Abstract
Individuals with pseudoexfoliation (PEX) syndrome exhibit various connective tissue pathologies associated with dysregulated extracellular matrix homeostasis. PEX glaucoma is a common, aggressive form of open-angle glaucoma resulting from the deposition of fibrillary material in the conventional outflow pathway. However, the molecular mechanisms that drive pathogenesis and genetic risk remain poorly understood. PEX glaucoma-associated single-nucleotide polymorphisms are located in and affect activity of the promoter of LOXL1-AS1, a long non-coding RNA (lncRNA). Nuclear and non-nuclear lncRNAs regulate a host of biological processes, and when dysregulated, contribute to disease. Here we report that LOXL1-AS1 localizes to the nucleus where it selectively binds to the mRNA processing protein, heterogeneous nuclear ribonucleoprotein-L (hnRNPL). Both components of this complex are critical for the regulation of global gene expression in ocular cells, making LOXL1-AS1 a prime target for investigation in PEX syndrome and glaucoma.
Introduction
Pseudoexfoliation (PEX) syndrome, also commonly known as exfoliation syndrome (XFS) (OMIM #177650), is a systemic disorder with many pathologic manifestations associated with dysregulation of extracellular matrix production and function in connective tissues (1), but it is most widely recognized as an age-related fibrillopathy linked to cataract and glaucoma (2). PEX glaucoma is an aggressive ocular manifestation of PEX syndrome characterized by fibrillary deposits found throughout the eye, including the conventional outflow pathway, which regulates intraocular pressure (IOP) (1,3–6). PEX glaucoma is a complex multifactorial disease, commonly presenting with elevated IOP and having an extremely strong genetic component (3,7,8). As a genetically distinct form of glaucoma, there is no overlap between genetic loci associated with PEX glaucoma and with other forms of glaucoma (9–12). Specific variants found within the LOXL1 locus are strongly associated with risk of PEX glaucoma, and are found in every population studied to date (7,13,14). Surprisingly, the same common alleles that increase risk for PEX glaucoma in one ethnic population reduce risk in other populations (15). This allelic reversal confounds our understanding of the role that the LOXL1 locus plays in the pathobiology of PEX glaucoma and suggests complex genetic interactions that contribute to disease. Importantly, there are multiple phenotypes that characterize PEX and PEX glaucoma, and there are multiple genes and pathways associated with the disease that can be altered to different extents in each patient, making PEX glaucoma an enigmatic disease to study.
Located within the LOXL1 locus is the gene encoding the LOXL1 protein and the LOXL1-AS1 lncRNA (ENSG00000261801). LOXL1 and LOXL1-AS1 (15) are transcribed from opposite strands of DNA, share no sequence homology and are distinct biological entities. Our initial study showed that variants located within the promoter region of LOXL1-AS1 associate with PEX glaucoma risk, that these variants affect the activity of the promoter and that the promoter activates in response to the glaucoma-relevant stressor, mechanical stretch (15). We have previously shown that there are two common isoforms of LOXL1-AS1, one expressed in fibroblasts (ENST00000566011), and a previously reported isoform that is expressed in all other patient tissues examined (Supplementary Material, Fig. S1) (15). LOXL1-AS1 regulates endothelial to mesenchymal transition as well as proliferation and metastasis in certain cancers (16–20). However, lncRNAs have different roles, depending upon the cell type. Thus, the structural and functional biology of LOXL1-AS1 warrants further investigation, as lncRNAs are emerging as critical regulators of cancer, ocular disease and neurodegenerative disease pathogenesis (18,21,22).
The lncRNAs are a class of abundant, non-coding, non-translated RNAs that are >200 base pairs in size (23). More than 27 000 lncRNAs have been identified in the human genome (24). lncRNAs perform a variety of molecular functions including regulation of gene expression. Importantly, lncRNAs often complex with protein-binding partners to perform their cellular functions (25–27). For example, recent studies have found significant interactions between hnRNPL and lncRNAs in regulating gene expression (26–28). Heterogeneous ribonucleoproteins (hnRNPs) are a large family of proteins that play a major role in the formation, packaging, processing and function of mRNA.
Since LOXL1-AS1 has not been identified in mice and there are no knockout cell lines or model organisms in which LOXL1-AS1 expression is ablated, human tissues or cell lines must be used for in vitro studies. In the present study, we investigated the structure, major binding partners and mechanistic role of LOXL1-AS1 in PEX glaucoma-relevant cells. HLE-B3 cells have been used in previous studies of glaucoma, and LOXL1-AS1 increases with an increase in oxidative stress to HLE-B3 cells (15). Significantly, our data indicate for the first time that the lncRNA, LOXL1-AS1, which is genetically associated with PEX glaucoma, binds hnRNPL and regulates gene expression in immortalized human lens cells.
Results
LOXL1-AS1 predicted secondary structures
The structure of lncRNAs is important for determining their functional roles as epigenetic regulators in biological pathways (29). LncRNA secondary structures include such formations as bulge loops, inner loops, multi-branch loops, stem, hairpins and pseudoknots (30). These formations, along with conserved regions of protein-binding sequences, direct the types of interactions that lncRNAs have with RNA, DNA and protein-binding partners. The novel isoform of LOXL1-AS1 includes two introns not present in the dermal fibroblast isoform of LOXL1-AS1 (ENST00000566011) due to alternative splicing, making it a slightly shorter lncRNA (see sequences in Supplementary Material, Fig. S1). Computationally predicted secondary structures of both isoforms are based on energetic favorability of covalent binding, and indicate regions of the many known secondary structure formations (Fig. 1) (30). These secondary structure formations, including hairpins and bulged nucleotides, can function as interactor elements or structural elements that mitigate interactions with other cellular proteins (31,32). The novel isoform of LOXL1-AS1 is the predominant isoform present in numerous ocular and systemic tissues (15), and thus the focus of the present study.
Figure 1.
