Abstract
Introduction:
Transferred emergency general surgery (EGS) patients are a vulnerable, high acuity population. Health and health care utilization outcomes among transferred (TRAN) as compared to directly admitted (DA) patients have primarily been studied using single institution or hospital system data limiting generalizability. We evaluated these outcomes among EGS patients using a national database.
Methods:
We identified encounters of patients aged ≥18 years with an American Association for the Surgery of Trauma-defined EGS diagnosis in the 2008–2011 Nationwide Inpatient Sample (NIS). Multivariable regression analyses determined if transfer status independently predicted in-hospital mortality (logistic regression) and morbidity (presence of any complication among those who survived to discharge; logistic regression), cost (log-linear regression), and length of stay (among those who survived to discharge; log-linear regression) accounting for the NIS sampling design.
Results:
We identified 274,145 TRAN (57,885 unweighted) and 10,456,100 DA (2,187,132 unweighted) encounters. On univariate analysis, TRAN patients were more likely to have higher comorbidity scores, have Medicare insurance, and reside in an area with lower median household income compared to DA patients (p<0.0001). Mortality was significantly higher in the TRAN vs DA groups (4.4% vs 1.6%; p<0.0001). Morbidity (presence of any complication) was also higher among TRAN patients (38.8% vs 26.1%; p<0.0001). Morbidity among TRAN patients was primarily due to urinary- (13.7%), gastrointestinal- (12.9%), and pulmonary-related (13.3%) complications. Median length of stay was 4.3 days for TRAN vs 3.0 days for DA (p<0.0001) patients. Median cost was higher for TRAN patients ($8,935 vs $7,167; p<0.0001). Regression analyses determined that TRAN patients after adjustment had significantly higher mortality, morbidity, and cost as well as longer lengths of stay.
Conclusions:
EGS patients who are transferred experience increased in-hospital morbidity and mortality as well as increased lengths of stay and cost. As the EGS population grows and ages while the EGS workforce declines, the need for interhospital transfers will increase. Identifying risk factors associated with worse outcomes among transferred patients can inform the design of performance improvement initiatives and direct the finite resources available to this vulnerable patient population.
Article Summary
Utilizing a large national database (the Nationwide Inpatient Sample), we demonstrated that EGS patients who are transferred experience significantly increased in-hospital morbidity and mortality as well as increased lengths of stay and cost. As the EGS population grows and ages while the EGS workforce declines, the need for interhospital transfers and performance improvement strategies to care for this vulnerable population with the finite resources available will increase.
Introduction
Patients with emergency general surgery (EGS) conditions, such as appendicitis, cholecystitis, and small bowel obstruction, are a large, growing, and high-acuity population. EGS diagnoses account for over 3 million hospitalizations annually and approximately 7% of all hospital admissions.1,2 In addition, EGS diagnoses are independently associated with poor outcomes, such as higher rates of mortality, complications, lengths of stay, and cost.3,4
Transferred EGS patients represent a particularly high-risk subset of the EGS population.5–9 However, studies of this patient population are limited because they have excluded non-operative EGS patients, have not examined the impact of interhospital transfer on morbidity or cost, or are single institution studies.5–9 While interhospital transfers currently account for only 2% of all EGS admissions,5 the rate of surgical patients undergoing interhospital transfer is increasing.5,6 Given the significant and growing burden of this patient population on the healthcare system, a comprehensive examination of the outcomes of transferred EGS patients is warranted.
In this study, we address the need for a national study to evaluate the impact of interhospital transfer on the range of health and health care utilization outcomes experienced by EGS patients. We include both operative and non-operative EGS patients and examine length of stay and cost in addition to in-hospital mortality and morbidity. We also contribute to the existing literature by developing models that account for organizational characteristics of the hospitals that care for EGS patients, as hospital characteristics impact care quality, handoffs, and the transfer process.10–13
Methods
Data Source
We analyzed the Nationwide Inpatient Sample (NIS). The NIS database was developed as part of the Healthcare Cost and Utilization Project (HCUP) and is maintained by the Agency for Healthcare Research and Quality. The NIS database is a systematic collection of discharge data from HCUP hospitals and is the largest source of all-payer hospital discharge information in the United States (US).14–16 This study was deemed exempt by the University of Wisconsin Institutional Review Board as the NIS is a publicly available database containing de-identified patient information.
Study Population
We identified adult patients (aged ≥18 years) admitted during 2008–2011 on a non-elective basis for EGS conditions as determined by the American Association for the Surgery of Trauma (AAST) International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) criteria.2 The re-design of the NIS sampling strategy, which was implemented in 2012, prevents users from identifying the same hospital in the data before and after 2012. Thus, data from 2008–2011 was utilized in this study. In order to focus specifically on patients who were admitted for an EGS diagnosis, and consistent with previous research, only patients with an AAST EGS-defined diagnosis code listed in the primary diagnosis field were included in our study.5,17,18 The AAST-defined EGS conditions include 309 unique ICD-9-CM codes, which correspond to eleven diagnosis groups (Supplemental Table I).1,2 While these diagnosis groups encompass emergent surgical conditions, included in these groups are vascular and cardiothoracic diagnoses, among others (Supplemental Table I), that may be treated by practitioners from various surgical specialties beyond that of general surgery. However, since the focus of this study was patient outcomes and not the specialty of the provider that administered the care, we elected to utilize the published, endorsed AAST diagnoses to identify our patient population of interest.
We compared health and health care utilization outcomes of EGS patients who were transferred in from another acute care hospital (TRAN) to those directly admitted (DA) (i.e., not transferred in from another acute care hospital). As the NIS database does not allow for patients and outcomes to be tracked between hospital encounters, we excluded patients from the transferred patient group whose discharge destination was to another hospital. Thus, our cohort represents EGS patients during their final encounter, i.e. patients who were either directly admitted or transferred to the hospital from which they were discharged or at which they died (Figure 1). All outcomes and patient- and hospital-level characteristics were assessed at this final patient encounter.
