Abstract
Background
Uncertainty exists about the optimal point at which multi‐component fortifier should be added to human milk for promoting growth in preterm infants. The most common practice is to start fortification when the infant’s daily enteral feed volume reaches 100 mL/kg body weight. Another approach is to commence fortification earlier, in some cases as early as the first enteral feed. Early fortification of human milk could increase nutrient intake and growth rates but may increase the risk of feed intolerance and necrotising enterocolitis (NEC).
Objectives
To assess effects on growth and safety of early fortification of human milk versus late fortification in preterm infants
To assess whether effects vary based upon gestational age (≤ 27 weeks; 28 to 31 weeks; ≥ 32 weeks), birth weight (< 1000 g; 1000 to 1499 g; ≥ 1500 g), small or appropriate for gestational age, or type of fortifier (bovine milk‐based human milk fortifier (HMF); human milk‐based HMF; formula powder)
Search methods
We used the standard strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8); OVID MEDLINE (R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (R) (1946 to 15 August 2019); MEDLINE via PubMed (1 August 2018 to 15 August 2019) for the previous year; and the Cumulative Index to Nursing and Allied Health Literatue (CINAHL) (1981 to 15 August 2019). We searched clinical trials databases and reference lists of included studies.
Selection criteria
We included randomised controlled trials that compared early versus late fortification of human milk in preterm infants. We defined early fortification as fortification started at < 100 mL/kg/d enteral feed volume or < 7 days postnatal age, and late fortification as fortification started at ≥ 100 mL/kg/d feeds or ≥ 7 days postnatal age.
Data collection and analysis
Both review authors assessed trial eligibility and risk of bias and independently extracted data. We analysed treatment effects in individual trials, and we reported risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We used the GRADE approach to assess the certainty of evidence.
Main results
We included two trials with a total of 237 infants. All participants were very low birth weight infants (birth weight < 1500 g). Early fortification was started at 20 mL/kg/d enteral feeds in one study and 40 mL/kg/d in the other study. Late fortification was started at 100 mL/kg/d feeds in both studies. One study used bovine milk‐based fortifier, and the other used human milk‐based fortifier.
Meta‐analysis showed that early fortification may have little or no effect on growth outcomes including time to regain birth weight (MD ‐0.06 days, 95% CI ‐1.32 to 1.20 days), linear growth (MD 0.10 cm/week, 95% CI ‐0.03 to 0.22 cm/week), or head growth (MD ‐0.01 cm/week, 95% CI ‐0.07 to 0.06 cm/week) during the initial hospitalisation period. Early fortification may have little or no effect on the risk of NEC (MD ‐0.01, 95% CI ‐0.07 to 0.06). The certainty of evidence was low for these outcomes due to risk of bias (lack of blinding) and imprecision (small sample size).
Early fortification may have little or no effect on incidence of surgical NEC, time to reach full enteral feeds, extrauterine growth restriction at discharge, proportion of infants with feed interruption episodes, duration of total parenteral nutrition (TPN), duration of central venous line usage, or incidence of invasive infection, all‐cause mortality, and duration of hospital stay. The certainty of evidence was low for these outcomes due to risk of bias (lack of blinding) and imprecision (small sample size).
We did not have data for other outcomes such as subsequent weight gain after birth weight is regained, parenteral nutrition‐associated liver disease, postdischarge growth, and neurodevelopmental outcomes.
Authors' conclusions
Available evidence is insufficient to support or refute early fortification of human milk in preterm infants. Further large trials would be needed to provide data of sufficient quality and precision to inform policy and practice.
Plain language summary
Earlier compared to later addition of human milk fortifier to human milk to promote growth in preterm infants
Review question
Does adding human milk fortifier (HMF) early promote growth and improve outcomes in preterm infants compared to adding it late?
Background
Uncertainty exists about the optimal point at which HMF should be added to human milk for promoting growth in preterm infants. The most common practice is to start HMF when the infant’s daily feed volume reaches 100 mL/kg body weight. Another approach is to commence HMF earlier, in some cases as early as the first feed. Adding HMF early could increase nutrient intake and growth rates but may increase the risk of feed intolerance and necrotising enterocolitis.
Study characteristics
Evidence is up‐to‐date as of August 2019. We identified two randomised controlled trials that evaluated the effects of adding HMF early for preterm infants.
Key results
We found only limited data from two trials. There is uncertainty as to whether adding HMF early for preterm infants has an effect on important outcomes such as growth during hospital stay, necrotising enterocolitis, death before discharge, presence of growth failure at discharge, and length of hospital stay.
Certainty of evidence
The available evidence is insufficient to support or refute early addition of HMF to human milk to promote growth in preterm infants. More trials are needed to examine whether adding HMF early is beneficial or harmful for preterm infants.
Summary of findings
Summary of findings 1. Early versus late fortification of human milk in preterm infants.
| Early versus late fortification of human milk in preterm infants | ||||||
| Patient or population: preterm infants Settings: neonatal unit Intervention: early fortification Comparison: late fortification | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Late fortification | Early fortification | |||||
| Time to regain birth weight (days) | Mean time to regain birth weight (days) in the intervention groups was 0.06 lower (1.32 lower to 1.2 higher) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | |||
| Linear growth (cm/week) | Mean linear growth (cm/week) in the intervention groups was 0.1 higher (0.03 lower to 0.22 higher) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | |||
| Increase in head circumference (cm/week) | Mean increase in head circumference (cm/week) in the intervention groups was 0.01 lower (0.07 lower to 0.06 higher) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | |||
| Necrotising enterocolitis stage 2 or 3 | Study population | RR 1.36 (0.44 to 4.16) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 43 per 1000 | 58 per 1000 (19 to 178) | |||||
| Moderate | ||||||
| 42 per 1000 | 57 per 1000 (18 to 175) | |||||
| Time to reach full enteral feeds | Mean time to reach full enteral feeds in the intervention groups was 0.27 higher (3.48 lower to 4.02 higher) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | |||
| Extrauterine growth restriction at discharge | Study population | RR 1.06 (0.81 to 1.39) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 342 per 1000 | 362 per 1000 (277 to 475) | |||||
| Moderate | ||||||
| 385 per 1000 | 408 per 1000 (312 to 535) | |||||
| Proportion of infants with feed interruption episodes | Study population | RR 0.99 (0.73 to 1.34) | 237 (2 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 402 per 1000 | 398 per 1000 (293 to 538) | |||||
| Moderate | ||||||
| 389 per 1000 | 385 per 1000 (284 to 521) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aLack of blinding. bSmall sample size.
Background
Description of the condition
Preterm birth places the infant at risk of nutritional deprivation and results in interruption of growth. It is a challenge to sustain in utero growth velocity in preterm infants due to difficulty in maintaining adequate protein‒energy supplementation and due to their catabolic state secondary to postnatal illnesses such as sepsis, necrotising enterocolitis (NEC), chronic lung disease, need for assisted ventilation, and exposure to postnatal steroids (Lima 2014).
Extremely low birth weight (ELBW) infants take 16 ± 7 days to regain birth weight (Steward 2002). The rate of extrauterine growth restriction (EUGR) at discharge is unacceptably high, ranging from 23% in infants born at 34 weeks' gestation to 71% in those born at 23 weeks' gestation (Clark 2003). Moreover, being small for gestational age (SGA) at birth increases the likelihood of EUGR at discharge by six times (Freitas 2016). Growth failure can continue even after discharge. Data from the National Institute of Child Health and Human Development (NICHD) cohort in the USA showed that 40% of ELBW‐SGA infants had weight, length, and head circumference less than the 10th percentile at 18 to 22 months' corrected age (Dusick 2003). Growth restriction in early infancy has long‐term consequences such as stunting, neurodevelopmental impairment, and early onset of adult diseases such as hypertension, diabetes, obesity, and hypercholesterolaemia (Barker 1989; Cooke 2004; Lucas 1994; Lucas 2004).
Early aggressive nutrition is the norm in the management of preterm infants. The nutritional requirement in the first few days is met by total parenteral nutrition (TPN), which is started soon after birth and is continued until adequate enteral feeds are established. However, TPN administration is technically demanding (need for trained staff, laminar flow, laboratory backup, and appropriate equipment) and expensive. It may cause adverse effects such as azotaemia, metabolic acidosis, hyperlipidaemia, cholestasis, and catheter‐related complications (Calkins 2014). Further, each day without enteral nutrition increases the likelihood of EUGR by 8% (Freitas 2016). Hence, enteral feeds should be started early, and full enteral feeds should be achieved as soon as possible.
