‐term | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐14.37, ‐5.63] |
| 6.4 Extension ROM short‐term | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐6.77, ‐1.23] |
| 6.5 Pain (VAS) at 6 months follow‐up | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐15.10 [‐32.84, 2.64] |
| 6.6 Back‐specific functional status (Oswestry) at 6 months follow‐up | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐5.20 [‐13.05, 2.65] |
6.2. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 2: Back‐specific functional status (Oswestry) short‐term
6.3. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 3: Flexion ROM short‐term
6.4. Analysis.

Comparison 6: Ultrasound versus spinal manipulative therapy, Outcome 4: Extension ROM short‐term
Comparison 7. Ultrasound versus osteopathic manual treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Low back pain reduction (threshold >= 30%) | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aziem 2012.
| Study characteristics | ||
| Methods | RCT conducted in outpatient physiotherapy clinic (secondary care setting) | |
| Participants | 150 patients with mechanical low back pain collected from the public and private hospitals, and student of College of Applied Medical Sciences in Taif, participated in this study. Participant were randomly assigned to 2 equal groups. Exclusion criteria: neurological disorders (as lumbar disc prolapse, polyneuropathy, sciatica), developmental, congenital or neuromuscular scoliosis and previous back or abdominal surgeries, also obese patients have been excluded | |
| Interventions | A) Laser treatment group: infrared laser therapy (904 N·m) 3 times per week at the level of low back paravertebral muscles at a power of 1 to 2 J/cm²; Low‐Intensity Laser Therapy (LILT). In combination with traditional exercise therapy. (B) Ultrasound treatment group: ultrasound therapy 3 times per week on low back paravertebral muscles, 1.5 W/cm² at a frequency of 1 MHz, in combination with traditional exercise therapy. | |
| Outcomes | VAS and ROM pre‐treatment, after 4 weeks and after 8 weeks. Both groups showed improvement in both outcomes after 4 and 8 weeks. | |
| Notes | The analysis and the reporting of the results are vague, so some results are not reported in the table due to ambiguity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports "randomly distributed"; method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Not reported in text, unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in text |
| Incomplete outcome data (attrition bias) Dropout rate described | High risk | ROM is not reported for different movements (flexion, extension, lateral flexion) and is reported by only 1 score. Dropouts not explained. |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | High risk | ROM is not reported for different movements (flexion, extension, lateral flexion) and is reported by only 1 score. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Unclear risk | No comparison is reported; only raw data. |
| Co‐interventions | High risk | Manipulation was delivered to all participants, which can be considered to be a co‐intervention. |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | High risk | Some timings are unclear in the text of the results section. |
Ansari 2006.
| Study characteristics | ||
| Methods | RCT conducted in outpatient physiotherapy clinic (secondary care setting) | |
| Participants | 15 participants were randomised, who were aged 18 to 65 years with non‐radiating, non‐specific low back pain lasting for more than 3 months. Exclusion criteria were: abnormal neurological status; concomitant severe disease; psychiatric illness; current psychotherapy; pathological lumbosacral X‐rays (except for minor degenerative changes); rheumatic inflammatory disease; planned hospitalisation; addiction to any kind of substance; and any contraindication to ultrasound therapy. | |
| Interventions | Intervention (I) group (n = 5) received 1 MHz continuous ultrasound, at 1.5 W/cm² for 10 sessions, 3 days per week. Duration of ultrasound application was calculated according to the formula: total treatment time = planned average local exposure time x (tissue area/effective radiation area of applicator). Control (C) group (n = 5) received placebo ultrasound for 10 sessions, 3 days per week. |
|
| Outcomes | Mean (SD) pre‐ and post‐treatment scores on Functional Rating Index were: (I) 56.5 (20.35), 34.5 (13.5); and (C) 46.95 (14.38), 39.9 (16.5). Mean (SD) pre‐ and post‐treatment degrees of flexion range of motion were: (I) 117.4 (2.5), 128.6 (14.3); and (C) 103.4 (13.39), 109.2 (10.6). Mean (SD) pre‐ and post‐treatment degrees of extension range of motion were: (I) 23.8 (4.15), 30 (6.4); and (C) 27.2 (3.03), 29 (4.2). No between‐group difference was seen for H‐reflex parameters (electroneurophysiological evaluation) or in lumbar spine lateral flexion (left and right) range of motion. |
|
| Notes | No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Participants blinded to group allocation. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measure is self‐reported, participants blind to group allocation. |
| Incomplete outcome data (attrition bias) Dropout rate described | High risk | Dropout rate described, 30% of sample dropped out. |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | High risk | Participants who dropped out were excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | High risk | Large differences in gender, age, BMI between groups |
| Co‐interventions | Low risk | Participants advised not to commence new treatments. |
| Compliance | High risk | High proportion of dropouts due to noncompliance. |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups. |
Durmus 2010a.
| Study characteristics | ||
| Methods | RCT conducted in unknown setting (not reported) | |
| Participants | 42 patients (29 females and 13 males) with chronic LBP lasting for at least 3 months were included in the study. Patients were excluded from the study for the following reasons: evidence of acute radiculopathy; the presence of an inflammatory disease, neoplastic disease, spondylolysis, spondylolisthesis, or sacroiliitis; lumbar disc herniation requiring surgical treatment; vertebral fractures; pregnancy. | |
| Interventions | Intervention (I) group (n = 21) participants received hot packs (15 minutes), ultrasound, and exercise. In this group, 1 MHz continuous ultrasound was applied to the lumbar paravertebral region at an intensity of 1 W/cm² for 10 minutes using a probe with an effective radiating area of 5 cm². Control (C) group (n = 21) participants received hot packs (15 minutes), placebo ultrasound, and exercise. Placebo ultrasound was applied to the same region for the same duration, with the same ultrasound device. No current was applied, but the device and the indicator lights were kept in the 'on' position. Both groups performed range of motion, stretching (hamstring, pelvic, and abdominal muscles), and strengthening (cervical, thoracic, and lumbar region muscles) exercises for 15 minutes. Participants were treated 5 days a week for 3 weeks. |
|
| Outcomes | Median (range) scores on the modified Oswestry Low Back Pain Disability Questionnaire pre‐ and post‐treatment were: (I) 38 (26 to 76), 12 (1 to 32); and (C) 44 (22 to 50), 17 (6 to 23). Median (range) scores on the VAS at rest pre‐ and post‐treatment were: (I) 6 (3 to 10), 2 (1 to 5); and (C) 6 (3 to 9), 4 (1 to 9). Significantly greater improvement was observed in (I) compared to (C) in Pain Disability Index scores, 6‐minute walk test, emotional and physical role functioning (SF‐36), functional performance, and depression. |
|
| Notes | Attempts made to contact authors for further data (as all data were reported as median (range)) were met with no response. No conflict of interest declared with regard to commercial funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Participants blinded to group allocation. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measures are self‐reported, participants blind to group allocation. |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | No dropouts reported. |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | Low risk | No dropouts reported, presumed complete outcome data were available. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Unclear risk | Not reported in text |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
Durmus 2010b.
