Abstract
Hair cortisol concentrations measured during pregnancy have emerged as a novel biomarker for prenatal stress exposure. However, associations between prenatal stress and distress, broadly defined, and hair cortisol concentrations during pregnancy are inconsistent. We examined relations among hair cortisol concentrations during the third trimester with (1) emotion dysregulation and (2) detailed measures of maternal prenatal stress. We also examined the predictive validity of maternal hair cortisol during pregnancy for adverse newborn health outcomes. Cortisol concentrations were derived from 6cm of hair during the third trimester of pregnancy. Mothers reported on their emotion dysregulation and stress at this time. A standardized newborn neurobehavioral exam was conducted shortly after birth and newborn birth weight and gestational age were assessed from medical records. All hypotheses were pre-registered on the Open Science Framework (osf.io/279ng). High levels of emotion dysregulation, but not stress, were predictive of high hair cortisol concentrations. Maternal prenatal BMI mediated the relation between maternal prenatal emotion dysregulation and hair cortisol concentrations. There was no association between hair cortisol and infant birth outcomes. This research supports the notion that transdiagnostic markers of psychopathology are important correlates of hair cortisol concentrations during pregnancy.
Research on the effects of prenatal stress on child outcomes is based on two critical hypotheses. First, prenatal exposure to stressors predicts adverse newborn neurodevelopmental or birth outcomes such as prematurity and low birth weight (Lobel, Dunkel-Schetter, & Scrimshaw, 1992; Rondó et al., 2003; Wadhwa, Sandman, Porto, Dunkel-Schetter, & Garite, 1993), pregnancy complications (Mulder et al., 2002), and later neurodevelopmental disorders (He et al., 2019; Kinney, Munir, Crowley, & Miller, 2008). Second, hypothalamic-pituitary-adrenal (HPA) axis functioning is one mechanism by which prenatal stress exposure can potentiate these neurodevelopmental outcomes. Researchers propose that high levels of prenatal cortisol may cross the placenta, disrupt brain growth and development, and increase risk for neurodevelopmental problems (Wadhwa, Sandman, & Garite, 2001).
Testing this HPA axis pathway hypothesis is difficult because cortisol output increases dramatically across pregnancy (Seth, Lewis, & Galbally, 2016). There is a two-to-four-fold increase in maternal cortisol during gestation and third trimester cortisol levels are essentially at ceiling (Glynn, Schetter, Chicz-DeMet, Hobel, & Sandman, 2007; Mastorakos & Ilias, 2003; Pokoly, 1973; Seth et al., 2016). High cortisol output is critical to fetal survival because glucocorticoid concentrations stimulate lung development (Mesiano, 1997), and corticotropin releasing hormone may stimulate the onset of labor (Liggins, 1994; Morsi, DeFranco, & Witchel, 2018; Sandman, Wadhwa, Chicz-DeMet, Dunkel-Schetter, & Porto, 1997). However, elevated third trimester cortisol, as measured via maternal saliva, poses a problem for researchers interested in studying associations between HPA axis functioning, prenatal stress and/or adverse newborn neurodevelopmental outcomes, because almost all women’s salivary cortisol levels are at ceiling.
Fortunately, technological advances allow for tests of cortisol concentrations in human hair (Braig et al., 2015; D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011). Cortisol measured in human hair reflects chronic output of the HPA axis during a 3 – 6 month period (Braig et al., 2015; D’Anna-Hernandez et al., 2011). Specifically, cortisol in blood is deposited in growing hair so that as hair grows from the scalp, there is a historical marker of cortisol exposure over time. Hair grows on average 1 cm per month (Pragst & Balikova, 2006), even during pregnancy (D’Anna-Hernandez et al., 2011). Consequently, a conservative estimate is that a 1cm segment of hair proximal to the scalp reflects exposure over the last 1–2 months. Indeed, this hypothesis has been confirmed by examining the convergent validity of hair cortisol with salivary cortisol measures during pregnancy. Both hair cortisol and salivary cortisol increase across pregnancy, and they are significantly and positively correlated during the second and third trimesters (D’Anna-Hernandez et al., 2011).
Prenatal Stress and Hair Cortisol Concentrations during Pregnancy
If stress is related to HPA axis output, and cortisol can be measured in hair, it follows that stress experienced during pregnancy should be related to hair cortisol concentrations. Indeed, self-reported prenatal stress and anxiety, and even stress experienced in childhood such as preconception stress, trauma exposure, and childhood physical and/or sexual abuse have all been associated with elevated hair cortisol concentrations during pregnancy (Hoffman, Mazzoni, Wagner, Laudenslager, & Ross, 2016; Kalra, Einarson, Karaskov, Van Uum, & Koren, 2007; Orta et al., 2018, 2019; Schreier et al., 2016; Swales et al., 2018). On the other hand, no associations were found between hair cortisol concentrations and maternal depression in the second trimester, pregnancy-related anxiety, somatization, or stress symptoms in other research (Braig et al., 2015; Kramer et al., 2009; Scharlau et al., 2018; Wikenius et al., 2016). A recent review concluded that associations between mild to moderate prenatal stress and hair cortisol concentrations are inconsistent (Mustonen et al., 2018). The authors also noted that hair cortisol assessed after delivery may not accurately measure stress experienced during pregnancy (Mustonen et al., 2018). Further, they reported that inconsistent prenatal distress and hair cortisol findings may be a consequence of heterogeneous “psychological distress” symptoms. In other words, transdiagnostic markers of psychopathology, ones that capture the full range of comorbidities women may experience during pregnancy, are needed to better understand whether prenatal psychological functioning is related to hair cortisol concentrations. We propose that a strong marker of prenatal distress during pregnancy may be emotion dysregulation, a transdiagnostic vulnerability factor for psychopathology (Beauchaine, 2015; Crowell, Puzia, & Yaptangco, 2015; Lin et al., 2019; Ostlund et al., 2019).
