Abstract
Germline DNA tests to identify pathogenic variants in genes linked to hereditary breast and ovarian cancer susceptibility have become widely available. However, the clinical utility of genetic testing depends on reliable evidence-based classification of sequence variants. Determination of pathogenicity traditionally relies on painstaking pedigree-based segregation analyses. However, the rapid increase in usage of germline DNA tests has led to the discovery of a large number of variants of uncertain clinical significance (VUS). For most VUS there is insufficient information for segregation analysis and therefore assessment of functional consequences is increasingly being used to support clinical annotation. Functional assays need to be accurate, robust, and reproducible to be used for clinical purposes. Here we use the lessons learned from BRCA1 and BRCA2 to identify best practices for the use of functional assays for clinical annotation of germline VUS in breast and ovarian cancer genes. We provide recommendations for the interpretation and use of established functional assays as well as for the development of new assays.
Keywords: Variants of Uncertain Significance, hereditary breast and ovarian cancer, functional assays, clinical annotation, cancer susceptibility, BRCA1, BRCA2
Introduction
Since the identification and cloning of BRCA1 in 1994 [1], and shortly thereafter of BRCA2 [2], genetic tests of germline DNA to identify pathogenic variants in genes linked to hereditary breast and ovarian cancer (HBOC) have become mainstream [3]. These tests are critical to identify women at increased risk relative to the general population. Women at moderate risk (2 ≤ Relative Risk (RR) < 4) may benefit from enhanced screening and chemoprevention while those at high risk (RR ≥ 4), including those with BRCA1 and BRCA2 pathogenic variants, may also benefit from preventive surgery. Germline mutation testing is also becoming increasingly relevant in the cancer treatment setting because carriers of pathogenic variants in BRCA1/2 may benefit from PARP (Poly-ADP Ribosyl Polymerase) inhibitors [4 5]. Importantly, genetic tests can identify individuals in HBOC families who do not carry the relevant predisposing allele and are not at elevated risk of cancer [6].
A significant fraction of documented variants in BRCA1 and BRCA2 are considered variants of uncertain clinical significance (VUS), for which cancer association has not been assessed or could not be determined due to insufficient information (Table 1). In ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), a clinically-oriented database, currently ~37% of BRCA1 and ~45% of BRCA2 unique variants recorded are VUS. Thus, there is a critical need to classify variants according to their pathogenicity.
Table 1.
Fraction of VUS in BRCA1 and BRCA2
| Databases: | BICa | ClinVarb | BRCA Exchangec | gnomADd | ||||
|---|---|---|---|---|---|---|---|---|
| n | % (%VUS) |
n | % (%VUS) |
n | % (%VUS) |
n | % | |
| BRCA1 Unique variants | 1781 | 100 | 5821 | 100 | 7898 | 100 | 2936 | 100 |
| BRCA1 VUS | 891 | 50.0 (100) |
2146 | 36.9 (100) |
5186 | 65.7 (100) |
n/a | n/a |
| BRCA1 Missense | 607 | 34.1 (68.1) |
1715 | 29.5 (79.9) |
1892 | 24.0 (36.5) |
938 | 31.9 |
| BRCA1 Missense VUS | 569 | 31.9 (63.9) |
1633 | 28.1 (76.1) |
1714 | 21.7 (33.1) |
n/a | n/a |
| BRCA2 Unique variants | 2000 | 100 | 8119 | 100 | 10422 | 100 | 4262 | 100 |
| BRCA2 VUS | 1065 | 53.3 (100) |
3615 | 44.5 (100) |
6980 | 67.0 (100) |
n/a | n/a |
| BRCA2 Missense | 891 | 44.6 (83.7) |
3111 | 38.3 (86.1) |
3484 | 33.4 (49.9) |
1909 | 44.8 |
| BRCA2 Missense VUS | 838 | 41.9 (78.7) |
3011 | 37.1 (83.3) |
3190 | 30.6 (45.7) |
n/a | n/a |
BIC, Breast Cancer Information Core (https://research.nhgri.nih.gov/bic/) is a locus specific database established in 1995 for BRCA1 and BRCA2 variants, including loci primarily found in clinical or research testing
ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) is a public archive of reports of the relationships among human variations and phenotypes and includes submissions reporting variants found in patient samples from clinical or research testing, and from the literature (note: ClinVar also includes BIC data)[69]. VUS counts also include conflicting assessments
BRCA Exchange (http://brcaexchange.org/) pools data on BRCA1/2 genetic variants and corresponding clinical data from around the world (including BIC, ClinVar, 1000 Genomes Project). BRCA Exchange is part of the Global Alliance for Genomics and Health. VUS counts also include ‘not yet reviewed’
gnomAD, The Genome Aggregation Database (http://gnomad.broadinstitute.org/), initially released as ExAC aggregates and harmonizes both exome and genome sequencing data from a wide variety of large-scale sequencing projects. It does not contain pathogenicity assessments. All searches were conducted in December 2017.
Over the past decade, functional assays have emerged that can be included as a source of evidence to classify variants according to their pathogenicity, with the potential to greatly accelerate classification [7]. Here we discuss several technical and conceptual aspects relevant for the use functional assays in the classification of variants. We present best practice recommendations to improve annotation quality and accuracy, and to provide a basis for the comparison and integration of functional data from different laboratories (Box 1). For the coming years, we anticipate that recent technological developments such as VAMP-Seq (variant abundance by massively parallel sequencing) or high-throughput CRISPR-based saturation mutagenesis will enable the functional assessment of every missense variant for all moderate and high risk HBOC genes [8–10]. Once established and validated, these catalogues of functional data will provide valuable information for clinical annotation. The recommendations proposed here are the result of a discussion that started at a Netherlands Cancer Institute (NKI) workshop on Functional Analysis of Sequence Variants in Hereditary Breast and Ovarian Cancer Genes (Amsterdam, Netherlands) and was followed by additional discussion and extensive refinement. It represents a consensus view that was self-developed by an international group of investigators (the authors) who have been active in this field.
Box 1. Summary recommendations for the development, reporting and interpretation of functional assays.
Assess the strength of evidence for association between each gene and HBOC risk to evaluate the potential clinical utility of a proposed functional assay
Consider the assumptions, biological characteristics, controls and limitations of each assay
Choose genomic DNA/cDNA/Protein sequences that correspond to the coding sequence of the most commonly found haplotype in non-affected individuals to be used as a reference (wild-type)
A minimal set of non-pathogenic and pathogenic variants should be used as internal reference for each run of an assay. Larger sets of reference variants should be used to assess the overall sensitivity and specificity of an assay
Verify that elements of the assay (reagents and data) have been through quality control, including periodical verification of cell line and strain identity (e.g. identity by short tandem repeat analysis for mammalian cells and phenotyping for yeast strains) and quality (mycoplasma testing)
Due to protein stability issues, exercise caution when developing and interpreting results from model organisms that are cultured at temperatures lower than 37°C
Inspect data to identify and correct batch effects
Do not assume that intermediate levels of activity necessarily reflects intermediate risks
In addition to loss-of-function effects also consider dominant negative and gain-of-function effects
When reporting results use explicitly defined terminology and aim for the development of a controlled vocabulary
Be explicit about assay’s limitations, performance metrics and thresholds used to classify variants
Assessment of the evidence for association of each gene with HBOC risk
The first step in developing or interpreting results from functional assays is to understand the level of evidence that links a particular gene to breast and ovarian cancer risk [3]. To date, there are nine genes for which an association between protein-truncating variants and breast cancer risk has been established (ATM, BRCA1, BRCA2, CDH1, CHEK2, PALB2, PTEN, STK11, and TP53) and several more (BARD1, FANCM, NBN, NF1, MLH1, MSH2, MSH6, PMS2, RAD51C, and RAD51D) [3 11–13] for which association has been suggested but not yet firmly established. At least twelve genes have been implicated in ovarian cancer risk (ATM, BRCA1, BRCA2, BRIP1, MLH1, MSH2, MSH6, PALB2, PMS2, RAD51C, and RAD51D) [14 15].
Development of functional tests for emerging genes provides opportunities to uncover new mechanistic aspects of their biology and identify functional domains. However, developers of functional assays should consider that clinical recommendations are unlikely to be made based on variants in genes for which the association has not been robustly established. Thus, a detailed understanding of the strength of evidence for association between each gene (and its variant alleles) and HBOC risk should be sought to evaluate the clinical utility of a proposed functional assay.
An additional aspect to consider when developing a functional assay is the proportion of missense VUS that are probably pathogenic. Missense variation is unlikely to significantly affect the overall protein function when located in disordered regions or in repeat motifs. Therefore, functional assays for these regions (or for a protein with a large portion of its coding sequence composed by these regions) may not be a priority.
Assessment of variant pathogenicity
Genes implicated in HBOC are tumor suppressor genes and therefore variants leading to disruption of function(s) are usually considered pathogenic for clinical purposes. Notable exceptions of variants with dominant negative or gain-of-function have also been reported [16]. Loss-of-function genetic alterations include frameshift and nonsense variants leading to truncation of a functionally important segment of the protein, alterations of donor and acceptor splice sites, and large genomic rearrangements altering segments of the coding region. Conversely, synonymous changes without effect on mRNA splicing are considered non-pathogenic. These variants can be reliably classified by a rule-based system that incorporates general DNA/RNA/protein rules and takes into account exceptions specific to each gene (ENIGMA rules for classification of BRCA1 and BRCA2 variants: https://enigmaconsortium.org/library/general-documents/enigma-classification-criteria/).