Description of the intervention
Human milk is the best enteral food for preterm infants. However, unfortified human milk may not provide adequate protein to support growth and lean body mass accretion in very low birth weight infants (Morales 2007). The amount of calcium and phosphorus provided by unfortified human milk is too low to match the in utero accretion rate (Boyd 2007; Lucas 1996). The method most commonly used to increase enteral supplementation of protein, calories, and minerals consists of adding multi‐component human milk fortifier (HMF) to human milk. On average, unfortified human milk provides 67 kcal and 1.1 g protein per 100 mL, while human milk with HMF provides 80 kcal and 2 g protein per 100 mL. A recent Cochrane meta‐analysis showed that fortification of human milk with multi‐component HMF improved in‐hospital growth rates; however, there was no significant difference in other major clinical outcomes (Brown 2016).
Definitive guidelines on when to start HMF are not available. The common practice is to start fortification when enteral feed volume reaches around 100 mL/kg/d (Berseth 2004; Gathwala 2007; Mukhopadhyay 2007). Fortification is delayed because of clinicians’ concern about the risk of NEC and feed intolerance. However, some studies have started fortification earlier, as early as the first feed (Alizadeh 2017; Maas 2013; Mimouni 2017; Shah 2016; Sullivan 2010; Tillman 2012).
How the intervention might work
Early fortification of human milk could improve protein, calorie, and mineral intake in preterm infants (Shah 2016; Tillman 2012). This may avoid the dip in nutrition and reduce the time needed to regain birth weight. It may also improve further postnatal growth and decrease the risk of EUGR (Steward 2002). Early fortification may be especially important for infants who receive pasteurised donor milk, which contains lower levels of protein, energy, and minerals than mother’s own milk (Arslanoglu 2010).
On the other hand, because most of the available HMFs have cow's milk as the base, and because HMF increases the osmolarity of feeds, early fortification may increase the risk of feed intolerance and NEC. This may result in interruption of feeds and delay in reaching full enteral feeds, which in turn may increase the duration of TPN and risk of parenteral nutrition‐associated liver disease (Calkins 2014). It may also increase the number of days of central venous line (CVL) usage, along with the risk of late‐onset sepsis and other CVL‐related complications (Hermansen 2005).
Why it is important to do this review
Given the potential use of early fortification of human milk to improve postnatal growth and other outcomes, as well as the possible risks, we performed this systematic review and meta‐analysis to identify and appraise data from RCTs with the goal of providing a synthesis of evidence to inform practice and research.
Objectives
To assess effects on growth and safety of early fortification of human milk versus late fortification in preterm infants
To assess whether effects vary based upon gestational age (≤ 27 weeks; 28 to 31 weeks; ≥ 32 weeks), birth weight (< 1000 g; 1000 to 1499 g; ≥ 1500 g), small or appropriate for gestational age, or type of fortifier (bovine milk‐based human milk fortifier (HMF); human milk‐based HMF; formula powder)
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in the review.
Types of participants
We included preterm infants (< 37 weeks' gestation).
Types of interventions
Intervention
Early fortification of human milk, started at < 100 mL/kg/d enteral feed volume or at < 7 days' postnatal age.
Comparison
Late fortification, started at ≥ 100 mL/kg/d feeds or at ≥ 7 days' postnatal age.
Fortification should be done with a multi‐component fortifier containing carbohydrate, protein, lipid, and micro‐nutrients. The fortifier could be bovine milk‐based HMF, human milk‐based HMF, or formula powder.
Types of outcome measures
Primary outcomes
Time to regain birth weight (days) and subsequent rate of weight gain (g/kg/d), linear growth (cm/week), and increase in head circumference (cm/week) during the initial hospitalisation period
Incidence of necrotising enterocolitis (NEC) stage 2 or 3 (modified Bell’s staging; Walsh 1986)
Secondary outcomes
Incidence of surgical NEC
Time to reach full enteral feeds ≥ 150 mL/kg/d
Incidence of extrauterine growth restriction at discharge (number of infants with weight < 10th percentile for the index population)
Proportion of infants with ≥ 1 episode of feed interruption lasting ≥ 12 hours
Duration of total parenteral nutrition (TPN) (days)
Incidence of parenteral nutrition‐associated liver disease
Duration of central venous line (CVL) usage (days)
Incidence of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine, or a normally sterile body space
All‐cause mortality before discharge or up to 44 weeks’ postmenstrual age
Duration of hospital stay (days)
Growth measures following discharge from hospital to latest follow‐up (weight, length, and head circumference)
Neurodevelopmental outcomes assessed after 12 months' corrected age: neurological evaluations; developmental scores; and classifications of disability, including auditory and visual disability. We defined neurodevelopmental impairment as the presence of one or more of the following: non‐ambulant cerebral palsy; developmental quotient more than 2 standard deviations below the population mean; blindness (visual acuity < 6/60), or deafness (any hearing impairment requiring or not improved by amplification)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8), in the Cochrane Library; OVID MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (R) (1946 to 15 August 2019); MEDLINE via PubMed (1 August 2018 to 15 August 2019) for the previous year; and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1981 to 15 August 2019). We have presented the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing and recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), as well as the US National Library of Medicine’s clinical trials registry (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not found through Cochrane CENTRAL.
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles. Trials reported only as abstracts were eligible if sufficient information was available from the report, or through contact with study authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of Cochrane Neonatal and Cochrane (Higgins 2017).
Selection of studies
Both review authors (ST and TA) screened the title and abstract of all studies identified by the search strategy and independently assessed the full‐text articles for potentially relevant trials. We excluded those studies that did not meet all inclusion criteria, and we stated the reasons for exclusion. We discussed disagreements until consensus was achieved.
We recorded the selection process in sufficient detail to complete a Characteristics of excluded studies table and a PRISMA flow diagram (Moher 2009).
Data extraction and management
Both review authors (ST and TA) extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We assessed each identified trial for methodological quality with respect to (a) inclusiveness of the population, (b) masking of allocation, (c) masking of intervention, (d) completeness of follow‐up, and (e) masking of outcome assessment. If data from the trial reports were insufficient, we contacted the trialists for further information. We sought clarification from at least one author of each trial considered for selection. We discussed any disagreements until we reached a consensus.
Assessment of risk of bias in included studies
Both review authors (ST and TA) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We discussed disagreements until we reached a consensus. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in individual trials using Review Manager 2014, and we reported the risk ratio (RR) for dichotomous data and the mean difference (MD) for continuous data, along with respective 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. We combined study results where there was little heterogeneity between study designs, and we considered interactions between effects of the intervention and the choice of randomisation unit to be unlikely.
Dealing with missing data
We requested and obtained from trial investigators additional data on important outcomes that were missing.
Assessment of heterogeneity
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each RR or MD analysis to quantify inconsistency across studies, and we described the percentage of variability in effect estimates that might be due to heterogeneity rather than to sampling error.
Assessment of reporting biases
Because only two trials were included in the meta‐analysis, we could not examine a funnel plot for possible publication bias.
Data synthesis
We analysed all infants randomised on an intention‐to‐treat basis and treatment effects in individual trials using a fixed‐effect model to combine the data. We calculated RRs for meta‐analyses of categorical outcomes and MDs for continuous outcomes, each with 95% CIs.
Certainty of evidence
