Abstract
Nitric oxide (NO) is an important part of the host defense mechanism; however, it displays both pro- and anti-inflammatory properties depending on its location and concentration. Importantly, excessive or inappropriate NO production can cause tissue damage. Systemic and local administration of NO synthase (NOS) inhibitors ameliorates and may exacerbate the inflammatory response, respectively. Here, we used a carrageenan-induced pleurisy model of acute inflammation in rats to confirm the location-dependent effects of NO and investigate the underlying mechanisms. As expected, localized suppression of NO production exacerbated inflammation, as evidenced by increased pleural exudate volumes and leukocyte counts and enhanced activity of enzymes related to oxidative stress. In contrast, local NO supplementation reduced leukocyte infiltration, vascular permeability, and the activity of oxidative stress-related enzymes. Interestingly, inhibition of heme oxygenase-1 (HO-1) reversed the anti-inflammatory effects of localized NO production, while the addition of hemin (HO-1 substrate) or carbon monoxide (CO; HO-1 metabolite) decreased leukocyte migration and exudation. Together, these findings confirm a protective role for NO at the inflammatory site, which appears to be mediated via NOS induction of the HO-1/CO pathway. Thus, NO supplementation may be a potential new treatment for oxidative stress-associated inflammatory diseases.
Keywords: Acute inflammation, Carrageenan, Nitric oxide, Pleurisy, Heme oxygenase-1, Carbon monoxide
Highlights
-
•
Systemic NOS inhibition ameliorated inflammation in a rat Cg-induced pleurisy model.
-
•
Conversely, localized NOS inhibition increased all examined markers of inflammation.
-
•
HO-1, hemin, and CO enhanced the localized anti-inflammatory effects of NO.
-
•
NOC-18, l-arginine, hemin, and CORM-3 decreased levels of inflammatory cytokines.
-
•
The localized anti-inflammatory effect of NO may be mediated via the HO-1/CO pathway.
Abbreviations
- AE-ITU
S-(2-aminoethyl) isothiourea
- CAT
catalase
- CDNB
1-chloro-2,4-dinitrobenzene
- Cg
carrageenan
- CO
carbon monoxide;
- CORM-3
carbon monoxide releasing molecule-3
- GPx
glutathione peroxidase
- GSH
glutathione
- GST
glutathione S-transferase
- HO-1
heme oxygenase-1
- iCORM-3
inactive carbon monoxide releasing molecule-3
- IL-1β
interleukin-1 beta
- L-NNA
NG-nitro-l-arginine;
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide;
- NOC-18
1-hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene
- NOS
nitric oxide synthase
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor alpha
- ZnPP-IX
zinc protoporphyrin IX
1. Introduction
Carrageenan (Cg)-induced pleurisy in rats is an acute inflammatory model characterized by fluid extravasation and phagocyte migration. Polymorphonuclear leukocytes and migrating mononuclear cells differentiating into macrophages dominate the reaction in the initial 12–24 h and up to its resolution at 48–72 h, respectively [1]. These cells synthesize and release inflammatory mediators, including nitric oxide (NO) [1,2]. NO is a pleiotropic mediator formed from l-arginine and oxygen by three NO synthase (NOS) isoforms: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) [3]. The constitutive NOS (cNOS), nNOS and eNOS, are constantly present and synthesize small amounts of NO in response to physical or receptor stimulation. In contrast, iNOS is induced by inflammatory-like stimuli and can produce large amounts of NO that predominate during inflammation [3].
eNOS-produced NO has both pro- and anti-inflammatory properties. Under physiological conditions, endothelium-released NO regulates vascular tone and maintains vessel patency by prevention of platelet aggregation and downregulation of adhesion molecules [4]. However, mediators released during the acute inflammatory phase evoke additional endothelial NO release, promoting vasodilatation and vascular permeability and facilitating edema formation and inflammatory cell trafficking [5].
NOS inhibitors, namely l-arginine analogs, are pharmacological agents utilized to inhibit NO production; however, they have poor NOS-isoform selectivity, thereby inhibiting both iNOS and eNOS during inflammation. In most reports, NOS inhibitors were administered systemically and ameliorated inflammation [[6], [7], [8], [9], [10]]. In some cases, the anti-inflammatory effects were reversed by vasodilators [11,12], suggesting that NOS inhibitors reduced inflammation largely through eNOS inhibition. However, we and others have demonstrated that direct administration of NOS inhibitors into the inflamed site, rather than systemically, instead exacerbated the inflammatory response in rat pleuritis [1,2]. Based on the above reports, we hypothesized that, in contrast to systemic NO production, local NO production could be protective during inflammatory insults.
Heme oxygenase-1 (HO-1) is a stress-inducible protein best known for its role in protecting cells from oxidative stress. However, as well as sharing many common features and an overlap in biological function, both exogenous NO and NO derived from iNOS activity strongly induce HO-1 expression [[13], [14], [15], [16]]. In addition, numerous studies have demonstrated that HO-1, its substrate heme, and its metabolite carbon monoxide (CO) can modulate the inflammatory process. For example, heme reportedly mediates oxidative stress and inflammation and is thought to be important in a wide variety of pathophysiological processes, triggering the production of reactive oxygen species and stimulating adhesion molecule expression and leukocyte infiltration at the site of inflammation [17]. However, a low concentration of heme can have anti-inflammatory and cytoprotective properties via the upregulation of HO-1 and increased formation of HO-1 metabolites such as CO, which were also shown to reduce cell migration, exudation, the release of pro-inflammatory mediators, and the expression of adhesion molecules [18,19], suggesting that HO-1 has a protective role during inflammation.
Models of Cg-induced pleurisy have been widely used to investigate the pathophysiology of acute inflammation and to evaluate the efficacy of drugs in inflammation. However, to date, no studies have examined whether HO-1 and its metabolite CO are involved in the inhibitory effects of NO using this model. Thus, we designed the current study to confirm the location-dependent contradictory effects of NO during inflammation and to investigate the underlying mechanisms, including the role of HO-1. Using NOS inhibitors NG-nitro-l-arginine (L-NNA; inhibits both cNOS and iNOS) and S-(2-aminoethyl) isothiourea (AE-ITU; inhibits iNOS only), we suppressed NO production both locally and systemically in a rat model of Cg-induced pleurisy, while l-arginine (substrate for NO formation) and 1-hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene (NOC-18; exogenous NO donor) were used to enhance NO levels. Rats were also pretreated with HO-1 inhibitor zinc protoporphyrin IX (ZnPP-IX) or carbon monoxide releasing molecule 3 (CORM-3) to investigate the involvement of the HO-1/CO pathway in the modulation of the immune response by NO. Edema formation, inflammatory cell migration, nitrite/nitrate levels, myeloperoxidase (MPO) activity, oxidative stress markers, and the levels of inflammatory cytokines and chemokines were then examined in exudates to better understand the site-specific effects of NO.
2. Methods
2.1. Reagents
L-NNA, AE-ITU, and NOC-18 were purchased from Dojindo (Kumamoto, Japan). Unless otherwise noted, all other chemicals were of the purest grade available and were obtained from Sigma-Aldrich (Tokyo, Japan).
ZnPP-IX was dissolved in 50 mM Na2CO3, while hemin was dissolved in 1 mM NaOH. L-NNA, AE-ITU, l-arginine, NOC-18, and CORM-3 were all dissolved in saline. Inactive CORM-3 (iCORM-3) served as a negative control and was generated by incubating CORM-3 in Dulbecco's phosphate-buffered saline (pH 7.4) at room temperature for 48 h to liberate all available CO gas from the molecule, as previously described [20].
2.2. Animals
Male Wistar rats (160 ± 10 g body weight; Japan SLC, Hamamatsu, Japan) were used in all experiments. Standard laboratory chow and fresh drinking water were available ad libitum. Rats were housed under controlled temperature (23 ± 1 °C) and lighting (8:00–20:00 light) conditions and were acclimatized for 4 days prior to experimentation. All experiments were carried out in accordance with the ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and were approved by the Ethics Committee for Animal Experimentation at the Nagoya University School of Health Science (approval number: 028–031).
2.3. Cg-induced pleurisy
Cg pleurisy induction and measurement of pleural exudate volumes and total leukocyte exudate numbers were performed as previously described [1]. A λ-Cg solution (1%, w/v) in physiological saline (0.15 ml total) was injected into the right pleural cavity. After 2, 6, 12, 24, 36, 54, and 72 h, pleural cavities were washed out with 1 ml of 3.15% (w/v) sodium citrate in saline as an anticoagulant. Edema formation was assessed by weighing the collected inflammatory exudate, and inflammatory cells were counted after trypan blue staining using an optical microscope with a Burker counting chamber. Exudate samples were centrifuged at 800×g for 10 min at 4 °C to separate inflammatory cells from exudate, with both fractions then stored at −80 °C for subsequent procedures.