For a significant fraction of rare variants pathogenicity cannot be predicted based on DNA changes alone. Primarily, these variants include small deletions or insertions that do not disturb the reading frame, missense changes, intronic and exonic variants that may lead to altered mRNA splicing and in-frame exon deletions or duplications. Missense variants represent the largest contributor to this class, making up to 79.9% and 86.1% of all BRCA1 and BRCA2 VUS in ClinVar, respectively (Table 1).
Classification of these BRCA1/2 variants for clinical use can be based on the ACMG/AMP (The American College of Medical Genetics and Genomics; Association for Molecular Pathology) guidelines in which pathogenicity is determined by the entire body of evidence [7]. In this proposed five-tier classification, variants with greater than 90% certainty of being pathogenic (which includes likely pathogenic and pathogenic variants), are considered actionable, and carriers are managed as high risk (RR ≥ 4). Evidence is qualitatively weighed as strong, moderate, supporting, or not used. Functional data provides strong (PS3: well established functional studies show a deleterious effect) and moderate (PM1: mutational hot spot or well-studied functional domain without benign variation) evidence for pathogenicity; and strong (BS3: well established functional studies show no deleterious effect) evidence for benign impact [7]. Reproducible and robust functional assays that have been validated are considered the most well-established source.
Alternatively, classification of BRCA1/2 variants can be based on a multifactorial statistical model that incorporates data on family history, co-segregation, and co-occurrence with another allele with a known pathogenic variant in the same gene (because biallelic inactivation is embryonic lethal while biallelic partial loss of function leads to Fanconi anemia) [17]. In the IARC proposed five-tier classification variants with greater than 95% certainty of being pathogenic are considered actionable [18]. Variants that reach greater than 1.0% allele frequency in the population are considered unlikely to be pathogenic on their own but there is simply insufficient clinic and family-based genetic information to determine the likelihood of pathogenicity of many uncommon (< 1.0%) variants. Currently, functional data is not integrated in these multifactorial statistical models.
The effects of these rare VUS can be predicted by a wide variety of publicly available in silico tools with variable performance [19 20]. For tools that use multiple sequence alignments, performance has been tied to the choice of alignments and calibration [21–23]. Reliance on multiple sequence alignment and evolutionary approaches may also generate false negatives. For example, Kondrashov et al. have estimated that approximately 10% of variants that are classified as “tolerated”, because a corresponding amino acid residue is found in the cognate position in another species, only score as “tolerated” because of compensatory variation elsewhere in the protein sequence [24]. Despite these limitations, algorithms are constantly improving and the concordance between some predictors and empirical data is sufficiently high (Figure 1) to guide prioritization of variants for functional assessment. However, empirical functional data will be necessary for the robust clinical annotation of uncommon variants.
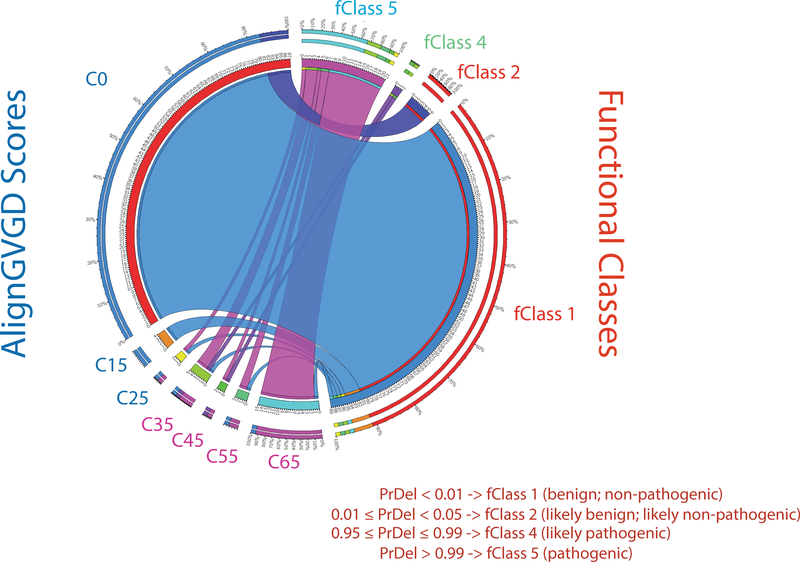
Figure 1. Circos plot illustrating the concordance between AlignGVGD predictions and experimental data derived from analysis of BRCA1 C-terminal variants (aa 1396–1863).
Variants were analyzed using the transcription activation assay [29] and assigned to functional classes by VarCall [57]. Blue ribbons show that all variants scoring C0 and C15 in Align GVGD and predicted to have no or little functional impact score as non-pathogenic or likely non-pathogenic (fClass1 or 2) in a validated functional assay. Conversely, most variants scoring as C65 and predicted to have a functional score as fClass5 (thick purple ribbon). Despite the strong concordance between alignGVGD and the transcriptional assay, a small but significant fraction of variants scoring as fClass1 were incorrectly predicted (C45-C65).
The spectrum of low, moderate, and high risk alleles in HBOC genes
When developing or interpreting a functional assay for VUS classification, the level of risk conferred by pathogenic variants should be considered. Findings of pathogenic variants in a low penetrance gene (RR < 2) currently do not trigger clinical recommendations making the development of a functional assay a low priority. It is also plausible that variants within the same gene may span the spectrum of low (RR < 2), moderate (2 ≤ RR < 4), and high (RR ≥ 4) risk. In other words, distinct “pathogenic” variants in the same gene may carry significantly different levels of risk.
Currently, the multifactorial statistical model for classification of BRCA1 or BRCA2 variants determines whether a variant is likely to be pathogenic. The clinical inference of the IARC classification is based on variants that typically are associated with a high cancer risk comparable to a truncating variant in BRCA1 or BRCA2 (RR ≥ 4) [18]. However, it is now clear that some pathogenic missense variants in BRCA1 (p.R1699Q and p.V1736A) and BRCA2 (p.Y3035S and p.G2508S) confer only moderate breast cancer risk (2 ≤ RR < 4) [25–27]. On the other hand, the BRCA2 p.K3326X, classified by the model as non-pathogenic, was shown to confer a mildly increased risk (RR = 1.4) of breast and ovarian cancer [28]. Although finding this variant would not trigger a change in clinical recommendation currently, this variant can contribute to polygenic risk scores based on common genetic variants that are now being used for risk stratification, and may prove effective for selection of screening and prevention options.
Some assays may have the ability to reflect different levels of risk depending on the dynamic range of the read-out but also on the specific biological assay being performed. It is important to stress that it should not be assumed that intermediate levels of activity in a biochemical or biological assay necessarily reflects intermediate risks. Several reference variants with known intermediate risks should be used to determine the ability of an assay to reflect the continuum of risk. While the transcription activation assay for BRCA1 does not seem to discriminate between variants with intermediate risks from variants associated with high risk [29], the BRCA2 homologous recombination (HR) assay may be able to distinguish high from moderate and low/neutral as suggested by functional assessment of variant p.Y3035S [30]. For genes in which pathogenic variants are clearly associated with disease risk, a two-stage reporting system has been proposed, i.e. the first stage would establish pathogenicity of the variant based on multiple criteria and the second stage would denote the likely severity or clinical consequence for that variant (high, moderate or low risk) [31]. Capturing the full spectrum of risk associated with distinct pathogenic variants is a critical challenge for assay development and for reporting laboratories.
Classification from both ACMG/AMP and multifactorial models are designed to distinguish high risk (RR ≥ 4; actionable) from not high risk (RR < 4) variants and are currently not suitable to identify moderate risk variants. From a clinical standpoint, while these models have a binary outcome (actionable vs. non-actionable), carriers of moderate risk variants (2 ≤ RR < 4) may also benefit from enhanced screening [32].
Functional assays for HBOC gene variant classification
For the purposes of our discussion, “function” is considered as any aspect defined by the Gene Ontology Consortium [33] molecular function, cellular component, and biological processes. A “functional assay” is generally defined as any in vitro and in vivo system able to determine the impact of a variant by assessing its effect on protein stability, conformation, and function. Thus, assays for splicing alterations are not considered functional assays for the purposes of this manuscript (for assessment of splice variants please refer to refs. [34 35]).
Several characterized functions of the BRCA1 and BRCA2 proteins have been exploited in the development of functional assays (reviewed in [36–38]). Reflecting their central role in DNA damage repair, many assays revolve around measuring the ability of the variant to promote survival following DNA insults, such as treatment with ionizing radiation or DNA damaging compounds. In addition to these viability assays, specific biochemical assays such as those measuring homologous recombination or ubiquitylation, are also rooted in the known biology of BRCA1 and BRCA2. Finally, more limited biochemical assays, measuring binding to specific interacting proteins have also been applied to the functional analysis of variants [36–38].