We used the GRADE approach, as outlined in the GRADE Handbook to assess the certainty of evidence for the following clinically relevant outcomes (Schünemann 2013).
Time to regain birth weight (days).
Linear growth (cm/week) during hospital stay.
Increase in head circumference (cm/week) during hospital stay.
Incidence of necrotising enterocolitis (NEC) stage 2 or 3.
Time to reach full enteral feeds (days).
Incidence of extrauterine growth restriction at discharge.
Proportion of infants with ≥ 1 episode of feed interruption lasting ≥ 12 hours.
Both review authors (ST and TA) independently assessed the certainty of evidence for each outcome. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and the presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create Table 1 to report the certainty of evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Subgroup analysis and investigation of heterogeneity
We planned to do subgroup analysis based on:
gestational age: ≤ 27 weeks; 28 to 31 weeks; ≥ 32 weeks' gestation;
birth weight: < 1000 g; 1000 to 1499 g; ≥ 1500 g;
small for gestational age or appropriate for gestational age infants (classified using birth weight relative to the reference population); and
type of human milk fortifier (HMF) (bovine milk‐based HMF; human milk‐based HMF; formula powder).
We did not perform the above mentioned subgroup analyses due to inadequate data. We assessed statistical heterogeneity using the I² statistic.
Sensitivity analysis
We planned to undertake sensitivity analyses to determine if findings were affected by including only studies reporting adequate methods (low risk of bias), defined as reporting adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up. However, we did not conduct sensitivity analyses because it was not required.
Results
Description of studies
Results of the search
We screened 2118 titles and abstracts that were identified via the search strategy. We carried out full‐text review of nine articles. We excluded six studies, and we reported details of the excluded studies. We identified one ongoing study. We identified two eligible studies for inclusion in qualitative and quantitative synthesis. See Figure 1.
1.
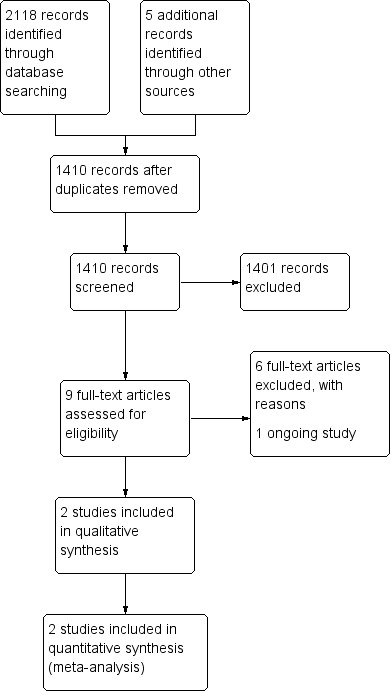
Study flow diagram.
Included studies
Two studies satisfied our inclusion criteria (Shah 2016; Sullivan 2010). Early fortification was started from 20 mL/kg/d feed volume in Shah 2016 and 40 mL/kg/d feed volume in Sullivan 2010. Late fortification was started from 100 mL/kg/d feed volume in both studies (Shah 2016; Sullivan 2010). One study used bovine milk‐based HMF (Shah 2016), and the other used human milk‐based HMF (Sullivan 2010). (See Characteristics of included studies.)
Shah 2016 was performed in the USA. Trialists randomised 100 infants with birth weight < 1500 g into the early fortification group (fortification starting from 20 mL/kg/d feeds) or the late fortification group (fortification starting from 100 mL/kg/d feeds). Fortification was done with bovine milk‐based liquid HMF (Enfamil); 5 mL HMF was added to 25 mL human milk to increase caloric density to 24 kcal/oz. Infants were given only trophic feeds for one to three days depending on their birth weight, followed by a gradual increase in feed volume. Feeds were delivered continuously (three hours on and one hour off). TPN was given until infants reached sufficient enteral feeds. Primary outcome was time to reach full enteral feeds (> 140 mL/kg/d). Secondary outcomes were feeding intolerance, NEC, daily weight gain, protein and caloric intake for the first four weeks of life, weight velocity at four weeks after birth and at 36 weeks' postmenstrual age, TPN days, length of hospital stay, metabolic acidosis, late‐onset sepsis, ventilation days, chronic lung disease, postnatal steroid treatment, patent ductus arteriosus, severe intraventricular haemorrhage (grade III and IV), periventricular leukomalacia, and retinopathy of prematurity.
Sullivan 2010 was a multi‐centre study done at 12 neonatal intensive care units ‐ 11 in the USA and 1 in Austria. Trialists included 207 infants with 500 to 1250 g birth weight. Infants were randomised into three arms: human milk‐based HMF fortification starting at 100 mL/kg/d feed volume (HM100), human milk‐based HMF fortification starting at 40 mL/kg/d feed volume (HM40), and bovine milk‐based HMF fortification or preterm formula feeding group (BOV). We have included only HM100 (late fortification) and HM40 (early fortification) groups in our analysis. Human milk‐based HMF (Prolact+ H2MF ) was used and calorie density was 24 kcal/oz. Trophic feeds were given for five days, followed by a gradual increase in feed volume up to a maximum of 160 mL/kg/d. The primary outcome was duration of TPN. Secondary outcomes were growth indices, late‐onset sepsis, NEC stage 2 or 3, feed intolerance, bronchopulmonary dysplasia, retinopathy of prematurity, duration of CVL usage, duration of hospital day, and duration of ventilation and oxygen therapy.
Excluded studies
We excluded six studies (see Characteristics of excluded studies).
Alizadeh 2017 was an RCT comparing early fortification (fortification from the first feed) and later fortification (fortification starting from 75 mL/kg/d feed volume). The trial recruited 80 preterm infants of 28 to 34 weeks' gestational age and < 2000 g birth weight. Fortification was done with bovine milk‐based HMF (Aptamil FMS HMF powder), 4.4 g for 100 mL of expressed breast milk, which gives 24 kcal/oz. We excluded this trial because late fortification was started at 75 mL/kg/d feed volume.
We excluded three studies as they were retrospective studies (Huston 2019; Maas 2013; Tillman 2012). Huston 2019 was a multi‐centre retrospective study that compared neonates with 500 to 1250 g birth weight receiving early fortification (starting at < 60 mL/kg/d) or later fortification (starting at > 60 mL/kg/d). Maas 2013 enrolled preterm babies at < 32 weeks' gestation and with birth weight < 1500 g born in 2006, 2007, and 2010. Babies who were born in 2006 and 2007 received later fortification (starting at 150 mL/kg/d feed volume), and babies who were born in 2010 received early fortification (starting at 100 mL/kg/d). Tillman 2012 used a retrospective pre–post design and compared early fortification (starting with the first feeding) in infants born before June 2009 versus later fortification (starting at 50 to 80 mL/kg/d) in infants born June 2009 and after.
We excluded Ghandehari 2012, as it was a post‐hoc analysis from Sullivan 2010.
We excluded Sajjadian 2014 because we found no published data and we could not obtain unpublished data from study authors.
Ongoing studies
We found one ongoing study (IRCT20171030037093N3).
This trial has randomised 90 preterm infants into three groups, with fortification started at 30, 70, and 100 mL/kg/d enteral feeds. The main outcome is weight, length, and head circumference at four weeks' postnatal age. The study has been completed. However, it remains to be published. See the Characteristics of ongoing studies table.
Risk of bias in included studies
See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both included studies have used block randomisation with a fixed block size of four. The random sequence was computer generated.
Sullivan 2010 was a multi‐centre study with central randomisation. In Shah 2016, the method of allocation concealment was not mentioned. Moreover, a fixed block size of four made allocation of every fourth infant predictable, as caregivers and trial investigators were not masked.
Blinding
Both studies were open‐label trials (Shah 2016; Sullivan 2010). Therefore, risk for performance bias and detection bias was high in both trials.
Incomplete outcome data
Both studies reported the outcomes of all participants (Shah 2016; Sullivan 2010).
Selective reporting
Both studies published all outcomes that were mentioned in the protocol (Shah 2016; Sullivan 2010).
Other potential sources of bias
No other potential source of bias was noted in either study (Shah 2016; Sullivan 2010).
Effects of interventions
See: Table 1
See Table 1.
We included two RCTs with 237 infants in the meta‐analysis to assess the benefits and safety of early fortification versus late fortification of human milk for various outcomes in preterm infants (Shah 2016; Sullivan 2010).
Primary outcomes
1. Time to regain birth weight (days) and subsequent rate of weight gain (g/kg/d), linear growth (cm/week), and increase in head circumference (cm/week) during the initial hospitalisation period
Data on time to regain birth weight were available from both included studies (Shah 2016; Sullivan 2010). The meta‐analysis did not show a difference in time to regain birth weight between early and late fortification groups (mean difference (MD) ‐0.06 days, 95% confidence interval (CI) ‐1.32 to 1.20 days; participants = 237; studies = 2). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.1; Figure 3). We did not find any data on subsequent rate of weight gain during the initial hospitalisation period beyond the time to regain birth weight. Meta‐analysis of data from both trials did not show a difference in linear growth between early and late fortification groups (MD 0.10 cm/week, 95% CI ‐0.03 to 0.22 cm/week; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.2). Meta‐analysis of data from both studies did not show a difference in the increase in head circumference between the two groups (MD ‐0.01 cm/week, 95% CI ‐0.07 to 0.06 cm/week; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). Heterogeneity was moderate (I² = 27%) (Analysis 1.3).
1.1. Analysis.

