Abstract
The COVID-19 outbreak has led to a focus by public health practitioners and scholars on ways to limit spread while facing unprecedented challenges and resource constraints. Recent COVID-19-specific enhanced Traffic Control Bundling (eTCB) recommendations provide a cogent framework for managing patient care pathways and reducing health care worker (HCW) and patient exposure to SARS-CoV-2. eTCB has been applied broadly and has proven to be effective in limiting fomite and droplet transmissions in hospitals and between hospitals and the surrounding community. At the same time, resource constrained conditions involving limited personal protective equipment (PPE), low testing availability, and variability in physical space can require modifications in the way hospitals implement eTCB. While eTCB has come to be viewed as a standard of practice, COVID-19 related resource constraints often require hospital implementation teams to customize eTCB solutions. We provide and describe a cross-functional, collaborative on-the-ground adaptive application of eTCB initially piloted at two hospitals and subsequently reproduced at 16 additional hospitals and health systems in the US to date. By effectively facilitating eTCB deployment, hospital leaders and practitioners can establish clearer ‘zones of risk’ and related protective practices that prevent transmission to HCWs and patients. We outline key insights and recommendations gained from recent implementation under the aforementioned constraints and a cross-functional team process that can be utilized by hospitals to most effectively adapt eTCB under resource constraints.
Keywords: Enhanced traffic control bundling, COVID-19, Health care workers, Nosocomial infection control, Pandemic, Mitigation
Background
The novel coronavirus SARS-CoV-2 has spread rapidly across the globe and has since infected over 8.6 million people worldwide as of June 19, 2020,1 , 2 including estimates of thousands of health care workers (HCWs).3 Neither the number of United States (US) HCW positive tests, nor the number of HCW deaths has been tracked centrally to date and such data is reported by only a few US states or territories—with Ohio and Minnesota respectively reporting more than 16% and approximately 28% positive cases to be HCWs.3 Similar to the emergence of two other coronavirus outbreaks, severe acute respiratory syndrome (SARS)4 in 2003, and Middle East respiratory syndrome (MERS)5 in 2012, the novel SARS-CoV-2 or COVID-19 pandemic has become one of the largest global public health crises. Based on prior experiences with SARS in Taiwan and key insights from early encounters with COVID-19, Schwartz et al.6 and Yen et al.7 , 8 recently recommended enhanced Traffic Control Bundling (eTCB) to be critically important to Health Care Worker (HCW) and patient safety.
Application of TCB in 18 Taiwanese hospitals during SARS proved effective in reducing nosocomial transmission of the coronavirus, eliminating nosocomial transmission to HCWs altogether compared to the 33 hospitals in the control group in which 115 HCWs tested positive.6 , 9 Based on these empirical findings, Yen et al. emphasized determination and management of “zones of risk—clearly delineating separate zones, including a contamination, transition, and clean zone each separated by checkpoints” (p. 2).7 There has been a dramatic, rapid increase in demand for health care resources, yet an absence of specific deployment-related guidance to hospitals and facilities implementing COVID-19 preparedness and responses.10 eTCB is emerging as the benchmark for establishing related hospital safety protocols.
