Graphical abstract
Keywords: Aflatoxins, Ochratoxin A, Wheat, Gamma irradiation, Estimated daily intake
Abstract
Aflatoxins and ochratoxin A occur frequently in grains and are associated with carcinogenic, and nephrotoxic properties. Therefore, the aim of this study was to determine the level of aflatoxins and ochratoxin A contamination in wheat samples obtained from different Egyptian governorates and to assess the effect of gamma irradiation on AFB1 in spiked wheat samples, as well as to evaluate the estimated daily intake and hazard index. Thirty-six wheat grain samples purchased from different sale points were analyzed by High-Performance Liquid Chromatography. Data revealed that 33.33 % of the wheat grain samples were contaminated by aflatoxin B1, whereas only 16.66 % of the wheat samples were above the maximum limits (2 μg/kg) set by the European Commission. Ochratoxin A was only detected in two wheat grain samples, and the results were considered below the maximum limit (5 μg/kg) set by the European Commission. On studying the effect of gamma irradiation on wheat samples spiked by aflatoxin B1 (20 μg/kg), results revealed that aflatoxin B1 was reduced to 1.22 and 0.94 μg/kg for samples gamma-irradiated at a dose of 10 and 20 KGy respectively. Estimated daily intake of ochratoxin A in wheat samples was found to be higher than that of the tolerable daily intake; however, hazard index values were below one, thus demonstrating no threat to human health.
1. Introduction
Wheat (Triticum sp.), is one of the most important food grains and cereal sources worldwide and is chiefly used for the manufacture of flour, bread, biscuits, cookies, noodles, and many other products [1]. Wheat is the only grain capable of producing strong and cohesive dough, which are qualities essential for a wide range of food products [2]. In Egypt, consumption per capita of bread products ranks among the highest in the world. Unfortunately, wheat grains are prone to fungal infection by many toxigenic fungal species that produce mycotoxins [3]. Mycotoxins that contaminate wheat grains may include aflatoxins (AFs), ochratoxin A (OTA), and Fusarium toxins [4].
Mycotoxins are considered a significant issue in the situation of food safety, due to their acute and chronic toxic eff ;ects on animals and humans [5]. Thus, most countries established and enforced maximum regulatory limits for mycotoxins in response to food safety threats [6]. Prolonged exposure to mycotoxins, mainly in low-income countries, even in low quantities, may cause immune disorders as well as liver damage and cancer [7].
Aflatoxins are mainly produced by Aspergillus flavus and Aspergillus parasiticus [8]. Among the known AFs, aflatoxins B1 (AFB1) is considered as the most potent one that is associated with both toxicity and carcinogenicity in humans and animals [9] and has been classified as a Group 1 human carcinogen by the International Agency for Research on Cancer [10]. On the other hand, ochratoxin A is produced by Penicillium and Aspergillus species [11], and possesses nephrotoxic, teratogenic, hepatotoxic, and immunosuppressive properties and is classified as a class 2B human carcinogen by the International Agency for Research on Cancer [10,12].
As human exposure to these mycotoxins occurs primarily through the consumption of wheat grain and their products, thus, it is important to emphasize on the procedures that may decrease these mycotoxins. An effective physical method for food preservation is the treatment with ionizing radiation [13], which is becoming more frequently used in the sterilization of a wide variety of foods. Gamma irradiation is established on the capability of high-energy photons generated by radiation of 60Co to prompt chemical changes of the target molecule by causing its degradation or remodeling [14]. In a study on naturally contaminated maize samples, Markov et al. [15] reported that gamma irradiation at a 10 KGy-dose reduced AFB1 by 94.5 %. Meanwhile, Patil et al. [16] indicated that AFB1 was reduced by 20–43% in artificially spiked peanuts using gamma irradiation (5–9 KGy). On the other hand, other studies showed conflicting results depending on the food matrix and the experimental setup [17,18]. Regardless of much public dispute on the safety of irradiated foods, ionization radiation is becoming more frequently used in the sterilization of a wide variety of foods [19,20]. Studies examining the impact of gamma irradiation on the nutritional quality of food concluded that gamma irradiation has no effects on the nutritional quality of food and has no adverse effect on human and animal health [21,22].
Studies considering food contamination and dietary exposure to mycotoxins are predominantly rare in the Middle East [23]. Therefore, the aim of this study was to determine the level of AFs and OTA contamination in wheat samples obtained from different Egyptian governorates and to assess the effect of gamma radiation on AFB1 in spiked wheat samples, as well as to estimate the possible threat of these mycotoxins on public health according to estimated daily intake and hazard index.
2. Materials and methods
2.1. Reagents
AFB1 and OTA standards were purchased from Sigma-Aldrich Chemical Company (Saint Louis, MO 63103, USA). Methanol and acetonitrile HPLC grade were purchased from Merck (Darmstadt, Germany). Filter papers (Whatman No. 4) were purchased from Whatman (Cytiva, Marlborough, MA 01752, USA). Pure water was obtained from a Milli-Q system (Millipore, Billerica, MA 01821, USA).
2.2. Preparation of standard solution
Aflatoxin B1 was dissolved in acetonitrile at a concentration of 1 mg/mL, whereas OTA was dissolved in methanol at a concentration of 1 mg/mL. Appropriate dilutions of the stock solutions were made for the preparation of a standard curve, and spiking of wheat samples. All the solutions were stored at−20◦C in amber glass vials in the dark and were brought to room temperature before use. Based on the average of three experiments, the LOD and LOQ values were determined according to the following equations for the calibration method.
Where σ is the standard deviation of the response or standard deviation of y-intercepts; s is the slope of the calibration curve,
2.3. Sample collection
A total of 36 wheat samples were purchased from different retailers in four Egyptian governorates (Alexandria, Cairo, El-Fayoum, and Giza) during November and December 2016. Sampling of wheat was done according to the guidelines provided by the European Commission and the International Organization for Standardization [24,25]. Wheat samples were stored under refrigeration conditions (<10 °C) in polythene bags until analysis [26].
