Abstract
Non-polio enteroviruses such as enterovirus A71 (EV-A71), EV-D68, and coxsackievirus B3 (CVB3) are significant human pathogens with disease manifestations ranging from mild flu-like symptoms to more severe encephalitis, myocarditis, acute flaccid paralysis/myelitis, and even death. There is currently no effective antivirals to prevent or treat non-polio enterovirus infection. In this study, we report our progress in developing potent and broad-spectrum antivirals against these non-polio enteroviruses. Starting from our previously developed lead compounds that had potent antiviral activity against EV-D68, we synthesized 43 analogs and profiled their broad-spectrum antiviral activity against additional EV-D68, EV-A71, and CVB3 viruses. Promising candidates were also selected for mouse microsomal stability test to prioritize lead compounds for future in vivo mouse model studies. Collectively, this multi-parameter optimization process revealed a promising lead compound 6aw that showed single-digit to submicromolar EC50 values against two EV-D68 strains (US/KY and US/MO), two EV-A71 strains (Tainan and US/AK), and one CVB3 strain, with a high selectivity index. Encouragingly, 6aw was stable in mouse microsomes with a half-life of 114.7 minutes. Overall, 6aw represents one of the most potent broad-spectrum antiviral against non-polio enteroviruses, rendering it a promising lead candidate for non-polio enteroviruses with translational potential.
Keywords: Enterovirus, A71, D68, coxsackievirus B3, quinoline, antiviral
Graphical Abstract

Enterovirus, a genus from the Picornaviridae family, is implicated in more than 10 million illnesses in the United States each year1. Among the various species of enteroviruses, 7 commonly infect humans: enterovirus A-D and rhinoviruses A-C. Since the successful eradication of poliovirus (PV) in the developed world, the main cause of enteroviral diseases are associated with non-polio enterovirus (EV) infections. Classification of enterovirus species are based principally on genomic and viral protein similarities. However, many species can have distinct cell surface receptors for entry, tissue tropism and pathogenesis. Enterovirus A serotype EV-A71 have been associated with large-scale epidemics since the early 1970s in Europe, the United States, Australia, and most aggressively, in the Asia-Pacific region2. EV-A71 is associated with many neurological complications similar to other EVs, including acute flaccid paralysis (AFM), aseptic meningitis, encephalitis or cardiorespiratory illness, but in most cases, EV-A71 is known for causing Hand Foot and Mouth Disease (HFMD) in children3. Enterovirus B serotype coxsackievirus B3 (CVB3) is commonly associated with viral heart disease, and may play a role in the onset of juvenile diabetes mellitus4. As of date, the most mysterious EV is the serotype enterovirus D68 (EV-D68) from the enterovirus D species. Unlike most enteroviruses, EV-D68 was largely unstudied until an unexpected epidemic occurred in 2014 in the United States5. Since then the outbreak appears to be reoccurring in a biennial pattern. Initial clinical manifestation of EV-D68 infection resembles rhinovirus infection, with mild-to-severe upper respiratory infection in children between the ages of 1–116. Unlike rhinovirus infection, recent EV-D68 strains have been linked with a large number of patients developing acute flaccid myelitis, or AFM7.
All EVs are non-enveloped positive-sense RNA viruses that are encapsulated by viral proteins VP1–4. The viral genome can be translated directly into a polyprotein which is processed by viral 2A and 3C proteases. Genome replication is mediated by the RNA-dependent RNA polymerase (RDRP) 3Dpol in replication organelles. The multifunctional viral protein 2C is involved in membrane rearrangement8, uncoating9, assembly9, 10, as well as viral RNA replication11. 2C contains Walker A,B, and C motifs that form a conserved ATPase domain that has helicase activity in vitro12. 2C also contains an N-terminal membrane-binding domain as well as a C-terminal domain that may play a role in the formation of dimers or higher oligomeric species13. Given the multifunctional roles of 2C in viral replication, it appears to be a high profile antiviral drug target14.
Until vaccines can be developed, antivirals are required to alleviate the burden of disease and reduce fatalities associated with non-polio enterovirus infections. As of date, no antiviral is approved for the treatment of any enterovirus infection. The most promising candidates are direct-acting antivirals targeting viral proteins such as viral VP1 capsid protein, 2A and 3C proteases, 2C protein, and the viral 3D polymerase1, 15–18. The viral 2C protein is a conserved viral protein, and has been validated as a drug target by a number of potent antivirals14, 19. Dibucaine, a quinoline derivative commonly used as a topical anesthetic, was found to inhibit the replication of CVB3 by targeting the viral 2C protein20. However, it was found that dibucaine was only moderately effective against other enteroviruses such as EV-A71 and EV-D6820. Recently, structure-activity relationship studies show that chemical modification of dibucaine increased its potency against contemporary strains of EV-D6814. However, their broad-spectrum antiviral activity against EV-A71 and coxsackievirus B3 virus remains to be profiled. In this work, we focus on optimizing the broad-spectrum antiviral activity and in vitro microsomal stability of quinoline analogs through structure-activity relationship (SAR) studies and structure-property (SPR) relationship studies. In addition, thermal shift binding assay was applied to quantify the direct binding between quinoline analogs and the viral 2C protein. Overall, this study revealed several promising quinoline analogs that had potent and broad-spectrum antiviral activity against EV-D68, EV-A71 and CVB3.