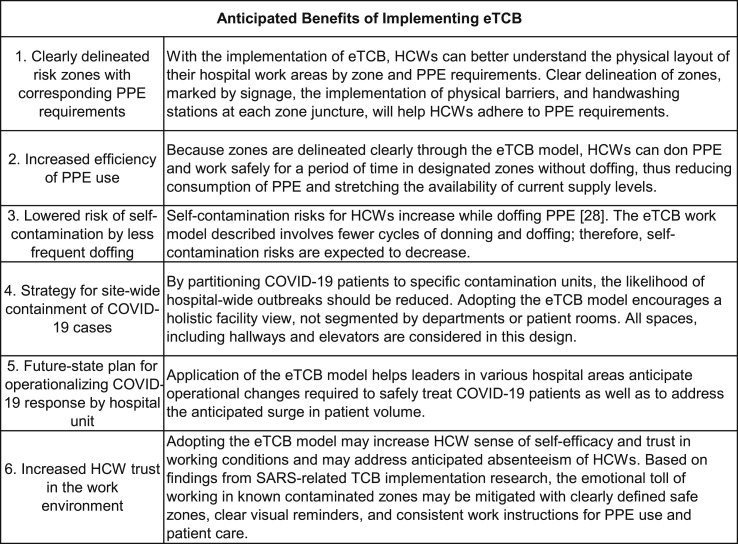
The primary purpose of this paper is to elaborate on steps taken to customize and deploy the eTCB framework. The anticipated benefits of eTCB implementation by cross-functional implementation teams (heretofore called “site teams”) are identified in Fig. 1 . Based on collaborative efforts by site teams at several hospital facilities and systems applying recommended eTCB principles, we share essential modifications developed in resource constrained COVID-19 focused environments—limited rapid-test capacity, finite bed and ventilator availability, insufficient supply of personal protective equipment (PPE)10—that create challenges for HCWs in “clearly delineating separate zones” as recommended. Another purpose of this paper is to contribute to emerging discussions in the literature regarding eTCB deployment from a variety of contexts, settings, and situations. The resource shortages US hospitals are facing present unique challenges to eTCB implementation. Understanding the impact of these resource constraints on the deployment of eTCB and modifications created on the ground contributes to the ongoing elaboration of eTCB research and practice toward protection of HCWs and patients.
Figure 1.
Anticipated benefits of eTCB implementation to HCWs and hospitals.
Literature overview
Yen et al.9 proposed the use of Traffic Control Bundling (TCB) as a method of containment during the 2003 SARS epidemic in Taiwan. In broad terms, the TCB model interrupts HCW exposure and the circular community-hospital-community transmission cycle by situating each patient in specific zones based on their diagnostic circumstances.9 , 11 Implementation of TCB has also proven effective in mitigating feelings of anxiety about the workplace environment, and increased HCW trust in the workplace due to the implementation of systems designed to protect them.6 , 7
To the extent that general TCB approaches involve a triage to sort and prioritize incoming patients,9 COVID-19 presents unique challenges in which carriers (including HCWs) may be asymptomatic for several days, are potentially communicable, and may present with a variety of symptoms.12 , 13 Reviews of general pandemic influenza triage protocols and TCB literature proved to be limited in application given unique elements of COVID-19.6 , 12, 13, 14 Combined with low availability of testing, prolonged turnaround times,9 and lack of direct evidence regarding efficacious treatment of COVID-19,15 HCWs are often unable to implement standard triage assessment procedures. Normal triage and general TCB11 , 16, 17, 18 provide limited solutions to COVID-19 as positive individuals may transform rapidly from asymptomatic to requiring urgent care,12 , 15 and there are increasing indications that asymptomatic carriers may expose others through talking and breathing.19 In contrast, eTCB was determined to be the most context-specific set of recommendations by the site teams described below.
At the same time, it must be noted that implementation related gaps in the aforementioned available literature (including on eTCB) provide opportunities for further elaboration regarding how hospitals should implement pivot screening and TCB.16 , 20 , 21 Recent COVID-19-specific publications reporting developments on pivot screening, TCB implementation,6 , 7 , 20 and general surge response guidelines22 , 23 provide few specifics regarding how hospitals collaborate internally to derive COVID-19 best practices. A recent article by Yen et al.,8 focusing on long-term care facilities, signals the importance that insights and recommendations regarding eTCB be extended for consideration, and emphasizes implementation results facilitated by practitioners and scholars be reported.
