Abstract
Purpose-
Crosslinked poly(vinyl alcohol) (PVA) is a biomaterial that can be used for multiple cardiovascular applications. The success of implanted biomaterials is contingent on the properties of the material. A crucial consideration for blood-contacting devices is their potential to incite thrombus formation, which is dependent on the material surface properties. The goal of this study was to quantify the effect of different crosslinking methods of PVA hydrogels on in vitro thrombogenicity.
Methods-
PVA was manufactured using three different crosslinking methods: 30% sodium trimetaphosphate (STMP), three 24hr freeze-thaw cycles (FT), and 2% glutaraldehyde-crosslinked (GA) to produce STMP-PVA, FT-PVA and GA-PVA, respectively. Expanded polytetrafluoroethylene (ePTFE) was used as a clinical control. As markers of thrombus formation, the degree of coagulation factor (F) XII activation, fibrin formation, and platelet adhesion were measured.
Results-
The GA-PVA material increased FXII activation in the presence of cofactors compared to vehicle and increase platelet adhesion compared to other PVA surfaces. The STMP-PVA and FT-PVA materials had equivalent degrees of FXII activation, fibrin formation and platelet adhesion.
Conclusion-
This work supports crosslinker dependent thrombogenicity of PVA hydrogels and advances our understanding of how the manufacturing of a PVA hydrogel affects its hemocompatibility.
Keywords: Poly(vinyl alcohol), crosslinking, hydrogel, vascular graft, thrombogenicity
Introduction
Synthetic vascular grafts are required to replace damaged or occluded vessels when autologous veins are unavailable [1]. Despite this beneficial treatment, small diameter vascular grafts have a tendency to fail due to thrombosis. The development of hemocompatible cardiovascular biomaterials remains an unmet clinical need. There has been an increasing interest in the use of hydrogels, including poly(vinyl alcohol) (PVA), for cardiovascular biomaterial purposes [2–5]. PVA is a water-soluble polymer that can be physically or chemically crosslinked into a hydrogel. This material has been used widely for biomedical applications due to its desirable properties including tunable mechanical properties, amenability to surface modifications and compliance [2–4].
The hemocompatibility of PVA has been widely deliberated [2,3,5–12]. However, there are stark differences in manufacturing of PVA hydrogels for different biomedical applications. The size, shape, and density of the materials all influence the interactions with the components of blood. The crosslinking method of hydrogels can potentially change the bulk and surface properties of the material [2], including thrombogenicity. Thrombogenicity is particularly important when developing blood-contacting biomaterials, such as vascular grafts.
There are multiple methods of crosslinking hydrogels, including physical and chemical processes. The common chemical crosslinking agents for PVA are aldehydes, such as glutaraldehyde (GA) [13]. The aldehyde functional group reacts with the hydroxyl group on PVA forming an ester bond. Another chemical crosslinking method was designed by Chaouat et al., who were the first to engineer a PVA-based vascular graft using sodium trimetaphosphate (STMP), a food grade crosslinker [5]. In this process, the hydroxyl groups on PVA undergo phosphoesterification by STMP, which leads to the availability of phosphate groups in PVA to react with other hydroxyl groups to form a crosslinked network. Additionally, PVA can be physically crosslinked through a series of freeze-thaw (FT) cycles, which is a process that relies on the hydrogen bonding and the crystallinity of the polymer [14,15].
The introduction of different chemical groups onto PVA can affect how blood components interact with the hydrogel, an important concern for blood-contacting devices. Thus, we hypothesized that the method of crosslinking PVA would alter the biomaterial thrombogenicity. It is widely accepted that the contact pathway of blood coagulation is activated by artificial surfaces. Blood coagulation factor (F) XII can bind to artificial surfaces, inducing a conformational change of FXII which leads to activation of the zymogen into its protease form, FXIIa [16,17]. FXIIa then activates prekallikrein (PK) into kallikrein, which along with high molecular weight kininogen (HK), amplifies FXIIa production [18]. FXIIa then activates FXI into FXIa, which ultimately leads to fibrin formation, one of the main structural components of a thrombus. In addition to fibrin, platelets are a major component of a thrombus. Platelets can bind to adsorbed fibrinogen and become activated, promoting thrombosis. This work compares the effects of the crosslinking method on the in vitro hemocompatibility, including the effect on the contact pathway, fibrin formation, and platelet attachment.
Materials and Methods
Hydrogel manufacturing
A final concentration of 10% (w/v) aqueous PVA (Sigma) was used for all hydrogels. The STMP-PVA was manufactured as previously described [5], 15% (w/v) sodium trimetaphosphate (STMP, Sigma) was added to aqueous PVA followed by 30% (w/v) sodium hydroxide and cured as films. The FT-PVA was manufactured by pouring a 10% aqueous PVA solution as a film, which was then frozen at −20°C for 18hr and thawed at RT for 6hr three times. The FT-PVA samples were then set at RT for 14h before placing in oven and increasing temperature by 10°C every 2hr until 80°C was reached. The FT-PVA samples remained at 80°C for 20hr before use. The glutaraldehyde crosslinked PVA (GA- PVA) was manufactured using a solution with a final concentration of 2% glutaraldehyde (Sigma) and 10% aqueous PVA. Ethanol (95%) treated expanded polytetrafluoroethylene (ePTFE, Gore) was dipped in the 2% glutaraldehyde crosslinking PVA solution three times with 30min of drying in between dips. The GA-PVA samples were dried overnight before additional drying in oven at 70°C for 2hr. After preparation all materials were punched with a 5mm biopsy punch. Control materials of glass (positive control for FXII activation) and unmodified ePTFE (clinical standard control) were used.
