Highlights
-
•
Azithromycin had better binding affinity scores than hydroxychloroquine and chloroquine.
-
•
The best binding affinity scores of azithromycin were ACE2 > CTSL > Mpro > RBD.
-
•
The best binding affinity scores of hydroxychloroquine were ACE2 > Mpro.
-
•
The best binding affinity score of chloroquine was Mpro.
-
•
Azithromycin appears to be a strong candidate for inhibition of SARS-CoV-2 replication.
Keywords: COVID-19, Coronavirus, Molecular docking, Azithromycin, Chloroquine, Hydroxychloroquine
Abstract
Coronavirus disease 2019 (COVID-19) is a highly transmissible viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical trials have reported improved outcomes resulting from an effective reduction or absence of viral load when patients were treated with chloroquine (CQ) or hydroxychloroquine (HCQ). In addition, the effects of these drugs were improved by simultaneous administration of azithromycin (AZM). The receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein binds to the cell surface angiotensin-converting enzyme 2 (ACE2) receptor, allowing virus entry and replication in host cells. The viral main protease (Mpro) and host cathepsin L (CTSL) are among the proteolytic systems involved in SARS-CoV-2 S protein activation. Hence, molecular docking studies were performed to test the binding performance of these three drugs against four targets. The findings showed AZM affinity scores (ΔG) with strong interactions with ACE2, CTSL, Mpro and RBD. CQ affinity scores showed three low-energy results (less negative) with ACE2, CTSL and RBD, and a firm bond score with Mpro. For HCQ, two results (ACE2 and Mpro) were firmly bound to the receptors, however CTSL and RBD showed low interaction energies. The differences in better interactions and affinity between HCQ and CQ with ACE2 and Mpro were probably due to structural differences between the drugs. On other hand, AZM not only showed more negative (better) values in affinity, but also in the number of interactions in all targets. Nevertheless, further studies are needed to investigate the antiviral properties of these drugs against SARS-CoV-2.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the highly transmissible coronavirus disease 2019 (COVID-19) [1]. This novel coronavirus, first reported in Wuhan, China, at the beginning of December 2019, had emerged rapidly worldwide. Following the implementation of human mobility control measures, such as extensive travel bans and quarantine in China, surveys showed that social restriction limited the spread of COVID-19 [2], however it remains a Public Health Emergency of International Concern (PHEIC).
Scientists from many countries are striving to understand, track and contain the COVID-19 pandemic. The spread of COVID-19 is increasing worldwide, with 14 043 176 confirmed cases and 597 583 deaths in less than 4 months (as of 18 July 2020) according to data from the World Heath Organization (WHO). Although drug repurposing has some limitations, repositioning of some drugs has been considered or suggested as potential candidates for treatment of the novel coronavirus disease [3], [4], [5].
Recent preliminary clinical trials conducted until now in China and France reported improved diseases outcomes with chloroquine (CQ) and hydroxychloroquine (HCQ) as shown by evidence of their effectiveness in reducing or eliminating the viral load of COVID-19 patients in a critical condition [6,7]. Moreover, their effects can be improved through their combination with azithromycin (AZM) [5,8]. Thus, CQ phosphate is already among the drugs with antiviral activity included in the latest version of the treatment guidelines issued by the National Health Commission of the People's Republic of China for the tentative treatment of novel coronavirus-induced pneumonia [4].
Some studies have discussed the possible mechanisms of AZM/CQ/HCQ against SARS-CoV-2 [9], [10], [11], [12]. It is known that the receptor-binding domain (RBD) of the spike (S) protein of the virus binds to the cell surface angiotensin-converting enzyme 2 (ACE2) receptor, allowing virus entry and replication inside host cells [9,10]. A recently published update has shown that the viral main protease (Mpro) might represent a suitable target for drugs inhibiting viral replication [11]. Preliminary data indicate that CQ interferes with SARS-CoV-2 by promoting an increase in the endosomal pH, the compartment where cleavage of the S protein is facilitated by the host protease cathepsin L (CTSL), which requires a low pH, thus impairing virus–endosome fusion and consequently preventing release into the cytosol [12], [13], [14].
