Abstract
Alcohol consumption may precede, or result from, behavioral inflexibility and contribute to individuals’ difficulties ceasing drinking. Attentional set-shifting tasks are an animal analog to a human behavioral flexibility task requiring recognition of a previous strategy as inappropriate, and the formation and maintenance of a novel strategy (Floresco et al., 2008). Abstinent individuals with AUD, nonalcoholic individuals with a family history of alcoholism, and mice exposed to chronic-intermittent alcohol vapor show impaired behavioral flexibility (Gierski et al., 2013; Hu et al., 2015; Oscar-Berman et al., 2009). Behavioral flexibility deficits can be linked to frontal cortical regions connected to the striatum (Ragozzino, 2007), and alterations to the endocannabinoid system, implicated in drug seeking and consumption (Economidou et al., 2006; Serrano & Parsons, 2011), may affect these behaviors. Alcohol preferring and non-preferring rodents exhibit differences in CB1 receptor expression (CB1R; Hansson et al., 2007; Hungund & Basavarajappa, 2000), but whether dorsal striatal CB1R are important for other alcohol-related behaviors such as attentional set-shifting tasks remains unclear. This study assesses whether selectively-bred High- vs. Low- Alcohol Preferring (HAP vs LAP) mice differ in an operant attentional set-shifting task or CB1R levels in the dorsal striatum, and whether a history of voluntary alcohol consumption in crossed HAP (cHAP) mice exacerbates inflexibility. Contrary to our hypothesis, neither genetic differences in alcohol preference nor drinking affected set shifting. However, HAP3 mice showed reduced levels of dorsal striatal CB1R compared to LAP3 mice, suggesting that genetic differences in alcohol consumption may be mediated in part by striatal CB1R.
Keywords: Behavioral Flexibility, Attentional Set-Shifting, Alcohol, Dorsal Striatum, CB1 Receptors
Drinking in spite of negative consequences is one of the critical problems associated with alcohol use disorder (AUD) (American Psychiatric Association, 2013) and is frequently associated with other problematic drinking behaviors such as binge drinking. These types of alcohol misuse result in substantial economic and public burdens (Sacks, Gonzales, Bouchery, Tomedi, & Brewer, 2015). Compulsive drinking, which may be defined as an inability to cease drinking despite problems, may be related to deficits in a specific form of executive function, referred to as behavioral flexibility. Impairments in behavioral flexibility could affect the decision to cease drinking in the presence of adverse consequences. However, it is currently unclear whether a history of drinking impairs the neural mechanisms related to behavioral flexibility, or whether behavioral inflexibility is a precursor to problem drinking, or both.
In humans, the Wisconsin Card Sorting Task (WCST) is used to measure impairments in behavioral flexibility. Although originally developed to detect impairments associated with neurological disorders, deficits in this task are observed in a variety of disorders such as addiction, schizophrenia, and depression (Anokhin, Golosheykin, Grant, & Heath, 2010; Gierski et al., 2013). In this task, human participants initially learn a rule for sorting cards by one of three dimensions (color, number, or shape), but the rule changes without instruction. Subjects are rated on whether they can set aside the initial rule and acquire the newly appropriate one. Impairments in behavioral flexibility tasks such as the WCST are assessed by examining the number of trials to reach a specific criterion as well as the number and types of errors committed when individuals are required to learn the new strategy. In rodents, behavioral flexibility is primarily assessed using reversal learning or attentional set-shifting tasks, the latter functioning as an analog to the WCST. Behavioral Flexibility tasks allow researchers to differentiate between multiple types of impairments, as these tasks require the ability to recognize a previously acquired strategy as being inappropriate, while simultaneously requiring the formation and maintenance of a new strategy (Floresco, Block, & Maric, 2008).
Research indicates that abstinent individuals previously diagnosed with alcoholism show impairments on neuropsychological tasks geared towards attentional set-shifting processes including the WCST (Oscar-Berman et al., 2009). However, as alcohol toxicity and drug abuse are thought to negatively affect executive function (Gierski et al., 2013), it is important to tease apart whether impairments are present prior to an alcohol use disorder (AUD) diagnosis, making them a candidate in the etiology of alcoholism, or if the AUD itself induces these deficits in behavioral flexibility. Interestingly, studies have shown that adolescents with high family histories of AUD but no personal AUD diagnosis performed worse on the WCST compared to adolescents with low family histories of AUD (Corral, Holguín, & Cadaveira, 2003). Considering the frontal cortex and executive function continues to develop into early adulthood, research assessing adults with high family histories of AUD for impairments in set-shifting has also been conducted. Gierski et al. (2013) found that nonalcoholic adults with high family histories of AUD performed worse on the WCST compared to nonalcoholic adults with a negative family history of AUD. Together these findings may implicate a genetic component to performance on WCST, wherein a family history of AUD predicts poorer performance on this attentional set-shifting task. Genetic vulnerabilities may help explain pre-existing deficits in behavioral flexibility, which has been indicated as a potential endophenotype for heavy drinking (Corral et al., 2003; Gierski et al., 2013; Shnitko, Gonzales, & Grant, 2018).
Similarly, genetic variance in the endocannabinoid system may be predictive of differences in addiction-related behaviors. Polymorphisms of the encoding gene for the CB1 receptor (Serrano & Parsons, 2011), in addition to reduced expression of postsynaptic fatty acid amide hydrolase which is primarily responsible for the enzymatic degradation of the endogenous endocannabinoid anandamide, have been linked to problematic drug use (Chiang, Gerber, Sipe,& Cravatt, 2004; Sipe, Chiang, Gerber, Beutler, & Cravatt, 2002). Furthermore, downregulation of CB1 receptors in multiple brain regions has also been observed in persons with AUD (Henderson-Redmond, Guindon, & Morgan, 2016). Moreover, animal research indicates the C57BL/6 mice, a commonly used alcohol-preferring strain, display lower CB1 receptor densities compared to DBA/2 mice, an alcohol-avoiding strain (Hungund & Basavarajappa, 2000), and CB1 receptor-deficient mice demonstrate normal ethanol tolerance and preference but fail to show ethanol withdrawal symptoms, a trait that may increase the risk for developing AUD’s in humans (Racz et al., 2003; Piasecki et al., 2012). In addition to its involvement in alcohol drinking, the endocannabinoid system could be involved in regulating behavioral flexibility. The dorsal striatum is one region to examine this possibility, as it is essential for reversal learning and attentional set-shifting tasks (Bissonette & Powell, 2012; Ragozzino, 2007), and contains some of the highest CB1 receptor expression levels in the brain (Pattij, Wiskerke, & Schoffelmeer, 2008; Zlebnik & Cheer, 2016). Therefore, it is possible that genetics predictive of drinking in HAP and LAP mice may be related both to genetic variation in CB1 receptor density, as observed in other alcohol preferring and non-preferring strains, and differences in behavioral flexibility.