Emotion dysregulation is characterized by problems with maintaining goal-directed behavior and accepting, identifying, and managing emotions (Beauchaine, 2015; Gratz & Roemer, 2004). Women with high levels of emotion dysregulation are more likely to experience depression, anxiety, and borderline personality disorder (Crowell et al., 2015), as well as varying degrees of prenatal stress, which can further exacerbate their symptoms (Lin et al., 2019). Thus, emotion dysregulation may be a more powerful predictor of hair cortisol concentrations than a specific stressor or disorder studied in isolation.
Hair Cortisol Concentrations during Pregnancy and Neonatal Outcomes
Hair cortisol concentrations measured prenatally may also predict important newborn neurodevelopmental and birth outcomes. However, evidence linking prenatal exposure to maternal hair cortisol and neonatal outcomes is contradictory. High prenatal stress exposure measured in hair was related to high birth weights and longer telomeres in females, but not males (Bosquet Enlow et al., 2019). In males, high prenatal maternal hair cortisol and high lifetime trauma predicted low birth weights (Flom et al., 2018). In both boys and girls, high prenatal maternal hair cortisol concentrations in the second trimester predicted a younger gestational age at delivery (Hoffman et al., 2016). In fact, high prenatal maternal hair cortisol was a stronger predictor of a younger gestational age than were maternal self-reports of psychological distress (Braig et al., 2015). In other research, however, high prenatal hair cortisol concentrations were associated with older gestational age at delivery (Kramer et al., 2009).
Studies examining prenatal hair cortisol concentrations as predictors of neurodevelopmental outcomes in infancy are limited. It is possible that prenatal programming influences could shape newborn neurodevelopmental outcomes via increased cortisol exposure (Conradt, Adkins, Crowell, Monk, & Kobor, 2018; O’Donnell, O’Connor, & Glover, 2009). For example, prenatal exposure to maternal salivary cortisol at 30–32 weeks gestation was related to greater newborn negative reactivity (Davis, Glynn, Dunkel Schetter, Hobel, Chicz-Demet, & Sandman, 2007). Infants with high anger and frustration were more likely to have mothers who experienced higher lifetime trauma and had high hair cortisol in the third trimester compared with infants whose mothers had a trauma history but low hair cortisol concentrations (Bosquet Enlow et al., 2017).
In the present study, we sought to determine whether emotion dysregulation, a transdiagnostic marker of psychopathology, was associated with hair cortisol concentrations during the second and third trimesters of pregnancy. We also test whether prenatal maternal hair cortisol concentrations predict newborn neurodevelopmental and birth outcomes. Consistent with calls for transparency and open science (Nosek et al., 2015), we pre-registered our hypotheses on the Open Science Framework (osf.io/279ng). In our first aim, we examined the association between prenatal exposure to maternal emotion dysregulation and prenatal maternal hair cortisol concentrations. We hypothesized that high maternal emotion dysregulation would be related to high prenatal hair cortisol concentrations. In the second aim, we examined the interactions between prenatal stress and emotion dysregulation on prenatal maternal hair cortisol concentrations. We expected that the combination of prenatal exposure to emotion dysregulation and stress would predict the highest levels of hair cortisol concentrations. For aim three, we tested the effects of prenatal maternal hair cortisol concentrations on newborn neurobehavior, birth weight, and gestational age. We hypothesized that prenatal exposure to high hair cortisol concentrations would predict poorer newborn attention, higher newborn arousal, lower birth weight, and a younger gestational age.
Method
Participants
Data come from 137 pregnant women who were recruited prenatally as part of a larger study on the intergenerational transmission of emotion dysregulation. Details on enrollment and exclusion criteria can be found elsewhere (Lin et al., 2019). In brief, women were recruited during or after their second trimester of pregnancy at local OB/GYN clinics and via local advertisement (e.g., posting flyers). Eligible women had a singleton pregnancy, were between the ages of 18 – 40 years, had not experienced major pregnancy complications at the time of enrollment (e.g., preeclampsia, gestational diabetes), and reported no substance use during their pregnancy. Table 1 includes demographic information. The mean age was 29 years (range 18–40 years). The majority of women (54%) were non-Hispanic white, and 9% identified as Asian, 6% multiracial, and less than 4% each as American Indian/Alaskan Native, Hawaiian Native/Pacific Islander, and Black/African American. Twenty-seven percent identified as Hispanic/Latina. The majority (72.2%) of newborns were delivered vaginally and 51.2% were female. Mothers were recruited in accordance with the Institutional Review Board of the University of Utah. All mothers gave written informed consent prior to participation.
Table 1.