| Study characteristics | ||
| Methods | RCT conducted in outpatient department (secondary care setting) | |
| Participants | 68 female patients who had been experiencing low back pain for at least 3 months were included. Exclusion criteria were: acute radicular signs or symptoms, radiographic evidence of inflammatory disease affecting the spine, tumour, spondylolysis, spondylolisthesis, or sacroiliitis, serious medical conditions for which exercise would be contraindicated, neuromuscular or dermatologic disease that involves the lumbar and abdominal area, were currently in an exercise programme, implanted cardiac pacemaker or defibrillator, contracture, previous trauma, history of spinal surgery, pregnancy, presence of severe structural deformity. | |
| Interventions | Group 1 (n = 20) was given an electrical stimulation programme and back and abdominal exercises (45 min). Group 2 (n = 19) was given a 10‐minute ultrasound treatment (1 MHz frequency and 1 W/cm² intensity and a transducer head with an area of 5 cm, an effective radiating area of 4 cm, and a BNR of 1:5) and back and abdominal exercises (45 min). Group 3 (n = 20) acted as the control group and was given only back and abdominal exercises (45 min). All of the programmes were 45 minutes per session, performed 3 days a week, for a duration of 6 weeks. Participants were evaluated pre‐treatment and in the third and sixth weeks of the therapy. |
|
| Outcomes | 59 participants completed the study. The post‐treatment (6‐week) between‐group comparison did not show a significant difference in Pain Disability Index, Oswestry Disability Questionnaire, range of motion (modified lumbar Schober, lumbar Schober, fingertip to floor distance), 6‐minute walk distance, or muscle strength between the 3 groups. There was a significantly greater improvement in physical function, energy, and social function scores of the SF‐36, VAS, and muscle endurance in groups 1 and 2 compared to group 3. There was no significant difference between groups 1 and 2 in these outcomes. |
|
| Notes | No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | High risk | Participants not blinded to intervention. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported primary outcome, participants not blinded to intervention |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | Described and acceptable |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | High risk | Dropouts excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Unclear risk | Not reported in text |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
Durmus 2013.
| Study characteristics | ||
| Methods | RCT conducted in outpatient department (secondary care setting) | |
| Participants | 64 female patients who had been experiencing low back pain for at least 3 months were included. Exclusion criteria were: acute radicular signs or symptoms, radiographic evidence of inflammatory disease affecting the spine, tumour, spondylolysis, spondylolisthesis, or sacroiliitis, serious medical conditions for which exercise would be contraindicated, neuromuscular or dermatologic disease that involves the lumbar and abdominal area, were currently in an exercise programme, implanted cardiac pacemaker or defibrillator, contracture, previous trauma, history of spinal surgery, pregnancy, presence of severe structural deformity. | |
| Interventions | Group 1 (n = 20) was given a group exercise programme for 60 min, 3 times per week for 6 weeks. Group 2 (n = 20) was given a 10‐minute ultrasound treatment (1 MHz frequency and 1.5 W/cm² intensity and a transducer head with an area of 5 cm, an effective radiating area of 4 cm, and a BNR of 1:5), 3 times per week for 6 weeks, and the same exercise programme as group 1. Group 3 (n = 20) was given phonophoresis therapy by applying 2 to 3 mm of capsaicin gel (10% capsicum oleoresin in 0.22% solution) then a 10‐minute ultrasound treatment (as per group 2), 3 times per week for 6 weeks, as well as the same exercise programme as group 1. Participants were evaluated pre‐treatment and after 6 weeks (post‐treatment). |
|
| Outcomes | 60 participants completed the study. The post‐treatment (6‐week) between‐group comparison showed a significant difference in VAS pain, walking performance (6‐minute walk test), and extensor muscle strength in favour of groups 2 and 3 compared to group 1. There was no significant difference between groups 2 and 3. There was a significantly greater improvement in pain, physical function, and energy subscales of the SF‐36 in group 3 compared to groups 1 and 2. |
|
| Notes | No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | High risk | Participants not blinded to intervention. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported primary outcome, participants not blinded to intervention |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | Described and acceptable |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | High risk | Dropouts excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Low risk | Medication controlled during intervention period. |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
Ebadi 2012.
| Study characteristics | ||
| Methods | RCT conducted in outpatient department (secondary care setting) | |
| Participants | 50 patients aged between 18 and 60 years with non‐specific chronic low back pain were randomised. Exclusion criteria were: nerve root symptoms; systemic disease and specific conditions such as neoplasm, fractures, spondylolisthesis, spondylolysis, spinal stenosis, ankylosing spondylitis; previous low back surgery; taking medication for specific psychological problems; and pregnancy. Participants were recruited from 3 university hospitals in Tehran, Iran. | |
| Interventions | The intervention (I) group (n = 24) received continuous ultrasound plus semi‐supervised exercise. Continuous ultrasound with a frequency of 1 MHz and an intensity of 1.5 W/cm² was applied to the painful paravertebral low back region. The duration of US was estimated for each participant using the formula: total treatment time = planned average local exposure time x (tissue area/effective radiation area of applicator). The (C) control group (n = 24) received placebo ultrasound plus semi‐supervised exercise. Placebo ultrasound involved the machine being turned on, with lights visible to the participant, but no current being applied. All participants in both groups received 10 sessions of treatment, 3 times a week, every other day. |
|
| Outcomes | 48 participants completed treatment sessions. Mean (SD) pre‐ and post‐treatment scores for VAS were: (I) 46.6 (17.7), 26.6 (13.8); and (C) 49 (16), 30.7 (13.1). Mean (SD) pre‐ and post‐treatment scores for Functional Rating Index were: (I) 40.8 (14.6), 23.4 (6.9); and (C) 43.9 (16.9), 31.1 (13.4). Changes in lumbar range of motion, muscle endurance, and median frequency slope of all measured paravertebral muscles were not significantly different between groups. | |
| Notes | No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Allocation was performed using opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Participants blinded to group allocation. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measure is self‐reported, participants blinded to group allocation. |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | Described and acceptable |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | Low risk | All randomised participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | Trial was pre‐registered and published protocol was available. All outcomes reported. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Low risk | Participants advised not to commence new treatments. |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
Grubisic 2006.