2.4. Aflatoxin analysis
Ground wheat samples (50 g) were mixed with NaCl (10 g) and methanol: water (80:20) (200 mL) in a blender jar and blended at high speed for 1 min. The extract was passed through a fluted filter paper. The filtered extract (10 mL) was diluted with purified water (20 mL) and mixed well. The diluted extract was filtered through a glass microfiber filter. The diluted filtered extract (2.0 mL) was completely passed through the immunoaffinity column (AflaTest, Vicam, Milford, MA 01757, USA) at a rate of about 1 drop/second. The column was washed with purified water (1.0 mL) at a rate of 1–2 drops/seconds twice. The eluate was eluted using HPLC grade methanol (1.0 mL) and then analyzed by HPLC.
2.5. Ochratoxin A analysis
Ground wheat samples (50 g) were mixed with acetonitrile: water (60:40) (100 mL) in a blender jar and blended at high speed for 1 min. The extract was passed through a fluted filter paper. The filtered extract (10 mL) was diluted with Phosphate Buffer Saline (PBS) (40 mL) and mixed well. The diluted extract was filtered through a glass microfiber filter. The diluted filtered extract (10 mL) was completely passed through the immunoaffinity column (OchraTes, Vicam, Milford, MA 01757, USA) at a rate of about 1 drop/second. After which PBS (10 mL) was passed at a rate of 1–2 drops/seconds, then purified water (10 mL) was passed at a rate of 1–2 drops/seconds. The eluate was eluted using HPLC grade methanol (1.5 mL), then purified water (1.5 mL) was added, vortexed, and analyzed by HPLC.
2.6. Effect of gamma irradiation on aflatoxin B1
Gamma irradiation was carried out in the National Center for Radiation Research and Technology (NCRRT), Cairo, Egypt. Wheat samples (50 g) free from AFB1 were ground, spiked with AFB1 at a concentration of 2, 10, and 20 μg/kg, packaged in polyethylene bags and gamma-irradiated in the dose of 5, 10, and 20 KGy at room temperature (25 °C). The irradiation was performed using a cobalt-60 γ-source irradiator with a dose rate of ∼ 2.77 KGy/h. After the gamma irradiation of spiked wheat samples, AFB1 was extracted as mentioned previously, and analyzed by HPLC.
2.7. HPLC system
HPLC system used was an Agilent 1200 series system (Agilent, Berks, UK) with a fluorescence detector (FLD G1321A), an autosampler (ALS G1329A), FC/ALS therm (G1330B), Degasser (G1379B), Bin Bump (G1312A) and a C18 (Phenomenex, Luna 5 μm, 150 × 4.6 mm) column joined to a pre-column (security guard, 4 × 3-mm cartridge, Phenomenex Luna). The mobile phase for AFB1 determination was water: acetonitrile: methanol (3:1:1, v/v/v). The mobile phase for OTA determination was acetonitrile: water: acetic acid (99:99:2, v/v/v).
2.8. Estimation of daily intake and hazard index
The estimated daily intake (EDI) of AFs and OTA were calculated as follows:
Where C is the concentration of mycotoxins in wheat (μg/kg); ADC is the average daily consumption of wheat (g/person/day), and BW is the body weight (kg). Based on WHO, [27], adults in the Middle East have an average daily consumption of 327.3 g/person/day for wheat. The bodyweight of an average adult was set to 70 kg.
The Hazard Index (HI) of AFB1 and OTA were calculated as follows:
EDI was divided by TD50, which was then divided by a safety factor of 50,000. TD50 is the dose (μg/kg/body weight/day) required to induce tumors in half of the tested animals that would have remained tumor-free at a zero dose as described by Taghizadeh et al. [28], Echodu et al. [29], and Kortei et al. [30].
2.9. Statistical analysis
All values were expressed as mean (95 % CI). Statistical analysis of the data was carried out using Microsoft Excel 2010 statistical program. A one-way analysis of variance (ANOVA) was performed, in which p < 0.05 was considered statistically significant. Fisher's Protected Least Significant Diff ;erence was also used to determine the diff ;erence between diff ;erent means.
3. Results and discussion
A total of 36 samples of wheat grains were analyzed for AFs and OTA using HPLC. The various wheat grain samples were collected from four governorates (samples were collected from three districts from each governorate). Wheat grain samples analyzed were contaminated with variable concentrations of AFs, whereas, OTA was the least detected. Data in Table 1 revealed that AFB1 was not detected in any of the districts of Alexandria and El-Fayoum governorates. On the other hand, in Cairo governorate, AFB1 was detected in low concentration recording 0.19, and 0.13 μg/kg in Helwan and Al-Maadi districts respectively (Table 1).
Table 1.
Concentrations of aflatoxins and ochratoxin A (μg/kg) in wheat samples collected from different Egyptian Governorates.
| Governorates | Districts | Mycotoxin concentration (μg/kg) |
||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | OTA | ||
| Alexandria | Al-Manshieh | <LOD | <LOD | 16.29 (15.74−16.84) |
3.13 (1.38−4.88) |
<LOD |
| Abu-Qir | <LOD | 1.47 (1.15−1.79) |
16.37 (15.25−17.49) |
3.17 (1.56−4.78) |
<LOD | |
| Al-Ajami | <LOD | 0.12 (0.08−0.16) |
12.05 (10.97−13.13) |
8.41 (6.53−10.30) |
<LOD | |
| Cairo | Helwan | 0.19 (0.18−0.20) |
0.09 (0.08−0.10) |
9.98 (8.97−10.99) |
7.12 (3.31−10.93) |
<LOD |
| Al-Maadi | 0.13 (0.05−0.21) |
<LOD | 4.16 (4.05−4.27) |
4.87 (2.68−7.06) |
<LOD | |
| Al-Shuruq | <LOD | 0.09 (0.05−0.13) |
4.10 (3.89−4.31) |
0.23 (0.19−0.27) |
1.37 (1.04–1.70) |
|
| El- Fayoum | Sinnures | <LOD | 1.93 (1.21−2.65) |
20.77 (17.78−23.76) |
1.48 (0.84−2.12) |
<LOD |
| Ibshway | <LOD | 2.44 (1.65−3.23) |
30.41 (28.98−31.84) |
1.18 (0.89−1.47) |
<LOD | |
| Etsa | <LOD | <LOD | 13.71 (12.08−15.35) |
3.28 (2.73−3.83) |
0.21 (0.09−0.34) |
|
| Giza | Al-Omraniyah | 35.21 (24.11−46.31) |
1.94 (1.46−2.43) |
21.71 (16.85−26.58) |
1.03 (0.96−1.10) |
<LOD |
| Dokki | <LOD | 0.22 (0.12−0.32) |
41.38 (34.17−48.59) |
2.05 (0.82−3.28) |
<LOD | |
| Atfih | 49.79 (37.41−62.17) |
2.96 (2.10−3.82) |
10.67 (7.94−13.40) |
0.20 (0.10−0.30) |
<LOD | |
| LOD | 0.04 | 0.12 | 0.09 | 0.11 | 0.02 | |
| LOQ | 0.10 | 0.40 | 0.30 | 0.36 | 0.07 | |
Results are expressed as mean (95 % CI).
