Human Immunodeficiency Virus (HIV) co-infection is the most critical risk factor for reactivation of latent tuberculosis infection (LTBI). While CD4+ T cell depletion has been considered the major cause of HIV-induced reactivation of LTBI, recent work in macaques co-infected with Mtb/SIV suggests that cytopathic effects of SIV resulting in chronic immune activation and dysregulation of T cell homeostasis correlates with reactivation of LTBI. This review builds on compelling data that the reactivation of LTBI during HIV co-infection is likely driven by the events of HIV replication and therefore highlights the need to have optimum translational interventions directed at reactivation due to co-infection.
TB/HIV co-infection: A global challenge
The dual epidemics of HIV and TB together pose one of the greatest modern-day public health challenges [1–4]. While the global control of TB is compounded by co-infection with HIV [5, 6], people living with HIV (PLHIV) are at high risk of reactivating LTBI [7, 8]. The problem has worsened due to the emergence of multidrug-resistant tuberculosis (MDR-TB) in PLHIV, leading to significantly higher mortality rates [9, 10]. Mtb infection is generally contained within granulomatous lesions by the immune system [11, 12]. However, chronic HIV co-infection may lead to progression of LTBI to active tuberculosis (ATB) and transmission through disruption of these organized granulomas; in addition to impairing Mtb-induced systemic proinflammatory cytokine/chemokine response [13, 14].
The most well characterized impact of HIV is the CD4+ T cell depletion in the lymphoid tissues and peripheral blood [15, 16]. However, studies using the nonhuman primate (NHP) model of Mtb/SIV co-infection has revealed protective CD4+ T cell-independent immune responses that suppress the reactivation of LTBI [17]. These were comprised of proliferating memory CD8+ T cell and the expanded presence of bronchus-associated lymphoid tissue (BALT). Additionally, indirect cytopathic effects of SIV resulting in chronic immune activation and dysregulation in T cell homeostasis have been implicated as critical mediators of reactivation of LTBI [18]. Chronic HIV-1 infection in human patients can lead to immune activation upon advent of antiretroviral therapy (ART), which can lead to a paradoxical worsening of TB in the form of immune-reconstituted inflammatory syndrome (TB-IRIS) [19–21]. Altogether, it appears that Mtb and HIV act in tandem to worsen the cognate disease condition by gradually deteriorating the immune functions (Box 1).
Box 1. Worsening of disease condition in Mtb/SIV co-infection.
TB is associated with increased mortality and morbidity in HIV-1 co-infection [22, 23]. A higher viral load along with a more rapid progression to AIDS is associated with ATB in humans [24, 25].
Though the underlying mechanisms need further elucidation, HIV-1 replicates in the tuberculous microenvironment rendered conducive by the release of proinflammatory cytokines, such as TNFα [26, 27].
Mtb driven HIV-1 infection of monocyte-derived macrophages has been observed in vitro, as well as ex-vivo, in macrophages isolated from HIV-1 infected human lungs [28, 29]. The TB-induced M(IL-10) macrophage activation program promotes HIV-1 infection in co-infected individuals [30].
Mtb is able to exacerbate HIV-1 pathogenesis through formation of membranous projections called tunneling nanotubes in an IL-10/STAT3 dependent manner in human macrophages [30].
Both pathogens infect macrophages in the pulmonary cavity [31, 32]. Some of the common pro-inflammatory mediators involved in disease control secreted by both Mtb and HIV include IFNγ, TNFα, and CCL2 (MCP-1) [33–35]. Secretion of CCL2 by Mtb-infected alveolar macrophages recruits HIV-1 permissive CCR2+ monocytes/macrophages and CD4+ T cells, thereby increasing the risk of HIV-1 infection.
As a result, the sustained signaling pathway activation due to the CCL2- mediated activation of HIV-LTR (long-terminal repeats) can lead to chronic inflammation in a Mtb/HIV-1 co-infection [35].
Overall, there is an aberrant in vivo T cell activation in both the diseases, as indicated by increased HLA-DR and CD38, decreased CD28 and IFN-γ production along with reduced macrophage viability and increased levels of proinflammatory cytokines [25, 36].
This review therefore highlights the role of chronic immune activation induced by Mtb/HIV co-infection in the reactivation of an otherwise contained TB infection (Figure 1, Key Figure). It describes the underlying mechanisms of the host-pathogen interactions with a focus on i) CD4+ T cell-independent factors responsible for HIV-induced reactivation of LTBI ii) critical contribution of the NHP model to understanding the mechanisms of immune activation and iii) translational interventions to treat chronic immune activation.
Figure 1. Chronic immune activation in Mtb/SIV co-infection.
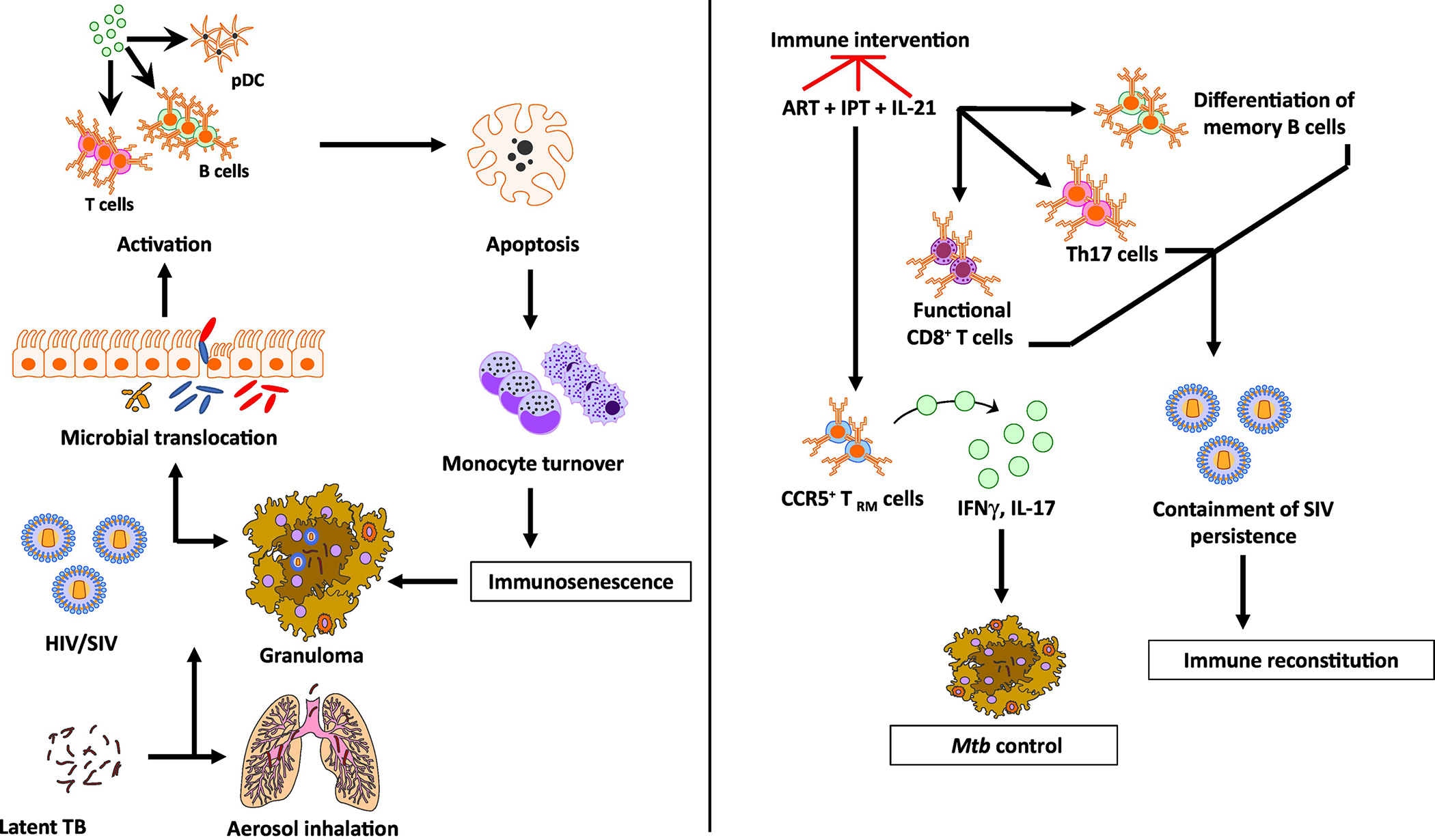
HIV co-infection of Mtb is characterized by immune activation encompassing a wide array of tissues and cells. HIV co-infection leads to a drastic depletion of CD4+ T cells by loss of mucosal integrity and in turn, a loss of immune function in the gastrointestinal tract. This causes a translocation of resident microbial products into the systemic circulation leading to activation of several cell types including T, B, NK cells, plasmacytoid dendritic cells and monocytes. In addition to producing pro-inflammatory cytokines, these activated cell subsets also demonstrate increased apoptosis and turnover. The integrity of the granuloma structure in a reactivated macaque is maintained by this increased monocyte turnover that replaces the apoptotic macrophages. The HIV infection promotes macrophage killing, leading to the breakdown of granulomas, which in turn, leads to a breach of Mtb containment and reactivation. While ART successfully contains the virus, it fails to resolve the chronic immune activation completely. Concurrent therapy with isoniazid and/or IL-21 could contain both bacterial containment and immune activation. While isoniazid treatment in conjunction with ART could restore CCR5+ TRM cells in the lung tissues leading to a better control of Mtb replication in the macrophages, IL-21 could serve to promote the maintenance and functionality of Th17 cells, B cells and CD8+ T cells. Together, this novel therapy could potentially lead to a better immune reconstitution and resolve virus-driven residual immune activation in a Mtb/HIV co-infection.
