Abstract
Background
Mycoplasma genitalium (MG) causes symptomatic urethritis in men, and can infect alone or together with other sexually transmitted infection (STI) agents.
Methods
The prevalence of MG and other STIs was determined in 1816 men with symptomatic urethritis. Resistance of MG to macrolides and fluoroquinolones was determined by sequencing; the impact of recent antimicrobial usage on the distribution of MG single or mixed infections was determined.
Results
Overall, prevalence of MG infection was 19.7% (358/1816). Fifty-four percent (166/307) of MG infections occurred alone in the absence of other STI agents. Men with single MG infection self-administered or were prescribed antibiotics more often in the 30 days prior to enrollment than subjects with urethritis caused by MG coinfection (P < .0001). Higher rates (96.7%) of infection with macrolide resistance in MG were identified in men who had taken macrolides prior to enrollment (P < .03). Overall, 88.9% (303/341) of 23S ribosomal RNA (rRNA) genes contained mutations responsible for macrolide resistance; 89.5% (308/344) of parC and 12.4% (42/339) of gyrA genes had mutations responsible for fluoroquinolone resistance. Approximately 88% (270/308) of MG had combined mutations in 23S rRNA and parC genes; 10.4% (32/308) had mutations in all 3 genes.
Conclusions
MG was the single pathogen identified in 11% of men with symptomatic urethritis. Overall, nearly 90% of MG infections were resistant to macrolides and fluoroquinolones. Men who took macrolides in the 30 days prior to enrollment had higher rates (97%) of macrolide-resistant MG. Resistance was associated with numerous mutations in 23SrRNA, parC, and gyrA genes.
Keywords: Mycoplasma genitalium, urethritis, macrolide/quinolone resistance
Mycoplasma genitalium (MG) can be the only cause of symptomatic urethritis in men who have taken ineffective antibiotics. In Nanjing, China, nearly 90% of MG infections were resistant to macrolides (and fluoroquinolones), implicating possible antibiotic selection that resulted in persistent infection.
(See the Editorial commentary by Singh and Manhart on pages 811–3.)
Mycoplasma genitalium (MG) is an established sexually transmitted infection (STI) agent and is associated with acute and chronic or persistent nongonococcal, nonchlamydial (NGNC) urethritis [1, 2] and proctitis in men who have sex with men [3]. In women, MG infection is correlated with cervicitis, endometritis, pelvic inflammation, infertility, and preterm delivery/spontaneous abortion [1, 4]. An association between MG and human immunodeficiency virus (HIV) infection also exists [5].
Increasing antibiotic resistance in MG has emerged [6]. Doxycycline treatment results in clearance rates as low as 30% [7]; clearance of MG following treatment with 1 g of azithromycin declined from 85% in 12 studies performed before 2009 to 67% in 9 studies after 2009 [8]. Treatment failures with azithromycin are documented in numerous countries [9–11]. Dual resistance to fluoroquinolones and macrolides has also been reported from at least 3 continents [11–14]. Macrolide resistance in MG is mediated by mutations in the 23S ribosomal RNA (rRNA) gene [9], and resistance to fluoroquinolones by mutations in the parC and gyrA genes [13, 15, 16].
In China, nucleic acid amplification testing for MG is not performed routinely in sexually transmitted disease (STD) or hospital clinics. The aims of our study were to highlight the prevalence of MG infections in male subjects with symptomatic urethritis, the distribution/pattern of macrolide and quinolone resistance in MG, and the association between prior antibiotic use and increased antimicrobial resistance of MG.
MATERIALS AND METHODS
Study Population
A total of 1816 male subjects were enrolled in a cohort study of gonococcal transmission from April 2011 to August 2015 at the Institute of Dermatology, Chinese Academy of Medical Sciences (CAMS), STD Clinic in Nanjing, China, a city of 8.3 million inhabitants. Men were eligible if they had symptomatic urethritis defined by urethral discharge and/or dysuria. Laboratory testing was performed on urethral specimens to identify causes of urethritis and to determine the degree of inflammation. The institutional review boards of the Institute of Dermatology, CAMS (approval number 2009-62), the University of Massachusetts Medical School (approval number 13448), and the Boston University School of Public Health (approval number H28858) approved the study; participants provided written informed consent. Demographic characteristics and sexual behavior, STI, and antibiotic histories were obtained and recorded in a centralized relational SQL (structured query language) database with an ASP.NET web front end using standardized data collection and sample tracking forms.
