Abstract
African and African American (AA) women have a higher incidence of triple negative breast cancers (TNBC) with high histological grade and aggressive clinical behavior, but the reasons are not fully understood. We recently found that the oncogenic protein EZH2 is overexpressed in Ghanaian breast cancer, with 16% of the tumors expressing cytoplasmic EZH2. Understanding the molecular underpinnings of these aggressive tumors lead to the identification of potential targetable oncogenic drivers. We characterized the copy number variations of 11 Ghanaian breast tumors by targeted multiplexed PCR-based DNA next generation sequencing (NGS) of over 130 cancer-relevant genes. While the DNA quality was not optimal for mutation analysis, 90% of the tumors had frequent recurrent copy number alterations (CNAs) of 17 genes: SDHC, RECQL4, TFE3, BCL11A, BCL2L1, PDGFRA, DEK, SMUG1, AKT3, SMARCA4, VHL, KLF6, CCNE1, G6PD, FGF3, ABL1, and CCND1, with the top oncogenic functions being mitotic G1–G1/S phase regulation, gene transcription, apoptosis, and PI3K/AKT pathway. The most common recurrent high level CNAs were gains of RECQL4 and SDHC, in 50% and 60% of cases, respectively. Network analyses revealed a significant predicted interaction among 12 of the 17 (70.6%) genes with high level CNAs (p=5.7E-07), which was highly correlated with EZH2 expression (r=0.4–0.75). By immunohistochemistry, RECQL4 and SDHC proteins were upregulated in 53 of 86 (61.6%) and 48 of 86 (56%) of Ghanaian invasive carcinomas tissue samples. In conclusion, our data show that invasive carcinomas from Ghana exhibit recurrent CNAs in 17 genes with functions in oncogenic pathways including PI3K/AKT and G1–G1/S regulation, which may have implications for the biology and treatment of invasive carcinomas in African and AA women.
Keywords: breast cancer, African, health disparities, metastasis, EZH2
INTRODUCTION
Breast cancer is the second most incident cancer worldwide, accounting for over 2 million new cases in 2018 (1). African women have a lower lifetime incidence of breast cancer than women from North American or Europe; however, age-standardized mortality is poorer, particularly for women from Western Africa (1). This likely reflects a combination of environmental and systematic factors as well as underlying differences in tumor biology. Women from sub-Saharan Africa tend to have earlier breast cancer presentation (2, 3). Histologically, invasive breast carcinomas from this population tend to be basal-like and display molecular expression patterns that are negative for estrogen receptor (ER), progesterone receptor (PR) and HER-2/neu amplification (i.e., triple-negative) (4). Similarly, African-American (AA) women, who are thought to share African ancestry, have significantly poorer outcomes than white patients after accounting for age, stage, and socioeconomic status and are more likely to have breast cancer earlier and ER- status (5–8). The underlying molecular alterations for the aggressive breast cancer phenotypes of women of African ancestry are not fully understood.
Basal-like breast cancers are a heterogeneous group of tumors comprising 15–20% of all breast cancers. They are more common in premenopausal, and in African and AA women (9). We previously reported on the incidence of triple-negative breast cancers in Ghanaian women (53.2%) which more closely resembled AA women (30%) than white American women (15.5%), suggesting that African ancestry may correlate with a higher likelihood of TNBC(10). Similar results have been found in other cohorts in women from Senegal and Nigeria (6). The higher proportion of TNBC in invasive breast tumors from African and AAs is associated with ALDH1 and AR expression, suggesting novel subtypes of TNBC in these populations (11), but the genetic landscapes need elucidation.
Enhancer of Zeste Homologue 2 (EZH2), an epigenetic regulator responsible for transcriptional repression, is overexpressed in multiple tumor types (12–17). In breast cancer, overexpression of EZH2 is significantly associated with negative estrogen and progesterone receptor status and distant metastasis (12, 17, 18). We previously showed that EZH2 is significantly associated with high grade and basal-like like tumors in a cohort of 169 breast tissues from Ghanaian women (16). We detected EZH2 expression in the cytoplasm of 16% of Ghanaian invasive carcinomas where it was significantly associated with TNBC status (16). While cytoplasmic EZH2 has been shown to increase breast cancer invasion, and metastasis, the relevance of cytoplasmic EZH2 to the aggressive behavior of breast cancer in African women has not been studied.
Despite advances in our understanding of the genomic landscape of breast and other cancers, the genetic alterations in invasive carcinomas from African women are far from understood. In this study, we define a set of somatic copy number alterations (CNAs) in a cohort of histologically well-characterized Ghanaian invasive breast carcinomas and investigate associations with patterns of EZH2 subcellular localization.
MATERIALS AND METHODS
Human Tissue Samples and TMA development
Tumor samples were collected from 100 women with breast cancer who underwent surgery at Komfo Anoyke Teaching Hospital (KATH) in Kumasi Ghana between 2006 and 2011. Clinicopathological features of these tumors, ER, PR, HER2 and EZH2 immunostaining were previously reported (16). Formalin-fixed, paraffin-embedded tissues were stained with H&E and reviewed independently by the study pathologists (MR and CK), and tumors were arrayed in a high density tissue microarray (TMA) with triplicate samples (n= 255 tissue microarray samples) following established protocols by our lab (12). We selected 20 cases for NGS analyses based on the following criteria: i) sufficient tissue on the block, ii) representative samples with EZH2 expression in the nucleus, cytoplasm, or no expression, iii) representative samples with different histology, tumor grade (2–3), and hormonal receptor and HER2/neu status.
Targeted next generation sequencing
Targeted NGS of breast tumor tissue was performed with IRB approval. For each specimen, 4 to 10 × 10 μm formalin-fixed, paraffin embedded sections of 20 breast tissue samples were cut from a single block and macrodissected with a scalpel under guidance of an H&E stained slide, to enrich for tumor content. We isolated DNA using the Qiagen Allprep FFPE DNA/RNA kit (Qiagen, Valencia, CA). DNA was quantified using the Qubit 2.0 fluorometer (Life Technologies, foster City, CA).