In general, there is enough evidence to tie each of these functions to the etiology of tumors arising in carriers. However their individual contribution to cancer risk is unclear, making it difficult to determine which assay is more or less biologically appropriate, or to assign different weights to results obtained from different assays. Rather, the determination of which assays should be used for clinical annotation relies on their accuracy, and not on their biological properties. Preliminary analysis has shown that these functional assays display very high (> 80%) sensitivity and specificity (Table 2) [39].
Table 2.
Categories and performance of functional assays for BRCA1 and BRCA2
| Gene | Assay | Set-up | Read-out | # of variants assessed [# of known non-pathogenic; pathogenic]a | Sensitivity (95%CI)b | Specificity (95%CI)b | Ref. |
|---|---|---|---|---|---|---|---|
| BRCA1 | Colony size | cell-based (yeast) | complementation/ perturbation | 40 [15; 25] | 0.96 (0.80–1.00) | 0.93 (0.68–1.00) | [58] |
| BRCA1 | Yeast localization | cell-based (yeast) | complementation/ perturbation | 40 [15; 25] | 0.84 (0.64–0.95) | 0.93 (0.68–1.00) | [58] |
| BRCA1 | Transcription activation | cell-based (HEK293T) | reporter system | 204 [25; 10] | 1.00 (0.75–1.00) | 1.00 (0.83–1.00) | [29] |
| BRCA1 | BARD1 binding | cell-based (yeast) | reporter system | 35 | n/a | n/a | [70] |
| BRCA1 | UbcH5a binding | cell-based (yeast) | reporter system | 35 | n/a | n/a | [70] |
| BRCA1 | Uniquitin ligase activity | cell-free (in vitro) | in vitro enzymatic activity | 35 | n/a | n/a | [70] |
| BRCA1 | Protease sensitivity | cell-free (in vitro) | in vitro binding activity | 117 [10; 14] | 0.79 (0.49–1.00) | 0.80 (0.44–0.98) | [56] |
| BRCA1 | Phosphopeptide binding activity | cell-free (in vitro) | in vitro binding activity | 117 [10; 14] | 0.86 (0.57–0.98) | 1.00 (0.69–1.00) | [56] |
| BRCA1 | Phosphopeptide binding specificity | cell-free (in vitro) | in vitro binding activity | 117 [10; 14] | 1.00 (0.77–1.00) | 0.99 (0.56–1.00) | [56] |
| BRCA1 | ES cell survival | cell-based (mouse ES cells) | complementation/ perturbation | 86 [25; 9]c | n/a | n/a | [62] |
| BRCA1 | Cisplatin sensitivity | cell-based (mouse ES cells) | complementation/ perturbation | 86 [25; 9] | 1.00 (0.63–1.00) | 1.00 (0.83–1.00) | [62] |
| BRCA1 | BARD1 binding | cell-based (yeast) | reporter system | 1,287 [3; 19] | n/a | n/a | [71] |
| BRCA1 | Uniquitin ligase activity | cell-free (in vitro) | in vitro enzymatic activity | 1,287 [3; 19] | n/a | n/a | [71] |
| BRCA1 | Haploid cell survival | cell-based (HAP1 cells) | complementation/ perturbation | 3,893 [22; 162]d | 0.967 | 0.982 | [10] |
| BRCA1 | Homologous recombination | cell-based (RG37-shBRCA1 cells) | complementation/ perturbation | 78 [6; 7] | 1.00 | 1.00 | [72] |
| BRCA1 | Localization | cell-based (RG37-shBRCA1 cells) | complementation/ perturbation | 78 [6; 7] | 0.714 | 1.00 | [72] |
| BRCA1 | Protein expression and stability | cell-free (in vitro) | In vitro solubility and thermostability | 78 [6; 7] | 0.714 | 0.83 | [72] |
| BRCA1 | Phosphopeptide binding activity | Cell-free (in vitro) | in vitro binding activity | 42 [5; 2] | n/a | n/a | [72] |
| BRCA1 | Homologous recombination | Cell-based (HeLa-DR-FRT) | complementation / perturbation | 1,056 [5; 8] | 0.875 | 1.00 | [9] |
| BRCA1 | Homologous recombination | Cell-based (HEK293T) | complementation / perturbation | 35 [23; 5]e | 1.00 | 1.00 | [73] |
| BRCA2 | Homologous recombination | Cell-based (V-C8 cells) | complementation / perturbation | 64 [18; 13] | 1.00 (0.75–1.00) | 1.00 (0.82–1.00) | [30] |
| BRCA2 | Homologous recombination | Cell-based (V-C8 cells) | complementation / perturbation | 139 [12; 13] | 1.00 (0.75–1.00) | 1.00 (0.69–1.00) | [74] |
| BRCA2 | Homologous recombination | Cell-based (mouse ES cells) | complementation/ perturbation | 43 [20; 15] | 1.00 (0.78–1.00) | 1.00 (0.83–1.00) | [75] |
Only assays in which more than 30 variants were tested are listed.
Known pathogenic and non-pathogenic variants used for estimating sensitivity and specificity are those classified using the multifactorial model as IARC Classes 1,2,4 or 5 (Ref.[18]), unless otherwise indicated.
As originally published, unless otherwise stated.
Used missense variants classified by multifactorial model as IARC Classes 1,2,4 or 5 (Ref.[18]) plus the recently classified G1770V variants as pathogenic.
Used ClinVar as a source of known pathogenic and non-pathogenic variants.
Used as non-pathogenic variants Align-GVGD grade of C0 and IARC Class 1, and as pathogenic variants Align-GVGD grade of C35-C65 and IARC Class 5).
After the development of a large number of functional assays for high risk genes (BRCA1, BRCA2 and TP53) [36 37 40], significant attention has been focused on developing assays for other high/moderate risk genes such as PALB2, ATM, and CHEK2 [41–46]. However, here we will focus on functional assays for missense variants of BRCA1 and BRCA2 as exemplars from which we have derived general guidelines.
Functional assays can be defined by three broad categories according to their experimental set-up (cell-free or cell-based), host (human or model organism) and read-out. Result interpretation requires careful consideration of the assumptions, the biological characteristics, and limitations of each assay.
Cell-free systems either test a specific biochemical activity in vitro (e.g. phosphopeptide binding, ubiquitin ligase activity, DNA combing, DNA binding, DNA recombination), proteinprotein interactions (e.g. yeast two-hybrid screening, co-immunoprecipitations), or the effect of different factors on protein structure and stability (e.g. protease sensitivity, calorimetry). Interpretation of results from cell-free assays should consider that they are restricted to specific functions, sometimes limited to specific regions of the protein, and may be particularly sensitive to temperature, buffer conditions, and concentrations of exogenous substrates.
Cell-based systems use a human or model organism (e.g. yeast, bacteria, or mouse) host cell as the basis for the assay. Cell-based systems can be further distinguished as in cellulo (when the assay context is a single cell) or in vivo (in the context of a whole metazoan organism), although there are currently no established in vivo functional assays for VUS. We recommend periodical authentication of cell line and strain identity by short tandem repeat analysis and phenotyping, respectively. Cell lines should be checked regularly for mycoplasma infection. Interpretation of results from assays performed in model systems should consider the degree of divergence of proteins from the host involved in the assay, differences in biology, and in growth conditions.
Cell-based assays can be further defined by read-out. Reporter systems include those in which the read-out for functional impact is an ectopic reporter (e.g. transcription activation or HR assays) or in which ectopic overexpression in a heterologous system leads to a defined phenotype (e.g. small colony phenotype in yeast). Limitations of reporter systems based on ectopic expression may include artifacts of over expression. Alternatively, assays in which the full length variant allele/protein replaces the endogenous gene/protein and defined biological processes are assessed are considered complementation/perturbation assays. Some assays may combine reporter systems and complementation.
Ultimately, the value of an assay will depend on its performance, defined using a set of known non-pathogenic and pathogenic control variants as reference (see below). Given the complexity of the interaction of multiple biochemical functions and breast and ovarian cancer phenotype it is uncertain that there will be a single comprehensive and highly accurate functional assay. Rather, the combination of approaches using diverse sources of data obtained with transparent methodology and careful interpretation is likely to solve the challenges of VUS in HBOC genes.
Mouse models, although not suitable for high throughput analysis, can be helpful in determining the effect of such variants on tumor predisposition and treatment response. Brca1 mutant mice expressing the p.I26A variant showed that the E3 ubiquitin ligase activity of BRCA1 is dispensable for tumor suppression and mice expressing 185delAG (c.68_69delAG; p.E23VfsTer17) revealed the hypomorphic nature of this pathogenic variant in response to therapy [47–49]. A knock-in mouse model of the BRCA2 p.G25R variant, which had no effect on ES cell viability but had subtle defect in HR, showed a significant increase in tumor formation in mutant animals [50]. Similarly, the effect on tumor predisposition of an alternatively spliced Brca2 transcript lacking exons 4–7 was revealed in mutant mice lacking these exons [51].
Requirements for a clinically relevant functional assay
The analytical validity is the degree of accuracy with which a functional assay correctly classifies variants as pathogenic or non-pathogenic. For each assay performance metrics (True Positive Rate or sensitivity; True Negative rate or specificity; False Positive and False Negative Rates; Positive and Negative Likelihood Ratios; False Discovery Rate; False Omission Rate; Positive Predicted Value or precision; Negative Predictive Value; Accuracy; and Diagnostic Odds Ratio) should be derived from testing a panel of known pathogenic and non-pathogenic variants. The recommendation is to chose a set of pathogenic and non-pathogenic missense controls whose likelihood of pathogenicity has been established by the multifactorial statistical model and can be found in a recent ENIGMA publication [36 37 52]..
