Abstract
Objective:
Stimulant medications are the most prevalent first-line pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD), but children with aggressive behavior often receive multi-agent treatment. There is sparse evidence for the benefits of adjunctive medications when stimulant monotherapy has proved inadequate, yet the adverse effects of common adjuncts are well-established. This study compared the efficacy in reducing aggressive behavior of risperidone (RISP), divalproex sodium (DVPX), and placebo (PBO) added to stimulant medication among children whose symptoms persisted after individually-optimized stimulant treatment.
Method:
This trial enrolled 6–12-year-olds with ADHD, a disruptive disorder, significant aggressive behavior, and prior stimulant treatment. Open, systematically titrated stimulant treatment identified patients with inadequate reductions in aggressive behavior, who were then randomized to receive adjunctive RISP, DVPX, or PBO under double-blind conditions for 8 weeks. Family-based behavioral treatment was offered throughout the trial. The primary outcome was the parent-completed Retrospective-Modified Overt Aggression Scale.
Results:
175 children participated, mean [SD] age 9.48 [2.04] years, 19% female. 151 participants completed the stimulant optimization phase, with aggression remitting among 96 (63%), and 45 were randomized to adjunctive treatment groups. The adjunctive RISP group showed greater reductions in aggression ratings than the PBO group; least squares means difference [∆LSM], −2.33 (95% confidence interval [CI], −3.83 – −0.82; effect size [ES], −1.32), as did those receiving DVPX (∆LSM, −1.60; 95% CI, −3.18 – −0.03; ES, −0.91). Mean standardized body mass index scores increased more among RISP-treated patients than those receiving PBO (∆LSM, 1.54; 95% CI, 0.68 – 2.40; ES = 0.58).
Conclusion:
High response rate during the trial’s open stimulant optimization phase suggests that rigorous titration of stimulant medication and concurrent behavioral therapy may avert the need for additional medications. Among nonremitters, RISP and DVPX were efficacious adjunctive treatments although RISP was associated with weight gain.
Clinical trial registration information:
Effectiveness of Combined Medication Treatment for Aggression in Children with Attention Deficit Hyperactivity Disorder (The SPICY Study); https://www.clinicaltrials.gov; NCT00794625
Keywords: attention deficit and disruptive behavior disorders, aggression, central nervous system stimulants, antipsychotic agents, anticonvulsants
INTRODUCTION
Aggressive behavior is among the most frequent reasons children obtain mental health care.1–3 The most prevalent psychiatric disorder with which childhood aggressive behavior co-occurs is attention-deficit/hyperactivity disorder (ADHD), usually combined with oppositional defiant disorder (ODD), conduct disorder (CD), or disruptive mood dysregulation disorder (DMDD).4–6 Impulsiveness is a cardinal element of both ADHD and childhood aggressive behavior, and patients often show deficits in behavioral restraint, cognitive control, and emotion regulation.5, 7 Recommended pharmacotherapy approaches for aggressive youth with ADHD therefore prioritize treatments that are effective for ADHD, particularly stimulants.8, 9
Children with ADHD and behavior disturbances that include aggression often receive other medications adjunctive to stimulants.10–16 Guidelines recommend that one consider adjunctive treatment when adequate trials of stimulant medication and behavioral interventions yield insufficient improvements.17–19 Monotherapy trials have shown efficacy for the use of risperidone (RISP) in reducing aggression,20 and use of risperidone and other second-generation antipsychotics (SGAs) in this context is widespread in U.S. outpatient care.15, 21–23 However, a study of risperidone vs. placebo added to study-titrated stimulant treatment24 yielded a smaller effect size than reported in RISP monotherapy trials.
SGAs have metabolic and cardiovascular risks to which youth are especially vulnerable; younger patients are also susceptible to neuromotor adverse effects, perhaps more so than adults.25–31 The propensity for SGAs to induce rapid weight gain in children is well-documented, and an increased risk for the emergence of insulin resistance has been confirmed.29, 32 The chronic nature of childhood aggression and behavioral volatility often entails lengthy treatment with medication, and the potential for prolonged exposure to SGAs further heightens concerns.
Despite consensus that first-line pharmacotherapy for ADHD and behavioral interventions should precede adjunctive medications for aggression, trials seldom evaluate cotherapy among patients who are demonstrably underresponsive to these initial treatments. A stepped-treatment trial33 for children who experienced inadequate response in community care provided an extensive stimulant optimization and behavioral therapy protocol (mean duration 6 weeks). Aggressive behavior remitted among half these participants after this treatment; adjunctive divalproex sodium (DVPX) was more effective than placebo in lowering aggression for the others whose symptoms were refractory to stimulant treatment alone. A trial24 that compared risperidone vs. placebo added after a briefer stimulant titration and behavioral treatment phase (3 weeks), reported that only 5% had an adequate response to stimulant treatment; however, those randomized to placebo showed further reductions in behavioral disturbances resulting in no difference in responder rates. Ratings of overall disruptive behavior symptoms favored risperidone, however both groups’ endpoint scores were within the normal range.24 These findings suggest that a longer period devoted to stimulant dose optimization and behavioral therapy may be required to determine that these treatments are insufficient for aggressive behavior.
It is important for clinical decision-making (a) to determine the effects of adjunctive treatments among children who are demonstrably under-responsive to thoroughly titrated first-line stimulant treatment; (b) to compare the efficacy of SGAs with alternative adjuncts for those with stimulant-refractory aggression; and (c) to compare adverse effects between these treatments. Stimulant medications are appealing first-line treatments because they have the largest effects sizes for ADHD symptoms, they frequently ameliorate other conduct problems, a regimen’s effects are evident right away, treatment can be adjusted rapidly, and their adverse effect profiles are well understood. SGAs ameliorate aggressive behavior, but their effects as adjunctive treatment to thoroughly titrated stimulant therapy is uncertain; moreover, their cardiometabolic and neuromotor risks are worrisome and require they be used sparingly. Among non-antipsychotic mood stabilizers, DVPX may contribute to behavioral stability when children have already gained benefit for ADHD symptoms from stimulant medication. 33 DVPX poses generally lower adverse effect liability for children than SGAs, but determining dose adequacy and safety requires serum levels. It is problematic among post-menarchial women due to its teratogenicity and possible anovulatory and androgenic effects. With these consideration in mind, the present study compared adjunctive risperidone, divalproex sodium, and placebo among 6- to 12-year-olds with ADHD, a disruptive disorder, and chronic aggressive behavior whose aggression did not resolve during an open stimulant optimization phase that also provided family-focused behavioral treatment. We hypothesized both active compounds would be superior to placebo. To increase the likelihood that participants would be candidates for adjunctive medication due to insufficient response after the open stimulant optimization phase, inclusion criteria required prior stimulant treatment during which the trials’ behavioral severity criteria were met.
METHOD
Study Design
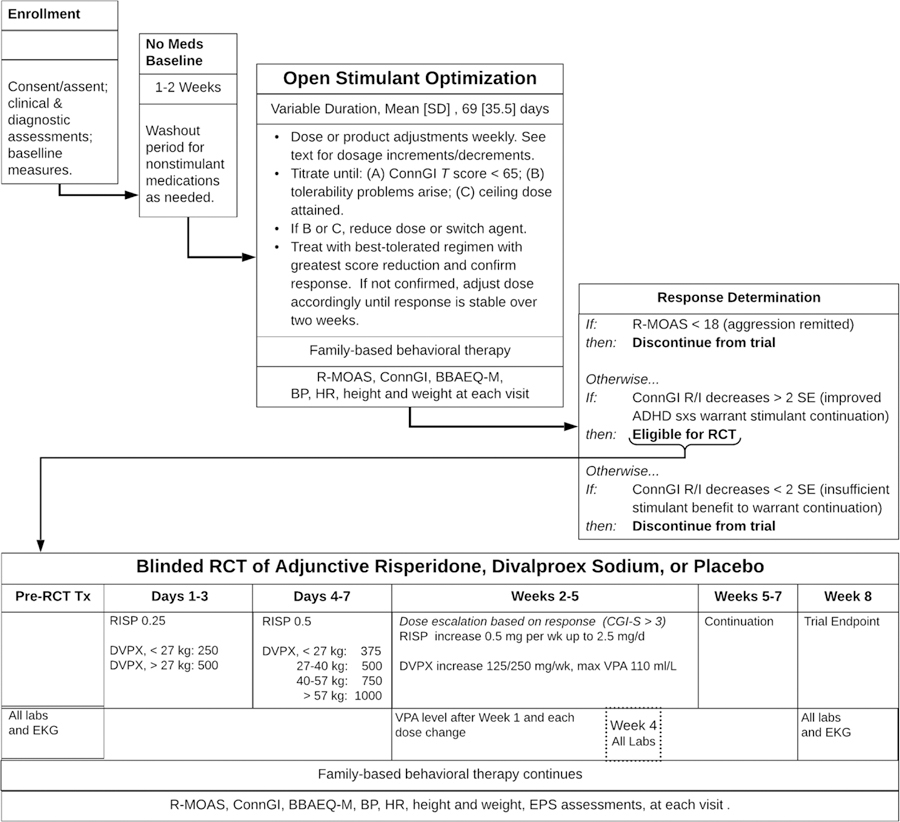
Figure 1 summarizes the trial’s design and procedures. The trial’s stepped-care approach began with open-label stimulant medication and behavioral therapy using a protocol to identify patients’ most effective, best-tolerated medication dose. Reassessment of aggressive behavior followed. Children whose aggressive behavior persisted were randomized to adjunctive treatment with either RISP, DVPX, or placebo (PBO), in a 2:2:1 ratio, for an eight-week double-blind controlled trial. Stimulant treatment remained constant and behavioral therapy continued. Children whose aggressive behavior remitted at the end of open stimulant optimization discontinued the trial.
