Graphical abstract
Keywords: Fucoidan, Sargassum wightii, Acute toxicity, Subacute toxicity
Highlights
-
•
Recent research proves fucoidan to be very effective in the management of cancer and diabetes.
-
•
There are only limited information available on safety data with long term treatment of fucoidan.
-
•
This study reports the toxicity profile of fucoidan derived from Sargassum wightii greville for the first time.
-
•
28 days repeated oral administration of fucoidan at the dose of 100, 200 and 400 mg/kg does not cause any significant organ toxicity.
Abstract
Objective
The present study aimed to investigate the acute and subacute toxicity profile of fucoidan obtained from Sargassum wightii Greville, a brown marine algae in order to assess its safety.
Methods
Fucoidan was isolated from Sargassum wightii Greviile and subjected to FTIR analysis to confirm the functional groups. In acute toxicity study, a single dose of fucoidan (2000 mg/kg) was orally administered to three female rats as per OECD guideline 423. OECD guidelines 407 was adopted for subacute toxicity study. Fucoidan was orally administered to male and female rats at doses of 100, 200 and 400 mg/kg. Hematological, biochemical and histopathological analyses were carried out.
Results
FTIR analysis confirmed the presence of major functional groups. The animals did not show any remarkable toxic signs or mortality in acute toxicity study at single oral administration of fucoidan at the dose of 2000 mg/kg bodyweight. In subacute toxicity, no statistically significant difference in body weight, relative weight of vital organs, food and water intake compared to the control group was observed. Serum glucose and cholesterol showed a statistically significant reduction at all the doses when compared to normal control and the reduction was in a dose dependent manner. There were no other changes observed in biochemical or haematological parameters. Histopathological analysis showed no significant toxic signs at organ levels in treated groups when compared to normal control.
Conclusions
Based on the results obtained from acute and subacute toxicity study, fucoidan is considered to be safe in the models tested, which encourages its long term administration for medicinal uses. This study supports the application of fucoidan as a traditional medicine.
1. Introduction
Marine seaweeds or algae constitute an important part in traditional medicine and diet since prehistoric time. Brown marine algae are the most intensely studied type of seaweeds. They are composed of potentially bioactive polysaccharides like fucoidan [1]. Fucoidan is chiefly derived from Fucus vesiculosus, Sargassum wightii, Undaria pinnatitinda, Ecklonia kurome [2]. Among the various brown marine algae, Sargassum species received more attention recently for its large amount of cell-wall polysaccharides that are mostly in the form of sulfated polysaccharides [3]. Sargassum species is extensively used in traditional chinese medicine to treat various diseases such as arteriosclerosis, skin diseases, neurosis, hepatomegaly, high blood pressure, acute esophagitis, chronic bronchitis, goitre [4].
Polysaccharides are biopolymers comprised of monosaccharides connected through glycosidic bonds which can be either continuous or surrounded by divided side chains that has common formula of Cx(H2O)y, where x is usually a large number between 200 and 2500 [5]. The structure of fucoidan consists chiefly of polymers formed by branched polysaccharide sulfate esters with l-fucose building blocks [6]. There are also other essential sugars including xylose, galactose, mannose present in minor amounts alongwith l-fuocse. The differences observed in the extent of these sugars and sulfate content make fucoidan unique from each source of brown marine algae. The differences known in the biological activities of F-fucoidan, U-fucoidan,and G-fucoidan further strengthen the exclusivity of fucoidan from each source of brown marine algae [2]. The bioactivity of fucoidan is related to their structural make-up, monosaccharide composition, sulfate content, position of sulfate ester groups, and molecular weight. Mild extraction technology maintains the structural integrity of the fucoidan polysaccharides and preserves their biological properties [1].
Currently, there is a global level research interest for fucoidan as it is revealed to possess a number of Pharmacological properties such as anti-tumor [7,8], antioxidant and anti-coagulant [9], anti-viral [10,11], neuroprotectant [12] and anti-inflammatory in the brain [13]. It is also part of dietary supplementary products, which has currently increased as it is evident with improved commercialization [14]. Fucoidan has been used as an anticancer drug in Chinese traditional medicine [15]. There is considerable research interest in the isolation of fucoidan from brown marine alge, identification of the bioactive components, and elucidation of the molecular mechanisms involved. Despite the pharmacological activities reported, the toxicology of fucoidan have not been thoroughly studied or reported. Kim et al., (2010) have reported the toxicity profile of fucidan obtained from Sporophyll of Undaria pinnatifida. However, there are no previous reports on toxicity profile of fucoidan derived from Sargassum wightii Greville (S. wightii). Thereby it is highly important to rule out any toxicity associated with fucoidan derived from S. wightii to strengthen its medicinal use. Thus, the present study is aimed to investigate the acute and subacute toxicity profile of fucoidan isolated from S. wightii.
2. Materials and methods
2.1. Collection and identification
The fresh S. wightii was collected from coastal regions of Rameshwaram, Tamil Nadu, India during October 2015. It was authenticated by Dr. Yoganarasimhan, Taxonomist and a voucher specimen (#52) was prepared as per the guidelines and deposited at the Herbarium of Faculty of Pharmacy, M. S. Ramaiah University of Applied Sciences, Bangalore, for future reference.
2.2. Processing of S. wightii
Seaweeds were washed thoroughly with sea water during collection to get rid of any redundant matter such as sand particles, salt, epiphytes or any other foreign materials. Later, the seaweeds were thoroughly washed with running tap water and then distilled water. It was shade dried at room temperature, powdered and then packed in airtight containers and stored in refrigerator for further study.