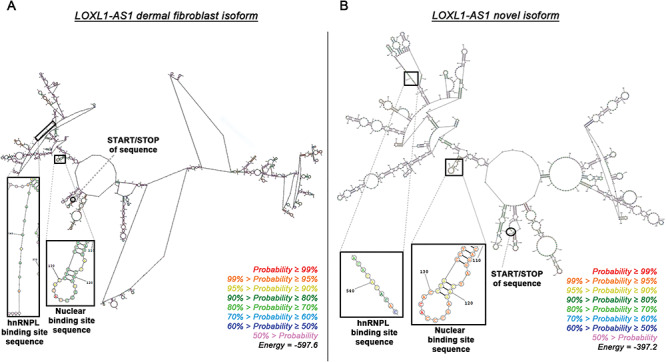
Predicted secondary structures of the LOXL1-AS1 isoforms. (A) The known isoform of LOXL1-AS1 (ENST00000566011) found in dermal fibroblast cells contains two more introns of sequence, and therefore has a vastly different predicted conformation from (B) the novel isoform of LOXL1-AS1 that is found in many major organ and ocular tissues. Individual nucleotides are colored based on the probability of forming the illustrated secondary structure. The overall illustrations are the predicted secondary structures based on the lowest free energy (Joules) required to create covalent bonds.
LOXL1-AS1 is a nuclear lncRNA
In comparison to mRNAs, lncRNAs are usually expressed at lower levels and are shorter in length (33). Importantly, lncRNAs often localize to the nucleus (33), with some forming nuclear bodies (33,34), indicating potential activity in regulating gene transcription. For this reason, we examined the subcellular localization of LOXL1-AS1. The consensus sequence (A/T)NNNN(G/C)NNAGCCC drives nuclear localization (35), and analysis of the LOXL1-AS1 sequence revealed a match, with base pairs 115–126 having the sequence ANNNNCNNAGCCC (Figs 1B and 2A). Using probes complimentary to LOXL1-AS1, fluorescence in situ hybridization analysis in HEK293 cells showed that the lncRNA is predominantly localized to the nucleus (Fig. 2B–I), while the cytoplasmic control, GAPDH, was excluded from the nucleus (Fig. 2B–I). Quantification of nuclear fluorescence from the confocal images confirmed there was significantly greater abundance of LOXL1-AS1 in the nucleus in comparison to the cytoplasm (P = 0.0139, n = 4) (Fig. 2J). This nuclear localization suggests a functional role of LOXL1-AS1 interacting with DNA or nuclear proteins.
Figure 2.

LOXL1-AS1 localizes to the nucleus in human embryonic kidney (HEK293) cells. (A) The nuclear localization motif and corresponding sequence in LOXL1-AS1 (red). (B–I) Immunofluorescence microscopy of HEK293 cells with fluorescence in situ hybridization (FISH) control and LOXL1-AS1-targeted FITC probes (green), DAPI nuclear labeling (blue) and GAPDH-TRITC cytoplasmic labeling (red). (J) Box and whisker plot showing fluorescence quantification of nuclear and cytoplasmic FITC fluorescence quantification indicates significantly higher nuclear localization of LOXL1-AS1 P = 0.0139. n = 4 biological replicates. Each data point is the average of cell compartment fluorescence measurements from 15 individual cells within one biological replicate. Student’s t-value (t) = 8.455. Degrees of freedom (df) = 73.54. Scale bar, 10 μm.
LOXL1-AS1 complexes with hnRNPL
lncRNAs commonly function within the nucleus in conjunction with proteins in ribonucleoprotein particles (RNPs) (26–28). Using the RBPmap server, the LOXL1-AS1 novel isoform is predicted to bind to a subset of 66 proteins (z-score < 2) based on binding sequences (data not shown) (36). To determine in vitro protein-binding partner(s), biotinylated LOXL1-AS1 and biotinylated scrambled control RNA of the same length were incubated with nuclear proteins from immortalized human lens epithelial (HLE-B3) cells, RNPs were isolated using streptavidin agarose and protein-binding partners were identified via mass spectrometry (MS) analysis (Supplementary Material, Fig. S2). Acceptance criteria for LOXL1-AS1 selectively bound proteins required identification of at least two peptides in each duplicate sample for each protein with a protein confidence interval percentage, CI% over 99%, corresponding to a false discovery rate of <1%. MS analysis revealed two candidates that met the selection criteria: heterogenous ribonucleoprotein L (hnRNPL) and eukaryotic peptide chain release factor GTP-binding subunit 3A (ERF3A), a molecule that mediates translation termination in eukaryotes (37). Western blot studies confirmed the complex of LOXL1-AS1 and hnRNPL, but ERF3A bound non-specifically to both the LOXL1-AS1 and control RNA (Fig. 3A and B). To verify the LOXL1-AS1/hnRNPL complex in a physiological system, endogenous hnRNPL was immunoprecipitated from HLE-B3 cell nuclear lysates (Fig. 3C). RNA was extracted from the pellet, cDNA was synthesized and PCR analysis confirmed a significant enrichment in levels of LOXL1-AS1 in the hnRNPL pulldown compared to control (P = 0.0297, n = 4) (Fig. 3D).
Figure 3.

LOXL1-AS1 binds hnRNPL via the CANACA binding motif in immortalized human lens (HLE-B3) cells. (A) Western blot analysis showing hnRNPL protein specifically bound to LOXL1-AS1 and not control lncRNA after streptavidin immunoprecipitation. (B) Western blot analysis of non-specific binding of ERF3a to LOXL1-AS1 and control lncRNA. (C) Immunoprecipitation of endogenous hnRNPL from nuclear extract of HLE-B3 cells with (D) RT-qPCR quantitation of endogenous LOXL1-AS1 in complex. P = 0.0297. n = 4 biological replicate HLE-B3 cell samples. Each data point represents the mean of technical triplicate qPCR reactions. t = 2.05. df = 3.268 (E) Canonical hnRNPL binding motif and the overlapping repeat region within the LOXL1-AS1 sequence. Western blot analysis shows recombinant hnRNPL binding to WT, not Δ14-LOXL1-AS1, and quantitation of (F) WT and Δ14-LOXL1-AS1 bound to hnRNPL indicates significantly more WT LOXL1-AS1 bound to hnRNPL P = 0.0457. n = 3 biological replicate HLE-B3 cell samples. Each data point is the mean of technical triplicate qPCR reactions. t = 2.813. df = 2.102.
hnRNPL typically binds RNA via a CANACA motif (38). Sequence analysis of LOXL1-AS1 revealed a 14-base pair region containing three overlapping CANACA motifs [bases 115–126 of LOXL1-AS1 (Figs 1B and 3E)]. To test for functionality of the hnRNPL binding site, biotinylated wild-type (WT) LOXL1-AS1 and a deletion construct (Δ14-LOXL1-AS1), which lacks the 14-base pair CANACA repeat region, were incubated with recombinant GST-tagged hnRNPL, complexes were pulled down with streptavidin agarose and subjected to western blot analysis. These co-precipitation experiments show that the 14-base pair CANACA region is required for binding of LOXL1-AS1 to hnRNPL (Fig. 3E and F).
