Abstract
Introduction
The central nervous system (CNS) is the most metabolically active organ characterized by high oxygen demand and relatively low anti-oxidative activity, which makes neurons and glia highly susceptible to damage by reactive oxygen and nitrogen byproducts as well as neurodegeneration. Free radicals are associated with secondary injuries that occur after a primary brain injury. Some of these free radical products include F2-Isoprostane (F2-IsoPs), malondialdehyde (MDA), 4-hydroxy-2-nonenal (4-HNE) and acrolein.
Methods
In this study we measured serum F2-IsoPs levels as markers of free radical activity in 10–12 week-old male Sprague-Dawley rats weighing 200–300 g, all rats (n = 10) subjected with a head injury according to the modified marmourou model, then divided into 2 groups, one group treated with CAPE (Caffeic Acid Phenethyl Ester) (n = 5) and the other not treated with CAPE (n = 5), serum levels in the two groups were compared starting from day-0 (before brain injury), day-4 and day-7.
Results
We found lower F2-IsoPs levels in the group that received the CAPE treatment compared to the group that did not receive the CAPE treatment.
Conclusion
CAPE is capable of significantly reducing oxidative stress in brain injury.
Keywords: Traumatic brain injury, F2-isoprostane, Oxidative stress
Highlights
-
•
The central nervous system (CNS) is the most metabolically active organ characterized by high oxygen demand and relatively low anti-oxidative activity.
-
•
Free radicals are associated with secondary injuries that occur after a primary brain injury.
-
•
Caffeic Acid Phenethyl Ester (CAPE) administration in a rat model with brain injury can reduce the formation of F2-Isoprostane (F2-IsoPs) as an indicator of oxidative stress in blood serum post-trauma.
1. Introduction
Oxidative stress is a condition caused by a shift between pro-oxidants to antioxidants that can create organic damage [1]. Pro-oxidant by definition is a free radical, single or grouped atoms can be either paired with electrons or unpaired, while antioxidants are chemical compounds that can bind to free radicals so as not to cause cell damage [1,2]. Oxidative stress can be caused by several conditions such as inflammation, carcinogenesis, neurodegeneration, growth process and trauma to the CNS [[1], [2], [3]].
The brain is a part of the CNS. It is very susceptible to oxidative stress due its high oxygen demand. About 20–30% of the oxygen of the inspired oxygen, is consumed by brain, its high levels of polyunsaturated fatty acids (PUFA) [4], as well as its weak antioxidant defense system [5]. This susceptibility is increased in several conditions especially in brain injury due to damage of the blood-brain barrier (BBB), release of neurotransmitters such as glutamate, mitochondrial dysfunction and increased production of reactive oxygen species (ROS) [6]. Over production of ROS is what will cause oxidative stress in cases like brain injury [[5], [6], [7]].
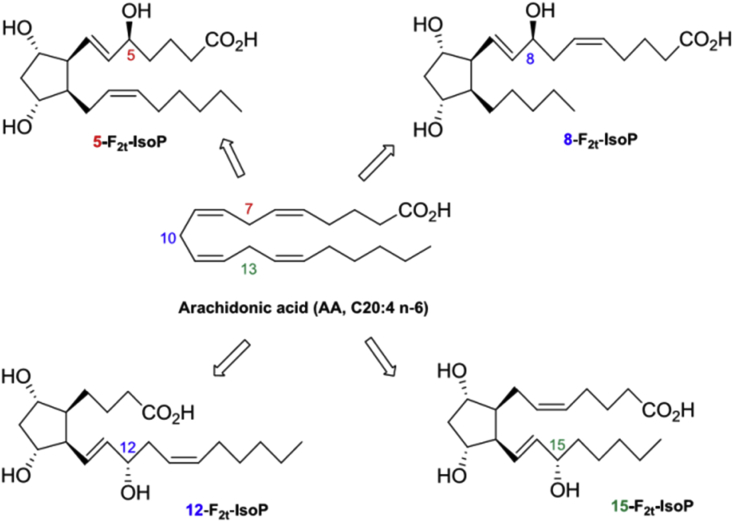
F2-Isoprostane (F2-IsoPs) is a unique series of prostaglandin-like compounds that are formed in vivo and in vitro through a non-enzymatic peroxidation mechanism mediated by free radicals of arachidonic acid (Fig. 1) [3,8]. This compound was first discovered by Morrow and colleagues who explained the formation of F2-IsoPs from arachidonic acid [3,[8], [9], [10]].
