Highlights
-
•
The relationship between surgery, neuroinflammation, and delirium remains unclear.
-
•
[11C]PBR28 positron emission tomography (PET) was used to image neuroinflammation.
-
•
Patients showed a global reduction of [11C]PBR28 binding one month after surgery.
-
•
[11C]PBR28 binding was not related to delirium or other markers of inflammation.
-
•
Post-operative reduction in [11C]PBR28 binding may reflect neuroimmune suppression.
Abbreviations: BIDMC, Beth Israel Deaconess Medical Center; BWH, Brigham and Women’s Hospital; HMS, Harvard Medical School; HSL, Hebrew SeniorLife; MGH, Massachusetts General Hospital; PI, principal investigator; UNL, University of Nebraska-Lincoln
Abstract
Major surgery is associated with a systemic inflammatory cascade that is thought, in some cases, to contribute to transient and/or sustained cognitive decline, possibly through neuroinflammatory mechanisms. However, the relationship between surgery, peripheral and central nervous system inflammation, and post-operative cognitive outcomes remains unclear in humans, primarily owing to limitations of in vivo biomarkers of neuroinflammation which vary in sensitivity, specificity, validity, and reliability. In the present study, [11C]PBR28 positron emission tomography, cerebrospinal fluid (CSF), and blood plasma biomarkers of inflammation were assessed pre-operatively and 1-month post-operatively in a cohort of patients (N = 36; 30 females; ≥70 years old) undergoing major orthopedic surgery under spinal anesthesia. Delirium incidence and severity were evaluated daily during hospitalization. Whole-brain voxel-wise and regions-of-interest analyses were performed to determine the magnitude and spatial extent of changes in [11C]PBR28 uptake following surgery. Results demonstrated that, compared with pre-operative baseline, [11C]PBR28 binding in the brain was globally downregulated at 1 month following major orthopedic surgery, possibly suggesting downregulation of the immune system of the brain. No significant relationship was identified between post-operative delirium and [11C]PBR28 binding, possibly due to a small number (n = 6) of delirium cases in the sample. Additionally, no significant relationships were identified between [11C]PBR28 binding and CSF/plasma biomarkers of inflammation. Collectively, these results contribute to the literature by demonstrating in a sizeable sample the effect of major surgery on neuroimmune activation and preliminary evidence identifying no apparent associations between [11C]PBR28 binding and fluid inflammatory markers or post-operative delirium.
1. Introduction
Major surgery is associated with a systemic inflammatory cascade that is thought, in some cases, to contribute to transient and/or sustained cognitive decline, possibly through neuroinflammatory mechanisms (Alam et al., 2018, Cortese and Burger, 2017, Maldonado, 2013, Marcantonio, 2012, Skvarc et al., 2018, Subramaniyan and Terrando, 2019, van Harten et al., 2012). Following surgical trauma, the innate immune system is activated and releases peripheral proinflammatory cytokines. These proinflammatory cytokines in turn disrupt the permeability of blood–brain barrier (e.g., via upregulation of cyclooxygenase 2 isozyme; Saxena and Maze, 2018) due to endothelial dysfunction, allowing them and leukocytes to enter the central nervous system. This causes circulating leukocytes that results in a cycle of neuroinflammation via cytokine expression and microglial activation. In addition, it is also possible for peripheral cytokines to enter the brain via activation of the vagus nerve or through the circumventricular regions (van Gool et al., 2010). Notably, cytokine elevation in the central nervous system following surgery has been associated with cognitive dysfunction in humans, broadly in domains of attention, memory, and executive functions (Hirsch et al., 2016, Ji et al., 2013). Moreover, recent meta-analytic evidence identified significantly elevated levels of plasma C-reactive protein (CRP) and interleukin-6 (IL-6) in patients who developed post-operative delirium compared with control patients (Liu et al., 2018), supporting the key role of proinflammatory cytokines in the pathogenesis of delirium following surgery.
The relationship between surgery, peripheral and central inflammation, and post-operative cognitive outcomes has been robustly demonstrated using animal models (e.g., Barrientos et al., 2012, Cibelli et al., 2010, Femenia et al., 2018, Hovens et al., 2015, Li et al., 2016, Terrando et al., 2011, Xu et al., 2017, Zhang et al., 2014, Zheng et al., 2017). However, much less evidence for these processes has been identified in humans, primarily owing to limitations of in vivo biomarkers of neuroinflammation which vary in sensitivity, specificity, validity, and reliability. Recent evidence points toward elevated levels of proteins such as S100B (Hov et al., 2017) and soluble triggering receptor expressed on myeloid cells 2 (TREM2) (Henjum et al., 2018) in cerebrospinal fluid (CSF) in patients with Alzheimer’s disease exhibiting symptoms of post-operative delirium (i.e., an acute state of confusion with fluctuating symptoms of disturbed attention and cognition; Bruce et al., 2007, Marcantonio, 2012). Additionally, a host of other CSF and peripheral inflammatory biomarkers also change post-operatively, including CSF monocyte chemoattractant protein 1 and various interleukins (Bromander et al., 2012, Buvanendran et al., 2006, Cape et al., 2014, Dong et al., 2017, Hirsch et al., 2016, Reinsfelt et al., 2012, Tang et al., 2011, Vasunilashorn et al., 2015) as well as CRP (Dillon et al., 2017, Vasunilashorn et al., 2017, Vasunilashorn et al., 2019). However, current evidence is mixed, with several studies reporting inconsistent findings, likely due to small sample sizes (Beishuizen et al., 2015, Hall et al., 2013, Westhoff et al., 2015).
In addition to CSF biomarkers, it is now possible to examine neuroinflammation in vivo using positron emission tomography (PET) radiotracers such as [11C]PBR28 that bind to the translocator protein 18kDa (TSPO), formerly known as the peripheral benzodiazepine receptor (PBR). TSPO is highly expressed in activated microglia and to a lesser degree in reactive astrocytes (Brown et al., 2007, Lavisse et al., 2012) and thus is typically considered an imaging biomarker of neuroinflammation (Alam et al., 2017, Albrecht et al., 2016, Cagnin et al., 2007, Loggia et al., 2015). [11C]PBR28 has been used to study neurodegenerative diseases including Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (Alshikho et al., 2016, Herranz et al., 2016; also reviewed in Dupont et al., 2017). Unlike CSF biomarkers, imaging biomarkers of neuroinflammation such as [11C]PBR28 might enable the localization of the inflammatory response, which could provide insights into abnormalities of brain regions or systems that contribute to cognitive complications following surgery. However, there exist very little data attempting to associate PET tracers of neuroinflammation following surgery to post-operative cognitive outcomes.
To our knowledge, only one published study has investigated neuroinflammation using [11C]PBR28 in the context of surgery (Forsberg et al., 2017). The authors measured TSPO expression in the brain via [11C]PBR28 PET in a sample of older adult patients (N = 8, all males) undergoing abdominal surgery under general anesthesia on three occasions: 1–3 days prior to surgery, on post-operative days 3–4, and again 3 months after surgery. The authors identified global downregulation of gray matter [11C]PBR28 binding 3–4 days post-operatively compared with pre-operative baseline, followed at 3 months by a return to or, in some cases, an increase above baseline. Patients showing a greater increase in [11C]PBR28 binding in the lateral frontal cortex from postoperative days 3–4 to 3 months also showed a greater decrease in cognitive performance (i.e., slower response times) on a test of executive function. It is important to note, however, that general anesthesia might be associated with reductions in [11C]PBR28 binding to TSPO in the human brain (Hines et al., 2013), thus leaving unclear whether Forsberg et al.’s (2017) findings reflect an effect of surgery per se vs. general anesthesia.
In the present study, [11C]PBR28 PET and magnetic resonance (MR) brain imaging, CSF, and blood plasma biomarkers of inflammation were assessed pre-operatively and approximately 1-month post-operatively in a cohort of patients undergoing major orthopedic surgery under spinal anesthesia (for a detailed description of the study design and protocol, see Hshieh et al., 2019). This study builds upon prior work by investigating the effect of surgery on [11C]PBR28 binding in a group of older adults of both sexes over the age of 70, who are at increased risk for post-operative cognitive decline. We focused on the investigation of imaging biomarkers of inflammation, with the following goals: (1) to determine whether changes in [11C]PBR28 binding are observed 1 month following surgery in a larger sample, and (2) to examine the possible association between symptoms of post-operative delirium and [11C]PBR28 binding. Based on prior work, we hypothesized that (H1) widespread changes in [11C]PBR28 binding in the brain would be identified between pre-operative baseline and at 1-month post-operative follow-up. If there was sustained neuroinflammation occurring at 1 month following surgery, we would expect to see increased [11C]PBR28 binding. Conversely, if a transient state of immunosuppression was still active at 1 month following surgery, we would expect to see decreased [11C]PBR28 binding. We further hypothesized that (H2) those patients with post-operative delirium would show elevated levels of [11C]PBR28 binding in the brain following surgery, relative to patients who do not develop post-operative delirium.
2. Materials and methods
The overall design and protocol of the study have been described in detail elsewhere (Hshieh et al., 2019), and will be briefly reported here.
