Abstract
Antibiotic-loaded bone cement (ALBC) is broadly used to treat orthopaedic infections based on the rationale that high-dose local delivery is essential to eradicate biofilm-associated bacteria. However, ALBC formulations are empirically based on drug susceptibility from routine laboratory testing, which is known to have limited clinical relevance for biofilms. There are also dosing concerns with non-standardized, surgeon-directed, hand-mixed formulations, which have unknown release kinetics. Based on our knowledge of in vivo biofilms, pathogen virulence, safety issues with non-standardized ALBC formulations, and questions about the cost-effectiveness of ALBC, there is a need to evaluate the evidence for this clinical practice. To this end, thought leaders in the field of musculoskeletal infection (MSKI) met on August 1, 2019 to review and debate published and anecdotal information, which highlighted four major concerns about current ALBC use: 1) substantial lack of Level 1 evidence to demonstrate efficacy; 2) ALBC formulations become subtherapeutic following early release, which risks induction of antibiotic resistance, and exacerbated infection from microbial colonization of the carrier; 3) the absence of standardized formulation protocols, and FDA-approved high-dose ALBC products to use following resection in MSKI treatment; and, 4) absence of a validated assay to determine the minimum biofilm eradication concentration (MBEC) to predict ALBC efficacy against patient specific microorganisms. Here we describe these concerns in detail, and propose areas in need of research.
Keywords: Biofilm Meeting, Musculoskeletal Infection (MSKI), Local Antibiotics, Antibiotic-loaded bone cement (ALBC)
Introduction and Background
As biofilm-associated implant-related musculoskeletal infections (MSKI) remain a major complication of orthopaedic surgery with catastrophic outcomes for patients and immense cost that is projected to exceed $1.62 billion in the USA by 2020 for treatment of periprosthetic joint infections (PJI) alone1. There has been a recent groundswell of investigators and clinical caregivers to address this critical issue2. A case in point was the 2018 International Consensus Meeting (ICM)3, which included 869 delegates from 92 countries, who derived recommendations with rationales for 652 consensus questions (https://icmphilly.com). Furthermore, specific 2018 ICM Research Workgroups (RW) addressed: high priority MSKI research questions (https://www.ors.org/icm-2018-research-agenda-workgroup/)4, questions pertaining specifically to PJI of the hip and knee (https://icmphilly.com/wp-content/uploads/2018/11/Hip-and-Knee.pdf)5–7, and biofilm questions (https://www.ors.org/icm-2018-biofilm-workgroup/)8. One clinically relevant and frequently discussed topic (https://www.ors.org/wp-content/uploads/2019/01/Question-8.pdf) that drew great attention by all three of these independent RW is the use of antibiotic-loaded methylmethacrylate bone cement (ALBC), and the use of bone graft extender/substitutes (calcium sulphate/calcium phosphate, CaS/CaP) as antibiotic carriers, in the treatment and prevention of surgical site infection (SSI), fracture-related infection (FRI) and PJI.
ALBC use can be categorized as either high-dose ALBC used in the first stage of MSKI treatment, or low-dose ALBC for use during primary arthroplasty as a prophylaxis, or in the second stage reconstruction for MSKI. A recent comprehensive review of ALBC use for prophylaxis in primary total joint replacement (TJR) included 24 qualifying studies9, focusing on three fundamental topics: 1) efficacy of ABLC to prevent PJI, 2) risks associated with ALBC for prophylaxis, and 3) economic justification for ALBC prophylaxis in primary TJR. The authors concluded that although contradictory results have been published, most clinical studies found similar infection rates between low-dose ABLC (≤2 g of antibiotic powder per 40 g batch of ALBC) and plain cement (both groups received systemic perioperative antibiotic prophylaxis). They also cited risks associated with routine use of ABLC including: dose-dependent degradation of material strength of the cement, adverse effects on local cells and healing, systemic toxicity when drug distribution out of the local site exceeds systemic clearance (Acute Kidney Injury, AKI), local tissue toxicity and development of antibacterial resistance. The authors also concluded that the cost of prophylactic use of low-dose ABLC for PJI (~ $234M annually) cannot be justified by cost savings from fewer PJIs.
On top of the aforementioned questions about low-dose prophylactic ALBC use in primary TJR, additional concerns with this clinical practice include: 1) well known limitations of commonly used antibiotics (Figure 1), 2) ineffectiveness for bacteria within biofilms (Figure 2), 3) unfavorable biodistribution patterns in which the antibiotic is dispersed away from the infection (Figure 3), 4) bacterial colonization of the ALBC after the antibiotic release decreases to subtherapeutic levels (i.e.; below the minimum inhibitory concentration (MIC)), 5) alterations to the host microbiome, 6) a limited number of commercially available products, 7) products available in the USA that contain an insufficient antibiotic load for the first stage treatment of biofilm-associated MSKI infections, and 8) complications from non-standardized, non-validated, surgeon-directed, high-dose ALBC (≥5 g of antibiotic powder per 40 g batch of ALBC) formulations with unknown antibiotic release, including AKI from systemic antibiotic exposure.
Figure 1. Limitations of gentamicin and opportunities towards an ideal drug for MSKI.
Although aminoglycosides remain an effective drug class, particularly for cystic fibrosis patients and treatment of Gram negative bacteria that are resistant to newer agents, their use in orthopaedic infections has declined ~80% over the last 30 years, largely due to toxicity issues. Interestingly, experimental use of gentamycin to study microbial pathogenesis and antibiotic resistance during this same time period, has identified important drug development opportunities. In 1973 G. L. Mandell reported that gentamicin does not penetrate mammalian cells67, which gave rise to the “Gentamicin Protection Assay” in the early1980s as a standard method to study intracellular bacterial persistence, replication, and unique virulence factors required for these behaviours68. A contemporary version of this assay is shown, in which a monolayer of murine primary bone marrow macrophages is labelled with a fluorescent dye (Lysotracker Red), exposed to S. aureus that express green fluorescent protein (GFP), and then incubated to allow intracellular infection of the macrophages in the absence (A), or presence of gentamicin (B). Note the clearance of the extracellular GFP+ bacteria by the gentamicin treatment, which allows for study of S. aureus within macrophages (arrows). As intracellular persistence is a mode of chronic infection, an ideal antibiotic treatment for MSKI must kill these intracellular pathogens. In 1979 Pelletier et al published methods to generate “Virulent gentamicin-induced small colony variants (SCVs) of Staphylococcus aureus”69, which has evolved into a well-established experimental approach to generate nonhemolytic SCVs (arrows in C) to study antibiotic resistance70. It is known that S. aureus resistance to antibiotics is mediated by cell wall thickening71, which can be observed in a murine model of implant-associated osteomyelitis72 by comparing TEM images of colonized osteocytic-canalicular networks of femurs from mice treated with saline (D) and gentamicin (E). While the ability of gentamicin to induce this bacterial phenotype indicates that the drug has access to this deep site of infection, it highlights the importance of achieving cytolytic drug levels.