The pre-COVID-19 TCB workflow has no quarantine zone and begins with patient screening outside of the hospital to assure direction to appropriate areas within the hospital to either contaminated or clean areas. Patients with a positive diagnosis are directed to the red contaminated zone which is separated from the rest of the hospital by a yellow transition zone and managed by HCWs in appropriate PPE. The remaining portion of the hospital is a designated green zone for patients with a negative diagnosis and where HCWs don PPE before transitioning to other zones.9
The SARS-focused TCB model has since been adapted, by the same research team, for COVID-19 and the name modified to enhanced Traffic Control Bundling (eTCB).6 , 7 The two enhancements of the original TCB model are as follows: (1) In eTCB, transitional yellow zones are expanded to include a quarantine unit where patients yielding inconclusive test results stay for the remainder of the incubation period; and (2) eTCB implements mandatory hand sanitization and use of face masks for all visitors of the hospital to prevent community to hospital spread via asymptomatic carriers. In the conceptual model of eTCB zones, patients are first screened in temporary structures outside the facility. Those with a positive test for COVID-19 are admitted to the isolation unit and those with inconclusive test results are admitted to the quarantine unit where they remain for the balance of the incubation period.7 Admitted patients follow a specific route from the pivot area to their unit which avoids clean zones and HCW routes. HCWs working in yellow or red zones don appropriate PPE in the green zone, pass through the transitional yellow zone, and work in the yellow quarantine unit or red isolation unit. When exiting yellow or red zones, HCWs undergo decontamination and remove PPE in the yellow transitional zone before proceeding into the green zone. Hand sanitization takes place at the junctions between all zones. To combat potential asymptomatic carriers, all visitors must sanitize their hands and wear a surgical mask before entering the hospital. Regular hand sanitization to mitigate virus exposure in HCWs has been shown to be a key factor in reducing transmission of infection24 as has comprehensive use of masks across all facilities.25
Through application of this recently updated eTCB model for COVID-19, we identified three adaptations which incorporated resource constraints common in the US and several countries worldwide, including—(a) current testing/diagnostic limitations, (b) constrained supply of PPE, and (c) needed customization based on inherent variation in each hospital's patient and staff workflow, and in each hospital's design and physical layout. First, while eTCB6 , 7 is increasingly considered to be the ‘gold standard’, some of it must be customized before being implemented in US hospitals—because current (and the foreseeable future) limited rapid-test capacity hinders the assignment of patients to confirmed contamination zones. Currently, a majority of patients are considered “persons under investigation” (PUIs) for COVID-19 and few hospitals have enough beds to admit all PUIs into a quarantine unit. Additionally, constraints on availability and the need to modify use of PPE impacts zone delineation for patient and HCW pathways. Second, key steps for implementation are not specified in the descriptions of the eTCB model. As a result, hospitals have difficulty rapidly adopting and implementing the recommended protocols. Third, each hospital environment has different workflows, physical layouts, and constraints; hence, each site requires a customized approach to adopting eTCB.
eTCB model implementation
In mid-March, a team of expert process consultants was deployed to assist several hospitals1 based in one of the twenty largest metropolitan areas in the US to assess their current COVID-19 patient care pathways and to prepare for the containment and surge of COVID-19 cases. The presenting problem was that area hospitals were not adequately prepared for the unique challenges presented by COVID-19 with key areas of concern being: (1) HCWs are at high risk for contracting COVID-19 and spreading the virus to co-workers and patients; (2) PPE is being used at alarmingly high rates without guidelines or confirmed timelines for replenishment; and (3) modifications to COVID-19 patient flow to mitigate HCW risk and effectively treat patients is unclear and constrained by each hospital's unique built environment.
Traffic Control Bundling (TCB) models were identified as possible approaches for addressing issues with HCW safety, rapid use of PPE, and establishing a logical flow for patient traffic with the eTCB appearing to be the most viable for addressing the presenting problem.6 , 7 However, no identified extant eTCB documentation proposes clear implementation steps. Each health care facility faced gaps between the eTCB model described by Yen et al. and constraints specific to each facility.