Figure 1:
Schematic of Stepped-Treatment Trial Design and Procedures
Note: BBAEQ-M = Barkley Behavior and Adverse Effects Questionnaire - Modified; BP = blood pressure; ConnG R/I = Conners Global Improvement Index Restless-Inattentive subscale; DVPX = divalproex sodium; EKG = electrocardiogram. EPS = extrapyramidal symptoms; HR = heart rate; mg/d = milligrams per day; mg/wk = milligrams per week; R-MOAS = Retrospective - Modified Overt Aggression Scale; RCT = randomized controlled trial; RISP = risperidone; SD = standard deviation; SE = standard error; sxs = symptoms; VPA, valproic acid.
Patients
Trial participants were boys and girls between 6 and 12 years old. Diagnostic inclusion criteria were ADHD (any subtype) and either ODD or CD, as defined in DSM-IV-TR. Severity inclusion criteria required parental ratings on the Restless/Inattentive subscale of the Conners Global Index34 (ConnGI-P) and on the Aggressive Behavior subscale of the Child Behavior Checklist35 (CBCL) at least 1.5 standard deviations above the mean for the normative reference group corresponding to the child’s age and sex. Aggressive behavior severity was measured by the parent-completed Retrospective - Modified Overt Aggression Scale (R-MOAS). Enrollment required an R-MOAS total score over 24, representing clinically significant aggressive behavior during the preceding week, at both the initial telephone screening and the in-person evaluation. Study enrollment also required recent or current treatment with stimulant medication at a minimum daily total dose equivalent of 30 mg of immediate-release methylphenidate (MPH; e.g., 15 mg of mixed amphetamine salts or dexmethylphenidate, 40 mg of lisdexamfetamine) for at least 30 days.
Exclusion criteria included these disorders as defined in DSM-IV-TR: current or previous major depressive, bipolar I or II, Tourette’s, autistic, or any psychotic disorder. IQ below 70 was also exclusionary. A current anxiety disorder was disqualifying if aggressive behavior was chiefly a complication of it (e.g., child with separation anxiety who became aggressive only when separated from attachment figures). Medical exclusions included seizure disorders, pregnancy, and contraindications to treatment with stimulants, RISP, or DVPX.
Recruitment and Enrollment
Three outpatient child and adolescent psychiatry centers recruited study participants from patients currently receiving or seeking care, as well as print and radio advertising. Initial telephone screenings were followed by an evaluation visit when indicated. Parents or legal guardians provided written informed permission, and children >8 years of age gave written assent. Institutional Review Boards at each site approved the protocol and conducted annual reviews.
Assessments and Outcomes
Diagnostic assessment included completion of the Schedule of Affective Disorders and Schizophrenia for School-Age Children36 (K-SADS) with a parent and the child by a clinical child psychologist or a child and adolescent psychiatrist. A second clinician (child and adolescent psychiatrist or advanced-practice nurse practitioner) conducted a separate clinical diagnostic evaluation and obtained a medical history from the parent. The K-SADS interviewer and the clinical assessor conferred to arrive at consensus diagnoses and eligibility to begin the trial.
The trials’ primary outcome, aggressive behavior, was assessed using the R-MOAS. This instrument is adapted from the Overt Aggression Scale37 so that informants rate the frequency of the child’s aggressive behaviors using predefined intervals, rather than clinician estimates of the number of incidents the original version acquires. Items are grouped into four categories of aggression (verbal, physical, toward property, and toward oneself). Scores are weighted to accord larger values to more severe aggressive behaviors. Psychometric data based on a similar cohort are available from an earlier trial.33 This form and its scoring guide are in Supplement 1, available online.
Secondary outcomes were the two subscales of the CBCL (Aggressive Behavior and Rule-Breaking Behavior) that form its Externalizing broadband component, and the ConnGI. These instruments yield standardized (T) scores based on their normative samples’ score distribution.
Other measures served to indicate the baseline severity of the patient sample. These measures were the teacher version of the Conners Global Index,34 the Teacher Report Form,35 and the parent-completed Columbia Impairment Scale38 (CIS).
Safety and adverse effects were assessed using the Barkley Behavioral and Adverse Effect Questionnaires39 (BBAEQ), amended to include effects associated with RISP and DVPX. Spontaneously reported adverse effects were also recorded and tracked. Height, weight, blood pressure, and pulse were measured at each visit. Standardized body mass index (z-BMI) was based on U.S. growth charts.40 Motor abnormalities were assessed at visits during the randomized controlled trial (RCT) phase.41–43 Laboratory tests for patients randomized to adjunctive medications included complete blood cell counts, glucose drawn during a fasting state, serum prolactin, liver enzymes, and trough valproic acid (VPA) levels.
Study Treatments
Stimulant Treatment
On trial entry, non-stimulant pharmacotherapy was discontinued on a washout schedule appropriate to its elimination half-life; the study only enrolled children for whom current treatment was ineffective and the provider concurred that discontinuation was in the child’s interest. Open-label stimulant titration was used to identify the child’s most effective and best tolerated regimen. This protocol began with MPH in an extended-release tablet whose coating contains the initial dose and later release of two internal drug layers is controlled by a reverse-osmosis mechanism (MPH-OROS) . Dosage adjustments occurred during weekly office visits based upon ConnGI-P (and ConnGI-T when available), R-MOAS, and BBAEQ-M data. Titration usually occurred in 18 mg increments until either (a) ConnGI-P ratings were within one standard deviation of the mean for the child’s age and sex, (b) adverse effects contraindicated the dose, or (c) the ceiling dose of 72 mg/d was attained, though clinicians had the option to increase to 90 if indicated and tolerated. When tolerability issues hindered continuation of a clinically more effective dose, reductions by 9 mg could be employed. Because earlier research indicated no association between stimulant dose and weight,44 titration was driven by response and tolerability rather than a target mg/kg approach.
If tolerability problems attributable to long duration of action occurred (sleep, appetite, etc.), patients could be switched from MPH-OROS to a beaded biphasic methylphenidate preparation (MPH-BI), adjusted by 10 mg per week, with a ceiling dose of 60 mg/d. Participants who experienced insufficient or adverse response to MPH could switch to extended-release mixed amphetamine salts (MAS-XR), titrated in 5 mg increments, with a ceiling dose of 35 mg/d.
Review of response by two clinicians at each site to all regimens determined the child’s best dose, which was continued or reinstated for another week to verify its efficacy. A final assessment determined eligibility for trial continuation. (1) Participants whose aggressive behavior remitted (RMOAS < 15) concluded the trial. (2) Participants whose aggression persisted and who experienced benefit for ADHD symptoms to warrant stimulant continuation (based on ConnGI-P improvement by at least 2 standard errors and clinical judgment) were randomly assigned to one of the double-blinded adjunctive medication treatment groups.
Randomization and Treatment with Adjunctive RISP, DVPX, or PBO
Group allocation was stratified by site and sex, and random orders using blocks of n=5 within each stratum was generated by a statistician using SAS (SAS Institute, Cary, NC). Investigational pharmacies at each site followed the sequence and dispensed study medications accordingly. Unblinded safety monitors at each site were the only other individuals aware of group assignment.
A compounding pharmacy produced capsules containing RISP (0.25, 0.5, and 1 mg), DVPX (125 and 250 mg), and matching PBOs containing cellulose filler. Medications were dispensed using a “double-dummy” technique; each patient took two sets of capsules, but only one contained active drug corresponding to his/her randomized treatment, or, if randomized to PBO, all capsules contained filler only.
