Highlights
-
•
Cognitive deficits, a core symptom of schizophrenia, are difficult to treat with available therapies.
-
•
Abnormal neural dynamics of frontal gamma oscillations contribute to these deficits.
-
•
Neurofeedback has been used previously to alter brain oscillations.
-
•
Gamma band neurofeedback can impact brain and behavioral markers of cognition.
Keywords: Schizophrenia, Neurofeedback, Cognitive remediation, Working memory, N-back, Gamma
Abbreviations: SCZ, Schizophrenia; NFB, Neurofeedback; EEG, electroencephalogram; GBR, gamma band response; WM, working memory
Abstract
Schizophrenia is a debilitating mental disorder that is associated with cognitive deficits. Impairments in cognition occur early in the course of illness and are associated with poor functional outcome, but have been difficult to treat with conventional treatments. Recent studies have implicated abnormal neural network dynamics and impaired connectivity in frontal brain regions as possible causes of cognitive deficits. For example, high-frequency, dorsal-lateral prefrontal oscillatory activity in the gamma range (30–50 Hz) is associated with impaired working memory in individuals with schizophrenia. In light of these findings, it may be possible to use EEG neurofeedback (EEG-NFB) to train individuals with schizophrenia to enhance frontal gamma activity to improve working memory and cognition. In a single-group, proof-of-concept study, 31 individuals with schizophrenia received 12 weeks of twice weekly EEG-NFB to enhance frontal gamma band response. EEG-NFB was well-tolerated, associated with increased gamma training threshold, and significant increases in frontal gamma power during an n-back working memory task. Additionally, EEG-NFB was associated with significant improvements in n-back performance and working memory, speed of processing, and reasoning and problem solving on neuropsychological tests. Change in gamma power was associated with change in cognition. Significant improvements in psychiatric symptoms were also found. These encouraging findings suggest EEG-NFB targeting frontal gamma activity may provide a novel effective approach to cognitive remediation in schizophrenia, although placebo-controlled trials are needed to assess the effects of non-treatment related factors.
1. Introduction
Schizophrenia (SCZ) is a heterogeneous, debilitating mental disorder that affects 2.4 million Americans and is a leading cause of disability worldwide. The disorder typically presents during late adolescence and disrupts multiple aspects of brain development (Silver, 2003, Mohamed, 1999). Cognitive deficits occur early in the course of the illness and are associated with poor functional outcome (Silver, 2003, Mohamed, 1999). Cognitive impairments have been difficult to treat using conventional treatment modalities. In this context, emerging evidence of aberrant neural dynamics in SCZ provides compelling opportunities to develop novel therapeutics to improve cognitive deficits.
Recent studies suggest that cognitive deficits in patients with SCZ may arise from desynchronization of distributed neural networks. Synchronization, or coordinated activation of neurons, specifically in the gamma band (30–50 Hz) has been shown to play a central role in top-down attention, multisensory processing, perceptual binding and working memory (WM). EEG recordings in patients with SCZ have been associated with abnormal gamma band responses (GBR), both in power and coherence relationships, during WM tasks. For instance, behavioral abnormalities in WM and aberrant gamma band response (GBR) have been noted in early (Haenschel, 2009) and chronic stages of SCZ (Cho et al., 2006, Minzenberg, 2010), across different tasks (Chen, 2014) (Kissler, 2000), and during various phases of WM including encoding, maintenance and retrieval (Chen, 2014). In contrast, resting state gamma power, however, appears to be a trait rather than state marker (Mitra, 2015), and does not change in response to antipsychotic treatment in individuals with SCZ. The degree of GBR impairment has also been associated with the degree of disorganization symptoms (Cho et al., 2006). These findings are also consistent with convergent data from molecular studies where parvalbumin expressing interneurons responsible for producing gamma oscillations are disrupted in SCZ patient (Dienel and Lewis, 2018, Lewis and Glausier, 2016, Rotaru et al., 2012).
These findings suggest that an intervention that enhances frontal GBR, such as EEG neurofeedback (NFB), may improve WM in schizophrenia. NFB is an operant conditioning technique for learning how to control one’s brain activity to improve cognitive performance, regulate stress levels, emotional functioning and behavior. NFB is based on visualization of brain activity to make it accessible. The subject is presented with a visual metaphor of brain activity, and then either up- or down- regulates it to make it fit within predetermined parameters. Brain activity can be measured via EEG and used to provide real-time feedback on brain function. EEG-NFB is a non-invasive, relatively inexpensive, well-tolerated and easily administered treatment. Modulation of brain activity via EEG-NFB has been employed in the treatment of a variety of disorders including attention deficit hyperactivity disorder (ADHD), autism, depression and post-traumatic stress disorder (PTSD) (Angelakis, 2007, Gevensleben, 2009, Gevensleben, 2009, Kluetsch, 2014, Levesque et al., 2006). Studies have shown that EEG-NFB has positive effects on cognitive function (Enriquez-Geppert et al., 2013, Gruzelier, 2014, Gruzelier, 2014, Gruzelier, 2014). At present, there are no studies employing EEG-NFB to modulate GBR in patients with SCZ.