2.1. Participants
The Role of Inflammation after Surgery for Elders (RISE) study was a prospective cohort of 65 older adults undergoing elective total joint arthroplasty under spinal anesthesia. Of this larger pool of patients, we analyzed data obtained from 36 patients (30 females, 6 males; knee [n = 17] or hip [n = 19]) who had undergone PET/MR imaging and phlebotomy both at pre-operative baseline (PREOP) and at 1-month follow-up (PO1MO)4. All patients were cognitively normal older adults, as determined by a series of neuropsychological tests at baseline assessing cognitive abilities in domains of memory, learning, attention, and executive functioning, which were used to compute the composite General Cognitive Performance (GCP) score (Jones et al., 2010) following our previous investigations (Dillon et al., 2017, Schmitt et al., 2012, Schmitt et al., 2015, Vasunilashorn et al., 2015). All patients had delirium assessments completed daily throughout hospitalization and also at PO1MO. A subset of these patients also had sampling of CSF at PREOP (n = 30) and/or PO1MO (n = 29). Demographic and clinical characteristics for the included RISE participants are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the study participants (N = 36).
| All (N = 36) | Non-delirious (n = 30) | Delirious (n = 6) | Group difference (p) | |
|---|---|---|---|---|
| Age at surgery (years) | 74.4 ± 3.9 | 74.3 ± 3.7 | 74.7 ± 5.3 | 0.808 |
| Education (years) | 16.0 ± 3.3 | 15.7 ± 3.4 | 17.3 ± 2.1 | 0.281 |
| Female sex (n, %) | 30, 83% | 25, 83% | 5, 83% | 1 |
| Proportion of knee/hip surgery | 17 (47%)/19(53%) | 13 (43%)/17 (57%) | 4 (67%)/2 (33%)) | 0.296 |
| Duration of anesthesia (mins) | 146.6 ± 32.4 | 141.1 ± 26.2 | 174.5 ± 47.5 | 0.019 |
| Duration of surgery (mins) | 92.9 ± 29.0 | 89.0 ± 27.5 | 112.7 ± 30.4 | 0.067 |
| Estimated blood loss (cc) | 203.5 ± 115.3 | 200.8 ± 123.3 | 216.7 ± 68.3 | 0.764 |
| Total length of stay (days) | 3.4 ± 0.9 | 3.3 ± 0.9 | 4.2 ± 1.0 | 0.037 |
| GCP (PREOP/PO1MO) | 61.5 ± 8.9/59.8 ± 9.3 | 62.4 ± 8.2/61.2 ± 8.3 | 56.1 ± 11.9/52.7 ± 11.2 | 0.148/0.037 |
| GDS (n, % ≥ 6) | 3, 0.083% | 1, 0.03% | 2, 33% | -* |
| CAM-S Peak | 3.1 ± 2.3 | 2.3 ± 1.2 | 6.8 ± 3.1 | <0.001 |
| CAM-S Sum | 5.6 ± 6.2 | 3.9 ± 2.7 | 14.2 ± 11.2 | <0.001 |
| Mean GM SUV60-90 (PREOP/PO1MO) | 1.0 ± 0.3/0.8 ± 0.2 | 1.1 ± 0.3/0.8 ± 0.2 | 0.9 ± 0.3/0.7 ± 0.2 | 0.118/0.230 |
| Mean WM SUV60-90 (PREOP/PO1MO) | 0.9 ± 0.2/0.7 ± 0.2 | 0.9 ± 0.2/0.7 ± 0.2 | 0.8 ± 0.3/0.7 ± 0.1 | 0.149/0.309 |
| Mean CSF SUV60-90 (PREOP/PO1MO) | 0.7 ± 0.2/0.7 ± 0.2 | 0.8 ± 0.2/0.7 ± 0.2 | 0.6 ± 0.2/0.6 ± 0.1 | 0.051/0.176 |
Note: PREOP = pre-operative baseline, PO1MO = post-operative 1-month follow-up, GCP = general cognitive performance, GSD = geriatric depression scale, CAM-S = Confusion Assessment Method-Severity, GM = gray matter, WM = white matter, CSF = cerebrospinal fluid, SUV = standardized uptake values. *p-value was not computed due to a small sample size.
Eligibility criteria for the RISE study enrollment required that each patient be 70 years of age or older, English speaking, and scheduled for hip or knee arthroplasty with planned spinal anesthesia conducted at the Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women’s Hospital (BWH), and Brigham and Women’s Faulkner Hospital (BWFH), all in Boston, MA. Inclusion criteria also required planned admission for at least 24 h and surgery scheduled at least 15 days in advance, to allow for baseline assessment and [11C]PBR28 PET/MR scan. Total hip and knee arthroplasties were targeted because of their delirium risk and frequent use of spinal anesthesia (Bruce et al., 2007), to avoid the potential confound of general anesthesia (Hines et al., 2013). Exclusion criteria included any active psychiatric disorders, total blindness, and contraindication to spinal anesthesia/lumbar puncture, MRI, or [11C]PBR28 PET (for details, see Hshieh et al., 2019). Importantly, all prospective patients were genotyped for the Ala147Thr polymorphism in the TSPO gene and those with predicted low-binding affinity for [11C]PBR28 (Thr/Thr) (Kreisl et al., 2013, Owen et al., 2012) were excluded. PET/MR data at both time points were collected at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH), Boston, MA. CSF data were obtained during the initiation of spinal anesthesia in the immediate pre-operative period at BIDMC, BWH, and BWFH; and at 1-month follow up at MGH. The Institutional Review Board of Partners Healthcare System (MGH, BWH, BWFH) approved all study procedures, with ceded review from BIDMC and Hebrew SeniorLife, the study coordinating center.
2.2. Neuropsychological and delirium assessment
Prior to the pre-operative baseline assessment, trained research staff conducted pre-screening evaluations involving telephone interview, medical record review, and safety screening for lumbar puncture and PET/MR imaging. The baseline face-to-face interview was performed in each patient’s home, which involved complete neuropsychological testing and delirium assessment. During their hospital stay, patients were assessed daily with 10–15 min interviews, which included brief cognitive testing and an adapted Delirium Symptom Interview (Albert et al., 1992) to rate delirium using the Confusion Assessment Method (CAM) (Inouye et al., 1990) and CAM-Severity (CAM-S) scoring (Inouye et al., 2014). The presence of delirium was determined by the CAM diagnostic algorithm, a standardized approach with high sensitivity (94–100%), specificity (90–95%) (Wei et al., 2008, Wong et al., 2010), and reliability. A follow-up interview with complete neuropsychological testing was conducted at 1 month after hospitalization. For a full list of measures collected during these sessions, see Hshieh et al., 2019). Delirium severity was determined using the CAM-S long form, which is based on the 10 features from the long CAM instrument to quantify the intensity of delirium features (Inouye et al., 2014). Scores on the CAM-S long form range from 0 to 19, with higher scores indicative of more severe delirium. Delirium severity was measured in the present study for each patient using both CAM-S peak (the highest single CAM-S rating observed during hospitalization) and CAM-S sum (the summed score across all hospital days), thereby capturing both delirium intensity and duration (Vasunilashorn et al., 2016).
2.3. PET/MR data acquisition
At pre-operative baseline (PREOP) and post-operative 1-month follow-up (PO1MO), all patients underwent integrated PET/MR scans using [11C]PBR28 as the radiotracer. Data were acquired on a Biograph mMR scanner (Siemens Healthineers, Erlangen, Germany) with a 16-channel head and neck receiver coil. [11C]PBR28 was synthesized on site (Imaizumi et al., 2007) and was administered as a slow bolus injection through an intravenous catheter. The mean (±standard deviation) dose of the radioligand was 535.02 ± 13.32 MBq for PREOP and 508.38 ± 72.15 MBq for PO1MO. PET images were acquired in list mode format for 60 min beginning 30 min post-injection. Simultaneously, 60 min of MRI data were collected using several sequences. Relevant to this study, morphological MR data were acquired using a T1-weighted 3D multi-echo magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (repetition time [TR] = 2530 ms, echo time [TE1-4] = 1.69/3.55/5.41/7.27 ms, inversion time [TI] = 1100 ms, flip angle = 7°, 256 mm field of view [FOV], 176 × 256 in-plane matrix, 1 mm isotropic voxels). Due to practical constraints, no arterial blood samples were acquired during the acquisition of PET/MR data.
2.4. PET/MR data analysis
PET images were reconstructed using the Ordinary Poisson Ordered Subset Expectation Maximization 3D algorithm (3 iterations, 21 subsets, 344 × 344 image matrix, with 2.1 mm in-plane pixel size and 2.0 mm slice thickness; 4 mm Gaussian filter) and applying all the required corrections using standard methods provided by the manufacturer. The head photon attenuation map was estimated from each patient’s MPRAGE volume using a combination of intensity- and prior-based tissue segmentation and atlas registration (Izquierdo-Garcia et al., 2014). [11C]PBR28 PET uptake images obtained from the data acquired 60–90 min post-radioligand injection were normalized to the injected dose and patient weight to obtain standardized uptake values (SUV60-90). We chose to use non-intensity-normalized SUV because of the within-subject nature of our study design and given the expectation that the signal changes due to surgery would be a global effect (Forsberg et al., 2017). Each patient’s MPRAGE data were used for automated volumetric segmentation of cortical and subcortical brain structures via the FreeSurfer image analysis suite, which is documented and freely available for download online (version 6.0, https://surfer.nmr.mgh.harvard.edu/). To correct for any head motion potentially occurring between the collection of the MPRAGE volume and PET data, the SUV60-90 image was co-registered to the T1 image acquired at the same time point for each patient.