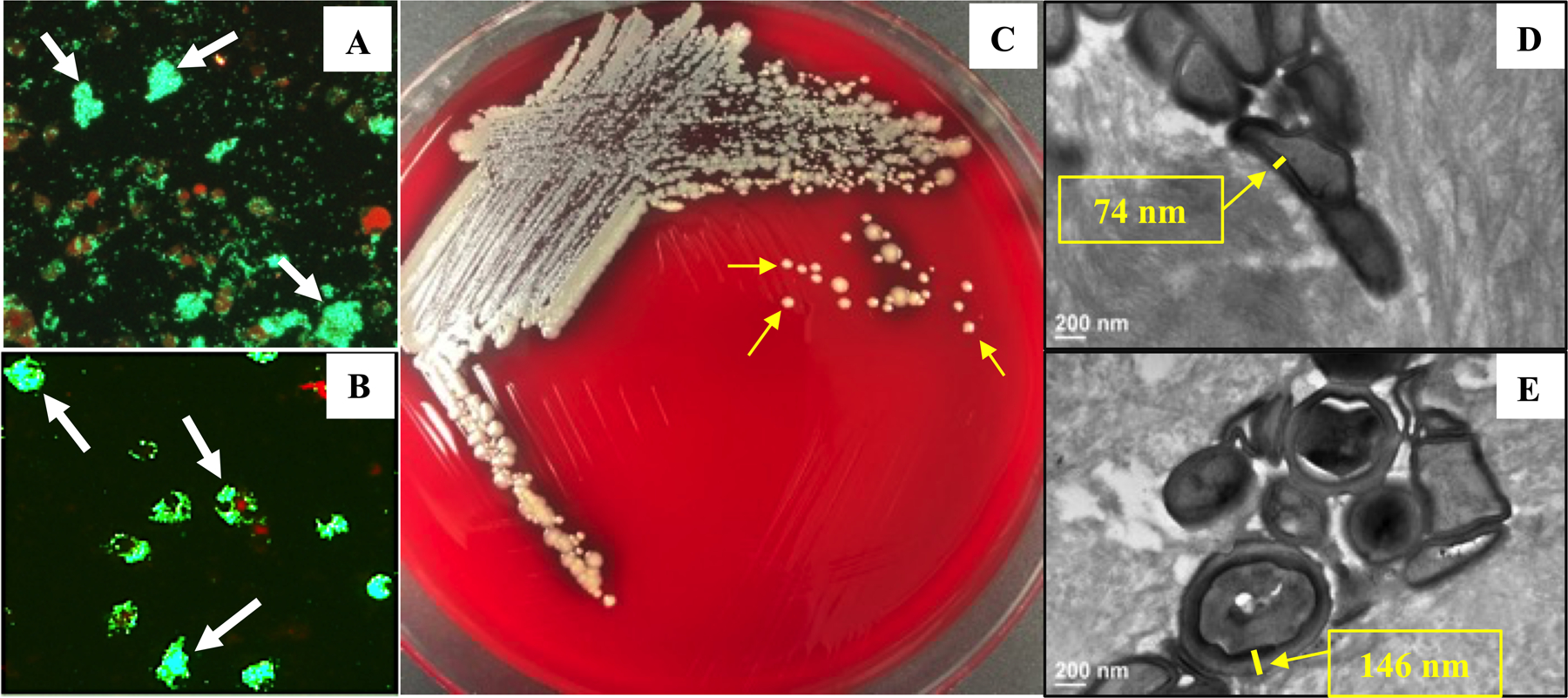
Figure 2. The four distinct biofilms in PJI.

Images of the four distinct microscopic biofilms known to exist in PJI are shown to illustrate the most challenging targets of ALBC. (A) Bacterial aggregates in synovial fluid (SEM x2.5k) described in Delaney et al73. (B) Biofilm on metal implant (SEM x150) described in Nishitani et al74. (C) Soft tissue abscess between infected necrotic bone and live bone (x40 Brown & Brenn stained histology) as described in Varrone et al75. And, (D) colonized osteocytic-canalicular networks (TEM x3.5k) as described in de Mesy Bentley et al72.
Figure 3. What do we know about the biodistribution of locally administered antibiotics and their concentration relative to MIC and MBEC?

Giers et al studied in vivo biodistribution of antibiotics following local release, using gadolinium diethylenetriamine pentaacetate (Gd-DTPA) discharged from bone cement as a surrogate for high-dose ALBC39. Published images from that study are show with permission form the publisher (Wiley Inc., Hoboken, NJ). 2D segmentation images from 7T MRI at 5.5 hr post-implantation in a rabbit quadriceps debridement model (A), were used to generate 3D renderings of the biodistribution in local tissues (B). On a 2D coronal MRI slice (C) through the thigh at the debridement/depot site two regions of interests (ROIs) are identified, Gd-DTPA concentration was calculated to be 33 ± 22 μg mL−1 at a distance in ROI 1, and 104 ± 63 μg mL−1 closer to the depot in ROI 2. Note that these concentrations are 30–100 fold higher than gentamicin/tobramycin MICs for many planktonic pathogens, but may not be as high as MBEC (100–1000 μg/mL sustained for 24 hrs)39; 76 for some pathogens in biofilms. The authors also documented that the concentration of released Gd-DTPA does not decrease exponentially with distance in all directions, as would be expected for diffusion dominated mass transport, and concluded that the anisotropic transport away from the implant is likely also driven by convection within fluid that exists in surgical wounds. Thus, contemporary experimental models of local antibiotic delivery that aim for MBEC concentration need to incorporate a combination of diffusion and convection within a post debridement site, which is where local delivery is commonly used.
Remarkably, despite the broad clinical use of ALBC since the 1980s10, this practice has not been successfully extended to other carriers. At the 2018 ICM there was a Strong Consensus (Super Majority agreement, Delegate Vote = Agree: 80%, Disagree: 13%, Abstain: 7%), that “The use of antibiotic-loaded ceramic bone graft substitutes, specifically calcium sulfate and calcium phosphate based materials, to locally deliver antibiotics at sites of musculoskeletal infection, specifically SSI, FRI and PJI11, has not been shown to be effective in the management of SSI/FRI/PJI.”4 This consensus is consistent with the most recent peer-reviewed literature on this topic, which has also not shown sufficient outcome evidence to prove the efficacy of ALBC in the management of osteoarticular infections5–7. This absence of efficacy data may be due to the lack of randomized clinical trials designed to answer this question.
Based on these and other questions, and controversies in the field, a Biofilm Symposium was held in New York City on August 1, 2019, the day before the Musculoskeletal Infection Society (MSIS) annual meeting. Attendees included MSKI investigators, infectious disease physicians, and orthopaedic surgeons. Primary goals of the meeting included granular discussions on: 1) current surgical debridement and post-debridement antibiotics for established MSKI and implant-related infections, 2) the effectiveness of these currently recommended treatment modalities to eradicate the four distinct biofilms known to exist in MSKI (Figure 2), 3) the basic science of ALBC in clinical use (antibiotic selection, antibiotic load, release kinetics, temporospatial biodistribution following local delivery), and 4) the potential of alternative local antibiotic delivery systems that are not currently available for clinical use (biodegradable controlled release carriers, anti-infective-coatings for implants, non-traditional anti-infectives, biofilm disruption and anti-quorum sensing strategies). After the meeting, a RW comprised of attendees and other experts in the field (the authors of this paper, termed the 2019 ALBC-RW) completed additional literature review to reach consensus about the science, clinical use, and future directions of local antibiotic therapy, as described below.
Methodology
Following the announcement of the 2019 Biofilm Symposia, the Editor-in-Chief of the Journal of Orthopaedic Research invited a Consensus Article submission on local versus systemic antibiotic therapy, based on the outcomes of the meeting. The Course Directors of the 2019 Biofilm Symposia (BB, EMS, ACM & TPS) defined the topics to be discussed, including: “Four Distinct Modes of Biofilm Formation During Implant-Associated Osteomyelitis” (presented by EMS), “What are the Goals of Debridement During 2-Stage Revision Surgery for PJI?” (presented by MB), “What is the Evidence for Local Antibiotics and Opportunities to Enhance Efficacy?” (presented by JP), and “Standard of Care Antibiotics. Their Efficacy and Limitations” (presented by ACM). The Course Directors also invited 61 MSKI experts to attend the meeting. Those with the most ALBC/local delivery expertise were invited to participate in the 2019 ALBC-RW, and contribute to this article. The 2019 ALBC-RW identified the key unknowns related to care for patients with biofilm-associated MSKI and framed ten questions based on those unknowns, three of which were combined to derive the final seven Consensus Questions. Primary responders to the questions were assigned from the 2019 ALBC-RW, and then consensus was sought through broad work group revision of those questions and responses.