RISP dose started at 0.25 mg each evening for three days, with a morning dose of 0.25 MG added on the fourth day. Further dose adjustments were elective and based on response and tolerability, but dose increases could not exceed 0.5 mg each week. Maximum allowable RISP dose was 2.5 mg in divided doses, achieved by week 5.
DVPX target dosing was weight-based and aimed to achieve approximately 18 mg/kg by the end of the first week (e.g., oral dose for those weighing 18–27 kg was 375 mg daily while for the heaviest weight stratum, 57–68 kg, dose was 1000 mg). Trough valproic acid levels were sent to the site’s safety monitor who advised blinded clinicians if a dose increase was permissible or if decrease was required; those on placebo DVPX received sham instructions according to a randomized schedule to preserve blinding. When permitted by VPA level, dose increases by 125 or 250 mg occurred based on clinical response through Week 5. Ceiling VPA level was 110 ml/L. Due to DVPX’s teratogenicity, the protocol required that all female participants of child-bearing potential be serum bHCG-negative, be counseled about the risks of pregnancy and that a method of continuous contraception be used for those who were sexually active. However, no individuals of child-bearing potential were randomized.
Cross-site teleconferences occurred week to biweekly throughout the trial during which participants’ treatment was reviewed to insure concordance with the protocol.
Family-Based Behavioral Therapy
All families had behaviorally-oriented psychosocial treatment during the stimulant medication optimization phase and the randomized, controlled trial phase. Therapists were licensed social workers or advanced graduate students in clinical child psychology. Families were seen weekly during the same visit that they met with the pharmacotherapy treatment clinicians. Treatment was based on the Community Parent Education Program,45 a group program adapted for use with individual families. Core components are 1) goal setting; 2) increasing positive interactions with the child and rewarding cooperative behavior and composure; 3) judicious ignoring of low-level misbehaviors; 4) communicating directions and feedback for behavior; 5) a system to reward cooperation and improved frustration tolerance; and 7) handling uncooperative and dyscontrolled behavior. The first author’s weekly supervision of therapists emphasized adherence and cross-site consistency in the application of the program’s principles and procedures.
Data Analysis
The primary outcome, R-MOAS total score, was positively skewed (Kolmogorov-Smirnov D = 0.12, p < 0.01). Analyses therefore used its square-root transformation.
Models for outcomes included the fixed effects of treatment group, time and their interaction. Patients nested within study sites were random effects. Model estimation and statistical tests were computed with PROC GLIMMIX46 running on SAS® v9.4. Estimates for outcomes are the marginal, least-squares means and standard errors these models generated. Analyses included all subjects who provided at least one post-randomization outcome measurement, following intention-to treat principles. Confidence intervals for the standardized differences were computed using the method for small sample sizes described by Hedges and Olkin.47, p. 86 Similarly, effect sizes (ES) were determined using Hedge’s g¸ which is interpretively identical to Cohen’s d, but its computation includes a correction for small samples (n < 50).48, p. 27 Pooled standard deviations for ES were derived from the groups’ baseline scores.
RESULTS
Participants and Progression through Trial Stages
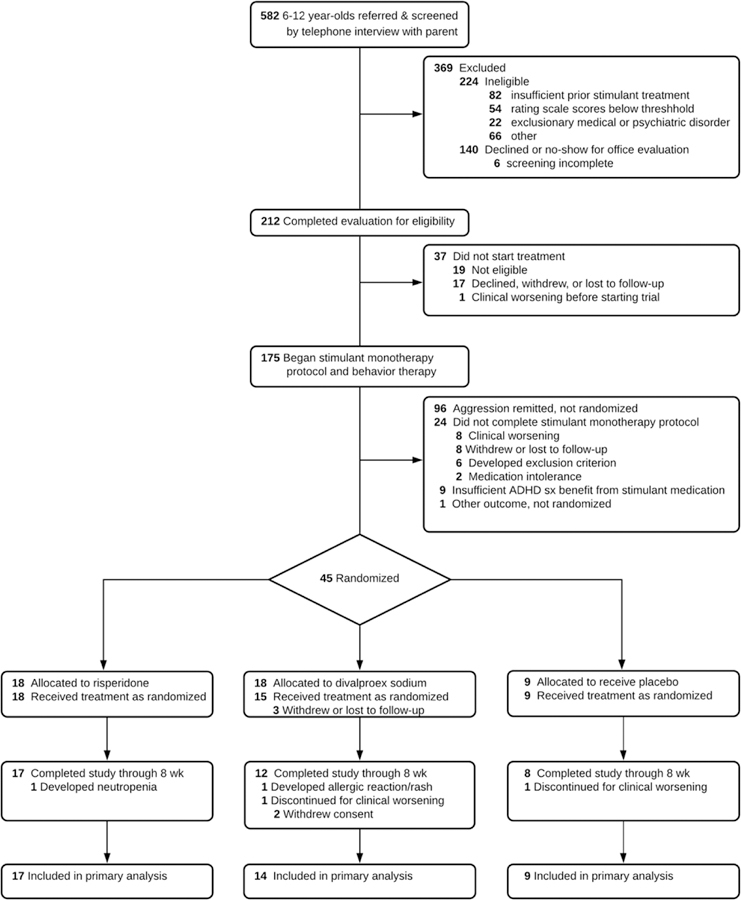
Figure 2 shows the enrollment and trial-completion status of all children screened for participation. Of the 175 who began study treatment, 24 (13.7%) did not complete the open stimulant optimization phase. Among those who did complete it, 96 (63.6%) fulfilled the criterion for remission of aggressive behavior, that is, three consecutive weeks with subthreshold R-MOAS ratings. Forty-five (29.6%) children were eligible for randomization to the adjunctive treatment conditions, and 42 began the blinded treatment to which they were allocated.
Figure 2:
CONSORT Flow Chart
Table 1 presents characteristics of the participants who started treatment in the trial. Ninety-two percent exceeded the CIS cutoff for impairment (for reference, 35–40% of youth receiving outpatient mental health care meet this criterion15). Almost all (94.3%) received special education services, and 31.4% were in specialized class settings. Twenty-four percent received prior antipsychotic treatment for behavioral disturbances, and 18.3% had at least one crisis that led to presentation at a hospital emergency department or admission to psychiatric inpatient treatment.