Factor XII Activation
The amount of factor XII (FXII) activation was quantified using a method modified from Puy et al [19]. A 200μL solution of FXII (200nM, Haematologic Technologies, Inc., Essex Junction, VT, USA) was incubated at 37°C alone in 25mM HEPES, pH 7.4, 150mM NaCl, and 0.1% BSA vehicle buffer or with the cofactors PK (50nM, Enzyme Research Laboratories, Inc., South, IN, USA) and HK (50nM, Enzyme Research Laboratories, Inc., South, IN, USA) in the vehicle buffer in the absence or presence of PVA biomaterials or glass as a control in a 96-well plate. Samples were removed and quenched with 50μg/ml soybean trypsin inhibitor (Sigma-Aldrich, St Louis, MO, USA) to limit further FXIIa production, followed by quantification of FXIIa generated by measuring the rates of Spectrozyme-FXIIa (American Diagnostica, Inc., Stamford, CT, USA) hydrolysis at 405nm absorbance using an Infinite M200 spectrophotometer (Tecan, Männedorf, Switzerland). A standard curve of FXIIa was used to convert the absorbance to FXIIa concentration. Six individual experiments were performed for this study.
Fibrin Generation and Fibrinolysis
Each PVA biomaterial, ePTFE as a clinical control, and a blank well were incubated in 50μl of pooled platelet-poor plasma (PPP, International Society of Thrombosis and Haemostasis Scientific and Standardization Committee Lot 4) with 50μl HEPES buffered saline (HBS, 25mM HEPES and 150mM NaCl, pH 7.4) and 3nM tissue plasminogen activator (tPA), followed by the addition of 50μl CaCl2 (8mM). Changes in turbidity of the solution were measured as absorbance at 405nm and documented over time at 37°C. Fibrin generation initiation times were defined by a 5% increase over the baseline absorbance. The 5% increase was chosen as a standard, comparable time point because it was a consistent point above the noise floor of the citrated plasma. The rates of fibrin generation were defined as the maximum slopes and the rates of fibrinolysis were characterized as the minimum slopes. Six individual experiments were performed for this study.
Washed platelet and platelet-rich plasma preparation
The work herein required human blood to isolate plasma and platelets, which was done in accordance with Oregon Health & Science University Institutional Review Board (IRB) approval. The subjects gave informed written consent to their participation in these studies and the work adheres to the Declaration of Helsinki.
Human venous whole blood was drawn from healthy donors into sodium citrate, as previously described [20]. In brief, the whole blood was centrifuged at 200g for 20min to obtain platelet-rich plasma (PRP). In select studies, purified platelets were isolated from PRP by further centrifugation at 1000g for 10min in the presence of 0.1μg/ml prostacyclin. The pellet was resuspended in HEPES/Tyrode buffer (129mM NaCl, 0.34mM Na2HPO4, 2.9mM KCl, 12mM NaHCO3, 20mM HEPES, 5mM glucose, 1mM MgCl2; pH 7.3) containing 0.1μg/ml prostacyclin. The platelets were washed once via centrifugation (1000g for 10min) and resuspended in HEPES-Tyrode buffer.
Platelet adhesion quantification
The static platelet adhesion was quantified using a method modified from Vaníčková et al [21]. A 200μL solution of PRP or purified washed platelets suspensions (5.0×108 platelets/mL) were incubated on each material in a microtiter plate for 1hr at room temperature. The samples were then rinsed three times with phosphate buffered saline (PBS, Thermo Fisher Scientific, Waltham, MA) to remove all non-adherent platelets. The amount of platelets attached to the surface of each material was measured using the platelet acid phosphatase activity with a calibration curve using platelet solutions (0–5.0×108 platelets/mL). Three individual experiments were performed using isolated purified platelets and six individual experiments were performed using platelet rich plasma.
Scanning Electron Microscopy
Samples were prepped identically to those in the purified platelet adhesion studies but were examined using scanning electron microscopy (SEM). After incubation with platelets, the samples were rinsed three times with PBS before fixing for 30min at room temperature using Karnovsky’s fixative (2% paraformaldehyde, 2.5% glutaraldehyde and 0.1M sodium phosphate buffer, pH 7.4 containing 0.1M sucrose), after which they were rinsed three times with PBS. The samples were then dehydrated using ethanol:water solutions (35, 50, 70, 80, 90, 95, 100% ethanol) for 10min each. The samples were further dehydrated with a treatment of hexamethyldisilazane (Sigma) solutions (50 and 100%) for 10min each, before drying overnight. The samples were mounted onto studs then sputter coated with gold palladium alloy at 48mA for 30sec in 50mTorr argon (Desk II, Denton Vacuum). The conformation of platelets adhered to each samples was observed using FEI ESEM Quanta 200 scanning electron microscope and was imaged at 10keV.
Atomic Force Microscopy
Atomic Force Microscopy (AFM) scans were performed on dry STMP-PVA samples using Bruker Fastscan-C silicon nitride cantilevers with silicon probe tips (5nm tip radius, 0.8N/m force constant) and a Dimension Fastscan Bio Icon System (Bruker Nano Surfaces, Santa Barbara, CA). Scans were acquired over a 5μm × 5μm area in Peakforce Tapping Mode. The samples were prepared using a 6mm biopsy punch and secured to a glass slide using double-sided tape before scanning.
Statistics
The data are shown as means ± SD. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparisons test in GraphPad Prism v 8.2.1 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com). Probability values of p < 0.05 were considered to be statistically significant.
Results
Effects of PVA biomaterials on factor XII activation
In purified systems, we first studied the ability of PVA biomaterials to both autoactivate FXII alone and amplify FXII activation in the presence of cofactors. FXII autoactivation was not detected on any of the crosslinked PVA hydrogels as compared to glass surfaces. In the presence of the cofactors PK and HK, an increase in the degree of FXII activation was observed on GA-PVA as compared to vehicle. A similar increase was observed for the clinical and positive controls, ePTFE and glass, respectively (Figure 1).
Figure 1.