Sample characteristics
| Characteristic | N | % | Mean | SD |
|---|---|---|---|---|
| Maternal age | 162 | --- | 28.99 | 5.16 |
| Maternal race/ethnicity | 162 | |||
| American Indian or Alaskan Native | 3.1 | |||
| Asian | 9.3 | |||
| Hawaiian or Pacific Islander | 1.2 | |||
| African American | 1.2 | |||
| Caucasian | 79.0 | |||
| More than 1 race | 6.2 | |||
| Hispanic | 27.2 | |||
| Maternal employment status | 161 | |||
| Full-time | 38.5 | |||
| Part-time | 18.7 | |||
| Not employed | 36.3 | |||
| Other | 6.5 | |||
| Maternal education | 159 | |||
| Less than 12th grade | 3.1 | |||
| High school or equivalent | 13.2 | |||
| Junior college or technical school | 32.1 | |||
| College graduate | 32.1 | |||
| Any post graduate school | 19.5 | |||
| Maternal marital status | 160 | |||
| Married | 76.8 | |||
| Single or never married | 16.9 | |||
| Separated or divorced | 6.3 | |||
| Prenatal maternal BMI | 152 | 30.62 | 6.68 | |
| Diagnosis of gestational diabetes (yes) | 160 | 3.1 | ||
| Diagnosis of gestational hypertension (yes) | 160 | 4.4 | ||
| Diagnosis of pre-eclampsia (yes) | 160 | 2.5 | ||
| Prenatal DERS | 160 | 79.93 | 26.74 | |
| UCLA life stress interview: Chronic stress | 162 | 2.36 | 0.45 | |
| UCLA life stress interview: Number of stress episodes | 162 | 3.58 | 2.48 | |
| Hair cortisol concentrations | 137 | 4.65 | 3.05 | |
| NNNS arousal | 155 | 0.00 | .79 | |
| NNNS attention | 155 | 0.01 | .93 | |
| Newborn gestational age | 159 | 38.89 | 1.33 | |
| Newborn birth weight (grams) | 156 | 3360.03 | 491.32 | |
| Newborn sex (female) | 160 | 51.2 |
Procedure
Eligible participants completed a self-report measure of emotion dysregulation during the recruitment process to determine eligibility for the study. Our goal was to recruit women along a uniform distribution of scores on the Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004). Because the DERS is typically normally distributed, this approach oversampled pregnant women with both high (scores ranging from 100–144) and low (scores ranging from 36–67) DERS values (Lin et al., 2019). Pregnant women completed questionnaires, including relevant demographic information prior to the laboratory visit. Women participated in the laboratory visit between 26 and 40 weeks (M = 33.58, SD = 2.99). They completed tasks designed to elicit an autonomic nervous system response to stress, completed additional questionnaires, and underwent prenatal stress and psychiatric interviews (see Lin et al., 2019 for a more complete description of our prenatal protocol). Between 24 hours and 2 months after birth trained graduate research assistants or clinicians completed the newborn neurobehavioral exam (M = 3.8 days, Mdn = 1.0 days, SD = 8.3 days, range = 1 – 59 days; see Ostlund et al., 2019 for more complete descriptions of our birth protocol). Below we describe the measures specific to this manuscript.
Measures
Emotion dysregulation
Participants completed the DERS (Gratz & Roemer, 2004) when recruited to determine eligibility for the study. DERS subscales include the degree to which respondents accept their emotions, have difficulty achieving goals when upset, have control over their impulses, are aware of their emotions, have clarity about their emotions, and have strategies to regulate emotions. Women identified how often each of 36 items applied to them on a scale ranging from 1 (almost never) to 5 (almost always). In this study, we used the total DERS score. Alpha scale reliability was α = 0.96.
Chronic and episodic life stress
Stress during the previous six months was assessed via the UCLA Life Stress Interview (Hammen et al., 1987) which was modified for use with pregnant women. Women were interviewed about their chronic stress in each of the following domains: close friendships, relationship with their partner, co-parenting with the baby’s father, dating, relationship with family (mother, father, and siblings), finances, work, neighborhood environment, school, and health (self and family). Trained interviewers rated women’s chronic stress in each domain on a 5-point scale tied to behaviorally specific anchor points. Higher scores reflected greater chronic stress. The chronic stress score was calculated by averaging scores across all domains. Episodic (i.e., acute) stress was assessed in each domain as well. Women were asked if they experienced any significant events in each domain in the last six months. Women then reported how stressful those acute events were on a scale from 1 (no or minimal impact) to 5 (severe impact). Consistent with standard scoring protocols, an episodic stress score was calculated by summing the total subjective rating across all episodes.
Prenatal maternal hair cortisol
Hair was collected from the posterior vertex region at an average of 33 weeks gestation (range 26 – 40 weeks). Following established procedures, hair was collected as close to the scalp as possible by trained experimenters from a 1cm2 patch using hair cutting shears (D’Anna-Hernandez et al., 2011). A 6cm length of hair was sampled to approximate hair cortisol concentrations during the previous six months of pregnancy, which was also the length of time during which women reported on their chronic and episodic life stress levels. The scalp end was marked and hair samples were wrapped in aluminum foil and stored in envelopes at room temperature until shipment to the Kirschbaum laboratory in Dresden, Germany, for analysis.
Hair cortisol was assayed based on procedures described in Braig et al. (2015) and Gao et al. (2013). In brief, hair samples were washed twice and cortisol was extracted from whole, unground hair and analyzed by a Shimadzu HPLC-tandem mass spectrometry system (Shimadzu, Canby, OR) coupled to an ABSciex API 5000 Turbio-ion-spray triple quadrupole tandem mass spectrometer (AB Sciex, Foster City, CA). The sensitivity, specificity, and reliability is well-established (intra and inter-assay CVs between 3.7 – 8.8%; Gao et al., 2013).
Newborn neurobehavior
Shortly after birth, infant behavior was assessed using the NICU Network Neurobehavioral Scale (NNNS; Lester, Tronick, & Brazelton, 2004). The NNNS includes 13 summary scales evaluating attention, self-regulation, amount of handling, habituation, stress signs, arousal, excitability, lethargy, nonoptimal reflexes, asymmetrical reflexes, hyper and hypotonicity, and quality of movement in the newborn. Our NNNS assessment and procedures are described in detail in Ostlund et al. (2019). Two factor scores, attention and arousal, were derived in this sample and used in the current study. Arousal comprised a higher handling score, decreased self-regulation, and more stress, arousal, and excitability signs. Attention comprised high attention scores and low lethargy scores. The α = 0.84 and 0.85 for the arousal and attention factors, respectively.