Fig 1.
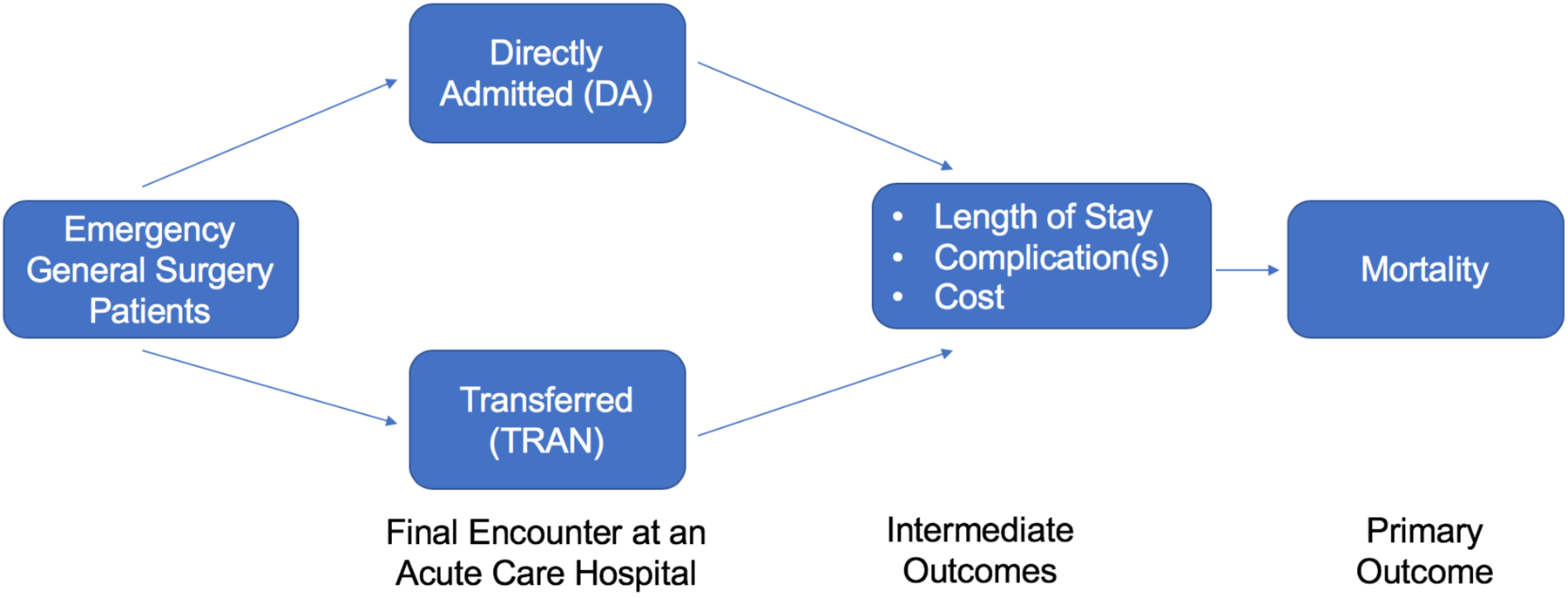
Study Population and Outcomes Based on Transfer Status
Outcomes Measured
Our primary outcome was in-hospital mortality. Secondary outcomes were presence of any in-hospital complication, median length of stay (LOS), and median cost. Thirty-three in-hospital complications relevant to EGS patients were identified based on previous literature19–28 and grouped into the following eight complication categories: urinary, mechanical wound, infections, pulmonary, gastrointestinal (GI) tract, cardiovascular, procedural, and systemic (Supplemental Table II).
Cost was determined using the total charges and the all-payer inpatient cost to charge (CPICC) ratio or the group average all-payer inpatient cost to charge ratio when CPICC was not available as previously described.18,29,30 Costs were transformed to 2011 US dollars using the price indices from the Gross Domestic Product from the US Department of Commerce Bureau of Economic Analysis.31 The cost multipliers used to estimate adjusted costs were 1.041 for 2008, 1.033 for 2009, and 1.021 for 2010.
Patient-Level Characteristics
Patient-level demographic variables included age, gender, race, expected primary payer, and median household income quartile (Table I). These variables were included to examine the impact of demographic and socioeconomic factors that have been previously shown to influence outcomes of EGS patients.3,17 Census-derived median household income according to a patient’s residential zip code was reported based upon percentage quartiles as predetermined by the NIS (0–25th, 26th–50th, 51st–75th, or 76th–100th). Because of the prevalence of missing race, encounters without race information were included as a ‘missing category.’ Patients with missing values for all other variables in the analysis including the four outcomes of interest (inhospital mortality and morbidity, length of stay, cost) were excluded (weighted n=335,056; 3.0%).
Table I.