2.4. Drug administration
In the first set of experiments, rats (n = 6–8 per group) received a single intraperitoneal injection of vehicle (saline), L-NNA (0.1, 0.3, or 1.0 mg/kg), or AE-ITU (1.0, 3.0, or 10 mg/kg) immediately prior to intrapleural Cg injection. Rats were sacrificed 6 h post-injection, and pleural exudates were collected and processed as previously described [1].
In the second set of experiments, immediately prior to intrapleural Cg injection, rats (n = 7–10 per group) received a single pleural injection of vehicle (saline), L-NNA (0.1, 0.3, or 1.0 mg/kg), AE-ITU (1.0, 3.0, or 10 mg/kg), l-arginine (10, 30, 100, or 300 mg/kg), or NOC-18 (1.0, 3.0, 10, or 30 mg/kg). A control group of six rats received saline instead of Cg. Six hours after the induction of pleurisy, pleural exudates were collected and processed as previously described [1].
In the third set of experiments, rats (n = 5–7 per group) treated subcutaneously with ZnPP-IX (0.3, 1.0, or 3.0 mg/kg) 1 h prior to Cg injection also received an intrapleural injection of l-arginine (30 mg/kg) or NOC-18 (10 mg/kg). Animals were sacrificed 6 h after the induction of pleurisy, and pleural exudates were collected and processed as previously described [1].
In the final set of experiments, immediately prior to intrapleural Cg injection, rats (n = 5–7 per group) received a single pleural injection of hemin (0.1, 0.3, or 1.0 mg/kg), CORM-3 (0.3, 1.0, or 3.0 mg/kg), or iCORM-3 (3.0 mg/kg). Six hours after the induction of pleurisy, pleural exudates were collected and processed as previously described [1].
In each set of experiments, the control group received only the vehicle(s) via the appropriate route of administration. The doses of L-NNA, AE-ITU, l-arginine, NOC-18, ZnPP-IX, hemin, CORM-3, and iCORM-3 were based on doses used in previous studies, with modification of the administration route as necessary [1,2,[21], [22], [23]].
Eight additional groups of rats (4–5 rats per group) were separately treated with AE-ITU (10 mg/kg), L-NNA (1.0 mg/kg), l-arginine (30 mg/kg), NOC-18 (10 mg/kg), ZnPP-IX (3.0 mg/kg), hemin (1.0 mg/kg), CORM-3 (3.0 mg/kg), or iCORM-3 (3.0 mg/kg) in the absence of Cg injection. After 6 h, the rats were sacrificed and pleural exudates were collected and processed as described above.
2.5. Inflammatory exudate levels of nitrite/nitrate (NOx), tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and monocyte chemoattractant protein-1 (MCP-1)
Nitric oxide is a labile free radical that is rapidly metabolized to nitrate and nitrite in the presence of oxygen. Therefore, after the reduction of nitrate to nitrite, we measured nitrite levels in the cell-free inflammatory exudate using a commercial colorimetric kit (Nitrite/Nitrate Assay Kit-C II, cat. no. NK-05; Dojindo) according to the manufacturer's instructions, the results of which were expressed in μM.
Total TNF-α, IL-1β, and MCP-1 concentrations were measured in the cell-free inflammatory exudates using enzyme-immunoassay kits (BioSource International, Camarillo, CA, USA) as per the manufacturer's instructions, the results of which were expressed in pg/ml.
2.6. Inflammatory cell iNOS activity
iNOS activity was assessed ex vivo in 96-well microtiter plates as described previously [24]. Briefly, inflammatory cells were sonicated at 4 °C in the presence of protease inhibitory buffer containing 1 mM phenylmethylsulphonyl fluoride, 1.5 mM pepstatin A, and 0.2 mM leupeptin. Protein concentrations were determined using the Bradford method [25]. Samples were then incubated with 20 mM Tris-HCl (pH 7.9) supplemented with 3 mM DTT, 4 μM flavin adenine dinucleotide, 2 mM l-arginine, 2 mM nicotinamide adenine dinucleotide phosphate (NADPH), and 4 μM 6R-5,6,7,8-tetrahydrobiopterin (final volume 100 μl) for 2 h at 37 °C. Lactate dehydrogenase (20 U/ml) was then added to each sample to stop the reaction via the oxidation of residual NADPH. The resulting nitrite was reacted with Griess reagent (Tokyo Kasei, Tokyo, Japan) and the absorbance of each sample at 550 nm was measured. An extra well per sample was incubated with all reagents except l-arginine and NADPH to assess nonspecific absorbance, which was then subtracted from that of the test wells. iNOS activity was expressed as pmol/mg protein/min.
2.7. MPO activity
MPO activity in pleural exudates was measured according to the method described by Bradley et al. [26] MPO activity was extrapolated from the standard curve, and the results were expressed as mU/ml.
2.8. Catalase, superoxide dismutase, glutathione peroxidase, and glutathione S-transferase activities
The supernatant from the centrifuged pleural exudate samples was used to determine catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST) activities. CAT activity was assessed via the rate of change of H2O2 concentration based on the absorbance at 240 nm in accordance with the method described by Aebi [27], and was expressed as mmol/min/ml. SOD activity was assayed via the inhibition of adrenaline auto-oxidation based on changes in absorbance at 480 nm over a 3-min period in accordance with the method described by Misra and Fridoovich [28], and was expressed as units of SOD (USOD)/ml. GPx activity was monitored based on the oxidation of NADPH at 340 nm in the presence of H2O2 as described by Flohé and Günzler [29], and was expressed as μmol/min/ml. GST activity was determined using glutathione (GSH) and 1-chloro-2,4-dinitrobenzene (CDNB) as substrates and monitoring the formation of S-(2,4-dinitrophenyl)-glutathione conjugate, calculated by changes in absorbance at 340 nm, in accordance with the method described by Habig and Jakoby [30], and was expressed as μmol/min/ml.
2.9. Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). The data were assessed for statistical significance using one-way analysis of variance with the Holm-Sidak post-hoc test. For transparency, both significant differences (P < 0.05) and trends (0.05 ≤ P < 0.1) are reported where appropriate. P-values less than 0.05 were considered significant. Statistical analysis was performed using Sigma Plot 13.0 software (Systat Software Inc., San Jose, USA).
3. Results
Time course of exudate NOx levels and inflammatory cell iNOS activity in the Cg-induced rat pleurisy model.
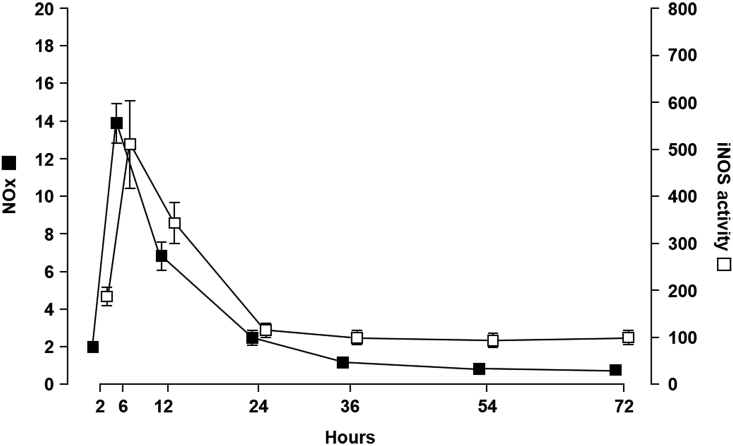
In this model, polymorphonuclear leukocytes are the principal cell type up to 12 h post-Cg injection when they are replaced by migrating mononuclear cells, which differentiate into macrophages and dominate the reaction up to resolution at 48–72 h post-injection [1,31]. NOx, as determined by measuring nitrite/nitrate levels in the cell-free inflammatory exudate, was detectable at 2 h and peaked at 6 h post-Cg injection before gradually decreasing (Fig. 1). Ex vivo measurement of inflammatory cell iNOS activity revealed a profile similar to that of exudate NOx levels (Fig. 1).
Fig. 1.
Correlation between nitrite/nitrate (NOx) levels and inducible nitric oxide synthase (iNOS) activity in carrageenan (Cg)-induced pleurisy. Exudate NOx (■, left vertical axis; μM) levels and inflammatory cell iNOS activity (□, right vertical axis; pmol/mg protein/min) throughout the inflammation period (time, horizontal axis). Data are expressed as mean ± SEM (n = 6 rats per time point).