2.3. Isolation of fucoidan
The processed seaweed was coarsely powdered using mixergrinder and sieved to remove seaweed fibre. Ethanol 1 L was added to 200 g of coarsely powdered seaweed and the mixture was constantly stirred using a mechanical stirrer for 12 h at room temperature in order to remove the proteins and pigments present in it. The mixture was then centrifuged and the residue was collected and dried at room temperature. From the dried residue, 5 g was taken and added with 100 mL of distilled water. The mixture was kept in a water bath at 65 °C for 1 h with constant stirring. The supernatant of this mixture was collected after centrifugation (18,500 x g) for about 10 min. To the supernatant, 1% CaCl2 was added and the solution was kept overnight (4 °C) to precipitate alginic acid. The mixture was then centrifuged (18,500 x g) for 10 min and the supernatant was collected.
To the supernatant, ethanol (99 %) was added to gain the final concentration of 30 % ethanol and the solution was placed at 4 °C for 4 h. The mixture was then centrifuged (18500 x g) for 10 min and the supernatant was collected. Further, some more ethanol was added to make the final concentration into 70 % ethanol and the solution was kept overnight at 4 °C. The mixture was then filtered through a nylon membrane and the obtained fucoidan was washed with ethanol and finally acetone and dried at room temperature overnight. The yield of the fucoidan was calculated and the dried fucoidan was packed in an airtight container until further use. FTIR analysis was performed to identify and confirm the functional groups present in fucoidan [16].
2.4. FTIR analysis
Fucoidan powder 2 mg was ground evenly with 100 mg KBr until particles measured <2.5 μm in size. The transparent KBr pieces were made at 500 kg/cm2. The fourier transform infrared spectroscopy (FTIR) spectra were obtained using a FT-730 spectrometer. The signals were automatically collected using 60n scans over the range of 4000−400 cm−1 at a resolution of 16 cm−1 and were compared to a background spectrum collected from the KBr alone at room temperature.
2.5. Toxicity studies
2.5.1. Experimental animals
All the animals (Wistar rats) used for this study were obtained from the Animal house facility, Faculty of Pharmacy, M. S. Ramaiah University of Applied Sciences (Reg No: 220/PO/ReBi/S/2000/CPCSEA/ 02.05.2016)
The animals were housed in polypropylene cages. The temperature in the experimental animal room was maintained at 22 °C (±3 °C), with the relative humidity 50–60 % and artificial lighting, the sequence being 12 h light and dark. The animals were maintained on normal diet and water. Food but not water was withheld overnight for experimental animals. Experimental procedures were conducted in accordance with the guidelines provided by Institutional Animal Ethics Committee and prior approval was obtained with the approval no. MSRFPH/PFP-59/2015.
2.5.2. Acute toxicity study
Acute toxicity study was performed as per OECD Guidelines 423 [17]. Fucoidan being a traditional medicine, with no reports of mortality [18,19] even in large doses, a limit test was carried out. Single oral dose (2000 mg/kg) of fucoidan was given by oral gavage to three female Wistar rats.
After dosing, for the first 30 min, animals were monitored individually. They were given special attention for the first 4 h for any toxic signs and observation was extended to first 24 h and daily there-after for a total of 14 days. The animals were observed for any toxic signs or pre-terminal deaths individually and recorded. Once in a week, individual body weight was monitored for all the animals to find out any drastic changes. The colour and consistency of faeces, changes in fur and skin, mucous membranes (nasal) and eyes of the animal were observed on a weekly basis.
Physical observation such as changes in circulatory (heart rate), respiratory (rate), autonomic (piloerection, lacrimation, salivation, urinary incontinence and defecation) and central nervous system (drowsiness, ptosis, gait, eye prominence, eyelid closure, convulsions, biting, straub’s test, motor in-coordination, writhing, stereotypy, aggression, righting reflex, pinnal reflex, and corneal reflex, tremors and convulsions) were also monitored.
2.5.3. Subacute toxicity
Subacute toxicity study was carried out as per OECD guidelines 407 [20]. Animals were divided into four groups, each group consisting of six animals. Group I served as normal control and received vehicle. Group II, III, IV received fucoidan 100 mg/kg, 200 mg/kg and 400 mg/kg respectively. All Animals were dosed orally once daily for 28 days. Dosing time was maintained constant to minimize the biological variation among animals.
At the end of the study, animals were euthanized using excess carbon dioxide anesthesia and blood sample was collected through retro orbital plexus into non-heparinized and heparinized tubes for biochemical and haematological parameters respectively. Biochemical analysis was carried out to explore major toxic effects in tissues. Specifically, effects on liver and kidney which included investigations of serum sodium, potassium [21], glucose [22], total protein and albumin [[23], [24], [25]], urea [26], 1962), creatinine [27], total cholesterol [28,29], alanine aminotransferase, aspartate aminotransferase [30]. Haematological parameters such as haemoglobin content, total leucocyte count, erythrocyte count and platelet were also determined [31].
All animals in the study were subjected to a complete, detailed gross necropsy. It included thorough examination of the external surface of the body, all orifices, thoracic, cranial and abdominal cavities. The wet weight of vital organs such as liver, lungs, spleen, brain, kidneys, heart was taken immediately after dissection to avoid drying. All the organs were also examined macroscopically for any gross lesions through naked eye. The tissues of testes, kidney, brain, pancreas, heart, lungs, spleen, ovary, stomach, intestine, liver were preserved in the fixation medium and subjected to histopathological examination.
3. Statistical analysis
Data were expressed as mean ± SEM. Significant difference between groups were determined using One-Way ANOVA followed by Tukey's multiple comparison. P < 0.05 was considered as significant.