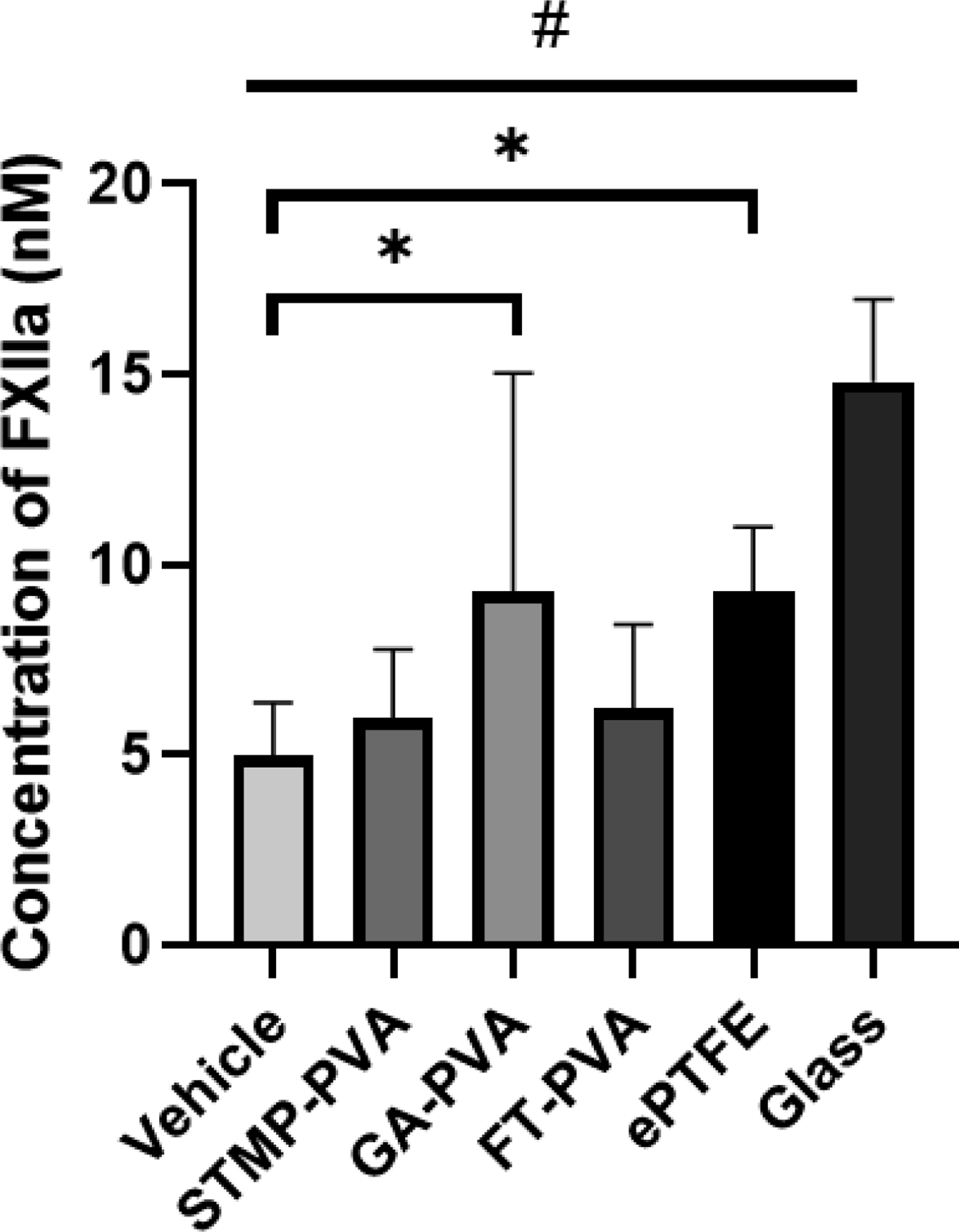
Time dependent FXII activation in the presence of PK and HK on crosslinked biomaterials after 60 min. Data are shown as means ± SD. Statistical analyses were performed using a one-way ANOVA with Tukey’s post hoc testing (n=6). * indicate significant increase from vehicle (p<0.05). # indicates an increase from all other sample means (p<0.01)
Effects of PVA biomaterials on fibrin generation and fibrinolysis
We next studied the effects of the PVA biomaterials on the formation and degradation of fibrin in platelet-poor plasma. The initiation time of fibrin formation remained roughly 1000sec for all three PVA biomaterials as well as plasma alone. An average initiation time of fibrin formation of 797 ± 33sec was recorded on ePTFE. There were no statistical significant differences in the rate of fibrin generation and fibrinolysis between ePTFE, the three crosslinked PVA biomaterials and the plasma control (Figure 2).
Figure 2.
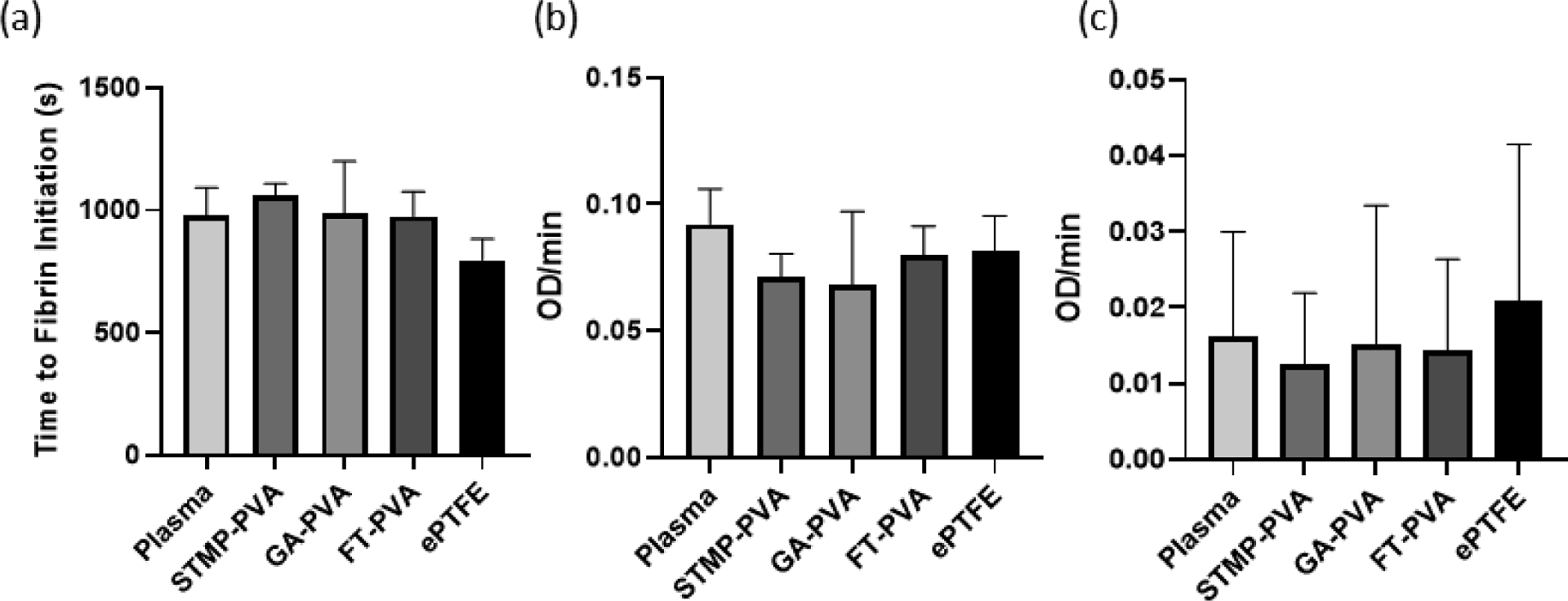
Fibrin formation dynamics in the presence of biomaterials. (a) Fibrin initiation time on crosslinked PVA hydrogels. (b) Rate of fibrin generation on biomaterials shown as optical density over time (OD/min). (c) tPA induced fibrinolysis in the presence of biomaterials shown as optical density over time (OD/min). The absolute value was taken to make decreasing rates positive for the fibrinolysis data. Data are shown as means ± SD. Statistical analyses were performed using a one-way ANOVA (n=6).