Targeted, multiplexed PCR-based next generation sequencing (NGS) was performed on each tumor. For samples with DNA concentration of > 40 ng, we used the Ion Ampliseq Comprehensive Cancer Panel (CCP), which targets 1,688,650 bases from 15,992 amplicons representing 409 cancer genes (Thermo Fisher Scientific). For samples with < 40 ng of DNA, we employed the Oncomine Comprehensive Panel (OCP) (Thermo Fisher Scientific), which is compatible with 20 ng of formalin fixed paraffin embedded isolated DNA and benchtop Ion Torrent sequencers (19). Barcoded libraries were generated from 40 ng of DNA per sample using the CCP or 20 ng of DNA per sample using the OCP, and the Ion Ampliseq library kit 2.0 (Life Technologies, Foster City, CA) according to manufacturer’s instructions with barcode incorporation. Sequencing of template libraries was then performed on an Ion Proton sequencer with Ion PI chips (Thermo Fisher Scientific) using the Ion PI Hi-Q Sequencing 200 Kit v3 (Life Technologies, Foster City, CA) according to the manufacturer’s instructions. Data analysis was performed as described previously in Torrent Suite (version 5.0.4) and in house previously validated bioinformatics pipelines (19, 20).
Copy number analysis
Amplicon-level read counts were determined using the coverage Analysis plugin. Briefly, normalized GC content corrected read counts per amplicon for each sample were divided by those from a composite normal male DNA sample (composed of multiple formalin-fixed, paraffin embedded and frozen tissues, individual and pooled samples) to generate amplicon-level copy number ratios, and weighted gene-level copy number estimates were determined as described previously (20). Genes with a log2 copy number ratio estimate of <−1 or >0.80 were considered to have high-level loss (deletion) or gain, respectively.
Network and bioinformatics analyses
For enrichment analyses, we performed gene ontology (GO) over-representation tests (GO annotations: biological process, molecular function, cellular compartment, protein domain) in PANTHER (v14.1), and STRING (v11.0) database for confirming enrichment results with topological features from protein/gene interaction networks. A p value of <0.05 was significant. Correlation between EZH2 and the 17 genes with CNAs was analyzed using Pearson Correlation Coefficient (r).
Immunohistochemistry for RECQL4 and SDHC
Immunohistochemistry was performed on the TMA containing 100 invasive carcinomas from Ghanaian women. Four-micron think sections of the TMA were prepared for staining with H&E and immunohistochemistry using a standard biotin-avidin complex technique, anti-RECQL4 (Abcam; ab188125, rabbit polyclonal, 1:50) and anti-SDHC (Abcam, ab155999 rabbit monoclonal, 1:2500) antibodies. Nuclear expression of RECQL4 and cytoplasmic expression of SDHC was interpreted independently by two pathologists (MR and CK). For each case the staining was graded as negative (score=1, no staining); weak (score 2, <25% of cells staining, any intensity); moderate (score=3, 25–75% of cells staining, any intensity); and strong (score=4, >75% of cells staining, any intensity) following previous studies (12, 21). High RECQL4 or SDHC expression was defined as scores 3 and 4; and low expression was defined as scores 1 and 2.
RESULTS
NGS identifies frequent copy number alterations in invasive breast carcinomas from Ghanaian women
Targeted NGS was performed on 20 formalin-fixed, paraffin-embedded breast tissue samples from Ghana identified with sufficient tissue, 9 were excluded due to poor DNA quality. The final cohort consisted of 11 samples comprising 10 invasive carcinomas and one fibroadenoma control. Representative micrographs are shown in Figure 1A–F.
Figure 1. Representative images of Ghanaian invasive carcinomas subjected to next generation sequencing (NGS).
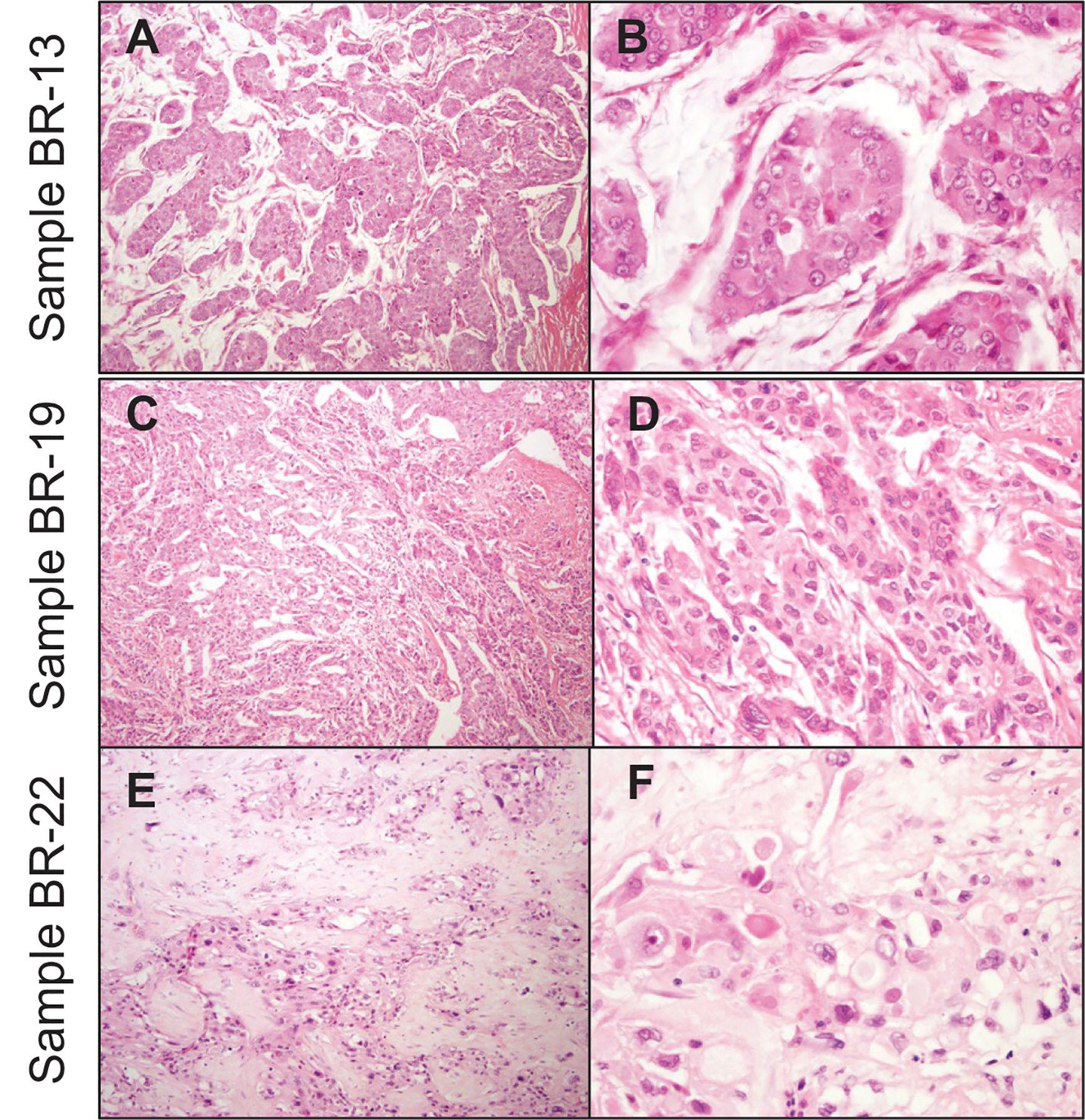
Low (A, C, and E) and high power (B, D, and F) hematoxylin and eosin stained (H&E) sections from three invasive carcinomas, Sample BR-13 (A&B) is an invasive ductal carcinoma of intermediate grade with mucinous differentiation, Sample BR-19 (C&D) is an invasive ductal carcinoma of grade 3, and Sample BR-22 (E&F) is metaplastic carcinoma with squamous differentiation and histological grade 3.