4. Results
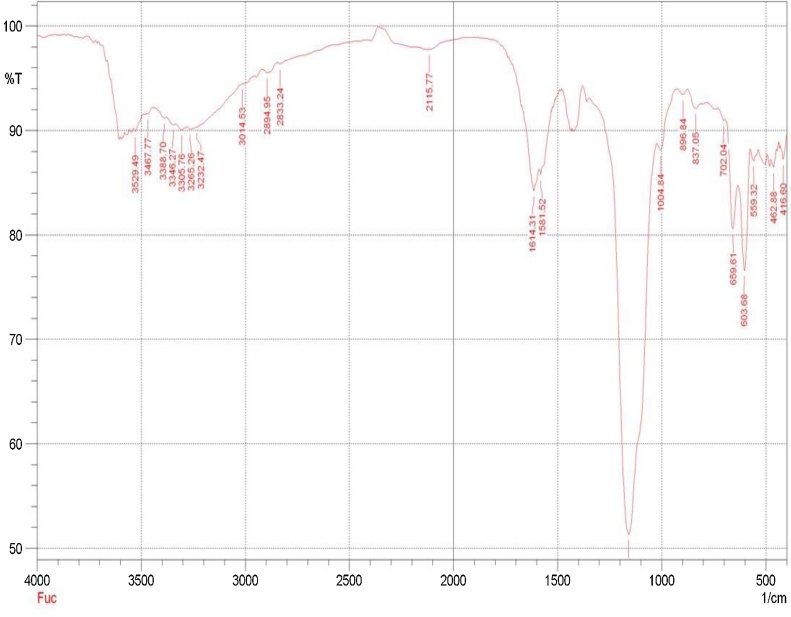
The percentage yield of isolated fucoidan was found to be in the range of 0.7–1 %. The FTIR analysis of Fucoidan from S.wightii is represented in Fig. 1. The band at 3467.77 is attributed to O—H stretching vibration. A weak band at 2115.77 is due to presence of C CO . A band at 1614.31 indicated the presence of O—CO— stretching vibration. The sulfate SO stretching is represented by a band 1159.14, Whereas C—OH deformation is represented at 1581.22 band whereas C—O stretching is indicated at 1004.84 band. C1-H deformation is represented at a band in 896.84. A band at 837.05 may be due to C—OS stretching of sulf—ate group, whereas band 603.66 indicated the presence of CC -H stretching vibration.
Fig. 1.
The characteristic peaks in FTIR representing the presence of various functional groups.
4.1. Acute toxicity study
Fucoidan did not induce any mortality or pre-terminal death in acute toxicity study. No changes were observed in salivation, lacrimation, perspiration, piloerection, micturition, and defecation. The animals were observed for ptosis, drowsiness, stereotypy, aggression, tremors, convulsion, Straub’s test, motor in-coordination, writhing and no abnormalities were observed in the treated animals. Gait, righting reflex and corneal reflex were found to be normal. Skin, fur, eyes, and body weight of animals were found to be normal. Tremors, lethargy, diarrhoea and coma were not observed throughout the study period.
4.2. Subacute toxicity study
Subacute toxicity study of fucoidan was performed according to OECD guidelines 407 in Wistar albino rats of either sex. The doses were derived from the results of acute toxicity limit test which was carried out at 2000 mg/kg. Since no toxic signs or mortality was observed, 1/5th, 1/10th, and 1/20th dose of 2000 mg/kg was selected for subacute toxicity study. During the course of study, animals did not show any aberrations in behaviour or autonomic activity. They were observed to have normal motor and sensory functions. They were also found to be free of any toxic signs during as well as at the end of the study.
The variations in body weight were monitored and recorded on a weekly basis and represented in Table 1. The body weight of animals treated with extract 100, 200 and 400 mg/kg were comparable with that of the standard and there were no significant changes between groups. Slight variations, either increase or decrease were noted in body weight overtime, which is correlated to normal physiological process.
Table 1.
Body weight changes on a weekly basis (g).
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV(400 mg/Kg) |
|---|---|---|---|---|
| 0th day | 178.5 ± 3.68 | 201.16 ± 3.29 | 191.66 ± 4.50 | 200.5 ± 7.48 |
| 7th day | 183.83 ± 3.36 | 204.5 ± 3.18 | 194.83 ± 4.94 | 201.83 ± 7.58 |
| 14th day | 188.33 ± 3.31 | 208 ± 3.69 | 197.5 ± 4.72 | 205 ± 7.49 |
| 21st day | 193.33 ± 3.94 | 211 ± 3.45 | 201 ± 5.07 | 207.83 ± 6.68 |
| 28th day | 198.16 ± 4.18 | 215.83 ± 2.94 | 203.66 ± 5.05 | 209.33 ± 6.30 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
Food and water intake was monitored and the results are represented in Table 2, Table 3 respectively on a weekly basis. There were no significant changes in food as well as water intake between control and fucoidan treated animals. Thereby it is suggested that fucoidan did not alter the appetite or thirstiness.
Table 2.
Food intake on a weekly basis (g).
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV(400 mg/Kg) |
|---|---|---|---|---|
| Week 1 | 24.5 ± 0.34 | 25.5 ± 0.76 | 23.66 ± 0.91 | 24 ± 1.15 |
| Week 2 | 25.16 ± 0.65 | 25.66 ± 1.22 | 24.66 ± 0.66 | 24.16 ± 0.60 |
| Week 3 | 24.66±.084 | 26.33 ± 1.16 | 24.5 ± 0.71 | 24.33 ± 0.42 |
| Week 4 | 23.16 ± 1.01 | 26.83 ± 0.70 | 25.33 ± 0.91 | 25.16 ± 0.54 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
Table 3.
Water Intake on a weekly basis (ml).