Laboratory Testing
First-void urine specimens and urethral swabs were collected. Urine specimens were examined for Chlamydia trachomatis (CT), MG, and Trichomonas vaginalis (TV) by polymerase chain reaction (PCR); urethral exudates were examined immediately by Gram stain for gram-negative intracellular diplococci to identify presumptive gonococci and directly swabbed onto culture plates for Neisseria gonorrhoeae (NG) (modified Thayer-Martin medium, Zhuhai DL Biotech Co Ltd); exudates were immediately inoculated onto liquid culture media (Mycoplasma IST2, bioMérieux) for Ureaplasma subspecies (including Ureaplasma urealyticum and Ureaplasma parvum)/Mycoplasma hominis), and urine specimens were examined for CT by PCR (DAAN Gene Co Ltd). Urine specimens were stored at –80°C; MG-positive urine specimens were analyzed further for macrolide and fluoroquinolone resistance. Polymorphonuclear leukocytes (PMNs) were enumerated microscopically per high-power field (HPF) on glass slides of Gram-stained urethral exudates.
Mycoplasma genitalium Testing
MG Identification
DNA was extracted from urine samples using the Rapid Bacterial Genomic DNA Isolation Kit (DNA Quick Extract Kit, Epicentre). Identification of MG was performed using nested PCR that targeted the mgpA gene. Outer primers have been described previously [17]; inner primers were designed to detect a 233-bp fragment of the mgpA gene (Supplementary Table 1). Positive samples were confirmed by DNA sequencing (Applied Biosystems 3730xl DNA analyzer).
Macrolide and Fluoroquinolone Resistance
Macrolide resistance–mediating mutations in region V of the 23S rRNA gene and fluoroquinolone resistance mutations in the parC and gyrA genes were detected by nested PCR and confirmed by DNA sequencing. Primers used for amplification of 23S rRNA have been described previously [9]; parC gene (nucleotides 164–377) and gyrA gene (nucleotides 172–402) primers were designed using online National Center for Biotechnology Information (NCBI) design tools (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Supplementary Table 1). Amplification products were sequenced in both directions (Nanjing Sai-Yinsi Biological Technology Co Ltd) and targeted genes were aligned with corresponding MG G37 sequences (GenBank accession number NC_00908.2; https://www.ncbi.nlm.nih.gov/genome /?term=mycoplasma+genitalium).
Trichomonas vaginalis Testing
Urine specimens were tested for TV by 2 different nested PCRs; primers for repetitive DNA sequence (TVC) [18] and a modification of β-tubulin primers (BTUBx [19]) were used (Supplementary Table 1).
Data Analysis
Clinical correlations and comparisons of data sets were examined for significance using standard online comparisons and χ2 or Fisher exact tests. Exact binomial confidence intervals (CIs) were estimated for rates. The association of antibiotic use and single MG infections was estimated and tested after adjusting for potentially confounding covariates. SPSS 19.0 (Chinese Edition) and Stata 15 (StataCorp, LLC, College Station, Texas) software programs were used for statistical analysis. Sequencing results were analyzed using NCBI blast and SnapGene 2.8.2 software. Comparisons of gene mutation rates over time were performed using a logistic regression test of trend. P ≤ .05 (2-sided) was considered significant. GraphPad Prism 6 software was used for the illustration.