Weighted Demographics of Encounters of Emergency General Surgery Patients Directly Admitted versus Transferred in from an Acute Care Hospital in the Nationwide Inpatient Sample (2008–2011)
| Variable | Study Group | ||
|---|---|---|---|
| Transferred | Directly Admitted | P value | |
| (n= 274,145) | (n= 10,456,100) | ||
| Age in years, mean (CI) | 60.1 (59.6–60.6) | 58.7 (58.5–58.9) | <0.0001 |
| Gender, % (n) | |||
| Male | 49.1 (134,702) | 45.6 (4,776,937) | <0.0001 |
| Female | 50.9 (139,442) | 54.3 (5,679,163) | |
| Race, % (n) | |||
| White | 60.5 (165,842) | 60.5 (6,324,274) | <0.0001 |
| Black | 8.0 (21,895) | 11.0 (1,154,965) | |
| Hispanic | 5.1 (13,876) | 10.2 (1,062,338) | |
| Other | 4.7 (12,930) | 4.7 (496,347) | |
| Missing | 21.7 (59,601) | 13.6 (1,418,176) | |
| Primary Insurance, % (n) | |||
| Medicare | 49.8 (136,627) | 44.9 (4,689,935) | <0.0001 |
| Medicaid | 10.7 (29,282) | 11.1 (1,158,967) | |
| Private insurance | 28.9 (79,106) | 30.8 (3,219,442) | |
| Other | 10.6 (29,130) | 13.3 (1,387,756) | |
| Median Household Income, % (n) | |||
| 0–25th percentile | 34.6 (94,966) | 27.4 (2,860,717) | <0.0001 |
| 26th–50th percentile | 31.5 (86,329) | 25.7 (2,692,022) | |
| 51st–75th percentile | 20.2 (55,480) | 24.6 (2,577,177) | |
| 76th–100th percentile | 13.6 (37,369) | 22.2 (2,326,184) | |
| Charlson Comorbidity Index, % (n) | |||
| 0 | 37.4 (102,419) | 46.5 (4,857,138) | <0.0001 |
| 1 | 23.4 (64,022) | 23.9 (2,503,132) | |
| 2 | 15.4 (42,238) | 12.8 (1,338,368) | |
| 3+ | 23.9 (65,466) | 16.8 (1,757,462) | |
| EGS Diagnosis Groups, % (n) | |||
| Hepatic-pancreatic-biliary | 24.6 (67,380) | 20.7 (2,165,405) | <0.0001 |
| Upper gastrointestinal tract | 19.7 (53,890) | 20.0 (2,094,072) | |
| Soft tissue | 11.9 (32,542) | 19.2 (2,004,296) | |
| Colorectal | 15.0 (41,223) | 18.2 (1,898,605) | |
| Intestinal obstruction | 11.0 (30,170) | 9.9 (1,034,131) | |
| General abdominal conditions | 5.5 (15,075) | 5.6 (588,989) | |
| Hernias | 3.4 (9,329) | 3.1 (321,540) | |
| Vascular | 5.7 (15,617) | 2.5 (266,230) | |
| Cardiothoracic | 2.6 (7,160) | 0.6 (62,792) | |
| Other | 0.5 (1,433) | 0.1 (15,430) | |
| Resuscitation | 0.1 (326) | 0.04 (4,610) | |
| Procedure Groups, % (n) | |||
| None | 64.5 (176,913) | 67.1 (7,016,306) | <0.0001 |
| Gallbladder | 7.5 (20,473) | 9.8 (1,023,444) | |
| Appendix | 2.7 (7,378) | 6.1 (638,656) | |
| Bone/soft tissue | 3.7 (10,213) | 5.1 (528,482) | |
| Colon | 4.2 (11,604) | 3.1 (320,812) | |
| Hernia | 1.7 (4,598) | 1.8 (183,288) | |
| Intra-abdominal adhesions | 2.1 (5,736) | 1.6 (167,973) | |
| Stomach | 3.0 (8,141) | 1.5 (161,234) | |
| Small bowel | 3.1 (8,446) | 1.5 (159,780) | |
| Blood vessel | 3.1 (8,518) | 0.7 (76,297) | |
| Rectum/anus | 0.3 (819) | 0.7 (71,124) | |
| Heart/lung/chest cavity | 1.7 (4,759) | 0.4 (45,229) | |
| Liver/bile duct | 0.3 (894) | 0.1 (8,447) | |
| Exploratory laparotomy | 0.4 (1,047) | 0.1 (14,035) | |
| Extremity (amputations) | 0.3 (744) | 0.1 (11,950) | |
| Laparoscopy | 0.1 (265) | 0.1 (8,881) | |
| Ear/nose/throat | 0.5 (1,261) | 0.1 (9,085) | |
| Pancreas | 0.8 (2,229) | 0.1 (8,500) | |
| Bladder | 0.03 (78) | 0.02 (2,374) | |
| Kidney | 0.01 (30) | 0.002 (202) | |
| Day of the week of admission, % (n) | |||
| Monday-Friday | 74.2 (203,466) | 75.4 (7,884,832) | <0.0001 |
| Saturday-Sunday | 25.8 (70,679) | 24.6 (2,571,268) | |
Patient-level clinical variables were informed by previous studies and included comorbidities, EGS diagnosis groups (Supplemental Table I), procedures performed, and the day of the week of admission (Table I). The previous literature suggests that transferred patients have more comorbidities.5,9 In our study, comorbidities were accounted for by constructing the Charlson Comorbidity Index from the ICD-9-CM diagnosis codes (up to 15) included on the inpatient discharge record.32,33 Previous studies have also demonstrated that the need for procedures can dictate the need for transfer.34,35 To describe the operative procedures performed, ICD-9CM procedure codes previously identified as pertaining to the AAST EGS-defined diagnoses were applied.1,5,17 Procedure groups included those related to the following organs/body regions: gallbladder, appendix, bone/soft issue, colon, hernia, intra-abdominal adhesions, stomach, small bowel, blood vessel, rectum/anus, heart/lung/chest cavity, liver/bile duct (noncholecystectomy), extremity, pancreas, bladder, kidney, and ear/nose/throat including both open and laparoscopic procedures.1,5,17 Finally, previous studies have shown that the day of the week (weekday vs weekend) of admission can affect outcomes for transferred patients due to resource availability and other systemic factors.35–38
Hospital-Level Characteristics
Hospital-level characteristics were defined by the NIS and included total number of discharges, hospital region, control/ownership (government, nonfederal; private, non-profit; private, investorown), bed size (small, medium, large), and hospital location/teaching status (rural, urban nonteaching, or urban teaching) (Table II). Both bed size and total number of discharges were included in the analysis as bed size accounts for the capacity or number of patients a hospital can treat while the total number of discharges quantifies how quickly a hospital can move patients through. These organizational factors may affect the hospital’s capacity to manage and accommodate transfers of patients and their care thereafter. The other hospital-level factors, such as region, teaching status, and ownership, outline geographic and structural characteristics that can affect hospital performance, resources, as well as the outcomes of a transfer.17,39,40
Table II.