To our knowledge, no attentional set-shifting research has utilized selectively bred lines such as the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice. The HAP3 and LAP3 selectively bred lines employed in this study were bred from heterogeneous HS/Ibg stock using bidirectional selection for differences in consumption of 10% v/v EtOH and water in a two-bottle choice (2BC) design (Oberlin, Best, Matson, Henderson, & Grahame, 2011). Repeated selection of the progenitor line has resulted in lines that are consistent with respect to this selection phenotype (Matson & Grahame, 2013), with HAP3 mice drinking over 23 g/kg/day and LAP3 mice drinking less than 1 g/kg/day. If behavioral flexibility deficits measured by an automated attentional set-shifting task is an endophenotype of high alcohol intake, we hypothesize that HAP mice would be impaired relative to LAP mice in this domain.
Alcoholism is often characterized by chronic, excessive alcohol intake, which is difficult to model in a translational manner in rodents (Matson & Grahame, 2013). For this reason, most of the current research on attentional set-shifting tasks in both rats and mice examining the effect of alcohol has utilized chronic intermittent EtOH (CIE) vapor administration instead of voluntary consumption (Hu et al., 2015; Kroener et al., 2012). CIE paradigms seek to repeatedly expose rodents to sufficient alcohol to induce dependence, typically for 16 hours a day, and an exposure history ranging anywhere from several weeks to several months (Gilpin, Richardson, Lumeng, & Koob, 2008; Hu et al., 2015; Kroener et al., 2012; Gass et al., 2014). Here we avoid the potential stress of vapor inhalation, utilizing simple 2BC drinking in cHAP mice, which have also been selectively bred for alcohol intake, drinking to average blood alcohol levels in excess of 250 mg/dl daily (Matson & Grahame, 2013). These levels are as high or higher than those typically reported in CIE studies. By using this population and procedure, we hoped to more accurately model chronic human alcohol consumption than CIE procedures, achieving similar dosing and exposure without weight loss or stress to the animals. We hypothesized that a history of drinking (2 weeks) would lead to impairments in behavioral flexibility.
The following experiments, utilizing an operant set shifting procedure adapted from Floresco et al. (2008), aim to better understand how genetic influences and understudied systems involved in addiction such as the endocannabinoid system, may affect performance on tasks examining behavioral flexibility. Drawing from the idea that impaired behavioral flexibility is an endophenotype of alcohol drinking, we hypothesized that HAP3 and alcohol-exposed cHAPs, relative to LAP3 and alcohol naive cHAPs, would display impaired attentional set-shifting, indicated by a greater number of trials and errors committed to reaching criterion following the shift. Additionally, HAP3, relative to LAP3, mice would exhibit lower dorsal striatal CBi receptor expression levels as measured by Western Blot.
Materials and methods
Subjects
For Experiment 1, a total of 33 HAP3 and 39 LAP3 mice were run in unbalanced cohorts (24 HAP3s, 24LAPs; Cohort 1; Table 1). A subset of animals completing the all stages of testing from Experiment 1, were used for Experiment 1a. For Experiment 2 a total of 30 cHAP mice were used (Table 1). All mice were approximately 60 days old and were individually housed in standard Plexiglas cages with pine bedding and accommodated to a 12-hour reverse light cycle with lights on from 1900- 0700 daily for at least 7 days prior to the beginning of the operant training. To encourage operant responding and to increase motivation for the liquid reinforcer, mice were water restricted and received approximately one hour of water access each day immediately following their experimental session. All work was approved by the IUPUI Institutional Animal Care and Use Committee.
Table 1:
Experimental n’s by sex and line.
| Experiment | Male n’s | Female n’s | Line |
|---|---|---|---|
| 1 – Line Differences | 16 | 17 | HAP3 |
| 19 | 20 | LAP3 | |
| 1a – Western Blots | 12* | 11* | HAP3 |
| 7* | 8* | LAP3 | |
| 2- EtOH Exposure | 15 | 15 | cHAP |
Animals used in experiment 1a are also accounted for in the n’s of experiment 1.
Apparatus
Twelve operant chambers (Med Associates, St. Albans, VT) were used for the operant testing in these experiments. Each chamber measured 21.6 x 19.7 x 12.7 cm (l x w x h) and was housed in a light- and sound-attenuating box. The operant boxes were equipped with green lights positioned above levers located to the left and right side of the center sipper-tube opening. Additionally, a nose-poke hole and accompanying green light was positioned above the sipper tube (Experiment 1). The 10mL sipper-tube containing 0.1% saccharin solution descended into the chamber’s opening upon a correct response. Intake for each animal was measured on the sipper tubes before and after the session. Session duration, trials, nose-pokes, reinforcers, omissions, and correct and incorrect lever presses were recorded using MED-PC IV software (Med Associates, St. Albans, VT).
