Highlights
-
•
Lactoferrin (Lf) is a naturally occurring, pleiotropic, non-toxic glycoprotein.
-
•
Lf has broad-spectrum antiviral, immunomodulatory and anti-inflammatory effects.
-
•
Lf shows in vitro antiviral activity against SARS-CoV, which is likely similar against SARS-CoV-2 via the same mechanism.
-
•
Lf's immunomodulatory and anti-inflammatory effects may be especially relevant as a potential adjunct for severe COVID-19.
Keywords: Lactoferrin, COVID-19, SARS-CoV-2, Antiviral, Viral prophylaxis
Abstract
The coronavirus disease 2019 (COVID-19) pandemic is rapidly advancing across the globe despite drastic public and personal health measures. Antivirals and nutritional supplements have been proposed as potentially useful against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that causes COVID-19, but few have been clinically established. Lactoferrin (Lf) is a naturally occurring, non-toxic glycoprotein that is orally available as a nutritional supplement and has established in vitro antiviral efficacy against a wide range of viruses, including SARS-CoV, a closely related coronavirus to SARS-CoV-2. Furthermore, Lf possesses unique immunomodulatory and anti-inflammatory effects that may be especially relevant to the pathophysiology of severe COVID-19 cases. Here we review the underlying biological mechanisms of Lf as an antiviral and immune regulator, and propose its unique potential as a preventative and adjunct treatment for COVID-19. We hope that further research and development of Lf nutritional supplementation would establish its role for COVID-19.
1. Introduction
Since its initial report in late 2019 [1], the coronavirus disease 2019 (COVID-19) outbreak has exploded from a few people suffering from a respiratory disease in the Chinese city of Wuhan to a surging pandemic affecting hundreds of thousands of people around the globe. Current methods of pandemic control are confined only to public containment and personal hygiene measures, whilst as yet there are no established antiviral treatments and most vaccines are still in the pre-clinical stage [2].
Lactoferrin (Lf) is a naturally occurring, non-toxic glycoprotein that has been studied against a broad range of viruses, including severe acute respiratory syndrome coronavirus (SARS-CoV), which is closely related to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 [3]. Furthermore, Lf has immunomodulatory and anti-inflammatory characteristics that can positively modify host responses to infections [4]. Lf is available as an oral supplement, and studies suggest that supplemental Lf may treat or prevent a host of microbial infections [5]. Here we examine the antiviral properties and immunomodulatory mechanisms of Lf within the context of its potential applications against SARS-CoV-2 and propose the possibility of supplemental Lf as a potential preventative and adjunct treatment for COVID-19, a condition whose pathophysiology involves both viral infection and an excessive host response [6].
2. Lactoferrin
Lf is a highly conserved, pleiotropic, iron-binding glycoprotein of the transferrin family that is expressed and secreted by glandular cells and is found in most body fluids [7]. It appears at especially high concentrations in mammalian milk and was first identified in bovine milk [8] and was subsequently isolated from human milk [9]. It is an 80-kDa glycoprotein containing 703 amino acid residues whose primary structure has been well characterised.
Since its discovery, Lf and its related peptides are mainly considered to be important non-specific host defence molecules against a variety of pathogens, including a range of viruses [5]. More recently, the anti-inflammatory and immunomodulatory roles of Lf have gained increasing scientific interest since it appears to be able to moderate the host response to infections and has the dual ability to stimulate the immune system to counteract pathogenic invasion while simultaneously preventing harmful host immune and inflammatory responses.
2.1. Lactoferrin as a broad-spectrum antiviral agent
The antiviral activity of Lf was first demonstrated in mice infected with a polycythemia-inducing strain of the Friend virus complex in the 1980s [10]. Since the 1990s, the list of Lf-susceptible pathogenic human viruses found to be inhibited by Lf have expanded to include naked and enveloped viruses as well as DNA and RNA viruses (Table 1 ), including cytomegalovirus, herpes simplex virus, human immunodeficiency virus (HIV), rotavirus, poliovirus, respiratory syncytial virus, hepatitis B virus, hepatitis C virus (HCV), parainfluenza virus, alphavirus, hantavirus, human papillomavirus, adenovirus, enterovirus 71, echovirus 6, influenza A virus and Japanese encephalitis virus, with in vitro EC50 values (half maximal effective concentration) generally in the micromolar range [11,12]. Particularly relevant to the current review is the ability of Lf to inhibit pseudotyped SARS-CoV with a 50% inhibitory concentration (IC50) of 0.7 μM [13] since it is the human coronavirus that is most closely related to SARS-CoV-2, which causes COVID-19.