95 % CI: 95 % Confidence interval of the mean.
LOD: Limit of detection; LOQ: Limit of quantification.
Statistical analysis was carried out for censored numbers (<LOD) given zero.
Within each column, means showed significant diff ;erence P < 0.05 (LSD=4.97).
Within each raw means showed no significant difference P > 0.05.
These concentrations were considered below the AFB1 maximum limits (2 μg/kg) set by both the European Commission [31] and the Egyptian Organization for Standardization and Quality Control [32]. Meanwhile, in Giza governorate, AFB1 was detected in high concentrations recording 35.21 and 49.79 μg/kg for Al-Omraniyah and Atfih districts respectively, whereas these concentrations were considered higher than the maximum limits (2 μg/kg) for AFB1 set by the European Commission [31] and Egyptian Organization for Standardization and Quality Control [32]. The wheat grain samples obtained from the Dokki district in Giza governorate were not contaminated by AFB1. Considering that the presence of AFs in wheat grain samples should not exceed 4 μg/kg according to the European Commission [31] and Egyptian Organization for Standardization and Quality Control [32], thus these wheat samples could be considered unfit for human consumption. Results also indicated that 33.33 % of the wheat grain samples were contaminated by AFB1, whereas only 16.66 % of the wheat samples were above 30 μg/kg.
Similar observations were reported by Toteja et al. [33] who noticed that 16 % of the wheat samples were contaminated by AFB1 at a concentration of 30 μg/kg. On the other hand, in Lebanon, 71.8 % of the wheat samples were contaminated by AFB1, among which 29.4 % exceeded the European Commission [34]. In a study on stored wheat, AFB1 was detected in four wheat samples at levels above the European Commission regulatory limit of 2 ppb in grains for human consumption [35]. In a recent study, Shi et al. [36] found that wheat samples grown under low heat unit climate conditions were not contaminated by AFB1.
Aflatoxin B2 was detected in 75 % of the wheat grain samples, whereas wheat grain samples obtained from the following districts; Al-Manshieh (Alexandria), Al-Maadi (Cairo), and Etsa (El-Fayoum) were free of AFB2. Data in Table 1 also revealed that AFB2 concentration was below 2 μg/kg in 77.77 % of the wheat grain samples. Results indicated that the wheat grain samples from Ibshway (El-Fayoum), and Atfih (Giza) recorded 2.44 and 2.96 μg/kg; respectively (Table 1). These results were considered different from those observed by Giray et al. [37] who mentioned that the incidence of AFB2 recorded 37 % in wheat samples analyzed in Turkey. On the other hand, Trombete et al. [38] found that AFB2 was not detected in any wheat samples in Brazil.
Both AFG1 and AFG2 were detected in 100 % of the wheat grain samples (Table 1), whereas wheat grain samples obtained from Dokki (Giza), and Ibshway (El-Fayoum) were highly contaminated by AFG1 which recorded 41.38 and 30.41 μg/kg respectively, followed by both Al-Omraniyah (Giza) and Sinnures (El-Fayoum) respectively. Data also showed that AFG1 concentrations were above 10 μg/kg in 75 % of the wheat grain samples, indicating the high AFG1 concentration. Although there are no regulations for AFG1 and AFG2 alone, the increase in AFG1 concentration is considered dangerous to public health, as it follows AFB1 in toxicity. Data in Table 1 revealed that wheat grain samples obtained from Al-Ajami (Alexandria) and Helwan (Cairo) were highly contaminated by AFG2 recording 8.41 and 7.12 μg/kg respectively, whereas 83.33 % of the wheat grain samples were below 5 μg/kg.
On the other hand, OTA was detected in two wheat grain samples obtained from Al-Shurug (Cairo), and Etsa (El-Fayoum) recording 1.37 and 0.21 μg/kg respectively. These results for OTA concentration were considered below the maximum limit (5 μg/kg) set by the Egyptian Organization for Standardization and Quality Control [32] and European Commission [39] for wheat grains. Several studies reported the occurrence of OTA in wheat in North African countries [[40], [41], [42], [43], [44], [45]]. Joubrane et al. [34] recorded that 28.21 % of wheat samples collected during the 2008 cultivation season from Lebanon were contaminated with OTA at concentrations (>3 μg/kg), while 19.20 % of wheat samples collected during the 2009 cultivation season were contaminated with OTA at concentrations (>3 μg/kg). Recently, Elaridi et al. [46] revealed that OTA was not detected in wheat samples obtained from Lebanon. The situations of the grains at harvest depend on, how carefully the grains are dried, and on the storage conditions [47].
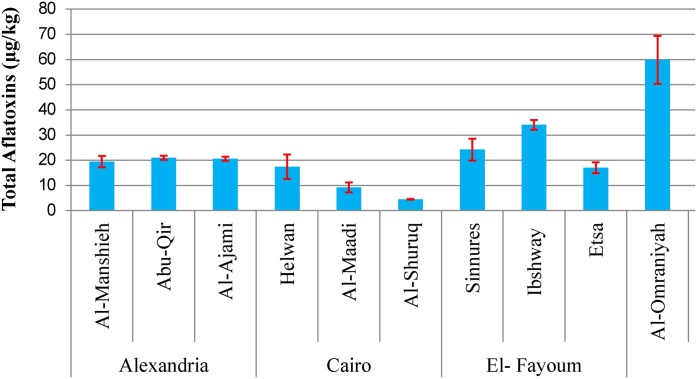
Results in Fig. 1 revealed the total AFs concentration in wheat grain samples obtained from different Egyptian governorates. Data revealed that wheat grain samples obtained from Giza governorate were highly contaminated and total AFs recorded 63.64, 59.89, and 43.66 μg/kg for Atfih, Al-Omraniyah, and Dokki districts respectively. These results were followed by El-Fayoum governorate, especially the Ibshway district, whereas total AFs recoded 34.04 μg/kg. On the other hand, wheat grain samples obtained from Cairo governorate showed the lowest total AFs concentration. Data clearly indicated that all wheat grain samples obtained from Egypt were highly contaminated, and they all exceeded the maximum limit (4 μg/kg) set by the European Commission [31] and Egyptian Organization for Standardization and Quality Control [32]. This increase in total AFs could be due to the increase in AFG1 concentration.