Additionally, only for Question 2, “Why do orthopaedic surgeons use local antibiotic delivery from ALBC when data to support it are from low level of evidence studies, conflicting or incomplete?”, the orthopaedic surgeons in the 2019 ALBC-WC (n=17) individually responded to the questions in Table 1, and the responses were tabulated.
Table 1:
Survey of Antibiotic Loaded Bone Cement Uses by Surgeons in the 2019 ALBC-RW
| Question 1 |
Do you use ALBC in total joint arthroplasty/PJI in your practice?
Response: Yes = 100% |
| Question 1a |
If yes, in which indication?
Response: 38% of the surgeons use it for all cemented primary arthroplasties, and 67% in primary arthroplasties with patients at risk for infection (diabetes, rheumatoid arthritis, obesity etc.). All surgeons use it for revision arthroplasty cases, particularly in periprosthetic joint infections. |
| Question 2 |
What is the best anecdotal evidence you have that ALBC in total joint arthroplasty/PJI is efficacious in your patients?
Response: “none” (27%), “low reinfection rates in my own case series” (27%), “good registry data confirming the benefit of ALBC in primary arthroplasty” (7%), “conflicting data from registries” (7%), “the Canadian register did not show any difference in PJI rates”, “I do not feel that ALBC is harmful and thus I use it on a theoretical advantage” (7%), other responses (28%). |
| Question 3 |
Do you think that more prospective randomized controlled trials (RCTs) data are required for ALBC for 2-stage revision surgery with ALBC?
Response: Yes = 47%; No = 29%; Undecided = 24% |
| Question 4 |
Do you think that more prospective randomized controlled trials (RCTs) data are required for primary arthroplasty with ALBC?
Response: Yes = 47%; No = 29%; Undecided = 24% |
The 2019 ALBC-RW accentuates that the consensus opinions in this article were achieved without unwarranted persuasive power or expressiveness, compulsion, incompetence to understand alternative actions, or exasperation with the debating process. Discussion at the meeting occurred in a controlled open forum in which all attendees had opportunities to ask questions, contribute information, voice their opinions, and make recommendations, which were used to derive the clinical unknowns and included in the responses reported in this article.
Results
Question 1: What is the rationale for using local antibiotic delivery for prevention and treatment of MSKI, especially biofilm-associated, implant-related infections?
Antibiotic-loaded carriers are used for prevention and treatment of SSI, FRI and PJI in particular, in which non-FDA-approved local treatments have become the standard of care in various settings to treat bone and implant-related infections. The idea was championed by Buchholz et al at the Endo Klinik in 198410, and since then a significant amount of experience has been acquired in this field12.
Local delivery of antibiotic represents an attractive therapeutic modality in the treatment of biofilm-associated, implant-related MSKI. A key advantage is that local delivery of antibiotics (via carriers or directly) can result in concentrations of antibiotics in the affected or targeted area, higher than achievable systemically, for organisms present at the site of infection, while serum antibiotic levels remain low13. This is important also as penetration of the systemically administrated antibiotics in implant-related MSKI might be suboptimal as a result of potentially compromised vascular perfusion and/or the presence of biofilms.
Local delivery could also provide continuous antibiotic release in affected compartments, optimizing the effect of most antibiotics, as time of exposure at adequate concentrations is one of the most important pharmacodynamic parameters for many antibiotic classes (i.e.; fT> MIC)14. Additionally, local delivery of antibiotics can be an advantage for individuals who are intolerant to systemic use of that antibiotic as the limited systemic absorption of the locally installed antibiotic reduces the risk of toxicity and avoids many of the adverse drug reactions caused by systemic antibiotics15. Furthermore, the effect on the human microbiome would be negligible.
There is consensus that bacterial attachment can occur on essentially all foreign material and injured or immune compromised biological surfaces, including surfaces of ALBC spacers utilized to locally deliver antibiotics8. As the antibiotic load in ALBC is released, the surrounding drug levels fall below the MIC, and thus the surface itself becomes susceptible to microbial colonization. Antibiotic levels can remain sub‐therapeutic for a prolonged time, which increases the risk for the emergence of microorganisms that are resistant to the incorporated antibiotic(s).
The use of commercially available ALBC for infection prophylaxis in primary THA and TKA remains prevalent in North America and internationally. Its use varies from less than 50% of cases in North America to greater than 90% of cases in some parts of Northern Europe16. Like other prophylactic modalities such as the use of antibiotics in surgical irrigation, or the use of laminar airflow in joint replacement, the use of ALBC in infection prophylaxis has a certain historical context, and was implemented in a time when there was a less rigorous standard required for proof of efficacy. Early elution studies for individual antibiotic or combinations from ALBC, and data on local directly measured antibiotic levels, suggested that antibiotic release from ALBC could achieve levels needed to prevent bacterial contamination from establishing infection. While many logical ideas have been subsequently proven false by clinical research, the concept of local antibiotic delivery seemed to be bolstered by advances in biofilm science in the field of MSKI. With the understanding that very high antibiotic concentrations are needed to treat biofilm-associated infections, the idea of local delivery to achieve high antibiotic concentrations seemed all the more appealing in treating and, by logical extension, preventing these infections. However, the proof of this concept is quite shallow. In the current era of evidence-based medicine, there is a glaring lack of Level 1 studies to support the use of ALBC for MSKI prophylaxis. A systematic review by Barth et al. showed that despite extensive experience with its use and the theoretical advantages, there are no well executed, prospective studies investigating the efficacy of ALBC in treating FRI and PJI17. Thus, while meta-analysis on the prevention of high-risk FRI suggests improved clinical outcomes when ALBC beads are used in open fractures18, the poor level of evidence from the cited studies, stemming from the great heterogeneity in prevention protocols between centers19, limits the conclusion. Therefore, the use of prophylactic ALBC remains a topic of debate that requires formal investigation to resolve.
Some institutional and registry data, particularly outside of the United States, do show benefit from prophylactic use of low-dose ALBC. For example, in 2017, Sanz-Ruiz et al. found that the use of ALBC in primary TJR in a single-institution, prospective, registry study reduced the risk of PJI by 57% and thereby resulted in a total cost savings of 992 € per patient20. In the Norwegian registry, the use of ALBC in primary THA appears to reduce infection rates relative to bone cement without antibiotics21. In contrast, data from the New Zealand registry did not support reduced infection rates with ALBC usage in primary TKA22. Most large institutional studies from the United States do not report lower infection rates with ALBC in primary prophylaxis23. A recent meta-analysis24, showed a similar risk of PJI in primary TKA fixed with ALBC or plain cement in both observational cohort studies and RTCs. Taken together, definitive support for the routine use of ALBC in primary total joint arthroplasty does not exist in the literature. Additionally, the cost of commercially available ALBC products may not be justified by the perceived benefits in PJI reduction23.
While the use of ALBC in all cases may not be clearly justified, it has been suggested that ALBC usage be reserved for high risk patients undergoing primary TKA or THA3. One small Taiwanese single-blinded RTC including 78 arthroplasties (surgery performed without the use of clean air operating theaters) found that cefuroxime impregnated bone cement reduced infection rates in patients with diabetes mellitus in primary TKA25. More recent studies, however, do not support the use of ALBC even in high risk primary THA and TKA. Qadir et al. found that selective ALBC use for high risk cohorts (patients with diabetes, inflammatory arthritis, previous joint infection, low albumin) did not reduce the rates of PJI in primary TKA26. In the Kaiser registry, the use of ALBC did not reduce the rate of infection in patients with diabetes mellitus after primary TKA27. Current data do not support the use of commercially available low-dose ALBC even in high risk patients.