Birth weight and gestational age
At delivery, newborns were weighed by nursing staff and their birth weight in grams was recorded in the delivery note. Newborn gestational age was also recorded in the delivery note. These data were abstracted from the medical record by trained research assistants and recorded in our database.
Results
Descriptive Statistics
Demographic information, means, and standard deviations of our key variables of interest, including our hair cortisol concentrations, can be found in Table 1. Hair cortisol concentrations were consistent with other published research from pregnant women processed at the Kirschbaum laboratory. Like other research, our hair cortisol data were positively skewed (Braig et al., 2015). We conducted a log-transformation prior to analyzing hair cortisol data, which resulted in normally distributed hair cortisol values. Table 2 includes correlations among our variables of interest. High prenatal hair cortisol concentrations were related to high prenatal maternal emotion dysregulation. High prenatal maternal emotion dysregulation was related to high chronic and episodic stress and low levels of newborn attention and arousal (as described previously in Ostlund et al., 2019). High prenatal episodic stress was related to high prenatal chronic stress and younger gestational age.
Table 2.
Correlations among study variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|
| 1. Prenatal maternal hair cortisol | --- | .18* | .16† | .09 | −.08 | −.03 | −.11 | .01 |
| 2. Prenatal maternal emotion dysregulation | --- | .29*** | .34*** | −.15 | −.17* | −.09 | −.06 | |
| 3. Prenatal chronic stress | --- | .37*** | .04 | −.14 | −.11 | −.14 | ||
| 4. Prenatal episodic stress | --- | −.17* | −.16† | −.24** | −.06 | |||
| 5. NNNS arousal | --- | −.13 | .08 | .11 | ||||
| 6. NNNS attention | --- | .09 | .13 | |||||
| 7. Gestational age | --- | .45*** | ||||||
| 8. Birthweight | --- |
Note:
p <.06
p <.05
p <.01
p <.001
Missing data
We recruited 162 women as part of a longitudinal study on the intergenerational transmission of emotion dysregulation (Lin et al., 2019; Ostlund et al., 2019). Sixteen participants had hair cortisol data that were excluded because they were extreme outliers (+/− 3SD from the sample mean). Four participants did not want their hair cut for cortisol analysis. Experimenter error resulted in missing data from five participants. The final sample size of mothers with hair cortisol data was 137. Mothers with missing hair cortisol data were more likely to be racial/ethnic minorities, t(160) = −2.39, p = .02.
Covariates
We examined the following covariates given their association with hair cortisol concentrations in the published literature: race/ethnicity, BMI (weight and height calculated by kg/m2 reported at the prenatal visit; Braig et al., 2015), gestational age in weeks at the prenatal visit, mode of delivery, hair color, hair texture, number of times hair was washed per week, use of hair dye and/or hair relaxers in the last 6 months, number of times per month hair dye or relaxers were used, number of times per week heating products (e.g., blow dry, hair straightener) were used, and number of times per week hair products for styling were used (Bosquet Enlow et al., 2019; Braig et al., 2015; Rippe et al., 2016; Schreier et al., 2016). Mothers who washed their hair more frequently had marginally lower hair cortisol concentrations, r = −.17, p = .06.
There were no significant differences in emotion dysregulation, chronic or episodic stress, or newborn neurobehavioral outcomes depending on maternal race/ethnicity. Maternal age and maternal gestational age at the prenatal assessment were not significantly associated with maternal emotion dysregulation, hair cortisol, chronic and episodic life stress, or newborn neurobehavior. There were no sex differences in newborn neurobehavior or hair cortisol concentrations.
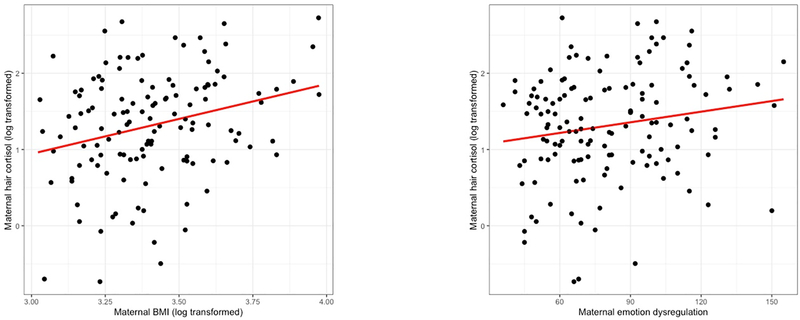
We also tested for the following covariates, given their association with prenatal stress: maternal education, income, marital, and employment status. The following significant covariates were included in our regression models. Mothers with higher incomes experienced significantly less chronic stress, F(11, 158 = 3.89, partial η2 = .23, p < .001). Maternal BMI at the prenatal visit was significantly and positively associated with emotion dysregulation, hair cortisol, and episodic stress, r(150) = .28, p < .001; r(134) = .27, p = .002, r(136) = .21, p = .01, respectively (and see scatterplot of emotion dysregulation and hair cortisol, and BMI and hair cortisol in Figure 1). Mothers who completed more years of education had significantly lower chronic stress scores and lower hair cortisol values than mothers with less education, F(4, 159) = 2.68, p = .03, partial η2 = .07; F(4, 134) = 2.51, p = .05, partial η2 = .07, respectively. Mothers who were not employed had significantly higher chronic stress scores and their newborns had significantly higher attention scores compared to mothers who were employed, F(3, 91) = 2.85, p = .04, partial η2 = .09; F (3, 86) = 3.17, p = .03, partial η2 = .10, respectively. Mothers who were separated or divorced experienced significantly greater episodic and chronic stress compared to mothers who were married, F(2, 146) = 4.79, p = .01, partial η2 = .06; F(2, 160) = 18.72, p < .001, partial η2 = .19, respectively. Mothers who used psychotropic medications reported significantly greater chronic stress compared to mothers who did not use psychotropic medications, F(1, 159) = 6.78, p = .01, partial η2 = .04.