Fig. 1.
Concentration of total aflatoxins in wheat samples collected from different Egyptian Governorates. Results are expressed as mean (95 % CI). Bars represent confidence interval.
Aflatoxins represent a public health problem due to their high toxicity, and because they remain partially stable during their industrial procedure [48,49]. On the other hand, during milling of wheat, mycotoxins are reallocated in the bran, thus minimizing mycotoxin concentration in fraction used for human consumption, as bran is only used for animal feed [50,51]. However, in recent years the human consumption of wheat bran has increased [52]. Therefore, mycotoxin concentration in wheat bran intended for human consumption must be reduced. Also, the co-occurrence of both nephrotoxic OTA and genotoxic-carcinogenic AFs in the wheat samples analyzed in this study may further increase the health risks. Therefore, there is a great need to detoxify cereal grains before human consumption to decrease mycotoxin concentration, as well as to decrease the health risks.
3.1. Effect of gamma irradiation
The effects of different radiation doses on wheat samples spiked with different concentrations of AFB1 are shown in Table 2. Data clearly showed that gamma irradiation had an impact on the reduction of AFB1, whereas higher reductions (2.35, 1.93 μg/kg) were recorded for spiked samples by AFB1 (10 μg/kg) radiated at a dose of 10 and 20 KGy respectively. Results also showed that by increasing the concentration of AFB1 in spiked samples, the reduction percentage increased. It was noticed that by increasing the radiation dose the reduction of AFB1 increased. Our results are considered similar to Mohamed et al. [53] who found that gamma radiation at a dose of 8 KGy reduced AFB1 in wheat samples by 69.29 %. In a similar study by Ghanem et al. [54], the authors reported that AFB1 was reduced by 85 and 69 % in unpeeled and peeled pistachio respectively. Recently, gamma irradiation (10KGy-10 min) treatments on AFB1 in spiked hazelnut were investigated, whereas AFB1 was reduced by 47 % [55]. These results indicate that there are several factors that influence the success of gamma irradiation; these include the type of mycotoxins, the concentration of mycotoxins, the radiation dose, and the presence of other compounds or matrix components [56].
Table 2.
Effect of gamma irradiation on the concentration of AFB1 (μg/kg) in spiked wheat samples.
| AFB1 concentration (μg/kg) in spiked wheat | Radiation Dose (KGy) |
||
|---|---|---|---|
| 5 | 10 | 20 | |
| 2 | 1.89 (1.80−1.98) |
1.77 (1.39−2.15) |
1.18 (1.02−1.34) |
| 10 | 8.73 (7.09−10.37) |
2.35 (0.36−4.34) |
1.93 (1.57−2.28) |
| 20 | 7.66 (5.52−9.80) |
1.22 (0.78–1.66) |
0.94 (0.67−1.22) |
Results are expressed as mean (95 % CI).
% CI: 95 % Confidence interval of the mean.
Within each column, means showed no significant diff ;erence P > 0.05.
Uses of irradiation for aflatoxin decontamination have shown promising results as the gamma rays are suitable due to their deep infiltration through food [57]. On the other hand, gamma irradiation at a dose of up to 10 KGy has been reported to have no adverse effect on human and animal health, and have no effects on the nutritional quality of food as reported by the World Health Organization [58].
3.2. Risk assessment of exposure to AFs and OTA via consumption of wheat
3.2.1. Estimated daily intake
The results of the estimated daily intakes of AFs and OTA using the consumption of wheat grains from the concentrations determined during the experiment (Table 3). Data revealed that the EDI for AFB1 for wheat samples obtained from the Giza district was very high, whereas the mean and the maximum EDI recorded 132.46 and 232.80 μg/kg body weight /day respectively, thus indicating that wheat samples obtained from Giza governorate are of public health concern. In a previous study in Egypt, Hussain et al. [59] indicated that many of the cereal grains including wheat obtained from Giza governorate were highly contaminated by fungi. Therefore, our results could be due to climate conditions which are one of the most important factors that have a great effect on fungal growth as well as mycotoxin production. On the other hand, Giza governorate was considered one of the highest governorates in temperature averages [60].
Table 3.
Estimated daily intake (EDI) for adults via consumption of wheat.
| Governorates | EDI (μg/kg body weight/day) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 |
AFB2 |
AFG1 |
AFG2 |
Total AFs |
OTA |
||||||||
| a | b | a | b | a | b | a | b | a | b | a | b | ||
| Alexandria | Mean | 0 | 0.09 | 2.48 | 2.57 | 69.67 | – | 22.91 | – | 23.75 | 23.80 | 0 | 0.05 |
| Maximum | 0 | – | 6.97 | – | 76.54 | – | 39.32 | – | 76.57 | – | 0 | – | |
| Cairo | Mean | 0.49 | 1.59 | 0.28 | 0.37 | 28.43 | – | 19.03 | – | 12.06 | 12.11 | 2.14 | 2.17 |
| Maximum | 0.88 | – | 0.42 | – | 46.66 | – | 33.29 | – | 46.66 | – | 5.41 | – | |
| El- Fayoum | Mean | 0 | 0.09 | 6.81 | 6.90 | 101.14 | – | 9.26 | – | 29.32 | 29.36 | 0.33 | 0.36 |
| Maximum | 0 | – | 11.41 | 142.19 | – | 15.34 | – | 142.19 | – | 0.98 | – | ||
| Giza | Mean | 132.46 | 132.51 | 7.99 | – | 114.60 | – | 5.09 | – | 65.13 | 65.14 | 0 | 0.05 |
| Maximum | 232.80 | – | 13.84 | – | 193.48 | – | 9.59 | – | 232.80 | – | 0 | – | |
| TDI | NE | – | – | – | 0.005 | ||||||||
EDI: Estimated Daily Intake; TDI: Tolerable Daily Intake; NE: Not established.
aCensored numbers (<LOD) were given zero.
bCensored numbers (<LOD) were given LOD/2.