Hospital-level Characteristics of Encounters of Emergency General Surgery Patients Directly Admitted versus Transferred in from an Acute Care Hospital in the Nationwide Inpatient Sample (2008–2011)
| Variable | Study Group | ||
|---|---|---|---|
| Transferred | Directly Admitted | P value | |
| (n= 274,145) | (n= 10,456,100) | ||
| Total number of discharges, median | |||
| (IQR) | 24,368 (16,346–36,692) | 16,132 (8,646–25,902) | <0.0001 |
| Bed size, % (n) | |||
| Small | 6.4 (17,434) | 12.3 (1,283,356) | <0.0001 |
| Medium | 18.4 (50,357) | 25.2 (2,634,351) | |
| Large | 75.3 (206,354) | 62.5 (6,538,393) | |
| Location/teaching status, % (n) | |||
| Rural | 6.8 (18,705) | 12.7 (1,332,222) | <0.0001 |
| Urban non-teaching | 20.8 (56,960) | 48.4 (5,062,333) | |
| Urban teaching | 72.4 (198,479) | 38.8 (4,061,545) | |
| Hospital control/ownership, % (n) | |||
| Government, nonfederal | 16.2 (44,350) | 12.8 (1,343,330) | <0.0001 |
| Private, nonprofit | 77.2 (211,735) | 72.4 (7,567,103) | |
| Private, investor-own | 6.6 (18,060) | 14.8 (1,545,667) | |
| Region, % (n) | |||
| Northeast | 10.5 (28,918) | 16.7 (1,750,921) | <0.0001 |
| Midwest | 38.0 (104,194) | 23.3 (2,437,958) | |
| South | 36.0 (98,676) | 40.9 (4,279,568) | |
| West | 15.5 (42,357) | 19.0 (1,987,654) | |
Statistical Analyses
Data analysis followed an approach that took into consideration the survey design of the NIS, which allowed for the calculation of national estimates.41 Hospital-level weights were chosen for this analysis to allow for hospital-level estimates (the unit of interest for variation among transfers). Hospital weights were calculated for each strata (US Census division, urban or rural location, teaching status, ownership, and bed size) such that a hospital’s weight was equal to the number of hospitals it represented for that data year.42 As twenty percent of the total American Hospital Association hospitals is sampled in each stratum (when possible), each hospital had a weight of approximately 5 (range=3.06 to 19.83). Hospitals were clustered by HCUP hospital identification number to determine similarity and dissimilarity in the outcomes across hospitals and account for the clustered sampling design.
Weighted summary statistics for patient- and hospital-level characteristics were reported as percentages for categorical variables and mean and standard error or median and interquartile range for continuous variables, as appropriate. We compared unadjusted differences by transfer status (DA vs TRAN) using the Rao-Scott chi-square test for categorical variables and the differences of least square means for continuous variables.
Weighted multivariable logistic or linear regression models were used to compare in-hospital mortality, presence of any complication, LOS, and cost between the DA group and TRAN group. Standard errors were estimated clustering on hospitals. Variables included in the regression models included patient-level factors and hospital-level factors (as described in Supplemental Table IV). As detailed above, these variables were selected a priori as they were expected to influence outcomes in EGS patients based on previous studies.3,21,22,25,43 As both LOS and cost had positively skewed distributions, log-transformed LOS and cost were used as the dependent variables in the linear regression models. Data analysis and statistics were performed with the SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
We identified 274,145 (57,885 unweighted) in the transferred (TRAN) group (49.1% male; mean age 60.1 years) and 10,456,100 patient encounters (2,187,132 unweighted) in the directly admitted (DA) group (45.6% male; mean age 58.7 years) with an AAST-defined EGS diagnosis (survey weighted). TRAN encounters accounted for 2.6% of all EGS encounters. Due to the large sample size, all baseline characteristics were statistically significant. However, there were several clinically notable baseline differences between the two groups (Table I). Compared to DA encounters, TRAN encounters were more likely to involve patients with Medicare insurance, less likely to have private insurance, more likely to live in an area with a lower median household income, and more likely to have a higher Charlson Comorbidity index (representing a larger burden of comorbid disease).
The two most common diagnosis groups were hepatic-pancreatic-biliary and upper gastrointestinal tract for both DA and TRAN. DA encounters were more likely than TRAN encounters to include diagnoses for soft tissue or colorectal diagnoses whereas hepatic-pancreatic-biliary, intestinal obstruction, hernia, vascular, and cardiothoracic diagnosis groups were more common among TRAN encounters. Patients in TRAN encounters were more likely to undergo any procedure compared to patients in DA encounters (35.5% vs 32.9%). However, there was a higher percentage of cholecystectomy, appendectomy, and bone/soft tissue procedures among the DA group. In contrast, colon, stomach, small bowel, blood vessel, and heart/lung/chest cavity related procedures were more frequent among the TRAN group (Table I).
There were several notable differences in hospital characteristics among the DA and TRAN groups (Table II). Patients in the TRAN group were more likely to have been transferred to larger hospitals with significantly higher numbers of discharges and beds. Additionally, a higher percentage of patients in the TRAN group received care at urban teaching hospitals and government or non-profit hospitals compared to DA patients whereas patients in DA encounters were more likely to be located at private, investor-owned hospitals. There were also significant regional differences with a higher percentage of patients in TRAN encounters in the Midwest.
Mortality and Morbidity
The TRAN group had significantly higher in-hospital mortality than the DA group (unadjusted rates: 4.4% vs 1.6%; adjusted odds ratio [AOR], 1.7; 95% confidence interval [CI], 1.6–1.8) (Table III). The TRAN group also had a significantly higher rate of complications compared to the DA group (unadjusted rates: 38.8% vs 26.1%; AOR, 1.4; 95% CI, 1.3–1.4) (Table III). In addition to excess complications, there were also significant differences in the proportion of encounters of patients with multiple complications with 6.4% of the TRAN group having three or more complications compared to only 2.1% of the DA group (p<0.0001) (Table III). This discrepancy in complications persisted across all eight of the complication categories in the TRAN vs DA groups (Fig 2) with the most pronounced differences in the following categories: urinary tract (13.7% vs 8.1%), pulmonary complications (13.3% vs 5.1%), and infections (9.9% vs 6.2%) (Supplemental Table III).