The viral 2C protein is relatively conserved among non-polio enteroviruses. Therefore, we hypothesized that 2C inhibitors will have broad-spectrum antiviral activity against non-polio enteroviruses. To test this hypothesis, we first started by profiling the broad-spectrum antiviral activity of literature reported 2C inhibitors20–24 against the non-polio enteroviruses including EV-D68, EV-A71 and CVB3 in cell culture using cytopathic effect (CPE) assay. It was found that dibucaine, fluoxetine, pirlindole mesylate, and formoterol all inhibited EV-D68 and CVB3, but not the EV-A71 virus. Moreover, they all had a low selectivity index (SI50 < 50). The only exception was guanidine, which showed broad-spectrum antiviral activity against all three viruses, but only at very high concentrations (200 μM–900 μM). Overall, the existing 2C inhibitors appears to lack the broad-spectrum antiviral activity against non-polio enteroviruses. Nevertheless, the results of guanidine suggested that it might be feasible to develop broad-spectrum antivirals against non-polio enteroviruses. As such, our focus of this study is to develop viral 2C inhibitors as broad-spectrum antivirals against not only EV-D68, but also EV-A71 and CVB3.
Synthesis of quinoline analogs started with Suzuki-Miyaura cross-coupling of 2-chloroquinoline-4-carboxylic acid 1 with various boronic acids 2. The carboxylic acid intermediate 3 was then reacted with various diamines to give the amide intermediate 5. Deprotection of Boc by TFA gave the final product 6. Overall, this is a highly efficient synthesis with an overall yield of 53.2 to 76.9%.
The purpose of the current structure-activity relationship studies was to provide additional lead compounds with potent antiviral activity (EC50 ≤ 1 μM) and a high selectivity index (SI50 > 100) for the following broad-spectrum antiviral activity profiling and mouse microsomal stability test. It is expected that majority of the compounds will be filtered during this multi-parameter optimization process; therefore it is imperative to obtain as many backup molecules as possible in order to increase the chance of success.
The antiviral activity of all compounds was initially tested against EV-D68 US/KY/14–18953 virus in RD cells using the CPE assay. The cytotoxicity assay was performed in parallel in RD cells using the neutral red method. Next, compounds with potent antiviral activity (EC50 ≤ 1 μM) and a high selectivity index (SI50 > 100) against EV-D68 US/KY/14–18953 were selected for further testing against additional EV-D68, EV-A71 and CVB3 viruses. In parallel, promising lead compounds were profiled for mouse microsomal stability. Overall, this is a multiparameter optimization approach with an aim of identifying lead compounds with broad-spectrum antiviral activity and favorable in vitro pharmacokinetic properties.
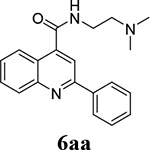
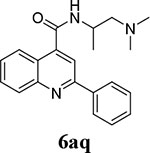
Compounds 6aa and 6bf were identified as potent EV-D68 antivirals from the previous round of structure-activity relationship studies of dibucaine14, and both compounds were included as references (Table 2). For the series of compounds with 1-position substitution being benzene (6aa – 6aq), it was found that replacing the 4-position amide linkage with ester abolished the antiviral activity (compound 6ab vs 6aa). Extending the linker between amide to terminal amine from ethyl to propyl also reduced the antiviral activity (6aa vs 6ac and 6ad). Compound 6ae with a terminal imidazole group was less active than compound 6aa that had a dimethyl amine group. Compounds 6ag, 6ah, 6ai, 6aj, 6ak, 6al, and 6am all had a single-digit micromolar EC50 value, but their low selectivity indexes (SI = 3.2 – 16.1) did not warrant further investigation of these compounds. Compounds 6af, 6an, and 6ap had similar antiviral activity as compound 6aa, but their selectivity indexes did not pass the selectivity threshold (SI > 100). The only compound met the criteria was compound 6aq (EC50 = 0.5 ± 0.3 μM; SI50 = 131.6). It is known that benzene substituent might be easily metabolized, especially through 4-hydroxylation. We therefore made an effort to block this metabolism by introducing either deuterium (6ar – 6au) or fluorine (6av – 6be). For compounds with deuterated benzene at 1-position (6ar – 6au), compound 6ar was the most potent and selective candidate (EC50 = 0.3 ± 0.2 μM; SI50 = 268.7), and it was selected for further characterization. For compounds with fluorine-substituted benzene at 1-position (6av – 6be), the four compounds 6av, 6aw, 6ax, and 6ba met the selection criteria (EC50 < 1 μM; SI50 > 100). For compounds containing thiophene at 1-position (6bf – 6bn), the five compounds 6bf, 6bg, 6bi, 6bj, and 6bl had the desired potency and selectivity index for further development. For compounds with furan at 1-position (6bo – 6bs), only compound 6bo met the selection criteria.