With this in mind, we designed an open label clinical trial of EEG-NFB to enhance gamma band response bilaterally across the frontal cortex in participants with SCZ. As a single group trial, the study was designed to explore if GBR can be modulated in participants with schizophrenia using EEG-NFB. Secondary exploratory aims included examining changes in WM, other cognitive domains, and symptoms during EEG-NFB. We hypothesized that gamma NFB would be associated with positive changes in neural markers (improved task-induced GBR), and behavior (gains in performance on the n-back task and WM domain of the MCCB). Additionally, we planned to estimate correlations between change in NFB training threshold (TT) and changes in cognition to test whether behavioral changes were associated with changes in brain metrics.
2. Materials and methods
Men and women of any race between the age of 18–65 years with a diagnosis of SCZ or schizoaffective disorder were recruited from a variety of outpatient clinics and board-and-care/group homes in San Diego County. A best estimate diagnostic approach was used in which information from the Structured Clinical Interview for DSM5 was supplemented by information from family, previous psychiatrists, and medical records to generate a diagnosis. Subjects prescribed the same antipsychotic for at least 60 days and a constant therapeutic dose for at least 30 days prior to study entry were included. Subjects with an organic brain disorder, seizure disorder, history of traumatic brain injury, or mental retardation, were excluded. Subjects who met DSM5 criteria for alcohol or substance dependence (except nicotine) within the last 6 months or DSM5 criteria for alcohol or substance abuse (except nicotine) within the last month were also excluded. Only those subjects competent to participate in the informed consent process and who provided voluntary informed consent were included. This study was approved by the Human Subjects Committee of UC San Diego.
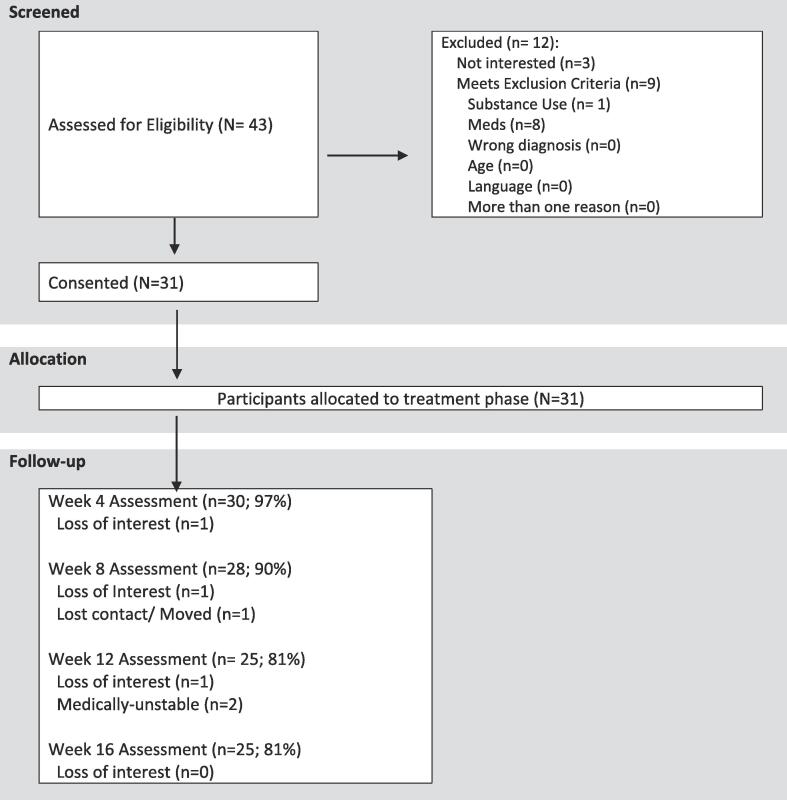
A total of 43 participants were screened, out of which 31 participants met inclusion criteria and consented for treatment (Fig. 1). Participants had a mean age at baseline of 45.5 years (SD = 9.4; range = 26–61) and 12.4 years (SD = 2.4; range = 8–19) of education. There were 16 female and 15 male participants. 71% of the sample was Caucasian. On average patients were on 419.1 mg (SD = 392.7) chlorpromazine equivalents of antipsychotic medication. At baseline subjects were moderately ill as characterized by the Positive and Negative Syndrome Scale (PANSS) symptom severity scores. Mean baseline total PANSS scores were 85.7 (SD = 6.5, range 73–98), positive symptom subscale scores were 20.0 (SD = 2.5, range 14–25), negative symptom subscale scores were 23.5 (SD = 4.5, range 15–33) and general psychopathology subscale scores were 42.3 (SD = 4.3, range 37–53), all within moderate severity range.
Fig. 1.
Study enrolment and subject flow.