Experiments 1 and 2: Operant Procedures
The first stage of pretraining required mice to learn to lever press. This was accomplished through one 30-minute session of fixed time (FT) schedule which delivered a noncontingent 20-second saccharin reward every 120 seconds. Additionally, any lever press on the animals randomly assigned correct lever also resulted in a 20-second reward delivery. Next, animals continued lever pressing on a fixed ratio 1 (FR1) schedule in 45-minute sessions, where only one lever was present at a time beginning with the same lever that was assigned for the FT training session. Reward length decreased in increments of 5 seconds every session for three sessions to increase responding, after which the final reward duration was reduced to 2s. After mice acquired stable responding on the initial lever (i.e., minimum of 20 lever presses and 0.2mL of reward consumption), training for the opposite lever ensued. For Experiment 1 the second pretraining stage required nose-pokes into an illuminated hole, situated above the sipper access slot. Nose-pokes were required to initiate trials using a FR1 nose-poke with a maximum 10-second latency leading to FR1 lever press with 10-second latency. Trials without a nose-poke or lever response were scored as omissions and the inter trial interval (ITI) was 4s. For Experiment 3, nose poke holes were removed and instead retractable levers were used. The ITI remained at 4s, at which time levers extended and mice had up to 10s to respond before a lever omission was scored and levers retracted. Upon a correct lever press, levers retracted and the 2s reward was delivered prior to the initiation of a new trial. For each pretraining phase, all animals moved to the next stage of training when 90% or more of animals acquired stable responding.
For both Experiments 1 and 2 the attentional set, which was a visual-cue discrimination, animals were required to always choose the active lever indicated by the illumination of the lever stimulus light, situated above each lever, where the stimulus light was randomized so that each lever was active ~50% of the time. In Experiment 1, criterion to move on to the next phase was 80% correct trial responses across the session, for Experiment 2 criterion was 8 out of 10 correct responses. The next phase was the attentional set-shift, which used an egocentric discrimination, in which mice were required to ignore the previously relevant stimulus light that continued to be presented randomly above each lever, and instead choose the assigned correct (left or right) lever. Lever presses during the final attentional set session were analyzed to determine if mice had a lever preference. If animals responded incorrectly for 10% or more of their total lever presses on a single lever during their final attentional set session, that lever was considered to be the animals lever bias and the opposite lever was assigned for the shift (a total of 24 animals from experiments 1 and 2 showed a lever bias). If no lever bias was present, animals were counterbalanced and randomly assigned a correct lever. Like the pretraining and the attentional set sessions, the attentional set-shifting sessions lasted up to 45-minutes. Criteria for completing the shift was 10 consecutive correct responses, upon which testing was terminated. In Experiment 1, neither nose-poke or lever omissions counted towards trials to reach criterion, and all correct responses resulted in a 2-second reward delivery. For Experiment 2 there were no nose-poke omissions, however lever omissions still did not count towards trials to reach criterion and all training and testing sessions were reduced to 30 minutes. Animals in Experiment 2 were retested on the attentional set for one session, following alcohol administration, prior to moving on to the attentional set-shift.
Drug Administration
In order to expose the animals in Experiment 2 to an alcohol history, a two-bottle choice procedure was used. Following the attentional set, animals were assigned to the experimental or control conditions counterbalanced by sex, family, and ensuring mean responses on the attentional set did not differ between groups. Sixteen mice (8 males) were given 24-hour access to one bottle of 10% EtOH (in a 50 mL graduated cylinder) and one bottle of water (25 mL graduated cylinder) for two weeks. 14 control (7 males) animals received two bottles of water (one in a 50 mL tube and another in a 25 mL tube). Intakes were measured on the cage without disturbing the bottles every Monday, Wednesday, and Friday in order to determine amount of ethanol and water consumed. On these days, the sides of the bottles were switched in order to deter animals from forming a side preference. All animals were weighed weekly.
Experiment 1a: Brain Extraction and Western Blot
On the day following the final day of behavioral testing, a subset of mice from Experiment 1 (n=23 HAP; 15 LAP3) were euthanized by cervical dislocation and brains were rapidly extracted. Bilateral punches of the dorsal striatum were extracted from a single 2mm coronal slice and rapidly frozen using liquid nitrogen. Western Blots were run to identify levels of CBi receptors following the method of Kasten et al. (2017). Briefly, the tissue samples were homogenized in RIP A buffer with protease inhibitor (1ml of RIP A buffer containing lOOul of 10X PI and lOul of 0.1M PMSF) (Thermo Fisher). Sample load was calculated using a Bio-Rad Protein Assay kit. Primary antibody (Anti-Cannabinoid Receptor 1, Rabbit polyclonal to Cannabinoid Receptor 1, Abeam) was added to the PBS buffer (5% nonfat milk in 1x PBS with 0.1% Tween 20) and a secondary antibody was added at a 1:5000 dilution (IRDye 800 CW Goat anti-Rabbit IgG (H+L), LI-COR). The image was then scanned from membrane with a CLx Odyssey scanner. B-actin was used as the reference (primary β-actin mouse monoclonal antibody, secondary antibody IRDye 680RD Donkey anti-Mouse IgG (H+L), LI-COR).
Statistical Analysis
Data were analyzed using SPSS software (SPSS, Version 26, Chicago, IL) and graphed using Prism software (GraphPad Prism, v. 6.0, La Jolla, CA). Significance was set at an α value of 0.05. For experiment 1, we used a 2x3 factorial analysis of variance (ANOVA) of Line (HAP3 vs. LAP3) x Sex (Male vs. Female) x Replicate (Cohort 1 vs. Cohort 2), as there were no effects of, or interactions with cohort for principle variables such as trials to criterion and total errors, subsequent analyses were collapsed across cohort, for Experiment 2, data were analyzed using a Group (EtOH v. Naive) x Sex (Male v. Female) factorial ANOVA. For experiment la, CBi protein expression for each mouse was calculated as the signal strength of CBi expression normalized to the signal strength of β-actin expression and assessed with an ANCOVA, where the covariate was the CBi protein expression normalized to the control for each gel, in order to account for potential between-gel variability.