Chronic immune activation in Mtb infection
While the role of ATB in immune activation with [37] or without HIV co-infection [38, 39] is well documented, Sullivan et al. have shown that PLHIV with LTBI also have a elevated levels of inflammation and immune activation, which impacts the progression of disease [25]. Plasma levels of immune activation markers such as sCD14, CRP, IL-6, IL-8, IP-10, HLA-DR and lymphocytic expression of CD38 were studied to assess the impact of TB infection status on the exacerbation of HIV. While LTBI did not contribute to elevated levels of soluble immune activation markers, subjects with LTBI had elevated CD38 expression on both CD4+ and CD8+ T cells compared to healthy controls. These results clearly indicate evidence for elevated T cell activation during LTBI, even before HIV coinfection. Immune activation in TB has been studied in the presence and absence of concurrent HIV infection using cellular and serum markers such as IL-2Rα, CD45RO and HLA-DR on CD4+ and CD8+ T cells. Interestingly, the expression of HLA-DR on both T cell subsets was already two-fold higher in the HIV− TB patients, indicating an elevated baseline immune activation in TB patients prior to HIV infection. While this increased expression was mirrored in the median serum TNFα and neopterin levels in HIV−TB cohort, it was not as significant in IL-2Rα and CD45RO expression. The interrelated changes observed in both macrophages and T cell lineages of TB patients ultimately led to a generalized immune activation that worsened upon HIV coinfection [40]. Characterization of the polyfunctional profile of ESAT-6/CFP-10-specific CD4+ T cells in LTBI individuals demonstrates a predominant proportion of IFNγ+IL-2+TNFα+ cells without HIV coinfection, indicating that the activation and polyfunctional profile of CD4+ T cells is solely dependent on the TB status [41]. In addition, there is an increased frequency of CD4+CD25+CD127− Treg in LTBI compared to healthy controls that can be controlled only modestly by preventive therapy [42]. Further studies on the role of TB-induced immune activation in individuals receiving ART and host-directed therapies could shed light on the impact of treatment on the disease outcome. In LTBI, the Mtb is contained with restricted growth within granulomas. The continued T cell activation in response to Mtb-specific antigens during LTBI seems to underline the possible ongoing antigen expression by the contained Mtb, a topic that has been the subject of intense debate. Although, the ESAT-6/CFP-10 may play a relatively lesser role than proteins from the DosR operon which is dominant during latency [43]. It could also be hypothesized that the contained bacteria resuscitate from time to time, given the oxygen supply favoring replication in the upper lung lobes where Mtb possibly resides, to maintain the adaptive immune response to the viable but dormant Mtb progeny [44]. HIV coinfection adds to the immune burden that eventually manifests as immune activation leading to reactivation of LTBI, increased inflammation and disease progression compared to HIV or Mtb infection alone.
Reactivation of TB due to SIV-induced immune activation
Rhesus-macaque adapted strains of SIV have been successfully used to cause a rapid decline in CD4+ T cells and cause reactivation of LTBI in an aerosol infection-based model [45]. Interestingly, a cohort of animals were able to prevent reactivation and maintain LTBI. Identification of correlates of protection from reactivation despite SIV co-infection in this cohort demonstrated increased CD8+ memory T cell proliferation, higher granzyme B production and expanded B cell follicles [17] (Figure 2). In order to better understand the mechanisms that lead to reactivation of LTBI due to HIV co-infection, we utilized the rhesus macaque model of coinfection. Since Mtb/SIV co-infected macaques all experience lymphopenia in the lung, but a subset of these animals did not experience reactivation, we hypothesized that the mere depletion of CD4+ T cells may be insufficient in leading to reactivation of LTBI. We depleted CD4+ T cells in macaques with LTBI, using a rhesusized-depleting antibody, leading to comparable lung depletion to that achieved by SIV co-infection, sans any serum sickness. These animals did not exhibit substantial reactivation, unlike the Mtb/SIV co-infected cohort [18]. The latter animals were characterized by the presence of immune activation signatures in the lung, while the animals in which CD4+ T cells were depleted by antibody were devoid of any inflammation. In these animals, the normal homeostasis of T cell repertoire was conserved, while this was clearly dysregulated in the Mtb/SIV co-infected macaques [18]. When a non-pathogenic strain of SIV, SIVmac239ΔGY, was used in our co-infection model that does not lead to tissue-specific viral replication, no reactivation was observed, nor was immune activation or bacterial dissemination to secondary sites of infection. We therefore conclude that i) the mere depletion of CD4+ T cells is not sufficient in leading to reactivation of LTBI and ii) that instead reactivation is correlated with chronic immune activation caused by the virus, which leads to T cell homeostasis dysregulation [18]. It is crucial to understand that while ATB is characterized by a strong cytokine storm, LTBI is itself associated with slightly higher than baseline inflammation [25]. The chronic immune activation observed during reactivation is, however, substantially higher than that observed during LTBI.
Figure 2. Pathogenesis of LTBI and its reactivation upon HIV co-infection.