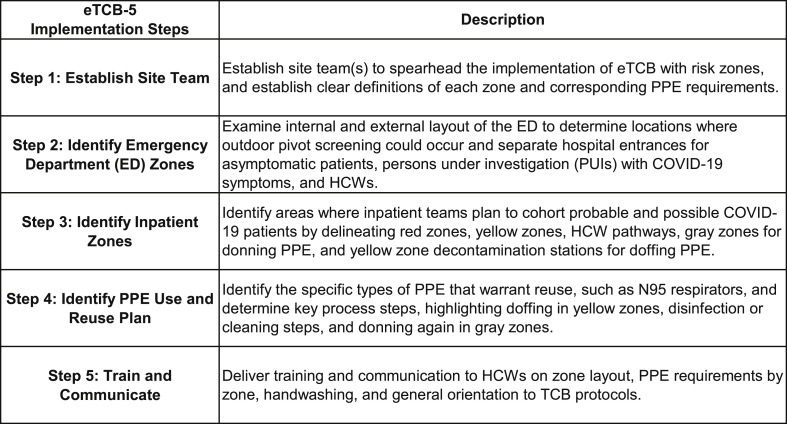
Based on the collaborative actions of the process consultants and on-location cross-functional HCW teams (“site teams” are typically composed of an MD lead, RN lead, a representative from facilities and/or operations, and input from Infection Prevention), a number of action steps were identified and undertaken. In total, these steps led to a general process for implementing the eTCB model (Fig. 2 ). This eTCB-5 Implementation Process led to the customized adoption of the eTCB model in a manner that was most functional given the available resources, opportunities and constraints presented in each physical location, and specific needs given the current and anticipated flow of patients and HCWs.
Figure 2.
eTCB-5 implementation steps.
Recommended eTCB implementation approach
Based on the collaborative work, site team learnings, and outcomes described above, we have three key findings from applying eTCB as described in the literature to our current environment. We have found that applying the eTCB model while using a cross-functional team allowed for (1) the creation of an eTCB blueprint and eventual application in specific work areas; (2) the visualization of health care worker, patient, and PPE flows; and (3) establishment of site-specific plans for surges in patient volume by mapping the expansion of red zones to meet increasing patient demand. Through our application of eTCB concepts in ED, inpatient, and ICU areas, these three findings have provided clarity to the construction of an effective implementation plan and strategies to customize the eTCB model to various hospital sites.
Visualization of three key flows: patients, HCW, and PPE
Application of the eTCB model facilitates the visualization of HCW, patient, and PPE flows in various hospital work areas. Before future-state flows can be visualized, operational definitions of each zone must be agreed to along with the PPE requirements by zone. Because efficient COVID-19 testing has not been available in the US during the first months of the COVID-19 outbreak the eTCB approach6 , 7 is not yet possible; therefore, modified zone definitions to the eTCB model were suggested to and accepted by the implementation teams.
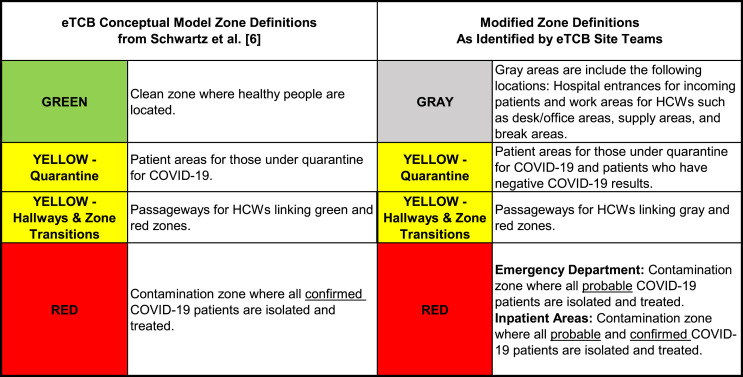
Through collaboration with each site, the “green zone,” as found in the original eTCB model, was renamed the “gray zone.” This color was chosen because of the common cultural understanding that a “gray” area commonly represents uncertainty or lack of clarity, while green is viewed as safe and requiring no additional precautions. As such, there are no green zones in our application of eTCB. Given the current testing constraints at each site (limited number of tests and long turnaround time for results), the use of the term gray zone helps to communicate that risk is always present with COVID-19 as it is currently not possible to identify asymptomatic positive COVID-19 patients or health care workers. Most of the hospital facilities had minimal gray zones in their designed floor plan, and these gray zone areas were designated as hospital entrances for patients prior to their pivot screen, or as other work and break areas for health care workers only. The “yellow” nomenclature was used to highlight that a pivot screened patient in this zone may not exhibit common signs of COVID-19; however, increasing community prevalence of the virus indicated that any patient could be a potential asymptomatic carrier. Yellow zones contained both temporarily isolated patients awaiting COVID-19 test results as well as patients who had negative COVID-19 test results with each type being roomed in separate yellow zone areas. Those patients with negative COVID-19 test results in yellow zones would be discharged for any additional recovery at home. Finally, the “red zone” definition was established for patients with a positive screen, a positive test result, or who otherwise were determined higher-risk and therefore probable COVID-19 based on presenting symptoms or known exposures. Fig. 3 summarizes the distinctions between the ideal eTCB zone definitions and the adapted zone definitions as applied locally.