Experiment 1 & 2: Error Analysis
For the attentional set-shift, there are three types of errors that can be committed: Perseverative, Regressive, Never-Reinforced. Perseverative errors scored are when the mouse chooses the lever under the active cue light when this lever was opposite the assigned correct egocentric lever. All consecutive trials in which a perseverative error could be committed were assessed in blocks of eight trials. After making fewer than five perseverative errors in a block of eight trials, the following errors were considered regressive errors, as the mouse was following an alternative strategy at least fifty percent of the time. Never Reinforced errors were scored when a mouse selected the lever opposite the correct response when the visual-cue stimulus was active above their assigned egocentric lever.
Results
Experiment 1: Line Differences
A total of 33 HAP3s and 26 LAP3s completed pretraining. Thirteen LAP3s were removed from the study prior to testing during various pre-training stages for failing to consume 0.2mL of the reinforcer or failing to make at least 20 responses within a session. All 59 animals that passed pretraining successfully completed the Attentional Set. Two LAP3s and one HAP3 failed the attentional shift, leaving a total of 32 HAP3s (15 males) 24 LAP3s (13 males) completing the shift. An additional LAP3 was excluded from behavioral analysis due to programing error during the shift. There were no observed sex differences for any of the measures.
The lines differed in number of nose poke omissions [F (1, 53) = 2.089, p =0.019] (Figure 1). However, the lines did not differ for the number of completed trials to reach the shift criterion [F (1, 53) = 0.384, p =0.226] (Figure 1). Additionally, contrary to our hypothesis, there were no observed differences for the total number of errors committed [F (1, 53) = .619 p =0.366] or for any of the error subtypes (Figure 1).
Figure 1.
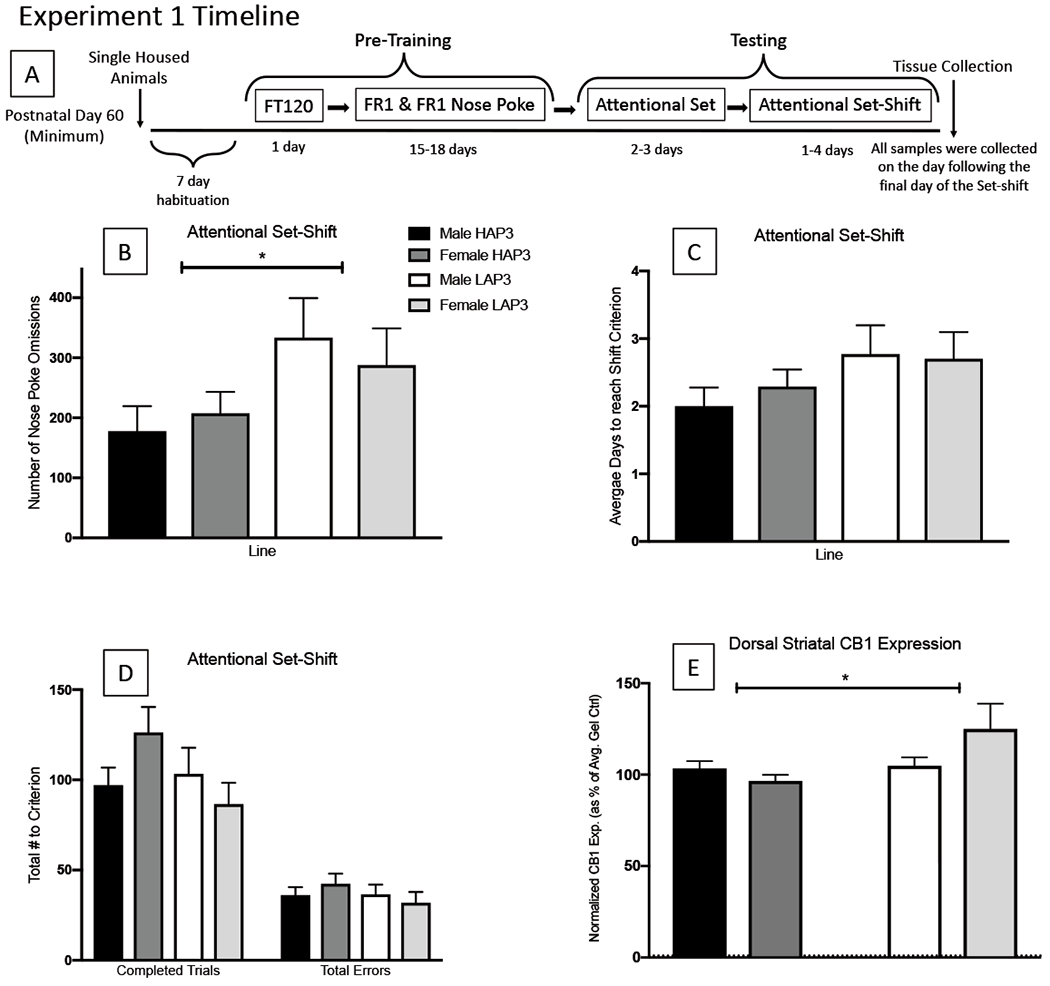
A: Schematic of experimental time line, FT120 = Fixed Time 120, FR = Fixed Ratio. B: LAP3’s committed more Nose Poke omissions than HAP3’s (p < .05). C: Although LAP3 animals took slightly longer to reach shift criterion, this difference was not significant (p < .05). D: Lines did not differ in the number of trials necessary to reach shift criterion (p > .05); nor were there significant differences between the lines in the number or types of errors to reach criterion (p > .05). E: Dorsal Striatal protein expression in HAP3’s and LAP3’s. Sample sizes are expressed within individual bars. ANCOVA results indicate lower levels of dorsal striatal CB1 expression in HAP3’s compared to LAP3’s (p < .05). Data were normalized to B-Actin and are displayed in this figure as % of averaged controls.
Experiment 1a: Western Blots
The ANCOVA results support our hypothesis that HAP3s, similar to other alcohol preferring rodents, have lower levels of dorsal striatal CB1 receptor protein expression compared to their non-preferring counterparts, the LAP3s [F (1,37) = 4.438, p =0.042] (Figure 1).
