Abstract
Cytogenetic and iFISH plays a major part in the diagnosis of the MM and have an important prognostic significance.
10–15% of patients with amyloidosis will also have multiple myeloma (MM). Few studies have addressed the clinical and cytogenetic features of patients with AL amyloidosis with concurrent multiple myeloma.
This study of MM case in which we found a near tetraploid complex karyotype with the t(11;14) (q13;q32) abnormality in cytogenetic analysis and the presence of t(4;14) and del(17p) by iFISH, referred to several studies which showed the translocation t(11;14) as the most frequent abnormality in both AL amyloidosis and MM.
Keywords: Multiple myeloma, AL amyloidosis, cytogenetic analysis, FISH, IGH/FGFR3, del 17p
1. Introduction
Systemic immunoglobulin light chain amyloidosis (AL) is a rare clonal plasma cell disorder characterized by the deposition of misfolded amyloid protein in vital organs, such as heart and kidney [1]. Fatal outcome in most AL-patients is due to cardiac events caused by amyloid involvement [2].
There is consensus that patients with AL and hypercalcemia, renal failure, anemia, and lytic bone lesions attributable to clonal expansion of plasma cells (CRAB criteria), have multiple myeloma (MM), as included in definition of symptomatic MM of the International Myeloma Working Group (IMWG) [3].
Traditionally, MM has been estimated to be present in approximately 10–20% of AL patients [2,4]. The symptoms and signs of AL in patients with MM are different according to organs involved [4].
Characterization of cytogenetic abnormalities by conventional karyotyping and interphase fluorescence in situ hybridization (iFISH) is essential for MM, because of the impacts on prognosis and disease characterization [5], [6]. Even complex karyotypes can be observed in MM, and are divided into hyperdiploid and non-hyperdiploid subtypes based on the number of chromosomes [6]. iFISH is an assay that is performed on non-dividing cells independently of cell proliferation. In MM efficiency can be increased by pre-sorting of CD138 positive plasma cells [7].
Chromosomal abnormalities (CA) detected by iFISH are a key element to define the biologic features of MM. High-risk diseases are characterized by the presence of at least one of these abnormalities: translocation t(4;14), deletion del(17p) and translocation t(14;16). Chromosomal translocations involving the immunoglobulin heavy-chain (IgH) locus in 14q32 are suggested to be initiating events in the development of several B-cell malignancies, including MGUS and MM [8]. Translocation t(11;14) (q13;q32) is associated with high prevalence of light-chain-only MM [9], and it is reported as the most common cytogenetic abnormality in AL, too, being associated with poor prognosis [5,10].
In this report, we describe a rare case of MM with primary systemic AL presenting translocation t(11;14), as well as translocation t(4;14), deletion del(17p) and gain in 1q and compare the case with other from the literature.
2. Case report and methods
2.1. Case history
A 68-year-old Moroccan woman was admitted to the oncology department of international university hospital Cheikh Khalifa at Casablanca, for abdominal pain management. Examination revealed dyspnea, tumor syndrome (splenomegaly 18 cm arriving at the navel), comorbid cardiopathy, anemia and renal failure.
Hematological examinations revealed a hemoglobin of 10.9 g/dl, total Leukocyte Count of 6830/mm3 and Platelet count of 130,000/mm3. The electrophoresis of serum proteins has shown the absence of an M band with hypogammaglobulinemia. The proteinuria over 24 h showed a rate of 2.486 g / 24 h of protein. The immunofluorescence light chain (Iflc) shows the presence of a light chain monoclonal Lambda protein (1292.00 mg / l). Urea examinations gave a rate of 1.98 g / l and a creatinine level of the order of 20.98 mg / l. Albumin was 36.2 g / l and serum 2-microglobulin 2 8.7 mg / l. Aspiration of bone marrow revealed 40% plasma cells. On the other hand, the immunohistochemical study on paraffin cut is in favor of myeloma with monotypic secretion of Lambda. Congo red staining of biopsied lip was negative for amyloid deposition.
Regarding the International Staging System (ISS) score based on the β2μ and albuminemia levels [2], the patient is classified in stage III.
The patient was put on cardio treatment: aldactone, lasilix, aspegic, cardioasperine, cordarone, after that, the bortezomib, dexamethasone, and thalidomide (VTD) treatment regimen with prophylaxis was administered with prophylaxis in 1 course. She died 25 days after her hospitalization.