Comparison 1: Early versus late fortification, Outcome 1: Time to regain birth weight (days)
3.

Forest plot of comparison: 1 Early versus late fortification, outcome: 1.1 Time to regain birth weight (days).
1.2. Analysis.

Comparison 1: Early versus late fortification, Outcome 2: Linear growth (cm/week)
1.3. Analysis.

Comparison 1: Early versus late fortification, Outcome 3: Increase in head circumference (cm/week)
The certainty of evidence was low (downgraded for lack of blinding and small sample size) for all three outcomes.
2. Incidence of necrotising enterocolitis (NEC) stage 2 or 3
Data for analysis of this outcome were available from both trials (Shah 2016; Sullivan 2010). The estimated risk ratio for this outcome was 1.36 (95% CI 0.44 to 4.16; participants = 237; studies = 2). The meta‐analysis did not show a difference in the incidence of NEC between early and late fortification groups. There was no evidence of heterogeneity (I² = 0%) (Analysis 1.4; Figure 4). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.4. Analysis.

Comparison 1: Early versus late fortification, Outcome 4: Necrotising enterocolitis stage 2 or 3
4.

Forest plot of comparison: 1 Early versus late fortification, outcome: 1.4 Necrotising enterocolitis stage 2 or 3.
Secondary outcomes
1. Incidence of surgical NEC
Data from both trials were available for analysis of this outcome (Shah 2016; Sullivan 2010). The meta‐analysis did not show a difference between the two groups in the incidence of surgical NEC (risk ratio (RR) 0.98, 95% CI 0.14 to 6.85; participants = 237; studies = 2). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.5). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.5. Analysis.

Comparison 1: Early versus late fortification, Outcome 5: Surgical NEC
2. Time to reach full enteral feeds ≥ 150 mL/kg/d
Two RCTs including 237 participants contributed data (Shah 2016; Sullivan 2010). Early fortification did not show a difference in the time to reach full enteral feeds (MD 0.27 days, 95% CI ‐3.48 to 4.02 days; participants = 237; studies = 2). Heterogeneity was moderate (I² = 39%) (Analysis 1.6). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.6. Analysis.

Comparison 1: Early versus late fortification, Outcome 6: Time to reach full enteral feeds
3. Incidence of extrauterine growth restriction at discharge (number of infants with weight < 10th percentile for the index population)
Meta‐analysis of two trials did not show a difference between groups in the incidence of extrauterine growth restriction at discharge (RR 1.06, 95% CI 0.81 to 1.39; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.7; Figure 5). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.7. Analysis.

Comparison 1: Early versus late fortification, Outcome 7: Extrauterine growth restriction at discharge
5.

Forest plot of comparison: 1 Early versus late fortification, outcome: 1.7 Extrauterine growth restriction at discharge.
4. Proportion of infants with feed interruption episodes
Meta‐analysis of two trials did not show a difference between early and late fortification groups in the proportion of infants with feed interruption episodes (RR 0.99, 95% CI 0.73 to 1.34; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.8; Figure 6). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.8. Analysis.

Comparison 1: Early versus late fortification, Outcome 8: Proportion of infants with feed interruption episodes
6.

Forest plot of comparison: 1 Early versus late fortification, outcome: 1.8 Proportion of infants with feed interruption episodes.
5. Duration of total parenteral nutrition (TPN) (days)
Meta‐analysis of two trials did not show a difference between groups in the duration of TPN (MD 0.08 days, 95% CI ‐3.07 to 3.24 days; participants = 237; studies = 2) (Analysis 1.9) (Shah 2016; Sullivan 2010). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.9. Analysis.