Experiment 2: Ethanol Exposure
All animals completed pretraining and the attentional set. Two EtOH animals were sacrificed during 2BC exposure due to illness and were removed from analyses. EtOH animals consumed an average of 22.74 g/kg/day of alcohol (Figure 2). A total of 14 EtOH and 14 control animals completed the attentional set retest and attentional set-shift. There were no Group x Sex interactions, nor were there any alcohol or retention interval effects on performance of the attentional set when examining the Retest (Figure 2), although this interval apparently increased the variability during the re-test. Contrary to our hypothesis, a history of alcohol did not impair attentional set-shifting compared to alcohol naive animals for the number of trials to reach criterion [F (1,26) = 0.00, p =0.761] or in the total number of errors [F (1,26) = 0.168, p =0.915] (Figure 2).
Figure 2.
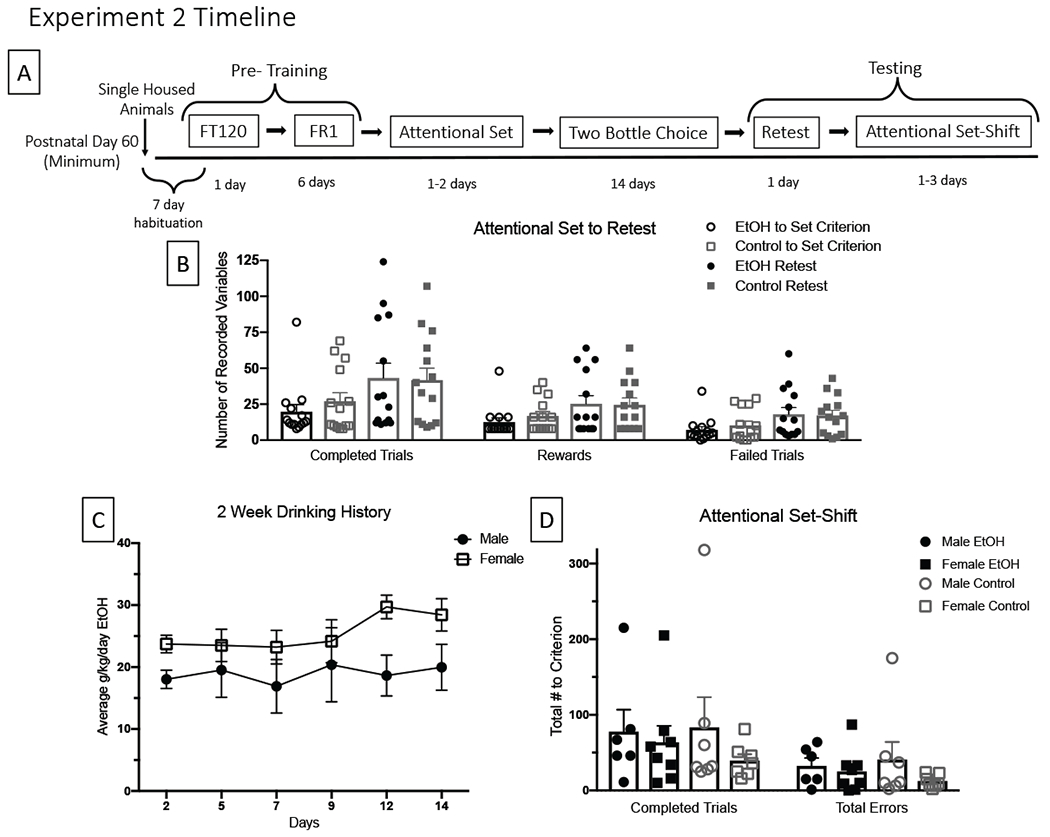
A: Schematic of experimental time line, FT120 = Fixed Time 120, FR = Fixed Ratio. B: There were no differences within or between the groups when comparing the Attentional Set to the Attentional Set Retest session (p > .05). C: Average of g/kg/day of 10% EtOH consumed across the 2-week drinking history by sex. D: There were no differences between alcohol exposed and alcohol naïve mice on the number of trials needed to reach criterion for the Attentional Set-Shift. Groups also did not differ in the number of errors committed during the Set-Shift (p > .05).
Discussion
We hypothesized that a genetic predisposition to drink alcohol would result in impaired behavioral flexibility as measured by an operant attentional set-shifting task, and that a history of free-choice alcohol drinking would induce behavioral flexibility deficits compared to alcohol-naive animals. Additionally, we theorized that similar to other alcohol-preferring strains, the HAP3 mice would exhibit lower CB1 receptor expression levels compared to their non-alcohol preferring counterparts; and that a difference of CBi receptor expression in the dorsal striatum may explain deficits in behavioral flexibility.
Contrary to our hypothesis, the HAP3s were not impaired on the attentional set-shift compared to the LAP3s, as there were no differences in the number of trials needed to reach criterion and both lines were able to master the shift with equivalent error rates (Figure 1). The only observed difference was of response rates (Figure 1); wherein, LAP3s were less likely to initiate trials via a nose-poke within the 10-s limit compared to HAP3s. Although this difference shows we are powered to detect some line differences in operant behavior, the results suggest that differences in response rates are not associated with the number of trials need to criterion or error rates. Rather, differential response rates may reflect line differences in avidity or locomotor activity that have previously been reported: for example, total distance traveled in an Open-Field Test is greater in HAP3 relative to LAP3 mice (Can, Grahame, & Gould, 2012), and HAP mice emitted more trials during a delay discounting task (also a cued task requiring a nosepoke to initiate trials) than low-drinking lines (Oberlin and Grahame, 2009). Differences in avidity may explain why many LAP3s fail pretraining, as they demonstrate lower saccharin preference (Oberlin et al., 2011) compared to the HAP3s and those failing pretraining may therefore not be as intrinsically motivated by the reinforcer used in these studies. Moreover, it is possible that we have excluded especially unmotivated LAP3 mice by not including those which did not acquire instrumental responding. Nonetheless, despite these factors no differences were observed for the main test measures among animals who completed pretraining. If reinforcer value did affect learning we would have expected the LAP3s to perform poorly relative to the HAP3s on the attentional set or set-shifting, which was not the case. Therefore, although differences in avidity are most likely the reason for divergent performance between the lines, avidity does not appear to associate with differences in behavioral flexibility.