Effects of PVA biomaterials on platelet adhesion
We then studied the extent to which PVA biomaterials supported platelet adhesion. Purified platelets or platelet-rich plasma was incubated on crosslinked biomaterials or ePTFE and the degree of adhesion quantified using an acid phosphatase assay. There was significantly higher platelet attachment to GA-PVA as compared to STMP-PVA in both the purified platelet system (Figure 3a) and in the platelet-rich plasma (Figure 3b). A similar degree of platelet adhesion was recorded on STMP-PVA and FT-PVA materials and ePTFE in both the purified platelet and platelet-rich plasma systems. These findings were further corroborated by visual inspection of the biomaterial surface by scanning electron microscopy. A robust degree of individual platelets and platelet aggregates were observed on GA-PVA and individual platelets on ePTFE (Figure 4). The analysis of the AFM scan (Figure 5) indicated the surface roughness average (Ra) was 4.60nm for STMP-PVA.
Figure 3.
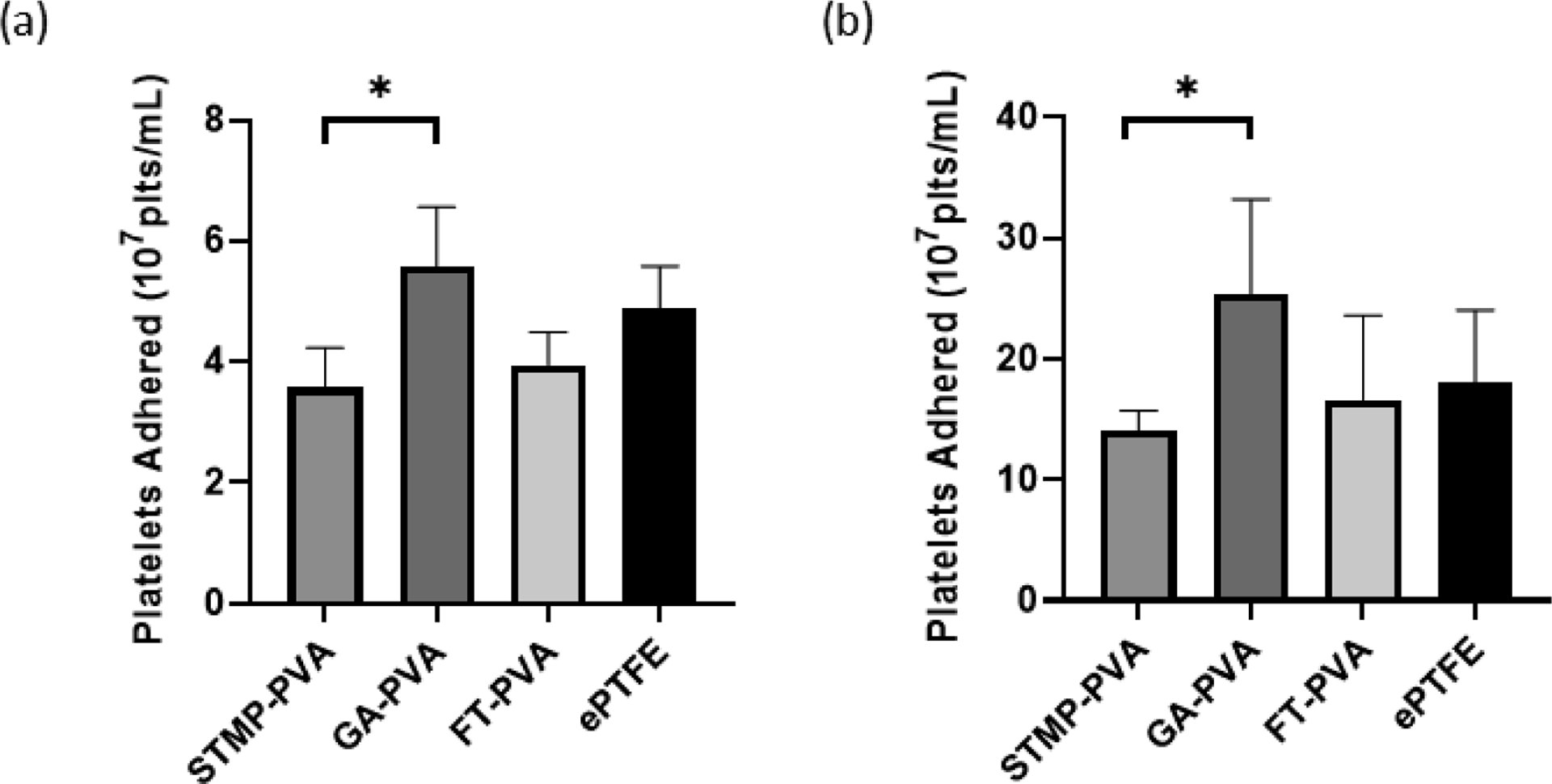
Static platelet adhesion presented as the number of platelets present per mL of lysis buffer. (a) Isolated platelet adhesion onto crosslinked PVA biomaterials (n=3). (b) Static platelet adhesion onto crosslinked biomaterials in platelet-rich plasma (n=6). Data are shown as means ± SD. Statistical analyses were performed using a one-way ANOVA with Tukey’s post hoc testing. * indicate significant difference (p<0.05)
Figure 4.