Nevertheless, recently some studies have raised questions about the clinical efficacy of the abovementioned drugs. A multicentre, open-label, randomised controlled clinical trial did not show additional benefits in virus elimination of HCQ in association with specifically standard of care in patients with mild to moderate COVID-19. It also promoted an increased frequency of adverse events [15]. Other studies in patients who received HCQ and/or AZM reported that they were not significantly associated with differences in in-hospital mortality [16,17]. Meanwhile, a retrospective study demonstrated that addition of HCQ, on top of basic treatments, reduced the death risk in severe COVID-19 patients [18].
Therefore, the aim of this study was to evaluate the molecular interactions of AZM, CQ and HCQ through ACE2, CTSL, Mpro and RBD using molecular docking. Hence, we also aim to provide results that can be useful in other studies on the mechanisms of action of these drugs in the therapeutic approach to COVID-19.
2. Methods
2.1. Obtaining and preparation of ligands
The ligands AZM (CSID: 10482163), CQ (CSID: 2618) and HCQ (CSID: 3526) were obtained from the virtual repository ChemSpider (http://www.chemspider.com/) in .mol format. ChemSpider is a free chemical structure database that provides quick access to more than 67 million structures, properties and associated information [19]. Using AvogadroⓇ 1.2.0 software, the molecules were optimised, calculated with force field using MMFF94 type and converted into.pdb format [20].
2.2. Preparation of receptors
For coronaviruses, a single region of the spike (S) protein called the RBD mediates the interaction with the host cell receptor [21]. Thus, the ACE2 receptor and the RBD region of the same structure were obtained in the Protein Data Bank (PDB) (https://www.rcsb.org) (PDB: 6VW1 – obtained by X-ray diffraction, 2.68 Å resolution), through structural separation using SWISS-MODELⓇ (https://swissmodel.expasy.org/), denominated ACE2 (PDB: 6VW1, chain A) and RBD (PDB: 6VW1, chain B) by the software itself. The structures PDB: 2XU3, obtained by X-ray diffraction with 0.9 Å resolution, and PDB: 6Y2E, also obtained by X-ray diffraction with 1.75 Å resolution, were used for CTSL and Mpro receptors, respectively. The structures were obtained in .pdb format for use in molecular docking studies.
2.3. Molecular docking
AutoDock VinaⓇ v.1.1.3 software was used in all docking experiments [22]. In AutoDock VinaⓇ, the proteins were optimised by removing water and other residues not important for the study, and then a polar hydrogen group was added to all structures. The automatic grid determined the position of the native ligand in the connection by organising the grid coordinates (X, Y and Z). The binding capacity of the ligands and their corresponding binding affinity scores (ΔG) were used to determine the best molecular interactions. During the experiment, all fittings were treated as flexible and the ligands were also flexible. Fitting analyses were performed using PyMOLⓇ v.1.7.4.5 Edu and Biovia Discovery StudioⓇ v.4.5.
3. Results and discussion
3.1. Evaluation of fitting score (binding affinity)
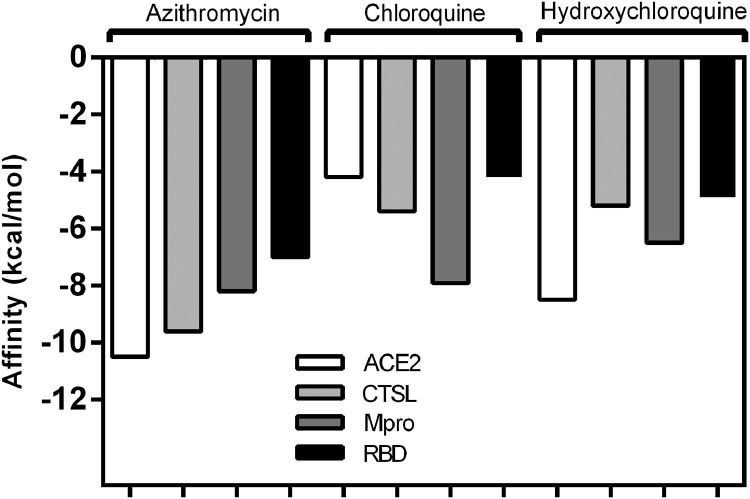
Before docking, the structures of ligands were prepared using their optimised form. At this stage, the ligands showed ten pre-established conformations for AZM, seven for CQ and eight for HCQ. Fig. 1 shows the values of the fitting score (binding affinity) for ACE2, CTSL, Mpro and RBD and their ligands.