There are no specific recommendations about which threshold of sensitivity or specificity should be used to consider the inclusion of data from a functional assay for variant classification. Plon and colleagues [18] have pointed out that clinical decisions based on predictive values of 80–85% are normally used in oncology. A more strict approach would require that the lower bound of the 95% confidence interval be above the suggested 80–85% threshold but that may be difficult to achieve for genes for which there are very few known pathogenic and non-pathogenic variants to use in a validation set, which will be reflected as wider 95% intervals.
Controls are critical for validation of assays, assessment of dynamic range, and to determine metrics of performance such as sensitivity and specificity. Some thought should be given to decide on genomic DNA/cDNA/Protein sequence that corresponds to the coding sequence in the most commonly found haplotype in non-affected individuals to be used as a reference (wild-type). Note that differences in frequency of common alleles may exist across different populations. This reference cDNA or genomic sequence must be included in every experiment. Variants are scored as having functional impact or not depending on how much they differ from the reference.
It is recommended that within each run of the assay, in addition to the reference sequence, at least one known missense pathogenic and one missense non-pathogenic variant is included. If possible, known missense variants for each protein domain are recommended. To account for the range of variation of non-pathogenic variants, additional known non-pathogenic variants should also be included. Addition of hypomorphic (attenuated) variants with established intermediate risk may help calibration of assay results. Concerning VUS in genes for which there are no known missense non-pathogenic and pathogenic variants, alternative approaches, such as the use of missense variants with greater than 1.0% allele frequency and truncating variants as benign and pathogenic controls, respectively, might provide a yardstick. Results from assays using only truncating variants as pathogenic control should be interpreted with caution as truncating variants may not produce detectable protein, with implications to measuring baseline activity.
Lessons from BRCA1 and BRCA2 functional assessment
BRCA1 and BRCA2 have 1,863 and 3,418 codons, respectively. If we consider all possible single nucleotide changes in these codons, 11,015 and 20,169 unique missense variants are generated (some changes will result in the same amino acid changes), respectively. Because many have never or only sparsely been observed, we expect that most are rare (< 0.01%) such that data from functional assays will be required to assess their likelihood of pathogenicity. In order to maximize the chances of identifying pathogenic variants, investigators have focused on functional domains and motifs in which it seems more likely that variants affect protein function. Thus far, most assays have focused on variants at the RING and BRCT domains of BRCA1 and at the DSS1 and DNA interaction domain of BRCA2 [9 10 36 37]. Several functional assays have been described for BRCA1 and BRCA2, but few have tested large (> 30) sets of variants (Table 2).
For specificity and sensitivity calculations, variants are classified according to a binary classification based on the functional data: functional impact versus no functional impact. Variants with intermediate scores are ignored. This classification is then compared to a binary classification of a reference panel which combines the non-actionable Classes 1 and 2 (benign and likely benign) or actionable Classes 4 & 5 (likely pathogenic and pathogenic). This simplification allows for the estimation of the assay’s ability to correctly identify actionable and non-actionable variants. Most published functional assays have reported high sensitivity and specificity, often close to 100% (Table 2). However, these numbers partially reflect the relatively low numbers of known variants used to assess specificity and sensitivity. To obtain a better sense of an assays performance, it is critical to record and report the lower bounds of the 95% confidence interval.
Several assays have been developed using yeast (Saccharomyces cerevisiae) which provides a cost-effective and practical platform to evaluate missense variants. However, caution is warranted when interpreting results from model organisms that are cultured at temperatures lower than 37°C. Some pathogenic variants are relatively stable at lower temperatures (30°C versus 37°C) and may score as false negatives [53–55], reflected in the assay’s slightly lower sensitivity (Table 2).
It is important to note that contradictory results, for example a variant scoring pathogenic in a functional assay while being classified by clinical and family data as non-pathogenic, provide opportunities for discovery. BRCA1 variant p.V1736A scored as pathogenic in several functional assays and by in silico prediction tools [56] despite being classified as non-pathogenic due to a co-occurrence with the known pathogenic p.D821Ifs*25 variant in the same patient. However, upon further examination it was found that the carrier presented several features (e.g. developmental delay, microcephaly, short stature, very early onset ovarian cancer) pointing at hypomorphic BRCA1 activity [25]. Detailed genetic and functional investigation of the p.V1736A variant led to the discovery of the first documented carrier of biallelic pathogenic variants in BRCA1. This analysis established the existence of variants with intermediate effects and highlighted the power of functional assessment [25].
Although there are several missense variants that have displayed intermediate effects in vitro or in mouse models, only three variants, in addition to p.V1736A, have been established as hypomorphic in humans [25 27]. BRCA1 variant p.R1699Q (c.5096G>A; OR = 4.29) and BRCA2 variants p.Y3035S (c.9104A>C; OR = 2.52) and p.G2508S (c.7522G>A; OR = 2.68), have been shown to confer moderate increased risks. There is currently no consensus guidelines about their clinical management but the ENIGMA consortium has recommended breast surveillance for female carriers of p.R1699Q based on mammogram annually from age 40 and bilateral salpingo-oophorectomy should be considered based on family history [32]. Care should be exercised in the choice of statistical treatment of the data generated in functional assays. Results from assays are usually normalized using the mean of the activity of the wild-type or reference sequence. Normalization allows for comparisons across multiple experiments and, in some cases across multiple assays since variant activity is being represented as a percentage of the wild-type activity. Batch effects may be problematic (variance of the wild-type activity across multiple batches should also be taken into account) and some statistical models have taken that in consideration [57].
A more difficult task is the decision of a specific threshold of activity to separate pathogenic from non-pathogenic variants. Several approaches use arbitrary thresholds (e.g. 20% or 50% of wild-type activity; number of standard deviations from the wild-type reference; highest activity of a pathogenic variant and lowest activity of a non-pathogenic variant) or linear regression. Recent approaches have moved towards providing a probabilistic interpretation (i.e. likelihood of pathogenicity of the variant given the functional data) [57 58].
Probabilistic approaches also provide a path for integration of functional data with other data sources used to classify variants. By generating likelihood ratios (LRs) from the raw or processed functional readouts (e.g. viability counts, luciferase activity, GFP intensity), these approaches allow for the incorporation of functional assays as a data source into traditional multifactorial models that have so far not integrated functional data [57].
Integration of functional data can also be achieved using the ACMG/AMP classification model. According to the ACMG/AMP, ‘well-established assays’ can be used to obtain a PS3 or BS3 (strong evidence) criterion but there are no specific guidelines, which are likely to be established by expert panels for each gene. For example, concordant results from at least three independent validated assays would be needed for PS3 or BS3 (strong) criteria; while concordant results in two independent validated assays would generate a PM1 (moderate) criterion.
Functional assays based on sensitivity to therapeutic compounds
Insight in the importance of BRCA1 and BRCA2 for HR led to the development of carrier-specific treatment modalities for breast and ovarian cancers [59–61], in particular with the use of poly ADP ribose polymerase inhibitors (PARPi) which are synthetic lethal with BRCA1 or BRCA2 deficiency. The therapeutic window for these types of treatment is greatly increased by the fact that BRCA1 and BRCA2 mutation carriers are generally heterozygous for the pathogenic allele, while tumor cells frequently undergo loss of the wild-type allele. Thus, while the tumor cells are not viable in the presence of PARPi, non-tumor cells survive, making the therapy highly effective yet well tolerated.
Because inactivation of the gene product is required both for disruption of HR and sensitivity to PARPi or platinum compounds, functional assays based on sensitivity to these compounds can be used as an indirect read-out for HR to classify germline variants according to their pathogenicity [62].
In addition to the classification of germline variants according to associated cancer risk there is an emerging need to classify germline and somatic variants according to their response to PARPi or DNA damaging compounds to predict drug response. PARPi have been approved in the US and Europe for treatment of advanced and metastatic breast and ovarian cancers. It is unclear the extent to which sensitivity to PARPi or platinum compounds measured in a functional assay predicts in vivo tumor sensitivity. Pathogenic variants conferring high cancer risk with hypomorphic activity towards PARPi response are known to exist. For example, mouse tumor cells carrying the pathogenic Brca1 p.C61G variant showed a poor response to platinum compounds and to PARPi, and resistance rapidly emerged [63]. Importantly, determining whether a BRCA1 or BRCA2 variant found in tumor tissue confers sensitivity to a given drug may need further clinical information to calibrate the functional assays for this purpose.
Many other genes implicated in HBOC (such as ATM, CHEK2, PALB2, and TP53) are involved in the DNA damage response, suggesting that associated tumors may also have a targetable DNA repair defect. Importantly, recent results from the NOVA, Study 19, and ARIEL3 studies have raised the possibility of a significant benefit of PARP inhibitors in ovarian cancer irrespective of BRCA mutation status [64–66].
