Highlights
-
•
The incidence rates of bone lymphoma had sharply increased for the early decades.
-
•
The incidence trend of bone lymphoma has stabilized in recent years.
-
•
The mortality had sharply increased from 1985 to 2016 without a turn point.
-
•
The incidence and mortality by various characteristics had similar patterns.
Keywords: Bone lymphoma, Incidence, Incidence-based mortality, Trend
Abstract
Background
In recent years, studies on bone lymphoma and its histologic types have reached a mature stage. However, reports on the incidence and incidence-based mortality trends of bone lymphoma are scanty.
Methods
Patients with bone lymphoma in the U.S. were selected from Surveillance, Epidemiology, and End Results (SEER) database (1975–2016), and categorized based on age, sex, race, tumor location, SEER Historic Stage A and histologic type. Data on the incidence (1975–2016) and incidence-based mortality (1985–2016) were directly obtained from the SEER program. Annual percentage change (APC) and 95% confidence intervals (CIs) were calculated using the joinpoint regression analysis program.
Results
Overall, 13,058 bone lymphoma cases diagnosed in resident patients of the U.S. were included in incidence analysis between 1975 and 2016 as follows: 6080 cases in 1975–1999, 3796 cases in 2000–2009, and 3182 cases in 2010–2016. Of these cases, 6888 died of bone lymphoma between 1985 and 2016. The overall incidence rates dramatically increased from 0.89 per 100,000 person-years in 1975 to 1.36 per 100,000 person-years in 2016. Incidence trend sharply increased from 1975 to 2009, and then stabilized between 2009 and 2016. Overall incidence-based mortality trends sharply increased from 1985 to 2016 without a joinpoint. Following the demographic and tumor characteristics, the trends of incidence and incidence-based mortality exhibited similar patterns.
Conclusion
Considering various characteristics (age, sex, race, tumor location, SEER Historic Stage A, and histologic type), we established that the incidence trend of bone lymphoma has sharply been increasing over the decades, however, in the recent years, the trend has stabilized. Besides, between 1985 and 2016, the incidence-based mortality had been sharply increasing without a turning point. These findings could give insights for clinicians to elaborately assess the epidemiology and risk factors of bone lymphoma.
1. Introduction
Lymphoma can infect any tissue, with approximately one-third of patients presenting with extranodal sites [1]. Bone lymphoma, a rare extranodal form, was initially reported by Oberlin [2]. Later, some researchers considered bone lymphoma as a distinct entity, which was acknowledged to be a malignant lymphoid infiltrate of the bone [3], [4], [5]. According to the last version of the World Health Organization (WHO) classification of tumors of soft tissue and bone, primary lymphomas of bone (PBL) is defined as a neoplasm composed of malignant lymphoid cells, producing one or more masses within the bone, excludes regional lymph node involvement or distant sites of extranodal disease [6]. On the contrary, bone lymphoma with systemic disease and a primary soft tissue mass secondarily involving a bone both are not considered as PBL and is defined as ‘‘secondary bone lymphoma” [7], [8], [9], [10]. Thus, based on the current understanding, bone lymphoma has two broad categories including, primary lymphomas of bone and secondary bone Lymphoma [8], [9].
Bone lymphoma is a rare tumor accounting for nearly 5% of extranodal lymphomas and between 3% and 7% of all bone malignancies [8], [9]. Considering the low incidence, previous assessments relied on results from small series or single-institution. The publicly available Surveillance, Epidemiology, and End Results (SEER) database provided plenty of data for population-level studies of bone lymphoma, which focused on prognostic factors and treatment outcomes. Unfortunately, existing reports failed to particularly describe the incidence and mortality trends associated with bone lymphoma, also, such studies did not conduct systemic analyses of the trends based on various characteristics [9], [11], [12]. Although the level of healthcare burden has continued to heighten in the U.S. over the past decade [13], the impacts of these policies remain unclear.
Hence, relying on the large population-based cancer registries in the U.S., this study purposed to assess the incidence rates and trends of bone lymphoma using demographic and tumor characteristics between 1975 and 2016. Also, we investigated the incidence-based mortality rates and trends by these characteristics in the U.S. between 1985 and 2016. To our knowledge, this is one of the largest epidemiological studies on primary bone lymphoma across the globe.
2. Methods
2.1. Data source and patients selection
All data of patients were obtained from the National Cancer Institute's SEER program (https://seer.cancer.gov), which was a deidentified and public use research file [14]. We used the SEER-9 cancer incidence file maintained by the United States National Cancer Institute between 1975 and 2016. The SEER database collected cancer data from 9 high-quality, population-based registries covering nearly 10% of the U.S. population in the following regions: California (San Francisco and Oakland), Connecticut, Georgia (Atlanta), Hawaii, Iowa, Michigan (Detroit), New Mexico, Utah, and Washington (Seattle and Puget Sound region) [15]. All data in the database were retrospective, therefore, we did not have to obtain the Institutional Review Board approval for this study.
With the SEER database, we relied on pathologically confirmed diagnosis and bone location (C40.0 to C41.9) in selecting patients diagnosed between 1975 and 2016 [12]. Lymphoma Subtype Recode ICD-O-3/WHO 2008 classification from the database was directly used to identify cases with lymphoma [16]. Age-adjusted incidence rates of bone lymphoma were calculated for the years 1975 through 2016. To avoid underestimation of mortality rates in the earlier years, incidence-based mortality analysis was restricted to deaths that occurred between 1985 and 2016. All incidence rates in our study were age-adjusted to the 2000 standard US population and expressed per 100,000 population. Besides, incidence-based mortality (IBM) rates were calculated as the number of specific tumors deaths among cases diagnosed over person-time at risk among people in the SEER areas (https://surveillance.cancer.gov/statistics). The IBM rate allowed for partitioning of mortality by variables associated with cancer onset. An accurate measure of IBM requires high-quality population-based cancer registry data and high-quality follow-up of cancer patients for vital status including the cause of death [11]. Consequently, to calculate “incidence-based mortality” rates, we captured age-adjusted population-level mortality rates by variables attributable to bone lymphoma reported to SEER registries which were expressed per 1000,000 population.