2.2. Cytogenetics analysis
Bone marrow (BM) was collected from the patient prior to treatment. BM was cultured in RPMI basal medium, containing l-glutamine and fetal bovine serum, for 96 h at 37°C in a CO2 incubator. The harvesting process was done acc. to standard procedures using a Metaphase Chromosome Harvester (HANABI PII). The spreading was done by the metaphase auto spreader mini (HANABI PV). R-banding was performed by standard techniques. Karyotype was described according to the International System for Human Cytogenetic Nomenclature ISCN (2016) [11].
3. Magnetic cell sorting
CD138 bone marrow plasma cells were purified by automagnetic activated cell sorting with CD138 immunobeads, according to the manufacturer's protocol (Auto Macs pro separator).
3.1. Flow cytometry analysis of magnetic cell sorting enrichment
The MACS enrichment was verified by flow cytometry analysis by using the Anti-CD138-PO, antiCD38-APC-H7 and anti-CD45-PE-Cy5 monoclonal antibodies. Plasmacells were identified by high expression of CD38.
3.2. Fluorescent in-situ hybridization (FISH) analysis
iFISH was performed on enriched plasma cells. The minimal panel of probes recommended at diagnosis of multiple myeloma was applied [7]: IGH/FGFR3 double fusion probe (Vysis), TP53/CEP 17 FISH probe (Vysis) and 1p36/1q25 FISH probe (Vysis), according standard protocols [12].
4. Results
4.1. Conventional karyotype
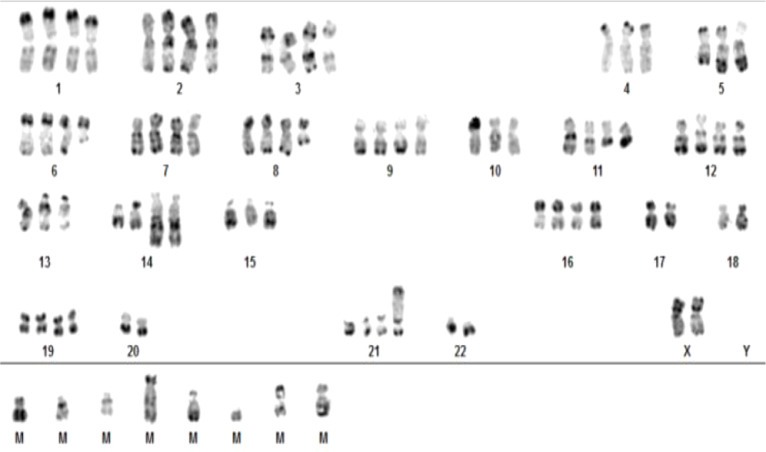
After analyzing 20 mitosis, a near tetraploid complex karyotype (82–87 chromosomes) with translocation t(11;14)(q13;q32) was found in 4/20 mitosis.
Karyotype was interpreted as 82–87,XX,−4,−5,del(5)(q13q33),del(6)(q13q23),del(8),−10,t(11;14)(q23;q23),t(11;14)(q23;q23),−13,−15,−17,−17,−18,−18,−20,−20,der(21)add(21)(p12),−22,−22,+1mar~8mar[cp4]/46,XX[16]. (Fig. 1)
Fig. 1.
Karyotype image from a cultured marrow sample of a patient with MM showing the near tetraploid karyotype with double t(11;14)(q13;q32).
4.2. Flow cytometry analysis of magnetic cell sorting enrichment
The percentage of plasma cell before sorting (in the specimen) was at 15%, and 82% after magnetic cell sorting enrichment.
4.3. iFISH analysis
FISH demonstrated the presence of a translocation t(4;14)(p16;q32)(IGH/FGFR3) in 20% of the plasma cells, a17p deletion in 80% and of 1q21 gain of copy numbers in 90% of the cells. (Fig. 2)
Fig. 2.
The iFISH technique for multiple myeloma showing different abnormalities: (A) polyploidy with t(4;14):(1 red, 1 green and 2 fusion signals); (B) polyploidy with 1q amplification (5 green signals) and 1p deletion (3 red signals); (C) polyploidy with monosomy of 17p (two red signal vs 4 green signals) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
5. Discussion
We report a rare case with AL and concomitant MM presented initially abdominal pain, dyspnea, splenomegaly and cardiopathy and complex karyotype.
AL is a systemic disorder characterized by widespread deposition of amyloid fibrils derived from monoclonal immunoglobulin light chains in organs and soft tissues. AL is typically caused by an underlying plasma cell clone disorder and occurs in 6–15% of patients with MM [13].