TABLE 1:
Characteristics of Patient Sample by Stimulant Optimization Trial Outcome Status
| Complete Sample (n = 175) | Stimulant Optimization Phase Aggression Remitters (n = 96) | Stimulant Optimization Non-Remitters Randomized to RCT (n = 45) | Stimulant Optimization Non-Completers or No Benefit (n = 34) | F or χ2 (p)a | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age in years, Mean (SD) | 9.47 (2.04) | 9.38 (2.11) | 9.63 (2.02) | 9.53 (1.93) | 0.26 (0.77) |
| Female / Male participants, No. (%) | 34 / 141 (19.4 / 80.6) | 20/76 (20.8 / 79.2) | 7/49 (15.6 / 84.4) | 7/27 (20.6 / 79.4) | 0.58 (0.76) |
| Race/Ethnicity, Number (%)b | |||||
| Black | 29 (16.57) | 19 (19.79) | 3 (6.67) | 7 (20.59) |  |
| Hispanic | 53 (30.29) | 33 (34.38) | 8 (17.78) | 12 (35.29) | |
| Other | 12 (6.86) | 5 (5.21) | 5 (11.11) | 2 (5.88) | |
| White | 81 (46.29) | 39 (40.63) | 29 (64.44) | 13 (38.24) | |
| Parents’ Marital Status, No. (%) | |||||
| Divorced/Separated | 38 (21.71) | 22 (22.92) | 7 (15.56) | 9 (26.47) | 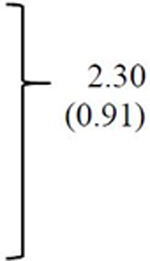 |
| Married | 96 (54.86) | 50 (52.08) | 28 (62.22) | 18 (52.94) | |
| Never married | 35 (20.00) | 20 (20.83) | 9 (20.00) | 6 (17.65) | |
| Other or Unknown | 6 (3.43) | 4 (4.17) | 1 (2.22) | 1 (2.94) | |
| Adults in home, No. (%) | |||||
| 1 | 45 (26.01) | 25 (26.04) | 10 (22.73) | 10 (30.30) |  |
| 2 | 98 (56.65) | 51 (53.13) | 28 (63.64) | 19 (57.58) | |
| 3 or more | 30 (17.34) | 20 (20.83) | 6 (13.64) | 4 (12.12) | |
| Clinical Characteristics | |||||
| Comorbid Disordersc (other than ADHD), No. (%) | |||||
| Oppositional defiant disorder | 148 (84.57) | 82 (85.42) | 39 (86.67) | 27 (79.41) | 0.90 (0.64) |
| Conduct disorder | 27 (15.43) | 14 (14.58) | 6 (13.33) | 7 (20.59) | 0.90 (0.64) |
| Anxiety disorder | 62 (35.43) | 40 (41.67) | 13 (28.89) | 9 (26.47) | 3.67 (0.18) |
| Depressive disorder | 36 (20.57) | 17 (17.71) | 10 (22.22) | 9 (26.47) | 1.28 (0.50) |
| Tic disorder | 6 (3.42) | 3 (3.13) | 1 (2.22) | 2 (5.88) | 0.84 (0.73) |
| Elimination disorder | 19 (10.86) | 9 (9.38) | 6 (13.33) | 4 (11.76) | 0.53 (0.76) |
| Learning disorder | 39 (22.29) | 25 (26.04) | 6 (13.33) | 8 (23.53) | 2.89 (0.21) |
| Clinical Severity, Mean (SD) | |||||
| Retrospective-Modified Overt Aggression Scale, total score | 62.79 (31.75) | 57.96 (29.79) | 67.71 (33.24) | 69.94 (33.69) | 2.56 (0.08) |
| Conners Global Index Restless/Inattentive, T score | 84.79 (7.63) | 85.15 (7.34) | 83 (9.28) | 86.15 (5.60) | 1.90 (0.15) |
| Conners Global Index Emotional Lability, T score | 82.4 (9.16) | 82.04 (9.14) | 81.60 (9.64) | 84.47 (8.50) | 1.12 (0.33) |
| Child Behavior Checklist, Total Problems, T score | 71.82 (5.63) | 71.87 (4.78) | 71.47 (6.69) | 72.15 (6.40) | 0.15 (0.86) |
| Child Behavior Checklist, Internalizing Problems, T score | 61.39 (8.26) | 60.62 (8.09) | 61.69 (8.93) | 63.15 (7.75) | 1.21 (0.30) |
| Child Behavior Checklist, Aggressive Behavior, T score | 76.95 (8.99) | 75.96 (8.56) | 78.18 (8.35) | 78.12 (10.77) | 1.29 (0.28) |
| Child Behavior Checklist, Rule Breaking Behavior, T | 72.37 (6.44) | 72.29 (6.61) | 71.87 (5.43) | 73.24 (7.26) | 0.45 (0.64) |
| Teacher Conners Global Index, Restless/Inattentive, T score | 75.63 (13.88) | 75.28 (14.17) | 73.96 (15) | 78.20 (12.03) | 0.62 (0.54) |
| Teacher Conners Global Index, Emotional Lability, T | 69.59 (17.36) | 68.37 (17.62) | 67.54 (18.55) | 74.88 (14.89) | 1.51 (0.23) |
| Columbia Impairment Scale, Total Impairment Score | 29.89 (9.35) | 29.43 (8.98) | 30.09 (10.55) | 30.94 (8.90) | 0.34 (0.71) |
| Columbia Impairment Scale, Severe Impairment (total score >=16), No. (%) | 161 (92.53) | 87 (90.63) | 41 (93.18) | 33 (97.06) | 1.54 (0.59) |
| Teacher Report Form, Total Problems, T score | 66.08 (9.91) | 66.49 (10.28) | 61.88 (10.53) | 69.04 (6.53) | 3.43 (0.04) |
| Teacher Report Form, Internalizing Problems, T score | 58.71 (9.39) | 58.85 (9.69) | 56.50 (10.94) | 60.50 (6.19) | 1.11 (0.33) |
| Teacher Report Form, Aggressive Behavior, T | 67.73 (12.11) | 68.64 (12.95) | 63.29 (11.46) | 69.42 (9.14) | 2.08 (0.13) |
| Teacher Report Form, Rule Breaking Behavior, T | 62.49 (8.05) | 62.93 (8.28) | 58.38 (6.47) | 65.29 (7.45) | 5.01 (0.01) |
| Prior Treatments & Services, No. (%) | |||||
| Special in-class school services | 110 (62.86) | 60 (62.50) | 29 (64.44) | 21 (61.76) | 0.07 (0.95) |
| Specialized class or school setting | 55 (31.43) | 32 (33.33) | 14 (31.11) | 9 (26.47) | 0.55 (0.78) |
| Prior antipsychotic treatment | 42 (24.00) | 16 (16.67) | 15 (33.33) | 11 (32.35) | 6.28 (0.04) |
| Prior mood stabilizer treatment | 6 (3.43) | 2 (2.08) | 2 (4.44) | 2 (5.88) | 1.28 (0.47) |
| Prior alpha-2-agonist treatment | 33 (18.86) | 18 (18.75) | 8 (17.78) | 7 (20.59) | 0.10 (0.93) |
| Prior selective serotonin reuptake inhibitor treatment | 17 (9.71) | 8 (8.33) | 7 (15.56) | 2 (5.88) | 2.53 (0.33) |
| Prior psychiatric emergency department visit or hospitalization | 32 (18.29) | 17 (17.71) | 9 (20) | 6 (17.65) | 0.12 (0.96) |
| Prior psychosocial treatment | 109 (62.29) | 58 (60.42) | 35 (77.78) | 16 (47.06) | 8.10 (0.02) |
Note:
Tests of frequencies are based on likelihood-ratio Χ2.
For consistency with text’s description of this result, values in parentheses for stimulant optimization outcome columns are percentages of each row. The Complete Sample column shows percentages of each race/ethic group in the sample.
Because the trial began before criteria for Disruptive Mood Dysregulation Disorder were established for DSM-5, it was not evaluated with the KSADS.
Table 1 also shows three background characteristics significantly associated with the likelihood that aggressive behavior would remit during the stimulant optimization phase. The significant overall race/ethnicity effect shown in the last column reflects the higher proportion of black children (65.52%) than white children (46.91%) who completed the open stimulant optimization phase and did not enter the randomized controlled trial because their aggressive behavior remitted (Χ2 [1] = 9.62, p < .002, for this comparison).
The remitter group had a smaller proportion of children with prior antipsychotic treatment (16.13%) than did the stimulant-refractory group (34.15%). However, children with prior antipsychotic treatment who completed the stimulant optimization phase had equal rates of remission and nonremission (15/29 and 14/29). Stimulant optimization phase non-remitters were also more likely to have had prior psychosocial treatment than remitters (78% vs. 60%).
Study Medication Doses and Psychosocial Treatment Attendance
At the end of their open stimulant optimization treatment, 104 children received MPH-OROS (69% of the aggression remitters, 55% of non-remitters in the RCT, and 29% of non-completers); 17 received MPH-BI (8% of remitters, 13% of non-remitters in RCT, 16% of non-completers), and 42 received MAS-XR (23% of remitters, 33% of non-remitters in RCT, and 25% of non-completers), with Χ2 [4] = 3.62, p =.46). The mean (sd) daily dose, in immediate-release methylphenidate equivalence units, was 41.6 (16.12) mg. Dose differences between stimulant optimization phase outcome groups were not significant (F [2, 160] = 1.68, p = .19). Remitters’ mean stimulant MPH dose equivalent was 42.03 (14.6), non-remitters RCT participants’ mean was 44.25 (16), and non-completers’ mean was 37.38 (19.9).
The duration of the stimulant optimization protocol varied because of the individualized nature of the dose optimization approach. Aggression remitters had a shorter stimulant optimization phase compared to those with persistent aggression who entered the RCT (mean [sd], 75.4 [32] days vs. 66.5 [36.4]), but this difference was not statistically significant, F (1,131) = 1.78, p =.18).
Nonremitters who entered the RCT phase had more psychosocial treatment sessions during the stimulant optimization phase than those whose aggression remitted by its end (mean [sd], 4.54 [2.5] vs. 3.48 [2.27]; F [1,123] =5.36, p = .023).