Fig. 1.
F2-isoprostane formation from arachidonic acid, which Leads to four F2-Isoprostane isomers [10].
Roberts and Marrow explained that there are six main advantages to using F2-IsoPs as a marker for oxidative damage in vivo [3,8,9,11,12].
2. F2 IsoPs are extremely stable compounds
-
2.
F2-IsoPs are the most specific markers of lipid peroxidation in vivo compared to other lipid peroxidation markers, such as malondialdehyde (MDA) or lipid hydroperoxide.
-
3.
F2-IsoPs are ready to be detected in all types of biological tissue and body fluids by the GC/NICI-MS method, which allows researchers to be able to determine a normal range or reference which is valuable for clinical studies in various conditions.
-
4.
It has been well proven that F2-IsoPs levels are elevated in various animal models of oxidative stress.
-
5.
F2-IsoPs levels are modulated by antioxidant system status.
-
6.
F2-IsoPs levels are not influenced by diet.
Since then, the quantification of F2-IsoPs has been considered as one of the most accurate approaches for assessing oxidative damage in vitro [3,4,13]. F2-IsoPs are more than just a biomarker or a monitor for physiological processes, but can also act as a lipid mediator in vasoconstriction and platelet aggregation and can play a role in intracellular signaling through activation of prostanoid receptors [4,14,15].
CAPE is obtained from propolis honey bee through extraction [16], the structure of CAPE contains catechol which is a powerful antioxidant [17]. Propolis has long been used for many years as traditional medicine [18]. The antioxidant effect of propolis which contain CAPE is stronger if compared to propolis without CAPE [17,18]. CAPE has also been reported to inhibit prostaglandin (PG) and leukotriene synthesis. Prostaglandins (PG) plays an important role in the progression of inflammation, CAPE's antioxidant activity is shown in its role in inhibiting the release of arachidonic acid and COX-1 and COX-2 activities [[19], [20], [21]].
3. Materials and Methods
This study examines the oxidative stress caused by brain trauma by evaluating the time course of the accumulation of F2-IsoPs in the serum, and comparing the responses between both groups; the group treated with CAPE and the group not treated with CAPE.
3.1. Surgical procedure
This study was approved by the ethics commission of Faculty of Medicine, Hasanuddin University, license number: 771/UN4.6.4.5.31/PP36/2019. Surgical procedures were performed aseptically. Ten male Sprague-Dawley rats free of viruses and other pathogens, (more than 2 months-old, weighing 280–300 g) had adequate access to standard food (Comfeed AD-2) and water until the time of the study. Rats were placed in two groups: (1) head injury with the CAPE treatment, (2) head injury without the CAPE treatment.
Rats were induced with ketamine which had been diluted at a dose of 3-10 mg/kg, a head injury in accordance with the modified Marmarou model was conducted [21,22]. The surgical procedure began with the aseptic procedure using povidone iodine, then a coronal incision was made through the center of the skull and then a burr holes was performed using a high-speed drill until the duramater was exposed; a 1.5 cm long craniectomy was done, then a mass of 20 g was dropped from a height of 20 cm by passing through a tube posing as a transport medium, the area with the exposed duramater was placed just below the opening of the tube, so that the mass [23] lands exactly on the area of the head which had the duramater exposed, to ensure the trauma model has caused damage to the brain, 1 rat was put down for pathology examination with hematoxylin staining, the results were hemorrhage in the brain tissue, while other rats were treated according to post-craniotomy procedure protocols, lastly all wounds were sutured using zyde 5.0 after antibiotic ointment was administered. All surgical procedures were performed aseptically by adhering to the principle of sterility. After the procedure, all rats were treated in a recovery room with room temperature settings before returned to their cages.
3.2. CAPE administration
CAPE obtained from Sigma-Aldrich Pte. ltd with Reagan Number 10454-70-9 was prepared in saline solution and given through an intraperitoneal injection (IP) administered 30 min post-trauma at a dose of 10 mg/kg, then repeated daily for 7 days, the control group was given a placebo with the same set-up as the CAPE group.
3.3. Sample collection and examination
Blood is drawn 24 h before treatment which is used as the base value of each sample, then is also taken on day-4 and day-7 of treatment. All blood samples were examined with an 8-iso-PGF2 Rat (8-isoprostane) ELISA kit with Catalog No. MBS7606827 which was bought from Mybiosource.com.