| Study characteristics | ||
| Methods | RCT conducted in specialist clinic (secondary care setting) | |
| Participants | 31 participants, aged 38 to 77 years, with low back pain lasting more than 3 months and intensity of pain on VAS at least 50 mm, were randomised. Exclusion criterion was non‐mechanical low back pain. | |
| Interventions | Intervention (I) group (n = 15) received ultrasound to the paravertebral muscles at an intensity of 1.2 W/cm² for 5 minutes plus kinesitherapy for 10 sessions over 2 weeks. Control (C) group (n = 16) received placebo ultrasound plus kinesitherapy for 10 sessions over 2 weeks. |
|
| Outcomes | Mean (SD) pre‐ and post‐treatment scores on VAS were: (I) 82.7 (14.0), 79.8 (12.2); and (C) 81.7 (12.1), 78.9 (12.1). Mean (SD) pre‐ and post‐treatment range of motion (cm) measures were: (I) 5.7 (0.8), 5.8 (0.9); and (C) 5.4 (0.9), 5.6 (1.0). There was no significant difference between groups regarding patient and physician global perceived efficacy. |
|
| Notes | Article originally published in Croatian. No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Participants blinded to group allocation. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measure is self‐reported, participants blind to group allocation. |
| Incomplete outcome data (attrition bias) Dropout rate described | Unclear risk | Not reported in text |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | Unclear risk | Not reported in text |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Unclear risk | Not reported in text |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
Khan 2013.
| Study characteristics | ||
| Methods | RCT conducted in outpatient physiotherapy clinic (secondary care setting) | |
| Participants | 30 patients from Raj Nursing Home (age 25 to 65 years) who were diagnosed with low back pain, with onset > 1 to 3 months (chronic) | |
| Interventions | Participants were randomly assigned to either group A receiving US and exercise combined or group B receiving exercise alone. Participants in both groups received 10 sessions of treatment, each about 20 minutes, over 4 weeks. In group A, US dose was 1 W/cm² with frequency of 1 MHz in continuous mode for 8 minutes over the paravertebral low back region. Group B received placebo US. Both groups received stretching and strengthening exercises. |
|
| Outcomes | VAS: both groups had significant reduction. McGill Pain Questionnaire (PRI/PPI): both groups had significant reduction. |
|
| Notes | No statistical analysis was performed to address the difference between groups regarding the amount of change. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for determining randomisation sequence not reported in text. |
| Allocation concealment (selection bias) | Unclear risk | No description of the method of group allocation |
| Blinding of participants and personnel (performance bias) Participants | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Not reported in text, unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in text |
| Incomplete outcome data (attrition bias) Dropout rate described | Unclear risk | Complete outcome data, but dropout not described |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | Unclear risk | Unclear if all cases were analysed or what method of analysis was used |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Unclear risk | Not reported in text |
| Co‐interventions | Unclear risk | Not reported in text |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Same across groups, before treatment and after 4 weeks |
Licciardone 2013.
| Study characteristics | ||
| Methods | RCT with 2 x 2 factorial design | |
| Participants | 455 patients aged 21 to 69 years with low back pain for at least 3 months recruited through newspaper advertisements, community agencies, and medical clinics. Exclusion criteria included pregnancy, red‐flag conditions (cancer, spinal osteomyelitis, spinal fracture, herniated disc, ankylosing spondylitis, or cauda equina syndrome); low back surgery in the past year; workers' compensation benefits in the past 3 months; ongoing litigation involving back problems; angina or congestive heart failure symptoms with minimal activity, history of a stroke, or transient ischaemic attack in the past year; implanted biomedical devices (such as cardiac pacemakers or artificial joints); active bleeding or infection in the lower back, or other conditions impeding protocol implementation; use of corticosteroids in the past month; or use of manual treatment (osteopathic or manual therapies delivered by chiropractors or physical therapists) or therapeutic ultrasound in the past 3 months or more than 3 times in the past year. Candidates whose screening was successful by telephone received a clinical screening to exclude those with a high probability of lumbar radiculopathy, a relative contraindication to osteopathic manual therapy (OMT). | |
| Interventions | Participants were allocated to 4 groups: OMT plus ultrasound, OMT plus placebo ultrasound, placebo OMT plus ultrasound, or placebo OMT plus placebo ultrasound. Treatments were scheduled at weeks 0, 1, 2, 4, 6, and 8 using 15 different physicians. Participants could self‐initiate low back pain co‐interventions, such as non‐prescription drugs, complementary and alternative medicine therapies, or usual care. |
|
| Outcomes | The current level of low back pain was measured before each treatment and at week 12 using a 100‐millimetre VAS. Secondary outcomes were measured at baseline and at weeks 4, 8, and 12 using the Roland‐Morris Disability Questionnaire, Medical Outcomes Study Short Form‐36 Health Survey general health scale, number of lost workdays in the past 4 weeks because of low back pain, and satisfaction with back care on a 5‐point Likert scale. | |
| Notes | Data are not published separately for the 4 randomised groups. We contacted the authors, who provided stratified outcome data (unpublished). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation site based on a computer program that generated pseudorandom numbers. |
| Allocation concealment (selection bias) | Low risk | Assignments were then conveyed directly to the physicians using numbered, opaque, sealed envelopes, which were subsequently placed in secured, segregated treatment files. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Participants blinded as placebo ultrasound and placebo OMT were used. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Providers unable to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported measure, participants blinded to intervention |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | Low rate of attrition, reasons provided in supplemental figure |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | Low risk | Intention‐to‐treat analysis presented. |
| Selective reporting (reporting bias) | Low risk | Trial was pre‐registered, and all relevant outcomes were presented. No published protocol available. |
| Similar groups | Low risk | Baseline measures similar across groups (Table 1). |
| Co‐interventions | Low risk | Measured co‐treatments reported by participants |
| Compliance | High risk | Relatively high contraindication and adverse event rate (4% serious adverse events) |
| Timing of outcome measures | Low risk | All groups measured at same time postrandomisation. |
Mohseni‐Bandpei 2006.
