Abstract
The complex pathophysiology of treatment-resistant schizophrenia (TRS) includes severe positive symptoms but also other symptom domains. The overlapping psychological profiles of schizophrenia and autistic spectrum disorder (ASD) are not established. We compared TRS patients (n = 30) with schizophrenia patients in remission (RemSZ, n = 28) and ASD patients (n = 28), focusing on both neurodevelopmental aspects and general and social cognitive impairments. The TRS group performed the worst on general neurocognition (measured by the MATRICS Consensus Cognitive Battery) and social cognition (measured by the theory of mind and emotional expression). The RemSZ group performed the best among the three groups. Regarding autistic traits, all measurements by the Autism-Spectrum Quotient/Autism Screening Questionnaire/Pervasive Developmental Disorder Assessment Rating Scale showed that (1) the ASD patients had the highest autistic traits (2) the TRS patients' scores were less severe than the ASD group's, but (3) the overall trends placed the TRS group between the ASD and the RemSZ group. These findings indicate that TRS patients and remitted patients could have distinctive neurodevelopmental and cognitive profiles. Further, the degrees of social cognitive dysfunction and autistic traits in TRS patients could be close to those of ASD patients, suggesting similarities between TRS and ASD.
Keywords: Autistic spectrum disorder, Remission, Social cognition, Theory of mind
1. Introduction
Antipsychotics are highly effective for positive symptoms in patients with schizophrenia (Snyder, 1981), but up to 30% of schizophrenia patients never show a significant response to the appropriate pharmacotherapy with antipsychotics; this subset of patients is said to have treatment-resistant schizophrenia (TRS) (Elkis, 2007; Kane et al., 1988). Clozapine (CLZ) is the only agent with efficacy established for these refractory patients. However, approx. 40%–70% of TRS patients do not respond sufficiently to medication even with CLZ (Lehman et al., 2004; Mouaffak et al., 2006).
Although the action mechanism of CLZ has not yet been clarified, several biological studies suggest that dopamine synthesized in presynaptic dopaminergic neurons of patients with TRS could be attenuated, in contrast to the increased dopamine synthesis seen in patients responding well to antipsychotics (Demjaha et al., 2012; Kim et al., 2017). Other glutamatergic and GABAnergic neurotransmitter systems have also been suggested to be involved in the pathology of patients with TRS and/or CLZ-resistance (Demjaha et al., 2014; Iwata et al., 2019; O'Connor and O'Shea, 2015). Better treatment options for individuals with TRS could be developed when precise etiology of TRS becomes clear.
Cognitive impairment and social cognitive impairment have been a recent focus in the schizophrenia clinical and research fields, and compared to an individual's positive symptoms, these two domains have more fundamental impacts on the individual's long-term prognosis (Green, 2016; Javed and Charles, 2018; Pinkham, 2014). It has been reported that patients with TRS have more severe neurocognitive and social cognitive dysfunctions compared to non-TRS patients (Joober et al., 2002; de Bartolomeis et al., 2013; Frydecka et al., 2016). These neurocognitive and social cognitive impairments might also be related to severe positive symptoms (particularly in TRS patients), as evidence of relationships between cognitive functions and positive symptoms in schizophrenia has been described, and because these two domains may have a shared genetic basis (Eack et al., 2010; Fett et al., 2013).
Neurocognitive and social cognitive impairments are also recognized as the core etiology of autism spectrum disorder (ASD) (Baron-Cohen et al., 1985; Lai et al., 2014). Bleuler (1950) described ASD as a child-onset form of schizophrenia, and prior to 1980, schizophrenia and ASD were categorized as the same disorder. It was not until the publication of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III in 1980 that schizophrenia and ASD were differentiated from the viewpoint of their different developmental trajectories (Kolvin, 1971).
Several studies have demonstrated commonalities between patients with schizophrenia and those with ASD, particularly in terms of the overlapping existence of autistic traits (Kincaid et al., 2017) and cognitive/social cognitive impairments (Sasson et al., 2011; Sugranyes et al., 2011). Other groups have observed that some children with ASD later transition to schizophrenia (Petty et al., 1984; Selten et al., 2015). Taken together, these findings have again raised questions about the essential difference between schizophrenia and ASD (Chisholm et al., 2015; King and Lord, 2011). In addition to commonalities in terms of symptomatology and cognitive dysfunctions, recent neuroimaging and genetic studies have detected certain similarities between the two disorders. Similar structural and functional alterations in the lateral and medical prefrontal regions, the temporal region and the amygdala have been identified (Baribeau and Anagnostou, 2013; Sugranyes et al., 2011). Additionally, several genetic studies using next-generation sequencing techniques have identified similar genetic profiles in these disorders (Khanzada et al., 2017).
Most patients with TRS may have a psychopathology that is closer to that of ASD patients than to that of non-TRS patients. This hypothesis is strongly supported by clinical evidences: some ASD patients present autistic symptoms that are quite similar to the negative symptoms observed in schizophrenia patients with persistent negative symptoms (Frith and Happé, 2005; Konstantareas and Hewitt, 2001). Earlier onset of disease such as that seen in childhood-onset schizophrenia could contribute to refractoriness, implying that any psychiatric abnormalities in TRS and ASD subjects would be noticed earlier than those in general schizophrenia patients (Asarnow et al., 2004; Short and Schopler, 1988). Taken together, these observations suggest that TRS patients have a complex psychopathology (Quintero et al., 2011).
In the present study, we hypothesized that TRS patients have more autistic traits and neurocognitive/social cognitive dysfunction compared to patients with other types of schizophrenia (i.e., non-TRS). To test this hypothesis, we evaluated whether autistic traits and social/neurocognitive functions were similar between TRS and ASD patients.