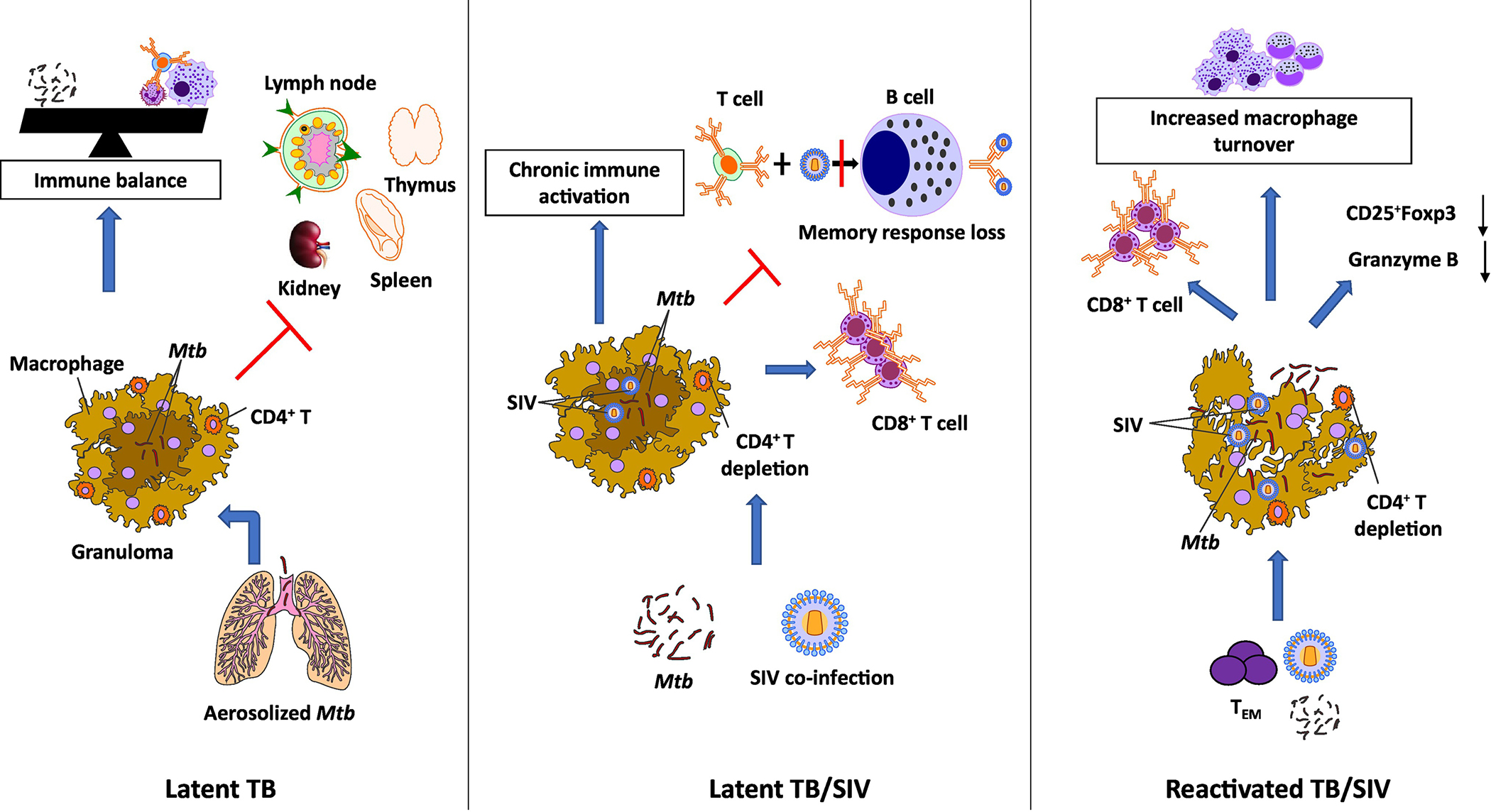
LTBI is characterized by a dynamic balance between the pathogen and the host as a consequence of limited bacterial replication due to its containment within granulomas. Inhaled droplet Mtb nuclei are engulfed by the macrophages and dendritic cells in the terminal alveoli in the lungs. In the latent phase, the replication is contained with the granuloma by the activated T lymphocytes and macrophages. This leads to an arrest of the disease progression and an immune balance is attained. Co-infection with SIV leads to a severe immunosuppression and a drastic decrease in CD4+ T cell counts in the granulomas. As a result, there is an increase in the number of CD8+ T cells with increased expression of activation markers, CD95, CD38 and HLA-DR. A reduced expression of CD25 on B cells during SIV infection results in perturbation of the B cell response to CD4+ T cells. A reduced antigen presentation from CD4+ T cells impairs the generation of memory B cells. Taken together, SIV co-infection of Mtb leads to chronic immune activation, immune dysbiosis and a skewed Treg/TH17 balance resulting in reactivation of LTBI. Following the SIV-induced immune perturbation, there is a reduction in the generation of lung homing Mtb-specific TEM CD4+ T cells. This preferred depletion of Mtb-specific CD4+ T cells and viral infection of the macrophages in the granulomas causes the integrity of the granuloma to disintegrate leaking the contained Mtb leading to dissemination.
NHP model to study Mtb/HIV co-infection
Modeling of Mtb/HIV co-infection in NHPs offers the advantage of being the genetically, physiologically and immunologically closest to humans. Indeed, the rhesus macaque (Macaca mulatta) and cynomolgus macaque (Macaca fascicularis) models have contributed immensely to our current understanding of the host-pathogen interactions in both Mtb and HIV pathogenesis [31, 45–48] (Box 2). Manipulating the bacterial and viral reservoirs in a macaque model of co-infection presents a valuable tool to dissect the local immune responses in a TB predominant microenvironment that is not possible in any other animal model. A recent study used positron emission tomography and computed tomography (PET CT) as a tool in a NHP model to monitor disease development in TB/SIV coinfected Mauritian cynomolgus macaques using [18F]fluorodeoxyglucose (FDG) to detect inflammation and disease progression [49]. Granulomas were quantified throughout the study using serial PET/CT imaging. This model enabled exploration of how a preexisting SIV infection impaired the resistance to Mtb. Further studies using FDG PET/CT in NHPs could identify ongoing low grade SIV infections that could be involved in LTBI reactivation and thus provide immune-therapeutic targets.
Box 2. The macaque model.
Upon exposure to a low dose of Mtb, macaques develop human-like LTBI marked by a positive tuberculin skin test (TST), absence of symptoms of active TB and absence of culturable Mtb bacilli [17, 45, 50, 51].
Macaques are susceptible to SIV, a surrogate of HIV. Importantly, SIV co-infection following the establishment of LTBI may reactivate LTBI, causing some animals to progress to ATB, mimicking human reactivation [17, 45].
The rhesus macaque model, using the low-virulence CDC1551 strain of Mtb, has been instrumental in elucidating the immunological mechanisms of TB latency and reactivation in a SIV co-infection [17, 31, 45, 47].
Besides, the macaques can be infected with Mtb via the inhalation route which is the natural route of infection in humans, allowing accurate delivery of the inoculum to the respiratory mucosa [52–54].
Though there are differences in susceptibility to Mtb in rhesus and cynomolgus macaques, both these models demonstrate the distinct structured granulomas in the lungs, a hallmark of human TB [55–59].
More recently, increased inducible bronchus-associated lymphoid tissue (iBALT) proximal to the granulomas has been associated with enhanced protection from reactivation of LTBI in a rhesus macaque model of Mtb/SIV co-infection [17].
These findings have shed light on crucial information on the role of B cells in Mtb/SIV co-infection. The macaque model offers the advantage of repeated sampling of bronchoalveolar lavage (BAL), allowing for longitudinal studies of both the local and systemic environments to further elaborate the specific mechanisms at each stage of Mtb/SIV co-infection [60, 61].
Lessons learnt from natural hosts of SIV
Immune activation characterized by an overall immune dysfunction is a hallmark of chronic HIV infection. Infection with HIV in humans or SIV in non-natural hosts leads to damage of the mucosal barrier of the gastrointestinal tract. This is accompanied by an injury to immune function that leads to an increased translocation of microbial products and a significant decrease in the activated memory mucosal CD4+ T cell counts at this anatomical site [62–64]. Breakdown of the integrity of the intestinal epithelial barrier leads to translocation of microbes and microbial byproducts from the intestine into the lamina propria, lymph nodes, liver and systemic circulation. Immune activation in the peripheral tissues and blood ensues as a result of the inability of intestinal macrophages to bind and clear the translocated microbial products [64].