2.2. Variables selection
Demographic and tumor characteristics included: age, year of diagnosis/death recode, sex (male and female), race (white, black, others and unknown), stage (localized, regional, distant and unknown), tumor location (appendicular, axial and unknown), and histological type (diffuse large B-cell lymphoma [DLBCL], other NHL, others and unclassified). Cases were categorized into four groups based on age as follows: (1) 0–19 years; (2) 20–39 years; (3) 40–59 years; (4) ≥60 years. The stage at diagnosis was identified in SEER from 1975 to 2015 as follows: SEER Historic Stage A, as localized (limited to the bone), regional (tumor extension beyond the limits of the bone), distant (distant metastasis), and unknown [17]. Tumor location was dichotomized as either appendicular or axial [12], the appendicular skeleton included upper limbs (including scapula) and lower limbs, while the axial skeleton included the spine, head, sternum, rib, and clavicle. Histological type was recorded from 1975 to 2016, by International classification of disease for oncology (ICD-O-3), as DLBCL, other NHL (NHL excluded DLBCL), and others and unclassified (HL, composite HL and NHL, unclassified). This classification was used because the incidence and mortality of HL and composite lymphoma were too low to show the data.
2.3. Statistical analysis
The SEER software (SEER*Stat, version 8.3.6) was used to calculate cancer incidence rates and incidence-based mortality rates. All incidence rates were age-adjusted to the US standard population and expressed per 100,000 person-years, whereas all mortality rates were expressed per 1,000,000 person-years. The joinpoint regression analysis program (version 4.4.0.0 [National Cancer Institute]) was used to calculate Annual Percentage Change (APC), mean APC, and corresponding 95% CIs. Compared with zero, the statistical significance of APCs was determined by t-test. Also, we selected calendar years (joinpoints) to identify the best-fitting log-linear regression model [18]. Two-sided P-values <0.05 were considered statistically significant.
3. Results
3.1. Incidence and mortality by characteristics
We included 13,058 cases of patients diagnosed with bone lymphoma in the U.S. between 1975 and 2016 for incidence analysis. The case counts, incidence rates, and incidence-based mortality rates based on demographic and tumor characteristics are shown in Table 1. Of them, the elderly (above 60 years old), male, white, and DLBCL patients comprised the most cases and the highest incidence rate in cases included from 1975 to 1999. Similar results were noted from 2000 to 2009 and 2010–2016. Interestingly, the incidence of localized stage, regional stage, and distant stage was equal among the three study durations. Also, the incidence of axial site and appendicular site was equal among the three study durations.
Table 1.
The age-adjusted incidence (1975–2016) and incidence-based mortality (1985–2016) of bone lymphoma.
| Characteristic | Age-adjusted Incidence |
Incidence-based mortality |
||||||
|---|---|---|---|---|---|---|---|---|
| 1975–1999 |
2000–2009 |
2010–2016 |
1985–2016 |
|||||
| Cases, No. (%) | Rate | Cases, No. (%) | Rate | Cases, No. (%) | Rate | Cases, No. (%) | Rate | |
| Overall | 6080 | 1.09 | 3796 | 1.38 | 3182 | 1.47 | 6000 | 7.21 |
| Age, y | ||||||||
| 0–19 | 1544 (25.4) | 0.89 | 731 (19.3) | 0.95 | 532 (16.7) | 0.95 | 575 (9.6) | 2.47 |
| 20–39 | 1349 (22.2) | 0.71 | 665 (17.5) | 0.84 | 496 (15.6) | 0.86 | 827 (13.8) | 3.22 |
| 40–59 | 1243 (20.4) | 1.97 | 1014 (26.7) | 1.29 | 810 (25.5) | 1.37 | 1002 (16.7) | 4.51 |
| ≥60 | 1944 (32.0) | 2.25 | 1388 (36.5) | 3.17 | 1344 (42.2) | 3.52 | 3596 (59.9) | 26.61 |
| Sex | ||||||||
| Male | 3451 (56.8) | 1.30 | 2115 (55.7) | 1.63 | 1836 (56.7) | 1.79 | 3500 (58.3) | 9.55 |