Figure 3.
eTCB zone conceptual model definitions compared to modified zone definitions for sites without rapid testing.
As with the eTCB model, patients are first pivot screened either outside the facility in a designated gray area immediately adjacent to the Emergency Department or in an entryway just inside the facility and within the Emergency Department. This workflow allows for separation of patients with COVID-19 symptoms from patients who are presenting for other causes. Patients whose pivot screen indicates possible or probable COVID-19 are dispatched for further assessment in the Emergency Department (ED) red zone through doors designated specifically for probable COVID-19 patients. Patients who pivot screen with symptoms not associated with COVID-19 are dispatched to yellow zone areas within the ED and enter through a door designated only for the yellow zone in the ED. Except for the pivot screen area inside the Emergency Department which is a gray zone, all other areas for patients are either yellow or red zones. As such, no patients remain in a gray zone either within the Emergency Department or in any inpatient unit. In addition, all patients are required to wear face masks once pivot screens are completed to reduce droplet transmission. For those requiring hospitalization, probable COVID-19 patients were admitted to red zone rooms in Med Surg or ICU areas as appropriate. Non-COVID-19 patients requiring hospitalization were dispatched to yellow zone rooms in Med Surg or ICU as appropriate. Admitted, probable COVID-19 patients follow designated routes, ideally separated from yellow pathways and HCWs transition zones. Because rapid COVID-19 testing has been unavailable, probable pretest diagnosis was the key indicator of where to dispatch patients both in ED and in all inpatient units while awaiting test results.
After modified operational definitions were adopted for each of the three zones, site teams reaffirmed that PPE would be donned in gray zones, and decontamination and doffing of PPE would occur in yellow zones. Similar to the eTCB model, our adapted approach then had HCWs moving from gray zones to work in yellow or red zones through yellow transitional hallways with hand sanitization taking place at all junctions between zones per the eTCB recommendations. Sites with available supplies began enforcing universal ear-loop masking of all patients regardless of symptomatic presentation to combat possible transmission. Additionally, many sites began to enforce universal masking of HCWs, regardless of their zone of work, to combat asymptomatic transmission.
After site teams reached consensus designations of gray, yellow, and red zones, the HCW, patient, and PPE flows became clear as the teams mapped zones onto existing blueprint floor plans. The demarcated workflow of HCWs was clearly identifiable including where HCWs enter the unit, don PPE, work by patient type in a specific zone, decontaminate and doff PPE, and leave the work area. These plans showed the flow of patients, starting with pivot screening either inside or outside the ED area, and transport through designated pathways to move patients from the ED into inpatient areas including Med Surg and ICU. Finally, under the assumption that limited supply of PPE warrants reuse, the application of the eTCB model helps to clarify the process which can include reuse of N95 respirators, beginning with doffing in a yellow zone, disinfecting of N95 respirators through a rotational system, and reuse of N95s with donning again in the gray zone. Many of the aforementioned hospital locations have issued several N95 respirators (usually 4–5 depending on work shift allocations) to each individual HCW. The masks are then used, one per day, with 4–5 days of rest between uses where respirators are stored in open brown paper bags so that they may dry out and decontaminate naturally.26 Masks are typically used 5–7 work days before they are replaced, but there have been reports of longer term use depending on supply/demand situation27 and the ongoing condition of the reused mask.
