Abstract
Numerous studies have been published regarding outcomes of cancer patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus causing the coronavirus disease 2019 (COVID-19) infection. However, most of these are single-center studies with a limited number of patients. To better assess the outcomes of this new infection in this subgroup of susceptible patients, we performed a systematic review and meta-analysis to evaluate the impact of COVID-19 infection on cancer patients. We performed a literature search using PubMed, Web of Science, and Scopus for studies that reported the risk of infection and complications of COVID-19 in cancer patients and retrieved 22 studies (1018 cancer patients). The analysis showed that the frequency of cancer among patients with confirmed COVID-19 was 2.1% (95% confidence interval [CI]: 1.3–3) in the overall cohort. These patients had a mortality of 21.1% (95% CI: 14.7–27.6), severe/critical disease rate of 45.4% (95% CI: 37.4–53.3), intensive care unit (ICU) admission rate of 14.5% (95% CI: 8.5–20.4), and mechanical ventilation rate of 11.7% (95% CI: 5.5–18). The double-arm analysis showed that cancer patients had a higher risk of mortality (odds ratio [OR] = 3.23, 95% CI: 1.71–6.13), severe/critical disease (OR = 3.91, 95% CI: 2.70–5.67), ICU admission (OR = 3.10, 95% CI: 1.85–5.17), and mechanical ventilation (OR = 4.86, 95% CI: 1.27–18.65) than non-cancer patients. Furthermore, cancer patients had significantly lower platelet levels and higher D-dimer levels, C-reactive protein levels, and prothrombin time. In conclusion, these results indicate that cancer patients are at a higher risk of COVID-19 infection-related complications. Therefore, cancer patients need diligent preventive care measures and aggressive surveillance for earlier detection of COVID-19 infection.
Keywords: Cancer, COVID-19, Mechanical ventilation, Meta-analysis, Mortality
Introduction
Life has dramatically changed since the global pandemic of the coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Lockdowns, social distancing, and curfews have become the new norm. Currently, there are no approved therapies (exception being Tocilizumab in China, and Remdesivir in the United States and Japan) and many uncertainties exist. Patients with comorbidities, including cancer patients, seem to be at a higher risk of a complicated course resulting in higher mortality [2], [3], [4]. The number of cancer patients with COVID-19 in published literature so far is small, with significant heterogeneity in studies, such as cancer types, different comorbidities, and treatment phases. Moreover, only a few reports compared COVID-19 patients with cancer to COVID-19 patients without cancer. Despite the limited evidence, hematological and oncological societies/organizations have promptly responded to these reports and provided detailed guidance or recommendations on the management of COVID-19 patients with different cancers [5], [6]. To better assess the impact of COVID-19 infection on cancer patients, we performed a pooled analysis of the published data regarding cancer patients suffering from COVID-19 infection.
Methods
This systematic review and meta-analysis was performed in compliance with the MOOSE statement (Supplementary File 1) [7].
Literature search
We used the following search strategy [“COVID-19” OR “SARS-CoV-2” OR “2019-nCov” AND “Cancer” OR “Neoplasm” OR “Carcinoma” OR “Tumor” OR “Malignancy”] to retrieve relevant results from Medline (via PubMed), Scopus, and Web of Science databases on April 20, 2020 (Updated May 27, 2020). We applied no restrictions to our electronic search process. Furthermore, we manually searched for the references of relevant articles, reviews, and guideline statements of major oncological societies/organizations for any relevant articles potentially missed during the online search. We did not restrict this analysis to a specific type of cancer.
Screening and data extraction
After retrieving citation records, these were imported to EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) to remove duplicates. The citation library was then uploaded to the Covidence website (Covidence, Melbourne, VIC, Australia) to select the eligible studies. We employed the following eligibility criteria: original studies that described the number/percentage of cancer patients in confirmed COVID-19 cases. No restrictions were based on country of origin, gender, type of cancer, or treatment received. We excluded studies with unavailable raw data for extraction, studies with fewer than 10 patients, reviews/opinion pieces, and articles for which the full text could not be retrieved. We also exerted efforts to ensure no duplicated data for the same cluster of patients were included in our analysis (we kept the report with the largest sample size). Following these criteria, the screening was executed in two steps: examining the title/abstract of the retrieved citations, followed by examining the full text of potentially eligible articles.
Subsequently, relevant data were extracted in a pre-formatted excel sheet to collect the following:
-
1.