In the RISP group, the mean dose (sd) of active RISP was 1.15 (0.81) mg/d (range, .05 to 3.0 mg/d). In the DVPX group, the mean dose of active drug was 713 (327) mg/d (range, 250 – 1750 mg/d and the mean (sd) serum valproic acid level was 77.75 (25.76) ml/L. The mean number (sd) of total behavioral therapy sessions for children randomized to adjunctive treatment was 8.22 (4.5)
Primary Outcome, Parent Ratings of Aggressive Behavior
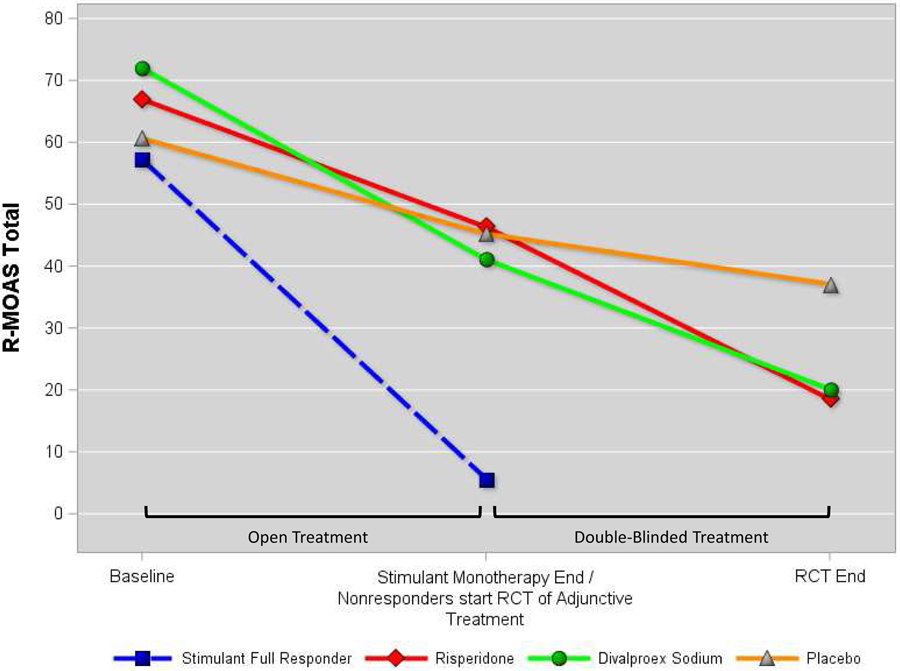
Figure 3 presents each study treatment group’s least-squares mean R-MOAS scores obtained at Baseline, Stimulant Optimization Endpoint, and, for children randomized to adjunctive treatments, their group’s controlled trial endpoint. The figure also shows the Baseline and Stimulant optimization endpoint values for children who experienced remission of aggressive behavior with after open stimulant optimization to indicate that the magnitude of improvement for randomized patients remained substantially less even after adjunctive treatments.
Figure 3:
Treatment Groups’ Retrospective-Modified Overt Aggression Scale Scores
Table 2 contains outcomes and statistical analysis results, also with stimulant optimization phase responders included for comparison though excluded from statistical tests. The treatment group-by-time interaction for RMOAS scores was significant (F [2, 105] = 2.77, p = .03). Pairwise comparisons of the group’s least-squares means at the RCT endpoint indicate larger reductions for both active treatments relative to placebo. However, the difference from placebo was larger for the risperidone group (−2.328; t[105] = −3.07, p < .003) than for the DVPX group (−1.6; t[105] = −2.02, p < .046), the latter excluding zero from its confidence interval only marginally.
TABLE 2.
Trial Outcomes
| Group Means Across Assessments Least-Squares Means (SD)a |
Fixed Effects F (df=105) P |
Between-Group Contrastsb Differences in Least-Squares Means [95% CI] Effect Sizec Significance test |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulant respondersd | Risperidone adjunctive to stimulant | Divalproex sodium adjunctive to stimulant | Placebo adjunctive to stimulant | Tx | Time | Tx by Time | RISP-PBO | DVPX-PBO | DVPX-RISP | ||||||||
| Baseline | Stim optim end | Baseline | Stim optim end | RCT end | Baseline | Stim optim end | RCT end | Baseline | Stim optim end | RCT end | |||||||
| R-MOAS, square-root | 7.42 (1.93) |
1.91 (1.94) |
7.91 (1.62) |
6.57 (1.61) |
3.33 (1.90) |
8.16 (1.58) |
6.12 (1.63) |
3.95 (1.91) |
7.56 (1.90) |
6.43 (1.90) |
5.41 (1.95) |
0.49 .62 |
58.34 <.001 |
2.77 .03 |
−2.33 [−3.83, −0.82] |
−1.60 [−3.18, −0.03] |
0.72 [−0.59, 2.04] |
| −1.32 | −0.91 | 0.45 | |||||||||||||||
|
t = −3.07, p = .003 |
t = −2.02, p = .046 |
t = 1.09, p = .277 |
|||||||||||||||
| CBCL Aggressive Behavior, T | 75.81 (8.96) |
61.16 (9.11) |
77.25 (6.89) |
72.62 (6.85) |
61.75 (8.19) |
78.67 (6.59) |
72.21 (6.86) |
63.88 (9.09) |
77.83 (7.64) |
73.03 (7.64) |
71.36 (8.17) |
1.30 .28 |
42.38 <.001 |
2.72 .03 |
−9.11 [−14.86, −36] |
−7.48 [−13.93, −1.03] |
1.63 [−3.90, 7.16] |
| −1.25 | −1.03 | 0.24 | |||||||||||||||
|
t = −3.15, p = .002 |
t = −2.30, p = .023 |
t = 0.58, p = .560 |
|||||||||||||||
| CBCL Rulebreaking Behavior, T | 72.42 (7.34) |
63.64 (7.47) |
71.83 (7.34) |
69.02 (7.47) |
60.07 (4.11) |
72.51 (4.09) |
68.68 (4.92) |
63.96 (3.94) |
71.85 (4.11) |
69.85 (5.48) |
68.07 (4.63) |
2.74 (4.63) |
48.83 (4.95) |
5.04 .07 |
−7.58 [−11.08, −4.09] |
−3.87 [−7.79, 0.06] |
3.72 [0.35, 7.08] |
| <.001 | <.001 | −1.73 | |||||||||||||||
|
t = −4.31, p = .000 |
t = −1.96, p = .053 |
t = 2.19, p = .031 |
|||||||||||||||
| CBCL Internalizing Behavior, T | 60.45 (9.06) |
51.05 (9.21) |
61.82 (7.07) |
57.53 (7.04) |
49.60 (8.36) |
61.90 (6.75) |
59.18 (7.01) |
51.21 (9.20) |
61.68 (7.78) |
59.38 (7.78) |
57.57 (8.30) |
1.07 0.35 |
22.91 <.001) |
1.78 0.14 |
−7.11 [−12.83, −1.39] |
−6.37 [−12.80, 0.05] |
0.74 [−4.77, 6.25] |
| −0.95 | −0.85 | 0.106 | |||||||||||||||
|
t = −2.47, p = .015 |
t = −1.97, p = .052 |
t = 0.27, p = .791 |
|||||||||||||||
| Conn-GI, Restless & Inattentive, T | 85.25 (14.09) |
58.43 (14.15) |
82.01 (8.32) |
68.84 (8.26) |
60.25 (9.70) |
83.66 (8.06) |
65.54 (8.32) |
64.27 (9.71) |
84.21 (9.67) |
70.61 (9.67) |
68.97 (9.89) |
1.17 .31 |
75.33 <.001 |
1.63 .17 |
−6.73 [−14.19, 0.73] |
−1.89 [−9.71, 5.93] |
4.84 [−1.68, 1.37] |
| −0.75 | −0.21 | 0.59 | |||||||||||||||
|
t = −1.79, p = .077 |
t = −0.48, p = .633 |
t = 1.47, p = .144 |
|||||||||||||||
| Conn-GI, Emotional Lability, T | 81.9 (11.92) |
55.37 (12.00) |
80.86 (9.21) |
71.87 (9.10) |
55.23 (11.01) |
81.80 (9.05) |
70.55 (9.46) |
64.28 (11.26) |
79.36 (10.94) |
75.76 (10.94) |
68.34 (11.35) |
1.87 .16 |
33.05 <.001 |
2.34 .06 |
−11.91 [−21.80, −2.01] |
−2.68 [−13.02, 7.67] |
9.23 [0.69,17.78] |
| −1.18 | −0.26 | 1.01 | |||||||||||||||
|
t = −2.39, p = .019 |
t = −0.51, p = .609 |
t = 2.14, p = .034 |
|||||||||||||||
| Child Depression Rating Scale | 34.53 (21.47) |
25.98 (21.49) |
36.62 (14.16) |
32.14 (14.14) |
26.18 (14.75) |
37.61 (13.06) |
33.74 (13.16) |
29.58 (14.29) |
37.52 (12.08) |
33.86 (11.82) |
35.12 (12.16) |
1.89 0.16 |
12.82 <.001 |
1.63 0.17 |
−7.62 [−13.58, −1.67] |
−5.51 [−12.06, 1.03] |
2.11 [−3.34, 7.56] |
| −0.58 | −0.42 | 0.15 | |||||||||||||||
|
t = −2.48, p = .015 |
t = −1.46, p = .148 |
t = 0.90, p = .369 |
|||||||||||||||
| Columbia Impairment Scale | 29.29 (10.23) |
16.24 (10.48) |
31.08 (8.06) |
24.48 (8.02) |
20.16 (9.85) |
30.93 (7.87) |
26.05 (7.94) |
23.62 (11.17) |
29.96 (9.52) |
28.09 (9.42) |
25.37 (9.80) |
0.64 0.53 |
11.50 <.001 |
0.79 0.54 |
−3.97 [−10.96, 3.03] |
−1.22 [−9.14, 6.70] |
2.75 [−4.09, 9.58] |
| (−0.4) | (−0.13) | (0.336) | |||||||||||||||
|
t = −1.13, p = .263 |
t = −0.31, p = .760 |
t = 0.80, p = .427 |
|||||||||||||||
| Body Mass Index, z-score | 0.47 (1.23) |
0.18 (1.23) |
0.05 (0.96) |
−0.18 (0.96) |
0.43 (1.00) |
0.14 (0.88) |
−0.17 (0.89) |
−0.17 (0.93) |
0.18 (0.84) |
−0.18 (0.82) |
−0.24 (0.84) |
1.57 0.21 |
8.30 <.001 |
6.44 <.001 |
0.74 [0.39, 1.09] |
0.09 [−0.28, 0.46] |
−0.65 [−0.96, −0.34] |
| 0.815 | 0.097 | −0.70 | |||||||||||||||
|
t = 4.16, p = .000 |
t = 0.47, p = .637 |
t = −4.18, p = .000 |
|||||||||||||||
| Weight, kg | 34.32 (11.91) |
34.08 (11.91) |
34.68 (8.73) |
35.07 (8.71) |
37.30 (8.83) |
35.13 (7.94) |
35.31 (7.96) |
35.96 (8.10) |
36.05 (6.91) |
34.48 (6.81) |
34.50 (6.90) |
0.24 0.79 |
2.47 0.09 |
3.28 0.01 |
3.18 [1.19, 5.17] |
1.50 [−0.61, 3.62] |
−1.68 [−3.38, 0.02] |
| 0.404 | 0.190 | −0.20 | |||||||||||||||
|
t = 3.17, p = .002 |
t = 1.41, p = .161 |
t = −1.96, p = .053 |
|||||||||||||||
NOTE: CBCL = Child Behavior Checklist; CI = confidence interval; ConnGI = Conners Global Index – Parent Version; DVPX = divalproex sodium; PBO = placebo; R-MOAS = Retrospective – Modified Overt Aggression Scale; RCT = randomized controlled trial; RISP = risperidone; Stim optim end = stimulant optimization phase endpoint; Tx = treatment.