Additionally, the CB1 receptor differences observed in this study (Figure 1) were not indicative of behavioral flexibility impairments in these selectively bred lines, but nonetheless add to the growing literature demonstrating the importance of CB1 receptors in a variety of alcohol-related behaviors, such as voluntary ethanol consumption (Colombo et al., 2002; Linsenbardt & Boehm, 2009; Wang, Liu, Harvey-White, Zimmer, & Kunos, 2003), reinstatement in alcohol seeking (Lopez-Moreno, Gonzalez-Cuevas, Fonseca, & Navarro, 2004; McGregor, Dam, Mallet, & Gallate, 2005), and reduced signs of withdrawal (Onaivi, 2008). One possibility is that in this population, dorsal striatal CB1 receptors are not involved in behavioral flexibility as measured by this attentional set-shifting task. Alternatively, dorsal striatal CB1 receptor differences between these lines may not have been large enough to produce behavioral flexibility deficits using this paradigm, as it is possible that other brain regions, such as the mPFC and OFC, which are involved in behavioral flexibility tasks (Ragozzino, 2007; Floresco et al., 2008; Brown & Tait, 2016), are able to compensate for potential minor deficits resulting from disparities in the endocannabinoid system in the dorsal striatum in this paradigm. Moreover, it is also possible that CB1 receptor differences may be more closely associated with reversal learning, which was not measured in this study, rather than attentional set-shifting. CB1 receptor agonists have been shown to negatively affect reversal learning when applied acutely prior to testing in adult rats (Egerton, Brett, & Pratt, 2005). Therefore, it is possible that utilizing a different task to assess behavioral flexibility such as reversal learning may be better suited to assessing whether dorsal striatal CB1 receptor differences in these lines relate to behavioral impairments.
While we hypothesized selectively bred lines would demonstrate differences on attentional set-shifting tasks similar to humans with and without a family history of alcoholism, behavioral impairments in the HAP3s compared to the LAP3s were not observed. Although these experiments failed to observe findings prevalent in the human literature which suggest that behavioral flexibility is an endophenotype of alcoholism, there are a number of possibilities as to why the hypothesized genetic deficits were not seen. A number of factors may have contributed to the WCST deficits in individuals with a family history of alcoholism detected by Gierski and colleagues (2013), such as higher rates of major depressive episodes and lifetime anxiety disorders, which may be more closely associated with either genetic or environmental differences between families with high and low histories of AUD, than these phenotypes are to genetic differences in alcohol drinking in rodents. Whereas it is important to bear in mind that this animal model is only capable of recapitulating some, but not all of the traits associated with AUD.
One could also argue that behavioral flexibility deficits observed in individuals with AUD arise not from genetic factors, but rather from a personal drinking history. Thus, we sought to recapitulate that history by using a population that has extremely high alcohol intake, reaching or exceeding levels found in rodent studies using forced alcohol vapor exposure. One possibility as to why this study failed to see deficits in cHAP mice with an alcohol drinking history is the route and means of chronic alcohol administration. CIE models typically produce BECs ranging from 175-225 mg/dl for the length of exposure (Hu et al., 2015; Kroener et al., 2012), comparable to BECs achieved by the 2BC administration in the selectively bred lines utilized here. Specifically, previous research has shown that cHAPs drinking ~24g/kg/day reached between 250-275 mg/dl, meaning that peak BECs from this model were higher than those typically achieved in CIE models (Matson & Grahame, 2013), but similar to levels observed in humans with AUD given ad libitum access to alcohol (Mello & Mendelson, 1970), which fall between 200 and 300 mg/dl. It is therefore perhaps unlikely that differences in BEC would explain why we were unable to see the hypothesized deficits in the attentional set-shifting task using drinking rather than CIE exposure. Another consideration is that withdrawal reactions have also been associated with structural brain changes (Fadda & Rossetti 1998). As CIE utilizes multiple withdrawal periods it is possible that these events resulted in an additive effect to produce even greater structural changes than those that may occur with alcohol administration alone. The cHAP mice used here are, like all high-drinking rodent populations (Metten et al., 1998), quite resistant to alcohol withdrawal. In fact, parent lines for cHAP mice, the HAP1 and HAP2 lines, show almost no measurable withdrawal after weeks of CIE (Lopez, Grahame & Becker, 2011), and none has been detected following voluntary alcohol consumption, so if changes in behavioral flexibility require repeated withdrawal, 2BC drinking in cHAP mice may not allow for these changes to occur. Another possibility is that CIE is more stressful compared to drinking models such as 2BC, as in addition to chronic exposure to high concentrations of alcohol vapor, it requires daily injections of alcohol along with pyrazole to inhibit alcohol metabolism. This stress could further exacerbate structural and behavioral changes resulting from alcohol administration and repeated withdrawal and may explain why deficits following 2BC were not observed in this study. Although we use 2BC as a way to evaluate the effects of alcohol consumption with minimal added stressors, our paradigm does require water restriction during pretraining and the attentional set-shifting task. This particular stressor occurs during a different period than the alcohol consumption but nonetheless may affect behavior in unforeseen ways. Although in natural settings rodents frequently deal with limited water access, and our own animals show no ill health effects of water deprivation, as evaluated by either animal welfare appearance guidelines or continued weight gain, our paradigm cannot be considered entirely stress-free.
In summary, genetic differences in alcohol intake within these lines are not predictive of impairments in this attentional set-shifting paradigm, nor does a short history of voluntary alcohol intake cause changes in behavioral flexibility. We would note that a 2-week duration of alcohol drinking is sufficient to induce compulsive, quinine-resistant alcohol intake in cHAP mice (Houck, Carron, Millie, & Grahame, 2019), and these animals acquire substantial tolerance to the ataxic effects of ethanol within 3 days (Matson et al., 2014), so alcohol drinking is not without profound effects on addiction-like behaviors in these animals. Furthermore, the amount of alcohol consumed within the 2-week duration of 2BC in these animals is comparable to Kroener et al (2011) CIE exposure which totaled 12 days and produced behavioral flexibility deficits. For these reasons we felt that 2 weeks of 2BC would be sufficient to produce behavioral impairments however, in the absence of an effect, we cannot rule out that a longer exposure period may produce deficits that were not observed in this experiment.