Table 2.
Antiviral activity against EV-D68 US/KY/14–18953.
  
|
Antiviral efficacy was determined in the CPE assay. Cytotoxicity was determined using the neutral red method. The results are the mean ± standard deviation of three repeats. SI = selectivity index (CC50/EC50). N.A. = not applicable.
Overall, in addition to the two reference compounds (6aa and 6bf), we identified 11 additional candidates (6aq, 6ar, 6av, 6aw, 6ax, 6ba, 6bg, 6bi, 6bj, 6bl, and 6bo) with high antiviral potency (EC50 < 1 μM) and an optimal selectivity index (SI50 > 100) that warrant further profiling of broad-spectrum antiviral activity and mouse microsomal stability.
Lead candidates with potent antiviral activity (EC50 < 1 μM) and a high selectivity index (SI50 > 100) against EV-D68 US/KY/14–18953 virus from Table 2 were selected for further testing against other enteroviruses, including EV-D68 US/MO/14–18947, EV-A71 Tainan/4643/98, EV-A71 US/AK/16–19516, and CVB3 viruses. In total 13 compounds met these criteria. In parallel, promising lead compounds were further tested for mouse microsomal stability. It was found that all 13 compounds had potent antiviral activity against both EV-D68 strains (US/KY and US/MO) with EC50 values ranging from single-digit to submicromolar. Encouragingly, all 13 compounds also had potent antiviral activity against CVB3 virus, and the EC50 values were very similar to that of EV-D68 viruses. When tested against the two EV-A71 strains, Tainan and US/AK, most of the compounds (6aa, 6ar, 6av, 6aw, 6ax, 6ba, 6bf, 6bg, 6bi) showed potent inhibition as well with low single-digit micromolar EC50 values. The four compounds 6aq, 6bj, 6bl and 6bo were less potent and had high single-digit to double-digit micromolar EC50 values. Overall, the pattern of broad-spectrum antiviral activity for compounds shown in Table 3 matches with that of known 2C inhibitors shown in Table 1: the 2C inhibitors have potent antiviral activity against EV-D68 and CVB3, but they are generally less active against EV-A71. Nevertheless, compounds 6ar, 6av, 6aw, 6bg, 6bi all had single-digit micromolar EC50 values against EV-A71, in addition to their potent antiviral activity against EV-D68 and CVB3 viruses.
Table 3.
Broad-spectrum antiviral activity against EV-D68, EV-A71, CVB3, and mouse microsomal stability.
| Structure | Anti-EV-D68 in RD cells (μM) | Anti-EV-A71 in RD cells (μM) | Anti-CVB3 in Vero C1008 cells (μM) | Mouse microsomal stability T1/2 (mins) | CLint(liver) (ml/min/kg) |
|---|---|---|---|---|---|
 |
US/KY/14–18953 EC50 = 0.4 ± 0.2 CC50 = 73.7 ± 19.1 SI50 = 184 US/MO/14–18947 EC50 = 0.2 ± 0.07 CC50 = 73.7 ± 19.1 SI50 = 369 |
Tainan EC50 = 4.2 ± 0.5 CC50 = 73.7 ± 19.1 SI50 = 18 US/AK EC50 > 10 |