All patients were female, ranging in age from 38 to 64 years old (mean, 48 years). Of the invasive carcinomas, 7 were invasive ductal of intermediate and high histological grade, and 3 were metaplastic carcinomas with squamous differentiation. EZH2 expression was high in the nucleus or cytoplasm (3 and 6 cases), and was negative in one tumor. Eight invasive carcinomas were TNBC including the metaplastic carcinomas, one was HER2/neu positive, and one was luminal B (positive for ER, PR, and HER2/neu). The fibroadenoma sample was negative for expression of EZH2. Details on the clinical and histologic characteristics of this cohort are listed in Table 1.
Table 1.
Clinical and pathological characteristics of the samples subjected to NGS.
| Sample | Age (years) | Diagnosis | Histological grade | ER | PR | HER2 | EZH2 |
|---|---|---|---|---|---|---|---|
| BR3 | 44 | Invasive ductal with papillary features | 3 | Neg | Neg | Neg | Nuclear |
| BR4 | 51 | Invasive ductal | 3 | Neg | Neg | Neg | Nuclear |
| BR5 | - | Fibroadenoma | N/A | N/A | N/A | N/A | Neg |
| BR6 | 42 | Invasive ductal | 3 | Neg | Neg | Pos | Nuclear |
| BR7 | 54 | Invasive ductal | 2 | Pos | Pos | Pos | Neg |
| BR13 | 64 | Invasive Ductal with mucinous features | 2 | Neg | Neg | Neg | Cyto |
| BR14 | 43 | Metaplastic with squamous differentiation | 3 | Neg | Neg | Neg | Cyto |
| BR17 | - | Invasive ductal | 2 | Neg | Neg | Neg | Cyto |
| BR18 | - | Metaplastic with squamous differentiation | 3 | Neg | Neg | Neg | Cyto |
| BR19 | 38 | Invasive ductal | 3 | Neg | Neg | Neg | Cyto |
| BR22 | - | Metaplastic with squamous differentiation | 3 | Neg | Neg | Neg | Cyto |
Using CCP and OPC NGS platforms according to the DNA concentration of each tumor, we successfully analyzed copy number alterations (CNAs) in 10 invasive carcinomas and one fibroadenoma tissue sample. The quality of the Ghanaian samples was not adequate for accurate sequencing studies. Copy number analysis of NGS data yielded a total of 17 high-level CNAs. Prioritized high level CNAs for each case are shown in an integrative heatmap (Figure 2).
Figure 2. Heatmap of copy number variations in 11 Ghanaian tumors.
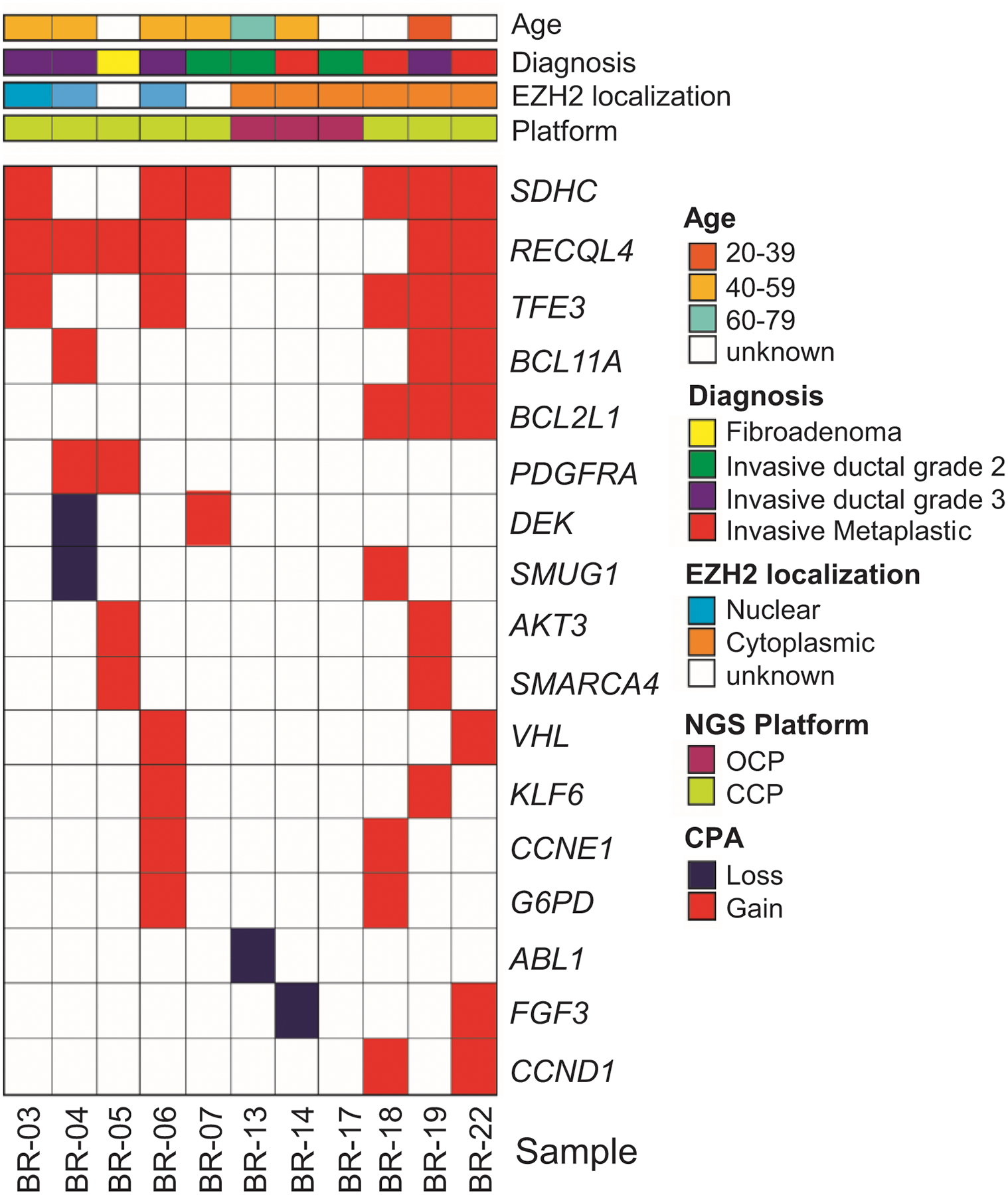
Shown are 17 high-level copy number alterations identified and color coded as indicated in the figure, using CCP and OCP NGS platforms. Ninety % of the invasive carcinomas show CNAs, largely gains, with the most frequent being RECQL4 and SDHC. Note that CNAs in PDGFRA and DEK are identified in tumors with nuclear EZH2, while CNAs in BCL2L1, AKT3, SMARCA4, CCND1, FGF3, and ABL1 are detected in tumors with cytoplasmic EZH2.