| Female | 2629 (43.2) | 0.89 | 1681 (44.3) | 1.16 | 1346 (42.3) | 1.19 | 2500 (41.7) | 5.39 |
| Race | ||||||||
| White | 5209 (85.7) | 1.13 | 3102 (81.7) | 1.46 | 2550 (80.1) | 1.57 | 5068 (84.4) | 7.57 |
| Black | 478 (7.9) | 0.86 | 388 (10.2) | 1.27 | 356 (11.2) | 1.44 | 551 (9.2) | 7.12 |
| Others | 366 (6.0) | 0.79 | 276 (7.3) | 0.87 | 250 (7.9) | 0.89 | 370 (6.2) | 4.33 |
| Unknown | 27 (0.4) | NA | 30 (0.8) | NA | 26 (0.8) | NA | 11 (0.2) | NA |
| SEER Historic Stage A | ||||||||
| Localized | 1805 (29.7) | 0.32 | 990 (26.1) | 0.36 | 667 (21.0) | 0.31 | 1145 (19.1) | 1.37 |
| Regional | 1714 (28.2) | 0.30 | 941 (24.7) | 0.34 | 612 (19.2) | 0.29 | 1466 (24.4) | 1.74 |
| Distant | 1190 (19.6) | 0.22 | 1064 (28.0) | 0.39 | 885 (27.8) | 0.40 | 1928 (32.1) | 2.32 |
| Unknown | 1371 (22.5) | 0.25 | 801 (21.1) | 0.29 | 1018 (32.0) | 0.46 | 1461 (24.4) | 1.78 |
| Tumor Location | ||||||||
| Appendicular | 3105 (51.1) | 0.54 | 1637 (43.1) | 0.59 | 1243 (39.1) | 0.59 | 2334 (40.1) | 2.79 |
| Upper limbs | 744 (12.7) | 0.13 | 411 (10.8) | 0.15 | 356 (11.2) | 0.17 | 562 (9.4) | 0.67 |
| Lower limbs | 2348 (38.6) | 0.41 | 1208 (31.8) | 0.44 | 869 (27.3) | 0.42 | 1750 (29.2) | 2.09 |
| Limbs, unknown | 13 (0.2) | <0.01 | 18 (0.5) | 0.01 | 18 (0.6) | 0.01 | 22 (0.4) | 0.03 |
| Axial | 2792 (45.9) | 0.51 | 2037 (53.7) | 0.74 | 1771 (55.7) | 0.80 | 3388 (56.5) | 4.08 |
| Spine | 1634 (26.9) | 0.30 | 1230 (32.4) | 0.45 | 1054 (33.1) | 0.47 | 2187 (36.4) | 2.64 |
| Head | 649 (10.7) | 0.12 | 484 (12.8) | 0.17 | 417 (13.1) | 0.19 | 667 (11.1) | 0.81 |
| Sternum, rib and Clavicle | 504 (8.3) | 0.09 | 313 (8.2) | 0.11 | 288 (9.1) | 0.13 | 521 (8.7) | 0.62 |
| Overlap | 5 (0.1) | <0.01 | 10 (0.3) | <0.01 | 12 (0.4) | 0.01 | 13 (0.2) | 0.01 |
| Unknown | 183 (3.1) | 0.03 | 122 (3.2) | 0.04 | 168 (5.3) | 0.08 | 278 (4.6) | 0.34 |
| Histologic type | ||||||||
| DLBCL | 388 (6.4) | 0.07 | 408 (10.7) | 0.95 | 342 (10.7) | 0.15 | 515 (8.6) | 0.63 |
| Other NPL | 594 (9.8) | 0.11 | 765 (20.1) | 0.28 | 809 (25.4) | 0.36 | 1170 (19.5) | 1.43 |
| Others and unclassified | 5098 (83.8) | 0.90 | 2623 (69.1) | 0.15 | 2031 (63.8) | 0.96 | 4315 (71.9) | 5.15 |
Abbreviations: APC, annual percent change; NA, not applicable; DLBCL, diffuse large B-cell lymphomas; NPL, non-Hodgkin’s lymphoma; SEER, Surveillance, Epidemiology, and End Results.
Out of the total cases, 6888 succumbed to bone lymphoma between1985 and 2016 and were identified in incidence-based mortality analysis. The largest proportion of the deaths were the elderly aged above 60 years (3596 [59.9%]), male (3500 [58.3%]), white (5068 [84.4%]), Distant (1928 [32.1%]), and DLBCL (515 [8.6%]). Similar incidence-based mortality of bone lymphoma occurred in axial (3388 [56.5%]) and appendicular (2334 [40.1%]) locations.
3.2. Overall incidence and mortality trends
Overall, the incidence of bone lymphoma dramatically increased from 0.89 per 100,000 person-years in 1975 to 1.36 per 100,000 person-years in 2016 (mean APC, 1.2%; 95% CI, 0.8%–1.6%). Joinpoint regression analysis results are shown in Fig. 1A and Table 2. Bone lymphoma incidence continuously rose from 1975 to 2009 (APC, 1.5%; 95% CI, 0.3%–1.8%), then stabilized from 2009 to 2016 (APC, −0.2%; 95% CI, [−2.3%–1.8%]). Intriguingly, the incidence-based mortality trend of bone lymphoma exhibited a continuously rising trend from 5.32 per 1,000,000 person-years in 1975 to 11.68 per 1,000,000 person-years in 2016 between 1985 and 2016 (APC, 1.5%; 95% CI, 1.3%–1.7%), without a stabilizing trend during the study duration (Fig. 1B & Table 3). The trends for incidence and incidence-based mortality by demographic and tumor characteristics are shown in Fig. 2 and Fig. 3, respectively.
Fig. 1.