Although differences in striatal CB1 receptor expression did not parallel behavioral flexibility here, future research implementing reversal learning may be better suited to detect any impairments related to the endocannabinoid system. Although this procedure was chosen to model both Floresco’s automated procedure and maze based set-shifting tasks which utilize a visual cue set and egocentric shift (Floresco, S. B., Magyar, O., Ghods-Sharifi, S., Vexelman, C., & Tse, M. T. L. 2006; Ragozzino, M. E., Ragozzino, K. E., Mizumori, S. J., & Kesner, R. P., 2002) it is also possible that increasing the difficulty of the attentional set by requiring animals to first learn the egocentric discrimination could produce impairments not seen here (Floresco et al., 2008). As our operant egocentric shift failed to produce deficits in behavioral flexibility, possibly due to the ease associated with remaining in front of the single correct lever, this extradimensional shift is likely more challenging in a maze procedure which requires substantially more effort from the mice to perform. While the neurophysiological differences observed in this study did not correspond to the attentional set-shifting task, these differences may be directly related to line differences in alcohol drinking, a theory supported by the emerging literature linking the endocannabinoid system, and CB1 receptors in particular, to alcohol-related behaviors.
Public Significance:
Individuals diagnosed with Alcohol Use Disorder have been observed to have deficits in flexibly responding to changed circumstances, a cognitive deficiency that may promote drinking. Unclear is whether this is a cause of, or effect of alcohol drinking. Here, we used mice that differ in alcohol consumption genetically, finding no differences in cognitive flexibility either before or after alcohol consumption, though the mice do differ in receptors for endogenous cannabinoids.
Acknowledgements:
This work was supported by AA P60 07611 to David Kareken. Thanks are due to Marian Logrip, Ph.D, for her help in training LAM on procedures for tissue punches.
This research was supported in part by AA07611 to David Crabb; AA07462 to Cristine Czachowski.
Footnotes
Conflict of Interest Statement: None to declare.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, & Heath AC (2010). Developmental and genetic influences on prefrontal function in adolescents: A longitudinal twin study of WCST performance. Neuroscience Letters, 472(2), 119–122. 10.1016/j.neulet.2010.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, & Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(11), 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, & Powell EM (2012). Reversal learning and attentional set-shifting in mice. Neuropharmacology, 62(3), 1168–1174. 10.1016/j.neuropharm.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, & Tait DS (2016). Attentional Set-Shifting Across Species. Current Topics in Behavioral Neurosciences, 28, 363–395. 10.1007/7854_2015_5002 [DOI] [PubMed] [Google Scholar]
- Can A, Grahame NJ, & Gould TD (2012). Affect-related related behaviors in mice selectively bred for high and low voluntary alcohol consumption. Behavior Genetics, 42(2), 313–322. 10.1007/s10519-011-9505-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, & Cravatt BF (2004). Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human Molecular Genetics, 75(18), 2113–2119. 10.1093/hmg/ddh216 [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, … Gessa G (2002). Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology, 159(2), 181–187. 10.1007/s002130100887 [DOI] [PubMed] [Google Scholar]
- Corral M, Holguín SR, & Cadaveira F (2003). Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. Journal of Studies on Alcohol, 64(2), 195–199. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, … Ciccocioppo R (2006). Effect of the cannabinoid CB<Subscript>1</Subscript> receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology, 183(4), 394–403. 10.1007/s00213-005-0199-9 [DOI] [PubMed] [Google Scholar]
- Egerton A, Brett RR, & Pratt JA (2005). Acute Δ9-Tetrahydrocannabinol-Induced Deficits in Reversal Learning: Neural Correlates of Affective Inflexibility. Neuropsychopharmacology, 30(10), 1895–1905. 10.1038/sj.npp.1300715 [DOI] [PubMed] [Google Scholar]
- Fadda F, & Rossetti ZL (1998). Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progress in Neurobiology, 56(4), 385–431. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, & Marie TL (2008). Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research, 190(1), 85–96. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, & Tse ΜTL (2006). Multiple Dopamine Receptor Subtypes in the Medial Prefrontal Cortex of the Rat Regulate Set-Shifting. Neuropsychopharmacology, 31(2), 297–309. 10.1038/sj.npp.1300825 [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, … Chandler LJ (2014). Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology, 39(11), 2570–2583. 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, … Limosin F (2013). Executive Functions in Adult Offspring of Alcohol-Dependent Probands: Toward a Cognitive Endophenotype? Alcoholism: Clinical and Experimental Research, 37(s1), E356–E363. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson ΗN, Lumeng L, & Koob GF (2008). Dependence-Induced Alcohol Drinking by Alcohol-Preferring (P) Rats and Outbred Wistar Rats. Alcoholism, Clinical and Experimental Research, 32(9), 1688–1696. 10.1111/j.1530-0277.2008.00678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Guimarães FS, & Grace AA (2015). Effects of Pubertal Cannabinoid Administration on Attentional Set-Shifting and Dopaminergic Hyper-Responsivity in a Developmental Disruption Model of Schizophrenia. International Journal of Nenropsychopharmacology, 18(2). 10.1093/ijnp/pyu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Bermúdez-Silva FJ, Malinen H, Hyytiä P, Sanchez-Vera I, Rimondini R, … Heilig M (2007). Genetic Impairment of Frontocortical Endocannabinoid Degradation and High Alcohol Preference. Neuropsychopharmacology, 32(1), 117–126. 10.1038/sj.npp.1301034 [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Guindon J, & Morgan DJ (2016). Roles for the endocannabinoid system in ethanol-motivated behavior. Process in Neuro-Psychopharmacology and Biological Psychiatry, 65, 330–339. 10.1016/j.pnpbp.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck CA, Carron CR, Millie LA, & Grahame NJ (n.d.). Innate and Acquired Quinine-Resistant Alcohol, but not Saccharin, Drinking in Crossed High Alcohol Preferring Mice. Alcoholism: Clinical and Experimental Research, 0(ja). 