In contrast, in natural hosts of SIV, e.g., sooty mangabeys (SM), there is no recorded evidence of either epithelial breakdown leading to microbial translocation, nor chronic immune activation. The non-progressive SIV infection of the natural hosts is characterized by a limited CD4+ T cell depletion and attenuation of the chronic immune activation. It is also possible to revert the chronic immune activation in natural hosts following repression of viral replication upon administration of ART [65]. African green monkeys (AGM), another natural host of SIV, display a rapid control of SIV replication in secondary lymphoid organs [66, 67]. A recent study characterized the capacity of NK cells to migrate into lymphoid follicles with CXCR5 expression [68]. Natural hosts are characterized by a weaker expression of proinflammatory cytokines like TNFα, IL-6, IFNγ and IL-8 upon SIV infection [69]. Though the macrophages in the lungs of AGM are infected with SIV, no SIV-infected macrophages are observed at peak viral production in early infection [70]. Overall, it appears that the natural hosts have developed mechanisms to control viral replication and maintain the integrity of the organs and cells needed to induce a stronger memory immune response while diminishing the immune activation at the same time.
Factors independent of CD4+ T cell–depletion contribute to chronic immune activation
In addition to the loss of CD4+ T cells, the systemic immune activation following HIV/SIV infection is also characterized by increased lymphocyte proliferation, increased T cell turnover [71], increased apoptosis of lymphocytes, increased activation of T cells and increased presence of proinflammatory cytokines in the serum. Indeed, an insight into the monocyte kinetics upon BrdU incorporation into Mtb/SIV co-infected rhesus macaques illustrated an increased monocyte turnover in the blood of the animals with reactivated TB compared to the SIV-infected macaques that were able to maintain LTBI [31]. Additionally, there was an increased number of BrdU+CD163+ macrophage turnover in the colon tissue from ATB/SIV macaques compared to that from SIV-infected macaques exhibiting LTBI. This increased tissue macrophage turnover correlated with increased apoptosis, confirming the hypothesis that there is a continuous macrophage destruction in the granulomas upon SIV-infection, perhaps due to the chronic immune activation that leads to spread of the contained Mtb. Additionally, LAG-3, an immune exhaustion marker, has been shown to be expressed at elevated levels on CD4+ T cells in reactivated LTBI rhesus macaques infected with SIV. Since the majority of the SIV-induced LAG-3 expression occurs at the periphery of lung granuloma in the reactivated animals, there could be a correlation between the increased turnover, apoptosis, spread of Mtb and exhaustion of immune resources that could be worth exploring [61]. CD4/CD8 ratio is an additional surrogate of HIV-1 induced immune activation and a predictor of disease progression that has been studied in the human samples. Since immunosenescence is a consequence of chronic immune activation, CD4/CD8 ratio can also be considered a marker for immunosenescence in HIV infection [72]. While CD4+T cells are the most evident cell type to be depleted during a chronic HIV infection, B cells and innate immune cells are also impacted [73] as discussed in the next section.
Role of innate immune response in chronic immune activation
A correlation between bioactive microbial lipopolysaccharide (LPS) and measures of innate and adaptive immune activation was demonstrated in a rhesus macaque model of intravenously infected SIVmac251 [63]. LPS, an immune-stimulatory bacterial component, can be quantified in the plasma and is a common measure of microbial translocation. Despite decreased CD4+ T cell counts during the acute phase of the infection in rhesus macaques, the plasma LPS levels did not increase until the chronic phase. Macaque models have also been critical in gaining a better understanding of the role of innate immune responses in driving the chronic immune activation with a specific interest in the role of innate leukocyte subsets such as macrophages, NKT cells, γδ T cells and plasmacytoid dendritic cells (pDCs). pDCs constitute a relatively smaller percentage (~0.2 – 0.5%) of cells in the peripheral blood mononuclear cells but secrete a wide array of inflammatory cytokines and chemokines such as TNFα, IL-6, CXCL10, CCL4 and CCL5 [74]. pDCs are reported to vigorously increase in blood during an acute SIV infection, but are depleted by ~50% during the chronic phase due to their migration to lymph nodes [75, 76]. The Type I IFN response initiated during the continued pDC activation during the chronic phase has now been shown to be detrimental as it promotes immune activation in the non-natural SIV hosts such as rhesus macaques compared to its timely and regulated resolution in natural hosts such as African green monkeys (AGM) and SM [77].
While type-1 interferons play a protective role during the acute phase of HIV-1, attenuation of antigen-specific CD4+ T cell activity is associated with it, and leads to a sustained immune activation in chronic HIV infection in humans. In the macaque model, IFN-simulated genes are expressed at higher levels in experimental SIV-infection of rhesus macaques and this response persists with the infection progressing to worsening of the disease [77, 78]. On the contrary, in the natural hosts, such as SM, the response starts to wane off during the transition from the acute to the chronic phase during a non-pathogenic SIV infection [79]. Type I IFN signaling is thus a double-edged sword in that it can induce a protective antiviral response in the host but can also induce systemic expansion through pathogen associated molecular pattern (PAMP) detection. A topical administration of recombinant human β-interferon protected against vaginal challenge of pathogenic SIV containing human env and reverse transcriptase genes (RT-SHIV) [80]. On the other hand, some studies have associated increased Type I IFN signaling to enhanced CD4+ T cell depletion [81] and decreased T cell proliferation [82]. In addition to Type I IFN signaling, the progression of HIV-1 infection to AIDS is characterized by a significant depletion in multiple subsets of dendritic cells (DCs), including DC-SIGN(+). HIV particles are known to bind dendritic cell specific intercellular adhesion molecule 3- grabbing non-integrin (DC-SIGN) through Gp120 in a chronic HIV infection. DC-SIGN+ cells have been implicated in the non-replicating SIV+ cells in a rhesus macaque model, underscoring their possible role in establishing viral persistence and/or reservoirs leading to sustained immune activation [83].
Chronic immune activation in antiretroviral therapy treated cohort
Whole genome transcriptional profiling of CD4+ T cells from HIV-1 elite controllers demonstrated significant similarity to that of the ART-treated patients but was different from HIV-1-negative individuals. In addition, a total of 978 transcripts showed differential expression in HIV-1 negative and ART-treated individuals. Further studies in NHP mimicking such a cohort of individuals that have a spontaneous control of the virus, and a transcriptional profile similar to virus-naïve animals, could prove critical in understanding the mechanisms involved in the relapse of the virus after a prolonged period of immune activation in a co-infection. Latent viral reservoirs in lymphoid tissues of patients on ART contribute to the immune activation by immune suppression leading to poor control of pathogens [84]. Indeed, persistent inflammation is associated with HIV persistence in this cohort (Box 3). A vicious cycle ensues, wherein, the immune activation in turn feeds the HIV reservoirs by generating activated T cells, target cells for HIV [84]. Studies focusing on raltegravir intensification demonstrated a drastic decrease in viral reservoirs of latently infected CD4+ T cells and a subsequent decrease in immune activation [85]. Defining mechanisms associated between immune activation and latent HIV reservoirs could therefore lead to development of targeted therapeutics. A recent study on elucidating the metabolic pathways of the gut microbiota in HIV+ patients on ART using metagenome sequencing revealed an altered metabolic activity of the microbiota with functional links to the host-pathogen interactions responsible for the sustained immune activation in the ART-treated patients [86]. Further studies should be designed to illustrate the specific pathways involved in correlation to the immune activation markers and how they impact co-infection with Mtb.
Box 3. Viral reservoirs during chronic SIV infection.
Macrophages in the lymph nodes and mucosal tissues have been implicated as the predominant viral reservoirs of SIV in rhesus macaques upon antibody-mediated depletion of CD4+ T cells.
A recent study in SIV-infected rhesus macaques presented a significant role of alveolar and interstitial macrophages in local viral infection [87]. Macrophages appear to have a shorter in vivo lifespan and exhibit a rapid turnover upon SIV infection [88].
A more recent study analyzed the macrophage reservoirs in latent SIV infection in macaques using quantitative viral outgrowth assay [89]. Macrophages in the blood, spleen and lungs of a majority of antiretroviral therapy (ART)-suppressed animals appeared to carry the latent viral genome.