2.1. Study assessment schedule
Behavioral and electrophysiological assessments were conducted at baseline and every 4 weeks of treatment (t = 0, 4, 8 and 12 weeks) to determine treatment-induced dose response relationships, as well as at 4 weeks post-treatment follow-up (t = 16 weeks), to examine durability of treatment effects. Symptom ratings and functional assessments were conducted at baseline, 12 and 16-week timepoints.
2.1.1. Electrophysiology/N-back task
Subjects were seated in a quiet room and asked to perform the n-back task while 32-channels of EEG were collected at 500 Hz sampling rate using the Cognionics Quick30 ®, wireless, dry electrode EEG recording cap. The n-back is a continuous performance, computerized task used to measure WM. The subject is presented with a sequence of stimuli (letters, number, or shapes), and the task consists of indicating when the current stimulus matches the one from n steps earlier in the sequence. The load factor n can be adjusted to make the task more or less difficult (Salari et al., 2014). Subjects performed the 0-back task as a positive control followed by the more demanding 1-back task, and the most demanding, 2-back task. Stimuli were presented on a computer monitor screen at a viewing distance of approximately 96 cm using Presentation software (version 20.3).
2.1.2. Neuropsychological functioning: the MATRICS Consensus Cognitive Battery (MCCB)
Consists of both paper and pencil and computerized cognitive tests (Green and Nuechterlein, 2004, Green, 2004). The MCCB was specifically designed to assess treatment-related changes in cognition in people with schizophrenia. The MCCB is comprised of 10 tests that assess seven cognitive domains: Speed of Processing, Attention/Vigilance, Working Memory (nonverbal), Working Memory (verbal), Verbal Learning, Visual Learning and Reasoning and Problem Solving. The MCCB composite score is a standardized mean of the seven domain scores. T-scores are standardized to normative data. For MCCB, larger scores indicate better performance.
2.1.3. Symptom ratings
The Positive and Negative Syndrome Scale (PANSS), including the Positive (7 items), Negative (7 items), and General Psychopathology (16 items) subscales, was used. Higher scores on total and subscales (positive, negative and general psychopathology) indicate higher severity of disease. Positive symptoms include items such as hallucinations, delusions and paranoid ideation, whereas negative symptoms include reduced affect, alogia, depression and amotivation.
2.1.4. Treatment tolerability/Patient satisfaction
Treatment tolerability was assessed every 4 weeks using a questionnaire. The survey used a scale from 0 to 20, where 20 represents highest satisfaction.
2.2. EEG-NFB training
Subjects were scheduled to receive 12 h of gamma-NFB training over 12 weeks (30 min of training, twice weekly for 12 weeks). Positive feedback was provided to reinforce an increase in synchronous GBR at both F3 and F4 electrodes, using the Thought Technologies, Procomp-2, 2-channel neurofeedback device.
Subjects were given a choice of visual metaphors (games) where success in the game was related to increasing the GBR. All subjects viewed a computer screen on which the game was displayed and instructed to make the game continuous and win as many points as possible. Games included flying a plane, riding a roller-coaster, or nature scenes that morphed as the game progressed. Baseline GBR was assessed on the first visit to compute an initial training threshold (TT). Initial TT was defined as the value required to achieve positive feedback 75–80% of the time during the first feedback session. On the next session, TT was increased by 5%, so that it was now 5% more difficult to receive positive feedback. This new threshold was maintained until the subject was able to achieve 75–80% success rate again. This process was repeated for the remainder of the study for a total of 12 weeks. This successive approximation was meant to operantly condition subjects to learn to modulate their frontal gamma activity. Change in TT was computed every 4 weeks throughout the duration of the study at t = 4, 8 and 12 weeks.
2.3. Data analysis
2.3.1. EEG data processing
Raw EEG was processed using EEGLAB (Delorme and Makeig, 2004) under Matlab 2018b. EEG data were high-pass filtered at 1.0 Hz and low-pass filtered at 50 Hz with FIR filters. EEGLAB plugins pop_rejchan() and clean_rawdata() were used to automatically remove and interpolate EEG channels with the following criteria: (1) spectral power between 1 and 50 Hz that was three standard deviations above or below that of other channels, (2) channels with flat signals longer than 5 s, (3) channels that were poorly correlated (r < 0.7) with their reconstructed versions based on adjacent channels, (4) channels with line noise power four standard deviations higher than their signals. EEG data were then re-referenced to common average reference.
For the remaining channels, line noise was further removed using cleanline in EEGLAB (Bigdely-Shamlo, 2015). Artifact subspace reconstruction (ASR) was applied to remove and interpolate non-stationary, high-amplitude bursts using clean_asr (σ = 20) (Chang, 2019). Independent component analysis was performed and an automatic IC classifier, ICLabel (Pion-Tonachini et al., 2019), was used to separate and label independent components (ICs) into seven categories (see https://labeling.ucsd.edu/tutorial/labels for details). The ICs labeled as muscle, eye, heart, line noise, and channel noise with probability higher than 0.5 were rejected. On average two to eight ICs, mostly labeled as eye and muscle, were rejected for each EEG recording. The final cleaned channel signals were reconstructed using the remaining ICs. The script of the processing pipeline is available online (https://github.com/goodshawn12/resteeg) and all the functions and plugins are available in EEGLAB.