3.1. Effects of L-NNA and AE-ITU on Cg-induced rat pleurisy
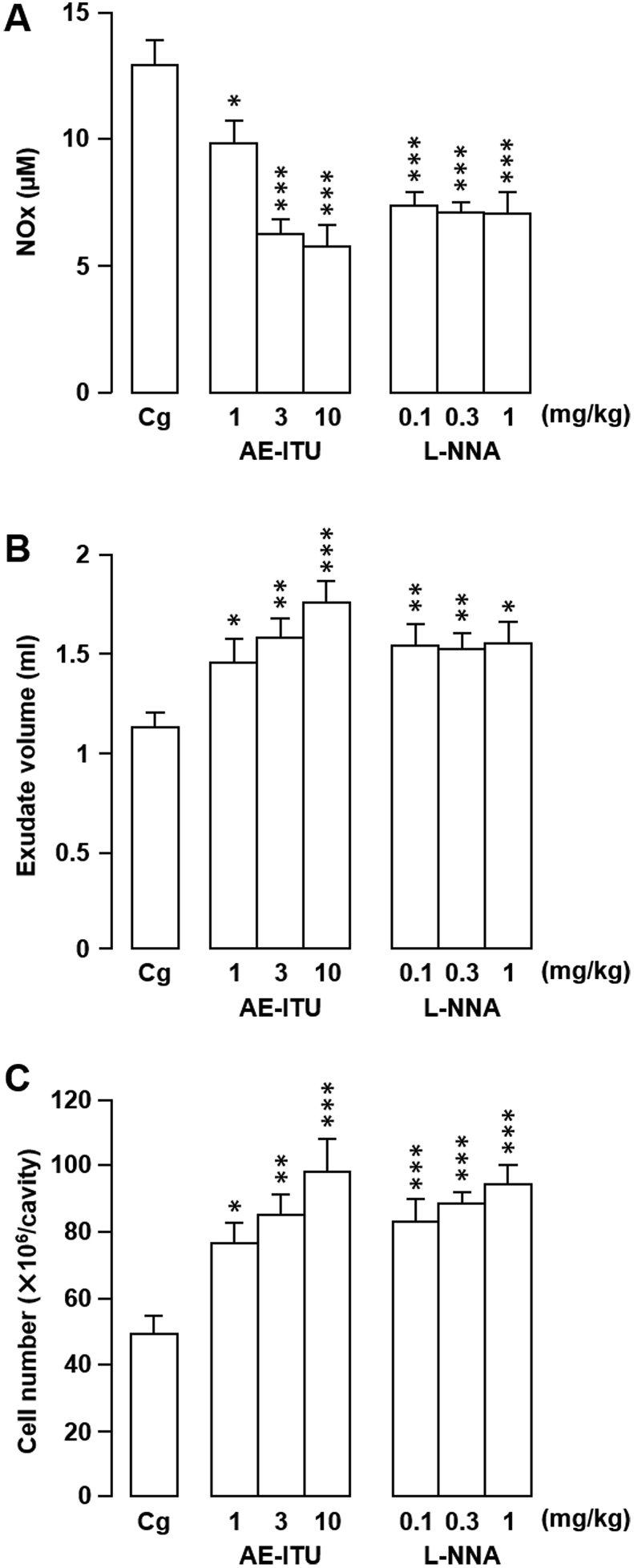
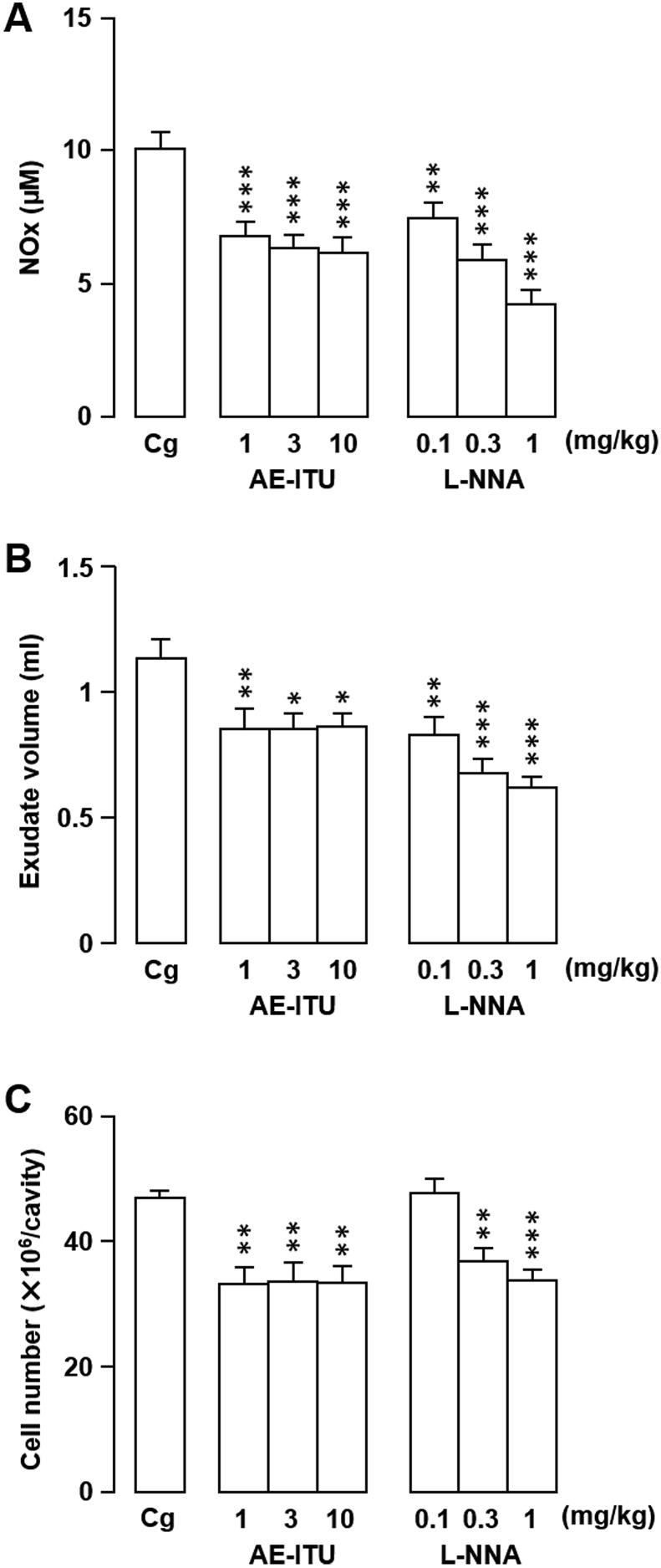
In the first experimental series, rats were treated with NOS inhibitors L-NNA or AE-ITU to confirm their effects on Cg-induced rat pleurisy [2] and to establish our model. We obtained similar results to those reported previously (Fig. 2, Fig. 3). Specifically, a single intrapleural injection of L-NNA or AE-ITU at a dose that inhibits NOx generation significantly exacerbated inflammation compared with vehicle-only inoculation, as shown by increased pleural exudate volumes and leukocyte influx into the pleural cavity (Fig. 2). In contrast, when injected systemically into the peritoneal cavity, both NOS inhibitors decreased inflammatory exudate NOx levels, exudate volumes, and inflammatory cell counts compared with the control (Fig. 3).
Fig. 2.
Effects of locally-injected S-(2-aminoethyl) isothiourea (AE-ITU) or NG-nitro-l-arginine (L-NNA) on rat carrageenan (Cg)-induced pleurisy and nitrite/nitrate (NOx) levels. (A–C) AE-ITU, L-NNA, or vehicle (saline) was injected directly into the pleural cavity immediately prior to intrapleural Cg injection. The effects of the treatments on NOx level (A), exudate volume (B), and cell number (C) in pleural exudates were evaluated 6 h after Cg challenge. Horizontal axes: doses (mg/kg) of AE-ITU and L-NNA. Cg indicates control animals injected with saline prior to Cg injection. Data are expressed as mean ± SEM (n = 6–8 rats). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Cg.
Fig. 3.
Effects of systemically-injected S-(2-aminoethyl) isothiourea (AE-ITU) or NG-nitro-l-arginine (L-NNA) on rat carrageenan (Cg)-induced pleurisy and nitrite/nitrate (NOx) levels. (A–C) AE-ITU, L-NNA, or vehicle (saline) was injected into the peritoneal cavity immediately prior to intrapleural Cg injection. The effects of the treatments on NOx level (A), exudate volume (B), and cell number (C) in pleural exudates were evaluated 6 h after Cg challenge. Horizontal axes: doses (mg/kg) of AE-ITU and L-NNA. Cg indicates control animals injected with saline prior to Cg injection. Data are expressed as mean ± SEM (n = 6–8 rats). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Cg.