Future challenges and opportunities
As we move forward, functional assays should be able to face a significant increase in the number of genes and variants to be analyzed for clinical use. The number of alleles for genes predisposing to breast and ovarian cancer is expected to be very large. BRCA1 and BRCA2 have 7,898 and 10,422 unique alleles documented (Table 1; BRCA Exchange: http://brcaexchange.org/) and other HBOC genes are expected to significantly add to the number of predisposing alleles. In addition, recent systematic germline sequencing efforts of breast and ovarian cancer cases are expected to reveal additional genes and variants associated with risk.
To face the rapid increase in variants, high throughput functional assays that can generate and analyze large numbers of variants have been developed for BRCA1 [9 10]. Initially these consisted of the large scale generation and analysis of ectopically expressed cDNA constructs. Recent development of CRISPR-assisted gene targeting has now also allowed saturated mutagenesis at endogenous loci, exemplified by high-troughput mutagenesis of the BRCA1 RING and BRCT domains in haploid cells [9 10]. Likewise, assays based on human primary cells from carriers may also be used to capture more subtle functional defects that depend on the carrier’s genetic background, but may be less amenable to high throughput approaches [42]. Such high-throughput approaches can provide catalogues of potentially pathogenic variants that can aid in their interpretation of newly observed VUS. CRISPR-assisted gene targeting is also expected to accelerated the pace at which mouse models can be generated [67 68].
The path to clinical implementation will necessarily involve the inclusion of clinically calibrated functional data into comprehensive multifactorial statistical models. Functional assays are specialized tests that most diagnostic testing laboratories are currently not able to provide. In the US, results from such tests cannot be directly used for clinical decisions unless they are conducted under Clinical Laboratory Improvement Amendments (CLIA) guidelines. However, if data have been validated (i.e. evaluated for a comprehensive series of performance metrics such as sensitivity, specificity, accuracy, precision, repeatability, and robustness), functional assays reported from research labs can be used as evidence for clinical annotation [7]. It is important to consider that mistakes may occur in the processing of a sample in the absence of standard operating procedures. Those mistakes include clerical mistakes in reports (e.g. reporting pathogenic when it should be non-pathogenic), sample swapping, errors in measurements due to lack of equipment calibration or improper staff training. Thus, evidence from functional studies performed in a research setting must be carefully verified to determine data quality, reliability and the degree of confidence in the results. In the context of risk assessment, we caution against the use of functional data as the sole source of information for clinical recommendations. Despite these challenges, the value of functional assessment of variants to improve cancer care, based on international and multidisciplinary collaborations, is expected to be high and therefore we envisage the clinical implementation of functional assays for classification of VUS to proceed at an accelerated pace.
ACKNOWLEDGEMENTS
The meeting was supported by funds from the Cancer Genomics Center Netherlands through the Netherlands Organization for Scientific Research (NWO), the Royal Netherlands Academy of Arts and Sciences (KNAW), the Danish Counsil for Independent Research (DFF), the Netherlands Cancer Institute, and from IDT and BIOKÉ. We thank Amanda Spurdle for critically reviewing the manuscript.
Abbreviations
- BIC
Breast Cancer Information Core
- BRCT
BRCA1 C-terminal domain
- HBOC
hereditary breast and ovarian cancer
- HR
homologous recombination
- IARC
International Agency for Research on Cancer
- OR
odds ratio
- PARP
poly ADP ribose polymerase
- RR
relative risk
- VUS
variant of uncertain clinical significance
Footnotes
Conflict of interest statement: No conflict of interest to disclose (A.N.M., M.P.G.V., D.M.E., G.A.M., P.B., A.N.K., J.Y.M., M.K.S., F.J.C., S.S., R.S.). L.W. is an inventor and owner of a patent on a test system for determining genotoxicities and cancer risk.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266(5182):66–71 [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378(6559):789–92 [DOI] [PubMed] [Google Scholar]
- 3.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, Devilee P, Meindl A, Couch FJ, Southey M, Goldgar DE, Evans DG, Chenevix-Trench G, Rahman N, Robson M, Domchek SM, Foulkes WD. Gene-panel sequencing and the prediction of breast-cancer risk. The New England journal of medicine 2015;372(23):2243–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narod SA, Offit K. Prevention and Management of Hereditary Breast Cancer. Journal of Clinical Oncology 2005;23(8):1656–63 [DOI] [PubMed] [Google Scholar]
- 5.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Human genetics 2008;124(1):31–42 [DOI] [PubMed] [Google Scholar]
- 6.Kurian AW, Gong GD, John EM, Johnston DA, Felberg A, West DW, Miron A, Andrulis IL, Hopper JL, Knight JA, Ozcelik H, Dite GS, Apicella C, Southey MC, Whittemore AS. Breast cancer risk for noncarriers of family-specific BRCA1 and BRCA2 mutations: findings from the Breast Cancer Family Registry. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011;29(34):4505–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matreyek KA, Starita LM, Stephany JJ, Martin B, Chiasson MA, Gray VE, Kircher M, Khechaduri A, Dines JN, Hause RJ, Bhatia S, Evans WE, Relling MV, Yang W, Shendure J, Fowler DM. Multiplex assessment of protein variant abundance by massively parallel sequencing. Nature genetics 2018;50(6):874–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starita LM, Islam MM, Banerjee T, Adamovich AI, Gullingsrud J, Fields S, Shendure J, Parvin JD. A Multiplex Homology-Directed DNA Repair Assay Reveals the Impact of More Than 1,000 BRCA1 Missense Substitution Variants on Protein Function. American journal of human genetics 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Findlay GM, Daza RM, Martin B, Zhang MD, Leith AP, Gasperini M, Janizek JD, Huang X, Starita LM, Shendure J. Accurate classification of BRCA1 variants with saturation genome editing. Nature 2018;562(7726):217–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Seifert BA, Shimelis H, Ghosh R, Crowley SB, Carter NJ, Doonanco K, Foreman AK, Ritter DI, Jimenez S, Trapp M, Offit K, Plon SE, Couch FJ. Clinical validity assessment of genes frequently tested on hereditary breast and ovarian cancer susceptibility sequencing panels. Genetics in medicine : official journal of the American College of Medical Genetics 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimelis H, LaDuca H, Hu C, Hart SN, Na J, Thomas A, Akinhanmi M, Moore RM, Brauch H, Cox A, Eccles DM, Ewart-Toland A, Fasching PA, Fostira F, Garber J, Godwin AK, Konstantopoulou I, Nevanlinna H, Sharma P, Yannoukakos D, Yao S, Feng BJ, Tippin Davis B, Lilyquist J, Pesaran T, Goldgar DE, Polley EC, Dolinsky JS, Couch FJ. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. Journal of the National Cancer Institute 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch FJ, Shimelis H, Hu C, Hart SN, Polley EC, Na J, Hallberg E, Moore R, Thomas A, Lilyquist J, Feng B, McFarland R, Pesaran T, Huether R, LaDuca H, Chao EC, Goldgar DE, Dolinsky JS. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol 2017;3(9):1190–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MR, Kamara D, Karlan BY, Pharoah PDP, Gayther SA. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecologic oncology 2017;147(3):705–13 [DOI] [PubMed] [Google Scholar]