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV(400 mg/Kg) |
|---|---|---|---|---|
| Week 1 | 49.66 ± 2.17 | 51 ± 1.88 | 50.5 ± 1.33 | 47.5 ± 2.63 |
| Week 2 | 51.16 ± 1.53 | 53 ± 1.84 | 51 ± 1.73 | 49.33 ± 2.76 |
| Week 3 | 51.66 ± 1.16 | 53.16 ± 1.70 | 52.33 ± 1.87 | 50.33 ± 2.27 |
| Week 4 | 54 ± 1.31 | 52.16 ± 1.44 | 51.5 ± 2.17 | 50.66 ± 2.06 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
4.2.1. Biochemical and haematological analysis
Haematological parameters including RBC, WBC, Platelets and Hb were determined at the end of 28 days and the results are presented in Table 4. There were no statistically significant differences in the level of RBC, WBC, Platelets or haemoglobin in the fucoidan treated animals compared to control. The results of 100, 200 and 400 mg/kg fucoidan treated animals were comparable to that of control.
Table 4.
Hematological Parameters.
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV(400 mg/Kg) |
|---|---|---|---|---|
| RBC(106/mm3) | 10.95 ± 0.19 | 11.75 ± 0.20 | 11.09 ± 0.29 | 10.45 ± 0.09 |
| WBC(103/mm3) | 10.4 ± 0.09 | 10.58 ± 0.37 | 10.94 ± 0.27 | 11.03 ± 0.23 |
| Platelets(Lakhs/mm3) | 4.23 ± 0.14 | 4.23 ± 0.15 | 4.5 ± 0.11 | 4.3 ± 0.13 |
| Hb(g/dl) | 13.1 ± 0.25 | 13.66 ± 0.34 | 13.38 ± 0.33 | 13.51 ± 0.23 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
Biochemical analyses on various parameters were performed namely serum glucose, cholesterol, albumin, total protein, creatinine, liver enzymes including alanine transaminase (ALT), aspartate transaminase (AST), urea and electrolytes including sodium, potassium. The results of biochemical analyses are reported in Table 5. The parameters such as albumin, total protein, creatinine, ALT, AST, urea sodium, potassium did not show any significant changes and were found to be normal at the end of 28 days treatment. There was a statistically significant decrease in the level of glucose with fucoidan treated animals with all the dose ranges i.e. 100, 200 and 400 mg/kg compared to normal group. Cholesterol level was found to be significantly reduced in fucoidan treated animals with all the dose ranges i.e. 100, 200 and 400 mg/kg compared to normal group.
Table 5.
Biochemical parameters.
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV (400 mg/Kg) |
|---|---|---|---|---|
| Glucose (mg/dL) | 82.81 ± 1.96 | 54.50 ± 1.52a | 48.21 ± 1.05a | 41.66 ± 0.38a |
| Cholesterol (mg/dL) | 74.5 ± 0.61 | 63.5 ± 1.25 a | 54 ± 1.80a | 47.33 ± 1.49a |
| Albumin (g/dL) | 4.03 ± 0.13 | 3.96 ± 0.10 | 4.03 ± 0.12 | 3.85 ± 0.08 |
| Total protein (g/dL) | 7.13 ± 0.23 | 7.06 ± 0.16 | 7.13 ± 0.12 | 7.13 ± 0.08 |
| Creatinine (mg/dL) | 0.59 ± 0.022 | 0.65 ± 0.016 | 0.60 ± 0.021 | 0.59 ± 0.01 |
| AST (U/L) | 217.83 ± 2.62 | 218 ± 2.26 | 212.33 ± 1.58 | 215 ± 1.00 |
| ALT (U/L) | 58.16 ± 2.28 | 54.16 ± 1.16 | 57.5 ± 1.99 | 52.66 ± 1.66 |
| Urea (mg/dL) | 36.33 ± 1.22 | 36 ± 0.68 | 35.33 ± 0.84 | 34.83 ± 0.79 |
| Sodium (mEq/L) | 151.83 ± 3.34 | 145 ± 1.03 | 146 ± 2.82 | 150.83 ± 2.34 |
| Potassium (mEq/L) | 4.58 ± 0.06 | 4.58 ± 0.09 | 4.6 ± 0.13 | 4.55 ± 0.09 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
P<0.001 (aP<0.001 indicates high significance with P value less than 0.001 when compared to control).
4.2.2. Organs weight
The organ weight of control and fucoidan treated animals were noted at the end of the study. The details of various organs weight are represented in Table 6. There were no significant changes in the wet weight of vital organs including heart, kidney, liver, spleen, lungs and brain. The gross anatomy of organs were found to be normal with all the treatment groups with no signs of damage.
Table 6.
Organ weight (g).
| Groups | Group I (Control) | Group II (100 mg/Kg) | Group III (200 mg/Kg) | Group IV (400 mg/Kg) |
|---|---|---|---|---|
| Heart | 0.815 ± 0.02 | 0.845 ± 0.01 | 0.77 ± 0.02 | 0.83 ± 0.01 |
| Kidney | 0.81 ± 0.01 | 0.83 ± 0.03 | 0.868 ± 0.03 | 0.898 ± 0.01 |
| Liver | 8.36 ± 0.31 | 7.81 ± 0.11 | 8.01 ± 0.18 | 8.3 ± 0.31 |
| Spleen | 0.87 ± 0.03 | 0.88 ± 0.03 | 0.89 ± 0.03 | 0.89 ± 0.05 |
| Brain | 1.79 ± 0.06 | 1.72 ± 0.04 | 1.82 ± 0.05 | 1.85 ± 0.02 |
| Lungs | 1.82 ± 0.02 | 1.78 ± 0.03 | 1.88 ± 0.02 | 1.97 ± 0.06 |
Values are expressed as Mean ± Standard Error of the Mean (SEM); (n = 6).