Whole-brain voxel-wise analysis. To perform voxel-wise analysis of SUV data at the group level comparing the two time points, we normalized each patient’s SUV60-90 images to MNI152 space via a combined volumetric and surface registration algorithm, which is particularly sensitive to both cortical and subcortical alignment (Zollei et al., 2010). The resulting spatially-normalized (resampled to 2 mm isotropic voxels) images were skull-stripped and volumetrically smoothed using a full width at half maximum (FWHM) of 8 mm. Finally, we performed group-level random-effects t-tests on these images in SPM12 (version 7487, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) to identify brain regions exhibiting significant changes in [11C]PBR28 binding between PREOP and PO1MO. A voxel-wise intensity threshold of p < .005 corrected for family-wise error rate was used to assess statistical significance.
Regions of interest (ROI) analysis. As noted above, we predicted that major surgery would be associated with widespread changes in [11C]PBR28 binding in the brain. Nevertheless, complementing the voxel-wise analysis in standard template space, we additionally analyzed SUV data in each patient’s native space to characterize changes in [11C]PBR28 binding between the time points with increased anatomical specificity. Such combination of whole-brain voxel-wise and ROI-based analyses are consistent with the approach employed in previous investigations from our groups (e.g., Alshikho et al., 2018) and others (Forsberg et al., 2017) performing within-subject comparisons of brain [11C]PBR28 uptake. Based on each patient’s high-resolution tissue segmentation derived by the standard Desikan-Killany atlas in FreeSurfer (Desikan et al., 2006), the symmetric geometric transfer matrix (GTM) method was used to correct for spill-in and spill-out effects between adjacent brain tissue types (Greve et al., 2016, Greve et al., 2014). The mean SUV60-90 was computed for each of the 48 individual ROIs (averaged across hemispheres), which was then used to compute the weighted mean SUV60-90 of cortical/subcortical gray matter, white matter, and CSF, separately for PREOP and PO1MO. We then performed repeated-measures analyses of variance (ANOVAs) and t-tests to evaluate regional variability and specificity in the magnitude of changes in [11C]PBR28 binding between PREOP and PO1MO.
Furthermore, to examine the effect of post-operative delirium on [11C]PBR28 binding, we also conducted ROI-based analyses comparing delirious and non-delirious participants. Given the unequal group size, we first identified two subsets of non-delirious participants matched to the delirious group on sex and age (delirious: Mage = 74.7, SDage = 5.3; non-delirious1: Mage = 75.8, SDage = 2.5; non-delirious2: Mage = 77.3, SDage = 6.1; 5 females in all groups). The delirious group did not differ in age from either non-delirious group (non-delirious1 p = .95, non-delirious2 p = .54). We then performed a three-way mixed ANOVA with Group (delirious vs. non-delirious), Time point (pre vs. post), and Tissue class (cortex, subcortical gray matter, white matter, and CSF) as factors, comparing the mean SUV60-90 separately for each group pair (i.e., delirious vs. non-delirious1, delirious vs. non-delirious2).
Correlation analysis. To explore the possible association between post-operative delirium severity and surgery-related changes in [11C]PBR28 binding, we calculated across patients the Pearson’s r between a) peak and sum CAM-S scores, and b) [11C]PBR28 SUV60-90 before surgery, 1 month after surgery, and the change in [11C]PBR28 SUV60-90 from baseline to 1 month after surgery.
2.5. Immunoassays
Specimen collection and storage. Immediately prior to the radiotracer injection at each time point, 20 mL of blood was collected into one heparinized and one ethylenediaminetetraacetic acid (EDTA) tube (10 mL in each). During processing, plasma and cellular material were separated using low-speed centrifugation (1500 relative centrifugal force) and stored at −80 °C. CSF was acquired in the immediate pre-operative period during induction of spinal anesthesia (PREOP) and at PO1MO via lumbar puncture. CSF was collected by aspiration or dropwise collection directly into the collection tubes. To minimize potential contamination of CSF sample with blood, the sample was centrifuged at 1000 relative centrifugal force for 10 min to separate before storage in 0.5 mL aliquot tubes at −80 °C. Samples were stored at −80 °C in polypropylene tubes until analyzed.
Protein measurement. Plasma and CSF concentrations of the inflammatory proteins CRP, IL-6, and Chitinase 3-Like 1 glycoprotein (CHI3L1, also known as YKL-40 [tyrosine (Y), lysine (K), leucine (L) with molecular weight of 40]) from heparinized plasma and CSF samples at PREOP and PO1MO were measured via enzyme-linked immunosorbent assay (ELISA) platforms (Ella System, ProteinSimple, San Jose, CA; Meso Scale Discovery [MSD], Gaithersburg, MD). Plasma and CSF biomarkers of inflammatory response were analyzed via t-tests corrected for multiple comparisons. We also performed exploratory correlation analyses between gray matter SUV60-90 and plasma and CSF analytes.
Correlation analysis. To explore the possible link between the observed change in [11C]PBR28 binding and plasma/CSF biomarkers of inflammation, we performed a series of correlation analyses. For these analyses, we calculated the mean change in [11C]PBR28 SUV60-90 in gray matter (ΔSUV60-90) as well as the change in plasma and CSF concentrations of the three aforementioned inflammatory proteins.
3. Results
3.1. Decreased brain binding of [11C]PBR28 following surgery
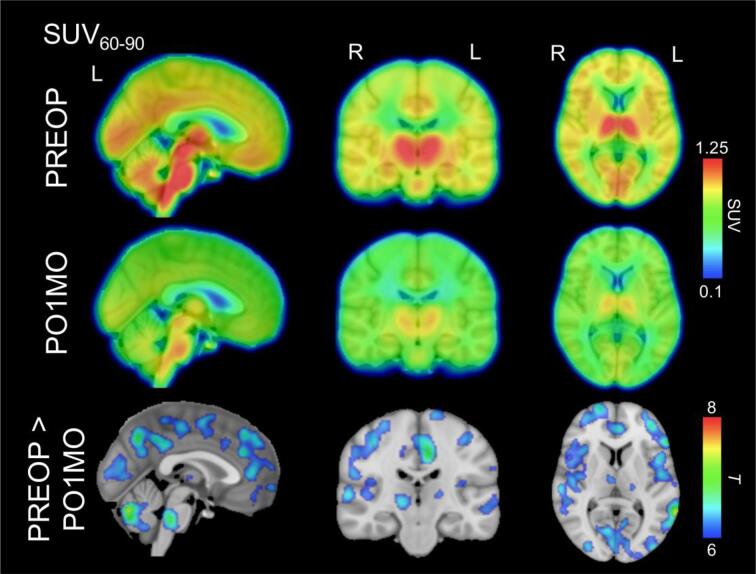
A paired t-test comparing the SUV60-90 images between the two time points revealed a number of regions exhibiting significant differences, including the thalamus, pons, cerebellum, medial frontal cortex, posteromedial cortex, middle and inferior frontal gyrus/insula, primary sensory and motor cortices, and superior temporal gyrus (Fig. 1).
Fig. 1.
[11C]PBR28 SUV60-90 images and statistical maps identifying differences between pre-operative and post-operative scans. The top and middle rows identify the mean [11C]PBR28 SUV60-90 images for the pre-operative baseline (PREOP) and post-operative 1-month follow-up visit (PO1MO), respectively, which are projected onto a high-resolution Montreal Neurological Institute (MNI152) template. The bottom row identifies brain regions exhibiting significantly reduced [11C]PBR28 binding for PO1MO compared with PREOP at pFWE < 0.005. For visualization purposes only, the SUV60-90 images and statistical maps were upsampled to match the underlay template resolution.
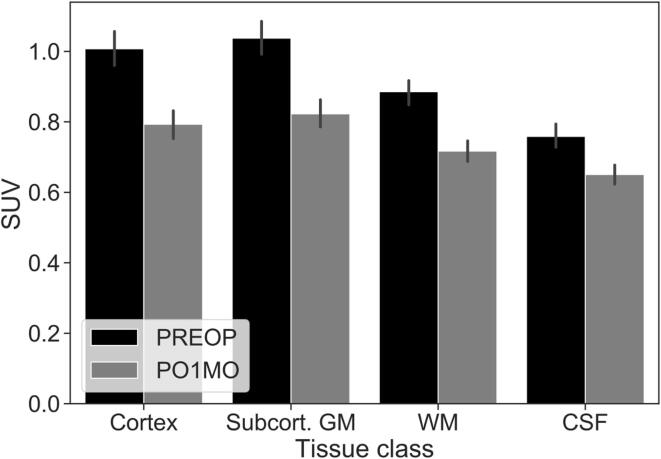
To complement the whole-brain voxel-wise analysis, we carried out ROI analysis to investigate the variability of the post-operative change in [11C]PBR28 binding across different brain tissue classes. A two-way ANOVA with tissue class and time point as factors revealed a significant main effect of tissue class [F(3, 105) = 123.00, p < .001, ηp2 = 0.778], of time point ([F(1, 35) = 30.94, p < .001, ηp2 = 0.469]), and a significant interaction between tissue class and time point ([F(3, 105) = 38.43, p < .001, ηp2 = 0.523]) (Fig. 2). To further interpret this interaction effect, we computed ΔSUV60-90 (i.e., PREOP – PO1MO) for each tissue class and analyzed the difference via post-hoc t-tests (Bonferroni corrected α = 0.05/6 tests = 0.008). The mean ± SD ΔSUV60-90 for each tissue class were as follows: Cortical gray matter (0.21 ± 0.21), subcortical gray matter (0.21 ± 0.22), white matter (0.17 ± 0.19), and CSF (0.11 ± 0.15). This analysis revealed that the mean ΔSUV60-90 was significantly different between every pair of tissue classes, except for cortical vs. subcortical gray matter (p = .955). Across all tissue classes, we observed a significant reduction in SUV60-90 from PREOP to PO1MO (Fig. 2).