Overall incidence (1975–2016) and incidence-based mortality (1985–2016) trends of bone lymphoma in the United States.
Table 2.
Bone lymphoma incidence trends by demographic and tumor characteristics (diagnosed 1975–2016).
| Characteristic | Overall, Mean APC, % (95% CI) | Period 1 |
Period 2 |
||
|---|---|---|---|---|---|
| Years | APC, % (95% CI) | Years | APC, % (95% CI) | ||
| Overall | 1.2 (0.8 to 1.6) | 1975–2009 | 1.5 (1.3 to 1.8) | 2009–2016 | −0.2 (−2.3 to 1.8) |
| Age, y | |||||
| 0–19 | 1 (−0.3 to 2.3) | 1975–1979 | 8.1 (−5.0 to 23.0) | 1979–2016 | 0.3 (−0.1 to 0.7) |
| 20–39 | 0.6 (−0.4 to 1.6) | 1975–2010 | 1.2 (0.7 to 1.7) | 2010–2016 | −3.0 (−8.9 to 3.3) |
| 40–59 | 1.7 (1.1 to 2.4) | 1975–1999 | 2.6 (1.7 to 3.5) | 1999–2016 | 0.5 (−0.5 to 1.5) |
| ≥60 | 1.6 (1.0 to 2.3) | 1975–2010 | 2.0 (1.6 to 2.4) | 2010–2016 | 0.5 (−4.5 to 3.7) |
| Sex | |||||
| Male | 1.5 (0.9 to 2.0) | 1975–1994 | 2.1 (1.1 to 3.0) | 1994–2016 | 1.0 (0.4 to 1.5) |
| Female | 1.2 (0.7 to 1.6) | 1975–2005 | 1.5 (1.2 to 1.9) | 2005–2016 | 0.1 (−1.2 to 1.5) |
| Race | |||||
| White | 1.2 (0.8 to 1.7) | 1975–2010 | 1.6 (1.4 to 1.8) | 2010–2016 | −0.9 (−3.4 to 1.8) |
| Black | 1.9 (0.8 to 3.1) | 1975–2010 | 2.6 (1.9 to 3.3) | 2010–2016 | −1.8 (−8.1 to 5.0) |
| Others | 0.4 (−0.8 to 1.7) | 1975–2010 | −0.2 (−0.1 to 0.7) | 2010–2016 | 4.1 (−3.3 to 12.0) |
| Unknown | NA | NA | NA | NA | NA |
| SEER Historic Stage A | |||||
| Localized | 0.7 (0.0 to 1.4) | 1975–1995 | 1.0 (−0.1 to 2.1) | 1995–2015 | 0.3 (−0.5 to 1.3) |
| Regional | 0.7 (−0.4 to 1.9) | 1975–2013 | 0.6 (0.3 to 0.8) | 2013–2015 | 4.2 (−17.0 to 30.9) |
| Distant | 3.1 (2.2 to 4.0) | 1975–2010 | 3.4 (2.9 to 4.0) | 2010–2015 | 0.5 (−5.4 to 6.8) |
| Unknown | NA | NA | NA | NA | NA |
| Tumor Location | |||||
| Appendicular | 0.5 (0.1 to 1.0) | 1975–1995 | 1.1 (0.3 to 1.8) | 1995–2016 | 0.0 (−0.6 to 0.6) |
| Axial | 1.7 (1.1 to 2.3) | 1975–2010 | 2.3 (2.0 to 2.7) | 2010–2016 | −1.8 (−5.2 to 1.7) |
| Unknown | NA | NA | NA | NA | NA |
| Histologic Type | |||||
| DLBCL | 2.9 (1.4 to 4.4) | 1975–2009 | 4.3 (3.1 to 5.5) | 2009–2016 | −3.7 (−10.3 to 3.4) |
| Other NPL | 7.6 (5.6 to9.6) | 1975–1991 | 15.1 (9.9 to 20.6) | 1991–2016 | 3.1 (2.1 to 4.0) |
| Others and unclassified | NA | NA | NA | NA | NA |
Abbreviations: APC, annual percent change; CI, confidence interval; NA, not applicable; DLBCL, diffuse large B-cell lymphomas; NPL, non-Hodgkin’s lymphoma; SEER, Surveillance, Epidemiology, and End Results.
Table 3.
Bone lymphoma incidence-based mortality trends by demographic and tumor characteristics (diagnosed 1985–2016).
| Characteristic | Overall, (1985–2016) |
|
|---|---|---|
| APC, % (95% CI) | P-value | |
| Overall | 1.5 (1.3–1.7) | <0.05 |
| Age, y | ||
| 0–19 | −1.7 (−2.7 to −0.7) | <0.05 |
| 20–39 | 0.4 (−0.4 to 1.3) | 0.3 |
| 40–59 | 1.7 (0.8 to 2.5) | <0.05 |
| ≥60 | 2.1 (1.7 to 2.5) | <0.05 |
| Sex | ||
| Male | 1.5 (1.1 to 1.9) | <0.05 |
| Female | 1.4 (1.0 to 1.9) | <0.05 |
| Race | ||
| White | 1.4 (1.3 to 1.6) | <0.05 |
| Black | 3.2 (2.1 to 4.3) | <0.05 |
| Others | 0.8 (−0.5 to 2.2) | 0.2 |
| Unknown | NA | NA |
| SEER Historic Stage A | ||
| Localized | 1.1 (0.6 to 1.6) | <0.05 |
| Regional | 0.3 (−0.2 to 0.8) | 0.2 |
| Distant | 2.9 (2.3 to 3.5) | <0.05 |
| Unknown | NA | NA |
| Tumor Location | ||
| Appendicular | 0.5 (0.0 to 1.0) | <0.05 |
| Axial | 2.0 (1.6 to 2.5) | <0.05 |
| Unknown | NA | NA |
| Histologic Type | ||
| DLBCL | 2.3 (1.2 to 3.5) | <0.05 |
| Other NPL | 3.7 (2.9 to 4.5) | <0.05 |
| Others and unclassified | NA | NA |
Abbreviations: APC, annual percent change; CI, confidence interval; NA, not applicable; DLBCL, diffuse large B-cell lymphomas; NPL, non-Hodgkin’s lymphoma; SEER, Surveillance, Epidemiology, and End Results.
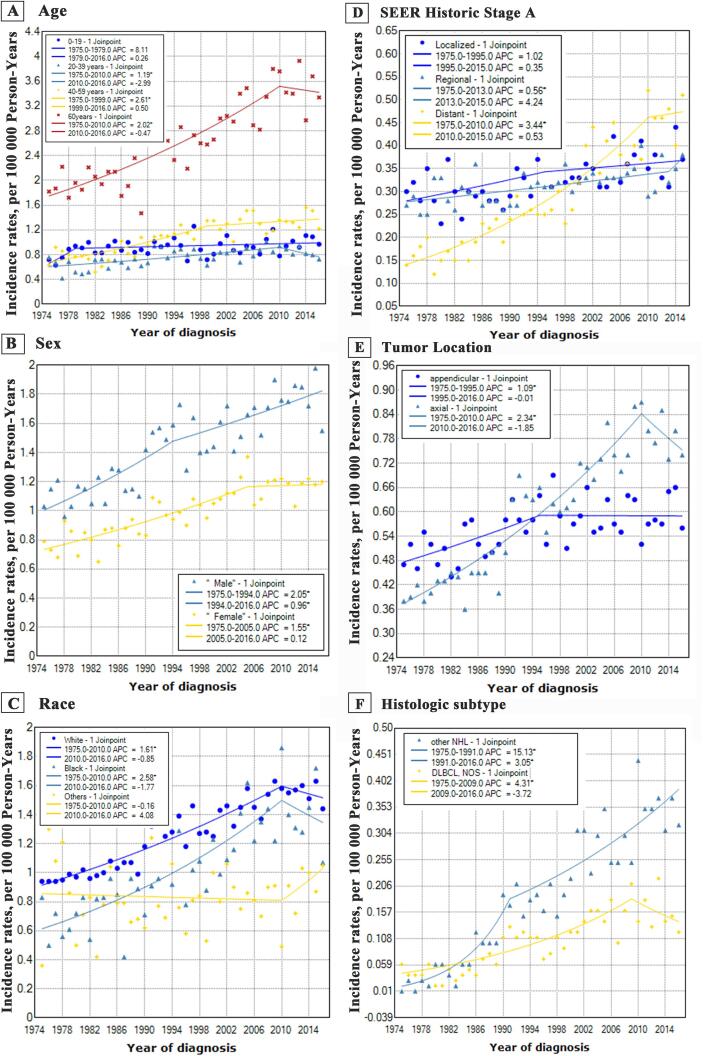
Fig. 2.