3.2. Effects of l-arginine and NOC-18 on Cg-induced rat pleurisy
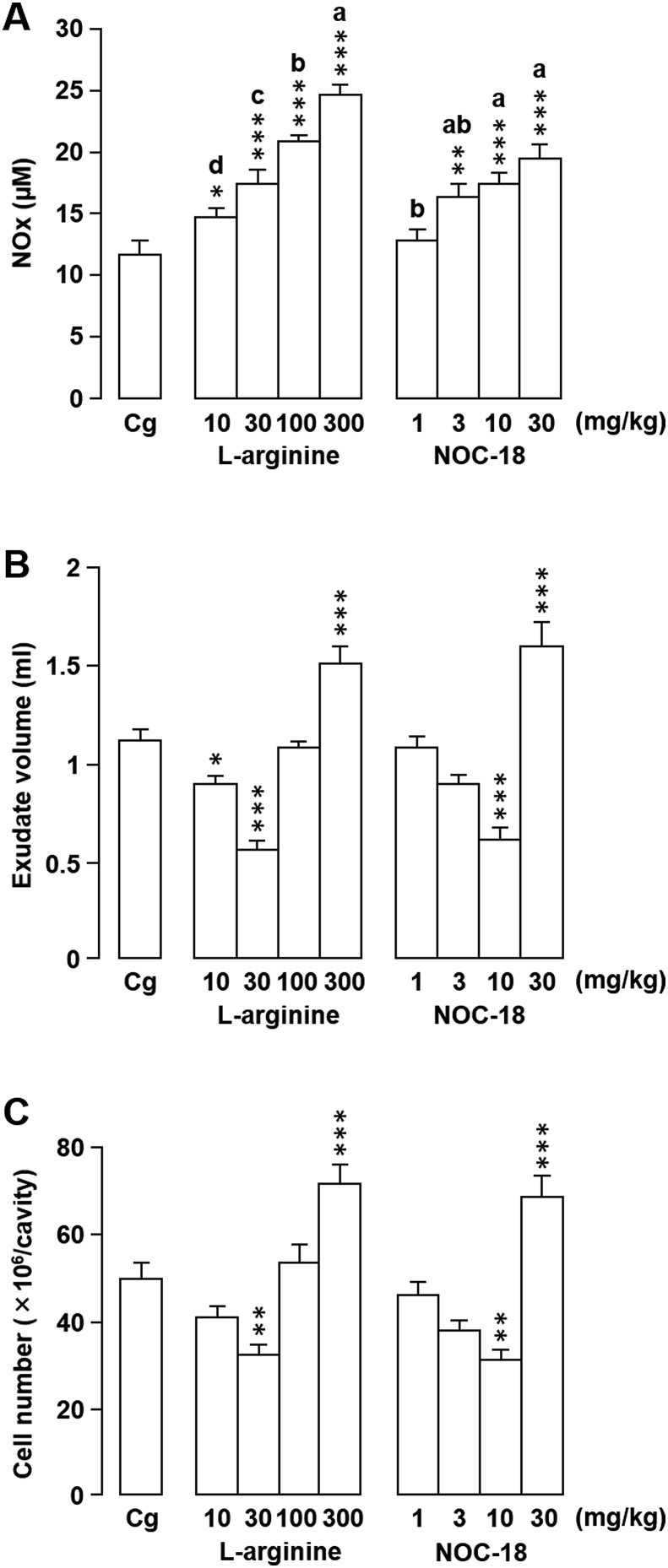
l-arginine is a substrate for NO formation and stimulates endogenous NO production in the area near its administration. Because the above results indicated that local NO production may have a protective effect at 6 h post-induction of pleurisy, we administered l-arginine to investigate the role of endogenous NO in Cg-induced pleurisy. An intrapleural l-arginine injection immediately prior to the establishment of pleurisy increased NOx concentration in pleural exudates in a dose-dependent manner (Fig. 4A). NOx levels were significantly higher in rats that received 10, 30, 100, or 300 mg/kg l-arginine compared with vehicle-treated rats, with observed increases of 26.0% (P = 0.027), 48.9% (P < 0.001), 79.0% (P < 0.001), and 110.1% (P < 0.001), respectively.
Fig. 4.
Effects of locally-injectedl-arginine or 1-hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene (NOC-18) on rat carrageenan (Cg)-induced pleurisy and nitrite/nitrate (NOx) levels. (A–C) l-arginine, NOC-18, or vehicle (saline) was injected directly into the pleural cavity immediately prior to intrapleural Cg injection. The effects of the treatments on NOx level (A), exudate volume (B), and cell number (C) in pleural exudates were evaluated 6 h after Cg challenge. Horizontal axes: doses (mg/kg) of l-arginine and NOC-18. Cg indicates control animals injected with saline prior to Cg injection. Data are expressed as mean ± SEM (n = 7–10 rats). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Cg. a, b, c, and d indicate significant differences among the designated groups (P < 0.05), where a > b > c > d and ab is not different from a or b.
In rats treated with 10 mg/kg l-arginine, the exudate volume was significantly decreased (P = 0.035) compared with that of vehicle-treated animals; however, there was no significant difference in leukocyte number between the two treatments (P = 0.190) (Fig. 4B and C). When l-arginine was administered at 30 mg/kg, both the exudate volume (P < 0.001) and leukocyte number (P = 0.003) were significantly decreased compared with the control (by 50.4% and 35.2%, respectively). In contrast, neither the exudate volume nor the extent of cell migration differed significantly from the vehicle-treated controls following an l-arginine dose of 100 mg/kg. However, exudate volume and leukocyte migration increased by 34.0% (P < 0.001) and 44.3% higher (P < 0.001), respectively, compared with the control following treatment with the highest tested l-arginine dose (300 mg/kg).
We next used NO donor NOC-18 as an exogenous sustained NO source to examine the role of exogenous NO in Cg-induced pleurisy. Following the induction of pleurisy, treatment with NOC-18 at 3.0, 10, or 30 mg/kg resulted in 39.1% (P = 0.005), 47.9% (P < 0.001), and 66.8% (P < 0.001) increases in NOx levels in the pleural exudates, respectively, compared with the vehicle-treated controls (Fig. 4A), although no difference was observed in rats treated with 1.0 mg/kg NOC-18. Similarly, the exudate volumes and leukocyte numbers were not affected by treatment with 1.0 mg/kg NOC-18 (Fig. 4B and C). While the exudate volume of rats treated with 3.0 mg/kg NOC-18 was lower than that of the controls, the difference was not significant (P = 0.069); however, administration of 10 mg/kg NOC-18 resulted in a 45.4% decrease in exudate volume compared with the vehicle treatment (P < 0.001). Similarly, the number of leukocytes in the pleural exudates was lower or significantly decreased (P = 0.002) compared with the control following the administration of 3.0 mg/kg NOC-18 (P = 0.051) or 10 mg/kg NOC-18, respectively. In contrast, following administration of 30 mg/kg NOC-18, 43.3% (P < 0.001) and 38.3% (P = 0.001) increases in exudate volume and leukocyte accumulation, respectively, were observed. These results suggest that both endogenous and exogenous NO play a critical role in modulating the inflammatory response.
For the remaining experiments, we selected l-arginine and NOC-18 doses that both significantly increased NO levels and most prominently suppressed the exudate volume and leukocyte number. Thus, 1.0 mg/kg L-NNA, 10 mg/kg AE-ITU, 30 mg/kg l-arginine, and 10 mg/kg NOC-18 were administered directly into the pleural cavity immediately prior to Cg injection to analyze the effects of these drugs on MPO and antioxidant enzyme (CAT, SOD, GPx, and GST) activities.
3.3. Effects of L-NNA, AE-ITU, l-arginine, and NOC-18 on Cg-induced oxidative stress parameters in the pleural cavity of rats
The inflammatory cascade produces, activates, and releases pro-inflammatory mediators, which can cause oxidative stress. When this phenomenon is not effectively resolved, it leads to an imbalance between the pro-oxidant and antioxidant status, aggravating the inflammatory condition [32]. To further investigate the consequences of local NO production, we assessed the effects of L-NNA, AE-ITU, l-arginine, and NOC-18 on Cg-induced oxidative stress parameters in the pleural cavity of rats. Using the doses and protocols described above to treat rats, we then measured MPO, CAT, SOD, GPx, and GST activity in pleural exudate samples (Table 1). The L-NNA (1.0 mg/kg) and AE-ITU (10 mg/kg) treatments resulted in significant increases in MPO (31.1% and 37.2%, respectively), CAT (34.6% and 30.4%, respectively), SOD (32.8% and 39.3%, respectively), GPx (25.3% and 25.8%, respectively), and GST (35.6% and 41.0%, respectively) activities compared with vehicle-treated controls (Table 1). In comparison, 30 mg/kg l-arginine resulted in significant decreases in the activity of MPO (38.7%), CAT (57.0%), SOD (47.9%), and GST (52.6%) compared with the control, with a non-significant (P = 0.062) decrease (14.7%) in GPx activity also observed in l-arginine-treated rats (Table 1). Similar decreases in enzyme activity compared with the control were observed following treatment with 10 mg/kg NOC-18 (MPO, 41.2%; CAT, 35.1%; SOD, 55.0%; GPx, 17.9%; GST, 51.9%) (Table 1).