2. Patients and methods
2.1. Subjects
A total of 86 patients in three groups participated: 28 adults with ASD, 28 adults with schizophrenia in remission (RemSZ), and 30 adults with TRS. All patients were recruited from July 2015 to July 2017 at Chiba University Hospital. All but one of the 86 patients were outpatients. The diagnoses of each disorder were based on DSM-5, and each patient's diagnosis was decided on a consensus basis between the patient's physician and one of the study's authors (Y.N.).
The inclusion criteria were (1) age 20–60 years; (2) meeting the DSM-5 criteria for ASD or schizophrenia; (3) no comorbidity of intelligence disability (DSM-5); (4) no history of serious head trauma or substance abuse; (5) Japanese ethnicity. All of the schizophrenia patients and nine ASD patients were taking prescribed antipsychotics. The criteria of the RemSZ and TRS groups are described in Table 1.
Table 1.
Participant demographic and clinical characteristics in the three groups
| Remission-SZ group (n = 28) |
TRS group (n = 30) |
ASD group (n = 28) |
Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ANOVA (F-value) |
ANOVA (p-value) |
Post-hoc (p-value) |
Rem vs TRSa (p-value) |
||||||
| Rem vs. TRS | Rem vs. ASD | TRS vs. ASD | |||||||
| Age (y) Range |
39.46 [8.46] 20–56 |
43.83 [10.39] 20–60 |
30.03[5.97] 20–45 |
19.67 | <0.001 | N.S. | <0.001 | <0.001 | – |
| Males/females: n | 15/13 | 16/14 | 23/5 | – | – | – | – | – | – |
| Japanese (%) | 100.00 | 100.00 | 100.00 | – | – | – | – | – | – |
| Illness duration (y) | 15.04 [7.66] | 22.70 [10.02] | – | – | – | – | – | – | 0.004 |
| Age at onset (y) | 24.43 [7.37] | 21.13 [6.02] | – | – | – | – | – | – | 0.091 |
| Estimate premorbid FIQ | 101.96 [9.59] | 97.97 [11.83] | 108.93 [8.92] | 8.46 | <0.001 | N.S. | 0.034 | <0.001 | – |
| PANSS-Total | 58.46 [7.05] | 83.33 [9.40] | – | – | – | – | – | – | <0.001 |
| PANSS-Positive | 13.14 [2.80] | 19.13 [3.13] | – | – | – | – | – | – | <0.001 |
| PANSS-Negative | 15.68 [2.54] | 24.13 [3.82] | – | – | – | – | – | – | <0.001 |
| APs dose (CP-eq.:mg) | 365.40 [211.87] | 778.75 [311.06] | 49.11 [84.30] | 76.70 | <0.001 | <0.001 | <0.001 | <0.001 | – |
Data are mean [SD].
Abbreviations: ANOVA: analysis of variance, ASD: autism spectrum disorder, APs: antipsychotics, CP-eq.: chlorpromazine-equivalent, N.S.: not significant, PANSS: Positive and Negative Syndrome Scale, SZ: schizophrenia, TRS: treatment-resistant schizophrenia, y: years.
Mann-Whitney U test.
The criteria for the RemSZ and TRS groups were as follows. We followed the Andreasen criteria (Andreasen et al., 2005) to define remission of schizophrenia. Patient scores for each of the 8 domains included in the PANSS (P1, delusion; P2, conceptual disorganization; P3, hallucinatory behavior; N1, blunted affect; N4, passive/apathetic and social withdrawal; N6, lack of spontaneity; G5, mannerisms and posturing; G9, unusual thought content), which was composed of a total 30 items, each with a point range of 1–7 (Kay et al., 1987), were <3 points in the 6 months preceding the study, and patients had not been hospitalized during the same period of time. For the diagnosis of TRS, we followed the Clozaril Patient Monitoring Service (CPMS) criteria. Briefly, the patient was deemed to have TRS if two different chemical classes of antipsychotic were unable to sufficiently relieve his/her positive symptoms with sufficient dosage (>chlorpromazine equivalent (CP-eq.) dose of 600 mg/day) for >4 weeks, and the patient did not exceed a mean GAF score of 41 within 1 year (the non-responder criteria). All 30 TRS patients met the non-responder criteria, and no patients met the intolerance criteria.
The study protocol was approved by the Ethics Committee of the Graduate School of Medicine at Chiba University and was conducted in accord with the Helsinki Declaration. Oral and written informed consent to participate in the study was obtained from all patients.
2.2. Neuropsychological comparisons of the TRS, RemSZ, and ASD groups
To assess autistic traits in the TRS, RemSZ and ASD groups, we used the Autism-Spectrum Quotient (AQ) Japanese version (range, 0–50; cut-off, <30) (Wakabayashi et al., 2004), the Autism Screening Questionnaire (ASQ) Japanese version (range, 0–39; cut-off, <13) (Dairoku et al., 2016), and the Pervasive Developmental Disorder Assessment Rating Scales Text Revision (PARS-TR) (infancy: range, 0–68; cut-off, <9; present: range, 0–66; cut-off, <20) (Adachi et al., 2008). The AQ is a self-rating scale, and the ASQ is completed by the patient's primary care-giver (in this study, the patient's mother or father). The PARS-TR is a semi-structured interview conducted with a parent or family member of the patient. We evaluated each patient's estimated premorbid IQ with the Japanese Adult Rating Scale-50 (JART-50) (Matsuoka et al., 2006).