Table 1.
Studies on the antiviral effects of lactoferrin (Lf) in vitro, in vivo and in humans.
| Virus | Effect | In vitro | In vivo | Clinical | Ref. |
|---|---|---|---|---|---|
| Adenovirus | Inhibition of cytopathic effect | √ | [11,12] | ||
| Avian flu (H5N1) | Antiviral activity | √ | [11,12] | ||
| Coxsackievirus A16 | Inhibition of cytopathic effect | √ | [12] | ||
| Cytomegalovirus | Inhibition of viral replication | √ | [11] | ||
| Echovirus 5 | Inhibition of binding and replication | √ | [11,12] | ||
| Enterovirus 71 (EV71) | Blocks viral adsorption; increases survival (mice) | √ | √ | √ | [11,12] |
| Hantavirus | Inhibition of viral adsorption | √ | [11] | ||
| hCoV-NL63 | Inhibition of viral entry | √ | [35] | ||
| Hepatitis B virus | Blocks viral entry | √ | [12] | ||
| Hepatitis C virus | Neutralises virus, blocks invasion; decreases viral titre (human) | √ | √ | [11,17,22] | |
| Herpes simplex virus 1 and 2 | Blocks viral entry, inhibits replication; prevents weight loss (mice) | √ | √ | [11,12,16] | |
| Human papillomavirus | Inhibition of cytopathic effect | √ | [12,15] | ||
| HIV | Blocks viral entry, inhibits replication | √ | [11,18] | ||
| Influenza A (H1N1) | Inhibition of cytopathic effect | √ | √ | [11] | |
| Norovirus | Inhibition of cytotoxic damage; reduction in gastroenteritis incidence and symptoms (children) | √ | √ | [12] | |
| Parainfluenza virus type 2 | Inhibition of viral entry | √ | [12] | ||
| Poliovirus | Inhibition of cytopathic effect | √ | [12] | ||
| Respiratory syncytial virus | Inhibition of virus entry and growth; no change in viral load or disease severity (mice) | √ | √ | [12] | |
| Rotavirus | Inhibition of cytopathic effect; decrease prevalence and severity (children) | √ | √ | [12,21] | |
| SARS-CoV (pseudotyped) | Inhibition of viral entry | √ | [13] | ||
| SARS-CoV-2 | Possible reduced severity and duration of infection, preventative in contacts (human) | √ | [38] |
HIV, human immunodeficiency virus; SARS-CoV, severe acute respiratory syndrome coronavirus.
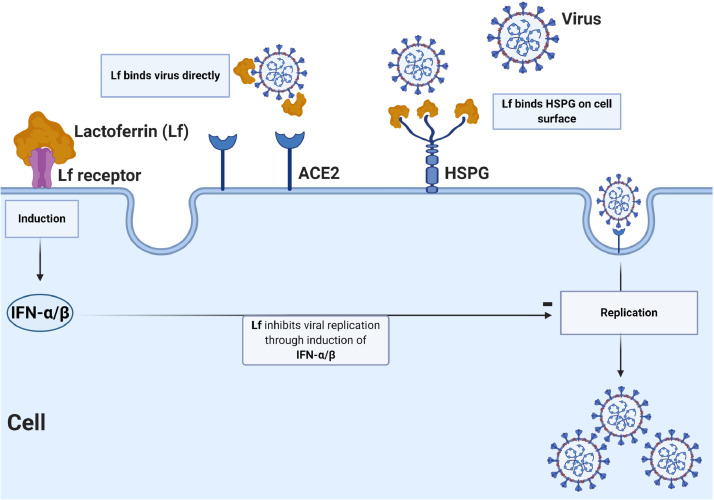
The antiviral mechanisms of Lf have been elucidated previously (Fig. 1 ). The ability of Lf to inhibit viral entry may be via binding to cell surface molecules or viral particles, or both. Current research has revealed that viral entry is a highly complex process involving cell surface molecules [14], with virus attachment followed by binding to a high-affinity cell surface receptor to initiate cell entry [15]. Heparan sulfate proteoglycans (HSPGs) have been identified as initial adhesion molecules for a number of viruses to increase their concentration at the cell surface and to improve their likelihood of binding a more specific entry receptor, and studies have demonstrated the role of Lf in preventing viral entry by binding to HSPGs [16]. Lf can also bind directly to virus particles, e.g. HCV, to divert them from target cells [17].