Least-squares means and SDs across assessment times are adjusted for random effect of site.
Contrasts are the differences in least-squares means in RCT endpoints between groups, adjusted for site, baseline and stimulant optimization endpoint assessments.
Effect sizes are Hedge’s g, which adjusts Cohen’s d for small sample sizes and is interpretively identical to it.
Data for those whose aggressive behavior remitted during stimulant optimization are Presented for reference; they were not included in the mixed effects analyses shown.
Secondary Outcomes
CBCL scales showed a similar pattern. Significant group-by-time interactions reflected greater average reductions for the risperidone group compared to the placebo group for Aggressive Behavior and Rulebreaking Behavior scores. DVPX differed reliably from placebo on the Aggressive Behavior scale.
The aggression-remission criterion (R-MOAS < 15) was met by 69% of the RISP group, 40% of the DVPX group, and 37% of the PBO group (Fisher’s exact test 49 p = .023).
The overall group-by-time interactions for the Conn-GI Emotional Lability and CDRS depression ratings were not significant. As shown in Table 2, however, between-group contrasts showed significant superiority for risperidone over placebo.
Table 2 shows that Columbia Impairment Scale ratings had a significant effect for time, reflecting improved mean scores overall, with no difference between treatment groups.
Tolerability
Standardized BMI scores increased significantly for the RISP group relative to the PBO and DVPX groups (Table 2). Weight gain in the RISP group averaged 2.23 kg, while those receiving DVPX and PBO averaged 0.65 kg and 0.02 kg, respectively.
There were no serious adverse events during this study. Table S1, available online, shows the rates of adverse events. During the stimulant optimization phase, there was one investigator-initiated discontinuation due to medication intolerance. During the controlled trial of adjunctive treatments, two children experienced adverse events that led to early discontinuation. One RISP-treated patient developed neutropenia and one DVPX-treated patient developed widespread skin rash; both resolved upon drug discontinuation and treatment by a separate, safety monitor physician. Two other patients, one each in the DVPX and PBO groups, exited the trial early because of symptom worsening. Study medications were judged unlikely to have caused their behavioral deterioration, but their clinical condition precluded continuing blinded treatment.
DISCUSSION
Among patients randomized to an adjunctive treatment because of inadequate response to open stimulant optimization, RISP showed an appreciable advantage over placebo on the primary outcome of aggressive behavior, the two CBCL externalizing behavior subscales, and on rates of remission of aggressive behavior. DVPX’s effect size over PBO was smaller but also statistically significant for reduced aggression and disruptive behavioral symptoms more broadly. Weight and z-BMI changes in the RISP group were significantly greater compared with PBO. The high rate of remitted aggressive behavior during the stimulant optimization phase reduced the RCT’s sample size, decreasing the trial’s power for the comparison of RISP and DVPX and rendering it inconclusive in comparing their efficacy and non-BMI safety profiles. After stimulant medication optimization, aggression remitted for 55% of enrollees and 63.6% of completers. This is noteworthy because participants were selected for their prior non-response to stimulant medication and elevated indices of severity (prior psychiatric emergencies, special education services, multi-medication treatment, etc.) were prevalent. By requiring prior stimulant treatment at moderate doses and high baseline severity, we aimed to increase the likelihood that enrollees would represent patients who need adjunctive medications because of inadequate response to first-line treatment. Nevertheless, a methodical approach to establishing these participants’ most beneficial stimulant regimen frequently culminated in behavioral improvements that made additional medications unnecessary. It is therefore important for future trials to plan for diminished sample sizes that may result when rigorous first-line treatment is followed by high rates of response that obviates the need for adjunctive medication. Although difficult and costly to conduct, stepped-care trials are necessary to determine the value of medication polytherapy, which is clinically pervasive but has very limited evidentiary support for children and adolescents.
We found that black children were more likely than white children to experience remission of aggressive behavior during the stimulant optimization. Bearing in mind studies reporting that black children less often receive appropriate evaluation and treatment for ADHD,50–53 it seems possible that regular contact during our trial’s lead-in treatment might have rectified deficiencies in prior care to which white children were less exposed. Other data suggest racial differences in parent ratings on common structured assessment tools, leading to inflated scores for black youth, which might yield baseline assessments that overestimate severity.54 These and other factors associating race and ethnicity with treatment outcomes indicate the importance of performing studies adequately powered to examine them.
Because the trial’s stimulant optimization phase provided open treatment, there is a risk that attributing its high aggression remission rate to its specific approach may be inaccurate. However, placebo and nonspecific effects stem largely from patients’ expectancies of a new medication or setting.55, 56 This study involved medications that children took at enrollment or earlier and most families had previous behavioral therapy in the same location as the trial sites. It is thus unlikely that this study’s open stimulant optimization phase embodied these elements of novelty that would have fostered expectancies favoring nonspecific effects. Moreover, placebo effects are generally small in childhood ADHD,57 and negligible among children who have had prior stimulant treatment58, 59
Another trial reported remitted aggression among just 4.5% of a similar patient population who completed a 3-week stimulant lead-in,24 suggesting that our more extensive titration and psychosocial treatment phase contributed to a more favorable outcome with first-line treatment. Because non-remitters attended more psychosocial therapy sessions than remitters, there is no evidence that underuse of that modality diminished behavioral response. Our study also based stimulant dose titration on weekly parent ratings of ADHD symptoms and adverse effects, allowed a wider range of doses, switched stimulant products for insufficient improvement of ADHD symptoms as well as for tolerability problems, and determined response based on three consecutive weekly assessments on a given regimen. Nevertheless, this trial was not designed to parse the contributions of the lead-in phase’s components. Further study is required to determine more conclusively the specific impact of well-monitored, measurement-based delivery of first-line treatments and other aspects of trial involvement.
Other study limitations include its short time frame for what are typically chronic problems that require extended treatment; we did not conduct systematic follow-up assessments of participants who discontinued because of satisfactory response during the initial stimulant optimization and behavior treatment phase. The trial’s outcomes were confined mainly to parent-reported behavior. Impairments outside the home setting are nevertheless significant for children with ADHD and conduct problems. Because we limited the patient cohort to children with ADHD and aggressive behavior our findings may not generalize to those who have significant conduct problems but do not have ADHD. Although we observed RISP’s propensity to raise BMI, our sample did not enable us to estimate the less common incidence of neuromotor adverse effects. We did not require that female participants be prepubertal, but just about all were. We recognize that DVPX’s teratogenicity disfavors its use among those who might become pregnant, and its potential association with hyperandrogenism and anovulation is also a deterrent. These factors restrict the generalizability of our findings for the treatment of postpubertal female individuals.
