Abstract
Empathy is a multidimensional construct including affective and cognitive components while maintaining the distinction between one-self and others. Our meta-analyses focused on shared and distinct networks underlying cognitive (taking somebody else’s perspective in emotional/painful situations) and affective (self-referentially feeling somebody else’s emotions/pain) empathy for various states including painful and emotional situations. Furthermore, a comparison with direct pain experience was carried out.
For cognitive empathy, consistent activation in the anterior dorsal medial frontal gyrus (dmPFG) and the supramarginal gyrus (SMG) occurred. For affective empathy, convergent activation of the posterior dmPFG and the inferior frontal gyrus (IFG) was found. Consistent activation of the anterior insula (AI), the anterior dmPFG and the SMG was observed for empathy for pain, while convergent recruitment of the temporo-parietal junction, precuneus, posterior dmPFG, and the IFG was revealed in the meta-analysis across empathy for emotion experiments. The AI and the dmPFG/mid-cingulate cortex (MCC) showed overlapping as well as distinct neural activation for pain processing and empathy for pain.
Taken together, we were able to show difference in the meta-analytic networks across cognitive and affective empathy as well as for pain and empathy processing. Based on the current results, distinct functions along the midline structures of the brain during empathy processing are apparent. Our data are lending further support for a multidimensional concept of empathy.
Keywords: cognitive and affective empathy, pain, emotions, ACC/MCC/dmPFG, TPJ, insula
1. Introduction
Empathy, a human trait that crucially affects social interaction, refers to adopting another person’s emotional state while maintaining the distinction between one-self and others (Decety & Jackson, 2004; Singer & Lamm, 2009). Empathy not only involves the affective experience of another person’s actual or inferred emotional state but also some minimal recognition and understanding of their emotional experience (Decety & Jackson, 2004). Previous research relied on different concepts and views of empathy and its components (e.g., compassion, sympathy or motivation for prosocial behavior (Kanske, Böckler, Trautwein, & Singer, 2015; Singer & Lamm, 2009; Singer & Klimecki, 2014; Zaki & Ochsner, 2012)). According to the multidimensional construct, empathy involves three components: i) emotion recognition, ii) affective empathy, which is sharing the emotional state of others (which is different from emotional contagion, such as mimicking other people’s emotions) (Hatfield, Cacioppo, & Rapson, 1993)) and iii) a cognitive component: taking (cognitively) the perspective of others (Decety & Jackson, 2004; Derntl et al., 2010). Therefore, it is generally agreed that empathy can be divided (at least) into a cognitive and an affective component (Walter, 2012). Besides allowing for a more thorough characterization and better understanding of the concept, assessing differences in affective and cognitive components of empathy is particularly of interest when it comes to social dysfunctions. Notably, the concept of empathy is closely related to that of theory-of-mind (Kanske et al., 2015; Stietz, Jauk, Krach, & Kanske, 2019), where one is explicitly inferring and reasoning about another person’s beliefs, thoughts or intentions. Theory-of-mind yields propositional knowledge of another person’s state, whereas empathy involves embodied sharing of a sensory, affective or bodily state (Singer, 2006). This crucial difference between these concepts is important to keep in mind for the current work. The here-defined “cognitive empathy” might be seen as a part of affective theory-of-mind (Kanske et al., 2015; Shamay-Tsoory & Aharon-Peretz, 2007), but the concept of theory-of-mind is much broader than it will be investigated here.
General or distinct deficits in the different components of empathic processing are common in various mental disorders. For instance, schizophrenia patients show deficits in cognitive and affective empathy (Derntl et al., 2012), while in autism-spectrum disorders, cognitive rather than affective aspects might be deviant (e.g., Dziobek et al., 2008). Thus, basic research is needed to further inform treatment options to tackle these burdening social impairments.
A large body of research is currently investigating the neural correlates of empathy. Bzdok and colleagues (2012) conducted a meta-analysis on empathy, including studies where subjects had to perceive and empathize with other persons’ emotion. The authors observed a broad network of regions including areas in the midline structure of the brain (comprising the dorso-medial prefrontal gyrus (dmPFG), the supplementary motor area (SMA), the anterior cingulate gyrus (ACC), and the anterior mid-cingulate cortex (aMCC)), the anterior insula (AI), the inferior frontal gyrus (IFG) and the temporo-parietal junction (TPJ) amongst others. Furthermore, findings from single neuroimaging studies strongly suggest that there are also differences in neural activity associated with different components of empathy such as affective and cognitive empathy (e.g., Völlm et al., 2006; Kanske et al., 2015). Fan and colleagues (2011) as well as Timmers and colleagues (2018), investigated a perceptual-affective and an evaluative-cognitive component of empathy. Studies of perceptual empathy included passively observing a picture/film-clip depicting another persons’ emotional experience, eliciting an automatic empathic response. In studies of evaluative empathy, participants evaluated another persons’ emotional state through an overt response. Again, regions along the midline structure of the brain (comprising MCC, dmPFG, SMA and dmPFG) and the AI were identified as significant regions for empathy. The dmPFG/aMCC seems to be a core region for evaluative empathy and the AI for perceptual empathy. Additionally, studies on gray matter/brain volume suggest that neural processing of different components of empathy is associated with the volume of different brain regions: In healthy women and men, increased gray matter volume in the IFG and the ACC/MCC was associated with less self-reported empathic concern, while higher personal distress ratings were correlated with increased gray matter volume in the AI (Banissy, Kanai, Walsh, & Rees, 2012). These two scales tap into affective empathy. The ACC/MCC was further positively associated with self-reported perspective taking, thus a measure of cognitive empathy.
Although pain is not a basic emotion, it elicits affective-emotional states and observing someone else in pain evokes similar responses as feeling the pain by oneself (Bzdok et al., 2012). Indeed, quite a large body of research has investigated empathic reactions to painful situations. A quite recent meta-analysis on empathy for pain and empathy for non-pain situations revealed that the AI and the aMCC seem to be involved in both empathic states (Timmers et al., 2018). Also, Lamm and colleagues (2011) found significant involvement of the AI and the aMCC for empathy for pain as well as for pain experience in their meta-analysis including studies of painful electrical stimulation of the hand. However, the authors didn’t differentiate between the affective and cognitive components of empathy and further didn’t include empathy for emotional situations.
It seems noteworthy here that within the empathy literature regions lying along the medial structure of the brain are often labelled heterogeneously, e.g. as ACC, dACC or aMCC (e.g., Cheng, Chen, Lin, Chou, & Decety, 2010; Danziger, Faillenot, & Peyron, 2009; de Greck et al., 2012; Lamm, Batson, & Decety, 2007; Singer et al., 2004). Furthermore, the observed clusters are often extending to the SMA, the dmPFG or even other regions (e.g., Greck et al., 2012; Lamm et al., 2007; Preis, Schmidt-Samoa, Dechent, & Kroener-Herwig, 2013a; Singer et al., 2004). Despite the heterogenic labeling for these specific regions along the midline structure of the brain, within the current manuscript we will stick to the labelling provided by the SPM Anatomy Toolbox version 2.2b for these regions (Eickhoff et al., 2007, 2005) to enable a more precise scientific exchange regarding specific brain areas associated with empathy processing.
Taken together, previous neuroimaging meta-analyses tested different aspects of empathy and highlighted the AI, brain areas along the midline structure and the IFG as core regions for empathy (Bzdok et al., 2012; Fan et al., 2011; Lamm, Decety, & Singer, 2011; Timmers et al., 2018). These meta-analyses on the one hand tested for a perceptual and an evaluative component of empathy and on the other hand for empathy for emotions and empathy for pain. However, so far no meta-analysis specifically targeted affective (“sharing the emotional state of others”) and cognitive (“taking the perspective of others”) empathy based on the multidimensional construct of empathy (Decety & Jackson, 2004). Furthermore, the literature base is lacking a direct comparison between cognitive as well as affective empathy for various empathic states including empathy for basic emotions and empathy for pain. Additionally, previous meta-analyses comparing direct pain experience with empathy for pain were conducted with a specific focus on electrical stimulation of the hand including only a few experiments (Lamm et al., 2011). Thus, direct comparisons of the meta-analytic derived neural networks of empathy for pain and pain experience across a broader range of studies testing direct painful experience under various conditions (e.g., cutaneous, muscle or visceral pain; pain induction on various body parts such as hands or feet), is missing.
The current study had three main interests that fundamentally distinguish it from previously performed meta-analyses: differentiating brain regions associated with (1) affective and cognitive empathy, (2) different empathic stimuli such as empathy for emotions and empathy for pain, and (3) empathy for pain and direct painful experience.
Within this study, we ran meta-analyses and meta-analytic contrasts (1) on i) affective empathy (self-referentially feeling someone else’s affective state; e.g., “Try to empathize with the depicted person. For each face that appears on the screen you should decide how you feel yourself when you look at that face.” (Schulte-Rüther et al., 2011)) and ii) cognitive empathy (taking someone else’s perspective in affective states/inferring about how the other person is feeling; e.g. “Try to empathize with the depicted person. For each face that appears on the screen you should decide how this person feels.” (Schulte-Rüther et al., 2011)). We differentiated these two forms of empathy based only on specific task instructions to empathize. In addition to previous meta-analyses (Fan et al., 2011; Timmers et al., 2018) we investigated the evaluative component of empathy in more detail. Therefore, for the current meta-analyses we only included studies directly asking participants to either (self-referentially) feel/empathize with another persons’ emotion (affective empathy) or to take another persons’ perspective (cognitive empathy) (see examples above).