Incidence trends of bone lymphoma by demographic characteristics (A–C) and tumor characteristics (D–F) in the United States from 1975 to 2016.
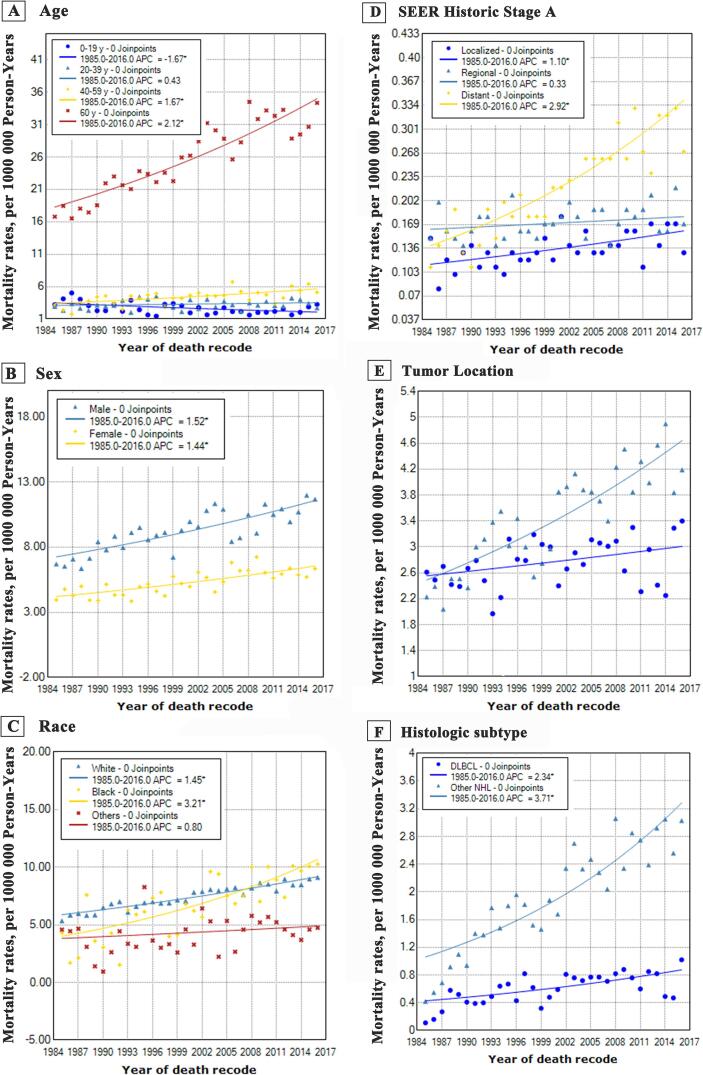
Fig. 3.
Incidence-based mortality trends of bone lymphoma by demographic characteristics (A–C) and tumor characteristics (D–F) in the United States from 1985 to 2016.
3.3. Trends by age
Incidence rates in the population aged below 19 years did not increased between 1975 and 2016 (1975–1979: APC, 8.1%; 95% CI, −5.0%–23.0%; 1979–2016: APC, 0.3%; 95% CI, −0.1%–0.7%), whereas the incidence-based mortality trend significantly decreased in 1985–2016 (APC, −1.7%; 95% CI, [-2.7% to −0.7%]). As for the cases more than 19 years, all the three groups exhibited similar incidence trends, with a dramatically increasing trend over the past decades and which stabilized in recent years. Besides, in terms of the incidence-based mortality, patients aged 20 to 39 years showed a stable trend (APC, 0.4%; 95% CI, [−0.4%–1.3%]), whereas patients aged above 40 years showed an increasing trend, and patients aged less than 19 years showed a decreasing trend.
3.4. Trends by sex
Here, a sharp increase in the trend of incidence was noted among males from 1975 to 1994 (APC, 2.1%; 95% CI, 1.1%–3.0%), then, a significantly increasing trend was reported from 1994 to 2016 (APC, 1.0%; 95% CI, 0.4%–1.5%). The trend of incidence rates in female showed an initial rise from 1975 to 2005 (APC, 1.5%; 95% CI, 1.2%–1.9%), followed by a stabilizing trend from 2005 to 2016 (APC, 0.1%; 95% CI, [−1.2%–1.5%]). It’s worth noting that the sex-based incidence-based mortality showed similar slopes between 1985 and 2016, with a sharp increase in male (APC, 1.5%; 95% CI, 1.1%–1.9%) and female (APC, 1.4%; 95% CI, 1.0%–1.9%).
3.5. Trends by race
Intriguingly, the trends of incidence and incidence-based mortality for different races exhibited highly similar patterns. For whites and blacks, the incidence-based mortality initially increased between 1975 and 2010 at 1.6% (95% CI, 1.4%–1.8%) and 2.6% (95% CI, 1.9%–3.3%) rates, respectively, followed by stabilizing trends between 2010 and 2016 at −0.9% (95% CI, [−3.4%–1.8%]) and −1.8% (95% CI, [−8.1%–3.3%]) rates. Besides, both the mortality of whites and blacks were described as sharply increasing trends. For other races, no significant increase or decrease in trend for either incidence or mortality were observed in the study period.
3.6. Trends by SEER Historic stage A
Among regional and distant groups, both stage cases were observed with an initial significantly increasing trend (rate: 0.6% and 3.4% respectively) which later stabilized. For localized cases between 1975 and 1995 (APC, 1.0%; 95% CI, [−0.1%–2.1%]) and between 1995 and 2015 (APC, 0.3%; 95% CI, [−0.5%–1.3%]), the incidence rate both did exhibit an increasing trend. In terms of incidence-based mortality between 1985 and 2016, the trends of localized group (APC, 1.1%; 95% CI, 0.6%–1.6%) and distant group (APC, 2.9%; 95% CI, 2.3%–3.5%) both exhibited a markedly increasing trend, whereas the trend of regional group was stable (APC, 0.3%; 95% CI, [−0.2%–0.8%]).