LOXL1-AS1 regulates gene expression
lncRNAs, particularly nuclear lncRNAs, often regulate the expression of multiple genes (33). To profile quantitatively the transcriptional targets of LOXL1-AS1, RNA-seq was performed on HLE-B3 cells after manipulation of LOXL1-AS1 levels. First, RNA-seq analysis was executed on HLE-B3 cells subjected to siRNA knockdown of LOXL1-AS1 (log2FC = −2.45, P = 3.22 × 10−136) or scrambled siRNA. Genes with a P-value of less than 0.01 were chosen, the expression of each gene was transformed to a z-score based on expression across all samples and the samples were clustered using correlation as a distance metric to produce heat maps (Fig. 4). Knockdown of LOXL1-AS1 by 82% significantly dysregulated over 450 RNAs (P < 0.01), including signaling and cytoskeletal targets such as natriuretic peptide B (NPPB), microfibril-associated protein 5 (MFAP5), Golgi-associated secretory pathway pseudokinase (FAM20A) and Selenoprotein P (SEPP1) (Fig. 4). Gene set enrichment analysis and gene ontology analysis revealed that pathways classically associated with PEX glaucoma were altered by reducing levels of LOXL1-AS1, including TGFβ-1 targets (upregulated) and MMP activity (downregulated) (Supplementary Material, Fig. S3). Using Ingenuity Pathway Analysis (IPA) software, top predicted canonical pathways, upstream regulators and causal networks were identified (Supplementary Material, Fig. S4). The top canonical pathways predicted to be involved in changes caused by LOXL1-AS1 knockdown including calcium signaling, caveolar-mediated endocytosis signaling and eNOS signaling, are all known to participate in the regulation of outflow resistance and thus IOP control (1,39,40).
Figure 4.
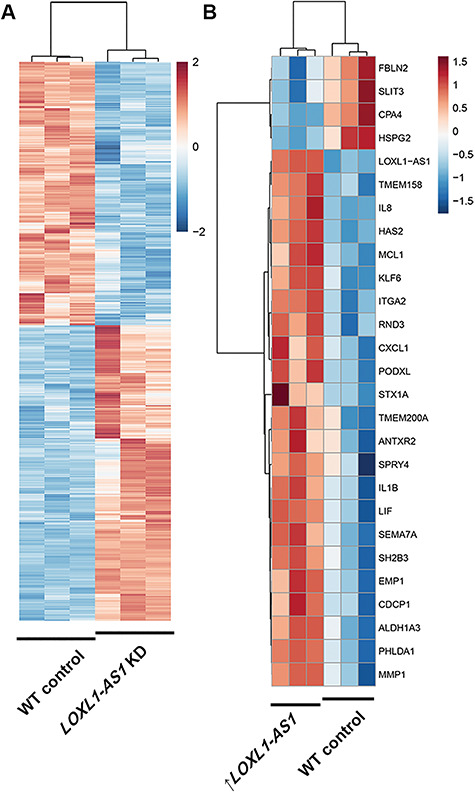
Alteration of LOXL1-AS1 lncRNA expression dysregulates downstream gene targets in immortalized HLE-B3 cells. RNAseq analysis heat maps with z-score thresholds set to +2 and − 2 for knockdown experiments and thresholds set to +1.5- and −1.5-fold change for overexpression experiments. (A) Knockdown of LOXL1-AS1 (log2FC = −2.45, P = 3.22 × 10−136) using a targeted siRNA leads to significant differential expression of over 450 gene targets in HLE-B3 cells. n = 3 biological replicate HLE-B3 samples per control or siRNA treatment. (B) Overexpression of LOXL1-AS1 also leads to significant changes in the expression of 27 genes. n = 3 biological replicate HLE-B3 samples per control or overexpression plasmid transfection treatment.
To determine whether increased expression of LOXL1-AS1 alters downstream target expression, RNA-seq was performed on HLE-B3 cells expressing additional copies of LOXL1-AS1. Notably, LOXL1-AS1 was driven by the native LOXL1 promoter resulting in a 2-fold increase in expression over controls (Supplementary Material, Fig. S5D). Overexpression of LOXL1-AS1 significantly altered 27 other genes including Fibulin 2 (FBN2), heparin sulfate proteoglycan 2 (HSPG2), Ras pathway-associated transmembrane protein 158 (TMEM158) and hyaluronan synthase 2 (HAS2) (P < 0.01) (Fig. 4B). Importantly, this modest increase in LOXL1-AS1 is within the inducible physiological range of LOXL1-AS1 in Schlemm’s canal endothelial cells under mechanical stress (15). Select RNAseq targets were validated using qPCR (Supplementary Material, Fig. S5). Gene expression changes of this magnitude likely lead to downstream post-translational modifications and signaling changes.
Discussion
This study elucidates for the first time the biological function of the novel isoform of LOXL1-AS1 in ocular cells. It was previously determined that single-nucleotide polymorphisms in the LOXL1-AS1 promoter impart risk for PEX glaucoma, a major cause of blindness (15). It was also found that LOXL1-AS1 expression in immortalized human lens cells significantly decreased in the presence of oxidative stress (15). In comparison to primary lens cells, immortalized human lens cells are more easily cultured, transfected and provide reliable quantities of sample for molecular analysis. Here, we found that LOXL1-AS1 localizes to the nucleus, binds hnRNPL and regulates the expression of a large gene set in immortalized lens cells.
LOXL1-AS1 binds hnRNPL in the nucleus and regulates downstream gene expression, but the mechanism by which this complex alters gene expression remains unknown. One possibility is that the LOXL1-AS1/hnRNPL complex binds directly to chromatin (Fig. 5A). The binding of LOXL1-AS1 to hnRNPL relies upon the CANACA binding site, which when deleted, prevents this binding (Figs 3F and 5B). LncRNA/hnRNP complexes are known to regulate gene expression via various mechanisms including alteration of gene transcription, regulation of mRNA stability and translation, plus modification of chromatin structure (28). It is possible that LOXL1-AS1 acts as a scaffold or tether for hnRNPL (and likely other protein-binding partners), localizing the complex to regulatory regions within the genome or to sites of critical premRNA/mRNA processing. Whether the LOXL1-AS1/hnRNPL complex induces or suppresses gene expression by directly altering transcription remains to be investigated, but our data show significant changes in large numbers of genes after altering the abundance of LOXL1-AS1 in PEX glaucoma-relevant cell types. In an attempt to get at this, we also conducted overexpression studies of Δ14-LOXL1-AS1; however, the presence of endogenous wtLOXL1-AS1 in the B3 cells made interpretation of these data impossible. A better understanding of the role hnRNPL protein plays in the regulation of gene expression will require the creation of a LOXL1-AS1 knockout cell line.