Furthermore, we categorized (2) whether the other person depicted felt i) basic-emotions (experiencing/inferring someone else’s emotions) or ii) pain (seeing someone else in painful situations). Additionally, within this study we investigated (3) differences and overlaps between the neural correlates for empathy for pain and pain experience. To the best of our knowledge, this is the first meta-analytic study directly comparing convergence across pain experience with empathy for pain experiments based on a large sample of neuroimaging results. In addition to previous meta-analyses comparing pain experience and empathy for pain, we included any kind of pain induction and application on any kind of body part for pain experience. Furthermore, we explicitly excluded those studies that investigated self-pain and other-pain experience for empathy within the same participants as direct pain experience may influence subsequent empathy for pain processing and vice-versa (Preis, Schmidt-Samoa, Dechent, & Kroener-Herwig, 2013b).
Based on above mentioned literature we expected to identify regions along the midline structure of the brain such as dmPFG/aMCC and the AI as core regions for empathy. For affective empathy, we expected the AI and the IFG to be the main structures involved, whereas the dmPFG/aMCC should rather be associated with cognitive empathy. For the comparison between empathy for pain and pain we hypothesized to find an overlap in the recruitment of the aMCC and the AI. Furthermore, we assumed to find IFG involvement to be specific for empathy compared to pain processing. To explore these contrast and conjunction analyses we ran activation-likelihood estimation (ALE) meta-analyses on affective empathy, cognitive empathy, empathy for basic emotions, empathy for pain and direct pain experience.
2. Materials and Methods
2.1. Selection criteria for used data
Literature research was conducted using PubMed (www.pubmed.com) searching for different combinations of the following keywords: “fMRI”, “PET”, “neuroimaging”, empathy“, „empathic“, „emotion contagion“, „affective theory of mind“, „affective mentalizing“, or “pain”. Additional studies were identified by review articles, other meta-analyses and by tracing references from retrieved studies. In case a study did not report the results sufficiently, corresponding authors were contacted and asked to provide more information on their data. In the following text, the term “experiment” refers to any single contrast analysis, and the term “study” refers to scientific publications sometimes reporting more “experiments” (Laird et al., 2011). Studies were evaluated by the in- and exclusion criteria of the current study (a PICOS table can be found in the supplement material). We included studies reporting results either for an empathy task for basic emotions or for pain or a task that directly manipulated pain experience (e.g., extreme heat or cold, electrical stimulation, etc.). Only results for healthy adults (aged 18 and older) with no prior report of neurological, psychiatric or pain-related disorders were considered for the current meta-analyses, while results for patients or for group effects (e.g., sex differences) were excluded. Furthermore, only neuroimaging studies, which utilized either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) on a whole-brain level and reported the coordinates of brain region activation in standard anatomical reference space (Talairach/Tournoux; Montreal Neurological Institute [MNI]), were included. Conversely, we excluded receptor-PET studies and articles that conducted solely region-of-interest (ROI) analyses (a-priori defined regions) or directed searches or did not report all significant peak-voxels at a specific threshold. Studies were excluded if they did not report original data in an English-written manuscript. We excluded reviews, meta-analyses and single case reports, studies where empathy assessment and direct pain stimulation was manipulated within the same participants (e.g., Singer et al., 2004, 2006; Morrison & Downing, 2007), any pharmacological/placebo manipulation, studies in which pain served as an independent factor affecting further cognitive domains (e.g., fear conditioning, decision making), any psychological intervention/manipulation (e.g., mood induction prior to pain experience, motor or cognitive tasks during pain experience, attention manipulation, acupuncture, hypnosis), ingroup-outgroup-comparison, priming, studies where data of the same sample was already reported in another included study, correlational and resting-state analyses.
Furthermore and this is in contrast to Fan and colleagues (2011) and Timmers and colleagues (2018), for empathy tasks, the study design had to include a clear instruction to empathize or to give feedback on how oneself or the observed person is feeling. In the end, we had to exclude studies where it was not possible to clearly distinguish between affective and cognitive empathy due to the study design. Therefore, studies were excluded if they i) did not report an empathy task which included a clear instruction to either empathize or to give feedback on how the participant (self) or the observed person (other) is feeling or if they ii) did not report on empathy for basic emotions or for pain. Studies directly asking participants to either (self-referentially) feel/empathize another persons’ emotion were coded as affective empathy (e.g., subjects should try to share the emotional state of a shown person (De Greck et al., 2012)), whereas studies asking participant to infer another persons’ perspective were coded as cognitive empathy (e.g., subjects had to infer the emotional expression of a masked face within a depicted scene (Derntl et al., 2012)). Additionally, each study was further classified whether it tested empathy for pain, empathy for emotions or direct pain experience (see also supplement material for a list of conducted contrast analyses). For the meta-analyses on empathy for emotions, empathy for different emotions could not be analyzed separately as some studies only reported results pooled across emotions. To prevent that multiple experiments from dependent samples included in one study influenced ALE values more than others (Turkeltaub et al., 2012) we coded contrasts from a single study reporting more contrasts from the same sample (e.g., 2 different emotions) as one experiment. This procedure resulted in the inclusion of 43 empathy studies with 57 experiments and 1193 participants (affective empathy: 19 experiments/428 participants; cognitive empathy: 38 experiments/765 participants; empathy for pain: 24 experiments/517 participants; empathy for emotions: 33 experiments/676 participants) and 68 pain studies with 72 experiments and 1019 participants (PRISMA flow charts of the identification flow and a list of the included studies (Table S1) can be found in the supplement material).
We performed the meta-analyses for the following concepts: affective empathy, cognitive empathy, empathy for emotions, empathy for pain, and pain experience. For cognitive empathy we were further able to divide experiments into cognitive empathy for pain (19 experiments) and cognitive empathy for emotion (19 experiments). To guarantee enough power for the analyses, it is recommended to include at least 17 experiments in an ALE meta-analysis (to detect moderate effects) (Eickhoff et al., 2016; Müller et al., 2018). Thus, we did not run separate analyses for affective empathy as the number of experiments was too low for each category (5 for affective empathy for pain/14 for affective empathy for emotions; Table S1).
2.2. Activation-likelihood (ALE) estimation
All meta-analyses were performed according to the standard analysis method used in previous studies (cf. Kogler et al., 2015) and following recently established best-practice guidelines (Müller et al., 2018) (see supplement material for a checklist for neuroimaging meta-analyses). In particular, analyses were based on the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). This algorithm identifies topographic clusters of activation showing significantly higher convergence across experiments than expected among random spatial distributions. Importantly, the reported foci are not treated as single points, but rather as centers of 3D Gaussian probability distributions. This acknowledges spatial uncertainty and reliability by weighting studies according to their sample sizes through the width of the 3D Gaussian probability distribution. Thus, larger sample sizes provide more reliable approximations of the true activation effect and are therefore modeled by smaller Gaussian distributions (Eickhoff et al., 2009). The resulting probabilities of all reported foci in a given experiment are combined for each voxel yielding a modeled activation (MA) map (Turkeltaub et al., 2012). The union of all MA maps from all experiments included in the analysis then results in voxel-wise ALE scores, which describe the convergence of results at each particular location in the brain. These ALE scores are then compared to an analytically derived null-distribution reflecting a random spatial association between experiments’ MA maps (Eickhoff et al., 2012). Hereby, a random-effects inference was invoked, focusing on inference on the above-chance convergence between studies, not the clustering of foci within a particular study.
Conceptually, the null-hypothesis was derived by sampling a random voxel from each of the MA maps and taking the union of these values. The p-value of a “true” ALE score is given by the proportion of equal or higher values obtained under the null-distribution. The resulting non-parametric p-values were then thresholded at a cluster-level family-wise error (FWE) corrected threshold of p<.05 (cluster-forming threshold at voxel-level p<0.001) (Bzdok et al., 2012; Eickhoff et al., 2010, 2016; Kogler et al., 2015).
Additionally, we conducted conjunction and contrast analyses between the meta-analyses of cognitive and affective empathy, of empathy for pain and empathy for emotions as well as of pain and empathy for pain. Minimum conjunction analyses (Nichols, Brett, Andersson, Wager, & Poline, 2005) were computed in order to isolate the intersection of the thresholded z-maps of two separate meta-analyses. Thus, any voxel determined to be significant by the conjunction analysis constitutes a region in the brain which survived inference corrected on cluster-level FWE in each of the individual meta-analyses. Differences between the different aspects of empathy (e.g., empathy for pain, empathy for emotions, cognitive empathy, affective empathy) as well as with pain were tested by comparing the difference between two ALEs to a random distribution of differences. First, the true difference between two individual analyses was determined by computing the voxel-wise difference between the non-thresholded ALE maps of each analysis (Eickhoff et al. 2012). Secondly, we determined a null-distribution of differences. This was done by pooling all experiments contributing to either analysis and randomly dividing them into two groups of the same size as the two original sets of experiments. ALE-scores for these two randomly assembled groups were calculated and the difference between these ALE-scores was recorded for each voxel in the brain. Repeating this process 25,000 times then yielded an expected distribution of ALE-score differences under the assumption of exchangeability. The “true” difference in ALE scores was then tested against this null-distribution yielding a posterior probability that the true difference was not due to random noise in an exchangeable set of labels, based on the proportion of lower differences in the random exchange. The resulting probability values were thresholded at p>.95 (95% chance for true difference) and inclusively masked by the respective main effects, i.e., the significant effects of the ALE analysis for the particular condition. For both, the conjunction and the contrast analyses, only clusters with 10 voxels or larger were considered. Anatomical labeling was conducted with SPM Anatomy Toolbox version 2.2b (Eickhoff et al., 2007, 2005).