EC50 = 0.3 ±
0.2 CC50 = 116.2 ± 56.3 SI50 = 387 |
22.4 | 245.5 |
 |
US/KY/14–18953 EC50 = 0.9 ± 0.3 CC50 = 47.8 ± 3.6 SI50 = 53 US/MO/14–18947 EC50 = 0.6 ± 0.3 CC50 = 47.8 ± 3.6 SI50 = 80 |
Tainan EC50 = 7.8 CC50 = 47.8 ± 3.6 SI50 = 6 US/AK EC50 > 10 |
EC50 = 1.1 ±
0.2 CC50 > 30.0 SI50 > 27 |
60.2 | 91.2 |
 |
US/KY/14–18953 EC50 = 0.5 ± 0.3 CC50 = 65.8 ± 16.8 SI50 = 132 US/MO/14–18947 EC50 = 0.3 ± 0.1 CC50 = 65.8 ± 16.8 SI50 = 219 |
Tainan EC50 = 18.3 ± 4.2 CC50 = 65.8 ± 16.8 SI50 = 4 US/AK EC50 = 11.7 ± 3.0 CC50 = 65.8 ± 16.8 SI50 = 6 |
EC50 = 2.0 ±
0.2 CC50 = 185.9 ± 13.9 SI50 = 93.0 |
N.T. | N.T. |
 |
US/KY/14–18953 EC50 = 0.3 ± 0.2 CC50 = 80.6 ± 3.3 SI50 = 269 US/MO/14–18947 EC50 = 0.2 ± 0.1 CC50 = 80.6 ± 3.3 SI50 = 403 |
Tainan EC50 = 5.2 ± 0.2 CC50 = 80.6 ± 3.3 SI50 = 16 US/AK EC50 = 4.9 ± 0.2 CC50 = 80.6 ± 3.3 SI50 = 16 |
EC50 = 0.4 ±
0.1 CC50 = 128.8 ± 25.8 SI50 = 322.0 |
23.6 | 232.2 |
 |
US/KY/14–18953 EC50 = 0.1 ± 0.1 CC50 = 36.2 ± 3.1 SI50 = 362 US/MO/14–18947 EC50 = 0.05 ± 0.02 CC50 = 36.2 ± 3.1 SI50 = 724 |
Tainan EC50 = 2.2 ± 0.1 CC50 = 36.2 ± 3.1 SI50 = 17 US/AK EC50 = 2.3 ± 0.1 CC50 = 36.2 ± 3.1 SI50 = 16 |
EC50 = 0.06 ±
0.04 CC50 = 48.8 ± 17.7 SI50 = 813 |
34.2 | 160.5 |
 |
US/KY/14–18953 EC50 = 0.2 ± 0.1 CC50 = 32.4 ± 4.1 SI50 = 162 US/MO/14–18947 EC50 = 0.1 ± 0.02 CC50 = 32.4 ± 4.1 SI50 = 324 |
Tainan EC50 = 3.1 ± 1.0 CC50 = 32.4 ± 4.1 SI50 = 11 US/AK EC50 = 3.6 ± 0.2 CC50 = 32.4 ± 4.1 SI50 = 9 |
EC50 = 0.2 ±
0.2 CC50 = 40.1 ± 13.2 SI50 = 200.5 |
114.7 | 47.9 |
 |
US/KY/14–18953 EC50 = 0.4 ± 0.1 CC50 = 48.2 ± 2.3 SI50 = 121 US/MO/14–18947 EC50 = 0.3 ± 0.2 CC50 = 48.2 ± 2.3 SI50 = 161 |
Tainan EC50 = 9.0 ± 0.3 CC50 = 48.2 ± 2.3 SI50 = 5 US/AK EC50 = 14.0 ± 2.1 CC50 = 48.2 ± 2.3 SI50 = 3 |
EC50 = 0.4 ±
0.1 CC50 = 52.3 ± 5.0 SI50 = 131 |
114.1 | 48.1 |
 |
US/KY/14–18953 EC50 = 0.3 ± 0.2 CC50 = 36.7 ± 5.6 SI50 = 122 US/MO/14–18947 EC50 = 0.2 ± 0.1 CC50 = 36.7 ± 5.6 SI50 = 184 |
Tainan EC50 = 7.9 ± 0.5 CC50 = 36.7 ± 5.6 SI50 = 5 US/AK EC50 > 10 |
EC50 = 0.4 ±
0.2 CC50 = 92.3 ± 3.2 SI50 = 231 |
N.T. | N.T. |
 |
US/KY/14–18953 EC50 = 0.04 ± 0.02 CC50 = 42.3 ± 8.3 SI50 = 1058 US/MO/14–18947 EC50 = 0.05 ± 0.03 CC50 = 42.3 ± 8.3 SI50 = 846 |
Tainan EC50 = 2.9 ± 0.2 CC50 = 42.3 ± 8.3 SI50 = 15 US/AK EC50 > 10 |
EC50 = 0.07 ± 0.0
μM CC50 = 94.5 ± 4.1 SI50 = 1350 |
26.6 | 206.6 |
 |
US/KY/14–18953 EC50 = 0.1 ± 0.1 CC50 = 20.6 ± 0.9 SI50 = 206 US/MO/14–18947 EC50 = 0.1 ± 0.03 CC50 = 20.6 ± 0.9 SI50 = 206 |
Tainan EC50 = 7.9 ± 0.4 CC50 = 20.6 ± 0.9 SI50 = 3 US/AK EC50 = 8.8 ± 0.8 CC50 = 20.6 ± 0.9 SI50 = 2 |
EC50 = 0.4 ±
0.2 CC50 = 111.8 ± 7.6 SI50 = 280 |
N.T. | N.T. |
 |