| Study characteristics | ||
| Methods | RCT conducted in outpatient physiotherapy department (secondary care setting) | |
| Participants | 120 individuals aged between 18 and 55 years with pain greater than 3 months were recruited and randomised into 2 groups of 60 participants. Individuals were excluded if they had an underlying disease such as malignancy, obvious disc herniation, osteoporosis, viscerogenic causes, infection or systemic disease of the musculoskeletal system; previous spinal manipulation therapy or ultrasound treatment; neurologic or sciatic nerve root compression, radicular pain, sensory disturbances, loss of strength and reflexes; previous back surgery; evidence of previous vertebral fractures or major structural abnormality; tumour of the spine; pregnancy; devices such as heart pacemakers that could be affected by electrical stimulation; or registered disabled or receiving any type of benefits because of their LBP. | |
| Interventions | The manipulation/exercise group (n = 56) received spinal manipulation with an exercise programme. On average, each participant was seen for 4 sessions (range 2 to 7 sessions), once or twice per week. The ultrasound/exercise group (n = 56) received ultrasound with the same exercise programme. Continuous ultrasound with a frequency of 1 MHz and intensity between 1.5 and 2.5 W/cm² for a period of 5 to 10 minutes was applied. On average, each participant was seen for 6 sessions (range 3 to 11 sessions), once or twice per week. |
|
| Outcomes | 112 participants completed the study. Post‐treatment, between‐group analysis showed that participants in the manipulation/exercise group demonstrated a significantly greater reduction in pain intensity and functional disability, as well as improved lumbar flexion and extension, than the ultrasound/exercise group. No significant difference was found between groups for measures of median frequency for either the multifidus or the iliocostalis lumborum muscle. A significant difference was found in the median frequency slope between groups for multifidus alone in favour of the manipulation/exercise group. These differences persisted at the 6‐month follow‐up. | |
| Notes | No conflict of interest declared with regard to commercial funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block style randomization scheme" with reference |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text |
| Blinding of participants and personnel (performance bias) Participants | High risk | Participants not blinded to intervention. |
| Blinding of participants and personnel (performance bias) Care providers | High risk | Care providers not blinded to group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded to group allocation, however primary outcome was self‐reported, and participants were not blinded to intervention. |
| Incomplete outcome data (attrition bias) Dropout rate described | Low risk | Described and acceptable |
| Incomplete outcome data (attrition bias) Intention‐to‐treat | High risk | Dropouts excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration or published protocol was available. |
| Similar groups | Low risk | Groups were well matched at baseline. |
| Co‐interventions | Unclear risk | Not reported in text |
| Compliance | Unclear risk | Not reported in text |
| Timing of outcome measures | Low risk | Similar timing of outcome assessment (post‐treatment) for both groups |
BMI: body mass index BNR: beam non‐uniformity ratio LBP: low back pain RCT: randomised controlled trial SD: standard deviation US: ultrasound VAS: visual analogue scale
ROM: Range of Motion
SF‐36:Short Form Health Survey questionnaire
PRI/PPI: Pain Rating Index/present pain intensity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acar 2012 | Not participants with low back pain |
| Allen 2006 | Not a randomised controlled trial |
| Bertocco 2002 | Ultrasound as part of treatment package |
| Borman 2003 | Not a randomised controlled trial |
| Brockow 1997 | All groups had ultrasound – no control intervention |
| Charlusz 2010 | Participants with acute low back pain |
| Chipchase 2003 | Not a randomised controlled trial |
| Cloonan 1987 | Not a randomised controlled trial |
| Draper 1993 | Not a randomised controlled trial |
| Fiore 2011 | Participants with acute low back pain |
| Foster 1999 | Not a randomised controlled trial |
| Gorbunov 1997 | Not a randomised controlled trial |
| Goren 2010 | Participants with lumbar spinal stenosis |
| Greenough 2009 | Not a randomised controlled trial |
| Gurer 2005 | Ultrasound as part of treatment package |
| Haas 2004 | Ultrasound as part of treatment package |
| Hamm 2003 | Not a randomised controlled trial |
| Hurwitz 2002 | Ultrasound as part of treatment package |
| Jia 2003 | Ultrasound as part of treatment package |
| Kiralp 2009 | Not a randomised controlled trial |
| Koes 1992a | Ultrasound as part of treatment package |
| Koes 1992b | Ultrasound as part of treatment package |
| Koes 1993 | Ultrasound as part of treatment package |
| Koldas 2008 | Ultrasound as part of treatment package |
| Kumar 2009a | Ultrasound as part of treatment package |
| Kumar 2009b | Ultrasound as part of treatment package |
| Kumar 2010 | Ultrasound as part of treatment package |
| Leistner 1989 | Not a randomised controlled trial |
| Li 2007 | Ultrasound as part of treatment package |
| Lopes 2009 | Not a randomised controlled trial |
| Morrisette 2004 | Not a randomised controlled trial |
| Nordin 1999 | Not a randomised controlled trial |
| Nwuga 1983 | Participants with acute low back pain |
| Onel 1993 | Not a randomised controlled trial |
| Pensri 2005 | Not a randomised controlled trial |
| Poitras 2005 | Not a randomised controlled trial |
| Poitras 2008 | Not a randomised controlled trial |
| Roman 1960 | Not a randomised controlled trial |
| Rush 1994 | Not a randomised controlled trial |
| Sahin 2004 | Not a randomised controlled trial |
| Santiesteban 1984 | Participants with acute low back pain |
| Scott 2010 | Not a randomised controlled trial |
| Si 2005 | Not a randomised controlled trial |
| Tajali 2009 | Not a randomised controlled trial |
| Tander 2005 | Not a randomised controlled trial |
| Timm 1994 | Ultrasound as part of treatment package |
| Tonev 2010 | Ultrasound as part of treatment package |
| Unlu 2008 | Participants with acute low back pain |
| Wagner 1995 | Not a randomised controlled trial |
| Whitman 2006 | Participants with lumber spinal stenosis; ultrasound as part of treatment package |
| Wiesinger 1997 | Not a randomised controlled trial |
Characteristics of studies awaiting classification [ordered by study ID]
Ojoawo 2019.
| Methods | Randomised controlled trial |
| Participants | 50 participants with low back pain for greater than 90 days |
| Interventions | Intervention (n = 25): pulsed ultrasound, stabilisation exercise, and Lofnac gel Control (n = 25): kneading massage, stabilisation exercise, and Lofnac gel |
| Outcomes | Pain intensity (VAS); back‐specific functional status (Roland Morris Disability Questionnaire) |
| Notes |
Rubira 2019.
| Methods | Randomised controlled trial |
| Participants | 100 women, 18 to 40 years of age, with chronic non‐specific low back pain (VAS between 4 and 7) for longer than 4 months |
| Interventions | Intervention (n = 28): pulsed ultrasound Intervention (n = 30): continuous ultrasound Control (n = 29): pulsed laser Control (n = 24): no treatment |
| Outcomes | Pain intensity (VAS); back‐specific functional status (Roland Morris Disability Questionnaire) |
| Notes | ClinicalTrials.gov NCT02150096 |
Tantawy 2019.