Copy number analysis of NGS data demonstrated recurrent CNAs, including most frequent gain of chromosomes 1q (SDHC, AKT3) 8q (RECQL4), and X (TFE3, G6PD) and loss of chromosome 9q (ABL1). Nine (90%) invasive carcinoma cases tissue samples demonstrated CNAs of at least one of these genes: SDHC, RECQL4, TFE3, BCL11A, BCL2L1, PDGFRA, DEK, SMUG1, AKT3, SMARCA4, VHL, KLF6, CCNE1, G6PD, FGF3, and CCND1. One case showed loss of ABL1. It is interesting to note that we identified gains in RECQL4, PDGFRA, AKT3 and SMARCA4 in the fibroadenoma, suggesting that these alterations occur in early neoplastic lesions. Together, these data identify novel CNAs in invasive carcinomas from Ghanaian women with biological and translational implications.
Bioinformatics reveals a predicted interaction network among genes with CNAs and EZH2 expression
Nine of 17 (53%) genes with CNAs were detected in invasive carcinomas with nuclear and cytoplasmic EZH2 expression. Of note, gains in PDGFRA oncogene and loss of DEK tumor suppressor were identified in invasive carcinomas with only nuclear EZH2, and gains in BCL2L1, AKT3, SMARCA4, and CCND1 as well as gains and losses of FGF3, and loss of ABL1 were detected in invasive carcinomas with only cytoplasmic EZH2 expression (Figure 2).
We next tested the hypotheses that the genes harboring frequent recurring CNAs may be functionally related, and that they may associate with EZH2 expression. Enrichment analysis in GO annotation and STRING databases revealed a significantly enriched predicted interaction network (p-value = 5.76e-07) among 12 of the 17 genes with CNAs (70.6%) as well as a correlation between these genes and EZH2 (Pearson correlation coefficient r =0.4–0.75) (Figure 3A). Analyses using GO annotation for the top 10 altered pathways (REACTOME) showed significant representation of mitotic G1–G1/s phases (ABL1, AKT3, CCND1, CCNE1), transcription pathway (ABL1, AKT3, CCND1, CCNE1, DEK, G6PD, SMARCA4), PI3K/AKT signaling in cancer (AKT3, FGF3, PDGFRA), and RMTs methylate histone arginines (CCND1, SMARCA4) (Figure 3B and Table 2). Collectively, these data suggest a relationship between the 17 genes with CNAs and EZH2 expression and localization, which warrants further investigation. Our results highlight major oncogenic pathways deregulated in Ghanaian invasive carcinomas that can be explored for therapeutic application.
Figure 3. Predicted interaction network for the 17 genes with recurrent copy number alterations (CNAs) and with EZH2 in Ghanaian invasive carcinomas.