RESULTS
Mycoplasma genitalium Infection: Prevalence
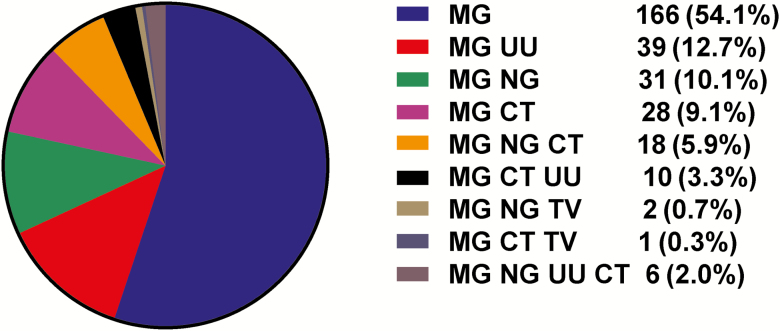
Overall, the prevalence of MG infection among 1816 male subjects with symptomatic urethritis was 19.7% (95% CI, 17.9%–19.7%), lower than rates of infection with NG (46.4% [95% CI, 44.1%–48.7%]) and CT (33.1% [95% CI, 30.9%–35.3%]) and higher than rates with TV (2.59% [95% CI, 1.9%–3.4%]) (Supplementary Table 2). There was no difference in percentage of MG positivity identified in 287 specimens that were not tested for Ureaplasma species and M. hominis compared to the 1816 specimens (total) or the 1529 that were fully characterized (Supplementary Table 2). Ureaplasma species were identified in 18.4% (95% CI, 16.5%–20.4%) of 1529 cases; infection with M. hominis was negligible (<1%). In the 1529 cases, prevalence of MG infection alone (single infection) was 10.9% (95% CI, 9.3%–12.5%), which was lower than rates of gonococcal (26.9% [95% CI, 24.7%–29.2%]) and chlamydial (16.7% [95% CI, 14.9%–18.7%]) single infections (Supplementary Table 3). Single infection with MG was seen in 54.1% (95% CI, 48.3%–59.7%) of all MG cases (Figure 1 and Supplementary Table 3). In particular, among commonly classified etiologic causes of urethritis (gonococcal alone, chlamydial alone, or gonococcal/chlamydial together), the rates of coinfection with MG were 8.4%, 9.8%, and 15.7%, respectively, compared with MG infection alone (41.4%) (P < .0001 comparing each coinfection with MG alone; Supplementary Table 4). MG is a common cause of symptomatic NGNC urethritis in this population and was less commonly associated with gonococcal and/or chlamydial coinfections.
Figure 1.
Distribution of single infections and coinfections among 307 men with urethritis and Mycoplasma genitalium infection. Abbreviations: CT, Chlamydia trachomatis; MG, Mycoplasma genitalium; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis; UU, Ureaplasma urealyticum.
Symptomatic Urethritis in Men With Single MG Infection
Demographic and Laboratory Characteristics
Among 166 men with single MG infection (no other STIs identified), the mean age was 36.5 years (range, 20–62 years); the median level of education completed was senior high school (see Supplementary Table 7B for stratification of educational levels). One hundred thirty-five (81.3%) were married and all but one were ethnic Han. All men with single MG infection reported that they were heterosexual; 57 of 165 (34.5%) reported ≥2 female sexual partners in the 30 days before their clinic visit, and 56 of 162 (34.5%) reported a previous episode of gonorrhea.
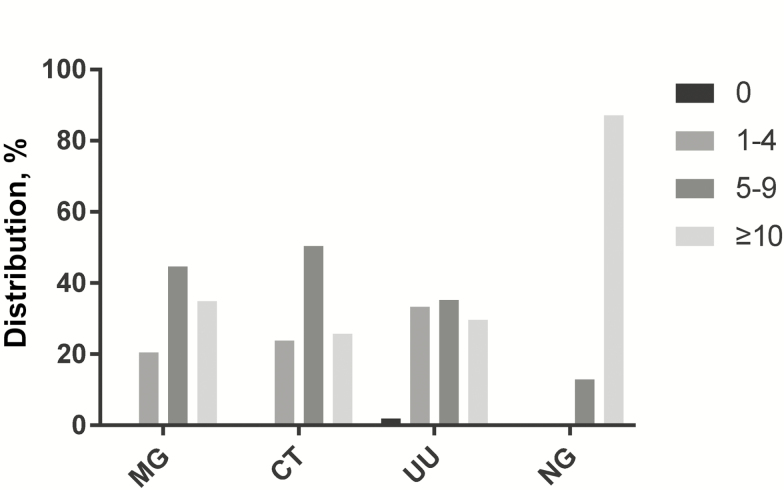
All 166 subjects with single MG infection had microscopic evidence of urethral inflammation, judged by PMN counts on Gram stains of urethral exudates (≥1 PMNs/HPF). The degree of inflammation, in men with single MG infection and urethritis, overall, was less than that in single infections with NG (χ2 = 183.233, P < .0001) and similar to single infections with CT (χ2 = 4.083, P = .138) and U. urealyticum (χ2 = 7.113, P = .068) (Figure 2 and Supplementary Table 5).