For EEG recordings during the working memory task, EEG data were epoched from −0.25 to 1.75 s relative to the presentation of the stimuli (i.e. letters on the screen). The epoched trials that contained absolute amplitude larger than 150 µV were rejected using pop_eegthresh function in EEGLAB. The trials with improbable events (e.g. artifacts), defined as having normalized joint probabilities that were three standard deviations above that of all trials were also rejected using pop_jointprob function in EEGLAB (for details, see Section “Rejecting improbable data” in https://sccn.ucsd.edu/wiki/Chapter_01:_Rejecting_Artifacts). Event-related spectrogram (ERS) was computed using continuous wavelet transform (CWT) with analytic Morlet and averaged across all the remaining trials for each recording. The ERS results were further divided into five frequency bands (delta, theta, alpha, beta, and gamma) and three time-windows (0–0.5, 0.5–1, and 1–1.5 s). Average EEG power in the region of interest (ROI), gamma frequency band (30–50 Hz) and each time window at the frontal locations F3, F4, and Fz were obtained. These values were averaged to generate frontal gamma power (FGP). It is worth noting that we did not use the event-related spectral perturbation (ERSP) by normalizing the ERS with the spectral power of pre-stimulus baseline EEG due to its unavailability in the n-back task. Although the relative power (e.g. ratio of gamma to other low frequencies) could be used as a normalized EEG metric to reduce the inter-session variability of FGP, it could be affected by other task-relevant activities (e.g. P300 response to the target stimuli) or task-irrelevant factors (e.g. drowsiness or noise due to sweat).
2.3.2. Statistical analyses
Hierarchical linear modeling (utilizing HLM v6.08) was used to assess change in outcome (EEG power, n-back performance, neuropsychological test performance, clinical measures) over the course of the 16-week study. Growth curve models predicting each level-1 outcome variable were estimated using time (measured in weeks centered at baseline), as a level-1 predictor. Significant results were followed up by paired t-tests. Cohen’s d effect sizes were also calculated for each t-test. Additionally, hypothesis driven Pearson correlations were estimated to ascertain the relationship between change in FGP, NFB training threshold and change in performance of the n-back task. Multiple imputation was used to fill in missing EEG data that resulted from technical difficulties, and ocassional recording failure during EEG recording. 71 missing EEG observations out of 310 possible observations (~23%), or 3.8% of the total dataset used for analysis was imputed.
3. Results
3.1. Treatment tolerability
Overall treatment completion at 12 weeks was 81%. Twenty-nine subjects (94%) completed at least one assessment point in addition to baseline (Fig. 1), and 81% completed all assessments. The patient satisfaction survey showed moderate to high levels of satisfaction (week 4: M = 16.7, SD = 2.6; week 8: M = 17.9, SD = 2.1; week 12: M = 18.0, SD = 1.7; week 16: M = 17.3, SD = 2.5) through the entirety of the study. The treatment was well tolerated, without any treatment related adverse events. One subject was hospitalized for reasons unrelated to study participation.
3.2. Training threshold
Was measured at baseline and subsequently trained via EEG-NFB at each treatment session. TT ranged between 0.7 and 28 (M = 6.1, SD = 7.6) at baseline, and increased by 136%, 202% and 157% on average, at 4, 8- and 12-week treatment timepoints, respectively.
3.3. Neurophysiology/N-back task
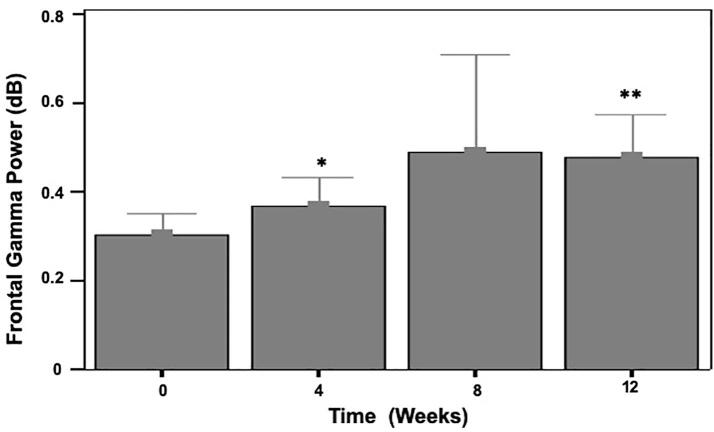
A significant time effect was found for task related FGP during the 2-back condition (γ = 0.02, t(29) = 2.19, p = .037), but not the 1-back condition (γ = 0.01, t(29) = 0.83, p = .413). Follow-up paired t-tests revealed significantly greater FGP dose effects during the 2-back task at 4 (t(23) = 2.55, p = .018, d = 0.5) and 12 (t(19) = 3.42, p = .003, d = 0.75) week doses (Fig. 2). Treatment effects on n-back performance were significant for 1-back performance over time (γ = 0.55, t(30) = 2.07, p = .047) and 2-back performance over time (γ = 0.46, t(30) = 2.17, p = .038). Follow-up paired t-tests were marginally significant for 1-back performance at the 4-week dose (t(28) = 1.86, p = .074, d = 0.35) and statistically significant at 8-weeks (t(27) = 3.36, p = .002, d = 0.65) and 12-weeks (t(24) = 3.37, p = .003, d = 0.65). For 2-back performance, follow-up paired t-tests were marginally significant at the 8-week dose (t(27) = 1.94, p = .063, d = 0.35) and statistically significant at the 12-week dose (t(24) = 2.33, p = .029, d = 0.45).