Statistical analysis was carried out for censored numbers (<LOD) given zero.
Within each column, means showed significant diff ;erence P < 0.05 (LSD=4.27).
Within each raw means showed no significant difference P > 0.05.
Within each column, maximum showed significant diff ;erence P < 0.05 (LSD=31.57).
Within each raw maximum showed significant difference P < 0.05(LSD=31.57).
Meanwhile, EDI for wheat samples obtained from Cairo governorate recorded 0.49 and 0.88 μg/kg body weight /day respectively for mean and maximum samples and thus was considered low. Although these low concentration might cause a risk to human health, as the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and Scientific Committee on Food (SCF) reports [61,62], revealed that a very low exposure level to aflatoxins at a concentration of 1 ng/kg body weight /day may induce liver cancer, and the “As Low as Reasonably Achievable” approach is recommended [63].
Results also revealed that the EDI for OTA in wheat samples obtained from Cairo governorate were considered high, whereas the mean and the maximum EDI recorded 2.14 and 5.41 μg/kg body weight /day respectively. Meanwhile, EDI for wheat samples obtained from El-Fayoum governorate was also high, although considered lower than those recorded for wheat samples obtained from Cairo governorate. These results were considered higher than the Tolerable Daily Intake (TDI) for OTA (0.005 μg/kg body weight/day) thus indicating the risk to public health. The risks of the increase in EDI for OTA to consumers have been evaluated by the Scientific Committee on Food [64], the Joint FAO/WHO Expert Committee on Food Additives [[65], [66], [67]] as well as the European Food Safety Authority [68].
3.2.2. Hazard index
Results in Table 4 indicated that the recorded hazard index values ranged from 5.63 × 10−7 to 1.46 × 10-3 and from 9.73 × 10-8 to 1.46 × 10-6 for AFB1 and OTA respectively. Data also clearly showed that HI results for AFB1 and OTA exposure via consumption of wheat is less than one, thus indicating that the intake of wheat will not pose any health risks, as it is well known that an HI ≤ 1 indicates no significant health risk. However, the possibility of long-term adverse health effects increases with increasing HI values as an HI between 1.1 and 10 reflects a moderate-risk [69], and HI > 10 indicates high risk [70].
Table 4.
Hazard Index (HI) values for adults via consumption of wheat samples.
| Governorates | HI (μg/kg body weight/day) |
||||
|---|---|---|---|---|---|
| AFB1 |
OTA |
||||
| a | b | a | b | ||
| Alexandria | Mean | 0 | 5.63 × 10−7 | 0 | 1.35 × 10−8 |
| Maximum | 0 | – | 0 | – | |
| Cairo | Mean | 3.06 × 10−6 | 9.94 × 10−6 | 5.78 × 10−7 | 5.86 × 10−7 |
| Maximum | 5.50 × 10−6 | – | 1.46 × 10−6 | – | |
| El- Fayoum | Mean | 0 | 5.63 × 10−7 | 8.92 × 10−8 | 9.73 × 10−8 |
| Maximum | 0 | – | 2.65 × 10−7 | – | |
| Giza | Mean | 8.28 × 10−4 | 8.28 × 10−4 | 0 | 1.35 × 10−8 |
| Maximum | 1.46 × 10−3 | – | 0 | – | |
The results of this study provided evidence of high levels of mycotoxin contamination and co-occurrence in wheat grain samples obtained from Egypt, thus indicating that consumers are at high risk due to the exposure to AFs and OTA. However, the hazard index values recorded less than one and thus consumers were at no risk.
4. Conclusions
The present study, carried out in Egypt, provided additional information related to the occurrence and exposure assessment of aflatoxins and ochratoxin A in wheat. About thirty-three percent of the wheat samples were contaminated with AFB1, whereas two samples exceeded the European Commission's maximum level for AFB1. Meanwhile, about sixteen percent of the wheat samples were contaminated with OTA, whereas no samples exceeded the European Commission's maximum level for OTA. Gamma irradiation had an impact on the reduction of AFB1, whereas higher reductions (2.35, 1.93 μg/kg) were recorded for spiked samples (10 μg/kg) irradiated at a dose of 10 and 20 KGy respectively. The estimated daily intake for both mycotoxins was found to be higher than that of the tolerable daily intake, however, the hazard index values were less than one and therefore the consumption of wheat was found to pose no health risk to consumers.
Authors statement
Ahmed Fouzy: managed and coordinated the responsibility for the research activity, and provided resources for the study; Amal Hathout, Shaaban Abel-Fattah, and Yehia Abou-Sree: developed and designed the methodology, conducted the research and investigation process, and wrote the original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was funded by the National Research Centre, Cairo, Egypt (Grant No. 11040303).