3.7. Trends by tumor location
A sharp rise in the incidence of patients with bone lymphoma occurred in appendicular site based on reports between 1975 and 1995 (APC, 1.1%; 95% CI, 0.3%–1.8%) and axial site from 1975 to 2010 (APC, 2.3%; 95% CI, 2.0%–2.7%), followed by stabilizing trend in the appendicular site from 1995 to 2016 (APC, 0.0%; 95% CI, [−0.6%–0.6%]) and axial site from 2010 to 2016 (APC, −1.8%; 95% CI, [−5.2%–1.7%]). Besides, the incidence-based mortality between 1985 and 2016 increased markedly in appendicular site at a rate of 0.5% (95% CI, 0.0%–1.0%) and in axial site at higher rate of 2.0% (95% CI, 1.6%–2.5%).
3.8. Trends by histologic type
We observed two different patterns when analyzing the incidence rates based on historic types. Incidence rates of DLBCL initially rose rapidly between 1975 and 2009 (APC, 4.3%; 95% CI, 3.1%–5.5%), a sustained trend occurred between 2009 and 2016 (APC, −3.7%; 95% CI, [−10.3%–3.4%]). Incidence rates of other NPL exhibited a significant increase between 1975 and 1991 (APC, 15.1%; 95% CI, 9.9%–20.6%). Similarly, an increasing trend was observed between 1991 and 2016 (APC, 3.1%; 95% CI, 2.1%–4.0%). Interestingly, the incidence-based mortality from 1991 to 2016 followed the similarly upward pattern without turn point.
4. Discussion
With the occurrence and advancement of public databases, in particular, the SEER database, a growing body of work was performed to study bone lymphoma, among them, ascribing the epidemiological data and analyzing the prognostic factors, which aimed at accounting for the clinical indicators [9], [12]. Previous assessments, however, lost sight of the vital significance in showing the trends of incidence and mortality of bone lymphoma to identify tumor-related risk factors. To our knowledge, this is the first study to uncover the incidence and incidence-based mortality rates for bone lymphoma using epidemiological and tumor characteristics. At the same time, this study systematically compared the corresponding trends based on these features, thus may help clinicians better manage bone lymphoma tumor. Being one of the largest population-based studies, it possesses the ability to including a larger collection of cases [19]. To our knowledge, it is not until the WHO classification that pathologists and clinicians unify the diagnosis criteria of lymphoma subtypes and DLBCL as a term corresponded to the WHO classification of 2000. From our study, it is evident that the proportion of cases with unclassified subtypes from 1975 to 1999 is significantly more compared to cases between 2000 and 2009 and between 2010 and 2016. Whereas, the proportions of DLBCL cases and NPL cases between 1975 and 1999 are significantly less than cases between 2000 and 2009 and those between 2010 and 2016. Above data suggested that the occurrence of WHO classification resulted in decreased unclassified cases and increased classified cases. Additionally, we reported that the overall incidence rate from 1975 to 1999 was significantly lower than that from 2000 to 2009 and from 2010 to 2016. This could be attributed to real increase and artefactual increase; also, artefactual increase could be attributed to occurrence of WHO classification, the improved diagnosis, and completeness of cancer registries. Further detailed analysis of increased incidence rates would be provided in the next trend analysis.
In our analysis, the increased trends of overall incidence and incidence-based mortality of bone lymphoma have been demonstrated for past decades, though the trend in recent years, has stabilized. A similar variational pattern with one joinpoint was e observed in most groups using various characteristics. Notably, four reasons may have contributed to the increasing trend in the early decades. First, advancement in detection methods and the subsequent emergence of early diagnosis enhanced the chance of disease detection. On the contrary, improved diagnosis can enhance the death cases of bone lymphoma registration to increase the incidence-based mortality. Second, the completeness of cancer registries in this period may result in an artefactual increase in bone lymphomas, thus may simply reflect an increase of registration rather than a factual increase [20]. Third, a change of diagnostic criteria throughout the study period, and the occurrence of diagnostic consensus between pathologists and clinicians according to WHO classification can lead to the recording of more diagnosed cases of bone lymphomas. This phenomenon might lead to a sudden increasing trend with a turning point in the early decades. However, we could observe that, in the early decades, the incidence rates exhibited a continual increase rather than a sudden increase. Furthermore, considering the standard data collection strategy, we tend to think that this might not be the main reason to potentially be attributed to the continued increase in incidence rates. Fourth, we did not exclude the possibility of a real increase of bone lymphoma patients in the general population, which was not associated with the detection at early diagnosis.
Of note, the trend of incidence-based mortality showed a significant rise without an inflection point, which mismatched the incidence trend. Also, based on the various characteristics, the trends of all sub-groups exhibited a sharply increasing trend. Over the past decade, the growing level of healthcare burden in the U.S. is of great concern [13]. Intuitively, the management and treatment of patients could be greatly improved. However, whether these enormous costs reaped the deserved outcome can be answered regarding the result of mortality. Given this, there is an urgent need to effectively improve the survival of patients with bone lymphoma. Moreover, based on the incidence and mortality cases by characteristics, the elderly, men and patients with distant metastasis or tumor occurring in axial location should be given more attention, which would be systematically analyzed in the next incidence analysis.
In this present study, the incidence and mortality rates of bone lymphoma both increased with age. Also, patients aged above 60 years accounted for more than half of bone lymphoma cases with an incidence and mortality rates rate 6 times and 15 times respectively, higher than each younger group. These findings are consistent with a previous report which ascribed a theory whereby the incidence rates exponentially rose with age in the multi-stage carcinogenic carcinogenesis of solid tumors [21]. Elsewhere, Yin et al. also reported a similar result, which only confined to extranodal diffuse large B-cell lymphoma from the SEER database between 1973 and 2015 [11]. In summary, the above observations can be explained as follows: (1) With age, the immune system in the elderly declines. (2) The possibility of virus invasion gradually increases which further exhausts T-cell, resulting in telomere attrition and immune senescence [6], [22], [23]. (3) Some genes and epigenetics might change with age. (4) The level of some micromolecules and the microenvironment would be abnormal, such as BCL2 [24].