Figure 1.
Scatterplots of the association between BMI and prenatal maternal hair cortisol concentrations, and emotion dysregulation and prenatal maternal hair cortisol concentrations.
Aim 1: Emotion dysregulation as a predictor of prenatal hair cortisol concentrations
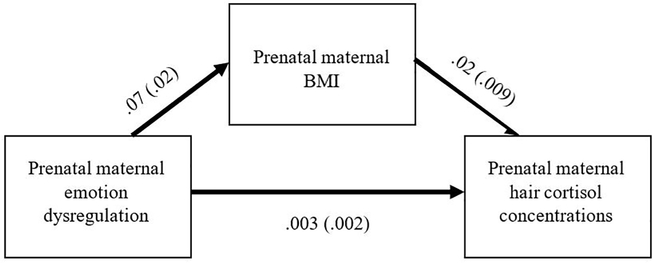
We ran a multiple linear regression to examine whether prenatal maternal emotion dysregulation predicted prenatal maternal hair cortisol. In the first model, maternal prenatal BMI and maternal education were included as covariates in step 1. Maternal prenatal maternal emotion dysregulation was included in step 2. Maternal prenatal BMI was the only significant predictor of hair cortisol concentrations, b = .21, p = .02 (Table 3). The main effect of prenatal maternal emotion dysregulation was not significant, b = .12, p = .20. In an exploratory analysis that was not pre-registered, we decided to test whether maternal prenatal BMI mediated the effect of prenatal maternal emotion dysregulation on prenatal hair cortisol using the PROCESS macro in SPSS with 1000 bootstrapped samples (Hayes, 2018). The indirect effect of prenatal maternal emotion dysregulation on hair cortisol through maternal BMI was significant (ab = .002; 95% CI = .0003, .0033; Figure 2). Prenatal maternal emotion dysregulation did not mediate the association between prenatal BMI on prenatal hair cortisol concentrations. We did not pre-register the mediation analysis because we did not anticipate that maternal prenatal BMI would have such a strong effect on prenatal maternal hair cortisol levels.
Table 3.
Predictors of 3rd trimester hair cortisol concentrations
| β | SE | b | p | |
|---|---|---|---|---|
| Covariates | ||||
| Log-transformed maternal prenatal BMI | .70 | .31 | .21 | .02 |
| Maternal education | −.08 | .06 | −.11 | .22 |
| Key predictor | ||||
| Maternal prenatal emotion dysregulation | .003 | .002 | .12 | .20 |
Note: Results from step 2 are presented; SE, standard error; R2 change = .013
Figure 2.
Path coefficients and standard errors depicting the indirect effect of prenatal maternal emotion dysregulation on hair cortisol concentrations through maternal prenatal BMI during the third trimester of pregnancy.
Aim 2: Prenatal maternal stress and emotion dysregulation as predictors of prenatal hair cortisol concentrations
Next we conducted a hierarchical linear regression to test if prenatal maternal BMI and prenatal maternal education (step 1), prenatal maternal emotion dysregulation, chronic stress, and episodic stress predicted prenatal maternal hair cortisol (step 2). In step 3, we added the interactions between chronic stress and emotion dysregulation, and episodic stress and emotion dysregulation. None of the main effects or interactions were significant.
Aim 3: Prenatal maternal hair cortisol concentrations as predictors of newborn neurodevelopmental and birth outcomes
For our third aim, we examined if prenatal maternal hair cortisol concentrations significantly predicted the newborn neurobehavioral outcomes of attention and arousal, newborn birth weight, and newborn gestational age. Four regressions were conducted to test these outcomes. There were no significant main effects of prenatal maternal hair cortisol on newborn neurobehavior, newborn birth weight, or newborn gestational age (all b’s < .11, all p’s > .20).
Discussion
In non-pregnant samples, chronic stress is related to a 22% higher median hair cortisol concentration value (Stalder et al., 2017). The association is weaker and inconsistent in pregnant women (Mustonen et al., 2018). In this study, the effect of prenatal maternal stress, assessed via maternal interview, was not a significant predictor of prenatal maternal hair cortisol. A significant, though small, effect was found for prenatal maternal emotion dysregulation, though this effect was mediated via maternal prenatal BMI. No significant associations were found between prenatal maternal hair cortisol and newborn neurodevelopmental and birth outcomes. We discuss the implications of these findings for perinatal stress and health research below.
High prenatal emotion dysregulation, but not prenatal stress measured via maternal interview, predicted elevated hair cortisol concentrations. Mothers who experience high levels of emotion dysregulation may not necessarily feel stressed, and vice-versa. Many women who experience high levels of prenatal stress may have well-developed coping strategies for regulating their emotions (Troy & Mauss, 2011). Our prenatal maternal stress interview captured a wide range of stressors experienced in the past six months; thus, we expected that it would relate to hair cortisol concentrations over the same time period. It may be that a mother’s daily experience managing and identifying her emotions provides a more robust association with chronic HPA axis functioning than subjective stress experiences. However, we cannot infer direction of effect with these findings; high cortisol concentrations could also contribute to dysregulated emotions. It is likely that multiple factors contribute to chronic HPA axis output during pregnancy, including complex daily transactions between stress exposure, physiological symptoms, one’s ability to notice and regulate emotions, and coping strategies.