Fig. 1.
Potential antiviral mechanisms of lactoferrin (Lf): (i) direct binding of virus by Lf; (ii) Lf binding heparan sulfate proteoglycans (HSPGs) on the host cell surface, reducing viral surfing and subsequent viral entry; and (iii) Lf inhibition of viral replication via induction of intracellular cell signals. ACE2, angiotensin-converting enzyme 2; IFN, interferon.
Besides reducing viral entry, Lf can also suppresses virus replication after the virus enters the cell, as in the case of HIV [18]. Thereafter, Lf can also exert an indirect antiviral effect on immune cells that play a crucial role in the early stages of viral infection.
In human oral supplementation studies against pathologic viruses, Lf given in the range of 100–1000 mg/day in humans was found to reduce the incidence of colds [19] and cold-like symptoms [20] as well as to ameliorate rotaviral gastroenteritis [21]. In HCV patients, a randomised controlled study involving 111 patients receiving Lf versus no Lf along with standard anti-HCV drugs demonstrated a significant decrease in HCV viral titres and sustained virological response in the Lf group [22].
2.2. Lactoferrin as an immunomodulatory and anti-inflammatory agent
Lf is a unique multifunctional moiety that is not only a broad-spectrum antiviral but also has immunomodulatory [23] and anti-inflammatory [24] actions that may play a role in the pathophysiology of severe infections. The key immunomodulatory role of this protein stems from its unique potential to maintain immune and physiological homeostasis and to limit tissue damage by modulation of cytokines, chemokines and cell surface receptors involved in cascades of signalling pathways [25]. The myriad of biological pathways of control and feedback interactions with Lf has been extensively reviewed [26]. Specific examples of the balancing and restorative roles of Lf are perhaps illustrated in the context of systemic inflammatory response syndrome (SIRS), which describes the complex physiological response to severe insults such as sepsis as defined by a consensus conference in 1991 [27]. An updated and overlapping concept of ‘cytokine storm’ similarly reflects hyperinduction of inflammatory responses resulting from unchecked immune activation [28], which Kruzel's group and others have proposed Lf might treat [25].
Injection of lipopolysaccharide (LPS) into animals reproduces the pathophysiological changes induced by bacteria and it is considered a standard model for sepsis. Using this model, it was demonstrated that Lf treatment reduced or eliminated many biological reactions normally seen upon LPS administration in a dose-dependent manner [29], and in an earlier study a single dose of lactoferrin prior to LPS injection reduced the mortality of mice to 16.7% compared with 83.3% in controls [30].
Given promising laboratory and animal studies, Lf has been investigated in a number of clinical settings against sepsis. Recently, a meta-analysis of 10 randomised controlled trials involving 3679 infants concluded that Lf reduces late-onset sepsis in pre-term infants [31]. Separately, a human recombinant Lf, talactoferrin, was studied in a phase 2 clinical trial to assess the outcome in severe sepsis and a 12.5% reduction in all-cause mortality was found in those treated with talactoferrin [32], but the results were regrettably not replicated in a follow-up phase 2/3 trial [33].
