Introduction
Waldenström macroglobulinemia (WM) is a rare subtype of lymphoplasmacytic lymphoma of B lymphocytes. It is characterized by monoclonal proliferation of lymphoplasmacytes in the bone marrow, lymph nodes, and spleen. Increased levels of circulating IgM monoclonal antibodies lead to their deposition in the skin and other organs.1
Cutaneous manifestations of WM are rare and can be classified into specific and nonspecific findings. Nonspecific findings are attributed to hyperviscosity or cryoglobulinemia, whereas specific manifestations are related to neoplastic B-cell infiltrates and monoclonal IgM deposition in the skin, which is referred to as cutaneous macroglobulinosis (CM).1
We report a case of a 50-year-old woman whose clinical, laboratory, and pathologic findings are consistent with the diagnosis of CM that developed after WM. To our knowledge, only 9 cases of CM in patients with an established diagnosis of WM have been previously reported (Table I).1, 2, 3, 4, 5, 6, 7, 8, 9
Table I.
Case reports of CM that developed after patients were previously diagnosed with WM
| Study | Year | Age (yrs)/Sex | Duration of WM (yrs) | Clinical presentation | Sites affected | IgM level | Lymph nodes/HSM | History of neuropathy | Special stains |
Treatment | Outcome of treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAS | Congo red | IgM | |||||||||||
| Mascaro et al4 | 1982 | 48/M | 4 | Asymptomatic, discrete, smooth, pink, translucent, pearly and shiny papules, each 1-5 mm in diameter. Some of them show central crust and erosion. |
Buttocks, thighs and legs | 3400 mg/dL | Bilateral cervical lymphadenopathy | NM | + | NM | + by DIF | Chlorambucil and prednisone (before onset of skin lesions) | NM |
| Cobb et al5 | 1992 | 59/M | 4 | Widespread eruption of 2-4 mm succulent erythematous excoriated papules, with confluence to plaques | Trunk, arms and legs | 1520 mg/dL | None | NM | NM | NM | + by DIF | Erythromycin and dapsone | Ineffective |
| 2% lindane lotion | Ineffective | ||||||||||||
| Daily prednisone | The eruptions improved. | ||||||||||||
| PUVA | The eruptions completely cleared. | ||||||||||||
| Gressier et al1 | 2010 | 71/M | NM | Asymptomatic hyperkeratotic flesh-colored papules, some with central crust | Both knees | 18.50 mg/dL | None | Peripheralneuropathy of all 4 limbs | + | - (for amyloid-specific stains) | + by DIF | Rituximab and chlorambucil | Clearance of cutaneous lesions |
| Marchand et al6 | 2011 | 67/M | NM | Multiple erythematous, nonpruriginous,1-2 mm papules | Anterior face of the knees and calves | NM | NM | NM | + | - | + by DIF | Bortezomib and rituximab | The skin lesions remained unchanged. |
| Camp and Magro2 | 2012 | 80/M | NM | Painful erythematous papules and nodules with central ulceration | Bilateral lower extremities and back of right hand | 3016 mg/dL | NM | NM | NM | NM | + by DIF | Patient received 2 doses of rituximab prior to the onset of the skin eruption. | NM |
| D'Acunto et al7 | 2014 | 70/M | 15 | Nodules covered by a thick hyperkeratotic layer. The lesions were extremely painful to pressure. |
Soles of the feet | 2290 mg/dL | NM | NM | + | - | + by IHC | NM | NM |
| Oshio-Yoshii et al8 | 2017 | 63/M | 1 | Small reddish papules, some of which developed into discrete blister-like nodules | On and around the right medial malleolus | NM | NM | NM | + | - | + by IHC | Intravenous immunoglobulin therapy | Ineffective |
| Rituximab | Clearance of the skin lesions leaving pigmented macules, but lesions recurred after 6 months | ||||||||||||
| Roupie et al9 | 2019 | 65/M | 3 | Papules covered by a thick hyperkeratotic layer | Soles of the feet | NM | NM | NM | + | - | + by IHC | Rituximab, cyclophosphamide and corticosteroids | Complete regression of skin lesions and a partial hematologic response |
| Fayne et al3 | 2019 | 56/F | 0.5 | Numerous crusted papules and nodules with eschars | Disseminated across the body, including the face and fingers | 280,200 g/dL | NM | NM | + | Weak + | + by IHC | R-CHOP, rituximab, bendamustine and ibrutinib | Failed |
| Current case | 2020 | 50/F | 0.5 | Multiple erythematous to hyperpigmented papules and nodules with central crust | Face, upper eyelids, and extensor aspects of both upper limbs | 2200 mg/dL | Generalized lymphadenopathy and HSM | Peripheral Sensory neuropathy of both upper and lower limbs |
+ | - | + by IHC | R-CHOP | Patient died after 2 sessions |
DIF, Direct immunofluorescence; HSM, hepatosplenomegaly; IHC, immunohistochemistry; NM, not mentioned; PUVA, psoralen and ultraviolet A.