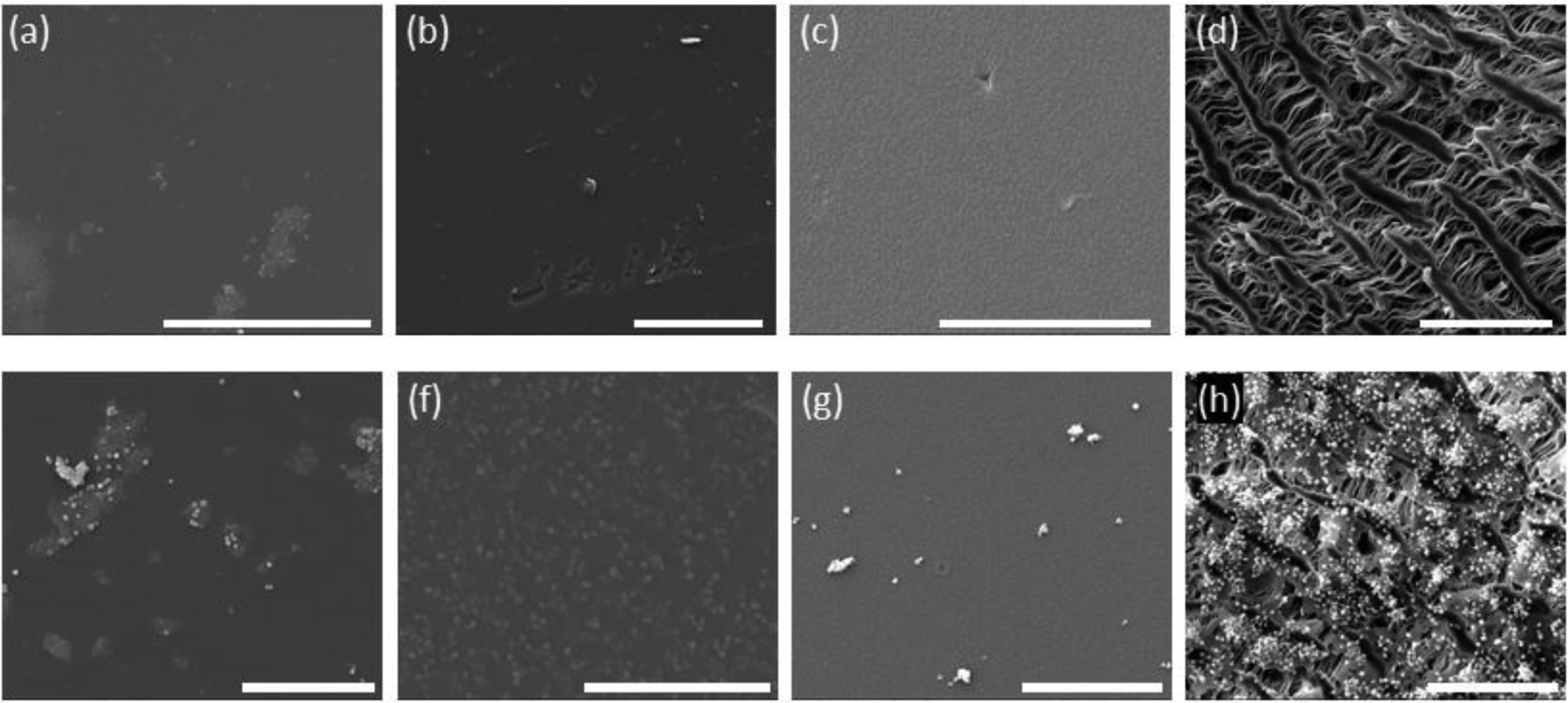
Representative SEM micrographs of each biomaterial before (top row) and after (bottom row) isolated platelet incubation. (a, e) STMP-PVA; (b, f) GA-PVA; (c, g) FT-PVA; and (d, h) ePTFE-PVA. All scale bars = 50nm.
Figure 5.