4.2.3. Histopathology
Histopathological evaluation was conducted on various organs such as testes, kidney, brain, pancreas, heart, lungs, testes, spleen, ovary, stomach, intestine, liver. All the organs showed normal architecture and there was no abnormality in the structure microscopically. Testes of fucoidan treated animals showed normal seminiferous tubules with spermatogenesis (Fig. 2a–d). Kidney showed no changes in all the groups by manifesting normal glomeruli and renal tubules (Fig. 3a–d). Brain showed normal nerve fibres with astrocytes in all the groups (Fig. 4a–d). Pancreas showed normal pancreatic acini with intercalated ducts (Fig. 5a–d). Heart showed normal cardiac muscle bundles with myocytes in all the groups (Fig. 6a–d). In lungs, 100 and 200 mg/kg treated groups showed mild congestion of alveolar tissue with normal alveolus. In 400 mg/kg treated groups, mild infiltrations were seen in alveolar tissue with normal alveolus (Fig. 7a–d). Spleen showed normal architecture with lymphoid aggregation in all the groups (Fig. 8a–d). Ovary showed normal ovarian stroma with corpus leuteum and follicles in all the groups (Fig. 9a–d). Stomach tissue showed normal gastric glands with normal parietal cells and gastric mucosa in all the groups (Fig. 10a–d). Intestine tissue showed normal columnar epithelial cells, intestinal villi, goblet cells with prominent mucus secretion (Fig. 11 a–d). In liver 100 mg/kg treated groups showed normal hepatocytes with central vein whereas 200 and 400 mg/kg treated groups showed normal hepatocytes with mild congestion of blood vessel (Fig. 12a–d).
Fig. 2.
Light microscope photography of testes morphology (H&E x100). (2a) Control group; (2b) Fucoidan 100 mg/kg treated group; (2c) Fucoidan 200 mg/kg treated group; (2d) Fucoidan 400 mg/kg treated group; all the groups show normal seminiferous tubules.
Fig. 3.
Light microscope photography of kidney morphology (H&E x100). (3a) Control group; (3b) Fucoidan 100 mg/kg treated group; (3c) Fucoidan 200 mg/kg treated group; (3d) Fucoidan 400 mg/kg treated group; all the groups show normal glomeruli and renal tubules.
Fig. 4.
Light microscope photography of brain morphology (H&E x100). (4a) Control group; (4b) Fucoidan 100 mg/kg treated group; (4c) Fucoidan 200 mg/kg treated group; (4d) Fucoidan 400 mg/kg treated group; all the groups show normal nerve fibres with astrocytes.
Fig. 5.
Light microscope photography of pancreas morphology (H&E x100). (5a) Control group; (5b) Fucoidan 100 mg/kg treated group; (5c) Fucoidan 200 mg/kg treated group; (5d) Fucoidan 400 mg/kg treated group; all the groups show normal pancreatic acini with intercalated ducts.
Fig. 6.
Light microscope photography of heart morphology (H&E x100). (6a) Control group; (6b) Fucoidan 100 mg/kg treated group; (6c) Fucoidan 200 mg/kg treated group; (6d) Fucoidan 400 mg/kg treated group; all the groups show normal cardiac muscle bundles with myocytes.
Fig. 7.
Light microscope photography of lungs morphology (H&E x100). (7a) Control group; (7b) Fucoidan 100 mg/kg treated group; (7c) Fucoidan 200 mg/kg treated group; (7d) Fucoidan 400 mg/kg treated group; 7b and 7c show mild congestion of alveolar tissue with normal alveolus; 7d show mild infiltrations in alveolar tissue with normal alveolus.
Fig. 8.
Light microscope photography of spleen morphology (H&E x100). (8a) Control group; (8b) Fucoidan 100 mg/kg treated group; (8c) Fucoidan 200 mg/kg treated group; (8d) Fucoidan 400 mg/kg treated group; all the groups show normal architecture with lymphoid aggregation.
Fig. 9.
Light microscope photography of ovarian morphology (H&E x100). (9a) Control group; (9b) Fucoidan 100 mg/kg treated group; (9c) Fucoidan 200 mg/kg treated group; (9d) Fucoidan 400 mg/kg treated group; all the groups show normal ovarian stroma with corpus leuteum and follicles.
Fig. 10.
Light microscope photography of stomach morphology (H&E x100). (10a) Control group; (10b) Fucoidan 100 mg/kg treated group; (10c) Fucoidan 200 mg/kg treated group; (10d) Fucoidan 400 mg/kg treated group; all the groups show normal gastric glands with normal parietal cells and gastric mucosa.
Fig. 11.
Light microscope photography of intestine morphology H&E (x100). (11a) Control group; (11b) Fucoidan 100 mg/kg treated group; (11c) Fucoidan 200 mg/kg treated group; (11d) Fucoidan 400 mg/kg treated group; all the groups show normal columnar epithelial cells, intestinal villi, goblet cells with prominent mucus secretion.
Fig. 12.
Light microscope photography of liver morphology (H&E x100). (12a) Control group; (12b) Fucoidan 100 mg/kg treated group; (12c) Fucoidan 200 mg/kg treated group; (12d) Fucoidan 400 mg/kg treated group; 12a and 12b show normal hepatocytes with central vein whereas 12c and 12d show normal hepatocytes with mild congestion of blood vessel.
5. Discussion
The research and development in toxicology with a notion of identifying the undisclosed toxicity of low toxic chemical factors is the current trend of modern science. It is achieved by toxicological models which mainly involves laboratory animals which are highly similar to humans in their biochemical, physiological and pathological aspect of view [32] which invigorated Wistar rats as the preferred model in the present study.
Natural products are indiscriminately considered as safe in health care system of developing countries [33]. They lack the actual details on their toxicological profile and mostly used as self-medication. Various studies have reported the toxicity of herbal compounds [34,35]. Increasing evidence highlights that not only delayed wash out of harmful chemicals from the body but also the chronic exposure to low doses of less harmful substances are reported to distort homeostasis and protective mechanisms of human body [36]. Thus the concept of ‘no side effects with natural products’ is becoming outdated, which necessitates the importance of toxicological profile of natural compounds.