Table III.
Outcomes of Encounters of Emergency General Surgery Patients by Transfer Status in the Nationwide Inpatient Sample (2008–2011)
| Outcome | Study Group | Transferred vs Directly Admitted | ||
|---|---|---|---|---|
| Transferred (n= 274,145) | Directly Admitted (n= 10,456,100) | Unadjusted OR (95%CI) | Adjusted OR (95%CI) | |
| Mortality, % (n) | 4.4 (12,086)* | 1.6 (164,978) | 2.9 (2.7–3.1)* | 1.7 (1.6–1.8)* |
| Any Complication, % (n) | 38.8 (106,312)* | 26.1 (2,724,217) | 1.7 (1.6–1.8)* | 1.4 (1.3–1.4)* |
| 1–2 | 32.4 (88,893) | 24.0 (2,505,959) | --- | ---- |
| ≥3 | 6.4 (17,419) | 2.1 (218,258) | --- | --- |
| 0 | 61.2 (167,833) | 73.9 (7,731,883) | --- | --- |
| Estimated Regression Coefficient | ||||
| LOS, days, median (IQR) | 4.3 (2.2–8.2)* | 3.0 (1.6–5.4) | 31.1* | 15.8* |
| Cost, $, median (IQR) | $8,687 ($4,595–17,774)* | $6,759 ($4,334 –10,977) | 26.1* | 8.2* |
p<0.0001;
OR = odds ratio; CI = confidence interval
Fig 2.
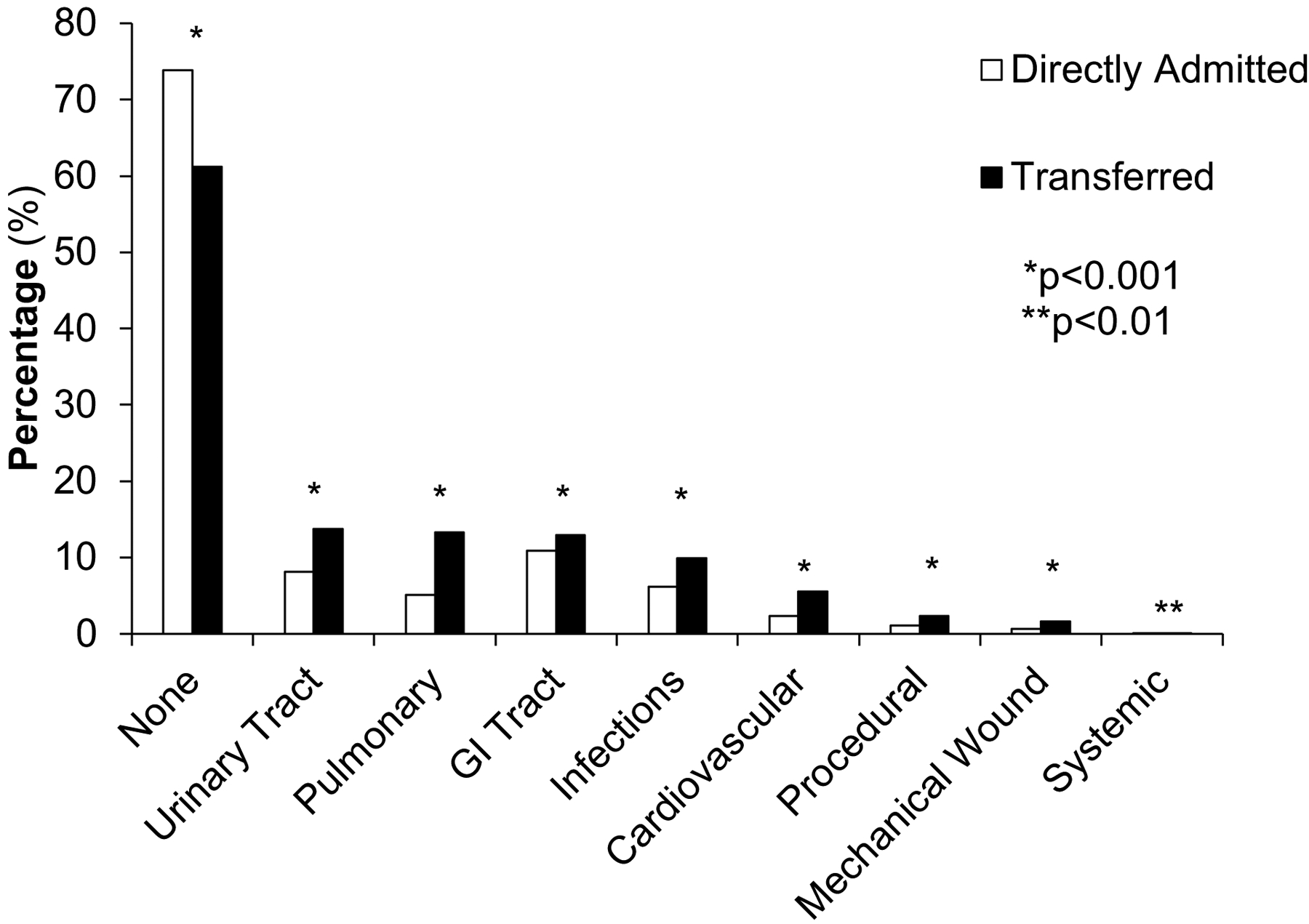
Frequency of complications in encounters of emergency general surgery patients who were directly admitted versus transferred in the Nationwide Inpatient Sample (2008–2011)
Results of the weighted multivariable logistic regression that include the association of patient- and hospital-level factors with in-hospital morbidity and mortality are available in Supplemental Table IV. In addition to transfer status (as detailed above), the majority of the variables in the model demonstrated a significant association with outcomes. As expected, complications have a significant, strong association with mortality (AOR: 36.0 for ≥3 complications and 4.5 for 1–2 compared to none). Similarly, the odds of mortality increased among patients with more comorbidities. Other patient factors demonstrating a significant association with mortality were presence in the resuscitation or vascular diagnosis groups (AOR: 6.0 and 3.3, respectively) and undergoing an exploratory laparotomy or laparoscopy compared to no procedure (AOR: 8.7 and 2.3, respectively).