It is important to note that when resource constraints are removed, the previously established eTCB approach7 , 8 , 28 should be implemented. The combination of reported innovations and new findings from the CDC, the WHO, and other organizations, along with any relief to current resource limitations, will influence team decision making continuously. More detailed descriptions of HCW doffing,29 “mass masking” efforts,30 drive-through31 and walk through testing32 schemes, telemedicine,33 and the expansion of outdoor tenting and facilities34 increase options for site teams to consider. For instance, as surge responses in some areas require that ER, ICU, and beds move to tents, temporary structures and new sites may be developed. The built environment constraints change from fixed structure concerns to engineering questions (e.g., access to adequate power, room/space temperature control, appropriate ventilation) of how to deploy this protocol. Site teams should review new information from identified online and media sources and incorporate ongoing innovations and medical findings to help guide workflow.
eTCB and surge preparation
Application of the eTCB model provides a baseline for site teams to estimate surge capacity and formulate surge responses to meet increasing patient demand. Through mapping gray, yellow, and red zones, teams can identify current state eTCB zones and anticipate future state designs, including plans for the expansion of contaminated red zones as COVID-19 patient volumes increase. As red zones expand due to increased numbers of probable and confirmed COVID-19 patients requiring hospitalization, gray or yellow zones are likely to shrink in the same area due to the finite amount of floor space in the physical environment, making room for incoming patients. Consequently, teams may be required to move donning and doffing locations to other floors in the facility or eventually outside as the red zone expands or if the entire indoor hospital building becomes a yellow or red zone under extreme surge conditions.
Generating shared meaning of eTCB through dialogue and collaboration
Repeated implementation efforts across several hospital settings (EDs, ICUs, inpatient units) and systems have led to the conclusion that cross-functional departmental teams35 , 36 are best-suited to make eTCB zoning decisions.6 As noted earlier, site teams including an MD lead, RN lead, Operations/Facilities representative, and Infection Prevention input are able to formulate “future state” eTCB blueprints and scenario plans for how flows of patients, HCWs, and PPE may occur. Initial meetings with these cross-functional site teams focused on agreement of operational zone definitions and associated PPE requirements. When teams struggle mapping the future state zones initially, labeling the current state zones first has been a helpful next step. When teams start with a current state map, they often discover areas of COVID-19 exposure that can be immediately remediated.
Preliminary results
Early findings for hospitals in our participation group who implemented resource-constrained eTCB are encouraging if difficult to compare due to the paucity of HCW infection rate data accessible in the US. Data from the Emergency Department teams of 11 hospitals in our participation group indicated that 16 COVID-19 HCW infections were confirmed of 1464 staff with direct patient care responsibilities since the COVID-19 pandemic began through June 12, 2020 for an overall infection rate of 1.14%. Although longitudinal comparative data between implementing hospitals and non-implementing hospitals will take more time to collect and analyze, these early results from the Emergency Departments of a number of the hospitals in our participation group are promising. For instance, the 1.14% infection rate for those sites implementing resource-constrained eTCB in our participation group is lower than rates reported by the CDC of other US-based hospitals which was ranged from 3% to as much as 12%.37 , 38 However, in order to better understand the outcomes on infection rates, improved comparisons need to be made. Given the dynamic impact of COVID-19 public health data collection, we anticipate the ability to access retrospective and future longitudinal data comparing “eTCB treatment groups” to hospitals who have not implemented eTCB concepts, similar to the work by Yen et al.9 Furthermore, standardized HCW definitions and how to treat full-time versus part-time staff in infection rate calculations must be normalized in future studies for more meaningful comparisons to be made. We must also note the conditions under which US infection rate data are collected and reported and the absence of a US-wide contact tracing protocol makes the determination of in-hospital versus community transmission difficult to ascertain.
Conclusion
Ideally, eTCB would be implemented worldwide in the context of available resources to support hospital and community mitigation of SARS-CoV-2. In the case of the US, taking on a “whole society approach”39 would increase the likelihood that the significant and enduring PPE, testing and contact tracing gaps currently creating challenges for both mitigation and data collection would be closed. Until desired resource levels are achieved, customization of eTCB related protocols may be necessary. Through implementation of eTCB in resource constrained US hospitals, several collaborative cross-functional hospital site teams have crafted modifications to their eTCB approach for COVID-19—to address the lack of rapid-test capabilities, limited bed capacity, and reduced PPE inventories available at most sites. We reported on eTCB model applications to customize patient and HCW flows, establish zones, and plan for containment and surge with a cross functional site team, adapting as innovations emerge and medical guidance is updated. Application of eTCB, through implementation steps that allow for adaptation to unique environmental or other circumstances, facilitates the goals of limiting HCW worker and patient exposure and promoting more efficient use of PPE. The authors encourage ongoing dialogue in the scholarly literature from a variety of national perspectives that detail the challenges and opportunities presented in the implementation of eTCB and contribute to the related evidence base.