Our findings confirm that RISP and DVPX may benefit patients with aggression refractory to first-line stimulant and behavioral treatments. However, Figure 3 shows that those receiving these adjunctive treatments remained more symptomatic than those who remitted with stimulant alone. It is unclear if insufficient response of aggression to stimulant medication denotes a distinct pathophysiology, a more pernicious course, or greater overall disorder severity. We did not find, however, that baseline indices of severity distinguished those experiencing robust stimulant response from others. It is possible that stimulant refractoriness marks a mechanism of disorder that eludes stimulants’ effect of enhanced synaptic availability of dopamine and norepinephrine. It is tempting, if simplistic, to suspect that these youngsters have greater mood-related (i.e., “bottom-up”) psychopathology, while strong stimulant responders have disturbances more restricted to impulse dyscontrol (i.e., “top-down” regulatory deficits). Disruptive mood dysregulation disorder (DMDD) is a clinical phenotype thought to involve a primary abnormality of mood that features severe behavioral outbursts alongside persistent and pervasive irritable or angry mood. However, our group reported earlier that children with these characteristics, as well as comorbid ADHD, were just as likely to benefit from stimulant medication as those without persistently negative mood. 60 Antidepressant controlled trials for DMDD are limited. One inpatient stepped-care trial in which either adjunctive citalopram or placebo was added to methylphenidate showed an advantage for citalopram on Clinical Global Impressions (CGI) Improvement ratings and modestly larger change in CGI Severity scores.61
Our results reinforce the clinical value of optimizing first-line stimulant pharmacotherapy for impaired youngsters with ADHD through measurement-based titration and family-based behavioral therapies, practices that remain uncommon in routine care.62, 63 Given the adverse effect liabilities of adjunctive medications, it is important to improve ADHD care so that clinicians initiate antipsychotic and other co-therapies as sparingly as possible. For those who do not experience sufficient benefit from thoroughly-implemented initial treatments, this study adds to the emerging evidence base that such adjunctive medications may be helpful.
Supplementary Material
Acknowledgments
This work was supported by grants R01MH080050 from the National Institute of Mental Health (NIMH; Blader), RR1059482 (Stony Brook General Clinical Research Center), and RR018535 (Feinstein Institute for Medical Research General Clinical Research Center) from the National Center for Research Resources.
The authors gratefully acknowledge the contributions of study coordinators, therapists, pharmacists, and Data and Safety Monitoring Board members. The authors are deeply thankful to the participating children and their families who made this work possible.
Dr. Blader has received research support from Texas Health and Human Services and Abbott Laboratories (now AbbVie) and consultant’s honoraria from Arbor Pharmaceuticals and Supernus Pharmaceuticals. He is affiliated with Northwell Health (unsalaried). Dr. Pliszka has received research support from Texas Health and Human Services, Ironshore Pharmaceuticals, and Shire (a Takeda company) and has anticipated funding from Otsuka Pharmaceuticals; consultant’s honoraria from Supernus Pharmaceuticals, Sunovion Pharmaceuticals, and Cingulate Therapeutics; and provided compensated expert testimony on behalf of AstraZeneca and NLS Pharma. Dr. Kafantaris has received investigational drug and placebo supplies for investigator-initiated research from Janssen Pharmaceuticals. Dr. Carlson has a family member on trial review boards for Pfizer and Lundbeck A/S.
Dr. Blader and Stephen Finch, PhD (planning), served as the statistical experts for this research.
Statement of Authors’ Financial Disclosures
Authors financial disclosures for the preceding two years and foreseeable future are as follows.
Joseph C. Blader, PhD
Employed by the University of Texas. Received funding from Texas Health and Human Services. Received consultant honorarium from Arbor Pharmaceuticals. Affiliated with (unsalaried) Northwell Health.
Steven R. Pliszka, MD
Employed by the University of Texas. Received funding from Texas Health and Human Services and Ironshore Pharmaceuticals. Anticipated funding from Otsuka Pharmaceuticals.
Vivian Kafantaris, MD
Employed by Northwell Health
Carmel A. Foley, MD
Employed by Northwell Health.
Gabrielle A. Carlson, MD
Employed by the State University of New York.
Judith A. Crowell, MD
Employed by the State University of New York. Affiliated with Boston Children’s Hospital.
Brigitte Y. Bailey, MD
Employed by the University of Texas.
Colin Sauder, PhD
Employed by Adams Clinical
W. Burleson Daviss, MD
Employed by the Geisel School of Medicine at Dartmouth.
Christa Sinha, MSN
Employed by the State University of New York.
Thomas L. Matthews, MD
Employed by the University of Texas.
David M. Margulies, MD
Employed by the State University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in a symposium at the American Academy of Child and Adolescent Psychiatry 65th Annual Meeting; October 22–27, 2018; Seattle, Washington.
Disclosure: Drs. Foley, Crowell, Bailey, Sauder, Daviss, Matthews, and Margulies and Mrs. Sinha have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Joseph C. Blader, University of Texas Health Science Center, San Antonio..
Steven R. Pliszka, University of Texas Health Science Center, San Antonio..
Vivian Kafantaris, Zucker Hillside Hospital and the Feinstein Institute for Medical Research, Divisions of Northwell Health, Manhasset, NY..
Carmel A. Foley, Cohen Children’s Medical Center of New York and Zucker Hillside Hospital, Divisions of Northwell Health, Manhasset, NY..
Gabrielle A. Carlson, Renaissance School of Medicine, Stony Brook University, NY..
Judith A. Crowell, Renaissance School of Medicine, Stony Brook University, NY..
Brigitte Y. Bailey, University of Texas Health Science Center, San Antonio..
Colin Sauder, Adams Clinical, Watertown, MA..
W. Burleson Daviss, Geisel School of Medicine at Dartmouth, Lebanon, NH..
Christa Sinha, Renaissance School of Medicine, Stony Brook University, NY..
Thomas L. Matthews, University of Texas Health Science Center, San Antonio..
David M. Margulies, Renaissance School of Medicine, Stony Brook University, NY..