3. Results
3.1. Main effect empathy
The analysis across all empathy experiments revealed convergent activity in left dorso-medial prefrontal cortex (dmPFG), the left IFG extending into the AI, and the bilateral precuneus (Table S2 and Figure 1 for details).
Figure 1.
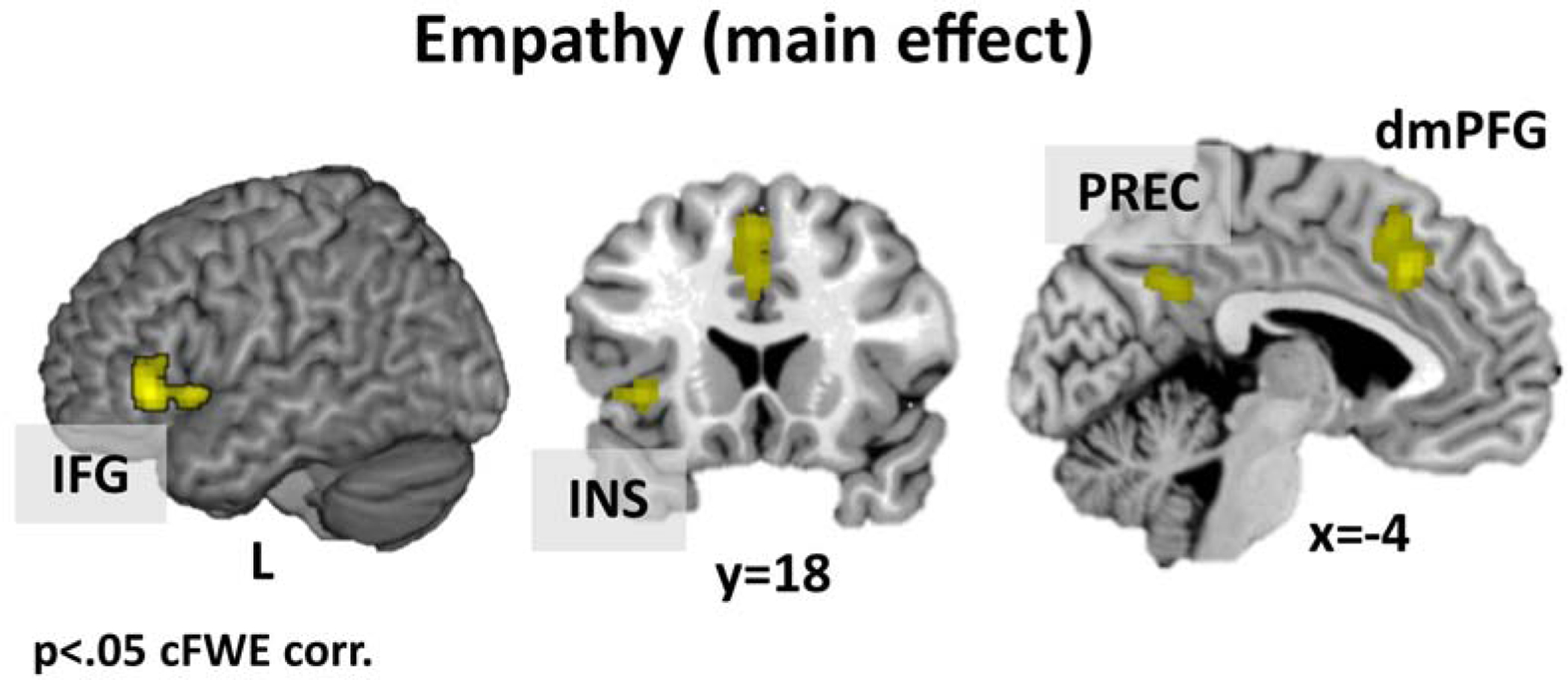
Activations across all empathy experiments. Results are cluster-level FWE corrected.
Note: dorso-medial prefrontal gyrus = dmPFG, inferior frontal gyrus = IFG, insula = INS, precuneus = PREC.
Empathy for pain.
Convergent activation across experiments for empathy for pain was found in the left dmPFG including the MCC, the left SMG and the bilateral AI (Table S2 and Figure 2).
Figure 2.
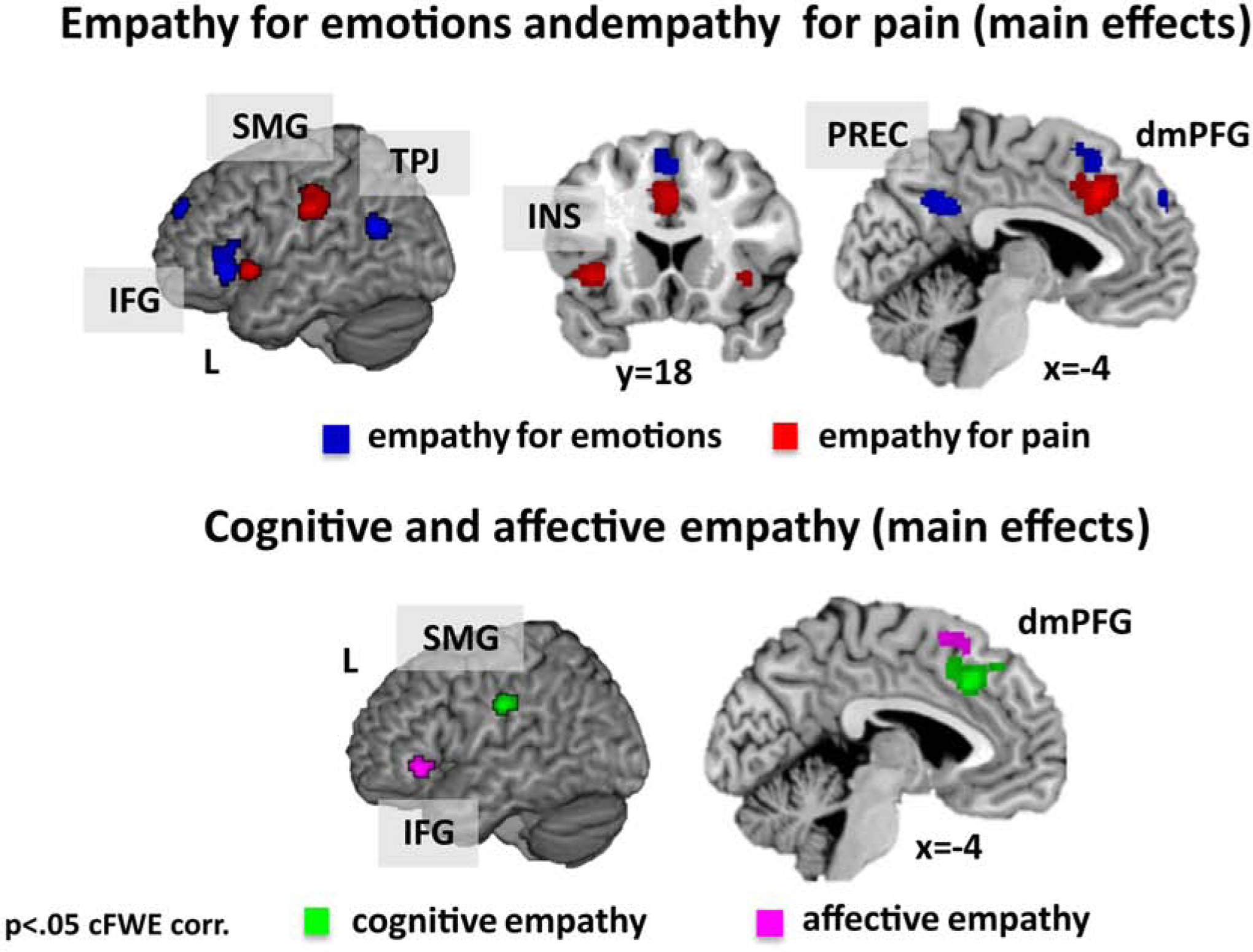
Activations for empathy for emotions (blue, upper panel) and empathy for pain (red, upper panel) as well as for cognitive empathy (green, lower panel) and affective empathy (pink, lower panel). Results are cluster-level FWE corrected.
Note: dorso-medial prefrontal gyrus = dmPFG, inferior frontal gyrus = IFG, insula = INS, precuneus = PREC, supramarginal gyrus = SMG, temporo-parietal-junction = TPJ.
Empathy for emotions.
For empathy for emotions consistent activation across experiments occurred in the bilateral precuneus, the left dmPFG, the left IFG and the left TPJ (Table S2 and Figure 2).
Empathy for pain and empathy for emotions.
Performing a conjunction analysis to identify joint networks for empathy for pain and empathy for emotion indicated no joint activation.
Empathy for pain vs. empathy for emotions.
Directly comparing empathy for pain vs. empathy for emotions revealed stronger convergence in the left SMG, the left dmPFG/MCC and the bilateral AI for empathy for pain and in the bilateral precuneus, the left dmPFG, the left TPJ extending into the angular gyrus and the left IFG for empathy for emotions.
3.2. Cognitive and affective empathy
3.2.1. Cognitive empathy (taking somebody else’s perspective).
Investigation of consistent activation across experiments of cognitive empathy revealed convergent activation in the left dmPFG and left SMG (Table S2 and Figure 2).
Cognitive empathy for pain.
The meta-analysis across experiments reporting cognitive empathy for pain revealed significant convergence in the left dmPFG extending into the MCC, the left SMG and the left AI.
Cognitive empathy for emotions.
Convergent activation for cognitive empathy for emotions was found in the bilateral precuneus.
Cognitive empathy for pain and cognitive empathy for emotions.
Performing a conjunction analysis to identify joint networks for cognitive empathy for pain and cognitive empathy for emotion indicated no joint activation.