Surprisingly, the frequency of SIV-infected macrophages was comparable to SIV-infected CD4+ T cells in these animals. These findings reiterate that the latently SIV-infected macrophages are capable of reactivating the disease upon treatment interruption in macaques [89].
Thus, rhesus macaques have been critical in bridging the gap between animal model and human immunology by providing indispensable data to identify therapeutic and vaccine targets in HIV research.
Mitigation of chronic immune activation
In the viral realm, there is a need to address the early events in HIV/SIV infection that lead to the sustained immune activation in the chronic viral phase. Specifically, it is imperative to understand the multiple causes of the immune activation such as T cell dysregulation in Mtb co-infection, viral infection of the macrophages and CD4+ T cells within the granulomas and the impact of the microbial translocation from the gut of co-infected subjects. The macaque model could shed light on the impact of earlier administration of ART in SIV and Mtb/SIV co-infection on mitigation of the immune activation. While ART has improved the life expectancy in PLHIV, the incidence of TB in this population remains high. We used a rhesus macaque model of LTBI to study the impact of ART on virus replication, virus-induced immune activation and CD4+ T cell restoration in Mtb/SIV coinfection (Ganatra S et al., JCI, in press). Though ART reduced the plasma and BAL viral load in the coinfected macaques significantly, there was a sustained Mtb burden in the BAL, lungs and bronchial lymph nodes (BrLN). It is possible that this maintained Mtb burden in the organs acts as a source of continued antigen stimulation leading to a sustained immune activation. Consistent with the findings in clinical settings [90–92], ART administered 4 weeks post-SIV challenge failed at preventing LTBI reactivation in the nonhuman primate model of Mtb/SIV co-infection (Ganatra S et al., JCI, in press). Initiation of ART in these coinfected animals resulted in a significant increase in the frequency of CXCR3+CCR6+CD4+ T cells that harbored high levels of integrated HIV DNA [93]. We hypothesized that the significant increase in Th1 associated cytokines following ART [94] leads to a compromise in the structural integrity of the granuloma thereby leading to dissemination of the contained latent Mtb. Additionally, a significant percentage of CD68+/CD163+ macrophages was observed in the BALT upon initiation of ART, suggesting a dysregulated homing of CD4+ T cells into the interstitial lung. There is a need to decipher the mechanisms by which the early onset of ART during acute phase of viral replication prior to peak viremia can dampen chronic immune activation. It is possible that the initiation of ART at an earlier stage of viral infection is able to maintain the gut integrity, thereby reducing the microbial translocation and long-term chronic immune activation, but this remains to be tested. It would also be interesting to follow through with concurrent treatment for both SIV and Mtb in this model to further suppress immune activation.
Utilizing an immune-based intervention such as treatment with IL-21 in conjunction with ART could be a promising approach to mitigating the chronic immune activation in a Mtb/HIV co-infection (Figure 1) as could be testing an anti-TB drug along with ART in co-infected subjects. IL-21 is known to regulate the T, NK and B cell functionality in addition to regulating the innate and adaptive immunity to both Mtb and SIV [95]. Administration of IL-21 with ART and anti-TB therapy (ATT) could (i) promote better immune reconstitution, (ii) resolve residual immune activation post ART and (iii) ensure better control of both bacterial replication within the lungs and viral persistence, thus significantly reducing the rate of LTBI reactivation.
Another promising strategy to suppress immune activation would be to supplement ART with a low dose of phytocannabinoids (delta-9-tetrahydrocannabinol (THC) or non-psychotropic cannabidiol (cannabidiol) or a high ratio CBD:THC combination [96]. It has been hypothesized that Tetrahydrocannabinol (Δ9-THC), the major psychoactive and anti-inflammatory cannabinoid in marijuana (Δ9-THC) administration will prevent chronic immune activation (CIA) in Mtb/SIV co-infection and prevent, or reduce, the incidence of SIV(HIV)-mediated LTBI reactivation. Although CD4+ T-cells are the primary targets of HIV/SIV, almost all lymphocyte populations are significantly impacted (6–9). Early and persistent B-cell dysfunction is a hallmark of HIV infection and based on studies in SIV-infected RMs, this occurs even before CD4+ T-cell depletion (10). While the mechanisms that cause B-cell dysfunction in HIV/SIV infection are unclear, mounting evidence in systemic lupus erythematosus (SLE)(11–15), multiple sclerosis (MS)(16, 17) and rheumatoid arthritis (RA)(18) (diseases of chronic inflammation) suggests that the ability of dysregulated microRNAs (miRNA) to modulate cellular functions through post-transcriptional gene silencing significantly contribute to B-cell dysfunction/hyperactivity. This indicates that inflammation-induced aberrant miRNA expression leads to their dysfunction and plays a role in the pathogenesis of HIV and potentially Mtb reactivation in coinfected individuals. Consistent with this, we showed a role for the miR-34a-SIRT1-acetyl p65 axis in causing hyperactivation and dysfunction of the intestinal B-cell system(19). Further, marked dysregulation of miRNAs previously linked to B-cell activation (miR-34a, miR-21& miR-30)(20) and lymphomagenesis (miR-21) was observed in CD20+ cells from chronic SIV-infected RMs(21). We recently showed that chronic administration of Δ9-THC to SIV-infected RMs inhibits T cell activation and exhaustion(22). This is accompanied by repression of miR-34a, miR-21, and miR-30 levels compared to uninfected controls(23) and downregulation of MMP8, a neutrophil-derived matrix metalloprotease strongly implicated in disease severity (cavitary TB)(24) at the levels of both mRNA and protein(22). Recent studies in ART naïve chronically SIV-infected rhesus macaques showed that controlled long-term THC dosing effectively attenuated intestinal inflammation, T cell proliferation/activation and lymphoid fibrosis without any adverse effects. Based on the above data, we propose that it should be possible to directly test if chronic THC administration can revert CIA in Mtb/SIV co-infected RMs, leading to either a reduction, or a complete ablation of LTBI reactivation. Because the gastrointestinal tract (GIT) is the central organ of cannabinoid signaling and also the primary target of HIV replication, dissemination and reservoir establishment, the protective effects of cannabinoids on the GIT can preserve intestinal epithelial integrity, prevent dysbiosis and microbial translocation [96]. Moreover, cannabinoids have been demonstrated to inhibit Th1 cytokine production [97] and matrix metallaprotease-8 (MMP8) protein expression [96], a key matrix metalloprotease implicated in cavitary TB [98]. Chronic THC administration could thus prevent non-CD4+ T and B-cell lymphocyte activation/dysfunction in Mtb/SIV coinfection and reduce the incidence of SIV-mediated LTBI reactivation. Therefore, we predict that phytocannabinoids as host-directed adjunct therapy can beneficially modulate the pathogenesis of HIV/SIV and Mtb co-infection by inhibiting local and systemic immune activation/inflammation, microbial translocation and lymph node fibrosis while simultaneously preventing LTBI reactivation and limiting Mtb dissemination by maintaining the structural integrity of the granuloma.
Furthermore, has recently been shown that inhibition of tryptophan catabolism using inhibitors of the enzyme IDO can improve immune activation caused by active TB [99]. IDO expression is induced in tissues following both Mtb and HIV infection and it is widely believed that this contributes to immune dysfunction. Catabolism of tryptophan by IDO has consequences additional to generation of kynurenine products which can directly result in T cell dysfunction. The IDO/Kyn pathway also represents a de novo NAD+ synthesis mechanism. NAD+ is required for the survival of host cells, particularly since Mtb infection increases the expression of CD38, a marker of inflammation on CD4+ and CD8+ T cells, as well as B cells. One of the activities of CD38 is its enzymatic activity as an NAD+ glycohydrolase [100]. We have previously shown that inhibition of Kynurenine production via the in vivo blockade of IDO activity in NHPs, improved anti-TB immune responses and granuloma-specific Mtb-killing [99]. It is therefore possible that treatment of Mtb/HIV co-infected individuals with certain efficacious IDO inhibitors will mitigate chronic immune activation, thereby boosting protective immune responses, and lead to better clinical outcomes. To optimize the impact of IDO blockade however, its inhibitors may be coupled with NAD supplements as well as Type I IFN pathway inhibitors.