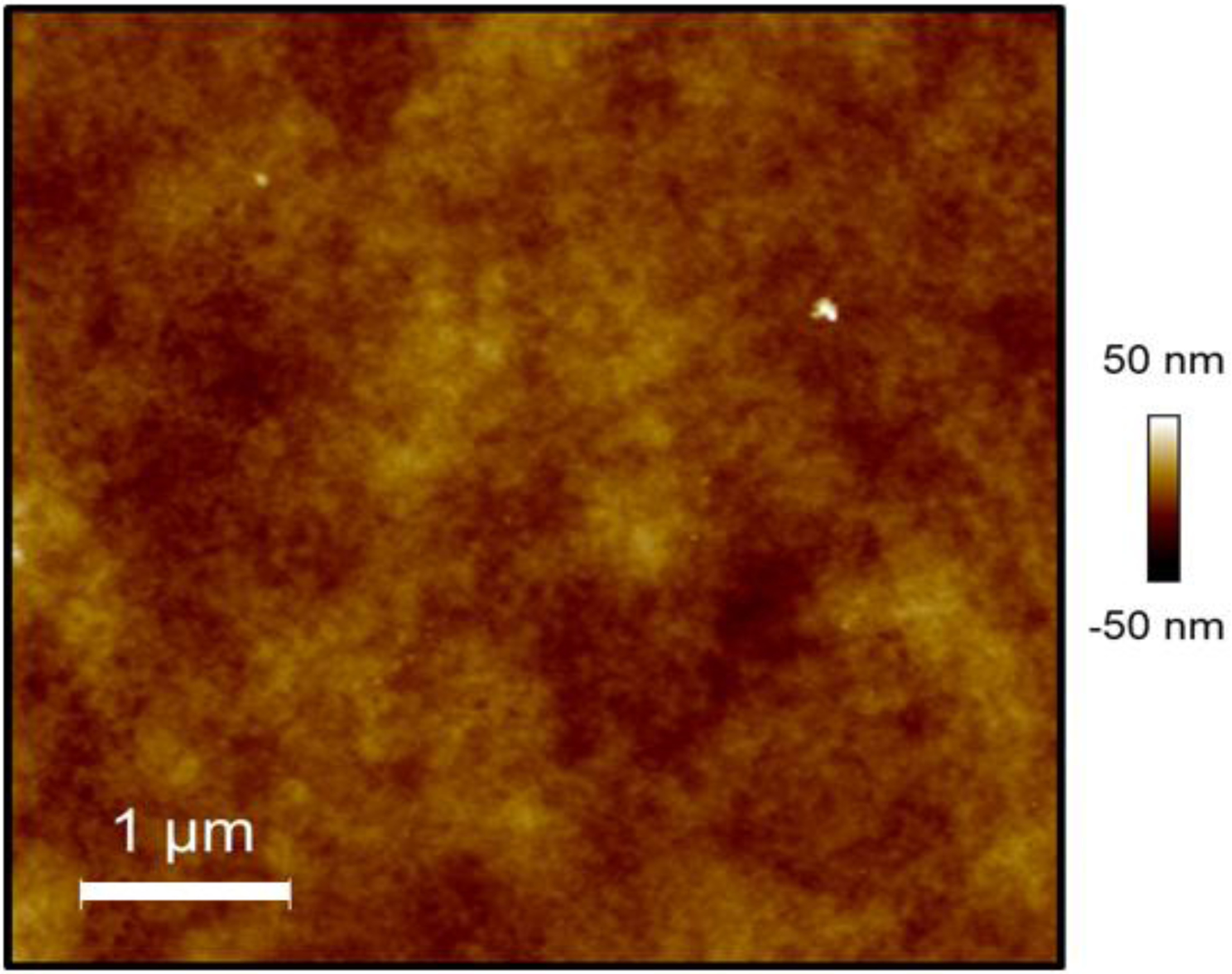
Topographic characterization of the surface of STMP-PVA using AFM. The dimensions of the scan are 5μm × 5μm. The surface had roughness average (Ra) is 4.6nm and the total surface area of the sample is 25.248μm2.
Discussion
PVA hydrogels have many desirable properties for cardiovascular applications including modifiable surfaces and tunable mechanical properties. However, the extent to which a change in the bulk properties of PVA hydrogels alters thrombogenicity remains poorly understood. This work investigated how FXII activation, fibrin generation, and platelet adhesion are affected in vitro due to physically and chemically crosslinked PVA hydrogels.
The crosslinking method of PVA can change the surface properties of the biomaterial, thus altering thrombogenicity. Here we studied the effects of chemically crosslinking the PVA with STMP and GA, as well as a physically crosslinking PVA with consecutive FT cycles, on hemocompatibility. The use of STMP as a crosslinking agent leads to the formation of durable PVA scaffolds with mechanical properties similar to native human arteries that are non-toxic to cells [9,22,23]. STMP crosslinked PVA biomaterials have demonstrated low platelet adhesion and long thrombin generation times [5]. The low platelet adhesion and low fibrin formation on the surface of STMP crosslinked PVA have also been shown in an ex vivo arteriovenous shunt model [2,11,12,24]. In vivo studies using STMP crosslinked PVA vascular prosthesis showed 100% patency after 7days of implantation in a rat abdominal aorta model [25]. Glutaraldehyde crosslinked PVA hydrogels have insufficient mechanical strength to act as a material alone and therefore have been studied as a coating [26]. These glutaraldehyde crosslinked PVA hydrogels, were reactive to platelets in a canine arteriovenous (AV) shunt model even with the addition of heparin to the material surface [26]. FT crosslinked PVA hydrogels are highly elastic, porous and spongy when compared to other crosslinking methods [14,15,27]. The preparation of PVA biomaterials via this cryotropic treatment has been a preferred method due to the absence of potentially toxic chemical crosslinking agents. Yet the effect of FT crosslinked PVA on thrombus formation was not known. There are advantages of chemically crosslinking PVA, including the prevention of hydrogel dissolution and controllable mechanical properties. However, the leaching of some chemical agents, such as glutaraldehyde and formaldehyde, can lead to toxic microenvironments and decreased hemocompatibility.
We studied activation of the contact pathway by the three different crosslinked PVA hydrogels. FXII autoactivation was not detected on any of the crosslinked PVA surfaces. Plasma proteins are adsorbed onto surfaces within seconds of encountering blood. Amongst these proteins is FXII, which is activated into FXIIa upon binding to a surface [18]. The PVA hydrogels are highly hydrophilic, which has been shown to minimize protein adsorption [28]. The surface of glass is negatively charged, which is known to activate FXII at a high rate [16]. The surface chemistry of ePTFE consists of a fluorinated carbon-hydrogen backbone making the material hydrophobic. Hydrophobic surfaces encourage protein adsorption. FXII is also activated on surfaces in the presence of kallikrein (K) and high molecular weight kininogen (HK). In these cofactor mediated FXII activation studies, GA-PVA and ePTFE significantly increased the amount of FXIIa after 1hr (Figure 1). The increased amount of FXIIa is likely due to the hydrophobicity of the ePTFE surfaces. The addition of glutaraldehyde into PVA polymer networks introduces small hydrophobic regions into the hydrogel, while the STMP crosslinker and physical crosslinking of the PVA maintain their hydrophilicity. Despite these findings, it must be noted that continuous activation of FXII by biomaterial surfaces does not always lead to continuous downstream activation of the coagulation system [18,19]. Thus, FXII activation alone in purified systems on biomaterial surfaces is not a complete predictor of biomaterial thrombogenicity. For a complete understanding of biomaterial thrombogenicity, the coagulation system must be studied in conjunction with platelet activation.