Baseline data, including the number of patients as per the study, age, gender, cancer site, received oncological treatment, and reported outcomes.
-
2.
Clinical/epidemiological outcome data: frequency of cancer diagnosis in COVID-19 cases, risk of mortality, severe/critical cases, intensive care unit (ICU) admission, and mechanical ventilation. Severe COVID-19 was defined as patients having an SpO2 ≤93% on room air at sea level, respiratory rate >30 breaths/minute, PaO2/FiO2 <300, or lung infiltrates >50%. Critical COVID-19 occurs in severe cases defined (in our study) as associated with acute respiratory distress syndrome, septic shock, or cardiac dysfunction [8].
-
3.
Biochemical/hematological test results (whenever available): counts of white neutrophils (×109/L), lymphocytes (×109/L), platelets (×109/L), and serum levels of D-dimer (mg/L), interleukin-6 (IL-6, pg/mL), C-reactive protein (CRP, mg/L), procalcitonin (ng/mL), and prothrombin time (seconds).
When the data were reported as median (range/interquartile range), they were converted to mean/standard deviation using the conversion equations provided by Hozo et al. [9] (only for studies with relatively large sample size). Whenever possible, the results of hematological and biochemical tests in cancer patients with COVID-19 were compared to non-cancer patients (platelets count, D-dimer, CRP, and prothrombin time). Otherwise, we performed an exploratory single-arm analysis to determine changes in lymphocytes, neutrophils count, and IL-6.
Data analysis
We employed two sets of analyses in the current review: single- and double-arm meta-analysis. The single-arm analysis was performed using OpenMeta[Analyst] software (Center for Evidence-Based Medicine, Brown University, Rhode Island, USA), and the double-arm analysis was performed using RevMan software version 5.3 (Cochrane Collaboration Nordic Enter, Oxford, UK). Categorical outcomes were summarized as odds ratios (ORs, 95% confidence interval [CI]) under the Mantel–Haenszel method, whereas continuous outcomes were summarized as mean differences (MD, 95% CI) under the inverse variance method. Heterogeneity was assessed using the Cochrane Q (interpreted that a p value < .1 indicates significant heterogeneity) and I2 tests to quantify the extent of heterogeneity [10]. When significant heterogeneity was confirmed, the analysis was conducted under the random effects model using the DerSimonian and Laird method. Finally, to identify the source of heterogeneity, we conducted a sensitivity analysis using the leave-one-out method.
Results
Literature search
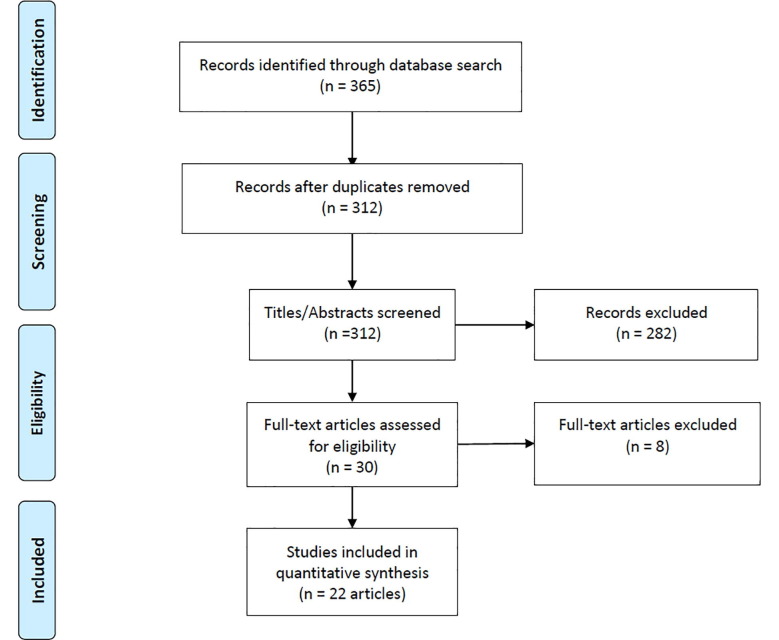
The initial database search retrieved 312 records, which was subsequently reduced to 30 after title and abstract screening. Further scrutiny led to 22 studies (1108 cancer patients vs. 10,135 non-cancer patients, all patients with confirmed COVID-19) that were included in our quantitative analysis; all these studies included patients with different types of cancers [2], [3], [4], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. The details of the literature search and study selection are summarized in Fig. 1 .