Fig. 1.
Graphical representation of binding energies (ΔG, in kcal/mol) of molecular docking between the ligands [azithromycin, chloroquine and hydroxychloroquine] and targets [angiotensin-converting enzyme 2 (ACE2), cathepsin L (CTSL), viral main protease (Mpro) and the receptor-binding domain (RBD)] calculated by AutoDock VinaⓇ software.
AZM is a macrolide antibiotic generally used to treat infections such as pneumonia and upper respiratory tract infections. Its antibacterial mechanism of action is through inhibition of bacterial protein synthesis by binding to the 50S ribosomal subunit and blocking messenger RNA-directed polypeptide synthesis [23]. Moreover, it has also been used for the treatment of cancer as well as autoimmune and inflammatory diseases [24]. We found that AZM affinity scores showed strong interactions of –10.5 kcal/mol (ACE2), –9.6 kcal/mol (CTSL), –8.2 kcal/mol (Mpro) and –7.0 kcal/mol (RBD).
Although the antiviral mechanism of action of AZM is still unclear in some previously tested viral infections, studies have shown anti-Zika virus activity in vitro by inhibiting viral replication [25,26]. In an in vivo study, AZM was administered intranasally to infected mice and reduced the viral load of influenza A virus (H1N1) in the lungs [27]. In an in vitro study with the same virus, it also showed effective blockade of viral internalisation as well as inactivation of the endocytic activity of host cell progeny virus [27]. Therefore, our results suggest that AZM affects internalisation of the virus as well as its binding on the host cell surface. Another study regarding respiratory syncytial virus, found in common colds, hypothesised that macrolides may reduce the expression of activated intracellular protein RhoA (Ras homologue gene family, member A) and inhibit subsequent Rho kinase activation in human airway epithelial cells. This receptor is important for the fusion of viral F glycoprotein with cell membranes and the transfer of viral genome material into the cell [28].
CQ and HCQ are aminoquinolines traditionally used to treat malaria and both have also shown a therapeutic effect in non-malarial infections [29]. CQ affinity scores showed three low-energy scores (less negative) of –4.2 kcal/mol (ACE2), –5.4 kcal/mol (CTSL) and –4.2 kcal/mol (RBD) and a firm bond score of –7.9 kcal/mol with Mpro. On other hand, HCQ was firmly bound to the targets ACE2 and Mpro, with scores of –8.5 kcal/mol and –6.5 kcal/mol, respectively. CTSL and RBD, however, showed low interaction energies (–5.2 kcal/mol and –4.9 kcal/mol, respectively).
An extensive survey of the literature showed the versatility of CQ effects against diverse viral infections [30], including several respiratory diseases caused by influenza A and B viruses, influenza A H5N1 virus, Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-1 [12,30]. Furthermore, there is an ongoing randomised controlled clinical trial using HCQ and AZM in combination in 630 hospitalised and non-critical patients with COVID-19 infection, which is currently expecting results [31].
Based on our data, we conclude that while the interactions of CQ and HCQ showed better results with one and two receptors, respectively, AZM showed strong binding with all tested receptors, demonstrating a great binding potential in several biological processes related to viral replication of SARS-CoV-2. These drugs with a score above –6.0 kcal/mol were able to firmly bind to the structures [32] that perform the SARS-CoV-2 molecular replication process and are therefore potential candidates for inhibiting processes and reinforcing those currently showing promising results in clinical trials.