In the contact pathway, FXIIa subsequently activates downstream coagulation factors, ultimately leading to fibrin production. This work showed ePTFE reduced fibrin generation initiation time compared to STMP-PVA in human PPP, indicating that the ePTFE more rapidly initiated fibrin formation (Figure 2a). All of the crosslinked PVA biomaterials had initiation times and rates of fibrin generation equivalent to the vehicle. Thus, FXIIa activity did not correlate to rate of fibrin generation on PVA crosslinked materials supporting previous work that FXII activation alone does not equate to clot formation. However, the shorter initiation time of fibrin generation for ePTFE did correlate with increased FXII activation in the presence of PK and HK. As expected, once fibrin formation is initiated, the fibrin clot grew at the same rate and had the same rate of fibrinolysis for all of the materials. Thus, in these static conditions, the density of the fibrin clot was likely unchanged by the underlying material.
When materials contact blood, the materials are immediately coated with blood proteins, particularly fibrinogen. Platelets can then bind to adsorbed fibrinogen via the glycoprotein IIb/IIIa receptor (GPIIb/IIIa), which leads to platelet activation. Activated platelets, along with fibrin, are the main structural components of a thrombus. In this work, we used a static acid phosphatase detection assay to quantify the amount of platelets attached to each material. There was increased platelet adhesion to GA-PVA compared to STMP-PVA (Figure 3), in both isolated platelet and platelet rich plasma assays, which supports the platelet reactivity reported in the literature [13,29]. In previous studies glutaraldehyde crosslinked PVA-coated polyethylene (PE) was shown to have higher platelet reactivity and attachment, when compared to the shunt tubing alone in a canine arteriovenous shunt model [26]. Whereas, STMP crosslinked PVA was shown to have reduced platelet attachment when compared to clinical and biological positive controls in a non-human primate arteriovenous shunt model [2,11]. The difference in the animal models of these previous studies is noteworthy. Canine platelets are known to be more reactive to procoagulants than human platelets, which could account for these differences. Additionally, the differences in platelet adhesion to the crosslinked PVA materials in these studies may be due to how platelet reactivity was measured. In the canine arteriovenous shunt model, platelets were indirectly measured by platelet regeneration as defined by cyclooxygenase activity after a single dose of aspirin. In the baboon model, platelets were radiolabeled, and real-time accumulation was measured using a nuclear imaging camera. However, this current work shows that the PVA crosslinker also has an effect on platelet adhesion.
The work presented here has shown that changes in the bulk PVA hydrogel network can lead to changes in platelet attachment to the surface, specifically the GA-PVA had increased platelet attachment; however, the absence of surface characterization of the crosslinked materials is a limitation of this study. The surface of each material was qualitatively examined using SEM. There were no visible differences in the STMP and GA crosslinked PVA surfaces. The FT-PVA samples did appear to have a more crystalline surface than the STMP-PVA and GA-PVA hydrogel surfaces. The surface morphology of FT-PVA surfaces has been quantified previously using AFM [30]. In these previous studies, hydrogels prepared from 10% PVA, which were subjected to 15 FT cycles, were found to have a roughness average of approximately 80nm and a significant decrease in roughness was observed as the number of FT cycles increased. If the data obtained by Pramanick et al. were extrapolated to 3 FT cycles, as was examined in the present work, the roughness of the PVA hydrogel surface would exceed a roughness average of 80nm. We quantified the surface roughness of STMP-PVA using AFM and have found that the surface has a roughness average approximately 4.6nm (Figure 5). The surface of ePTFE has been reported to have a roughness average of approximately 150nm [31]. While these data cannot be statistically compared, taken together, they suggest that the increase in platelet adhesion to GA-PVA is unlikely due to an increase in surface area due to surface roughness.
Conclusion
This work supports the hypothesis that the crosslinker in PVA hydrogel networks affects individual components of coagulation in vitro. A limitation of this study is that the quantities of FXIIa in solution compared to protein adsorbed onto the material surfaces were not measured directly. Additionally, these studies were all performed in vitro in the absence of flow. Future studies should incorporate the crosslinked PVA materials into flow systems to study hemocompatibility. Furthermore, differentially crosslinked PVA materials can be investigated in shunt models or whole blood systems, which are more physiologically representative to predict in vivo performance. This work is an important step toward understanding how the design of cardiovascular biomaterials can impact thrombogenicity.
Acknowledgments
This work was supported by the Achievement Rewards for College Scientists (ARCS) Foundation and the National Institutes of Health grants R01HL130274 and R01HL144113
Dr. Owen JT McCarty received research grant number R01HL144113 from the National Institutes of Health.
Dr. Monica T Hinds received research grants number R01HL130274 and R01HL144113 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Novella M Bates, Dr. Cristina Puy, and Dr. Patrick L Jurney declare that they have no conflict of interest.