US/KY/14–18953 EC50 = 0.1 ± 0.2 CC50 = 39.0 ± 3.4 SI50 = 390 US/MO/14–18947 EC50 = 0.06 ± 0.03 CC50 = 39.0 ± 3.4 SI50 = 650 |
Tainan EC50 = 6.3 ± 0.6 CC50 = 39.0 ± 3.4 SI50 = 6 US/AK EC50 = 8.0 ± 0.5 CC50 = 39.0 ± 3.4 SI50 = 5 |
EC50 = 0.2 ±
0.1 CC50 = 90.3 ± 5.9 SI50 = 452 |
N.T. | N.T. |
 |
US/KY/14–18953 EC50 = 0.3 ± 0.1 CC50 = 103.1 ± 49.5 SI50 = 344 US/MO/14–18947 EC50 = 0.2 ± 0.1 CC50 = 103.1 ± 49.5 SI50 = 516 |
Tainan EC50 = 8.4 ± 2.6 CC50 = 103.1 ± 49.5 SI50 = 12 US/AK EC50 = 11.5 ± 0.8 CC50 = 103.1 ± 49.5 SI50 = 9 |
EC50 = 0.3 ±
0.05 CC50 = 70.7 ± 4.8 SI50 = 236 |
75.4 | 72.8 |
 |
US/KY/14–18953 EC50 = 0.4 ± 0.1 CC50 = 42.1 ± 13.4 SI50 = 105 US/MO/14–18947 EC50 = 0.2 ± 0.1 CC50 = 42.1 ± 13.4 SI50 = 211 |
Tainan EC50 = 38.3 ± 6.5 CC50 = 42.1 ± 13.4 SI50 = 1 US/AK EC50 > 30 |
EC50 = 0.6 ±
0.2 CC50 = 63.7 ± 3.2 SI50 = 106 |
100.8 | 54.5 |
 |
US/KY/14–18953 EC50 = 1.1 ± 0.4 CC50 = 198.6 ± 9.4 SI50 = 181 US/MO/14–18947 EC50 = 1.1 ± 0.1 CC50 = 198.6 ± 9.4 SI50 = 181 |
Tainan EC50 = 30.9 ± 1.3 CC50 = 198.6 ± 9.4 SI50 = 6 US/AK EC50 = 20.9 ± 8.2 CC50 = 198.6 ± 9.4 SI50 = 10 |
EC50 = 1.2 ±
0.1 CC50 > 300.0 SI50 > 250 |
N.T. | N.T. |
Antiviral efficacy was determined in the CPE assay. Cytotoxicity was determined using the neutral red method. The results are the mean ± standard deviation of three repeats. SI = selectivity index (CC50/EC50). T1/2 was obtained using mouse liver microsomes. N.T. = not tested.
Table 1.
Broad-spectrum antiviral activity of known 2C inhibitors against non-polio enteroviruses.
| Structure | Antiviral activity and selectivity index against EV-D68 US/KY/14-18953 in RD cells (μM) | Antiviral activity and selectivity index against EV-A71 Tainan in RD cells (μM) | Antiviral activity and selectivity index against CVB3 in Vero cells (μM) |
|---|---|---|---|
 |
EC50 = 5.3 ±
1.2 CC50 = 56.6 ± 28.7 SI50 = 11 |
EC50 >
10.0 CC50 = 56.6 ± 28.7 SI50 = N.A. |
EC50 = 1.3 ±
0.2 CC50 = 64.4 ± 2.1 SI50 = 50 |
 |
EC50 = 1.0 ±
0.1 CC50 = 11.9 ± 4.9 SI50 = 12 |
EC50 >
10.0 CC50 = 11.9 ± 4.9 SI50 = N.A. |
EC50 = 2.1 ±
1.0 CC50 = 11.0 ± 3.1 SI50 = 5 |
 |
EC50 = 8.4 ±
1.1 CC50 = 19.0 ± 1.6 SI50 = 2 |
EC50 > 20 CC50 = 19.0 ± 1.6 SI50 = N.A. |
EC50 = 3.6 ±
0.8 CC50 = 40.5 ± 3.5 SI50 = 11 |
 |
EC50 = 24.7 ±
1.1 CC50 = 146.3 ± 27.8 SI50 = 6 |
EC50 >
100 CC50 = 146.3 ± 27.8 SI50 = N.A. |
EC50 = 9.9 ±
1.1 CC50 > 300.0 SI50 > 30 |
 |
EC50 = 219.4 ±
15.0 CC50 > 1000.0 SI50 > 4 |
EC50 =
901.6 CC50 > 1000.0 SI50 > 1 |
EC50 = 224.5 ±
57.9 CC50 > 3000.0 SI50 > 13 |
Antiviral efficacy was determined in the CPE assay. Cytotoxicity was determined using the neutral red method. The results are the mean ± standard deviation of three repeats. SI50 = selectivity index (CC50/EC50). N.A. = not applicable.