Fig. 1.
PRISMA flow diagram of literature search and study selection. Note. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
Baseline characteristics
Most studies reported only the number of cancer patients among their overall COVID-19 cohort, whereas 11 studies presented detailed characteristics of their cancer patients. The patient characteristics in these 11 studies are illustrated in Table 1 . To summarize, the number of cancer patients in these reports ranged from 12 to 334 (8 of these studies were from China). Lung cancer was the most prevalent, followed by hematological, colorectal, and breast cancers. Patients were at various stages of receiving oncological treatments.
Table 1.
Characteristics of cancer patients in the included studies with details of our target population.
| Study | Country | N | Age (y) | Gender (Male) | Cancer site | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| Liang et al. [14] | China | 18 | 63.1 ± 12.1 | 12 (66.6) | 5 lung, 3 breast, 2 bladder, 4 colon/colorectal, other cancers | Most patients were in the postoperative period of tumor resection, while few were still undergoing chemo/targeted therapy | Severe events occurred in 9/18 patients |
| He et al. [4] | China | 13 | 35 (23–53) | 7.00 | All hematological malignancy (M/C acute lymphoblastic AND myeloid leukemias) | 6 patients received chemotherapy, 3 received allotransplant, among other modalities | 4 moderate, 4 severe, and 5 critical |
| Martín-Moro et al. [17] | Spain | 34 | 72.5 (35–94) | 19 (55.9) | 7 acute leukemia, 1 plasma cell dyscrasia, 6 CLL, 5 NHL, among others (most patients were in remission) | 16 patients were receiving treatment at the time of COVID diagnosis and 3 patients received most recent TTT 6 months before COVID diagnosis | 17 patients developed moderate/severe ARDS, 4 mechanical ventilation, and 2 ICU admission |
| Miyashita et al. [3] | USA | 334 | Most patients (50–80) | NA | 57, 56, 23, 18, and 16 patients with breast, prostate, lung, urothelial, and colon cancer, respectively | NA | Cancer patients had higher risk of intubation than non-cancer patients, while the death rate was similar |
| Zhang et al. [27] | China | 28 | 65 (56–70) | 17 (60.7) | Most common were lung (7), esophagus (4), and breast (3) | Most patients underwent operations 21 (75%) or chemo/radiotherapy 25 (89.3%) | 8 patients died, 8 developed ARDS, 6 ICU admission, and 15 had severe events |
| Mehta et al. [18] | USA | 218 | 76 (10–92) | 117.00 | 54 hematologic malignancies and 164 solid malignancies; metastasis (42) and active cancer (92) | Most patients were on active chemotherapy/radiotherapy and few were on targeted immunotherapy | 61 patients died, 23 ICU admission, and 45 mechanical ventilation |
| Yang et al. [25] | China | 52 | 63 (34–98) | 28.00 | Lung cancer (19.2%), breast cancer (17.3%), rectal cancer (15.4%), colon cancer (9.6%), cervical cancer (7.7%), and thyroid carcinoma (5.8%) | 33 mild cases and 19 severe/critical cases (11 died) | |
| Guan et al. [2] | China | 18 | 63.1 ± 12.1 | 11 (61.1) | NA | NA | 9 severe cases, 3 died, 5 ICU admissions, 2 mechanical ventilation |
| Yu et al. [26] | China | 12 | Most patients > 60 | 10.00 | 7 lung cancer, 3 gastrointestinal cancers, 1 breast cancer, and 1 urothelial cancer | Most patients were receiving radiochemotherapy; three patients were receiving best supportive care only | 3 patients died and 6 were discharged alive |
| Ma et al. [16] | China | 37 | 62 (59–70) | 20 (54.1) | 11 colorectal cancer, 8 lung cancer, 7 breast cancer, and 5 gynecological cancer | 13 had received antitumor therapy within 1 month of diagnosis | 20 severe/critical cases, of which 5 died |
| Dai et al. [12] | China | 105 | 64 (IQR: 14) | 57 (54.72) | 22 lung cancer , 13 gastrointestinal cancer, 11 breast cancer, 11 thyroid cancer, and 9 hematologic cancer | 12 died, 20 ICU admission, and 10 mechanical ventilation |
Note. ARDS = acute respiratory distress syndrome; CLL = chronic lymphocytic leukemia; ICU = intensive care unit; IQR = interquartile range; NA = not available; NHL = non-Hodgkin lymphoma.