While proponents for or against the routine use of ALBC can quote studies to support their position, there remains a lack of Level 1 data supporting the use of ALBC for PJI prophylaxis. The studies that are available in this area have concerning issues. National registries and large observational cohort studies suffer from selection bias. In most cases, it is difficult to determine why some patients received ALBC versus other patients who had fixation with plain bone cement. It is possible that the ALBC group was a higher risk group of patients, which can bias the results to suggest no benefit to ALBC when there indeed may be one. Most of these studies do not control for these factors because underlying patient characteristics that contribute to this risk, including body mass index, nutritional concerns, smoking status, and blood glucose control for diabetic patients, are not reported or controlled for. Additionally, certain surgeons or hospitals may use ALBC routinely, but the results across surgeons and hospitals are not uniform. Other measures instituted over the past decades including medical optimization, preoperative antibiotic administration, staphylococcus screening, and decolonization, may confound the observational cohort studies of ALBC usage.
A perceived lack of harm may be another reason why surgeons use ALBC despite the lack of strong evidence to support its use. Most low-dose ALBC doses do not lead to systemic complications such as AKI or hypersensitivity reactions. Aside from cost, surgeons may perceive that there is no downside to low-dose ALBC usage. Hansen et al. did not find a change in antibiotic resistance patterns after the introduction of routine ALBC in primary TKA28. Data from the National Joint Registry, however, have shown that the use of gentamicin-loaded bone cement in primary TKA and THA increases the risk of gentamicin resistance and methicillin resistance at the time of revision for subsequent PJI29. Further studies are warranted to assess the consequences of antibiotic resistance in the setting of ALBC use in primary joint replacement.
Question 2: Why do orthopaedic surgeons use local antibiotic delivery from ALBC when data to support it are low level, conflicting or incomplete?
Given the aforementioned theoretical and scientific concerns with ALBC, and the absence of Level 1 evidence to support this practice after three decades of clinical experience, we attempted to answer Question 2 ourselves. A survey based on 4 questions (Table 1) was sent to the 17 MSKI expert orthopaedic surgeons in the 2019 ALBC-RW to generate an overview on their current clinical practice with ALBC in primary and revision total joint arthroplasty. One-hundred percent of the surgeons stated that they use ALBC in their current practice. Thirty-eight percent of the surgeons use it for all cemented primary arthroplasties, and 67% for primary arthroplasties in patients with increased risk for infection (e.g. diabetes, rheumatoid arthritis, obesity). All surgeons use it for revision arthroplasty cases, particularly in PJIs. Answers for the question “What is the best anecdotal evidence you have that ALBC in TJR/PJI is efficacious in your patients” range from “none” (27%), “low reinfection rates in my own case series” (27%), “good registry data confirming the benefit of ALBC in primary arthroplasty” (7%), “conflicting data from registries” (7%), “the Canadian registry did not show any difference in PJI rates”, “I do not feel that ALBC is harmful and thus I use it on a theoretical advantage” (7%) and others (28%). Forty-seven percent of the surgeons stated that more high-level data are required to evaluate the value of ALBC for 2-stage PJI treatment, whereas 29% stated that RCTs for this indication are not required and 24% were undecided. While RCTs for the use of ALBC for primary arthroplasty was also desired by 47% of the surgeons, 29% did not find this necessary and 24% were undecided as well.
Based on these responses and our review of the literature, the 2019 ALBC-RW surmised that surgeons currently use ALBC because: 1) this is the standard of care in their hospital, and 2) there is consensus belief that ALBC has prophylactic effects in high risk patients (diabetes, rheumatoid arthritis, obesity) and therapeutic effects in revision cases, particularly in the setting of infection. However, there is no consensus that convincing prophylactic or therapeutic efficacy data supporting the use of ALBC exists, nor is there strong enthusiasm to test this hypothesis in RTCs. Additionally, there are no data on impact of selecting resistant bacteria.
Question 3: Is commercially formulated, low-dose ALBC more effective than surgeon-directed, hand-mixed, low-dose ALBC?
The use of low-dose ALBC in primary hip and knee arthroplasty procedures has declined drastically in the United States, with rates decreasing from 90% in 2006 to 34.5% in 201730. A key reason for this decline is a growing body of literature questioning the efficacy of low-dose ALBC in preventing PJI. When surgeons do consider using ALBC for primary arthroplasty, they can choose to either utilize commercially prepared low-dose ALBC or manually add antibiotic powder to non-antibiotic loaded cement. Proponents of commercially prepared ALBC point out that these products, in comparison to hand-mixed ALBC, reduce operating time and claim that they provide ALBC with more consistent, homogenously mixed antibiotics that improve drug delivery and produce a cement mantle that has a better mechanical profile31. However, such advantages have not been consistently verified. While formulated with fine particulate antibiotic powders and industrial mixing, mechanical strength and elution from commercial ALBC was not consistently better than hand-mixed low-dose ALBC across disparities between products and various mixing techniques (Supplement 1).
Question 4: How can surgeon-directed hand-mixed ALBC formulation be standardize to maximize consistency, minimize systemic exposure, prevent systemic toxicity and adverse effects on local tissues and healing?
When considering hand-mixed high-dose ALBC for the treatment of PJI, surgeons must balance the need to deliver a sufficient antibiotic dose that can act against infective organisms, and the risk that absorption of the local antibiotic may exceed systemic clearance, leading to systemic toxicity. The risk of organ toxicity should not be taken lightly, with the incidence of AKI ranging from 5 to 24% in cases of PJI treated with antibiotic spacers32. Variation in AKI occurrence is multifactorial partly explained by discordance in the literature regarding how nephrotoxic states are defined. AKI is associated with the use of aminoglycosides and vancomycin which are nephrotoxic antibiotics32. Yet, in addition to selecting an antibiotic and its dose, surgeons must also consider the brand of cement they use, how the cement and antibiotics are mixed, and whether supplementary agents should be utilized to improve antibiotic elution as detailed in Supplement 1.
Studies have proposed that the mechanism of release of antibiotics from the largely impermeable PMMA relies on diffusion-driven release from small cracks and micropores in the material, meaning that only antibiotics within 50–100 μm from the surface are released33. Increasing the number of larger pores through incorporation of porogens, increasing surface area to volume ratio, or other modifications to mixing protocols have all been proposed as mechanisms to increase antibiotic elution from high-dose ALBC34(Supplement 1). The antibiotic powder itself is considered a porogen within PMMA, as it is intended to dissolve away after the material is set. It has been suggested that molecular weight of the antibiotics included may affect the diffusion of the drug through the PMMA35, with the aminoglycosides (tobramycin and gentamicin) having approximately 1/3 of the molecular weight of vancomycin. However, the volume of powdered antibiotics was found to play a larger role than molecular weight in the elution kinetics of antibiotics36. The volume of the antibiotic powder is particularly relevant for hand-mixed ALBC since generic and proprietary formulations for the same antibiotics have been found to have dramatically different mass/volume ratios, particularly for tobramycin. Others have found that adding antibiotics in combination, such as the common practice of including vancomycin with tobramycin, can increase elution of both. Contradictory results have been reported in studies using different commercially available cements34. For surgeon-directed hand-mixing of antibiotics, it is important to understand that the use of excipients in commercially supplied antibiotics, such as cyclodextrin, surfactant stabilized emulsifiers, or liposomal formulations for hydrophobic antibiotics can increase elution of drug from PMMA37. Liposome formulated antifungal has been shown to increase elution compared to formulation with a solubilizing agent, without some of the reported compromises in mechanical properties that other antibiotic formulations are known to induce37. Antibiotic loading through liposomes theoretically could enable delivery of lipophilic drugs that are currently considered incompatible with PMMA, such as the radical-scavenging rifampin38.