To assess the patients' neurocognitive function, we used the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) Japanese version (Kaneda et al., 2013). The MCCB has been shown to be valid for comparing neurocognition between patients with ASD and those with schizophrenia (Kuo et al., 2019).
We assessed the patients' emotional perception by using the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) (Mayer et al., 2003) which is included in the MCCB, and we determined the patients' theory of mind (ToM) by using five false-belief (FB) tasks. This evaluation was performed using the animation CD “Theory of mind tasks ver. 2” (DIK Educational Publications, Saitama, Japan). The FB task set consisted of a total of 5 tasks: the Sally-Anne's task, the Smarties task, the Strange stories test, the Sabotage/Deception task, and the John and Mary task. Each task was scored 0 for wrong and 1 for right, so that total score ranged from 0 to 5 points.
2.3. Statistical analysis
We used SPSS ver. 23.0 software (IBM, New York, NY) for the statistical analyses of the demographic, neurodevelopmental, and cognitive data. We applied Student's t-test or the Mann-Whitney U test, checking homoscedasticity and normality to analyze differences between pairs of groups. The Shapiro-Wilk test was used to confirm the normality of the data. To analyze the differences in the variables of demographic features, treatment-related factors, and the measurements of neurocognitive/social cognitive domains among the three patient groups, we performed an analysis of variance (ANOVA). Post-hoc tests were performed with Tukey's test for values with equal distribution, and with the Games-Howell test for values with non-equal distribution. The significance threshold was set at p = 0.05 (both tails) for all comparisons.
3. Results
The ASD patients were significantly younger than both the RemSZ patients and TRS patients (Table 1). The three groups were also significantly different in terms of estimated IQ and CP-eq. dose: the estimated IQ was higher in the ASD group than either of the schizophrenia groups, and the antipsychotic dosages were highest in the TRS group, followed in order by the RemSZ group and ASD group. As expected, all psychopathological measures determined by the PANSS were significantly higher in the TRS group than the RemSZ group. The most major frequently prescribed antipsychotics in the schizophrenia patients were aripiprazole (oral, n = 13; aripiprazole once-monthly injectable, n = 1) in the RemSZ group, and CLZ (n = 11) in the TRS group.
3.1. Neuropsychological comparisons of the TRS, RemSZ, and ASD patients
3.1.1. Autistic traits
Autistic traits as represented by the AQ, ASQ, and PARS results showed significant differences among the three patient groups (Table 2). The post hoc analysis revealed that all of the autistic traits were highest in the ASD group with the exception of the ASQ scores, which were similar in the ASD and TRS groups. Regarding the differences between the TRS and RemSZ groups, although only the PARS TR-present scores of the TRS group were significantly higher than those of the RemSZ group, other parameters demonstrated similar trends of difference with the TRS group demonstrating higher degrees of autistic traits compared to the RemSZ group. These results overall indicated that the ASD group was highest in autistic traits, followed by the TRS group and the RemSZ group in descending order.
Table 2.
Neurocognitive and social cognitive performance and autistic traits in the three groups
| Remission-SZ group (n = 28) |
TRS group (n = 30) |
ASD group (n = 28) |
Analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| ANOVA (F-value) |
ANOVA (p-value) |
Post-hoc (p-value) |
||||||
| Rem vs. TRS | Rem vs. ASD | TRS vs. ASD | ||||||
| Autistic traits: | ||||||||
| AQ [range 0–50: cutoff < 33] | 24.32 [7.19] | 27.67 [7.75] | 33.29[6.17] | 11.47 | <0.001 | 0.177 | <0.001 | 0.009 |
| ASQa [range 0–34: cutoff < 13] | 6.83 [4.64] | 9.09 [5.92] | 11.15 [5.57] | 3.90 | 0.025 | 0.338 | 0.018 | 0.381 |
| PARS TR-infancya [range 0–68: cutoff < 9] | 4.10 [3.23] | 6.23 [4.07] | 13.70 [5.72] | 27.33 | <0.001 | 0.287 | <0.001 | <0.001 |
| PARS TR-presenta [range 0–66: cutoff < 20] | 9.25 [5.66] | 15.86 [6.32] | 20.87 [7.01] | 17.33 | <0.001 | 0.004 | <0.001 | 0.029 |
| MCCB (T score): | ||||||||
| Speed of process | 42.00 [8.63] | 28.53 [13.26] | 46.14[13.70] | 16.89 | <0.001 | <0.001 | 0.411 | <0.001 |
| Attention/vigilance | 47.43 [11.49] | 35.67 [10.97] | 51.46 [9.07] | 17.61 | <0.001 | <0.001 | 0.331 | <0.001 |
| Working memory | 42.86 [10.66] | 30.90 [14.45] | 44.32 [11.27] | 10.50 | <0.001 | 0.001 | 0.896 | <0.001 |
| Verbal learning | 35.50 [8.50] | 30.70 [5.92] | 46.61 [10.48] | 26.74 | <0.001 | 0.043 | 0.001 | <0.001 |
| Visual learning | 49.04 [8.57] | 39.07 [14.26] | 50.39 [7.25] | 10.00 | <0.001 | 0.006 | 0.799 | <0.001 |
| Problem solving | 53.29 [11.64] | 43.77 [10.60] | 53.11 [9.93] | 7.53 | 0.001 | 0.003 | 0.998 | 0.004 |
| Social cognition (MSCEIT) | 36.07 [11.73] | 27.37 [12.85] | 37.11 [11.77] | 5.68 | 0.005 | 0.021 | 0.945 | 0.008 |
| False-belief tasks (5 tasks) [range 0–5] | 4.29 [0.94] | 3.50 [0.86] | 3.75 [1.08] | 5.02 | 0.009 | 0.007 | 0.098 | 0.584 |
Data are mean [SD].