Fig. 2.
Gamma-NFBs effects on frontal gamma power over time in the 2-back condition. *p < 0.1; **p < 0.01.
3.4. Neuropsychological functioning
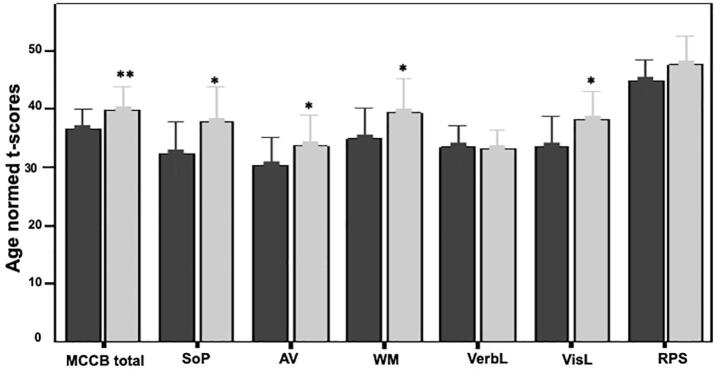
A statistically significant time effect was found for MCCB age-normed T-scores (γ = 0.19, t(30) = 3.61, p = .001). Follow-up paired t-tests showed significant effects as early as 4 weeks (week 4: t(29) = 2.74, p = .010, d = 0.5; week 8: t(27) = 3.46, p = .002, d = 0.65; week 12: t(24) = 3.64, p = .001, d = 0.75), that continued to be significant until the end of training at 12 weeks. Follow-up testing revealed near significant time effects for WM (γ = 0.21, t(30) = 2.01, p = .053), and significant effects for reasoning and problem solving domain (γ = 0.26, t(30) = 3.07, p = .005), and speed of processing domain (γ = 0.34, t(30) = 3.06, p = .005). A trend (p < .10) for the effect of time was also found visual learning (γ = 0.24, t(30) = 1.90, p = .067). Treatment effects on MCCB cognitive domains are shown in Fig. 3.
Fig. 3.
Gamma-NFBs effect on neuropsychological variables from baseline to12-weeks of treatment; *p < 0.1; **p < 0.01; MCCB: MATRICS Consensus Cognitive Battery; SoP: Speed of Processing; AV: audiovisual; WM: Working memory; VerbL: Verbal learning; VisL: Visual learning; RPS: Reasoning and problem solving domains.
3.5. Symptoms
Significant time effects were found indicating improvements in PANSS Positive (γ = -0.08, t(30) = -3.56, p = .002), PANSS Negative (γ = -0.13, t(30) = -4.47, p < .001), PANSS General (γ = -0.16, t(30) = -4.73, p < .001) and PANSS Total (γ = -0.37, t(30) = -6.63, p < .001) scores.
3.6. Durability of treatment effects
At the 16-week follow-up, FGP during 2-back condition remained signficantly greater than baseline (t(20) = 2.18, p = .041, d = 0.5), as did MCCB (t(24) = 3.35, p = .003, d = 0.65), SoP (t(24) = 2.81, p = .010, d = 0.55), RPS (t(24) = 2.75, p = .011,d = 0.55), and (marginally) 2-back performance (t(23) = 1.82, p = .082, d = 0.35). In addition, PANSS total (t(22) = 7.07, p < .001, d = 1.45), positive (t(22) = 3.28, p = .003, d = 0.7), negative (t(22) = 3.77, p = .001, d = 0.8) and general (t(22) = 4.57, p < .001, d = 0.95) symptom ratings remained significantly lower than baseline. A summary of treatment effects on all variables is presented in Table 1.
Table 1.