Cognitive empathy for pain vs. cognitive empathy for emotions.
Directly comparing cognitive empathy for pain vs. cognitive empathy for emotions revealed stronger convergence in the left MCC, left SMG and left AI for pain, while cognitive empathy for emotions was accompanied by stronger convergence of the bilateral precuneus (Table S2).
3.2.2. Affective empathy (feeling somebody else’s emotions/pain).
Investigation of consistent activation across affective empathy revealed cluster in the left posterior dmPFG and left IFG (Table S2 and Figure 2).
3.2.3. Cognitive and affective empathy.
For the conjunction of cognitive and affective empathy no joint activation occurred.
Cognitive vs. affective empathy.
The direct comparison of cognitive and affective empathy revealed stronger convergent activation for cognitive empathy in the bilateral anterior dmPFG and the left SMG, while affective empathy showed stronger convergence of a more posterior part of the left dmPFG (Table S2 and Figure 3).
Figure 3.
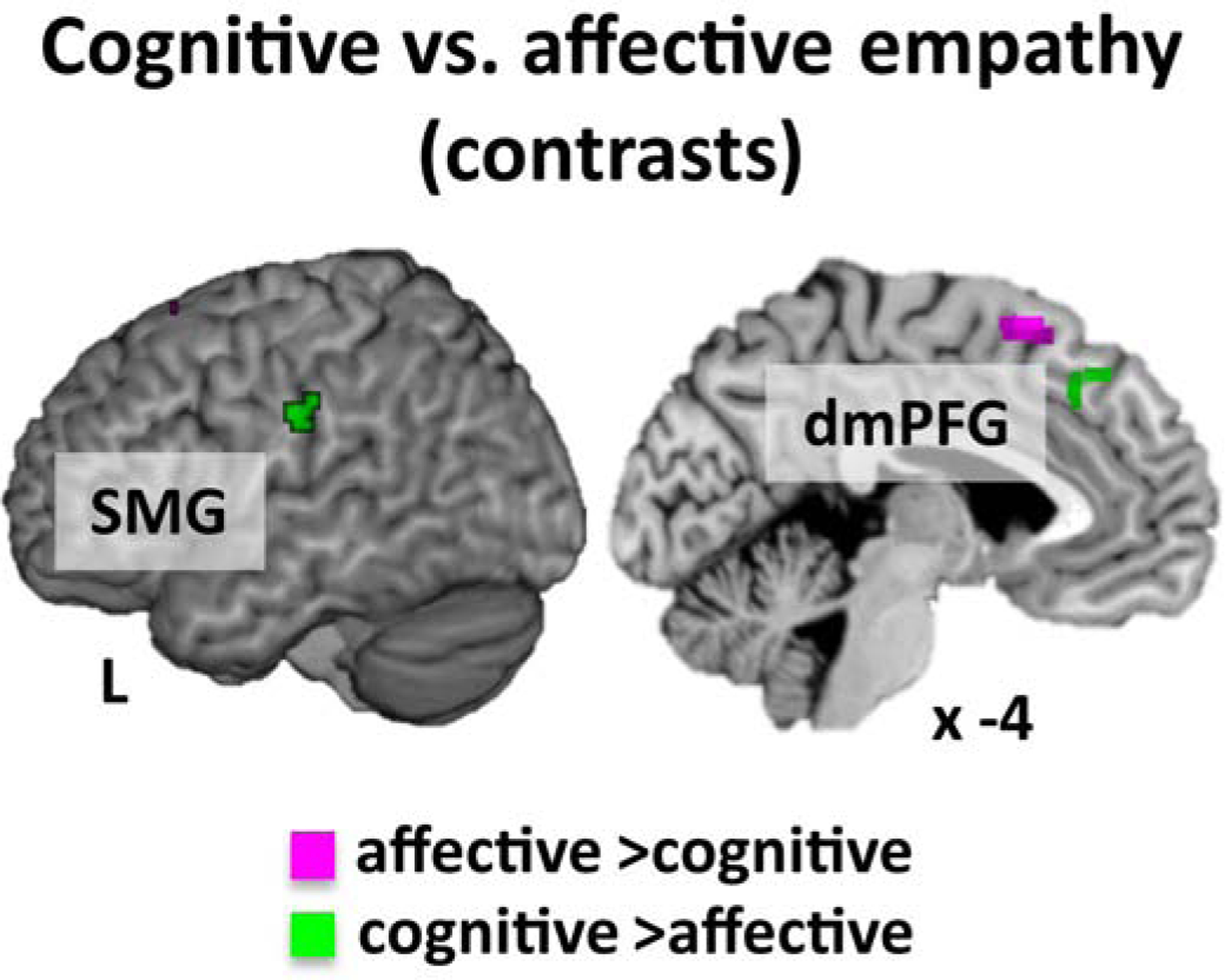
Contrast analyses between experiments for cognitive vs. affective empathy.
Note: dorso-medial prefrontal gyrus = dmPFG, supramarginal gyrus = SMG.
3.3. Pain and empathy for pain
3.3.1. Pain.
Investigation of consistent activation across experiments of pain processing revealed activation in the left postcentral gyrus and right SMG, both extending to the insula, to the putamen and to the thalamus, bilateral MCC/ACC/dmPFG, bilateral MFG/IFG and left cerebellum (Table S3 and Figure 4).
Figure 4.
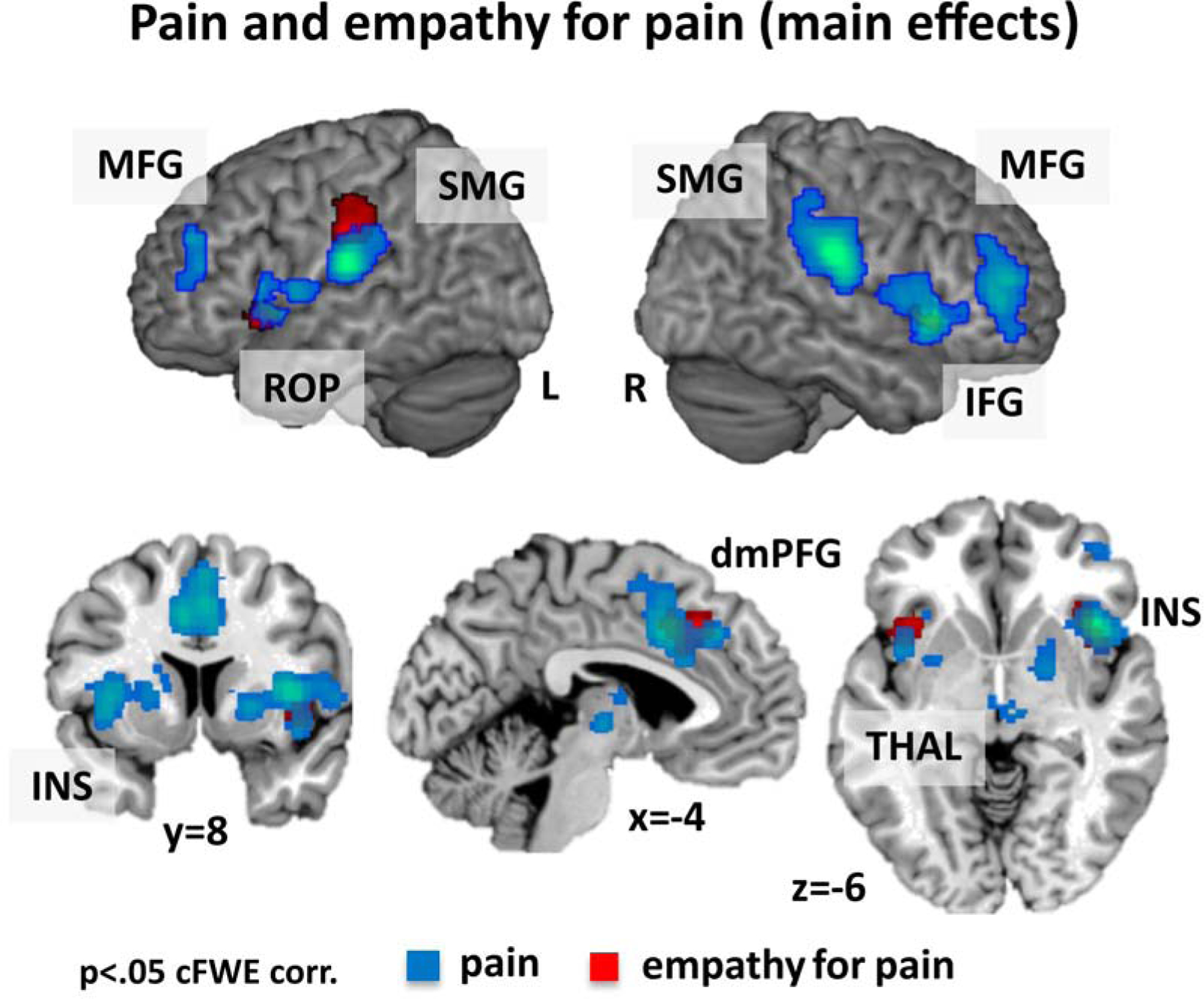
Activation for the main effect of pain (blue) and empathy for pain (red).
Note: dorso-medial prefrontal gyrus = dmPFG, inferior frontal gyrus = IFG, insula = INS, supramarginal gyrus = SMG, middle frontal gyrus = MFG, rolandic operculum = ROP, thalamus = THAL.
3.3.2. Pain and empathy for pain.
Joint activation appeared for pain and empathy for pain in bilateral AI and the left MCC (Table S3 and Figure 5).
Figure 5.