Figure 2.
Microscopic signs of urethral inflammation (polymorphonuclear lymphocytes per high-power field [PMNs/HPF]) in subjects having Mycoplasma genitalium (MG) infection alone (n = 166), Chlamydia trachomatis (CT) infection alone (n = 256), Ureaplasma urealyticum (UU) infection alone (n = 54), and Neisseria gonorrhoeae (NG) infection alone (n = 412). Statistical comparison of the distribution of PMNs/HPF in MG infection vs other single infections is described in Supplementary Table 5.
Antibiotic Usage
Of 166 men with single MG infection, 63.9% reported that they had taken antibiotics (self-administered or prescribed) in the 30 days prior to their STD clinic visit compared with 46 of the 141 (32.6%) remaining subjects with MG infection coinfected with other STIs (P < .0001) and 36.6% of 1222 non-MG-infected subjects (P < .0001) (Supplementary Table 6). Among the 166 men with single MG infection, 44 (26.5%) had taken macrolides (including azithromycin, clarithromycin, and roxithromycin); 41 (24.7%) cephalosporins; 22 (13.3%) quinolones, and 17 (10.2%) tetracycline class antibiotics (doxycycline and minocycline).
The prevalence of MG single infection in subjects who had taken antibiotics in the 30 days before their STD clinic visit was 17.7% (106/599) vs 6.5% (59/910) in those who had not taken antibiotics (χ2 = 45.5, P < .0001; unadjusted odds ratio [OR], 3.101 [95% CI, 2.214–4.345]) (Supplementary Table 7A). When adjusted for age, educational level, marital status, having ≥2 sexual partners in the 30 days before the clinic visit, a previous episode of gonorrhea, and PMN counts in the urethral smear, there was minimal effect (adjusted OR, 2.903 [95% CI, 2.048–4.116]) (Supplementary Table 7B).
Macrolide Resistance–associated Mutations in MG
The 23S rRNA gene was successfully amplified and sequenced in 341 of 358 (95.3%) mgpA-positive MG samples; mutations in the 23S rRNA gene were detected in 303 (88.9%) samples. The mutation sites were located at positions 2071 and 2072 (corresponding to positions 2058 and 2059 in Escherichia coli 23S rRNA). The most common mutation was A2059G, accounting for 211 of 341 (61.9%) of mutations, followed by A2058G (17.6%), A2059T (8.8%), A2059C (0.3%), and A2058C (0.3%) (Table 1).
Table 1.
Frequency of Mutations Identified in the 23S Ribosomal RNA, parC, and gyrA Genes
| Gene and Positiona | Mutation | Amino Acid Change | No. of Samples |
|---|---|---|---|
| 23S rRNA | |||
| 2071 (2058)a | A-C | No amino acid product | 1 |
| A-G | No amino acid product | 60 | |
| 2072 (2059)a | A-C | No amino acid product | 1 |
| A-G | No amino acid product | 211 | |
| A-T | No amino acid product | 30 | |
| WT | … | … | 38 |
| parC | |||
| 225 | G-A | No change | 1 |
| 234 | C-T | No change | 2 |
| 241 | G-T | Gly81Cys | 1 |
| 248 | G-A | Ser83Asn | 4 |
| 248; 259 | G-A; G-T | Ser83Asn; Asp87Tyr | 2 |
| 248 | G-T | Ser83Ile | 288 |
| 259 | G-A | Asp87Asn | 4 |
| 259 | G-T | Asp87Tyr | 8 |
| 260 | A-G | Asp87Gly | 1 |
| WT | … | … | 33 |
| gyrA | |||
| 240 | G-A | No change | 1 |
| 244; 285 | G-A; G-A | Val82Ile; Met95Ile | 1 |
| 249 | T-C | No change | 1 |
| 277 | G-T | Gly93Cys | 1 |
| 283 | A-G | Met95Val | 1 |
| 285 | G-A | Met95Ile | 18 |
| 285 | G-C | Met95Ile | 3 |
| 285 | G-T | Met95Ile | 1 |
| 295 | G-A | Asp99Asn | 6 |
| G-T | Asp99Tyr | 4 | |
| 296 | A-G | Asp99Gly | 4 |
| 285; 309 | G-A; A-G | Met95Ile; No change | 1 |
| 285; 317 | G-A; A-G | Met95Ile; Glu106Arg | 2 |
| WT | … | … | 295 |
Abbreviations: rRNA, ribosomal RNA; WT, wild type.
a Escherichia coli numbering is indicated in parentheses for 23S rRNA mutations.