2.3. Lactoferrin as a potential preventative and adjunct treatment for COVID-19
Lf has been found experimentally to inhibit viral entry by binding to host cell surface HSPGs in murine coronavirus [34] as well as human coronaviruses hCoV-NL63 [35] and pseudotyped SARS-CoV [13]. There are as yet no published studies on the effects of Lf on SARS-CoV-2 and its entry into host cells. Nevertheless, given the currently accepted ‘viral surfing’ model for the role of cell surface HSPGs [36], which the invading virion particles ‘surf’ from low-affinity HSPG anchoring sites to high-affinity entry receptors in an invasion, together with the homology of SARS-CoV and SARS-CoV-2 spike protein structures, as well as both viruses depending on the same angiotensin-converting enzyme 2 (ACE2) receptor for cell entry [37], we feel safe to postulate a similar mechanism whereby HSPGs serve as SARS-CoV-2 attachment sites that congregate the virus on the cell surface and facilitate specific entry receptors such as ACE2. It is thus likely that Lf can inhibit SARS-CoV-2 invasion at micromolar concentrations and in a dose-dependent manner just as in the case of SARS-CoV [13].
More relevant to our thesis are the recent results reported by Serrano et al. that a liposomal bovine Lf supplement containing 32 mg of Lf administered at four to six doses per day for 10 days with zinc 10 mg two to three times daily resulted in 100% recovery of 75 symptomatic SARS-CoV-2-positive patients within 4–5 days, and the same treatment at lower dose appeared to prevent the disease in healthy contacts [38].
Since another major aspect of Lf bioactivity relates to its immunomodulatory and anti-inflammatory functions, in the case of viral infections in particular, it may often be the magnitude of immune response and inflammation that contributes to disease severity, and this is particularly relevant for COVID-19.
Current thinking suggests that mortality from COVID-19 is not simply due to viral infection but is a result of a cytokine storm syndrome in select patients associated with hyperinflammation leading to acute respiratory distress and subsequent mortality [39]. A cytokine profile in severe COVID-19 cases is characterised by increases in cytokines and acute-phase reactants such as interleukin 6 (IL-6), tumour necrosis factor-alpha (TNFα) and ferritin. In this regard, Lf is demonstrated to reduce IL-6 and TNFα [40] and to downregulate ferritin [41] in experimental settings simulating sepsis. If the hypothesis that Lf can modulate an overactive immune and inflammatory response to viral infection is correct, then Lf could be a candidate adjunct treatment for more severe cases of COVID-19.
3. Discussion
Lf can be recombinant or derived naturally from bovine or mammalian sources and is considered by the US Food and Drug Administration (FDA) as ‘generally recognised as safe’ (GRAS) with no contraindications. It is widely used as a nutritional additive in infant formula, and clinical studies employed Lf doses ranging from 100 mg to 4.5 g a day for various indications without apparent toxicities. Newer formulations of Lf including encapsulation and liposomalisation have been explored [38,42], and Lf derivatives and related peptides such as lactoferricin and lactoferrampin with more potent antiviral properties are being explored and developed [5].
One observation regarding the clinical epidemiology of the current COVID-19 pandemic that may be relevant to Lf is the relatively low incidence of infection in children. Indeed, it has been reported that the incidence of COVID-19 in children aged 0–10 years was only 0.9% in the Chinese cases reported [42]. COVID-19 cases were rarer still in neonates and infants, with a total of only nine infected and hospitalised cases in China between 8 December 2019 and 6 February 2020 out of a total 31 211 reported cases nationwide [43]. Moreover, the course of COVID-19 in infants was mild even upon infection, with none of the nine reported cases requiring intensive care unit (ICU) admission or ventilation support, with infection rarely progressing to lower respiratory tract infection [44]. We postulate that breast-feeding or wide use of Lf-containing infant formula in this population may account for the above observation, but this remains to be validated.
Another interesting observation is that zinc-saturated lactoferrin can apparently exert a more potent antiviral effect. In experiments with poliovirus, it was observed that only zinc-saturated lactoferrin, and not iron-saturated lactoferrin, inhibited viral infection when incubated with cells after viral attachment, and the inhibition directly correlated with the degree of zinc saturation [45]. This is of particular relevance in COVID-19 as zinc supplementation has been proposed as a possible supplemental intervention for the disease [46].