- 15.Lilyquist J, LaDuca H, Polley E, Davis BT, Shimelis H, Hu C, Hart SN, Dolinsky JS, Couch FJ, Goldgar DE. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecologic oncology 2017;147(2):375–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2010;2(2):a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, len-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. AmJ HumGenet 2007;81(5):873–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Human mutation 2008;29(11):1282–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst C, Hahnen E, Engel C, Nothnagel M, Weber J, Schmutzler RK, Hauke J. Performance of in silico prediction tools for the classification of rare BRCA1/2 missense variants in clinical diagnostics. BMC medical genomics 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart SN, Hoskin T, Shimelis H, Moore RM, Feng B, Thomas A, Lindor NM, Polley EC, Goldgar DE, Iversen E, Monteiro ANA, Suman VJ, Couch FJ. Comprehensive annotation of BRCA1 and BRCA2 missense variants by functionally validated sequence-based computational prediction models. Genetics in medicine : official journal of the American College of Medical Genetics 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavtigian SV, Greenblatt MS, Lesueur F, Byrnes GB, Group IUGVW. In silico analysis of missense substitutions using sequence-alignment based methods. Human mutation 2008;29(11):1327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson BA, Greenblatt MS, Vallee MP, Herkert JC, Tessereau C, Young EL, Adzhubey IA, Li B, Bell R, Feng B, Mooney SD, Radivojac P, Sunyaev SR, Frebourg T, Hofstra RM, Sijmons RH, Boucher K, Thomas A, Goldgar DE, Spurdle AB, Tavtigian SV. Calibration of multiple in silico tools for predicting pathogenicity of mismatch repair gene missense substitutions. Human mutation 2013;34(1):255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks S, Wheeler DA, Plon SE, Kimmel M. Prediction of missense mutation functionality depends on both the algorithm and sequence alignment employed. Human mutation 2011;32(6):661–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondrashov AS, Sunyaev S, Kondrashov FA. Dobzhansky-Muller incompatibilities in protein evolution. Proceedings of the National Academy of Sciences 2002;99(23):14878–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domchek SM, Tang J, Stopfer J, Lilli DR, Hamel N, Tischkowitz M, Monteiro AN, Messick TE, Powers J, Yonker A, Couch FJ, Goldgar DE, Davidson HR, Nathanson KL, Foulkes WD, Greenberg RA. Biallelic deleterious BRCA1 mutations in a woman with early-onset ovarian cancer. Cancer discovery 2013;3(4):399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spurdle AB, Whiley PJ, Thompson B, Feng B, Healey S, Brown MA, kConFab Pettigrew C, Van Asperen CJ, Ausems MG, Kattentidt-Mouravieva AA, van den Ouweland AM, Dutch Belgium UVC, Lindblom A, Pigg MH, Schmutzler RK, Engel C, Meindl A, German Consortium of Hereditary B, Ovarian C, Caputo S, Sinilnikova OM, Lidereau R, French Cgc, Couch FJ, Guidugli L, Hansen T, Thomassen M, Eccles DM, Tucker K, Benitez J, Domchek SM, Toland AE, Van Rensburg EJ, Wappenschmidt B, Borg A, Vreeswijk MP, Goldgar DE, Consortium E. BRCA1 R1699Q variant displaying ambiguous functional abrogation confers intermediate breast and ovarian cancer risk. Journal of medical genetics 2012;49(8):525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimelis H, Mesman RLS, Von Nicolai C, Ehlen A, Guidugli L, Martin C, Calleja F, Meeks H, Hallberg E, Hinton J, Lilyquist J, Hu C, Aalfs CM, Aittomaki K, Andrulis I, Anton-Culver H, Arndt V, Beckmann MW, Benitez J, Bogdanova NV, Bojesen SE, Bolla MK, Borresen-Dale AL, Brauch H, Brennan P, Brenner H, Broeks A, Brouwers B, Bruning T, Burwinkel B, Chang-Claude J, Chenevix-Trench G, Cheng CY, Choi JY, Collee JM, Cox A, Cross SS, Czene K, Darabi H, Dennis J, Dork T, Dos-Santos-Silva I, Dunning AM, Fasching PA, Figueroa J, Flyger H, Garcia-Closas M, Giles GG, Glendon G, Guenel P, Haiman CA, Hall P, Hamann U, Hartman M, Hogervorst FB, Hollestelle A, Hopper JL, Ito H, Jakubowska A, Kang D, Kosma VM, Kristensen V, Lai KN, Lambrechts D, Marchand LL, Li J, Lindblom A, Lophatananon A, Lubinski J, Machackova E, Mannermaa A, Margolin S, Marme F, Matsuo K, Miao H, Michailidou K, Milne RL, Muir K, Neuhausen SL, Nevanlinna H, Olson JE, Olswold C, Oosterwijk JJC, Osorio A, Peterlongo P, Peto J, Pharoah PDP, Pylkas K, Radice P, Rashid MU, Rhenius V, Rudolph A, Sangrajrang S, Sawyer EJ, Schmidt MK, Schoemaker MJ, Seynaeve C, Shah M, Shen CY, Shrubsole M, Shu XO, Slager S, Southey MC, Stram DO, Swerdlow A, Teo SH, Tomlinson I, Torres D, Truong T, van Asperen CJ, van der Kolk LE, Wang Q, Winqvist R, Wu AH, Yu JC, Zheng W, Zheng Y, Leary J, Walker L, Foretova L, Fostira F, Claes KBM, Varesco L, Moghadasi S, Easton DF, Spurdle A, Devilee P, Vrieling H, Monteiro ANA, Goldgar DE, Carreira A, Vreeswijk MPG, Couch FJ, for kConFab AI, for NC. BRCA2 Hypomorphic Missense Variants Confer Moderate Risks of Breast Cancer. Cancer research 2017;77(11):2789–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeks HD, Song H, Michailidou K, Bolla MK, Dennis J, Wang Q, Barrowdale D, Frost D, Embrace, McGuffog L, Ellis S, Feng B, Buys SS, Hopper JL, Southey MC, Tesoriero A, kConFab I, James PA, Bruinsma F, Campbell IG, Australia Ovarian Cancer Study G, Broeks A, Schmidt MK, Hogervorst FB, Hebon, Beckman MW, Fasching PA, Fletcher O, Johnson N, Sawyer EJ, Riboli E, Banerjee S, Menon U, Tomlinson I, Burwinkel B, Hamann U, Marme F, Rudolph A, Janavicius R, Tihomirova L, Tung N, Garber J, Cramer D, Terry KL, Poole EM, Tworoger SS, Dorfling CM, van Rensburg EJ, Godwin AK, Guenel P, Truong T, Collaborators GS, Stoppa-Lyonnet D, Damiola F, Mazoyer S, Sinilnikova OM, Isaacs C, Maugard C, Bojesen SE, Flyger H, Gerdes AM, Hansen TV, Jensen A, Kjaer SK, Hogdall C, Hogdall E, Pedersen IS, Thomassen M, Benitez J, Gonzalez-Neira A, Osorio A, Hoya Mde L, Segura PP, Diez O, Lazaro C, Brunet J, Anton-Culver H, Eunjung L, John EM, Neuhausen SL, Ding YC, Castillo D, Weitzel JN, Ganz PA, Nussbaum RL, Chan SB, Karlan BY, Lester J, Wu A, Gayther S, Ramus SJ, Sieh W, Whittermore AS, Monteiro AN, Phelan CM, Terry MB, Piedmonte M, Offit K, Robson M, Levine D, Moysich KB, Cannioto R, Olson SH, Daly MB, Nathanson KL, Domchek SM, Lu KH, Liang D, Hildebrant MA, Ness R, Modugno F, Pearce L, Goodman MT, Thompson PJ, Brenner H, Butterbach K, Meindl A, Hahnen E, Wappenschmidt B, Brauch H, Bruning T, Blomqvist C, Khan S, Nevanlinna H, Pelttari LM, Aittomaki K, Butzow R, Bogdanova NV, Dork T, Lindblom A, Margolin S, Rantala J, Kosma VM, Mannermaa A, Lambrechts D, Neven P, Claes KB, Maerken TV, Chang-Claude J, Flesch-Janys D, Heitz F, Varon-Mateeva R, Peterlongo P, Radice P, Viel A, Barile M, Peissel B, Manoukian S, Montagna M, Oliani C, Peixoto A, Teixeira MR, Collavoli A, Hallberg E, Olson JE, Goode EL, Hart SN, Shimelis H, Cunningham JM, Giles GG, Milne RL, Healey S, Tucker K, Haiman CA, Henderson BE, Goldberg MS, Tischkowitz M, Simard J, Soucy P, Eccles DM, Le N, Borresen-Dale AL, Kristensen V, Salvesen HB, Bjorge L, Bandera EV, Risch H, Zheng W, Beeghly-Fadiel A, Cai H, Pylkas K, Tollenaar RA, Ouweland AM, Andrulis IL, Knight JA, Ocgn, Narod S, Devilee P, Winqvist R, Figueroa J, Greene MH, Mai PL, Loud JT, Garcia-Closas M, Schoemaker MJ, Czene K, Darabi H, McNeish I, Siddiquil N, Glasspool R, Kwong A, Park SK, Teo SH, Yoon SY, Matsuo K, Hosono S, Woo YL, Gao YT, Foretova L, Singer CF, Rappaport-Feurhauser C, Friedman E, Laitman Y, Rennert G, Imyanitov EN, Hulick PJ, Olopade OI, Senter L, Olah E, Doherty JA, Schildkraut J, Koppert LB, Kiemeney LA, Massuger LF, Cook LS, Pejovic T, Li J, Borg A, Ofverholm A, Rossing MA, Wentzensen N, Henriksson K, Cox A, Cross SS, Pasini BJ, Shah M, Kabisch M, Torres D, Jakubowska A, Lubinski J, Gronwald J, Agnarsson BA, Kupryjanczyk J, Moes-Sosnowska J, Fostira F, Konstantopoulou I, Slager S, Jones M, genome PRcAgTICAait, Antoniou AC, Berchuck A, Swerdlow A, Chenevix-Trench G, Dunning AM, Pharoah PD, Hall P, Easton DF, Couch FJ, Spurdle AB, Goldgar DE. BRCA2 Polymorphic Stop Codon K3326X and the Risk of Breast, Prostate, and Ovarian Cancers. Journal of the National Cancer Institute 2016;108(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods NTB R; Golubeva V; Jhuraney A; De-Gregoriis G; Vaclova T; Goldgar DE; Couch FJ; Carvalho MA; Iversen ES; Monteiro AN Functional assays provide a robust tool for the clinical annotation of genetic variants of uncertain significance. NPJ Genomic Medicine 2016;1(1):16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidugli L, Pankratz VS, Singh N, Thompson J, Erding CA, Engel C, Schmutzler R, Domchek S, Nathanson K, Radice P, Singer C, Tonin PN, Lindor NM, Goldgar DE, Couch FJ. A classification model for BRCA2 DNA binding domain missense variants based on homology-directed repair activity. Cancer research 2013;73(1):265–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spurdle AB, Greville-Heygate S, Antoniou AC, Brown M, Burke L, de la Hoya M, Domchek S, Dork T, Firth HV, Monteiro AN, Mensenkamp A, Parsons MT, Radice P, Robson M, Tischkowitz M, Tudini E, Turnbull C, Vreeswijk MP, Walker LC, Tavtigian S, Eccles DM. Towards controlled terminology for reporting germline cancer susceptibility variants: an ENIGMA report. Journal of medical genetics 2019 [DOI] [PubMed] [Google Scholar]