Fucoidan is known for its ample availability and numerous biological properties such as anticancer, immunomodulatory, anti-inflammatory, antcoagulant and antioxidant justifying the considerable research interest of fucoidan among scientists [37]. However, there is a lack of detailed toxicity profile of fucoidan from different sources. The detailed toxicological investigation provides scientific information with regards to the safety for the use of natural products as alternative medicine. According to a study conducted by Kim et al., (2010) fucoidan derived from U. pinnatifida was reported to be non-toxic at the dose of 150, 450, and 1350 mg/kg bw/day for 4 weeks with no significant disruption to haematological, biochemical or histopathological parameters. However, fucoidan is known to have varied proportions of monosaccharides and sulfate depending upon the brown marine algae species. Thereby, in the present study fucoidan specifically isolated from S. wightii was subjected to acute and sub-acute toxicity study in wistar rats inorder to assess its safety.
FTIR analysis revealed and confirmed the presence of major functional groups such as O—H, CCO, OCO, SO, COH, CO, C —— ——1-H, COS, CC—— -H in the isolated fucoidan. The findings of the present study was in accordance with the FTIR analysis of fucoidan from S.wightii conducted by Immanuel et al., (2012) which ensures candidness of fucoidan isolated from S. wightii [16].
Animals were found to be free from major toxic signs or death during as well as at the end of acute toxicity study. There were no abnormal signs of any motor and sensory functions at the dose of 2000 mg/kg. Therefore it may be suggested that fucoidan is practically non-toxic via oral route up to single dose of 2000 mg/kg.
In subacute toxicity study, there were no significant changes observed in biochemical, haematological and histopathological analysis except a significant reduction in the level of glucose and cholesterol.
A reduction in 10 % of animal’s original body weight is known to affect survival, which is an indication of adverse effect [33]. The non-significant differences in body weight of animals taken on weekly basis in the present study indicates the normal physiology of growth with proportionate food and water intake at the dose of 100, 200 and 400 mg/kg. Any significant changes in food and water intake indicates the negative impact of test drug on the metabolism [38]. Non-significant differences in food and water intake indicate that fucoidan was safe on long term administration and did not induce any alteration to metabolic system.
Elevated level of creatinine and urea are considered to have strong correlations to renal impairment as they are the indicators of renal damage [39]. In our study, the normal range of electrolytes, creatinine and urea level indicates that fucoidan has no negative effect on kidneys. Thus it may suggested that fucoidan did not interfere with the physiology of kidneys and did not cause any renal impairment or damage. Generally Transaminases (AST and ALT) are considered to be indices of liver damage. Changes in hepatic cellular permeability, necrosis or damage to hepatocytes is known to be associated with a raise in the transaminases level [40]. In the present study, statistically insignificant results on elevation of ALT and AST ruled out any such toxic effects to liver. Our findings take the sustenance of a study conducted by Arumugam et al., (2019) [37] where the authors suggested that fucoidan does not predispose any carcinogenicity to liver from in vitro studies.
Reduction in glucose level in animals treated with fucoidan may be correlated to the previously reported hypoglycemic potential particularly alpha glucosidase and alpha amylase inhibitory activity of both S. wightii extract and isolated fucoidan [[41], [42], [43]]. Alterations in the level of cholesterol can indicate any changes associated with lipid metabolism [44]. Fucoidan is previously reported to possess hypolipidemic property through reduction in HMG CoA reductase expression and an upregulation of LDL receptor. The anti-hyperlipidemic effect observed in the present study complies with the previous studies available on hypolipidemic property of fucoidan [45].
Haemopoietic system is one of the most easy and sensitive target for the toxic compounds. It serves as a general index for the overall physiological and pathological status of the body [46]. Fucoidan showed no deleterious effect on haemopoietic system. However details on clotting time and related parameters are lacking in the present study, which needs further evaluation.
Natural products have high possibility of being toxic to important organs due to their wide distribution and diverse role [33]. Organ weight is known to be one of the main indices to derive any targeted organ toxicity. As the wet weight of vital organs showed no significant differences in groups treated with 100, 200 and 400 mg/kg of fucoidan compared to normal control, the organ level toxicity of fucoidan in the models selected is ruled out.
No signs of abnormality or organ level damages were observed in macroscopic examination of gross anatomy. In histopathological examination, all the organs showed normal architecture. Though, some changes were observed, they were minimal and comparable with the observations in the control group. The results obtained from histopathological study corroborates the claim of fucoidan to be non-toxic.
Additional genotoxicity, carcinogenicity and teratogenicity studies are also necessary [47] as they would strengthen the safety profile. However, this limitation is proposed to be future direction of our study.
Our study supports the previous findings on toxicity profile of fucoidan by revealing fucoidan to be safe at the dose of 100, 200 and 400 mg/kg bw/day on 28 days repeated oral administration.
6. Conclusion
The animals did not show any remarkable toxic signs or mortality in acute toxicity study at single oral administration of fucoidan at the dose of 2000 mg/kg bodyweight. In subacute toxicity, no statistically significant difference in body weight, relative weight of vital organs, food and water intake compared to the control group was observed. Serum glucose and cholesterol showed a statistically significant reduction at all the doses when compared to normal control and the reduction was in a dose dependent manner. There were no other changes observed in biochemical or haematological parameters. Histopathological analysis showed no significant toxic signs at organ levels in treated groups when compared to normal control. Based on the results obtained from acute and subacute toxicity study, fucoidan is considered to be safe in the models tested, which encourages its long term administration for medicinal uses. However, our study recommends a detailed chronic and special toxicity studies to further strengthen the safety profile of fucoidan.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ramu Sathiya: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Visualization, Writing - review & editing, Investigation. Anita Murali: Conceptualization, Supervision, Validation, Writing - review & editing. Geetha Narasimhaiah: . Jayaraman Anbu: Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to Faculty of Pharmacy, Ramaiah University of Applied Sciences for the required support and facility.