Fig. 2.
Mean [11C]PBR28 SUV60-90 in the four major tissue classes. N = 36. SUV = standardized uptake values, PREOP = pre-operative baseline, PO1MO = post-operative 1-month follow-up, GM = gray matter, WM = white matter, CSF = cerebrospinal fluid. Error bars represent the standard error of the mean. The difference between PREOP and PO1MO is significant at p < .001 for every tissue class.
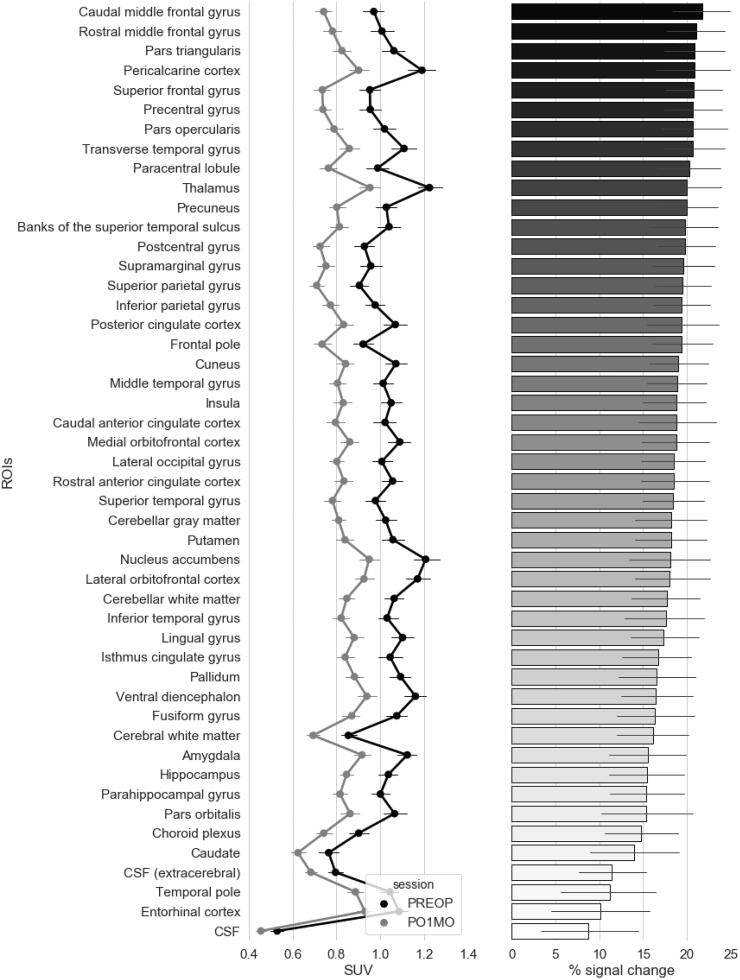
Next, we calculated the mean SUV60-90 for each individual ROI based on the standard Desikan-Killany atlas in FreeSurfer (Desikan et al., 2006) assembled into the GTM volumetric segmentation (Greve et al., 2016), which was then averaged across the hemispheres. A series of paired t-tests (Bonferroni corrected α = 0.05/48 tests = 0.001) revealed significant reduction in [11C]PBR28 binding in all regions, with the entorhinal cortex and CSF being the only ROIs that did not formally survive the threshold for significance after correction for multiple comparisons (p’s = 0.0015 and 0.008, respectively) (Fig. 3).
Fig. 3.
Mean [11C]PBR28 SUV60-90 and percent signal change in individual gray matter, white matter, and CSF regions of interest. The point and line plots on the left panel illustrate the magnitude of [11C]PBR28 SUV60-90 at pre-operative baseline (PREOP) and 1-month post-operative follow-up (PO1MO) in various regions of interest (ROIs) across the brain. These ROIs are rank-ordered by the magnitude of change in [11C]PBR28 SUV60-90 across the two timepoints expressed as percent, i.e., (PREOP – PO1MO) / PREOP × 100, from largest (top) to smallest (bottom). The corresponding % signal change for each ROI is depicted on the right panel. N = 36. Error bars represent the standard error of the mean.
3.2. Effect of post-operative delirium severity
Six patients developed delirium post-operatively (assessed during hospitalization); the delirium ratings were negative for all patients at post-operative 1-month follow-up. Characteristics of the non-delirious (n = 30) vs. delirious (n = 6) patients are summarized in Table 1. To examine potential group differences in [11C]PBR28 binding, we first conducted a three-way mixed ANOVA with Group (delirious vs. non-delirious), Time point (pre vs. post), and Tissue class (cortex, subcortical gray matter, white matter, and CSF) as factors. Results revealed neither a significant main effect of Group nor interactions involving this factor, suggesting that post-operatively delirious participants and non-delirious participants had similar [11C]PBR28 binding regardless of time point or tissue type (Table 2 and Table 3).
Table 2.
Mean [11C]PBR28 SUV60-90 in the four major tissue classes by delirium groups.
| Delirious | Non-delirious1 | Non-delirious2 | ||
|---|---|---|---|---|
| PREOP | ||||
| Cortex | 0.85 ± 0.32 | 0.92 ± 0.13 | 0.99 ± 0.23 | |
| Subcort. GM | 0.86 ± 0.30 | 0.96 ± 0.14 | 1.03 ± 0.22 | |
| WM | 0.77 ± 0.26 | 0.81 ± 0.10 | 0.89 ± 0.15 | |
| CSF | 0.61 ± 0.20 | 0.73 ± 0.12 | 0.76 ± 0.18 | |
| PO1MO | ||||
| Cortex | 0.69 ± 0.17 | 0.81 ± 0.21 | 0.82 ± 0.16 | |
| Subcort. GM | 0.72 ± 0.13 | 0.83 ± 0.19 | 0.85 ± 0.14 | |
| WM | 0.65 ± 0.10 | 0.71 ± 0.14 | 0.75 ± 0.12 | |
| CSF | 0.56 ± 0.08 | 0.66 ± 0.13 | 0.65 ± 0.13 | |
Note: n = 6 (5 females) in all subgroups. PREOP = pre-operative baseline, PO1MO = post-operative 1-month follow-up, subcort. GM = subcortical gray matter, WM = white matter, CSF = cerebrospinal fluid.
Table 3.
Results of three-way mixed analysis of variance.
| Delirious vs. Non-delirious1 | Delirious vs. Non-delirious2 | |||||
|---|---|---|---|---|---|---|
| Effect | F (df) | p | η2p | F (df) | p | η2p |
| Group | 1.10 (1,10) | 0.32 | 0.1 | 1.95 (1,10) | 0.19 | 0.16 |
| Time point | 3.65 (1,10) | 0.085 | 0.27 | 5.29 (1,10) | 0.04 | 0.35 |
| Tissue class | 35.63 (3,30) | <0.001 | 0.78 | 66.67 (3,30) | <0.001 | 0.87 |
| Group*Time point | 0.01 (1,10) | 0.91 | 0.001 | 0.08 (1,10) | 0.79 | 0.01 |
| Group*Tissue class | 0.82 (3,30) | 0.49 | 0.08 | 0.46 (3,30) | 0.71 | 0.04 |
| Time point*Tissue class | 8.11 (3,30) | <0.001 | 0.45 | 7.84 (3,30) | 0.001 | 0.44 |
| Group*Time point*Tissue class | 0.98 (3,30) | 0.42 | 0.09 | 1.22 (3,30) | 0.32 | 0.11 |
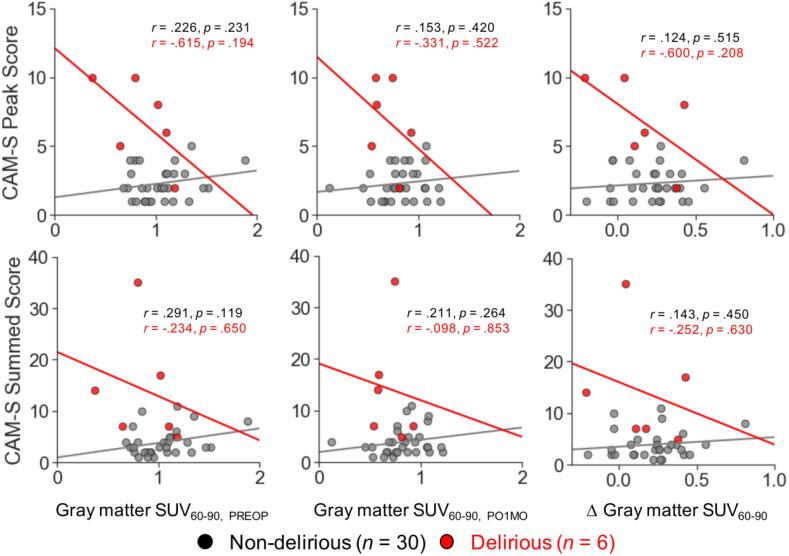
To explore the possible association between the magnitude of [11C]PBR28 binding and severity of post-operative delirium, we calculated the Pearson’s r between the mean gray matter SUV60-90 (computed based on all cortical and subcortical regions) and the CAM-S ratings. Following our previous work (Racine et al., 2017), we examined both CAM-S peak and sum scores. Upon visual inspection of scatter plots, we observed heteroscedasticity across the patient subgroups (i.e., delirious and non-delirious). Therefore, correlation analyses were performed separately for the non-delirious and delirious groups. These analyses identified no significant relationship between the mean gray matter SUV60-90 and delirium severity in either patient subgroup (Fig. 4). It is interesting to note, however, that the patient with the most severe delirium symptoms was among those showing the least reduction in [11C]PBR28 binding following surgery (top right, Fig. 4).