Clinical outcomes
Frequency of cancer in patients with COVID-19
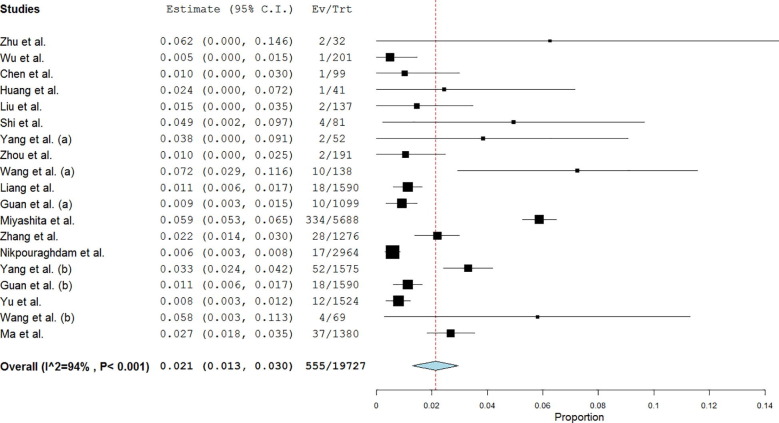
Single-arm analysis of 19 studies showed that the proportion of cancer among confirmed COVID-19 cases was 2.1% (95% CI: 1.3–3; Fig. 2 ). Leave-one-out analysis showed risk estimates that ranged between 1.6% and 2.3% upon removal of individual studies (Supplementary File 2).
Fig. 2.
Forest plot showing single-arm analysis of cancer frequency among patients with COVID-19. Note. COVID-19 = coronavirus disease 2019.
Mortality
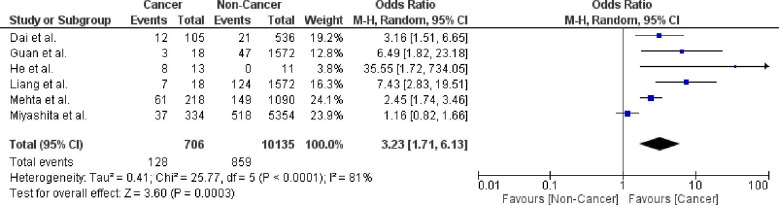
The risk of mortality in cancer patients with COVID-19 infection is 21.1% (95% CI: 14.7–27.6). Leave-one-out analysis showed risk estimates that ranged between 19.1% and 22.8% upon removal of individual studies. Comparative analysis using six studies showed that cancer patients had a significantly higher risk of mortality (OR = 3.23; 95% CI: 1.71–6.13; p = .0003; n = 10,841) than non-cancer patients (Fig. 3 ). Pooled studies were heterogeneous (p < .0001). Heterogeneity was resolved by excluding the study by Miyashita et al. [3] while maintaining the significance of the effect estimate (OR = 4.11; 95% CI: 2.28–7.41; p < .00001).
Fig. 3.
Forest plot showing double-arm analysis comparing the risk of mortality between cancer and non-cancer groups. Note. OR = odds ratio; 95% CI = confidence interval.
Severe/critical COVID-19 disease
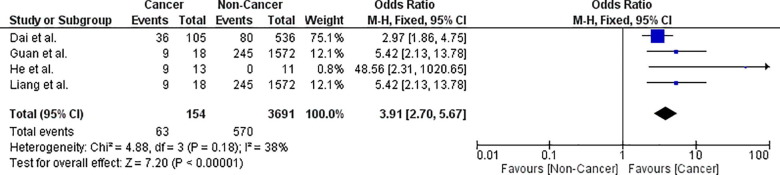
The risk of severe/critical disease in cancer patients with COVID-19 infection is 45.4% (95% CI: 37.4–53.3). Leave-one-out analysis showed risk estimates that ranged between 43.1% and 47.9% upon removal of individual studies. Comparative analysis using four studies showed that cancer patients had a significantly higher risk of severe/critical disease (OR = 3.91; 95% CI: 2.70–5.67; p < .00001; n = 3845) than non-cancer patients (Fig. 4 ). Pooled studies were homogenous (p = .18).
Fig. 4.
Forest plot showing double-arm analysis comparing the risk of severe/critical COVID-19 disease between cancer and non-cancer groups. Note. OR = odds ratio; 95% CI = confidence interval.