Examination of maternal prenatal BMI as a mediator in this study was exploratory and was not included in our pre-registered document. We found it to be a strong and robust predictor of prenatal maternal hair cortisol concentrations, consistent with previous research with pregnant women that included this variable as a covariate (Braig et al., 2015). One of the functions of the HPA axis is to transport glucose, and glucocorticoids likely affect lipid and carbohydrate metabolism (Stalder et al., 2013). For instance, individuals with metabolic syndrome have higher levels of hair cortisol concentrations than do those without the syndrome. Also, elevated hair cortisol concentrations predict HbA1C, which indexes chronic blood glucose concentrations (Stalder et al., 2013). It is therefore not surprising that high maternal prenatal BMI predicted high hair cortisol concentrations. In addition to height and weight, high BMI also likely indexes the physical activity levels and diet of the participants.
The main effect of prenatal maternal emotion dysregulation on elevated maternal hair cortisol concentrations was no longer significant when accounting for maternal BMI. However, BMI mediated the effect of emotion dysregulation on hair cortisol concentrations, providing insight into associations between prenatal maternal emotion dysregulation hair cortisol concentrations. It may be that mothers who are more emotionally dysregulated have maladaptive coping strategies (Hayaki, 2009; Lavender et al., 2015) such as poor nutritional intake and low physical activity, which can contribute to elevations in BMI. In fact, emotional brain training that helps women cope with negative emotions and mindfulness training designed to increase awareness and acceptance of emotions have been found to reduce stress and improve eating behaviors in pregnant women (Laraia et al., 2018). BMI elevations could then contribute to high prenatal cortisol concentrations, as high glucose levels may stimulate more HPA axis activity, leading to the elevated hair cortisol concentrations observed in our study and in other research with pregnant (Braig et al., 2015) and non-pregnant adults (Stalder et al., 2013). However, we cannot infer causality and our results warrant further investigation with larger sample sizes and multiple measurements of emotion dysregulation, BMI, and hair cortisol concentrations over time. In addition it is important to note that our effect size estimates were small. Emotion dysregulation thus likely explains only a small amount of variance in hair cortisol concentrations during pregnancy.
While the concurrent validity of prenatal hair cortisol concentrations was bolstered in this study by its associations with emotion dysregulation and BMI, the predictive validity of hair cortisol with respect to newborn outcomes was poor. Prenatal hair cortisol concentrations were not related to newborn neurobehavior, birth weight, or gestational age, which is consistent with past research. Our findings are strengthened given that this study was pre-registered. However, the pre-registered hypothesis, that prenatal hair cortisol concentrations would be significantly associated with newborn birth outcomes, was not supported. In our sample, the majority of infants (94%) were born at term so we may not have had the variability in gestational age and birth weight necessary to detect associations with prenatal hair cortisol concentrations. In other research, the negative association between hair cortisol concentrations and birth weight was only found in male infants whose mothers had high lifetime trauma (Flom et al., 2018; Schreier et al., 2016). Furthermore, in previous work, associations were found between maternal plasma cortisol and cortisol measured in amniotic fluid (Glover, Bergman, Sarkar, & O’Connor, 2009), which may be a more accurate marker of fetal exposure to cortisol than maternal hair cortisol concentrations. It could also be that prenatal exposure to cortisol measured via maternal hair is moderated by other factors such as maternal trauma exposure (Bosquet Enlow et al., 2017), a possibility we will explore in future studies.
A limitation of this study is that we chose to collect a maximum of 6cm of hair from the maternal scalp, which is the longest recommended length for conducting hair cortisol analyses (Kirschbaum, Tietze, Skoluda, & Dettenborn, 2009). In previous research, hair collected at delivery 6 – 9cm from the scalp had lower hair cortisol concentrations than did 3cm of hair collected during the first trimester of pregnancy (Orta et al., 2018). Hair cortisol concentrations can decrease up to 30 – 40% from one 3cm segment to the next due to hair washing and hair treatments (Wester, van der Wulp, Koper, de Rijke, & van Rossum, 2016). We controlled for these factors in our analyses and collected hair cortisol during pregnancy rather than the postpartum, given previous research showed that studies may have more power if they examine associations between maternal distress during pregnancy and prenatal hair cortisol at around the same time of pregnancy (Mustonen et al., 2018). In addition, while hair grows on average 1cm per month, some women may experience slightly more or less hair growth per month (Pragst & Balikova, 2006). Nevertheless, we have plans to replicate this study in an independent sample of pregnant women to strengthen the validity of our findings. We also cannot infer direction of effect with these findings given that our prenatal predictors were measured cross-sectionally during the third trimester of pregnancy.
We tested the validity of hair cortisol concentrations as a marker of both prenatal stress and emotion dysregulation and predictor of impaired newborn neurodevelopmental and birth outcomes. We highlighted the importance of measuring both transdiagnostic markers of psychopathology as well as common medical markers, like BMI, in future studies. We also provide evidence that one pathway from elevated emotion dysregulation to elevated hair cortisol concentrations may be via high BMI levels. Transdiagnostic markers of psychopathology may have stronger prognostic value for stress biomarkers than chronic or acute stress exposure during pregnancy. Research that leverages these transdiagnostic mechanisms may prove more fruitful for identifying children and mothers most in need of developmental support.