Declaration of Competing Interest
None.
Acknowledgement
Appreciation is extended to the many individuals on various teams who applied the eTCB model in their hospital areas. Co-authors would also like to extend appreciation to their colleagues in each of their respective organizations.
Footnotes
“several hospitals” refers to the two focal hospitals located in one of the 20 largest metropolitan areas in the United States in which the eTCB implementation process, reported herein, was initiated and deployed--along with 16 additional hospitals in the same metropolitan area who began deploying the implementation approach as described. The feedback to and from each facility is ongoing as the implementation approach contributes to growing validation of the eTCB model. Replication of the SARS Taiwan study7,8 is in early stage planning.
References
- 1.Center for Systems Science and Engineering Johns Hopkins University. COVID-19 interactive online dashboard. https://coronavirus.jhu.edu/map.html Available at:
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30120-1/fulltext Available at: [DOI] [PMC free article] [PubMed]
- 3.Bellisle M. How Many Medical Workers Have Contracted COVID-19? States Lack Key Data. Oregon Public Broadcasting. https://www.opb.org/news/article/:coronavirus-covid-19-data-health-care-medical-workers-infection/ Available at:
- 4.Yen M.Y., Chiu A.W.H., King J., King C.C., Lin Y.E., Chang S.C. From SARS in 2003 to H1N1 in 2009: lessons learned from Taiwan in preparation for the next pandemic. J Hosp Infect. 2014;87:185–193. doi: 10.1016/j.jhin.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen M.Y., Schwartz J., Wu J.S.J., Hsueh P.R. Controlling MERS: lesson learned from SARS. Clin Infect Dis. 2015;61:1761–1762. doi: 10.1093/cid/civ648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz J., King C.C., Yen M.Y. Protecting health care workers during the COVID-19 coronavirus outbreak–lessons from Taiwan's SARS response. Clin Infect Dis. 2020;71(15):858–860. doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen M.Y., Schwartz J., Chen S.Y., King C.C., Yang G.Y., Hsueh P.R. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: implications for global prevention and control efforts. J Microbiol Immunol Infect. 2020;53(3):377. doi: 10.1016/j.jmii.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen M.Y., Schwartz J., King C.C. Recommendations for protecting against and mitigating the COVID-19 pandemic in long-term care facilities. J Microbiol Immunol Infect. 2020;53(3):447–453. doi: 10.1016/j.jmii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen M.Y., Lin Y.E., Lee C.H., Ho M.-S., Huang F.-Y., Chang S.-C. Taiwan's traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2011;77:332–337. doi: 10.1016/j.jhin.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Department of Health and Human Services . 2020. Office of the inspector general. Hospital experiences responding to the COVID-19 pandemic: results of a national pulse survey march 23–27.https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf Available at: [Google Scholar]
- 11.Yen M.Y., Chiu A.W.-H., Schwartz J., King C.-C., Lin Y.E., Chang S.-C. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2006;62:195–199. doi: 10.1016/j.jhin.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan B., Lingsheng Y., Tao W. Presumed asymptomatic carrier transmission of COVID-19. https://jamanetwork.com/journals/jama/fullarticle/2762028 Available at: [DOI] [PMC free article] [PubMed]
- 13.Eliezer M., Hautefort C., Hamel A., Verilland B., Herman P., Houdart E., Eloit C. Sudden and complete olfactory loss function as a possible symptom of COVID-19. https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2764417 Available at: [DOI] [PubMed]
- 14.Toner E., Waldhorn R. What hospitals should do now to prepare for a COVID-19 pandemic. Clinicians' Biosecurity News. http://www.centerforhealthsecurity.org/cbn/2020/cbnreport-02272020.html Available at: [DOI] [PubMed]
- 15.Centers for Disease Control Information for clinicians on therapeutic options for patients with COVID-19. https://www.cdc.gov/coronavirus Available at:
- 16.World Health Organization Hospital preparedness checklist for pandemic influenza. https://www.who.int/publications/i/item/hospital-preparedness-checklist-for-pandemic-influenza