ICU admission
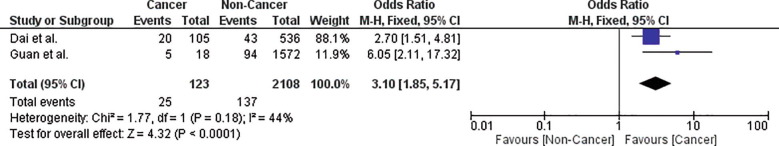
The risk of ICU admission in cancer patients with COVID-19 infection is 14.5% (95% CI: 8.5–20.4). Leave-one-out analysis showed risk estimates that ranged between 13.1% and 16.7% upon removal of individual studies. Comparative analysis using two studies showed that cancer patients had a significantly higher risk of ICU admission (OR = 3.10; 95% CI: 1.85–5.17; p < .0001; n = 2231) than non-cancer patients (Fig. 5 ). Pooled studies were homogenous (p = .18).
Fig. 5.
Forest plot showing double-arm analysis comparing the risk of ICU admission between cancer and non-cancer groups. Note. OR = odds ratio; 95% CI = confidence interval.
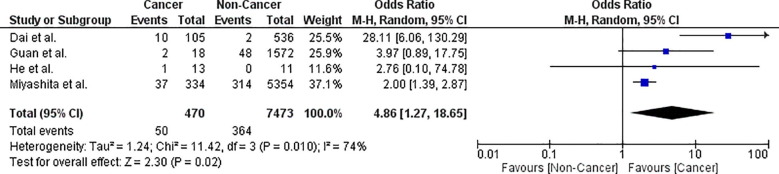
Mechanical (invasive) ventilation
The risk of requiring mechanical ventilation in cancer patients with COVID-19 infection is 11.7% (95% CI: 5.5–18). Leave-one-out analysis showed risk estimates that ranged between 19.1% and 22.8% upon removal of individual studies. Comparative analysis using four studies showed that cancer patients had a significantly higher risk of requiring mechanical ventilation (OR = 4.86; 95% CI: 1.27–18.65; p = .02; n = 7943) than non-cancer patients ( Fig. 6 ). Pooled studies were heterogeneous (p = .01). Heterogeneity was resolved by excluding either the studies by Miyashita et al. [3] or Dai et al. [12] while maintaining the significance of the effect estimate.
Fig. 6.
Forest plot showing double-arm analysis comparing the risk of mechanical ventilation between cancer and non-cancer groups. Note. OR = odds ratio; 95% CI = confidence interval.
Biochemical/hematological results
Blood cell counts
In cancer patients with confirmed COVID-19 diagnosis, the mean counts of lymphocytes and neutrophils (×109/L) were 9.4 (95% CI: −0.48 to 19.2; n = 136 patients/4 studies) and 20.7 (95% CI: −6 to 47.5; n = 136 patients/4 studies), respectively. The study by He et al. [4] (24 patients) showed that cancer patients had significantly lower platelet counts than non-cancer patients (Table 2 ).
Table 2.
Biochemical outcomes in cancer versus non-cancer groups infected with COVID-19.
| Biochemical outcome | n | Effect estimate (IV, fixed) |
|---|---|---|
| Platelets (×109/L) | 24 | −186.66 (−222.62, −150.70) |
| D-Dimer (mg/L) | 24 | 0.37 (0.06, 0.68) |
| C-reactive protein (mg/L) | 24 | 73.44 (37.61, 109.27) |
| Procalcitonin (ng/mL) | 24 | 1.82 (0.50, 3.15) |
| Prothrombin time (s) | 24 | 3.00 (1.72, 4.28) |
Note. Data presented are the mean difference, 95% confidence interval of the comparison between cancer and non-cancer patients. Analysis was conducted under the fixed effect model, inverse variance method.
Other biochemical tests
Cancer patients had significantly higher serum D-dimer (MD = 0.37; 95% CI: 0.06–0.68; p = .02), CRP (MD = 73.44; 95% CI: 37.61–109.27; p < .0001), procalcitonin (MD = 1.82; 95% CI: 0.50–3.15; p = .007) levels, as well as prothrombin time (MD = 3; 95% CI: 1.72–4.82; p < .00001) than non-cancer patients (Table 2). Moreover, single-arm meta-analysis showed that cancer patients had high serum IL-6 levels (18.86 pg/mL; 95% CI: 3.43–34.3) compared with reference values (5–15 pg/mL).