The breadth of innovation in formulation and mixing strategies for ALBC over the course of 50 years raises the question about the lack of standardized mixing methods or FDA-approved formulations making use of porogens. Low-dose ALBC was approved by the FDA in 2003, and no other combination devices for antibiotic release have been approved since. Thus, high-dose formulations of ALBC are typically determined using an empirical approach, as describe in Supplement 2. The release properties of PMMA make it unavoidable that over the course of implantation, decreased release leads to sub-inhibitory concentrations, and models of drug release into tissue indicate that diffusion into tissue is anisotropic and may be limited to tissues where fluid flow is greater39. Medical devices that release antibiotic as a secondary mode of action are considered “combination products” by the FDA, and must undergo rigorous testing to evaluate both safety and efficacy. Recommendations for use as a preventative measure in primary arthroplasties presents challenges in this regard, as powering a multi-center RTC to determine differences in infection rate that may be less than 1% would require many thousands of patients, placing a high financial burden on industry. As a corollary, research on degradable calcium sulfate as a carrier for antibiotics for prevention of orthopaedic infections has been ongoing for decades, with no FDA approval for using calcium-based bone graft substitutes with antibiotic loading. Jurisdictional issues between Centers that regulate drugs and those that regulate devices within the FDA may also present obstacles, as well as the “credibility gap” between positive research findings in preclinical in vitro studies and animal models and demonstration of clear clinical benefit. The profitability of seeking FDA approval for antibiotic combination devices limits the incentive for industry to pursue innovative antibiotic delivery systems. However, innovative regulatory pathways similar to the Limited Population Antibacterial Drug (LPAD) approval mechanism may be a valuable pathway to seek approval for use of new antibiotic-loaded devices in smaller more targeted populations with high risk of infection, such as patients with a history of infection, diabetes, or obesity, or those who are unable to undergo two-stage revisions.
Question 5: What levels and durations are needed for clinical effectiveness?
High antibiotic tolerance (even if the cultured pathogen is susceptible by conventional clinical tests) is arguably the best recognized and well-characterized trait of bacterial biofilms for many types of pathogens against all classes of antibiotics. Antibiotic tolerance has been reported in many in vitro experiments performed over decades by many independent labs, including failure of the antibiotic to access intracellular microorganisms, and phenotypic adaption by pathogens (Figure 1). This tolerance seen in vitro, is associated with four distinct biofilm adaptions in bacterial growth to avoid host defenses (Figure 2), and is reflected in the clinic where clearing biofilm infections with antibiotic therapy is often challenging or unsuccessful. However, many of these studies were conducted by assessing log reductions of bacterial loads (measured in colony forming units (cfu)/mL) after antibiotic exposure of 24 hrs40. Time-kill assays are typically performed over 24 hrs41. Several studies that have evaluated the effects of longer antibiotic exposure times on the killing of biofilm bacteria have shown that greater than 3-log reductions or even complete eradication (often referred to as the “minimum biofilm eradication concentration”, MBEC) can be achieved, but sometimes such results are not seen until exposure to high concentrations of antibiotics has occurred for many days40. Further, biofilm age is an important factor and a study on Enterococcus biofilms showed that younger (24 hrs) biofilms were more susceptible to antibiotics than older (120 hrs) biofilms, but even the young biofilms were highly tolerant compared to planktonic cultures42. Host factors such as plasma and heme also increase the tolerance to antibiotics, as the same aged biofilm in normal media versus media with human plasma has been shown to have up to 100-fold difference in tolerance43. In vitro studies suggest that the high antibiotic concentrations and durations required to treat biofilm infections cannot be achieved in patients systemically due to toxicity concerns44. Thus, local delivery through ABLC and other carriers is an alternative strategy. Clearly more appropriate studies are needed in order to develop standardized methods for assessing biofilm susceptibility over extended exposure times.
Question 6: What are the most promising technologies for local antibiotic delivery?
Local antibiotic delivery has been be attempted with intrawound vancomycin powder. Pre-clinical in vivo data have shown that this approach significantly reduces bacterial load and MSKI in rats45. The local application of vancomycin powder is proposed to be a rapidly available and inexpensive intra-operative strategy to decrease bacterial colonization that leads to biofilm formation. Although clinical observational studies have shown significant efficacy using intrawound vancomycin powder in spine surgery, a meta-analysis indicated that prospective randomized data do not support this conclusion46. In the arthroplasty literature, while several retrospective observational studies have reported significantly decreased infection and re-operation rates in wounds that have been treated with vancomycin powder47, others have found significantly increased aseptic wound complications attributed to the vancomycin powder, possibly related to local adverse response48. Currently there is no high-level evidence to direct the use of intrawound vancomycin powder. Although there may be some efficacy, adverse effects should be considered.
Absorbable calcium based mineral bone fillers are used to deliver antibiotics locally in an attempt to achieve the sustained high concentrations required to kill biofilms. In addition, since they slowly resorb over periods of weeks they end up releasing all of the antibiotic. The release from PMMA is limited by insoluble polymer, such that antibiotic is retained with long term sub therapeutic release. Each of these delivery methods has advantages and limitations and it is possible that optimal combinations can be found for the prevention of biofilm formation or the treatment of biofilm that might remain after debridement.
Anti-bacterial coating of implants may serve to inhibit both bacterial adhesion and biofilm formation. Anti-bacterial silver coatings have been assessed in vitro and in vivo and have been found to be anti-infective and biocompatible49. This approach has been slow to be adopted in practice due to concerns related to cost; however some have argued that the reduction in implant infection-related costs far outweigh the increased costs of the coated implant50. Unfortunately, there is very limited clinical data to support the use of silver-coated implants51; this may be due to the lack of available devices and published studies. There is also a poly(D,L-lactide) (PDLLA)/gentamicin coated nail undergoing FDA approval for tibial fractures. The coating releases gentamicin over a period of two weeks, following a burst release in the initial days. Two clinical studies evaluated these gentamicin-coated tibia nails in acute complex fractures and revision cases, in which no postoperative infectious complications were documented52; 53.
There are many promising strategies being developed in the laboratory that have applications for orthopedic infections, and the subject has been reviewed elsewhere54. Transforming these strategies from the laboratory bench and small animals, to clinically relevant size devices and wounds, and the mechanical performance criteria required by real implants, remains a challenge.
Question 7: What antibiotic level and duration of antibiotic exposure is needed for local antibiotic delivery in the clinical setting?
The antibiotic levels should be high enough to kill bacteria, irrespective of their living condition (i.e.; planktonic versus stationary versus biofilm form). Because host killing within biofilm is markedly limited and unreliable, the goal for local antibiotic treatment duration should be long enough to eliminate all the microorganisms within the biofilm. This concept implies microbiological variables that are not typically used in clinical practice. Firstly, instead of the minimal inhibitory concentrations (MICs, reported from the clinical microbiology laboratory) minimal biofilm eradication concentrations (MBECs) must be considered. The MBEC of an antibiotic agent is the concentration of antibiotic substances needed to kill all viable bacteria within an established biofilm, including persister cells and cells growing within host tissue cells. Secondly, while MICs and MBCs are determined on planktonic bacteria, the antibiotic treatment concepts need to focus on bacteria in biofilm form. Thirdly, our current understanding of antibiotic mechanisms in killing bacteria (e.g.; fT>MIC, Cmax>MIC or AUC24h>MIC) derive from in-vitro experiments with planktonic bacteria. The determination of MBEC is dependent on killing of bacteria in biofilm. However, MBEC determination is not offered clinically, is not standardized, and antibiotic exposure time is not reported. In addition, methods for MBEC determination have technical difficulties, leading to a considerable variability of results (Supplement 2). On the basis of these uncertainties, providers aim for high antibiotic concentrations that cannot be achieved in bone and the neighboring compartments with systemic administration but may be possible if administered locally. Conversely, the local level of antibiotic concentrations must not be so high that absorption leads to systemic toxic effects, and should not be detrimental to neighboring host tissue cells55; 56.