Results of the multivariable logistic regression model predicting in-hospital morbidity were similar to the results for mortality (Supplemental Table IV). In addition to comorbidities, the following diagnosis groups were demonstrated to be strong predictors of morbidity: resuscitation, upper gastrointestinal tract, and cardiothoracic. Similarly, the following procedures increased the risk of morbidity compared to no procedure: ear/nose/throat, exploratory laparotomy, liver/bile duct, pancreas, kidney, small bowel, and blood vessel.
Length of Stay and Cost
The TRAN encounter group had significantly longer median LOS compared to the DA encounter group (4.3 vs 3.0 days, p<0.0001) (Table III). Multivariable log-linear regression demonstrated that TRAN status confers a 15.8% increase in LOS compared to DA status (Table III). Similarly, there was a significantly higher median cost in the TRAN group compared to the DA group ($8,687 vs $6,759, p<0.001) (Table III). Multivariable linear regression demonstrated TRAN status conferred a significant 8.2% increase in cost compared to DA status (Table III). In particular, a higher comorbidity burden (i.e., higher Charlson Comorbidity Index) and higher numbers of complications were associated with greater length of stay and cost in the multivariable log-linear regression models (Supplemental Table IV).
Discussion
This large, nationwide, observational cohort study of EGS patients demonstrates that interhospital transfer, compared to direct admission, is associated with poorer outcomes, despite controlling for patient- and hospital-level characteristics. Specifically, transfer status is an independent risk factor for increased mortality, morbidity, LOS, and cost.
Our analyses are consistent with previously published studies of transferred EGS patients from NIS cohorts5,18 and studies of cohorts of transferred EGS patients who specifically underwent surgery.6,7 Yelverton, et al. recently demonstrated higher odds of in-hospital mortality for transferred patients compared to directly admitted patients using the 2002–2011 NIS.5 However, they did not use the full list of AAST EGS diagnoses and excluded vascular and cardiovascular diagnoses, reasoning that they are outside the scope of a general surgeon. We provide a more complete picture of EGS practice, having (1) analyzed morbidity and cost and (2) utilized the full list of AAST-defined EGS diagnoses which was developed through a comprehensive consensus panel in 2012. We also study the role of household income and complications in regression models, finding that transferred patients were more likely to live in an area with a lower median household income and to have excess complications. Furthermore, in our study, hospital-level variables, such as a higher number of discharges and beds, urban teaching status, government ownership or non-profit hospitals, and Midwestern location, are also associated with higher morbidity. While the Yelverton study did not include day of the week admission, we found that weekend admissions are associated with higher costs.
Beyond identifying transfer status as an independent predictor of both morbidity and mortality, we quantified the effect of patient- and hospital-level characteristics on outcomes in the transferred EGS population. The current trends of regionalizing complex patients to larger hospitals,44–47 reducing the surgeon workforce,48–51 and surgical trainees pursuing more specialized careers52 have likely contributed to the growing trend to transfer EGS patients.6 Given these trends, transfer status is likely to continue to be a growing and non-modifiable risk factor in the EGS population. Therefore, identifying the patient- and hospital-level factors that contribute to poor outcomes in this population is critical to creating focused quality improvement initiatives that can reduce the outcome gap between transferred and directly admitted EGS patients. Our findings clearly highlight the need to better understand and act upon the factors that are modifiable and contribute to poor outcomes in transferred EGS patients. For example, using a large national sample, our results demonstrate that transferred EGS patients are a particularly medically complex, at-risk population with rates of in-hospital complications higher than 25%. Our regression models account for the number of complications and show that the development of complications has a significant effect on mortality, LOS, and cost, highlighting the potential for future quality improvement efforts focused on “failure to rescue” to improve outcomes in this population.53 Further studies focused on improving outcomes in transferred EGS patients as discussed above is a critical area of future work and will likely necessitate research, such as qualitative analyses and mixed-methods studies, that provides detailed targets for intervention that cannot be gleaned from national database studies.
Although our study provides new information on the transferred EGS patient population, our results should be interpreted in the context of several limitations. First, the NIS, due to its nature as an administrative database of discharge records from US hospitals, lacks information on the physiological status of the patient, among other factors, that may contribute to unmeasured confounding. As such, details of the patients’ clinical status, such as physical condition (e.g., disease severity) and vital signs, were not available for inclusion in this study. Second, while transfer status was available, other information regarding the transfer process, such as reason for transfer or delays in transfer, were not available. Third, determining the timing of procedures relative to presentation and tracking patients between hospitals in the NIS is not possible. Thus, we were unable to include in the analysis details of the timing of procedures performed at the referring or accepting hospitals as well as the capabilities of the referring hospital. Thus, delay in receiving definitive care (as represented by a surgical procedure) is a cofounding factor that potentially contributes to the poor outcomes observed the transferred EGS patients. Additionally, we were also unable to consider longer-term outcomes, such as rates of readmission or complications occurring after discharge. Fourth, while the median income of individual patient zip codes is available in the NIS and included in our analysis as described above, individual patient zip codes are not available. Thus, we were unable to evaluate the distance between the patient’s home and the hospital to which they were transferred. Increased distance between a patient’s home and the hospital to which they were transferred likely impacts discharge planning and coordination and thus acts as a potential confounding variable when studying length of stay as an outcome.