ANOVA: analysis of variance, AQ: Autism-Spectrum Quotient, ASD: autism spectrum disorder, ASQ: Autism Screening Questionnaire, MCCB: MATRICS Consensus Cognitive Battery, Rem: remission schizophrenia, PARS TR: Pervasive Developmental Disorder Assessment Rating Scales Text Revision., SZ: schizophrenia, TRS: treatment-resistant schizophrenia.
PARS-TR (infancy and present data) and ASQ were available from 65 patients (RemSZ, n = 20; TRS, n = 22; ASD, n = 23) and 72 patients (RemSZ, n = 23; TRS, n = 23; ASD, n = 26), respectively.
3.1.2. Neurocognition
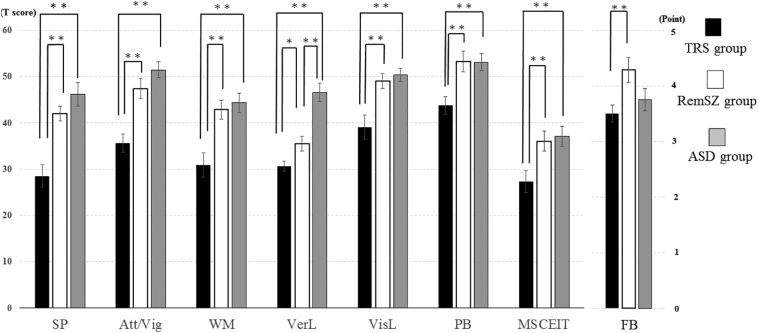
The ANOVA results indicated significant differences for all neurocognitive domains of the MCCB (Table 2, Fig. 1). The post hoc analysis revealed that the TRS group had lower scores compared to the RemSZ and ASD groups for all domains. In addition, post hoc comparisons showed that the RemSZ group had lower verbal learning scores compared to the ASD group. When age, estimated IQ, the CP-eq. dose, or all three factors were used as covariate(s), the results were the same. However, the following items alone did change the results: when IQ was the sole covariate, the significant differences in verbal learning between the RemSZ and TRS groups and in problem solving between the TRS and ASD groups disappeared.
Fig. 1.
Cognitive function results (by MCCB) and theory of mind (FB task) in the three groups.
*: p < 0.05, **: p < 0.001.
Abbreviations: Att/Vig: attention/vigilance, FB: false-believe task, MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test, PB: problem-solving, SP: speed of process, VerL: verbal learning, VisL: visual learning, WM: working memory.
Further, when we conducted additional analyses to compare the patients with CLZ treatment (n = 11) and the patients without CLZ (n = 19; by other antipsychotics), we observed that verbal learning and visual learning differed significantly between these groups, as follows. (Verbal learning, TRS patients with CLZ: 27.3 ± 6.75 points; TRS patients without CLZ: 32.7 ± 14.5 points; t = −2.65, p = 0.013; visual learning, TRS patients with CLZ: 32.4 ± 14.5 points; TRS patients without CLZ: 42.9 ± 13.0 points; t = −2.07, p = 0.048). However, for other neurocognitive measurements, there was no significant difference between the patients who were treated versus not treated with CLZ (p > 0.05).
3.1.3. Social cognition
The MSCEIT and FB tasks scores revealed significantly differences among the ASD, TRS, and RemSZ groups. For the MSCEIT scores, the post hoc comparisons indicated the following relationship: ASD = RemSZ > TRS. For the FB tasks, the relationship was as follows: RemSZ > ASD = TRS (Table 2, Fig. 1). The significance of the results was not almost changed when age, estimated IQ, the CP-eq. dose or all three factors were used for as covariate(s). When IQ was the sole covariate, the significant difference in MSCEIT between the TRS and ASD groups disappeared. Regarding FB tasks, the difference between the RemSZ and ASD groups reached statistical significance, as it also did when age was the sole covariate.
Additional comparisons between only the TRS patients with and without CLZ treatment did not show any significant differences on the MSCEIT and FB tasks.
4. Discussion
This is the first study to compare TRS patients and ASD patients in an attempt to clarify the etiology of schizophrenia and/or ASD by using established neurocognitive/social cognitive assessments. The results revealed that the TRS patients had slightly higher levels of autistic traits than the RemSZ group, but slightly lower levels of autistic traits than the ASD patients (Table 2). The TRS group had broadly severe non-social and social cognitive impairments compared to both the ASD and RemSZ groups (Table 2, Fig. 1). Overall, these results partly support our hypothesis since TRS patients were found to differ greatly in their neurocognitive and social cognitive impairments compared to remitted patients, and since it was found that TRS patients could have any developmental abnormalities prior to the onset of schizophrenia. However, we found no evidence that TRS patients showed prominent autistic traits similar to those of ASD patients since the autistic traits of ASD patients were much more severe than those of TRS patients.
The ASD group presented the highest scores for all autistic trait measures, and the RemSZ group showed the lowest scores for all measures. These results clearly indicated that patients reaching the remission state have fewer autistic traits, confirming that not all schizophrenia patients display autistic traits (Barlati et al., 2019). The scores in the present TRS group did not reach the cutoff points for any measures of autistic traits, but several measures indicated that a considerably wide range of autistic traits was present in this group (Table 2). These results are consistent with those of previous studies showing that patients with schizophrenia have a certain level of autistic traits (Bastiaansen et al., 2011; Matsuo et al., 2015; Naito et al., 2010). Our results showed that there was a significant level of difference only in the PARS TR-present scores between the two schizophrenia subgroups of TRS and RemSZ. However, the results measuring autistic traits overall showed that the TRS patients had more severe autistic traits compared to the RemSZ patients, since all scores of the TRS group were in the middle position between the ASD and RemSZ groups (Table 2). These subtle but clear differences between the TRS and RemSZ groups might be explained by the small size of our study population, but this finding also may reflect that the TRS patients did not have a high degree of autistic traits, unlike ASD patients.