Acknowledgements
We would like to thank all of the families who generously donated their time to participate in our study. We would also like to thank the dedicated research assistants from the CAN Lab. We are indebted to Mike Varner and Bob Silver for their support of the BABY study and for providing their dedicated OB/GYN Research Network staff to help with screening and recruitment. We thank Connie Hammen for her assistance with training and scoring the UCLA Life Stress Interview. We would also like to thank the University of Utah Vice President’s Clinical Translational Research Scholars program for their mentorship and grantsmanship assistance.
Funding sources: This manuscript was supported by the National Institute of Mental Health under Award Number R21MH109777 (to S.C. and E.C.), a Career Development Award from the National Institute on Drug Abuse 7K08DA038959 (to E.C.) and grants from the University of Utah Consortium for Families and Health Research and Interdisciplinary Research Pilot Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Data Availability Statement: The data that support the findings have been uploaded to the National Institute of Mental Health (NIMH) repository. Data have also been pre-registered at osf.io/279ng.
References
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Devick KL, Brunst KJ, Lipton LR, Coull BA, & Wright RJ (2017). Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22, 492–513. 10.1111/infa.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Sideridis G, Bollati V, Hoxha M, Hacker MR, & Wright RJ (2019). Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology, 102, 225–235. 10.1016/j.psyneuen.2018.12.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, … Rothenbacher D (2015). Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology, 52, 289–296. 10.1016/j.psyneuen.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Conradt E, Adkins DE, Crowell SE, Monk C, Kobor MS (2018). An epigenetic pathway approach to investigating associations between prenatal exposure to maternal mood disorder and newborn neurobehavior. Development and Psychopathology, 30, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Puzia ME, & Yaptangco M (2015). The ontogeny of chronic distress: Emotion dysregulation across the life span and its implications for psychological and physical health. Current Opinion in Psychology, 3, 91–99. 10.1016/j.copsyc.2015.03.023 [DOI] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, & Laudenslager ML (2011). Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology and Behavior, 104, 348–353. 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Flom JD, Chiu Y-HM, Hsu H-HL, Devick KL, Brunst KJ, Campbell R, … Wright RJ (2018). Maternal lifetime trauma and birthweight: Effect modification by in utero cortisol and child sex. The Journal of Pediatrics, 203, 301–308. 10.1016/j.jpeds.2018.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, & Kirschbaum C (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. Journal of Chromatography B, 928, 1–8. 10.1016/j.jchromb.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, & O’Connor TG (2009). Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology, 34, 430–435. 10.1016/j.psyneuen.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Glynn LM, Dunkel-Schetter C, Chicz-DeMet A, Hobel CJ, & Sandman CA (2007). Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides, 28, 1155–1161. 10.1016/j.peptides.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment, 26, 41–54. 10.1023/B:JOBA.0000007455.08539.94 [DOI] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, & Hiroto D (1987). Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology, 96, 190–198. 10.1037/0021-843X.96.3.190 [DOI] [PubMed] [Google Scholar]
- Hayaki J (2009). Negative reinforcement eating expectancies, emotion dysregulation, and symptoms of bulimia nervosa. International Journal of Eating Disorders, 42, 552–556. 10.1002/eat.20646 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (Second edition). New York, NY: Guilford Press. [Google Scholar]
- He P, Luo Y, Guo C, Chen G, Song X, & Zheng X (2019). Prenatal war exposure and schizophrenia in adulthood: Evidence from the Sino-Japanese War of 1937–1945. Social Psychiatry and Psychiatric Epidemiology, 54, 313–320. 10.1007/s00127-018-1584-0 [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, & Ross RG (2016). Measures of maternal stress and mood in relation to preterm birth. Obstetrics and Gynecology, 127, 545–552. 10.1097/AOG.0000000000001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Einarson A, Karaskov T, Van Uum S, & Koren G (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clinical and Investigative Medicine, 30, 103–107. 10.25011/cim.v30i2.986 [DOI] [PubMed] [Google Scholar]
- Kinney D, Munir K, Crowley D, & Miller A (2008). Prenatal stress and risk for autism. Neuroscience and Biobehavioral Reviews, 32, 1519–1532. 10.1016/j.neubiorev.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, & Dettenborn L (2009). Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34, 32–37. 10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, … Platt RW (2009). Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal of Epidemiology, 169, 1319–1326. 10.1093/aje/kwp061 [DOI] [PubMed] [Google Scholar]
- Laraia BA, Adler NE, Coleman-Phox K, Vieten C, Mellin L, Kristeller JL, … Epel E (2018). Novel interventions to reduce stress and overeating in overweight pregnant women: A feasibility study. Maternal and Child Health Journal, 22, 670–678. 10.1007/s10995-018-2435-z [DOI] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, & Mitchell JE (2015). Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical Psychology Review, 40, 111–122. 10.1016/j.cpr.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, & Brazelton TB (2004). The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics, 113, 641–667. [PubMed] [Google Scholar]
- Liggins G (1994). The role of cortisol in preparing the fetus for birth. Reproduction, Fertility and Development, 6, 141–150. 10.1071/RD9940141 [DOI] [PubMed] [Google Scholar]