Next, selected lead compounds with potent and broad-spectrum antiviral activity against EV-D68, EV-A71, and CVB3 were profiled for mouse microsomal stability25, 26. Compound 6af was included to investigate the influence of the terminal amine substitution on microsomal stability. In general, compounds with terminal monoalkylamine and dialkylamine had longer half-lives than compounds with trialkylamine (6af vs 6aa; 6aw, 6ax vs 6av; 6bj, 6bl vs 6bf). Installing a fluorine at the 4-position of benzene indeed increased the microsomal stability (6aw vs 6af). Collectively, the most promising candidate is 6aw, which not only had potent antiviral activity against EV-D68, EV-A71 and CVB3 viruses, but also had a long half-life in mouse microsomes with T1/2 of 114.7 minutes.
To provide direct evidence that the quinoline compounds target the viral 2C protein, we expressed the CVB3 2C protein and quantified the drug binding using the differential scanning fluorimetry (DSF), or thermal shift assay (TSA)16, 24. In this assay, a temperature gradient is applied to unfold a given protein, and when the protein unfolds, it exposes the hydrophobic region that can bind to a fluorescence dye, resulting in increased fluorescence emission. When the protein is stabilized by a small molecule binder, the melting temperature, Tm, will increase. For this experiment, we included two known 2C inhibitors, fluoxetine and guanidine as positive controls, and we tested the most promising lead candidate 6aw in three different concentrations. As shown in Fig. 1, CVB3 2C protein was stabilized by both fluoxetine and guanidine with Tm shifts of 1.31 and 1.77 °C when tested at 100 μM and 1 mM, respectively (Figs. 1A&1B). Similarly, compound 6aw also increased the thermal stability of CVB3 2C in a concentration dependent manner and had a more profound effect than fluoxetine at 100 μM (Figs. 1C&1D), which suggests that compound 6aw binds with a higher affinity than fluoxetine to 2C protein. This result is consistent with the higher antiviral efficacy of 6aw compared to fluoxetine. Overall, the TSA assay provided direct evidence that quinoline compound 6aw binds directly to the viral 2C protein and the binding affinity is correlated with the cellular antiviral efficacy.
Figure 1.

Binding of fluoxetine, guanidine, and 6aw to CVB3 2C protein using differential scanning fluorimetry. CVB3 (4.5 μM) 2C was incubated with DMSO (black), 100 μM fluoxetine (A), 1 mM guanidine (B), or different concentrations of 6aw (C), and the raw DSF data were plotted and curve fitting were performed using the Boltzmann sigmoidal equation in Prism (v5) software to determine the Tm and ΔTm (D).
Non-polio enteroviruses are significant human pathogens for which we do not have any treatment available for now. More alarmingly, there have been an increasing trend of severe infections including EV-A71 and EV-D68 associated neurological complications such as acute flaccid myelitis in recently years27, 28. Although the detailed mechanism regarding how these contemporary viruses lead to neurological infections is still under debate29, 30, there is nevertheless a consensus among the scientific community that EV-A71 and EV-D68 are the etiological agents causing the neurological complications based on clinical data as well as animal model studies31, 32. As such, developing effective antivirals against these non-polio enteroviruses is a valid approach to prevent and treat these viral infections.
In this study, we aim to develop broad-spectrum antivirals with a high selectivity index as well as a long half-life in mouse microsomes. This is a multi-parameter optimization approach and the focus was on balancing different properties. To achieve this goal, we adapted a stepwise optimization strategy. Specifically, we started with structure-activity relationship studies of a quinoline lead compound 6aa, and generated a number of candidates with potent antiviral activity and a high selectivity index against the EV-D68 US/KY/14–18953 virus. Next, 13 prioritized lead compounds were further profiled for broad-spectrum antiviral activity against an additional strain of EV-D68, the EV-D68 US/MO/14–18947 virus, as well as two EV-A71 viruses (Tainan/4643/98 and US/AK/16–19516) and one CVB3 virus. In parallel, compounds with potent and broad-spectrum antiviral activity were evaluated for mouse liver microsomal stability. Overall, this multi-parameter optimization strategy yielded the promising candidate 6aw that not only showed potent and broad-spectrum antiviral activity against all five strains of non-polio enteroviruses tested, but also displayed a high microsomal stability with a T1/2 of 114.7 minutes. In summary, this study is a step forward towards developing the urgently needed antivirals against non-polio enteroviruses, and the results presented herein might be informative in guiding other medicinal chemistry projects, such as optimization of microsomal stability.
Supplementary Material
Scheme 1.

Synthesis of quinoline analogs
Highlights.