Case report
A 50-year-old diabetic woman presented to our department with itchy skin lesions affecting the face and both upper limbs of 2 months duration.
One year ago, she complained of chronic anemia and peripheral sensory neuropathy of both upper and lower limbs that was diagnosed at that time as chronic inflammatory polyradiculopathy. The patient was treated with iron and vitamin B complex supplements without significant improvement. After 6 months, a bone marrow biopsy found marked reduction in hematopoietic stem cells, CD20+ lymphoplasmacytic infiltrate and markedly elevated IgM assay. The diagnosis of WM was proposed, and few months later, skin lesions appeared.
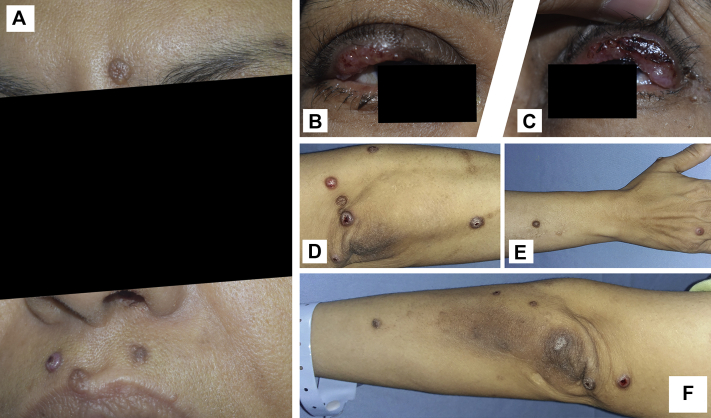
At the time of admission, skin examination showed multiple erythematous to hyperpigmented papules and nodules with central crust. The lesions were distributed on the face (Fig 1, A), upper eyelids (Fig 1, B and C) and extensor aspects of both upper limbs (Fig 1, D to F). General examination revealed generalized lymphadenopathy and hepatosplenomegaly. Abdominal ultrasound scan confirmed the presence of hepatosplenomegaly. Blood workup found severe normochromic anemia (hemoglobin, 4 gm/dL) and marked leukocytosis (31,000 /mm³). Serum electrophoresis exhibited a monoclonal peak in the β2 region. Serum IgM level was markedly elevated (2200 mg/dL; reference range, 40-220 mg/dL) and whole blood viscosity was also high (7.8 cP).
Fig 1.
Multiple erythematous to hyperpigmented papules and nodules, with central crust located on the face (A), upper eyelids (B and C), and extensor aspects of the upper limbs (D to F).
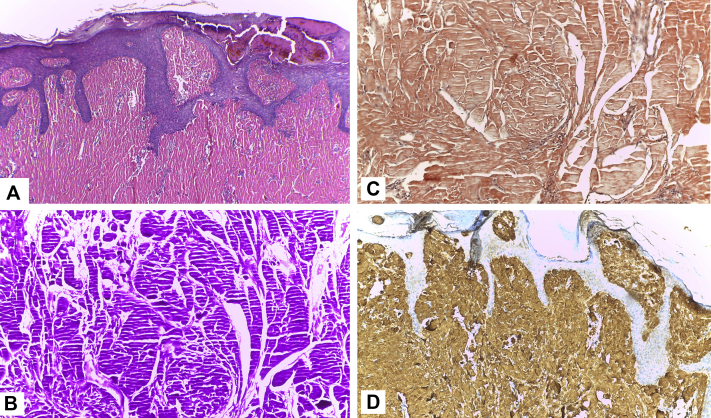
The clinical differential diagnosis included Kyrle disease, multiple keratoacanthoma, and nodular prurigo. A skin biopsy specimen of one of those nodules showed dermal occupation by homogenous bright eosinophilic materials (Fig 2, A) that stained positive for periodic acid–Schiff (PAS) (Fig 2, B), but Congo red staining was negative (Fig 2, C). Immunohistochemistry showed positive staining for IgM with κ light chain restriction (Fig 2, D). A mild lymphocytic infiltrate without plasma cells was seen. These histologic and immunohistochemical findings were consistent with the diagnosis of CM.
Fig 2.
Skin biopsy shows homogenous bright eosinophilic materials with parallel cleft artifacts characteristic of hyaline deposits (A), positive staining for PAS (B), negative staining for Congo red (C), and positive staining for IgM (D). (A, Hematoxylin-eosin stain; original magnifications: A, ×100; B to D, ×200.)
The patient was referred to oncology department where she received 2 sessions of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. Unfortunately, she died after rapid deterioration of her general condition.