Figure 5.

Illustration of LOXL1-AS1/hnRNPL complex gene regulation. Schematic of gene expression regulation by the LOXL1-AS1/hnRNPL complex. LOXL1-AS1 binds hnRNPL via the CANACA binding motif to regulate downstream gene expression.
Many downstream effectors of LOXL1-AS1 are known participants in ECM/cytoskeletal homeostasis. We identified TGFβ-1, MMPs and myocardin (MYOCD) as predicted targets of LOXL1-AS1-mediated gene regulation. Predicted upstream activating regulators included TGFβ-1 and MYOCD (Supplementary Material, Fig. S4), both known to be involved in regulating glaucoma-related ocular hypertension and cytoskeletal reorganization (41–43). The molecular mechanism(s) by which LOXL1-AS1 drives these changes are currently unknown but represent potential therapeutic targets for the treatment of PEX glaucoma. TGFβ-1 is upregulated with stress in outflow tissue cells (42), and is upregulated in fibrosis (44). Additionally, TGFβ-1 and MMP2 are upregulated in the aqueous humor of PEX glaucoma patients (45,46), indicating a potential link with their activity and progression of disease. Lastly, MYOCD is known to play a role in regulating conjunctival fibrosis (41), smooth muscle cell differentiation and gene expression (47), and is involved in regulating cell plasticity, myofibroblast activation and fibrogenesis in outflow pathway tissue (48).
An identifying feature of PEX glaucoma is ocular hypertension due to dysfunction in the conventional outflow tract, where IOP is regulated. Thus, defects in mechanosensing are likely involved in conventional outflow dysfunction. Our IPA implicated three cellular processes important for mechanosensing: calcium signaling, caveolar-mediated endocytosis signaling and eNOS signaling. Significantly, the PEX-associated gene CACNA1A encodes a calcium ion channel (1), and caveolar-mediated endocytosis signaling via Cav1 (39) and shear stress-induced eNOS signaling (40) modulate outflow cell mechanotransduction properties that alter IOP. In previous work, Schlemm’s canal cells were shown to have increased levels of LOXL1-AS1 in response to mechanical stretch, a process known to induce cellular stress (15).
PEX glaucoma is a disease hallmarked by fibrous material accumulation and increased rigidity of the outflow pathway, which will also affect mechanosensing (49). To date, no therapeutic treatment is available to combat this pathology, which affects ~7 million people worldwide. Here, we describe LOXL1-AS1 as a hub regulator of several processes critical for proper functioning of the conventional outflow pathway. These include ECM, inflammatory and fibrogenic components, all of which have been implicated in PEX glaucoma.
Materials and Methods
LOXL1-AS1 RNA structure and binding partner predictions
Comparing the RNA sequences for ENST00000566011 and the novel isoform of LOXL1-AS1, RNA structure predictions were determined using the online web server: https://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html (50). The structure with the lowest energy (J) conformation was chosen for Figure 1. To determine potential RNA-binding protein partners, the LOXL1-AS1 RNA sequences were entered into the RBPmap web server: http://rbpmap.technion.ac.il/ (36). Predicted binding partners were determined by analyzing nucleotide sequence stretches on the LOXL1-AS1 RNA, and 66 protein hits with a z-score of greater than +2 or less than −2 were found. These data are available upon request.
Cell culture
Human embryonic kidney (HEK293) cells were cultured in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% fetal bovine serum (FBS) (#s11550) (Atlanta Biologicals, Flowery Branch, GA) and incubated at 37°C and 5% CO2. HEK293 cells were plated to 70–80% confluency on 18 mm round #1 coverglass in a 12-well culture plate prior to fixation and FISH. Immortalized human lens epithelial (HLE-B3) cells were cultured in high glucose DMEM (−) sodium pyruvate, HEPES (#11965092, Thermo Fisher Scientific, Raleigh, NC) supplemented with 20% FBS and incubated at 37°C and 5% CO2. Cells were grown to at least 70–80% confluency prior to harvest for analysis.
Fluorescence in situ hybridization (FISH)
Probes complimentary to LOXL1-AS1 were generated using Stellaris® Probe Designer version 4.2. Target sequences can be found in Supplementary Material, Table S1. Control Q-570 conjugated GAPDH probes and fluorescein (FITC)-conjugated LOXL1-AS1 probes were purchased from LGC Biosearch Technologies (Petaluma, CA). FISH was performed on human embryonic kidney (HEK293A) cells (#CRL-11268™) from ATCC® (Manassas, VA) following the manufacturer’s specific Stellaris RNA FISH alternative protocol for adherent cells. To visualize nuclei, coverslips were mounted using 10 μl of mowiol 4-88 containing 1:5000 4′,6-diamidino-2-phenylindole (DAPI) from Sigma-Aldrich (St. Louis, MO). Cells were viewed and imaged using a Nikon Eclipse 90i confocal laser-scanning microscope from Nikon Instruments (Melville, NY) equipped with three lasers with light emission at 405, 488 and 561 nm to detect DAPI, LOXL1-AS1-FITC and GAPDH-TRITC respectively. Images were generated using all three lasers to detect the specific probes at identical optimized settings at the total magnification of 1000× (oil immersion objective). Images were taken of cells hybridized with probes against GAPDH and LOXL1-AS1. A total of 60 cells were chosen at random for quantification of area. The nucleus and the cytoplasm of each cell were manually identified and the intensity of pixels of fluorescence present was separately quantified for each randomly chosen cell using Fiji imaging software (51).