Comparison 1: Early versus late fortification, Outcome 9: Duration of TPN (days)
6. Incidence of parenteral nutrition‐associated liver disease
We did not find any data on this outcome.
7. Duration of central venous line (CVL) usage (days)
Data from two studies did not show a difference between early and late fortification groups in the duration of CVL usage (MD 1.04 days, 95% CI ‐3.13 to 5.20; participants = 237; studies = 2) (Analysis 1.10) (Shah 2016; Sullivan 2010). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.10. Analysis.

Comparison 1: Early versus late fortification, Outcome 10: Duration of CVL usage (days)
8. Incidence of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine, or a normally sterile body space
Data for analysis of this outcome were available from both trials (Shah 2016; Sullivan 2010). The meta‐analysis did not show a difference between groups in the incidence of invasive infection (RR 0.69, 95% CI 0.40 to 1.18; participants = 237; studies = 2). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.11). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.11. Analysis.

Comparison 1: Early versus late fortification, Outcome 11: Incidence of invasive infection
9. All‐cause mortality before discharge or up to 44 weeks’ postmenstrual age
Meta‐analysis of two trials did not show a difference between groups for this outcome (RR 1.32, 95% CI 0.30 to 5.77; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.12). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.12. Analysis.

Comparison 1: Early versus late fortification, Outcome 12: All‐cause mortality before discharge
10. Duration of hospital stay (days)
Meta‐analysis of data from both studies did not show a difference between groups in the duration of hospital stay (MD 2.33, 95% CI ‐6.44 to 11.11; participants = 237; studies = 2) (Shah 2016; Sullivan 2010). There was no evidence of heterogeneity (I² = 0%) (Analysis 1.13). The certainty of evidence was low, downgraded for lack of blinding and small sample size.
1.13. Analysis.