The required levels of local antibiotic treatment depend on the antibiotic agent and the target organisms. Table 2 illustrates the MBEC:MIC-ratio of selected compounds and their relations to Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. For Gram-positive organisms, MBECs of beta-lactams and glycopeptides are typically several 100 times higher than the corresponding MICs. The MBEC:MICs-ratio for aminoglycosides and rifampin is typically lower than the ones for beta-lactams. However, Staphylococcus spp., Streptococcus spp. and Enterococcus spp. may display high level gentamicin resistance. The delivery of local rifampin in PMMA has raised concerns in terms of compression strength of the PMMA carrier (i.e., mechanical properties, see above)57, and concerns about development of rapid resistance when used as a single agent. The addition of fosfomycin in ABLC for staphylococcal infections has shown high concentrations with promising data in experimental biofilm models58–60. In Gram-negative rods, treatment options are currently limited to local aminoglycosides. Clinical reports have suggested the successful use of colistin in ALBC61; 62.
Table 2.
Antibiotic susceptibility of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa as a planktonic population (MIC) and as a biofilm population (MBEC). Adapted from85–87.
| Microorganisms | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain number | ATCC 2921385,86 ATCC 3555687 | ATCC 2592285 | ATCC 2785385 | ||||||
| Antimicrobial Agents | MIC | MBEC | Ratio | MIC | MBEC | Ratio | MIC | MBEC | Ratio |
| Oxacillin | 0.12–0.25 | >1024 | >1000 | ||||||
| Piperacillin | 1 – 4 | 32 | 8 – 32 | 2 – 16 | >1024 | >64 | |||
| Aztreonam | 2 – 4 | >1024 | >250 | ||||||
| Cefazolin | 0.5 | >1024 | >1000 | 1 – 4 | >1024 | >250 | |||
| Ceftazidime | 1 – 2 | >1024 | >500 | ||||||
| Imipenem | 1 – 4 | >1024 | >250 | ||||||
| Clindamycin | 0.12–0.25 | 128–256 | >1000 | ||||||
| >256(Ref87) | |||||||||
| Linezolid | 8 | >128(Ref86) | >16 | ||||||
| Rifampin | <0.02 | 4(Ref86) | 200 | ||||||
| Ciprofloxacin | 0.25–0.5 | 512 | >1000 | 0.004 – 0.008 | 8 | >1000 | 0.25 | 4 | 16 |
| Amikacin | 2 – 4 | 16 | 4 – 8 | ||||||
| Gentamicin | 0.5 | 2 | 4 | 2 – 4 | 16 | 4 – 8 | 2 – 4 | 128 | 32 – 64 |
| Tobramycin | 1 – 4 | 2 | 2 | 0.5 – 1 | 2 | 2 – 4 | |||
| Trimethoprim-sulfamethoxazole | |||||||||
| 1.14 – 2.28 | 608 – 1216 | >500 | |||||||
MIC, minimal inhibitory concentration; MBEC minimal biofilm eradication concentration. The unit of the values is μg/mL. Determination of MBEC were established using the MBEC device, formerly called Calgary device, according to the manufacturer’s protocol or with modification of the protocol.
When interpreting MBEC results and corresponding in-vitro studies there are technical limitations to consider. Methods used to measure MBEC are research based, vary by investigator and there are important variables that can change the result dramatically8. A major downside of research based MBEC assays is the missing validation as a diagnostic to use in the clinical setting, and the lack of therapeutic drug monitoring in the local compartment. For MBECs to be clinically useful, a standardized, clinically relevant assay is needed. MBEC is specific to the microorganism, the drug, the site of infection and the methodology used to measure it. Knowing the MBEC of the patient’s pathogen(s) has the potential to improve outcomes. Treatment failures, unusual pathogens and pathogens expected to have extremely high-MBECs may be good initial clinical indications to assay the MBEC.
The ideal treatment duration to treat clinical biofilm infection is unknown, and depends on numerous factors. In MBEC assays, the antibiotic exposure time has frequently not been evaluated for longer than 1 day. Time is an important consideration during three steps in the assay40. First, biofilms grown for up to 24 hours are usually considered immature biofilms with cells in the exponential phase of growth, which may not represent the dormant profile of cells found in mature biofilms63. Mature biofilms are heterogeneous raising the possibility that it may be necessary to use several time points for biofilm growth to assay MBEC throughout the biofilm life cycle40. Second is a progressive decrease in MBEC over 5 days of exposure to antibiotics, by as much as 32-fold. Third, time for viability assessment by subculture is important because persister cells require more than 24 hours incubation to be detected on subculture.
Experimental models of foreign body infection confirm that biofilm bacteria are reduced but not eradicated after 6 days of local antibiotic exposure to systemically achievable levels and synergy did not occur between penicillin and gentamicin for group B strep64. While both found decreasing MBEC with increasing antibiotic exposure, Post et al.65 found that biofilms under mechanical stress have higher MBECs than static biofilms and Badha et al.66 found tobramycin-vancomycin combined therapy had lower MBECs, within the range clinically achievable by local delivery. Again, these experiments have their limitations from a clinical perspective. Based on systemic therapy, it appears reasonable to target an exposure time between 1 and 4 weeks; however, the plausible duration that MBEC levels can be achieved clinically with current local delivery techniques may only be a few days40.
Conclusions
Currently, high quality clinical evidence of ALBC efficacy does not exist, and Level 1 evidence would require large, prohibitively expensive RCTs, and not all surgeons are willing to enroll patients into a non-ALBC control group (for non-evidenced-based reasons). Economically, there is no evidence that ALBC is cost-effective to prevent or treat MSKI. Scientifically, established limitations of commercial ALBC exist, including inability to achieve MBEC in vivo due to their release kinetics and biodistribution following implantation, the risk of inducing antibacterial resistance from prolonged subtherapeutic release and bacterial colonization of the retained ALBC. For surgeon directed high-dose formulations, systemic toxicity, adverse effects on local cells and healing, and loss of material strength of the ALBC are the main concerns. Practically, all surgeons that treat MSKI use ALBC for some indications. However, standardization of ALBC use may only be possible with data to establish formulation specifications. Nevertheless, opportunities exist for disruptive technologies that markedly improve the safety and efficacy of local delivery. These include: novel antibiotics that achieve MBEC with current delivery system (e.g. ceramics, implant coatings), and hydrogel carriers with dramatically different release kinetics and biomaterial properties. Research is needed to develop these technologies, faithful assays of in vivo MBEC, and clinical trial designs that facilitate Level 1 evidence.
Supplementary Material
Acknowledgments
The authors thank Whitney Arons, Marlisa Veridiano, Wanda Napolitano and Allina Nocon for their assistance with administrative tasks. We also thank Karen de Mesy Bentley, Yugo Morita and Elysia Masters for their help generating the figures.