Generation of biotinylated RNA
Biotinylated RNA was generated using pUC57 plasmids from Origene (Rockville, MD) that contained a T7 promoter followed immediately by the LOXL1-AS1 sequence, or control RNA terminating with an EcoRV site. Generation of the deletion construct was completed by sequential site-directed mutagenesis using the QuickChange II XL Site Directed Mutagenesis Kit (#200521) from Agilent Technologies (Santa Clara, CA) following the manufacturer’s protocols. Each reaction removed seven base pairs resulting in the deletion of base pairs 532–545 in the LOXL1-AS1 sequence. Sequence verification was performed by Genewiz (South Plainfield, NJ). Plasmids were linearized using EcoRV following manufacturer’s protocols (NEB) and they were gel purified using Zymoclean Gel DNA recovery Kit (Zymo Research). The biotinylated RNA was generated using the AmpliScribe™ T7-Flash™ Biotin-RNA Transcription Kit (#ASB71110) from LGC Biosearch Technologies (Petaluma, CA) following the manufacturer’s protocols.
RNA–protein complex isolation
RNA–protein complexes were isolated following a previously described protocol (52). Briefly, immortalized human lens epithelial (HLE-B3) (#CRL-11421™) (ATCC®, Manassas, VA) cells were trypsinized, washed with PBS, and re-suspended in nuclear isolation buffer, incubated on ice for 20 min, and nuclei were harvested by centrifugation at 2000 RCF for 15 min at 4°C. The nuclear fraction was re-suspended in RIPA buffer including proteinase and RNAse inhibitors, and then it was mechanically homogenized through Dounce shearing. The cell membrane and debris fraction was separated by centrifugation at 15 000 RCF for 15 min at 4°C. Nuclear lysate was then pre-cleared by incubating 60 μl of streptavidin agarose (Pierce, Waltham, MA) for 1 h. Then 10 pmol of biotinylated RNA was incubated with the nuclear lysate for 2 h. A volume of 60 μl of streptavidin agarose was then incubated with the RNA/nuclear proteins for 1 h. Agarose was spun down at 950 RCF for 3 min and washed five times using NT2 buffer (Tris-HCl, pH 7.4 (50 mm), NaCl (150 mm), MgCl2 (1 mm), Nonidet P-40 (0.05%) and H2O. Proteins were eluted using Laemmli buffer and run on an SDS page followed by mass spectrometry, or proteins were analyzed via western blot. Then, 5 μg of hnRNPL or 5 μg of isotype antibody control (anti-GST) was incubated with nuclear lysate and Pierce™ protein G agarose (#20398) (Thermo Fisher Scientific, Raleigh, NC). Agarose was spun down and washed four times, and an aliquot was removed and used for confirmation of pulldown via western blot analysis. RNA was isolated from the remaining agarose using TRIZol™ reagent (#15596026) (Thermo Fisher Scientific, Raleigh, NC) following the manufacturer’s protocols.
Mass spectrometry
LOXL1-AS1 and control RNA–protein complexes were isolated as described above (in duplicate), except streptavidin-conjugated dynabeads were used in place of agarose. Protein elution was completed by incubating the samples in 2% SDS and 10 mm DTT for 15 min at 50°C. Samples for mass spectrometry analysis were prepared as previously described (53). Proteins were alkylated with 1 M iodoacetamide for 30 min. Then, 2 μl of hydrophobic and hydrophilic carboxylate modified Sera-Mag Speed beads (#24152105050250 and #44152105050250) (GE Healthcare Life Sciences, Marlborough, MA) were added. The mixture was acidified with 0.25% formic acid and equal volumes of acetonitrile was added and incubated for 10 min at RT. Using a magnet, beads were washed twice with 70% EtOH and then acetonitrile. Proteins were digested overnight using 300 ng trypsin (Promega, Madison, WI) in 50 mm ammonium bicarbonate at 37°C. The resulting peptides were eluted in 2% DMSO/0.2% formic acid and dried using a speedvac. Peptide mixes obtained from in-on-bead-digest were analyzed using a nanoAcquity UPLC system coupled to a Synapt G2 HDMS mass spectrometer (Waters Corporation, Milford, MA). Peptides were separated on a 75 μm × 100 mm column with 1.7 μm C18 BEH (Ethylene Bridged Hybrid) particles (Waters Corporation) using a 30 min gradient of 6–32% acetonitrile with 0.1% formic acid at a flow rate of 0.3 μl/min and 45°C column temperature. For each sample, we conducted a data-dependent analysis (DDA) using a 0.8 s mass spectrometry (MS) scan followed by MS/MS acquisition on the top three ions with charge greater than one. MS/MS scans for each ion used an isolation window of ∼3 Da, a maximum of 3 s per precursor and dynamic exclusion for 120 s within 1.2 Da. DDA data were converted to searchable files using ProteinLynx Global Server 2.5 (Waters Corporation) and searched against the Uniprot human database (2016) using Mascot server 2.2 with the following parameters: maximum of one missed cleavage site, carbamidomethylation at Cys residues as fixed modification and Met oxidation, Asn, Gln deamidation as variable modifications. Precursor ion mass tolerance was set to 20 ppm, while fragment mass tolerance to 0.2 Da. Mascot data were imported into Scaffold 4.4 (Proteome Software Inc., Portland, OR) to merge all the data for a sample, identify a false discovery rate for protein identification, group proteins and perform spectral counting-based protein quantification. Acceptance criteria for protein identification required identification of at least two peptides in each duplicate sample for each protein with a protein confidence interval percentage, CI% over 99%, corresponding to a false discovery rate of <1%.
RNA extraction and RNA-seq
RNA extraction was completed using either the mirVANA kit or TRIZol (Thermo Fisher Scientific, Raleigh, NC) following manufacturer’s protocol. RNA-seq data were processed using the TrimGalore toolkit (24), which employs Cutadapt (54) to trim low-quality bases and Illumina sequencing adapters from the 3′ end of the reads. Only reads that were 20 nt or longer after trimming were kept for further analysis. Reads were mapped to the GRCh37v73 version of the human genome and transcriptome (55) using the STAR RNA-seq alignment tool (56). Reads were kept for subsequent analysis if they mapped to a single genomic location. Gene counts were compiled using the HTSeq tool (28). Only genes that had at least 10 reads in any given library were used in subsequent analysis. Normalization and differential expression was carried out using the DESeq2 (57) Bioconductor (58) package with the R statistical programming environment (55). The false discovery rate was calculated to control for multiple hypothesis testing. Gene set enrichment analysis (59) was performed to identify differentially regulated pathways and gene ontology terms for each of the comparisons performed, and the FDR q-value limit was set at 0.05. Data were also analyzed for predicted upstream regulators through the use of Ingenuity Pathway Analysis (QIAGEN Inc.), https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis. Significantly enriched pathways and predicted upstream regulators were selected based on z-scores greater than 2 or less than −2, with P-values less than 0.05.
