Abstract
Immune‐related adverse events (irAEs) are induced by immune checkpoint inhibitors (ICIs) which are administered for many cancers. There are many irAEs such as endocrine abnormalities, interstitial lung disease, and colitis. However, irAEs associated with type 2 (T2) inflammation are less known. We herein report a 71‐year‐old woman who developed eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis (ECRS) simultaneously during combination therapy with nivolumab and ipilimumab for renal cell carcinoma. After two cycles of therapy, she developed cough and nasal congestion with high level of fractioned exhaled nitric oxide and blood eosinophil count, and nasal polyps with eosinophil infiltration in bilateral nasal cavities. She was diagnosed with eosinophilic airway inflammation and ECRS, and treated with corticosteroid inhalation, steroid nasal spray, and nasal irrigation, resulting in symptom reduction. Although they are relatively rare irAEs of ICIs, clinicians should consider these diseases associated with T2 inflammation and treat appropriately.
Keywords: Cytotoxic T‐lymphocyte‐associated antigen‐4, eosinophilic chronic rhinosinusitis, immune checkpoint inhibitor, immune‐related adverse event, programmed cell death‐1
There are many immune‐related adverse events (irAEs) induced by immune checkpoint inhibitors; however, irAEs associated with type 2 inflammation are less known. We report a case of eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis simultaneously during combination therapy with nivolumab and ipilimumab for renal cell carcinoma.
Introduction
Immune checkpoint inhibitors (ICIs) are anti‐cancer drugs that are used for many cancers. Although multiple immune‐related adverse events (irAEs) have been reported, irAEs associated with type 2 (T2) inflammation are relatively rare. We herein report the case of a patient with a clinical diagnosis of eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis (ECRS) simultaneously during combination of nivolumab and ipilimumab for renal cell carcinoma.
Case Report
A 71‐year‐old woman presented to the hospital with a two‐week history of cough and nasal congestion. Since two months previously, she had been treated with a combination of nivolumab and ipilimumab as the first‐line treatment for stage IV (T4N0M1) renal cell carcinoma. There were multiple lung metastasis (Fig. 1A). Regimen of drugs consisted of nivolumab 240 mg and ipilimumab 45 mg on day 1; the treatment was repeated every three weeks. After the administration of two cycles, infiltrates appeared on both the upper lobes on chest computed tomography (CT), suggesting drug‐induced interstitial lung disease (Fig. 1B); following this, the drugs were discontinued. On drug withdrawal, the infiltration disappeared in two weeks (Fig. 1C). However, dry cough and nasal congestion appeared, and CT scan showed the thickness of bronchial wall and mucus plugs (Fig. 1C). She had a history of childhood asthma. However, since childhood, she never experienced respiratory symptoms, including nasal congestion. No medications were used except anti‐cancer drugs, and she had no smoking history. She was afebrile and her respiratory rate was 20/min with 95% O2 saturation in room air. Rhonchi was audible on the bilateral side. There was no skin eruption or neurological findings. The laboratory results were as follows: white blood cells, 6500/μL with 21.9% eosinophils (1423/μL); and C‐reactive protein, 0.09 mg/dL. Her immunoglobulin E (IgE) level was 436 IU/mL. Specific IgE was negative. Myeloperoxidase anti‐neutrophil cytoplasmic antibody (ANCA) and proteinase 3 ANCA were negative. The eosinophil fraction of blood was 7.8% (429/μL) before treatment with ICIs; however, it increased immediately after administration. The eosinophil fraction in the sputum was 12.5% and fractioned exhaled nitric oxide (FENO) was 84 ppb. Spirometric values were as follows: forced expiratory volume in 1 sec (FEV1), 1.50 L (87.7% of predicted); forced vital capacity (FVC), 1.92 L; and FEV1/FVC, 78.1%. In an airway reversibility test, FEV1 changed to 1.52 L (improvement of 1.3%). Although airway reversibility was poor and she could not be diagnosed with asthma, the presence of eosinophilic airway inflammation was suggested from these tests. Moreover, otolaryngological examination revealed nasal polyps with infiltration of eosinophils (more than 70 eosinophils with 400× field of view) in both the nasal cavities and CT showed soft density shading especially in the bilateral ethmoid sinus (Fig. 2). On the basis of these examinations, she was also diagnosed with ECRS, according to the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) score criteria [1]. The JESREC scoring system is well known for the diagnosis of ECRS especially in Japan, consists of bilateral disease sites, nasal polyps, CT findings (soft density shading especially in the ethmoid sinus), eosinophilia in peripheral blood (more than 10%), and infiltration of eosinophils in tissue. After starting fluticasone furoate/vilanterol trifenatate (100/45 μg) for eosinophilic airway inflammation and fluticasone furoate nasal spray and nasal irrigation for ECRS, dry cough and nasal congestion reduced. Her symptoms were controllable; therefore, combination of nivolumab and ipilimumab was resumed and two more cycles were administered; after a total of four cycles, nivolumab was administered every two weeks as maintenance therapy. Even after ICIs were resumed, her symptoms did not worsen. The FENO level decreased to 16 ppb and FEV1 improved to 1.62 L after treatment for eosinophilic airway inflammation and ECRS in two months. Owing to combination therapy, renal cell carcinoma could be controlled.
Figure 1.