10.1111/acer.14196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, & Kroener S (2015). Effects of Acamprosate on Attentional Set-Shifting and Cellular Function in the Prefrontal Cortex of Chronic Alcohol-Exposed Mice. Alcoholism: Clinical and Experimental Research, 39(6), 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, & Basavarajappa BS (2000). Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. Journal of Neuroscience Research, 60(1), 122–128. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Zhang Y, & Boehm SL (2017). Acute and Long-Term Effects of Δ9-tetrahydrocannabinol on Object Recognition and Anxiety-Like Activity are Age- and Strain-Dependent in Mice. Pharmacology, Biochemistry, and Behavior, 163, 9–19. 10.1016/j.pbb.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, & Chandler LJ (2012). Chronic Alcohol Exposure Alters Behavioral and Synaptic Plasticity of the Rodent Prefrontal Cortex. PLOS ONE, 7(5), e37541 10.1371/journal.pone.0037541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, & Boehm SL (2009). AGONISM OF THE ENDOCANNABINOID SYSTEM MODULATES BINGE-LIKE ALCOHOL INTAKE IN MALE C57BL/6J MICE: INVOLVEMENT OF THE POSTERIOR VENTRAL TEGMENTAL AREA. Neuroscience, 164(2), 424–434. 10.1016/j.neuroscience.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, & Becker HC (2011). Development of Ethanol Withdrawal-Related Sensitization and Relapse Drinking in Mice Selected for High or Low Ethanol Preference. Alcoholism, Clinical and Experimental Research, 35(5), 953–962. 10.1111/j.1530-0277.2010.01426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Fonseca F. R. de, & Navarro M (2004). Long-Lasting Increase of Alcohol Relapse by the Cannabinoid Receptor Agonist WIN 55,212–2 during Alcohol Deprivation. Journal of Neuroscience, 24(38), 8245–8252. 10.1523/JNEUROSCI.2179-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, & Grahame NJ (2013). Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology, 18(6), 921–929. 10.1111/j.1369-1600.2011.00412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL, & Grahame NJ (2014). Selectively Bred Crossed High Alcohol Preferring Mice Drink To Intoxication And Develop Functional Tolerance, But Not Locomotor Sensitization During Free-Choice Ethanol Access. Alcoholism, Clinical and Experimental Research, 38(1), 267–274. 10.1111/acer.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 2003;146:97–103. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Dam KDB, Mallet PE, & Gallate JE (2005). Delta9-THC reinstates beer- and sucrose-seeking behaviour in abstinent rats: comparison with midazolam, food deprivation and predator odour. Alcohol and Alcoholism (Oxford, Oxfordshire), 40(1), 35–45. 10.1093/alcalc/agh113 [DOI] [PubMed] [Google Scholar]
- Mello NK and Mendelson JH (1970). Experimentally. induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. The Journal of Pharmacology and Expermental Therapeutics, 173, 101–116. [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, … Belknap JK (1998). High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mammalian Genome: Official Journal of the International Mammalian Genome Society, 9(12), 983–990. 10.1007/s003359900911 [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcoholism, Clinical and Experimental Research. 2009. July;33(7):1294–1303. DOI: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, & Grahame N (2011). Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behavior Genetics, 41(2), 288–302. 10.1007/s10519-010-9394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES (2008). An Endocannabinoid Hypothesis of Drug Reward and Drug Addiction. Annals of the New York Academy of Sciences, 1139(1), 412–421. 10.1196/annals.1432.056 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer K. s, Kirkley SM, Gansler DA, Merritt D, & Couture A (2009, May 25). Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. 10.2147/NDT.S4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, & Schoffelmeer ANM (2008). Cannabinoid modulation of executive functions. European Journal of Pharmacology, 585(2), 458–463. 10.1016/j.ejphar.2008.02.099 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KJ, Shiffman S, & Heath AC (2012). Low Sensitivity to Alcohol: Relations With Hangover Occurrence and Susceptibility in an Ecological Momentary Assessment Investigation. Journal of Studies on Alcohol and Drugs, 73(6), 925–932. 10.15288/jsad.2012.73.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, & Zimmer A (2003). A Critical Role for the Cannabinoid CB1 Receptors in Alcohol Dependence and Stress-Stimulated Ethanol Drinking. Journal of Neuroscience, 23(6), 2453–2458. 10.1523/JNEUROSCI.23-06-02453.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, & Kesner RP (2002). Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral neuroscience, 116(1), 105–115. doi: 10.1037//0735-7044.116.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME (2007). The Contribution of the Medial Prefrontal Cortex, Orbitofrontal Cortex, and Dorsomedial Striatum to Behavioral Flexibility. Annals of the New York Academy of Sciences, 1121(1), 355–375. 10.1196/annals.1401.013 [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, & Brewer RD (2015). 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine, 49(5), e73–e79. 10.1016/j.amepre.2015.05.031 [DOI] [PubMed] [Google Scholar]
- Serrano A, & Parsons LH (2011). Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacology & Therapeutics, 132(3), 215–241. 10.1016/j.pharmthera.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Gonzales SW, & Grant KA (2018). Low cognitive flexibility as a risk for heavy alcohol drinking in non-human primates. Alcohol. 10.1016/j.alcohol.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, & Cravatt BF (2002). A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of the National Academy of Sciences of the United States of America, 99(12), 8394–8399. 10.1073/pnas.082235799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, & Kunos G (2003). Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proceedings of the National Academy of Sciences, 100(3), 1393–1398. 10.1073/pnas.0336351100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, & Cheer JF (2016). Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions. Journal of Neuroscience, 36(40), 10230–10238. 10.1523/JNEUROSCI.1712-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


