Summary
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spready globally. This report describes the person-to-person transmission of the virus in a hospital setting.
Presentation of case
A 63-year-old man with pneumonia and a 70-year-old man without symptoms were admitted to a tertiary hospital with SARS-CoV-2 infection. Both men were accompanied by their wives, who stayed with their husbands during their hospitalisation. The wives of Patient 1 and Patient 2 tested positive and negative for SARS-CoV-2, respectively. Of the environmental samples tested, 1/21 and 0/25 from the rooms of Patient 1 and Patient 2, respectively, tested positive for SARS-CoV-2. Patient 1’s wife appeared to have acquired infection during her husband’s hospitalisation.
Discussion
The study had several limitations, including methodology inconsistencies. Additionally, a viral culture was not performed to demonstrate the viability of the virus identified in the environmental sample. Finally, the wife of Patient 1 stayed on the Diamond Princess cruise ship for 4 days before being transferred to the hospital and may have been infected on the ship and not while in the hospital.
Conclusion
Our study suggests that airborne transmission of SARS-CoV-2 may be limited. However, owing to the abovementioned limitations, the results should be interpreted with caution.
Keywords: Severe acute respiratory syndrome coronavirus 2, Environment, Contamination, Transmission, Hospital
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has now infected tens of thousands of people in China and has spread rapidly around the globe [1]. The World Health Organization has declared a Public Health Emergency of International Concern, and released interim guidelines on patient management [2]. Community transmission of SARS-CoV-2 is occurring across the globe [[3], [4], [5]]; however, there is still much more to learn about how SARS-CoV-2 spreads [6]. While we took care of SARS-CoV-2-infected patients who were transferred from the Diamond Princess Cruise Ship quarantined at Yokohama Port in Japan, there were 2 occasions where a SARS-CoV-2 polymerase chain reaction (PCR)-negative spouse had to accompany a SARS-CoV-2 PCR-positive patient. We assessed the potential for SARS-CoV-2 transmission from person to person in a hospital setting by means of repeat testing of two patients hospitalised with confirmed SARS-2-CoV infection, their wives, and environmental samples from their rooms.
Methods
Case summaries
The patients had tested positive for SARS-CoV-2 PCR, and had been transferred from the Diamond Princess Cruise Ship to the National Centre for Global Health and Medicine in Tokyo.
Patient 1
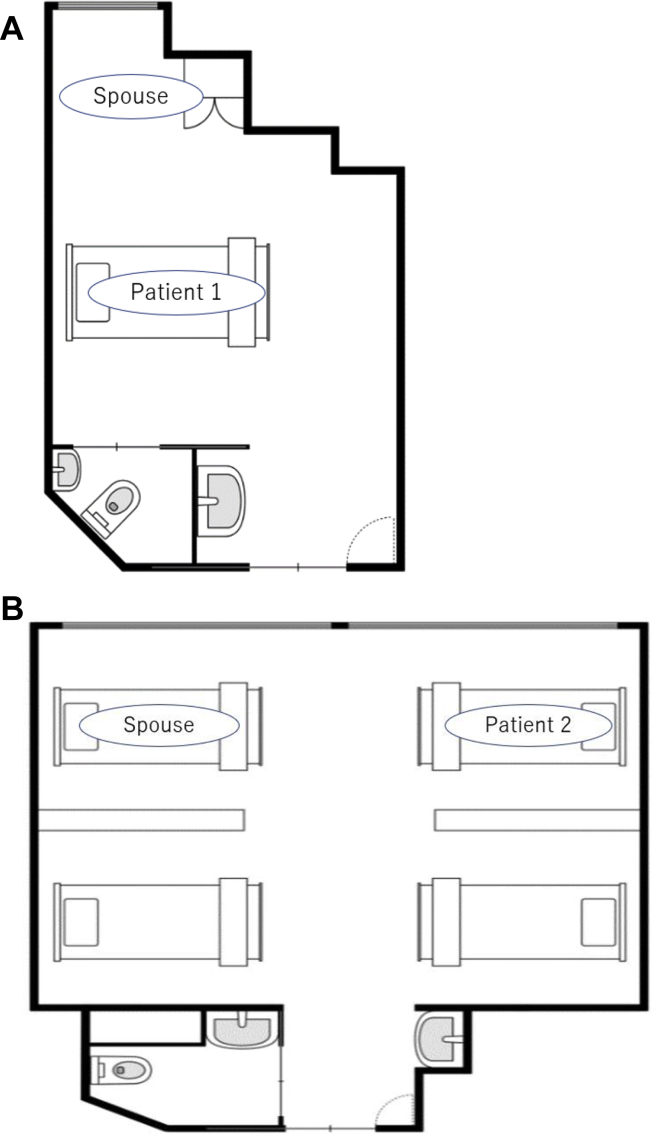
Patient 1 was a 63-year-old man with pneumonia. Sampling of respiratory specimen and environment was conducted on Day 13 from diagnosis when he was on mechanical ventilation. He was admitted to a general ward of our hospital due to SARS-CoV-2 pneumonia on Day 7, and was intubated on Day 10 from diagnosis. He had been staying in a single room in our hospital (Figure 1A), and used the toilet by himself before intubation. His 65-year old wife, who did not have any symptoms and was negative for SARS-CoV-2 before admission, refused to stay apart from her husband. Thus, she stayed with Patient 1 in a single room during the day, and stayed alone in the room next door at night. The healthcare providers advised her to wear a mask at all times and to wash her hands frequently, and did not allow her to provide any direct patient care. She developed mild cough on Day 12 from her husband's diagnosis, and was tested for SARS-CoV-2 on Day 13.