3.2. Differences in binding energy
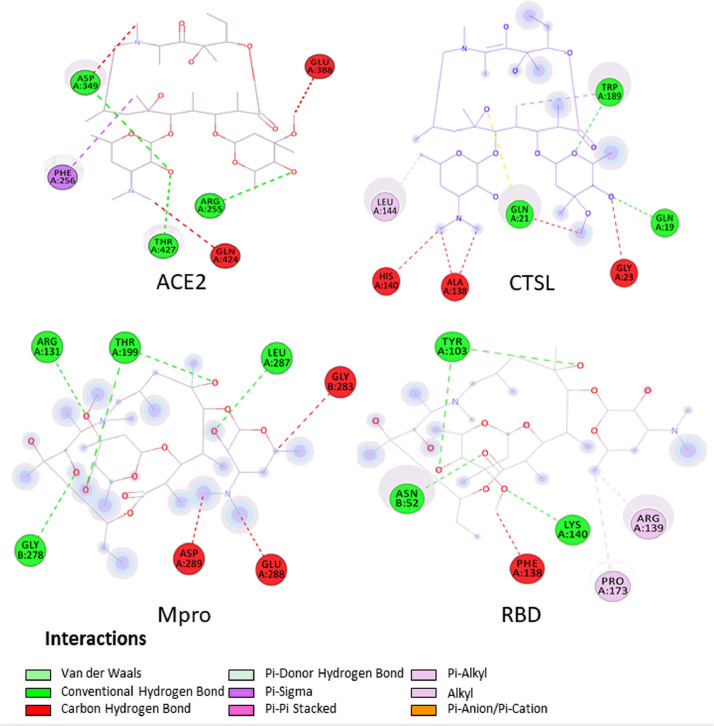
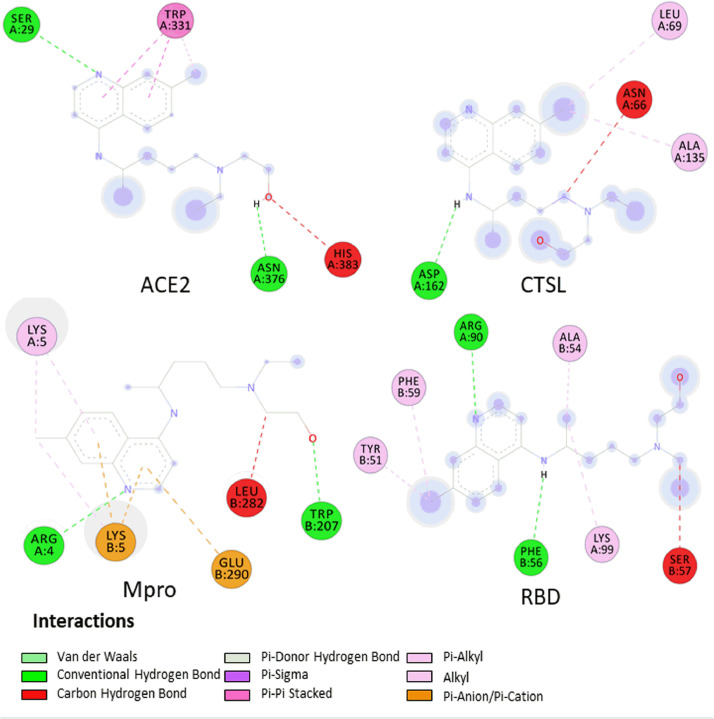
To analyse the possible reason for the differences in binding energies, the interactions formed after coupling were assessed using Biovia Discovery StudioⓇ v.4.5. Figs 2 , Fig. 3, Fig. 4 show the interactions formed between the drugs AZM, CQ and HCQ, respectively, and the four targets (ACE2, CTSL, Mpro and RBD) after two-dimensional coupling to better visualise the formed interactions and the types of constituent amino acids in the interactions.
Fig. 2.
Interactions established in two dimensions in Biovia Discovery StudioⓇ 4.5 software after docking between azithromycin and angiotensin-converting enzyme 2 (ACE2), cathepsin L (CTSL), viral main protease (Mpro) and the receptor-binding domain (RBD). Coupling scores are listed on each complex to reflect the binding power. Receptor amino acids are represented by spheres of different colours around the structure. The H-bonds are shown as dashed green lines (darker colour), while the other dashed lines represent hydrophobic interactions and other types of intermolecular interactions.
Fig. 3.
Interactions established in two dimensions in Biovia Discovery StudioⓇ 4.5 software after docking between chloroquine and angiotensin-converting enzyme 2 (ACE2), cathepsin L (CTSL), viral main protease (Mpro) and the receptor-binding domain (RBD). Coupling scores are listed on each complex to reflect the binding power. Receptor amino acids are represented by spheres of different colours around the structure. The H-bonds are shown as dashed green lines (darker colour), while the other dashed lines represent hydrophobic interactions and other types of intermolecular interactions.
Fig. 4.