- 17.Moskop J.C., Iserson K.V. Triage in medicine, part I: concept, history and types. Ann Emerg Med. 2006;48:275–281. doi: 10.1016/j.annemergmed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Moskop J.C., Iserson K.V. Triage in medicine, part II: underlying values and principles. Ann Emerg Med. 2006;49:282–287. doi: 10.1016/j.annemergmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phua J., Weng L., Ling L., Egi M. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. https://www.thelancetcom/journals/lanres/article/PIIS2213-2600(20)30161-2/fulltext Available at: [DOI] [PMC free article] [PubMed]
- 21.World Health Organization World health report--primary healthcare (now more than ever) http://www.who.int/whr/2008/en/index.html
- 22.Paganini M., Conti A., Weinstein E., della Corte F., Ragazzoni L. Disaster Med Public; 2020. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Physician's Weekly Management of elective surgery reviewed in setting of COVID-19. https://www.physiciansweekly.com/management-of-elective-surgery-reviewed-in-setting-of-covid-19/ Accessed on 23 March 2020. Available at:
- 24.Kratzel A., Todt D., V’kovski P., Steiner S., Gultrom M., Thao T.T.N. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020 doi: 10.3201/eid2607.200915. (online prepublication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Advice on the use of masks in the context of COVID-19: Interim guidance 5 June 2020. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO-2019-nCov-IPC_Masks-2020.4-eng.pdf
- 26.The National Institute for Occupational Safety and Health (NIOSH) Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html
- 27.Centers for Disease Control Strategies for Optimizing the Supply of N95 Respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html
- 28.Yang C.J., Chen Y.H. The preventive strategies of community hospital in the battle of fighting pandemic COVID-19 in Taiwan. J Microbiol Immunol Infect. 2020;53(3):381–383. doi: 10.1016/j.jmii.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baloh J., Reisinger H.S., Dukes K., da Silva J.P., Salehi H.P., Ward M. Healthcare workers' strategies for doffing personal protective equipment. Clin Infect Dis. 2019;69:S192–S198. doi: 10.1093/cid/ciz613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung C.C., Lam T.H., Cheng K.K. Mass masking in the COVID-19 epidemic: people need guidance. https://aphn.org/wp-content/uploads/2020/04/Mitigate-the-effects-of-home-confinement-on-childrenduring-the-COVID19-outbreak.pdf Available at: [DOI] [PMC free article] [PubMed]
- 31.Kwon K.T., Ko J., Shin H. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. https://jkms.org/DOIx.php?id=10.3346/jkms.2020.35.e123 Available at: [DOI] [PMC free article] [PubMed]
- 32.Shim H. Korea's evolving virus tests--from drive-through to walk-through. http://www.koreabiomed.com/news/articleView.html?idxno=7767 Available at:
- 33.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. https://www.nejm.org/doi/full/10.1056/NEJMp2003539 Available at: [DOI] [PubMed]
- 34.Chen S., Zhang Z., Yang J. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30744-3/fulltext Available at: [DOI] [PMC free article] [PubMed]
- 35.Reeves S., Lewin S. Interprofessional collaboration in the hospital: strategies and meanings. J Health Serv Res Pol. 2004;9(4):218–225. doi: 10.1258/1355819042250140. [DOI] [PubMed] [Google Scholar]
- 36.Abraham J., Reddy M.C. Challenges to inter-departmental coordination of patient transfers: a workflow perspective. Int J Med Inf. 2010;79:112–122. doi: 10.1016/j.ijmedinf.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control Coronavirus Disease (COVID-19): Cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 38.National Public Radio COVID-19 Has Killed Close To 300 U.S. Health Care Workers, New Data From CDC Shows. https://www.npr.org/sections/health-shots/2020/05/28/863526524/covid-19-has-killed-close-to-300-u-s-health-care-workers-new-data-from-cdc-shows
- 39.Schwartz J., Yen M.Y. Toward a collaborative model of pandemic preparedness and response: Taiwan's changing approach to pandemics. J Hosp Infect. 2017;87:125–132. doi: 10.1016/j.jmii.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]