Figure 1.
Plan of the hospital rooms of Patients 1 and 2. (A) A single room where Patient 1 and his spouse stayed; and (B) A large room with 4 beds where Patient 2 and his spouse stayed.
Patient 2
Patient 2 was an asymptomatic 70-year-old man with Alzheimer's disease. His score on the Mini-Mental State Examination during admission was 25/30. He was admitted 3 days after diagnosis. The nasopharyngeal and environmental sampling was done on admission (Day 3). He had been staying in a large room with 4 beds (Figure 1B). He was mobile and used the toilet. His 58-year-old wife, who did not have any symptoms and was negative for SARS-CoV-2 before admission, needed to stay with Patient 2 due to his dementia. The healthcare providers advised Patient 2 and his wife to wear masks at all times and to wash their hands frequently, and did not allow her to provide any direct patient care. She developed mild cough on Day 13 from her husband's diagnosis, and was tested for SARS-CoV-2 on the same day.
The rooms for Patient 1 and Patient 2 were designed to manage patients with tuberculosis, lacked anterooms, and had negative pressure ventilation.
This study was reviewed and approved by the Ethics Committee of the Center Hospital of the National Center for Global Health and Medicine on the condition that a document that declares an opt-out policy by which any possible patient and/or relatives could refuse to be included in this study was uploaded on the Web page of the Center Hospital of the National Center for Global Health and Medicine.
Sample collection
Air was sampled using an MD8 airscan sampling device (Sartorius, Goettingen, Germany) and sterile gelatin filters (80 mm diameter and 3-μm pores; Sartorius). Air was sampled twice at a speed of 50 L/minute for 20 minutes in the negative-pressure room and its associated bathrooms [7]. The filters were dissolved aseptically and stored at −80°C until analysed. Dacron swabs, premoistened with viral transport medium (VTM), were used to swab surfaces aseptically. The following types of surface were swabbed: fomites (including smart phones, masks, stethoscopes, blood pressure cuffs, intubation tube, pillows and TV remote controllers); (2) fixed structures in the rooms and their associated bathrooms (including door knobs, bed guardrails, over tables, a ventilator touch screen, monitoring devices, nurse call buttons, televisions, curtains, toilet seats, and hand soap dispensers); and the ventilation exits on the ceilings in the negative-pressure rooms. Cotton swabs with polystyrene shafts (FB57835) were moistened with VTM and then rubbed across a maximum area of 4 × 5 cm2 in three different directions, applying even pressure. Immediately after sampling, swabs in VTM were put in the refrigerator before being frozen at –80°C. This sampling method had been validated during a previous study [8]. All the environmental samples were collected 6–8 hours after once-daily cleaning and disinfection of the rooms. Surface swabbing was focused especially on surfaces such as ventilator exits and the tops of television sets, which are easily missed during daily cleaning [7].