Comparison 1: Early versus late fortification, Outcome 13: Duration of hospital stay (days)
11. Growth measures following discharge from hospital to latest follow‐up (weight, length, and head circumference)
We did not find any data on this outcome.
12. Neurodevelopmental outcomes assessed after 12 months' corrected age: neurological evaluations; developmental scores; and classifications of disability, including auditory and visual disability
We did not find any data on this outcome.
Discussion
Summary of main results
Evidence from two studies including 237 infants that contributed data to the outcomes of this review showed that early fortification of human milk compared to late fortification may have little or no effect on growth outcomes during the initial hospitalisation period nor on the incidence of necrotising enterocolitis (NEC). Similarly, the meta‐analysis showed that early fortification may have little or no effect on other important outcomes such as surgical NEC, time to reach full enteral feeds, extrauterine growth restriction at discharge, proportion of infants with feed interruption episodes, duration of total parenteral nutrition (TPN), duration of central venous line usage, incidence of invasive infection, all‐cause mortality, and duration of hospital stay. The quality of evidence was low for all outcomes, downgraded for lack of blinding and small sample size. We did not find any data on other important outcomes such as subsequent weight gain after birth weight was regained, parenteral nutrition‐associated liver disease, postdischarge growth, and neurodevelopmental outcomes.
Overall completeness and applicability of evidence
Early fortification may improve protein, calorie, and mineral intake in preterm infants and thus may reduce extrauterine growth restriction. However, adding human milk fortifier (HMF) to human milk early may increase the risk of feed intolerance and NEC, and thus increase the time to full enteral feeds. This review identified only two studies for meta‐analysis (Shah 2016; Sullivan 2010). Shah 2016 used bovine milk‐based HMF, and Sullivan 2010 used human milk‐based HMF, both with the same calorie density of 24 kcal/oz. Thus the results may be applicable to both commonly used fortifiers, namely, bovine milk‐based and human milk‐based HMF.
Both studies were done in very low birth weight infants; inclusion criteria were birth weight < 1500 g in Shah 2016 and birth weight 500 to 1250 g in Sullivan 2010. Hence, the results may not be applicable to larger preterm infants, who have a biologically more mature gastrointestinal system and lower risk of NEC.
Feed increment was started only after the first few days in both studies ‐ after one to three days in Shah 2016, and at five days in Sullivan 2010. Only trophic feeds were given to the babies until then. Hence, even in the early fortification group, fortification was started on or after day 2 in Shah 2016, and on or after day 6 in Sullivan 2010. Hence, the results are not applicable to neonatal intensive care units (NICUs), where fortification is started on day 1 of postnatal life.
Both studies were conducted in high‐income countries.
Quality of the evidence
The methodological quality of both included trials was good (Shah 2016; Sullivan 2010). Trialists used computer‐generated randomisation and reported all intended outcomes, and there was no attrition. However, both were open‐label trials, hence risk of performance and detection bias was high. One trial did not mention the method of allocation concealment (Shah 2016).
The certainty of evidence was low for all outcomes such as time to regain birth weight (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), linear growth (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), increase in head circumference (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), NEC stage 2 or 3 (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), time to reach full enteral feeds (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), extrauterine growth restriction at discharge (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size), and feed interruption episodes among infants (downgraded for serious risk of bias due to lack of blinding and serious imprecision due to small sample size).
Potential biases in the review process
We have no financial or other conflicts of interest.
We found only two small trials for inclusion in this review. Although we conducted a comprehensive search, we cannot exclude fully the possibility of publication bias because we do not know whether other published (but not indexed) or unpublished trials have been conducted. We did not have a sufficient number of trials to explore symmetry of funnel plots as a means of identifying possible publication or reporting bias.
Agreements and disagreements with other studies or reviews
Three systematic reviews have compared early versus late fortification in preterm infants (Alyahya 2020; Godden 2019; Mimouni 2017). None of these reviews predefined early and late fortification by using a specific feed volume. They intended to include all studies that started fortification at two different feed volumes.
Two systematic reviews included only clinical trials (Alyahya 2020; Mimouni 2017); Godden 2019 included retrospective cohort studies as well. Mimouni 2017 included the two randomised controlled trials (RCTs) that were included in our study (Shah 2016; Sullivan 2010). The studies included in the other two reviews were different (Alyahya 2020; Mimouni 2017). Alyahya 2020 included two RCTs (Alizadeh 2017; Shah 2016). Godden 2019 included three RCTs (Alizadeh 2017; Shah 2016; Sullivan 2010), as well as two retrospective studies (Lapointe 2016; Tillman 2012). Two systematic reviews concluded that early fortification had no significant impact on any clinical outcomes (Alyahya 2020; Mimouni 2017). Godden 2019 concluded that early fortification is safe and well tolerated but has no impact on growth outcomes.
Alizadeh 2017 was an RCT done in 80 infants at 28 to 34 weeks' gestational age with birth weight < 2000 g. This trial compared early fortification (starting from first feed) to late fortification (starting from 75 mL/kg/d feed volume) and showed no difference in clinical outcomes between groups.
Huston 2019 is a large multi‐centre retrospective study done in 394 infants with birth weight of 500 to 1250 g. Early fortification (starting at < 60 mL/kg/d) was compared to late fortification (starting at > 60 mL/kg/d). This study showed that early fortification improved weight gain velocity and head growth and decreased the occurrence of chronic lung disease without increasing the risk of NEC.
Tillman 2012 is a retrospective study done in 95 infants at < 31 weeks' gestational age. The study compared early fortification (fortification from first feed) and late fortification (fortification from 50 to 80 mL/kg/d). Early fortification did not increase the incidence of feed intolerance but did not increase weight gain at 34 weeks' postmenstrual age as well. Babies in the early fortification group had less alkaline phosphatase from 33 weeks' postmenstrual age.
Thus, the results of our review matched the results of almost all previous studies and showed that there is no difference in important clinical outcomes between early and late fortification groups, and that limited data are available.
Authors' conclusions
Implications for practice.
We found only limited data from two unblinded trials on the effects on growth and safety of early fortification compared to late fortification of human milk in preterm infants. The certainty of evidence was low for all outcomes due to lack of blinding and small sample size. Hence, available evidence is insufficient to either support or refute early fortification of human milk to promote growth in preterm infants.
Implications for research.
Further randomised controlled trials adequately powered to detect meaningful differences in outcomes are needed to assess whether early fortification compared to late fortification of human milk improves important clinical outcomes for preterm infants. These trials should provide more precise estimates on important outcomes such as in‐hospital growth, time to reach full enteral feeds, time to regain birth weight, incidence of extrauterine growth restriction (EUGR), duration of total parenteral nutrition (TPN), incidence of parenteral nutrition‐associated liver disease, duration of central venous line (CVL) usage, incidence of invasive infection, and duration of hospital stay. Trials should also provide data on postdischarge growth and neurodevelopmental outcomes. Trialists should aim to include extremely preterm infants and infants with intrauterine growth restriction, so that subgroup analyses can be planned for this population, which is at higher risk of necrotising enterocolitis (NEC).
We identified one ongoing study (IRCT20171030037093N3). This trial has randomised 90 preterm infants into three groups, with fortification started at 30, 70, and 100 mL/kg/d enteral feeds. The main outcome is weight, length, and head circumference at four weeks' postnatal age. This study has been completed, but it remains to be published. See the Characteristics of ongoing studies table.
History
Protocol first published: Issue 8, 2019 Review first published: Issue 7, 2020
Acknowledgements
The methods section of this protocol is based on a standard template used by the Cochrane Neonatal Review Group.
We are grateful to Drs Shah, Sullivan, and Lee for providing further details and data from their trials.
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating Editor; and Bill McGuire, Co‐coordinating Editor, all of whom provided editorial and administrative support. Carol Friesen, Information Specialist, designed and ran the literature searches.
As a Cochrane Neonatal Associate Editor, Dr Jon Dorling has peer‐reviewed and offered feedback for this review.
Appendices
Appendix 1. Search methods
The RCT filters were created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2017). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
Cochrane CENTRAL via CRS Web
Date searched: 15 August 2019 Terms: 1. MESH DESCRIPTOR Milk, Human EXPLODE ALL AND CENTRAL:TARGET 2. MESH DESCRIPTOR Food, Fortified EXPLODE ALL AND CENTRAL:TARGET 3. MESH DESCRIPTOR Dietary Supplements EXPLODE ALL AND CENTRAL:TARGET 4. #3 OR #2 5. #1 AND #4 6. (fortif* OR supplement* OR enrich*) ADJ4 (human OR breast OR expressed OR mother* OR maternal OR donor*) ADJ2 milk* AND CENTRAL:TARGET 7. (fortif* OR supplement* OR enrich*) ADJ4 (DHM OR HM OR breastmilk*) AND CENTRAL:TARGET 8. #5 OR #6 OR #7 9. MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 10. infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 11. #10 OR #9 AND CENTRAL:TARGET 12. #8 AND #11