Part of this work was supported by the Stavros Niarchos Foundation and the Hospital for Special Surgery, New York, NY. Part of the work on this publication was supported by the National Institutes of Health (NIH) under award numbers R01 AR069119 (Hickok), R01 AR072513 (Hickok, Parvizi), P50 AR072000 (Schwarz), R21AR073321 (Oh), and R01GM124436 (Stoodley). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Hospital for Special Surgery 2019 Biofilm Symposium Workgroup:
Edward M. Schwarz, Alex C. McLaren, Thomas P. Sculco, Barry Brause, Mathias Bostrom, Stephen L. Kates, Javad Parvizi, Volker Alt, William V. Arnold, Alberto Carli, Antonia F. Chen, Hyonmin Choe, Débora C. Coraça-Huber, Michael Cross, Michelle Ghert, Noreen Hickok, Jessica Amber Jennings, Manjari Joshi, Willem-Jan Metsemakers, Mark Ninomiya, Kohei Nishitani, Irvin Oh, Douglas Padgett, Benjamin Ricciardi, Kordo Saeed, Dr. Parham Sendi, Bryan Springer, Paul Stoodley, and Joseph C. Wenke
References
- 1.Kurtz SM, Lau E, Watson H, et al. 2012. Economic burden of periprosthetic joint infection in the United States. The Journal of arthroplasty 27:61–65. e61. [DOI] [PubMed] [Google Scholar]
- 2.Masters EA, Trombetta RP, de Mesy Bentley KL, et al. 2019. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parvizi J, Gehrke T, Mont MA, et al. 2018. Introduction: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz EM, Parvizi J, Gehrke T, et al. 2019. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. J Orthop Res 37:997–1006. [DOI] [PubMed] [Google Scholar]
- 5.Abdel MP, Barreira P, Battenberg A, et al. 2019. Hip and Knee Section, Treatment, Two-Stage Exchange Spacer-Related: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34:S427–S438. [DOI] [PubMed] [Google Scholar]
- 6.de Beaubien B, Belden K, Bell K, et al. 2019. Hip and Knee Section, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34:S477–S482. [DOI] [PubMed] [Google Scholar]
- 7.Fillingham Y, Greenwald AS, Greiner J, et al. 2019. Hip and Knee Section, Prevention, Local Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34:S289–S292. [DOI] [PubMed] [Google Scholar]
- 8.Saeed K, McLaren AC, Schwarz EM, et al. 2019. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res 37:1007–1017. [DOI] [PubMed] [Google Scholar]
- 9.Sultan AA, Samuel LT, Umpierrez E, et al. 2019. Routine use of commercial antibiotic-loaded bone cement in primary total joint arthroplasty: a critical analysis of the current evidence. Ann Transl Med 7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz HW, Elson RA, Heinert K. 1984. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res:96–108. [PubMed] [Google Scholar]
- 11.Metsemakers WJ, Fragomen AT, Moriarty TF, et al. 2019. Evidence-based recommendations for Local antimicrobial strategies and Dead space management in Fracture-Related Infection. J Orthop Trauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vugt TA, Geurts J, Arts JJ. 2016. Clinical Application of Antimicrobial Bone Graft Substitute in Osteomyelitis Treatment: A Systematic Review of Different Bone Graft Substitutes Available in Clinical Treatment of Osteomyelitis. Biomed Res Int 2016:6984656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butini ME, Cabric S, Trampuz A, et al. 2018. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces 161:252–260. [DOI] [PubMed] [Google Scholar]
- 14.Gunderson BW, Ross GH, Ibrahim KH, et al. 2001. What do we really know about antibiotic pharmacodynamics? Pharmacotherapy 21:302S–318S. [DOI] [PubMed] [Google Scholar]
- 15.Dale AP, Saeed K. 2015. Novel negative pressure wound therapy with instillation and the management of diabetic foot infections. Curr Opin Infect Dis 28:151–157. [DOI] [PubMed] [Google Scholar]
- 16.Bohm E, Zhu N, Gu J, et al. 2014. Does adding antibiotics to cement reduce the need for early revision in total knee arthroplasty? Clin Orthop Relat Res 472:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth RE, Vogely HC, Hoepelman AI, et al. 2011. ‘To bead or not to bead?’ Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int J Antimicrob Agents 38:371–375. [DOI] [PubMed] [Google Scholar]
- 18.Morgenstern M, Vallejo A, McNally MA, et al. 2018. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Joint Res 7:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puetzler J, Zalavras C, Moriarty TF, et al. 2019. Clinical practice in prevention of fracture-related infection: An international survey among 1197 orthopaedic trauma surgeons. Injury 50:1208–1215. [DOI] [PubMed] [Google Scholar]
- 20.Sanz-Ruiz P, Matas-Diez JA, Sanchez-Somolinos M, et al. 2017. Is the Commercial Antibiotic-Loaded Bone Cement Useful in Prophylaxis and Cost Saving After Knee and Hip Joint Arthroplasty? The Transatlantic Paradox. J Arthroplasty 32:1095–1099. [DOI] [PubMed] [Google Scholar]
- 21.Engesaeter LB, Espehaug B, Lie SA, et al. 2006. Does cement increase the risk of infection in primary total hip arthroplasty? Revision rates in 56,275 cemented and uncemented primary THAs followed for 0–16 years in the Norwegian Arthroplasty Register. Acta Orthop 77:351–358. [DOI] [PubMed] [Google Scholar]
- 22.Tayton ER, Frampton C, Hooper GJ, et al. 2016. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J 98-B:334–340. [DOI] [PubMed] [Google Scholar]
- 23.Yayac M, Rondon AJ, Tan TL, et al. 2019. The Economics of Antibiotic Cement in Total Knee Arthroplasty: Added Cost with No Reduction in Infection Rates. J Arthroplasty. [DOI] [PubMed] [Google Scholar]
- 24.Kunutsor SK, Wylde V, Whitehouse MR, et al. 2019. Influence of Fixation Methods on Prosthetic Joint Infection Following Primary Total Knee Replacement: Meta-Analysis of Observational Cohort and Randomised Intervention Studies. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu FY, Lin CF, Chen CM, et al. 2001. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J Bone Joint Surg Br 83:691–695. [DOI] [PubMed] [Google Scholar]
- 26.Qadir R, Sidhu S, Ochsner JL, et al. 2014. Risk stratified usage of antibiotic-loaded bone cement for primary total knee arthroplasty: short term infection outcomes with a standardized cement protocol. J Arthroplasty 29:1622–1624. [DOI] [PubMed] [Google Scholar]
- 27.Namba RS, Chen Y, Paxton EW, et al. 2009. Outcomes of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty 24:44–47. [DOI] [PubMed] [Google Scholar]
- 28.Hansen EN, Adeli B, Kenyon R, et al. 2014. Routine use of antibiotic laden bone cement for primary total knee arthroplasty: impact on infecting microbial patterns and resistance profiles. J Arthroplasty 29:1123–1127. [DOI] [PubMed] [Google Scholar]
- 29.Holleyman RJ, Deehan DJ, Walker L, et al. 2019. Staphylococcal resistance profiles in deep infection following primary hip and knee arthroplasty: a study using the NJR dataset. Arch Orthop Trauma Surg 139:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly MP, Illgen RL, Chen AF, et al. 2018. Trends in the Use of High-Viscosity Cement in Patients Undergoing Primary Total Knee Arthroplasty in the United States. The Journal of arthroplasty 33:3460–3464. [DOI] [PubMed] [Google Scholar]
- 31.Yayac M, Rondon AJ, Tan TL, et al. 2019. The Economics of Antibiotic Cement in Total Knee Arthroplasty: Added Cost with No Reduction in Infection Rates. The Journal of arthroplasty. [DOI] [PubMed] [Google Scholar]
- 32.Filippone EJ, Yadav A. 2019. Acute kidney injury after hip or knee replacement: Can we lower the risk? Cleve Clin J Med 86:263–276. [DOI] [PubMed] [Google Scholar]
- 33.Seeley SK, Seeley JV, Telehowski P, et al. 2004. Volume and surface area study of tobramycin-polymethylmethacrylate beads. Clin Orthop Relat Res:298–303. [DOI] [PubMed] [Google Scholar]
- 34.Anagnostakos K, Kelm J. 2009. Enhancement of antibiotic elution from acrylic bone cement. Journal of Biomedical Materials Research Part B: Applied Biomaterials 90:467–475. [DOI] [PubMed] [Google Scholar]
- 35.Klekamp J, Dawson JM, Haas DW, et al. 1999. The use of vancomycin and tobramycin in acrylic bone cement: biomechanical effects and elution kinetics for use in joint arthroplasty. J Arthroplasty 14:339–346. [DOI] [PubMed] [Google Scholar]