References
- 1.Belderok B., Mesdag J., Donner D.A. Springer, Kluwer Academic Publishers; Dordrecht, Netherlands: 2008. Bread-Making Quality of Wheat. [Google Scholar]
- 2.Kamboj U., Guha P., Mishra S. Changes in rheological properties of wheat due to storage. J. Sci. Food Agric. 2018;98:1374–1380. doi: 10.1002/jsfa.8603. [DOI] [PubMed] [Google Scholar]
- 3.Pakfetrat S., Amiri S., Radi M., Abedi E., Torri L. Reduction of phytic acid, aflatoxins, and other mycotoxins in wheat during germination. J. Sci. Food Agric. 2019;99:4695–4701. doi: 10.1002/jsfa.9710. [DOI] [PubMed] [Google Scholar]
- 4.Miraglia M., Brera C. Mycotoxins in grains and related products. In: Nollet L.M.L., editor. Food Analysis by HPLC. Marcel Dekker; New York: 2000. pp. 493–522. [Google Scholar]
- 5.Shanakhat H., Sorrentino A., Raiola A., Romano A., Masia P., Cavellaa S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: an overview. J. Sci. Food Agric. 2018;98(11):4003–4013. doi: 10.1002/jsfa.8933. [DOI] [PubMed] [Google Scholar]
- 6.Van Egmond H.P., Schothorst R.C., Jonker M.A. Regulations relating to mycotoxins in food: perspectives in a global and European context. Anal. Bioanal. Chem. 2007;389:147–157. doi: 10.1007/s00216-007-1317-1319. [DOI] [PubMed] [Google Scholar]
- 7.Emmott A. International Food Policy Research Institute; Washington, DC: 2013. Market-Led Aflatoxin Interventions: Smallholder Groundnut Value Chains in Malawi, in 2020 Focus Brief, 20 (16) [Google Scholar]
- 8.Kostarelou P., Kanapitsas A., Pyrri I., Kapsanaki-Gotsi E., Markaki P. Aflatoxin B1 production by Aspergillus parasiticus and strains of Aspergillus section Nigri in currants of Greek origin. Food Control. 2014;43:121–128. doi: 10.1016/j.foodcont.2014.02.011. [DOI] [Google Scholar]
- 9.Aly S.E., Hathout A.S. Fate of aflatoxin B1 in contaminated corn gluten during acid hydrolysis. J. Sci. Food Agric. 2011;91(3):421–427. doi: 10.1002/jsfa.4201. [DOI] [PubMed] [Google Scholar]
- 10.Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as human carcinogens- the IARC Monographs classification. Mycotoxin Res. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 11.Gallo A., Knox B.P., Bruno K.S., Solfrizzo M., Baker S.E., Perrone G. Identification and characterization of the polyketide synthase involved in ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2014;179:10–17. doi: 10.1016/j.ijfoodmicro.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 13.Dhanya R., Mishra B.B., Khaleel K.M., Cheruth A. Shelf life extension of fresh turmeric (Curcuma longa L.) using gamma radiation. Radiat. Phys. Chem. 2009;78:791–795. doi: 10.1016/j.radphyschem.2009.05.011. [DOI] [Google Scholar]
- 14.Domijan A.M., Marjanović Čermak A.M., Vulić A., Tartaro B., Pavičić I., Pleadin J., Markov K., Mihaljević B. Cytotoxicity of gamma-irradiated aflatoxin B1 and ochratoxin A. J. Environ. Sci. Health B. 2019;54(3):155–162. doi: 10.1080/03601234.2018.1536578. [DOI] [PubMed] [Google Scholar]
- 15.Markov K., Mihaljevic B., Domijan A.-M., Pleadin J., Delas F., Frece J. Inactivation of aflatoxigenic fungi and the reduction of aflatoxin B1in vitro and in situ using gamma irradiation. Food Control. 2015;54:79–85. doi: 10.1016/j.foodcont.2015.01.036. [DOI] [Google Scholar]
- 16.Patil H., Shah N.G., Hajare S.N., Gautam S., Kumar G. Combination of microwave and gamma irradiation for reduction of aflatoxin B1 and microbiological contamination in peanuts (Arachis hypogaea L.) World Mycotoxin J. 2019;12:269–280. doi: 10.3920/WMJ2018.2384. [DOI] [Google Scholar]
- 17.Hooshmand H., Klopfenstein C.F. Effects of gamma irradiation on mycotoxin disappearance and amino acid contents of corn, wheat, and soybeans with different moisture contents. Plant Foods Hum. Nutr. 1995;47(3):227–238. doi: 10.1007/BF01088331. [DOI] [PubMed] [Google Scholar]
- 18.Prado G., De Carvalho E.P., Oliveira M.S., Madeira J.G.C., Morais V.D., Correa R.F., Cardoso V.N., Soares T.V., da Silva J.F.M., Goncalves R.C.P. Effect of gamma irradiation on the inactivation of aflatoxin B1 and fungal flora in peanut. Braz. J. Microbiol. 2003;34(Suppl.1):138–140. doi: 10.1590/S1517-83822003000500047. [DOI] [Google Scholar]
- 19.Rosa J., Barbosa-Canovas G.V. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food Sci. Nutr. 2003;43:265–285. doi: 10.1080/10408690390826527. [DOI] [PubMed] [Google Scholar]
- 20.Kabak B., Dobson A.D.W., Var I. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit. Rev. Food Sci. Nutr. 2006;46:593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- 21.Aziz N.H., Souzan R.M., Azza A. Effect of gamma-irradiation on the occurrence of pathogenic microorganisms and nutritive value of four principal cereal grains. Appl. Radiat. Isot. 2006;64:1555–1562. doi: 10.1016/j.apradiso.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Hamza R.G., Afifi S., Abdel-Ghaffar A.-R.B., Borai I.H. Effect of gamma-irradiation or/and extrusion on the nutritional value of soy flour. Biochem. Anal. Biochem. 2012;1:118. doi: 10.4172/2161-1009.1000118. [DOI] [Google Scholar]
- 23.Raad F., Nasreddine L., Hilan C., Bartosik M., Parent-Massin D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem. Toxicol. 2014;73:35–43. doi: 10.1016/j.fct.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 24.EC (European Commission) Off. J. Eur. Union; 2006. Commission Regulation No 401/2006 of 23 February 2006, Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; pp. 12–34. L70. [Google Scholar]
- 25.ISO (International Organization for Standardization) 2009. ISO-24333: Cereal and Cereal Products-Sampling, Geneva, Switzerland. [Google Scholar]
- 26.Pekmez H. Cereal storage techniques: a review. J. Agric. Sci. Technol. B. 2016;6 doi: 10.17265/2161-6264/2016.02.001. [DOI] [Google Scholar]