Table 1.
Effects of local nitric oxide inhibition or induction on myeloperoxidase activity and antioxidant enzyme activities in the pleural exudates from 6 h post-carrageenan challenge.
| Group | MPOa (mU/ml) | CATb (mmol/min/ml) | SODc (USOD/ml) | GPxd (μmol/min/ml) | GSTe (μmol/min/ml) |
|---|---|---|---|---|---|
| Saline | 73.1 ± 3.8 | 11.5 ± 1.0 | 82.8 ± 1.5 | 1.54 ± 0.09 | 30.4 ± 1.6 |
| Vehiclef + Cgg | 295.8 ± 8.1 *** | 34.5 ± 1.3 *** | 115.4 ± 3.0 *** | 3.52 ± 0.10 *** | 106.4 ± 1.6 *** |
| L-NNAh + Cg | 387.9 ± 11.2 ***, ### | 46.5 ± 2.8 ***, ### | 153.2 ± 6.3 ***, ### | 4.41 ± 0.21 ***, ## | 144.4 ± 3.8 ***, ### |
| AE-ITUi + Cg | 405.9 ± 10.3 ***, ### | 45.0 ± 1.3 ***, ### | 160.8 ± 5.2 ***, ### | 4.42 ± 0.11 ***, ### | 150.1 ± 2.9 ***, ### |
| l-arginine + Cg | 181.4 ± 6.0 ***, ### | 14.9 ± 0.6 ### | 60.1 ± 4.0 **, ### | 3.00 ± 0.21 | 50.4 ± 1.7 ***, ### |
| NOC-18 + Cg | 174.0 ± 6.5 ***, ### | 22.4 ± 1.7 ***, ### | 51.9 ± 3.5 ***, ### | 2.89 ± 0.19 *, # | 51.2 ± 2.0 *, ### |
Each value represents the mean ± SEM of 6–10 rats. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. saline controls. #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. vehicle + Cg.
MPO, myeloperoxidase activity.
CAT, catalase activity.
SOD, superoxide dismutase activity.
GPx, glutathione peroxidase activity.
GST, glutathione S-transferase activity.
Vehicle, saline.
Cg, carrageenan.
L-NNA, NG-nitro-l-arginine.
AE-ITU, S-(2-aminoethyl) isothiourea.
3.4. Mechanisms by which l-arginine and NOC-18 may ameliorate inflammation
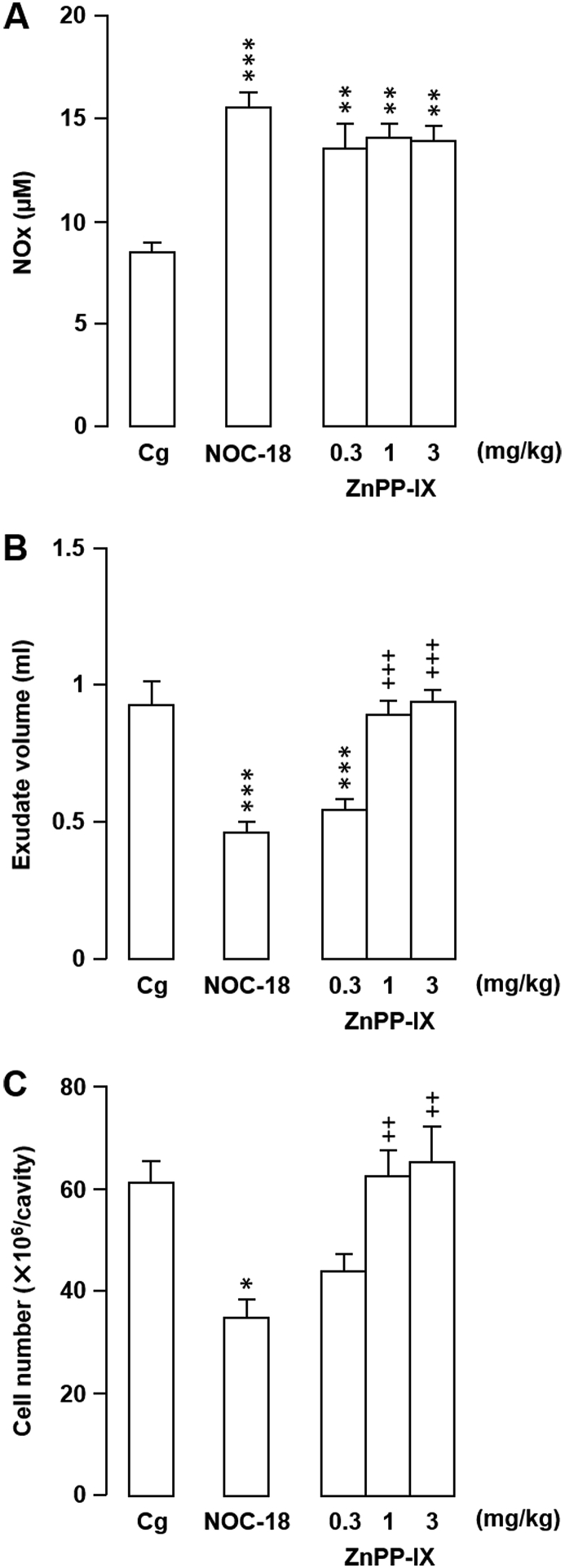
In an attempt to elucidate the mechanism(s) underlying the amelioration of acute inflammation by local NO production, we investigated the involvement of HO-1 in the observed inhibitory effects of l-arginine (30 mg/kg) and NOC-18 (10 mg/kg) on Cg-induced inflammation. Inhibition of HO-1 activity by pretreatment of rats with 1.0 or 3.0 mg/kg ZnPP-IX appeared to completely block the inhibitory effects of NOC-18, with exudate volumes and leukocyte numbers of ZnPP-IX + NOC-18-treated rats similar to those of the Cg-treated pleurisy controls (Fig. 5). Interestingly, ZnPP-IX had no effect on NOx levels (Fig. 5). Similar results were obtained following ZnPP-IX pretreatment of rats then administered l-arginine (data not shown).
Fig. 5.
Effects of zinc protoporphyrin IX (ZnPP-IX) on the influence of 1-hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene (NOC-18) on rat carrageenan (Cg)-induced pleurisy and nitrite/nitrate (NOx) levels. (A–C) ZnPP-IX or vehicle (Na2CO3) was injected subcutaneously 1 h prior to intrapleural Cg injection. Intrapleural injection of NOC-18 (10 mg/kg) was performed immediately prior to Cg injection. The effects of the treatments on NOx level (A), exudate volume (B), and cell number (C) in pleural exudates were evaluated 6 h after Cg challenge. Horizontal axes: doses (mg/kg) of ZnPP-IX. Cg indicates control animals injected with vehicle prior to Cg injection. Data are expressed as mean ± SEM (n = 5–7 rats). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Cg. ++, P < 0.01; +++, P < 0.001 vs. NOC-18.