Conclusion
This study evaluated the association between interhospital transfer and outcomes, including mortality, complications, cost, and LOS, in a nationally representative cohort of operative and non-operative EGS patients. Transfer status is an independent risk factor for poor outcomes. Additional studies are needed to understand the mechanisms by which patient transfers may lead to poorer outcomes and ameliorate the significant morbidity and mortality experienced by these patients.
Supplementary Material
Acknowledgements
This research is supported by grants from the Agency for Healthcare Research Quality (1K08HS025224-01A1) to Dr. Ingraham and (R01HS022694) to Dr. Santry as well as the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373, at the University of Wisconsin-Madison. The content represents the thoughts and opinions of the authors and not the funding agencies.
COI/Disclosure Statement and a Funding/Support Statement
The authors have no personal or financial conflicts of interest. The following are the authors’ financial disclosures. Dr. Ingraham works as a clinical consultant for American College of Surgeons (ACS) Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery; the research being submitted for publication was done independently of that position, is on an unrelated topic, and does not reflect the thoughts and opinions of the ACS. Dr. Santry is a consultant on a Fragility Fracture Advisory Board for the Johnson & Johnson Company; the research being submitted for publication was done independently of that position, is on an unrelated topic, and does not reflect the thoughts and opinions of the company.
References
- 1.Gale SC, Shafi S, Dombrovskiy VY, Arumugam D, Crystal JS. The public health burden of emergency general surgery in the United States: A 10-year analysis of the Nationwide Inpatient Sample--2001 to 2010. J Trauma Acute Care Surg. 2014;77(2):202–208. [DOI] [PubMed] [Google Scholar]
- 2.Shafi S, Aboutanos MB, Agarwal S Jr., et al. Emergency general surgery: definition and estimated burden of disease. J Trauma Acute Care Surg. 2013;74(4):1092–1097. [DOI] [PubMed] [Google Scholar]
- 3.Havens JM, Peetz AB, Do WS, et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg. 2015;78(2):306–311. [DOI] [PubMed] [Google Scholar]
- 4.Wandling MW, Ko CY, Bankey PE, et al. Expanding the scope of quality measurement in surgery to include nonoperative care: Results from the American College of Surgeons National Surgical Quality Improvement Program emergency general surgery pilot. J Trauma Acute Care Surg. 2017;83(5):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yelverton S, Rozario N, Matthews BD, Reinke CE. Interhospital transfer for emergency general surgery: An independent predictor of mortality. Am J Surg. 2018;216(4):787–792. [DOI] [PubMed] [Google Scholar]
- 6.Huntington CR, Cox TC, Blair LJ, et al. Acuity, outcomes, and trends in the transfer of surgical patients: a national study. Surg Endosc. 2016;30(4):1301–1309. [DOI] [PubMed] [Google Scholar]
- 7.Crippen CJ, Hughes SJ, Chen S, Behrns KE. The impact of interhospital transfers on surgical quality metrics for academic medical centers. The American Surgeon. 2014;80(7):690–695. [PubMed] [Google Scholar]
- 8.Leberer D, Elliott JO, Dominguez E. Patient characteristics, outcomes and costs following interhospital transfer to a tertiary facility for appendectomy versus patients who present directly. The American Journal of Surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Limmer AM, Edye MB. Interhospital transfer delays emergency abdominal surgery and prolongs stay. ANZ J Surg. 2017;87(11):867–872. [DOI] [PubMed] [Google Scholar]
- 10.Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital Facility Transfers in the United States: A Nationwide Outcomes Study. J Patient Saf. 2017;13(4):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511–2517. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care. 2013;51(7):567–574. [DOI] [PubMed] [Google Scholar]
- 14.Houchens RL, Ross DN, Elixhauser A, Jiang J. Nationwide Inpatient Sample Redesign Final Report. 2014; http://www.hcupus.ahrq.gov/db/nation/nis/nisrelatedreports.jsp.
- 15.Stulberg JJ, Haut ER. Practical Guide to Surgical Data Sets: Healthcare Cost and Utilization Project National Inpatient Sample (NIS). JAMA surgery. 2018;153(6):586–587. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality. Introduction to the HCUP National Inpatient Sample (NIS) 2011. 2011; https://www.hcupus.ahrq.gov/db/nation/nis/NIS_Introduction_2011.jsp.