Fluoroquinolone Resistance–associated Mutations in MG
The parC gene was successfully amplified and sequenced in 344 of 358 (96.1%) mgpA-positive MG samples; 90.4% (311/344) of MG samples showed a mutation(s). Missense mutation(s) were seen in 89.5% (308/344). Six separate missense mutations were identified in the parC gene, at positions 241, 248, 259, and 260 (Table 1). Most samples (85% [294/344]) had a single-nucleotide substitution at position 248; 83.7% (288/344) displayed a G248T single-nucleotide substitution, which results in an aa change at position 83 (Ser83→Ile) in ParC. A double parC gene mutation (G248A and G259T) was seen in 2 samples.
The gyrA gene was successfully amplified and sequenced in 339 of 358 (94.7%) mgpA-positive MG samples; 13.0% of MG samples showed a mutation(s); missense mutation(s) were seen in 12.4%. Ten separate missense mutations were identified in the gyrA gene, at positions 244, 277, 283, 285, 295, 296, and 317 (Table 1). Among 42 samples with missense mutations, 26 (61.9%) had a single-nucleotide substitution at position 285, which would result in an aa change at position 95 (Met95→Ile) in GyrA. Ten (23.8%) samples had mutations at position 295 (Asp99→Asn or Tyr). Double mutations in the gyrA gene were detected in 4 samples. All samples that possessed gyrA gene missense mutations also had parC gene mutation(s).
Three hundred eight samples had all 3 resistance genes successfully amplified and analyzed; 87.7% had mutations in both the 23S rRNA and parC genes and 10.4% had mutations in all 3 genes. The rates of mutations in 23S RNA, parC, and gyrA genes were unchanged across the 5 calendar years of the study; no significant trends were identified (Supplementary Table 8).
Prior Antibiotic Use and Macrolide/Quinolone Resistance in MG Infection
We examined the association of antibiotic use in the 30 days before enrollment and specimen collection with macrolide/quinolone resistance in MG infections. Eight subjects were unable to identify their antibiotic(s) and were excluded. Among the remaining 333 MG-infected subjects who had urine samples sequenced for the 23S rRNA gene, 60 had taken macrolides in the prior 30 days; 96.7% possessed mutations in the 23S rRNA gene compared to 86.8% of 273 infected men who had not taken macrolides (P = .026, Fisher exact test; Supplementary Table 9). Among 337 subjects who had urine samples sequenced for the parC gene, 35 had taken quinolones in the prior 30 days; 88.6% had parC mutations compared with 89.4% of specimens from 302 subjects who had not taken quinolones (P = .778, Fisher exact test; Supplementary Table 9). Among 332 subjects with urine samples sequenced for the gyrA gene, 35 had taken quinolones in the prior 30 days; 14.3% had gyrA mutations, compared with 12.1% in 297 specimens from subjects who had not taken quinolones (P = .785, Fisher exact test; Supplementary Table 9).
DISCUSSION
Urethritis is the most common syndrome in men attending STD clinics and is caused by numerous pathogens; mixed infections are common [20]. Because symptomatic urethritis was required for entry into the study, identification of NG (46.4%), CT (33.1%), and MG (19.7%) was common. To our knowledge, this represents the largest number of male subjects with symptomatic urethritis who have been tested/reported as a single cohort for these and other causes of urethral infections, thereby affording the opportunity to separate and analyze a large group of men with MG infections alone. MG is an important cause of urethritis; MG prevalence in subjects with NGNC urethritis was 41.4% (95% CI, 37.3%–45.6%), higher than rates in symptomatic men with NGNC urethritis in Japan (22.7%) [20] and Belgium (27%) [21]. Overall prevalence of MG infection in men with symptomatic urethritis was 19.7% (95% CI, 17.9%–21.6%), similar to other reported rates (11%–19.3%) in the same time period from 3 Western countries [21–23], but lower than 28% reported from another Chinese STD clinic (Guangxi) [24].