Interactions established in two dimensions in Biovia Discovery StudioⓇ 4.5 software after docking between hydroxychloroquine and angiotensin-converting enzyme 2 (ACE2), cathepsin L (CTSL), viral main protease (Mpro) and the receptor-binding domain (RBD). Coupling scores are listed on each complex to reflect the binding power. Receptor amino acids are represented by spheres of different colours around the structure. The H-bonds are shown as dashed green lines (darker colour), while the other dashed lines represent hydrophobic interactions and other types of intermolecular interactions.
ACE2 is widely distributed in the heart, kidneys, lungs and testicles and plays a vital role in the renin–angiotensin–aldosterone system (RAAS) and homeostasis [33]. In silico and in vivo studies have suggested a potential deleterious effect of the RAAS [10,34], and a pilot trial using soluble human recombinant ACE2 (APN01) has recently been initiated in patients with COVID-19 (Clinicaltrials.gov ID: NCT04287686) [35,36].
The SARS-CoV-2 mRNA encodes essential proteins, including the 4 main structural proteins [small envelope (E) protein, matrix (M) protein, nucleocapsid (N) protein and spike (S) glycoprotein] and 16 non-structural proteins (NSPs). The M and E proteins play a role in particle assembly and release [10,13]. The S glycoprotein is responsible for a critical step in virus entry as it binds to host cell receptor (ACE2) and fuses the viral membrane with the cell membrane. This glycoprotein has two subunits, S1 (key function domain – RBD, cell tropism) and S2 [mediates virus–cell membrane fusion through heptad repeat 1 (HR1) and heptad repeat (HR2) domains] [37].
However, a recent study has shown that SARS-CoV-2 interaction with ACE2 alone is not sufficient to allow host cell entry, and preliminary studies aim to identify proteolytic systems involved in S protein activation by SARS-CoV-2 [38]. The pH-dependent endosomal cell factors, such as cysteine protease, are determinant to SARS-CoV membrane fusion, especially CTSL [39]. Unlike other proteases, CTSL is ubiquitously expressed; the cleavage site is reported to be close to the predicted S1/S2 boundary, a critical site for proteolysis [40]. Since it is most commonly associated with the activation of viral glycoproteins (MERS-CoV, HCoV-229E and MHV-2) [41], several studies have suggested that cathepsin inhibitors are possible virus therapeutic targets and might have broad applicability [42,43].
Another important step for SARS-CoV-2 replication is the cysteine protease Mpro. Mpro participates in the proteolysis process, cleaving polyproteins that are encoded by the coronavirus genome to mature NSPs, assisting viral replication and transcription [44]. Mpro (or Nsp5) cleaves the polyproteins at 11 conserved sites [45]. During infection, the replication/transcription complex is anchored to double‐membrane vesicles that are derived from the endoplasmic reticulum or lysosomal membrane [46].
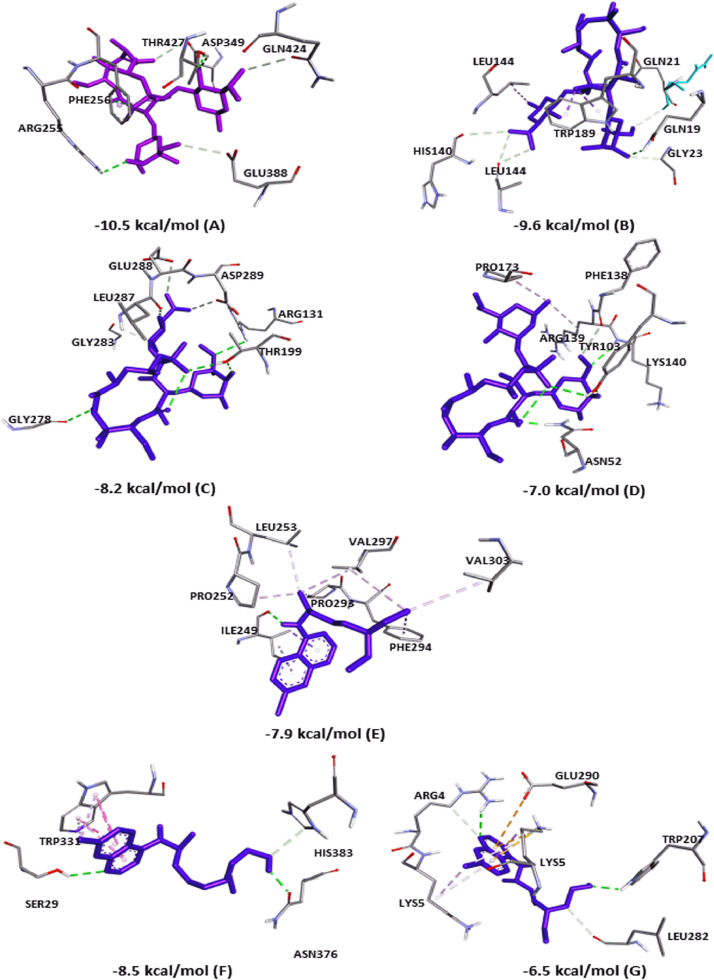
In the current study, AZM showed H-bonds in all couplings, with three interactions in ACE2 (ASP349, ARG255, THR427) and CTSL (TRP189, GLN19, GLN21). However, there were a greater number of interactions in the docking with Mpro (ARG131, LEU287, GLY278 and two interactions with THR199) and RBD (Chain A: LYS140 and two interactions with TYR103; Chain B: ASN52). In addition, the fittings showed other types of bonds, such as carbon–hydrogen bonds, π–sigma bonds, alkyl bonds and Van der Waals forces. Fig. 5 shows the three-dimensional (3D) bonds of AZM between the residues of the receptor structures. The affinity value of each coupling is also shown.