REFERENCES
- 1.Costello EJ, Egger H, Angold A. 10-Year Research Update Review: The epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry 2005;44(10):972–986. [DOI] [PubMed] [Google Scholar]
- 2.Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatr 2011;127(3):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in U.S. adolescents: Results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2011;50(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamshere ML, Langley K, Martin J, Agha SS, Stergiakouli E, Anney RJ, et al. High loading of polygenic risk for ADHD in children with comorbid aggression. Am J Psychiatry 2013;170(8):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blader JC, Connor DF. Aggression in children: An integrative approach In: Martin A, Volkmar FR, Bloch MH, eds. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook. 5th ed. Philadelphia: Wolters Kluwer; 2017:399–410. Available at [Google Scholar]
- 6.Graziano PA, Garcia A. Attention-deficit hyperactivity disorder and children’s emotion dysregulation: A meta-analysis. Clin Psychol Rev 2016;46:106–123. [DOI] [PubMed] [Google Scholar]
- 7.Côté SM, Vaillancourt T, LeBlanc JC, Nagin DS, Tremblay RE. The development of physical aggression from toddlerhood to pre-adolescence: A nation wide longitudinal study of Canadian children. J Abnorm Child Psychol 2006;34(1):71–85. [DOI] [PubMed] [Google Scholar]
- 8.Pliszka SR, Crismon ML, Hughes CW, Conners CK, Emslie GJ, Jensen PS, et al. The Texas Children’s Medication Algorithm Project: Revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry 2006;45(6):642–657. [DOI] [PubMed] [Google Scholar]
- 9.Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS, on behalf of the T-MAY Steering Group. Treatment of Maladaptive Aggression in Youth: CERT guidelines I. Engagement, assessment, and management. Pediatr 2012;129(6):e1562–1576. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum ML, Saito E, Gerhard T, Winterstein A, Olfson M, Kane JM, et al. Pharmacoepidemiology of antipsychotic use in youth with ADHD: Trends and clinical implications. Curr Psychiatry Rep 2013;15(8):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiatry 2010;49(10):1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelsohn GA, Karpov I, Parthasarathy M, Hutchison SL, Castelnovo K, Ghuman J, et al. Trends in antipsychotic prescribing in Medicaid-eligible youth. J Am Acad Child Adolesc Psychiatry 2017;56(1):59–66. [DOI] [PubMed] [Google Scholar]
- 13.Goddard AW, Schwartz K, Hendrix K, Aalsma MC, Slaven J, Hancock EF, et al. Trends in use and cost of second-generation antipsychotics among children and teens in Indiana Medicaid, 2004–2012. Psychiatr Serv 2016;67(9):1030–1034. [DOI] [PubMed] [Google Scholar]
- 14.Hoagwood KE, Kelleher K, Zima BT, Perrin JM, Bilder S, Crystal S. Ten-year trends in treatment services for children with attention deficit hyperactivity disorder enrolled in Medicaid. Health Aff 2016;35(7):1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olfson M, Druss BG, Marcus SC. Trends in mental health care among children and adolescents. N Engl J Med 2015;372(21):2029–2038. [DOI] [PubMed] [Google Scholar]
- 16.Sikirica V, Pliszka SR, Betts KA, Hodgkins P, Samuelson T, Xie J, et al. Comparative treatment patterns, resource utilization, and costs in stimulant-treated children with ADHD who require subsequent pharmacotherapy with atypical antipsychotics versus non-antipsychotics. J Manag Care Pharm 2012;18(9):676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappadopulos E, Macintyre JC II, Crismon ML, Findling RL, Malone RP, Derivan A, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145–161. [DOI] [PubMed] [Google Scholar]
- 18.Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007;46(7):894–921. [DOI] [PubMed] [Google Scholar]
- 19.Scotto Rosato N, Correll CU, Pappadopulos E, Chait A, Crystal S, Jensen PS, et al. Treatment of maladaptive aggression in youth: CERT Guidelines II. Treatments and ongoing management. Pediatr 2012;129(6):e1577–e1586. [DOI] [PubMed] [Google Scholar]
- 20.Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev 2012;9:CD008559. [DOI] [PubMed] [Google Scholar]
- 21.Crystal S, Mackie T, Fenton MC, Amin S, Neese-Todd S, Olfson M, et al. Rapid growth of antipsychotic prescriptions for children who are publicly insured has ceased, but concerns remain. Health Aff 2016;35(6):974–982. [DOI] [PubMed] [Google Scholar]
- 22.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 2015;72(9):867–874. [DOI] [PubMed] [Google Scholar]
- 23.Medicaid Medical Directors Learning Network, Rutgers Center for Education and Research on Mental Health Therapeutics. Antipsychotic Medication Use in Medicaid Children and Adolescents: Report and Resource Guide from a 16-State Study. New Brunswick, NJ: MMDLN/Rutgers CERTs; 2010. (July). Available at: http://rci.rutgers.edu/~cseap/MMDLNAPKIDS/Antipsychotic_Use_in_Medicaid_Children_Report_and_Resource_Guide_Final.pdf Accessed September 20, 2017. [Google Scholar]
- 24.Aman MG, Bukstein OG, Gadow KD, Arnold LE, Molina BS, McNamara NK, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry 2014;53(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobo WV, Cooper WO, Stein CM, Olfson M, Graham D, Daugherty J, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 2013;70(10):1067–1075. [DOI] [PubMed] [Google Scholar]
- 26.Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 2009;19(2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: A different story? J Clin Psychiatry 2005;66(10):1331–1332. [DOI] [PubMed] [Google Scholar]
- 28.Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: A systematic review. J Child Adolesc Psychopharmacol 2007;17(5):647–656. [DOI] [PubMed] [Google Scholar]
- 29.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009;302(16):1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adol Med 2008;162(10):929–935. [DOI] [PubMed] [Google Scholar]
- 31.Ray WA, Stein CM, Murray KT, Fuchs DC, Patrick SW, Daugherty J, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry 2019;76(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8(2):114–126. [DOI] [PubMed] [Google Scholar]
- 33.Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry 2009;166(12):1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conners CK. Conners 3rd EditionTM Manual. Toronto: Multi-Health Systems; 2008. [Google Scholar]
- 35.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 36.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity. J Am Acad Child Adolesc Psychiatry 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 37.Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986;143(1):35–39. [DOI] [PubMed] [Google Scholar]
- 38.Bird HR, Shaffer D, Fisher P, Gould MS, et al. The Columbia Impairment Scale (CIS): Pilot findings on a measure of global impairment for children and adolescents. Int J Methods Psychiatr Res 1993;3(3):167–176. [Google Scholar]
- 39.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatr 1990;86(2):184–192. [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [computer program]. Atlanta: CDC National Center for Health Statistics; 2016. update Available at https://www.cdc.gov/growthcharts/computer_programs.htm. Accessed April 8, 2020. [Google Scholar]
- 41.Barnes T. A rating scale for drug-induced akathisia. Br J Psychiatry 1989;156:672–676. [DOI] [PubMed] [Google Scholar]
- 42.Guy W, ed. Assessment Manual for Psychopharmacology. Washington, DC: U.S. Department of Health, Education and Welfare; 1976. Assessment Manual for Psychopharmacology [Google Scholar]
- 43.Simpson G, Angus J. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970;212(Suppl):11–19. [DOI] [PubMed] [Google Scholar]
- 44.Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: Is body mass predictive of clinical response? J Am Acad Child Adolesc Psychiatry 1997;36(4):523–530. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham CE, Bremner R, Secord M, Harrison R. COPE The Community Parent Education Program: A school-based family systems oriented workshop for parents of children with disruptive behavior disorders. 2nd ed. Hamilton, Ontario: COPE Works!; 2009. [Google Scholar]
- 46.SAS Institute. The GLIMMIX Procedure In: SAS/STAT® 14.1 User’s Guide. Cary, NC: SAS Institute, Inc; 2015:3188–3530. Available at [Google Scholar]
- 47.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- 48.Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2009. [Google Scholar]
- 49.Mehta CR, Patel NR. A network algorithm for performing Fisher’s Exact Test in r × c contingency tables. J. Am. Stat. Assoc. 1983;78(382):427–434. [Google Scholar]
- 50.Cummings JR, Ji X, Allen L, Lally C, Druss BG. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatr 2017;139(6):e20162444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan PL, Staff J, Hillemeier MM, Farkas G, Maczuga S. Racial and ethnic disparities in ADHD diagnosis from kindergarten to eighth grade. Pediatr 2013;132(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hervey-Jumper H, Douyon K, Franco KN. Deficits in diagnosis, treatment and continuity of care in African-American children and adolescents with ADHD. Journal of the National Medical Association 2006;98(2):233. [PMC free article] [PubMed] [Google Scholar]
- 53.Coker TR, Elliott MN, Toomey SL, Schwebel DC, Cuccaro P, Emery ST, et al. Racial and ethnic disparities in ADHD diagnosis and treatment. Pediatr 2016;138(3):e20160407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillemeier MM, Foster EM, Heinrichs B, Heier B, and the Conduct Problems Prevention Research Group. Racial differences in parental reports of attention-deficit/hyperactivity disorder behaviors. J Dev Behav Pediatr 2007;28(5):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry 2013;170(7):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol 2008;48(1):33–60. [DOI] [PubMed] [Google Scholar]
- 57.Vitiello B, Severe JB, Greenhill LL, Arnold E, Abikoff HB, Bukstein O, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: Lessons from the MTA. J Am Acad Child Adolesc Psychiatry 2001;40:188–196. [DOI] [PubMed] [Google Scholar]
- 58.Klein RG, Abikoff H, Hechtman L, Weiss G. Design and rationale of controlled study of long-term methylphenidate and multimodal psychosocial treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry 2004;43(7):792–801. [DOI] [PubMed] [Google Scholar]
- 59.Abikoff H, Hechtman L, Klein RG, Weiss G, Fleiss K, Etcovitch J, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry 2004;43(7):802–811. [DOI] [PubMed] [Google Scholar]
- 60.Blader JC, Pliszka SR, Kafantaris V, Sauder C, Posner J, Foley CA, et al. Prevalence and treatment outcomes of persistent negative mood among children with attention-deficit/hyperactivity disorder and aggressive behavior. J Child Adolesc Psychopharmacol 2016;26(2):164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towbin K, Vidal-Ribas P, Brotman MA, Pickles A, Miller KV, Kaiser A, Vitale AD, et al. A double-blind randomized placebo-controlled trial of citalopram adjunctive to stimulant medication in youth with chronic severe irritability. J Am Acad Child Adolesc Psychiatry. 2020;59(3):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinkman WB, Baum R, Kelleher KJ, Peugh J, Gardner W, Lichtenstein P, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry 2016;55(4):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epstein JN, Kelleher KJ, Baum R, Brinkman WB, Peugh J, Gardner W, et al. Variability in ADHD care in community-based pediatrics. Pediatr 2014;134(6):1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.