Summary of Gamma-NFB induced changes in all variables over time.
| Baseline |
4 Weeks |
8 Weeks |
12 Weeks |
16 Weeks |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | N | M (SD) | N | M (SD) | N | M (SD) | |
| FGP-1 back | 26 | 0.39 (0.38) | 26 | 0.37 (0.18) | 21 | 0.42 (0.22) | 22 | 0.45 (0.19)** | 23 | 0.49 (0.31)* |
| FGP-2 back | 28 | 0.30 (0.12) | 26 | 0.37 (0.16)* | 22 | 0.49 (0.50) | 22 | 0.48 (0.22)** | 23 | 0.43 (0.24)* |
| 1-back | 31 | 52.4 (28.3) | 29 | 61.2 (29.1)† | 28 | 65.8 (26.2)** | 25 | 64.5 (26.6)** | 25 | 61.7 (26.5)† |
| 2-back | 31 | 43.4 (23.7) | 29 | 47.6 (27.1) | 28 | 51.8 (28.1)† | 25 | 51.3 (25.5)* | 24 | 50.6 (23.6)† |
| MCCB | 31 | 36.6 (8.2) | 30 | 38.8 (8.7)* | 28 | 39.4 (9.1)** | 25 | 39.8 (9.7)** | 25 | 39.5 (11.1)** |
| SoP | 31 | 32.0 (12.5) | 30 | 35.5 (14.0)* | 28 | 36.8 (16.3)* | 25 | 37.8 (14.5)* | 25 | 38.0 (19.3)** |
| AV | 29 | 31.2 (12.5) | 30 | 34.1 (13.6) | 28 | 32.6 (11.6) | 25 | 33.8 (14.4)* | 25 | 32.7 (14.4) |
| WM | 31 | 35.2 (12.7) | 30 | 36.5 (10.5) | 28 | 38.6 (13.4)* | 25 | 39.3 (14.4)* | 25 | 36.6 (14.8) |
| Verb Learn | 30 | 34.4 (8.6) | 30 | 34.4 (7.6) | 28 | 34.1 (6.7) | 25 | 33.2 (7.2) | 25 | 33.6 (8.9) |
| Vis Learn | 31 | 33.3 (12.8) | 30 | 40.1 (12.2)*** | 28 | 37.1 (14.2) | 25 | 38.2 (11.5)* | 25 | 38.9 (14.0)** |
| RPS | 31 | 44.8 (8.8) | 30 | 45.5 (9.9) | 28 | 46.7 (10.1)† | 25 | 47.6 (11.9) | 25 | 48.7 (11.0)* |
| PANSS Total | 31 | 85.7 (6.5) | – | – | 24 | 80.1 (7.7)*** | 23 | 80.4 (7.2)*** | ||
| PANSS Pos | 31 | 20.0 (2.5) | – | – | 24 | 18.7 (2.6)** | 23 | 19.0 (2.5)** | ||
| PANSS Neg | 31 | 23.5 (4.5) | – | – | 24 | 21.3 (3.9)*** | 23 | 21.6 (3.5)** | ||
| PANSS Gen | 31 | 42.3 (4.3) | – | – | 24 | 40.1 (4.3)*** | 23 | 39.9 (4.5)*** | ||
.* p < 0.1; ** p < 0.01; *** p < 0.001; †p < 0.10 for comparisons relative to baseline; FGP: Frontal gamma power; MCCB: MATRICS Consensus Cognitive Battery global T-score; SoP: Speed of Processing; AV: audiovisual; WM: Working memory; Verb Learn: Verbal learning; Vis Learn: Visual learning; RPS: Reasoning and problem solving; PANSS: Positive and Negative Symptom Scale; PANSS pos: positive symptom subscale; PANSS Neg: negative symptom subscale; PANSS gen: general symptom subscale.
3.7. Correlations
To further examine the relationship between NFB training, change in frontal electrical activity and behavior, correlations between change in NFB TT, FGP and n-back performance from baseline to treatment completion (12 weeks) were examined. Moderate strength, statistically significant correlations were found between change in NFB TT and change in 1-back (r = 0.47, p = .020) and 2-back performance (r = 0.53, p = .008). Additionally, change in FGP was also statistically significantly correlated with change in 2-back performance at 8 weeks (r = 0.40, p = .047) and 12 weeks of treatment (r = 0.40, p = .046), with moderate strength correlations.
4. Conclusions and discussion
The primary aim of the study was to assess target engagement to test whether gamma oscillatory activity is a viable target for EEG NFB, and our proof-of-concept study is the first to demonstrate that EEG-NFB can be used to influence gamma oscillations in individuals with SCZ. The study also aimed to obtain preliminary data on dose response relationships of NFB sessions needed to improve FGP and WM, as well as relationships between behavioral change and neural markers. We hypothesized that treatment would influence both neural markers and cognitive performance, and that change in these domains would be correlated with each other. The EEG-NFB intervention was associated with significant improvements in frontal gamma power and working memory on the n-back task with medium effect sizes, as well as generalization to improvements in working memory, speed of processing, and reasoning and problem solving on the MCCB. Moreover, improvements in gamma power were associated with improvements in cognition that persisted four weeks after treatment ended. These findings are consistent with NFB effects in other studies where treatment effects persisted well beyond the end of treatment. For instance, a recent meta-analysis of 10 studies in Attention Deficit Hyperactivity Disorder showed persistent treatment effects up to 6 months (Van Doren, 2019), and changes have been observed for as long as 22 months post treatment in patients with schizophrenia (Surmeli, 2016).