Laboratory procedures
Respiratory specimen samples, air and environmental swab samples were sent to the National Institute of Infectious Diseases, Tokyo, Japan, for real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing for SARS-CoV-2. Real-time RT-PCR was performed using Quantitect probe one-step RT-PCR kit (Qiagen, Hilden, Germany) with following probe and primer sets: WuhanCoV-N1f 5′-GGCCGCAAATTGCACAAT-3′, WuhanCoV-N1r 5′-CCAATGCGCGACATTCC-3′, and WuhanCoV-N1pr-fam 5′-FAM-CCCCCAGCGCTTCAGCGTTCT-TAMRA-3′ targeting nucleoprotein gene (29175–29235 in MN908947.3) for analysis of respiratory samples; NIID_2019-nCOV_N_F2 5′-AAATTTTGGGGACCAGGAAC-3′, NIID_2019-nCOV_N_R2 5′-TGGCAGCTGTGTAGGTCAAC-3′, and NIID_2019-nCOV_N_P2 5′-FAM--ATGTCGCGCATTGGCATGGA-TAMRA-3′ targeting nucleoprotein gene (29125–29282 in GenBank accession MN908947.3) for environmental samples.
Results
A summary of the two patients' case status; the real-time RT-PCR results of respiratory samples from the two patients and their wives; and the RT-PCR results of environmental samples from the two patients' rooms is shown in Table I. Patient 1's wife tested negative for SARS-CoV-2 on Day 1 and positive on Day 13 after her husband's diagnosis. Patient 2's wife tested negative for SARS-CoV-2 on Days 1 and 13 after her husband's diagnosis. Of the environmental samples tested, 1 of 21, and 0 of 25 samples from the rooms of Patients 1 and 2, respectively, tested positive for SARS-CoV-2.
Table I.
A summary of the patients' case status, real-time RT-PCR results of respiratory and environmental samples in the 2 SARS-CoV-2-infected patients
| Sample source | Patient data |
Environmental data |
|||||
|---|---|---|---|---|---|---|---|
| Case status at time of environmental sampling | Days after diagnosis and real-time RT-PCR∗ results of respiratory samples |
Environmental sampling | Number of positive real-time RT-PCR from samples/total number of samples | Viral load | |||
| Day 1 | Day 6 | Day 13 | |||||
| Patient 1 | Pneumonia on mechanical ventilation | positive | positive (at the time of sampling) | Air Fomites Fixed structure |
0/2 1/6 0/11 |
4.62 x 105/ml | |
| Patient 1's wife | asymptomatic | negative | positive | Ventilation exits | 0/2 | ||
| Patient 2 | asymptomatic | positive | positive (at the time of sampling) | Air Fomites Fixed structure |
0/2 0/7 0/14 |
||
| Patient 2's wife | asymptomatic | negative | negative | Ventilation exits | 0/2 | ||
Real-time RT-PCR, real-time reverse transcription-polymerase chain reaction.
Respiratory samples
Patients 1 and 2 both tested positive for SARS-CoV-2 using real-time RT-PCR. The viral RNA loads were 3.86 x 103 copies/mL and 3.76 x 103 copies/mL, respectively. Patient 1's wife tested positive for SARS-CoV-2 using real-time RT-PCR on Day 7 of her husband's hospitalisation, while Patient 2's wife remained negative on Day 10 of her husband's hospitalisation.
Air samples
To examine the possibility of airborne transmission of SARS-CoV-2, 4 samples air in the patients' rooms were collected and tested for SARS-CoV-2 using real-time RT-PCR. Both rooms were occupied by the patients during the sampling. Intubation was performed for patient 1 three days before the sampling. Patient 2 was asymptomatic when the sampling was conducted. All air samples were negative for SARS-CoV-2.
Environmental samples
Of the 42 environmental samples collected from fomites, fixed structures in the rooms and the attached bathrooms, and the ventilation exits on the ceilings of the negative-pressure rooms, only the intubation tube of Patient 1 tested positive for SARS-CoV-2 (viral RNA load: 4.62 x 105 copies/swab).