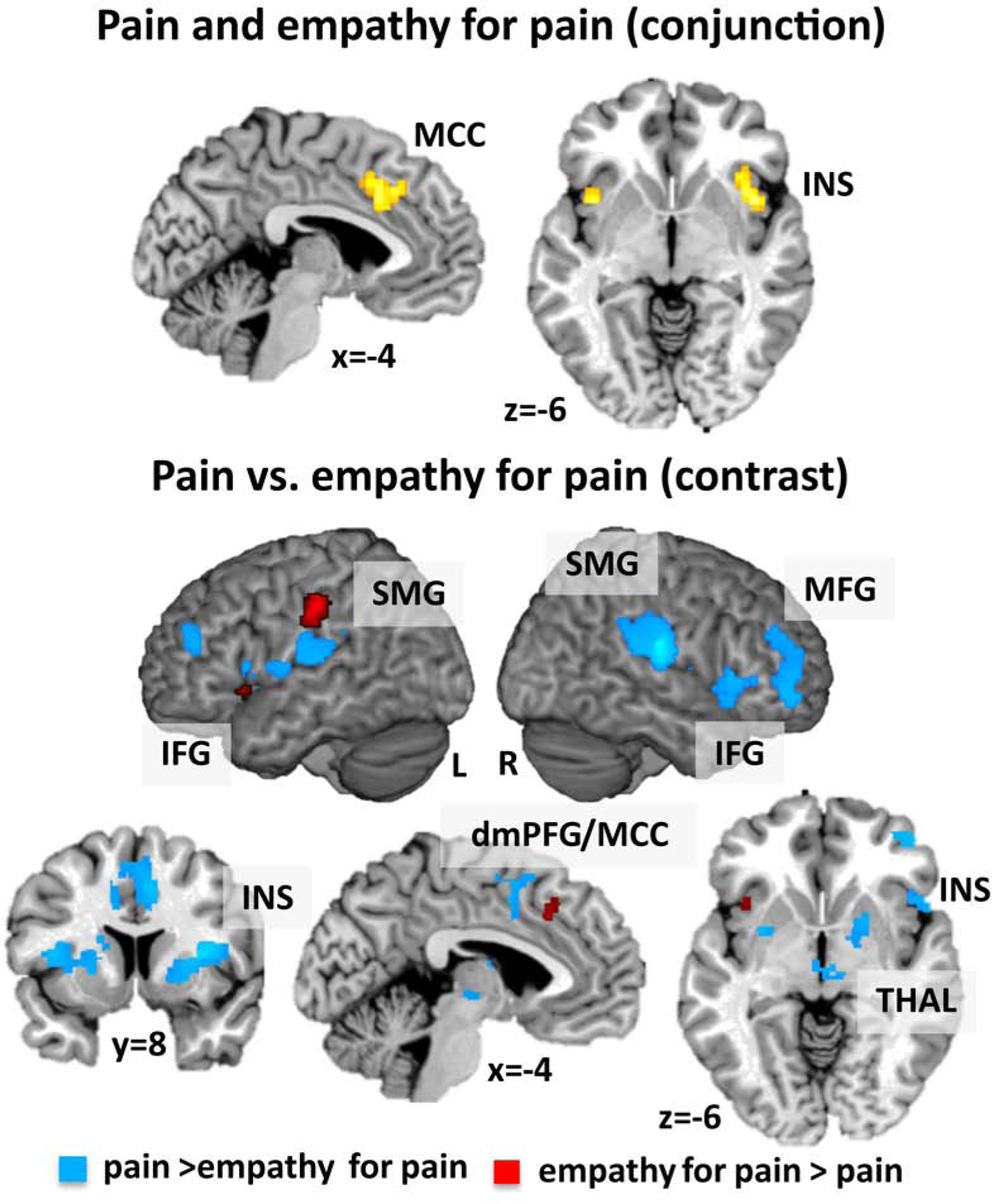
Conjunction and contrast analyses between experiments for pain and empathy for pain.
Note: dorso-medial prefrontal gyrus = dmPFG, middle cingulate cortex = MCC, inferior frontal gyrus = IFG, insula = INS, supramarginal gyrus = SMG, middle frontal gyrus = MFG, thalamus = THAL.
Pain vs. empathy for pain.
The direct comparison of pain and empathy for pain revealed stronger convergence for pain in the bilateral rolandic operculum extending to the insula, IFG and putamen, the right MCC/dmPFG, right middle orbital gyrus, bilateral MFG, bilateral thalamus, and left cerebellum (Table S3 and Figure 5). In turn, this analysis showed stronger convergence for empathy for pain in the left SMG, left MCC and left AI (Table S3 and Figure 5).
4. Discussion
The main objective of this study was to investigate specific and general neural underpinnings for cognitive and affective empathy. So far, no study has explicitly focused on shared and distinct networks underlying the two facets of empathy for diverging states divided into empathy for pain and empathy for basic emotions. Therefore, we conducted analyses on subjectively feeling the emotions/pain of others (affective empathy) and on inferring how somebody else is feeling in emotional/painful situations (cognitive empathy). To confirm the neural correlates of empathy for pain and empathy for emotions, we also performed analyses on these aspects separately.
Additionally, we undertook comparisons of studies on pain experience with studies on empathy for pain. To our knowledge, this is the first study directly comparing studies on pain experience with those on empathy for pain in a large sample of coordinate-based neuroimaging data. The results shall be discussed further in detail.
4.1. Empathy
The analysis across both, cognitive and affective empathy revealed convergent activity in the left dorso-medial prefrontal gyrus, left inferior frontal gyrus extending to the anterior insula and bilateral precuneus. Thus, global empathy relied on a network, which is in accordance with previous literature (Bzdok et al., 2012; Fan et al., 2011; Lamm et al., 2011). Additionally, we now show that neural regions are recruited differently depending on the different aspects of empathy, which will be discussed in the following.
4.1.1. Cognitive empathy
Cognitive empathy, mainly defined as taking over the perspective of another person and inferring how the other person is feeling, is associated with consistent activation of the anterior part of the left anterior dorso-medial prefrontal gyrus (dmPFG) and the left supramarginal gyrus (SMG). Both regions are associated with perspective-taking in social situations (Banissy et al., 2012; Eres, Decety, Louis, & Molenberghs, 2015; Fan et al., 2011) and self-other distinction (van der Heiden, Scherpiet, Konicar, Birbaumer, & Veit, 2013) (see Figure 2).
The anterior dmPFG seems to be specific for complex social-emotional processes in affective and social situations as it shows an overlap in activation with moral cognition, theory-of-mind and empathy (Bzdok et al., 2012). Importantly, in our data, we found an anatomical distinction within the dmPFG for cognitive and affective empathy processing: While the meta-analysis for cognitive empathy revealed stronger convergence in the anterior part, for affective empathy a more posterior part was convergently involved. This finding is partly in accordance with results from Fan and colleagues (2011) who found the anterior part of the dmPFG to be significantly involved in evaluative empathy. Interestingly and further supporting the role of this anterior dmPFG region in cognitive empathy, the gray matter density of this region shows a positive correlation with self-report measures tapping cognitive empathy (Banissy et al., 2012; Eres et al., 2015). Digging deeper into cognitive empathy, we observed stronger convergence in this anterior part for cognitive empathy for pain than for cognitive empathy for emotions. This corroborates previous reports that the dmPFG is involved when participants were watching painful visual stimuli from a self-perspective or imagined a loved-one in pain (Cheng et al., 2010) or when seeing in-group members in pain (Xu, Zuo, Wang, & Han, 2009). It further correlates positively with subjective unpleasantness and pain intensity ratings (Cheng et al., 2007). Thus, the anterior part of the dmPFG seems to be significantly involved when we indicate how someone else is feeling, and particularly when somebody else is in a painful situation.
The left supramarginal gyrus (SMG) is part of a network of higher order somatosensory processing (Eickhoff et al., 2010) and has been found to become activated during somatosensory and pain perception (Bingel, Lorenz, Schoell, Weiller, & Büchel, 2006). Interestingly, the left SMG is also activated when observing others telling negative stories, whereas the right SMG is activated when beliefs of others have to be inferred (Kanske et al., 2015). In light of the current results on left SMG activation during cognitive empathy processing, lateralization of SMG activation might be related to task requirements such as indicating the emotional state of someone else or inferring someone else’s beliefs. Moreover, the SMG was more convergently activated for cognitive empathy in painful than in emotional situations in our study. Hence, the SMG seems to critically contribute when inferring somebody else’s feelings especially in painful situations.
4.1.2. Affective empathy
In contrast to cognitive empathy, for affective empathy a posterior part of the dmPFG as well as the left inferior frontal gyrus (IFG) showed significant convergence across experiments. The posterior dmPFG is associated with observation and imitation of emotions (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Leslie, Johnson-Frey, & Grafton, 2004), in particular when indicating how oneself feels in response to an emotional face (Schulte-Rüther et al., 2011) or an emotional video (Klimecki, Leiberg, Lamm, & Singer, 2013) as well as when empathizing with another person (De Greck et al., 2012). Therefore, this posterior region of the dmPFG is associated with subjective feelings in response to emotional situations.
With the current study design, we were able to find distinct neural activation for cognitive and affective empathy within the midline structure of the brain. The anterior part of the dmPFG is recruited when we attempt to indicate the feelings/pain of others, while the posterior part is associated with the subjective emotional experience in response to emotions of others.