References
- [1].Anderson DEJ, Glynn JJ, Song HK, Hinds MT, Engineering an endothelialized vascular graft: a rational approach to study design in a non-human primate model., PLoS One. 9 (2014) e115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cutiongco MFA, Anderson DE, Hinds MT, Yim EK, In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts, Acta Biomater. 25 (2015) 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cutiongco MFA, Kukumberg M, Peneyra JL, Yeo MS, Yao JY, Rufaihah AJ, Le Visage C, Ho JP, Yim EKF, Submillimeter Diameter Poly(Vinyl Alcohol) Vascular Graft Patency in Rabbit Model, Front. Bioeng. Biotechnol 4 (2016) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu Y, Yu C, Xing M, Wang L, Guan G, Surface modification of polyvinyl alcohol (PVA)/polyacrylamide (PAAm) hydrogels with polydopamine and REDV for improved applicability, J. Biomed. Mater. Res. - Part B Appl. Biomater (2019) 1–11. [DOI] [PubMed] [Google Scholar]
- [5].Chaouat M, Le Visage C, Baille WE, Escoubet B, Chaubet F, Mateescu MA, Letourneur D, A novel cross-linked poly(vinyl alcohol) (PVA) for vascular grafts, Adv. Funct. Mater (2008). [Google Scholar]
- [6].Walker J, Young G, Hunt C, Henderson T, Multi-centre evaluation of two daily disposable contact lenses, Contact Lens Anterior Eye. (2007). [DOI] [PubMed] [Google Scholar]
- [7].Pal K, Banthia AK, Majumdar DK, Preparation and Characterization of Polyvinyl Alcohol-Gelatin Hydrogel Membranes for Biomedical Applications, AAPS PharmSciTech. 8 (2007) E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang S-H, Lee Y-SJ, Lin F-H, Yang J-M, Chen K, Chitosan/Poly(vinyl alcohol) Blending Hydrogel Coating Improves the Surface Characteristics of Segmented Polyurethane Urethral Catheters, J. Biomed. Mater. Res. B. Appl. Biomater 83B (2007) 304–313. [DOI] [PubMed] [Google Scholar]
- [9].Ino JM, Sju E, Ollivier V, Yim EKF, Letourneur D, Le Visage C, Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films, J. Biomed. Mater. Res. - Part B Appl. Biomater 101 (2013) 1549–1559. [DOI] [PubMed] [Google Scholar]
- [10].Gorbet M, Sperling C, Maitz MF, Siedlecki CA, Werner C, Sefton MV, The blood compatibility challenge. Part 3: Material associated activation of blood cascades and cells, Acta Biomater. 94 (2019) 25–32. [DOI] [PubMed] [Google Scholar]
- [11].Anderson DEJ, Truong KP, Hagen MW, Yim EKF, Hinds MT, Biomimetic modification of poly(vinyl alcohol): Encouraging endothelialization and preventing thrombosis with antiplatelet monotherapy, Acta Biomater. 86 (2019) 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jurney PL, Anderson DEJ, Pohan G, Yim EKF, Hinds MT, Reactive Ion Plasma Modification of Poly(Vinyl-Alcohol) Increases Primary Endothelial Cell Affinity and Reduces Thrombogenicity, Macromol. Biosci 18 (2018) 1800132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Llanos MV, Sefton Gerard, Immobilization of ploy(ethylene glycol) onto a poly(vinyl alcohol) hydrogel: 2. Evaluation of thrombogenicity, J. Biomed. Mater. Res 27 (1993) 1383–1391. [DOI] [PubMed] [Google Scholar]
- [14].Hassan CM, Peppas NA, Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods, Adv. Polym. Sci 153 (2000) 37–65. [Google Scholar]
- [15].Hassan CM, Peppas NA, Structure and Morphology of Freeze/Thawed PVA Hydrogels, Macromolecules. 33 (2000) 2472–2479. [Google Scholar]
- [16].Stavrou E, Schmaier AH, Factor XII: What Does It Contribute To Our Understanding Of The Physiology and Pathophysiology of Hemostasis & Thrombosis, Thromb Res. 125 (2010) 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chatterjee K, Guo Z, Vogler EA, Siedlecki CA, Contributions of contact activation pathways of coagulation factor XII in plasma, J. Biomed. Mater. Res. Part A 90A (2009) 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ivanov I, Matafonov A, Sun M-F, Cheng Q, Dickeson SK, Verhamme IM, Emsley J, Gailani D, Proteolytic properties of single-chain factor XII: a mechanism for triggering contact activation, 129 (2017) 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Puy C, Tucker EI, Wong ZC, Gailani D, Smith SA, Choi SH, Morrissey JH, Gruber A, Mccarty OJT, Factor XII promotes blood coagulation independent of factor XI in the presence of long chain polyphosphate, J Thromb Haemost. 11 (2013) 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McCarty OJT, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VLJ, Machesky LM, Watson SP, Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow, J. Biol. Chem 280 (2005) 39474–39484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vaníčková M, Suttnar J, Dyr JE, Vaníč M, Vaníč V, Ková K, Jiř K, Suttnar I´, The adhesion of blood platelets on fibrinogen surface: Comparison of two biochemical microplate assays, Platelets. 17 (2006) 470–476. [DOI] [PubMed] [Google Scholar]
- [22].Moscato S, Mattii L, D’Alessandro D, Cascone MG, Lazzeri L, Serino LP, Dolfi A, Bernardini N, Interaction of human gingival fibroblasts with PVA/gelatine sponges, Micron. 39 (2008) 569–579. [DOI] [PubMed] [Google Scholar]
- [23].Liu Y, Vrana NE, Cahill PA, McGuinness GB, Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility, J. Biomed. Mater. Res. Part B Appl. Biomater 90B (2009) 492–502. [DOI] [PubMed] [Google Scholar]
- [24].Yao Y, Zaw AM, Anderson DEJ, Hinds MT, Yim EKF, Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility, Biomaterials. 249 (2020) 120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uchida N, Emoto H, Kambic H, Harasaki H, Chen J-F, Hsu S-H, Murabayashi S, Nose Y, Compliance Effect on Patency of Small Diameter, Trans Am Soc Artif Intern Organs. 35 (1989) 556–558. [DOI] [PubMed] [Google Scholar]
- [26].V Cholakis M, Cynthia, Zingg, Walter, Sefton, Effect of heparin-PVA hydrogel on platelets in a chronic canine arterio-venous shunt., J. Biomed. Mater. Res 23 (1989) 417–441. [DOI] [PubMed] [Google Scholar]
- [27].Peppas NA, Stauffer SR, Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: a short review, J. Control. Release 16 (1991) 305–310. [Google Scholar]
- [28].Zhuo R, Siedlecki CA, Vogler EA, Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces, Biomaterials. 27 (2006) 4325–4332. [DOI] [PubMed] [Google Scholar]
- [29].V Cholakis M, Cynthia, Sefton, In vitro platelet interactions with a heparin-polyvinyl alcohol hydrogel., J. Biomed. Mater. Res 23 (1989) 399–415. [DOI] [PubMed] [Google Scholar]
- [30].Pramanick AK, Gupta S, Mishra T, Sinha A, Topographical heterogeneity in transparent PVA hydrogels studied by AFM, Mater. Sci. Eng. C 32 (2012) 222–227. [Google Scholar]
- [31].Doble M, Makadia N, Pavithran S, Kumar RS, Analysis of explanted ePTFE cardiovascular grafts (modified BT shunt), Biomed. Mater 3 (2008) 034118. [DOI] [PubMed] [Google Scholar]