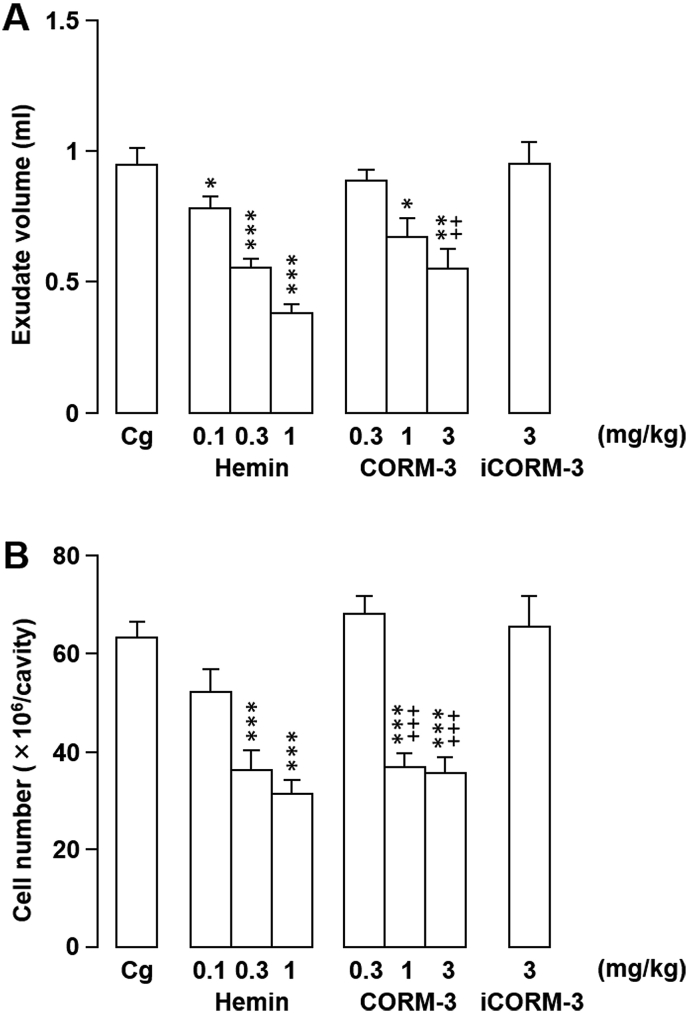
We next investigated whether hemin, the substrate of HO-1, or CO, a metabolite, were also involved in the inflammatory response. As shown in Fig. 6, pretreatment with hemin (0.3 or 1.0 mg/kg) significantly decreased the degree of exudation (P < 0.001) and leukocyte migration (P < 0.001) in Cg-induced inflammation. Similarly, pretreatment of rats with the water-soluble exogenous CO donor CORM-3 at 1.0 or 3.0 mg/kg decreased both inflammatory cell numbers and exudate formation compared with the untreated controls (Fig. 6A and B). Interestingly, the negative control, iCORM-3 (3.0 mg/kg), which is unable to release CO, had no effect on the assessed inflammatory parameters (Fig. 6).
Fig. 6.
Effects of locally-injected hemin, carbon monoxide releasing molecule-3 (CORM-3), or inactive carbon monoxide releasing molecule-3 (iCORM-3) on rat carrageenan (Cg)-induced pleurisy. (A, B) Hemin, CORM-3, iCORM-3, or vehicle (NaOH) was injected directly into the pleural cavity immediately prior to intrapleural Cg injection. The effects of the treatments on exudate volume (A) and cell number (B) in pleural exudates were evaluated 6 h after Cg challenge. Horizontal axes: doses (mg/kg) of hemin, CORM-3, and iCORM-3. Cg indicates control animals injected with vehicle prior to Cg injection. Data are expressed as mean ± SEM (n = 5–7 rats). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Cg. ++, P < 0.01; +++, P < 0.001 vs. iCORM-3.
3.5. Effects of l-arginine, NOC-18, hemin, CORM-3, and iCORM-3 on Cg-induced release of pro-inflammatory cytokines and chemokines in the pleural cavity of rats
In the final series of experiments, we investigated the possible interference of l-arginine (30 mg/kg), NOC-18 (10 mg/kg), hemin (1.0 mg/kg), CORM-3 (3.0 mg/kg), and iCORM-3 (3.0 mg/kg) in the release of cytokines TNF-α and IL-1β and the chemokine MCP-1 in the pleural exudates of Cg-treated rats 6 h post-injection. As shown in Table 2, induction of pleural inflammation by Cg treatment significantly increased the levels of TNF-α, IL-1β, and MCP-1 in the pleural exudate compared with the saline control group. In contrast, pretreatment with l-arginine, NOC-18, hemin, or CORM-3 resulted in a marked decrease in TNF-α, IL-1β, and MCP-1 levels compared with the vehicle-treated controls. Administration of negative control iCORM-3 failed to significantly modify the levels of these mediators.
Table 2.
Effects of l-arginine, 1-hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene (NOC-18), hemin, carbon monoxide releasing molecule-3 (CORM-3), or inactive carbon monoxide releasing molecule-3 (iCROM-3) on the release of pro-inflammatory cytokines and chemokines in the pleural exudates 6 h after carrageenan (Cg) challenge.
| Group | TNF-αa (pg/ml) | IL1-βb (pg/ml) | MCP-1c (pg/ml) |
|---|---|---|---|
| Saline | 36.0 ± 0.6 | 167.3 ± 9.8 | 517.6 ± 27.6 |
| Vehicled + Cg | 1412.2 ± 109.4 *** | 1897.4 ± 165.7 *** | 2889.6 ± 183.6 *** |
| l-arginine + Cg | 778.2 ± 100.6 ***, ### | 1231.5 ± 220.3 **, # | 1756.4 ± 176.9 ***, ### |
| NOC-18 + Cg | 792.2 ± 106.3 ***, ### | 883.7 ± 152.6 ### | 1806.5 ± 161.7 ***, ### |
| Hemin + Cg | 721.2 ± 72.8 ***, ### | 930.5 ± 171.1 *, ## | 1758.9 ± 205.8 ***, ### |
| CORM-3 + Cg | 598.9 ± 88.9 **, ### | 723.6 ± 131.0 ### | 993.4 ± 125.1 ***, ### |
| iCORM-3 + Cg | 1475.2 ± 89.3 *** | 1844.9 ± 174.8 *** | 3167.3 ± 178.4 *** |
Each value represents the mean ± SEM of 5–10 rats. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. saline controls. #, P < 0.05; ##, P < 0.01, ###, P < 0.001 vs. vehicle + Cg.
TNF-α, tumor necrosis factor-α.
IL-1β, interleukin-1β.
MCP-1, monocyte chemoattractant protein-1.
Vehicle, saline.
3.6. Effects of L-NNA, AE-ITU, l-arginine, NOC-18, ZnPP-IX, hemin, CORM-3, and iCORM-3 on pleural inflammation in the absence of Cg
To confirm that AE-ITU, L-NNA, l-arginine, NOC-18, ZnPP-IX, hemin, CORM-3, and iCORM-3 did not affect inflammation simply as a result of direct irritation, these compounds, as well as the appropriate vehicles, were injected into rats in the absence of Cg. None of the compounds caused exudate formation or an increase in inflammatory cells (data not shown).
4. Discussion
NO is responsible for numerous physiological and pathophysiological processes, although excess concentrations are toxic and can cause tissue damage [33]. As such, NOS inhibitors have shown anti-inflammatory effects in both acute and chronic models of inflammation. For example, in a rat trinitrobenzene sulfonic acid-induced colitis model, NOS inhibitor NG-nitro-l-arginine methyl ester hydrochloride reduced the influx of both neutrophils and macrophages [6], while a second NOS inhibitor, NG-l-monomethylarginine, significantly inhibited exudate formation and cellular influx in a Cg-induced pleurisy model [10]. However, in contrast to these findings, a single intrapleural injection of the selective iNOS inhibitors AE-ITU and N-(3-(aminomethyl)-benzyl) acetamidine or the selective eNOS inhibitor L-N [5](1-iminoethyl)-ornithine not only exacerbated inflammation but also prevented the resolution of inflammation [2]. The apparently contradictory and site-specific effects of NO on inflammation were confirmed in the current study, where intrapleural injection of NOS inhibitors L-NNA or AE-ITU significantly exacerbated inflammation, while systemic injection of the inhibitors into the peritoneal cavity decreased inflammatory exudate NOx levels, exudate volumes, and inflammatory cell counts compared with the control. These findings were further supported by the decreased leukocyte infiltration, vascular permeability, and oxidative stress-associated enzyme activity observed in the rats following treatment with NO substrate l-arginine or NO donor NOC-18.
While our results supported a protective role for low levels of NO at the site of inflammation, the underlying mechanism remained elusive. HO is a member of the heat shock protein family that catalyzes the conversion of heme to biliverdin/bilirubin, ferrous iron, and CO. The inducible isoform of HO, HO-1, is activated in response to cellular stress, with NO shown to be a powerful inducer of HO-1 expression [[14], [15], [16]]. For example, Motterlini et al. showed that iNOS activity significantly induced the expression of HO-1 in porcine aortic endothelial cells, providing protection against hydrogen peroxide stress [14]. Subsequent studies confirmed the protective role of NO-mediated HO-1 expression under both hypoxia and nitrosative stress conditions [15,16], which can impair the cellular redox status, leading to inflammation and cell injury [34,35]. As such, numerous studies have demonstrated that HO-1 expression and the concomitant production of its metabolite CO have an anti-inflammatory effect [22,[36], [37], [38]]. Therefore, we explored the interplay between the HO-1 and NOS systems. Pretreatment of rats with HO-1-specific inhibitor ZnPP-IX completely blocked the beneficial effects of NOC-18 on the degree of exudation and leukocyte migration triggered by Cg challenge, without affecting NOx levels. In contrast, HO-1 substrate hemin and CO-releasing molecule CORM-3 significantly decreased leukocyte migration and exudation, indicating that NO-induced HO-1 expression is likely to be at least partly responsible for the anti-inflammatory effects of localized NO expression. In addition, the reduction of inflammatory parameters promoted by CO was dependent on the production of pro-inflammatory cytokines and chemokines. There is substantial evidence that pro-inflammatory cytokines TNF-α and IL-1β, along with chemokine MCP-1, propagate both local and systemic inflammatory processes [39,40]. Our results confirm that the inflammatory process induced by Cg injection into the pleural cavity leads to substantial increases in the levels of TNF-α, IL-1β, and MCP-1 in the exudate. In contrast, NOC-18 and l-arginine prevented the increase in these inflammatory molecules in the exudate, which is consistent with our previous findings [1].