3.4. Statistical analysis
The data is presented as mean ± SD. All data were processed and analyzed using Excel 2013 and SPSS version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). F2-IsoPs levels were analyzed with Independent T test. P-values less than 0.05 were considered statistically significant.
4. Results
All rats survived after trauma until a predetermined experimental time point was reached.
4.1. Characteristics of research subjects
Characteristics of Sprague-Dawley rats are shown in Table 1.
Table 1.
Sprague dawley rat body weight data.
| Mice Body Weight (gram) | p-Value | |
|---|---|---|
| Mean | 290.07 | 0.155 |
| SD | ± 10.48 |
The homogeneity test of Sprague Dawley rats as a head injury model using the levene homogeneity test yielded a p > 0.05 was obtained, and it can be concluded that each rat's weight was not significantly different.
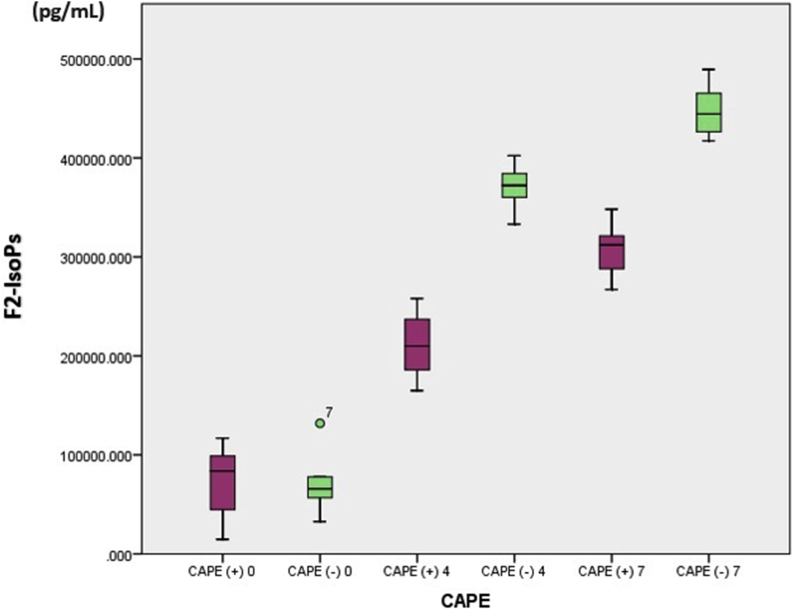
The average F2-IsoPs levels in rats with brain injury that were given CAPE compared to those not given CAPE on day 4 were 211210.376 ± 37559.206 pg/mL versus 370435.934 ± 25982.905 pg/mL (p < 0.05) and day 7 were 307346.562 ± 31119.798 pg/mL versus 448546.585 ± 29328.062 pg/mL (p < 0.05). The values were found to be significantly lower in the treatment group compared to the non-treated group (Table 2). On the seventh day, the F2-IsoPs levels in both groups were higher than on the fourth day. This finding can also be is clearly illustrated in the Boxplot graph below (Fig. 2):
Table 2.
Levels of F2-isoprostane in rats with experimental brain injury.
| Day- | CAPE Treatment | F2-isoprostane (pg/mL) |
Mean difference | P-value | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| 0 | (+) | 71812.907 | 41519.641 | −1201.702 | 0.963 |
| (−) | 73014.609 | 36831.245 | |||
| 4 | (+) | 211210.376 | 37559.206 | −159225.56 | <0.001 |
| (−) | 370435.934 | 25982.905 | |||
| 7 | (+) | 307346.562 | 31119.798 | −141200.023 | <0.001 |
| (−) | 448546.585 | 29328.062 | |||
Abbreviations: SD = standard deviation; Independent T-test with a p value < 0.05 was significant, CAPE: Caffeic acid phenyl ester.
Fig. 2.
Daily F2-isoprostane serum levels in rats with and without caffeic acid phenylethyl ester (CAPE).
5. Discussion
The results of this study showed a significant decrease in the amount of F2-IsoPs in the Sprague Dawley blood serum, in previous studies it was found that in cases of brain injury, an increase in oxidative stress would occur [1,4,24]. The oxidative stress that is formed is characterized by an increase in ROS (hydrogen peroxide, superoxide anions, and other free radicals) that is not matched by antioxidant defenses [4,25,26]. ROS is a normal substrate of aerobic metabolism derived from oxygen reduction, important for intracellular signaling systems under physiological conditions [1,27].