As there is currently neither an established treatment regimen for COVID-19 nor an established preventative for SARS-CoV-2 infection, one can contemplate the use of Lf both as a non-toxic health supplement to prevent the infection as well as an adjunct treatment for those who have developed COVID-19. Its successful combined use to enhance conventional antiviral drug treatments in viral disease has been demonstrated against HCV [22] as well as in a recent study against SARS-CoV-2 [38], and its potential to reduce mortality due to cytokine-induced inflammation and respiratory failure in severe COVID-19 is also suggested by laboratory, animal and clinical studies.
4. Conclusion
Much progress has been achieved to elucidate the multifaceted function of Lf in the past 30 years as an antiviral as well as a unique anti-inflammatory and immunomodulatory molecule. We have presented the experimental as well as clinical rationale for its use in COVID-19, but further experiments to verify its inhibition of SARS-CoV-2 as well as clinical trials to elucidate dosage and efficacy are necessary to confirm the potential of Lf for SAR-CoV-2 prevention and COVID-19 treatment.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calina D, Docea AO, Petrakis D, Egorov AM, Ishmukhametov AA, Gabibov AG. Towards effective COVID-19 vaccines: updates, perspectives and challenges (review) Int J Mol Med. 2020;46:3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand D, Elass E, Carpentier M, Mazurier J. Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol. 2006;84:282–290. doi: 10.1139/o06-045. [DOI] [PubMed] [Google Scholar]
- 5.Bruni N, Capucchio MT, Biasibetti E, Pessione E, Cirrincione S, Giraudo L. Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine. Molecules. 2016;21:752. doi: 10.3390/molecules21060752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 8.Sorensen M, Sorensen SPL. The proteins in whey. C R Trav Lab Carlsb Ser Chim. 1940;23:55–99. [Google Scholar]
- 9.Johanson B, Virtanen AI, Tweit RC, Dodson RM. Isolation of an iron-containing red protein from human milk. Acta Chem Scand. 1960;14:510–512. doi: 10.3891/acta.chem.scand.14-0510. [DOI] [Google Scholar]
- 10.Lu L, Hangoc G, Oliff A, Chen LT, Shen RN, Broxmeyer HE. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 1987;47:4184–4188. [PubMed] [Google Scholar]
- 11.Ng TB, Cheung RCF, Wong JH, Wang Y, Ip DTM, Wan DCC. Antiviral activities of whey proteins. Appl Microbiol Biotechnol. 2015;99:6997–7008. doi: 10.1007/s00253-015-6818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi H, Oda H, Yamauchi K, Abe F. Lactoferrin for prevention of common viral infections. J Infect Chemother. 2014;20:666–671. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Lang J, Yang N, Deng J, Liu K, Yang P, Zhang G. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6:e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 15.Sapp M, Bienkowska-Haba M. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 2009;276:7206–7216. doi: 10.1111/j.1742-4658.2009.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen JH, Jenssen H, Sandvik K, Gutteberg TJ. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J Med Virol200474:262–71. doi: 10.1002/jmv.20171. [DOI] [PubMed]
- 17.Nozaki A, Ikeda M, Naganuma A, Nakamura T, Inudoh M, Tanaka K. Identification of a lactoferrin-derived peptide possessing binding activity to hepatitis C virus E2 envelope protein. J Biol Chem. 2003;278:10162–10173. doi: 10.1074/jbc.M207879200. [DOI] [PubMed] [Google Scholar]
- 18.Puddu P, Borghi P, Gessani S, Valenti P, Belardelli F, Seganti L. Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int J Biochem Cell Biol. 1998;30:1055–1062. doi: 10.1016/s1357-2725(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 19.Vitetta L, Coulson S, Beck SL, Gramotnev H, Du S, Lewis S. The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: a double blind randomized study. Complement Ther Med201321:164–71. doi: 10.1016/j.ctim.2012.12.006. [DOI] [PubMed]
- 20.Oda H, Nakano M, Wakabayashi H, Yamauchi K, Toida T, Iwatsuki K. Questionnaire survey on the subjective effects of a lactoferrin supplement. J Jpn Soc Complement Altern Med. 2012;9:121–128. doi: 10.1625/jcam.9.121. [DOI] [Google Scholar]