Quantitative PCR
Template cDNA was generated using approximately 1 μg of isolated RNA per sample. cDNA was generated using iSCRIPT cDNA synthesis reaction kit (Bio-Rad, Hercules, CA) following manufacturer’s protocols. Quantitative PCR was completed using iQ SYBR green (BioRAD, Hercules, CA) using the CFX96 Real Time System on a C1000 Thermal cycler (Bio-Rad), using Taqman assays (Hs00173746, LOXL1-AS1; Hs006110320, TGFβR1; Hs01548727_m1, MMP2; Hs00957562_m1, MMP9; Hs00420895, RPLP0), or using Qiagen ECM target array plates (PAHS-013ZD) on a Viia7 (Thermo Fisher Scientific, Raleigh, NC) both following manufacturer’s protocols.
Knockdown of LOXL1-AS1
Immortalized human lens epithelial (HLE-B3) cells (#CRL-11421) from ATCC (Manassas, VA) were seeded in 6-well cell culture plates at a density of 3 × 105 cells/well. After 18 h of growth, a siRNA targeted to LOXL1-AS1 (#SI05724243) and a negative control scramble siRNA (#SI03650318) from QIAGEN (Germantown, MD) were transfected using the Lipofectamine® RNAiMAX reagent from Invitrogen (Waltham, MA) according to the manufacturer’s protocol. Each siRNA was tested in triplicate. The LOXL1-AS1 siRNA targeted a sequence that was present in the novel LOXL1-AS1 splice variant that we previously isolated in multiple ocular and systemic tissues. Total RNA was extracted 48 h post-transfection using the mirVANA™ miRNA isolation kit (#AM1560) from Thermo Fisher Scientific (Raleigh, NC) and cDNA was synthesized using the iScript™ cDNA synthesis kit (#1708890) from Bio-Rad (Philadelphia, PA) as per manufacturers’ protocols.
Overexpression of LOXL1-AS1
To obtain vectors carrying LOXL1-AS1, Δ14-LOXL1-AS1 or the compliment sequence, pENTR1A plasmids containing the LOXL1 promoter followed immediately by the novel isoform of LOXL1-AS1 (Supplementary Material, Fig. S1B), Δ14-LOXL1-AS1 or the compliment sequence of LOXL1-AS1 followed by the BGH polyA tail were purchased from Genewiz (South Plainfield, NJ). Prior to plasmid transfection, 4 × 105 HLE-B3 cells were seeded into each well of a 6-well dish. Then, 1.5 μg of plasmid DNA per well was transfected using Lipofectamine 2000 following the manufacturer’s protocols. After 48 h, RNA was harvested using the miRVana RNA extraction kit (#AM1560) (Thermo Fisher Scientific, Raleigh, NC) following manufacturer’s protocols.
Endogenous LOXL1-AS1/hnRNPL isolation
Nuclear lysates were obtained in the same manner as described above except the input for this experiment required 10-fold more nuclear lysate. Then, 5 μg of hnRNPL or 5 μg of isotype antibody control (anti-GST) was incubated with nuclear lysate and Pierce™ protein G agarose (#20398) (Thermo Fisher Scientific, Raleigh, NC). Agarose was spun down and washed four times, and an aliquot was removed and used for confirmation of pulldown via western blot analysis. RNA was isolated from the remaining agarose using TRIZol™ reagent (#15596026) (Thermo Fisher Scientific, Raleigh, NC) following the manufacturer’s protocols.
Statistics
The sample size of each experiment is displayed in the figures or in the corresponding figure legends. No statistical methods were used to determine sample size, rather sample sizes were chosen based on population sizes known to yield a power high enough to detect biological changes. Investigators were not blinded to sample conditions. Heat maps were generated with fold change cutoffs at ±1.5 or ±2.0 with P < 0.01. All other quantified data are expressed using box and whisker plots with the min, median and max displayed when n = 3. When n ≥ 4, box and whisker plots detail the min/max, median and upper/lower quartiles of the data set. Thresholding and significance for RNAseq and pathway analysis is described above under RNA extraction and RNAseq. Significance was determined by Welch’s (assuming unequal variance) two-tailed Student’s t-test with P < 0.05 for FISH, western blot and qPCR data. The t-values and degrees of freedom (df) for significant differences are listed in the figure legends.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNAseq data are attached as a supplemental Excel document, and this will be deposited to NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) upon publication of this manuscript.
Supplementary Material
Acknowledgements
We thank the Duke University School of Medicine core research facilities for mass spectrometry analysis and confocal microscopy and the Duke Genomic Analysis and Bioinformatics Shared Resource for RNAseq analysis.
Conflict of Interest Statement. The authors claim no conflict of interest.
Funding
Fight for Sight (to W.M.J.); Research to Prevent Blindness (to W.D.S., R.R.A., M.A.H.); LC Industries (to W.D.S., R.R.A., M.A.H.); Glaucoma Foundation (to W.D.S., R.R.A.); National Institutes of Health (R01EY028608 to M.A.H., R01EY022359 to W.D.S., R01EY019696 to W.D.S., R01EY030617 to W.D.S., M.A.H., P30EY005722 to W.D.S., R.R.A., M.A.H.).