Discussion
One of the most important findings of this study is that of 46 environmental samples tested, SARS-CoV-2 was detected from only one sample: Patient 1's intubation tube. This suggests that there was minimal environmental contamination by SARS-CoV-2. This finding is in direct contrast to that of another study conducted in Singapore, which found extensive environmental contamination of the space occupied by non-intubated patients with mild SARS-CoV-2 upper respiratory tract infection [9]. This suggests that intubation prevented environmental contamination, and that the environment of asymptomatic patients does not become contaminated. For more comprehensive infection prevention and control, further studies are required to further explore the risk of environmental contamination in other clinical settings, including non-intubated patients with severe respiratory symptoms; patients on mechanical ventilators; patients treated with extra-corporeal membrane oxygenation.
The second important finding is that during the period in which Patient 1 was receiving mechanical ventilation, his wife tested positive for SARS-CoV-2, while Patient 2's wife remained negative during her husband's hospitalization. At the time of sampling of Patient 1's environment, SARS-CoV-2 was not detected from the environmental surfaces that his wife was likely to touch, such as smart phone, mask, TV remote controller, door knobs, bed guardrails, over tables, nurse call buttons, curtains, toilet seats, and hand soap dispensers. However, the environmental sampling was done 3 days after Patient 1's intubation, so the possibility of environmental contamination prior to his intubation cannot be determined. Therefore, route of transmission from Patient 1 to his wife is unclear.
The third important finding was that air samples and the surface samples of the ventilation exits were negative for SARS-CoV-2 though aerosol-generating procedures such as suctioning and intubation were performed in Patient 1's room. This implied that the aerosol-generating procedures did not always create a risk of airborne transmission. There is evidence supporting aerosol/airborne transmission of Middle East respiratory syndrome coronavirus and the severe acute respiratory syndrome coronavirus [7,10]. Further more detailed studies are required to assess the possibility of airborne transmission of SARS-CoV-2 so that appropriate infection prevention and control measures can be implemented in resource-limited settings.
This study had several limitations. First, viral culture was not performed to demonstrate viability of the virus identified in the environmental sample. Second, due to operational limitations during an outbreak response, the methodology was inconsistent in terms of timing of respiratory and environmental sampling. Lastly, Patient 1's wife, who initially tested negative for SARS-CoV-2, stayed in the Diamond Princess Cruise Ship for 4 days before transferring to our hospital. Therefore, there were three possibilities for it. The first one was that she was infected with SARS-CoV-2 in the last 4 days of her stay in the ship, and not while she was staying in the hospital. The second one was that both husband and wife were infected with SARS-CoV-2 with a longer incubation period in the wife. The last one was that a single negative throat swab did not exclude the disease, due to low sensitivity, so the wife's initial test may have been a false negative result.
Financial support
This study was supported in part by a Grant-in Aid from the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP19fk0108104 and JP19fk0108110.
Conflicts of interest
All authors report no potential conflicts.
Credit author statement
Shinichiro Morioka: Methodology, Formal analysis, Data Curation, Writing - Original Draft, Project administration. Keiji Nakamura: Methodology, Shun Iida: Investigation, Resources, Writing - Review & Editing. Satoshi Kutsuna: Conceptualization. Noriko Kinoshita: Tetsuya Suzuki: Tadaki Suzuki: Investigation, Resources, Funding acquisition. Kei Yamamoto: Resources. Kayoko Hayakawa: Writing - Review & Editing. Sho Saito: Writing - Review & Editing, Funding acquisition. Norio Ohmagari: Supervision.
Acknowledgements
None.
References
- 1.Paules C.I., Marston H.D., Fauci A.S. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. New Eng J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. New Eng J Med. 2020 doi: 10.1056/nejmc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caribbean Public Health Agency . 2020. Outbreak of 2019 novel coronavirus (2019-nCoV) in wuhan, China. Situation report – No. 1. 22 january 2020. [Google Scholar]
- 7.Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H. Extensive Viable Middle East Respiratory Syndrome (MERS) Coronavirus Contamination in Air and Surrounding Environment in MERS Isolation Wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killingley B., Greatorex J., Cauchemez S., Enstone J.E., Curran M., Read R.C. Virus shedding and environmental deposition of novel A (H1N1) pandemic influenza virus: interim findings. Health Technol Assess. 2010;14:237–254. doi: 10.3310/hta14460-04. [DOI] [PubMed] [Google Scholar]
- 9.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New Eng J Med. 2004;350:1731–1739. doi: 10.1056/nejmoa032867. [DOI] [PubMed] [Google Scholar]