Therefore, the refractory process TRS patients experience during their long-term disease course cannot be explained only by the subtle autistic traits observed in future TRS patients. However, some longitudinal studies suggested that future TRS patients could have a more difficult clinical course after their first episode of psychosis compared to that of non-TRS patients (Demjaha et al., 2017; Kanahara et al., 2018; Lally et al., 2016). It is uncertain how the subtle autistic traits identified in TRS patients could affect their severe psychotic symptoms as part of the TRS etiology. However, our findings suggest a need for greater focus on the early stages of the disorder, including prior to and following the onset of schizophrenia, in future TRS patients.
Compared to the RemSZ and ASD groups, the TRS group showed distinctively lower scores on all cognitive measures, and the TRS and RemSZ groups had lower verbal learning than the ASD group (Table 2, Fig. 1), suggesting global deficits in the TRS group (Schaefer et al., 2013). Our observation of greater neurocognitive deficits in TRS patients compared to non-TRS patients is consistent with previous studies (de Bartolomeis et al., 2018; Frydecka et al., 2016; Joober et al., 2002), with one exception (Anderson et al., 2015). In these previous studies, the poor neurocognitive impairments in TRS patients remained at a significant level after several clinical parameters were removed from the statistical analyses (e.g., severe psychopathology and high antipsychotics dose), suggesting that this neurocognitive dysfunction is part of the core pathology of TRS (Schaefer et al., 2013). Our findings are statistically robust and are consistent with results in the literature (de Bartolomeis et al., 2018; Frydecka et al., 2016; Joober et al., 2002). Both when these factors are dealt with together as covariates and when they are treated separately as individual covariates, the overall results are the same. This indicates that differences in neurocognitive and social cognitive impairments could be derived from the diagnosis and/or from disease severity, although several confounding factors (IQ, age or drug dose) might have an impact on some.
However, it has been demonstrated that antipsychotic agents, atypical antipsychotics in particular, could provide some beneficial effects on cognition in schizophrenia patients (Woodward et al., 2005). Therefore, CLZ, the major antipsychotic taken by our TRS patients, might have the ability to improve cognitive dysfunction in some domains to some degree (Woodward et al., 2005). Our comparison of the patients under CLZ treatment and those under other antipsychotic treatment showed significant differences in verbal learning and visual learning (i.e., worse in the patients treated with CLZ). For other neurocognitive domains, the patients treated with CLZ tended to present a poor cognitive performance, although this did not reach significance. These results may indicate that our TRS patients did not overcome their neurocognitive impairments even with the introduction of CLZ, and this possibility is consistent with some other reports showing lesser advantage in cognitive improvement with CLZ treatment (Czepielewski et al., 2018; Nielsen et al., 2015). This result might indicate that our TRS group had some patients with quite severe TRS, whose cognitive deficits would have been unlikely to respond to even CLZ.
Studies comparing neurocognition between patients with ASD and those with schizophrenia-generally, almost all of those studies included clinically stable patients-showed that these two patient groups were comparably impaired overall, although other studies reported inconsistent results in terms of specific domains (Eack et al., 2013; Marinopoulou et al., 2016; Øie et al., 2020). Our present finding that the remitted patients with a high degree of clinical stability had almost the same level of neurocognitive impairment as the ASD patents except for verbal learning, is in line with the previous studies (Eack et al., 2013; Marinopoulou et al., 2016; Øie et al., 2020). Furthermore, it seems that our remitted patients with slightly but significantly lower levels on several MCCB domains are similar to a schizophrenia group identified in a recent meta-analysis showing slightly more deficits in several specific cognitive domains (Kuo and Eack, 2020).
We evaluated social cognition by using the MSCEIT and FB tasks. On the MSCEIT, the TRS patients showed significantly lower mean scores than both the ASD and RemSZ patients, and there was no significant difference between the ASD and RemSZ groups. In the FB tasks, the TRS and ASD patients achieved significantly lower scores than the RemSZ patients. Although several studies have attempted to clarify which disorder is more severe in terms of social cognitive dysfunction, no definitive conclusion has been reached. Our present findings indicate that the TRS patients performed the worst on social cognition as well as general cognition. These results also suggested that the MSCEIT task scores showed greater deficits than the ToM scores for the RemSZ and TRS groups compared to the ASD group. Two recent meta-analyses on this issue suggest that the ASD group shows lower levels in emotional recognition and social perception than the schizophrenia group, although the two groups showed similar levels in mentalizing including ToM tasks and attributional style (Fernandes et al., 2018; Pinkham et al., 2019). Another meta-analysis demonstrated that, in schizophrenia patients, the degrees of impairment in affect recognition (measured by the MSCEIT) and ToM (measured by an FB task) had similar effect sizes (Savla et al., 2013). These results paint a quite puzzling picture of the relationship between the two disorders with respect to social cognition, since our study had two separate two schizophrenia subgroups (the remitted and TRS groups), which was different from the literatures performing meta-analysis (Fernandes et al., 2018; Pinkham et al., 2019); it should also be noted that both the reported meta-analyses and our study lacked normal healthy subjects. But our present results may support that finding in part, but patients in remission or treatment resistance may be affected differently by domains of social cognition. ToM ability is generally acquired in 4- to 6-year-olds, and affective recognition is acquired at a later age (Baron-Cohen, 2000). Thus, an individual's failure to sequentially acquire different social cognitive domains during childhood may lead to a greater negative influence of the disease onset in TRS patients.