Compound 6aw inhibits multiple strains of EV-D68, EV-A71 and CVB3
Compound 6aw has a high selectivity index
Compound 6aw is stable in mouse liver microsomes
Compound 6aw targets viral 2C protein as confirmed by thermal shift binding assay
Funding
This research is supported by the NIH grant AI147325, and the Arizona Biomedical Research Centre Young Investigator grant ADHS18-198859 to J.W.
ABBREVIATIONS
- AFM
acute flaccid myelitis
- EV-D68
enterovirus D68
- EV-A71
enterovirus A71
- SI
selectivity index
- TSA
thermal shift assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting information
The supporting information is available free of charge on the ACS Publications website: synthesis procedures; characterization of compounds; antiviral and cytotoxicity assays; mouse microsomal stability assay; thermal shift assay.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Baggen J; Thibaut HJ; Strating JRPM; van Kuppeveld FJM The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol 2018, 16, 368–381. [DOI] [PubMed] [Google Scholar]
- 2.Solomon T; Lewthwaite P; Perera D; Cardoso MJ; McMinn P; Ooi MH Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis 2010, 10, 778–790. [DOI] [PubMed] [Google Scholar]
- 3.Ooi MH; Wong SC; Lewthwaite P; Cardosa MJ; Solomon T Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [DOI] [PubMed] [Google Scholar]
- 4.Baboonian C; Davies MJ; Booth JC; McKenna WJ Coxsackie B viruses and human heart disease. Curr. Opin. Microbiol. Immunol 1997, 223, 31–52. [DOI] [PubMed] [Google Scholar]
- 5.Holm-Hansen CC; Midgley SE; Fischer TK Global emergence of enterovirus D68: a systematic review. Lancet Infect. Dis 2016, 16, E64–E75. [DOI] [PubMed] [Google Scholar]
- 6.Midgley CM; Watson JT; Nix WA; Curns AT; Rogers SL; Brown BA; Conover C; Dominguez SR; Feikin DR; Gray S; Hassan F; Hoferka S; Jackson MA; Johnson D; Leshem E; Miller L; Nichols JB; Nyquist AC; Obringer E; Patel A; Patel M; Rho B; Schneider E; Schuster JE; Selvarangan R; Seward JF; Turabelidze G; Oberste MS; Pallansch MA; Gerber S; Grp E-DW Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir. Med 2015, 3, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greninger AL; Naccache SN; Messacar K; Clayton A; Yu GX; Somasekar S; Federman S; Stryke D; Anderson C; Yagi S; Messenger S; Wadford D; Xia DX; Watt JP; Van Haren K; Dominguez SR; Glaser C; Aldrovandi G; Chiu CY A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect. Dis 2015, 15, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teterina NL; Gorbalenya AE; Egger D; Bienz K; Ehrenfeld E Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J. Virol 1997, 71, 8962–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asare E; Mugavero J; Jiang P; Wimmer E; Paul AV A Single Amino Acid Substitution in Poliovirus Nonstructural Protein 2C(ATPase) Causes Conditional Defects in Encapsidation and Uncoating. J. Virol 2016, 90, 6174–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CL; Jiang P; Sand C; Paul AV; Wimmer E Alanine Scanning of Poliovirus 2C(ATPase) Reveals New Genetic Evidence that Capsid Protein/2C(ATPase) Interactions Are Essential for Morphogenesis. J. Virol 2012, 86, 9964–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton DJ; Flanegan JB Synchronous replication of poliovirus RNA: Initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J. Virol 1997, 71, 8482–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia HJ; Wang PP; Wang GC; Yang J; Sun XL; Wu WZ; Qiu Y; Shu T; Zhao XL; Yin L; Qin CF; Hu YY; Zhou X Human Enterovirus Nonstructural Protein 2C(ATPase) Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone. Plos Pathog. 2015, 11, e1002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan HX; Tian J; Zhang C; Qin B; Cui S Crystal structure of a soluble fragment of poliovirus 2C(ATPase). Plos Pathog. 