Contributor Information
Sathiya Ramu, Email: sathya.pharma@gmail.com.
Anita Murali, Email: anita.murali4@gmail.com.
Geetha Narasimhaiah, Email: ngeetha971@gmail.com.
Anbu Jayaraman, Email: cologist2019@gmail.com.
References
- 1.Ale M.T., Meyer A.S. Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013;3:8131. [Google Scholar]
- 2.Kui-Jin K., Ok-Hwan L., Hee-Hyun L., Boo-Yong L. A 4-week repeated oral dose toxicity study of fucoidan from the Sporophyll of Undaria pinnatifida in Sprague–Dawley rats. Toxicology. 2010;267:154–158. doi: 10.1016/j.tox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Suresh V., Senthilkumar N., Thangam R., Rajkumar M., Anbazhagan C., Rengasamy R., Gunasekaran P., Kannan S., Palani P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochem. 2013;48:364–373. [Google Scholar]
- 4.Liu L., Heinrich M., Myers S., Dworjanyn S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 2012;142:591–619. doi: 10.1016/j.jep.2012.05.046. https://www.ncbi.nlm.nih.gov/pubmed/22683660 [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Cuellar-Bermudez S.P., Aguilar-Hernandez I., Cardenas-Chavez D.L., Ornelas-Soto N., Romero-Ogawa M.A., Parra-Saldivar R. Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015;8(2):190–209. doi: 10.1111/1751-7915.12167. https://www.ncbi.nlm.nih.gov/pubmed/252238677 [PubMed] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis T.A., Volesky B., Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. https://www.ncbi.nlm.nih.gov/pubmed/14511705 [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Koyanagi S., Tanigawa N., Nakagawa H., Soeda S., Shimeno H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003;65:173–179. doi: 10.1016/s0006-2952(02)01478-8. https://www.ncbi.nlm.nih.gov/pubmed/12504793 [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Maruyama H., Tamauchi H., Hashimoto M., Nakano T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo (Brooklyn) 2003;17:245–249. https://www.ncbi.nlm.nih.gov/pubmed/12929574 [PubMed] [PubMed] [Google Scholar]
- 9.Wang J., Zhang Q., Zhang Z., Song H., Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010;46:6–12. doi: 10.1016/j.ijbiomac.2009.10.015. https://www.ncbi.nlm.nih.gov/pubmed/19883681 [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Prokofjeva M.M., Imbs T.I., Shevchenko N.M., Spirin P.V., Horn S., Fehse B., Zvyagintseva T.N., Prassolov V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs. 2013;11:3000–3014. doi: 10.3390/md11083000. https://www.ncbi.nlm.nih.gov/pubmed/23966033 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuy T.T., Ly B.M., Van T.T., Quang N.V., Tu H.C., Zheng Y., Seguin-Devaux C., Mi B., Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. https://www.ncbi.nlm.nih.gov/pubmed/25439876 [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Uhm C.S., Kim K.B., Lim J.H., Pee D.H., Kim Y.H., Kim H., Eun B.L., Tockgo Y.C. Effective treatment with fucoidin for perinatal hypoxic–ischemic encephalopathy in rats. Neurosci. Lett. 2003;353:21–24. doi: 10.1016/j.neulet.2003.09.013. https://www.ncbi.nlm.nih.gov/pubmed/14642428 [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Del-Bigio M.R., Yan H.J., Campbell T.M., Peeling J. Effect of fucoidan treatment on collagenase-induced intracerebral hemorrhage in rats. Neurol. Res. 1999;21:415–419. doi: 10.1080/01616412.1999.11740953. https://www.ncbi.nlm.nih.gov/pubmed/10406016 [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Fitton J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs. 2011;9:1731–1760. doi: 10.3390/md9101731. https://www.ncbi.nlm.nih.gov/pubmed/22072995 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pozharitskaya O.N., Shikov A.N., Faustova N.M., Obluchinskaya E.D., Kosman V.M., Vuorela H., Makarov V. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs. 2018;16:132. doi: 10.3390/md16040132. https://www.ncbi.nlm.nih.gov/pubmed/29669995 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Immanuel G., Sivagnanavelmurugan M., Marudhupandi T., Radhakrishnan S., Palavesam A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon. Fish Shellfish Immunol. 2012;32:551–564. doi: 10.1016/j.fsi.2012.01.003. https://www.ncbi.nlm.nih.gov/pubmed/22245839 [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.OECD Guideline for testing of chemicals . 2001. Acute Oral Toxicity - Fixed Dose Procedure. [Google Scholar]
- 18.Li N., Zhang Q., Song J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in wistar Rats. Food Chem. Toxicol. 2005;43(3):421–426. doi: 10.1016/j.fct.2004.12.001. https://www.ncbi.nlm.nih.gov/pubmed/15680677 [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Lakshmanasenthil S., Vinothkumar T., Geetharamani D., Marudhupandi T., Suja G., Sindhu N.S. Fucoidan – a novel alpha amylase inhibitor from Turbinaria ornate with relevance to NIDDM therapy. Biocatal. Agric. Biotechnol. 2014;3(3):66–70. [Google Scholar]
- 20.OECD Guideline for testing of chemicals . 2008. Repeated Dose 28-Day Oral Toxicity Study in Rodents. [Google Scholar]
- 21.Steve O., Florence E., Anyika E.N. Evaluation of subchronic toxicity of Staachytarphaecta angustifolia extract in animals. Afr. J. Biotechnol. 2009;8(9):1793–1799. [Google Scholar]