It has been suggested that some alterations in the oxytocin system and/or GABA system could be involved in the neurocognitive and social cognitive impairments in ASD (Cataldo et al., 2018; Feldman et al., 2016). It is not yet known whether the cognitive/social cognitive dysfunction in the present study's TRS group had already appeared prior to the onset of full-brown psychosis. However, since a majority of patients with future TRS show severe psychopathology from the time point of their first-episode psychosis as mentioned above, our TRS patients could have exhibited some cognitive/social cognitive dysfunction prior to their disease onset.
Our study's limitations are as follows. First, a relatively small number of patients was examined, and we included remitted patients who are quite unlike TRS patients as a comparative group. Furthermore, although similar previous studies have used the PANSS to compare schizophrenia and ASD patients, we did not use this scale to assess our ASD group in the present study. These factors of the study design preclude generalized conclusions about our results. Future investigations should consider more general types of patients (i.e., non-TRS and non-remitted), and the interpretation of the present results might need be corrected to some degree. Third, we use screening tools such as the AQ and ASQ in the evaluation of autistic traits. Forth, although a wide range of neurocognitive domains was evaluated by the MCCB, for social cognition we used only FB tasks (theory of mind) and the MSCEIT (emotional perception) included in the MCCB. Due to this procedural limitation, our results did not determine whether TRS patients have a wide range of social cognitive impairments or have only impairments in theory of mind and emotional perception. Moreover, we cannot rule out the possibility that extremely poor neurocognitive function could affect the patients' poor social cognition.
Nevertheless, this is the first study to report a high degree of autistic traits in TRS patients, who are poor responders to antipsychotics and have serious psychotic symptoms from the initial phase of their clinical courses. Our findings emphasize the importance of discussing autistic traits in schizophrenia, and they indicate a new direction for further research.
CRediT authorship contribution statement
Yusuke Nakata: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Nobuhisa Kanahara: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Atsushi Kimura: Investigation.Tomihisa Niitsu: Investigation. Hideki Komatsu: Investigation. Yasunori Oda: Investigation. Masatomo Ishikawa: Investigation. Tadashi Hasegawa: Investigation.Yu Kamata: Investigation. Atsushi Yamauchi: Investigation. Kazuhiko Inazumi: Investigation. Hiroshi Kimura: Supervision. Masaomi Iyo: Conceptualization, Writing - original draft, Supervision.
Declaration of competing interest
N.K. reports honoraria from Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Janssen Pharmaceutical K.K., Meiji Seika Pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd. M.I. reports honoraria from Mochida Pharmaceutical Co., Ltd. M.I. received consultant fees from Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Japan Inc., Abbott Japan Co., Ltd. and Janssen Pharmaceutical K.K., and reports honoraria from Janssen Pharmaceutical K.K., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Takeda Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Eisai Co. Ltd., Daiichi-Sankyo Co. Ltd., Novartis Pharma K.K., Teijin Ltd., Shionogi & Co., Ltd., Hisamitsu Pharmaceutical Co., Inc. and Asahi Kasei Corporation. Y.N., A.K., T.N., H.K., Y.O., M.N., T.H., Y.K., A.Y., K.I., and H.K. have no conflict of interest to declare.
Acknowledgements
The present study was not supported by any profitable organization and was conducted with our internal funding.
The authors thank Dr. N. Yamanouchi, Dr. H. Sasaki and Dr. M. Shimagami for supporting subjects' recruitment.
Footnotes
Location of work: The Department of Psychiatry, Graduate School of Medicine, the Department of Psychiatry, Chiba University Hospital.
References
- Adachi J., Yukihiro R., Inoue M. Reliability and validity of short version of Pervasive Developmental Disorders Autism Society Japan Rating Scale (PARS); a behavior checklist for people with PDD. Seishin Igaku. 2008;50:431–438. (Article in Japanese) [Google Scholar]
- Anderson V.M., McIlwain M.E., Kydd R.R., Russell B.R. Does cognitive impairment in treatment-resistant and ultra-treatment-resistant schizophrenia differ from that in treatment responders? Psychiatry Res. 2015;230:811–818. doi: 10.1016/j.psychres.2015.10.036. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Carpenter W.T., Jr., Kane J.M., Lasser R.A., Marder S.R., Weinberger D.R. Remission in schizopherenia: proposed criteria and rationale for consensus. Am. J. Psychiatr. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Asarnow J.R., Thompson M.C., McGrath E.P. Annotation: childhood-onset schizophrenia: clinical and treatment issues. J. Child Psychol. Psychiatry. 2004;45:180–194. doi: 10.1111/j.1469-7610.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- Baribeau D.A., Anagnostou E. A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: a review of the literature. Frontier in Psychiatry. 2013;4:175. doi: 10.3389/fpsyt.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlati S., Deste G., Gregorelli M., Vita A. Autistic traits in a sample of adult patients with schizophrenia: prevalence and correlates. Psychol. Med. 2019;49:140–148. doi: 10.1017/S0033291718000600. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Is Asperger syndrome/high-functioning autism necessarily a disability? Dev. Psychopathol. 2000;12:489–500. [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bastiaansen J.A., Meffert H., Hein S. Diagnosing autism spectrum disorders in adults: the use of Autism Diagnostic Observation Schedule (ADOS) module 4. J. Autism Dev. Disord. 2011;41:1256–1266. doi: 10.1007/s10803-010-1157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin J., editor. International Universities Press; New York: 1950. translator. [Google Scholar]