Fig. 4.
Correlation between delirium severity and [11C]PBR28 binding in gray matter. Regression lines identify the least-squares fit in the non-delirious (n = 30, gray) and delirious (n = 6, red) patients. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Effect of surgery on plasma and CSF biomarkers of inflammation
Finally, although our main focus was to examine changes in [11C]PBR28 binding following surgery, we also performed post hoc exploratory analyses of the plasma and CSF inflammatory biomarkers in the current sample. These analyses focused on plasma and CSF concentrations of three inflammatory proteins (CRP, IL-6, and YKL-40) and their measurements at PREOP and PO1MO. Intriguingly, compared to PREOP, elevated post-operative levels of IL-6 and YKL-40 were identified in plasma, and elevated post-operative levels of CRP were identified in CSF (Table 4). To examine the possible link between the observed reduction in [11C]PBR28 binding in the brain and these inflammatory biomarkers, we performed correlation analyses of gray matter ΔSUV60-90 with ΔIL-6 in plasma, ΔYKL-40 in plasma, and ΔCRP in CSF. These analyses identified no significant relationships (gray matter ΔSUV60-90 × ΔIL-6 in plasma: r = -0.061, p = .724; gray matter ΔSUV60-90 × ΔYKL-40 in plasma: r = -0.068, p = .694; gray matter ΔSUV60-90 and ΔCRP in CSF: r = -0.137, p = .517).
Table 4.
Concentrations of inflammatory proteins.
| PREOP | PO1MO | p-value | ||
|---|---|---|---|---|
| Plasma | ||||
| IL-6 (pg/mL) | 4.1 ± 1.7 | 7.0 ± 6.7 | 0.012 | |
| CRP (pg/mL) | 7991272.5 ± 12107737.0 | 9285735.6 ± 10495969.1 | 0.644 | |
| YKL-40 (pg/mL) | 95619.8 ± 86595.0 | 152204.2 ± 121945.4 | <0.001* | |
| CSF | ||||
| IL-6 (pg/mL) | 3.9 ± 1.6 | 8.3 ± 23.9 | 0.325 | |
| CRP (pg/mL) | 15132.4 ± 17809.2 | 33586.5 ± 39374.3 | 0.039 | |
| YKL-40 (pg/mL) | 291233.2 ± 101711.7 | 276455.6 ± 62818.2 | 0.401 | |
Note: IL-6 = interleukin-6, CRP = C-reactive protein, YKL-40 = also known as CHI3L1 [Chitinase 3-Like 1 glycoprotein], PREOP = pre-operative baseline, PO1MO = post-operative 1-month follow-up. *Significant at p < .008 Bonferroni-corrected for multiple comparisons.
4. Discussion
Because of the aging of the population and a burgeoning number of older persons undergoing major surgery (Hamel et al., 2005, Turrentine et al., 2006), substantial numbers of people are at risk for post-operative delirium and long-term cognitive decline. Given that neuroinflammation is thought to contribute to the pathogenesis of both (Cunningham, 2011, Marcantonio, 2012, Safavynia and Goldstein, 2019, Vasunilashorn et al., 2015), there is an urgent need to better understand the neural mechanisms underlying the inflammatory response triggered by major surgery, and how this relates to short- and long-term cognitive dysfunction. The present study contributes to the literature by demonstrating in a relatively sizeable sample for this type of imaging that, 1 month following major orthopedic surgery under spinal anesthesia, [11C]PBR28 binding in the brain is globally downregulated compared with pre-operative baseline. Converging evidence from the whole-brain voxel-wise and ROI analyses suggest that, although some tissue-related and regional variability exist, the observed reduction does not appear to be localized to specific regions. No significant associations were identified between [11C]PBR28 binding and delirium or between [11C]PBR28 binding and CSF/plasma biomarkers of inflammation. These findings are discussed further below.
Our whole-brain voxel-wise analysis identified a widespread reduction in [11C]PBR28 binding from pre-operative baseline to 1-month post-operative follow-up. Global downregulation of brain [11C]PBR28 binding was similarly identified in a small sample of older adult patients 3–4 days following abdominal surgery under general anesthesia (Forsberg et al., 2017). This has been interpreted as reflecting a period of anti-inflammatory responses following the initial pro-inflammatory phase and prior to the return to or increase above baseline at 3-month post-operative follow-up. It is therefore possible that the downregulation of [11C]PBR28 at 1-month post-operative follow-up observed in the present study reflects this state of immunosuppression persistent at this time point following surgery. Notably, there is evidence showing that reduction in [11C]PBR28 binding might reflect inhibition of neuroprotective microglial activation. In the presence of inflammation or disruption of blood–brain barrier, microglia that are usually inactive can differentiate into one of two activated phenotypes, pro-inflammatory M1 and anti-inflammatory M2 (Gordon, 2003, Martinez et al., 2009, Szalay et al., 2016, Thériault et al., 2015). This raises the intriguing possibility that higher TSPO levels/[11C]PBR28 binding do not always reflect a neuroinflammatory response. Indeed, there is evidence demonstrating that M1-predominant microglial activation did not increase TSPO levels in in-vitro human microglia (Owen et al., 2017), whereas M2-predominant microglial activation resulted in overexpression of TSPO in rodent microglial cells (Bae et al., 2014). These findings are in line with the interpretation that downregulation of [11C]PBR28 binding following surgery might reflect reduced activation or depletion of neuroprotective microglia leading to neuroimmune suppression. Moreover, these finding are also consistent with the conclusion of a recent study in which the authors identified decreased [11C]PBR28 binding (quantified as total volume of distribution, VT) among patients with post-traumatic stress disorder (Bhatt et al., 2020), a clinical condition typically associated with elevated levels of peripheral inflammatory markers (Passos et al., 2015). Post-operative impairments in immune activity have important clinical implications as they are associated with a heightened risk of developing post-operative infections and sepsis (Albertsmeier et al., 2017, O'Dwyer et al., 2015). The current results shed light on the potential importance of developing pharmacological interventions aimed at restoring microglial activation subserving neuroimmune system function during a post-operative recovery period.
Alternatively, the present findings may also in part be explained by other possibilities. For instance, in addition to the neuroimmunosuppression account above, it is possible that surgery and/or the use of anesthesia contributed to alterations in mitochondrial physiology. In neonatal rats, exposures to sevoflurane (an inhalational anesthetic commonly used in humans) have been shown to decrease mitochondrial density in hippocampal axon terminals by 20% (Amrock et al., 2015). In vitro studies have also confirmed that a number of common general and ancillary anesthetic agents inhibit mitochondrial function (Hsieh et al., 2017). Given that TSPO is a transmembrane protein located in the outer mitochondrial membrane (Papadopoulos et al., 2006), decreased [11C]PBR28 binding following surgery might reflect reduced mitochondrial activity.
Yet another possibility is that the reduction in [11C]PBR28 binding might reflect decreased availability of the radioligand passing through blood–brain barrier, thus highlighting the importance of concomitantly assessing plasma concentrations of [11C]PBR28. SUV is considered a reliable metric of [11C]PBR28 binding that shows high correlation with VT (Matheson et al., 2017, Yoder et al., 2015), which is the standard for quantification of [11C]PBR28 binding when arterial plasma data are available (Innis et al., 2007). However, the use of SUV depends on the assumption of no significant differences in radioligand delivery to the brain between time points of interest. It is possible that major surgery and ensuing hospitalization led to changes in brain blood flow, metabolism, protein binding, and/or peripheral TSPO binding, which cannot be completely ruled out as possibilities without arterial plasma sampling. Indeed, one study showed that brain [11C]PBR28 binding was reduced 4 h following the systemic administration of lipopolysaccharide (LPS; known to induce global inflammatory response) in a sample of non-human primates compared to pre-LPS baseline (Hannestad et al., 2012). However, when the authors accounted for the radioligand concentration in the arterial plasma, which had decreased to a greater extent due to the robust peripheral metabolism of [11C]PBR28, whole-brain [11C]PBR28 binding was found to increase from baseline to 4 h post-LPS. Given this evidence, it is possible that due to prolonged peripheral inflammation at post-operative 1-month follow-up, the amount of [11C]PBR28 that is available to enter the brain is decreased compared with pre-operative baseline, thus resulting in a relative reduction of [11C]PBR28 binding. We cannot formally test this possibility given that we were not able to obtain arterial blood samples from the participants in this study due to practical constraints. However, it is important to note that none of the peripheral inflammatory marker levels was significantly correlated with the observed change in gray matter [11C]PBR28 binding, supporting our interpretation that the observed effects are not merely driven by the limited availability of [11C]PBR28 to be detected by brain PET. Nevertheless, future work should examine the replicability of the current results through techniques that can account for the aforementioned factors that might influence overall [11C]PBR28 binding in the brain (Innis et al., 2007).