- 36.McLaren RL, McLaren AC, Vernon BL. 2008. Generic tobramycin elutes from bone cement faster than proprietary tobramycin. Clin Orthop Relat Res 466:1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham B, McLaren AC, Pauken C, et al. 2012. Liposomal formulation increases local delivery of amphotericin from bone cement: a pilot study. Clin Orthop Relat Res 470:2671–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funk GA, Menuey EM, Cole KA, et al. 2019. Radical scavenging of poly(methyl methacrylate) bone cement by rifampin and clinically relevant properties of the rifampin-loaded cement. Bone Joint Res 8:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giers MB, McLaren AC, Schmidt KJ, et al. 2014. Distribution of molecules locally delivered from bone cement. J Biomed Mater Res B Appl Biomater 102:806–814. [DOI] [PubMed] [Google Scholar]
- 40.Castaneda P, McLaren A, Tavaziva G, et al. 2016. Biofilm Antimicrobial Susceptibility Increases With Antimicrobial Exposure Time. Clin Orthop Relat Res 474:1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber KE, Werth BJ, McRoberts JP, et al. 2014. A novel approach utilizing biofilm time-kill curves to assess the bactericidal activity of ceftaroline combinations against biofilm-producing methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:2989–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmberg A, Rasmussen M. 2016. Mature biofilms of Enterococcus faecalis and Enterococcus faecium are highly resistant to antibiotics. Diagn Microbiol Infect Dis 84:19–21. [DOI] [PubMed] [Google Scholar]
- 43.Cardile AP, Sanchez CJ Jr., Samberg ME, et al. 2014. Human plasma enhances the expression of Staphylococcal microbial surface components recognizing adhesive matrix molecules promoting biofilm formation and increases antimicrobial tolerance In Vitro. BMC Res Notes 7:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandell JB, Orr S, Koch J, et al. 2019. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res 37:1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelstein AI, Weiner JA, Cook RW, et al. 2017. Intra-Articular Vancomycin Powder Eliminates Methicillin-Resistant S. aureus in a Rat Model of a Contaminated Intra-Articular Implant. J Bone Joint Surg Am 99:232–238. [DOI] [PubMed] [Google Scholar]
- 46.Evaniew N, Khan M, Drew B, et al. 2015. Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis. Eur Spine J 24:533–542. [DOI] [PubMed] [Google Scholar]
- 47.Patel NN, Guild GN 3rd, Kumar AR. 2018. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today 4:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanada M, Nishikino S, Hotta K, et al. 2019. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc 27:2322–2327. [DOI] [PubMed] [Google Scholar]
- 49.Kuehl R, Brunetto PS, Woischnig AK, et al. 2016. Preventing Implant-Associated Infections by Silver Coating. Antimicrob Agents Chemother 60:2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romano CL, Tsuchiya H, Morelli I, et al. 2019. Antibacterial coating of implants: are we missing something? Bone Joint Res 8:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmolders J, Koob S, Schepers P, et al. 2017. Lower limb reconstruction in tumor patients using modular silver-coated megaprostheses with regard to perimegaprosthetic joint infection: a case series, including 100 patients and review of the literature. Arch Orthop Trauma Surg 137:149–153. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs T, Stange R, Schmidmaier G, et al. 2011. The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch Orthop Trauma Surg 131:1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metsemakers WJ, Reul M, Nijs S. 2015. The use of gentamicin-coated nails in complex open tibia fracture and revision cases: A retrospective analysis of a single centre case series and review of the literature. Injury 46:2433–2437. [DOI] [PubMed] [Google Scholar]
- 54.Moriarty TF, Kuehl R, Coenye T, et al. 2016. Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Rev 1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldschmidt E, Rasmussen J, Chabot JD, et al. 2016. The effect of vancomycin powder on human dural fibroblast culture and its implications for dural repair during spine surgery. J Neurosurg Spine 25:665–670. [DOI] [PubMed] [Google Scholar]
- 56.Chu S, Chen N, Dang ABC, et al. 2017. The Effects of Topical Vancomycin on Mesenchymal Stem Cells: More May Not Be Better. Int J Spine Surg 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galvez-Lopez R, Pena-Monje A, Antelo-Lorenzo R, et al. 2014. Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis 78:70–74. [DOI] [PubMed] [Google Scholar]
- 58.Presterl E, Hajdu S, Lassnigg AM, et al. 2009. Effects of azithromycin in combination with vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 53:3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajdu S, Lassnigg A, Graninger W, et al. 2009. Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J Orthop Res 27:1361–1365. [DOI] [PubMed] [Google Scholar]
- 60.Hajdu S, Holinka J, Reichmann S, et al. 2010. Increased temperature enhances the antimicrobial effects of daptomycin, vancomycin, tigecycline, fosfomycin, and cefamandole on staphylococcal biofilms. Antimicrob Agents Chemother 54:4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zachiu C, Ries M, Moonen C, et al. 2017. An Adaptive Non-Local-Means Filter for Real-Time MR-Thermometry. IEEE Trans Med Imaging 36:904–916. [DOI] [PubMed] [Google Scholar]
- 62.Crane DP, Gromov K, Li D, et al. 2009. Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J Orthop Res 27:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johani K, Malone M, Jensen SO, et al. 2018. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: from in vitro to in vivo. J Antimicrob Chemother 73:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruppen C, Mercier T, Grandgirard D, et al. 2018. Is Penicillin Plus Gentamicin Synergistic Against Sessile Group B Streptococcal Isolates? An in Vivo Study With an Experimental Model of Foreign-Body Infection. Front Microbiol 9:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Post V, Wahl P, Richards RG, et al. 2017. Vancomycin displays time-dependent eradication of mature Staphylococcus aureus biofilms. J Orthop Res 35:381–388. [DOI] [PubMed] [Google Scholar]
- 66.Badha V, Moore R, Heffernan J, et al. 2019. Determination of Tobramycin and Vancomycin Exposure Required to Eradicate Biofilms on Muscle and Bone Tissue In Vitro. J Bone Jt Infect 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandell GL. 1973. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest 52:1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaudaux P, Waldvogel FA. 1979. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother 16:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pelletier LL Jr., Richardson M, Feist M. 1979. Virulent gentamicin-induced small colony variants of Staphylococcus aureus. J Lab Clin Med 94:324–334. [PubMed] [Google Scholar]
- 70.Tuchscherr L, Kreis CA, Hoerr V, et al. 2016. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J Antimicrob Chemother 71:438–448. [DOI] [PubMed] [Google Scholar]
- 71.Yuan W, Hu Q, Cheng H, et al. 2013. Cell wall thickening is associated with adaptive resistance to amikacin in methicillin-resistant Staphylococcus aureus clinical isolates. J Antimicrob Chemother 68:1089–1096. [DOI] [PubMed] [Google Scholar]
- 72.de Mesy Bentley KL, Trombetta R, Nishitani K, et al. 2017. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J Bone Miner Res 32:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delaney LJ, MacDonald D, Leung J, et al. 2019. Ultrasound-triggered antibiotic release from PEEK clips to prevent spinal fusion infection: Initial evaluations. Acta Biomater 93:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. 2015. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res 33:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, et al. 2014. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res 32:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McLaren A, Giers MB, Fraser J, et al. 2014. Antimicrobial distribution from local delivery depends on dose : a pilot study with MRI. Clin Orthop Relat Res 472:3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