The NHP model could therefore be utilized to test these novel therapies in longitudinal studies that would allow to follow up the effect of the treatment on i) an existing LTBI/HIV co-infection, ii) repeated and/or multiple exposures to Mtb in highly endemic regions and iii) reactivation of HIV-induced LTBI.
Concluding remarks and future perspectives
The NHP model has proven indispensable in the ongoing quest to understand the TB/HIV syndemic. The Mtb/SIV co-infection model provides a useful resource to study reliable correlates of protection when designing vaccines (Box 4) and drugs to elicit sustained protective immune responses, e.g., treatment of ATB and LTBI, impact of ART, as well as concurrent ART and TB therapy on the progression of both, infection and immunity, host-directed therapeutics and preclinical testing of vaccines that are efficacious and safe in the face of TB/HIV co-infection. The focus of basic research should also be to fast-track novel candidates that facilitate pre-exposure immune responses and protection. Recent advances in the field led to the development of promising candidates such as MTBVAC, the BCG revaccination approach, H4:IC31, H56:IC31 and M72/AS01 [101–105]. The NHP model represents a key tool in identifying the immune correlates of this dual pandemic as evidenced by the recent findings following intravenous administration of BCG. Several candidates tested for safety and immunogenicity in this preclinical model have moved onto human clinical trials, such as ID93+GLA-SE and MVA85A [106, 107]. The model also serves as an important check point for possible side effects and risk monitoring of the vaccine candidates.
Box 4. Vaccine strategies for Mtb/HIV co-infection.
Development of a vaccine, preferably a single vaccine to combat both diseases is an ambitious but plausible goal. The underlying hypothesis of a recombinant BCG (rBCG) vaccine is to have a second vaccine generation with a backbone expressing antigens from both Mtb and HIV and administered post-birth to prevent both diseases simultaneously.
Since the prerequisites of a successful HIV vaccine is elicitation of neutralizing antibodies, stimulation of CD4+ and CD8+ T cells and a long-lasting innate and adaptive immune response, rBCG is an excellent candidate as a vaccine vehicle [108–110].
Several NHP studies have been conducted to study both safety and efficacy of Mycobacterium-based HIV-1/SIV vaccines [111–114]. Alternatively, there is a need to identify new vaccine candidates with potential to combat HIV-related TB in highly TB endemic regions.
The challenge would be to be able to induce sustained protective immunity against Mtb in the presence of HIV-associated immune activation and associated immune deficiencies. While ART restores the CD4+ T cell response partially, HIV also impacts Mtb antigen presentation by dendritic cells, impairs B cell and antibody function that is not reversed by ART and has a significant role to play in the vaccine’s immunogenicity and efficacy.
We have utilized the rhesus macaque model to validate protective immunity against Mtb in the context of SIV co-infection. Aerosol vaccination of rhesus macaques with MtbΔsigH prior to SIV infection induced bronchus-associated lymphoid tissue (iBALT) and CD8+ effector memory T cells, in addition to reducing the bacterial burden, clinical manifestations and granulomatous pathology [47].
Vaccination of infant rhesus macaques with a pediatric combination vaccine containing an auxotroph Mtb strain co-expressing HIV antigens that demonstrated enhanced myeloid cell responses and a possible attenuation of immune activation [115].
In another study, vaccination of rhesus macaques with BCG vectors expressing SIV-gag elicited baseline humoral and cellular immune responses to Mtb. In addition to the mycobacterial response, the vaccinated primates also elicited a strong response to SIV gag and this response was independent from the baseline mycobacterial immunity [116].
As discussed in this review, chronic immune activation is a key player in the unfolding of immune cascade in Mtb/HIV co-infection that warrants a closer and deeper look (see outstanding questions). Finally, there is a need to have optimum translational interventions directed at reactivation due to co-infection such as i) a cost-effective and efficient vaccine that targets both Mtb and HIV induced chronic immune activation ii) concurrent therapies to suppress the chronic immune activation in Mtb/HIV co-infection, iii) early initiation of ART to maintain the gut integrity and reduce microbial translocation thereby reducing the virus-driven immune activation.
Outstanding questions box.
Can we design a joint TB-HIV vaccine design based off of a i) mycobacterial based live vaccine vehicle, ii) induction of potent Th1 immune responses and iii) antibiotic-free plasmid selection system?
Can we utilize the NHP model to better understand the impact of this vaccine-induced immunity on immune activation and ultimately the final outcome of co-infection?
Can the future studies factor in pre-existing immune responses to the rBCG vectors being used in the vaccine? The vaccine candidate should be safe for use in humans, should be able to demonstrate a relatively low level of replication and should be able to be measured by a soluble marker in blood or urine.
When designing a dual Mtb-HIV vaccine candidate, are we considering the impact of genetic manipulations of Mtb on the overall immune spectrum and the impact of HIV immunogens on the metabolic burden?
Can the dual Mtb/HIV vaccine candidate induce either i) an immune response in Mtb/HIV co-infected individuals similar to the response induced in a natural Mtb infection in resistant individuals, ii) a complete eradication of the pathogens or iii) sustain the LTBI by preventing its reactivation?
Can earlier administration of ART mitigate the immune activation in Mtb/SIV co-infection?
Can ART and concurrent treatment with IL-21 or isoniazid preventive treatment (IPT) result in better immune reconstitution and resolve residual immune activation in Mtb/HIV co-infection?
Highlights.
A third of all 1.5 million annual deaths due to Tuberculosis (TB) have a Human immunodeficiency virus (HIV) component.
The mere depletion of lung CD4+ T cells is insufficient in causing reactivation in macaques with latent tuberculosis infection (LTBI).
These findings suggest a critical role for CD4+ T cell independent factors such as chronic immune activation and altered effector T cell phenotypes in the reactivation of LTBI in Mtb/SIV co-infection.
The approaches discussed herein, represent the ideal use of knowledge gained from decades of work on two of the deadliest microbes known to humanity – Mtb and HIV, to develop translational approaches towards the control of the TB/AIDS syndemic.