Contrary to our hypothesis, we did not find evidence in support of a relationship between delirium status or severity and [11C]PBR28 binding. The small number of patients who developed delirium post-operatively (n = 6) made it difficult to examine a potential association with [11C]PBR28 binding. Future studies should make every effort to balance the distribution of non-delirious vs. delirious cases by, for instance, oversampling patients at higher risk for post-operative delirium based on prior risk stratification approaches (Devore et al., 2017, Marcantonio et al., 1994) and neuropsychological characteristics (Fong et al., 2015). Another approach might be to employ a nested case-control study design in which all delirium cases would be studied with a matched set of non-delirious control participants.
Finally, our exploratory analyses of plasma and CSF biomarkers of inflammation identified significant increases in the concentrations of IL-6 and YKL-40 in plasma and of CRP in CSF from pre-operative baseline to post-operative follow-up at 1 month. In our previous work in the Successful Aging after Elective Surgery (SAGES) Study, in which we examined a larger pool of patients (N = 566) undergoing wider types of surgery under general or spinal anesthesia (Schmitt et al., 2012, Schmitt et al., 2015), we did not observe a significant change in plasma IL-6 from pre-operative baseline to post-operative follow-up at 1 month. Instead, among the four time points (at pre-operative baseline, post-anesthesia care unit, post-operative day 2, and post-operative 1-month follow-up), post-operative increases in plasma IL-6 and CRP peaked at post-operative day 2 and then returned to baseline levels at the post-operative 1-month follow-up (Dillon et al., 2017, Vasunilashorn et al., 2015). It is unclear why levels of some inflammatory biomarkers remained elevated post-operatively at 1 month in the present sample. It should be noted that the levels of plasma IL-6 at the post-operative 1-month follow-up were comparable to those we previously identified in a larger sample at the same time point (Vasunilashorn et al., 2015). This allows us to rule out the possibility that the current sample who had undergone [11C]PBR28 PET scans exhibited particularly sustained levels of peripheral inflammation 1 month following surgery relative to an independent, larger sample. Nevertheless, these seemingly disparate findings underscore the importance of further examining the role of systemic inflammation and neuroinflammation in post-operative delirium, with a particular focus on elucidating the complex relationship between [11C]PBR28 PET, plasma, and CSF biomarkers of inflammation across different time points. Clarification of this issue is particularly important given that the concentration of YKL-40 in CSF, which has recently emerged as a neuroinflammatory biomarker (reviewed in Baldacci et al., 2019), did not show changes across time points in the current study.
A few potential limitations of the present study should be acknowledged. Although incidentally, the present sample included a disproportionately greater number of females than males. It is important to note, however, that prior work examining the effect of surgery on [11C]PBR28 binding in human involved only males (Forsberg et al., 2017). Thus, our results identifying a significant effect of major orthopedic surgery based on a mixed-sex sample make an important addition to the literature. Nevertheless, future work should make every effort to include similar numbers of females and males in the sample, given recent evidence identifying significant differences between sexes in [11C]PBR28 binding among healthy subjects (Tuisku et al., 2019). In addition, ROI analyses comparing differences in [11C]PBR28 binding revealed that a significant reduction from pre-operative baseline to post-operative 1 month follow up was also observed in CSF, although the magnitude of change was significantly smaller compared with both gray and white matter. This suggests that some of the signal difference observed in the parenchyma may reflect non-specific binding and/or unbound radioligand, which may be due to the limited radioligand availability for the central nervous system as discussed above. It would be critical for future work to obtain dynamic PET data with radiometabolite-corrected arterial input function and a correction for plasma free fraction to address this potential concern. Another important limitation is the lack of information on other medications received in the perioperative period, which may have confounded the results. Finally, despite our use of a well-validated approach for detection, we acknowledge that delirium is a fluctuating condition and any measurement approach may have false negatives, contributing to potential measurement error.
Notwithstanding these potential limitations, the new evidence identified in the present study sheds light on the possible mechanisms through which surgery contributes to prolonged suppression of TSPO expression and highlights the need for future work to assess the time-dependent interaction of central vs. peripheral indices of inflammation as well as its modulation in a balanced sample of delirious and non-delirious participants.
CRediT authorship contribution statement
Yuta Katsumi: Software, Formal analysis, Data curation, Writing - original draft, Visualization. Annie M. Racine: Investigation, Data curation, Writing - original draft. Angel Torrado-Carvajal: Software, Resources, Writing - review & editing. Marco L. Loggia: Resources, Writing - review & editing. Jacob M. Hooker: Resources, Writing - review & editing. Douglas N. Greve: Software, Resources. Baileigh G. Hightower: Data curation. Ciprian Catana: Resources, Software, Writing - review & editing. Michele Cavallari: Resources, Writing - review & editing. Steven E. Arnold: Conceptualization, Methodology, Investigation, Resources, Data curation. Tamara G. Fong: Conceptualization, Methodology, Investigation. Sarinnapha M. Vasunilashorn: Resources, Writing - review & editing. Edward R. Marcantonio: Conceptualization, Methodology, Investigation, Writing - review & editing. Eva M. Schmitt: Conceptualization, Supervision, Project administration. Guoquan Xu: Investigation, Data curation. Towia A. Libermann: Conceptualization, Methodology, Investigation. Lisa Feldman Barrett: Resources. Sharon K. Inouye: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision, Funding acquisition. Bradford C. Dickerson: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision, Funding acquisition. Alexandra Touroutoglou: Formal analysis, Visualization, Writing - original draft, Supervision, Project administration. Jessica A. Collins: Formal analysis, Visualization, Writing - original draft, Supervision, Project administration.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Role of Inflammation after Surgery in Elders (RISE) study. This work was supported by grants from the Alzheimer’s Drug Discovery Foundation (SKI) and 2P01AG031720 (SKI) from the National Institute on Aging. Other support of investigator time was provided by grants no. K07AG041835 (SKI), R24AG054259 (SKI), R01AG056015 (BCD), R01AG051658 (ERM/TAL), K24AG035075 (ERM), K01AG057836 (SMV), and R03AG061582 (SMV) from the National Institute on Aging, grant no. AARF-18- 560786 (SMV) from the Alzheimer’s Association, and grant no. 1R01NS094306-01A1 (MLL) from the National Institute of Neurological Disorders and Stroke, and the JSPS Overseas Research Fellowships, Japan Society for the Promotion of Science (YK). Dr. Inouye holds the Milton and Shirley F. Levy Family Chair at Hebrew SeniorLife/Harvard Medical School.
RISE Study Group
[Presented in alphabetical order; individuals listed may be part of multiple groups, but are listed only once under major activity, listed in parentheses].
Principal Investigator: Sharon K. Inouye, MD, MPH (Overall PI; HSL, BIDMC, HMS).
Executive Committee: Steven Arnold, MD (MGH); Bradford Dickerson, MD (MGH Site PI, HMS); Tamara Fong, MD, PhD (HMS, HSL, BIDMC); Richard Jones, ScD (Brown University); Towia A. Libermann, PhD (HMS, BIDMC); Edward R. Marcantonio, MD, SM (BIDMC Site PI, HMS); Thomas Travison, PhD (HSL, HMS).
Co-Investigators: Becky C. Carlyle, PhD (HMS, MGH); Michele Cavallari, MD (BWH); Simon T. Dillon, PhD (HMS, BIDMC); Jacob Hooker, PhD, (MGH, HMS); Tammy Hshieh, MD, MPH (BWH); Savannah Kandigian, BA (MGH); Pia Kivisakk-Webb, MD, PhD (MGH), Long Ngo, PhD (HMS, BIDMC), Hasan Otu, PhD (UNL); Eva M. Schmitt, PhD (Overall Project Director, HSL); Alexandra Touroutoglou, PhD (HMS, MGH); Bianca Trombetta (MGH); Sarinnapha Vasunilashorn, PhD (BIDMC).
Surgical Leaders: Ayesha Abdeen, MD (HMS, BIDMC); Douglas Ayres, MD (HMS, BIDMC); Brandon Earp, MD (HMS, BWH); Jeffrey Lange, MD (HMS, BWH).
Surgeons: Gregory Brick, MBCHB (HMS, BWH); Antonia Chen, MD (HMS, BWH); Robert Davis, MD (HMS, BIDMC); Jacob Drew, MD (HMS, BIDMC); Richard Iorio, MD (HMS, BWH); Fulton Kornack, MD (HMS, BWH); Michael Weaver, MD (HMS, BWH); Anthony Webber, MD (HMS, BWH); Richard Wilk, MD (HMS, BWH).
Anesthesiology Leaders: Lisa Kunze, MD (BIDMC, HMS); David Shaff, MD (BWH, HMS); Kamen Vlassakov, MD (BWH, HMS).
Epidemiology Core: Brett Armstrong, MPH (BIDMC); Angelee Banda, MA (BIDMC); Sylvie Bertrand, BS (HSL); Madeline D’Aquila (HSL); Jacqueline Gallagher, MS (BIDMC); Baileigh Hightower, BA (MGH); Shannon Malloy, MA (BIDMC); Jacqueline Nee, BA (HSL); Chloe Nobuhara (MGH); Abigail Overstreet, MA (BIDMC); Annie Racine, PhD (HSL); David Urick (MGH); Guoquan Xu, MD, PhD (HSL).
Biomedical Imaging Core: Grae Arabasz (MGH); Michael Brickhouse (MGH); Regan Butterfield (MGH); Shirley Hsu (MGH); Sara Makaretz (MGH); Judit Sore (MGH).
Data Management and Statistical Analysis Core: Fan Chen, MPH, MS (HSL); Yun Gou, MA (HSL); Douglas Tommet, MS (Brown University).