References
- 1. Aboobakar I.F., Johnson W.M., Stamer W.D., Hauser M.A. and Allingham R.R. (2017) Major review: exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp. Eye Res., 154, 88–103. [DOI] [PubMed] [Google Scholar]
- 2. Schlötzer-Schrehardt U. (2009) Molecular pathology of pseudoexfoliation syndrome/glaucoma--new insights from LOXL1 gene associations. Exp. Eye Res., 88, 776–785. [DOI] [PubMed] [Google Scholar]
- 3. Johnson W.M., Finnegan L.K., Hauser M.A. and Stamer W.D. (2018) lncRNAs, DNA methylation, and the pathobiology of exfoliation glaucoma. J. Glaucoma, 27, 202–209. [DOI] [PubMed] [Google Scholar]
- 4. Quigley H.A. (1996) Number of people with glaucoma worldwide. Br. J. Ophthalmol., 80, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritch R. and Schlotzer-Schrehardt U. (2001) Exfoliation syndrome. Surv. Ophthalmol., 45, 265–315. [DOI] [PubMed] [Google Scholar]
- 6. Challa P. and Johnson W.M. (2018) Composition of exfoliation material. J. Glaucoma, 27, S29–S31. [DOI] [PubMed] [Google Scholar]
- 7. Aung T., Ozaki M., Lee M.C., Schlötzer-Schrehardt U., Thorleifsson G., Mizoguchi T., Igo R.P. Jr., Haripriya A., Williams S.E., Astakhov Y. et al. (2017) Genetic association study of exfoliation syndrome identifies a protective rare variant at LOXL1 and five new susceptibility loci. Nat. Genet., 49, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thorleifsson G., Magnusson K.P., Sulem P., Walters G.B., Gudbhartsson D.F., Stefansson H., Johnsson T., Jonasdottir A., Jonasdottir A. and Stefansdottir G. (2007) Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science, 317, 1397–1400. [DOI] [PubMed] [Google Scholar]
- 9. Chakrabarti S., Kaur K., Rao K.N., Mandal A.K., Kaur I., Parikh R.S. and Thomas R. (2009) The transcription factor gene FOXC1 exhibits a limited role in primary congenital glaucoma. Invest. Ophthalmol. Vis. Sci., 50, 75–83. [DOI] [PubMed] [Google Scholar]
- 10. Rao K.N., Ritch R., Dorairaj S.K., Kaur I., Liebmann J.M., Thomas R. and Chakrabarti S. (2008) Exfoliation syndrome and exfoliation glaucoma-associated LOXL1 variations are not involved in pigment dispersion syndrome and pigmentary glaucoma. Mol. Vis., 14, 1254–1262. [PMC free article] [PubMed] [Google Scholar]
- 11. Liu L. (2008) Australia and New Zealand survey of glaucoma practice patterns. Clin. Exp. Ophthalmol., 36, 19–25. [DOI] [PubMed] [Google Scholar]
- 12. Wolf C., Gramer E., Müller-Myhsok B., Pasutto F., Gramer G., Wissinger B. and Weisschuh N. (2010) Lysyl oxidase-like 1 gene polymorphisms in German patients with normal tension glaucoma, pigmentary glaucoma and exfoliation glaucoma. J. Glaucoma, 19, 136–141. [DOI] [PubMed] [Google Scholar]
- 13. Challa P., Schmidt S., Liu Y., Qin X., Vann R.R., Gonzalez P., Allingham R.R. and Hauser M.A. (2008) Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol. Vis., 14, 146–149. [PMC free article] [PubMed] [Google Scholar]
- 14. Tanito M., Minami M., Akahori M., Kaidzu S., Takai Y., Ohira A. and Iwata T. (2008) LOXL1 variants in elderly Japanese patients with exfoliation syndrome/glaucoma, primary open-angle glaucoma, normal tension glaucoma, and cataract. Mol. Vis., 14, 1898–1905. [PMC free article] [PubMed] [Google Scholar]
- 15. Hauser M.A., Aboobakar I.F., Liu Y., Miura S., Whigham B.T., Challa P., Wheeler J., Williams A., Santiago-Turla C., Qin X. et al. (2015) Genetic variants and cellular stressors associated with exfoliation syndrome modulate promoter activity of a lncRNA within the LOXL1 locus. Hum. Mol. Genet., 24, 6552–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao R., Zhang R., Zhang C., Liang Y. and Tang W. (2018) LncRNA LOXL1-AS1 promotes the proliferation and metastasis of medulloblastoma by activating the PI3K/AKT pathway. Anal. Cell. Pathol., 2018, 9275685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long B., Li N., Xu X.X., Li X.X., Xu X.J., Liu J.Y. and Wu Z.H. (2018) Long noncoding RNA LOXL1-AS1 regulates prostate cancer cell proliferation and cell cycle progression through miR-541-3p and CCND1. Biochem. Biophys. Res. Commun., 505, 561–568. [DOI] [PubMed] [Google Scholar]
- 18. Wang C., Li S., Xu J., Niu W. and Li S. (2018) microRNA-935 is reduced in non-small-cell lung cancer tissue, is linked poor outcome, and acts on signal transduction mediator E2F7 and the AKT pathway. Br. J. Biomed. Sci., 76, 17–23. [DOI] [PubMed] [Google Scholar]
- 19. Chen S., Li W. and Guo A. (2019) LOXL1-AS1 predicts poor prognosis and promotes cell proliferation, migration, and invasion in osteosarcoma. Biosci. Rep., 39, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bai T., Liu Y. and Li B. (2019) LncRNA LOXL1-AS1/miR-let-7a-5p/EGFR-related pathway regulates the doxorubicin resistance of prostate cancer DU-145 cells. IUBMB Life, 71, 1537–1551. [DOI] [PubMed] [Google Scholar]
- 21. Fu Q., Qin Z., Zhang L., Lyu D., Tang Q., Yin H., Chen X. and Yao K. (2017) A new long noncoding RNA ALB regulates autophagy by enhancing the transformation of LC3BI to LC3BII during human lens development. Mol Ther Nucleic Acids, 9, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou W., Wang J., Qi Q., Feng Z., Huang B., Chen A., Zhang D., Li W., Zhang Q., Bjerkvig R. et al. (2018) Matrine induces senescence of human glioblastoma cells through suppression of the IGF1/PI3K/AKT/p27 signaling pathway. Cancer Med., 7, 4729–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long Y., Wang X., Youmans D.T. and Chech T.R. (2017) How do lncRNAs regulate transcription? Sci. Adv., 3, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hon C.-C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J.L., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J. et al. (2017) An atlas of human long non-coding RNAs with accurate 5′ ends. Nature, 543, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y.W., Flynn R.A., Chen Y., Qu K., Wan B., Wang K.C., Lei M. and Chang H.Y. (2014) Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. elife, 3, e02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klingenberg M., Groß M., Goyal A., Polycarpou-Schwarz M., Miersch T., Ernst A., Leupold J., Patil N., Warnken U., Allgayer H. et al. (2018) The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein L form a complex and coregulate genes linked to AKT signaling. Hepatology, 68, 1817–1832. [DOI] [PubMed] [Google Scholar]
- 27. Li Z., Chao T.-C., Chang K.-Y., Lin N., Patil V.S., Shimizu C., Head S.R., Burns J.C. and Rana T.M. (2014) The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. USA, 111, 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun X., Haider Ali M.S.S. and Moran M. (2017) The role of interactions of long non-coding RNAs and heterogeneous nuclear ribonucleoproteins in regulating cellular functions. Biochem. J., 474, 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L.K., Chen X.F., He D.D., Li Y. and Fu J. (2017) Dissection of functional lncRNAs in Alzheimer’s disease by construction and analysis of lncRNA-mRNA networks based on competitive endogenous RNAs. Biochem. Biophys. Res. Commun., 485, 569–576. [DOI] [PubMed] [Google Scholar]