In line with Bzdok and colleagues (2012), the left inferior frontal gyrus (IFG) also demonstrated significant activation during empathy processing, particularly affective empathy. The IFG is essential for emotional perspective taking (Schulte-Rüther, Markowitsch, Fink, & Piefke, 2007), perception, evaluation and comprehension of emotions (Carr et al., 2003; Seitz et al., 2008), emotion regulation (Morawetz, Bode, Derntl, & Heekeren, 2017), and during social exclusion (Cacioppo et al., 2013). Additionally, the activation of the IFG has been shown to positively correlate with emotional empathy scores (Schulte-Rüther et al., 2007). Recruitment of this region during affective empathy might mirror the degree of subjective emotional distress or involvement (Schulte-Rüther, Markowitsch, Shah, Fink, & Piefke, 2008). The IFG has also been suggested to be part of the mirror neuron system (Caspers, Zilles, Laird, & Eickhoff, 2010). Patients with lesions within the IFG show diminished affective empathy scores for personal distress and diminished external emotion recognition abilities (Shamay-Tsoory, Aharon-Peretz, & Perry, 2009).
Strikingly, our results for cognitive and affective empathy are mainly left-lateralized. Hypotheses on withdrawal- and approach behavior suggest that the left hemisphere is associated with approach behavior for negative and positive emotions (Harmon-Jones & Gable, 2018), which fits nicely with the current results. We selected studies based on the instruction to feel with or take over the perspective of an observed person. These instructions might have led to an implicit, motivational approach behavior. Left-lateralization was also shown by Fan and colleagues (2012) for evaluative-cognitive empathy. However, Timmers and colleagues (2018) reported left and right activation for evaluative empathy. An association between empathy and motivational approach traits was reported previously (Balconi & Bortolotti, 2012). Whether the instruction to empathize with someone else also leads to approach behavior needs to be tested in future research.
4.1.3. Empathy for pain
Interestingly, besides showing stronger convergence for cognitive than for affective empathy, both regions that appeared to be significant for cognitive empathy (dmPFG, SMG) also show stronger convergence for empathy for painful rather than for emotional situations. These results are in accordance with previous reports (Timmers et al. 2018). As already mentioned before, the recruitment of the anterior part of the dmPFG points to the attempt to indicate the feelings of others, particularly when indicating whether the observed person feels pain (Ma, Wang, & Han, 2011).
Additionally, the bilateral anterior insula (AI) appeared to be consistently activated during empathic processing in general and for empathy for pain. This has been reported previously and consistently for empathy (Bzdok et al., 2012; Fan et al., 2011) and empathy for pain (Lamm et al., 2011; Timmers et al., 2018). The AI is associated with interoceptive processing and consciousness (Craig, 2009; Craig, 2003; Critchley, 2004; Kurth, Zilles, Fox, Laird, & Eickhoff, 2010) as well as with negative affective processing (Kogler et al., 2015). A mindfulness meditation, thus a training to reduce personal distress, can down-regulate AI activation during perception of social embarrassing situations (Laneri et al., 2017), further reflecting the association of the AI with negative affective states. Our results show that the AI is more consistently activated when participants should indicate the perspective of a person in painful situations rather than in emotional situations, which is also in line with the results of Timmers and colleagues (2018). This result highlights the significance of interoceptive processing when inferring negative affective states of others.
4.1.4. Empathy for emotions
Besides activation of the dmPFG, during empathic processing for emotional situations, the precuneus plays a crucial role. We additionally observed more consistent activation for cognitive empathy for emotions than cognitive empathy for pain in this region, which also fits with previous results showing activation of the precuneus when evaluating emotional states (Terasawa, Fukushima, & Umeda, 2013), inferring another person’s emotion (Atique, Erb, Gharabaghi, Grodd, & Anders, 2011) and the attribution of emotions of the self and of others (Ochsner et al., 2004). Activation of the precuneus is further correlated with self-esteem (Eisenberger, Inagaki, Muscatell, Haltom, & Leary, 2011; Kogler et al., 2017), self-descriptive (Kircher et al., 2002), and self-referential processing (Cavanna & Trimble, 2006b). Recruitment of the precuneus may suggest pronounced self-related memory retrieval and self-referential as well as interoceptive processing during empathic engagement (Addis, Pan, Vu, Laiser, & Schacter, 2009; Cavanna & Trimble, 2006a; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; Gusnard & Raichle, 2001; Martinelli, Sperduti, & Piolino, 2013). Another region that appeared to be essential for empathy, particularly for empathic processing of emotions, is the left temporo-parietal junction (TPJ), which is in line with previous results on general empathy (Bzdok et al., 2012; van der Heiden et al., 2013). The left TPJ might also be seen as part of the human mirror neuron system (Caspers et al., 2010) that helps facilitating social interactions by providing a sense of acting with others (Iacoboni, 2009; Schilbach et al., 2016). The left TPJ becomes activated when subjects have to indicate changes in feelings for oneself or for others (Schnell, Bluschke, Konradt, & Walter, 2011), when other people’s beliefs have to be inferred (Saxe & Kanwisher, 2003) and when directly contrasting self-related narratives with inferring beliefs of others (Vogeley et al., 2001). Furthermore, it has been shown to become activated during empathy, theory-of-mind and moral cognition (Bzdok et al., 2012; Kanske et al., 2015), revealing its significance for empathy, particularly in emotional situations as shown in our study.
Taken together, the current data highlight the complexity of empathy processing. The AI and the dmPFG are part of the salience network (Menon & Uddin, 2010) and might transpose information between internally driven self-referential information and externally driven cognitive information (Menon, 2015; Sridharan, Levitin, & Menon, 2008) and are further thought to convey information to other brain regions to assist them in the generation of appropriate behavioral responses (Menon & Uddin, 2010). It was shown that the dmPFG and the AI are involved in egocentric decision making in social situations. Both are recruited when someone is rejecting undeserved disadvantageous outcomes compared to when accepting undeserved advantageous outcome (Feng et al., 2019). Thus, the dmPFG and the AI are key regions when differentiating one’s own needs and sensations from those of others in order to further adapt behavioral reactions (Lamm et al., 2011; Lamm & Singer, 2010). Additionally, the IFG, AI and TPJ are part of the ventral attention network that drives attention towards salient information (Fox et al., 2006; Viviani, 2013). Cross-talk and interplay between large-scale brain networks is therewith likely in empathy processing. Furthermore, clustering across all empathy studies showed different converging regions than running separate analyses on cognitive and affective empathy. This difference in results might be driven by the heterogeneity between cognitive and affective empathy. Our data therewith emphasize that cognitive and affective empathy should be treated as different functions in neuroimaging research. In particular, our results show that cognitive and affective empathy are processed in different sub-regions across the midline structures of the brain, with empathy for pain being processed in a more anterior part and empathy for emotions in a more posterior part of the dmPFG. These different functions of the midline structures of the brain should be considered in future empathy research. We not only hope that our results help to improve characterization of different functions involved in empathy processing but also that they lead to a more appropriate labeling of the involved regions.
4.2. Pain processing
One further goal of the current study was the direct comparison of empathy for pain and pain studies to test for neural differences and overlaps. To the best of our knowledge, this is the first meta-analysis comparing empathy for pain with direct pain experience in a large sample of neuroimaging studies on pain experience including different painful states (such as cutaneous, muscular or visceral pain). The meta-analysis of activation during pain revealed convergence in regions typically activated during pain processing (Friebel, Eickhoff, & Lotze, 2011; Kogler et al., 2015; Peyron, Laurent, & García-Larrea, 2000). The detection of sensory qualities, the handling of affective information and the integration of those sensations are particularly significant in pain processing. The medial and lateral frontal cortices as well as thalamic nuclei belong to the affective-cognitive-evaluative, and motor and somatosensory cortices to the discriminative-sensory pain system (Friebel et al., 2011; Iannetti & Mouraux, 2010).
4.2.1. Pain and empathy for pain
Apparently, the MCC/dmPFG and the AI show convergent activation for pain and empathy for pain processing. Additionally, both regions appear in the direct contrasts pain vs. empathy for pain. These contradictory findings were already reported by Lamm and colleagues (2011), who described the dmPFG and the AI (at a slightly lowered threshold) to be activated in a conjunction of self-pain experience and seeing others in pain and the MCC/dmPFG to be more convergently activated during pain experience than during empathy for pain (Lamm et al., 2011). The current results again indicate differential functional processing within the midline structure of the brain: While an anterior part of the dmPFG is associated with empathy for pain processing, a posterior part is strongly involved in processing of self-pain experience. Moreover, there seems to be a middle intersection that is significant for both, pain and empathy for pain processing. The MCC is a structure commonly reported in imaging studies of nociceptive stimulation (Farrell, Laird, & Egan, 2005; Peyron et al., 2000), and it is associated with response selection and motor inhibition (Friebel et al., 2011; Palomero-Gallagher, Vogt, Schleicher, Mayberg, & Zilles, 2009). The AI is associated with interoceptive processing, consciousness (Craig, 2009; Craig, 2003; Critchley, 2004; Kurth et al., 2010) and specifically the right AI with stress experience (Kogler et al., 2015). Interoceptive, affective and stressful processing are overlapping functions of self-pain experience and observing pain in others. As part of the salience network (Menon & Uddin, 2010), both regions might integrate interoceptive sensations and affective states of others. The comparison of empathy for pain with pain processing revealed consistent activation of the left SMG during empathy for pain as an indicator for inferring somebody else’s feelings in painful situations (Kanske et al., 2015). In contrast, more convergent activation for pain emerged in brain areas associated with somatosensory processing and pain perception such as the bilateral operculum covering somatosensory and secondary somatosensory regions (Eickhoff, Amunts, Mohlberg, & Zilles, 2006; Eickhoff et al., 2010). Involvement of somatosensory regions was also previously reported by Lamm and colleagues (2011). Additionally, recruitment of the middle frontal gyrus indicates a modulatory effect on pain experience (Apkarian, Bushnell, Treede, & Zubieta, 2005; Casey, 1999; Friebel et al., 2011; Ingvar, 1999).