Importantly, hemin and CORM-3 also decreased the levels of the pro-inflammatory mediators in the exudate. This supports the findings of Vicente et al. [19], who demonstrated that HO-1 induction by hemin reduced the levels of TNF-α and IL-1β in a zymosan-induced model of inflammation. Another study revealed that CORM-3-derived CO reduces the concentrations of TNF-α and IL-1β in joint tissues in a mouse model of collagen-induced arthritis [41]. In vitro studies have also shown that NO donors (NONOate compounds, 3-morpholino-sydnonimine (SIN-1), and nitroprusside) inhibit TNF-α- or IL-1β-induced expression and production of MCP-1 in human endothelial and mesangial cells [42,43], suggesting that NO modulates MCP-1 expression and activity in vitro. These findings, together with the current results showing lower levels of TNF-α, IL-1β, and MCP-1 in the l-arginine + Cg, NOC-18 + Cg, hemin + Cg, and CORM-3 + Cg groups, confirm the protective effects of both NO and HO-1 induction, leading us to hypothesize that the NOS/HO-1/CO pathway is involved in blunting the inflammatory response.
Our results showed that both l-arginine and NOC-18 significantly increase vascular permeability and cell migration at the highest tested doses, which may be associated with blood pressure reduction or additional tissue damage caused by the high concentrations of NO at the inflammatory site [21,33]. Conversely, low l-arginine or NOC-18 doses, which are not likely to affect vascular tone [21], significantly suppressed leukocyte migration, suggesting that NO primarily modulates Cg-induced pleurisy by reducing cell accumulation at the inflammation site. The modulatory role of l-arginine depends on NO formation, which can be generated at the inflammatory site by either cNOS or iNOS [7]. However, the observed dose-dependent effects of l-arginine in this study indicate that iNOS expression plays an important role in rat Cg-induced pleurisy. Intracellular l-arginine levels are inadequate to sustain the large amounts of NO produced by iNOS, meaning that NO synthesis from iNOS depends on substrate replenishment from extracellular stores [44], and that NO generation from l-arginine is dose-dependent. In contrast, we did not observe any significant difference in NOx concentrations in pleural exudates of rats treated with NOC-18 at 3, 10, or 30 mg/kg. This indicates that the mechanisms of NO formation from l-arginine and NOC-18 may differ, which is supported by the previously demonstrated blood pressure drop induced by 30 mg/kg NOC-18 [21].
GST is a detoxification enzyme that plays a pivotal role in cellular responses to oxidative stress along with CAT, SOD, and GPx [45]. GST, CAT, SOD, and GPx activities are increased in various models of acute inflammation, including Cg-induced pleurisy [[46], [47], [48]]. In the current study, the Cg-induced overactivity of these four antioxidant enzymes reverted to baseline levels following co-treatment with l-arginine or NOC-18. In contrast, both L-NNA and AE-ITU increased enzymatic activity. Therefore, we hypothesize that because leukocytes are largely responsible for the formation of reactive oxygen species as a host defense, this effect is a consequence of the inhibitory activity of NO on leukocyte migration. The enzyme MPO is another major pro-inflammatory mediator that participates in neutrophil phagocytosis and is responsible for the conversion of H2O2, generated by SOD, into hypochlorous acid. Although it stimulates the host immune response to microbial invasion, continuous and unbalanced MPO activity is closely related to tissue damage as a consequence of oxidative stress [49]. Our results demonstrate that the administration of a NO substrate or NO donor can inhibit MPO activity, which supports previous findings by Fernandes et al. [50] who showed that two different NO donors inhibited MPO activity in a Cg-induced paw edema mouse model.
In summary, using a Cg-induced pleurisy model, we explored the critical involvement of NO in leukocyte migration to the inflammatory site and the interplay between the HO-1 and NOS systems, finding evidence that a molecular cascade formed by NOS/NO/HO-1/CO downregulates leukocyte migration during an acute inflammatory reaction. Importantly, we also showed that HO-1 activity and CO are involved in the inhibitory effect of NO on the production of pro-inflammatory cytokines and chemokines. To our knowledge, this is the first time that local production of NO has been shown to be protective by virtue of its ability to regulate the release of typical pro-inflammatory mediators, and that the effect of NO is likely to be mediated via the HO-1/CO pathway. Although our findings are currently limited to the Cg model of rat pleurisy and need confirmation in other inflammation models, they suggest that NO supplementation may be a promising treatment for inflammatory diseases involving oxidative stress.
Author contributions
MI, TI, and SS conceived and designed the study. MI, TI, and KH performed the experiments. MI, TI, KH, YA, MF, SM, WT, and SS analyzed and interpreted the data. MI, YA, and SS wrote, reviewed, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Public Advertisement Research Project of Nihon Fukushi University. The funding body had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Declaration of competing interest
The authors have no conflicts of interests to declare.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. We thank Tamsin Sheen, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100790.
Contributor Information
Masahiro Iwata, Email: iwata-m@n-fukushi.ac.jp.
Takayuki Inoue, Email: takagoingmyway@med.nagoya-u.ac.jp.
Yuji Asai, Email: asai@n-fukushi.ac.jp.
Kiyomi Hori, Email: kiyo@med.kanazawa-u.ac.jp.
Mitsuhiro Fujiwara, Email: 32fujiwara@gmail.com.
Shingo Matsuo, Email: matsuo@n-fukushi.ac.jp.
Wakako Tsuchida, Email: w-tsuchida@aist.go.jp.