- 30. Talkish J., May G., Lin Y., Woolford J.L. Jr. and McManus C.J. (2014) Mod-seq: high-throughput sequencing for chemical probing of RNA structure. RNA, 20, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fabbri M., Girnita L., Varani G. and Calin G.A. (2019) Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res., 29, 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helder S., Blythe A.J., Bond C.S. and Mackay J.P. (2016) Determinants of affinity and specificity in RNA-binding proteins. Curr. Opin. Struct. Biol., 38, 83–91. [DOI] [PubMed] [Google Scholar]
- 33. Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G. et al. (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res., 22, 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ip J.Y. and Nakagawa S. (2012) Long non-coding RNAs in nuclear bodies. Develop. Growth Differ., 54, 44–54. [DOI] [PubMed] [Google Scholar]
- 35. Zhang B., Gunawardane L., Niazi F., Jahanbani F., Chen X. and Valadkhan S. (2014) A novel RNA motif mediates the strict nuclear localization of a long noncoding RNA. Mol. Cell. Biol., 34, 2318–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paz I., Kosti I., Ares M. Jr., Cline M. and Mandel-Gutfreund Y. (2014) RBPmap: a web server for mapping binding sites of RNA-binding proteins. Nucleic Acids Res., 42, W361–W367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chauvin C., Salhi S., Goff C.L., Viranaicken W., Diop D. and Jean-Jean O. (2005) Involvement of human release factors eRF3a and eRF3b in translation termination and regulation of the termination complex formation. Mol. Cell. Biol., 25, 5801–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ray D., Kazan H., Cook K.B., Wirauch M.T., Najafabadi H.S., Li X., Gueroussov S., Albu M., Zheng H., Yang A. et al. (2013) A compendium of RNA-binding motifs for decoding gene regulation. Nature, 499, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elliott M.H., Ashpole N.E., Gu X., Hernberger L., McClellan M.E., Griffith G.L., Reagan A.M., Boyce T.M., Tanito M., Tamm E.R. et al. (2016) Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci. Rep., 6, 37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashpole N.E., Overby D.R., Ethier C.R. and Stamer W.D. (2014) Shear stress-triggered nitric oxide release from Schlemm’s canal cells. Invest. Ophthalmol. Vis. Sci., 55, 8067–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernando O., Tagalakis A.D., Awwad S., Brocchini S., Khaw P.T., Hart S.L. and Yu-Wai-Man C. (2018) Development of targeted siRNA nanocomplexes to prevent fibrosis in experimental glaucoma filtration surgery. Mol. Ther., 26, 2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liton P.B., Liu X., Challa P., Epstein D.L. and Gonzalez P. (2005) Induction of TGF-beta1 in the trabecular meshwork under cyclic mechanical stress. J. Cell. Physiol., 205, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sethi A., Mao W., Wordinger R.J. and Clark A.F. (2011) Transforming growth factor-β induces extracellular matrix protein cross-linking Lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci., 52, 5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimbori C., Bellaye P.-S., Xia J., Gauldie J., Ask K., Ramos C., Becerril C., Pardo A., Selman M. and Kolb M. (2016) Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J. Pathol., 240, 197–210. [DOI] [PubMed] [Google Scholar]
- 45. Garweg J.G., Zandi S., Gerhardt C. and Pfister I.B. (2017) Isoforms of TGF-β in the aqueous humor of patients with pseudoexfoliation syndrome and a possible association with the long-term stability of the capsular bag after cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol., 255, 1763–1769. [DOI] [PubMed] [Google Scholar]
- 46. Gayathri R., Coral K., Sharmila F., Sripriya S., Sripriya K., Manish P., Shantha B., Ronnie G., Vijaya L. and Narayanasamy A. (2016) Correlation of aqueous humor lysyl oxidase activity with TGF-ß levels and LOXL1 genotype in pseudoexfoliation. Curr. Eye Res., 41, 1331–1338. [DOI] [PubMed] [Google Scholar]
- 47. Tan Z., Li J., Zhang X., Yang X., Zhang Z., Yin K.-J. and Huang H. (2018) P53 promotes retinoid acid-induced smooth muscle cell differentiation by targeting myocardin. Stem Cells Dev., 27, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pattabiraman P.P., Maddala R. and Rao P.V. (2014) Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by rho GTPase signaling. J. Cell. Physiol., 229, 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou E.H., Krishnan R., Stamer W.D., Perkumas K.M., Rajendran K., Nabhan J.F., Lu Q., Fredberg J.J. and Johnson M. (2012) Mechanical responsiveness of the endothelial cell of Schlemm’s canal: scope, variability and its potential role in controlling aqueous humour outflow. J. R. Soc. Interface, 9, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reuter J.S. and Mathews D.H. (2010) RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics, 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsc T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012) Fiji—an open source platform for biological image analysis. Nat. Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feng Y., Hu X., Zhang Y., Zhang D., Li C. and Zhang L. (2014) Methods for the study of long noncoding RNA in cancer cell signaling. Methods Mol. Biol., 1165, 115–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hughes C.S., Foehr S., Garfield D.A., Furlong E.E., Steinmetz L.M. and Krijgsveld J. (2014) Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol., 10, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J, 17, 10–12. [Google Scholar]
- 55. Kersey P.J., Staines D.M., Lawson D., Kulesha E., Derwent P., Humphrey J.C., Hughes D.S.T., Keenan S., Kerhornou A., Koscielny G. et al. (2012) Ensembl genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res., 40, D91–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M. and Gingeras T.R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Love M.I., Huber W. and Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol., 15, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huber W., Carey V.J., Gentleman R., Anders S., Carlson M., Carvalho B.S., Bravo H.C., Davis S., Gatto L., Girke T. et al. (2015) Orchestrating high-throughput genomic analysis with bioconductor. Nat. Methods, 12, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E. et al. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet., 34, 267–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNAseq data are attached as a supplemental Excel document, and this will be deposited to NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) upon publication of this manuscript.