4.3. Limitations
The current study has some limitations that might influence data interpretation. In the current meta-analyses, studies using simple emotion recognition paradigms (e.g., viewing of emotional pictures) were excluded. This was done in contrast to previous meta-analyses on empathy processing (e.g. Fan et al. 2011; Timmers et al. 2018) to avoid confusion of simple emotion recognition or emotion processing with affective or cognitive empathy. Differences in paradigm selection probably influenced outcome differences such as missing amygdala activation in the current study compared to Timmers and colleagues (2018). Assessing the overlap between passive viewing of emotions and actively getting involved in the expressed emotion would be worth evaluating in future research. We also want to point out that the analyses might be biased based on the number of included experiments. Analyses with a higher number of included experiments can have a higher power (Eickhoff et al., 2016). Imbalances in the included analyses therewith might influence the results.
Furthermore, a lot of studies on empathy were only reporting a consortium of different emotions (e.g., happiness, anger and disgust) (e.g., De Greck et al. 2012). Thus, in the current meta-analysis we were not able to additionally distinguish between empathy for positive and negative emotions. Findings may be different for positive and negative emotions. This is especially relevant in the comparison of empathy for emotions and empathy for pain as well as in the comparison of global empathy with pain experience. We hope that with the increasing literature it will be possible to understand neural empathy processing for positive and negative emotions in the future.
The amount of studies for affective empathy is modest and we were not able to conduct separate analyses on affective empathy of pain and of emotions. Inclusion of studies reporting whole-brain analyses was one precondition of the current study. We therefore excluded a fair amount of studies due to region-of-interest analyses or small-volume corrections. Furthermore, we excluded studies that did not explicitly ask participants to feel with or take over the perspective of another person. Additionally, we had to pool across different task instructions, such as “Share the emotional state of someone else” and “How do you feel when observing this person?”. Due to the minor amount of studies for each subcategory, we were not able to explore differences due to these instructions separately. We hope that studies on affective empathy will emerge within the coming years to enable further and robust results on these data.
4.4. Summary and conclusion
The goal of the current meta-analysis was to assess the neural correlates of empathy and its cognitive and affective subcomponents for pain and for basic emotions (see Figure 2). With the current study design, we were able to replicate findings of previous meta-analyses on empathy (Bzdok, et al., 2012; Lamm et al., 2011; Fan et al., 2011; Timmers et al., 2018) and to further distinguish affective and cognitive empathy components in more detail. The analyses revealed a distinction within the midline structures of the brain for cognitive and affective empathy. For cognitive empathy, convergence over studies was found in the anterior dmPFG and the SMG, thus regions associated with inferring feelings of others (Kanske et al., 2015). Contrarily, consistent activation of the posterior dmPFG and the IFG during affective empathy points to the subjective emotional involvement when seeing others in emotional/painful situations.
Additionally, our data show that the specific situations – empathic engagement for emotional or painful situations – should be considered. On the one hand, empathy for emotions reliably shows activation of the precuneus, the posterior dmPFG, and the TPJ, thus pointing to a subjective affective involvement and self-referential processing. On the other hand, during empathy for pain the anterior dmPFG, the SMG and the AI, regions associated with interoceptive, negative affective processing and inferring feelings of others, are specifically recruited (see Figures 2 & 3).
The dmPFG/MCC and the insula are convergently activated for pain and empathy processing. Furthermore, the same regions appear when contrasting pain with empathy and empathy for pain. Thus, a distinction of the functions of these regions is apparent. In empathy for pain we additionally see an overlap with direct pain experience again within the insula and the MCC/dmPFG (see Figure 5). Distinct activation in empathy and empathy for pain in comparison to direct pain experience is seen in the SMG, MCC, AI, IFG and the precuneus. These regions are associated with emotion processing and regulation as well as self-referential processing.
With the current study design, we were able to show distinct processing of cognitive and affective empathy as well as for pain and empathy processing within the midline structures of the brain. Our data is lending further support for a multidimensional concept of empathy. No part of the study was pre-registered prior to the research being conducted. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Analysis codes and results are publicly available at http://anima.fz-juelich.de/studies/Kogler_Empathy_2020 or can be requested by contacting the authors.
Supplementary Material
Acknowledgments
This work was supported by the fORTÜNE-program of the Medical Faculty, University of Tübingen (2393-0-0 to LK), by the Deutsche Forschungsgemeinschaft (DFG, IRTG-1328), by the National Institute of Mental Health (R01-MH074457 to SBE), as well as the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 720270 (HBP SGA1 to SBE) and 785907 (HBP SGA2 to SBE). We thank Teresa Luther for her support in literature research. We thank the following researchers for providing additional information on their work: Corrado Corradi-Dell’Acqua, Nicolas Danziger, Moritz de Greck, Tom Farrow, Claus Lamm, Delia Lenzi, Yina Ma, Naomi Eisenberger, Yoshiya Moriguchi, Daniella Perry, Mira Preis, Martin Schulte-Rüther, Diana Torta, Etienne Vachon-Presseau.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure and conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest in relation to the manuscript.
References
- Addis DR, Pan L, Vu M-A, Laiser N, & Schacter DL (2009). Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia, 47, 2222–2238. 10.1016/j.neuropsychologia.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Atique B, Erb M, Gharabaghi A, Grodd W, & Anders S (2011). Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. NeuroImage, 55(4), 1899–1911. 10.1016/j.neuroimage.2010.12.036 [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Kanai R, Walsh V, & Rees G (2012). Inter-individual differences in empathy are reflected in human brain structure. NeuroImage, 62(3), 2034–2039. 10.1016/j.neuroimage.2012.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, & Büchel C (2006). Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain, 120, 8–15. 10.1016/j.pain.2005.08.027 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, & Eickhoff SB (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function, 217(4), 783–796. 10.1007/s00429-012-0380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, & Cacioppo JT (2013). A quantitative meta-analysis of functional imaging studies of social rejection. Scientific Reports, 3, 2027 10.1038/srep02027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, & Lenzi GL (2003). Neural mechanisms of empathy in humans: A relay froom neural systems for imitations to limbic areas. Proceedings of the National Academy of Sciences, 100(9), 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, & Eickhoff SB (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–1167. 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006a). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129, 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006b). The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, & Decety J (2010). Love hurts: An fMRI study. NeuroImage, 51(2), 923–929. 10.1016/j.neuroimage.2010.02.047 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, & Decety J (2007). Expertise Modulates the Perception of Pain in Others. Current Biology, 17(19), 1708–1713. 10.1016/j.cub.2007.09.020 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). Howdo you feel—now? The anterior insula and human awareness. Nat Rev Neurosci, 10, 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig ADB (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13, 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Critchley HD (2004). The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences of the United States of America, 101(17), 6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, & Peyron R (2009). Can We Share a Pain We Never Felt? Neural Correlates of Empathy in Patients with Congenital Insensitivity to Pain. Neuron, 61(2), 203–212. 10.1016/j.neuron.2008.11.023 [DOI] [PubMed] [Google Scholar]
- de Greck M, Shi Z, Wang G, Zuo X, Yang X, Wang X, … Han S (2012). Culture modulates brain activity during empathy with anger. Neuroimage, 59(3), 2871–2882. 10.1016/j.neuroimage.2011.09.052 [DOI] [PubMed] [Google Scholar]
- De Greck Moritz, Scheidt L, Bölter AF, Frommer J, Ulrich C, Stockum E, … Northoff G (2012). Altered brain activity during emotional empathy in somatoform disorder. Human Brain Mapping, 33(11), 2666–2685. 10.1002/hbm.21392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Jackson PL (2004). The functional architecture of human empathy. Behav Cogn Neurosci Rev, 3(2), 71–100. 10.1177/1534582304267187 [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, Schneider F, & Habel U (2010). Multidimensional assessment of empathic abilities : Neural correlates and gender differences. Psychoneuroendocrinology, 35, 67–82. 10.1016/j.psyneuen.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Voss B, Eickhoff SB, Kellermann T, Schneider F, & Habel U (2012). Neural correlates of the core facets of empathy in schizophrenia. Schizophrenia Research, 136(1–3), 70–81. 10.1016/j.schres.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, & Convit A (2008). Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). Journal of Autism and Developmental Disorders, 38(3), 464–473. 10.1007/s10803-007-0486-x [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, & Fox PT (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage, 59, 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, & Behrens TEJ (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. The Journal of Neuroscience, 30(18), 6409–6421. 10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, … Eickhoff CR (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M, Evans AC, Zilles K, & Amunts K (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage, 36, 511–521. 10.1016/j.neuroimage.2007.03.060 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, & Zilles K (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25, 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Laird A, Grefkes C, Wang LE, Zilles K, & Fox PT (2009). Coordinate-based ALE meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. 10.1002/hbm.20718.Coordinate-based [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Byrne Haltom KE, & Leary MR (2011). The Neural Sociometer: Brain Mechanisms Underlying State Self-esteem. [DOI] [PubMed]