Both AL and MM are plasma cell dyscrasias with distinct clinically features, but few studies have addressed the clinical and cytogenetic features of patients with AL and concomitant MM [5].
Patients with t(11;14) have traditionally been classified as standard risk MM, based on studies conducted before novel agents were available . Recent observations suggest that patients with t(11;14) may have unfavorable outcomes when compared to other standard risk patients, casting doubt on the traditional view [9].
The study of Hiroki Kobayashi et al, reported that patients with AL amyloidosis who had t(11;14) tended to have shorter OS, irrespective of the presence or absence of concurrent MM [5].
Importance of interphase fluorescent in situ hybridization (FISH) on bone marrow is not well understood in light chain amyloidosis (AL) [14], this study has demonstrated that: - 81% of patients had an abnormal FISH, whose common abnormalities involved were translocations of chromosome 14q32 (52%), specifically: t(11;14) (43%), t(14;16) (3%) and t(4;14) (2%). - An abnormal FISH at diagnosis is prognostic for survival and advanced cardiac disease. -trisomies and t(11;14) affect survival when degree of plasma cell burden is considered (>10%).
In addition to t(11;14) founed by karyotype, the patient had the t(4;14) in 20% of cells, del (17p) in 80%, gain 1q in 90% and del 1p in 90%.
The t(4;14)(p16;q32) translocation is detected in 10%−15% of the patients with myeloma [6], it is regarded as primary cytogenetic events[8]
Chromosome 17p deletion is considered as a secondary event. It is observed in around 10% of patients with newly diagnosed MM [15].
1q21 gain is the most frequent structural abnormality, observed in 35%−40% of the patients with MM. It is considered to be a secondary event that influences tumor progression. The number of 1q21 copies is correlated with both disease progression and prognosis [6].
Other study has showed that patients harboring t(11;14) have adverse outcomes in both AL amyloidosis and MM when treated with bortezomib-containing regimens [10].
Perhaps the greatest obstacle to treating both multiple myeloma and amyloidosis with high-dose chemotherapy is that patients with organs damaged by amyloid deposition will be more susceptible to toxicity and less able to tolerate the chemotherapy.
6. Conclusion
This case of this patient is an example that supports the results of several studies. This is evidence which strongly suggests that the t(11;14) is the most frequent abnormality in patients with AL amyloidosis with MM defined either by CRAB or by more than 10% BMPCs have a similarly poor prognosis. The iFISH results are important independent prognostic factors in AL amyloidosis.
7. Statement of ethics
The study was approved by an ethics committee and written informed consent was given.
Authors’ contribution
Hamdaoui Hasna carried out the cytogenetic study and drafted the manuscript. Abdelhafid Natiq, Thomas Liehr revised the work critically for important intellectual content. Oumaima Benlarroubia was helping in carried out the cytogenetic study. Hind Dehbi reviewed the paper. Latifa Loukhmas is a doctor who consulted and followed the patient. Fatima Chegdani reviewed the article before submission not only for spelling and grammar but also for its intellectual content.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to thank Dr AL Rikabi Ahmed for language revision and relevant comments.
References
- 1.Badar T., Cornelison A.M., Shah N.D., Bashir Q., Parmar S., Patel K., Hosing C., Popat U., Weber D.M., Thomas S.K., Shah J.J., Orlowski R.Z., Champlin R.E. Qazilbash MH: outcome of patients with systemic light chain amyloidosis with concurrent renal and cardiac involvement. Eur. J. Haematol. 2016;97(4):342–347. doi: 10.1111/ejh.12736. [DOI] [PubMed] [Google Scholar]
- 2.Dinner S., Witteles W., Witteles R., Lam A., Arai S., Lafayette R., George T.I., Schrier S.L., Liedtke M. The prognostic value of diagnosing concurrent multiple myeloma in immunoglobulin light chain amyloidosis. Br. J. Haematol. 2013;161(3):367–372. doi: 10.1111/bjh.12269. [DOI] [PubMed] [Google Scholar]