Discussion
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with monoclonal IgM gammopathy. It represents approximately 2% of all hematologic malignancies. It is more common in men, with a median age of 60 to 70 years. The disease is characterized by an indolent course in most patients, with a median survival of about 5 years. IgM paraprotein can cause various symptoms resulting from systemic amyloidosis, paraprotein depositions in the organs, cryoglobulinemia, peripheral neuropathy, and hyperviscosity syndrome.10
Tichenor et al first described cutaneous macroglobulinosis in 1978 as macroglobulinemia cutis. Most reported presentations are skin-colored and pink papules, sometimes with central crust, on the knees, buttocks, and extensor aspects of the extremities. Uncommon clinical presentations of nodules, plaques, or ulcerated lesions along with involvement of the trunk, face, neck, and scalp were occasionally reported.2
Patients can develop CM before, concurrent with, or—as in our reported case—after diagnosis of the underlying lymphoplasmacytic lymphoma. Hence, CM can predict a latent plasma cell dyscrasia before any other clinical or pathologic evidence.3
Nine previous case reports exist of CM that developed in patients after WM was diagnosed (Table I).1, 2, 3, 4, 5, 6, 7, 8, 9 Of these 9 cases, only one female case was reported, and this makes our case the second reported female case of CM in a patient with a history of WM.3 Our case had peripheral neuropathy, which is also a rare association with CM.1 The development of neuropathy is thought to be related to the accumulation of IgM in myelin sheaths.2 A unique clinical finding in our case is the involvement of the eyelids, which, to our knowledge, has not been previously reported in any case of CM.
Finding dermal deposits of eosinophilic amorphous material is the pathologic hallmark of CM. The deposited material is usually positive for PAS, but negative for Congo red stain. Only 1 report showed weak positive staining for Congo red.3 The most relevant diagnostic test is the detection of IgM by immunohistochemistry and/or direct immunofluorescence (Table I). Immunoelectron microscopy was found to clearly demonstrate the presence of large amounts of IgM in the dermis, which were found in the lesions of CM and in normal skin. These results suggest that the IgM storage papules result from a greater density of deposits rather than a site-specific accumulation.11
Treatment for WM is symptom directed. There are neither guidelines for initial therapy nor trials assessing a primary outcome of improvement in cutaneous involvement. Therefore, alkylator-based therapy, purine nucleoside analogue agents, and rituximab may all be considered for initial therapy for newly diagnosed patients.2
We present a new rare case of CM associated with WM. The clinical features of this case are interesting, as it affected a female patient with involvement of eyelids and associated with peripheral neuropathy. IgM deposits are a characteristic histologic feature that helps pathologists differentiate CM from other PAS-positive depositional disorders such as lipoid proteinosis. In clinical practice, knowledge of different clinical manifestations related to IgM can be useful for early diagnosis of lymphoid hemopathies associated with it.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Gressier L., Hotz C., Lelièvre J.D. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146(2):165–169. doi: 10.1001/archdermatol.2009.359. [DOI] [PubMed] [Google Scholar]
- 2.Camp B.J., Magro C.M. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39(10):962–970. doi: 10.1111/j.1600-0560.2012.01983.x. [DOI] [PubMed] [Google Scholar]
- 3.Fayne R., Rosenberg M., White K. Disseminated cutaneous immunoglobulin M macroglobulinosis associated with cryoglobulinemia and minimal residual disease of Waldenström macroglobulinemia. JAAD Case Rep. 2019;5(10):918–922. doi: 10.1016/j.jdcr.2019.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascaro J.M., Montserrat E., Estrach T. Specific cutaneous manifestations of Waldenstrom's macroglobulinaemia. A report of two cases. Br J Dermatol. 1982;106(2):217–222. doi: 10.1111/j.1365-2133.1982.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 5.Cobb M.W., Domloge-Hultsch N., Frame J.N. Waldenstrom macroglobulinemia with an IgM-kappa antiepidermal basement membrane zone antibody. Arch Dermatol. 1992;128(3):372–376. [PubMed] [Google Scholar]
- 6.Marchand T., Tas P., Houot R. Cutaneous macroglobulinosis treated with bortezomib and rituximab. Eur J Haematol. 2011;87(1):98. doi: 10.1111/j.1600-0609.2011.01610.x. [DOI] [PubMed] [Google Scholar]
- 7.D’Acunto C., Nigrisoli E., Liardo E.V. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71(6):e251–e252. doi: 10.1016/j.jaad.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Oshio-Yoshii A., Fujimoto N., Shiba Y. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31(1):e30–e31. doi: 10.1111/jdv.13613. [DOI] [PubMed] [Google Scholar]
- 9.Roupie A.L., Battistella M., Talbot A. Coexisting cutaneous macroglobulinosis and scleredema of Buschke in a patient with a Waldenstrom macroglobulinemia. J Eur Acad Dermatol Venereol. 2019;33(3):e104–e106. doi: 10.1111/jdv.15268. [DOI] [PubMed] [Google Scholar]
- 10.Yun S., Johnson A., Okolo O. Waldenstrom macroglobulinemia: review of pathogenesis and management. Clin Lymphoma Myeloma Leuk. 2017;17(5):252–262. doi: 10.1016/j.clml.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipsker D., Cribier B., Spehner D. Examination of cutaneous macroglobulinosis by immunoelectron microscopy. Br J Dermatol. 1996;135(2):287–291. [PubMed] [Google Scholar]