Improvements in cognition were noted on both the computerized n-back task and the more generalized cognitive tests of the MCCB. Time effects revealed significant improvement in performance on the 1-back task at 4, 8 and 12 weeks of doses, and significant changes in the 2-back task at 12 weeks of treatment, suggesting that a greater dose of treatment is needed for more difficult tasks. Significant effects on the MCCB battery total score were noted at the 4, 8 and 12-week timepoints, with a large effect by week 12 and significant improvements in SoP, WM and RPS domains. Since the present study did not have a placebo arm, it is possible that changes in neuropsychological variables resulted from practice effects. In their recent study, however, Keefe et al. examined data from 813 subjects (Keefe, 2017) and found minimal practice effects on the MCCB in longitudinal treatment studies in patients with SCZ.
Furthermore, FGP was also improved over time, with significant effects at the 4- and 12-week doses. Although several outcomes showed significant improvement by 4 or 8 weeks, the most robust improvements across measures were found by 12 weeks, suggesting 12 weeks (12 h) of gamma NFB treatment may be the optimal dose for our treatment protocol. A recent review of published EEG-NFB studies in SCZ between 1964 and 2019 by our group identified 7 studies with empirical data, none of which targeted gamma oscillations (accepted for publication: https://doi.org/10.1093/schizbullopen/sgaa005). The studies revealed a wide range of effective treatment doses between 2.75 (Schneider and Pope, 1982) hours to 58.5 h (Surmeli, 2012), suggesting that treatment dose may depend on protocol, as well as underlying illness severity. Additionally, change in both FGP and TT was associated with change in n-back performance with moderate strength correlation coefficients. Although the current study cannot rule out the effects of non-treatment factors or medications, these relationships suggest that improvements in GBR may contribute to change in cognition.
In general, antipsychotic medications have shown small effects on cognition that are similar to practice effects in healthy controls in large clinical trials such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial of 57 sites that analyzed neurocognition in 817 subjects (Keefe, 2007). Additionally, animal models of SCZ indicate that antipsychotic medications maintain or worsen hypofrontality, a state of decreased blood flow and glucose metabolism in the prefrontal cortex, which worsens function of brain regions that support cognition (Krzystanek and Palasz, 2019). In the study by Minzenberg et al. in first episode psychosis, no relationship was found between gamma power and medications (Minzenberg, 2010). Therefore, it is unlikely that medication changes alone can account for improved cognition in the present study. Nonetheless, medication effects and other non-NFB factors need to be explored further in a larger randomized, placebo-controlled clinical trial, as potential contributors to treatment efficacy.
Positive and negative symptoms were not a direct treatment target. Nonetheless, significant changes were observed in general psychopathology, positive and negative subscales of the PANSS at the conclusion of the study. These gains continued to be present at the 4-week post-treatment completion timepoint. The general principle that activating cognitive areas can improve positive and negative symptoms has been frequently reported in the literature on cognitive remediation (CR) studies, as confirmed by two recent meta analyses. In the first one, 67 studies (n = 4067) demonstrated significant effects of cognitive remediation not only on cognitive domains, but also symptoms. Subsequent statistical modeling suggested that improving cognitive symptoms led to improvements in psychosocial functioning, which in turn facilitated improvements in symptoms (Kambeitz-Ilankovic, 2019). In another meta-analysis on CR’s effects, 15 studies showed small to moderate effects on negative symptoms compared to treatment as usual, again, suggesting that activation of cognitive resources may lead to reductions in symptoms (Cella, 2017).
Our encouraging initial findings must be considered in the context of some limitations. These include the need for a larger sample, and placebo-controlled trial to ascertain the effects of non-treatment factors such as behavioral activation and contact with study staff, as well as any effects of changes in medication adherence and administration. Such studies will also provide needed statistical power to identify mediators and moderators of treatment that can guide further refinement of treatment protocols. Additional questions pertaining to the treatment protocol itself also remain unanswered- Would higher treatment doses lead to greater gains? Would participants experience boredom, burnout and lack of motivation with longer protocols? How might delivering the same treatment dose over a shorter time period (e.g. 12 h of EEG-NFB delivered over 6 weeks) influence efficacy? And what about long-term effects? What length of follow-up period is ideal to assess NFB effects? These and many other important questions will need to be addressed with studies that incorporate larger sample sizes, placebo arms, and uniform well thought out study designs (Ros et al., Brain, in press). Ultimately, developing and refining direct brain modulating treatments has the potential to innovate treatments and teach us more about the human brain, and most importantly, improve the lives of those suffering with serious mental illness.
5. Funding sources
This work was supported by a grant from the National Institute of Mental Health (R61MH112793); and University of California at San Diego, Chancellor’s Research Excellence Scholarship (UCSD-CRES).