Acknowledgements:
The authors were supported by National Institutes of Health (NIH) awards R01AI134240, R01AI135726, R01AI123047, R01AI111914, R01AI111943, UH3AI122320, U19AI1112111, and P51OD0111133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zumla A, et al. (2015) The WHO 2014 global tuberculosis report--further to go. The Lancet. Global health 3, e10–12 [DOI] [PubMed] [Google Scholar]
- 2.Bekker LG, et al. (2018) Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society-Lancet Commission. Lancet (London, England) 392, 312–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deribew A, et al. (2019) The Burden of HIV/AIDS in Ethiopia from 1990 to 2016: Evidence from the Global Burden of Diseases 2016 Study. Ethiopian journal of health sciences 29, 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean E, et al. (2019) Diagnosing active tuberculosis in people living with HIV: an ongoing challenge. Current opinion in HIV and AIDS 14, 46–54 [DOI] [PubMed] [Google Scholar]
- 5.Eshetie S, et al. (2018) Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: A systematic review and meta-analysis. PloS one 13, e0194675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magis-Escurra C, et al. (2017) Treatment outcomes of MDR-TB and HIV co-infection in Europe. The European respiratory journal 49 [DOI] [PubMed] [Google Scholar]
- 7.Sanderson JM, et al. (2014) Re: “Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup”. American journal of epidemiology 180, 556–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfein RS, et al. (2010) Latent tuberculosis among persons at risk for infection with HIV, Tijuana, Mexico. Emerging infectious diseases 16, 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaakidis P, et al. (2015) Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 19, 969–978 [DOI] [PubMed] [Google Scholar]
- 10.Efsen AMW, et al. (2018) Management of MDR-TB in HIV co-infected patients in Eastern Europe: Results from the TB:HIV study. The Journal of infection 76, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautam US, et al. (2015) DosS Is required for the complete virulence of mycobacterium tuberculosis in mice with classical granulomatous lesions. American journal of respiratory cell and molecular biology 52, 708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marakalala MJ, et al. (2016) Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nature medicine 22, 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassa D, et al. (2016) The effect of HIV coinfection, HAART and TB treatment on cytokine/chemokine responses to Mycobacterium tuberculosis (Mtb) antigens in active TB patients and latently Mtb infected individuals. Tuberculosis (Edinburgh, Scotland) 96, 131–140 [DOI] [PubMed] [Google Scholar]
- 14.Devalraju KP, et al. (2019) Transforming Growth Factor-beta Suppresses Interleukin (IL)-2 and IL-1beta Production in HIV-Tuberculosis Co-Infection. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 39, 355–363 [DOI] [PubMed] [Google Scholar]
- 15.Mattapallil JJ, et al. (2005) Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, et al. (2016) HIV-TB co-infection: mechanisms that drive reactivation of Mycobacterium tuberculosis in HIV infection. Oral diseases 22 Suppl 1, 53–60 [DOI] [PubMed] [Google Scholar]
- 17.Foreman TW, et al. (2016) CD4+ T-cell-independent mechanisms suppress reactivation of latent tuberculosis in a macaque model of HIV coinfection. Proceedings of the National Academy of Sciences of the United States of America 113, E5636–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucsan AN, et al. (2019) Mechanisms of reactivation of latent tuberculosis infection due to SIV co-infection. The Journal of clinical investigation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveira-Mattos PS, et al. (2019) Differential expression of CXCR3 and CCR6 on CD4(+) T-lymphocytes with distinct memory phenotypes characterizes tuberculosis-associated immune reconstitution inflammatory syndrome. Scientific reports 9, 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanc FX, et al. (2011) Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. The New England journal of medicine 365, 1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goovaerts O, et al. (2015) Lower Pre-Treatment T Cell Activation in Early-and Late-Onset Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. PloS one 10, e0133924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford N, et al. (2016) TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. Journal of the International AIDS Society 19, 20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi NR, et al. (2010) HIV coinfection in multidrug-and extensively drug-resistant tuberculosis results in high early mortality. American journal of respiratory and critical care medicine 181, 80–86 [DOI] [PubMed] [Google Scholar]
- 24.Toossi Z, et al. (2001) Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clinical and experimental immunology 123, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan ZA, et al. (2015) Latent and Active Tuberculosis Infection Increase Immune Activation in Individuals Co-Infected with HIV. EBioMedicine 2, 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nusbaum RJ, et al. (2016) Pulmonary Tuberculosis in Humanized Mice Infected with HIV-1. Scientific reports 6, 21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas-Taraco AG, et al. (2006) Mycobacterium tuberculosis upregulates coreceptors CCR5 and CXCR4 while HIV modulates CD14 favoring concurrent infection. AIDS research and human retroviruses 22, 45–51 [DOI] [PubMed] [Google Scholar]
- 28.Toossi Z, et al. (1997) Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-infected individuals in vitro. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 15, 325–331 [DOI] [PubMed] [Google Scholar]
- 29.Ranjbar S, et al. (2012) Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway. PLoS pathogens 8, e1002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souriant S, et al. (2019) Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell reports 26, 3586–3599.e3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda MJ, et al. (2018) High Turnover of Tissue Macrophages Contributes to Tuberculosis Reactivation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. The Journal of infectious diseases 217, 1865–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino Y, et al. (2007) Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. The Journal of infectious diseases 195, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 33.Gutlapalli VR, et al. (2016) High levels of plasma interferon gamma and +874T/A gene polymorphism is associated with HIV-TB co-infection. Human immunology 77, 1264–1270 [DOI] [PubMed] [Google Scholar]
- 34.de Noronha AL, et al. (2008) Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathology, research and practice 204, 155–161 [DOI] [PubMed] [Google Scholar]
- 35.Ansari AW, et al. (2006) Host chemokine (C-C motif) ligand-2 (CCL2) is differentially regulated in HIV type 1 (HIV-1)-infected individuals. International immunology 18, 1443–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hertoghe T, et al. (2000) T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clinical and experimental immunology 122, 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toossi Z, et al. (2013) Systemic immune activation and microbial translocation in dual HIV/tuberculosis-infected subjects. The Journal of infectious diseases 207, 1841–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloom CI, et al. (2013) Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PloS one 8, e70630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloom CI, et al. (2012) Detectable changes in the blood transcriptome are present after two weeks of antituberculosis therapy. PloS one 7, e46191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanham G, et al. (1996) Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clinical and experimental immunology 103, 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson KA, et al. (2016) Activation Profile of Mycobacterium tuberculosis-Specific CD4(+) T Cells Reflects Disease Activity Irrespective of HIV Status. American journal of respiratory and critical care medicine 193, 1307–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wergeland I, et al. (2011) T regulatory cells and immune activation in Mycobacterium tuberculosis infection and the effect of preventive therapy. Scandinavian journal of immunology 73, 234–242 [DOI] [PubMed] [Google Scholar]
- 43.Mack U, et al. (2009) LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. The European respiratory journal 33, 956–973 [DOI] [PubMed] [Google Scholar]
- 44.Hudock TA, et al. (2017) Hypoxia Sensing and Persistence Genes Are Expressed during the Intragranulomatous Survival of Mycobacterium tuberculosis. American journal of respiratory cell and molecular biology 56, 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehra S, et al. (2011) Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. Journal of medical primatology 40, 233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaushal D, et al. (2015) Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nature communications 6, 8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foreman TW, et al. (2017) Nonpathologic Infection of Macaques by an Attenuated Mycobacterial Vaccine Is Not Reactivated in the Setting of HIV Co-Infection. The American journal of pathology 187, 2811–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diedrich CR, et al. (2010) Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One 5, e9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodgers MA, et al. (2018) Preexisting Simian Immunodeficiency Virus Infection Increases Susceptibility to Tuberculosis in Mauritian Cynomolgus Macaques. Infection and immunity 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehra S, et al. (2015) The DosR Regulon Modulates Adaptive Immunity and Is Essential for Mycobacterium tuberculosis Persistence. American journal of respiratory and critical care medicine 191, 1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slight SR, et al. (2013) CXCR5(+) T helper cells mediate protective immunity against tuberculosis. The Journal of clinical investigation 123, 712–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayner EL, et al. (2015) Early lesions following aerosol challenge of rhesus macaques (Macaca mulatta) with Mycobacterium tuberculosis (Erdman strain). Journal of comparative pathology 152, 217–226 [DOI] [PubMed] [Google Scholar]
- 53.Sibley L, et al. (2016) Route of delivery to the airway influences the distribution of pulmonary disease but not the outcome of Mycobacterium tuberculosis infection in rhesus macaques. Tuberculosis (Edinburgh, Scotland) 96, 141–149 [DOI] [PubMed] [Google Scholar]
- 54.Sharpe S, et al. (2016) Ultra low dose aerosol challenge with Mycobacterium tuberculosis leads to divergent outcomes in rhesus and cynomolgus macaques. Tuberculosis (Edinburgh, Scotland) 96, 1–12 [DOI] [PubMed] [Google Scholar]