MEDLINE via Ovid
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present: Date ranges: 1946 to 15 August 2019 Terms: 1. exp Milk, Human/ 2. exp Food, Fortified/ 3. exp Dietary Supplements/ 4. 2 or 3 5. 1 and 4 6. (fortif* adj4 ((human or breast or expressed) adj2 milk*)).mp. 7. (fortif* adj4 ((mother* or maternal or donor*) adj2 milk*)).mp. 8. (supplement* adj4 ((human or breast or expressed) adj2 milk*)).mp. 9. (supplement* adj4 ((mother* or maternal or donor*) adj2 milk*)).mp. 10. (enrich* adj4 ((human or breast or expressed) adj2 milk*)).mp. 11. (enrich* adj4 ((mother* or maternal or donor*) adj2 milk*)).mp. 12. ((fortif* or supplement* or enrich*) adj4 DHM).mp. 13. ((fortif* or supplement* or enrich*) adj4 HM).mp. 14. ((fortif* or supplement* or enrich*) adj4 breastmilk*).mp. 15. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. 5 or 15 17. exp infant, newborn/ 18. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 19. 17 or 18 20. randomized controlled trial.pt. 21. controlled clinical trial.pt. 22. randomized.ab. 23. placebo.ab. 24. drug therapy.fs. 25. randomly.ab. 26. trial.ab. 27. groups.ab. 28. or/20‐27 29. exp animals/ not humans.sh. 30. 28 not 29 31. 19 and 30 32. 16 and 31
MEDLINE via PubMed
Date ranges: 01 August 2018 to 15 August 2019 Terms: (((("Milk, Human"[Mesh] AND ("Food, Fortified"[Mesh] OR "Dietary Supplements"[Mes2h]))) OR ((fortif*[TW] OR supplement*[TW] OR enrich*[TW]) AND (human[TW] OR breast[TW] OR expressed[TW] OR mother*[TW] OR maternal[TW] OR donor*[TW]) AND milk*[TW])) OR ((fortif*[TW] OR supplement*[TW] OR enrich*[TW]) AND (DHM[TW] OR HM[TW] OR breastmilk*[TW]))) AND (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))) Filters activated: Publication date from 2018/08/01
CINAHL via EBSCOhost
Date ranges: 1981 to 15 August 2019 Terms: S1MH milk, human S2MH Food, Fortified S3MH Dietary Supplementation S4S2 OR S3 S5S1 AND S4 S6(fortif* OR supplement* OR enrich*) AND (human OR breast OR expressed OR mother* OR maternal OR donor*) AND milk* S7(fortif* OR supplement* OR enrich*) AND (DHM OR HM OR breastmilk*) S8S5 OR S6 OR S7 S9((infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW)) AND ((randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)) S10S8 AND S9
ISRCTN
Date searched: 15 August 2019 Search terms: milk AND Interventions: fortification AND Participant age range: Neonate Retrieved: 2 (1 was retrieved in the CRS, so the remaining record was saved in the text file) milk AND Interventions: supplementation AND Participant age range: Neonat
Appendix 2. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of trials. For each trial, we sought information regarding the method of randomisation and blinding and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as being at low, high, or unclear risk of bias. Both review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table ‘Characteristics of included studies’. We evaluated the following issues and entered the findings into the ‘Risk of bias’ table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
Data and analyses
Comparison 1. Early versus late fortification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to regain birth weight (days) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.32, 1.20] |
| 1.2 Linear growth (cm/week) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.03, 0.22] |
| 1.3 Increase in head circumference (cm/week) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.06] |
| 1.4 Necrotising enterocolitis stage 2 or 3 | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.44, 4.16] |
| 1.5 Surgical NEC | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.85] |
| 1.6 Time to reach full enteral feeds | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.48, 4.02] |
| 1.7 Extrauterine growth restriction at discharge | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 1.8 Proportion of infants with feed interruption episodes | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 1.9 Duration of TPN (days) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐3.07, 3.24] |
| 1.10 Duration of CVL usage (days) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐3.13, 5.20] |
| 1.11 Incidence of invasive infection | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.18] |
| 1.12 All‐cause mortality before discharge | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.77] |
| 1.13 Duration of hospital stay (days) | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 2.33 [‐6.44, 11.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Shah 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 100 preterm infants were recruited Inclusion criteria: infants with birth weight < 1500 g Exclusion criteria: death or expected to die within 72 hours, major congenital or chromosomal abnormalities, when mother could not provide her own milk and refused the use of donor breast milk |
|
| Interventions | Early fortification group ‐ fortification starting from 20 mL/kg/d feeds Late fortification group ‐ fortification starting from 100 mL/kg/d feeds Fortification was done with bovine milk‐based liquid HMF (Enfamil); 5 mL HMF was added to 25 mL human milk to increase caloric density to 24 kcal/oz |
|
| Outcomes | Primary outcome: time to reach full enteral feeds (≥ 140 mL/kg/d) Secondary outcomes: feed intolerance, NEC, time to regain birth weight, daily weight gain, protein and caloric intake for the first 4 weeks of life, weight velocity at 4 weeks after birth and at 36 weeks' postmenstrual age, TPN days, length of hospital stay, metabolic acidosis, late‐onset sepsis, ventilation days, chronic lung disease, postnatal steroid treatment, patent ductus arteriosus, severe intraventricular haemorrhage (grade III and IV), periventricular leukomalacia, retinopathy of prematurity |
|
| Notes | Infants were given only trophic feeds for 1 to 3 days depending on their birth weight, followed by a gradual increase in feed volume. Feeds were delivered continuously (3 hours on and 1 hour off). TPN was given until infants reached sufficient enteral feeds Definition of full‐volume enteral feeds differed from our definition (≥ 140 vs ≥ 150 mL/kg/d, respectively) Definition of feed intolerance in the trial differed from our definition (≥ 24 hours of feed interruption vs ≥ 12 hours of feed interruption, respectively) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed by computerised software. Block size was 4 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention was not blinded to allow proper handling of mother’s own milk and appropriate fortification |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Proper handling of mother’s own milk and appropriate fortification prevented masking of infants’ caregivers and research investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants' outcomes were analysed, except 1 infant in the early fortification group. Reason was not stated. This was not considered a significant source of bias |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were reported |
| Other bias | Low risk | Nil |
Sullivan 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: preterm infants with birth weight 500 to 1250 g were recruited Exclusion criteria: major congenital malformations, high likelihood of transfer to a non‐study institution during the study period |
|
| Interventions | Infants were randomised into 3 arms: HM100 ‐ human milk‐based HMF fortification starting at 100 mL/kg/d feed volume HM40 ‐ human milk‐based HMF fortification starting at 40 mL/kg/d feed volume BOV ‐ bovine milk‐based HMF fortification or preterm formula feeding We have included only the HM100 (late fortification) and HM40 (early fortification) groups in our analysis. Human milk‐based HMF (Prolact+ H2MF ) was used, and calorie density was 24 kcal/oz |
|
| Outcomes | Primary outcome: duration of TPN Secondary outcomes: growth indices (weight, length, and head circumference), late‐onset sepsis, NEC stage 2 or 3, feed intolerance, bronchopulmonary dysplasia, retinopathy of prematurity, duration of CVL usage, duration of hospital day, duration of ventilation and oxygen therapy |
|
| Notes | Trophic feeds were given for 5 days, followed by a gradual increase in feed volume up to a maximum of 160 mL/kg/d As per protocol, the study was planned with 2 groups ‐ Group 1 with 3 arms (HM100, HM40, bovine‐based HMF) and Group 2 with 2 arms (HM100 vs Preterm/term formula). However, only Group 1 outcomes were published; Group 2 outcomes were not published Triple blinding was mentioned in the protocol but was not followed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Separate block randomisation schemes were prepared for each of the strata and were performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The need to ensure proper handling of mother’s own milk precluded true blinding of infants’ caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all enrolled infants were published |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were published |
| Other bias | Low risk | Nil |
BOV = bovine.
CVL = central venous line.
HMF = human milk fortifier.
NEC = necrotising enterocolitis.
TPN = total parenteral nutrition.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alizadeh 2017 | Late fortification was started at 75 mL/kg/d feed volume. |
| Ghandehari 2012 | Post‐hoc analysis of Sullivan 2010 |
| Huston 2019 | Retrospective study |
| Maas 2013 | Retrospective study including multiple interventions |
| Sajjadian 2014 | No published data available |
| Tillman 2012 | Retrospective study |
Characteristics of ongoing studies [ordered by study ID]
IRCT20171030037093N3.
| Study name | Investigation and comparison of the effects of early and late breast milk enrichment in preterm infants |
| Methods | Clinical trial with parallel groups |
| Participants | Inclusion criteria: infants with gestational age of 28 to 32 weeks with birth weight less than 2000 g Exclusion criteria: presence of any congenital anomaly and formula feeding Planned to recruit 90 preterm infants |
| Interventions | Group 1: fortification started at 30 mL/kg/d feed volume Group 2: fortification started at 70 mL/kg/d feed volume Group 3: fortification started at 100 mL/kg/d feed volume Fortification is done with 4.4 g of Aptamil human milk fortifier in 100 mL human milk |