| Methods | Randomised controlled trial |
| Participants | 45 participants with chronic non‐specific low back pain, aged 30 to 40 years |
| Interventions | Intervention (n = 15): continuous ultrasound plus exercise Control (n = 15): laser photobiomodulation therapy plus exercise Control (n = 15): exercise |
| Outcomes | Pain intensity (VAS); back‐specific functional status (Modified Oswestry Low Back Pain Disability Questionnaire, Pain Disability Index); lumbar range of motion |
| Notes |
Yurdakul 2019.
| Methods | Randomised controlled trial |
| Participants | 69 participants with chronic low back pain |
| Interventions | Intervention (n = 23): ultrasound plus hot pack and TENS Control (n = 23): hot pack and TENS Control (n = 23): no intervention |
| Outcomes | Pain intensity (numerical rating scale); back‐specific functional status (Oswestry Low Back Pain Disability Questionnaire); well‐being (SF‐36) |
| Notes | All participants were prescribed paracetamol 500 mg 3 times a day during the study period and were advised not to exercise until the end of the treatment. |
TENS: transcutaneous electrical nerve stimulation SF‐36: 36‐item Short Form Health Survey VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
NCT03835182.
| Study name | Efficacy of ultrasound versus short wave diathermy in the treatment of a slipped disc of the lower back |
| Methods | Randomised controlled trial |
| Participants | 93 participants aged 20 to 60 years old, with low back pain for greater than 3 months |
| Interventions | Group 1: ultrasound plus TENS, hot pack, exercise Group 2: short‐wave diathermy plus TENS, hot pack, exercise Group 3: TENS, hot pack, exercise |
| Outcomes | Pain intensity, disability, health‐related quality of life |
| Starting date | 14 September 2018 |
| Contact information | Selin Ozen; selinhassan@hotmail.com |
| Notes | Estimated completion date 30 June 2020 |
TENS: transcutaneous electrical nerve stimulation
Differences between protocol and review
Adverse events were not listed as an outcome in the protocol, but have been included in this update of the review.
The protocol listed PyscLIT as a database to be searched, but this was removed, and CINAHL, Index to Chiropractic Literature, ClinicalTrials.gov, and the WHO ICTRP were added as databases since publication of the protocol.
The timing of the outcome measures have been slightly modified from those stated in the protocol to align with recommendations from Cochrane Back and Neck.
'Risk of bias' assessments are now expressed by domain to align with current methodological recommendations.
The latest version of Review Manager (5.3.5) was used for this update.
Due to the small number of included studies, there were insufficient data to perform any subgroup or sensitivity analyses. In addition, funnel plots were not created.
Methods for this review update were revised according to published recommendations from Cochrane Back and Neck (Furlan 2015).
'Summary of findings' tables were created for two main comparisons: therapeutic ultrasound compared to placebo (Table 1) and therapeutic ultrasound plus exercise compared to exercise alone (Table 2).
We added additional information on unit of analysis issues with regard to cluster and cross‐over trials.
Contributions of authors
Review authors SE, NH, and MvT designed the protocol. SE and NH screened the studies, extracted the data, and performed the analyses. SE drafted the manuscript with help from the other review authors. All review authors read and approved the final version.
Declarations of interest
SE: no conflicts of interest to declare
NH: declares no conflicts of interest. NH works for Cochrane Response, an evidence consultancy initiative from Cochrane, but did not receive any payment for his contribution to this review.
BF: no conflicts of interest to declare
NNA: no conflicts of interest to declare
MvT: At the time of conducting this systematic review, MvT was a Co‐ordinating Editor of the Cochrane Back and Neck Review Group. He is currently on the Editorial Board and therefore was not part of the peer review or publication decision‐making process. Editorial Board members are required to author reviews to remain current in methods. MvT has also received grants and money to his institution from the Netherlands Organisation for Health Research and Development and The Dutch Health Insurance Council. He has also received travel, accommodation, or meeting expenses unrelated to the activities listed from EFIC (European Pain Federation) and the Danish Occupational Therapy Association. He declares no competing interest; all research funding comes from nonprofit, governmental funding agencies, and all funding including travel and stay expenses were paid to the Vrije Universiteit.
ABG: no conflicts of interest to declare
EF: no conflicts of interest to declare
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdel‐Aziem 2012 {published data only}
- Abdel-aziem AA, Ahmed ET, Youssief MA. LASER versus ultrasound in treatment of mechanical low back pain. Journal of American Science 2012;8(4):7. [Google Scholar]
Ansari 2006 {published data only}
- Ansari NN, Ebadi S, Talebian S, Naghdi S, Mazaheri H, Olyaei G, et al. A randomized, single blind placebo controlled clinical trial on the effect of continuous ultrasound on low back pain. Electromyography and Clinical Neurophysiology 2006;46:329-36. [PubMed] [Google Scholar]
Durmus 2010a {published data only}
- Durmus D, Akyol Y, Cengiz K, Terzi T, Cantürk F. Effects of therapeutic ultrasound on pain, disability, walking performance, quality of life, and depression in patients with chronic low back pain: a randomized, placebo controlled trial. Turkish Journal of Rheumatology 2010;25:82-7. [DOI] [PubMed] [Google Scholar]
Durmus 2010b {published data only}
- Durmus D, Durmaz Y, Canturk F. Effects of therapeutic ultrasound and electrical stimulation program on pain, trunk muscle strength, disability, walking performance, quality of life, and depression in patients with low back pain: a randomized-controlled trial. Rheumatology International 2010;30:901-10. [DOI] [PubMed] [Google Scholar]
Durmus 2013 {published data only}
- Durmus D, Alayli G, Goktepe AS, Taskaynatan MA, Bilgici A, Kuru O. Is phonophoresis effective in the treatment of chronic low back pain? A single-blind randomized controlled trial. Rheumatology International 2013;33:1737-44. [DOI] [PubMed] [Google Scholar]
Ebadi 2012 {published data only}