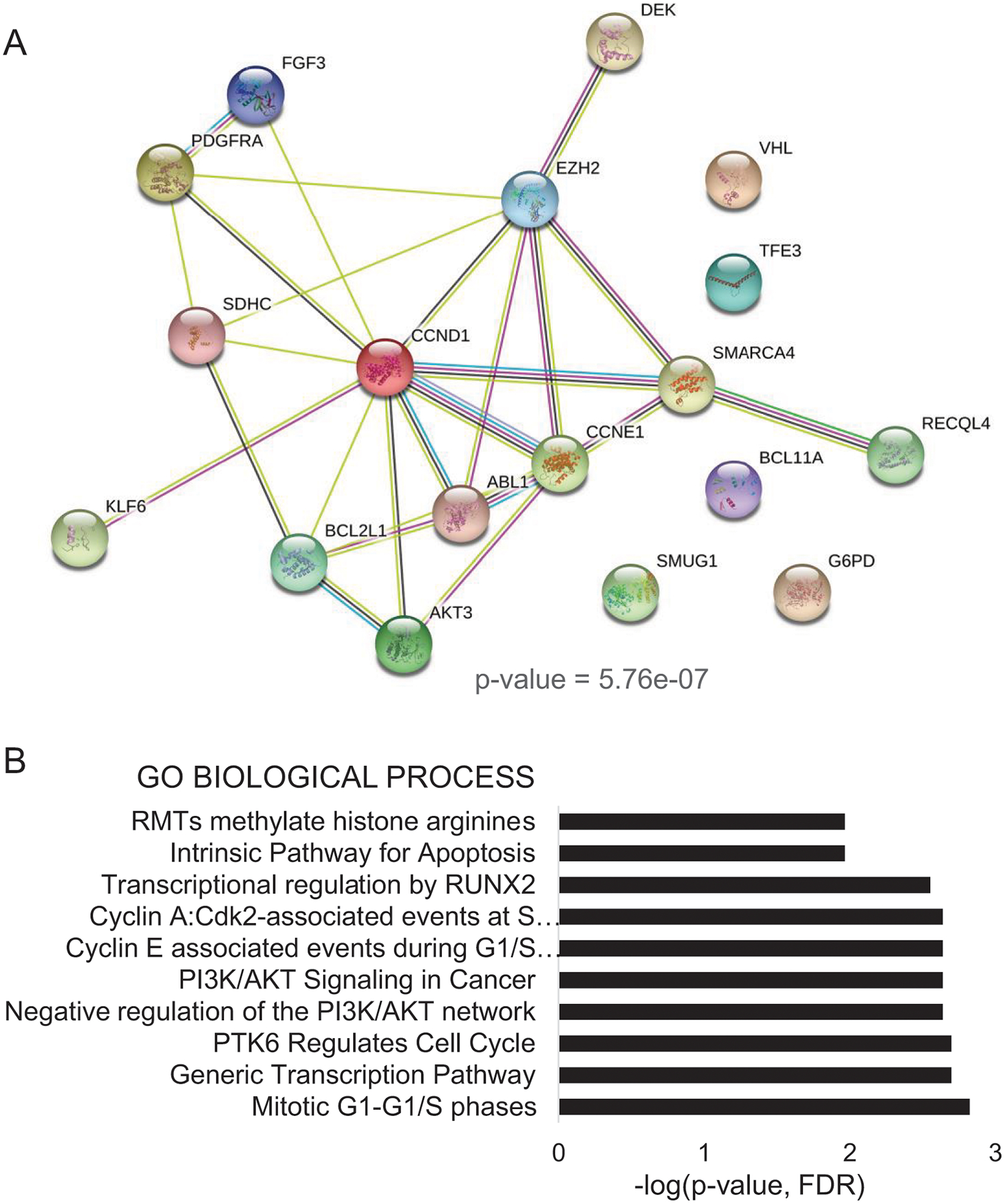
A. Network graph shows enrichment (p-value = 5.7E-07) of topological features of the 17 genes with CNAs. Enrichment analysis in STRING database (v11.0) predicts interactions between EZH2 and 12 of the 17 genes with CNAs (r=0.4 – 0.75). B. Enrichment analyses with biological functions of top 10 altered biological pathways (REACTOME). Legend (bars: -log(p-value)
Table 2.
Top 10 altered pathways associated with the 17 genes with CNAs in Ghanaian invasive carcinomas obtained using enrichment analysis using GO annotation (REACTOME).
| GO Term | Biological Process | FDR | Genes |
|---|---|---|---|
| HSA-453279 | Mitotic G1–G1/S phases | 0.0015 | ABL1,AKT3,CCND1,CCNE1 |
| HSA-212436 | Generic Transcription Pathway | 0.002 | ABL1,AKT3,CCND1,CCNE1, DEK,G6PD,SMARCA4 |
| HSA-8849470 | PTK6 Regulates Cell Cycle | 0.002 | CCND1,CCNE1 |
| HSA-199418 | Negative regulation of the PI3K/AKT network | 0.0023 | AKT3,FGF3,PDGFRA |
| HSA-2219528 | PI3K/AKT Signaling in Cancer | 0.0023 | AKT3,FGF3,PDGFRA |
| HSA-69202 | Cyclin E associated events during G1/S transition | 0.0023 | AKT3,CCND1,CCNE1 |
| HSA-69656 | Cyclin A:Cdk2-associated events at S phase entry | 0.0023 | AKT3,CCND1,CCNE1 |
| HSA-8878166 | Transcriptional regulation by RUNX2 | 0.0028 | ABL1,AKT3,CCND1 |
| HSA-109606 | Intrinsic Pathway for Apoptosis | 0.0108 | AKT3,BCL2L1 |
| HSA-3214858 | RMTs methylate histone arginines | 0.0108 | CCND1,SMARCA4 |
RECQL4 and SDHC show frequent copy number gains and protein overexpression in Ghanaian invasive carcinomas
The genes demonstrating the highest frequency of copy number gains in our cohort were SDHC (1q23.3) and RECQL4 (8q24.3), neither of which has been previously considered in the context of breast carcinoma in African patients. SDHC, which encodes for the Succinate Dehydrogenase Complex Subunit C, was amplified in 6 of 10 (60%) of invasive carcinomas, and RECQ4L encoding for a DNA helicase and functions in homologous recombination-mediated double-stranded break repair, was amplified in 5 of 10 (50%) carcinoma cases sequenced (Figures 2 and 4).
Figure 4. Copy number variations Ghanaian breast tumors.