| Outcomes | Weight, length, and head circumference at 4 weeks' postnatal age |
| Starting date | 11 November 2017 |
| Contact information | Name of organisation: Shahre‐kord University of Medical Sciences Name of responsible person: Majid Hamidi Street address: Shahrekord University of Medical Sciences, Building No. 2, University headquarters, Ayatollah Kashani Blvd City: Shahrekord Province: Chahar‐Mahal‐va‐Bakhtiari Postal code: 8815713471 Phone: +98 38 3227 4004 Email: majid.hamidi@yahoo.com |
| Notes | Trial completion date: 1 November 2018 Trial results are not yet published |
Differences between protocol and review
We made the following changes to the published protocol (Thanigainathan 2019).
We did not prespecify in the protocol outcomes for Table 1. We defined feed interruption as interruption for ≥ 12 hours. However, in Shah 2016, feed interruption was defined as interruption for ≥ 24 hours, which we accepted and included the outcome data in our meta‐analysis.
As of July 2019, the Cochrane Neonatal Review Group no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated its searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, the Cochrane Neonatal Review Group no longer searches for RCTs and CCTs from ClinicalTrials.gov nor from the World Health Organization’s ICTRP (http://International Clinical Trials Registry Platform), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN at http://www.isrctn.com/, formerly Controlled‐trials.com, is searched separately.
For the 2019 update, we developed a new search strategy, which we ran without applying date limits (Appendix 1).
Contributions of authors
Both authors (ST and TA) developed the protocol, screened search outputs, assessed study eligibility, and extracted and synthesised data. Both authors (ST and TA) assessed risk of bias across key domains and undertook GRADE assessment. Both authors revised the final review.
Sources of support
Internal sources
-
Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India
to ST
-
Sri Ramachandra Institute of Higher Education and Research (SRIHER), Chennai, India
to TA
External sources
-
National Institute of Health Research, UK
This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from the Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
ST has no conflict of interest to declare.
TA has no conflict of interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation that is independent from the Gerber Products Company. The grantor has no input on the content of the review nor on the editorial process (Sources of support).
New
References
References to studies included in this review
Shah 2016 {published and unpublished data}
- Shah SD, Dereddy N, Jones TL, Dhanireddy R, Talati AJ. Early versus delayed human milk fortification in very low birth weight infants - a randomized controlled trial. Journal of Pediatrics 2016;174:126-31. [DOI: 10.1016/j.jpeds.2016.03.056] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sullivan 2010 {published and unpublished data}
- Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. Journal of Pediatrics 2010;156(4):562-7. [DOI: 10.1016/j.jpeds.2009.10.040] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alizadeh 2017 {published data only}
- Alizadeh Taheri P, Sajjadian N, Asgharyan Fargi M, Shariat M. Is early breast milk fortification more effective in preterm infants?: a clinical trial. Journal of Perinatal Medicine 2017;45(8):953-7. [DOI: 10.1515/jpm-2015-0375] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghandehari 2012 {published data only}
- Ghandehari H, Lee ML, Rechtman DJ, H2MF Study Group. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Research Notes 2012;5:188. [DOI: 10.1186/1756-0500-5-188] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huston 2019 {published data only}
- Huston RK, Lee ML, Rider ED, Stawarz ML, Hedstrom DM, Pence MM, et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk based fortifier. Journal of Neonatal-Perinatal Medicine 2019:Epub ahead of print. [DOI: 10.3233/NPM-190300] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maas 2013 {published data only}
- Maas C, Wiechers C, Bernhard W, Poets CF, Franz AR. Early feeding of fortified breast milk and in-hospital growth in very premature infants: a retrospective cohort analysis. BMC Pediatrics 2013;13(4):178. [DOI: 10.1186/1471-2431-13-178] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sajjadian 2014 {published data only}
- Sajjadian N, Alizadeh Taheri P, Asgharyan Fargi M, Shariat M. A comparison between early and late breast milk fortification in preterm infants: a clinical trial study. Iranian Journal of Pediatrics 2014;24(2 (Suppl)):45. [Google Scholar]
Tillman 2012 {published data only}
- Tillman S, Brandon DH, Silva SG. Evaluation of human milk fortification from the time of the first feeding: effects on infants of less than 31 weeks gestational age. Journal of Perinatology 2012;32(7):525-31. [DOI: 10.1038/jp.2011.140] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT20171030037093N3 {published data only}
- IRCT20171030037093N3. Effect of breast milk enrichment on early infants [Investigation and comparison of the effect of early and late breast milk enrichment on preterm infants]. en.irct.ir/trial/37163 (first received 23 February).
Additional references
Alyahya 2020
- Alyahya W, Simpson J, Garcia AL, Mactier H, Edwards CA. Early versus delayed fortification of human milk in preterm infants: a systematic review. Neonatology 2020;117(1):24-32. [DOI: 10.1159/000501279] [DOI] [PubMed] [Google Scholar]
Arslanoglu 2010
- Arslanoglu S, Moro GE, Ziegler EE, The Wapm Working Group on Nutrition. Optimization of human milk fortification for preterm infants: new concepts and recommendations. Journal of Perinatal Medicine 2010;38(3):233-8. [DOI: 10.1515/JPM.2010.073] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barker 1989
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2(8663):577–80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berseth 2004
- Berseth CL, Van Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004;114(6):e699-e706. [DOI: 10.1542/peds.2004-0911] [PMID: 15545616 ] [DOI] [PubMed] [Google Scholar]
Boyd 2007
- Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(3):F169-75. [DOI: 10.1136/adc.2005.089490] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016
- Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD000343. [DOI: 10.1002/14651858.CD000343.pub3] [DOI] [PubMed] [Google Scholar]
Calkins 2014
- Calkins KL, Venick RS, Devaskar SU. Complications associated with parenteral nutrition in the neonate. Clinics in Perinatology 2014;41(2):331-45. [DOI: 10.1016/j.clp.2014.02.006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2003
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986–90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cooke 2004
- Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(5):F428-30. [PMID: 15321963 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusick 2003
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Seminars in Perinatology 2003;27(4):302–10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freitas 2016
- Freitas BA, Priore SE, Lima LM, Franceschini SC. Extrauterine growth restriction: universal problem among premature infants [Restrição de crescimento extrauterino: problema universal entre os]. Revista de Nutricao 2016;29(1):53-64. [DOI: dx.doi.org/10.1590/1678-98652016000100006 ]
Gathwala 2007
- Gathwala G, Chawla M, Gehlaut VS. Fortified human milk in the small for gestational age neonate. Indian Journal of Pediatrics 2007;74(9):815-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Godden 2019
- Godden B, Collins CT, Hilditch C, McLeod G, Keir A. Does early compared to late fortification of human milk for preterm infants improve clinical outcomes? Journal of Paediatrics and Child Health 2019;55(7):867-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hermansen 2005
- Hermansen MC, Hermansen MG. Intravascular catheter complications in the neonatal intensive care unit. Clinics in Perinatology 2005;32(1):141-56, vii. [DOI: 10.1016/j.clp.2004.11.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from https://training.cochrane.org/handbook.
Lapointe 2016
- Lapointe M, Barrington Kj, Savaria M, Janvier A. Preventing postnatal growth restriction in infants with birthweight less than 1300 g. Acta Paediatrica 2016;105(2):e54-9. [DOI] [PubMed] [Google Scholar]
Lima 2014
- Lima PA, Carvalho Md, Costa AC, Moreira ME. Variables associated with extra uterine growth restriction in very low birth weight infants. Jornal de Pediatria 2014;90(1):22-7. [DOI: 10.1016/j.jped.2013.05.007] [PMID: 24156833 ] [DOI] [PubMed] [Google Scholar]
Lucas 1994
- Lucas A. Role of nutritional programming in determining adult morbidity. Archives of Disease in Childhood 1994;71(4):288–90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1996
- Lucas A, Fewtrell MS, Morley R, Lucas PJ, Baker BA, Lister G, et al. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. American Journal of Clinical Nutrition 1996;64(2):142–51. [DOI: 10.1093/ajcn/64.2.142] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucas 2004
- Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, et al. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. American Journal of Respiratory and Critical Care Medicine 2004;170(5):534–40. [PMID: 15172897 ] [DOI] [PubMed] [Google Scholar]
Mimouni 2017
- Mimouni FB, Nathan N, Ziegler EE, Lubetzky R, Mandel D. The use of multinutrient human milk fortifiers in preterm infants: a systematic review of unanswered questions. Clinics in Perinatology 2017;44(1):173-8. [DOI: 10.1016/j.clp.2016.11.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morales 2007
- Morales Y, Schanler RJ. Human milk and clinical outcomes in VLBW infants: how compelling is the evidence of benefit? Seminars in Perinatology 2007;31(2):83–8. [DOI: 10.1053/j.semperi.2007.02.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mukhopadhyay 2007
- Mukhopadhyay K, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatrics 2007;44(4):286-90. [PMID: ] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Steward 2002
- Steward DK, Pridham KF. Growth patterns of extremely low-birth-weight hospitalized preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2002;31(1):57-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Thanigainathan 2019
- Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013392. [DOI: 10.1002/14651858.CD013392] [DOI] [PMC free article] [PubMed] [Google Scholar]