Shigeyuki Suzuki, Email: suzuki@met.nagoya-u.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Iwata M., Suzuki S., Asai Y., Inoue T., Takagi K. Involvement of nitric oxide in a rat model of carrageenin-induced pleurisy. Mediat. Inflamm. 2010:682879. doi: 10.1155/2010/682879. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul-Clark M.J., Gilroy D.W., Willis D., Willoughby D.A., Tomlinson A. Nitric oxide synthase inhibitors have opposite effects on acute inflammation depending on their route of administration. J. Immunol. 2001;166:1169–1177. doi: 10.4049/jimmunol.166.2.1169. [DOI] [PubMed] [Google Scholar]
- 3.Lee M., Rey K., Besler K., Wang C., Choy J. Immunobiology of nitric oxide and regulation of inducible nitric oxide synthase. Results Probl. Cell Differ. 2017;62:181–207. doi: 10.1007/978-3-319-54090-0_8. [DOI] [PubMed] [Google Scholar]
- 4.Kubes P., Suzuki M., Granger D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii E., Irie K., Uchida Y., Tsukahara F., Muraki T. Possible role of nitric oxide in 5-hydroxytryptamine-induced increase in vascular permeability in mouse skin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994;350:361–364. doi: 10.1007/BF00178952. [DOI] [PubMed] [Google Scholar]
- 6.Hogaboam C.M., Jacobson K., Collins S.M., Blennerhassett M.G. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am. J. Physiol. 1995;268:G673–G684. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- 7.Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992;211:177–182. doi: 10.1016/0014-2999(92)90526-A. [DOI] [PubMed] [Google Scholar]
- 8.Kozan A., Kilic N., Alacam H., Guzel A., Guvenc T., Acikgoz M. The effects of dexamethasone and L-NAME on acute lung injury in rats with lung contusion. Inflammation. 2016;39:1747–1756. doi: 10.1007/s10753-016-0409-0. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi Y., Shirahase H., Kunishiro K., Ichikawa A., Kanda M., Uehara Y. Effect of combination of nitric oxide synthase and cyclooxygenase inhibitors on carrageenan-induced pleurisy in rats. Life Sci. 2006;79:442–447. doi: 10.1016/j.lfs.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Tracey W.R., Nakane M., Kuk J., Budzik G., Klinghofer V., Harris R., Carter G. The nitric oxide synthase inhibitor, L-NG-monomethylarginine, reduces carrageenan-induced pleurisy in the rat. J. Pharmacol. Exp. Therapeut. 1995;273:1295–1299. [PubMed] [Google Scholar]
- 11.Najafipour H., Ferrell W.R. Nitric oxide modulates sympathetic vasoconstriction and basal blood flow in normal and acutely inflamed rabbit knee joints. Exp. Physiol. 1993;78:615–624. doi: 10.1113/expphysiol.1993.sp003710. [DOI] [PubMed] [Google Scholar]
- 12.Ridger V.C., Pettipher E.R., Bryant C.E., Brain S.D. Effect of the inducible nitric oxide synthase inhibitors aminoguanidine and L-N6-(1-iminoethyl)lysine on zymosan-induced plasma extravasation in rat skin. J. Immunol. 1997;159:383–390. [PubMed] [Google Scholar]
- 13.Vicente A.M., Guillén M.I., Alcaraz M.J. Modulation of haem oxygenase-1 expression by nitric oxide and leukotrienes in zymosan-activated macrophages. Br. J. Pharmacol. 2001;133:920–926. doi: 10.1038/sj.bjp.0704145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motterlini R., Foresti R., Intaglietta M., Winslow R.M. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am. J. Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 15.Foresti R., Clark J.E., Green C.J., Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. J. Biol. Chem. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 16.Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE and Green CJ: Endothelial heme oxygenase-1 induction by hypoxia. J. Biol. Chem. 275: 13613–13620. [DOI] [PubMed]
- 17.Wagener F.A., van Beurden H.E., von den Hoff J.W., Adema G.J., Figdor C.G. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102:521–528. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S., Takamiya R., Yamaguchi T., Matsumoto K., Tojo S.J., Tamatani T., Kitajima M., Makino N., Ishimura Y., Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ. Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 19.Vicente A.M., Guillén M.I., Habib A., Alcaraz M.J. Beneficial effects of heme oxygenase-1 up-regulation in the development of experimental inflammation induced by zymosan. J. Pharmacol. Exp. Therapeut. 2003;307:1030–1037. doi: 10.1124/jpet.103.057992. [DOI] [PubMed] [Google Scholar]
- 20.Clark J.E., Naughton P., Shurey S., Green C.J., Johnson T.R., Mann B.E., Foresti R., Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 21.Ialenti A., Ianaro A., Maffia P., Sautebin L., Di Rosa M. Nitric oxide inhibits leucocyte migration in carrageenin-induced rat pleurisy. Inflamm. Res. 2000;49:411–417. doi: 10.1007/s000110050609. [DOI] [PubMed] [Google Scholar]
- 22.Chaves H.V., do Val D.R., Ribeiro K.A., Lemos J.C., Souza R.B., Gomes F.I.F., da Cunha R.M.S., de Paulo Teixeira Pinto V., Filho G.C., de Souza M.H.L.P., Bezerra M.M., de Castro Brito G.A. Heme oxygenase-1/biliverdin/carbon monoxide pathway downregulates hypernociception in rats by a mechanism dependent on cGMP/ATP-sensitive K+ channels. Inflamm. Res. 2018;67:407–422. doi: 10.1007/s00011-018-1133-z. [DOI] [PubMed] [Google Scholar]
- 23.Mizuguchi S., Stephen J., Bihari R., Markovic N., Suehiro S., Capretta A., Potter R.F., Cepinskas G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H920–H929. doi: 10.1152/ajpheart.00305. 2009. [DOI] [PubMed] [Google Scholar]
- 24.Fritsche G., Dlaska M., Barton H., Theurl I., Garimorth K., Weiss G. Nramp1 functionality increases inducible nitric oxide synthase transcription via stimulation of IFN regulatory factor 1 expression. J. Immunol. 2003;171:1994–1998. doi: 10.4049/jimmunol.171.4.1994. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 29.Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 30.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/S0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 31.Willis D., Moore A.R., Frederick R., Willoughby D.A. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 32.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;5698931 doi: 10.1155/2016/5698931. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskin J.D., Heck D.E., Laskin D.L. Multifunctional role of nitric oxide in inflammation. Trends Endocrinol. Metabol. 1994;5:377–382. doi: 10.1016/1043-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 34.Motterlini R., Green C.J., Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxidants Redox Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- 35.Foresti R., Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic. Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- 36.Steiner A.A., Branco L.G., Cunha F.Q., Ferreira S.H. Role of the haeme oxygenase/carbon monoxide pathway in mechanical nociceptor hypersensitivity. Br. J. Pharmacol. 2001;132:1673–1682. doi: 10.1038/sj.bjp.0704014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freitas A., Alves-Filho J.C., Secco D.D., Neto A.F., Ferreira S.H., Barja-Fidalgo C., Cunha F.Q. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br. J. Pharmacol. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dal-Secco D., Freitas A., Abreu M.A., Garlet T.P., Rossi M.A., Ferreira S.H., Silva J.S., Alves-Filho J.C., Cunha F.Q. Reduction of ICAM-1 expression by carbon monoxide via soluble guanylate cyclase activation accounts for modulation of neutrophil migration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010;381:483–493. doi: 10.1007/s00210-010-0500-2. [DOI] [PubMed] [Google Scholar]
- 39.Fröde T.S., Souza G.E., Calixto J.B. The modulatory role played by TNF-α and IL-1β in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2001;13:162–168. doi: 10.1006/cyto.2000.0816. [DOI] [PubMed] [Google Scholar]
- 40.Lansley S.M., Cheah H.M., Lee Y.C. Role of MCP-1 in pleural effusion development in a carrageenan-induced murine model of pleurisy. Respirology. 2017;22:758–763. doi: 10.1111/resp.12951. [DOI] [PubMed] [Google Scholar]
- 41.Ferrándiz M.L., Maicas N., Garcia-Arnandis I., Terencio M.C., Motterlini R., Devesa I., Joosten L.A., van den Berg W.B., Alcaraz M.J. Treatment with a CO-releasing molecule (CORM-3) reduces joint inflammation and erosion in murine collagen-induced arthritis. Ann. Rheum. Dis. 2008;67:1211–1217. doi: 10.1136/ard.2007.082412. [DOI] [PubMed] [Google Scholar]
- 42.Desai A., Miller M.J., Huang X., Warren J.S. Nitric oxide modulates MCP-1 expression in endothelial cells: implications for the pathogenesis of pulmonary granulomatous vasculitis. Inflammation. 2003;27:213–223. doi: 10.1023/a:1025036530605. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.K., Kim C.S., Yang W.S., Kim S.B., Park S.K., Park J.S. Exogenous nitric oxide inhibits tumor necrosis factor-alpha- or interleukin-1-beta-induced monocyte chemoattractant protein-1 expression in human mesangial cells. Role of IkappaB-alpha and cyclic GMP. Nephron. 2002;92:780–787. doi: 10.1159/000065441. [DOI] [PubMed] [Google Scholar]
- 44.Griffith O.W., Park K.H., Aisaka K., Levi R., Gross S.S. The role of plasma arginine in nitric oxide synthesis: studies with arginase-treated Guinea pigs and rats. In: Moncada S., Marlette M.A., Hibbs J.B. Jr., Higgs E.A., editors. The Biology of Nitric Oxide. 1. Physiological and Clinical Aspects. Portland Press; London: 1992. pp. pp6–10. [Google Scholar]
- 45.Sánchez-Gómez F.J., Díez-Dacal B., García-Martín E., Agúndez J.A., Pajares M.A., Pérez-Sala D. Detoxifying enzymes at the cross-roads of inflammation, oxidative stress, and drug hypersensitivity: role of glutathione transferase P1-1 and aldose reductase. Front. Pharmacol. 2016;7:237. doi: 10.3389/fphar.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalmarco E.M., Budni P., Parisotto E.B., Wilhelm Filho D., Fröde T.S. Antioxidant effects of mycophenolate mofetil in a murine pleurisy model. Transpl. Immunol. 2009;22:12–17. doi: 10.1016/j.trim.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Halici Z., Dengiz G.O., Odabasoglu F., Suleyman H., Cadirci E., Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur. J. Pharmacol. 2007;566:215–221. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 48.Valença S.S., Pimenta W.A., Rueff-Barroso C.R., Ferreira T.S., Resende A.C., Moura R.S., Porto L.C. Involvement of nitric oxide in acute lung inflammation induced by cigarette smoke in the mouse. Nitric Oxide. 2009;20:175–181. doi: 10.1016/j.niox.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Odobasic D., Yang Y., Muljadi R.C., O'Sullivan K.M., Kao W., Smith M., Morand E.F., Holdsworth S.R. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheum. 2014;66:907–917. doi: 10.1002/art.38299. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes D., Da Silva-Santos J.E., Assreuy J. Nitric oxide-induced inhibition of mouse paw edema: involvement of soluble guanylate cyclase and potassium channels. Inflamm. Res. 2002;51:377–384. doi: 10.1007/PL00000318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.