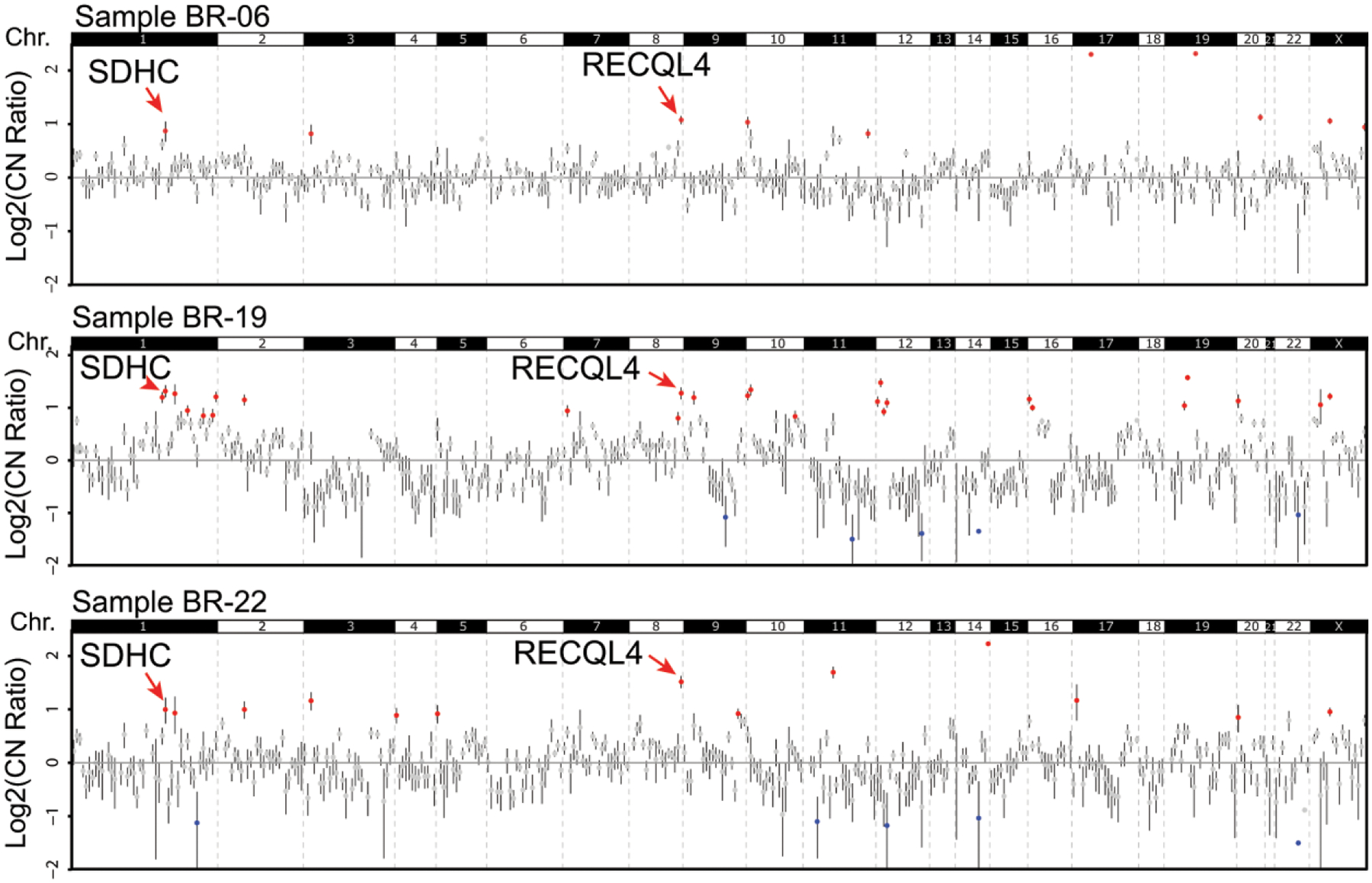
Copy number profiles for three Ghanaian invasive carcinomas with high-level CNAs highlighting gains at SDHC (1q23.3) and RECQL4 (8q24.3). Log2 copy-number ratios per amplicon are plotted, with each individual amplicon represented by a single dot.
To explore whether the CNAs in these genes are associated with protein overexpression, we next tested RECQL4 and SDHC protein expression in 100 tissue samples of Ghanaian invasive carcinomas arranged in TMAs. Of the 86 tumors with sufficient tissue to evaluate in the TMA, positive nuclear RECQL4 staining was detected in 53/86 (61.6%) and positive SDHC cytoplasmic expression in 48/86 (56%) tumors (Figure 5A–C). Further supporting our data, analysis of the TCGA breast tissue datasets using UALCAN showed that RECQL4 and SDHC mRNA expression is significantly upregulated in invasive carcinomas compared to normal breast tissue samples (Supplementary Figure 1A). When analyzed according to patient race, RECQL4 mRNA expression is significantly higher in invasive carcinomas in AA women compared to Caucasians and Asians and to normal tissues, while SDHC was significantly higher in Caucasians and Asians compared to AAs (Supplementary Figure 1B). Analysis of TCGA breast cancer database suggests that high mRNA levels of RECQL4 and SDHC may be associated with worse overall survival (Supplementary Figure 1C).
Figure 5. RECQL4 and SDHC proteins are overexpressed in a substantial number of Ghanaian invasive carcinomas.
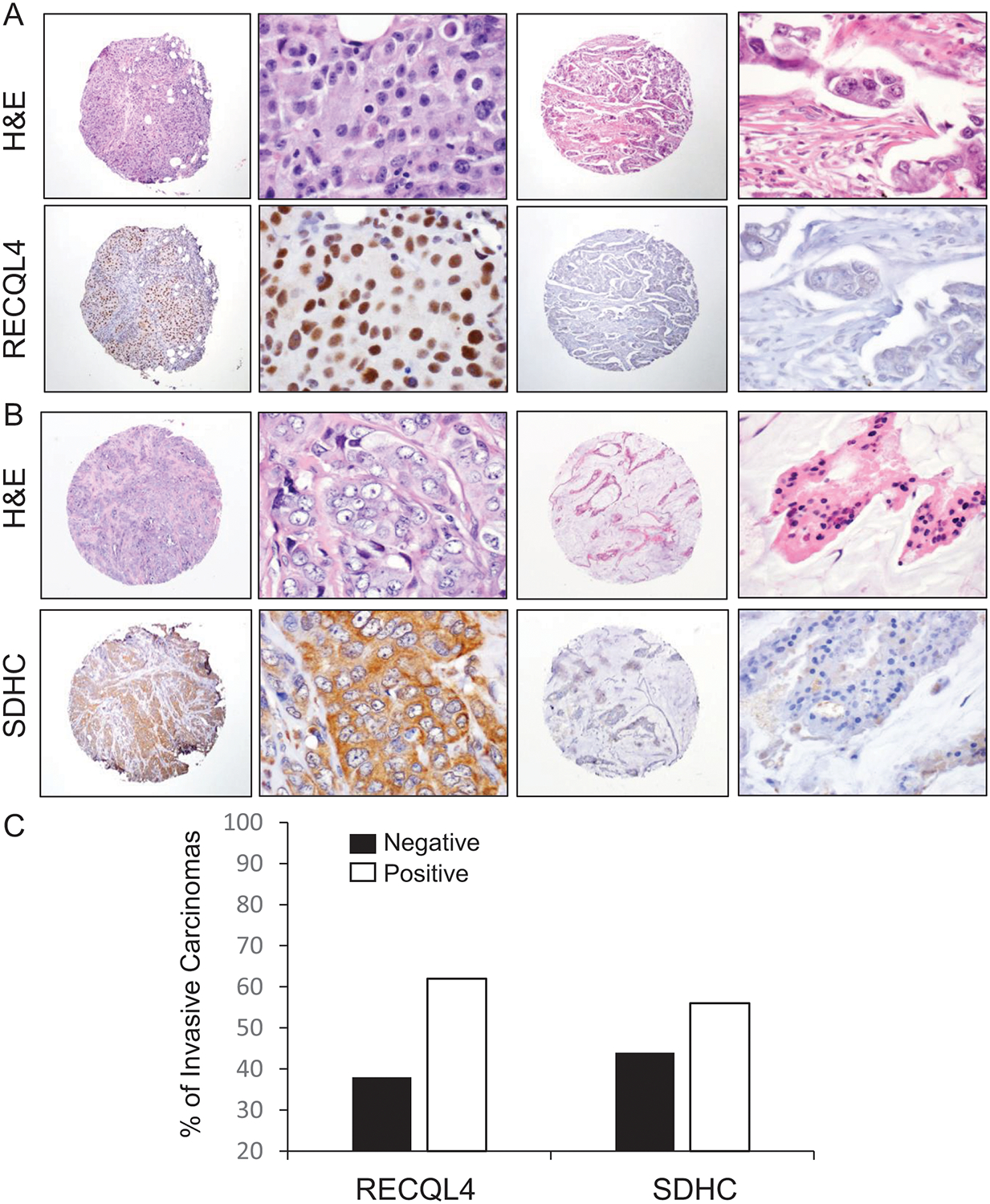
A-B. Representative images of invasive carcinomas with negative and positive nuclear RECQL4 (A) and cytoplasmic SDHC (B) expression, from the TMA containing 86 invasive carcinomas from Ghana. C. Quantification of the percentage of invasive carcinomas negative and positive for each protein.