- 55.Maiello P, et al. (2018) Rhesus Macaques Are More Susceptible to Progressive Tuberculosis than Cynomolgus Macaques: a Quantitative Comparison. Infection and immunity 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kauffman KD, et al. (2018) Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal immunology 11, 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattila JT, et al. (2015) Granzyme B-expressing neutrophils correlate with bacterial load in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Cellular microbiology 17, 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehra S, et al. (2010) Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PloS one 5, e12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehra S, et al. (2013) Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. The Journal of infectious diseases 207, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green AM, et al. (2010) CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. The Journal of infectious diseases 202, 533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips BL, et al. (2015) LAG3 expression in active Mycobacterium tuberculosis infections. The American journal of pathology 185, 820–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Picker LJ, et al. (2004) Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. The Journal of experimental medicine 200, 1299–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brenchley JM, et al. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine 12, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 64.Estes JD, et al. (2010) Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS pathogens 6, e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon SN, et al. (2007) Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. Journal of immunology (Baltimore, Md. : 1950) 179, 3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cumont MC, et al. (2008) Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. Journal of virology 82, 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gueye A, et al. (2004) Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. Journal of medical primatology 33, 83–97 [DOI] [PubMed] [Google Scholar]
- 68.Huot N, et al. (2017) Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nature medicine 23, 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huot N, et al. (2016) Innate immune cell responses in non pathogenic versus pathogenic SIV infections. Current opinion in virology 19, 37–44 [DOI] [PubMed] [Google Scholar]
- 70.Pandrea IV, et al. (2007) Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. Journal of immunology (Baltimore, Md. : 1950) 179, 3035–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasegawa A, et al. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serrano-Villar S, et al. (2013) The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. The Journal of infection 66, 57–66 [DOI] [PubMed] [Google Scholar]
- 73.Klatt NR, et al. (2013) Microbial translocation, immune activation, and HIV disease. Trends in microbiology 21, 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teleshova N, et al. (2013) Simian immunodeficiency virus interactions with macaque dendritic cells. Advances in experimental medicine and biology 762, 155–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reeves RK and Fultz PN (2007) Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology 365, 356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown KN, et al. (2007) Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. Journal of immunology (Baltimore, Md. : 1950) 178, 6958–6967 [DOI] [PubMed] [Google Scholar]
- 77.Harris LD, et al. (2010) Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. Journal of virology 84, 7886–7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandler NG, et al. (2014) Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511, 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bosinger SE, et al. (2009) Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of clinical investigation 119, 3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veazey RS, et al. (2016) Prevention of SHIV transmission by topical IFN-beta treatment. Mucosal immunology 9, 1528–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Utay NS and Douek DC (2016) Interferons and HIV Infection: The Good, the Bad, and the Ugly. Pathogens & immunity 1, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marshall HD, et al. (2011) Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. Journal of virology 85, 5929–5939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pahar B, et al. (2017) A significant productive in vivo infection of resting cells with simian immunodeficiency virus in a macaque with AIDS. Journal of medical primatology 46, 59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douek DC (2013) Immune activation, HIV persistence, and the cure. Topics in antiviral medicine 21, 128–132 [PMC free article] [PubMed] [Google Scholar]
- 85.Llibre JM, et al. (2012) Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antiviral therapy 17, 355–364 [DOI] [PubMed] [Google Scholar]
- 86.Vazquez-Castellanos JF, et al. (2015) Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal immunology 8, 760–772 [DOI] [PubMed] [Google Scholar]
- 87.Cai Y, et al. (2014) In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. Journal of immunology (Baltimore, Md. : 1950) 192, 2821–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Micci L, et al. (2014) CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS pathogens 10, e1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abreu CM, et al. (2019) Infectious Virus Persists in CD4(+) T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. Journal of virology 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawn SD, et al. (2006) Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 42, 1040–1047 [DOI] [PubMed] [Google Scholar]
- 91.Lawn SD, et al. (2006) Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS (London, England) 20, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 92.Ahmed A, et al. (2018) Incidence and determinants of tuberculosis infection among adult patients with HIV attending HIV care in north-east Ethiopia: a retrospective cohort study. BMJ open 8, e016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khoury G, et al. (2016) Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS (London, England) 30, 1511–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourgarit A, et al. (2006) Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS (London, England) 20, F1–7 [DOI] [PubMed] [Google Scholar]
- 95.Micci L, et al. (2015) Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. The Journal of clinical investigation 125, 4497–4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar V, et al. (2019) Cannabinoid Attenuation of Intestinal Inflammation in Chronic SIV-Infected Rhesus Macaques Involves T Cell Modulation and Differential Expression of Micro-RNAs and Pro-inflammatory Genes. Frontiers in immunology 10, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molina PE, et al. (2011) Cannabinoid neuroimmune modulation of SIV disease. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 6, 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ong CW, et al. (2015) Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS pathogens 11, e1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gautam US, et al. (2018) In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America 115, E62–e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aksoy P, et al. (2006) Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochemical and biophysical research communications 349, 353–359 [DOI] [PubMed] [Google Scholar]
- 101.Tameris M, et al. (2019) Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double-blind dose-escalation trial. The Lancet. Respiratory medicine 7, 757–770 [DOI] [PubMed] [Google Scholar]
- 102.Suliman S, et al. (2016) Bacillus Calmette-Guerin (BCG) Revaccination of Adults with Latent Mycobacterium tuberculosis Infection Induces Long-Lived BCG-Reactive NK Cell Responses. Journal of immunology (Baltimore, Md. : 1950) 197, 1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nemes E, et al. (2018) Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. The New England journal of medicine 379, 138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suliman S, et al. (2019) Dose Optimization of H56:IC31 Vaccine for Tuberculosis-Endemic Populations. A Double-Blind, Placebo-controlled, Dose-Selection Trial. American journal of respiratory and critical care medicine 199, 220–231 [DOI] [PubMed] [Google Scholar]
- 105.Kumarasamy N, et al. (2016) A Randomized, Controlled Safety, and Immunogenicity Trial of the M72/AS01 Candidate Tuberculosis Vaccine in HIV-Positive Indian Adults. Medicine 95, e2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.White AD, et al. (2013) Evaluation of the safety and immunogenicity of a candidate tuberculosis vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clinical and vaccine immunology : CVI 20, 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Penn-Nicholson A, et al. (2018) Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. The Lancet. Respiratory medicine 6, 287–298 [DOI] [PubMed] [Google Scholar]
- 108.Aldovini A and Young RA (1991) Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351, 479–482 [DOI] [PubMed] [Google Scholar]
- 109.Kim BJ, et al. (2018) Development of a Live Recombinant BCG Expressing Human Immunodeficiency Virus Type 1 (HIV-1) Gag Using a pMyong2 Vector System: Potential Use As a Novel HIV-1 Vaccine. Frontiers in immunology 9, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venkataswamy MM, et al. (2014) Improving Mycobacterium bovis bacillus Calmette-Guerin as a vaccine delivery vector for viral antigens by incorporation of glycolipid activators of NKT cells. PloS one 9, e108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chege GK, et al. (2013) Robust immunity to an auxotrophic Mycobacterium bovis BCG-VLP prime-boost HIV vaccine candidate in a nonhuman primate model. Journal of virology 87, 5151–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chege GK, et al. (2009) A prime-boost immunisation regimen using recombinant BCG and Pr55(gag) virus-like particle vaccines based on HIV type 1 subtype C successfully elicits Gag-specific responses in baboons. Vaccine 27, 4857–4866 [DOI] [PubMed] [Google Scholar]
- 113.Falk LA, et al. (2000) Recombinant bacillus Calmette-Guerin as a potential vector for preventive HIV type 1 vaccines. AIDS research and human retroviruses 16, 91–98 [DOI] [PubMed] [Google Scholar]
- 114.Honda M, et al. (1995) Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guerin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proceedings of the National Academy of Sciences of the United States of America 92, 10693–10697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jensen K, et al. (2017) Balancing Trained Immunity with Persistent Immune Activation and the Risk of Simian Immunodeficiency Virus Infection in Infant Macaques Vaccinated with Attenuated Mycobacterium tuberculosis or Mycobacterium bovis BCG Vaccine. Clinical and vaccine immunology : CVI 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korioth-Schmitz B, et al. (2015) Rhesus immune responses to SIV Gag expressed by recombinant BCG vectors are independent from pre-existing mycobacterial immunity. Vaccine 33, 5715–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]