- 32.Moghadasi S, Meeks HD, Vreeswijk MP, Janssen LA, Borg A, Ehrencrona H, Paulsson-Karlsson Y, Wappenschmidt B, Engel C, Gehrig A, Arnold N, Hansen TVO, Thomassen M, Jensen UB, Kruse TA, Ejlertsen B, Gerdes AM, Pedersen IS, Caputo SM, Couch F, Hallberg EJ, van den Ouweland AM, Collee MJ, Teugels E, Adank MA, van der Luijt RB, Mensenkamp AR, Oosterwijk JC, Blok MJ, Janin N, Claes KB, Tucker K, Viassolo V, Toland AE, Eccles DE, Devilee P, Van Asperen CJ, Spurdle AB, Goldgar DE, Garcia EG. The BRCA1 c. 5096G>A p.Arg1699Gln (R1699Q) intermediate risk variant: breast and ovarian cancer risk estimation and recommendations for clinical management from the ENIGMA consortium. Journal of medical genetics 2018;55(1):15–20 [DOI] [PubMed] [Google Scholar]
- 33.The Gene Ontology C Expansion of the Gene Ontology knowledgebase and resources. Nucleic acids research 2017;45(D1):D331–D38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomassen M, Blanco A, Montagna M, Hansen TV, Pedersen IS, Gutierrez-Enriquez S, Menendez M, Fachal L, Santamarina M, Steffensen AY, Jonson L, Agata S, Whiley P, Tognazzo S, Tornero E, Jensen UB, Balmana J, Kruse TA, Goldgar DE, Lazaro C, Diez O, Spurdle AB, Vega A. Characterization of BRCA1 and BRCA2 splicing variants: a collaborative report by ENIGMA consortium members. Breast cancer research and treatment 2011 [DOI] [PubMed] [Google Scholar]
- 35.Walker LC, Whiley PJ, Couch FJ, Farrugia DJ, Healey S, Eccles DM, Lin F, Butler SA, Goff SA, Thompson BA, Lakhani SR, Da Silva LM, kConFab Investigators, Tavtigian SV, Goldgar DE, Brown MA, Spurdle AB. Detection of splicing aberrations caused by BRCA1 and BRCA2 sequence variants encoding missense substitutions: implications for prediction of pathogenicity. Human mutation 2010;31:E1484–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidugli L, Carreira A, Caputo SM, Ehlen A, Galli A, Monteiro AN, Neuhausen SL, Hansen TV, Couch FJ, Vreeswijk MP, consortium E. Functional assays for analysis of variants of uncertain significance in BRCA2. Human mutation 2014;35(2):151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millot GA, Carvalho MA, Caputo SM, Vreeswijk MP, Brown MA, Webb M, Rouleau E, Neuhausen SL, Hansen T, Galli A, Brandao RD, Blok MJ, Velkova A, Couch FJ, Monteiro AN. A guide for functional analysis of BRCA1 variants of uncertain significance. Human mutation 2012;33(11):1526–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhuraney A, Velkova A, Johnson RC, Kessing B, Carvalho RS, Whiley P, Spurdle AB, Vreeswijk MP, Caputo SM, Millot GA, Vega A, Coquelle N, Galli A, Eccles D, Blok MJ, Pal T, van der Luijt RB, Santamarina Pena M, Neuhausen SL, Donenberg T, Machackova E, Thomas S, Vallee M, Couch FJ, Tavtigian SV, Glover JN, Carvalho MA, Brody LC, Sharan SK, Monteiro AN, Evidence-based Network for the Interpretation of Germline Mutant Alleles C. BRCA1 Circos: a visualisation resource for functional analysis of missense variants. Journal of medical genetics 2015;52(4):224–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes VC, Golubeva VA, Di Pietro G, Shields C, Amankwah K, Nepomuceno TC, de Gregoriis G, Abreu RBV, Harro C, Gomes TT, Silva RF, Suarez-Kurtz G, Couch F, Iversen ES, Monteiro ANA, Carvalho MA. Impact of amino acid substitutions at secondary structures in the BRCT domains of the tumor suppressor BRCA1: Implications for clinical annotation. The Journal of biological chemistry 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, Ishioka C. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. ProcNatlAcadSciUSA 2003;100(14):8424–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell DW, Kim SH, Godwin AK, Schiripo TA, Harris PL, Haserlat SM, Wahrer DC, Haiman CA, Daly MB, Niendorf KB, Smith MR, Sgroi DC, Garber JE, Olopade OI, Le Marchand L, Henderson BE, Altshuler D, Haber DA, Freedman ML. Genetic and functional analysis of CHEK2 (CHK2) variants in multiethnic cohorts. International journal of cancer Journal international du cancer 2007;121(12):2661–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keimling M, Volcic M, Csernok A, Wieland B, Dork T, Wiesmuller L. Functional characterization connects individual patient mutations in ataxia telangiectasia mutated (ATM) with dysfunction of specific DNA double-strand break-repair signaling pathways. FASEB J 2011;25(11):3849–60 [DOI] [PubMed] [Google Scholar]
- 43.Desrichard A, Bidet Y, Uhrhammer N, Bignon YJ. CHEK2 contribution to hereditary breast cancer in non-BRCA families. Breast cancer research : BCR 2011;13(6):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caleca L, Catucci I, Figlioli G, De Cecco L, Pesaran T, Ward M, Volorio S, Falanga A, Marchetti M, Iascone M, Tondini C, Zambelli A, Azzollini J, Manoukian S, Radice P, Peterlongo P. Two Missense Variants Detected in Breast Cancer Probands Preventing BRCA2-PALB2 Protein Interaction. Front Oncol 2018;8:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obermeier K, Sachsenweger J, Friedl TW, Pospiech H, Winqvist R, Wiesmuller L. Heterozygous PALB2 c.1592delT mutation channels DNA double-strand break repair into error-prone pathways in breast cancer patients. Oncogene 2016;35(29):3796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pauty J, Couturier AM, Rodrigue A, Caron MC, Coulombe Y, Dellaire G, Masson JY. Cancer-causing mutations in the tumor suppressor PALB2 reveal a novel cancer mechanism using a hidden nuclear export signal in the WD40 repeat motif. Nucleic acids research 2017;45(5):2644–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakya R, Reid LJ, Reczek CR, Cole F, Egli D, Lin CS, deRooij DG, Hirsch S, Ravi K, Hicks JB, Szabolcs M, Jasin M, Baer R, Ludwig T. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science 2011;334(6055):525–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drost R, Dhillon KK, van der Gulden H, van der Heijden I, Brandsma I, Cruz C, Chondronasiou D, Castroviejo-Bermejo M, Boon U, Schut E, van der Burg E, Wientjens E, Pieterse M, Klijn C, Klarenbeek S, Loayza-Puch F, Elkon R, van Deemter L, Rottenberg S, van de Ven M, Dekkers DH, Demmers JA, van Gent DC, Agami R, Balmana J, Serra V, Taniguchi T, Bouwman P, Jonkers J. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest 2016;126(8):2903–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Krais JJ, Bernhardy AJ, Nicolas E, Cai KQ, Harrell MI, Kim HH, George E, Swisher EM, Simpkins F, Johnson N. RING domain-deficient BRCA1 promotes PARP inhibitor and platinum resistance. J Clin Invest 2016;126(8):3145–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartford SA, Chittela R, Ding X, Vyas A, Martin B, Burkett S, Haines DC, Southon E, Tessarollo L, Sharan SK. Interaction with PALB2 Is Essential for Maintenance of Genomic Integrity by BRCA2. PLoS genetics 2016;12(8):e1006236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thirthagiri E, Klarmann KD, Shukla AK, Southon E, Biswas K, Martin BK, North SL, Magidson V, Burkett S, Haines DC, Noer K, Matthai R, Tessarollo L, Loncarek J, Keller JR, Sharan SK. BRCA2 minor transcript lacking exons 4–7 supports viability in mice and may account for survival of humans with a pathogenic biallelic mutation. Human molecular genetics 2016;25(10):1934–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons MT, Tudini E, Li H, Hahnen E, Wappenschmidt B, Feliubadalo L, Aalfs CM, Agata S, Aittomaki K, Alducci E, Alonso-Cerezo MC, Arnold N, Auber B, Austin R, Azzollini J, Balmana J, Barbieri E, Bartram CR, Blanco A, Blumcke B, Bonache S, Bonanni B, Borg A, Bortesi B, Brunet J, Bruzzone C, Bucksch K, Cagnoli G, Caldes T, Caliebe A, Caligo MA, Calvello M, Capone GL, Caputo SM, Carnevali I, Carrasco E, Caux-Moncoutier V, Cavalli P, Cini G, Clarke EM, Concolino P, Cops EJ, Cortesi L, Couch