- Ebadi S, Ansari NN, Naghdi S, Jalaei S, Sadat M, Bagheri H, et al. The effect of continuous ultrasound on chronic non-specific low back pain: a single blind placebo-controlled randomized trial. BMC Musculoskeletal Disorders 2012;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grubisic 2006 {published data only}
- Grubisic F, Grazio S, Jajic Z, Nemcic T. Therapeutic ultrasound in chronic low back pain treatment. Reumatizam 2006;53(1):18-21. [PubMed] [Google Scholar]
Khan 2013 {published and unpublished data}
- Khan S, Shamsi S, Alyaemni AAA, Abdelkader S. Effect of ultrasound and exercise combined and exercise alone in the treatment of chronic back pain. Indian Journal of Physiotherapy & Occupational Therapy 2013;7(2):6. [Google Scholar]
Licciardone 2013 {published and unpublished data}
- Licciardone JC, Minotti DE, Gatchel RJ, Kearns CM, Singh KP. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Annals of Family Medicine 2013;11(2):122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohseni‐Bandpei 2006 {published data only}
- Mohseni-Bandpei MA, Critchley J, Staunton T, Richardson B. A prospective randomised controlled trial of spinal manipulation and ultrasound in the treatment of chronic low back pain. Physiotherapy 2006;92:34-42. [Google Scholar]
References to studies excluded from this review
Acar 2012 {published data only}
- Acar B, Yilmaz OT. Effects of different physiotherapy applications on pain and mobility of connective tissue in patients with myofascial pain syndrome. Journal of Back and Musculoskeletal Rehabilitation 2012;25(4):261-7. [DOI] [PubMed] [Google Scholar]
Allen 2006 {published data only}
- Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Physical Medicine and Rehabilitation Clinics of North America 2006;17:315-45. [DOI] [PubMed] [Google Scholar]
Bertocco 2002 {published data only}
- Bertocco P, Montesano A, Baccalaro G, Parisio C, Vismara L. Controlled study on the efficacy of two different treatments in obese patients affected by chronic low back pain, assessed by an isokinetic device: analysis of muscle strength and spine mobility. Europa Medicophysica 2002;38(4):187-93. [Google Scholar]
Borman 2003 {published data only}
- Borman P, Keskin D, Bodur H. The efficacy of lumbar traction in the management of patients with low back pain. Rheumatology International 2003;23(2):82-6. [DOI] [PubMed] [Google Scholar]
Brockow 1997 {published data only}
- Brockow T, Schreiber U, Smolenski U, Frohlich A. Pain intensity and power densities of therapeutic ultrasound - a serial, comparative pilot study in patients with low back pain. Schmerz 1997;11(6):396-9. [DOI] [PubMed] [Google Scholar]
Charlusz 2010 {published data only}
- Charlusz M, Gasztych J, Irzmanski R, Kujawa J. Comparative analysis of analgesic efficacy of selected physiotherapy methods in low back pain patients. Ortopedia Traumatologia Rehabilitacja 2010;12(3):225-36. [PubMed] [Google Scholar]
Chipchase 2003 {published data only}
- Chipchase LS, Trinkle D. Therapeutic ultrasound: clinician usage and perception of efficacy. Hong Kong Physiotherapy Journal 2003;21:5-14. [Google Scholar]
Cloonan 1987 {published data only}
- Cloonan MA, Wagstaff PS. A pilot study to compare the efficacy of diadynamic and a combined treatment of diadynamic and ultrasound on the relief of chronic low back pain. Iranian Journal of Medical Science 1987;156(10):292. [Google Scholar]
Draper 1993 {published data only}
- Draper DO, Sunderland S, Kirkendall DT, Ricard M. A comparison of temperature rise in human calf muscles following applications of underwater and topical gel ultrasound. Journal of Orthopaedic and Sports Physical Therapy 1993;17(5):247-51. [DOI] [PubMed] [Google Scholar]
Fiore 2011 {published data only}
- Fiore P, Panza F, Cassatella G, Russo A, Frisardi V, Solfrizzi V, et al. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of low back pain: a randomized controlled trial. European Journal of Physical & Rehabilitation Medicine 2011;47(3):367-73. [PubMed] [Google Scholar]
Foster 1999 {published data only}
- Foster NE, Thompson KA, Baxter GD, Allen JM. Management of nonspecific low back pain by physiotherapists in Britain and Ireland: a descriptive questionnaire of current clinical practice. Spine 1999;24(13):1332-42. [DOI] [PubMed] [Google Scholar]
Gorbunov 1997 {published data only}
- Gorbunov FE, Vinnikov AA, Krupennikov AI, Kubalova MN. Methods of instrumental physiotherapy in the rehabilitative treatment of pareses caused by nerve compression of the extremities and spinal nerve root compression. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 1997;5:22-4. [PubMed] [Google Scholar]
Goren 2010 {published data only}
- Goren A, Yildiz N, Topuz O, Findikoglu G, Ardic F. Efficacy of exercise and ultrasound in patients with lumbar spinal stenosis: a prospective randomized controlled trial. Clinical Rehabilitation 2010;24:623-31. [DOI] [PubMed] [Google Scholar]
Greenough 2009 {published data only}
- Greenough CG. Degenerative disc and vertebral disease - clinical. Surgery 2009;27(7):301-5. [Google Scholar]
Gurer 2005 {published data only}
- Gurer G, Sendur OF, Beydag OB. The effect of physical therapy on pain and activity of daily life in patients with low back pain. Journal of Rheumatology and Medical Rehabilitation 2005;16(4):237-42. [Google Scholar]
Haas 2004 {published data only}
- Haas M, Groupp E , Kraemer DF. Dose-response for chiropractic care of chronic low back pain. Spine Journal: Official Journal of the North American Spine Society 2004;4(5):574-83. [DOI] [PubMed] [Google Scholar]
Hamm 2003 {published data only}
- Hamm L, Mikkelsen B, Kuhr J, Stovring H, Munck A, Kragstrup J. Danish physiotherapists' management of low back pain. Advances in Physiotherapy 2003;5(3):109-13. [Google Scholar]
Hurwitz 2002 {published data only}
- Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Belin TR, Yu F, et al. The effectiveness of physical modalities among patients with low back pain randomized to chiropractic care: findings from the UCLA low back pain study. Journal of Manipulative and Physiological Therapeutics 2002;25(1):10-20. [DOI] [PubMed] [Google Scholar]
Jia 2003 {published data only}
- Jia J, Zhang X. Combination of physical therapies on 80 cases of protrusion of lumbar intervertebral discs. Chinese Journal of Clinical Rehabilitation 2003;7(4):704. [Google Scholar]
Kiralp 2009 {published data only}