Based on our analysis, the incidence and mortality rates of male patients were higher than females. It is worth noting that the incidence trend in the male population showed a remarkable increase in recent years, whereas, in the female population, a similar trend was not observed. This phenomenon has in most cases been reported for most cancers [9], [11], also, it was reminiscent of the fact that male sex often was considered as risk and prognostic factors [9], [17]. Unfortunately, the underlying reasons have not been deeply evaluated. Further, we hypothesized that the differences in health awareness and lifestyle mainly contributed to the phenomenon. Smoking and social stress which have been regarded as predisposing factors for lymphoma might be the main reason [25]. In addition, females exert stronger humoral and cellular immunity, which are closely linked to lymphomagenesis [26], [27]. On mortality, some treatment drugs such as rituximab offer more beneficial effects in females, thus might extend the survival time [28], [29].
In terms of race, whites had the most proportion of the highest incidence and mortality rates in the U.S. in our study. However, the increasing trend in blacks was more significant than that in other races. These findings might strongly explain that in the early decades, whites comprise most populations in the U.S., and they could acquire more medical resources for tumor detection and management, therefore, whites were included in medical recode. With the rise in social and healthcare expenses, blacks were also able to get diagnosis and management into the medical record [30], which contributed to the dramatic increase in the incidence and mortality trends. Furthermore, this is reminiscent of the fact that racial diversity for most tumors was still existing and blacks were diagnosed with more advanced cancers than other races in the U.S. [11], [17]. This could explain why the mortality rates in blacks were higher than that in whites from 2008.
Moreover, we found that the overall incidence rates of localized, regional, and distant diseases were similar, however, the data revealed higher overall mortality rates as the historic stages progressed. Also, distant diseases had significantly higher mortality trends compared with localized and regional diseases which was closely associated with the sharply increasing incidence trends. As such, we concluded that early detection and early treatment of bone lymphoma patients can reduce the patients with advanced stages at initial diagnosis, which could decrease the mortality rates in the U.S.
A number of previous researchers reported contradicting results on the location at which bone lymphoma occurs. For instance, in the Japanese population, bone lymphoma most occurred in the pelvis in two different series [31], [32]. However, some studies ascribed that the long bones were the most common site of this tumor [33], [34]. Besides, other studies in the U.S. and Britain reported the similarly equal frequency between axial and extremity sites [12], [35], which was consistent with our findings. Furthermore, we found a markedly increasing trend of incidence and mortality, however, the rising trends in axial involvement were significantly more rapid than that in appendicular involvement.
DLBCL was acknowledged as the most common type of lymphoma, accounting for 59.8% [9]. In our study of bone lymphoma, our data revealed a similar result with the previous report. Considering the low incidence of other historic types, we included the other types of NPL into one group, then the PL was incorporated into an unclassified group because of the extremely low incidence. As for incidence, in recent years, the bone DLBCL exhibited a slightly decreasing trend, while other NPL showed a continuously increasing sharp trend. Also, the increased mortality trend of other NPL was significantly more rapid than that in bone DLBCL. According to our result, except for bone DLBCL, the other NPL occurring in the bone as fairly rare tumors should be deeply explored.
A few limitations existed in the present study. Firstly, because of the limitation of the SEER database, some basic information was not enrolled, including environmental exposures and other risk factors. Secondly, selection bias might exist in the retrospective trial because of its inherent flaws. Thirdly, Ann Arbor Stage is an important tumor characteristic for bone lymphoma, however, several unclassified cases in the database might affect the results, therefore, we did not estimate the incidence and incidence-based mortality rates. Fourthly, the SEER database does not have 100% coverage, and the population covered may not be the same during the study period, thus it may lack generalizability to the U.S. population. Until now, the SEER database always was reliable for assessing rare tumors in a preliminary analysis, but further study based better databases were deserved.
5. Conclusion
This study revealed that the incidence trend of bone lymphoma had been sharply increasing in the past decades, but has plateaued in recent years. And the joinpoint is approximately in 2009. Besides, the incidence-based mortality exhibited a sharply increasing trend between 1985 and 2016 without a turning point. Notably, these findings could help clinicians to elaborately determine the epidemiology and risk factors of bone lymphoma, and provide new insight into improving healthcare quality and better bone lymphoma management.
Funding statement
This work was supported by grants of the National Natural Science Foundation of China (No. 81871806) and Basic Public Welfare Project of Zhejiang Province (No. LGF19H060008).
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the Surveillance, Epidemiology and End Results database.
CRediT authorship contribution statement
Daoliang Xu: Data curation, Writing - original draft. Ben Wang: Data curation, Writing - original draft. Lijie Chen: Data curation, Writing - original draft. Huawei Zhang: Data curation. Xiangyang Wang: Writing - review & editing. Jiaoxiang Chen: Conceptualization, Methodology, Software, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Xiangyang Wang, Email: xiangyangwang@wmu.edu.cn.
Jiaoxiang Chen, Email: chenjiaoxiang@126.com.