Fig. 5.
Best affinity interactions established after coupling the drugs azithromycin (AZM), chloroquine (CQ) and hydroxychloroquine (HCQ) with receptors for the proliferation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a three-dimensional format. The H-bonds are shown as green lines, while hydrophobic interactions are the remaining lines. Residues are identified by abbreviations/numbering in each field, and the fitting scores are listed in each complex. (A–D) Interaction between AZM and ACE2 (A), CTSL (B), Mpro (C) and RBD (D); (E) interaction between CQ and Mpro; and (F,G) interaction between HCQ and ACE2 (F) and Mpro (G). ACE2, angiotensin-converting enzyme 2; CTSL, cathepsin L; Mpro, viral main protease; RBD, receptor-binding domain.
Regarding CQ, only two couplings showed H-bonds, a single interaction with CTSL (ASN66) and an interaction with Mpro (ILE249). In this coupling, eight alkyl bonds were verified owing to the capacity of several saturated carbons in the structures. In the two dockings that did not show an H connection (ACE2 and RBD), many interactions of the carbon–hydrogen type, π–sigma and Van der Waals forces were also observed. With HCQ, four couplings showed H-bonds, two interactions with ACE2 (ASN376, SER29) and a π–π stacked interaction. That latter occurs when two aromatic rings interact with each other, in this case the two HCQ rings bonded with the TRP331 ring [47]. There was an H-bond (ASP162) in the interaction with CTSL, two H-bonds (Chain A: ARG90; Chain B: PHE56) with RBD, and two H-bond interactions (Chain A: ARG4; Chain B: TRP207) between HCQ and Mpro. Furthermore, π–anion, π–cation and carbon–hydrogen types of bonds were observed.
The structures of CQ and HCQ that showed the highest binding affinity are shown in Fig. 5 in a 3D format. HCQ has one hydroxyl group more than CQ. This difference allows HCQ to have a greater role of regioselectivity and binding character in molecular simulations, because oxygen is an atom with greater regioselectivity [48]. This may explain the difference in docking results between these drugs.
Modulation of autophagy may be the mechanism responsible for the success of preliminary studies against SARS-CoV-2 [49]. It was reported that HCQ and CQ are lysosomotropic agents. Their effect on inhibition of autophagy is due to the impact of lysosomal acidification, inhibiting autophagosome–lysosome fusion and inactivating enzymes that several viruses require for replication, which in the case of SARS-CoV-2 may be Mpro [50]. During infection, autophagy can play either a proviral or antiviral role depending on the virus, the cell type and the cell environment [51,52]. In case of the SARS‐CoV-1, autophagy inhibition is necessary for the success of treatment [52].
However, understanding these molecular details requires further investigation. In fact, this assumption has been investigated in relation to other viral infections [49]. A recent study showed that the SARS-CoV-2 Mpro has 96% homology to SARS-CoV-1 [53]. Among the targets related to coronavirus diseases, a greater number of patents of SARS-CoV-1 Mpro inhibitor complexes have been registered and more potential drug candidates are emerging [54]. Some of these inhibitors (peptidic or peptidomimetic) have been used to attain covalent binding to the active-site cysteine of Mpro [55,56].