- Lin B, Kaliush PR, Conradt E, Terrell S, Neff D, Allen AK, … Crowell SE (2019). Intergenerational transmission of emotion dysregulation: Part I. Psychopathology, self-injury, and parasympathetic responsivity among pregnant women. Development and Psychopathology, 1–15. 10.1017/S0954579419000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M, Dunkel-Schetter C, & Scrimshaw SC (1992). Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychology, 11, 32–40. 10.1037/0278-6133.11.1.32 [DOI] [PubMed] [Google Scholar]
- Mastorakos G, & Ilias I (2003). Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences, 997, 136–149. 10.1196/annals.1290.016 [DOI] [PubMed] [Google Scholar]
- Mesiano S (1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews, 18, 378–403. 10.1210/er.18.3.378 [DOI] [PubMed] [Google Scholar]
- Morsi A, DeFranco D, & Witchel SF (2018). The hypothalamic-pituitary-adrenal axis and the fetus. Hormone Research in Paediatrics, 89, 380–387. 10.1159/000488106 [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Robles De Medina PG, Huizink A, Van den Bergh BR, Buitelaar J, & Visser GH (2002). Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Human Development, 70, 3–14. 10.1016/S0378-3782(02)00075-0 [DOI] [PubMed] [Google Scholar]
- Mustonen P, Karlsson L, Scheinin NM, Kortesluoma S, Coimbra B, Rodrigues AJ, & Karlsson H (2018). Hair cortisol concentration (HCC) as a measure for prenatal psychological distress — A systematic review. Psychoneuroendocrinology, 92, 21–28. 10.1016/j.psyneuen.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, … Yarkoni T (2015). Promoting an open research culture. Science, 348, 1422–1425. 10.1126/science.aab2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, & Glover V (2009). Prenatal stress and neurodevelopment of the child: Focus on the HPA Axis and role of the placenta. Developmental Neuroscience, 31, 285–292. 10.1159/000216539 [DOI] [PubMed] [Google Scholar]
- Orta OR, Tworoger SS, Terry KL, Coull BA, Gelaye B, Kirschbaum C, … Williams MA (2018). An evaluation of distal hair cortisol concentrations collected at delivery. Stress, 21, 355–365. 10.1080/10253890.2018.1458088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orta OR, Tworoger SS, Terry KL, Coull BA, Gelaye B, Kirschbaum C, … Williams MA (2019). Stress and hair cortisol concentrations from preconception to the third trimester. Stress, 22, 60–69. 10.1080/10253890.2018.1504917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Vlisides-Henry RD, Crowell SE, Raby KL, Terrell S, Brown MA, … Conradt E (2019). Intergenerational transmission of emotion dysregulation: Part II. Developmental origins of newborn neurobehavior. Development and Psychopathology, 1–14. 10.1017/S0954579419000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrullo L, & Lu A (2019). Natural variation in fetal cortisol exposure is associated with neonatal body mass in captive vervet monkeys (Chlorocebus aethiops). American Journal of Primatology, 81, 20–28. 10.1002/ajp.22943 [DOI] [PubMed] [Google Scholar]
- Pokoly TB (1973). The role of cortisol in human parturition. American Journal of Obstetrics and Gynecology, 117, 549–553. 10.1016/0002-9378(73)90119-1 [DOI] [PubMed] [Google Scholar]
- Pragst F, & Balikova MA (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370, 17–49. 10.1016/j.cca.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Rippe RCA, Noppe G, Windhorst DA, Tiemeier H, van Rossum EFC, Jaddoe VWV, … van den Akker ELT (2016). Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology, 66, 56–64. 10.1016/j.psyneuen.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Rondó PHC, Ferreira RF, Nogueira F, Ribeiro MCN, Lobert H, & Artes R (2003). Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. European Journal of Clinical Nutrition, 57, 266–272. 10.1038/sj.ejcn.1601526 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, & Porto M (1997). Maternal stress, HPA activity, and fetal/infant outcome. Annals of the New York Academy of Sciences, 814, 266–275. 10.1111/j.1749-6632.1997.tb46162.x [DOI] [PubMed] [Google Scholar]
- Scharlau F, Pietzner D, Vogel M, Gaudl A, Ceglarek U, Thiery J, … Kiess W (2018). Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress, 21, 43–50. 10.1080/10253890.2017.1392507 [DOI] [PubMed] [Google Scholar]
- Schreier HMC, Bosquet Enlow M, Ritz T, Coull BA, Gennings C, Wright RO, & Wright RJ (2016). Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress, 19, 45–52. 10.3109/10253890.2015.1117447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth> S, Lewis AJ, & Galbally M (2016). Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: A systematic literature review. BMC Pregnancy and Childbirth, 16 10.1186/s12884-016-0915-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, … Fischer JE (2013). Cortisol in hair and the metabolic syndrome. The Journal of Clinical Endocrinology and Metabolism, 98, 2573–2580. 10.1210/jc.2013-1056 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, … Miller R (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. 10.1016/j.psyneuen.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Swales DA, Stout-Oswald SA, Glynn LM, Sandman C, Wing DA, & Davis EP (2018). Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biological Psychology, 139, 186–192. 10.1016/j.biopsycho.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AS, & Mauss IB (2011). Resilience in the face of stress: Emotion regulation as a protective factor In Southwick SM, Litz BT, Charney D, & Friedman MJ (Eds.), Resilience and mental health: Challenges across the lifespan (pp. 30–44). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Wadhwa PD, Sandman CA, & Garite TJ (2001). The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research, 133, 131–142. 10.1016/S0079-6123(01)33010-8 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, & Garite TJ (1993). The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. American Journal of Obstetrics and Gynecology, 169, 858–865. 10.1016/0002-9378(93)90016-C [DOI] [PubMed] [Google Scholar]
- Wester VL, van der Wulp NRP, Koper JW, de Rijke YB, & van Rossum EFC (2016). Hair cortisol and cortisone are decreased by natural sunlight. Psychoneuroendocrinology, 72, 94–96. 10.1016/j.psyneuen.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Wikenius E, Moe V, Kjellevold M, Smith L, Lyle R, Waagbø R, … Myhre AM (2016). The association between hair cortisol and self-reported symptoms of depression in pregnant women. PLoS ONE, 11, e0161804 10.1371/journal.pone.0161804 [DOI] [PMC free article] [PubMed] [Google Scholar]