- Kiralp MZ, Cakar E, Dincer U, Durmus O. Effectiveness of the physical therapy agents on lumbar spondylosis treatment. Arthritis and Rheumatism 2009;60:1185. [Google Scholar]
Koes 1992a {published data only}
- Koes BW, Bouter LM, Mameren H, Essers AH, Verstegen GM, Hofhuizen DM, et al. The effectiveness of manual therapy, physiotherapy, and treatment by the general practitioner for nonspecific back and neck complaints. A randomized clinical trial. Spine 1992;17(1):28-35. [DOI] [PubMed] [Google Scholar]
Koes 1992b {published data only}
- Koes BW, Bouter LM, Mameren H, Essers AH, Verstegen GM, Hofhuizen DM, et al. Randomised clinical trial of manipulative therapy and physiotherapy for persistent back and neck complaints: results of one year follow up. BMJ 1992;304(6827):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koes 1993 {published data only}
- Koes BW, Bouter LM, Mameren H, Essers AH, Verstegen GJ, Hofhuizen DM, et al. A randomized clinical trial of manual therapy and physiotherapy for persistent back and neck complaints: subgroup analysis and relationship between outcome measures. Journal of Manipulative & Physiological Therapeutics 1993;16(4):211-9. [PubMed] [Google Scholar]
Koldas 2008 {published data only}
- Koldas DS, Sonel TB, Kurtais Y, Atay MB. Comparison of three different approaches in the treatment of chronic low back pain. Clinical Rheumatology 2008;27(7):873-81. [DOI] [PubMed] [Google Scholar]
Kumar 2009a {published data only}
- Kumar S, Negi MP, Sharma VP, Shukla R, Dev R, Mishra UK. Efficacy of two multimodal treatments on physical strength of occupationally subgrouped male with low back pain. Journal of Back & Musculoskeletal Rehabilitation 2009;22(3):179-88. [DOI] [PubMed] [Google Scholar]
Kumar 2009b {published data only}
- Kumar S, Sharma VP, Negi MP. Efficacy of dynamic muscular stabilization techniques (DMST) over conventional techniques in rehabilitation of chronic low back pain. Journal of Strength & Conditioning Research 2009;23(9):2651-9. [DOI] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar S, Sharma VP, Shukla R, Dev R. Comparative efficacy of two multimodal treatments on male and female sub-groups with low back pain (part II). Journal of Back & Musculoskeletal Rehabilitation 2010;23(1):1-9. [DOI] [PubMed] [Google Scholar]
Leistner 1989 {published data only}
- Leistner K, Wessel G, Braunig E, Jeremies C, Jeremies J. Type and frequency of physiotherapy in patients with rheumatic diseases in the country of Gera. Results of an epidemiological study. Zeitschrift fur Physiotherapie 1989;41(5):323-7. [Google Scholar]
Li 2007 {published data only}
- Li XY, Huang ZM, Zhang CJ, Chen XW, Lin QL, Li TR. Therapeutic effect of compositive rehabilitation on lumber disc herniation. Journal of Central South University (Medical Sciences) 2007;32(1):144-7. [PubMed] [Google Scholar]
Lopes 2009 {published data only}
- Lopes AD, Barreto HJ, Aguiar RC, Gondo FB, Neto JG. Brazilian physiotherapy services in the 2007 Pan-American Games: injuries, their anatomical location and physiotherapeutic procedures. Physical Therapy in Sport 2009;10(2):67-70. [DOI] [PubMed] [Google Scholar]
Morrisette 2004 {published data only}
- Morrisette DC, Brown D, Saladin ME. Temperature change in lumbar periarticular tissue with continuous ultrasound. Journal of Orthopaedic & Sports Physical Therapy 2004;34(12):754-60. [DOI] [PubMed] [Google Scholar]
Nordin 1999 {published data only}
- Nordin M, Campello M. Physical therapy: exercises and the modalities: when, what, and why? Neurologic Clinics 1999;17(1):75-89. [DOI] [PubMed] [Google Scholar]
Nwuga 1983 {published data only}
- Nwuga VC. Ultrasound in treatment of back pain resulting from prolapsed intervertebral disc. Archives of Physical Medicine & Rehabilitation 1983;64(2):88-9. [PubMed] [Google Scholar]
Onel 1993 {published data only}
- Onel D, Sari H, Donmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients: conservative treatment or surgical intervention? Spine 1993;18(2):291-8. [PubMed] [Google Scholar]
Pensri 2005 {published data only}
- Pensri P, Foster NE, Srisuk S, Baxter GD, McDonough SM. Physiotherapy management of low back pain in Thailand: a study of practice. Physiotherapy Research International 2005;10(4):201-12. [DOI] [PubMed] [Google Scholar]
Poitras 2005 {published data only}
- Poitras S, Blais R, Swaine B, Rossignol M. Management of work-related low back pain: a population-based survey of physical therapists. Physical Therapy 2005;85(11):1168-81. [PubMed] [Google Scholar]
Poitras 2008 {published data only}
- Poitras S, Brosseau L. Evidence-informed management of chronic low back pain with transcutaneous electrical nerve stimulation, interferential current, electrical muscle stimulation, ultrasound, and thermotherapy. Spine Journal 2008;8(1):226-33. [DOI] [PubMed] [Google Scholar]
Roman 1960 {published data only}
- Roman MP. A clinical evaluation of ultrasound by use of a placebo technic. Physical Therapy Review 1960;40:649-52. [DOI] [PubMed] [Google Scholar]
Rush 1994 {published data only}
- Rush PJ, Shore A. Physician perceptions of the value of physical modalities in the treatment of musculoskeletal disease. British Journal of Rheumatology 1994;33(6):566-8. [DOI] [PubMed] [Google Scholar]
Sahin 2004 {published data only}
- Sahin O, Donmez-Altuntas H, Hizmetli S, Hamurcu Z, Imamoglu N. Investigation of genotoxic effect of ultrasound in cases receiving therapeutic ultrasound by using micronucleus method. Ultrasound in Medicine & Biology 2004;30(4):545-8. [DOI] [PubMed] [Google Scholar]
Santiesteban 1984 {published data only}
- Santiesteban AJ. Comparison of electroacupuncture and selected physical therapy for acute spine pain. American Journal of Acupuncture 1984;12(3):257-61. [Google Scholar]
Scott 2010 {published data only}
- Scott NA, Moga C, Harstall C. Managing low back pain in the primary care setting: the know-do gap. Pain Research & Management 2010;15(6):392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Si 2005 {published data only}
- Si RS, Xiong CM, Han DJ, Zhu FJ, Ning L, Zhang M. Clinical observation on McKenzie mechanics principle plus ultrashort wave in treatment of lumbar and leg pain. Zhongguo Linchuang Kangfu 2005;9(26):210-2. [Google Scholar]
Tajali 2009 {published data only}
- Tajali SB, Azari A, Elahi F, Javadi S, Abbasi S. The clinical effects of high and low frequency ultrasound therapy on chronic low back pain. Pain Practice 2009;9:166. [Google Scholar]
Tander 2005 {published data only}