Fiscal Management Committee: Sabrina Carretie (HSL); Katherine Tasker (Chair, HSL).
Footnotes
The one month time period was chosen for a follow-up for both conceptual and logistical reasons. Conceptually, our prior studies have shown cognitive decline at one month post-surgery (Inouye et al., 2016), and investigating the possibility of neuroinflammation at this time point appeared relevant. Logistically, patients reported that returning sooner than one month would have been prohibitive due to their rehabilitation needs and mobility concerns during the post-operative period.
References
- Alam A., Hana Z., Jin Z., Suen K.C., Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.M., Lee J., Lee S.Y. Recent progress in the development of TSPO PET ligands for neuroinflammation imaging in neurological diseases. Nucl. Med. Mol. Imaging. 2017;51(4):283–296. doi: 10.1007/s13139-017-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.S., Levkoff S.E., Reilly C., Liptzin B., Pilgrim D., Cleary P.D., Rowe J.W. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J. Geriatric Psychiatry Neurol. 1992;5(1):14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- Albertsmeier M., Prix N.J., Winter H., Bazhin A., Werner J., Angele M.K. Monocyte-dependent suppression of T-cell function in postoperative patients and abdominal sepsis. Shock. 2017;48(6):651–656. doi: 10.1097/shk.0000000000000924. [DOI] [PubMed] [Google Scholar]
- Albrecht D.S., Granziera C., Hooker J.M., Loggia M.L. In vivo imaging of human neuroinflammation. ACS Chem. Neurosci. 2016;7(4):470–483. doi: 10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshikho M.J., Zurcher N.R., Loggia M.L., Cernasov P., Chonde D.B., Izquierdo Garcia D., Atassi N. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology. 2016;87(24):2554–2561. doi: 10.1212/wnl.0000000000003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshikho M.J., Zürcher N.R., Loggia M.L., Cernasov P., Reynolds B., Pijanowski O., Atassi N. Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Ann. Neurol. 2018;83(6):1186–1197. doi: 10.1002/ana.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrock L.G., Starner M.L., Murphy K.L., Baxter M.G. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122(1):87–95. doi: 10.1097/aln.0000000000000477. [DOI] [PubMed] [Google Scholar]
- Bae K.R., Shim H.J., Balu D., Kim S.R., Yu S.W. Translocator protein 18 kDa negatively regulates inflammation in microglia. J. Neuroimmune Pharmacol. 2014;9(3):424–437. doi: 10.1007/s11481-014-9540-6. [DOI] [PubMed] [Google Scholar]
- Baldacci F., Lista S., Palermo G., Giorgi F.S., Vergallo A., Hampel H. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: advances in development. Expert Rev Proteomics. 2019;16(7):593–600. doi: 10.1080/14789450.2019.1628643. [DOI] [PubMed] [Google Scholar]
- Barrientos R.M., Hein A.M., Frank M.G., Watkins L.R., Maier S.F. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J. Neurosci. 2012;32(42):14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beishuizen S.J., Scholtens R.M., Vellekoop A.E., Vrouenraets B.C., Westhoff D., van de Beek D., van Munster B.C. Timing Is Critical in Determining the Association Between Delirium and S100 Calcium-Binding Protein B. J. Am. Geriatr. Soc. 2015;63(10):2212–2214. doi: 10.1111/jgs.13696. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Hillmer A.T., Girgenti M.J., Rusowicz A., Kapinos M., Nabulsi N., Traumatic Stress Brain Study G. PTSD is associated with neuroimmune suppression: evidence from PET imaging and postmortem transcriptomic studies. Nat. Commun. 2020;11(1):2360. doi: 10.1038/s41467-020-15930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromander S., Anckarsater R., Kristiansson M., Blennow K., Zetterberg H., Anckarsater H., Wass C.E. Changes in serum and cerebrospinal fluid cytokines in response to non-neurological surgery: an observational study. J Neuroinflammation. 2012;9:242. doi: 10.1186/1742-2094-9-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.K., Fujita M., Fujimura Y., Liow J.S., Stabin M., Ryu Y.H., Innis R.B. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J. Nucl. Med. 2007;48(12):2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- Bruce A.J., Ritchie C.W., Blizard R., Lai R., Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int. Psychogeriatr. 2007;19(2):197–214. doi: 10.1017/S104161020600425X. [DOI] [PubMed] [Google Scholar]
- Buvanendran A., Kroin J.S., Berger R.A., Hallab N.J., Saha C., Negrescu C., Tuman K.J. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104(3):403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Cagnin A., Kassiou M., Meikle S.R., Banati R.B. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics. 2007;4(3):443–452. doi: 10.1016/j.nurt.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape E., Hall R.J., van Munster B.C., de Vries A., Howie S.E., Pearson A., MacLullich A.M. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1beta in delirium after hip fracture. J. Psychosom. Res. 2014;77(3):219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M., Fidalgo A.R., Terrando N., Ma D., Monaco C., Feldmann M., Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese G.P., Burger C. Neuroinflammatory challenges compromise neuronal function in the aging brain: Postoperative cognitive delirium and Alzheimer's disease. Behav. Brain Res. 2017;322(Pt B):269–279. doi: 10.1016/j.bbr.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem. Soc. Trans. 2011;39(4):945–953. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devore E.E., Fong T.G., Marcantonio E.R., Schmitt E.M., Travison T.G., Jones R.N., Inouye S.K. Prediction of Long-term Cognitive Decline Following Postoperative Delirium in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72(12):1697–1702. doi: 10.1093/gerona/glx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S.T., Vasunilashorn S.M., Ngo L., Otu H.H., Inouye S.K., Jones R.N., Libermann T.A. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: A longitudinal nested case-control study. Biol. Psychiatry. 2017;81(2):145–153. doi: 10.1016/j.biopsych.2016.03.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R., Sun L., Lu Y., Yang X., Peng M., Zhang Z. NeurimmiRs and postoperative delirium in elderly patients undergoing total hip/knee replacement: A pilot study. Front. Aging Neurosci. 2017;9:200. doi: 10.3389/fnagi.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont A.C., Largeau B., Santiago Ribeiro M.J., Guilloteau D., Tronel C., Arlicot N. Translocator protein-18 kDa (TSPO) positron emission tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int. J. Mol. Sci. 2017;18(4) doi: 10.3390/ijms18040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T., Gimenez-Cassina A., Codeluppi S., Fernandez-Zafra T., Katsu-Jimenez Y., Terrando N., Gomez-Galan M. Disrupted neuroglial metabolic coupling after peripheral surgery. J. Neurosci. 2018;38(2):452–464. doi: 10.1523/JNEUROSCI.1797-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T.G., Hshieh T.T., Wong B., Tommet D., Jones R.N., Schmitt E.M., Inouye S.K. Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J. Am. Geriatr. Soc. 2015;63(5):977–982. doi: 10.1111/jgs.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Cervenka S., Jonsson Fagerlund M., Rasmussen L.S., Zetterberg H., Erlandsson Harris H., Eriksson L.I. The immune response of the human brain to abdominal surgery. Ann. Neurol. 2017;81(4):572–582. doi: 10.1002/ana.24909. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Salat D.H., Bowen S.L., Izquierdo-Garcia D., Schultz A.P., Catana C., Johnson K.A. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–343. doi: 10.1016/j.neuroimage.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Svarer C., Fisher P.M., Feng L., Hansen A.E., Baare W., Knudsen G.M. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–236. doi: 10.1016/j.neuroimage.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R.J., Ferguson K.J., Andrews M., Green A.J., White T.O., Armstrong I.R., MacLullich A.M. Delirium and cerebrospinal fluid S100B in hip fracture patients: a preliminary study. Am J Geriatr Psychiatry. 2013;21(12):1239–1243. doi: 10.1016/j.jagp.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Hamel M.B., Henderson W.G., Khuri S.F., Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J. Am. Geriatr. Soc. 2005;53(3):424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- Hannestad J., Gallezot J.D., Schafbauer T., Lim K., Kloczynski T., Morris E.D., Cosgrove K.P. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63(1):232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjum K., Quist-Paulsen E., Zetterberg H., Blennow K., Nilsson L.N.G., Watne L.O. CSF sTREM2 in delirium-relation to Alzheimer's disease CSF biomarkers Abeta42, t-tau and p-tau. J Neuroinflammation. 2018;15(1):304. doi: 10.1186/s12974-018-1331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz E., Gianni C., Louapre C., Treaba C.A., Govindarajan S.T., Ouellette R., Mainero C. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann. Neurol. 2016;80(5):776–790. doi: 10.1002/ana.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines C.S., Fujita M., Zoghbi S.S., Kim J.S., Quezado Z., Herscovitch P., Innis R.B. Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J. Nucl. Med. 2013;54(1):64–69. doi: 10.2967/jnumed.112.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Vacas S., Terrando N., Yuan M., Sands L.P., Kramer J., Leung J.M. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J. Neuroinflamm. 2016;13(1):211. doi: 10.1186/s12974-016-0681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hov K.R., Bolstad N., Idland A.V., Zetterberg H., Blennow K., Chaudhry F.A., Watne L.O. Cerebrospinal Fluid S100B and Alzheimer's Disease Biomarkers in Hip Fracture Patients with Delirium. Dement. Geriatr. Cogn. Dis. Extra. 2017;7(3):374–385. doi: 10.1159/000481853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens I.B., van Leeuwen B.L., Nyakas C., Heineman E., van der Zee E.A., Schoemaker R.G. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol. Learn. Mem. 2015;118:74–79. doi: 10.1016/j.nlm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Hshieh T.T., Vasunilashorn S.M., D'Aquila M.L., Arnold S.E., Dickerson B.C., Fong T.G., Group R.S. The Role of Inflammation after Surgery for Elders (RISE) study: Study design, procedures, and cohort profile. Alzheimers Dement. (Amst) 2019;11:752–762. doi: 10.1016/j.dadm.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh V.C., Krane E.J., Morgan P.G. Mitochondrial Disease and Anesthesia. J. Inborn Errors Metab. Screen. 2017;5 doi: 10.1177/2326409817707770. 2326409817707770. [DOI] [Google Scholar]