- 3.Kourelis T.V., Kumar S.K., Gertz M.A., Lacy M.Q., Buadi F.K., Hayman S.R., Zeldenrust S., Leung N., Kyle R.A., Russell S., Dingli D., Lust J.A., Lin Y., Kapoor P., Rajkumar S.V., McCurdy A., Dispenzieri A. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J. Clin. Oncol. 2013;31(34):4319–4324. doi: 10.1200/JCO.2013.50.8499. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu H., Huang D., Ji M., Zhang S. Multiple myeloma with primary amyloidosis presenting with digestive symptoms: a case report and literature review. Arab. J. Gastroenterol. 2020 doi: 10.1016/j.ajg.2020.01.002. pii: S1687-1979(20)30002-2. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H., Abe Y., Miura D., Narita K., Kitadate A., Takeuchi M., Matsue K. Prevalence and clinical implications of t(11;14) in patients with amyloid light-chain amyloidosis with or without concurrent multiple myeloma. Jpn. J. Clin. Oncol. 2019;49(2):195–198. doi: 10.1093/jjco/hyy202. 1. [DOI] [PubMed] [Google Scholar]
- 6.Saxe D., Seo E.J., Bergeron M.B., Han J.Y. Recent advances in cytogenetic characterization of multiple myeloma. Int. J. Lab. Hematol. 2019;41(1):5–14. doi: 10.1111/ijlh.12882. [DOI] [PubMed] [Google Scholar]
- 7.Daudignon A., Quilichini B., Ameye G., Poirel H., Bastard C., Terré C. place de cytogenetique dans la prise en charge du myeloma multiple: actualisation par le groupe francophone de cytogenetique hematologique (GFCH) Ann. Biol. Clin. 2016;74(5):588–595. doi: 10.1684/abc.2016.1178. [DOI] [PubMed] [Google Scholar]
- 8.Hayman S.R.1., Bailey R.J., Jalal S.M., Ahmann G.J., Dispenzieri A., Gertz M.A., Greipp P.R., Kyle R.A., Lacy M.Q., Rajkumar S.V., Witzig T.E., Lust J.A., Fonseca R. Translocations involving the immunoglobulin heavy-chain locus are possible early genetic events in patients with primary systemic amyloidosis. Blood. 2001;98(7):2266–2268. doi: 10.1182/blood.v98.7.2266. [DOI] [PubMed] [Google Scholar]
- 9.Lakshman A., Alhaj Moustafa M., Rajkumar S.V. Natural history of t(11;14) multiple myeloma. Leukemia. 2018;32:131–138. doi: 10.1038/leu.2017.204. [DOI] [PubMed] [Google Scholar]
- 10.Bochtler T., Hegenbart U., Kunz C. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J. Clin. Oncol. 2015;33:1371–1378. doi: 10.1200/JCO.2014.57.4947. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto R.K., de Freitas S.L., Ratis C.A., Borri D., Sitnik R., Velloso E.D. Validation of interphase fluoresecence in situ hybridization (iFISH) for multiple myeloma using CD138 positive cells. Rev. Bras. Hematol. Hemoter. 2016;38(2):113–120. doi: 10.1016/j.bjhh.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann L., Biggerstaff J.S., Chapman D.B., Scott J.M., Johnson K.R., Ghirardelli K.M., Fritschle W.K., Martinez D.L., Bennington R.K., de Baca M.E., Wells D.A., Loken M.R., Zehentner B.K. Detection of genomic abnormalities in multiple myeloma: the application of FISH analysis in combination with various plasma cell enrichment techniques. Am. J. Clin. Pathol. 2011;136(5):712–720. doi: 10.1309/AJCPF7NFLW8UAJEP. [DOI] [PubMed] [Google Scholar]
- 13.Liu D., Uqdah H.T., Gordy A.D. An unusual case of chronic lymphocytic leukemia, multiple myeloma and cardiac amyloidosis. J. Community Hosp. Intern. Med. Perspect. 2017;7(4):230–233. doi: 10.1080/20009666.2017.1370940. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warsame R., Kumar S.K., Gertz M.A., Lacy M.Q., Buadi F.K., Hayman S.R., Leung N., Dingli D., Lust J.A., Ketterling R.P., Lin Y., Russell S., Hwa L., Kapoor P., Go R.S., Zeldenrust S.R., Kyle R.A., Rajkumar S.V., Dispenzieri A. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015;5(5):e310. doi: 10.1038/bcj.2015.34. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avet-Loiseau H., Attal M., Moreau P., Charbonnel C., Garban F., Hulin C., Leyvraz S., Michallet M., Yakoub-Agha I., Garderet L., Marit G., Michaux L., Voillat L., Renaud M., Grosbois B., Guillerm G., Benboubker L., Monconduit M., Thieblemont C., Casassus P., Caillot D., Stoppa A.M., Sotto J.J., Wetterwald M., Dumontet C., Fuzibet J.G., Azais I., Dorvaux V., Zandecki M., Bataille R., Minvielle S., Harousseau J.L., Facon T., Mathiot C. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489‐3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]