The prevalence of single MG infection was 10.9% (95% CI, 9.3%–12.5%) in men tested for all 6 organisms and 54.1% (95% CI, 48.3%–59.7%) in men with MG infections, overall. These rates were lower than the 31% and 75% respectively, reported in 155 male subjects attending family medicine/planning, public health, and STD clinics, which included asymptomatic men, all of whom had tests performed for MG, CT, NG, and TV [22]. This suggests that our study underestimates the proportion of MG infections seen in the general male population because our subjects were symptomatic whereas MG infections, generally, are skewed to asymptomatic men [2].
We did not find that macrolide resistance of MG increased significantly across the 5 calendar years of our study; however, single MG infection was more common among subjects who took antibiotics in the 30 days prior to presentation. We speculate that selection of MG as a single infection may have resulted from use of antimicrobials that did not effectively treat MG or may not be efficient because of MG resistance, while also treating and possibly eradicating other STIs. An association between antibiotic use and resultant macrolide-resistant MG infections has been reported [9, 25, 26].
We found that 79.5% of men with MG single infection had ≥5 PMNs/HPF on urethral Gram stain, and the number of PMNs enumerated was similar to single infections with CT and Ureaplasma species. Several studies have found that MG infection mirrored clinical features of NGNC urethritis [27, 28] and also elicited ≥5 PMNs/HPF on urethral Gram stain [28], the European guideline for objective evidence of inflammation [29] (the US Centers for Disease Control and Prevention guideline uses ≥2 PMNs/HPF) [30].
We documented a high level (88.9%) of macrolide resistance of MG in male subjects with symptomatic urethritis. We report 5 separate mutations in the 23S rRNA gene also reported in earlier studies [9, 31, 32]. A2059G and A2058G substitutions accounted for 89.4% of mutations in the 23S rRNA gene, resulting in high-level macrolide resistance that has been associated with failure of azithromycin treatment [9, 31, 32]. High rates (58%–82.4%) of macrolide-resistant MG have been reported in the Asia-Pacific region, Europe, and North America [10, 33–35]. Identification of macrolide-resistant mutants during treatment with a single 1-g dose of azithromycin or a 5-day regimen has also been reported [26, 31]. Azithromycin treatment during the prior 12-month period has been associated with macrolide resistance in Spain [14].
We also found a high level (89.5% [95% CI, 85.1%–92.6%]) of parC fluoroquinolone resistance mutations in MG from men with symptomatic urethritis. Mutations at position G248 were present in 85.5% (95% CI, 81.3%–89.0%) of MG-positive samples; the majority, 83.7% (95% CI, 79.4%–87.5%), displayed G248T that would result in a missense mutation at position 83 (Ser→Ile). Treatment failures of MG infection with fluoroquinolones have been reported from 4 continents [10–13, 16, 33]. The prevalence of MG with fluoroquinolone resistance–associated parC mutations has been low in Europe (≤10.5%) [14, 34, 36] and higher in the Asia-Pacific region [10, 35]. parC missense mutations at aa 83, including S83I and S83R, have been associated with failure of moxifloxacin therapy in several countries [11–13, 15, 16]. Whole-genome sequencing of 28 MG isolates also confirmed a correlation between the S83I amino acid substitution resulting from G248T mutations in parC and resistance to ciprofloxacin and moxifloxacin [37]. Yamaguchi et al [38] reported that a single “upstream” mutation in G241T (resulting in the amino acid change G81C) led to an 11-fold increase in the 50% minimum inhibitory concentration of moxifloxacin; this mutation was found in 1 (of 344) of our samples. The rate of gyrA mutations in our study was 13% (95% CI, 9.6%–17.0%), similar to the 10% reported in Japan [36], but higher than reported in Australia (5%) [13]. We found that the predominant mutations in the gyrA gene were G285A, G285C, and G285T (that would result in an M95I aa mutation), which were detected in 7.7% (95% CI, 5.1%–11.0%) of MG-infected samples. The M95I mutation has been associated with failure of moxifloxacin to clear MG from 1 patient in Australia [16].
Multidrug-resistant MG infections have been reported in the Asia-Pacific region and Europe [10–13, 16]. Deguchi et al reported that dual resistance rates of MG from Japanese subjects with nongonococcal urethritis increased from 0 in 2011 to 30.8% in 2014 [39]. We identified a high rate (87.7% [95% CI, 83.5%–91.1%]) of combined mutations in 23S rRNA and parC genes. The rate of mutations in 23S rRNA and both parC and gyrA genes was 10.4% (95% CI, 7.2%–14.3%), similar to that reported in Japan [35].