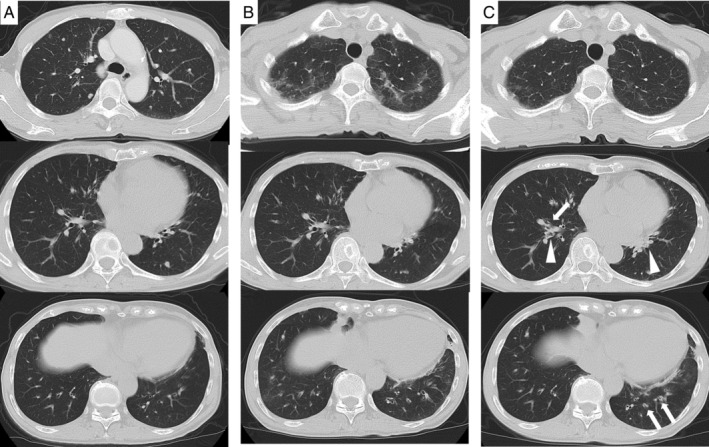
(A) Computed tomography shows multiple minimal lung metastasis before treatment with anti‐cancer drugs. There is no abnormal infiltration in the lung. (B) Consolidation in the upper lung field appeared after two cycles of immune checkpoint inhibitor administration. (C) Lung infiltrations disappeared two weeks after drug withdrawal, while thickness of bronchial wall (arrow) and mucus plugs (arrowhead) appeared especially in the lower lobe.
Figure 2.
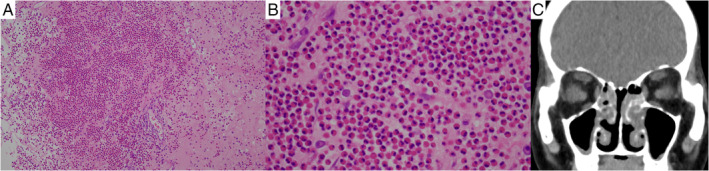
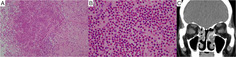
(A) Infiltration of eosinophils with 100× field of view in the nasal polyp tissue (haematoxylin and eosin staining). (B) Infiltration of more than 70 eosinophils with 400× field of view. (C) Computed tomography shows soft density shading especially in the bilateral ethmoid sinus.
Discussion
We herein presented a patient who developed eosinophilic airway inflammation and ECRS simultaneously during ICIs.
Nivolumab is the antibody that acts against programmed cell death‐1 (PD‐1) and ipilimumab acts against cytotoxic T‐lymphocyte‐associated antigen‐4 (CTLA‐4). These drugs are administered for some carcinomas, and combination therapy is currently mainly used for renal cell carcinoma and malignant melanoma. During ICI therapy, some mechanisms are known about disease associated with T2 inflammation. Activated Th2 cells produce interleukin (IL)‐4, IL‐5, and IL‐13 in the lungs and enhance eosinophil recruitment. PD‐1 and the ligand, PD‐L1/PD‐L2, play an important role in not only cancer immunity, but also respiratory T2 inflammation. Matsumoto et al. demonstrated that PD‐L2 is highly expressed on the pulmonary dendritic cells and antibodies against PD‐L2, enhancing the airway hypersensitiveness [2]. PD‐1–PD‐L2 interaction inhibits Th2 cells; therefore, blocking this connection leads to T2 inflammation. The role of CTLA‐4 in T2 inflammation is less known; however, some reports suggest an association [3, 4].
It is improbable that nasal polyps were formed in a short period of time; therefore, they seem to exist before the appearance of symptoms that may be aggravated with the use of ICIs. We are unsure as to why eosinophilic airway inflammation and ECRS occurred simultaneously in the patient; however, it is well established that eosinophilic airway inflammation including asthma, allergic rhinitis, and rhinosinusitis are closely related diseases. Upper and lower respiratory tracts are anatomically continuous and share common defence structures and immune functions [5].
There was a case report of eosinophilic pneumonia associated with T2 inflammation caused by nivolumab [6]. In the present case, bronchoscopy was not performed and consolidation disappeared spontaneously after discontinuation of ICIs; therefore, infiltration of eosinophils into lung tissue was not clear. However, examinations such as bronchial wall thickening on CT scan, high level of FENO, and sputum eosinophil fraction revealed the presence of eosinophilic airway inflammation. The combination of nivolumab and ipilimumab is being used for some cancers, including lung cancer; therefore, clinicians should pay attention to the development of T2 inflammation of the respiratory tract. When they are treated appropriately, continuous ICI treatment can be possible. Further investigations are needed to clarify whether patients with a history of inflammation of respiratory tract, such as childhood asthma, are susceptible to these irAEs.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Watanabe, H , Asada, K , Shirai, T , Torii, H , Yoshimura, K , Kusafuka, K . (2020) Eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis during nivolumab and ipilimumab. Respirology Case Reports, 8(7), e00638 10.1002/rcr2.638
Associate Editor: Bei He
References
- 1. Tokunaga T, Sakashita M, Haruna T, et al. 2015. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 70:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsumoto K, Inoue H, Nakano T, et al. 2004. B7‐DC regulates asthmatic response by an IFN‐gamma‐dependent mechanism. J. Immunol. 172:2530–2541. [DOI] [PubMed] [Google Scholar]
- 3. Hellings PW, Vandenberghe P, Kasran A, et al. 2002. Blockade of CTLA‐4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur. J. Immunol. 32:585–594. [DOI] [PubMed] [Google Scholar]
- 4. Tsuyuki S, Tsuyuki J, Einsle K, et al. 1997. Costimulation through B7‐2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J. Exp. Med. 185:1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caimmi D, Marseglia A, Pieri G, et al. 2012. Nose and lungs: one way, one disease. Ital. J. Pediatr. 38:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jodai T, Yoshida C, Sato R, et al. 2019. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti‐PD‐1 immune checkpoint antibody in a lung cancer patient. Immun. Inflamm. Dis. 7:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