- 22.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- 23.Brobeck J.R. nineth ed. Wilkins and Wilkins; Baltomore: 1973. Physiological Basis of Medical Practice. [Google Scholar]
- 24.Doumas B.T. Standards for total serum protein assays – a collaborative study. Clin. Chem. 1975:1159–1166. [PubMed] [Google Scholar]
- 25.Webster B.T. Estimation of protein in liquid and solid sample. Clin. Chem. 1977;21:1159. [Google Scholar]
- 26.Chaney A.L., Marbach E.P. Determination of urea in liquid samples. Clin. Chem. 1962;8:130. [PubMed] [Google Scholar]
- 27.Tietz N.W. WB Saunders Co; Philadelphia: 1976. Fundamentals of Clinical Chemistry. [Google Scholar]
- 28.Assmann G. At what levels of total low or high density lipoprotein cholesterol should diet/drug therapy be initiated European guidelines. Am. J. Cardiol. 1990;65:11. doi: 10.1016/0002-9149(90)91248-5. [DOI] [PubMed] [Google Scholar]
- 29.Assmann G., Schriewer H., Schmitz G. Qualification of high density lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin. Chem. 1983;29:2026–2030. [PubMed] [Google Scholar]
- 30.Bergmayer H.U. Academic press; United States: 1974. Methods of Enzymatic Analysis. [Google Scholar]
- 31.Ghai C.L. Jaypee brothers Medical publishers; New Delhi: 2003. A Text Book of Practical Physiology. [Google Scholar]
- 32.Tyshko N.V., Shestakova S.I. Model of vitamin and mineral deficiency for toxicological research: apoptosis activity under conditions of CCL4 intoxication. Toxicol. Rep. 2019;1(6):151–154. doi: 10.1016/j.toxrep.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adewale O.B., Onasanya A., Anadozie S.O., Abu M.F., Akintan I.A., Ogbole C.J., et al. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J. Ethnopharmacol. 2016;188:153–158. doi: 10.1016/j.jep.2016.05.003. https://www.ncbi.nlm.nih.gov/pubmed/27154407 [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Vaghasiya Y.K., Shukla V.J., Chanda S.V. Acute oral toxicity Study of Pluchea arguta boiss Extract in mice. J. Pharmacol. Toxicol. 2011;6:113–123. [Google Scholar]
- 35.Christapher P.V., Parasuraman S., Asmawi M.Z., Murugaiyah V. Acute and subchronic toxicity studies of methanol extract of Polygonum minus leaves in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2017;86:33–41. doi: 10.1016/j.yrtph.2017.02.005. https://www.ncbi.nlm.nih.gov/pubmed/28229903 [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Tsatsakis A., Docea A.O., Constantin C., Calina D., Zlatian O., Nikolouzakis T.K., Stivaktakis P.D., Kalogeraki A., Liesivuori J., Tzanakakis G., Neagu M. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol. Lett. 2019;316:154–170. doi: 10.1016/j.toxlet.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam P., Arunkumar K., Sivakumar L., Murugan M., Murugan K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019;6:556–563. doi: 10.1016/j.toxrep.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attanayake A.P., Jayatilaka K., Pathirana C., Mudduwa L. Efficacy and toxicological evaluation of Coccinia grandis (Cucurbitaceae) extract in male Wistar rats. Asian Pac. J. Trop. Dis. 2013;3(6):460–466. [Google Scholar]
- 39.Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A.K., Vernekar S.N. Markers of renal function tests, North Am. J. Med. Sci. 2010;2(4):170–173. [PMC free article] [PubMed] [Google Scholar]
- 40.Sujith K., Darwin R., Suba V. Toxicological evaluation of ethanol extract of Anacyclus pyrethrum in albino wistar rats. Asian Pac. J. Trop. Dis. 2012;2(6):437–441. [Google Scholar]
- 41.Senthil L.S., Kumar T.V., Geetharamani D., Suja G., Yesudas R., Chacko A. Fucoidan – an alpha amylase inhibitor from S. Wightii with relevance to NIDDM. Int. J. Biol. Macromol. 2015;81:644–647. doi: 10.1016/j.ijbiomac.2015.08.065. https://www.ncbi.nlm.nih.gov/pubmed/26325677 [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Kim K., Yoon K., Lee B. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+ db and C57BL/KSJ db/db mice. Fitoterapia. 2012;83(6):1105–1109. doi: 10.1016/j.fitote.2012.04.027. https://www.ncbi.nlm.nih.gov/pubmed/22580164 [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Hu S., Xia G., Wang J., Wang Y., Li Z., Xue Fucoidan from sea cucumber protects against high-fat high sucrose diet induced hyperglycemia and insulin resistance in mice. J. Funct. Foods. 2014;10:128–138. [Google Scholar]
- 44.Poornima K., Krishnan R., Aswathi K.V., Gopalakrishnan V.K. Toxicological evaluation of ethanol extract of Tabernaemontana coronaria (L) R. Br. Asian Pac. J. Trop. Dis. 2012:S679–S684. [Google Scholar]
- 45.Park J., Yeoam M., Hahm D. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016;131(2):84–92. doi: 10.1016/j.jphs.2016.03.007. https://www.ncbi.nlm.nih.gov/pubmed/27094367 [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Olson H.G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., et al. Concordance of toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 47.Yoshino S., Awa R., Ohto N., Miyake Y., Kuwahara H. Toxicological evaluation of standardized Kaempferia parviflora extract: sub-chronic and mutagenicity studies. Toxicol. Rep. 2019;6:544–549. doi: 10.1016/j.toxrep.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]