References
- 1.Zucca E., Roggero E., Bertoni F., Cavalli F. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann. Oncol. 1997;8(8):727–737. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 2.Oberlin C. Les reticulosarcomes at les reticuloenotheliosarcomes de la moelle osseuse sarcomes dEwing. Bull. Assoc. Fr. Etud. Cancer. 1928;17:259–296. [Google Scholar]
- 3.Pettit C.K., Zukerberg L.R., Gray M.H. Primary lymphoma of bone. A B-cell neoplasm with a high frequency of multilobated cells. Am. J. Surg. Pathol. 1990;14(4):329–334. [PubMed] [Google Scholar]
- 4.Parker J., Jackson H. Primary reticulum cell sarcoma of bone. Surg. Gynecol. Obstet. 1939;68:45–53. [Google Scholar]
- 5.Mccormack L.J., Ivins J.C., Dahlin D.C. Primary reticulum-cell sarcoma of bone. Cancer. 1952;5(6):1182–1192. doi: 10.1002/1097-0142(195211)5:6<1182::aid-cncr2820050615>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.L. Zhang, Clinical characteristics and prognostic factors of bone lymphomas: focus on the clinical significance of multifocal bone involvement by primary bone large B-cell lymphomas, BMC Cancer 14(1): 900. [DOI] [PMC free article] [PubMed]
- 8.Messina C., Christie D., Zucca E. Primary and secondary bone lymphomas. Cancer Treat. Rev. 2015;41(3):235–246. doi: 10.1016/j.ctrv.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Li J., Wei R., Liu C., Nataraj A., Yan J. Prognostic factors associated with bone lymphoma primarily presenting in the spine. Spine (Phila Pa 1976) 2019;44(3):185–194. doi: 10.1097/BRS.0000000000002844. [DOI] [PubMed] [Google Scholar]
- 10.Wu H., Zhang L., Shao H. Prognostic significance of soft tissue extension, international prognostic index, and multifocality in primary bone lymphoma: a single institutional experience. Br. J. Haematol. 2014;166(1):60–68. doi: 10.1111/bjh.12841. [DOI] [PubMed] [Google Scholar]
- 11.Yin X., Xu A., Fan F. Incidence and mortality trends and risk prediction nomogram for extranodal diffuse large B-cell lymphoma: an analysis of the surveillance, epidemiology, and end results database. Front. Oncol. 2019;9:1198. doi: 10.3389/fonc.2019.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs A.J., Michels R., Stein J. Socioeconomic and demographic factors contributing to outcomes in patients with primary lymphoma of bone. J. Bone Oncol. 2015;4(1):32–36. doi: 10.1016/j.jbo.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada G.K., Basques B.A., Samartzis D. Development and validation of a novel scoring tool for predicting facility discharge after elective posterior lumbar fusion. Spine J. 2020 doi: 10.1016/j.spinee.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Duggan M.A., Anderson W.F., Altekruse S. The Surveillance, Epidemiology, and End Results (SEER) program and pathology: toward strengthening the critical relationship. Am. J. Surg. Pathol. 2016;40(12):e94–e102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian Z.J., Jin M.C., Meister K.D. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973–2013. JAMA Otolaryngol. Head Neck Surg. 2019;145(7):617–623. doi: 10.1001/jamaoto.2019.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlader N., Shiels M.S., Mariotto A.B., Engels E.A. Contributions of HIV to Non-hodgkin lymphoma mortality trends in the United States. Cancer Epidemiol. Biomarkers Prev. 2016;25(9):1289–1296. doi: 10.1158/1055-9965.EPI-16-0273. [DOI] [PubMed] [Google Scholar]
- 17.Wang B., Chen L., Huang C. The homogeneous and heterogeneous risk factors for occurrence and prognosis in lung cancer patients with bone metastasis. J. Bone Oncol. 2019;17 doi: 10.1016/j.jbo.2019.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.J., Fay M.P., Feuer E.J. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Matikas A., Briasoulis A., Tzannou I. Primary bone lymphoma: a retrospective analysis of 22 patients treated in a single tertiary center. Acta Haematol. 2013;130(4):291–296. doi: 10.1159/000351051. [DOI] [PubMed] [Google Scholar]
- 20.Parkin D.M., Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur. J. Cancer. 2009;45(5):756–764. doi: 10.1016/j.ejca.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Armitage P., Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer. 2004;91(12):1983–1989. doi: 10.1038/sj.bjc.6602297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellon M., Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses. 2017;9(10) doi: 10.3390/v9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuno Y., Murata T., Sato Y. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat. Microbiol. 2019;4(3):404–413. doi: 10.1038/s41564-018-0334-0. [DOI] [PubMed] [Google Scholar]
- 24.Reiter A., Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br. J. Haematol. 2008;142(3):329–347. doi: 10.1111/j.1365-2141.2008.06988.x. [DOI] [PubMed] [Google Scholar]
- 25.Taborelli M., Montella M., Libra M. The dose-response relationship between tobacco smoking and the risk of lymphomas: a case-control study. BMC Cancer. 2017;17(1):421. doi: 10.1186/s12885-017-3414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor J.G., Gribben J.G. Microenvironment abnormalities and lymphomagenesis: immunological aspects. Semin. Cancer Biol. 2015;34:36–45. doi: 10.1016/j.semcancer.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouman A., Heineman M.J., Faas M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11(4):411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 28.Riihijärvi S., Taskinen M., Jerkeman M., Leppä S. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur. J. Haematol. 2011;86(2):124–128. doi: 10.1111/j.1600-0609.2010.01541.x. [DOI] [PubMed] [Google Scholar]
- 29.Pfreundschuh M., Schubert J., Ziepert M. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9(2):105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 30.Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 2016; 66(4): 290-308. [DOI] [PubMed]
- 31.Maruyama D., Watanabe T., Beppu Y. Primary bone lymphoma: a new and detailed characterization of 28 patients in a single-institution study. Jpn. J. Clin. Oncol. 2007;37(3):216–223. doi: 10.1093/jjco/hym007. [DOI] [PubMed] [Google Scholar]
- 32.Ueda T., Aozasa K., Ohsawa M. Malignant lymphomas of bone in Japan. Cancer. 1989;64(11):2387–2392. doi: 10.1002/1097-0142(19891201)64:11<2387::aid-cncr2820641132>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Heyning F.H., Hogendoorn P.C., Kramer M.H. Primary non-Hodgkin's lymphoma of bone: a clinicopathological investigation of 60 cases. Leukemia. 1999;13(12):2094–2098. doi: 10.1038/sj.leu.2401582. [DOI] [PubMed] [Google Scholar]
- 34.Zinzani P.L., Carrillo G., Ascani S. Primary bone lymphoma: experience with 52 patients. Haematologica. 2003;88(3):280–285. [PubMed] [Google Scholar]
- 35.Ramadan K.M., Shenkier T., Sehn L.H. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann. Oncol. 2007;18(1):129–135. doi: 10.1093/annonc/mdl329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: the Surveillance, Epidemiology and End Results database.