Treatment of antibiotic-resistant MG is problematic in the face of high resistant rates to fluoroquinolones and macrolides. Newer classes of antimicrobials and advanced versions of older ones [6] have not been systematically tested in controlled efficacy trials. Antibiotic resistance testing, together with combination antimicrobial therapy, potentially is an approach to control progression of antimicrobial resistance and is used to manage antimicrobial-resistant bacterial infections caused by NG [6, 40] and Mycobacterium tuberculosis [41, 42].
In conclusion, our study confirms that MG is associated with sexually acquired urethritis when present as a single pathogen [22] and when prior antibiotic therapy may select for MG single infection. We observed an unprecedented high (~90%) prevalence of both macrolide and quinolone resistance in men with symptomatic MG urethritis. Furthermore, an even higher rate of macrolide resistance (97%) in MG infection was associated with macrolide usage in the 30 days prior to identifying infection, suggesting that resistance of MG to macrolides can develop rapidly.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the sexually transmitted disease clinic staff in the Institute of Dermatology, Chinese Academy of Medical Sciences, for their contributions to the enrollment of subjects and performance of laboratory testing.
Financial support. This work was supported by the US National Institutes of Health (grant number U19 AI084048) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (grant number 2016-I2M-3–021).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horner PJ, Martin DH. Mycoplasma genitalium infection in men. J Infect Dis 2017; 216(Suppl 2):S396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bissessor M, Tabrizi SN, Bradshaw CS, et al. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin Microbiol Infect 2016; 22:260–5. [DOI] [PubMed] [Google Scholar]
- 4. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 5. Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–20. [DOI] [PubMed] [Google Scholar]
- 6. Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017; 14:139–52. [DOI] [PubMed] [Google Scholar]
- 7. Seña AC, Lensing S, Rompalo A, et al. Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis infections in men with nongonococcal urethritis: predictors and persistence after therapy. J Infect Dis 2012; 206:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis 2015; 61:1389–99. [DOI] [PubMed] [Google Scholar]
- 9. Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium–positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 2008; 47:1546–53. [DOI] [PubMed] [Google Scholar]
- 10. Anderson T, Coughlan E, Werno A. Mycoplasma genitalium macrolide and fluoroquinolone resistance detection and clinical implications in a selected cohort in New Zealand. J Clin Microbiol 2017; 55:3242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soni S, Parkhouse A, Dean G. Macrolide and quinolone-resistant Mycoplasma genitalium in a man with persistent urethritis: the tip of the British iceberg? Sex Transm Infect 2017; 93:556–7. [DOI] [PubMed] [Google Scholar]
- 12. Deguchi T, Ito S, Yasuda M, et al. Emergence of Mycoplasma genitalium with clinically significant fluoroquinolone resistance conferred by amino acid changes both in GyrA and ParC in Japan. J Infect Chemother 2017; 23:648–50. [DOI] [PubMed] [Google Scholar]
- 13. Murray GL, Bradshaw CS, Bissessor M, et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 2017; 23:809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbreá MJ, Fernández-Huerta M, Jensen JS, Caballero E, Andreu A. Mycoplasma genitalium macrolide and fluoroquinolone resistance: prevalence and risk factors among a 2013–2014 cohort of patients in Barcelona, Spain. Sex Transm Dis 2017; 44:457–62. [DOI] [PubMed] [Google Scholar]
- 15. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents 2010; 36:255–8. [DOI] [PubMed] [Google Scholar]
- 16. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS 2013; 24:822–8. [DOI] [PubMed] [Google Scholar]
- 17. Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 1991; 29:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bandea CI, Joseph K, Secor EW, et al. Development of PCR assays for detection of Trichomonas vaginalis in urine specimens. J Clin Microbiol 2013; 51:1298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madico G, Quinn TC, Rompalo A, McKee KT Jr, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol 1998; 36:3205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito S, Hanaoka N, Shimuta K, et al. Male non-gonococcal urethritis: from microbiological etiologies to demographic and clinical features. Int J Urol 2016; 23:325–31. [DOI] [PubMed] [Google Scholar]