- 27.WHO (World Health Organization) 2003. GEMS/Food Regional Diets: Regional Per Capita Consumption of Raw and Semi-processed Agricultural Commodities / Prepared by the Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme (GEMS/Food), Geneva, Switzerland. [Google Scholar]
- 28.Taghizadeh S.F., Rezaee R., Davarynejad G., Asili J., Nemati S.H., Goumenou M., Tsakiris I., Tsatsakis A.M., Shirani K., Karimi G. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different Pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ. Toxicol. Pharmacol. 2018;61:61–66. doi: 10.1016/j.etap.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Echodu R., Malinga G.M., Kaducu J.M., Ovuga E., Haesaert G. Prevalence of aflatoxin, ochratoxin, and deoxynivalenol in cereal grains in northern Uganda: implication for food safety and health. Toxicol. Rep. 2019;6:1012–1017. doi: 10.1016/j.toxrep.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kortei N., Agyekum A., Akuamoa F., Kyei-Baffour V., Alidu H. Risk assessment and exposure to levels of naturally occurring aflatoxins in some packaged cereals and cereal-based foods consumed in Accra, Ghana. Toxicol. Rep. 2018;6:34–41. doi: 10.1016/j.toxrep.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EC (European Commission) Off. J. Eur. Union; 2010. Regulation No 165/2010 of 26 February 2010, Amending Regulation (EC) No 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs As Regards Aflatoxins; pp. 8–12. 50. [Google Scholar]
- 32.(EOSQC) Egyptian Organization for Standardization and Quality Control . 2010. Egyptian Standard No. 7136/2010, Cairo, Egypt. [Google Scholar]
- 33.Toteja G., Mukherjee A., Diwakar S., Singh P., Saxena N., Sinha K., Sinha A., Kumar N., Nagaraja K., Bai G., Prasad C., Vanchinathan S., Roy R., Parkar S. Aflatoxin B1contamination in wheat grain samples collected from different geographical regions of India: A multicenter study. J. Food Prot. 2006;69:1463–1467. doi: 10.4315/0362-028X-69.6.1463. [DOI] [PubMed] [Google Scholar]
- 34.Joubrane K., El-Khoury A., Lteif R., Rizk T., Awad M., Hilan C., Maroun R. Occurrence of aflatoxin B1 and ochratoxin A in Lebanese cultivated wheat. Mycotoxin Res. 2011;27:249–257. doi: 10.1007/s12550-011-0101-z. [DOI] [PubMed] [Google Scholar]
- 35.Sadhasivam S., Britzi M., Zakin V., Kostyukovsky M., Trostanetsky A., Quinn E. Rapid detection and identification of mycotoxigenic fungi and mycotoxins in stored wheat grain. Toxins. 2017;9(10):302. doi: 10.3390/toxins9100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H., Schwab W., Yu P. Natural occurrence and co-contamination of twelve mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition. Toxins. 2019;11(3):160. doi: 10.3390/toxins11030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giray B., Girgin G., Engin A.B., Aydin S., Sahin G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control. 2007;18:23–29. doi: 10.1016/j.foodcont.2005.08.002. [DOI] [Google Scholar]
- 38.Trombete F.M., Moraes D., Porto Y.D., Santos T.B., Direito G.M., Fraga M.E., Saldanha T. Determination of aflatoxins in wheat and wheat by-products intended for human consumption, Marketed in Rio de Janeiro. Brazil. J. Food Nutr. Res. 2014;2(10):671–674. doi: 10.12691/jfnr-2-10-3. [DOI] [Google Scholar]
- 39.EC (European Commission) Off. J. Eur. Union; 2006. Regulation No 1881/2006 of 19 December 2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs; pp. 1–28. L364. [Google Scholar]
- 40.Hajjaji A., El Otmani M., Bouya D., Bouseta A., Mathieu F., Collin S., Lebrihi A. Occurrence of mycotoxins (ochratoxin A, deoxynivalenol) and toxigenic fungi in Moroccan wheat grains: impact of ecological factors on the growth and ochratoxin A production. Mol. Nutr. Food Res. 2006;50:494–499. doi: 10.1002/mnfr.200500196. [DOI] [PubMed] [Google Scholar]
- 41.Riba A., Mokrane S., Mathieu F., Lebrihi A., Sabaou N. Mycoflora and ochratoxin A producing strains of Aspergillus in Algerian wheat. Int. J. Food Microbiol. 2008;122:85–92. doi: 10.1016/j.ijfoodmicro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 42.Zaied C., Abid S., Zorgui L., Bouaziz C., Chouchane S., Jomaa M., Bacha H. Natural occurrence of ochratoxin A in Tunisian cereals. Food Control. 2009;20:218–222. doi: 10.1016/j.foodcont.2008.05.002. [DOI] [Google Scholar]
- 43.Zinedine A., Blesa J., Mahnine N., El-Abidi A., Montesano D., Mañes J. Pressurized liquid extraction coupled to liquid chromatography for the analysis of ochratoxin A in breakfast and infants cereals from Morocco. Food Control. 2009;4:009. doi: 10.1016/j.foodcont.2009.04.009. [DOI] [Google Scholar]
- 44.Jedidi I., Cruz A., González-Jaén M.T., Said S. Aflatoxins and ochratoxin A and their Aspergillus causal species in Tunisian cereals. Food Addit. Contam., B. 2016;10:51–58. doi: 10.1080/19393210.2016.1247917. [DOI] [PubMed] [Google Scholar]
- 45.Zebiri S., Mokrane S., Verheecke C., Choque É., Reghioui H., Sabaou N., Mathieu F., Riba A. Occurrence of ochratoxin A in Algerian wheat and its milling derivatives. Toxin Rev. 2018;37:1–6. doi: 10.1080/15569543.2018.1438472. [DOI] [Google Scholar]
- 46.Elaridi J., Yamani O., Al Matari A., Dakroub S., Attieh Z. Determination of ochratoxin A (OTA), ochratoxin B (OTB), T-2, and HT-2 toxins in wheat grains, wheat flour, and bread in Lebanon by LC-MS/MS. Toxins. 2019;11(8):471. doi: 10.3390/toxins11080471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eskola M. National Veterinary and Food Research Institute (EELA), University of Helsinki; 2002. Study on Trichothecenes Zearalenone and Ochratoxin a in Finnish Cereals: Occurrence and Analytical Techniques. Doctoral Dissertation. [Google Scholar]