The reaction of the hydroxyl radical (OH) with unsaturated fatty acids produces lipid radicals that can react with oxygen molecules (O2) to form peroxyl lipid radicals. Peroxyl lipid radicals can take hydrogen from adjacent fatty acids to form lipid hydroperoxide (LOOH) and other lipid radicals. Alkoxyl and peroxyl radicals trigger lipid peroxidation chain reactions by removing hydrogen atoms. Lipid peroxidation is one of the main causes of cell damage. The process of fatty acid peroxidation mainly occurs in the phospholipid membrane. Various products are produced due to lipid peroxidation such as F2-IsoPs, MDA (Malondialdehyde), and F4-Neuroprostane [28]. In this study we analyzed the F2-IsoPs levels in Sprague Dawleys with brain injury.
CAPE and its metabolites, are efficient in detoxification of reactive oxygen species (ROS), for example, HOˉ and H2O2 and they also interact directly with reactive nitrogen species (RNS), as shown in Table 2 [[19], [20], [21]]. Quantification of F2-IsoPs is a sensitive index of neural oxidative damage, which can represent oxidation status occurring in the CNS [29]. At present, the detection of F4-NeuroPs and F2-dihomo-IsoPs is examined mainly in brain tissues and/or body fluids [1,4,30]. This process is in line with a study conducted on athletes who train in high altitudes for 2 weeks, found that F4-NeuroPs were detected in the athletes’ urine, this shows that the height factor can be related to the production of DHA (Docosahexaenoic Acid), through lipid peroxidation. As a main conclusion, exercise conducted at a moderate altitude increases F4-NeuroPs and F2-dihomo-IsoPs in relation to oxidative damage from CNS [1,4,31].
CAPE is a molecule with antioxidant and cytoprotective properties and plays a role in immunomodulation, is obtained from propolis extracted from honey bees [16,17]. At first propolis was known as a traditional medicine [16]. The ischemic process that follows a head injury causes accumulation of lactic acid due to anaerobic glycolysis, increases membrane permeability, and edema [1,2,26]. This is followed by cell membrane depolarization and excessive release of glutamate and aspartate neurotransmitters, activation of N-methyl-d-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolpropionate and changes in voltage-dependent Ca2+ and Na+ channel [4,26]. Constant influx of Ca2+ and Na+ triggers the process of intracellular catabolism. Ca2+ activates lipid peroxidase, protease, and phospholipase which increase intracellular concentrations of free fatty acids and free radicals (ROS) [1,2,4,32].
6. Conclusion
From the results and description above, it can be concluded that the administration of CAPE in a rat model with brain injury can reduce the formation of F2-IsoPs as an indicator of oxidative stress in blood serum post-trauma.
Author contribution
RAN, AAI, MH, AT, NAL, and PRI wrote the manuscript and participated in the study design. RAN, AAI, AT, NAL and PRI drafted and revised the manuscript. RAN, AAI, AT, NAL and MF performed head trauma treatment and surgery. AAI, MH, PRI and MF performed bioinformatics analyses and revised the manuscript. All authors read and approved the final manuscript.
Registration of research studies
None.
Guarantor
Rizha Anshori Nasution
Funding
No funding or sponsorship
Ethical approval
All procedure for Animal experiment has been approved by Ethics Commission Faculty of Medicine, Hasanuddin University, Number: 771/UN4.6.4.5.31/PP36/2019.
Consent
This manuscript does not involve human participants, human data, or human tissue.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
The authors declare that they have no conflict of interests
Acknowledgements
A higher appreciation to all staff from the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.07.036.
Contributor Information
Rizha Anshori Nasution, Email: rizha_nst@yahoo.com.
Andi Asadul Islam, Email: andiasadul@yahoo.com.
Mochammad Hatta, Email: hattaram@yahoo.com.
Prihantono, Email: prihantono.md@gmail.com.
Agus Turchan, Email: agusturchan@gmail.com.
Nasrullah, Email: nasrullah.makassar@gmail.com.