Several published studies with inhibitors of viral proteases have supported the theory that the SARS-CoV-2 Mpro could be a good target for therapeutic agents [54,[57], [58], [59], [60]]. Furthermore, remdesivir and CQ, alone and in combination, are under investigation, showing that they significantly blocked SARS-CoV-2 replication and that patients were declared to be clinically recovered [61]. These data may also be useful to research potential inhibitors of this protease, aiming to block viral replication in COVID-19 [62].
The quinoline-based drugs, such as CQ and HCQ, accumulate in the acidic lysosomes, aggravating endoplasmic reticulum stress [50]. In this context, the proteasomes and inhibitors of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) might be a strategy [63], a theory that was recently reinforced by Wang et al. by showing the CQ effectively inhibits SARS-CoV-2 in vitro [14]. Zhou et al. showed that teicoplanin blocks Ebolavirus entry by specifically inhibiting the activity of CTSL, a tetrahydroquinoline oxocarbazate, on Ebola virus and SARS-CoV-1 [64].
A recent study using molecular modelling showed that CQ and HCQ bind to sialic acid-containing gangliosides, a site responsible for viral primary attachment along the respiratory tract in coronavirus diseases, besides ACE2, which strengthens the hypothesis that these drugs could act as antivirals (against SARS-CoV-2) [65]. The same study demonstrated that HCQ is more potent than CQ. Similar results were found in other studies with SARS-CoV and SARS-CoV-2, respectively [66,67]. Thus, our results corroborate these findings, showing that HCQ had better interactions and affinity when compared with the same target (ACE2 and Mpro).
The CQ and HCQ combination has been reported in an early clinical trial conducted in COVID-19 Chinese patients and was shown to be efficient against SARS-CoV-2 [4,5,7]. Another preliminary study also suggested promising results of the HCQ and AZM combination. At Day 6 post-inclusion, 100% of patients treated with the HCQ and AZM combination were virologically cured compared with 57.1% of patients treated with HCQ only and 12.5% in untreated patients [8]. Consistent with this idea, our molecular modelling study has simultaneously identified the binding of ACE2, Mpro, CTSL and RBD (to AZM/CQ/HCQ) against SARS-CoV-2, to surmise the molecular mechanisms underlying the antiviral mechanisms. Interestingly, our simulations indicated that AZM has better affinity than HCQ, and a possible association with this drug might increase the antiviral activity of HCQ against SARS-CoV-2. Further studies will help clarify this point.
4. Conclusion
To date, no drug or vaccine has been approved for clinical use as an antiviral agent against COVID-19 or against any human coronavirus infection. Due to the need for therapeutic intervention against COVID-19, several efforts have been made to identify appropriate targets to develop specific antivirals or to repurpose drugs against this newly emerging pathogen. Our results showed that HCQ achieved better interactions and affinity with ACE2 and Mpro, whilst CQ achieved better results with Mpro and CTSL. This is probably due to structural differences between the drugs. AZM, on other hand, not only showed more negative (better) values in affinity, but also regarding the number of interactions. AZM showed more promising results than HCQ and CQ in all targets. Thus, it is suggested as a better candidate for inhibition of the processes that contribute to viral replication. However, further studies are needed to validate the antiviral properties of these drugs against SARS-CoV-2.