- 48.Bullerman L.B., Bianchini A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007;119(2):140–146. doi: 10.1016/j.ijfoodmicro.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Giménez I., Herrera M., Escobar J., Ferruz E., Lorán S., Herrera A., Ariño A. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control. 2013;34(2):268–273. doi: 10.1016/j.foodcont.2013.04.033. [DOI] [Google Scholar]
- 50.Herrera M., Juan T., Estopaña G., Ariño A. Comparison of deoxynivalenol, ochratoxin A and aflatoxin B1 levels in conventional and organic durum semolina and the effect of milling. J. Food Nutr. Res. 2009;48(2):92–99. [Google Scholar]
- 51.Cheli F., Pinotti L., Rossi L., Dell’Orto V. Effect of milling procedures on mycotoxin distribution in wheat fractions: a review. LWT - Food Sci. Technol. 2013;54(2):307–314. doi: 10.1016/j.lwt.2013.05.040. [DOI] [Google Scholar]
- 52.Vidal A., Marní S., Ramos A.J., Cano-sancho G., Sanchis V. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in wheat and oat-based bran supplements sold in the Spanish market. Food Chem. Toxicol. 2013;53(1):133–138. doi: 10.1016/j.fct.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Mohamed N.F., Shams El-Dine R.S., Kotb M.A.M., Saber A. Assessing the possible effect of gamma irradiation on the reduction of aflatoxin B1, and on the moisture content in some cereal grains. Am. J. Biomed. Sci. 2015;7(1):33–39. doi: 10.5099/aj150100033. [DOI] [Google Scholar]
- 54.Ghanem I., Orfi M., Shamma M. Effect of gamma radiation on the inactivation of aflatoxin B1 in food and feed crops. Braz. J. Microbiol. 2008;39(4):787–791. doi: 10.1590/S1517-838220080004000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen Y., Onal-Ulusoy B., Mutlu M. Detoxification of hazelnuts by different cold plasmas and gamma irradiation treatments. Innov. Food Sci. Emergy Technol. 2019;54:252–259. doi: 10.1016/j.ifset.2019.05.002. [DOI] [Google Scholar]
- 56.Calado T., Venancio A., Abrunhosa L. Irradiation for mold and mycotoxin control: a review. Compr. Rev. Food Sci. 2014;13(5):1049–1061. doi: 10.1111/1541-4337.12095. [DOI] [Google Scholar]
- 57.Pankaj S.K., Shi H., Keener K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018;71:73–83. doi: 10.1016/j.tifs.2017.11.007. [DOI] [Google Scholar]
- 58.WHO (World Health Organization) 1999. High-Dose Irradiation: Wholesomeness of Food Irradiated With Doses Above 10 KGy. Joint FAO/IAEA/WHO Study Group. Technical Report Series, No. 890, Geneva, Switzerland. [PubMed] [Google Scholar]
- 59.Hussain O.A., Sobhy H.M., Hathout A.S., Fouzy A.S.M. Isolation and molecular identification of Fusarium fungi from some Egyptian grains. Asian J. Plant Sci. 2018;17:182–190. doi: 10.3923/ajps.2018.182.190. [DOI] [Google Scholar]
- 60.Badr A.N., Abdel-Fatah S.M., Abu Sree Y.H., Amra H.A. Mycotoxigenic fungi and mycotoxins in Egyptian barley under climate changes. Res. J. Environ. Toxicol. 2017;11:1–10. [Google Scholar]
- 61.JECFA (Joint FAO/WHO Expert Committee on Food Additives) WHO; Geneva, Switzerland: 1999. Evaluation of Certain Food Additives and Contaminants: Forty-ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No 884.http://whqlibdoc.who.int/trs/WHO_TRS_884.pdf [PubMed] [Google Scholar]
- 62.SCF (Scientific Committee on Food) 1994. European Commission DG XXIV Unit B3. Thirty-fifth Report. Opinion on Aflatoxins B1, B2, G1, G2, M1, and Patulin. Expressed on 23 September 1994. [Google Scholar]
- 63.JECFA (Joint FAO/WHO Expert Committee on Food Additives) WHO; Geneva, Switzerland: 2001. Evaluation of Certain Food Additives and Contaminants: Fifty-fifth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No 901.http://whqlibdoc.who.int/trs/WHO_TRS_901.pdf [Google Scholar]
- 64.SCF (Scientific Committee on Food) 1998. European Commission DG XXIV Unit B8. Opinion on Ochratoxin A. [Google Scholar]
- 65.FAO/WHO (Food and Agriculture Organization/World Health Organization) 1991. Evaluation of Certain Food Additives and Contaminants (Thirty-seventh Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, N° 806, and Corrigenda. [PubMed] [Google Scholar]
- 66.FAO/WHO (Food and Agriculture Organization/World Health Organization) 1995. Evaluation of Certain Food Additives and Contaminants (Forty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, N° 859. [Google Scholar]
- 67.FAO/WHO (Food and Agriculture Organization/World Health Organization) World Health Organization; Geneva: 2001. Safety Evaluation of Certain Mycotoxins in Food, Prepared by the Fifty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) - WHO Food Additives Series 47 - FAO Food and Nutrition Paper - IPCS - International Programme on Chemical Safety. [Google Scholar]
- 68.EFSA (European Food Safety Authority) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to ochratoxin A in food, adopted on 4 April 2006. EFSA J. 2006;365:1–56. http://www.efsa.europa.eu/en/scdocs/doc/contam_opej365_ochratoxin_a_food_en.pdf [Google Scholar]
- 69.Lemly A.D. Evaluation of the hazard quotient method for risk assessment of selenium. Ecotoxicol. Environ. Saf. 1996;35:156–162. doi: 10.1006/eesa.1996.0095. [DOI] [PubMed] [Google Scholar]
- 70.Ogunkunle C.O., Fatoba P.O. Potential health risk assessment for soil heavy metal contamination of Sagamu, South-West Nigeria due to cement production. Int. J. Appl. Sci. Technol. 2013;3(2):89–96. [Google Scholar]
- 71.2020. CPDB (The Carcinogenic Potency Database)https://files.toxplanet.com/cpdb/cpdb.html last updated: September 1, 2011, accessed on June 28, 2020. [Google Scholar]
- 72.Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: aflatoxin, ochratoxin, and zearalenone. Can. J. Physiol. Pharmacol. 1990;68(7):1017–1024. doi: 10.1139/y90-155. [DOI] [PubMed] [Google Scholar]