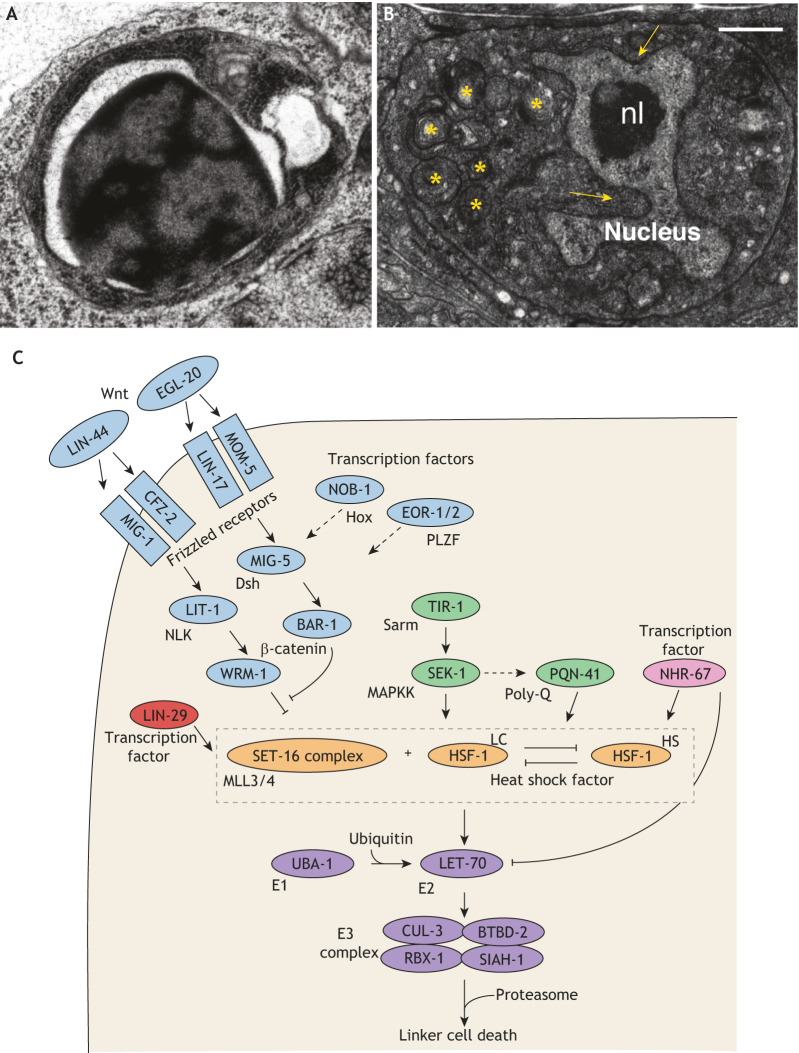
Fig. 2.
Regulation of linker cell-type death in C. elegans. (A) Electron micrograph of a C. elegans apoptotic cell. (B) Electron micrograph of a C. elegans linker cell dying by LCD. nl, nucleolus. Arrows, nuclear invaginations/crenellations. Asterisks, swollen organelles. (C) Pathway for LCD in C. elegans. LCD in worms is subject to both cell-autonomous transcriptional control and non-autonomous control through multiple control pathways. Death onset is controlled by two opposing Wnt pathways (blue). The Wnt ligand LIN-44 acts non-autonomously in conjunction with the Frizzled receptors MIG-1 and CFZ-2, the Nemo-like kinase LIT-1 and the β-catenin WRM-1 in the linker cell to possibly prevent premature death. A second Wnt pathway inhibits the LIN-44/Wnt pathway. Here, EGL-20/Wnt acts non-autonomously, and LIN-17/Frizzled and MOM-5/Frizzled act in the linker cell with MIG-5/Disheveled and BAR-1/β-catenin, promoting death. This second pathway is likely controlled by two additional transcription factors, NOB-1/Hox and the EOR-1/-2/PLZF complex. In a parallel pathway (green), death is promoted by TIR-1, the C. elegans ortholog of mammalian Sarm, which activates the MAPKK protein SEK-1, which in turn may promote expression of the polyglutamine-repeat protein PQN-41 in the linker cell. The zinc-finger protein LIN-29 (red), which regulates developmental timing, and the nuclear hormone receptor NHR-67 (pink) appear to act independently of and in parallel with the Wnt and MAPKK pathways to promote death. In addition, HSF-1 may transcriptionally activate components of the ubiquitin proteasome system (purple).The pro-death function of HSF-1 (LC) competes with the pro-survival function (HS). Adapted from Kutscher and Shaham (2017). Scale bars: 0.5 μm.