Abstract
The immune landscape of cancer determines its responsiveness to immunotherapy. Tumors infiltrated with CD8+ T cells (immune-active tumors) are more likely to respond to immunomodulatory agents. However, immune activation often is counterbalanced by strong immunosuppressive mechanisms that are necessary to maintain homeostasis but consequentially can facilitate the survival of cancer cells in the immunocompetent host, a concept defined as compensatory immune suppression. TReg cells contribute to compensatory immune suppression, and therapies targeting the immunosuppressive TReg population are being actively explored. Wang et al. characterize a subset of peripheral circulating CD45–FOXP3hi TReg II cells that phenotypically and functionally parallel the activity of their intratumoral counterparts. The findings are paradigm shifting and may provide a potential liquid002Dbased tool to evaluate the immunosuppressive activity of intratumoral TReg cells; they may also allow temporal assessment of whether the fine balance between immune rejection versus tolerance is achieved with various applied therapies.
Tumors infiltrated with CD8+ T cells (immune-active tumors) are more likely to respond to immunotherapy than tumors where such infiltrates are absent (immune-desert tumors) or are limited to the margins of the cancer nests (immune-excluded). Immune activation, in turn, is evolutionarily dependent upon the coexistence of immune-suppressive mechanisms that inadvertently can facilitate the survival of an immunogenic entity in an immunocompetent host, a concept defined as compensatory immune resistance [1,2]. Wang et al. describe a subset of circulating CD45–FOXP3hi TReg II cells that phenotypically and functionally parallel intratumoral TReg cells a finding that will have several important clinical implications [3]. (i) TReg II (CD45RA–FOXP3hi) cells are increased in the tumor microenvironment (TME) compared with the broader distribution of TReg cell subsets in the peripheral circulation. (ii) The T cell receptor (TCR) repertoire of intratumoral TReg overlaps with that of circulating TReg II T cells but not of other TReg subtypes. (iii) CD25 expression is higher in both the circulating and intratumoral TReg II cells, and may represent a principal mechanism of immune suppression by competing for and/or consuming interleukin (IL)-2. (iv) Localization of TReg II cells is mediated by the CCL1/CCR8 axis, although other chemokine receptors are selectively overexpressed, including CCR4, CCR5, and CXCR6, but not CCR2, CCR10, or CXCR3. (v) TReg II cells are more sensitive to stimulation with immunosuppressive cytokines such as transforming growth factor (TGF)-β or IL-10, and are less sensitive to immunostimulatory cytokines such as IL-6 and inter-feron (IFN)-γ. Sensitivity to these cytokines in pretreatment conditions is associated with worse relapse-free survival (RFS) in breast cancer patients. The predictive value is enhanced when parameters from the four cytokines are combined into a cytokine signaling index (CSI), whereas no correlation with RFS was noted when the CSI of other TReg subpopulations was tested. (vi) The percentage of intratumoral TReg II cells interacting with tumor-associated macrophages (TAMs) is higher among patients with worse RFS and was strongly associated with the CSI of circulating TReg II cells. Moreover, TReg II cell frequency correlates with a reduced prevalence of CD+ T cells, suggesting that interactions between TReg II cells and TAMs cooperate in creating a strong immune-sup-pressive TME, perhaps by augmenting the production of CCL2. (vii) The ratio of CD8 T cells/TReg II cells is inversely correlated with the percentage of TReg II cells adjacent to TAMs. This last observation may represent a driving mechanism of immune suppression because low CD8/TReg ratios have previously been associated with poor clinical outcomes [4,5].
Several thought-provoking questions arise from this study. First, what is the role of the circulating tumor-derived TReg II cells? TReg II cells express CCR8 – does the chemotactic CCL1/CCR8 axis play an ongoing role in peripheral circulating TReg II cells? The authors [3] suggest that ‘peripheral blood TReg II cells represent a major source of intratumoral TReg II cells’. Given that the TCR repertoire of circulating TReg II cells parallels that of intratumoral TReg cells, the origin of the TReg II cells could be through primary exposure to the cancer cells and/or to antigen-presenting cells (APCs) within the TME, and tumor-derived TReg II cells then enter the circulation after being ‘educated’ in the TME and/or tumor-involved draining lymph nodes. Given the continued expression of CCR8 by the circulating immunosuppressive TReg II cells, these specific TReg cells could then be recruited to cancer cells seeded at distant metastatic sites and may play an important role in propagating a systemic immune suppressive microenvironment (Figure 1). If this is the case, are the circulating TReg II cells a crucial immune cell population that should be targeted to reverse systemic immune suppression? Furthermore, the observation that the peripheral TReg II cells and intratumoral TRegs have shared TCR clonal populations brings forth another interesting consideration: can immunogenic tumor antigens expressed by the tumor be identified and tracked over time by characterization of the TCRs expressed on the peripheral circulating TReg II population? This would have significant implications for cellular and/or vaccine-based therapies without a need for invasive tumor biopsy.