- 21.Egashira M, Takayanagi T, Moriuchi M, Moriuchi H. Does daily intake of bovine lactoferrin-containing products ameliorate rotaviral gastroenteritis. Acta Paediatr200796:1242–4. doi: 10.1111/j.1651-2227.2007.00393.x. [DOI] [PubMed]
- 22.Kaito M, Iwasa M, Fujita N, Kobayashi Y, Kojima Y, Ikoma J. Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin. J Gastroenterol Hepatol. 2007;22:1894–1897. doi: 10.1111/j.1440-1746.2007.04858.x. [DOI] [PubMed] [Google Scholar]
- 23.Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35:557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 25.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a context of inflammation-induced pathology. Front Immunol. 2017;8:1438. doi: 10.3389/fimmu.2017.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA. The ACCP/SCCM Consensus Conference Committee. Vol. 101. American College of Chest Physicians/Society of Critical Care Medicine; Chest: 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis; pp. 1644–1655. [DOI] [PubMed] [Google Scholar]
- 28.Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 29.Kruzel ML, Actor JK, Radak Z, Bacsi A, Saavedra-Molina A, Boldogh I. Lactoferrin decreases LPS-induced mitochondrial dysfunction in cultured cells and in animal endotoxemia model. Innate Immun. 2010;16:67–79. doi: 10.1177/1753425909105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24:33–44. doi: 10.1023/a:1006935908960. [DOI] [PubMed] [Google Scholar]
- 31.Razak A, Hussain A. Lactoferrin supplementation to prevent late-onset sepsis in preterm infants: a meta-analysis. Am J Perinatol. 2019 Sep 17 doi: 10.1055/s-0039-1696676. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Guntupalli K, Dean N, Morris PE, Bandi V, Margolis B, Rivers E. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit Care Med. 2013;41:706–716. doi: 10.1097/CCM.0b013e3182741551. [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Marshall JC, Dellinger RP, Simonson SG, Guntupalli K, Levy MM, et al. Talactoferrin in severe sepsis: results from the phase II/III Oral tAlactoferrin in Severe sepsis Trial. Crit Care Med201543:1832–8. doi: 10.1097/CCM.0000000000001090. [DOI] [PubMed]
- 34.de Haan CAM, Li Z, te Lintelo E, Bosch BJ, Haijema BJ, Rottier PJM. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J Virol. 2005;79:14451–14456. doi: 10.1128/jvi.79.22.14451-14456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol201488:13221–30. doi: 10.1128/jvi.02078-14. [DOI] [PMC free article] [PubMed]
- 36.Burckhardt CJ, Greber UF.Virus movements on the plasma membrane support infection and transmission between cells. PLoS Pathog20095:e1000621. doi: 10.1371/journal.ppat.1000621. [DOI] [PMC free article] [PubMed]
- 37.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano G, Kochergina I, Albors A, Diaz E, Oroval M, Hueso G. Liposomal lactoferrin as potential prevention and cure for COVID-19. Int J Res Health Sci. 2020;8:8–15. doi: 10.5530/ijrhs.8.1.3. [DOI] [Google Scholar]
- 39.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimecki M, Właszczyk A, Zagulski T, Kübler A. Lactoferrin lowers serum interleukin 6 and tumor necrosis factor alpha levels in mice subjected to surgery. Arch Immunol Ther Exp (Warsz) 1998;46:97–104. [PubMed] [Google Scholar]
- 41.Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci. 2017;18:1985. doi: 10.3390/ijms18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishikado A, Imanaka H, Takeuchi T, Harada E, Makino T. Liposomalization of lactoferrin enhanced it's anti-inflammatory effects via oral administration. Biol Pharm Bull. 2005;28:1717–1721. doi: 10.1248/bpb.28.1717. [DOI] [PubMed] [Google Scholar]
- 43.Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020;61:131–132. doi: 10.1016/j.pedneo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti M, Superti F, Ammendolia MG, Rossi P, Valenti P, Seganti L. Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin. Med Microbiol Immunol. 1999;187:199–204. doi: 10.1007/s004300050093. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]