DISCUSSION
While African women have a lower lifetime incidence in breast cancer, age-standardized mortality is poorer (1). African American (AA) women, like women from Ghana, have a higher incidence of TNBC and basal-like tumors than white American women, suggesting that African ancestry may be associated with differences in tumor biology (10). Our laboratory reported that invasive carcinomas from Ghana have a significantly higher frequency of high histological grade, triple negative status, and EZH2 overexpression (16). However, the genetic alterations that underlie the aggressive biological behavior of invasive carcinomas in African women have not been fully elucidated, in part due to the limited availability of tissue samples from these patients. Here, we used NGS to discover high frequency copy number alterations in invasive carcinomas from Ghana, which may influence their biological behavior and advance our understanding of these tumors.
Our studies revealed the presence of recurrent CNAs in 17 genes in 90% of invasive carcinomas from Ghana studied, including gains in SDHC, RECQL4, TFE3, BCL11A, BCL2L1, PDGFRA, AKT3, SMARCA4, VHL, KLF6, CCNE1, G6PD, FGF3, and CCND1, gains and losses in DEK, SMUG1, and FGF3, and loss of ABL1. Functionally, these genes belong to critical signaling pathways in breast tumorigenesis including PI3K-Akt, transcriptional, and cell cycle regulatory pathways, which have not been previously considered in African breast cancer. These data pave the way to investigating the role of these signaling pathways in the aggressive clinical behavior of breast cancer in this population, and to studies testing the potential utility of inhibiting these pathways on halting breast cancer progression.
Emerging data suggest that the oncogenic function of EZH2 in breast cancer is mediated by transcriptional repression through trimethylation of histone H3 at lysine 27 and, in a subset of triple negative breast cancers by interacting with cytoplasmic proteins that regulate cell migration and invasion (18). Our lab has reported that EZH2 is overexpressed in the nucleus in 42% and in the cytoplasm in 16% of Ghanaian invasive carcinomas, where it is associated significantly with TNBC status (16). We have recently reported that activation of p38 mitogen activated kinase leads to EZH2 phosphorylation at threonine 367 with resulting accumulation of EZH2 protein in the cytoplasm and interaction with vinculin and other cytoskeletal proteins (18). In this study we detected that of the 17 genes with CNAs, 6 (35%) are associated with cytoplasmic (gains of BCL2L1, AKT3, SMARCA4, and CCND1 as well as gains and losses of FGF3, and loss of ABL1) and 2 (12%) with nuclear EZH2 expression (gains in PDGFRA oncogene and loss of DEK tumor suppressor). Network analyses demonstrated a robust predicted interaction between EZH2 and these genes, especially strong with DEK, SMARCA4, CCNE1, ABL1 and CCND1. These data are intriguing as there are several reports of EZH2-mediated regulation of these genes. For example, EZH2 has been reported to directly repress DEK in fibroblasts (22), and EZH2 and PDGFRA were found inversely associated in Merkel cell carcinomas (23), EZH2 inhibition was found to selectively kill SMARCA4 deficient cells in small cell carcinoma of the ovary (24), and EZH2 was shown to regulate cell cycle progression and the levels of cyclins in various cancer types, including our studies showing that EZH2 regulates CCND1 (25–28).
Our study shows that the most frequently altered genes in Ghanaian breast cancers are RECQL4 and SDHC, harboring copy number gains in 50% and 60% of invasive carcinomas in our cases. These findings were validated in a histopathologically well-characterized cohort of invasive carcinomas treated at the Komfo Anoyke Teaching Hospital in Kumasi, Ghana, where RECQL4 and SDHC proteins were overexpressed in 61.6% and 56% of 86 tumors with sufficient tissue for evaluation.
RECQL4 is mapped to 8q24, a gene desert flanked by MYC and PVT1. Amplification of this region is frequently associated with susceptibility to multiple tumor types, including breast and prostate (29, 30). The mechanism of this susceptibility has been thought to occur through the amplification of MYC (31) or ncRNA PVT1 (32). In our study, we observed MYC amplification in 2/11 samples compared to RECQL4 amplification in 6/11 samples, suggesting that in this cohort, gains of RECQL4 occur independently of MYC. Several studies have demonstrated through loss-of-function in vitro studies that RECQL4 contributes to breast cancer proliferation and chemoprotection (33, 34). Succinate dehydrogenase protein C (SDHC) is part of a family of metabolic enzymes that function in the citric acid cycle and electron transport chains. Germline inactivating mutations in SDHC result in accumulation of succinate and are associated with paragangliomas, renal cell carcinomas, and gastrointestinal stromal tumors (35). While RECQL4 and SDHC have been studied in the context of breast cancer, they have not been previously considered in African populations.