Figure 1. Schematic of Hypothesized Systemic Immunosuppressive Effects of T Regulatory (TReg) II Cells.
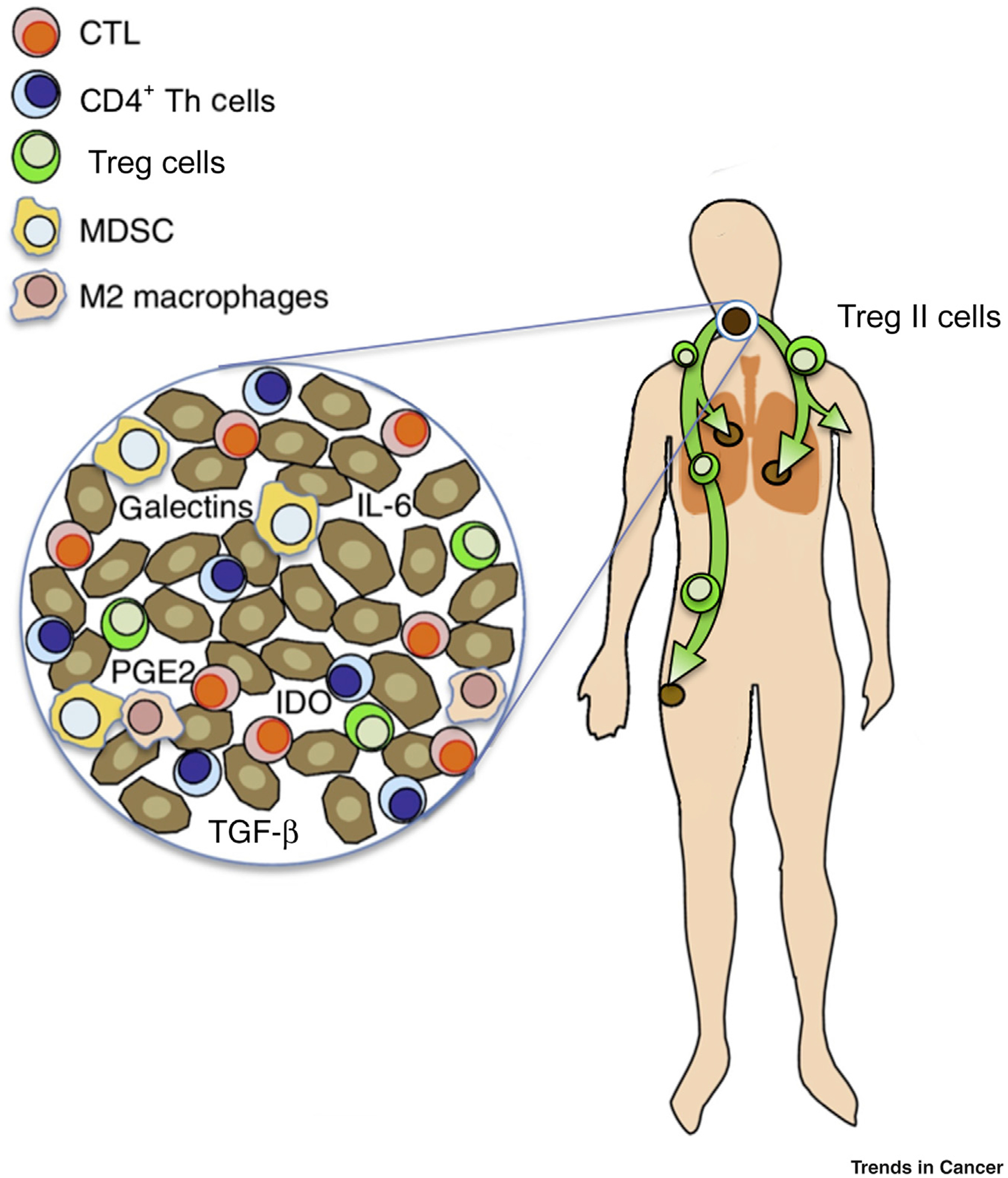
The T cell receptor (TCR) repertoire of circulating TReg II cells parallels that of intratumoral TReg cells. Thus, it is hypothesized that TReg II cells could be primed through primary exposure to the cancer cells and/or antigen-presenting cells (APCs) within the tumor microenvironment (TME), and tumor-derived TReg II cells then enter the circulation after being ‘educated’ in the TME and/or tumor-involved draining lymph nodes. Given the continued expression of CCR8 by the circulating immunosuppressive TReg II cells, these specific TReg cells could then be recruited to cancer cells seeded at distant metastatic sites and may play an important role in propagating a systemic immune-suppressive microenvironment. Abbreviations: CTL, cytotoxic T lymphocyte; IDO, indoleamine 2,3-dioxygenase; IL-6, interleukin 6; MDSC, myeloid-derived suppressor cell; PGE2, prostaglandin E2; Th cell, T helper cell.