- Cataldo I., Azhari A., Esposito G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Frontier in Molecular Neuroscience. 2018;11:27. doi: 10.3389/fnmol.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K., Lin A., Abu-Akel A., Wood S.J. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci. Biobehav. Rev. 2015;55:173–183. doi: 10.1016/j.neubiorev.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Czepielewski L.S., Londero M.D.B., de Sousa M.H. Long-term treatment with clozapine and verbal memory performance in schizophrenia. Schizophrenia Research Cognition. 2018;12:40–41. doi: 10.1016/j.scog.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairoku H., Senjyu A., Hayashi E., Toujyou Y., Ichikawa H. Kokuritu tokusyu kyouiku sougou kennkyuujyo bunnsitu ippan kennkyu houkokusyuo. vol. 3. 2016. Jiheisyou sukuriining situmonsi (ASQ) nihonngobann no kaihatu; pp. 19–34. (Article in Japanese) [Google Scholar]
- de Bartolomeis A., Balletta R., Giordano S., Buonaguro E.F., Latte G., Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res. 2013;210:387–395. doi: 10.1016/j.psychres.2013.06.042. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A., Prinzivalli E., Callovini P. Treatment resistant schizophrenia and neurological soft signs may converge on the same pathology: evidence from explanatory analysis on clinical, psychopathological and cognitive variables. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;81:356–366. doi: 10.1016/j.pnpbp.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Demjaha A., Murray R.M., McGuire P.K., Kapur S., Howes O.D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatr. 2012;169:1203–1210. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- Demjaha A., Egerton A., Murray R.M. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol. Psychiatry. 2014;75:e11–e13. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Demjaha A., Lappin J.M., Stahl D. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol. Med. 2017;47:1981–1989. doi: 10.1017/S0033291717000435. [DOI] [PubMed] [Google Scholar]
- Eack S.M., Mermon D.E., Montrose D.M. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr. Bull. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S.M., Bahorik A.L., McKnight S.A. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr. Res. 2013;148:24–28. doi: 10.1016/j.schres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkis H. Treatment-resistant schizophrenia. Psychiatr. Clin. N. Am. 2007;30:511–533. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Feldman R., Monakhov M., Pratt M., Ebstein R.P. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatry. 2016;79:174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Fernandes J.M., Cajão R., Lopes R., Jerónimo R., Barahona-Corrêa J.B. Social cognition in schizophrenia and autism spectrum disorders: a systematic review and meta-analysis of direct comparisons. Frontiers in Psychiatry. 2018;9:504. doi: 10.3389/fpsyt.2018.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A.K.J., Maat A., GROUP Investigators Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophr. Bull. 2013;39:77–85. doi: 10.1093/schbul/sbr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U., Happé F. Autism spectrum disorder. Curr. Biol. 2005;15:R786–R790. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Frydecka D., Beszłej J.A., Gościmski P., Kiejna A., Misiak B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res. 2016;235:133–138. doi: 10.1016/j.psychres.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Green M.F. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J. Clin. Psychiatry. 2016;77(Suppl. 2):8–11. doi: 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- Iwata Y., Nakajima S., Plitman E. Glutamatergic neurometabolite levels in patients with ultra-treatment-resistant schizophrenia: a cross-sectional 3T proton magnetic resonance spectroscopy study. Biol. Psychiatry. 2019;85:596–605. doi: 10.1016/j.biopsych.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Javed A., Charles A. The importance of social cognition in improving functional outcomes in schizophrenia. Frontier in Psychiatry. 2018;9:157. doi: 10.3389/fpsyt.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joober R., Rouleau G.A., Lal S. Neuropsychological impairments in neuroleptic-responder vs. -nonresponder schizophrenic patients and healthy volunteers. Schizophr. Res. 2002;53:229–238. doi: 10.1016/s0920-9964(01)00279-1. [DOI] [PubMed] [Google Scholar]
- Kanahara N., Yamanaka H., Suzuki T., Takase M., Iyo M. First-episode psychosis in treatment-resistant schizophrenia: a cross-sectional study of a long-term follow-up cohort. BMC Psychiatry. 2018;18:274. doi: 10.1186/s12888-018-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J., Honigfeld G., Singer J., Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Ohmori T., Okahisa Y. Measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery: validation of the Japanese version. Psychiatry and Clinical Neuroscience. 2013;67:182–188. doi: 10.1111/pcn.12029. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Khanzada N.S., Butler M.G., Manzardo A.M. Gene analytics pathway analysis and genetic overlap among autism spectrum disorder, bipolar disorder and schizophrenia. International Journal of Molecular Science. 2017;18:527. doi: 10.3390/ijms18030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Howes O.D., Veronese M. Presynaptic dopamine capacity in patients with treatment-resistant schizophrenia taking clozapine: an [(18)F]DOPA PET study. Neuropsychopharmacology. 2017;42:941–950. doi: 10.1038/npp.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid D.L., Doris M., Shannon C., Mulholland C. What is the prevalence of autism spectrum disorder and ASD traits in psychosis? A systematic review. Psychiatry Res. 2017;250:99–105. doi: 10.1016/j.psychres.2017.01.017. [DOI] [PubMed] [Google Scholar]