Muhammad Faruk, Email: faroex8283@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Signorini C., De Felice C., Durand T., Galano J.-M., Oger C., Leoncini S., Ciccoli L., Carone M., Ulivelli M., Manna C., Cortelazzo A., Lee J.C.-Y., Hayek J. Relevance of 4-F(4t)-neuroprostane and 10-F(4t)-neuroprostane to neurological diseases. Free Radic. Biol. Med. 2018;115:278–287. doi: 10.1016/j.freeradbiomed.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Muneer P.M., Chandra N., Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015;51:966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proudfoot J.M., Murrey M.W., McLean S., Greenland E.L., Barden A.E., Croft K.D., Galano J.-M., Durand T., Mori T.A., Pixley F.J. F2-isoprostanes affect macrophage migration and CSF-1 signalling. Free Radic. Biol. Med. 2018;126:142—152. doi: 10.1016/j.freeradbiomed.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 4.García-Flores L.A., Medina S., Cejuela R., Martínez-Sanz J.M., Oger C., Galano J.-M., Durand T., Casas-Pina T., Martínez-Hernández P., Ferreres F., Gil-Izquierdo Á. Assessment of oxidative stress biomarkers - neuroprostanes and dihomo-isoprostanes - in the urine of elite triathletes after two weeks of moderate-altitude training. Free Radic. Res. 2016;50:485–494. doi: 10.3109/10715762.2015.1111514. [DOI] [PubMed] [Google Scholar]
- 5.Foret M.K., [Do Carmo] S., Lincoln R., Greene L.E., Zhang W., Cuello A.C., Cosa G. Effect of antioxidant supplements on lipid peroxidation levels in primary cortical neuron cultures. Free Radic. Biol. Med. 2019;130:471–477. doi: 10.1016/j.freeradbiomed.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Hiebert J.B., Shen Q., Thimmesch A.R., Pierce J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015;350:132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 7.Dan Dunn J., Alvarez L.A.J., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiśniewski K., Jóźwik-Pruska J., Bieńkowski M., Bobeff E.J., Bryl M., Kałużna-Czaplińska J., Jaskólski D.J. Isoprostanes as potential cerebral vasospasm biomarkers. Neurol. Neurochir. Pol. 2018;52:643–651. doi: 10.1016/j.pjnns.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Yen H.-C., Chen T.-W., Yang T.-C., Wei H.-J., Hsu J.-C., Lin C.-L. Levels of F2-isoprostanes, F4-neuroprostanes, and total nitrate/nitrite in plasma and cerebrospinal fluid of patients with traumatic brain injury. Free Radic. Res. 2015;49:1419–1430. doi: 10.3109/10715762.2015.1080363. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaidis M.G., Kyparos A., Vrabas I.S. F2-isoprostane formation, measurement and interpretation: the role of exercise. Prog. Lipid Res. 2011;50:89–103. doi: 10.1016/j.plipres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Proudfoot J.M., Barden A.E., Croft K.D., Galano J.-M., Durand T., Bultel-Poncé V., Giera M., Mori T.A. F(2)-Isoprostanes in HDL are bound to neutral lipids and phospholipids. Free Radic. Res. 2016;50:1374–1385. doi: 10.1080/10715762.2016.1250262. [DOI] [PubMed] [Google Scholar]
- 12.Yen H.-C., Wei H.-J., Lin C.-L. Unresolved issues in the analysis of F 2 -isoprostanes, F 4 -neuroprostanes, isofurans, neurofurans, and F 2 -dihomo-isoprostanes in body fluids and tissue using gas chromatography/negative-ion chemical-ionization mass spectrometry. Free Radic. Res. 2015;49:861–880. doi: 10.3109/10715762.2015.1014812. [DOI] [PubMed] [Google Scholar]
- 13.McCullough P.A., Vasudevan A., Lopez L.R., Swift C., Peterson M., Bennett-Firmin J., Schiffmann R., Bottiglieri T. Oxidative stress reflected by increased F(2)-isoprostanes is associated with increasing urinary 11-dehydro thromboxane B(2) levels in patients with coronary artery disease. Thromb. Res. 2016;148:85–88. doi: 10.1016/j.thromres.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Melton C.D., Luo R., Wong B.J., Spasojevic I., Wagenknecht L.E., D'Agostino R.B.J., Il’yasova D. Urinary F(2)-isoprostanes and the risk of hypertension. Ann. Epidemiol. 2017;27:391–396. doi: 10.1016/j.annepidem.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosavi F., Hosseini R., Rajaian H., Silva T., Magalhaes E Silva D., Saso L., Edraki N., Miri R., Borges F., Firuzi O. Derivatives of caffeic acid, a natural antioxidant, as the basis for the discovery of novel nonpeptidic neurotrophic agents. Bioorg. Med. Chem. 2017;25:3235–3246. doi: 10.1016/j.bmc.