- Imaizumi M., Kim H.J., Zoghbi S.S., Briard E., Hong J., Musachio J.L., Fujita M. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neurosci. Lett. 2007;411(3):200–205. doi: 10.1016/j.neulet.2006.09.093. [DOI] [PubMed] [Google Scholar]
- Innis R.B., Cunningham V.J., Delforge J., Fujita M., Gjedde A., Gunn R.N., Carson R.E. Consensus Nomenclature for in vivo Imaging of Reversibly Binding Radioligands. J. Cereb. Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Inouye S.K., Kosar C.M., Tommet D., Schmitt E.M., Puelle M.R., Saczynski J.S., Jones R.N. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann. Intern. Med. 2014;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S.K., Marcantonio E.R., Kosar C.M., Tommet D., Schmitt E.M., Travison T.G., Jones R.N. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Garcia D., Hansen A.E., Forster S., Benoit D., Schachoff S., Furst S., Catana C. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J. Nucl. Med. 2014;55(11):1825–1830. doi: 10.2967/jnumed.113.136341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M.H., Yuan H.M., Zhang G.F., Li X.M., Dong L., Li W.Y., Yang J.J. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2013;27(2):236–242. doi: 10.1007/s00540-012-1506-3. [DOI] [PubMed] [Google Scholar]
- Jones R.N., Rudolph J.L., Inouye S.K., Yang F.M., Fong T.G., Milberg W.P., Marcantonio E.R. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J. Clin. Exp. Neuropsychol. 2010;32(10):1041–1049. doi: 10.1080/13803391003662728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl W.C., Jenko K.J., Hines C.S., Lyoo C.H., Corona W., Morse C.L., Innis R.B. A Genetic Polymorphism for Translocator Protein 18 Kda Affects both in Vitro and in Vivo Radioligand Binding in Human Brain to this Putative Biomarker of Neuroinflammation. J. Cereb. Blood Flow Metab. 2013;33(1):53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S., Guillermier M., Herard A.S., Petit F., Delahaye M., Van Camp N., Escartin C. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Pan K., Chen L., Ning J.L., Li X., Yang T., Tao G. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J Neuroinflammation. 2016;13(1):268. doi: 10.1186/s12974-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yu Y., Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS ONE. 2018;13(4) doi: 10.1371/journal.pone.0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia M.L., Chonde D.B., Akeju O., Arabasz G., Catana C., Edwards R.R., Hooker J.M. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado J.R. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Marcantonio E.R. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E.R., Goldman L., Mangione C.M., Ludwig L.E., Muraca B., Haslauer C.M. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. https://www.ncbi.nlm.nih.gov/pubmed/8264068 Retrieved from. [PubMed] [Google Scholar]
- Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Matheson G.J., Plavén-Sigray P., Forsberg A., Varrone A., Farde L., Cervenka S. Assessment of simplified ratio-based approaches for quantification of PET [(11)C]PBR28 data. EJNMMI Res. 2017;7(1):58. doi: 10.1186/s13550-017-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dwyer M.J., Owen H.C., Torrance H.D. The perioperative immune response. Curr Opin Crit Care. 2015;21(4):336–342. doi: 10.1097/mcc.0000000000000213. [DOI] [PubMed] [Google Scholar]
- Owen D.R., Narayan N., Wells L., Healy L., Smyth E., Rabiner E.A., Moore C.S. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J. Cereb. Blood Flow Metab. 2017;37(8):2679–2690. doi: 10.1177/0271678x17710182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.R., Yeo A.J., Gunn R.N., Song K., Wadsworth G., Lewis A., Rubio J.P. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Baraldi M., Guilarte T.R., Knudsen T.B., Lacapere J.J., Lindemann P., Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27(8):402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Kauer-Sant'Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/s2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Racine A.M., Fong T.G., Travison T.G., Jones R.N., Gou Y., Vasunilashorn S.M., Dickerson B.C. Alzheimer's-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol. Aging. 2017;59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsfelt B., Ricksten S.E., Zetterberg H., Blennow K., Freden-Lindqvist J., Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann. Thorac. Surg. 2012;94(2):549–555. doi: 10.1016/j.athoracsur.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Safavynia S.A., Goldstein P.A. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front. Psychiatry. 2019;9:752–10.3389/fpsyt.2018.00752. doi: 10.3389/fpsyt.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Maze M. Impact on the brain of the inflammatory response to surgery. La Presse Médicale. 2018;47(4):e73–e81. doi: 10.1016/j.lpm.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E.M., Marcantonio E.R., Alsop D.C., Jones R.N., Rogers S.O., Jr., Fong T.G., Inouye S.K. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J. Am. Med. Dir. Assoc. 2012;13(9) doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E.M., Saczynski J.S., Kosar C.M., Jones R.N., Alsop D.C., Fong T.G., Inouye S.K. The successful aging after elective surgery (SAGES) Study: cohort description and data quality procedures. J. Am. Geriatr. Soc. 2015;63(12):2463–2471. doi: 10.1111/jgs.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvarc D.R., Berk M., Byrne L.K., Dean O.M., Dodd S., Lewis M., Gray L. Post-Operative Cognitive Dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Subramaniyan S., Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 2019;128(4):781–788. doi: 10.1213/ane.0000000000004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., Dénes Á. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.X., Baranov D., Hammond M., Shaw L.M., Eckenhoff M.F., Eckenhoff R.G. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115(4):727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N., Eriksson L.I., Ryu J.K., Yang T., Monaco C., Feldmann M., Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011;70(6):986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thériault P., ElAli A., Rivest S. The dynamics of monocytes and microglia in Alzheimer's disease. Alzheimers Res Ther. 2015;7(1):41. doi: 10.1186/s13195-015-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuisku J., Plavén-Sigray P., Gaiser E.C., Airas L., Al-Abdulrasul H., Brück A., Cervenka S. Effects of age, BMI and sex on the glial cell marker TSPO - a multicentre [(11)C]PBR28 HRRT PET study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(11):2329–2338. doi: 10.1007/s00259-019-04403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrentine F.E., Wang H., Simpson V.B., Jones R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. The Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- van Harten A.E., Scheeren T.W., Absalom A.R. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S.M., Dillon S.T., Inouye S.K., Ngo L.H., Fong T.G., Jones R.N., Marcantonio E.R. High C-reactive protein predicts delirium incidence, duration, and feature severity after major noncardiac surgery. J. Am. Geriatr. Soc. 2017;65(8):e109–e116. doi: 10.1111/jgs.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S.M., Marcantonio E.R., Gou Y., Pisani M.A., Travison T.G., Schmitt E.M., Inouye S.K. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. J. Gen. Intern. Med. 2016;31(10):1164–1171. doi: 10.1007/s11606-016-3671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S.M., Ngo L., Inouye S.K., Libermann T.A., Jones R.N., Alsop D.C., Marcantonio E.R. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J. Gerontol. Series a-Biol. Sci. Med. Sci. 2015;70(10):1289–1295. doi: 10.1093/gerona/glv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S.M., Ngo L.H., Chan N.Y., Zhou W., Dillon S.T., Otu H.H., Marcantonio E.R. Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(2):261–268. doi: 10.1093/gerona/gly036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.A., Fearing M.A., Sternberg E.J., Inouye S.K. The Confusion Assessment Method: a systematic review of current usage. J. Am. Geriatr. Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff D., Witlox J., van Aalst C., Scholtens R.M., de Rooij S.E., van Munster B.C., Koenderman L. Preoperative protein profiles in cerebrospinal fluid in elderly hip fracture patients at risk for delirium: A proteomics and validation study. BBA Clin. 2015;4:115–122. doi: 10.1016/j.bbacli.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.L., Holroyd-Leduc J., Simel D.L., Straus S.E. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779–786. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- Xu J., Dong H., Qian Q., Zhang X., Wang Y., Jin W., Qian Y. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav. Brain Res. 2017;332:145–153. doi: 10.1016/j.bbr.2017.05.066. [DOI] [PubMed] [Google Scholar]
- Yoder K.K., Territo P.R., Hutchins G.D., Hannestad J., Morris E.D., Gallezot J.D., Cosgrove K.P. Comparison of standardized uptake values with volume of distribution for quantitation of [(11)C]PBR28 brain uptake. Nucl. Med. Biol. 2015;42(3):305–308. doi: 10.1016/j.nucmedbio.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Jiang W., Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience. 2014;261:1–10. doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Lai R., Li J., Zuo Z. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. Brain Behav. Immun. 2017;61:365–374. doi: 10.1016/j.bbi.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollei L., Stevens A., Huber K., Kakunoori S., Fischl B. Improved tractography alignment using combined volumetric and surface registration. Neuroimage. 2010;51(1):206–213. doi: 10.1016/j.neuroimage.2010.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]