Discussion
In the current systematic review and meta-analysis, we identified 22 studies that evaluated the prevalence of cancer among patients infected with COVID-19 and COVID-19 complications in this subgroup of patients. Our analysis shows that cancer patients are at a higher risk of severe/critical COVID-19 disease, mortality, ICU admission, and mechanical ventilation. We performed an exploratory analysis to understand the differences between cancer and non-cancer patients in terms of inflammatory (CRP) and coagulopathy (D-dimer, prothrombin time) markers, which are increasingly recognized as mechanisms of COVID-19-induced organ injury. We found that CRP, D-dimer, and prothrombin time were significantly higher in cancer patients than in non-cancer patients, and single-arm analysis showed high serum IL-6 levels in cancer patients compared with reference values.
The meta-analysis by Desai et al. [30] showed a similar prevalence of cancer among patients with COVID-19 (pooled prevalence 2%; 95% CI: 2–3). However, the meta-analysis only assessed the prevalence of cancer among patients with COVID-19 but did not evaluate the outcomes of these patients. A report from New York showed that cancer patients may not be at an increased risk of a severe course from COVID-19; however, the number of patients was small and the majority were young patients [3]. Our findings showed that cancer patients are at an increased risk of a complicated course. However, drawing robust conclusions is difficult for many reasons, including but not limited to the small number of cancer patients, the absence of other cancer-related information like staging, grading, phase of therapy, comorbidities, type of cancer therapy, type of treatment provided for COVID-19 infection, and the capacity of the healthcare system caring for these patients, as many of these factors will have an impact on the outcomes of patients. This issue of confounders is highlighted by another related publication by Wang and Zhang [31].
Several reports recently indicated that D-dimer levels correlate with COVID-19 severity (especially at levels >2.0 µg/mL) and that it can potentially be used to predict the outcomes from COVID-19 [32], [33], [34]. A recent meta-analysis showed that early and serial measurements of serum procalcitonin could predict the evolution of COVID-19 disease [35]. Another study showed that serum CRP and interleukin-6 levels correlate with disease severity and can predict outcomes in patients with COVID-19 [36]. Our findings regarding the poor prognosis of cancer patients can be further interpreted in light of the altered serum levels of the aforementioned biochemical parameters.
In addition to the higher severity in cancer patients, COVID-19 infection may affect this subgroup of patients in many other ways, such as diagnosis and therapy delays, limited transfusion support, and saturation of the healthcare system and supportive services [5], [37].
Limitations
Our meta-analysis had several limitations. All the included studies were retrospective in nature, the number of cancer patients was small, and many important data were not reported in these studies (cancer types, stages, and treatments).
Recommendations
Various cancer organizations worldwide have released guidelines to care for cancer patients suffering from COVID-19. Given the findings from our meta-analysis in the absence of prospective data, we recommend diligent preventive care measures, full supportive care for immunosuppressed patients to minimize the risk of infection, limiting patient visits to the hospital when possible, and using telecommunication technology. Future studies should focus on collecting all the baseline characteristics of cancer patients suffering from COVID-19, all cancer- and chemotherapy/radiation-related variables, the detailed COVID-19 care protocol followed in these patients, and the dynamic biochemical and inflammatory profile of these patients during the infection.
Authors’ contributions
G.M.E. wrote the initial draft; S.H. and R.E.F. thoroughly revised the initial draft; all other coauthors contributed to subsequent revisions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hemonc.2020.07.005.
Contributor Information
Ghada M. ElGohary, Email: ghelgohary@gmail.com.
Riad El Fakih, Email: riadfakih@homtail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020 doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W., Chen L., Chen L., Yuan G., Fang Y., Chen W. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/; 2020 [accessed May 25, 2020]. [PubMed]
- 9.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P., Thomas J. 2nd ed. John Wiley & Sons; 2019. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 11.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín-Moro F., Marquet J., Piris M., Michael B.M., Sáez A.J., Corona M. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190:e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikpouraghdam M., Farahani A.J., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019, in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 25.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J., Ouyang W., Chua M.L., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W., Xie K., Lu H., Xu L., Zhou S., Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. 2020 doi: 10.1002/jmv.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai A., Sachdeva S., Parekh T., Desai R. COVID-19 and Cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21 doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y., Li T., Han M., Li X., Wu D., Xu Y. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. 201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavillet M., Klappert J.C., Spertini O., Blum S. Acute leukemia in the time of COVID-19. Leuk Res. 2020;92 doi: 10.1016/j.leukres.2020.106353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.