- 21. Libois A, Hallin M, Crucitti T, Delforge M, De Wit S. Prevalence of Mycoplasma genitalium in men with urethritis in a large public hospital in Brussels, Belgium: an observational, cross-sectional study. PLoS One 2018; 13:e0196217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54:2278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58:632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng BJ, Yin YP, Xiang Z, et al. An epidemiological study of Mycoplasma genitalium infections among males attending a sexually transmitted disease clinic in Guangxi, China. Jpn J Infect Dis 2014; 67:17–21. [DOI] [PubMed] [Google Scholar]
- 25. Chra P, Papaparaskevas J, Papadogeorgaki E, et al. Prevalence of Mycoplasma genitalium and other sexually-transmitted pathogens among high-risk individuals in Greece. Germs 2018; 8:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Read TR, Fairley CK, Tabrizi SN, et al. Azithromycin 1.5 g over 5 days compared to 1 g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis 2017; 64:250–6. [DOI] [PubMed] [Google Scholar]
- 27. Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium is associated with symptomatic and asymptomatic non-gonococcal urethritis in men. Sex Transm Infect 2009; 85:15–8. [DOI] [PubMed] [Google Scholar]
- 28. Leung A, Eastick K, Haddon LE, Horn CK, Ahuja D, Horner PJ. Mycoplasma genitalium is associated with symptomatic urethritis. Int J STD AIDS 2006; 17:285–8. [DOI] [PubMed] [Google Scholar]
- 29. Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS 2016; 27:928–37. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. 2015 sex ually transmitted diseases treatment guidelines. Available at: http://www.cdc.gov/std/tg2015/default.htm. Accessed 5 August 2018. [Google Scholar]
- 31. Twin J, Jensen JS, Bradshaw CS, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One 2012; 7:e35593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chrisment D, Charron A, Cazanave C, Pereyre S, Bébéar C. Detection of macrolide resistance in Mycoplasma genitalium in France. J Antimicrob Chemother 2012; 67:2598–601. [DOI] [PubMed] [Google Scholar]
- 33. Gesink D, Racey CS, Seah C, et al. Mycoplasma genitalium in Toronto, Ont: estimates of prevalence and macrolide resistance. Can Fam Physician 2016; 62:e96–101. [PMC free article] [PubMed] [Google Scholar]
- 34. Pitt R, Fifer H, Woodford N, Alexander S. Detection of markers predictive of macrolide and fluoroquinolone resistance in Mycoplasma genitalium from patients attending sexual health services in England. Sex Transm Infect 2018; 94:9–13. [DOI] [PubMed] [Google Scholar]
- 35. Deguchi T, Ito S, Yasuda M, et al. Surveillance of the prevalence of macrolide and/or fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Japan. J Infect Chemother 2018; 24:861–7. [DOI] [PubMed] [Google Scholar]
- 36. Dumke R, Thürmer A, Jacobs E. Emergence of Mycoplasma genitalium strains showing mutations associated with macrolide and fluoroquinolone resistance in the region Dresden, Germany. Diagn Microbiol Infect Dis 2016; 86:221–3. [DOI] [PubMed] [Google Scholar]
- 37. Fookes MC, Hadfield J, Harris S, et al. Mycoplasma genitalium: whole genome sequence analysis, recombination and population structure. BMC Genomics 2017; 18:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamaguchi Y, Takei M, Kishii R, Yasuda M, Deguchi T. Contribution of topoisomerase IV mutation to quinolone resistance in Mycoplasma genitalium. Antimicrob Agents Chemother 2013; 57:1772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deguchi T, Kikuchi M, Yasuda M, Shin Ito. Multidrug-resistant Mycoplasma genitalium is increasing. Clin Infect Dis 2016; 62:405–6. [DOI] [PubMed] [Google Scholar]
- 40. Singh V, Bala M, Bhargava A, Kakran M, Bhatnagar R. In vitro efficacy of 21 dual antimicrobial combinations comprising novel and currently recommended combinations for treatment of drug resistant gonorrhoea in future era. PLoS One 2018; 13:e0193678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuen CM, Kurbatova EV, Tupasi T, et al. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med 2015; 12:e1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drobniewski F, Cooke M, Jordan J, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess 2015; 19:1–188, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.