- King B.H., Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Kolvin I. Studies in the childhood psychoses. I. Diagnostic criteria and classification. Br. J. Psychiatry. 1971;118:381–384. doi: 10.1192/bjp.118.545.381. [DOI] [PubMed] [Google Scholar]
- Konstantareas M.M., Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J. Autism Dev. Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- Kuo S.S., Eack S.M. Meta-analysis of cognitive performance in neurodevelopmental disorders during adulthood: comparisons between autism spectrum disorder and schizophrenia on the Wechsler Adult Intelligence Scales. Frontiers in Psychiatry. 2020;11:187. doi: 10.3389/fpsyt.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S.S., Wojtalik J.A., Mesholam-Gately R.I., Keshavan M.S., Eack S.M. Transdiagnostic validity of the MATRICS Consensus Cognitive Battery across the autism-schizophrenia spectrum. Psychol. Med. 2019:1–10. doi: 10.1017/S0033291719001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Lally J., Ajnakina O., Di Forti M. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol. Med. 2016;46:3231–3240. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- Lehman A.F., Lieberman J.A., Dixon L.B. Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am. J. Psychiatr. 2004;161(Suppl. 2):1–56. [PubMed] [Google Scholar]
- Marinopoulou M., Lugnegård T., Hallerbäck M.U., Gillberg C., Billstedt E. Asperger syndrome and schizophrenia: a comparative neuropsychological study. Journal of Autism Developmental Disorder. 2016;46:2292–2304. doi: 10.1007/s10803-016-2758-9. [DOI] [PubMed] [Google Scholar]
- Matsuo J., Kamio Y., Takahashi H. Autistic-like traits in adult patients with mood disorders and schizophrenia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Uno M., Kasai K., Koyama K., Kim Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry and Clinical Neuroscience. 2006;60:332–339. doi: 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Salovey P., Caruso D.R., Sitarenios G. Measuring emotional intelligence with the MSCEIT v 2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Mouaffak F., Tranulis C., Gourevitch R. Augmentation strategies of clozapine with antipsychotics in the treatment of ultraresistant schizophrenia. Clin. Neuropharmacol. 2006;29:28–33. doi: 10.1097/00002826-200601000-00009. [DOI] [PubMed] [Google Scholar]
- Naito K., Matsui Y., Maeda K., Tanaka K. Evaluation of the validity of the Autism Spectrum Quotient (AQ) in differentiating high-functioning autistic spectrum disorder from schizophrenia. Kobe Journal of Medical Science. 2010;56:E116–E124. [PubMed] [Google Scholar]
- Nielsen R.E., Levander S., Kjaersdam Telléus G. Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015;131:185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- O’Connor W.T., O’Shea S.D. Clozapine and GABA transmisstion in schizophrenia disease models establishing principles to guide treatments. Pharmacol. Ther. 2015;150:47–80. doi: 10.1016/j.pharmthera.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Øie M.G., Andersen P.N., Hovik K.T., Skogli E.W., Rund B.R. Similar impairments shown on a neuropsychological test battery in adolescents with high-functioning autism and early onset schizophrenia: a two-year follow-up study. Cognitive Neuropsychiatry. 2020;25((3):163–178. doi: 10.1080/13546805.2020.1713736. [DOI] [PubMed] [Google Scholar]
- Petty L.K., Ornitz E.M., Michelman J.D., Zimmerman E.G. Autistic children who become schizophrenic. Arch. Gen. Psychiatry. 1984;41:129–135. doi: 10.1001/archpsyc.1984.01790130023003. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E. Social cognition in schizophrenia. J. Clin. Psychiatry. 2014;75(Suppl. 2):14–19. doi: 10.4088/JCP.13065su1.04. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E., Morrison K.E., Penn D.L., Harvey P.D., Kelsven S., Ludwig K., Sasson N.J. Comprehensive comparison of social cognitive performance in autism spectrum disorder and schizophrenia. Psychol. Med. 2019:1–9. doi: 10.1017/S0033291719002708. [DOI] [PubMed] [Google Scholar]
- Quintero J., Barbudo del Cura E., López-Ibor M.I., López-Ibor J.J. The evolving concept of treatment-resistant schizophrenia. Actas Esp. Psiquiatr. 2011;39:236–250. [PubMed] [Google Scholar]
- Sasson N.J., Pinkham A.E., Carpenter K.L., Belger A. The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J. Neurodev. Disord. 2011;3:87–100. doi: 10.1007/s11689-010-9068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savla G.N., Vella L., Armstrong C.C., Penn D.L., Twamley E.W. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr. Bull. 2013;39:979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Giangrande E., Weinberger D.R., Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr. Res. 2013;150:42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten J.P., Lundberg M., Rai D., Magnusson C. Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: a population-based study. JAMA Psychiatry. 2015;72:483–489. doi: 10.1001/jamapsychiatry.2014.3059. [DOI] [PubMed] [Google Scholar]
- Short A.B., Schopler E. Factors relating to age of onset in autism. J. Autism Dev. Disord. 1988;18:207–216. doi: 10.1007/BF02211947. [DOI] [PubMed] [Google Scholar]
- Snyder S.H. Dopamine receptors, neuroleptics, and schizophrenia. Am. J. Psychiatr. 1981;138:460–464. doi: 10.1176/ajp.138.4.460. [DOI] [PubMed] [Google Scholar]
- Sugranyes G., Kyriakopoulos M., Corrigall R., Taylor E., Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A., Tojo Y., Baron-Coen S., Wheelwright S. Autism-Spectrum Quotient (AQ) Japanese version: evidence from high-functioning clinical group and normal adults. Sinrigaku Kenkyu. 2004;75:78–84. doi: 10.4992/jjpsy.75.78. (Article in Japanese) [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Purdon S.E., Meltzer H.Y., Zald D.H. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]