- Eres R, Decety J, Louis WR, & Molenberghs P (2015). Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. NeuroImage, 117, 305–310. 10.1016/j.neuroimage.2015.05.038 [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 903–911. 10.1016/j.neubiorev.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Feng C, Feng X, Wang L, Wang L, Gu R, Ni A, … Luo YJ (2019). The neural signatures of egocentric bias in normative decision-making. Brain Imaging and Behavior, 13(3), 685–698. 10.1007/s11682-018-9893-1 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, & Christoff K (2015). The wandering brain : Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, & Raichle ME (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–10051. 10.1073/pnas.0604187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel U, Eickhoff SB, & Lotze M (2011). Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage, 58(4), 1070–1080. 10.1016/j.neuroimage.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, & Raichle ME (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience, 2, 685–694. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Cacioppo JT, & Rapson RL (1993). Emotional contagion. Current Directions in Psychological Science, 2(3), 96–99. [Google Scholar]
- Iacoboni M (2009). Imitation, Empathy, and Mirror Neurons. Annual Review of Psychology, 60(1), 653–670. 10.1146/annurev.psych.60.110707.163604 [DOI] [PubMed] [Google Scholar]
- Iannetti GD, & Mouraux A (2010). From the neuromatrix to the pain matrix (and back). Experimental Brain Research, 205, 1–12. 10.1007/s00221-010-2340-1 [DOI] [PubMed] [Google Scholar]
- Kanske P, Böckler A, Trautwein FM, & Singer T (2015). Dissecting the social brain: Introducing the EmpaToM to reveal distinct neural networks and brain-behavior relations for empathy and Theory of Mind. NeuroImage, 122, 6–19. 10.1016/j.neuroimage.2015.07.082 [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Brammer M, Bullmore E, Simmons A, Bartels M, & David AS (2002). The neural correlates of intentional and incidental self processing. Neuropsychologia, 40(6), 683–692. 10.1016/S0028-3932(01)00138-5 [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, & Singer T (2013). Functional Neural Plasticity and Associated Changes in Positive Affect After Compassion Training. Cereb Cortex, 23(7), 1552–1562. 10.1093/cercor/bhs142 [DOI] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, & Derntl B (2015). Psychosocial versus physiological stress - Meta-analyses on the deactivations and activations of the neural correlates of stress reactions. Neuroimage, 119, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Seidel E, Metzler H, Thaler H, Boubela RN, Pruessner JC, … Windischberger C (2017). Impact of self-esteem and sex on stress reactions. Scientific Reports, (November), 1–9. 10.1038/s41598-017-17485-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, & Eickhoff SB (2010). A link between the systems : functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function, 214, 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, … Fox PT (2011). The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Research Notes, 4, 349 10.1186/1756-0500-4-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, & Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lamm Claus, Batson CD, & Decety J (2007). The Neural Substrate of Human Empathy: Effects of Perspective-taking and Cognitive Appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. 10.1162/jocn.2007.19.1.42 [DOI] [PubMed] [Google Scholar]
- Lamm Claus, Decety J, & Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Laneri D, Krach S, Paulus FM, Kanske P, Schuster V, Sommer J, & Müller-Pinzler L (2017). Mindfulness meditation regulates anterior insula activity during empathy for social pain. Human Brain Mapping, 38(8), 4034–4046. 10.1002/hbm.23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Serra L, Perri R, Pantano P, Lenzi GL, Paulesu E, … Macaluso E (2011). Single domain amnestic MCI: A multiple cognitive domains fMRI investigation. Neurobiology of Aging, 32(9), 1542–1557. 10.1016/j.neurobiolaging.2009.09.006 [DOI] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, & Grafton ST (2004). Functional imaging of face and hand imitation: Towards a motor theory of empathy. NeuroImage, 21(2), 601–607. 10.1016/j.neuroimage.2003.09.038 [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang C, & Han S (2011). Neural responses to perceived pain in others predict real-life monetary donations in different socioeconomic contexts. NeuroImage. 10.1016/j.neuroimage.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, & Piolino P (2013). Neural substrates of the self-memory system: New insights from a meta-analysis. Human Brain Mapping, 34, 1515–1529. 10.1002/hbm.22008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0.Saliency [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, & Heekeren HR (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, … Eickhoff SB (2018). Ten simple rules for neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 84(November 2017), 151–161. 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, & Poline J-B (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–660. 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Nomi JS, Scherfeld D, Friederichs S, Schäfer R, Franz M, Wittsack HJ, … Seitz RJ (2008). On the neural networks of empathy: A principal component analysis of an fMRI study. Behavioral and Brain Functions, 4, 1–13. 10.1186/1744-9081-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, & Mackey SC (2004). Reflecting upon Feelings: An fMRI Study of Neural Systems Supporting the Attribution of Emotion to Self and Other. Journal of Cognitive Neuroscience, 16(10), 1746–1772. 10.1162/0898929042947829 [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, & García-Larrea L (2000). Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiologie Clinique, 30, 263–288. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11126640 [DOI] [PubMed] [Google Scholar]
- Preis MA, Schmidt-Samoa C, Dechent P, & Kroener-Herwig B (2013a). The effects of prior pain experience on neural correlates of empathy for pain: An fMRI study. Pain, 154(3), 411–418. 10.1016/j.pain.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Preis MA, Schmidt-Samoa C, Dechent P, & Kroener-Herwig B (2013b). The effects of prior pain experience on neural correlates of empathy for pain: An fMRI study. Pain, 154(3), 411–418. 10.1016/j.pain.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Saxe R, & Kanwisher N (2003). People thinking about thinking peopleThe role of the temporo-parietal junction in “theory of mind.” NeuroImage, 19(4), 1835–1842. 10.1016/S1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Derntl B, Aleman A, Caspers S, Clos M, Diederen KMJ, … Eickhoff SB (2016). Differential Patterns of Dysconnectivity in Mirror Neuron and Mentalizing Networks in Schizophrenia. Schizophrenia Bulletin, 42(5), 1135–1148. 10.1093/schbul/sbw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, & Walter H (2011). Functional relations of empathy and mentalizing: An fMRI study on the neural basis of cognitive empathy. NeuroImage, 54(2), 1743–1754. 10.1016/j.neuroimage.2010.08.024 [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, & Piefke M (2011). Dysfunctions in brain networks supporting empathy: An fMRI study in adults with autism spectrum disorders. Social Neuroscience, 6(1), 1–21. 10.1080/17470911003708032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Fink GR, & Piefke M (2007). Mirror Neuron and Theory of Mind Mechanisms Involved in Face-to-Face Interactions: A Functional Magnetic Resonance Imaging Approach to Empathy. Journal of Cognitive Neuroscience, 19(8), 1354–1372. 10.1162/jocn.2007.19.8.1354 [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Shah NJ, Fink GR, & Piefke M (2008). Gender differences in brain networks supporting empathy. NeuroImage, 42(1), 393–403. 10.1016/j.neuroimage.2008.04.180 [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Schäfer R, Scherfeld D, Friederichs S, Popp K, Wittsack HJ, … Franz M (2008). Valuating other people’s emotional face expression: a combined functional magnetic resonance imaging and electroencephalography study. Neuroscience, 152(3), 713–722. 10.1016/j.neuroscience.2007.10.066 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Aharon-Peretz J (2007). Dissociable prefrontal networks for cognitive and affective theory of mind : A lesion study. Neuropsychologia, 45(13), 3054–3067. 10.1016/j.neuropsychologia.2007.05.021 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Singer T, & Lamm C (2009). The social neuroscience of empathy. Annals of the New York Academy of Science, 1156, 81–96. 10.1111/j.1749-6632.2009.04418.x [DOI] [PubMed] [Google Scholar]
- Singer Tania. (2006). The neuronal basis and ontogeny of empathy and mind reading : Review of literature and implications for future research. Neuroscience & Biobehavioral Reviews, 30, 855–863. 10.1016/j.neubiorev.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Singer Tania, & Klimecki OM (2014). Empathy and compassion. Current Biology, 24(18), R875–R878. 10.1016/j.cub.2014.06.054 [DOI] [PubMed] [Google Scholar]
- Singer Tania, Seymour B, O’Doherty J, Kaube H, Dolan RJ, & Frith CD (2004). Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science, 303(5661), 1157–1162. 10.1126/science.1093535 [DOI] [PubMed] [Google Scholar]
- Stietz J, Jauk E, Krach S, & Kanske P (2019). Dissociating empathy from perspective-taking: Evidence from intra- And inter-individual differences research. Frontiers in Psychiatry, 10(MAR), 1–8. 10.3389/fpsyt.2019.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y, Fukushima H, & Umeda S (2013). How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Human Brain Mapping, 34(3), 598–612. 10.1002/hbm.21458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers I, Park AL, Fischer MD, Kronman CA, Heathcote LC, Hernandez JM, & Simons LE (2018). Is Empathy for Pain Unique in Its Neural Correlates ? A Meta-Analysis of Neuroimaging Studies of Empathy, 12(November), 1–12. 10.3389/fnbeh.2018.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, & Fox P (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heiden L, Scherpiet S, Konicar L, Birbaumer N, & Veit R (2013). Inter-individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: An fMRI study. NeuroImage, 65, 387–394. 10.1016/j.neuroimage.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Viviani R (2013). Emotion regulation, attention to emotion, and the ventral attentional network. Frontiers in Human Neuroscience, 7, 746 10.3389/fnhum.2013.00746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, … Zilles K (2001). Mind reading: Neural mechanisms of theory of mind and self-perspective. NeuroImage, 14(1 I), 170–181. 10.1006/nimg.2001.0789 [DOI] [PubMed] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, Corcoran R, Stirling J, McKie S, … Elliott R (2006). Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. NeuroImage, 29(1), 90–98. 10.1016/j.neuroimage.2005.07.022 [DOI] [PubMed] [Google Scholar]
- Walter H (2012). Social cognitive neuroscience of empathy: Concepts, circuits, and genes. Emotion Review. 10.1177/1754073911421379 [DOI] [Google Scholar]
- Xu X, Zuo X, Wang X, & Han S (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience, 29(26), 8525–8529. 10.1523/jneurosci.2418-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, & Ochsner K (2012). The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci, 15(5), 675–680. 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