Data availability statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding: This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraemer MUG, Yang CH, Gutierrez B, Wu CH, Klein B, Pigott DM. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368:493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 5.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/J.JCRC.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14 doi: 10.5582/bst.2020.01047. 73–73. [DOI] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;67:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ton AT, Gentile F, Hsing M, Ban F, Cherkasov A. Rapid identification of potential inhibitors of SARS‐CoV‐2 main protease by deep docking of 1.3 billion compounds. Mol Inform. 2020 Mar 11 doi: 10.1002/minf.202000028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo D. Old weapon for new enemy: drug repurposing for treatment of newly emerging viral diseases. Virol Sin. 2020;35:253–255. doi: 10.1007/s12250-020-00204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020:369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;82:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu B, Li C, Chen P, Zhou N, Wang L, Li J. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020 May 15 doi: 10.1007/s11427-020-1732-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pence HE, Williams A. ChemSpider: an online chemical information resource. J Chem Educ. 2010;87:1123–1124. doi: 10.1021/ed100697w. [DOI] [Google Scholar]
- 20.Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints. 2020 doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- 21.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelić D, Antolović R. From erythromycin to azithromycin and new potential ribosome-binding antimicrobials. Antibiotics (Basel) 2016;5:E29. doi: 10.3390/antibiotics5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel PH, Hashmi MF. StatPearls. StatPearls Publishing; Treasure Island, FL: 2020. Macrolides [updated 2019 Nov 28] [Google Scholar]
- 25.Bosseboeuf E, Aubry M, Nhan T, De Pina JJ, Rolain JM, Raoult D. Azithromycin inhibits the replication of Zika virus. J Antivir Antiretrovir. 2018;10:6–11. doi: 10.4172/1948-5964.1000173. [DOI] [Google Scholar]
- 26.Li C, Zu S, Deng Y-Q, Li D, Parvatiyar K, Quanquin N. Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.00394-19. e00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo) 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- 28.Asada M, Yoshida M, Suzuki T, Hatachi Y, Sasaki T. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 2009;83:191–200. doi: 10.1016/j.antiviral.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Alessandro S, Scaccabarozzi D, Signorini L, Perego F, Ilboudo DP, Ferrante P. The use of antimalarial drugs against viral infection. Microorganisms. 2020;8:85. doi: 10.3390/microorganisms8010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov. Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-Cov-2 virus (COVID-19) (Coalition-I). ClinicalTrials.gov ID NCT04322123. https://clinicaltrials.gov/ct2/show/NCT04322123[accessed 20 June 2020].
- 32.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ocaranza MP, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catapang JK, Billones JB. On the generation of novel ligands for SARS-CoV-2 protease and ACE2 receptor via constrained graph variational autoencoders. ChemRxiv. 2020 Mar 20 doi: 10.26434/chemrxiv.12011157.v1. [DOI] [Google Scholar]
- 39.Gierer S, Bertram S, Kaup F, Wrensch F, Heurich A, Krämer-Kühl A. The spike protein of the emerging Betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. 2013;87:5502–5511. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Nunneley JW. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/J.ANTIVIRAL.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Báez-Santos YM, St John SE, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye G, Wang X, Tong X, Shi Y, Fu ZF, Peng G. Structural basis for inhibiting porcine epidemic diarrhea virus replication with the 3C-like protease inhibitor GC376. Viruses. 2020;12:240. doi: 10.3390/v12020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang WR, Wang Y, Cui PF, Xing L, Lee J, Kim D. Applications of π–π stacking interactions in the design of drug-delivery systems. J Control Release. 2019;294:311–326. doi: 10.1016/j.jconrel.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Sun YJ, Liu L, Cheng L. Regioselective synthesis and anticancer evaluation of H2O2-activatable nucleosides. Chem Commun (Camb) 2020;56:6484–6487. doi: 10.1039/d0cc02245d. [DOI] [PubMed] [Google Scholar]
- 49.Choi Y, Bowman JW, Jung JU. Autophagy during viral infection—a double-edged sword. Nat Rev Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ. Chloroquine inhibits autophagic flux by decreasing autophagosome–lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdoli A, Alirezaei M, Mehrbod P, Forouzanfar F. Autophagy: the multi-purpose bridge in viral infections and host cells. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1973. e1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabaan AA, Alahmed SH, Bazzi AM, Alhani HM. A review of candidate therapies for Middle East respiratory syndrome from a molecular perspective. J Med Microbiol. 2017;66:1261–1274. doi: 10.1099/jmm.0.000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Zhou Q, Li Y, Garner L V, Watkins SP, Carter LJ. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anand J, Ziebuhr P, Wadhwani JR, Mesters R, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Yang M, Ding Y, Liu Y, Lou Z, Zhou Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou K-C, Wei D-Q, Zhong W-Z. Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem Biophys Res Commun. 2003;308:148–151. doi: 10.1016/S0006-291X(03)01342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 59.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golden EB, Cho H-Y, Hofman FM, Louie SG, Schönthal AH, Chen TC. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38:E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 64.Zhou N, Pan T, Zhang J, Li Q, Zhang X, Bai C. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J Biol Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55 doi: 10.1016/J.IJANTIMICAG.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 67.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding: This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Competing interests: None declared.
Ethical approval: Not required.