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Ayla S., Tunali G., Bilgic B.E., Sofuoglu K., Ozdemir A.A., Tanriverdi G., Ozdemir S., Soner B.C., Ozturk B., Karahuseyinoglu S., Aslan E.G., Seckin I. Antioxidant activity of CAPE (caffeic acid phenethyl ester) in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Acta Histochem. 2018;120:117–121. doi: 10.1016/j.acthis.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Salmas R.E., Gulhan M.F., Durdagi S., Sahna E., Abdullah H.I., Selamoglu Z. Effects of propolis, caffeic acid phenethyl ester, and pollen on renal injury in hypertensive rat: an experimental and theoretical approach. Cell Biochem. Funct. 2017;35:304–314. doi: 10.1002/cbf.3277. [DOI] [PubMed] [Google Scholar]
- 18.Cetin A., Deveci E., Çetin A., Deveci E. Evaluation of PECAM-1 and p38 MAPK expressions in cerebellum tissue of rats treated with caffeic acid phenethyl ester: a biochemical and immunohistochemical study. Folia Morphol. 2019;78:221–229. doi: 10.5603/FM.a2018.0085. [DOI] [PubMed] [Google Scholar]
- 19.Aloutaibi G., Gashlan H., Moselhy S., Malki A.L.A., Khan J. Possible cardioprotective action OF pomegranate juice punica granatum and propolis against myocardial infarction induced IN rats. Afr. J. Tradit., Complementary Altern. Med. 2017;14 doi: 10.21010/ajtcam.v14i5.17. [DOI] [Google Scholar]
- 20.Chu J., Zhang X., Jin L., Chen J., Du B., Pang Q. Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats. Environ. Toxicol. Pharmacol. 2015;39:683–689. doi: 10.1016/j.etap.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Marmarou A., Fatouros P.P., Barzo P., Portella G., Yoshihara M., Tsuji O., Yamamoto T., Laine F., Signoretti S., Ward J.D., Bullock M.R., Young H.F. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J. Neurosurg. 2000;93:183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 22.Nasution R.A., Islam A.A., Hatta M., Prihantono Decreased neutrophil levels in mice with traumatic brain injury after cape administration. Ann. Med. Surg. 2020;54:89–92. doi: 10.1016/j.amsu.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branton A. The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. Int. J. Clin. Dev. Anat. 2017;3:9. doi: 10.11648/j.ijcda.20170303.11. [DOI] [Google Scholar]
- 24.Schoneich C., Hewarathna A., Pal R., Jiang L., Michaelis E. P 236. Oxidative stress markers of alzheimer's disease in peripheral cell mitochondria. Free Radic. Biol. Med. 2017;108 doi: 10.1016/j.freeradbiomed.2017.04.321. [DOI] [Google Scholar]
- 25.Corps K.N., Roth T.L., McGavern D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokum J.A., Gerzanich V., Simard J.M. Molecular pathophysiology of cerebral edema. J. Cerebr. Blood Flow Metabol. 2016;36:513–538. doi: 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoiber W., Obermayer A., Steinbacher P., Krautgartner W.-D. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules. 2015;5:702–723. doi: 10.3390/biom5020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoubnasabjafari M., Ansarin K., Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;5:123–127. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galano J.-M., Lee Y.Y., Oger C., Vigor C., Vercauteren J., Durand T., Giera M., Lee J.C.-Y. Isoprostanes, neuroprostanes and phytoprostanes: an overview of 25years of research in chemistry and biology. Prog. Lipid Res. 2017;68:83–108. doi: 10.1016/j.plipres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Niedzielska E., Smaga I., Gawlik M., Moniczewski A., Stankowicz P., Pera J., Filip M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016;53:4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Tolba M.F., Omar H.A., Azab S.S., Khalifa A.E., Abdel-Naim A.B., Abdel-Rahman S.Z. Caffeic acid phenethyl ester: a review of its antioxidant activity, protective effects against ischemia-reperfusion injury and drug adverse reactions. Crit. Rev. Food Sci. Nutr. 2016;56:2183–2190. doi: 10.1080/10408398.2013.821967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.