- 17.Shah AA, Haider AH, Zogg CK, et al. National estimates of predictors of outcomes for emergency general surgery. J Trauma Acute Care Surg. 2015;78(3):482–490; discussion 490–481. [DOI] [PubMed] [Google Scholar]
- 18.Reinke CE, Thomason M, Paton L, Schiffern L, Rozario N, Matthews BD. Emergency general surgery transfers in the United States: a 10-year analysis. J Surg Res. 2017;219:128–135. [DOI] [PubMed] [Google Scholar]
- 19.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250(6):1029–1034. [DOI] [PubMed] [Google Scholar]
- 20.Zogg CK, Najjar P, Diaz AJ, et al. Rethinking Priorities: Cost of Complications After Elective Colectomy. Ann Surg. 2016;264(2):312–322. [DOI] [PubMed] [Google Scholar]
- 21.LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252(3):544–550; discussion 550–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele SR, Brown TA, Rush RM, Martin MJ. Laparoscopic vs open colectomy for colon cancer: results from a large nationwide population-based analysis. J Gastrointest Surg. 2008;12(3):583–591. [DOI] [PubMed] [Google Scholar]
- 23.Guller U, Hervey S, Purves H, et al. Laparoscopic versus open appendectomy: outcomes comparison based on a large administrative database. Ann Surg. 2004;239(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guller U, Jain N, Hervey S, Purves H, Pietrobon R. Laparoscopic vs open colectomy: outcomes comparison based on large nationwide databases. Archives of Surgery. 2003;138(11):1179–1186. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MM, Ng SC, Simons JP, Csikesz NG, Shah SA, Tseng JF. Predictors of major complications after laparoscopic cholecystectomy: surgeon, hospital, or patient? J Am Coll Surg. 2010;211(1):73–80. [DOI] [PubMed] [Google Scholar]
- 26.Simons JP, Shah SA, Ng SC, Whalen GF, Tseng JF. National complication rates after pancreatectomy: beyond mere mortality. J Gastrointest Surg. 2009;13(10):1798–1805. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Elixhauser A, Zhan C, Meyer GS. Patient Safety Indicators: using administrative data to identify potential patient safety concerns. Health Serv Res. 2001;36(6 Pt 2):110–132. [PMC free article] [PubMed] [Google Scholar]
- 28.Nowygrod R, Egorova N, Greco G, et al. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43(2):205–216. [DOI] [PubMed] [Google Scholar]
- 29.Reimer AP, Schiltz N, Koroukian SM, Madigan EA. National incidence of medical transfer: patient characteristics and regional variation. Journal of health and human services administration. 2016;38(4):509. [PubMed] [Google Scholar]
- 30.Haider AH, Gupta S, Zogg CK, et al. Beyond incidence: Costs of complications in trauma and what it means for those who pay. Surgery. 2015;158(1):96–103. [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality. USING APPROPRIATE PRICE INDICES FOR ANALYSES OF HEALTH CARE EXPENDITURES OR INCOME ACROSS MULTIPLE YEARS. https://meps.ahrq.gov/about_meps/Price_Index.shtml. Accessed January, 2019. [Google Scholar]
- 32.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods of information in medicine. 1993;32(5):382–387. [PubMed] [Google Scholar]
- 33.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. Journal of clinical epidemiology. 1996;49(12):1429–1433. [DOI] [PubMed] [Google Scholar]
- 34.Sharp SP, Ata A, Valerian BT, Canete JJ, Chismark AD, Lee EC. Complications and surgical outcomes after interhospital transfer vs direct admission in colorectal surgery: A National Surgical Quality Improvement Program analysis. Am J Surg. 2017;213(6):10311037. [DOI] [PubMed] [Google Scholar]
- 35.Philip JL, Saucke MC, Schumacher JR, et al. Characteristics and Timing of Interhospital Transfers of Emergency General Surgery Patients. J Surg Res. 2019;233:8–19. [DOI] [PubMed] [Google Scholar]
- 36.Vedantam A, Hansen D, Briceño V, Moreno A, Ryan SL, Jea A. Interhospital transfer of pediatric neurosurgical patients. Journal of Neurosurgery: Pediatrics. 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 37.Ingraham A, Wang X, Havlena J, et al. Factors Associated With the Interhospital Transfer of Emergency General Surgery Patients. J Surg Res. 2019;240:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mell MW, Wang NE, Morrison DE, Hernandez-Boussard T. Interfacility transfer and mortality for patients with ruptured abdominal aortic aneurysm. Journal of vascular surgery. 2014;60(3):553–557. [DOI] [PubMed] [Google Scholar]
- 39.van Groningen JT, Eddes EH, Fabry HFJ, et al. Hospital Teaching Status and Patients’ Outcomes After Colon Cancer Surgery. World J Surg. 2018;42(10):3372–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozdemir BA, Sinha S, Karthikesalingam A, et al. Mortality of emergency general surgical patients and associations with hospital structures and processes. Br J Anaesth. 2016;116(1):54–62. [DOI] [PubMed] [Google Scholar]
- 41.Khera R, Angraal S, Couch T, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA. 2017;318(20):2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Healthcare Cost and Utilization Project (HCUP). Producing National HCUP Estimates - Accessible Version. https://www.hcupus.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp.
- 43.Broman KK, Hayes RM, Kripalani S, et al. Inter-hospital Transfer for Acute Surgical Care: Does Delay Matter? The American Journal of Surgery. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menchine MD, Baraff LJ. On-call specialists and higher level of care transfers in California emergency departments. Acad Emerg Med. 2008;15(4):329–336. [DOI] [PubMed] [Google Scholar]
- 45.Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290(20):2703–2708. [DOI] [PubMed] [Google Scholar]
- 46.Block EF, Rudloff B, Noon C, Behn B. Regionalization of surgical services in central Florida: the next step in acute care surgery. J Trauma. 2010;69(3):640–643; discussion 643–644. [DOI] [PubMed] [Google Scholar]
- 47.American College of Surgeons Committee on Trauma. Regional Trauma Systems: Optimal Elements, Integration, and Assessment Systems Consultation Guide. In:2008.
- 48.Rao MB, Lerro C, Gross CP. The shortage of on-call surgical specialist coverage: a national survey of emergency department directors. Acad Emerg Med.17(12):13741382. [DOI] [PubMed] [Google Scholar]
- 49.ACS Health Policy Research Institute and the American Association of Medical Colleges. The Surgical Workforce in the United States: Profile and Recent Trends. . Chapel Hill, NC: April 2010. [Google Scholar]
- 50.Lynge DC, Larson EH, Thompson MJ, Rosenblatt RA, Hart LG. A longitudinal analysis of the general surgery workforce in the United States, 1981–2005. Arch Surg. 2008;143(4):345–350; discussion 351. [DOI] [PubMed] [Google Scholar]
- 51.Voelker R Experts say projected surgeon shortage a “looming crisis” for patient care. Jama. 2009;302(14):1520–1521. [DOI] [PubMed] [Google Scholar]
- 52.Borman KR, Vick LR, Biester TW, Mitchell ME. Changing demographics of residents choosing fellowships: longterm data from the American Board of Surgery. Journal of the American College of Surgeons. 2008;206(5):782–788. [DOI] [PubMed] [Google Scholar]
- 53.Sheetz KH, Krell RW, Englesbe MJ, Birkmeyer JD, Campbell DA Jr., Ghaferi AA. The importance of the first complication: understanding failure to rescue after emergent surgery in the elderly. J Am Coll Surg. 2014;219(3):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