Credit authorship contribution statement
Fiza Singh: Conceptualization, Writing. I-Wei Shu: EEG task design and interpretation, editing. Sheng-Hsiou Hsu: EEG data reduction and analysis. Peter Link: Statistical analysis. Jaime A. Pineda: Neurofeedback design, editing. Eric Granholm: Neuropsychological test interpretation, clinical trial design.
References
- Silver H. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am. J. Psychiatry. 2003;160(10):1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Mohamed S. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch. Gen. Psychiatry. 1999;56(8):749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Haenschel C. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J. Neurosci. 2009;29(30):9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R.Y., Konecky R.O., Carter C.S. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2006;103(52):19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35(13):2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.M. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin. Neurophysiol. 2000;111(11):2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Mitra S. Evaluation of resting state gamma power as a response marker in schizophrenia. Psychiatry Clin. Neurosci. 2015;69(10):630–639. doi: 10.1111/pcn.12301. [DOI] [PubMed] [Google Scholar]
- Dienel S.J., Lewis D.A. Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol. Dis. 2018 doi: 10.1016/j.nbd.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Glausier J.R. Alterations in prefrontal cortical circuitry and cognitive dysfunction in schizophrenia. Nebr. Symp. Motiv. 2016;63:31–75. doi: 10.1007/978-3-319-30596-7_3. [DOI] [PubMed] [Google Scholar]
- Rotaru D.C., Lewis D.A., Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev. Neurosci. 2012;23(1):97–109. doi: 10.1515/revneuro-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E. EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin. Neuropsychol. 2007;21(1):110–129. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- Gevensleben H. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int. J. Psychophysiol. 2009;74(2):149–157. doi: 10.1016/j.ijpsycho.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Gevensleben H. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry. 2009;50(7):780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- Kluetsch R.C. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand. 2014;130(2):123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J., Beauregard M., Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci. Lett. 2006;394(3):216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S., Huster R.J., Herrmann C.S. Boosting brain functions: Improving executive functions with behavioral training, neurostimulation, and neurofeedback. Int. J. Psychophysiol. 2013;88(1):1–16. doi: 10.1016/j.ijpsycho.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014;44:124–141. doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. II: creativity, the performing arts and ecological validity. Neurosci. Biobehav. Rev. 2014;44:142–158. doi: 10.1016/j.neubiorev.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. III: a review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 2014;44:159–182. doi: 10.1016/j.neubiorev.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Salari N., Buchel C., Rose M. Neurofeedback training of gamma band oscillations improves perceptual processing. Exp. Brain Res. 2014;232(10):3353–3361. doi: 10.1007/s00221-014-4023-9. [DOI] [PubMed] [Google Scholar]
- Green M.F., Nuechterlein K.H. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr. Res. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Green M.F. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bigdely-Shamlo N. The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Front Neuroinform. 2015;9:16. doi: 10.3389/fninf.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.Y. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-channel EEG Recordings. IEEE Trans. Biomed. Eng. 2019 doi: 10.1109/TBME.2019.2930186. [DOI] [PubMed] [Google Scholar]
- Pion-Tonachini L., Kreutz-Delgado K., Makeig S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage. 2019;198:181–197. doi: 10.1016/j.neuroimage.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren J. Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry. 2019;28(3):293–305. doi: 10.1007/s00787-018-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeli T. Quantitative EEG Neurometric Analysis-Guided Neurofeedback Treatment in Dementia: 20 Cases. How Neurometric Analysis Is Important for the Treatment of Dementia and as a Biomarker? Clin. EEG Neurosci. 2016;47(2):118–133. doi: 10.1177/1550059415590750. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E. Placebo Response and Practice Effects in Schizophrenia Cognition Trials. JAMA Psychiatry. 2017;74(8):807–814. doi: 10.1001/jamapsychiatry.2017.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S.J., Pope A.T. Neuroleptic-like electroencephalographic changes in schizophrenics through biofeedback. Biofeedback Self Regul. 1982;7(4):479–490. doi: 10.1007/BF00998888. [DOI] [PubMed] [Google Scholar]
- Surmeli T. Schizophrenia and the efficacy of qEEG-guided neurofeedback treatment: a clinical case series. Clin. EEG Neurosci. 2012;43(2):133–144. doi: 10.1177/1550059411429531. [DOI] [PubMed] [Google Scholar]
- Keefe R.S. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Krzystanek M., Palasz A. NMDA receptor model of antipsychotic drug-induced hypofrontality. Int. J. Mol. Sci. 2019:20(6). doi: 10.3390/ijms20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz-Ilankovic L. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci. Biobehav. Rev. 2019;107:828–845. doi: 10.1016/j.neubiorev.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M. Cognitive remediation for negative symptoms of schizophrenia: a network meta-analysis. Clin. Psychol. Rev. 2017;52:43–51. doi: 10.1016/j.cpr.2016.11.009. [DOI] [PubMed] [Google Scholar]