The study also highlights the need to investigate the mechanism of TReg interactions with TAMs. Is there a spatial polarization of such interactions that could explain the phenomenon of immune exclusion, a frequent immune phenotype whose etiology remains largely elusive [6]. According to the immunologic constant of rejection (ICR) signature [7], CCR5 and CXCR3 and their respective chemokine ligands are coordinately expressed in immunogenic tumors and are also enriched with immune-suppressive compensatory mechanisms [1,2,8]. However, the selective expression of CCR8 and coex-pression of CCR5, but not CXCR3, by TReg II cells – and the reverse in CD8+ T cells – suggests that these fundamental chemoattractive vectors in the TME may play different roles by attracting distinct immune cell populations, respectively immune-suppressive TReg II cells through CCR5 versus immune-effector CD8+ T cells and other immune-effector cells through CXCR3. This may result in different TReg II cells/CD8+ T cell ratios in distinct patients experiencing various degrees of clinical response.
Wang et al. report their findings in the context of breast cancer patients, and it would be of interest to assess the applicability of their findings to other tumor types. It is increasingly appreciated that CD4+ FoxP3hi T cells are prevalent in areas of tumor inflammation, often outnumbering CD8+ T cells [9,10], suggesting that they may play a dominant role in immune suppression. In oral tongue squamous cell carcinoma (OTSCC) we observed a high prevalence of programmed cell death protein-1 (PD-L1)-expressing cancer and/or stromal cells, whereas programmed cell death (PD)-1 expression was observed in CD4+ tumor-infil-trating lymphocytes in the presence of PD-L1+ TAMs [9]. In OTSCC as well as in human papilloma virus-positive oropharyngeal carcinomas [11], congregates of PD1/PD-L1 expressing cells are observed at the periphery of the cancer nests in immune-excluded tumors [9]. This pattern is presumed to result from inductive expression of PD-L1 by cancer and/or stromal cells caused by IFN-γ secreted by T cells upon encounter with cancer cells at the tumor margins as a dynamic functional barrier to T cell infiltration. It can be hypothesized that the TReg II cells described by Wang et al. [3] may also explain observations in head and neck cancers [9,11]. Specifically, chemokine secretion in parallel with checkpoint upregulation in response to IFN-γ secretion by tumor antigen-specific T cells may result in the accumulation of different subtypes of immune cells in the TME, and that by targeting the TReg population one can overcome the immune-excluded phenotype. Studies on crosstalk between CD8+ T cells and myeloid cells to assess immune responsiveness are actively being explored [12]; however, the work of Wang et al. brings forward the concept of whether TReg II cells and TAM crosstalk may be at the crux of changing the immune landscape. Finally, several common and distinctive markers have been suggested that connect or distinguish intra-tumoral and circulating TReg cells (Table 1). These will need to be validated by future studies in other tumor types.
Table 1.
Comparative Markers between Peripheral TReg II Cells and Intratumoral TReg Cells
| Shared markers | |
|---|---|
| Increased expression | Decreased expression |
| CD25 | LAG 3 |
| CD39 | CCR2 |
| CTLA4 | CCR10 |
| TIGIT | CXCR3 |
| ICOS | |
| OX40 | |
| GITR | |
| HLA-DR | |
| Helios | |
| CCR8 | |
| TCR clonal overlap | |
| Distinct marker expression by circulating TReg II cells | |
| IL-2RG | |
| pSTAT | |
| PD1 | |
| TIM3 | |
| CCR4 | |
| CCR5 | |
| CXCR6 | |
Acknowledgments
We would like to thank Kayla Roche for generating the illustration.
References
- 1.Turan T et al. (2018) Immune oncology, immune responsiveness and the theory of everything. J. Immunother. Cancer 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedognetti D et al. (2019) Toward a comprehensive view of cance immune responsiveness: a synopsis from the SITC workshop. J. Immunother. Cancer 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L et al. (2019) Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat. Immunol 20, 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang B et al. (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci. Rep 5, 15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendry S et al. (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group. Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv. Anat. Pathol 24, 311–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kather JN et al. (2018) Topography of cancer-associated immune cells in human solid tumors. Elife 7, e36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang E et al. (2008) The immunologic constant of rejection. Trends Immunol 29, 256–262 [DOI] [PubMed] [Google Scholar]
- 8.Cesano A et al. (2020) Status of immune oncology: challenges and opportunities. Methods Mol. Biol 2055, 3–21 [DOI] [PubMed] [Google Scholar]
- 9.Mattox AK et al. (2017) PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4+ TILs in the presence of PD-L1+ TAMs. Cancer Res 77, 6365–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karim R et al. (2009) Tumor-expressed B7–H1 and B7–DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin. Cancer Res 15, 6341–6347 [DOI] [PubMed] [Google Scholar]
- 11.Lyford-Pike S et al. (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 73, 1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garris CS et al. (2018) Successful anti-PD-1 cancer immunotherapy requires cell–dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity 49, 1148–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]