- Tander B, Canturk F, Cengiz K, Durmus D, Akyol Y. Are the physical therapeutic modalities really safe? Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2005;51(4):131-3. [Google Scholar]
Timm 1994 {published data only}
- Timm KE. A randomized-control study of active and passive treatments for chronic low back pain following L5 laminectomy. Journal of Orthopaedic & Sports Physical Therapy 1994;20(6):276-86. [DOI] [PubMed] [Google Scholar]
Tonev 2010 {published data only}
- Tonev D, Radeva S, Toncheva A. Non-pharmacological treatment of subacute and chronic low back pain without radiculopathy: acupuncture versus physiotherapy. Rheumatology 2010;18(2):46-50. [Google Scholar]
Unlu 2008 {published data only}
- Unlu Z, Tasci S, Tarhan S, Pabuscu Y, Islak S. Comparison of 3 physical therapy modalities for acute pain in lumbar disc herniation measured by clinical evaluation and magnetic resonance imaging. Journal of Manipulative & Physiological Therapeutics 2008;31(3):191-8. [DOI] [PubMed] [Google Scholar]
Wagner 1995 {published data only}
- Wagner UA, Diedrich V, Schmitt O. Determination of skeletal maturity by ultrasound: a preliminary report. Skeletal Radiology 1995;24(6):417-20. [DOI] [PubMed] [Google Scholar]
Whitman 2006 {published data only}
- Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill HE, Ryder MG, et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine 2006;31(22):2541-9. [DOI] [PubMed] [Google Scholar]
Wiesinger 1997 {published data only}
- Wiesinger GF, Quittan M, Ebenbichler G, Kaider A, Fialka V. Benefit and costs of passive modalities in back pain outpatients: a descriptive study. European Journal of Physical Medicine and Rehabilitation 1997;7(6):182-6. [Google Scholar]
References to studies awaiting assessment
Ojoawo 2019 {published data only}
- Ojoawo AO, Malomo EO, Olusegun EO, Olaogun BM. Effects of pulse ultrasound and kneading massage in managing individual with incessant pain at lower region of back using random allocation. Journal of Exercise Rehabilitation 2018;14(3):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubira 2019 {published data only}
- Rubira AP, Rubira MC, Rubira LD, Comachio J, Magalhães MO, Marques AP. Comparison of the effects of low-level laser and pulsed and continuous ultrasound on pain and physical disability in chronic non-specific low back pain: a randomized controlled clinical trial. Advances in Rheumatology 2019;59(1):57. [DOI] [PubMed] [Google Scholar]
Tantawy 2019 {published data only}
- Tantawy SA, Abdelbasset WK, Kamel DM, Alrawaili SM, Alsubaie SF. Laser photobiomodulation is more effective than ultrasound therapy in patients with chronic nonspecific low back pain: a comparative study. Lasers in Medical Science 2019;34(4):793-800. [DOI] [PubMed] [Google Scholar]
Yurdakul 2019 {published data only}
- Yurdakul OV, Beydoğan E, Yalçınkaya EY. Effects of physical therapy agents on pain, disability, quality of life, and lumbar paravertebral muscle stiffness via elastography in patients with chronic low back pain. Turkish Journal of Physical Medicine and Rehabilitation 2019;65(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT03835182 {published data only}
- NCT03835182. Efficacy of ultrasound versus short wave diathermy in the treatment of a slipped disc of the lower back. clinicaltrials.gov/ct2/show/nct03835182 (first received 8 February 2019).
Additional references
Bekkering 2003
- Bekkering GE, Hendriks HJM, Koes BW, Oostendorp RAB, Ostelo RWJG, Thomassen JMC, et al. Dutch physiotherapy guidelines for low back pain. Physiotherapy 2003;89:82-96. [Google Scholar]
Chou 2017
- Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine 2017;4:166. [DOI] [PubMed] [Google Scholar]
Dyson 1976
- Dyson M, Franks C, Suckling J. Stimulation of healing of varicose ulcers by ultrasound. Ultrasonics 1976;14:232-6. [DOI] [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al from the Editorial Board of the Cochrane Back, Neck Group. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660-73. [DOI] [PubMed] [Google Scholar]
Gay 2001
- Gay RE, Brault JS. Evidence-informed management of chronic low back pain with traction therapy. Spine Journal 2001;8:234-42. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 1 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Haldeman 2008
- Haldeman S, Dagenais S. What have we learned about the evidence informed management of chronic low back pain? Spine Journal 2008;8:266-77. [DOI] [PubMed] [Google Scholar]
Henschke 2015
- Henschke N, Kamper SJ, Maher CG. The epidemiology and economic consequences of pain. Mayo Clinic Proceedings 2015;90(1):139-47. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Krismer 2007
- Krismer M, Tulder MW. Low back pain (non-specific). Best Practice & Research in Clinical Rheumatology 2007;21:77-91. [DOI] [PubMed] [Google Scholar]
Maher 2017
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389(10070):736-747. [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellence. Low back pain and sciatica in over 16s: assessment and management. NICE guideline [NG59]. www.nice.org.uk/guidance/ng59 (accessed prior to 2 June 2020).
O'Brien 2007
- O'Brien WD. Ultrasound - biophysics mechanisms. Progress in Biophysics and Molecular Biology 2007;93:212-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2017
- Qaseem Amir , Wilt Timothy J , McLean Robert M, Forcie aMary Ann. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine 2017;166(7):514-30. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2006
- Robertson VJ, Ward A, Low J, Reed A. Electrotherapy Explained: Principles and Practice. 4th edition. Butterworth Heinemann, 2006. [Google Scholar]
Saragiotto 2016
- Saragiotto BT, Maher CG, Yamato TP, Costa LC, Ostelo RW, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Waddell 2004
- Waddell G. The Back Pain Revolution. 2nd edition. Churchill Livingstone, 2004. [Google Scholar]
Watson 2008
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008;48:321-9. [DOI] [PubMed] [Google Scholar]
Wegner 2013
- Wegner I, Widyahening IS, Tulder MW, Blomberg SE, Vet HC, Brønfort G, et al. Traction for low-back pain with or without sciatica. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003010.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ebadi 2011
- Ebadi S, Henschke N, Ansari NN, Fallah E, Tulder MW. Therapeutic ultrasound for chronic low-back pain. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009169.pub2] [DOI] [PubMed] [Google Scholar]