FJ, Darder E, de la Hoya M, Dean M, Debatin I, Del Valle J, Delnatte C, Derive N, Diez O, Ditsch N, Domchek SM, Dutrannoy V, Eccles DM, Ehrencrona H, Enders U, Evans DG, Farra C, Faust U, Felbor U, Feroce I, Fine M, Foulkes WD, Galvao HCR, Gambino G, Gehrig A, Gensini F, Gerdes AM, Germani A, Giesecke J, Gismondi V, Gomez C, Gomez Garcia EB, Gonzalez S, Grau E, Grill S, Gross E, Guerrieri-Gonzaga A, Guillaud-Bataille M, Gutierrez-Enriquez S, Haaf T, Hackmann K, Hansen TVO, Harris M, Hauke J, Heinrich T, Hellebrand H, Herold KN, Honisch E, Horvath J, Houdayer C, Hubbel V, Iglesias S, Izquierdo A, James PA, Janssen LAM, Jeschke U, Kaulfuss S, Keupp K, Kiechle M, Kolbl A, Krieger S, Kruse TA, Kvist A, Lalloo F, Larsen M, Lattimore VL, Lautrup C, Ledig S, Leinert E, Lewis AL, Lim J, Loeffler M, Lopez-Fernandez A, Lucci-Cordisco E, Maass N, Manoukian S, Marabelli M, Matricardi L, Meindl A, Michelli RD, Moghadasi S, Moles-Fernandez A, Montagna M, Montalban G, Monteiro AN, Montes E, Mori L, Moserle L, Muller CR, Mundhenke C, Naldi N, Nathanson KL, Navarro M, Nevanlinna H, Nichols CB, Niederacher D, Nielsen HR, Ong KR, Pachter N, Palmero EI, Papi L, Pedersen IS, Peissel B, Perez-Segura P, Pfeifer K, Pineda M, Pohl-Rescigno E, Poplawski NK, Porfirio B, Quante AS, Ramser J, Reis RM, Revillion F, Rhiem K, Riboli B, Ritter J, Rivera D, Rofes P, Rump A, Salinas M, Sanchez de Abajo AM, Schmidt G, Schoenwiese U, Seggewiss J, Solanes A, Steinemann D, Stiller M, Stoppa-Lyonnet D, Sullivan KJ, Susman R, Sutter C, Tavtigian SV, Teo SH, Teule A, Thomassen M, Tibiletti MG, Tischkowitz M, Tognazzo S, Toland AE, Tornero E, Torngren T, Torres-Esquius S, Toss A, Trainer AH, Tucker KM, van Asperen CJ, van Mackelenbergh MT, Varesco L, Vargas-Parra G, Varon R, Vega A, Velasco A, Vesper AS, Viel A, Vreeswijk MPG, Wagner SA, Waha A, Walker LC, Walters RJ, Wang-Gohrke S, Weber BHF, Weichert W, Wieland K, Wiesmuller L, Witzel I, Wockel A, Woodward ER, Zachariae S, Zampiga V, Zeder-Goss C, Investigators K, Lazaro C, De Nicolo A, Radice P, Engel C, Schmutzler RK, Goldgar DE, Spurdle AB. Large scale multifactorial likelihood quantitative analysis of BRCA1 and BRCA2 variants: An ENIGMA resource to support clinical variant classification. Human mutation 2019;40(9):1557–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worley T, Vallon-Christersson J, Billack B, Borg A, Monteiro AN. A naturally occurring allele of BRCA1 coding for a temperature-sensitive mutant protein. Cancer Biol Ther 2002;1(5):497–501 [DOI] [PubMed] [Google Scholar]
- 54.Lovelock PK, Spurdle AB, Mok MT, Farrugia DJ, Lakhani SR, Healey S, Arnold S, Buchanan D, Investigators K, Couch FJ, Henderson BR, Goldgar DE, Tavtigian SV, Chenevix-Trench G, Brown MA. Identification of BRCA1 missense substitutions that confer partial functional activity: potential moderate risk variants? Breast cancer research : BCR 2007;9(6):R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millot GA, Berger A, Lejour V, Boule JB, Bobo C, Cullin C, Lopes J, Stoppa-Lyonnet D, Nicolas A. Assessment of human Nter and Cter BRCA1 mutations using growth and localization assays in yeast. Human mutation 2011;32(12):1470–80 [DOI] [PubMed] [Google Scholar]
- 56.Lee MS, Green R, Marsillac SM, Coquelle N, Williams RS, Yeung T, Foo D, Hau DD, Hui B, Monteiro AN, Glover JN. Comprehensive analysis of missense variations in the BRCT domain of BRCA1 by structural and functional assays. Cancer research 2010;70(12):4880–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iversen ES, Couch FJ, Goldgar DE, Tavtigian SV, Monteiro ANA. A Computational Method to Classify Variants of Uncertain Significance Using Functional Assay Data with Application to BRCA1. Cancer Epidemiology Biomarkers & Prevention 2011;20(6):1078–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thouvenot P, Ben Yamin B, Fourriere L, Lescure A, Boudier T, Del Nery E, Chauchereau A, Goldgar DE, Houdayer C, Stoppa-Lyonnet D, Nicolas A, Millot GA. Functional Assessment of Genetic Variants with Outcomes Adapted to Clinical Decision-Making. PLoS genetics 2016;12(6):e1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de BM, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. ProcNatlAcadSciUSA 2008;105(44):17079–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434(7035):917–21 [DOI] [PubMed] [Google Scholar]
- 61.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434(7035):913–17 [DOI] [PubMed] [Google Scholar]
- 62.Bouwman P, van der Gulden H, van der Heijden I, Drost R, Klijn CN, Prasetyanti P, Pieterse M, Wientjens E, Seibler J, Hogervorst FB, Jonkers J. A high-throughput functional complementation assay for classification of BRCA1 missense variants. Cancer discovery 2013;3(10):1142–55 [DOI] [PubMed] [Google Scholar]
- 63.Drost R, Bouwman P, Rottenberg S, Boon U, Schut E, Klarenbeek S, Klijn C, van der Heijden I, van der Gulden H, Wientjens E, Pieterse M, Catteau A, Green P, Solomon E, Morris JR, Jonkers J. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer cell 2011;20(6):797–809 [DOI] [PubMed] [Google Scholar]
- 64.Lheureux S, Lai Z, Dougherty BA, Runswick S, Hodgson DR, Timms KM, Lanchbury JS, Kaye S, Gourley C, Bowtell D, Kohn EC, Scott C, Matulonis U, Panzarella T, Karakasis K, Burnier JV, Gilks CB, O’Connor MJ, Robertson JD, Ledermann J, Barrett JC, Ho TW, Oza AM. Long-Term Responders on Olaparib Maintenance in High-Grade Serous Ovarian Cancer: Clinical and Molecular Characterization. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(15):4086–94 [DOI] [PubMed] [Google Scholar]
- 65.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Madry R, Christensen RD, Berek JS, Dorum A, Tinker AV, du Bois A, Gonzalez-Martin A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA, Investigators E-ON. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England journal of medicine 2016;375(22):2154–64 [DOI] [PubMed] [Google Scholar]
- 66.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O’Malley DM, Armstrong DK, Garcia-Donas J, Swisher EM, Floquet A, Konecny GE, McNeish IA, Scott CL, Cameron T, Maloney L, Isaacson J, Goble S, Grace C, Harding TC, Raponi M, Sun J, Lin KK, Giordano H, Ledermann JA, investigators A. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390(10106):1949–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339(6121):819–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013;153(4):910–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic acids research 2016;44(D1):D862–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris JR, Pangon L, Boutell C, Katagiri T, Keep NH, Solomon E. Genetic analysis of BRCA1 ubiquitin ligase activity and its relationship to breast cancer susceptibility. Human molecular genetics 2006;15(4):599–606 [DOI] [PubMed] [Google Scholar]
- 71.Starita LM, Young DL, Islam M, Kitzman JO, Gullingsrud J, Hause RJ, Fowler DM, Parvin JD, Shendure J, Fields S. Massively Parallel Functional Analysis of BRCA1 RING Domain Variants. Genetics 2015;200(2):413–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petitalot A, Dardillac E, Jacquet E, Nhiri N, Guirouilh-Barbat J, Julien P, Bouazzaoui I, Bonte D, Feunteun J, Schnell JA, Lafitte P, Aude JC, Nogues C, Rouleau E, Lidereau R, Lopez BS, Zinn-Justin S, Caputo SM. Combining Homologous Recombination and Phosphopeptide-binding Data to Predict the Impact of BRCA1 BRCT Variants on Cancer Risk. Molecular cancer research : MCR 2018 [DOI] [PubMed] [Google Scholar]
- 73.Anantha RW, Simhadri S, Foo TK, Miao S, Liu J, Shen Z, Ganesan S, Xia B. Functional and mutational landscapes of BRCA1 for homology-directed repair and therapy resistance. Elife 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guidugli L, Shimelis H, Masica DL, Pankratz VS, Lipton GB, Singh N, Hu C, Monteiro ANA, Lindor NM, Goldgar DE, Karchin R, Iversen ES, Couch FJ. Assessment of the Clinical Relevance of BRCA2 Missense Variants by Functional and Computational Approaches. American journal of human genetics 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mesman RLS, Calleja F, Hendriks G, Morolli B, Misovic B, Devilee P, van Asperen CJ, Vrieling H, Vreeswijk MPG. The functional impact of variants of uncertain significance in BRCA2. Genetics in medicine : official journal of the American College of Medical Genetics 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]