2018, 14, e1007304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musharrafieh R; Zhang JT; Tuohy P; Kitamura N; Bellampalli SS; Hu YM; Khanna R; Wang J Discovery of Quinoline Analogues as Potent Antivirals against Enterovirus D68 (EV-D68). J. Med. Chem 2019, 62, 4074–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JY; Kung YA; Shih SR Antivirals and vaccines for Enterovirus A71. J. Biomed. Sci 2019, 26, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musharrafieh R; Ma C; Zhang J; Hu Y; Diesing JM; Marty MT; Wang J Validating Enterovirus D68–2A(pro) as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68–2A(pro) Inhibitor. J. Virol 2019, 93, e02221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egorova A; Ekins S; Schmidtke M; Makarov V Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses. Eur. J. Med. Chem 2019, 178, 606–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C; Hu Y; Zhang J; Musharrafieh R; Wang J A Novel Capsid Binding Inhibitor Displays Potent Antiviral Activity against Enterovirus D68. ACS Infect. Dis 2019, 5, 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer L; Lyoo H; van der Schaar HM; Strating J; van Kuppeveld FJM Direct-acting antivirals and host-targeting strategies to combat enterovirus infections. Curr. Opin. Virol 2017, 24, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulferts R; de Boer SM; van der Linden L; Bauer L; Lyoo HR; Mate MJ; Lichiere J; Canard B; Lelieveld D; Omta W; Egan D; Coutard B; van Kuppeveld FJM Screening of a Library of FDA-Approved Drugs Identifies Several Enterovirus Replication Inhibitors That Target Viral Protein 2C. Antimicrob. Agents. Chemother 2016, 60, 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoden E; Zhang M; Nix WA; Oberste MS In Vitro Efficacy of Antiviral Compounds against Enterovirus D68. Antimicrob. Agents. Chemother 2015, 59, 7779–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L; Meijer A; Froeyen M; Zhang L; Thibaut HJ; Baggen J; George S; Vernachio J; van Kuppeveld FJ; Leyssen P; Hilgenfeld R; Neyts J; Delang L Antiviral Activity of Broad-Spectrum and Enterovirus-Specific Inhibitors against Clinical Isolates of Enterovirus D68. Antimicrob. Agents. Chemother 2015, 59, 7782–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smee DF; Evans WJ; Nicolaou KC; Tarbet EB; Day CW Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res. 2016, 131, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer L; Manganaro R; Zonsics B; Strating J; El Kazzi P; Lorenzo Lopez M; Ulferts R; van Hoey C; Mate MJ; Langer T; Coutard B; Brancale A; van Kuppeveld FJM Fluoxetine Inhibits Enterovirus Replication by Targeting the Viral 2C Protein in a Stereospecific Manner. ACS Infect. Dis 2019, 5, 1609–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y; Hu Y; Xu S; Zhang Y; Musharrafieh R; Hau RK; Ma C; Wang J In Vitro Pharmacokinetic Optimizations of AM2-S31N Channel Blockers Led to the Discovery of Slow-Binding Inhibitors with Potent Antiviral Activity against Drug-Resistant Influenza A Viruses. J. Med. Chem 2018, 61, 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y; Hau RK; Wang Y; Tuohy P; Zhang Y; Xu S; Ma C; Wang J Structure-Property Relationship Studies of Influenza A Virus AM2-S31N Proton Channel Blockers. ACS Med. Chem. Lett 2018, 9, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morens DM; Folkers GK; Fauci AS Acute Flaccid Myelitis: Something Old and Something New. Mbio 2019, 10, e00521–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassidy H; Poelman R; Knoester M; Van Leer-Buter CC; Niesters HGM Enterovirus D68-The New Polio? Frontiers Microbiol. 2018, 9, 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld AB; Warren AL; Racaniello VR Neurotropism of Enterovirus D68 Isolates Is Independent of Sialic Acid and Is Not a Recently Acquired Phenotype. mBio 2019, 10, e02370–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DM; Hixon AM; Oldfield LM; Zhang Y; Novotny M; Wang W; Das SR; Shabman RS; Tyler KL; Scheuermann RH Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells. mBio 2018, 9, e01954–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert RD; Hawes IA; Ramachandran PS; Ramesh A; Crawford ED; Pak JE; Wu W; Cheung CK; O’Donovan BD; Tato CM; Lyden A; Tan M; Sit R; Sowa GA; Sample HA; Zorn KC; Banerji D; Khan LM; Bove R; Hauser SL; Gelfand AA; Johnson-Kerner BL; Nash K; Krishnamoorthy KS; Chitnis T; Ding JZ; McMillan HJ; Chiu CY; Briggs B; Glaser CA; Yen C; Chu V; Wadford DA; Dominguez SR; Ng TFF; Marine RL; Lopez AS; Nix WA; Soldatos A; Gorman MP; Benson L; Messacar K; Konopka-Anstadt JL; Oberste MS; DeRisi JL; Wilson MR Pan-viral serology implicates enteroviruses in acute flaccid myelitis. Nat. Med 2019, 25, 1748–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra N; Ng TFF; Marine RL; Jain K; Ng J; Thakkar R; Caciula A; Price A; Garcia JA; Burns JC; Thakur KT; Hetzler KL; Routh JA; Konopka-Anstadt JL; Nix WA; Tokarz R; Briese T; Oberste MS; Lipkin WI Antibodies to Enteroviruses in Cerebrospinal Fluid of Patients with Acute Flaccid Myelitis. MBio 2019, 10, e01903–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