The current study also evidences the difficulties encountered in studying invasive breast tumors from western sub-Saharan populations. While we began our study with 20 FFPE blocks of Ghanaian breast tumors, 9 of which were excluded due to low quality DNA and 3 additional had low DNA content requiring the use of a smaller sequencing panel. Despite these limitations, our team has generated a cohort of breast tissue samples from Ghana with adequate material for molecular studies. We have identified novel high level CNAs in 17 genes with functions in tumorigenic pathways with associations to the oncoprotein EZH2. We validated the frequent overexpression of RECQL4 and SDHC tumors in this patient population. Collectively, our data provide the basis for further sequencing and clinical studies to better understand the pathobiology and offer therapeutic opportunities for breast cancer in African and AA women.
Supplementary Material
Acknowledgements.
This work was supported by National Institute of Health (NIH) grants R01CA125577 and R01CA107469 (C.G.K.), F30CA19084 (T.A.), and University of Michigan Rogel Cancer Center support grant P30CA046592. We thank members of the Kleer lab for useful discussions during the execution of the study.
Footnotes
This study was presented as a platform presentation at the 2019 108th USCAP Annual meeting
Supplemental material is available on Modern Pathology’s web site.
Disclosure/Conflict of Interest. Scott Tomlins has had a prior sponsored research agreement with Thermo Fisher Scientific. He is co-founder of, prior consultant to, equity holder in, and current employee of Strata Oncology. All other authors have no relevant conflicts of interest to disclose.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer 2005;103:1540–50. [DOI] [PubMed] [Google Scholar]
- 3.Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am 2014;23:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark A, Kleer CG, Martin I, Awuah B, Nsiah-Asare A, Takyi V, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 2010;116:4926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer 2003;97:134–47. [DOI] [PubMed] [Google Scholar]
- 6.Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 2009;27:4515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, et al. Comparison of Breast Cancer Molecular Features and Survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol 2017;3:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman LA. Breast cancer in African-American women. Oncologist 2005;10:1–14. [DOI] [PubMed] [Google Scholar]
- 9.Toft DJ, Cryns VL. Minireview: Basal-like breast cancer: from molecular profiles to targeted therapies. Mol Endocrinol 2011;25:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiagge E, Oppong JK, Bensenhaver J, Aitpillah F, Gyan K, Kyei I, et al. Breast Cancer and African Ancestry: Lessons Learned at the 10-Year Anniversary of the Ghana-Michigan Research Partnership and International Breast Registry. J Glob Oncol 2016;2:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proctor E, Kidwell KM, Jiagge E, Bensenhaver J, Awuah B, Gyan K, et al. Characterizing Breast Cancer in a Population with Increased Prevalence of Triple-Negative Breast Cancer: Androgen Receptor and ALDH1 Expression in Ghanaian Women. Ann Surg Oncol 2015;22:3831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 2003;100:11606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 2006;24:268–73. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez ME, DuPrie ML, Krueger H, Merajver SD, Ventura AC, Toy KA, et al. Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res 2011;71:2360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell 2011;19:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang J, Toy KA, Griffith KA, Awuah B, Quayson S, Newman LA, et al. Invasive breast carcinomas in Ghana: high frequency of high grade, basal-like histology and high EZH2 expression. Breast Cancer Res Treat 2012;135:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding M, Zhang H, Li Z, Wang C, Chen J, Shi L, et al. The polycomb group protein enhancer of zeste 2 is a novel therapeutic target for cervical cancer. Clin Exp Pharmacol Physiol 2015;42:458–64. [DOI] [PubMed] [Google Scholar]
- 18.Anwar T, Arellano Garcia C, Ropa J, Chen YC, Kim HS, Yoon E, et al. p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis. Nat Commun 2018;9:2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovelson DH, McDaniel AS, Cani AK, Johnson B, Rhodes K, Williams PD, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia 2015;17:385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani AK, Hovelson DH, McDaniel AS, Sadis S, Haller MJ, Yadati V, et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol Cancer Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624–9. [DOI] [PubMed] [Google Scholar]
- 22.Iannetti A, Ledoux AC, Tudhope SJ, Sellier H, Zhao B, Mowla S, et al. Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence. PLoS Genet 2014;10:e1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veija T, Koljonen V, Bohling T, Kero M, Knuutila S, Sarhadi VK. Aberrant expression of ALK and EZH2 in Merkel cell carcinoma. BMC Cancer 2017;17:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan-Penebre E, Armstrong K, Drew A Grassian AR, Feldman I, Knutson SK, et al. Selective Killing of SMARCA2- and SMARCA4-deficient Small Cell Carcinoma of the Ovary, Hypercalcemic Type Cells by Inhibition of EZH2: In Vitro and In Vivo Preclinical Models. Mol Cancer Ther 2017;16:850–60. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, et al. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 2009;28:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG. Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol 2009;175:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J, Pontes KC, Heijkants RC, Brouwer NJ, Groenewoud A, Jordanova ES, et al. Overexpression of EZH2 in conjunctival melanoma offers a new therapeutic target. J Pathol 2018;245:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao W, Feng Z, Cui Z, Zhang C, Sun Z, Mao L, et al. Up-regulation of enhancer of zeste homolog 2 is associated positively with cyclin D1 overexpression and poor clinical outcome in head and neck squamous cell carcinoma. Cancer 2012;118:2858–71. [DOI] [PubMed] [Google Scholar]
- 29.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 2008;40:316–21. [DOI] [PubMed] [Google Scholar]
- 30.Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 2007;39:631–7. [DOI] [PubMed] [Google Scholar]
- 31.Letessier A, Sircoulomb F, Ginestier C, Cervera N, Monville F, Gelsi-Boyer V, et al. Frequency, prognostic impact, and subtype association of 8p12, 8q24, 11q13, 12p13, 17q12, and 20q13 amplifications in breast cancers. BMC Cancer 2006;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res 2007;13:5745–55. [DOI] [PubMed] [Google Scholar]
- 33.Fang H, Nie L, Chi Z, Liu J, Guo D, Lu X, et al. RecQL4 helicase amplification is involved in human breast tumorigenesis. PLoS One 2013;8:e69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora A, Agarwal D, Abdel-Fatah TM, Lu H, Croteau DL, Moseley P, et al. RECQL4 helicase has oncogenic potential in sporadic breast cancers. J Pathol 2016;238:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldera AP, Govender D. Gene of the month: SDH. J Clin Pathol 2018;71:95–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


